Summary
Emerging infectious diseases (EIDs) are recognized as having significant social, economic and ecological costs, threatening human health, food security, wildlife conservation and biodiversity. We review the processes underlying the emergence of infectious disease, focusing on the similarities and differences between conceptual models of disease emergence and biological invasions in general.
Study of the IUCN's list of the world's worst invaders reveals that disease is cited as a driver behind the conservation, medical or economic impact of nearly a quarter of the species on the data base.
The emergence of novel diseases in new host species are, in essence, examples of invasions by parasites. Many of the ecological and anthropogenic drivers of disease emergence and classical invasions are also shared, with environmental change and global transport providing opportunities for the introduction and spread of invaders and novel parasites.
The phases of disease emergence and biological invasions have many parallels; particularly the early and late phases, where demographic and anthropogenic factors are key drivers. However, there are also differences in the intermediate phases, where host–parasite co‐evolution plays a crucial role in determining parasite establishment in novel hosts.
Similar opportunities and constraints on control and management occur at the different phases of invasions and disease emergence. However, exploitation of host immune responses offers additional control opportunities through contact control and vaccination against EIDs. We propose that cross‐fertilization between the disciplines of disease emergence and invasion biology may provide further insights into their prediction, control and management.
Keywords: emerging infectious disease, host jump, host switch, introduced species, invasive species, parasite, parasite‐mediated, world's worst invaders

Short abstract
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2435.2012.02031.x/suppinfo
Introduction
There is increasing awareness of the need to consider the diverse roles of parasites at all scales in ecology, from immune interactions within hosts (Tompkins et al. 2011) through ecological interactions (Hatcher, Dick & Dunn 2006) to impacts on community structure (Hudson et al. 2002; Thomas, Renaud & Guegan 2005) and ecosystem function (Hatcher, Dick & Dunn 2012). Parasites are often key players in invasions; they may mediate the outcome and impact of invasions or may themselves be invasive species (Dunn 2009; Tompkins et al. 2011). Examination of the IUCN list of the 100 World's Worst Invasive Alien Species illustrates the importance of parasitic disease in invasion processes (Table 1). Disease is cited as a driver behind the economic, medical or conservation impact for 24 species on this list. Eight species listed are parasites; for example, rinderpest (caused by a morbillivirus) led to loss of domestic cattle as well as declines in wild ungulates in Africa, whilst banana bunchytop virus causes loss of crops and the death of infected plants. Four species are intermediate hosts/vectors for parasitic diseases; for example, the tiger mosquito Aedes albopictus is a vector for malaria, West Nile virus and dengue fever, whilst the whitefly Bemisia tabaci transmits several viruses affecting crop plants. Finally, for 12 species, capacity to act as disease reservoirs is cited either as the major impact or as one of several influences on invaded communities. For example, the American bullfrog (Rana catesbiana) is a reservoir for Batrachochytrium dendrobatidis (this parasite is itself on the list) which causes chytrid disease, one of the drivers of global declines in amphibian abundance and diversity, whilst the invasive grey squirrel (Sciurus carolinensis) is causing local extinction of the European red squirrel (Sciurus vulgaris), through competition and transmission of a pox virus that is lethal to the native species.
Table 1.
Species from the IUCN list of 100 of the World's Worst Invasive Species for which disease is a driver behind the impact of the invasion
| Species | Impact | Description | Jumped ship? |
|---|---|---|---|
| Parasites | |||
| Aphanomyces astaci, fungus, causes crayfish plague | A,B | In Europe, the invasive signal crayfish acts as a reservoir for A. astaci. Outbreaks of the disease have caused economic losses to fish farms and have led to extinction of populations of endangered noble and white clawed crayfish in Europe | J |
| Babuvirus, banana bunchy top virus | A | Spread through agriculture and by aphids, causes economic loss of banana crops and plants | ? |
| Batrachochytrium dendrobatidis, fungus, causes chytrid disease | B | First reported in 1998. The fungus has been spread through amphibian trade and is spread locally by the invasive American bullfrog (see below). The fungus is a key factor in the decline in amphibian populations and extinction of at least one species | J |
| Cryphonectria parasitica, fungus, causes chestnut blight | B | Introduced to N. America from Japan in 1900s by nursery trade and is spread locally by windborne spores. Outbreaks led to loss of chestnuts (the dominant overstorey species), changing the forest landscape and the wildlife it supports. | J |
| Ophiostoma ulmi, fungus, causes Dutch elm disease | B | Probably native to Asia, introduced to America, Europe and New Zealand, transmitted by bark beetles and tree grafts. Death of elms (a dominant overstorey species) in Europe had cascading effects on biodiversity | J |
| Phytophthora cinnamomi, cinnamon fungus | A B | Native to S.E. Asia, introduced to Europe, N. America and Australasia via agriculture, forestry and the nursery trade. Locally spread by spore dispersal. It has led to declines in forestry and fruit production and to declines in native woody perennials including several endangered species | J |
| Plasmodium relicta, protist, causes avian malaria | B | Native to Africa, bird introductions brought malaria to Hawaii, Europe and N. America. The disease played a role in the extinction of approximately half of the endemic bird species in Hawaii | J |
| Morbillivirus, causes rinderpest | A B | Loss of cattle, famine. Transmission from domestic cattle led to declines in wild ungulates. As a result of global vaccination programme, rinderpest was eradicated in 2010 | J |
| Reservoirs | |||
| Oncorhynchus mykiss, rainbow trout | B | Native to N. America, widespread introduction for sport and aquaculture. Rainbow trout is a reservoir for whirling disease (caused by the myxozoan Myxobolus cerebralis) causing outbreaks in wild fish* | J |
| Sciurus carolinensis, grey squirrel | B | Native to N. America, introduced to N. Europe and S. Africa. The grey squirrel is a reservoir for squirrel poxvirus and its invasion has led to local extinction of the native red squirrel (Sciurus vulgaris) | J |
| Lithobates catesbeianus (Rana catesbeiana), American bullfrog | B | Widespread introductions for food and insect biocontrol. The bullfrog is a reservoir for chytrid disease (B. dendrobatidis, see above), outbreaks of which have caused widespread amphibian declines | J |
| Rattus rattus, black rat | H | Native to India, now widespread through global transport. The black rat is a reservoir for the bacteria Leptospirosis (which causes Weil's disease) and Yersinia pestis (which causes bubonic plague) and has been implicated in recent plague outbreaks in Madagascar* | J |
| Pueraria montana var. lobata, kudzu vine | A | Native to Asia, invasive in N. America. Kudzu is a reservoir for soybean rust and Phytophthora species* | J |
| Sturnus vulgaris, starling | B H | Native range Africa, Asia and Europe, anthropogenic introductions world‐wide. The starling is a reservoir for a number of diseases including Plasmodium relicta (avian malaria, see above) and Chlamydophila psittaci which causes psittacosis and can infect humans | J |
| Trichosurus vulpecula, brush tail possum | A | Native range Australia, introduced to New Zealand. The possum is a reservoir for Mycobacterium bovis which causes bovine tuberculosis, affecting cattle and deer farming* | J |
| Euglandina rosea, rosy wolf snail | H | Introduced as a biocontrol agent for the giant African land snail Achatina fulica) on Indian and Pacific islands. This snail can act as an intermediate host for the rat lungworm Angiostrongylus cantonensis, which can cause eosinophilic meningitis in humans* | J |
| Trachemys scripta elegans, red eared slider turtle | H | Native to N. America, introduced to Europe by pet trade. Slider turtles may transmit Salmonella to humans* | J |
| Sus scrofa, feral pig | A H | Feral pigs may transmit a number of diseases that affect farmed animals (including M. bovis, which causes bovine tuberculosis, and Aphtae epizooticae, which causes foot and mouth disease) and diseases of humans (including Leptospirosis which causes Weil's disease)* | J |
| Gambusia affinis, mosquito fish | B | Introduced from N. America to many regions as predatory biocontrol for mosquitoes. Mosquito fish may act as a reservoir for helminths* | J |
| Herpestes auropunctatus, small Indian mongoose | B H | Introduced to S. America and several islands to control rats and snakes. The mongoose is a reservoir for parasites of wildlife and humans including Lyssavirus (rabies) and Leptospira (Weil's disease)* | J |
| Macaca fascicularis, crab eating macaque | H | Native to South‐east Asia, introduced into Mauritius, Palau Hong Kong and Indonesia. The macaque can act as a reservoir for human diseases including Macacine herpesvirus 1 (B virus) and Lyssavirus (rabies)* | J |
| Vectors | |||
| Aedes albopictus, tiger mosquito | H | Anthropogenic spread, for example, via tyre trade, range increase with climate change. A potential vector for many parasites of humans including Flavivirus spp. (including west Nile virus and Dengue fever) and Dirofilaria immitis (causing filariasis) | G |
| Anopheles quadrimaculatus, mosquito | B H | Native to N. America, linked to disease outbreaks in America. Vector for malaria (Plasmodium falciparum, Plasmodium vivax and Plasmodium malariae) and west Nile virus in the United States. Although malaria was eradicated in the 1950s, A. quadrimaculatus is responsible for occasional outbreaks in the United States | GJ |
| Bemisia tabaci, whitefly | A | Spread by transport of infected plant products. Local spread following establishment. Vector for numerous plant viruses leading to economic losses of agricultural crops including cassava | GJ |
| Habitat modifier | |||
| Lantana camara, lantana shrub | H | Originated in tropics in the old world, introduced world‐wide as an ornamental plant. Lantana provides habitat for disease vectors including tsetse fly and mosquitoes* | G |
The list was compiled by the IUCN Invasive Species Specialist Group (http://www.issg.org/database/species/search.asp?st=100ss), and species were selected on the basis of either their impact on biodiversity or human activities, and their illustration of issues surrounding invasions. The list includes 24 species that either cause parasitic disease or are reservoirs or vectors for parasites. In some cases, the major impact of the invader is through parasitic disease, for others, disease acts in concert with other effects (e.g. competition/predation). These diseases may affect human health (H), human activities such as agriculture, livestock, forestry (A), and biodiversity (B). For those examples marked with *, the main impact of the invasion is through the predatory or competitive impact of the invader on native species, with disease transmission cited as a secondary factor. The majority of the diseases have emerged as a result of spill over to novel host species (J‐ jumped ship), whilst others have emerged in new geographical areas/host populations, but have not jumped to new host species (G).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Other papers in this special issue examine the role of parasites in biological invasions of (or concerning) hosts, considering the effects such as enemy release and the impact of parasites on native/invader interactions. In this paper, we focus on scenarios where parasites themselves are the invaders: in this context, the invading species is an emerging infectious disease (EID). The World Health Organization defines an emerging disease as one that has appeared in a population for the first time, or that may have existed previously but is rapidly increasing in incidence or geographical range (http://www.who.int/topics/emerging_diseases/en/, accessed on 15.5.2012). Emerging diseases are an important consideration for community ecologists, for several reasons (Hatcher & Dunn 2011). Some EIDs are regarded as significant threats for the long‐term conservation of species. For instance, chytrid disease is listed as the main cause of extinction for at least one amphibian species and is a serious threat for many others, causing particularly sudden and extreme population collapses (e.g. Vredenburg et al. 2010). EIDs that move between wildlife and farmed species may have significant economic costs and can result in control procedures that are at odds with the aims of wildlife conservation (Mathews 2009). Even EIDs of humans (the majority of which are zoonotic in origin; Woolhouse, Haydon & Antia 2005) have a community ecology component if they involve parasites of more than one host species, with prediction and control requiring knowledge of the ecological interactions within host communities.
Emerging diseases can have potentially global impacts socially, ecologically and economically, for public health, conservation, veterinary science and agriculture. For example, the cost to the UK of preparedness and response to the 2009 swine flu pandemic was estimated at £1·2 billion (http://www.dhsspsni.gov.uk/the2009influenzapandemic_acc.pdf). The economic and social costs of the Nipah virus outbreak in Malaysia in 1998–1999 included >100 deaths, $35 million in compensation, evacuation of homes and 36 000 job losses (http://www.fao.org/DOCREP/005/AC449E/ac449e04.htm). Attempts to control bovine tuberculosis (caused by Mycobacterium bovis) in the UK by culling badgers (Meles meles), regarded as the chief wildlife reservoir for this disease, are projected to cost >£1 bn over the next decade, yet these programs have been ineffective or indeed counter‐productive, because movements of remaining badgers can lead to spread of the disease to new subpopulations (Macdonald & Laurenson 2006; Mathews 2009).
There is a strong perception that the frequency of emergence is increasing as a result of increasing rates of environmental change (e.g. Childs, Richt & Mackenzie 2007; Plowright et al. 2008). For instance, Jones et al. (2008) identify 335 human EID first records between 1940 and 2004 (including novel antibiotic resistant strains of bacteria and opportunistic infections associated with the pandemic spread of HIV), and Dobson & Foufopoulos (2001) identify 31 wildlife EIDs in North America over a 2‐year period. Analyses of the literature have also identified increases in the frequency of EIDs in both marine and freshwater ecosystems (reviewed in Rohr et al. 2011; Okamura & Feist 2011). Similarly, increasing rates of biological invasions have been linked to environmental change (Walther et al. 2009), often as a result of human activity, which brings together novel combinations of species. In the case of parasites, this can result in exposure to putative novel host species to which the parasite may become adapted, resulting in an (evolutionary) host jump (Woolhouse, Haydon & Antia 2005). Although in general many EIDs are of the endemic but currently increasing variety (discussed in Okamura & Feist 2011), the majority of those that have obtained ‘World's Worst’ status are ‘novel’ parasites, those that have ‘jumped ship’ to novel host species (Table 1). We examine EIDs in the framework of biological invasions, using examples from wildlife and human EIDs to look for parallels and differences between these processes. We examine the successive phases of disease emergence and ask whether parallels with the processes identified in invasion biology can inform our understanding of disease emergence and provide insight into control and management approaches. We focus on EIDs that have switched hosts; however, as many other EIDs are the result of classical biological invasions (by the parasites or their hosts) of the kind usually considered by invasion biologists, comparison of approaches should be of broad relevance to EID and invasion biology.
Invasion and emergence: parallel definitions and processes
There has been much debate on the terminology surrounding ‘invasive species’ (Collauti & MacIsaac 2004; Davis 2009), particularly as to whether ‘invasive’ implies the existence of measurable impact (itself an anthropocentric concept; Ricciardi & Cohen 2007). Here, we take the view that an invasive species (or more correctly an invasive population of a species; Collauti & MacIsaac 2004) is one that is introduced (transported), becomes established, spreads and in doing so causes impact in the recipient location. This is in line with several frameworks for invasion (e.g. Kolar & Lodge 2001; Collauti & MacIsaac 2004; Blackburn et al. 2011). EIDs, as a result of their increasing incidence and impact on host fitness, are thus an important subset of biological invaders.
From an ecological perspective, the processes of disease emergence and biological invasion share many similarities. For biological invasions, four distinct phases have been suggested (Fig. 1): translocation, introduction, establishment and invasive spread (Kolar & Lodge 2001). There are variations and extensions of this basic framework (e.g. Blackburn et al. 2011), but all recognize the four phases represented in Fig. 1. This bears a close resemblance to some models of disease emergence where multiple host species are implicated (Childs, Richt & Mackenzie 2007; Hatcher & Dunn 2011), where the phases of contact, spillover, local persistence and pandemic spread map onto the sequence for invasions. Other classifications of EIDs emphasize evolutionary (as opposed to demographic) aspects (e.g. Wolfe, Dunavan & Diamond 2007); although the terminology differs, parallels are still evident (Fig. 1). As we explore in this review, these conceptually similar phases are based upon similar underlying processes such as propagule pressure (see phase I), sampling loss (Phases II and III) and boom and bust (phase III). However, evolutionary factors in particular differ between the phases and thus not all aspects of disease emergence map exactly within the frameworks of biological invasion.
Figure 1.
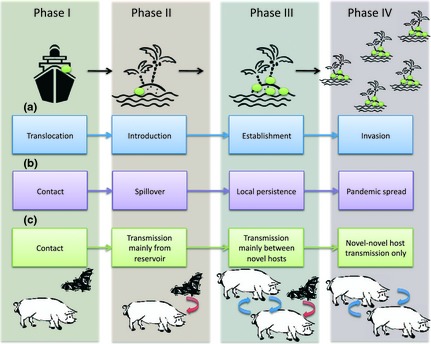
Phases of invasion and emergence. Arrow‐linked boxes show the sequence of processes involved in (a) biological invasion (e.g. Kolar & Lodge 2001); (b,c) emerging infectious diseases (EID): (b) the demographic perspective (e.g. Childs, Richt & Mackenzie 2007; Hatcher & Dunn 2011), (c) the evolutionary perspective (e.g. Wolfe, Dunavan & Diamond 2007). Vertically, the aligned phases (as shaded) correspond; we will refer to these as Phases I to IV. Transition from one phase to the next is influenced by different factors; these factors are to some extent common to both invasions and EIDs, and an examination of parallels may shed light on potential control opportunities. Phase 1: contact between reservoir and novel hosts may be frequent; it may arise or be increased as a result of introduction of a new reservoir host; contact rate may increase as a result of environmental change; contact rate may be enhanced through parasite manipulation of host behaviour. Phase 2: spillover/introduction is affected by rate of bombardment with the novel parasite (propagule pressure), the suitability of the novel host or habitat (i.e. evolutionary considerations, such as local adaptation, and environmental characteristics, such as climate suitability). Phase 3: establishment/local persistence/novel intraspecific transmission; for EIDs, this requires that each infected host of the novel species generates more than one secondary case of infection (i.e. R 0 for the parasite >1). This will be affected by evolutionary factors (local adaptation; host or habitat competency) and also depends on (host) population or habitat structure. Phase 4: pandemic spread/invasion/novel intraspecific‐only transmission. For EIDs, this also requires R 0 > 1, but the distribution of R 0 also matters (skewed distributions with superspreaders can lead to more rapid spread); successful transition also depends on parasite (or host) evolution; for instance adaptation to the novel host/environment. Pandemic emergence is strongly linked to global transport and travel.
Phase I contact/translocation
A prerequisite for all biological invasions is that the potential invader comes into contact with a novel habitat, not previously part of its range, where it may potentially become established. In the case of an EID that has switched host species, the novel habitat is the new host species. In invasion terminology, this part of the process is commonly referred to as translocation, reflecting the frequent influence of third parties (often man) in bringing this contact about (Blackburn et al. 2011). For instance, the North American Great lakes had been invaded by at least 182 species by 2005, with 65% of all invasions in these lakes attributable to ballast water translocation (Ricciardi 2006). For parasites, the routes to translocation may be more complex. First, parasites may be introduced to a new host through movement of the original host into the range of the new host (e.g. co‐introduction of the grey squirrel and squirrel poxvirus in England; Gurnell et al. 2006). Secondly, parasites may be introduced as free‐living or environmentally resistant stages (e.g. transportation of crayfish plague spores on fishing gear (Alderman 1996). Finally, novel hosts, when introduced to a new range, may come into contact with parasites endemic to that range; this might be an increasingly important route for acquiring EIDs as a result of increased human activities in pristine environments (Dunn 2009).
Environmental change, much of it anthropogenic, is an important factor creating opportunities for novel contacts and translocations (Peeler & Feist 2011). For EIDs, land use changes (such as encroachment of farming; see Fig. 2 for example), transport systems and trade in wildlife are frequently involved. For instance, longitudinal mapping studies suggest chytrid disease has been translocated within and between continents by human transport (Lips et al. 2008), and timber trading is thought to have introduced Chestnut blight (Cryphonectria parasitica) to the US and Dutch elm disease (Ophiostoma ulmi and O. novo‐ulmi) to the UK (Loo 2009). Combined transport and climate mapping have revealed a close association between the world‐wide distribution of the tiger mosquito and traffic volumes to ports with favourable climatic conditions (Tatem, Hay & Rogers 2006); as climate conditions change, further range expansion of this species and other important disease vectors are anticipated (Table 1; Rohr et al. 2011).
Figure 2.
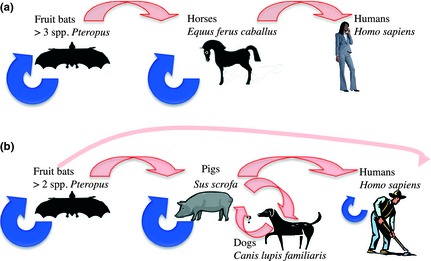
Spillover of two human emerging infectious diseases (EIDs) involving multiple host species. (a) Hendra and (b) Nipah viruses have spilled over into humans from domestic animal reservoirs (horses and pigs respectively), which themselves became infected via spillover from fruit bats (Pteropus spp); pale arrows depict interspecific (including spillover) transmission; dark arrows depict intraspecific transmission (including human intraspecific transmission in the case of Nipah virus). In both cases, increasing contact with bats as a result of farm encroachment and human settlement combined with range expansion of bats fuelled by local habitat destruction are the suspected ultimate causes. Control for both focuses on reducing the contact between the bat reservoir species and domestic stock, for instance, by siting orchards (which attract bats) away from farms. Additional transmission routes for Nipah virus require further control strategies, including education about the risks associated with raw fruit where bats have fed, and rigorous farm and hospital hygiene measures (reviewed in Field, Mackenzie & Daszak 2007).
These patterns have parallels with the facilitation of invasive species by environmental and climate change. For example, degradation of water quality of large rivers such as the Rhine facilitated multiple waves of invasions, and global warming has facilitated many animal and plant invasions (Walther et al. 2009). Enhanced connectivity (via increased shipping, canal building and irrigation) increases translocation of invaders, and disturbance (e.g. from agriculture or human settlement) correlates with numbers of establishing invaders (reviewed in Lockwood, Hoopes & Marchetti 2007). One problem, however, is establishing precise cause and effect, as disturbance and agriculture are themselves correlated with human movements and the construction of transport routes. Similarly, it is difficult to generalize about the exact drivers of disease emergence, with most studies reviewing evidence on a case‐by‐case basis (Childs, Richt & Mackenzie 2007; Plowright et al. 2008); unravelling cause and effect would allow more effective control or mitigation of EIDs and invaders.
Phase II spillover/introduction
The contact/translocation phase can transition to the next phase, spillover (in EID terminology), or introduction (the term used in invasion biology). Spillover is dependent on contact/translocation, but does not necessarily follow from it. Other things being equal, spillover is more likely in associations where contact with a novel host is more frequent (Childs, Richt & Mackenzie 2007), but evolutionary factors are also important (see below). In invasion biology, the likelihood of introduction success depends upon propagule pressure, which has two key elements: propagule size (the number or ‘dose’ of individuals released) and propagule number (the number of distinct release events; Lockwood, Cassey & Blackburn 2005). These concepts can be applied to EIDs in the process of switching to novel hosts: propagule size broadly equates to the initial parasite dose on spillover to novel hosts, and propagule number to the number of spillover events. A further element, propagule ‘health’ (the condition of the individuals released; Lockwood, Cassey & Blackburn 2005; Lockwood, Hoopes & Marchetti 2007), is not obviously paralleled in EIDs. In invasion biology, propagule pressure (or elements of this) has been shown to be critical in determining the probability of introduction and establishment phases (reviewed in Lockwood, Cassey & Blackburn 2005; Lockwood, Hoopes & Marchetti 2007). For example, Marchetti, Moyle & Levine (2004) show that spread of fish invaders is best predicted by propagule pressure. Control or mitigation of this second phase, for both introductions and EIDs, focuses on strategies for decreasing the elements of propagule pressure (Table 2).
Table 2.
Opportunities for the control of invasive species and EIDs, with representative examples. Many of the control strategies apply to more than one phase. Options for the control of both EIDs and invaders decrease through the phases
| Phase | Invasion control | EID control and selected examples |
|---|---|---|
| I Introduction/translocation |
Transport control, for example, ballast water exchange/treatment (Ricciardi 2006) Fumigation/sanitary measures, for example, using synthetic pyrethroids in aircraft, methyl bromide and phosphine in cargo containers |
Transport control, for example, reduce/educate on contact limitation and hygiene measures (e.g. human simian contact, Wolfe et al. 2007); vector control (see invasion control for this phase) |
| II Contact/spillover |
Quarantine, import/export regulations/rapid response protocols, for example, GB Non‐native Species Secretariat; http://https//secure.fera.gov.uk/nonnativespecies/ |
Reduce reservoir populations and cross‐species contact (e.g. vaccinating reservoir hosts, culling grey squirrels to limit pox transmission) |
| III Establishment/Local persistence | Culling, trapping, eradication, biocontrol. Sterile male release, immunocontraception (inducing Allee effects; Taylor & Hastings 2005). Control corridors/manage individual invaded/native subpopulations (Chades et al. 2011) |
Contact control (within new species), for example, condoms to limit HIV transmission. Local eradication through infected host culling (e.g. culling pigs to limit Nipah virus spread). Local eradication through quarantine and isolation (SARS), hygiene, vaccination (e.g. bovine TB vaccination for cattle; Mathews 2009). Control corridors/manage individual infected or uninfected subpopulations (Chades et al. 2011) |
| IV Invasion/Pandemic spread |
Selective culling at pinch points Habitat management (e.g. limit corridors), for example, grey squirrels (Gurnell et al. 2006) |
Control movement at bottlenecks in dispersal (e.g. airport screening during SARS epidemic; cull grey squirrels in habitat corridors). Selective vaccination at population corridors International quarantine, hygiene, isolation, for example, cattle movement only if bovine TB free |
EID, emerging infectious disease; SARS, Severe Acute Respiratory Syndrome.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The degree of host specificity of a parasite can influence the effectiveness of spillover to new hosts (phase II) and transition to effective intraspecific transmission in the novel host (phase III). Reviews of EIDs have found that microparasites with indirect transmission (including vector borne parasites) frequently jump to new hosts (Woolhouse, Haydon & Antia 2005). Such parasites are likely to encounter a range of potential hosts, so a generalist strategy should maximize opportunities for transmission. For intracelluar parasites such as rabies, the use of cell receptors that are conserved across host species may increase the ability to switch to new hosts (Woolhouse, Haydon & Antia 2005). Unfortunately, the drive to find species traits (such as generalist vs. specialists in resource utilization) as reliable predictors of invasive species has been less successful (Davis 2009). Success in invasion is dependent on the integration of species traits (such as high reproductive output), the environmental context (disturbance, for example) and propagule pressure. Add to this the variation in relative importance of such factors across taxonomic groups, and the search for useful trait predictors of invasion success is perhaps a dead‐end. Thus, this may be one area where parallels between classic biological invasions and EIDs are not evident. There are, however, some parallel patterns in the type of habitat (host) most vulnerable to invasion/EIDs. Cox & Lima (2006) demonstrate that islands and freshwaters are more vulnerable to invasive predators as these habitats tend to support prey species that are naïve to functionally different predator ‘archetypes’. In contrast, as continental prey are exposed to all the main predator archetypes, they are less vulnerable to introduced predators. This has parallels with cross‐immunity, where hosts have (partial) immunity to some parasites after infection with related species (Tompkins et al. 2011). For instance, recent outbreaks of canine distemper in East African carnivores may have been exacerbated by the successful control (now eradication) of rinderpest, exposure to which may have provided cross‐immunity to this closely related morbillivirus (reviewed in Thomas, Renaud & Guegan 2005).
Parasite spillover is often a transient phenomenon; spillover events may be very frequent, but the majority, occurring in isolated populations and failing to transition further towards emergence, will remain unnoticed. This is particularly likely in wildlife populations where health monitoring is difficult to perform (Macdonald & Laurenson 2006). For viral diseases, which arguably comprise the majority of EIDs in humans, spillover transience has led to the use of terms such as ‘viral chatter’ (e.g. Wolfe, Dunavan & Diamond 2007). Many human EIDs cluster in the early spillover stage of emergence, with frequent spillover events but no evidence of human–human transmission. For example, antibody seroprevalence for simian retroviruses is high in bushmeat hunters, indicating frequent spillover of different strains, but no evidence of sustained transmission or maintenance in human populations (discussed in Wolfe, Dunavan & Diamond 2007). Sequence data for HIV1 and 2 suggest repeated transmission events into humans before global spread, as well as a complex history of prior spillovers and recombination of simian immunodeficiency virus variants in chimps before spillover into man (Sharp & Hahn 2008). In invasions, the majority of introductions are thought to fail, with only subsets progressing to establishment and other phases. This is sometimes referred to as the Tens Rule (which posits that about 10% of invaders will progress to the next phase from the previous phase; Williamson 1996), but debate over its applicability continues (Lockwood, Hoopes & Marchetti 2007).
A subset of EIDs evolve intraspecific transmission in the new host. Hendra virus (Fig. 2a), spillover of which has occurred on at least 11 occasions in Australia, has no record of human–human transmission. The same was originally true for the related Nipah virus, which first emerged in Malaysia and Singapore (Fig. 2b). However, more recent Nipah outbreaks in Bangladesh and India indicate bat–human and human–human transmission without the involvement of pigs (reviewed in Field, Mackenzie & Daszak 2007). When parasites infect multiple host species and can be transmitted between them, several different host species may act as partial reservoirs, and the parasite is not reliant on a single host population for persistence. The dynamics of spillover (where the reservoir host is the original host species) and spillback (the reservoir is a more recently acquired host; Kelly et al. 2009; Poulin et al. 2011) are important in EIDs of this nature, and control measures must focus on identifying and controlling important reservoir hosts, or controlling contact between reservoir and novel host species. For instance, in the United States, the risk of contracting Lyme disease in humans is a complicated function of wildlife community composition and lagged climatic variables; many species are potential reservoirs for the virus or its tick vector, but vary in their competence to amplify or transmit the virus or vector (Ostfeld et al. 2006). High densities of domestic stock can provide opportunities for disease spillover or spillback to wild animals. For example, domestic birds (novel, but competent hosts) act as a reservoir for spillback of influenza virus which originated in wild birds (Woolhouse, Haydon & Antia 2005). Conversely, bovine tuberculosis, which probably originated in cattle, has spilled over into a number of wildlife species that now act as reservoirs for its spillback into cattle (for instance, in the UK, badgers M. meles; in New Zealand, the brush tail possum Trichosurus vulpecula, itself on the World's Worst list). In addition to being invaders themselves, parasite spillover/spillback can also influence native–invader interactions. For example, the invasive signal crayfish (Pasifastacus leniusculus) in the UK acts as a reservoir for Anaphomyces astacus (crayfish plague, Table 1), whilst in Argentina, introduced salmonid fish act as reservoirs for spillback of the acanthocephalan parasite Acanthocephalus tumescens to native fish (Poulin et al. 2011). Other examples are considered elsewhere in this special issue (e.g. Dunn et al. this issue).
Phase III persistence/establishment
Spillover events may result in persistence when the parasite becomes locally established in a novel host population, but has yet to spread to other populations (broadly equivalent to the establishment phase for invasions). For parasites of a single host species, theory predicts that the parasite can spread in a large population of susceptible hosts if, on average, each primary case of infection results in more than one secondary case (the parasite's basic reproductive number, R 0, must exceed 1; Anderson & May 1982). The same criterion must also be met for spread of an emerging disease; however, here R 0 is a composite of transmission within and between multiple host species. Hence, it is possible for parasites to persist despite R 0 in the novel host being <1, if repeated spillover from the reservoir host can make up this shortfall. Parallels with invasion are evident when considering deliberate releases of species in attempts to ‘force’ them into establishment, such as during ‘naturalization’ and biological control projects. For example, exotic species often only persist because of their repeated releases by man (e.g. grass carp Ctenopharyngodon idella in UK), and many biological control agents require multiple releases to be effective (Lockwood, Hoopes & Marchetti 2007). The value of R 0 and hence the likelihood of transition to phase III is influenced by demographic factors (e.g. host population structure, propagule pressure), evolutionary factors (on parasite and host traits) and environmental factors, as discussed below.
Demographic factors
For parasites of a single host species and with density‐dependent transmission, theory predicts a threshold host population density below which R 0 < 1 resulting in (deterministic) extinction of the parasite. This parallels strong Allee effects in invasions where propagule size is insufficient for population establishment (Taylor & Hastings 2005). This result has implications for disease emergence; for instance, ‘crowd diseases’ did not emerge before human populations reached critical sizes following the agricultural revolution (Wolfe, Dunavan & Diamond 2007). In the United States, successful conservation measures in the Greater Yellowstone Area have led to increasing population densities of elk (Cervus elaphus), with the unintended consequence that elk populations are now of sufficient size to maintain brucellosis, which now spills back into cattle and occasionally humans (Cross et al. 2010). Stochastic effects also influence extinction probability; outbreaks in the novel host will often stutter to extinction, even if R 0 > 1, if successive transmission events fail by chance in small populations (May, Gupta & McLean 2001). Such stochastic extinction depends crucially on the number of individuals infected at the point of spillover (the propagule size). Probabilistic models demonstrate that even when R 0 is substantially >1, if the initial propagule of infected individuals is small (<5), the chances of extinction are very high (May, Gupta & McLean 2001). These processes are paralleled in invasion biology, where the importance of initial propagule size has been demonstrated theoretically and empirically (Shigesada & Kawasaki 1997; Lockwood, Cassey & Blackburn 2005; Lockwood, Hoopes & Marchetti 2007), and stochasticity is also shown to influence establishment success (reviewed in Taylor & Hastings 2005).
Parasites of a single host species are also predicted to go extinct locally if they are too virulent, as the supply of susceptible hosts becomes depleted and infected hosts die before they can pass on the infection (Anderson & May 1982). Indeed, an outbreak of crayfish plague in Ireland killed all of its hosts rapidly in the location such that the disease was extinguished and has not been seen in Ireland since (Reynolds 1988). This contrasts with the situation in England, where invasive signal crayfish provide a reservoir for reinfection (Table 1). Squirrel poxvirus in red squirrels is also highly pathogenic, and models suggest it will ‘burn‐out’ in pure red squirrel populations without repeated spillover from grey squirrels, the reservoir hosts (Gurnell et al. 2006). Burnout of virulent diseases is analogous to the ‘bust’ phase that some ‘boom and bust’ invaders undergo as a result of exhaustion of resources in the novel habitat. For instance, reindeer (Rangifer tarandus) introduced to Alaskan islands initially increased rapidly but crashed as a result of over‐exploitation of their lichen food sources (Simberloff & Gibbons 2004). For invaders, boom and bust cycles can also be caused by other mechanisms (disease, adaptation of native predators, interspecific competition; Simberloff & Gibbons 2004). An alternative mechanism that may apply to EIDs involves replacement by novel parasite strains (‘escape mutants’) that evade host immune responses (Bull & Ebert 2008). From a control perspective, decline and burnout are of interest; in such cases (if they can be predicted with accuracy), a potential epidemiological (if not necessarily ethical) strategy for control could be that of non‐intervention (Simberloff & Gibbons 2004).
The overall distribution of EIDs or invaders may be the product of source‐sink dynamics, with populations growing in some areas but declining in others but for immigration, for example, with zebra mussels Dreissena polymorpha in lakes (sources) and streams (sinks; Horvath et al. 1996). Population structure can influence the apparent rate at which newly emerging diseases spread; for instance, models for HIV emergence within a network of villages indicate that the infection may have spread very slowly in the initial stages, potentially even declining within initial seed villages whilst being gradually propagated throughout the network (May, Gupta & McLean 2001). Similar processes might apply in invasion biology; Simberloff & Gibbons (2004) note frequent cases of ‘lagged’ invasions wherein introduced species remained at low population densities for decades before sudden, explosive population growth.
Evolutionary factors
For EIDs, it has been suggested that the combination of spillover and transmission in source‐sink systems can result in local persistence in populations in contact with the reservoir host, allowing time for evolutionary adaptation to the novel host (Dennehy et al. 2006). In contrast, arguments for invasions suggest that, with source‐sink dynamics, gene flow from source to sink might dilute local adaptation (Holt & Gaines 1992). Hence, there is a need for further work to examine whether processes in invasions apply to EIDs (depending on the likelihood of recombination between parasite strains, for instance).
Even when R 0 in the novel host is below 1, some parasites will achieve appreciable chains of successful transmission; these ‘stuttering chains’ of transmission provide an opportunity for selection on parasite replication traits (Antia et al. 2003). Rapid evolution following a jump to a novel host species may increase the likelihood of transition through stages II and III, and studies of some human EIDs suggest that viral evolution can indeed occur on the time‐scale of these initial stuttering transmission chains. For instance, the Severe Acute Respiratory Syndrome (SARS) coronavirus shows evidence of rapid sequence evolution in its multiple novel hosts, including man (Fig. 3). The Influenza A virus has the potential for rapid evolution as a result of both mutation and reassortment if a host individual is infected by more than one strain. For example, the 2009 outbreak of H1N1 swine flu in humans resulted from the recombination of two types of swine flu virus, one of which was itself a reassorted strain containing regions originating from avian, swine and human influenza (Smith et al. 2009). Evolution of host resistance or tolerance may also occur on an ecological time‐scale. For example, the termination of outbreaks of Metchinikowia bicuspidata in populations of Daphnia dentifera has been attributed to rapid evolution of host resistance (Duffy et al. 2009). Interestingly, the evolution of host tolerance to infection (i.e. a reduction in virulence effects of the parasite) may in fact help to sustain an EID in the host population (Penczykowski, Forde & Duffy 2011). Rapid evolution in novel habitats by invasive species, and by natives in response to invaders, has also been documented for plant and animal systems, and more work is needed to establish the frequency and speed of such processes (Strauss, Lau & Carroll 2006).
Figure 3.
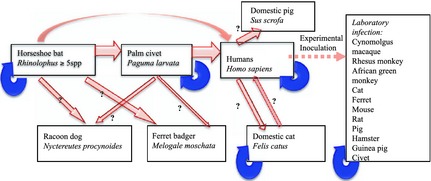
Severe Acute Respiratory Syndrome (SARS) was caused by a coronavirus thought to have spilled over from bats to palm civets then on to humans as a result of trading wild and farmed species at markets, which achieved pandemic spread (including outbreaks in China, Singapore, Taiwan and Toronto, among others) in 2002 and 2003. Pale arrows depict interspecific (spillover) transmission; dark arrows show amplification by intraspecific transmission (including transmission between humans). Genome sequence analyses of the virus suggest it adapted rapidly to each of its novel hosts; sequences from early human cases showed strong homology to virus from civets and bats, but sequences from later cases had diverged (reviewed in Wang & Eaton 2007). Adapted from Hatcher & Dunn (2011).
For both EIDs and invaders, the probability of establishment increases with increasing propagule pressure; however, the shape of this ‘dose–response’ relationship is critical to understanding and predicting invasion success and emergence. In invasion biology, few studies have either established this shape or disentangled the relative roles of propagule size, number or ‘health’ (see Lockwood, Cassey & Blackburn 2005; Lockwood, Hoopes & Marchetti 2007). Similar issues hold for EIDs; in particular, identifying the relationship between parasite virulence and transmission efficiency (the virulence‐transmission trade‐off), and how transmission varies with host population density, are key to assessing ‘virulence management’ strategies (Bull & Ebert 2008). Both invasion biology and the study of EIDs require elucidation of these aspects if effective control is to be developed. For instance, in the longer term, selection on parasites is predicted to optimize intraspecific transmission, trading off infection and replication processes vs. virulence effects on the host. However, in the early stages of emergence (including the emergence of drug‐resistant ‘escape mutants’), substantially higher transmission and virulence traits can be favoured (Bull & Ebert 2008). This may explain why some newly emerging diseases are strongly detrimental to the host and may have parallels in invasion biology with respect to some ‘boom and bust’ invasions where the evolutionary responses of natives ameliorate the impacts of invaders (Simberloff & Gibbons 2004; Strauss, Lau & Carroll 2006).
Environmental factors
Environmental change may also affect transmission opportunities, often with multiple environmental factors contributing to EIDs (Lafferty 2009; Okamura & Feist 2011). There is much current debate over whether disease emergence is increasing in parallel with anthropogenic and environmental change (e.g. Jones et al. 2008; Plowright et al. 2008). Climate and seasonality affect transmission of many diseases of wildlife (e.g. coral disease, chytrid disease) and humans (e.g. malaria, dengue, cholera, plague; Rohr et al. 2011). For example, a combination of warming and eutrophication have led to outbreaks of proliferative kidney disease (caused by the myxozoan Tetracapsuloidae bryosalmonae) in freshwater fish, as a result of increased densities of the intermediate bryozoan host, as well as increased spore production (Okamura et al. 2011). The distribution of malaria, and its emergence in new regions, result from multiple factors including climate change, control strategies and socio‐economic factors, which interact to affect the life cycles of both the Plasmodium parasite and its mosquito vector, as well as transmission opportunities to new hosts (Lafferty 2009). This parallels examples from the invasion literature, where climate change (warming) has facilitated invasions through a number of mechanisms, such as removing temperature barriers to invasion of temperate regions and altering dispersal behaviour (Walther et al. 2009).
The processes of disease emergence and invasion may also be linked to changes in biodiversity in similar ways. Reduction in biodiversity may fuel increased incidence of parasitism (Keesing et al. 2010; Okamura & Feist 2011). Furthermore, increased incidence of parasitism can under some circumstances reduce biodiversity, further fuelling parasite spread (Hatcher, Dick & Dunn 2012). Invasion biologists also recognize the importance of biodiversity in determining invasion success, and in potential interdependence of invasion and biodiversity. For instance, the ‘biodiversity‐invasibility’ hypothesis postulates that more diverse communities are better buffered from invasion as there are fewer unexploited ecological niches, and through the process of ‘invasion meltdown’, it is argued that invasion by some species facilitates successful invasion by others (discussed in Davis 2009).
Phase IV pandemic spread/invasion
This final phase of emergence is termed pandemic emergence. There has been historical and current variation in the use and meaning of the term pandemic (Morens, Folkers & Fauci 2009). The dictionary definition of ‘pandemic’ is an epidemic (i.e. an outbreak) over a very large area (http://www.oed.com/). For human EIDs, WHO defines a pandemic as global spread affecting more than one WHO region (http://www.who.int/topics/emerging_diseases/en). Morens, Folkers & Fauci (2009) point out that the broader meaning (and the only consistently met characteristic) of pandemic is that of widespread (not necessarily world‐wide) geographical extension. Although the term has usually been restricted to human EIDs, it is also applicable to EIDs of wildlife or farmed species that have attained a widespread geographical range, such as bovine tuberculosis in cattle, chytrid disease in amphibians and West Nile virus in birds. There is also variation in the use and meaning of the term invasive, but here the debate is over the impact of an invader; whilst all define invasive species as introduced species that have established and spread, not all definitions require impact on the recipient community and, indeed, invasiveness does not predict impact (Ricciardi & Cohen 2007).
For parasites that jump to novel hosts, pandemic emergence may occur provided the parasite can be maintained in the novel host alone (i.e. it has an R 0 > 1 through intraspecific transmission) and transmission routes enable extensive within and between population spread. This occurs when local chains of transmission from populations of established infection link into larger populations with stronger transmission routes. This phase bears similarity to the invasive stage reached by some introduced species that spread rapidly throughout the introduced range. However, the intercontinental spread of an EID and invasive species are potentially subject to different processes. For a (non‐parasitic) species, this requires an additional round of translocation (and subsequent phases) for each successful introduction into a discrete habitat. However, for EIDs of novel hosts, repetition of prior phases in subsequent populations is not required for phase IV. Because spillover from an ancestral host and adaptation to the new host species have already occurred, there is no evolutionary barrier for the parasite to overcome following introduction into a new geographical region. Hence, the stage is set for rapid pandemic spread (as seen in the 2009 swine flu pandemic, for example; Smith et al. 2009).
Pandemic spread may be facilitated by scale‐dependent aspects of population structure, host movement and contact rates resulting in increased transmission once infection reaches larger populations. For instance, in the case of HIV, initial contact, spillover and persistence phases occurred in remote villages in the Congo basin, and it was several decades before cases reached the population centre of Kinshasa. Proliferation and viral evolution occurred for several more decades in this city, before translocation to other continents; once this phase was reached, world‐wide dissemination was very rapid (reviewed in Sharp & Hahn 2008). Similarly, there is evidence of scale‐dependent transmission of chytrid disease, with 200‐fold higher rates of intercontinental than local transmission (Lips et al. 2008). The scale‐dependent nature of invasions has also been noted; intercontinental spread of invasives is necessarily dependent on human transport and will operate at different rates to local dispersal within a novel habitat (Lockwood, Cassey & Blackburn 2005; Lockwood, Hoopes & Marchetti 2007). Indeed, the balance between the processes of local diffusive spread of invaders and long‐distance migrations means that the scale of observation will often determine the perceived rate of invasion (Shigesada & Kawasaki 1997).
Heterogeneity in parasite transmission rates is another important factor determining rapid and pandemic spread. Superspreaders are individuals that are responsible for many more than the average number of transmission events and have been documented for a number of human EIDs (Lloyd‐Smith et al. 2005). EIDs with an important component of superspreading are predicted to go extinct more frequently compared to those with homogenous transmission (for a given average R 0), but when disease outbreaks do occur, they will be more prolonged and severe. For instance, contact‐tracing data demonstrated the importance of superspreaders in fuelling SARS epidemics in Hong Kong and Singapore, and models demonstrate that nearly 90% of secondary cases resulted from a minority of superspreading individuals (Lloyd‐Smith et al. 2005). A more general application of the superspreading concept to classic invasion biology is difficult because of the fundamental difference that EIDs are contained within hosts whilst other invaders are not.
Conclusions
There are several broad parallels between the processes of invasion and disease emergence, although distinct differences are also evident. From a process perspective, there is arguably greater similarity between the first (contact/translocation) and final (pandemic/invasive spread) phases than in the intermediate (spillover/introduction and persistence/establishment) phases. In both invasion and emergence scenarios, the early and late phases are more strongly determined by demographic processes in which anthropogenic factors (human transport, economic/agricultural activity and settlement) play a primary role. However, in the intermediate phases, the ecological and evolutionary factors that underpin particular host–parasite and native–invader interactions are of greater importance. During these intermediate phases, the parallels between emergence and invasion tend to be less strong as the co‐evolutionary interactions between host and parasite become important in determining persistence and spread of EIDs.
For both EIDs and invasions, the processes determining transition between phases are complex, involving multiple demographic, evolutionary and environmental factors. Consequently, identifying specific factors or traits involved in successful invasion or emergence is difficult. One key area is that of propagule pressure, which has been well elucidated for invasions (Lockwood, Cassey & Blackburn 2005; Lockwood, Hoopes & Marchetti 2007) and the processes are also relevant to EIDs. Propagule number equates to the frequency of spillover events and has been recognized as a key factor in determining frequency of emergence (e.g. Childs, Richt & Mackenzie 2007). Propagule size can be considered equivalent to initial parasite dose in a novel host individual and is shown theoretically to be an important determiner of persistence (May, Gupta & McLean 2001). In addition to the areas of overlap, examination of process differences might provide further insight. Reporting bias (and environmental constraints) may differ between EIDs and free‐living invaders. Many biological invasions probably go unnoticed, particularly if their impacts do not affect agriculture or other human activity; given their size and habitat within the host, parasites may be even more likely to escape detection (Macdonald & Laurenson 2006). Conversely, Okamura & Feist (2011) argue that many apparent examples of aquatic EIDs are actually indigenous parasite species that were under‐reported or have begun to produce novel disease symptoms, perhaps as a result of environmental change.
The similarities between parts of the invasion and disease emergence process suggest that there should be overlap in prediction and management approaches. Indeed, some risk assessment models of disease emergence or spread to new locations borrow specific techniques developed in invasion biology and some recognize that general approaches can be applied to invasion and disease management (Childs, Richt & Mackenzie 2007; see also Jeschke, J.M., Keesing, F. & Ostfeld R.S., Submitted). For instance, some approaches use risk analysis methodologies borrowed from invasion biology to examine risks of translocation (phase I) or pandemic spread (IV) for EIDs or their vectors (e.g. Tatem, Hay & Rogers 2006; see also Thrush et al. 2011 for risk assessment models applied to aquatic EIDs). Some papers recognize structural similarities between invasions and emergence; for instance, Chades et al. (2011) use network techniques to examine control strategies in small, sparsely connected metapopulations; their ‘infected’ populations can equally represent the presence of an EID, an invasive species or a threatened species. In cases where diseases emerge or increase along with an introduced host species, control measures for the EID may focus on control of the invading host (squirrel pox and grey squirrels, for example; Boots et al. 2012).
At a general level, there is overlap in the type of control most appropriate across the phases of invasion and disease emergence (Table 2). However, infection and immunological processes offer additional control opportunities for emerging diseases. For instance, one can take advantage of latent periods (after exposure of the host to the parasite, but prior to becoming infectious) to limit disease spread via contact control. Isolation of exposed individuals played a pivotal role in controlling the spread of SARS, for example (Lloyd‐Smith et al. 2005). In some cases, the host immune system can provide defence via vaccination; there are numerous examples of its application to limit human infectious diseases, but it is also an option for some diseases of wildlife and domestic animals (e.g. Gurnell et al. 2006; Mathews 2009). Options for control of both invaders and EIDs diminish through the phases (Childs, Richt & Mackenzie 2007; Hatcher & Dunn 2011), with efficacy also tending to decrease whilst costs increase. It is therefore no surprise that one area of agreement concerning approaches to control is over current calls for more effective, timely and global surveillance and reporting programs for both EIDs and invaders (e.g. Childs, Richt & Mackenzie 2007; Plowright et al. 2008; Mathews 2009). Further to this, we require realism in the efforts to eradicate EIDs and invasive species at crucial points in their emergence, balancing issues such as animal welfare, legal considerations and costs (see Blackburn et al. 2010). In practice, it may be more realistic to aim for mitigation and management of EIDs and invasions guided by robust risk assessment on a case‐by‐case basis (Thrush et al. 2011). We thus encourage those engaged in EID and invasion research to facilitate this with further cross‐fostering of ideas and approaches.
Supporting information
LaySummary
Acknowledgements
The authors acknowledge support from the Natural Environment Research Council (NERC NE/G015201/1 and NE/J00630/1). We thank the anonymous referees for their thought‐provoking comments on an earlier draft of the manuscript.
References
- Alderman, D.J. (1996) Geographical spread of bacterial and fungal diseases of crayfish. Revue Scientifique et Technique, 15, 603–642. [DOI] [PubMed] [Google Scholar]
- Anderson, R.M. & May, R.M. (1982) Coevolution of hosts and parasites. Parasitology, 85, 411–426. [DOI] [PubMed] [Google Scholar]
- Antia, R. , Regoes, R.R. , Koella, J.C. & Bergstrom, C.T. (2003) The role of evolution in the emergence of infectious diseases. Nature, 426, 658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn, T.M. , Pettorelli, N. , Katzner, T. , Gompper, M.E. , Mock, K. , Garner, T.W.J. , Altwegg, R. , Redpath, S. & Gordon, I.J. (2010) Dying for conservation: eradicating invasive alien species in the face of opposition. Animal Conservation, 13, 227–228. [Google Scholar]
- Blackburn, T.M. , Pysek, P. , Bacher, S. , Carlton, J.T. , Duncan, R.P. , Jarosik, V. , Wilson, J.R.U. & Richardson, D.M. (2011) A proposed unified framework for biological invasions. Trends in Ecology & Evolution, 26, 333–339. [DOI] [PubMed] [Google Scholar]
- Bull, J.J. & Ebert, D. (2008) Invasion thresholds and the evolution of nonequilibrium virulence. Evolutionary Applications, 1, 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chades, I. , Martin, T.G. , Nicol, S. , Burgman, M.A. , Possingham, H.P. & Buckley, Y.M. (2011) General rules for managing and surveying networks of pests, diseases, and endangered species. Proceedings of the National Academy of Sciences of the United States of America, 108, 8323–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs, J.E. , Richt, J.A. & Mackenzie, J.S. (2007) Introduction: Conceptualizing and partitioning the emergence process of zoonotic viruses from wildlife to humans Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross‐Species Transmission (eds Childs J.E., Richt J.A. & Mackenzie J.S.), pp. 1–31. Springer, Berlin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collauti, R.I. & MacIsaac, H.J. (2004) A neutral terminology to define ‘invasive’ species. Diversity and Distributions, 10, 135–141. [Google Scholar]
- Cox, J.G. & Lima, S.L. (2006) Naivete and an aquatic‐terrestrial dichotomy in the effects of introduced predators. Trends in Ecology and Evolution, 21, 674–680. [DOI] [PubMed] [Google Scholar]
- Cross, P.C. , Cole, E.K. , Dobson, A.P. , Edwards, W.H. , Hamlin, K.L. , Luikart, G. , Middleton, A.D. , Scurlock, B.M. & White, P.J. (2010) Probable causes of increasing brucellosis in free‐ranging elk of the Greater Yellowstone Ecosystem. Ecological Applications, 20, 278–288. [DOI] [PubMed] [Google Scholar]
- Davis, M.A. (2009) Invasion Biology, Oxford University Press, Oxford. [Google Scholar]
- Dennehy, J.J. , Friedenberg, N.A. , Holt, R.D. & Turner, P.E. (2006) Viral ecology and the maintenance of novel host use. The American Naturalist, 167, 429–439. [DOI] [PubMed] [Google Scholar]
- Dobson, A. & Foufopoulos, J. (2001) Emerging infectious pathogens of wildlife. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 356, 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy, M.A. , Hall, S.R. , Caceres, C.E. & Ives, A.R. (2009) Rapid evolution, seasonality, and the termination of parasite epidemics. Ecology, 90, 1331–1448. [DOI] [PubMed] [Google Scholar]
- Dunn, A.M. (2009) Parasites and biological invasions. Advances in Parasitology, 68, 161–184. [DOI] [PubMed] [Google Scholar]
- Dunn, A.M. , Torchin, M.E. , Hatcher, M.J. et al (2012) Indirect effects of parasites in biological invasions. Functional Ecology, 26, 1262–1274. [Google Scholar]
- Field, H.E. , Mackenzie, J.S. & Daszak, P. (2007) Henipaviruses: Emerging Paramyxoviruses associated with fruit bats Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross‐Species Transmission (eds Childs J.E., Richt J.A. & Mackenzie J.S.), pp. 133–159. Springer, Berlin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnell, J. , Rushton, S.P. , Lurz, P.W.W. , Sainsbury, A.W. , Nettleton, P. , Shirley, M.D.F. , Bruemmer, C. & Geddes, N. (2006) Squirrel poxvirus: landscape scale strategies for managing disease threat. Biological Conservation, 131, 287–295. 0006‐3207. [Google Scholar]
- Hatcher, M.J. , Dick, J.T.A. & Dunn, A.M. (2006) How parasites affect interactions between competitors and predators. Ecology Letters, 9, 1253–1271. [DOI] [PubMed] [Google Scholar]
- Hatcher, M.J. , Dick, J.T.A. & Dunn, A.M. (2012) Diverse effects of parasites in ecosystems: linking interdependent processes. Frontiers In Ecology and the Environment, 10, 186–194. [Google Scholar]
- Hatcher, M.J. & Dunn, A.M. (2011) Parasites in Ecological Communities: From Interactions to Ecosystems. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Holt, R.D. & Gaines, M.S. (1992) Analysis of adaptation in heterogeneous landscapes: implications for the evolution of fundamental niches. Evolutionary Ecology, 6, 433–447. [Google Scholar]
- Horvath, T.G. , Lamberti, G.A. , Lodge, D.M. & Perry, W.L. (1996) Zebra mussel dispersal in lake‐stream systems: source‐sink dynamics? Journal of the North American Benthological Society, 15, 564–575. [Google Scholar]
- Hudson P.J., Rizzoli A., Grenfell B., Heesterbeck H. & Dobson A.P. (eds) (2002) The Ecology of Wildlife Diseases. Oxford University Press, Oxford, UK. [Google Scholar]
- Jones, K.E. , Patel, N.G. , Levy, M.A. , Storeygard, A. , Balk, D. , Gittleman, J.L. & Daszak, P. (2008) Global trends in emerging infectious diseases. Nature, 451, 990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing, F. , Belden, L.K. , Daszak, P. , Dobson, A.P. , Harvell, C.D. , Holt, R.D. , Hudson, P. , Jolles, A. , Jones, K.E. , Mitchell, C.E. , Myers, S.S. , Bogich, T. & Ostfeld, R.S. (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature, 468, 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, D.W. , Paterson, R.A. , Townsend, C.R. , Poulin, R. & Tompkins, D.M. (2009) Parasite spillback: a neglected concept in invasion ecology? Ecology, 90, 2047–2056. [DOI] [PubMed] [Google Scholar]
- Kolar, C.S. & Lodge, D.M. (2001) Progress in invasion biology: predicting invaders. Trends in Ecology & Evolution, 16, 199–204. [DOI] [PubMed] [Google Scholar]
- Lafferty, K.D. (2009) The ecology of climate change and infectious diseases. Ecology, 90, 888–900. [DOI] [PubMed] [Google Scholar]
- Lips, K.R. , Diffendorfer, J. , Mendelson, J.R. & Sears, M.W. (2008) Riding the wave: reconciling the roles of disease and climate change in amphibian declines. Plos Biology, 6, 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd‐Smith, J.O. , Schreiber, S.J. , Kopp, P.E. & Getz, W.M. (2005) Superspreading and the effect of individual variation on disease emergence. Nature, 438, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood, J.L. , Cassey, P. & Blackburn, T. (2005) The role of propagule pressure in explaining species invasions. Trends in Ecology and Evolution, 20, 223–228. [DOI] [PubMed] [Google Scholar]
- Lockwood, J.L. , Hoopes, M.F. & Marchetti, M.P. (2007) Invasion Ecology. Blackwell Publishing, Oxford. [Google Scholar]
- Loo, J. (2009) Ecological impacts of non‐indigenous invasive fungi as forest pathogens. Biological Invasions, 11, 81–96. [Google Scholar]
- Macdonald, D.W. & Laurenson, M.K. (2006) Infectious disease: inextricable linkages between human and ecosystem health. Biological Conservation, 131, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti, M.P. , Moyle, P.B. & Levine, R. (2004) Alien fishes in California watersheds: characteristics of successful and failed invaders. Ecological Applications, 14, 587–596. [Google Scholar]
- Mathews, F. (2009) Zoonoses in wildlife: integrating ecology and management. Advances in Parasitology, 68, 185–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, R.M. , Gupta, S. & McLean, A.R. (2001) Infectious disease dynamics: what characterizes a successful invader? Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 356, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens, D.M. , Folkers, G.K. & Fauci, A.S. (2009) What is a pandemic? Journal of Infectious Diseases, 200, 1018–1021. [DOI] [PubMed] [Google Scholar]
- Okamura, B. & Feist, S.W. (2011) Emerging diseases in freshwater systems. Freshwater Biology, 56, 627–637. [Google Scholar]
- Okamura, B. , Hartikainen, H. , Schmidt‐Posthaus, H. & Wahli, T. (2011) Life cycle complexity, environmental change and the emerging status of salmonid proliferative kidney disease. Freshwater Biology, 56, 735–753. [Google Scholar]
- Ostfeld, R.S. , Canham, C.D. , Oggenfuss, K. , Winchcombe, R.J. & Keesing, F. (2006) Climate, deer, rodents, and acorns as determinants of variation in Lyme‐disease risk. Plos Biology, 4, 1058–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeler, E.J. & Feist, S.W. (2011) Human intervention in freshwater ecosystems drives disease emergence. Freshwater Biology, 56, 705–716. [Google Scholar]
- Penczykowski, R.M. , Forde, S.E. & Duffy, M.A. (2011) Rapid evolution as a possible constraint on emerging infectious diseases. Freshwater Biology, 56, 689–704. [Google Scholar]
- Plowright, R.K. , Sokolow, S.H. , Gorman, M.E. , Daszak, P. & Foley, J.E. (2008) Causal inference in disease ecology: investigating ecological drivers of disease emergence. Frontiers in Ecology and the Environment, 6, 420–429. [Google Scholar]
- Poulin, R. , Paterson, R.A. , Townsend, C.R. , Tompkins, D.M. & Kelly, D.W. (2011) Biological invasions and the dynamics of endemic diseases in freshwater ecosystems. Freshwater Biology, 56, 676–688. [Google Scholar]
- Reynolds, J.D. (1988) Crayfish extinctions and crayfish plague in central Ireland. Biological Conservation, 45, 279–285. [Google Scholar]
- Ricciardi, A. (2006) Patterns of invasion of the Laurentian Great Lakes in relation to changes in vector activity. Diversity and Distributions, 12, 425–433. [Google Scholar]
- Ricciardi, A. & Cohen, J. (2007) The invasiveness of an introduced species does not predict its impact. Biological Invasions, 9, 309–315. [Google Scholar]
- Rohr, J.R. , Dobson, A.P. , Johnson, P.T.J. , Kilpatrick, A.M. , Paull, S.H. , Raffel, T.R. , Ruiz‐Moreno, D. & Thomas, M.B. (2011) Frontiers in climate change‐disease research. Trends in Ecology and Evolution, 26, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp, P.M. & Hahn, B.H. (2008) Aids – prehistory of HIV‐1. Nature, 455, 605–606. [DOI] [PubMed] [Google Scholar]
- Shigesada, N. & Kawasaki, K. (1997) Biological Invasions: Theory & Practice. Oxfrod University Press, Oxford UK. [Google Scholar]
- Simberloff, D. & Gibbons, L. (2004) Now you see them, now you don't! – population crashes of established introduced species. Biological Invasions, 6, 161–172. [Google Scholar]
- Smith, G.J.D. , Vijaykrishna, D. , Bahl, J. , Lycett, S.J. , Worobey, M. , Pybus, O.G. , Ma, S.K. , Cheung, C.L. , Raghwani, J. , Bhatt, S. , Peiris, J.S.M. , Guan, Y. & Rambaut, A. (2009) Origins and evolutionary genomics of the 2009 swine‐origin H1N1 influenza A epidemic. Nature, 459, 1122–1125. [DOI] [PubMed] [Google Scholar]
- Strauss, S.Y. , Lau, J.A. & Carroll, S.P. (2006) Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecology Letters, 9, 357–374. [DOI] [PubMed] [Google Scholar]
- Tatem, A.J. , Hay, S.I. & Rogers, D.J. (2006) Global traffic and disease vector dispersal. Proceedings of the National Academy of Sciences of the United States of America, 103, 6242–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, C.M. & Hastings, A. (2005) Allee effects in biological invasions. Ecology Letters, 8, 895–908. [Google Scholar]
- Thomas, F. , Renaud, F. & Guegan, J.F. (2005) Parasitism and Ecosystems. Oxford University Press, Oxford, UK. [Google Scholar]
- Thrush, M.A. , Murray, A.G. , Brun, E. , Wallace, S. & Peeler, E.J. (2011) The application of risk and disease modelling to emerging freshwater diseases in wild aquatic animals. Freshwater Biology, 56, 658–675. [Google Scholar]
- Tompkins, D.M. , Dunn, A.M. , Smith, M.J. & Telfer, S. (2011) Wildlife diseases – from individuals to ecosystems. Journal of Animal Ecology, 80, 19–38. [DOI] [PubMed] [Google Scholar]
- Vredenburg, V.T. , Knapp, R.A. , Tunstall, T.S. & Briggs, C.J. (2010) Dynamics of an emerging disease drive large‐scale amphibian population extinctions. Proceedings of the National Academy of Sciences of the United States of America, 107, 9689–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther, G.R. , Roques, A. , Hulme, P.E. , Sykes, M.T. , Pysek, P. , Kühn, I. , Zobel, M. , Bacher, S. , Botta‐Dukát, Z. , Bugmann, H. , Czúcz, B. , Dauber, J. , Hickler, T. , Jarosik, V. , Kenis, M. , Klotz, S. , Minchin, D. , Moora, M. , Nentwid, W. , Ott, J. , Panov, V.E. , Reineking, B. , Robinet, C. , Semenchenko, V. , Solarz, W. , Thuiller, W. , Vila, M. , Vohland, K. & Settele, J. (2009) Alien species in a warmer world: risks and opportunities. Trends in Ecology and Evolution, 24, 686–693. [DOI] [PubMed] [Google Scholar]
- Wang, L.F. & Eaton, B.T. (2007) Bats, Civets and the emergence of SARS Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross‐Species Transmission (eds Childs J.E., Richt J.A. & Mackenzie J.S.), pp. 325–344. Springer, Berlin. [Google Scholar]
- Williamson, M. (1996) Biological Invasions, Chapman & Hall, London. [Google Scholar]
- Wolfe, N.D. , Dunavan, C.P. & Diamond, J. (2007) Origins of major human infectious diseases. Nature, 447, 279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhouse, M.E.J. , Haydon, D.T. & Antia, R. (2005) Emerging pathogens: the epidemiology and evolution of species jumps. Trends in Ecology and Evolution, 20, 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LaySummary


