Abstract
Rapid response to environmental changes is achieved by uni‐ and multicellular organisms through a series of molecular events, often involving modification of macromolecules, including proteins, nucleic acids and lipids. Amongst these, ADP‐ribosylation is of emerging interest because of its ability to modify different macromolecules in the cells, and its association with many key biological processes, such as DNA‐damage repair, DNA replication, transcription, cell division, signal transduction, stress and infection responses, microbial pathogenicity and aging. In this review, we provide an update on novel pathways and mechanisms regulated by ADP‐ribosylation in organisms coming from all kingdoms of life.
Keywords: ADP‐ribosylation, cellular pathways, metabolism of ADP‐ribosylation, poly(ADP‐ribose) polymerase, post‐translational modification
ADP‐ribosyltransferases (ARTs) utilise NAD+ as a substrate to modify different molecular targets and control wide variety of processes in organisms of all domains of life.
Abbreviations
- ADPr
ADP‐ribose
- ART
ADP‐ribosyltransferase
- ARTC
Cholera toxin‐like ART
- ARTD
Diphtheria toxin‐like ART
- MARylation
mono(ADP‐ribosyl)ation
- monoARTs
mono(ADP‐ribosyl)transferases
- NAD+
nicotinamide adenine dinucleotide
- NUDIX
Nucleoside Diphosphate linked to X‐moiety hydrolases
- OAADPr
O‐acetyl‐ADP‐ribose
- PARG
poly(ADP‐ribose) glycohydrolase
- PAR
poly‐ADP‐ribose
- PARP
poly(ADP‐ribose) polymerase
- PARylation
poly(ADP‐ribosyl)ation
- PR
phosphoribosylation
- PTM
post‐translational modification
Introduction
Evolution shows remarkable examples of how living species adapt and survive in response to natural and environmental changes 1. All living organisms have evolved molecular mechanisms that enable them to quickly adapt to nutritional, chemical or physical alterations. These adaptations are induced by cascades of molecular events involving qualitative and quantitative changes in the basic, cellular macromolecules, such as proteins, nucleic acids and lipids. Ultimately, these signalling events will trigger the appropriate response. One of the most common tools to induce a rapid change in the cellular environment is the post‐translational modification (PTM) of proteins by addition of chemical moieties, such as phosphate, acyl (most commonly methyl and acetate), small proteins or sugars 2. One highly conserved PTM system is the ADP‐ribosylation, the addition of ADP‐ribose (ADPr) groups from nicotinamide adenine dinucleotide (NAD+) to proteins 3 (Fig. 1). Interestingly, ADP‐ribosylation can happen not only on proteins but also on other macromolecules such as DNA, or small chemical groups 4, 5, 6, 7 (Fig. 1). The first discovered ADP‐ribosyltransferase (ART) enzymes were identified as bacterial toxins, such as the Cholera and Diphtheria toxins 7, 8. These toxins are released from bacterial pathogens to irreversibly modify host proteins to gain an advantage over the infected host 7, 8, 9. Later on, homologous transferases and modification‐reversing hydrolytic enzymes have been discovered in organisms from all kingdoms of life 3. Moreover, many recent observations show how viral genomes evolved the genetic tools that enable them to modulate ADP‐ribosylation signalling of infected cells 10, 11, 12, 13. ADP‐ribosylation seems to be particularly prominent in the highest organisms and it is best studied for the poly(ADP‐ribose) polymerase (PARP) superfamily of ART enzymes 3, 7, 14, 15, 16. Altogether, ADP‐ribosylation is a widespread modification that controls a vast number of cellular processes, including DNA damage repair, transcription, cell‐cycle progression, cell division, unfolded protein response, aging, nitrogen fixation, microbial pathogenicity, cell death and many others 7, 14, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30. However, our understanding of ADP‐ribosylation is still in its infancy, as can be seen from the current rapid rate of discoveries of previously unknown pathways regulated by ADP‐ribosylation.
Figure 1.
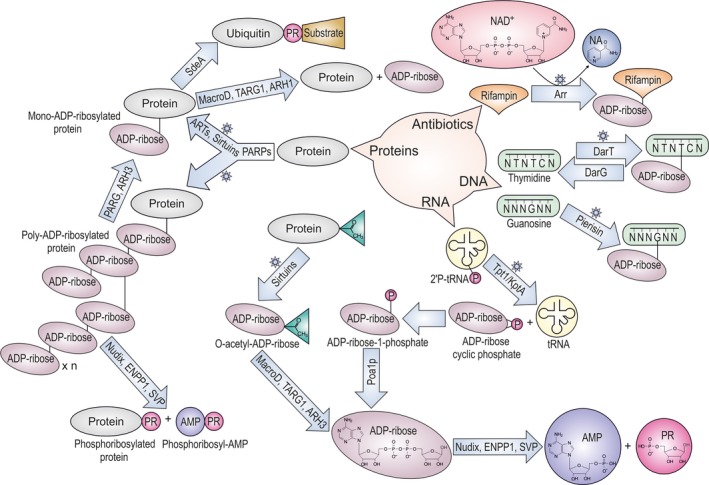
Targets and pathways involved in the metabolism of ADP‐ribose. Scheme is simplified to show only the main products and ADP‐ribose metabolites. Stars indicate reactions that use NAD +. PR, ribose‐5′‐phosphate.
ADP‐ribosyltransferases
All so far characterized ARTs use NAD+ cofactor and transfer a single or multiple ADPr moieties onto an acceptor molecule (termed mono‐ and poly(ADP‐ribosyl)ation, also called MARylation and PARylation respectively) combined with the release of nicotinamide (NA; Fig. 1) 3, 7, 16. The most widespread super families of ARTs contain transferase folds evolutionary related to bacterial toxins. These proteins can be grouped into: (a) PARP‐like proteins, alternatively called Diphtheria toxin‐like ART (ARTD) superfamily; and (b) Cholera toxin‐like ART (ARTCs) superfamily. Most of the transferases from these groups are known to modify proteins, however, some of them modify DNA or small chemical groups such as phosphate 3, 4, 5, 6, 7, 8, 9, 16, 17, 31 (Fig. 1).
Eighteen mammalian genes with a sequence homology to PARPs/ARTDs have been described 3, 7, 14, 16, 27, 28. The first and best characterized of these proteins was originally noted for its ability to synthesize ADPr polymers upon DNA damage and called poly(ADP‐ribose) polymerase 1 (PARP1) 15, 23, 26, 32, 33, 34. Human PARP1, PARP2 and Tankyrases (PARP5a and PARP5b), and close homologues from lower organisms and bacteria are able to produce long repeating chains of ADPr on target proteins 3, 7, 16, 23, 35, 36, 37. PARP1 and PARP2 can also catalyse the formation of branched chains of poly(ADP‐ribose) (PAR) on proteins and possibly DNA 6, 35. Most of the other characterized PARPs are mono(ADP‐ribosyl) transferases (monoARTs) 16, 24, 35. Another, highly diverged ART may also belong to PARP‐like proteins, but it transfers a phosphate group from RNA onto ADPr (Fig. 1). This is KptA/Tpt1 protein, RNA phosphotransferase found in all three domains of life 7, 38. In yeast, Tpt1 catalyses a NAD+‐dependent dephosphorylation of tRNA‐splicing intermediates, generating ADPr‐1‐phopshate through a cyclic intermediate 38 (Fig. 1).
Among the ARTC subfamily of transferases, pierisins are the only transferases able to act on DNA 4, all the other enzymes in this group characterised so far act as transferases for proteins 7, 17. Several bacterial toxins can be included in this family, such as the C3 ectotoxin from Staphylococcus aureus, VIP2 from Bacillus cereus, and SpvB from Salmonella typhimurium 7, 39, 40, 41. Mammalian ARTC family includes four human proteins (hARTC1, 3, 4, 5) that are glycosylphosphatidylinositol (GPI)‐anchored or secreted proteins 17, 42. hARTCs have been reported to modify soluble and plasma membrane‐associated protein targets and thus they are proposed to be involved in intercellular signalling, immune responses and inflammation 17, 42, 43.
Evolutionary unrelated ART enzymes to the previous group are sirtuins. Sirtuins are best known as NAD+‐dependent protein deacetylaeses and they are found in proteins of all kingdoms of life 44, 45 (Fig. 1). There are seven sirtuin proteins operating in human cells 46, their primary enzymatic activity is protein deacetylation producing O‐acetyl‐ADP‐ribose (OAADPr) metabolite as a by‐product of its ART reaction 47. Sirtuins can sometimes directly modify proteins 48, 49 (Fig. 1).
It has been suggested that the nonenzymatic ADP‐ribosylation of proteins may reach significant levels in vivo. This is due to the chemical reactivity of the free ADPr with side chains of variety of amino acids, most notably lysine and cysteine 50.
ADPr‐binding domains
As with other PTMs, ADP‐ribosylation tags are recognized by cellular proteins in a timely manner in order to activate downstream events in the relevant signalling pathways 16, 26, 30, 51. Therefore, many proteins involved in these pathways possess ADPr‐binding domains within their protein structure 16, 30, 51. Among the evolutionary widespread ADPr binding domains, the macrodomain has been studied the most extensively. Macrodomains are found in proteins from all kingdoms of life supporting different cellular processes 3, 16, 51, 52, 53, 54. Macrodomain‐containing proteins can recognize variety of substrates, including MARylated and PARylated proteins, different ADPr metabolites (such as OAADPr) 16, 53, 54, 55 and RNA 54, 56, 57 (Fig. 1). Some macrodomains have also evolved enzymatic activity and are capable of hydrolysing ADP‐ribosylation (see below) 36, 49, 54, 58, 59. As a consequence, macrodomain‐containing proteins are involved in a diverse set of cellular functions, such as chromatin remodelling and DNA‐damage repair, oxidative stress response, metabolic processes and pathogenic mechanisms 3, 5, 10, 11, 12, 13, 30, 37, 53, 54, 59, 60, 61. In addition to macrodomain, several other widely distributed domains have been described as readers for ADP‐ribosylation, such as the PAR‐binding zinc finger (PBZ) 62, the WWE (named after three of its conserved residues) 63, the oligonucleotide/oligosaccharide‐binding (OB) domain 64 and the PAR‐binding motifs (PBM) which is abundant in DNA‐damage repair proteins 65.
Hydrolases
As mentioned before, the ADP‐ribosylation is a reversible modification 66. Two evolutionary unrelated protein domains are known to support this catalytic activity: already mentioned macrodomains and the DraG‐like fold containing proteins 3, 67, 68. The catalytic macrodomain fold is found in a number of proteins coming from all the kingdoms of life 54. In humans four catalytic macrodomains have been identified: poly(ADP‐ribose) glycohydrolase (PARG), MacroD1, MacroD2 and terminal ADP‐ribosyl glycohydrolase 1 (TARG1/C6orf130) 16, 36, 54, 59, 69, 70. PARG efficiently cleaves the PAR‐specific O‐glycosidic ribose–ribose bonds, however, it is unable to remove the terminal ADPr unit directly linked to a protein (Fig. 1) 36, 58. The existence of PARG‐splicing variants ensure the presence of the enzyme both in the nucleus and in the cytoplasm or in membranous organelles 71, 72, 73 and allows for rapid turnover of PAR, ensuring tight control of this modification 66. The terminal ADPr moiety is removed by MARylation preferring hydrolases, such as MacroD1, MacroD2 or TARG1 58, 59, 69, 70 (Fig. 1). The latter enzymes can also hydrolyse some other ADPr metabolites, such as OAADPr 54, 55, 74 (Fig. 1). An additional macrodomain containing protein is Poa1p (YBR022) from Saccharomyces cerevisiae, functionally characterized as a specific phosphatase that removes the phosphate group of ADPr‐1‐phosphate in the tRNA‐splicing pathway in yeast 75.
Another class of de‐ADP‐ribosylation enzymes includes the dinitrogenase reductase‐activating glycohydrolase (DraG) and related proteins 67, 68, 73, 76, 77, 78, 79. DraG is known to regulate, in conjunction with ART called DraT, the central enzyme of nitrogen fixation in several bacterial species 68, 76. Mammals carry distant homologues of DraG (called ARH1‐3 in humans), whose functions are so far not fully understood. The ARH1 protein shows efficient hydrolytic activity against MARylated proteins on arginine residues 77. ADP‐ribosylated proteins on arginine are found on cellular plasma membrane, in the lumen of endoplasmic reticulum (ER) 42, 43, 78, 79, 80, 81 and in cytoplasm 82, 83. ARH3 was shown to hydrolyse the O‐glycosidic bond of PAR chains and OAADPr 79, 84, 85, 86. ARH2 is believed to be catalytically inactive 79, 84, 85.
The released ADPr by all the active hydrolases can be further recycled and eventually converted to ATP by enzymes such as members of the Nucleoside Diphosphate linked to X‐moiety hydrolases (NUDIX) family 87, 88, 89.
Other enzymes that cleave protein ADP‐ribosylation
Noncanonical enzymes able to perform the hydrolysis of ADPr linked to proteins have been recently identified. These include two unrelated protein families, the NUDIX 87, 88 and Ectonucleotide Pyrophosphatase/Phosphodiesterase (ENPP) 90, both of which hydrolyse the ADPr phosphodiester bond in mono‐ADP‐ribose and PAR linked to proteins, thus liberating adenosine monophosphate (AMP) and phosphoribose‐AMP and leaving ribose‐5′‐phosphate (phosphoribosylation; PR) tags bound to the protein 91, 92, 93, 94 (Fig. 1). The NUDIX enzymes able to perform this reaction are human NUDT16 and Escherichia coli RppH 91, 92. Within the ENPP family of enzymes, vertebrate ENPP1 proteins and Phoshodiesterase I found in the poison glands of rattlesnakes (Snake Venom Phosphodiesterase) exhibit the same activity 93, 94. The physiological relevance of the activities of ENPP1 and NUDT16 enzymes against the protein ADP‐ribosylation remains unclear.
Phosphoribosylation of proteins is also a consequence of the activity of Sde, an enzyme that couples the ART with a phosphodiesterase domain, associated with the control of the host ubiquitination signalling by human pathogen Legionella pneumophila (see detailed description below) 95, 96.
Mammalian ADP‐ribosylation signalling
ADP‐ribosylation in mammals is known to regulate a number of different processes 14, 17, 24, 26, 27, 28, 30. Best understood is regulation of DNA‐damage repair pathways by PARP1‐3 that are activated upon binding to DNA breaks 14, 26, 27, 28, 97, 98, 99, 100, 101, 102 PARP1 also plays roles in transcription and metabolism 29, 30, 103. The functions of other PARPs are comparatively much less understood 27, 28. PARP4 (also called VPARP) is a component of the cytosolic ribonucleoprotein vault complex, however, its biological functions are unknown 104. PARPs 5a and 5b (tankyrases) are best understood for their roles in mitosis 19, 25 and Wnt signalling 105, 106, 107, 108, but they also have roles at telomere and DNA‐damage repair 108, 109, 110. PARP6 and PARP8 are poorly understood, however, PARP6 has been shown to be involved in hippocampus neuronal development 111. Several PARPs (PARP7 PARP10, PARP12 and PARP13) are involved in mechanisms of post‐transcriptional regulation of mRNA, mediated either by RNA‐binding domains 112 or by ADP‐ribosylation of RNA‐binding proteins 20. In addition, PARP10 has been implied in the regulation of NF‐kB 113, 114, GSK3β 113, 115 and transcription 103, 113, 116. Also, PARP9 and PARP14 are suggested to act on transcription, in particular of genes required for macrophage activation 117. PARP16 regulates the unfolded protein response 18. Concurrent with its nuclear pore localization, PARP11 modifies targets involved in the coordination of the nuclear envelope and the organization of nuclear pores 118, 119.
Future work is needed to properly understand the physiological functions of most of the PARPs and the new potential functions for PARPs and other ARTs are continuously arising 120.
Compared to PARPs, much less research has been conducted on members of the ARTC family in mammals. This family includes four proteins in humans (hARTC1, 3, 4, 5) and six in mice (mARTC1, 2.1, 2.2, 3, 4, 5) that are glycosylphosphatidylinositol (GPI)‐anchored (hARTC1 and mARTC1) or ecto‐proteins (hARTC3, 4, 5 and mARCT2.1, 2.2, 3, 4, 5) 42. mARTC1, 2, and 5 have been reported to modify soluble and plasma membrane‐associated protein targets on arginine residues, including the P2X7 purinergic receptor, and thus they can affect cellular processes such as intercellular signalling, immune responses and inflammation 43. hARTC1 has been detected in the lumen of ER and its function in stress response has been suggested 81.
Amino acid specificity of mammalian ARTs
Although all the ARTC proteins in mammals characterized so far modify substrate proteins on arginine residues 42, the situation for PARP family is more complex and there is still no strong consensus in the field on the preferred amino acid targets for many PARPs. In this respect, the progress has been additionally hampered in the case of poly(ADP‐ribosyl)ating PARPs, as the current methods to identify sites do not make the difference between mono and poly(ADP‐ribosyl)ation for specific amino acid position. Overall, acidic residues might be the main targets for most of the PARPs 35, 121, 122, 123, 124 but cysteine 35, 120, 123, arginine 121, 123, 124, lysine 83, 121, 123, 124 and serine residues 125 have been suggested as well. ADP‐ribosylation of acidic residues and lysines has been shown to be induced by oxidative stress 83. However, additional evidence has revealed that many of these lysine residues may have been mis‐annotated as modification sites, and that actual modification sites are proximal serine residues, that usually follow immediately after these lysine residues 126. Indeed, the KS motif has been identified as a preferred target for serine ADP‐ribosylation by several studies 125, 126, 127. Notably, serine ADP‐ribosylation seems to be specific for regulation of DNA damage response and other pathways important for genome stability such as regulation of chromatin structure, transcription and mitosis 127. HPF1/C4orf27 is the first protein identified acting as a specificity factor for the serine ADP‐ribosylation 127, 128. It acts in conjunction with PARP1 and PARP2 proteins and directs modification of histones, PARP1 itself, high‐mobility group proteins and likely many other proteins 127.
ADP‐ribosylation in bacteria
The first discovered ARTs were secreted toxins that are found sporadically in bacteria and that irreversibly modify crucial host cell proteins 129. However, the genomic evidence suggests that intracellular, reversible ADP‐ribosylation is much more common amongst bacteria, yet, there is little evidence on its physiological relevance. A notable exception is the DraT/DraG system of nitrogen‐fixating bacteria from the Azospirillum and Rhodospirillum genera. DraT homologues are restricted to several nitrogen‐fixing bacteria, while DraG homologues are distributed across all three domains of life 67. Endogenous ADP‐ribosylation has also been reported for some other bacterial species where this process probably regulates important cellular functions such as sporulation in Bacillus subtilis 130, development and cell–cell interaction in Myxococcus xanthus 131, 132, as well as differentiation and secondary metabolism in Streptomyces 133, 134, 135, 136.
Streptomyces – bacterial model organism for the study of ADP‐ribosylation
So far, the most evidence for intracellular endogenous protein ADP‐ribosylation has been found in Streptomyces species. Streptomyces are soil‐inhabiting Gram‐positive bacteria best known for their complex life cycle that includes morphological differentiation and the production of various secondary metabolites including antibiotics, anti‐cancer drugs and immunosuppressors. ADP‐ribosylation has been discovered in Streptomyces over 20 years ago 137. First reports demonstrated considerable ADP‐ribosylating activities in cell extracts and suggested a role for ADP‐ribosylation in growth and differentiation processes in Streptomyces griseus 133, 134. In both Str. griseus and Streptomyces coelicolor, ADP‐ribosylation patterns change with morphological differentiation 138, 139 and several identified ADP‐ribosylated proteins in Str. coelicolor suggested a connection between protein ADP‐ribosylation and the regulation of metabolic requirements of the cells 135.
Streptomyces coelicolor genomic data (Table 1) suggest that ADP‐ribosylation should be prominent in Streptomyces. Two ARTs have been characterized in Streptomyces, SCO5461 from Str. coelicolor 136, 140 and Scabin from plant pathogen Streptomyces scabies 141. These proteins are homologues of pierisins 4 and possess guanine‐specific DNA ART activity, but they are not conserved across the Streptomyces species and cannot be found in Str. griseus, suggesting that the major protein ARTs in Streptomyces have yet to be discovered. Disruption of SCO5461 leads to conditional pleiotropic phenotype characterized by defects of morphological differentiation, antibiotic production and secretion 136.
Table 1.
Enzymes potentially involved in ADP‐ribosylation process in Streptomyces coelicolor
| ADP‐ribosyltransferases | SCO2860 (Arr homologue) |
| SCO3953 (Tpt1/KptA homologue) | |
| SCO5461 (Pierisin homologue) | |
| Macrodomain hydrolases | SCO0909 (bacterial‐type PARG) |
| SCO6450 (MacroD homologue) | |
| SCO6735 (TARG1‐like) | |
| DraG/ARH ‐like hydrolases | SCO0086 |
| SCO1766 | |
| SCO2028 | |
| SCO2029 | |
| SCO2030 | |
| SCO2031 | |
| SCO4435 | |
| SCO5809 | |
| Sirtuins | SCO0452 |
| SCO6464 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The SCO3953 protein is a homologue of yeast tRNA 2′‐phosphotransferase Tpt1, an essential enzyme in yeast that catalyses the final step in tRNA splicing. This reaction includes dephosphorylation of tRNA 2′‐phosphate in two steps; transfer of ADPr from NAD+ to tRNA 2′‐phosphate that generates a 2′‐phospho‐ADPr‐RNA intermediate and release of mature tRNA together with ADPr 1″‐2″‐cyclic phosphate 142 (Fig. 1). Tpt1 homologues are found distributed across all domains of life including bacterial species that have no known intron‐containing tRNAs (Str. coelicolor and E. coli whose orthologue is called KptA are among them). Therefore, bacterial Tpt1/KptA homologues should have some yet uncovered substrate(s) and function(s).
The SCO2860 is a homologue of Mycobacterium smegmatis ART Arr that modifies antibiotic rifampicin (Fig. 1) and causes antibiotic resistance. Mycobacterium smegmatis Arr gene has been acquired from horizontal gene transfer 143. It is upregulated after exposure to different kinds of stress and its endogenous cellular function has been proposed in a general stress response 144.
There is evidence of a much larger number of potential ADPr hydrolases in Str. coelicolor. Eight of them are uncharacterized DraG homologues and three are macrodomain proteins representing three different classes within the macrodomain superfamily (Table 1).
The SCO0909 is a bacterial‐type PARG that cleaves the PARylation in the same manner as mammalian PARGs 36. Nothing is known about the function of SCO0909, but in the radiation‐resistant bacterium Deinococcus radiodurans the Sco0909 gene is one of the most highly induced genes after DNA damage caused by ionizing radiation 145.
The SCO6450 is a MacroD homologue and it is predicted to remove protein MARylation. SCO6450 orthologues are found in most of the bacteria 54. Escherichia coli homologue YmdB appears to be a multifunctional protein that regulates variety of cellular processes; deacetylates OAADPr, hydrolyses MARylated protein substrates, regulates RNAse III activity and modulates bacterial biofilm formation 55, 69, 146, 147.
SCO6735 is a macrodomain protein closest to human proteins ALC1 and TARG1 54, 148. In vitro SCO06735 can remove MARylation from glutamate residues, yet structural and biochemical characterization indicate a mechanism distinct from any other known macrodomain hydrolases 148. Although SCO6735 physiological substrate is still unknown, its expression is under the control of a RecA‐independent DNA damage inducible promoter 149, 150 and upregulated upon UV‐induced DNA damage 148, thus indicating a role in DNA damage response. Moreover, SCO6735 is possibly involved in the regulation of antibiotic production and disruption of the Sco6735 gene was shown to increase actinorhodin production 148.
Two sirtuins, CobB1 (SCO0452) and CobB2 (SCO6464), have been identified in Str. coelicolor. CobB1 is a SIRT4 homologue that exhibits deacetylase activity on acetyl‐CoA synthetase and consequently regulates its activity 151. Auto‐ADP‐ribosylation was demonstrated for the SIRT4 homologue of M. smegmatis 152. CobB2 appears to be related to SIRT5 and its overexpression suppresses production of two pigmented antibiotics, thus creating a loss‐of‐colouration phenotype 153.
Altogether, Streptomyces represent a good model for the study of ADP‐ribosylation in bacteria and future studies on this model should help deciphering players and mechanisms of reversible ADP‐ribosylation process. Since ADP‐ribosylation is involved in the control of antibiotic production in Streptomyces, a better understanding of this process will also enable better exploitation of Streptomyces biotechnological potential.
Other notable ADP‐ribosylation systems in bacteria
Studies looking either at the genomic context of ADP‐ribosylating systems or their evolution in bacteria suggest that ADP‐ribosylation might be involved in the regulation of many crucial cellular processes including bacterial persistence, oxidative stress response and adaptation to the host environment in general 5, 9, 49, 154.
Reversible DNA ADP‐ribosylation
A novel DNA‐ribosylating toxin‐antitoxin (TA) system has been identified in a variety of different bacterial species including the human pathogens Mycobacterium tuberculosis and enterohemorrhagic E. coli 5. The toxin component of the TA system is a DNA ART (DarT), which catalyses the modification of the second thymidine base in the TNTC motif of ssDNA. This modification is reversed by the DNA ADPr glycohydrolase (DarG) activity of the antitoxin. The substrate specificity of DarT led to the discovery that the ADP‐ribosylation interferes with DNA replication and induces DNA damage signalling via the SOS response 5. DarG belongs to the ALC1‐like class of macrodomains and is structurally most similar to human TARG1. In addition to the reversal of the DNA ADP‐ribosylation by DarG macrodomain hydrolytic activity, protein–protein interaction between DarT and DarG (resembling a type II TA system) revealed a second layer of DarT regulation 5. All available data including the fact that DarG is essential in M. tuberculosis suggest that targeting this ADP‐ribosylating TA system may have a therapeutic potential 5, 155.
Sirtuin dependent ADP‐ribosylation in regulation of oxidative stress
Protein ADP‐ribosylation carried out by a distinct class of sirtuins (SirTM) was described in Sta. aureus and Streptococcus pyogenes and was suggested to regulate oxidative stress response in these pathogens. SirTM is encoded within an operon containing a modification carrier protein (GcvH‐L). GcvH‐L becomes doubly modified by two different PTMs through the actions of SirTM (ADP‐ribosylation) and another component of the operon that acts as a lipoate ligase (synthesising the protein lipoylation) 2, 49, 156. Yet another protein product of the same operon is a macrodomain protein (belonging to the MacroD‐type class), which specifically reverses the ADP‐ribosylation of GcvH‐L 49. It was suggested that the lipoylation acts as a scavenger of reactive oxygen species (either host derived or environmentally induced), while the reversible ADP‐ribosylation may regulate interactions with other proteins involved in the oxidative stress response that are part of this protein complex 49. SirTM homologues are found in a number of fungal pathogens 49.
ADP‐ribosylation as precursor for ligase‐independent ubiquitination
Bacterial‐induced ADP‐ribosylation has been implicated in regulation of host ubiquitination signalling, a eukaryotic‐specific PTM via attachment of a small protein ubiquitin and associated with modulation of the target protein function or degradation 2, 157. The pathogenic bacterium L. pneumophila uses ubiquitin effector proteins of the Sde family, a new class of ubiquitin‐specific monoART, to modulate the host ubiquitin signalling and create a favourable growth environment within the host cell 158. One of the Sde proteins, SdeA, contains a monoART domain as well as phosphodiesterase domain (PDE) 95, 96. The monoART catalyses the ADP‐ribosylation of ubiquitin on Arg42, while the PDE hydrolizes the ADPr‐phosphodiester bond, thus establishing a 5′‐phosphoribosyl modification (Fig. 1). Subsequently, the phosphoribosylated ubiquitin is linked to a serine residue within target proteins, thus completing an E2/3 ligase‐independent ubiquitination system. Sde‐mediated ubiquitination of several ER‐associated Rab proteins and reticulon 4 impairs several cellular processes, such as mitophagy, TNF signalling, tubular endoplasmic reticulum functions and proteasomal degradation, allowing better bacterial growth 95, 96.
ADP‐ribosylation in archaea
Among archaea only the Tpt1/KptA ART type can be found widely spread. Considering its wide distribution in all three domains of life and structural simplicity, this protein could represent the ancestral version of the entire ART superfamily 9. Other representatives of the ART superfamily can be found, but these are limited to only a few species per homologue 9. In the two methane‐producing archaea Methanobrevibacter smithii and Methanospirillum hungatei homologues of the exotoxin Alt/VIP2 were identified. A gene encoding a fusion‐protein homologue of the DarT‐DarG TA system was found in Nitrosopumilus maritimus. Protein ADP‐ribosylating sirtuins (SirTM) have been found in the genomes of Sulfolobus solfataricus and Methanobrevibacter species 54, 159. Although PARPs‐encoding genes could not been found in archaeal genomes, a PARP‐like protein ADP‐ribosylation activity has been detected in Su. solfataricus 160.
Enzymes capable of removing ADP‐ribosylation are represented in archaea by two classes, MacroD‐type and TARG1‐like. The best‐studied archaeal macrodomain protein is Af1521 from thermophile archaea Arhaeoglobus fulgidus. This protein is capable of binding both ADPr and PAR, and possesses enzymatic activity capable of hydrolysing Appr‐1‐P and MARylated protein substrates 52, 69, 70. Af1521 is also used as a tool to enrich ADP‐ribosylated protein for mass‐spectrometry analyses of modification sites 83, 161. TARG1‐like enzymes, as well as DraG homologues are sporadically found in some archaeal species such as Methanococcus janaschii (PDB code http://www.rcsb.org/pdb/search/structidSearch.do?structureId=1T5J).
ADP‐ribosylation in viruses
Viruses can manipulate host ADP‐ribosylation machinery and MARylation has been recognized as an efficient weapon in the bacteriophage arsenal that is successfully used against bacterial antiphage defence 155, 162. Alt, ModA and ModB are T4 phage ARTC‐like monoARTs that modify the E. coli host proteins shortly after infection to overtake the control of the host transcriptional and translational machinery. These enzymes together modify over 30 E. coli proteins, including RNA polymerase, ribosomal protein S1, EF‐Tu and MazF 162, 163. Of these MazF belongs to one of the best‐studied type II TA systems (MazE/MazF), which is involved in bacteriophage defence. MazE is a rapidly degraded antitoxin and MazF is a stable toxin with RNA cleavage activity (specific to ACA RNA sequence) that blocks protein synthesis. Using Alt, the T4 phage defends itself against this system by ADP‐riboysilating MazF, impairing the RNA cleavage activity, and thus enables phage growth 163.
Another type of ARTs that can be identified in a limited number of dsDNA viruses are PARP‐like proteins. These are most likely acquired by horizontal gene transfer and their physiological role remains yet to be studied 3.
Proteins encoding macrodomains are more frequently distributed in viral genomes and several different types of macrodomains can be found in both dsDNA and positive‐strand ssRNA 10, 54. Viral macrodomains are usually part of larger proteins that contain additional domains. Biochemical, structural and phylogenetic evidences showed that viral and cellular macrodomains are closely related. Viral macrodomains bind ADPr and PAR and can perform activities characteristic for cellular macrodomains. Most viral macrodomains belong to MacroD‐type class, but besides their basic de‐MARylation activity, they are also capable to remove the whole PAR chain from PARylated substrates resembling TARG1 activity 10. In coronaviruses, the MacroD‐type macrodomain is a part of the multidomain nonstructural protein 3 (nsP3). It has been shown that this macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome (SARS) coronavirus infection 11. In addition to the MacroD‐type of macrodomains, a highly diverged macrodomain SUD‐M was found as a part of the nsP3 in SARS coronaviruses. This unique macrodomain binds nucleic acids, preferentially RNA, and is crucial for viral genome replication/transcription 164. Numerous other examples show that viral macrodomains affect virus replication and interferon‐response in humans 12, 13. Viral macrodomains may act against mammalian PARPs that are known to possess antiviral activity 10. PARPs involved in the antiviral defence are interferon‐inducible, bear the signature of accelerated evolution and inhibit virus replication 165. Specifically, PARP7, PARP10 and PARP12 have been experimentally shown to act as inhibitors of virus replication 166. Another rapidly evolving PARP with broad antiviral activity is PARP13 (zinc finger antiviral protein), which specifically binds to viral RNA sequences targeting them for degradation 167. Evidences for positive selection have also been found in macro‐PARPs (PARP9, PARP14 and PARP15) and PARP4 168. In some cases, cellular PARP activity can be beneficial for viral infection rather than inhibitory 169, 170.
Concluding remarks and future work
Numerous studies investigating ADP‐ribosylation have been performed in the last several decades. Yet, our understanding of the molecular mechanisms governing ADP‐ribosylation signalling and the physiological and pathophysiological importance of the pathways regulated by ADP‐ribosylation are still poorly understood. Thus, there are many exciting findings waiting to be discovered in this field of research and the scientific community researching the ADP‐ribosylation has been steadily growing in recent years. Many researchers are now also investing great efforts in developing new platforms, tools, methods and pipelines to study the ADP‐ribosylation 83, 91, 122, 123, 125, 161, 171, 172, 173, 174, 175 these should greatly facilitate further understanding of the complexity of molecular and cellular mechanisms controlled by ADP‐ribosylation.
Author contributions
LP, AM and IA cowrote the manuscript and designed the figures.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgements
The authors are grateful to Johannes Rack and Kerryanne Crawford (University of Oxford) for their constructive comments on the manuscript. Ahel laboratory is funded by the Wellcome Trust (grant 101794), Cancer Research UK (grant C35050/A22284), and the European Research Council (grant 281739). This work is also supported by the Croatian Science Foundation (Project No. IP–2016–06–4242).
References
- 1. Darwin C (1859) On the Origin of Species by Means of Natural Selection, or, the Preservation of Favoured Races in the Struggle for Life. J Murray, London. [PMC free article] [PubMed] [Google Scholar]
- 2. Walsh C (2006) Posttranslational Modification of Proteins: Expanding Nature's Inventory. Roberts and Co. Publishers, Englewood, CO: XXI, pp. 490. [Google Scholar]
- 3. Perina D, Mikoč A, Ahel J, Ćetković H, Žaja R & Ahel I (2014) Distribution of protein poly(ADP‐ribosyl)ation systems across all domains of life. DNA Repair (Amst) 23, 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakano T, Takahashi‐Nakaguchi A, Yamamoto M & Watanabe M (2015) Pierisins and CARP‐1: ADP‐ribosylation of DNA by ARTCs in butterflies and shellfish. Curr Top Microbiol Immunol 384, 127–149. [DOI] [PubMed] [Google Scholar]
- 5. Jankevicius G, Ariza A, Ahel M & Ahel I (2016) The toxin‐antitoxin system DarTG catalyzes reversible ADP‐ribosylation of DNA. Mol Cell 64, 1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Talhaoui I, Lebedeva NA, Zarkovic G, Saint‐Pierre C, Kutuzov MM, Sukhanova MV, Matkarimov BT, Gasparutto D, Saparbaev MK, Lavrik OI et al (2016) Poly(ADP‐ribose) polymerases covalently modify strand break termini in DNA fragments in vitro. Nucleic Acids Res 44, 9279–9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hottiger MO, Hassa PO, Lüscher B, Schüler H & Koch‐Nolte F (2010) Toward a unified nomenclature for mammalian ADP‐ribosyltransferases. Trends Biochem Sci 35, 208–219. [DOI] [PubMed] [Google Scholar]
- 8. Collier RJ (2001) Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 39, 1793–1803. [DOI] [PubMed] [Google Scholar]
- 9. Aravind L, Zhang D, de Souza RF, Anand S & Iyer LM (2015) The natural history of ADP‐ribosyltransferases and the ADP‐ribosylation system. Curr Top Microbiol Immunol 384, 3–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li C, Debing Y, Jankevicius G, Neyts J, Ahel I, Coutard B & Canard B (2016) Viral macro domains reverse protein ADP‐ribosylation. J Virol 90, 8478–8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fehr AR, Channappanavar R, Jankevicius G, Fett C, Zhao J, Athmer J, Meyerholz DK, Ahel I & Perlman S (2016) The conserved coronavirus macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome coronavirus infection. MBio 7, pii: e01721‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McPherson RL, Abraham R, Sreekumar E, Ong SE, Cheng SJ, Baxter VK, Kistemaker HA, Filippov DV, Griffin DE & Leung AK (2017) ADP‐ribosylhydrolase activity of Chikungunya virus macrodomain is critical for virus replication and virulence. Proc Natl Acad Sci USA 114, 1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eckei L, Krieg S, Bütepage M, Lehmann A, Gross A, Lippok B, Grimm AR, Kümmerer BM, Rossetti G, Lüscher B et al (2017) The conserved macrodomains of the non‐structural proteins of Chikungunya virus and other pathogenic positive strand RNA viruses function as mono‐ADP‐ribosylhydrolases. Sci Rep 7, 41746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gibson BA & Kraus WL (2012) New insights into the molecular and cellular functions of poly(ADP‐ribose) and PARPs. Nat Rev Mol Cell Biol 13, 411–424. [DOI] [PubMed] [Google Scholar]
- 15. Kraus WL (2015) PARPs and ADP‐ribosylation: 50 years … and counting. Mol Cell 58, 902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barkauskaite E, Jankevicius G & Ahel I (2015) Structures and mechanisms of enzymes employed in the synthesis and degradation of PARP‐dependent protein ADP‐ribosylation. Mol Cell 58, 935–946. [DOI] [PubMed] [Google Scholar]
- 17. Corda D & Di Girolamo M (2003) Functional aspects of protein mono‐ADP‐ribosylation. EMBO J 22, 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jwa M & Chang P (2012) PARP16 is a tail‐anchored endoplasmic reticulum protein required for the PERK‐ and IRE1α‐mediated unfolded protein response. Nat Cell Biol 14, 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang P, Coughlin M & Mitchison TJ (2005) Tankyrase‐1 polymerization of poly(ADP‐ribose) is required for spindle structure and function. Nat Cell Biol 7, 1133–1139. [DOI] [PubMed] [Google Scholar]
- 20. Leung AK, Vyas S, Rood JE, Bhutkar A, Sharp PA & Chang P (2011) Poly(ADP‐ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol Cell 42, 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH et al (2011) PARP‐1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13, 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bai P, Canto C, Brunyánszki A, Huber A, Szántó M, Cen Y, Yamamoto H, Houten SM, Kiss B, Oudart H et al (2011) PARP‐2 regulates SIRT1 expression and whole‐body energy expenditure. Cell Metab 13, 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Langelier MF & Pascal JM (2013) PARP‐1 mechanisms for coupling DNA damage detection to poly(ADP‐ribose) synthesis. Curr Opin Struct Biol 23, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feijs KL, Verheugd P & Lüscher B (2013) Expanding functions of intracellular resident mono‐ADP‐ribosylation in cell physiology. FEBS J 280, 3519–3529. [DOI] [PubMed] [Google Scholar]
- 25. Palazzo L, Della Monica R, Visconti R, Costanzo V & Grieco D (2014) ATM controls proper mitotic spindle structure. Cell Cycle 13, 1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tallis M, Morra R, Barkauskaite E & Ahel I (2014) Poly(ADP‐ribosyl)ation in regulation of chromatin structure and the DNA damage response. Chromosoma 123, 79–90. [DOI] [PubMed] [Google Scholar]
- 27. Bai P (2015) Biology of poly(ADP‐ribose) polymerases: the factotums of cell maintenance. Mol Cell 58, 947–958. [DOI] [PubMed] [Google Scholar]
- 28. Bock FJ & Chang P (2016) New directions in poly(ADP‐ribose) polymerase biology. FEBS J 283, 4017–4031. [DOI] [PubMed] [Google Scholar]
- 29. Gibson BA, Zhang Y, Jiang H, Hussey KM, Shrimp JH, Lin H, Schwede F, Yu Y & Kraus WL (2016) Chemical genetic discovery of PARP targets reveals a role for PARP‐1 in transcription elongation. Science 353, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Posavec Marjanović M, Crawford K & Ahel I (2017) PARP, transcription and chromatin modeling. Semin Cell Dev Biol 63, 102–113. [DOI] [PubMed] [Google Scholar]
- 31. Culver GM, McCraith SM, Consaul SA, Stanford DR & Phizicky EM (1997) A 2′‐phosphotransferase implicated in tRNA splicing is essential in Saccharomyces cerevisiae . J Biol Chem 272, 13203–13210. [DOI] [PubMed] [Google Scholar]
- 32. Nishizuka Y, Ueda K, Nakazawa K & Hayaishi O (1967) Studies on the polymer of adenosine diphosphate ribose. I. Enzymic formation from nicotinamide adenine dinuclotide in mammalian nuclei. J Biol Chem 242, 3164–3171. [PubMed] [Google Scholar]
- 33. Reeder RH, Ueda K, Honjo T, Nishizuka Y & Hayaishi O (1967) Studies on the polymer of adenosine diphosphate ribose. II. Characterization of the polymer. J Biol Chem 242, 3172–3179. [PubMed] [Google Scholar]
- 34. Fujimura S, Hasegawa S, Shimizu Y & Sugimura T (1967) Polymerization of the adenosine 5′‐diphosphate‐ribose moiety of nicotinamide‐adenine dinucleotide by nuclear enzyme. I. Enzymatic reactions. Biochim Biophys Acta 145, 247–259. [DOI] [PubMed] [Google Scholar]
- 35. Vyas S, Matic I, Uchima L, Rood J, Zaja R, Hay RT, Ahel I & Chang P (2014) Family‐wide analysis of poly(ADP‐ribose) polymerase activity. Nat Commun 5, 4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slade D, Dunstan M, Barkauskaite E, Weston R, Lafite P, Dixon N, Ahel M, Leys D & Ahel I (2011) The structure and catalytic mechanism of a poly(ADP‐ribose) glycohydrolase. Nature 477, 616–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunn AR, Banos‐Pinero B, Paschke P, Sanchez‐Pulido L, Ariza A, Day J, Emrich M, Leys D, Ponting CP, Ahel I et al (2016) The role of ADP‐ribosylation in regulating DNA interstrand crosslink repair. J Cell Sci 129, 3845–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lopes RR, Kessler AC, Polycarpo C & Alfonzo JD (2015) Cutting, dicing, healing and sealing: the molecular surgery of tRNA. Wiley Interdiscip Rev RNA 6, 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han S, Craig JA, Putnam CD, Carozzi NB & Tainer JA (1999) Evolution and mechanism from structures of an ADP‐ribosylating toxin and NAD complex. Nat Struct Biol 6, 932–936. [DOI] [PubMed] [Google Scholar]
- 40. Tsuge H, Nagahama M, Oda M, Iwamoto S, Utsunomiya H, Marquez VE, Katunuma N, Nishizawa M & Sakurai J (2008) Structural basis of actin recognition and arginine ADP‐ribosylation by Clostridium perfringens iota‐toxin. Proc Natl Acad Sci USA 105, 7399–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Margarit SM, Davidson W, Frego L & Stebbins CE (2006) A steric antagonism of actin polymerization by a Salmonella virulence protein. Structure 14, 1219–1229. [DOI] [PubMed] [Google Scholar]
- 42. Glowacki G, Braren R, Firner K, Nissen M, Kuhl M, Reche P, Bazan F, Cetkovic‐Cvrlje M, Leiter E, Haag F et al (2002) The family of toxin‐related ecto‐ADP‐ribosyltransferases in humans and the mouse. Protein Sci 11, 1657–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F & Koch‐Nolte F (2003) NAD‐induced T cell death: ADP‐ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 19, 571–582. [DOI] [PubMed] [Google Scholar]
- 44. Frye RA (2000) Phylogenetic classification of prokaryotic and eukaryotic Sir2‐like proteins. Biochem Biophys Res Commun 273, 793–798. [DOI] [PubMed] [Google Scholar]
- 45. Verdin E (2015) NAD⁺ in aging, metabolism, and neurodegeneration. Science 350, 1208–1213. [DOI] [PubMed] [Google Scholar]
- 46. Vassilopoulos A, Fritz KS, Petersen DR & Gius D (2011) The human sirtuin family: evolutionary divergences and functions. Hum Genomics 5, 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Michan S & Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bheda P, Jing H, Wolberger C & Lin H (2016) The substrate specificity of sirtuins. Annu Rev Biochem 85, 405–429. [DOI] [PubMed] [Google Scholar]
- 49. Rack JG, Morra R, Barkauskaite E, Kraehenbuehl R, Ariza A, Qu Y, Ortmayer M, Leidecker O, Cameron DR, Matic I et al (2015) Identification of a class of protein ADP‐ribosylating sirtuins in microbial pathogens. Mol Cell 59, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cervantes‐Laurean D, Jacobson EL & Jacobson MK (1996) Glycation and glycoxidation of histones by ADP‐ribose. J Biol Chem 271, 10461–10469. [DOI] [PubMed] [Google Scholar]
- 51. Kalisch T, Amé JC, Dantzer F & Schreiber V (2012) New readers and interpretations of poly(ADP‐ribosyl)ation. Trends Biochem Sci 37, 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karras GI, Kustatscher G, Buhecha HR, Allen MD, Pugieux C, Sait F, Bycroft M & Ladurner AG (2005) The macro domain is an ADP‐ribose binding module. EMBO J 24, 1911–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Feijs KL, Forst AH, Verheugd P & Lüscher B (2013) Macrodomain‐containing proteins: regulating new intracellular functions of mono(ADP‐ribosyl)ation. Nat Rev Mol Cell Biol 14, 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rack JG, Perina D & Ahel I (2016) Macrodomains: structure, function, evolution, and catalytic activities. Annu Rev Biochem 85, 431–454. [DOI] [PubMed] [Google Scholar]
- 55. Chen D, Vollmar M, Rossi MN, Phillips C, Kraehenbuehl R, Slade D, Mehrotra PV, von Delft F, Crosthwaite SK, Gileadi O et al (2011) Identification of macrodomain proteins as novel O‐acetyl‐ADP‐ribose deacetylases. J Biol Chem 286, 13261–13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tan J, Vonrhein C, Smart OS, Bricogne G, Bollati M, Kusov Y, Hansen G, Mesters JR, Schmidt CL & Hilgenfeld R (2009) The SARS‐unique domain (SUD) of SARS coronavirus contains two macrodomains that bind G‐quadruplexes. PLoS Pathog 5, e1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Neuvonen M & Ahola T (2009) Differential activities of cellular and viral macro domain proteins in binding of ADP‐ribose metabolites. J Mol Biol 385, 212–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barkauskaite E, Brassington A, Tan ES, Warwicker J, Dunstan MS, Banos B, Lafite P, Ahel M, Mitchison TJ, Ahel I et al (2013) Visualization of poly(ADP‐ribose) bound to PARG reveals inherent balance between exo‐ and endo‐glycohydrolase activities. Nat Commun 4, 2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sharifi R, Morra R, Appel CD, Tallis M, Chioza B, Jankevicius G, Simpson MA, Matic I, Ozkan E, Golia B et al (2013) Deficiency of terminal ADP‐ribose protein glycohydrolase TARG1/C6orf130 in neurodegenerative disease. EMBO J 32, 1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Golia B, Moeller GK, Jankevicius G, Schmidt A, Hegele A, Preißer J, Tran ML, Imhof A & Timinszky G (2017) ATM induces MacroD2 nuclear export upon DNA damage. Nucleic Acids Res 45, 244–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saikatendu KS, Joseph JS, Subramanian V, Clayton T, Griffith M, Moy K, Velasquez J, Neuman BW, Buchmeier MJ, Stevens RC et al (2005) Structural basis of severe acute respiratory syndrome coronavirus ADP‐ribose‐1″‐phosphate dephosphorylation by a conserved domain of nsP3. Structure 13, 1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ahel I, Ahel D, Matsusaka T, Clark AJ, Pines J, Boulton SJ & West SC (2008) Poly(ADP‐ribose)‐binding zinc finger motifs in DNA repair/checkpoint proteins. Nature 451, 81–85. [DOI] [PubMed] [Google Scholar]
- 63. Wang Z, Michaud GA, Cheng Z, Zhang Y, Hinds TR, Fan E, Cong F & Xu W (2012) Recognition of the iso‐ADP‐ribose moiety in poly(ADP‐ribose) by WWE domains suggests a general mechanism for poly(ADP‐ribosyl)ation‐dependent ubiquitination. Genes Dev 26, 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang F, Chen Y, Li M & Yu X (2014) The oligonucleotide/oligosaccharide‐binding fold motif is a poly(ADP‐ribose)‐binding domain that mediates DNA damage response. Proc Natl Acad Sci USA 111, 7278–7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pleschke JM, Kleczkowska ME, Strohm M & Althaus FR (2000) Poly(ADP‐ribose) binds to specific domains in DNA damage checkpoint proteins. J Biol Chem 275, 40974–40980. [DOI] [PubMed] [Google Scholar]
- 66. Barkauskaite E, Jankevicius G, Ladurner AG, Ahel I & Timinszky G (2013) The recognition and removal of cellular poly(ADP‐ribose) signals. FEBS J 280, 3491–3507. [DOI] [PubMed] [Google Scholar]
- 67. Moure VR, Costa FF, Cruz LM, Pedrosa FO, Souza EM, Li XD, Winkler F & Huergo LF (2015) Regulation of nitrogenase by reversible mono‐ADP‐ribosylation. Curr Top Microbiol Immunol 384, 89–106. [DOI] [PubMed] [Google Scholar]
- 68. Berthold CL, Wang H, Nordlund S & Högbom M (2009) Mechanism of ADP‐ribosylation removal revealed by the structure and ligand complexes of the dimanganese mono‐ADP‐ribosylhydrolase DraG. Proc Natl Acad Sci USA 106, 14247–14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jankevicius G, Hassler M, Golia B, Rybin V, Zacharias M, Timinszky G & Ladurner AG (2013) A family of macrodomain proteins reverses cellular mono‐ADP‐ribosylation. Nat Struct Mol Biol 20, 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosenthal F, Feijs KL, Frugier E, Bonalli M, Forst AH, Imhof R, Winkler HC, Fischer D, Caflisch A, Hassa PO et al (2013) Macrodomain‐containing proteins are new mono‐ADP‐ribosylhydrolases. Nat Struct Mol Biol 20, 502–507. [DOI] [PubMed] [Google Scholar]
- 71. Winstall E, Affar EB, Shah R, Bourassa S, Scovassi IA & Poirier GG (1999) Preferential perinuclear localization of poly(ADP‐ribose) glycohydrolase. Exp Cell Res 251, 372–378. [DOI] [PubMed] [Google Scholar]
- 72. Meyer‐Ficca ML, Meyer RG, Coyle DL, Jacobson EL & Jacobson MK (2004) Human poly(ADP‐ribose) glycohydrolase is expressed in alternative splice variants yielding isoforms that localize to different cell compartments. Exp Cell Res 297, 521–532. [DOI] [PubMed] [Google Scholar]
- 73. Niere M, Mashimo M, Agledal L, Dölle C, Kasamatsu A, Kato J, Moss J & Ziegler M (2012) ADP‐ribosylhydrolase 3 (ARH3), not poly(ADP‐ribose) glycohydrolase (PARG) isoforms, is responsible for degradation of mitochondrial matrix‐associated poly(ADP‐ribose). J Biol Chem 287, 16088–16102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Peterson FC, Chen D, Lytle BL, Rossi MN, Ahel I, Denu JM & Volkman BF (2011) Orphan macrodomain protein (human C6orf130) is an O‐acyl‐ADP‐ribose deacylase: solution structure and catalytic properties. J Biol Chem 286, 35955–35965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shull NP, Spinelli SL & Phizicky EM (2005) A highly specific phosphatase that acts on ADP‐ribose 1″‐phosphate, a metabolite of tRNA splicing in Saccharomyces cerevisiae . Nucleic Acids Res 33, 650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nordlund S & Högbom M (2013) ADP‐ribosylation, a mechanism regulating nitrogenase activity. FEBS J 280, 3484–3490. [DOI] [PubMed] [Google Scholar]
- 77. Kato J, Zhu J, Liu C & Moss J (2007) Enhanced sensitivity to cholera toxin in ADP‐ribosylarginine hydrolase‐deficient mice. Mol Cell Biol 27, 5534–5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mueller‐Dieckmann C, Kernstock S, Lisurek M, von Kries JP, Haag F, Weiss MS & Koch‐Nolte F (2006) The structure of human ADP‐ribosylhydrolase 3 (ARH3) provides insights into the reversibility of protein ADP‐ribosylation. Proc Natl Acad Sci USA 103, 15026–15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oka S, Kato J & Moss J (2006) Identification and characterization of a mammalian 39‐kDa poly(ADP‐ribose) glycohydrolase. J Biol Chem 281, 705–713. [DOI] [PubMed] [Google Scholar]
- 80. Bazan JF & Koch‐Nolte F (1997) Sequence and structural links between distant ADP‐ribosyltransferase families. Adv Exp Med Biol 419, 99–107. [DOI] [PubMed] [Google Scholar]
- 81. Fabrizio G, Di Paola S, Stilla A, Giannotta M, Ruggiero C, Menzel S, Koch‐Nolte F, Sallese M & Di Girolamo M (2015) ARTC1‐mediated ADP‐ribosylation of GRP78/BiP: a new player in endoplasmic‐reticulum stress responses. Cell Mol Life Sci 72, 1209–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Matic I, Ahel I & Hay RT (2012) Reanalysis of phosphoproteomics data uncovers ADP‐ribosylation sites. Nat Methods 9, 771–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Martello R, Leutert M, Jungmichel S, Bilan V, Larsen SC, Young C, Hottiger MO & Nielsen ML (2016) Proteome‐wide identification of the endogenous ADP‐ribosylome of mammalian cells and tissue. Nat Commun 7, 12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mashimo M, Kato J & Moss J (2014) Structure and function of the ARH family of ADP‐ribosyl‐acceptor hydrolases. DNA Repair (Amst) 23, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ono T, Kasamatsu A, Oka S & Moss J (2006) The 39‐kDa poly(ADP‐ribose) glycohydrolase ARH3 hydrolyzes O‐acetyl‐ADP‐ribose, a product of the Sir2 family of acetyl‐histone deacetylases. Proc Natl Acad Sci USA 103, 16687–16691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kasamatsu A, Nakao M, Smith BC, Comstock LR, Ono T, Kato J, Denu JM & Moss J (2011) Hydrolysis of O‐acetyl‐ADP‐ribose isomers by ADP‐ribosylhydrolase 3. J Biol Chem 286, 21110–21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mildvan AS, Xia Z, Azurmendi HF, Saraswat V, Legler PM, Massiah MA, Gabelli SB, Bianchet MA, Kang LW & Amzel LM (2005) Structures and mechanisms of Nudix hydrolases. Arch Biochem Biophys 433, 129–143. [DOI] [PubMed] [Google Scholar]
- 88. McLennan AG (2006) The Nudix hydrolase superfamily. Cell Mol Life Sci 63, 123–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wright RH, Lioutas A, Le Dily F, Soronellas D, Pohl A, Bonet J, Nacht AS, Samino S, Font‐Mateu J, Vicent GP et al (2016) ADP‐ribose‐derived nuclear ATP synthesis by NUDIX5 is required for chromatin remodeling. Science 352, 1221–1225. [DOI] [PubMed] [Google Scholar]
- 90. Bollen M, Gijsbers R, Ceulemans H, Stalmans W & Stefan C (2000) Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol 35, 393–432. [DOI] [PubMed] [Google Scholar]
- 91. Palazzo L, Thomas B, Jemth AS, Colby T, Leidecker O, Feijs KL, Zaja R, Loseva O, Puigvert JC, Matic I et al (2015) Processing of protein ADP‐ribosylation by Nudix hydrolases. Biochem J 468, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Daniels CM, Thirawatananond P, Ong S, Gabelli SB & Leung AK (2015) Nudix hydrolases degrade protein‐conjugated ADP‐ribose. Sci Rep 5, 18271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Palazzo L, Daniels CM, Nettleship JE, Rahman N, McPherson RL, Ong SE, Kato K, Nureki O, Leung AK & Ahel I (2016) ENPP1 processes protein ADP‐ribosylation in vitro. FEBS J 283, 3371–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Oka J, Ueda K & Hayaishi O (1978) Snake venom phosphodiesterase: simple purification with Blue Sepharose and its application to poly(ADP‐ribose) study. Biochem Biophys Res Commun 80, 841–848. [DOI] [PubMed] [Google Scholar]
- 95. Bhogaraju S, Kalayil S, Liu Y, Bonn F, Colby T, Matic I & Dikic I (2016) Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination. Cell 167, 1636–1649. [DOI] [PubMed] [Google Scholar]
- 96. Kotewicz KM, Ramabhadran V, Sjoblom N, Vogel JP, Haenssler E, Zhang M, Behringer J, Scheck RA & Isberg RR (2017) A single legionella effector catalyzes a multistep ubiquitination pathway to rearrange tubular endoplasmic reticulum for replication. Cell Host Microbe 21, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schultz N, Lopez E, Saleh‐Gohari N & Helleday T (2003) Poly(ADP‐ribose) polymerase (PARP‐1) has a controlling role in homologous recombination. Nucleic Acids Res 31, 4959–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. De Vos M, Schreiber V & Dantzer F (2012) The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem Pharmacol 84, 137–146. [DOI] [PubMed] [Google Scholar]
- 99. Langelier MF, Planck JL, Roy S & Pascal JM (2012) Structural basis for DNA damage‐dependent poly(ADP‐ribosyl)ation by human PARP‐1. Science 336, 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hu Y, Petit SA, Ficarro SB, Toomire KJ, Xie A, Lim E, Cao SA, Park E, Eck MJ, Scully R et al (2014) PARP1‐driven poly‐ADP‐ribosylation regulates BRCA1 function in homologous recombination‐mediated DNA repair. Cancer Discov 4, 1430–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Xie S, Mortusewicz O, Ma HT, Herr P, Poon RY, Helleday T & Qian C (2015) Timeless interacts with PARP‐1 to promote homologous recombination repair. Mol Cell 60, 163–176. [DOI] [PubMed] [Google Scholar]
- 102. Rulten SL, Fisher AE, Robert I, Zuma MC, Rouleau M, Ju L, Poirier G, Reina‐San‐Martin B & Caldecott KW (2011) PARP‐3 and APLF function together to accelerate nonhomologous end‐joining. Mol Cell 41, 33–45. [DOI] [PubMed] [Google Scholar]
- 103. Vida A, Márton J, Mikó E & Bai P (2017) Metabolic roles of poly(ADP‐ribose) polymerases. Semin Cell Dev Biol 63, 135–143. [DOI] [PubMed] [Google Scholar]
- 104. Kickhoefer VA, Siva AC, Kedersha NL, Inman EM, Ruland C, Streuli M & Rome LH (1999) The 193‐kD vault protein, VPARP, is a novel poly(ADP‐ribose) polymerase. J Cell Biol 146, 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S et al (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620. [DOI] [PubMed] [Google Scholar]
- 106. Fearon ER (2009) PARsing the phrase “all in for Axin”‐ Wnt pathway targets in cancer. Cancer Cell 16, 366–368. [DOI] [PubMed] [Google Scholar]
- 107. Mariotti L, Templeton CM, Ranes M, Paracuellos P, Cronin N, Beuron F, Morris E & Guettler S (2016) Tankyrase requires SAM domain‐dependent polymerization to support Wnt‐β‐catenin signaling. Mol Cell 63, 498–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Haikarainen T, Krauss S & Lehtio L (2014) Tankyrases: structure, function and therapeutic implications in cancer. Curr Pharm Des 20, 6472–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Smith S, Giriat I, Schmitt A & de Lange T (1998) Tankyrase, a poly(ADP‐ribose) polymerase at human telomeres. Science 282, 1484–1487. [DOI] [PubMed] [Google Scholar]
- 110. Nagy Z, Kalousi A, Furst A, Koch M, Fischer B & Soutoglou E (2016) Tankyrases promote homologous recombination and check point activation in response to DSBs. PLoS Genet 12, e1005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Huang JY, Wang K, Vermehren‐Schmaedick A, Adelman J & Cohen MS (2016) PARP6 is a regulator of hippocampal dendritic morphogenesis. Sci Rep 6, 18512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bock FJ, Todorova TT & Chang P (2015) RNA regulation by poly(ADP‐ribose) polymerases. Mol Cell 58, 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bütepage M, Eckei L, Verheugd P & Lüscher B (2015) Intracellular mono‐ADP‐ribosylation in signaling and disease. Cells 4, 569–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Verheugd P, Forst AH, Milke L, Herzog N, Feijs KL, Kremmer E, Kleine H & Lüscher B (2013) Regulation of NF‐κB signalling by the mono‐ADP‐ribosyltransferase ARTD10. Nat Commun 4, 1683. [DOI] [PubMed] [Google Scholar]
- 115. Feijs KL, Kleine H, Braczynski A, Forst AH, Herzog N, Verheugd P, Linzen U, Kremmer E & Lüscher B (2013) ARTD10 substrate identification on protein microarrays: regulation of GSK3β by mono‐ADP‐ribosylation. Cell Commun Signal 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yu M, Schreek S, Cerni C, Schamberger C, Lesniewicz K, Poreba E, Vervoorts J, Walsemann G, Grötzinger J, Kremmer E et al (2005) PARP‐10, a novel Myc‐interacting protein with poly(ADP‐ribose) polymerase activity, inhibits transformation. Oncogene 24, 1982–1993. [DOI] [PubMed] [Google Scholar]
- 117. Iwata H, Goettsch C, Sharma A, Ricchiuto P, Goh WW, Halu A, Yamada I, Yoshida H, Hara T, Wei M et al (2016) PARP9 and PARP14 cross‐regulate macrophage activation via STAT1 ADP‐ribosylation. Nat Commun 7, 12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Meyer‐Ficca ML, Ihara M, Bader JJ, Leu NA, Beneke S & Meyer RG (2015) Spermatid head elongation with normal nuclear shaping requires ADP‐ribosyltransferase PARP11 (ARTD11) in mice. Biol Reprod 92, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Carter‐O'Connell I, Jin H, Morgan RK, Zaja R, David LL, Ahel I & Cohen MS (2016) Identifying family‐member‐specific targets of mono‐ARTDs by using a chemical genetics approach. Cell Rep 14, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Westcott NP, Fernandez JP, Molina H & Hang HC (2017) Chemical proteomics reveals ADP‐ribosylation of small GTPases during oxidative stress. Nat Chem Biol 13, 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chapman JD, Gagné JP, Poirier GG & Goodlett DR (2013) Mapping PARP‐1 auto‐ADP‐ribosylation sites by liquid chromatography‐tandem mass spectrometry. J Proteome Res 12, 1868–1880. [DOI] [PubMed] [Google Scholar]
- 122. Zhang Y, Wang J, Ding M & Yu Y (2013) Site‐specific characterization of the Asp‐ and Glu‐ADP‐ribosylated proteome. Nat Methods 10, 981–984. [DOI] [PubMed] [Google Scholar]
- 123. Daniels CM, Ong SE & Leung AK (2015) The promise of proteomics for the study of ADP‐ribosylation. Mol Cell 58, 911–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Daniels CM, Ong SE & Leung AK (2014) Phosphoproteomic approach to characterize protein mono‐ and poly(ADP‐ribosyl)ation sites from cells. J Proteome Res 13, 3510–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Leidecker O, Bonfiglio JJ, Colby T, Zhang Q, Atanassov I, Zaja R, Palazzo L, Stockum A, Ahel I & Matic I (2016) Serine is a new target residue for endogenous ADP‐ribosylation on histones. Nat Chem Biol 12, 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bilan V, Leutert M, Nanni P, Panse C & Hottiger MO (2017) Combining Higher‐Energy Collision Dissociation and Electron‐Transfer/Higher‐Energy Collision Dissociation Fragmentation in a Product‐Dependent Manner Confidently Assigns Proteome wide ADP‐Ribose Acceptor Sites. Anal Chem 89, 1523‐1530. [DOI] [PubMed] [Google Scholar]
- 127. Bonfiglio JJ, Fontana P, Zhang Q, Colby T, Gibbs‐Seymour I, Atanassov I, Bartlett E, Zaja R, Ahel I & Matic I (2017) Serine ADP‐ribosylation depends on HPF1. Mol Cell 65, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gibbs‐Seymour I, Fontana P, Rack JG & Ahel I (2016) HPF1/C4orf27 Is a PARP‐1‐interacting protein that regulates PARP‐1 ADP‐ribosylation activity. Mol Cell 62, 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Holbourn KP, Shone CC & Acharya KR (2006) A family of killer toxins – exploring the mechanism of ADP‐ribosylating toxins. FEBS J 273, 4579–4593. [DOI] [PubMed] [Google Scholar]
- 130. Huh JW, Shima J & Ochi K (1996) ADP‐ribosylation of proteins in Bacillus subtilis and its possible importance in sporulation. J Bacteriol 178, 4935–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Eastman D & Dworkin M (1994) Endogenous Adp‐ribosylation during development of the prokaryote Myxococcus xanthus . Microbiology 140, 3167–3176. [DOI] [PubMed] [Google Scholar]
- 132. Hildebrandt K, Eastman D & Dworkin M (1997) ADP‐ribosylation by the extracellular fibrils of Myxococcus xanthus . Mol Microbiol 23, 231–235. [DOI] [PubMed] [Google Scholar]
- 133. Ochi K, Penyige A & Barabas G (1992) The possible role of ADP‐ribosylation in sporulation and streptomycin production by Streptomyces griseus . J Gen Microbiol 138, 1745–1750. [DOI] [PubMed] [Google Scholar]
- 134. Penyige A, Deak E, Kalmanczhelyi A & Barabas G (1996) Evidence of a role for NAD(+)‐glycohydrolase and ADP‐ribosyltransferase in growth and differentiation of Streptomyces griseus NRRL B‐2682: inhibition by m‐aminophenylboronic acid. Microbiology 142, 1937–1944. [DOI] [PubMed] [Google Scholar]
- 135. Penyige A, Keseru J, Fazakas F, Schmelczer I, Szirak K, Barabas G & Biro S (2009) Analysis and identification of ADP‐ribosylated proteins of Streptomyces coelicolor M145. J Microbiol 47, 549–556. [DOI] [PubMed] [Google Scholar]
- 136. Szirak K, Keseru J, Biro S, Schmelczer I, Barabas G & Penyige A (2012) Disruption of SCO5461 gene coding for a mono‐ADP‐ribosyltransferase enzyme produces a conditional pleiotropic phenotype affecting morphological differentiation and antibiotic production in Streptomyces coelicolor . J Microbiol 50, 409–418. [DOI] [PubMed] [Google Scholar]
- 137. Penyige A, Barabás G, Szabó I & Ensign JC (1990) ADP‐ribosylation of membrane proteins of Streptomyces griseus strain 52‐1. FEMS Microbiol Lett 57, 293–297. [DOI] [PubMed] [Google Scholar]
- 138. Penyige A, Saido‐Sakanaka H & Ochi K (1996) Endogenous ADP‐ribosylation of proteins during development of Streptomyces griseus . Actinomycetologica 10, 98–103. [Google Scholar]
- 139. Shima J, Penyige A & Ochi K (1996) Changes in patterns of ADP‐ribosylated proteins during differentiation of Streptomyces coelicolor A3(2) and its developmental mutants. J Bacteriol 178, 3785–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nakano T, Matsushima‐Hibiya Y, Yamamoto M, Takahashi‐Nakaguchi A, Fukuda H, Ono M, Takamura‐Enya T, Kinashi H & Totsuka Y (2012) ADP‐ribosylation of guanosine by SCO5461 protein secreted from Streptomyces coelicolor . Toxicon 63, 55–63. [DOI] [PubMed] [Google Scholar]
- 141. Lyons B, Ravulapalli R, Lanoue J, Lugo MR, Dutta D, Carlin S & Merrill AR (2016) Scabin, a novel DNA‐acting ADP‐ribosyltransferase from Streptomyces scabies . J Biol Chem 291, 11198–11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sawaya R, Schwer B & Shuman S (2005) Structure‐function analysis of the yeast NAD+‐dependent tRNA 2′‐phosphotransferase Tpt1. RNA 11, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Baysarowich J, Koteva K, Hughes DW, Ejim L, Griffiths E, Zhang K, Junop M & Wright GD (2008) Rifamycin antibiotic resistance by ADP‐ribosylation: structure and diversity of Arr. Proc Natl Acad Sci USA 105, 4886–4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Stallings CL, Chu LD, Li LX & Glickman MS (2011) Catalytic and non‐catalytic roles for the mono‐ADP‐ribosyltransferase Arr in the mycobacterial DNA damage response. PLoS One 6, e21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liu YQ, Zhou JZ, Omelchenko MV, Beliaev AS, Venkateswaran A, Stair J, Wu LY, Thompson DK, Xu D, Rogozin IB et al (2003) Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc Natl Acad Sci USA 100, 4191–4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kim KS, Manasherob R & Cohen SN (2008) YmdB: a stress‐responsive ribonuclease‐binding regulator of E. coli RNase III activity. Genes Dev 22, 3497–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kim T, Lee J & Kim KS (2013) Escherichia coli YmdB regulates biofilm formation independently of its role as an RNase III modulator. BMC Microbiol 13, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lalic J, Posavec Marjanovic M, Palazzo L, Perina D, Sabljic I, Zaja R, Colby T, Plese B, Halasz M, Jankevicius G et al (2016) Disruption of macrodomain protein SCO6735 increases antibiotic production in Streptomyces coelicolor . J Biol Chem 291, 23175–23187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Ahel I, Vujaklija D, Mikoc A & Gamulin V (2002) Transcriptional analysis of the recA gene in Streptomyces rimosus: identification of the new type of promoter. FEMS Microbiol Lett 209, 133–137. [DOI] [PubMed] [Google Scholar]
- 150. Gamulin V, Cetkovic H & Ahel I (2004) Identification of a promoter motif regulating the major DNA damage response mechanism of Mycobacterium tuberculosis . FEMS Microbiol Lett 238, 57–63. [DOI] [PubMed] [Google Scholar]
- 151. Mikulik K, Felsberg J, Kudrnacova E, Bezouskova S, Setinova D, Stodulkova E, Zidkova J & Zidek V (2012) CobB1 deacetylase activity in Streptomyces coelicolor . Biochem Cell Biol 90, 179–187. [DOI] [PubMed] [Google Scholar]
- 152. Tan Y, Xu Z, Tao J, Ni J, Zhao W, Lu J & Yao YF (2016) A SIRT4‐like auto ADP‐ribosyltransferase is essential for the environmental growth of Mycobacterium smegmatis . Acta Biochim Biophys Sin (Shanghai) 48, 145–152. [DOI] [PubMed] [Google Scholar]
- 153. Moore JM, Bradshaw E, Seipke RF, Hutchings MI & McArthur M (2012) Use and discovery of chemical elicitors that stimulate biosynthetic gene clusters in Streptomyces bacteria. Methods Enzymol 517, 367–385. [DOI] [PubMed] [Google Scholar]
- 154. Harms A, Maisonneuve E & Gerdes K (2016) Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 354, pii: aaf4268, doi: 10.1126/science.aaf4268. [DOI] [PubMed] [Google Scholar]
- 155. Sberro H, Leavitt A, Kiro R, Koh E, Peleg Y, Qimron U & Sorek R (2013) Discovery of functional toxin/antitoxin systems in bacteria by shotgun cloning. Mol Cell 50, 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Spalding MD & Prigge ST (2009) The amidase domain of lipoamidase specifically inactivates lipoylated proteins in vivo. PLoS One 4, e7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Welchman RL, Gordon C & Mayer RJ (2005) Ubiquitin and ubiquitin‐like proteins as multifunctional signals. Nat Rev Mol Cell Biol 6, 599–609. [DOI] [PubMed] [Google Scholar]
- 158. Qiu J, Sheedlo MJ, Yu K, Tan Y, Nakayasu ES, Das C, Liu X & Luo ZQ (2016) Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 533, 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Bell SD, Botting CH, Wardleworth BN, Jackson SP & White MF (2002) The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science 296, 148–151. [DOI] [PubMed] [Google Scholar]
- 160. Faraone‐Mennella MR, Gambacorta A, Nicolaus B & Farina B (1998) Purification and biochemical characterization of a poly(ADP‐ribose) polymerase‐like enzyme from the thermophilic archaeon Sulfolobus solfataricus . Biochem J 335, 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Dani N, Stilla A, Marchegiani A, Tamburro A, Till S, Ladurner AG, Corda D & Di Girolamo M (2009) Combining affinity purification by ADP‐ribose‐binding macro domains with mass spectrometry to define the mammalian ADP‐ribosyl proteome. Proc Natl Acad Sci USA 106, 4243–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Uzan M & Miller ES (2010) Post‐transcriptional control by bacteriophage T4: mRNA decay and inhibition of translation initiation. Virol J 7, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Alawneh AM, Qi D, Yonesaki T & Otsuka Y (2016) An ADP‐ribosyltransferase Alt of bacteriophage T4 negatively regulates the Escherichia coli MazF toxin of a toxin‐antitoxin module. Mol Microbiol 99, 188–198. [DOI] [PubMed] [Google Scholar]
- 164. Kusov Y, Tan J, Alvarez E, Enjuanes L & Hilgenfeld R (2015) A G‐quadruplex‐binding macrodomain within the “SARS‐unique domain” is essential for the activity of the SARS‐coronavirus replication‐transcription complex. Virology 484, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Kuny CV & Sullivan CS (2016) Virus‐host interactions and the ARTD/PARP family of enzymes. PLoS Pathog 12, e1005453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Atasheva S, Frolova EI & Frolov I (2014) Interferon‐stimulated poly(ADP‐Ribose) polymerases are potent inhibitors of cellular translation and virus replication. J Virol 88, 2116–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Guo X, Ma J, Sun J & Gao G (2007) The zinc‐finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc Natl Acad Sci USA 104, 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Daugherty MD, Young JM, Kerns JA & Malik HS (2014) Rapid evolution of PARP genes suggests a broad role for ADP‐ribosylation in host‐virus conflicts. PLoS Genet 10, e1004403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Tempera I, Deng Z, Atanasiu C, Chen CJ, D'Erme M & Lieberman PM (2010) Regulation of Epstein‐Barr virus OriP replication by poly(ADP‐ribose) polymerase 1. J Virol 84, 4988–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Grady SL, Hwang J, Vastag L, Rabinowitz JD & Shenk T (2012) Herpes simplex virus 1 infection activates poly(ADP‐ribose) polymerase and triggers the degradation of poly(ADP‐ribose) glycohydrolase. J Virol 86, 8259–8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Carter‐O'Connell I & Cohen MSI (2015) Identifying direct protein targets of poly‐ADP‐ribose polymerases (PARPs) using engineered PARP variants‐orthogonal nicotinamide adenine dinucleotide (NAD+) analog pairs. Curr Protoc Chem Biol 7, 121–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Bartolomei G, Leutert M, Manzo M, Baubec T & Hottiger MO (2016) Analysis of chromatin ADP‐ribosylation at the genome‐wide level and at specific loci by ADPr‐ChAP. Mol Cell 61, 474–485. [DOI] [PubMed] [Google Scholar]
- 173. Kistemaker HA, Lameijer LN, Meeuwenoord NJ, Overkleeft HS, van der Marel GA & Filippov DV (2015) Synthesis of well‐defined adenosine diphosphate ribose oligomers. Angew Chem Int Ed Engl 54, 4915–4918. [DOI] [PubMed] [Google Scholar]
- 174. Lambrecht MJ, Brichacek M, Barkauskaite E, Ariza A, Ahel I & Hergenrother PJ (2015) Synthesis of dimeric ADP‐ribose and its structure with human poly(ADP‐ribose) glycohydrolase. J Am Chem Soc 137, 3558–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Kistemaker HA, Nardozza AP, Overkleeft HS, van der Marel GA, Ladurner AG & Filippov DV (2016) Synthesis and macrodomain binding of mono‐ADP‐ribosylated peptides. Angew Chem Int Ed Engl 55, 10634–10638. [DOI] [PubMed] [Google Scholar]


