Abstract
BACKGROUND & AIMS
Low serum levels of vitamin D have been associated with Crohn’s disease (CD). However, it is unclear whether low vitamin D levels cause CD or CD reduces serum vitamin D.
METHODS
United States military personnel with CD (n [ 240) and randomly selected individuals without CD (controls, n [ 240) were matched by age, sex, race, military branch, and geography. We measured 25-hydroxyvitamin D in sera 8–3 years (pre-2) and 3 years to 3 months before diagnosis (pre-1) and 3 months before through 21 months after diagnosis (pre-0). We genotyped VDR and GC vitamin D related polymorphisms. We used conditional logistic regression, including adjustments for smoking, season, enlistment status, and deployment, to estimate relative odds of CD according to vitamin D levels and interactions between genetic factors and levels of vitamin D.
RESULTS
Levels of vitamin D before diagnosis were not associated with CD in pre-2 (P trend = .65) or pre-1 samples (P trend = .84). However, we found an inverse correlation between CD and highest tertile of vitamin D level in post-diagnosis samples (P trend = .01; odds ratio, 0.51; 95% CI, 0.30–0.86). Interactions were not detected between vitamin D levels and VDR or GC polymorphisms. We observed an association between VDR Taq1 polymorphism and CD (independent of vitamin D) (P = .02).
CONCLUSIONS
In serum samples from military personnel with CD and matched controls, we found no evidence for an association between CD and vitamin D levels up to 8 years before diagnosis. However, we observed an inverse-association between post-diagnosis vitamin D levels and CD. These findings suggest that low vitamin D does not contribute to development of CD—instead, CD leads to low vitamin D.
Keywords: Inflammatory Bowel Disease, IBD, Nutrition, Genetics, Autoimmune, Epidemiology, Etiology, Reverse Causation
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract with poorly defined etiologies. Epidemiologic studies have shown greater incidence in higher latitudes with less sunlight exposure (and less natural synthesis of vitamin D) than in lower latitudes.1 These observations led to the hypothesis that low vitamin D increases CD risk, supported by multiple reports that 25-hydroxyvitamin D (25[OH]D; heretofore also referred to as vitamin D) concentrations are lower among CD patients versus controls. However, the converse may also be true, where CD itself might result in lower vitamin D, a concept known as reverse causation.
To overcome limitations of reverse causation, longitudinal vitamin D concentrations need to be measured both before and after CD onset. An analysis of the Nurses’ Health Study (NHS) found higher prediagnosis 25[OH]D levels associated with lower CD risk2; however, vitamin D levels were not measured but estimated on the basis of physical activity, diet, and other surrogate measures of vitamin D status. The relationship between vitamin D and CD measured directly, both before and after diagnosis, remains unstudied. Our objective was to clarify whether low vitamin D is a cause of CD or if low vitamin D occurs after disease development by directly measuring 25(OH) D levels in sera collected before, in the period of, and within 21 months after CD diagnosis or corresponding time points in matched controls. We also evaluated whether vitamin D and CD associations may depend on interaction with vitamin D–related gene polymorphisms.
Methods
Study Population
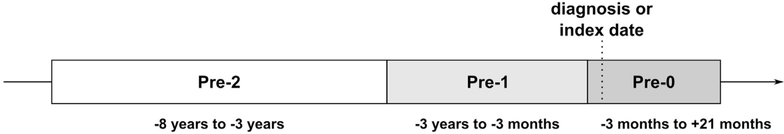
The source population included US military personnel who served between 1998 and 2011. We began with an evaluation of 400 individuals with at least 2 International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes for CD (555) and no codes for ulcerative colitis (UC) (ICD-9-CM 556). Controls were randomly selected from the same population but without a diagnosis of CD, UC, or nonspecific intestinal inflammation (ICD-9-CM 558). Subjects were individually matched for age, sex, race, service branch, and geography at the time of CD diagnosis (for cases) or index time (for controls). Incidence density sampling was used to match cases and controls. The diagnosis times for cases and corresponding index time for controls were matched within a year from each other. Three sera specimens were obtained from the Department of Defense Serum Repository (DoDSR) at separate time points leading up to CD diagnosis or index time. Time points were 3–8 years before diagnosis or index time (pre-2); 3 months to 3 years before diagnosis or index time (pre-1); or 3 months before and up to 21 months after diagnosis or index time (pre-0) (Figure 1). Samples 3 months before diagnosis were included in pre-0 because Walter Reed National Military Medical Center (WRNMMC) inflammatory bowel disease gastroenterologists asserted it would be highly unusual to have a diagnosis of CD within 3 months of symptom onset; widening pre-0 made the pre-1 time period more of a “prediagnostic” period with fewer samples from CD patients who already developed clinical symptoms awaiting diagnostic confirmation. Smoking status was identified by ICD-9 code.3 Data on deployment status and timing (quarter) of serum collection were provided by the DoDSR. Further timing granularity was not available because of security restrictions.
Figure 1.
Time points for collected serum samples. Three serum specimens were obtained for each participant. Time points were 3 to 8 years before diagnosis or index date (pre-2), 3 months to 3 years before diagnosis or index date (pre-1), and 3 months before up to 21 months after diagnosis or index date (pre-0).
After completion of vitamin D measurements on the 2400 serum samples from the 400 matched case-control pairs, we received permission for the WRNMMC inflammatory bowel disease specialists to perform chart review for 300 cases and 100 controls, of which 284 had available medical records. A more specific case definition was developed from the chart review, and this case definition was applied to the remaining 116 non–chart-reviewed cases.
Vitamin D Measurements
Serum 25(OH)D concentrations were measured by using DiaSorin 25-Hydroxyvitamin D 125I radioimmunoassay kit (Saluggia, Italy). All 6 sera samples from each case-control pair were distributed randomly on the same plate to minimize batch effects. Laboratory personnel were blinded to sample case-control status and time points. To assess sample reliability, we assayed 20 duplicates placed on consecutive plates.
Genotyping
DNA was purified from sera by using QIAGEN QIAmp DNA Kit (Germantown, MD). Primers and TaqMan probes were designed for rs731236 (TaqI site of vitamin D receptor [VDR]) and rs2282679 (vitamin D binding protein [DBP] or group specific component [GC]). Genotyping was by TaqMan method on an Applied Biosystems (Foster City, CA) 7900HT (and validated using 10 sequenced controls).
Statistical Analyses
Vitamin D levels were categorized by using predefined vitamin D thresholds as vitamin D sufficiency (≥30 ng/mL), insufficiency (20–30 ng/mL), and deficiency (≤20 ng/mL).4 Conditional logistic regression was used to estimate the relative odds of CD according to vitamin D levels when comparing cases and controls at each time point. In addition to matching factors, regression models were adjusted for rank (enlisted or officer), education at enlistment, smoking status (ever or never), deployment before diagnosis or index time, and quarter of serum collection. Sensitivity analyses evaluated the association between vitamin D and CD, where levels were categorized as tertiles or quintiles, per distribution of 25(OH)D concentrations among controls. Genetic associations with CD were evaluated by χ2 test for minor alleles or carrier genotypes. Gene-environment interactions were evaluated by carrier or wild-type genotypes (using the binary gene categories) and vitamin D levels coded as tertiles; the wild-type genotype at the highest vitamin D tertile was used as the references. Missing 25(OH)D data were rare (<1%), and the case-control pair was excluded from the analysis for that time period. Statistical significance was defined as two-tailed α of less than 0.05. All analyses were performed by using Stata MP 13.1 (College Station, TX).
Ethics and Study Subject Anonymity
This study was approved by the Institutional Review Boards of Johns Hopkins University and WRNMMC and by the DoDSR.
Results
We initially evaluated 390 matched case-control pairs for which we had vitamin D measurements at all 3 time points (Supplementary Table 1). Greater 25(OH)D concentrations were observed among white (n = 312) vs black (n = 35) controls at all time points (pre-2: 33.1 vs. 22.1 ng/mL, P < .01; pre-1: 32.1 vs. 23.0 ng/mL, P < .01; pre-0: 33.1 vs. 24.0 ng/mL, P < .01).5 Low mean absolute differences in 25(OH)D concentrations between duplicates placed on separate plates (2.7 ± 2.0 ng/mL; range, 0.3–7.5; excluding one clear outlier [difference 41.7 ng/mL]) indicated excellent assay reliability. Interassay coefficient of variation was 7.9%, comparable with 10%–13% in a similar study.5
Vitamin D Levels and Crohn’s Disease
When categorizing 25(OH)D concentrations as predefined vitamin D thresholds for pre-0, vitamin D sufficiency relative to deficiency was associated with significantly lower odds (odds ratio [OR], 0.62; 95% confidence interval [CI], 0.38–1.00) of being a case (Supplementary Table 1). A similar but nonsignificant association was observed for insufficiency relative to deficiency (OR, 0.62; 95% CI, 0.38–1.03). P trend for these predefined thresholds was .10. There was no protective effect of higher levels in pre-1 or pre-2 periods (P trend .80 and .96, respectively). Vitamin D levels examined as equally distributed tertiles showed an inverse CD and vitamin D association at pre-0 (highest vs lowest tertile: OR, 0.62; 95% CI, 0.41–0.93; P trend = .02). However, again no associations were observed for pre-1 or pre-2. Parallel results were observed when vitamin D levels were examined as equally distributed quintiles.
High-Specificity Study Population
Chart review of 284 cases confirmed CD in 196 (positive predictive value [PPV], 69%). When selecting cases with 3 CD diagnostic encounters and requiring these encounters come from a gastroenterologist or general surgeon, PPV was 84% (sensitivity, 71%; specificity, 86%). We therefore performed our remaining evaluations on the combined set of 196 chart-confirmed cases plus 44 cases from the non–chart-reviewed set that met the higher PPV definition. PPV estimated for all 240 cases was 97%. No chart-reviewed controls had evidence of CD or UC. The a priori power was estimated to be 0.87 on the basis of the original assumptions that 50% of controls had 25(OH)D concentrations ≥30 ng/mL (actual, 48%),6 vitamin D sufficiency–associated CD risk was 0.55,2 and the correlation coefficient was ±0.1 (actual, –0.09).
A description of these 240 case-control pairs is provided in Table 1. Mean age at diagnosis/index time was 28.2 ± 6.2 years (range, 20–51). Most participants were male (83.8%) and white (81.7%). There were more smokers among cases (44.2%) than controls (35.4%) (P = .05). There were fewer cases (61.2%) than controls (72.9%) ever deployed overseas by diagnosis or index time point (P < .01). Mean 25(OH)D concentrations among controls were 31.6 ng/mL, 31.4 ng/mL, and 32.4 ng/mL in pre-2, pre-1, and pre-0, respectively.
Table 1.
Patient Characteristics at Time of Diagnosis or Index Time
| Characteristic | Cases (n = 240) | Controls (n = 240) | P value |
|---|---|---|---|
| Age, mean y (SD)a | 28.2 (6.2) | 28.3 (6.2) | |
| Sex (%) | 1.00 | ||
| Male | 201 (83.8) | 201 (83.8) | |
| Female | 39 (16.2) | 39 (16.2) | |
| Race (%) | 1.00 | ||
| White | 196 (81.7) | 196 (81.7) | |
| Black | 22 (9.2) | 22 (9.2) | |
| Other | 22 (9.2) | 22 (9.2) | |
| Rank (%) | .80 | ||
| Enlisted | 202 (84.2) | 200 (83.3) | |
| Officer | 38 (15.8) | 40 (16.7) | |
| Highest degree (%) | .93 | ||
| High school | 194 (80.8) | 191 (79.6) | |
| Bachelor’s | 29 (12.1) | 29 (12.1) | |
| Graduate school | 9 (3.8) | 12 (5.0) | |
| Other | 8 (3.3) | 8 (3.3) | |
| Ever smokers (%) | .05 | ||
| No | 134 (55.8) | 155 (64.6) | |
| Yes | 106 (44.2) | 85 (35.4) | |
| Ever deployed? (%) | <.01 | ||
| No | 93 (38.8) | 65 (27.1) | |
| Yes | 147 (61.2) | 175 (72.9) |
SD, standard deviation.
Age presented as mean.
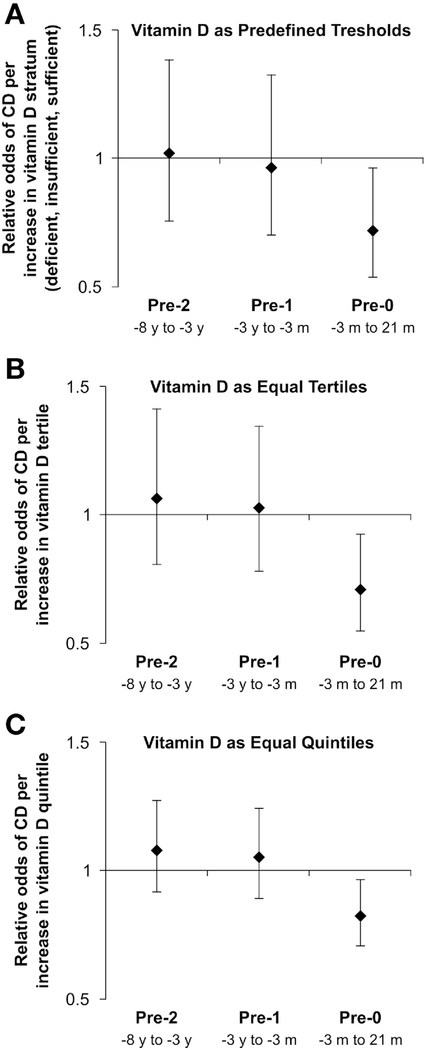
In this set of 240 case-control pairs with 97% PPV, again at pre-0, vitamin D sufficiency relative to insufficiency was inversely and significantly associated with CD (OR, 0.53; 95% CI, 0.28–0.999) (Table 2). P trend at pre-0 for these more restrictive cases was significant (P trend = .03). No associations were observed for pre-2 (P trend = .90) or pre-1 (P trend = .81). When categorizing 25(OH)D levels as tertiles or quintiles, results were similar (Table 2). As tertiles, vitamin D levels were not associated with CD in pre-2 (P trend = .65) and pre-1 (P trend = .84). However, vitamin D levels were inversely associated with CD in pre-0 (P trend = .01). Compared with the lowest vitamin D tertile (5.84–27.57 ng/mL), the highest tertile (35.61–71.96 ng/mL) had significantly lower odds of CD (OR, 0.51; 95% CI, 0.30–0.86). As quintiles, vitamin D levels were not associated with CD in pre-2 (P trend = .37) and pre-1 (P trend = .55), whereas vitamin D levels were inversely associated with CD in pre-0 (P trend = .02). Compared with the lowest quintile (5.84–23.25 ng/mL), the highest quintile (39.20–71.96 ng/mL) also had significantly lower CD odds (OR, 0.48; 95% CI, 0.24–0.96). Relative odds of CD per increase in vitamin D pre-defined stratum, vitamin D tertile, or vitamin D quintile showed parallel results with significance likewise observed only for Pre-0 data in all analyses (Figure 2). Sensitivity analyses for pre-0 excluding 56 case-control pairs with serum samples for cases collected in the 3 months before diagnosis generated similar results (Supplementary Table 2).
Table 2.
Vitamin D Concentrations and Relative Odds of Crohn’s Disease at Each Time Point
| Serum Pre-2 |
Serum Pre-1 |
Serum Pre-0 |
|
|---|---|---|---|
| OR (95% CI)a | OR (95% CI)a | OR (95% CI)a | |
| Predefined thresholds | |||
| Deficiency (≤20 ng/mL) | 1.0 | 1.0 | 1.0 |
| Insufficiency (21–29 ng/mL) | 0.67 (0.32–1.37) | 0.90 (0.46–1.76) | 0.79 (0.42–1.51) |
| Sufficiency (≥30 ng/mL) | 0.88 (0.45–1.71) | 0.90 (0.46–1.77) | 0.53 (0.28–0.999) |
| P trend | .90 | .81 | .03 |
| Equal tertile distribution | |||
| 5.84–27.57 ng/mL | 1.0 | 1.0 | 1.0 |
| 27.58–35.60 ng/mL | 0.81 (0.50–1.33) | 0.90 (0.56–1.46) | 0.67 (0.41–1.09) |
| 35.61–71.96 ng/mL | 1.13 (0.64–2.00) | 1.06 (0.61–1.82) | 0.51 (0.30–0.86) |
| P trend | .65 | .84 | .01 |
| Equal quintile distribution | |||
| 5.84–23.25 ng/mL | 1.0 | 1.0 | 1.0 |
| 23.26–29.09 ng/mL | 0.66 (0.35–1.27) | 1.32 (0.71–2.44) | 0.76 (0.43–1.36) |
| 29.10–34.10 ng/mL | 0.80 (0.41–1.53) | 1.16 (0.60–2.23) | 0.70 (0.38–1.30) |
| 34.11–39.19 ng/mL | 1.01 (0.50–2.04) | 0.89 (0.45–1.78) | 0.48 (0.25–0.92) |
| 39.20–71.96 ng/mL | 1.16 (0.55–2.45) | 1.51 (0.73–3.15) | 0.48 (0.24–0.96) |
| P trend | .37 | .55 | .02 |
CI, confidence interval; OR, adjusted conditional odds ratio.
Cases and controls were matched by age, sex, race, service branch, and geography at the index time. Models were also adjusted for rank, highest degree achieved, smoking, deployment history, and quarter of serum collection.
Figure 2.
Vitamin D levels and relative odds of Crohn’s disease (CD) at each time point. When vitamin D levels were stratified according to predefined thresholds (A), equally distributed tertiles (B), or quintiles (C), vitamin D was not associated with CD at pre-2 and pre-1 time points. Levels were inversely associated with CD at pre-0. Error bars indicate 95% confidence intervals.
Gene–Vitamin D Interactions
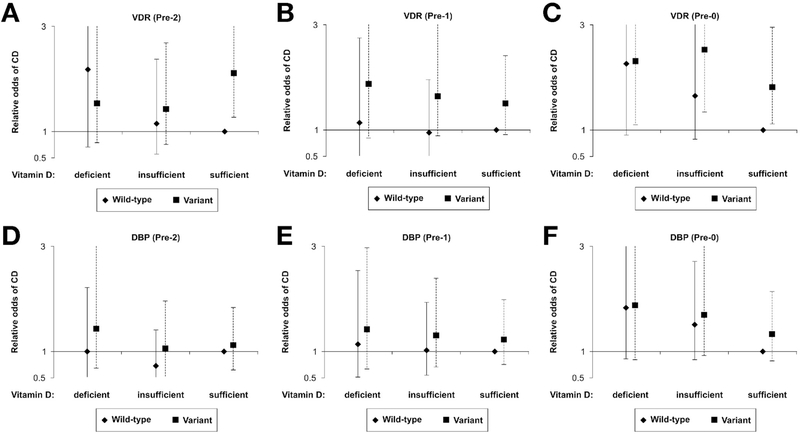
We evaluated gene–vitamin D interactions only for the 240 high-specificity case-control pairs. The genotype completion rate was 99.0%. TaqI VDR polymorphism was significantly increased in cases vs controls (P = .02 for C allele or carrier; Table 3). However, there was no difference in distributions of DBP alleles between cases and controls. We found no evidence of interactions between predefined vitamin D levels (deficient, insufficient, sufficient) and either vitamin D–related genetic polymorphisms (Figure 3). Vitamin D levels, stratified by VDR or DBP variant carrier status, were not significantly associated with CD in pre-2 or pre-1 (with the exception of variant VDR genotypes and sufficient vitamin D levels in pre-2). For pre-0 samples, those with variant VDR genotypes were associated with CD at all vitamin D levels (deficient: OR, 2.30; 95% CI, 1.10–4.81; insufficient: OR, 2.53; 95% CI, 1.35–4.75; sufficient: OR, 1.81; 95% CI, 1.12–2.95) compared with those with VDR wild-type and sufficient vitamin D levels. DBP carrier status did not appear to have any association with CD.
Table 3.
Gene Polymorphisms in N Crohn’s Disease Cases and N Matched Controls
|
VDR |
DBP |
|
|---|---|---|
| Variant | Taql (T → C) | rs2282679 (A → C) |
| Case | ||
| WW | 80 | 126 |
| MW | 125 | 94 |
| MM | 34 | 19 |
| Carrier (%) | 66.5 | 47.3 |
| MAF | 0.40 | 0.28 |
| Control | ||
| WW | 106 | 138 |
| MW | 93 | 78 |
| MM | 37 | 20 |
| Carrier (%) | 55.1 | 41.5 |
| MAF | 0.35 | 0.25 |
| P-AF | .02 | .36 |
| Carrier risk | ||
| OR of Crohn’s disease (95% CI) | 1.57 (1.09–2.27) | 1.25 (0.87–1.80) |
| P carrier | .02 | .23 |
CI, confidence interval; DBP, vitamin D binding protein; MAF, minor allele frequency; MM, minor allele homozygote; MW, heterozygote; OR, conditional odds ratio; P-AF, P value of allele frequency distribution; VDR, vitamin D receptor; WW, wild-type genotype.
Figure 3.
VDR and DBP polymorphisms and relative odds of Crohn’s disease (CD) across vitamin D tertiles. Relative odds of CD were compared between cases and controls at predefined vitamin D levels using the wild-type in those who have sufficient vitamin D levels as the reference. (A and B) In the pre-2 and pre-1 periods, the odds of CD remained similar across vitamin D levels for both VDR wild-type and variant genotypes, except for those with variant VDR genotypes and sufficient vitamin D levels in pre-2. (C) In the pre-0 period, the odds of CD were higher for those with VDR variant (P < .02) genotypes. (D–F) The odds of CD remained similar across vitamin D levels for both DBP wild-type and variant genotypes. Error bars indicate 95% confidence intervals. DBP, vitamin D binding protein; VDR, vitamin D receptor.
Discussion
This study directly measured prediagnosis and postdiagnosis vitamin D concentrations and evaluated their longitudinal relationships with CD risk. Using a highly matched case-control population, we found that prediagnosis vitamin D levels were not associated with CD. By contrast, CD was associated with lower postdiagnosis vitamin D levels. These findings were consistently observed when stratifying vitamin D levels as standard thresholds (deficiency, insufficiency, and sufficiency), equally distributed tertiles, and quintiles. These data suggest that vitamin D is not a risk factor in CD pathogenesis, although the presence of CD contributes to lower vitamin D levels.
Our observation that vitamin D is not a risk factor for later development of CD has important implications for CD pathogenesis. Vitamin D deficiency was initially proposed as a risk factor for CD primarily to explain consistently greater CD incidence in higher latitude populations. If lower vitamin D is not the cause of this association, other environmental factors must be present. Chief among them would be the “cold chain” hypothesis that suggests certain bacteria that grow better in colder environments, notably Yersinia and Listeria, may give rise to CD.7 Conversely, potentially protective parasite exposures are more frequent in more temperate climates. Dietary differences (eg, more fruits, vegetables, and fiber) may also play a role. Our findings suggest that all of these factors should be examined more intensely to explain the correlation between latitude and CD incidence.
Although our finding that vitamin D levels in pre-0 were significantly lower among CD cases was expected from previous studies, it is nonetheless important. This is the only study to examine vitamin D in case-control pairs within 21 months of diagnosis, whereas other studies would normally include patients several years after diagnosis. These findings are consistent with current assumptions that CD is associated with lower vitamin D levels in patients after disease onset8 and affirm that when CD is clinically established, it is important to evaluate and correct vitamin D insufficiency. Low vitamin D post diagnosis may stem from dietary alterations (eg, reduced fortified milk consumption), reduced sunlight exposure (eg, decreased outdoor activity from illness and sunlight avoidance while on immunomodulators), and intestinal malabsorption. Conceivably, low vitamin D may not lie along the causal pathway of pathogenesis but is influenced by inflammation.9 Pappa et al10 observed that only 26% of children administered high-dose vitamin D (ie, 2.7 times age-recommended levels) maintained vitamin D sufficiency (>20 ng/mL) throughout the year, suggesting that CD or inflammation results in lower vitamin D.
Our study is consistent with the 2 other studies that directly measured vitamin D before CD diagnosis. In a case-control study nested within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, Opstelten et al11 found no difference in the risk of CD when comparing quartiles or predefined categories (deficiency, insufficiency, sufficiency) of 25(OH)D concentrations in 72 cases who developed CD on average 4.7 years later versus 144 matched controls. In a Danish cohort, Lund-Nielsen et al12 found no difference in the cumulative incidence of CD over 30 years when comparing baseline 25(OH)D concentrations among 58 cases that developed CD during follow-up as compared with 35,468 persons at risk, and they did not observe evidence for a CD risk associated with genetically predicted vitamin D levels or allelic predicted increase in vitamin D risk among 2 case-control populations (using Mendelian randomization). Altogether, these findings and ours (the largest case cohort directly measuring vitamin D and the only one measuring vitamin D both before and after diagnosis), the only studies to directly evaluate vitamin D before diagnosis, make a prediagnostic effect of vitamin D even less likely.
Because the effect of vitamin D is influenced by its binding to DBP and interaction with VDR, we evaluated 25(OH)D levels stratified by polymorphisms known to influence DBP and VDR activities. There was no evidence of vitamin D interactions with these polymorphisms on CD risk, and significant risk was only observed in pre-0 among participants subgrouped by carrier status. Interestingly, in the genetic analyses alone, we found an association between the TaqI site in VDR with CD. This relationship was previously described in European and Chinese CD cohorts, but less strongly among those from other racial and ethnic backgrounds.13–16 TaqI association with CD may benefit from further evaluation in larger cohorts. We chose to evaluate the rs2282679 variant because it was associated with vitamin D deficiency in genome-wide analyses.17 However, rs2282679 was not associated with CD in our case-control cohort. Although its lack of association might be seen as additional supportive evidence against innate influence of vitamin D on CD risk, our study size was not designed to render such a conclusion.
There were some limitations of our study. First, although subjects were matched geographically at diagnosis or index time and we adjusted for prediagnostic deployments, there was lack of other factors that can affect vitamin D status such as dietary patterns or vitamin D supplementation. However, regardless of vitamin D source, the resulting 25(OH)D concentration is the critical variable to measure to directly evaluate the relationship between 25(OH)D and CD incidence. Although unknown and unmeasured confounders causing unexpectedly higher vitamin D levels in cases relative to controls before diagnosis could potentially exist, this was unlikely for several reasons. Our study was carefully matched on multiple parameters by design. The inclusion or exclusion of smoking, deployment status, and time of serum collection in the multivariable models did not alter the results, demonstrating the robustness of the primary analysis. Body mass index and physical activity were not measured; however, greater body mass index and reduced physical activity, both proposed as CD risk factors, would lower vitamin D in cases prediagnostically rather than normalize it. Statistically, on the basis of the “E-value,”18 an unmeasured confounder would theoretically need a 2.3-fold greater effect (OR, ≥2.3) on vitamin D concentrations in cases than controls to overcome the negative findings in pre-2 and pre-1. Most importantly, because we indeed did observe a significant association with CD at and after diagnosis, any confounder would have had to exert an effect only before diagnosis, which would be quite unusual.
Second, because of limited sera availability, 25(OH)D levels were measured as single measurements. Duplicate samples on different plates showed only minor variability, and all case-control pair sera were assayed together in sets of 6 to optimize reliability and remove potential batch effects from paired analyses. When comparing 25(OH)D levels between black and white controls, blacks expectedly maintained a mean difference of –10.3 to –9.2 ng/mL across time points. Our measurements were also consistent with concentrations found in a multiple sclerosis study using a similar military cohort.6 Third, because of the general composition of military personnel, there was a male predominance in our study population, which may affect the generalizability of our findings. Because the analysis of the NHS suggesting an inverse association between vitamin D levels and risk of CD only included women, it is possible that different findings may relate to gender or age differences. The mean age at diagnosis for the NHS was 64.0 years, an atypical age for CD onset, whereas ours was 28.2 years, similar to that reported in multiple population-based studies.19 Finally, although the null findings of our study could theoretically have been limited by insufficient power, we had twice the sample size of CD patients than the NHS.2 Comparatively, our study had an a priori power of 0.87 to test prediagnosis vitamin D association with CD using the risks observed in the NHS. Furthermore, unlike all of the pre-0 data, which consistently demonstrated an inverse gradient relationship between vitamin D levels and CD, the point OR estimates for both the tertile and quartile analyses for pre-1 and pre-2 samples appeared random relative to increasing vitamin D levels, with the highest strata nonprotective.
In summary, this prospective case-control study is thus far the most direct and comprehensive evaluation of the relationship between longitudinal 25(OH)D levels and CD incidence. We found no association between prediagnosis vitamin D and CD, thus calling into question prior assessments that vitamin D deficiency may lead to the development of CD. We nonetheless found an association between CD and postdiagnosis vitamin D, suggesting that the directionality of effect may instead involve CD lowering vitamin D concentrations. Our study also has clinical implications; vitamin D supplementation to prevent future development of CD would not be recommended, because it may unlikely provide the desired effects and vitamin D supplementation is not completely risk-free.20,21 In contrast, our observation of lower vitamin D levels within the first 21 months of diagnosis demonstrates that at CD onset it is important to measure and correct vitamin D levels to reduce the potential untoward effects of vitamin D deficiency.
Supplementary Material
What You Need to Know.
Background
Low serum levels of vitamin D have been reported to increase the risk of Crohn’s disease (CD). However, it is unclear whether low levels of vitamin D cause CD or CD reduces serum levels of vitamin D.
Findings
In an analysis of serum samples from 240 military personnel with CD and matched controls, we found no evidence for an association between CD and level of vitamin D up to 8 years before diagnosis CD. However, we did observe an inverse association between postdiagnosis level of vitamin D and CD. These findings suggest that low vitamin D does not contribute to development of CD; instead, CD leads to low vitamin D.
Implications for patient care
Vitamin D supplementation is not likely to prevent CD. However, patients with CD should be monitored for low levels of vitamin D, which appear to be a result of the disease.
Acknowledgments
Funding
Supported by an American College of Gastroenterology Clinical Research Award (BNL) and an IBD Working Group GI Fellows Research Award (BNL). These funds were used to cover the costs of reagents, assays, and statistical software. This study was also partly supported by the Congressionally Directed Medical Research Programs grant #PR110833 (SRB). These funds were used to purchase the serum samples and cover in part pipetting expenses, data management, and a portion of research efforts of 2 investigators (SMH, SRB). No funds were used for the study design or reporting.
Abbreviations used in this paper
- CD
Crohn’s disease
- CI
confidence interval
- DBP
vitamin D binding protein
- DoDSR
Department of Defense Serum Repository
- 25[OH]D
25-hydroxyvitamin D
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- NHS
Nurses’ Health Study
- OR
odds ratio
- PPV
positive predictive value
- UC
ulcerative colitis
- VDR
vitamin D receptor
- WRNMMC
Walter Reed National Military Medical Center
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2019.09.034.
References
- 1.Limketkai BN, Bechtold ML, Nguyen DL. Vitamin D and the pathogenesis of inflammatory bowel disease. Curr Gastroenterol Rep 2016;18:52. [DOI] [PubMed] [Google Scholar]
- 2.Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology 2012;142:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiley LK, Shah A, Xu H, et al. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc 2013;20:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281. [DOI] [PubMed] [Google Scholar]
- 5.Middleton JP, Bhagavathula AP, Gaye B, et al. Vitamin D status and bone mineral density in African American children with Crohn disease. J Pediatr Gastroenterol Nutr 2013;57:587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006; 296:2832–2838. [DOI] [PubMed] [Google Scholar]
- 7.Hugot JP, Alberti C, Berrebi D, et al. Crohn’s disease: the cold chain hypothesis. Lancet 2003;362:2012–2015. [DOI] [PubMed] [Google Scholar]
- 8.Farraye FA, Nimitphong H, Stucchi A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis 2011;17:2116–2121. [DOI] [PubMed] [Google Scholar]
- 9.Autier P, Boniol M, Pizot C, et al. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol 2014;2:76–89. [DOI] [PubMed] [Google Scholar]
- 10.Pappa HM, Mitchell PD, Jiang H, et al. Maintenance of optimal vitamin D status in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing two regimens. J Clin Endocrinol Metab 2014;99:3408–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opstelten JL, Chan SSM, Hart AR, et al. Prediagnostic serum vitamin D levels and the risk of Crohn’s disease and ulcerative colitis in European populations: a nested case-control study. Inflamm Bowel Dis 2018;24:633–640. [DOI] [PubMed] [Google Scholar]
- 12.Lund-Nielsen J, Vedel-Krogh S, Kobylecki CJ, et al. Vitamin D and inflammatory bowel disease: Mendelian randomization analyses in the Copenhagen studies and UK Biobank. J Clin Endocrinol Metab 2018;103:3267–3277. [DOI] [PubMed] [Google Scholar]
- 13.Simmons JD, Mullighan C, Welsh KI, et al. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut 2000;47:211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naderi N, Farnood A, Habibi M, et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J Gastroenterol Hepatol 2008; 23:1816–1822. [DOI] [PubMed] [Google Scholar]
- 15.Dresner-Pollak R, Ackerman Z, Eliakim R, et al. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genet Test 2004; 8:417–420. [DOI] [PubMed] [Google Scholar]
- 16.Xia SL, Lin XX, Guo MD, et al. Association of vitamin D receptor gene polymorphisms and serum 25-hydroxyvitamin D levels with Crohn’s disease in Chinese patients. J Gastroenterol Hepatol 2016;31:795–801. [DOI] [PubMed] [Google Scholar]
- 17.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017; 167:268–274. [DOI] [PubMed] [Google Scholar]
- 19.Hovde O, Moum BA. Epidemiology and clinical course of Crohn’s disease: results from observational studies. World J Gastroenterol 2012;18:1723–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korgavkar K, Xiong M, Weinstock MA. Review: higher vitamin D status and supplementation may be associated with risks. Eur J Dermatol 2014;24:428–434. [DOI] [PubMed] [Google Scholar]
- 21.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med 2016; 176:175–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.