Abstract
The reversible post‐translational modification of proteins by ubiquitin and ubiquitin‐like proteins regulates almost all cellular processes, by affecting protein degradation, localization, and complex formation. Deubiquitinases (DUBs) are proteases that remove ubiquitin modifications or cleave ubiquitin chains. Most DUBs are cysteine proteases, which makes them well suited for study by activity‐based probes. These DUB probes report on deubiquitinase activity by reacting covalently with the active site in an enzyme‐catalyzed manner. They have proven to be important tools to study DUB selectivity and proteolytic activity in different settings, to identify novel DUBs, and to characterize deubiquitinase inhibitors. Inspired by the efficacy of activity‐based probes for DUBs, several groups have recently reported probes for the ubiquitin conjugation machinery (E1, E2, and E3 enzymes). Many of these enzymes, while not proteases, also posses active site cysteine residues and can be targeted by covalent probes. In this review, we will discuss how features of the probe (cysteine‐reactive group, recognition element, and reporter tag) affect reactivity and suitability for certain experimental applications. We will also review the diverse applications of the current probes, and discuss the need for new probe types to study emerging aspects of ubiquitin biology.
Keywords: activity‐based probe, activity‐based protein profiling, chemoproteomics, deubiquitinase, E1, E3, protease, ubiquitin, ubiquitin–proteasome system
E1, E2, E3, and deubiquitinating enzymes regulate protein ubiquitination, with profound consequences for protein stability and function. Therefore, there is considerable interest in developing approaches to study their activity. Activity‐based probes are valuable tools to understand the biochemical mechanisms and cellular functions of these enzymes.

Abbreviations
- ABP
activity‐based probe
- ABPP
activity‐based protein profiling
- AOMK
acyloxymethyl ketone
- Dha
dehydroalanine
- DUB
deubiquitinase
- HECT
homologous to the E6‐AP carboxyl terminus
- MS
mass spectrometry
- NEDD8
neural precursor cell expressed, developmentally down‐regulated 8
- OTU
ovarian tumor domain protease
- RBR
RING‐between‐RING
- RING
really interesting new gene
- SENP
SUMO‐specific protease
- SUMO
small ubiquitin‐like modifier
- Ubl
ubiquitin‐like protein
- Ub
ubiquitin
- UCH
ubiquitin C‐terminal hydrolase
- ULP
Ubl protease
- UPS
ubiquitin–proteasome system
- USP
ubiquitin‐specific protease
- VME
vinyl methyl ester
- VS
vinyl sulfone
Ubiquitination: a dynamic multipurpose post‐translational modification
Since the discovery in the late 1970s and early 1980s that modification by ubiquitin targets proteins for degradation by the proteasome, the ubiquitin–proteasome system (UPS) has emerged as an essential regulator of almost all cellular processes in eukaryotes. Aside from maintaining proteostasis by degrading damaged or unwanted proteins, ubiquitination and subsequent degradation regulates the activity of many short‐lived proteins, including cell cycle regulators and transcription factors, and produces antigenic peptides for presentation on the cell surface 1, 2. Ubiquitination also has many proteasome‐independent roles, including regulating DNA damage repair, receptor endocytosis, and inflammation 3. Not surprisingly, dysregulation of the UPS contributes to many illnesses, including cancer, neurodegenerative diseases, and immunological disorders.
Ubiquitin (Ub) is a small (8.5 kDa) globular protein of 76 amino acids that adopts a beta‐grasp fold (Fig. 1A). It is highly conserved and found in all eukaryotes. Ub is attached to substrates through its terminal glycine residue, a process referred to as ubiquitination (or ubiquitylation) 4. Typically, Ub is attached to proteins via an isopeptide bond to a lysine side chain, but proteins may also be ubiquitinated on their N terminus via a peptide bond. Ub itself can be ubiquitinated (polyubiquitination), giving rise to Ub chains 5. This can occur through one of seven Lys residues (K6, K11, K27, K29, K33, K48, and K63) or the N‐terminal Met residue (M1). K48‐linked polyubiquitination is the best‐known and most‐studied modification, as it targets proteins to the proteasome for degradation 6. Other chain types typically have signaling roles: for example, M1‐linked (also called linear) chains are important in several immune signaling pathways 7, 8.
Figure 1.

Ubiquitin conjugation and deconjugation machinery. (A) X‐ray crystal structure of human ubiquitin (PDB: http://www.rcsb.org/pdb/search/structidSearch.do?structureId=1UBI). Lysine side chains are shown in magenta, N‐terminal methionine (M1) shown in cyan, and C‐terminal di‐Gly shown in blue; (B) overview of enzymes involved in Ub conjugation and deconjugation, with catalytic Cys residues indicated; (C) the ‘three Rs’ of an activity‐based probe (ABP)—reactive group, recognition element, and reporter tag—and the reaction of an ABP with an enzyme containing an active site Cys residue, such as a Cys protease.
Ubiquitin is the archetypal example of a group of beta‐grasp proteins, referred to as ubiquitin‐like proteins (UBLs). In humans, these include the SUMO (small ubiquitin‐like modifier) family of proteins, NEDD8 (neural precursor cell expressed, developmentally down‐regulated 8), and several homologs of yeast Atg8 (autophagy‐related protein 8). Different conjugation and deconjugation machineries exist for the UBLs although there appears to be considerable overlap, with some enzymes functioning on several classes of UBL 9, 10. Prokaryotes do not encode for Ub, but do produce Ub‐like small protein modifiers that can be attached enzymatically to target proteins. The function of these modifiers is not fully understood 11, but in some instances, such as the prokaryotic ubiquitin‐like protein (Pup) of Mycobacterium tuberculosis, they can direct proteins for proteasomal degradation 12.
Ubiquitination is a dynamic modification, with a dedicated system of enzymes responsible for transferring Ub to substrates, and proteases responsible for removing it. Ub conjugation requires the sequential action of three classes of enzymes (Fig. 1B). E1 enzymes initially activate free ubiquitin in a two‐step mechanism. In the first step, the E1 enzyme binds Ub and ATP, and catalyzes Ub C‐terminal acyl‐adenylation to form E1:Ub~AMP. (The tilde [~] is used to indicate a reactive covalent bond, while the colon [:] indicates a noncovalent complex.) In the second step, Ub is transferred onto a Cys residue to form a thioether linkage (E1~Ub). Activated Ub is next transferred to the Cys residue of a Ub‐conjugating (sometimes referred to as Ub‐carrier) enzyme, E2, to give E2~Ub. E2s, together with Ub ligases (E3), then transfer Ub to the substrate. E3s confer substrate specify to the E2 enzymes. The cullin‐RING ligases, which are the largest group of E3s, do not form a covalent bond to Ub. However, two smaller groups, the HECT ligases (~ 30 members) and RBR ligases (~ 12 members) accept Ub from E2s to form a Ub‐thioester (E3~Ub), which then transfers Ub to a substrate 13, 14.
Deubiquitinases (DUBs) are proteases that hydrolyze the amide bond between the C‐terminal Gly residue of Ub and ubiquitinated proteins, thereby antagonizing the Ub conjugation machinery 15. As well as rescuing proteins from degradation and reversing signaling events induced by ubiquitination, certain DUBs cleave Ub gene products into individual Ub monomers prior to activation by E1s 16. The proteasome 19S regulatory particle also contains three DUBs (POH1, USP14, and UCHL5), which ‘recycle’ Ub by removing it from proteins prior to degradation. POH1 (PSMD14, RPN11) is located close to the entrance to the 20S core, and promotes substrate degradation by cleaving entire Ub chains from substrates, allowing the protein to enter the pore for degradation. By contrast, USP14 and UCHL5 are located further from the 20S core and antagonize degradation by removing Ub in a stepwise manner from the distal end, promoting substrate dissociation from the proteasome 17.
The human genome encodes for approximately 90 DUBs, which fall into six classes 18, 19. Five classes are papain‐type Cys proteases: the ubiquitin‐specific proteases (USPs), ubiquitin C‐terminal hydrolases (UCHs), ovarian tumor domain proteases (OTUs), Machado–Joseph disease domain proteases (MJDs or Josephins), and motif interacting with ubiquitin‐containing novel DUB family (MINDY) DUBs. The sixth class of DUBs, JAB1/MPN/MOV34 (JAMMs, also known as MPN+), are zinc‐dependent metalloproteases.
Enzymes that attach and remove Ub have attracted significant interest as therapeutic targets. The NEDD8 E1 inhibitor MLN4924 (pevonedistat) is in Phase II trials for leukemia (ClinicalTrials.gov identifier: NCT02610777), and Ub E1 inhibitor MLN7243 has also recently entered clinical trials (ClinicalTrials.gov identifier: NCT02045095). Immunomodulatory imide drugs (IMiDs) such as thalidomide are known to target the cullin‐RING E3 cereblon, and are approved in hematological malignancies 20. Several classes of compound that target the Ub ligase MDM2 are now in clinical trials 21. These compounds antagonize the interaction of MDM2 with its substrate, the tumor suppressor p53, thereby increasing p53 stability. Antagonists of the inhibitor of apoptosis (IAP) family of E3 proteins have also entered clinical trials 22. Recently, a DUB inhibitor (VLX1570) has entered Phase I trials for multiple myeloma (ClinicalTrials.gov identifier: NCT02372240). Tools to study the activity of the Ub conjugation and deconjugation machinery are therefore valuable not only to better understand the functions of these enzymes but also to identify and characterize new inhibitors.
Activity‐based probes
Enzyme activity is tightly controlled at a post‐translational level. This allows cells to respond rapidly to stimuli, and to avoid unwanted activation of enzymatic activity. Many proteases are secreted in inactive forms and must undergo activation, either irreversibly (e.g., by proteolysis) or reversibly (e.g., by phosphorylation, formation of complexes with other proteins, pH change, or change in localization). Protein expression, as indicated by western blot or proteomics techniques, cannot therefore be used reliably to infer enzyme activity. Turnover of the natural substrate provides a direct measurement of activity but requires detection of the reaction products, which may be challenging in a complex cellular environment. Additionally, different enzymes often process the same substrate, making it difficult to assign activity to a particular enzyme. Activity‐based probes (ABPs) mimic substrate, but rather than being processed by the enzyme, they become covalently attached to the active site in an enzyme‐catalyzed reaction. As ABPs do not react with inactive enzymes, the extent of probe labeling is an indirect measure of enzyme activity. ABPs are particularly well suited for studying enzymes with nucleophilic catalytic residues, as a reactive electrophile can be incorporated into the probe to react covalently with the nucleophile. Consequently, cysteine, threonine, and serine proteases have been extensively studied with ABPs 23.
An ABP consists of three components—‘three Rs’ (Fig. 1C):
A reactive group. For enzymes with active site nucleophiles, this is an electrophile, often referred to as a ‘warhead’. The choice of reactive group will affect both the reactivity and selectivity of the probe.
A recognition element (targeting group). This confers selectivity toward the target of interest, and may be a small‐molecule inhibitor, a short peptide, or a full‐length protein.
A reporter tag (also referred to as a handle or label), for detection of probe‐labeled proteins. Fluorophores facilitate rapid and sensitive detection, while affinity labels (such as biotin or a peptide epitope) also allow for isolation and enrichment of labeled proteins. Alternatively, a small bioorthogonal group 24 such as an alkyne or azide may be included in the probe to allow subsequent attachment of a reporter. This is often referred to as ‘two‐step’ labeling, and may be particularly advantageous when larger tags interfere with reactivity, selectivity, or physiochemical properties of the probe. Some large ABPs, particularly the DUB probes described below, substantially increase the molecular weight of the target protein, so a mass shift on an SDS/PAGE gel can be used as an indication of probe labeling.
Activity‐based probes differ from substrate‐based probes, which lack the reactive group that becomes covalently attached to the active site. Fluorogenic substrates are frequently used as protease probes, and as they are turned over by the enzyme, they are very useful for kinetic studies. However, it is difficult to assign substrate turnover to the activity of a particular protease. ABPs overcome this limitation by forming a covalent bond to the target enzyme, and are therefore a powerful tool to identify and isolate the labeled protease. This is a major advantage when studying enzyme families with overlapping substrate selectivity, such as DUBs. A more detailed comparison of substrate‐based and activity‐based probes has been presented in a recent review 23. Broad‐spectrum ABPs can be used to study activity of many members of a protein class simultaneously, a technique referred to as activity‐based protein profiling (ABPP) 25.
The Ub conjugation and deconjugation machinery is uniquely suited for study using ABPs, due to the prevalence of enzymes with active site nucleophiles. Most DUBs are Cys proteases, and ABPs bearing Cys‐targeting electrophiles have proven to be powerful tools to identify DUBs, study the activity and selectivity of DUB inhibitors, characterize DUB enzymatic activity, and determine the physiological roles of this protease family. Additionally, E1, E2, and some E3 enzymes employ catalytic Cys residues to transfer Ub, which has recently been exploited to develop ABPs. In this review, we will focus on the design and application of ABPs for DUBs. We will also discuss the recent development of ABPs for the ubiquitin‐conjugating machinery (E1, E2, and E3 enzymes). Although these enzymes are not proteases, their probes share many similar features to the DUB ABPs.
Deubiquitinases
In the 15 years since the development of the first covalent DUB ABP 26, many different probe designs have been reported, differing in reporter tag and reactive group, but typically using one or more full‐length ubiquitin moieties as the recognition element. Four configurations of recognition element, electrophile, and label have been described (Fig. 2A). The development of ABPs with such a variety of structures has occurred concurrently with the development of new techniques to synthesize modified ubiquitin, which have been the subject of several excellent reviews 27, 28, 29, 30. Recently, a DUB ABP employing a small molecule as a recognition element has been described, and is discussed in more detail below 31.
Figure 2.

(A) DUB probe designs. Almost all DUB ABPs contain one (designs i and ii) or two (designs iii and iv) full‐length Ub proteins, with an electrophile (E) positioned at the site of the scissile bond. The reporter tag is typically at the N terminus (designs i, iii, and iv) but may be located at the C terminus, attached to the electrophile (design ii); (B) reaction between a DUB active site Cys and probe electrophiles. Mechanistically, the reactions can be classified as direct addition (e.g., Ub‐PA), conjugate addition (e.g., Ub‐VS), or nucleophilic displacement (e.g., Ub‐Br2).
Mono‐ubiquitin probes
Reactive group
The first ABPs to be developed for DUBs contain a single full‐length ubiquitin with an electrophile in place of the C‐terminal Gly residue (G76) 26, 32. Ubiquitin‐aldehyde (Ubal) and ubiquitin‐nitrile (Ub‐CN) were important tools in early mechanistic studies of DUBs 33, notably being used to demonstrate that ubiquitin C‐terminal hydrolases form a covalent intermediate during catalysis 34, 35. Ubal was also used to solve the first structure of a DUB in complex with Ub 36, revealing the conformational changes associated with Ub binding. However, the modification of DUBs by Ubal and Ub‐CN is reversible, and is not compatible with the strongly reducing conditions of SDS/PAGE 33.
To overcome the limitations of these probes, Borodovsky et al. developed the first irreversible DUB ABPs by introducing a C‐terminal vinyl methyl sulfone (VS). The probe labeled DUBs in cell lysates, and could be detected following SDS/PAGE due to the presence of an HA tag on the probe 26. Related Ubl‐VS probes for NEDD8, ISG‐15, SUMO‐1, GATE‐16, GABARAP, MAP1‐LC3, and Apg8L were reported shortly thereafter 10, 37. A wide range of C‐terminal electrophiles have since been investigated, as summarized in Table 1. Electrophiles are classified according to the nature of the reaction with the catalytic Cys (Fig. 2B). Vinyl methyl sulfones and vinyl methyl esters (VME) have been widely employed, with Ub‐VME showing slightly greater reactivity 32. Propargyl amides also react with DUB active site Cys residues, forming a vinyl thioether 38, 39. This surprising reaction appears to proceed via direct nucleophilic attack on the internal alkyne carbon, facilitated by stabilization of the developing carbanion by the ‘oxyanion hole’ of the active site 40. The resulting adduct was stable to denaturing and reducing conditions, but could be cleaved in acid, which is useful for proteomic studies. (An acid‐cleavable conjugate formed between an E1 and an ABP is discussed below 41.) Note that conjugate addition to electrophiles such as VS or VME is in principle reversible under basic conditions, but this does not appear to occur under the conditions in which these probes are used.
Table 1.
Cysteine‐reactive electrophiles employed in mono‐Ub/Ubl ABPs. Widely employed, commercially available probes are highlighted in red. Ub implies Ub1–75 (i.e., without the C‐terminal Gly), unless otherwise indicated. Note that two probes have been referred to as ‘Ub‐CN’. For some SUMO probes, only short peptides have been used, rather than full‐length protein: i: QTGG; ii: FQQQTGG
Native ubiquitin conjugate

| ||||
|---|---|---|---|---|
| Reaction type | Cys‐reactive electrophile | Structure | Abbreviation | References |
| Direct (1,2) addition | Aldehyde |

|
Ubal | 33 |
| Nitrile |

|
Ub‐CN | 33 | |
| Propargyl amide |

|
Ub‐Prg/Ub‐PA | 38, 39 | |
| Conjugate (1,4) addition | Vinyl methyl sulfone |

|
Ub‐VS | 26, 32 |
| Vinyl phenyl sulfone |

|
Ub‐VSPh | 32 | |
| Vinyl ethylsulfonate |

|
Ub‐OEtVS | 42 | |
| Vinyl methyl ester |

|
Ub‐VME | 32 | |
| Vinyl cyanide |

|
Ub‐VCN | 32 | |
| Thioacrylonitrile |

|
Ub‐CN | 114 | |
| Dehydroalanine |

|
Ub‐Dha‐N‐methylamide | 123 | |
| Nucleophilic substitution | Chloroethylamine |

|
Ub‐Cl | 32 |
| Bromoethylamine |

|
Ub‐Br2 | 32 | |
| Bromopropylamine |

|
Ub‐Br3 | 32 | |
| Acyloxymethyl ketone (AOMK) |

|
Ub‐TF3BOK, VEA505i | 42, 43 | |
| Fluoromethyl ketone |

|
SUMO‐FMKii | 44 | |
| α‐Amino‐β‐lactone |

|
Ub‐Lac | 42 | |
| Chloromethyl ketone |

|
– | 31 | |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Probes that react with the catalytic Cys by nucleophilic substitution have not been used widely, although a variety of leaving groups have been investigated 32, 42, 43, 44. Alkyl halides label a distinct, though more restricted, subset of DUBs compared to VS/VME electrophiles, and were important in the identification of OTU DUBs (discussed below) 32. Acyloxymethyl ketones (AOMKs) are more reactive than VS/VME electrophiles, but show similar activity in lysate, perhaps due to their instability 42, 43. Consistent with its high reactivity toward Cys nucleophiles, the AOMK HA‐UbTF3BOK labeled many E1, E2, and E3 enzymes in addition to DUBs 42.
As Ub‐electrophile conjugates react with Cys residues on many non‐DUB proteins, should these molecules truly be regarded as ABPs? That is to say, do they target only active DUBs, or will they label DUBs regardless of whether the enzyme is in a catalytically active form? In several instances, DUB reactivity toward a probe has been shown to change after a stimulus without a change in DUB expression, suggesting the probe does indeed target only the active subpopulation of the enzyme. For example, treating cells with hydrogen peroxide reduces the labeling of some DUBs by Ub‐VS or Ub‐VME, due to oxidation of the catalytic Cys residue 45, 46. More revealingly, a study by Naik and Dixit showed that the catalytic activity of USP9X is activated by phosphorylation upon T‐cell receptor activation. Brief stimulation of T cells increased the labeling of USP9X by HA‐Ub‐VS without an increase in USP9X levels, while the phospho‐site mutant, which still possess a potentially reactive active site Cys residue, was much less reactive toward the probe following stimulation. Hence, the probe can distinguish between active and inactive forms of USP9X 47.
Probe labeling could also give a misleading indication of activity if the reaction occurs on noncatalytic residues. In the case of OTUB1, HA‐Ub‐VS reacts preferentially at a noncatalytic Cys, so probe labeling does not indicate deubiquitinating activity 48, but it is not clear if this is a more widespread phenomenon. Therefore, when interpreting experiments using Ub‐electrophile conjugates, researchers should be aware of the possibility of activity‐independent labeling arising from the electrophilic nature of the probe. As such, the proteins labeled with Ub‐electrophile conjugates are perhaps best referred to as ‘probe‐reactive’ rather than ‘active’.
Reporter tag
Radioiodination 26 or nonspecific biotinylation 49 of a probe was initially used to detect probe‐reactive proteins. However, preparing the labeled probe is technically challenging and gives inhomogeneous labeling. Furthermore, radiolabeling does not allow for retrieval and identification of probe‐reactive proteins. These techniques have been supplanted by the use of N‐terminal epitope tags. The HA tag is widely used and has little effect on Ub recognition 32, but other epitope tags have been used successfully: for example, FLAG‐NEDD8‐VS was used as an ABP to identify deneddylases, as HA‐NEDD8‐VS was insoluble 50.
Epitope tags facilitate both detection and isolation of labeled proteins. For analysis of DUBs in lysates, detection of the tag by western blot allows rapid visualization of DUB activity. However, to relate the patterns seen in these blots to the activity of individual DUBs, proteins labeled in specific bands must be identified, usually by tandem MS. Additionally, the resolution of proteins by this technique is poor, and one apparent labeled protein in a gel may contain many labeled DUBs. To study the activity of specific DUBs, investigators frequently take advantage of the ~ 10 kDa increase in MW on probe labeling: by blotting for an individual DUB, and comparing the intensities of the larger (labeled) band to the smaller (unlabeled) band, the reactivity of the DUB toward the probe can be inferred. Affinity purification employing the epitope tag can be used to enrich labeled proteins, facilitating detection and identification of DUBs. The purified DUBs can be digested in solution and identified by tandem MS 51, or initially resolved by SDS/PAGE followed by in‐gel digestion and MS identification 32. In‐solution digestion procedures have the advantage of improved quantitation and detection of low‐abundance proteins, but require more complex analysis. A review of MS proteomic techniques in UPS research has been published recently 52.
Developments in peptide synthesis and protein chemistry have allowed the incorporation of a range of labels at both the C and N termini. McGouran et al. introduced probes containing a fluorophore (Cy5 or fluorescein) at the C terminus. In‐gel fluorescence gave better resolution of labeled proteins than anti‐HA immunoblotting, particularly for high MW proteins, as no transfer step is necessary prior to visualization, but the probe was somewhat less reactive than unmodified HA‐Ub‐VME. This was attributed to the size and charge of the fluorophore, which is positioned close to the DUB active site 53. By contrast, probes with an N‐terminal fluorophore, as reported by de Jong et al. 54, showed the same activity toward DUBs as unmodified HA‐Ub‐VME. These N‐terminal conjugates were prepared by total chemical synthesis, allowing great flexibility in the choice of modification: a variety of dyes (Cy5 and TMR), affinity tags (HA, His6, and biotin), and linkers (aminohexanoic acid, lysine, and photocleavable linker) could be incorporated by this method. Additionally, dual‐function probes were prepared, for example, containing both a biotin (for affinity purification of labeled DUBs) and a Cy5 (for fluorescence imaging). Claessen et al. devised an alternative strategy for N‐terminal functionalization, which used ‘sortagging’ (i.e., sortase‐mediated ligation between appropriately tagged protein/peptide fragments) to introduce a biotin label. Additionally, they incorporated a chemically cleavable linker between biotin and the Ub N terminus. These linkers allowed elution and MS identification of probe‐reactive proteins after streptavidin–biotin affinity purification 55.
Recognition element
All DUBs must bind Ub close to the catalytic active site. Therefore, mono‐Ub can be employed as the recognition element in ABPs to target many DUBs, even those DUBs for which mono‐Ub is not the preferred substrate 32. Full‐length ubiquitin is necessary for an effective reaction between probe and DUBs. This is in contrast to ABPs for many proteases, where only short peptide recognition elements are required. The requirement for full‐length ubiquitin results from several factors:
The catalytic domains of DUBs contain large Ub‐binding surfaces 56. Many interactions therefore contribute to the affinity of DUBs for Ub, which are difficult to mimic with a small recognition element;
There is a short channel leading from the Ub‐binding surface to the active site. This provides selectivity for the C‐terminal tail of Ub and Ubls, but contributes only weakly to binding. For USP2, both the Ub ‘core’ and tail are required for binding: Ub C‐terminal peptides or Ub truncation mutants lacking any more than the C‐terminal Gly‐Gly motif showed negligible binding to the catalytic domain 57;
Structural rearrangements in the DUB catalytic domain occur upon Ub binding. Many apo DUB catalytic domains are in catalytically inactive conformations 18, and would be unreactive toward ABPs. Ub binding can bring active site residues into the correct alignment for catalysis, as occurs for USP7 58, or cause the translocation of surface loops that otherwise block the active site, as seem for USP14 59.
Borodovsky et al. have investigated Ub and Ubl C‐terminal peptides as recognition elements for ABPs. They synthesized a range of VS‐containing peptides of varying lengths, based on the C termini of Ubls. Labeling in lysate by [125I]‐Ub‐VS could only be inhibited by preincubation with high concentrations (> 50 μm) of a 12‐mer peptide based on the Ub C terminus, but shorter sequences were ineffective. By contrast, short recognition elements may be suitable for probes of SENP proteases that remove the ubiquitin‐like molecule SUMO from proteins 60. It is not immediately apparent why SENP probes would require a much shorter recognition element than DUB probes. Borodovsky et al. 60 note that SENPs recognize a smaller surface area of their substrate in the catalytic site compared to DUBs, and hence may be able to bind to shorter peptide sequences.
Cellular permeability
Deubiquitinase/Ubl protease (ULP) ABPs are generally not cell‐permeable, due to their large recognition elements. Probe treatment must therefore be carried out on cell lysates. Preferably, DUBs would be labeled in their native cellular environment, so as not to disrupt subcellular localization, weak protein–protein interactions, and other factors that may affect DUB activity. Both pore‐forming toxins 55 and electroporation 61 have been used to deliver Ub‐containing probes to cells. Subsequent ABPP experiments were successful in both cases, but it is not clear whether this technique is more sensitive than labeling in lysates. Shorter recognition elements may improve cell permeability, but disappointingly small peptidic AOMK‐based inhibitors for SENPs did not appear to be cell‐permeable 43.
Ward et al. 31 recently described the first cell‐permeable DUB ABP. A high‐throughput screen for DUB inhibitors identified a chloromethylketone‐containing inhibitor of USP activity. To convert this into an ABP, the authors prepared an alkyne‐tagged analog (Table 1). Competitive ABPP, in which intact U2OS cells were pretreated with varying concentrations of the untagged inhibitor followed by labeling with 125 nm of probe, was used to identify specific probe targets. Twelve USPs were identified, of which nine could be competed away by excess unlabeled probe. The probe also labeled several recombinant USPs that are not expressed in U2OS cells. However, the probe targets many non‐DUB proteins, presumably through nonspecific reaction with reactive Cys residues.
Diubiquitin probes
The mono‐Ub ABPs described above rely solely on the interaction between Ub and the S1 site (which binds the Ub distal to the scissile bond) for affinity and specificity. While some DUBs, notably those of the UCH family, prefer to process Ub with only short C‐terminal extensions, most DUBs cleave Ub from ubiquitinated substrates. These substrates may be ubiquitin itself, or other proteins. DUBs can display specificity for position in the Ub chain (exo, endo, or base cleavage) or linkage type using additional Ub‐binding sites, which can recognize Ub on the distal side (S1, S2 sites) or proximal side (in S1′ sites) (Fig. 3). Di‐Ub APBs have therefore been developed in order to study activity and linkage specify for such DUBs. Table 2 summarizes the di‐Ub probes reported to date, along with the linkage types that were studied with each probe type.
Figure 3.
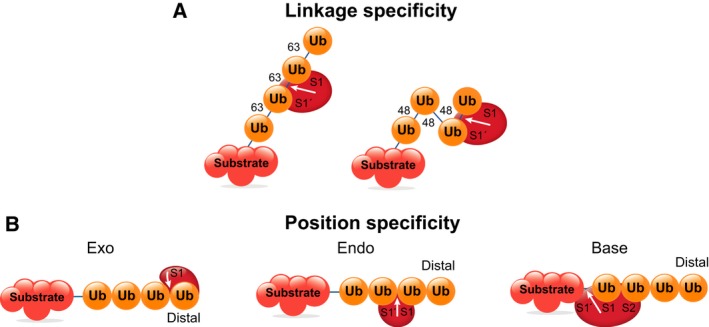
Deubiquitinases specificity. Ub‐binding sites in the DUB can convey specificity for: a particular linkage type such as K63‐ or K48‐linked chains (A); or position within the Ub chain (B) 18.
Table 2.
Designs of di‐Ub‐based ABPs
| Electrophile structure | Linkages | Reference | |
|---|---|---|---|
| 1 |

|
K48, K63 | 62 |
| 2 |

|
K48, K63 | 64 |
| 3 |

|
M1a, K6, K11, K27, K29, K33, K48, K63 | 65 |
| 4 |

|
M1a, K48, K63 | 66 |
| 5 |

|
K6, K11, K27, K29, K33, K48, K63 | 68 |
| 6 |

|
K6, K11, K27, K29, K33, K48, K63 | 67 |
Not an effective mimic of linear M1 linkage for DUBs (see discussion in text).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The first di‐Ub ABPs were reported by Iphöfer et al., who used short peptides conjugated through Lys isopeptide bonds to mimic the proximal Ub (structure 1, Table 2) 62. Probes mimicking K48‐ and K63‐linked di‐Ub showed the expected reactivity toward a small number of recombinant DUBs, and labeled different sets of DUBs in lysate, as determined by gel‐ and MS‐based ABPP experiments. It is apparent from structural studies, however, that some DUBs can make extensive contacts with proximal Ub in the S1′ site 63; probes containing short peptides are therefore unlikely to recapitulate the reactivity and selectivity of full‐length di‐Ub. Subsequent efforts have therefore focused on making di‐Ub probes containing two intact Ub monomers.
Several groups have recently reported strategies to make full‐length di‐Ub probes, either by intein‐based semisynthesis using recombinant protein (structure 2, Table 2) 64 or total chemical synthesis (structures 4–6, Table 2) 65, 66, 67. Generally, these probes show the expected reactivity toward DUBs with known linkage selectivity: for example, the generally unselective USP DUBs react with most probes, while the K48‐selective DUB OTUB1 reacts preferentially with K48‐mimicking probes. The activity of most probes was verified in lysate 62, 64, 65, 67, 68.
While it appears these probes can effectively mimic chains linked via Ub Lys residues, it has proved challenging to develop probes mimicking M1 linear Ub. The triazole‐containing linkage employed by McGouran et al. (structure 3, Table 2) was designed to mimic an isopeptide linkage and is significantly longer and more flexible than the native M1 peptide linkage. It did not react with known M1‐specific DUB OTULIN, but did react with USP13, USP25, and USP40, which show little activity toward the M1 chains in vitro 65. The dehydroalanine‐containing M1 probe reported by Haj‐Yahya et al. (structure 4, Table 2) was cleaved by the M1‐specific DUBs OTULIN and USP2, rather than reacting covalently with the electrophile. The dehydroalanine was in the P1′ site, and it appears to be inaccessible to the catalytic Cys 66.
Uses of DUB ABPs
Deubiquitinase and ULP APBs have found extensive use in a wide variety of studies, but a comprehensive analysis of all the reported uses of such probes is beyond the scope of this review. Instead, we summarize the principal applications of these probes, highlighting some key publications and recent developments. In addition to the uses described below, covalent DUB inhibitors such as Ubal and Ub‐VME have been used in crystallographic studies to determine the structures of DUBs bound to their substrate 36, 58, 69. However, only the recognition element and warhead are needed for this application. As a label is not required, these tools are not considered ABPs and will not be discussed further here.
Identification of novel DUBs
Activity‐based probes have proven to be invaluable tools to identify new DUBs and ULPs in a variety of organisms. In one of the earliest applications of ABPs to identify DUBs, Borodovsky et al. used [125I]‐Ubal to label DUBs in yeast and human cell lysates. The authors compared labeling in mutants lacking putative DUBs to identify labeled proteases. Comparison with labeling in human cells led to the identification of USP14 (the homolog of yeast Ubp6p) as a proteasome‐associated DUB 26. However, this approach to identifying DUBs is cumbersome and requires prior knowledge of candidate proteins. The combination of tandem mass spectrometry (LC‐MS/MS) for protein identification, and tagged probes for enrichment of labeled proteins, has allowed DUBs to be identified with high sensitivity in an unbiased manner. Notably, ABPs were used to identify the OTU family of DUBs 32, 70.
Mono‐Ub ABPs have also been widely used to identify DUBs in pathogenic organisms, including viruses (Adenoviridae 49 and Herpesviridae 71, 72), bacteria (Chlamydia trachomatis 55, 73, Legionella pneumophilia 74, and Escherichia coli 75), parasitic protozoa (Toxoplasma gondii 76 and Plasmodium falciparum 77), and nematodes (Trichinella spiralis 78). Prokaryotes and viruses, unlike eukaryotic pathogens, lack their own ubiquitination machinery 79. Therefore, pathogens have probably evolved this function in order to ‘hijack’ the host UPS, although some of these apparent DUBs may have preferred pathogenic substrates that happen to share some similarity with Ub/Ubl conjugates.
A recent study by Pruneda et al. 80 has examined the deubiquitinating activity of multiple bacterial proteases. Using a combination of ABPs (Ub‐PA) 38 and fluorescent substrates 81, the authors verified the deubiquitinase and/or deneddylase activity of several previously proposed bacterial DUBs and identified three novel enzymes (in Rickettsia bellii, Shigella flexneri, and Xanthomonas campestris). Notably, these bacterial DUBs all belonged to the CE protease clan, which in humans is limited to deneddylases and desumoylases. As bacterial members of the CE clan can also act as acetyltransferases, it appears that a single catalytic fold has evolved in different organisms to perform remarkably diverse functions.
Characterization of DUB selectivity
Using ABPs to examine DUB selectivity has only become possible since the development of di‐Ub ABPs. McGouran et al. profiled DUB activity in cell lysate by tandem MS with a panel of di‐Ub probes, but differences in geometry between the native isopeptide linkage and the triazole linkage employed in the probe (structure 3, Table 2) may alter reactivity 65. Comprehensive MS‐based ABPP experiments with newer di‐Ub probes have not yet been reported.
Several recent examples highlight the potential of di‐Ub ABPs to study the linkage selectivity of DUBs. Studies on substrate selectivity have used di‐Ub 82, or more recently, di‐Ub‐based ABPs with an internal electrophile (structure 5, Table 2) 83, 84. These substrates allow analysis of S1–S1′ preferences, but do not provide any information on the effect of S2 sites (Fig. 2A). Flierman et al. used di‐Ub ABPs with a terminal electrophile (structure 6, Table 2) in combination with di‐Ub substrate probes with a terminal fluorophore to study the effect of S2 sites 67. They observed significant differences between S1–S1′ and S1–S2 selectivity for OTU DUBs. For example, OTUD2 is fairly promiscuous in cleaving di‐Ub substrates, but shows a preference for the K11‐linked di‐Ub terminal ABP, suggesting that the S2 pocket refines the selectivity of this DUB toward K11‐linked chains. OTUD3 can cleave both K6 and K11‐linked di‐Ub, but only reacts with the K11 di‐Ub probe. Kinetic studies using di‐Ub substrate probes (di‐Ub‐AMC) suggested that the selectivity of OTUD2 for longer K11‐linked chains is driven by affinity (lower KM), while for OTUD3, it appears to be driven by vmax, hinting that binding of K11 chains to OTUD3 may optimally reorganize the active site for catalysis. As the authors note, different selectivities across the S1–S1′ and S1–S2 sites suggest that the preferred endogenous substrates for these DUBs may be heterotypic chains. Testing this hypothesis will require the development of tri‐Ub reagents with mixed linkage types.
A similar influence of S1–S2 preference is observed in the papain‐like protease of severe acute respiratory syndrome coronavirus (SARS PLpro). The enzyme is known to cleave K48‐linked polyubiquitin chains with a minimal preferred substrate of three Ub units, leading to the accumulation of diUbK48 85. To explore this striking selectivity, Békés et al. compared reactivity of the enzyme toward K48‐linked di‐Ub probes with either an internal (structure 5, Table 2) or a distal electrophile (structure 6, Table 2), and a mono‐Ub‐PA probe 86. The enzyme reacted rapidly with the distal K48‐linked di‐Ub probe, slowly with Ub‐PA and very slowly with the internal di‐Ub probe. This led the authors to conclude that SARS PLpro recognizes the K48 linkage between two distal Ub (S1 and S2), while cleaving between S1 and S1′. (K48 specificity across S1–S1′ is much weaker than across S1–S2.) The distal K48‐linked di‐Ub probe was subsequently used in structural studies to understand how SARS PLpro binds K48‐linked di‐Ub. Taken together, these studies highlight the value of using ABPs in combination with other tools, including substrate probes, to study the complexities of DUB selectivity.
Profiling DUB activity across different conditions or stimuli
Deubiquitinase ABPs can be used to identify changes in DUB activity under different conditions or in different cell types. Some of the first studies employing DUB APBs profiled DUB activity in tumor, normal, and virus‐infected cells 87, and in pairs of normal and tumor biopsies from cervical carcinoma patients 88. However, identifying consistent changes in the patterns of DUB activity was challenging, and the biological significance of the observed differences was unclear. Other studies have taken a more focused approach: applying a stimulus to cells, monitoring changes in DUB activity, identifying DUBs with large changes in activity, then validating their role in the system of interest. A notable example of this strategy is the identification of USP7 as a regulator of adipogenesis 89. HA‐Ub‐VS was used to monitor changes in DUB activity in an in vitro adipogenesis model, which identified a substantial increase in USP7 activity. Further experiments confirmed that USP7 was required for adipogenesis, and that it exerted its effect through the deubiquitniation and stabilization of the acetyltransferase Tip60, a key regulator of adipocyte differentiation.
This approach has also been used to study the effects of infection on host DUBs. Kummari et al. 90 used HA‐Ub‐VS to profile DUB activity in Salmonella‐infected chicken macrophages, and identified several DUBs that change substantially in activity following infection. UCHL3 activity decreased (although protein levels remained similar), while the proteasome‐associated DUB UCHL5 increased in both activity and protein level. The authors went on to demonstrate that UCHL5 expression increases IL‐1β secretion, and increases the levels of caspase‐1, a key member of the inflammasome that processes pro‐IL‐1β into its active form. This study, and others 91, point toward a role for DUBs such as UCHL5 in the innate immune response to bacterial infection, through modulation of inflammasome formation, cytokine section, and pyroptosis. The molecular mechanisms underlying the possible role of UCHL5 in inflammasome regulation, including the targets of deubiquitinase activity, are as yet unclear.
The studies highlighted here demonstrate the potential of ABPP to identify DUBs involved in cellular processes, exemplified here by differentiation or the innate immune response. ABPP offers an alternative approach to functional genomics techniques, such as overexpression 92 or siRNA 93 screens for DUBs. (Notably, overexpression screens have also identified USP7 as a Tip60 deubiquitinase 94, and have identified DUBs involved in inflammasome formation 95.) As ABPP measures endogenous DUB activity, it avoids the problems introduced by artificially overexpressing or reducing gene expression, such as changes in viability or off‐target effects. ABPP also has the advantage of experimental simplicity, as no transfection is required and DUB activity is measured in a single experiment, either a gel‐based assay or tandem MS. However, the activity of many DUBs may change between experimental and control conditions, and identifying those that are functionally relevant can be challenging. Additionally, ABPs are not equally reactive toward all DUBs, and so changes in activity of DUBs that are unreactive toward the probe (such as the metalloprotease JAMM/MPN+ family) will be missed.
DUB inhibitor screening
Activity‐based protein profiling is widely used to assess inhibitor selectivity 96. In a typical experiment, often referred to as competitive ABPP, cells are incubated with compounds of interest, then lysed and treated with an HA‐tagged APB. (Compound treatment can also be performed using lysate 97.) Comparison with an untreated control indicates the level of inhibition. As with experiments to profile DUB activity in cells, gel‐based methods provide a rapid readout 97, but this approach is at best semiquantitative, and identifying the compound's targets is challenging. Quantitative MS can overcome these limitations, at the expense of experimental simplicity 51.
Notably, ABPP has been used to assess selectivity during the development of VLX1570, the only DUB inhibitor so far to progress to clinical trials 98. The hit compound b‐AP15 was initially identified as an inducer of the lysosomal apoptosis pathway 99, and was subsequently shown to inhibit the proteasome 100. Gel‐based analysis of labeling by HA‐Ub‐VS showed little global effect of this compound on DUB activity at 1 μm, but labeling of the isolated 26S proteasome or 19S regulatory particle was inhibited. The 19S regulatory particle contains two Cys‐protease DUBs, USP14 and UCHL5, both of which are inhibited by this compound, with slightly greater inhibitory activity toward USP14 100. VLX1570 showed similar selectivity toward USP14 and UCHL5, a finding confirmed by conventional inhibition assays using recombinant DUBs and a fluorogenic substrate 101. b‐AP15 (and presumably VLX1570) are believed to be reversible inhibitors of proteasomal DUBs; nonetheless, they are able to inhibit labeling by irreversible Ub‐VS probes 102. It should be noted that reversible and irreversible inhibitors behave quite differently in competitive ABPP experiments: the degree of inhibition by irreversible inhibitors will be sensitive to the time between inhibitor treatment and labeling, while reversible inhibitors will be affected by changes in concentration between inhibitor treatment and labeling. For a detailed discussion of DUB inhibitors and their selectivity, several recent reviews are available 103, 104, 105, 106.
E1, E2, and E3 enzymes
Activity‐based probes for the E1, E2, and E3 enzymes have received much less attention than ABPs for DUBs. It has been suggested that it is more difficult to trap the active E1, E2, and E3 enzymes with electrophiles due to the lower reactivity of the catalytic Cys residue 61, but it is difficult to compare the relative reactivity of Cys residues across different enzymes. There are also many fewer E1 and E2 enzymes than DUBs, so there may be less interest in studying the activity of all members of the enzyme class simultaneously. For E3s, only the HECT and RBR ligases have a catalytic Cys residue, and so most E3s cannot be studied using ABPs. Nonetheless, recent ABPs for the Ub conjugation machinery have provided insights that would have been difficult to obtain using more conventional techniques. Furthermore, the entry of E1 inhibitors and E3 antagonists and inhibitors into the clinic provides additional motivation for developing techniques to study the activity of these enzymes.
E1
Prior to the development of ABPs for E1 enzymes, tools were designed for use in structural studies to capture the covalent intermediates in the E1 reaction mechanism. This closely parallels the use of Ubal to obtain Ub‐bound DUB structures, prior to the development of DUB ABPs. In an elegant study, Olsen et al. developed a SUMO~AMP mimic to trap the tetrahedral intermediate (E1~SUMO~AMP) formed by the catalytic Cys residue (Fig. 4A) 107. In this compound, SUMO1‐AVSN, the acyl‐phosphate linkage of SUMO1~AMP is replaced with a vinyl sulfonamide. They also developed an unreactive SUMO~AMP mimic incorporating an unreactive acyl‐sulfamide (SUMO1‐AMSN). Structures of SUMO E1 bound to the two compounds revealed major differences in protein architecture. The initial E1:SUMO‐AMSN complex adopts an ‘open’ conformation, in which the active site is organized for ATP binding and the catalytic Cys is buried and far from the SUMO adenylate. By contrast, the covalent complex E1~SUMO‐AVSN adopts a ‘closed’ conformation with a radically different active site: residues involved in catalyzing the first step have been ‘swapped’ for those required for the transesterification reaction. Therefore, E1 enzymes appear not to have two spatially distinct active sites; rather, the region around the SUMO C terminus and AMP is remodeled after the adenylation reaction in order to catalyze transesterification. These synthetic SUMO adenylate mimics were vital for crystallizing the E1 enzymes in otherwise transient states, illuminating the complex dynamics of these enzymes during catalysis. Similar probes will also react with and inhibit Ub E1 enzymes 108.
Figure 4.

Activity‐based probes for the Ub conjugation machinery. (A) E1 probes that label the enzyme active site (Ubl~AMP shown for comparison): Ubl‐AVSN resembles Ubl~AMP but reacts with E1 to form a covalent mimic of the E1:Ubl~AMP complex; Ubl‐AMSN is an unreactive Ubl~AMP analog 107, 108; Ub‐Probe3 reacts in a similar way to Ubl‐AVSN, but incorporates an alkyne tag for detection 109; (B) an E1 ABP, ABP1 41, which labels Ub, and the NEDD8 E1 inhibitor MLN4924 (pevonedistat) which shares a similar structure and mechanism; (C) transthiolation probes for the E1‐E2 reaction 114 and the E2‐E3 reaction 115; (D) ‘cascading’ ABP Ub‐Dha, which can label E1, E2, and some E3 enzymes 61.
Despite the utility of SUMO1‐AVSN in trapping the tetrahedral intermediate in the E1~SUMO~AMP complex, it has several drawbacks as a broadly applicable ABP, including lengthy synthesis, a requirement for a fixed, non‐native sequence at the C terminus of the Ubl, and a lack of a label for detection. To overcome these limitations, An and Statsyuk developed an alternative probe design which used a dehydroalanine (Dha) moiety in place of a vinyl sulfone to trap the catalytic Cys, simplifying synthesis and preserving the C‐terminal sequence of the Ubl (‘Ub‐Probe3’, Fig. 4A) 109. Additionally, they incorporated an alkyne tag into the adenine moiety for detection. A wide variety of Ubl‐containing probes could be prepared by their method, and they validated the reactivity of Ub‐ and LC3‐containing probes toward the relevant E1 enzymes (UBA1 and ATG7). Unfortunately, a Ub‐containing probe was cleaved by the DUB IsoT (USP5). The reactivity of other Ubl‐containing probes toward ULPs was not investigated, but this may limit their applicability in studies where DUBs/ULPs are present.
In a separate publication, An and Statsyuk reported an ABP for E1 enzymes that labels the substrate (Ub/Ubl) rather than the enzyme 41. The probe, referred to as ABP1 (Fig. 4B), takes advantage of the reversibility of the transthiolation step, in which the E1:AMP~Ub complex transfers Ub to the catalytic Cys to form E1~Ub + AMP (Fig. 1B). The authors designed an AMP mimic which would react with the E1~Ub complex to form a stable AMP~Ub analog. As both the adenylation and transthiolation steps must have occurred prior to probe reaction, formation of the AMP~Ub analog reports on E1 activity. ABP1 is an analog of pevonedistat (MLN4924), an inhibitor of the NEDD8 E1 enzyme NAE which is currently in clinical trials 110 (Fig. 4B). ABP1 differs from pevonedistat in showing pan‐E1 reactivity, and additionally includes an alkyne for detection or enrichment (though ‘click’ reactions to fluorophore‐azide or biotin‐azide, respectively). The probe will react with a variety of E1 enzymes in vitro, in the presence of their cognate Ub/Ubl protein and ATP. Significantly, ABP1 is cell‐permeable, and was used to characterize the intracellular potency and selectivity of E1 inhibitors. A close analog of ABP1, ABP3, selectively labeled Ub and NEDD8 over other Ubls, suggesting selectivity toward the Ub and NEDD8 E1 enzymes 111. An unexpected advantage of these probes is that the N‐acylsulfamate linker, formed between the Ub/Ubl and the probe, is cleaved under acidic conditions. This facilitates subsequent proteomic studies, as proteins can be eluted under relatively mild conditions after biotin–streptavidin affinity purification, reducing background from nonspecifically bound proteins 112, 113.
The probes described above are useful for studying the Ub‐activating activity of E1 enzymes. However, E1 enzymes are also involved in a second process, the transfer of Ub to E2 enzymes. Stanley et al. 114 have developed ABPs to profile this E1‐E2 transthiolation reaction, which consist of an E2 enzyme tagged with a electrophile on the catalytic Cys. These probes undergo a proximity‐dependent reaction with the catalytic Cys of active E1 enzymes (Fig. 4C). A probe derived from the E2 UBE2N selectively labeled the E1 UBA1 in cell lysate, and could be used in a competitive ABPP experiment to test E1 inhibitors. Perhaps surprisingly, E2‐derived probes did not label E3s. The authors suggest that Ub in E2~Ub is required to induce a transthiolation‐competent conformation of the E3. Indeed, the authors have subsequently reported probes containing a Ub moiety as RBR ligase ABPs 115, which are discussed below.
E3
Although the majority of E3 ligases do not form a covalent intermediate with Ub, those of the HECT and RBR families accept Ub from E2 enzymes in a transthiolation reaction, and subsequently transfer it to substrates in an aminolysis step. The covalent nature of the intermediate makes the HECT and RBR E3s suitable candidates for the development of ABPs. Only recently have the first ABPs for the transthiolation step been described 115, which build on the E2‐derived probes for E1‐E2 transthiolation described above 114. In those probes, the E2 active site was modified to trap cognate E1 enzymes, but they did not react with E3s, which additionally require E2‐bound Ub to activate E3 enzymes for transthiolation. Pao et al. extended these probes with a Ub moiety to generate an E2~Ub conjugate with an internal electrophile (Fig. 4C). For ease of detection, the E2 enzyme could be prepared with a His6 epitope tag and an alkyne tag, allowing conjugation to a fluorophore for rapid detection of labeled species by in‐gel fluorescence. A UBE2L3‐containing probe labeled the E3 ligase parkin, a member of the RBR family encoded by the PARK6 gene, which is mutated in an autosomal‐recessive form of Parkinson's disease (PD). The authors tested the transthiolation activity of recombinant parkin mutants derived from patients with PD, and found that 10 of 12 mutants tested had reduced transthiolation activity. The probes were also used to investigate parkin activation in cells. Mitochondrial depolarization activates the kinase PINK1 (encoded by PARK6), which in turn activates parkin. In cells derived from PD patients with mutations in PARK2 or PARK6, chemical induction of mitochondrial depolarization did not activate parkin (as detected by probe labeling), in contrast to the activation observed in wild‐type cells. While the number of samples tested here was small, this observation suggests the possibility of using these probes to diagnose familial forms of PD in which the PINK1/parkin pathway is mutated.
We anticipate that the E3 ligase probes described by Pao et al. will find applications beyond the detailed dissection of a single E2‐E3 interaction. For example, the probes could be used to identify novel E3s that interact with a given E2, or could be used in competitive ABPP experiments to identify inhibitors or activators of E2‐E3 transthiolation. With the increasing sophistication of techniques to produce modified or fusion proteins, and to introduce reactive functionality, we expect that new methods to study E1, E2, and E3 enzymes will emerge over the next few years.
Probes to study multiple members of the Ub conjugation/deconjugation machinery
Deubiquitinase ABPs such as HA‐Ub‐VME cross‐react with E1, E2, and E3 enzymes, and for one HECT E3 ligase, this reaction was shown to occur on Cys residues 42. A Ub‐VS probe has been used to infer reactivity of a HECT E3 115, but clearly these probes are not optimized for studying multiple types of enzyme from the UPS simultaneously. Mulder et al. recently published an elegant method to target E1, E2, and E3 enzymes with a single ABP 61. The probe, Ub‐Dha (Fig. 4D), can be activated by E1 enzymes in the same manner as Ub and passed sequentially along E2 and E3 enzymes. At each transthiolation step, the probe also has the option of reacting irreversibly with the active site Cys. Importantly, the probe does not become conjugated to target proteins, and appears rather unreactive toward DUBs. The probe could be labeled with a fluorophore or biotin, the latter being used to enrich labeled proteins for proteome‐wide profiling of the Ub conjugation machinery by tandem MS. The authors also delivered the probe to cells by electroporation, potentially overcoming the limitations of performing labeling experiments in lysate, where disruption of intracellular structures and dilution of the cytosol may well reduce the ability of the probe to rapidly label many proteins in the Ub conjugation machinery.
Perspective
There now exists an extensive ‘toolbox’ of ABPs for DUBs, and ABPs for E1, E2, and E3 enzymes have recently been reported. Nevertheless, several challenges remain:
Cell permeability. Most ABPs for DUBs probes and the Ub conjugation machinery are not cell‐permeable. Studies on the proteasome have shown substantial differences between labeling in lysate and labeling in live cells. Cell‐permeable DUB and E1‐E3 ABPs would open up substantial new opportunities, including the exciting possibility of studying the activity of these enzymes in living organisms. Ward et al. have recently reported a cell‐permeable ABP for USP DUBs by modifying a covalent DUB inhibitor 31, and this approach could be extended by attaching reporter tags to other inhibitors 104, 106.
Subtype selectivity of DUB probes. Broad‐spectrum DUB probes are extremely useful for ABPP experiments, but more selective probes would be useful to study a single enzyme or small subset of enzymes, for example, when examining the pharmacodynamics of a DUB inhibitor. Substrate‐selective DUB probes are likely to have increased sensitivity, and deconvolution of individual proteins becomes unnecessary. Selective small molecules or engineered Ub variants 116, 117 could provide suitable recognition elements.
M1‐linked di‐Ub probes. There is an increasing interest in M1‐linked chains and their corresponding deubiquitinases, which have important roles in inflammation 118, 119. ABPs for M1‐specific DUBs would be valuable tools for studying this physiologically intriguing linkage.
Probes for JAMM/MPN+ DUBs. The JAMM/MPN+ family DUBs are zinc‐dependent metalloproteases, and so do not form a covalent intermediate with the substrate. It is therefore challenging to develop ABPs that become covalently attached to the enzyme. Several groups have reported strategies to develop ABPs for metalloproteases: Cravatt incorporated a photocrosslinker and alkyne tag into a metal‐chelating inhibitor 120, while we have introduced a Cys residue into the protein of interest that could react with an electrophile on the probe 121. Similar strategies could be applied to develop covalent ABPs for JAMM/MPN+ DUBs.
E2 probes. Aside from the ‘cascading’ ABP reported by Mulder et al., there are no ABPs for E2 enzymes. E2‐based probes for the transthiolation activity of E1 and E3 enzymes have been described 114, 115 (Fig. 4C), and analogous probes based on E1 or E3 enzymes may be useful to study the transthiolation activity of E2s.
Effect of post‐translational modification of Ub. Recent studies have shown that Ub can be modified by phosphorylation or acetylation, with significant biological consequences (reviewed in 122). However, the mechanisms by which these modifications affect the function of Ub are poorly understood. Synthetic and semisynthetic methods to produce homogeneously modified Ub or Ub chains have been reported 29 but have not yet been applied to ABPs. Probes for the Ub conjugation or deconjugation machinery incorporating defined modifications could provide insights into how modification of Ub affects its function.
Ubiquitination is a versatile process, and correspondingly versatile techniques are required to study it. ABPs, by physically coupling protein to probe in an enzyme‐catalyzed reaction, are especially suited for studying the ubiquitinating and deubiquitinating enzymes. By recognizing what features are shared between enzymes (e.g., nucleophilic Cys residues, a Ub‐binding site close to the catalytic center), and what features differ (e.g., number of Ub‐binding sites), probes have been developed which can interrogate specific components of the ubiquitination/deubiquitination system. New probes will no doubt continue to emerge, inspired both by innovative methods in protein chemistry, and the fascinating biology and clinical importance of ubiquitin modification.
Acknowledgements
We thank Norman Cyr (Department of Pathology, Stanford University) for assistance with figures.
Author contributions
DSH, JAF, MB and IEW discussed and developed the concept of the review and edited the manuscript. DSH wrote the review.
Contributor Information
David S. Hewings, Email: hewingsd@gene.com.
Matthew Bogyo, Email: mbogyo@stanford.edu.
Ingrid E. Wertz, Email: ingrid@gene.com.
References
- 1. Hershko A & Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67, 425–479. [DOI] [PubMed] [Google Scholar]
- 2. Wilkinson KD (2005) The discovery of ubiquitin‐dependent proteolysis. Proc Nat Acad Sci USA 102, 15280–15282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ikeda F & Dikic I (2008) Atypical ubiquitin chains: new molecular signals. “Protein Modifications: Beyond the Usual Suspects” review series. EMBO Rep 9, 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Komander D (2009) The emerging complexity of protein ubiquitination. Biochem Soc Trans 37, 937–953. [DOI] [PubMed] [Google Scholar]
- 5. Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D & Gygi SP (2003) A proteomics approach to understanding protein ubiquitination. Nat Biotechnol 21, 921–926. [DOI] [PubMed] [Google Scholar]
- 6. Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK & Varshavsky A (1989) A multiubiquitin chain is confined to specific lysine in a targeted short‐lived protein. Science 243, 1576–1583. [DOI] [PubMed] [Google Scholar]
- 7. Hu H & Sun S‐C (2016) Ubiquitin signaling in immune responses. Cell Res 26, 457–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shimizu Y, Taraborrelli L & Walczak H (2015) Linear ubiquitination in immunity. Immunol Rev 266, 190–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herrmann J, Lerman LO & Lerman A (2007) Ubiquitin and ubiquitin‐like proteins in protein regulation. Circ Res 100, 1276–1291. [DOI] [PubMed] [Google Scholar]
- 10. Hemelaar J, Lelyveld VS, Kessler BM & Ploegh HL (2003) A single protease, Apg4B, is specific for the autophagy‐related ubiquitin‐like proteins GATE‐16, MAP1‐LC3, GABARAP, and Apg8L. J Biol Chem 278, 51841–51850. [DOI] [PubMed] [Google Scholar]
- 11. Maupin‐Furlow JA (2014) Prokaryotic ubiquitin‐like protein modification. Annu Rev Microbiol 68, 155–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearce MJ, Mintseris J, Ferreyra J, Gygi SP & Darwin KH (2008) Ubiquitin‐like protein involved in the proteasome pathway of Mycobacterium tuberculosis . Science 322, 1104–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scheffner M & Kumar S (2014) Mammalian HECT ubiquitin‐protein ligases: biological and pathophysiological aspects. Biochim Biophys Acta 1843, 61–74. [DOI] [PubMed] [Google Scholar]
- 14. Berndsen CE & Wolberger C (2014) New insights into ubiquitin E3 ligase mechanism. Nat Struct Mol Biol 21, 301–307. [DOI] [PubMed] [Google Scholar]
- 15. Heideker J & Wertz IE (2015) DUBs, the regulation of cell identity and disease. Biochem J 465, 1–26. [DOI] [PubMed] [Google Scholar]
- 16. Grou CP, Pinto MP, Mendes AV, Domingues P & Azevedo JE (2015) The de novo synthesis of ubiquitin: identification of deubiquitinases acting on ubiquitin precursors. Sci Rep 5, 12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee MJ, Lee B‐H, Hanna J, King RW & Finley D (2011) Trimming of ubiquitin chains by proteasome‐associated deubiquitinating enzymes. Mol Cell Proteomics 10, R110.003871–R1111000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Komander D, Clague MJ & Urbé S (2009) Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol 10, 550–563. [DOI] [PubMed] [Google Scholar]
- 19. Abdul Rehman SA, Kristariyanto YA, Choi S‐Y, Nkosi PJ, Weidlich S, Labib K, Hofmann K & Kulathu Y (2016) MINDY‐1 is a member of an evolutionarily conserved and structurally distinct new family of deubiquitinating enzymes. Mol Cell 63, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito T & Handa H (2016) Cereblon and its downstream substrates as molecular targets of immunomodulatory drugs. Int J Hematol 104, 293–299. [DOI] [PubMed] [Google Scholar]
- 21. Kojima K, Ishizawa J & Andreeff M (2016) Pharmacological activation of wild‐type p53 in the therapy of leukemia. Exp Hematol 44, 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fulda S (2015) Promises and challenges of Smac mimetics as cancer therapeutics. Clin Cancer Res 21, 5030–5036. [DOI] [PubMed] [Google Scholar]
- 23. Sanman LE & Bogyo M (2014) Activity‐based profiling of proteases. Annu Rev Biochem 83, 249–273. [DOI] [PubMed] [Google Scholar]
- 24. Prescher JA & Bertozzi CR (2005) Chemistry in living systems. Nat Chem Biol 1, 13–21. [DOI] [PubMed] [Google Scholar]
- 25. Cravatt BF, Wright AT & Kozarich JW (2008) Activity‐based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem 77, 383–414. [DOI] [PubMed] [Google Scholar]
- 26. Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD & Ploegh HL (2001) A novel active site‐directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J 20, 5187–5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gopinath P, Ohayon S, Nawatha M & Brik A (2016) Chemical and semisynthetic approaches to study and target deubiquitinases. Chem Soc Rev 45, 4171–4198. [DOI] [PubMed] [Google Scholar]
- 28. Hameed DS, Sapmaz A & Ovaa H (2016) How chemical synthesis of ubiquitin conjugates helps to understand ubiquitin signal transduction. Bioconjugate Chem. doi: 10.1021/acs.bioconjchem.6b00140. [DOI] [PubMed] [Google Scholar]
- 29. van Tilburg GB, Elhebieshy AF & Ovaa H (2016) Synthetic and semi‐synthetic strategies to study ubiquitin signaling. Curr Opin Struct Biol 38, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weller CE, Pilkerton ME & Chatterjee C (2014) Chemical strategies to understand the language of ubiquitin signaling. Biopolymers 101, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ward JA, McLellan L, Stockley M, Gibson KR, Whitlock GA, Knights C, Harrigan JA, Jacq X & Tate EW (2016) Quantitative chemical proteomic profiling of ubiquitin specific proteases in intact cancer cells. ACS Chem Biol 11, 3268–3272. [DOI] [PubMed] [Google Scholar]
- 32. Borodovsky A, Ovaa H, Kolli N & Gan‐Erdene T (2002) Chemistry‐based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol 9, 1149–1159. [DOI] [PubMed] [Google Scholar]
- 33. Lam YA, Xu W, DeMartino GN & Cohen RE (1997) Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature 385, 737–740. [DOI] [PubMed] [Google Scholar]
- 34. Pickart CM & Rose IA (1986) Mechanism of ubiquitin carboxyl‐terminal hydrolase. Borohydride and hydroxylamine inactivate in the presence of ubiquitin. J Biol Chem 261, 10210–10217. [PubMed] [Google Scholar]
- 35. Hershko A & Rose IA (1987) Ubiquitin‐aldehyde: a general inhibitor of ubiquitin‐recycling processes. Proc Nat Acad Sci USA 84, 1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnston SC, Riddle SM, Cohen RE & Hill CP (1999) Structural basis for the specificity of ubiquitin C‐terminal hydrolases. EMBO J 18, 3877–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hemelaar J, Borodovsky A, Kessler BM, Reverter D, Cook J, Kolli N, Gan‐Erdene T, Wilkinson KD, Gill G, Lima CD et al (2004) Specific and covalent targeting of conjugating and deconjugating enzymes of ubiquitin‐like proteins. Mol Cell Biol 24, 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ekkebus R, van Kasteren SI, Kulathu Y, Scholten A, Berlin I, Geurink PP, de Jong A, Goerdayal S, Neefjes J, Heck AJR et al (2013) On terminal alkynes that can react with active‐site cysteine nucleophiles in proteases. J Am Chem Soc 135, 2867–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sommer S, Weikart ND, Linne U & Mootz HD (2013) Covalent inhibition of SUMO and ubiquitin‐specific cysteine proteases by an in situ thiol‐alkyne addition. Bioorg Med Chem 21, 2511–2517. [DOI] [PubMed] [Google Scholar]
- 40. Arkona C & Rademann J (2013) Propargyl amides as irreversible inhibitors of cysteine proteases–a lesson on the biological reactivity of alkynes. Angew Chem Int Ed Engl 52, 8210–8212. [DOI] [PubMed] [Google Scholar]
- 41. An H & Statsyuk AV (2013) Development of activity‐based probes for ubiquitin and ubiquitin‐like protein signaling pathways. J Am Chem Soc 135, 16948–16962. [DOI] [PubMed] [Google Scholar]
- 42. Love KR, Pandya RK, Spooner E & Ploegh HL (2009) Ubiquitin C‐terminal electrophiles are activity‐based probes for identification and mechanistic study of ubiquitin conjugating machinery. ACS Chem Biol 4, 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Albrow VE, Ponder EL, Fasci D, Békés M, Deu E, Salvesen GS & Bogyo M (2011) Development of small molecule inhibitors and probes of human SUMO deconjugating proteases. Chem Biol 18, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dobrotă C, Fasci D, Hădade ND, Roiban GD, Pop C, Meier VM, Dumitru I, Matache M, Salvesen GS & Funeriu DP (2012) Glycine fluoromethylketones as SENP‐specific activity based probes. ChemBioChem 13, 80–84. [DOI] [PubMed] [Google Scholar]
- 45. Lee J‐G, Baek K, Soetandyo N & Ye Y (2013) Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat Commun 4, 1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cotto‐Rios XM, Békés M, Chapman J, Ueberheide B & Huang TT (2012) Deubiquitinases as a signaling target of oxidative stress. Cell Rep 2, 1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Naik E & Dixit VM (2016) Usp9X is required for lymphocyte activation and homeostasis through its control of ZAP70 ubiquitination and PKCβ kinase activity. J Immunol 196, 3438–3451. [DOI] [PubMed] [Google Scholar]
- 48. Wang T, Yin L, Cooper EM, Lai M‐Y, Dickey S, Pickart CM, Fushman D, Wilkinson KD, Cohen RE & Wolberger C (2009) Evidence for bidentate substrate binding as the basis for the K48 linkage specificity of otubain 1. J Mol Biol 386, 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Balakirev MY, Jaquinod M, Haas AL & Chroboczek J (2002) Deubiquitinating function of adenovirus proteinase. J Virol 76, 6323–6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gan‐Erdene T, Nagamalleswari K, Yin L, Wu K, Pan Z‐Q & Wilkinson KD (2003) Identification and characterization of DEN1, a deneddylase of the ULP family. J Biol Chem 278, 28892–28900. [DOI] [PubMed] [Google Scholar]
- 51. Altun M, Kramer HB, Willems LI, McDermott JL, Leach CA, Goldenberg SJ, Kumar KGS, Konietzny R, Fischer R, Kogan E et al (2011) Activity‐based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem Biol 18, 1401–1412. [DOI] [PubMed] [Google Scholar]
- 52. Lill JR & Wertz IE (2014) Toward understanding ubiquitin‐modifying enzymes: from pharmacological targeting to proteomics. Trends Pharmacol Sci 35, 187–207. [DOI] [PubMed] [Google Scholar]
- 53. McGouran JF, Kramer HB, Mackeen MM, di Gleria K, Altun M & Kessler BM (2012) Fluorescence‐based active site probes for profiling deubiquitinating enzymes. Org Biomol Chem 10, 3379–3383. [DOI] [PubMed] [Google Scholar]
- 54. de Jong A, Merkx R, Berlin I, Rodenko B, Wijdeven RHM, El Atmioui D, Yalçin Z, Robson CN, Neefjes JJ & Ovaa H (2012) Ubiquitin‐based probes prepared by total synthesis to profile the activity of deubiquitinating enzymes. ChemBioChem 13, 2251–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Claessen JHL, Witte MD, Yoder NC, Zhu AY, Spooner E & Ploegh HL (2013) Catch‐and‐release probes applied to semi‐intact cells reveal ubiquitin‐specific protease expression in Chlamydia trachomatis infection. ChemBioChem 14, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ronau JA, Beckmann JF & Hochstrasser M (2016) Substrate specificity of the ubiquitin and Ubl proteases. Cell Res 26, 441–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Renatus M, Parrado SG, D'Arcy A, Eidhoff U, Gerhartz B, Hassiepen U, Pierrat B, Riedl R, Vinzenz D, Worpenberg S et al (2006) Structural basis of ubiquitin recognition by the deubiquitinating protease USP2. Structure 14, 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu M, Li P, Li M, Li W, Yao T, Wu J‐W, Gu W, Cohen RE & Shi Y (2002) Crystal structure of a UBP‐family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 111, 1041–1054. [DOI] [PubMed] [Google Scholar]
- 59. Hu M, Li P, Song L, Jeffrey PD, Chernova TA, Wilkinson KD, Cohen RE & Shi Y (2005) Structure and mechanisms of the proteasome‐associated deubiquitinating enzyme USP14. EMBO J 24, 3747–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Borodovsky A, Ovaa H, Meester WJN, Venanzi ES, Bogyo MS, Hekking BG, Ploegh HL, Kessler BM & Overkleeft HS (2005) Small‐molecule inhibitors and probes for ubiquitin‐ and ubiquitin‐like‐specific proteases. ChemBioChem 6, 287–291. [DOI] [PubMed] [Google Scholar]
- 61. Mulder MPC, Witting K, Berlin I, Pruneda JN, Wu K‐P, Chang J‐G, Merkx R, Bialas J, Groettrup M, Vertegaal ACO et al (2016) A cascading activity‐based probe sequentially targets E1‐E2‐E3 ubiquitin enzymes. Nat Chem Biol 12, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Iphöfer A, Kummer A, Nimtz M, Ritter A, Arnold T, Frank R, van den Heuvel J, Kessler BM, Jänsch L & Franke R (2012) Profiling ubiquitin linkage specificities of deubiquitinating enzymes with branched ubiquitin isopeptide probes. ChemBioChem 13, 1416–1420. [DOI] [PubMed] [Google Scholar]
- 63. Keusekotten K, Elliott PR, Glockner L, Fiil BK, Damgaard RB, Kulathu Y, Wauer T, Hospenthal MK, Gyrd‐Hansen M, Krappmann D et al (2013) OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1‐linked polyubiquitin. Cell 153, 1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li G, Liang Q, Gong P, Tencer AH & Zhuang Z (2013) Activity‐based diubiquitin probes for elucidating the linkage specificity of deubiquitinating enzymes. Chem Commun 50, 216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McGouran JF, Gaertner SR, Altun M, Kramer HB & Kessler BM (2013) Deubiquitinating enzyme specificity for ubiquitin chain topology profiled by di‐ubiquitin activity probes. Chem Biol 20, 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haj‐Yahya N, Hemantha HP, Meledin R, Bondalapati S, Seenaiah M & Brik A (2014) Dehydroalanine‐based diubiquitin activity probes. Org Lett 16, 540–543. [DOI] [PubMed] [Google Scholar]
- 67. Flierman D, van der Heden van Noort GJ, Ekkebus R, Geurink PP, Mevissen TET, Hospenthal MK, Komander D & Ovaa H (2016) Non‐hydrolyzable diubiquitin probes reveal linkage‐specific reactivity of deubiquitylating enzymes mediated by S2 pockets. Cell Chem Biol 23, 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mulder MPC, El Oualid F, ter Beek J & Ovaa H (2014) A native chemical ligation handle that enables the synthesis of advanced activity‐based probes: diubiquitin as a case study. ChemBioChem 15, 946–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Misaghi S, Galardy PJ, Meester WJN, Ovaa H, Ploegh HL & Gaudet R (2005) Structure of the ubiquitin hydrolase UCH‐L3 complexed with a suicide substrate. J Biol Chem 280, 1512–1520. [DOI] [PubMed] [Google Scholar]
- 70. Balakirev MY, Tcherniuk SO, Jaquinod M & Chroboczek J (2003) Otubains: a new family of cysteine proteases in the ubiquitin pathway. EMBO Rep 4, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kattenhorn LM, Korbel GA, Kessler BM, Spooner E & Ploegh HL (2005) A deubiquitinating enzyme encoded by HSV‐1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol Cell 19, 547–557. [DOI] [PubMed] [Google Scholar]
- 72. Schlieker C, Korbel GA, Kattenhorn LM & Ploegh HL (2005) A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J Virol 79, 15582–15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Misaghi S, Balsara ZR, Catic A, Spooner E, Ploegh HL & Starnbach MN (2006) Chlamydia trachomatis‐derived deubiquitinating enzymes in mammalian cells during infection. Mol Microbiol 61, 142–150. [DOI] [PubMed] [Google Scholar]
- 74. Sheedlo MJ, Qiu J, Tan Y, Paul LN, Luo Z‐Q & Das C (2015) Structural basis of substrate recognition by a bacterial deubiquitinase important for dynamics of phagosome ubiquitination. Proc Nat Acad Sci USA 112, 15090–15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Catic A, Misaghi S, Korbel GA & Ploegh HL (2007) ElaD, a deubiquitinating protease expressed by E. coli . PLoS One 2, e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Frickel E‐M, Quesada V, Muething L, Gubbels M‐J, Spooner E, Ploegh H & Artavanis‐Tsakonas K (2007) Apicomplexan UCHL3 retains dual specificity for ubiquitin and Nedd8 throughout evolution. Cell Microbiol 9, 1601–1610. [DOI] [PubMed] [Google Scholar]
- 77. Artavanis‐Tsakonas K, Misaghi S, Comeaux CA, Catic A, Spooner E, Duraisingh MT & Ploegh HL (2006) Identification by functional proteomics of a deubiquitinating/deNeddylating enzyme in Plasmodium falciparum . Mol Microbiol 61, 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. White RR, Miyata S, Papa E, Spooner E, Gounaris K, Selkirk ME & Artavanis‐Tsakonas K (2011) Characterisation of the Trichinella spiralis deubiquitinating enzyme, TsUCH37, an evolutionarily conserved proteasome interaction partner. PLoS Negl Trop Dis 5, e1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ponder EL & Bogyo M (2007) Ubiquitin‐like modifiers and their deconjugating enzymes in medically important parasitic protozoa. Eukaryot Cell 6, 1943–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pruneda JN, Durkin CH, Geurink PP, Ovaa H, Santhanam B, Holden DW & Komander D (2016) The molecular basis for ubiquitin and ubiquitin‐like specificities in bacterial effector proteases. Mol Cell 63, 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Geurink PP, El Oualid F, Jonker A, Hameed DS & Ovaa H (2012) A general chemical ligation approach towards isopeptide‐linked ubiquitin and ubiquitin‐like assay reagents. ChemBioChem 13, 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ritorto MS, Ewan R, Perez‐Oliva AB, Knebel A, Buhrlage SJ, Wightman M, Kelly SM, Wood NT, Virdee S, Gray NS et al (2014) Screening of DUB activity and specificity by MALDI‐TOF mass spectrometry. Nat Commun 5, 4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mevissen TET, Hospenthal MK, Geurink PP, Elliott PR, Akutsu M, Arnaudo N, Ekkebus R, Kulathu Y, Wauer T, El Oualid F et al (2013) OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell 154, 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mevissen TET, Kulathu Y, Mulder MPC, Geurink PP, Maslen SL, Gersch M, Elliott PR, Burke JE, van Tol BDM, Akutsu M et al (2016) Molecular basis of Lys11‐polyubiquitin specificity in the deubiquitinase Cezanne. Nature 538, 402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Békés M, Rut W, Kasperkiewicz P, Mulder MPC, Ovaa H, Drag M, Lima CD & Huang TT (2015) SARS hCoV papain‐like protease is a unique Lys48 linkage‐specific di‐distributive deubiquitinating enzyme. Biochem J 468, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Békés M, van der Heden van Noort GJ, Ekkebus R, Ovaa H, Huang TT & Lima CD (2016) Recognition of Lys48‐linked di‐ubiquitin and deubiquitinating activities of the SARS coronavirus papain‐like protease. Mol Cell 62, 572–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ovaa H, Kessler BM, Rolen U, Galardy PJ, Ploegh HL & Masucci MG (2004) Activity‐based ubiquitin‐specific protease (USP) profiling of virus‐infected and malignant human cells. Proc Nat Acad Sci USA 101, 2253–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rolén U, Kobzeva V, Gasparjan N, Ovaa H, Winberg G, Kisseljov F & Masucci MG (2006) Activity profiling of deubiquitinating enzymes in cervical carcinoma biopsies and cell lines. Mol Carcinog 45, 260–269. [DOI] [PubMed] [Google Scholar]
- 89. Gao Y, Koppen A, Rakhshandehroo M, Tasdelen I, van de Graaf SF, van Loosdregt J, van Beekum O, Hamers N, van Leenen D, Berkers CR et al (2013) Early adipogenesis is regulated through USP7‐mediated deubiquitination of the histone acetyltransferase TIP60. Nat Commun 4, 2656. [DOI] [PubMed] [Google Scholar]
- 90. Kummari E, Alugubelly N, Hsu C‐Y, Dong B, Nanduri B & Edelmann MJ (2015) Activity‐based proteomic profiling of deubiquitinating enzymes in salmonella‐infected macrophages leads to identification of putative function of UCH‐L5 in inflammasome regulation. PLoS One 10, e0135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lopez‐Castejon G, Luheshi NM, Compan V, High S, Whitehead RC, Flitsch S, Kirov A, Prudovsky I, Swanton E & Brough D (2013) Deubiquitinases regulate the activity of caspase‐1 and interleukin‐1β secretion via assembly of the inflammasome. J Biol Chem 288, 2721–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bingol B, Tea JS, Phu L, Reichelt M, Bakalarski CE, Song Q, Foreman O, Kirkpatrick DS & Sheng M (2014) The mitochondrial deubiquitinase USP30 opposes parkin‐mediated mitophagy. Nature 510, 370–375. [DOI] [PubMed] [Google Scholar]
- 93. Aressy B, Jullien D, Cazales M, Marcellin M, Bugler B, Burlet‐Schiltz O & Ducommun B (2010) A screen for deubiquitinating enzymes involved in the G2/M checkpoint identifies USP50 as a regulator of HSP90‐dependent Wee1 stability. Cell Cycle 9, 3839–3846. [DOI] [PubMed] [Google Scholar]
- 94. Dar A, Shibata E & Dutta A (2013) Deubiquitination of Tip60 by USP7 determines the activity of the p53‐dependent apoptotic pathway. Mol Cell Biol 33, 3309–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Py BF, Kim M‐S, Vakifahmetoglu‐Norberg H & Yuan J (2013) Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell 49, 331–338. [DOI] [PubMed] [Google Scholar]
- 96. Kramer HB, Nicholson B, Kessler BM & Altun M (2012) Detection of ubiquitin‐proteasome enzymatic activities in cells: application of activity‐based probes to inhibitor development. Biochim Biophys Acta 1823, 2029–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reverdy C, Conrath S, Lopez R, Planquette C, Atmanene C, Collura V, Harpon J, Battaglia V, Vivat V, Sippl W et al (2012) Discovery of specific inhibitors of human USP7/HAUSP deubiquitinating enzyme. Chem Biol 19, 467–477. [DOI] [PubMed] [Google Scholar]
- 98. Wang X, Mazurkiewicz M, Hillert E‐K, Olofsson MH, Pierrou S, Hillertz P, Gullbo J, Selvaraju K, Paulus A, Akhtar S et al (2016) The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin‐specific protease‐14 and induces apoptosis of multiple myeloma cells. Sci Rep 6, 26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Berndtsson M, Beaujouin M, Rickardson L, Havelka AM, Larsson R, Westman J, Liaudet Coopman E & Linder S (2009) Induction of the lysosomal apoptosis pathway by inhibitors of the ubiquitin‐proteasome system. Int J Cancer 124, 1463–1469. [DOI] [PubMed] [Google Scholar]
- 100. D'Arcy P, Brnjic S, Olofsson MH, Fryknäs M, Lindsten K, De Cesare M, Perego P, Sadeghi B, Hassan M, Larsson R et al (2011) Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med 17, 1636–1640. [DOI] [PubMed] [Google Scholar]
- 101. Wang X, D'Arcy P, Caulfield TR, Paulus A, Chitta K, Mohanty C, Gullbo J, Chanan Khan A & Linder S (2015) Synthesis and evaluation of derivatives of the proteasome deubiquitinase inhibitor b‐AP15. Chem Biol Drug Des 86, 1036–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang X, Stafford W, Mazurkiewicz M, Fryknäs M, Brjnic S, Zhang X, Gullbo J, Larsson R, Arnér ESJ, D'Arcy P et al (2014) The 19S Deubiquitinase inhibitor b‐AP15 is enriched in cells and elicits rapid commitment to cell death. Mol Pharmacol 85, 932–945. [DOI] [PubMed] [Google Scholar]
- 103. D'Arcy P, Wang X & Linder S (2015) Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol Ther 147, 32–54. [DOI] [PubMed] [Google Scholar]
- 104. Ndubaku C & Tsui V (2015) Inhibiting the deubiquitinating enzymes (DUBs). J Med Chem 58, 1581–1595. [DOI] [PubMed] [Google Scholar]
- 105. Farshi P, Deshmukh RR, Nwankwo JO, Arkwright RT, Cvek B, Liu J & Dou QP (2015) Deubiquitinases (DUBs) and DUB inhibitors: a patent review. Expert Opin Ther Pat 25, 1191–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kemp M (2016) Recent advances in the discovery of deubiquitinating enzyme inhibitors In Progress in Medicinal Chemistry (Lawton G. & Witty DR, eds), pp. 149–192. Elsevier, Amsterdam. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Olsen SK, Capili AD, Lu X, Tan DS & Lima CD (2010) Active site remodelling accompanies thioester bond formation in the SUMO E1. Nature 463, 906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lu X, Olsen SK, Capili AD, Cisar JS, Lima CD & Tan DS (2010) Designed semisynthetic protein inhibitors of Ub/Ubl E1 activating enzymes. J Am Chem Soc 132, 1748–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. An H & Statsyuk AV (2016) Facile synthesis of covalent probes to capture enzymatic intermediates during E1 enzyme catalysis. Chem Commun 52, 2477–2480. [DOI] [PubMed] [Google Scholar]
- 110. Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S et al (2009) An inhibitor of NEDD8‐activating enzyme as a new approach to treat cancer. Nature 458, 732–736. [DOI] [PubMed] [Google Scholar]
- 111. An H & Statsyuk AV (2015) An inhibitor of ubiquitin conjugation and aggresome formation. Chem Sci 6, 5235–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fonović M, Verhelst SHL, Sorum MT & Bogyo M (2007) Proteomics evaluation of chemically cleavable activity‐based probes. Mol Cell Proteomics 6, 1761–1770. [DOI] [PubMed] [Google Scholar]
- 113. Yang Y, Hahne H, Kuster B & Verhelst SHL (2013) A simple and effective cleavable linker for chemical proteomics applications. Mol Cell Proteomics 12, 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Stanley M, Han C, Knebel A, Murphy P, Shpiro N & Virdee S (2015) Orthogonal thiol functionalization at a single atomic center for profiling transthiolation activity of E1 activating enzymes. ACS Chem Biol 10, 1542–1554. [DOI] [PubMed] [Google Scholar]
- 115. Pao K‐C, Stanley M, Han C, Lai Y‐C, Murphy P, Balk K, Wood NT, Corti O, Corvol J‐C, Muqit MMK et al (2016) Probes of ubiquitin E3 ligases enable systematic dissection of parkin activation. Nat Chem Biol 12, 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang Y, Zhou L, Rougé L, Phillips AH, Lam C, Liu P, Sandoval W, Helgason E, Murray JM, Wertz IE et al (2012) Conformational stabilization of ubiquitin yields potent and selective inhibitors of USP7. Nat Chem Biol 9, 51–58. [DOI] [PubMed] [Google Scholar]
- 117. Ernst A, Avvakumov G, Tong J, Fan Y, Zhao Y, Alberts P, Persaud A, Walker JR, Neculai A‐M, Neculai D et al (2013) A strategy for modulation of enzymes in the ubiquitin system. Science 339, 590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Damgaard RB, Walker JA, Marco‐Casanova P, Morgan NV, Titheradge HL, Elliott PR, McHale D, Maher ER, McKenzie ANJ & Komander D (2016) The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell 166 (1215–1230), e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Elliott PR & Komander D (2016) Regulation of Met1‐linked polyubiquitin signalling by the deubiquitinase OTULIN. FEBS J 283, 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Saghatelian A, Jessani N, Joseph A, Humphrey M & Cravatt BF (2004) Activity‐based probes for the proteomic profiling of metalloproteases. Proc Nat Acad Sci USA 101, 10000–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Morell M, Nguyen Duc T, Willis AL, Syed S, Lee J, Deu E, Deng Y, Xiao J, Turk BE, Jessen JR et al (2013) Coupling protein engineering with probe design to inhibit and image matrix metalloproteinases with controlled specificity. J Am Chem Soc 135, 9139–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Herhaus L & Dikic I (2015) Expanding the ubiquitin code through post‐translational modification. EMBO Rep 16, 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Whedon SD, Markandeya N, Rana ASJB, Senger NA, Weller CE, Tureček F, Strieter ER & Chatterjee C (2016) Selenocysteine as a latent bioorthogonal electrophilic probe for deubiquitylating enzymes. J Am Chem Soc 138, 13774–13777. [DOI] [PMC free article] [PubMed] [Google Scholar]


