Abstract
The extent to which the fitness costs of infection are mediated by key life‐history traits such as age or social status is still unclear. Within populations, individual heterogeneity in the outcome of infection is the result of two successive processes; the degree of contact with the pathogen (exposure) and the immune response to infection. In social mammals, because individuals holding high social status typically interact more frequently with group members, they should be more often in contact with infected individuals than those of low social status. However, when access to resources is determined by social status, individuals with a high social status are often better nourished, have a greater opportunity to allocate resources to immune processes and therefore should have a smaller chance of succumbing to infection than individuals with low social status.
We investigated the risk and fitness costs of infection during a virulent epidemic of canine distemper virus (CDV) in a social carnivore, the spotted hyena, in the Serengeti National Park. We analysed two decades of detailed life‐history data from 625 females and 816 males using a multi‐event capture–mark–recapture model that accounts for uncertainty in the assignment of individual infection states.
Cubs of mothers with a high social status had a lower probability of CDV infection and were more likely to survive infection than those with low social status. Subadult and adult females with high social status had a higher infection probability than those with low social status. Subadult females and pre‐breeder males that had recovered from CDV infection had a lower survival than susceptible ones.
Our study disentangles the relative importance of individual exposure and resource allocation to immune processes, demonstrates fitness costs of infection for juveniles, particularly for those with low social status, shows that patterns of infection can be driven by different mechanisms among juveniles and adults and establishes a negative relationship between infection and fitness in a free‐ranging mammal.
A http://onlinelibrary.wiley.com/doi/10.1111/1365-2435.13059/suppinfo is available for this article.
Keywords: canine distemper virus, exposure, fitness costs, infection risk, multi‐event capture–mark–recapture model, resource allocation, social status, spotted hyena
http://onlinelibrary.wiley.com/doi/10.1111/1365-2435.13059/suppinfo

1. INTRODUCTION
Infectious diseases pose a particular threat to high‐density populations and group‐living species with high contact rates (Hawley & Altizer, 2011). There may be substantial variation among members of a group in their degree of exposure to a given pathogen and in the outcome of infection (Beldomenico & Begon, 2010; VanderWaal & Ezenwa, 2016). Body condition and the ability to allocate resources to immune processes can profoundly affect the outcome of infection as can sex, age and social status (East et al., 2015; Schmid‐Hempel, 2003). Quantifying individual differences in the outcome of infection and determining the impact of infection on Darwinian fitness in wildlife populations may be a challenge because diagnosing the infection status for large numbers of individuals is difficult in practice, particularly if only non‐invasive methods are available (Gimenez, Lebreton, Gaillard, Choquet, & Pradel, 2012; McClintock et al., 2010). This probably explains why little is known about these processes in natural environments. Obtaining such information is, however, crucial to improve knowledge of host–pathogen dynamics and predict the likely impact of diseases, particularly during epidemics, on the most vulnerable classes of individuals and on population long‐term viability (Gervasi, Civitello, Kilvitis, & Martin, 2015; Hawley & Altizer, 2011; Kappeler, Cremer, & Nunn, 2015; Oli, Venkataraman, Klein, Wendland, & Brown, 2006).
In social mammals with stable dominance hierarchies, an individual's social status regulates its access to (food) resources and is thus often positively correlated with both body condition and fitness (Clutton‐Brock & Huchard, 2013). Even so, the relationship between an individual's social status and the outcome of infection with a given pathogen is less straightforward. Several studies suggest that socially dominant animals are more frequently exposed to pathogens because they are “valuable” social partners which experience higher contact rates with conspecifics than subordinates, for instance when forming and maintaining social bonds (Seyfarth, 1977). Such a positive relationship between social status and exposure has been shown in several mammals (e.g. MacIntosh et al., 2012). On the other hand, subordinate individuals may be less exposed but more likely to contract diseases than dominants and experience a more severe outcome when infected. This is because subordinates, whose access to resources is limited, have a higher allostatic load, i.e. a higher cumulative energetic cost of maintaining homeostasis (Cavigelli & Chaudhry, 2012; Goymann & Wingfield, 2004; Kappeler et al., 2015; Sapolsky, 2005), and thus their allocation of resources to immune processes is more often curtailed. Despite considerable research into the influence of social processes on disease risk and spread, often with the use of social network analyses (e.g. Cauchemez et al., 2011; Duboscq, Romano V., & MacIntosh, 2016; Kappeler et al., 2015; MacIntosh et al., 2012; Nunn, Jordán, McCabe, Verdolin, & Fewell, 2015), to our knowledge few studies on wildlife populations have investigated the effect of social status on fitness by explicitly accounting for the effect of both these processes: (1) variation in exposure to a pathogen (“exposure” hypothesis) and (2) variation in the outcome of infection determined by the opportunity to allocate resources to immune processes (“allocation” hypothesis).
Multi‐event capture–mark–recapture (MECMR) models are a recent and powerful advance in statistical methods that make it possible to disentangle the effect of both processes on the infection probability and to determine the impact of infection on survival (Chambert et al., 2012; Conn & Cooch, 2009; Gimenez et al., 2012; Pradel, 2005). Here, we used this approach to assess the effect of social status in a highly social carnivore, the spotted hyena Crocuta crocuta (hereafter “hyena”) on canine distemper virus (CDV) infection during an epidemic when a strain pathogenic to this species circulated (Nikolin et al., 2017). CDV is a highly contagious virus of a taxonomically broad range of carnivores (Beineke, Baumgärtner, & Wohlsein, 2015; Deem, Spelman, Yates, & Montali, 2000). The outcome of CDV infection ranges from subclinical to fatal, and as with other morbilliviruses, individuals surviving the infection acquire lifelong immunity (Appel & Summers, 1995). After transmission to a susceptible host, CDV targets lymphocytes, macrophages, dendritic cells and lymphatic tissue and induces immunosuppression, which enhances the spread of CDV through the bloodstream (asymptomatic and non‐contagious stage, Tatsuo, Ono, & Yanagi, 2001). In the next stage, CDV enters epithelial cells, which results in the manifestation of clinical disease and virus shedding (Sawatsky, Wong, Hinkelmann, Cattaneo, & von Messling, 2012) and finally, CDV attacks the central nervous system, causing neurological signs (symptomatic and contagious stages). During all stages, CDV profoundly suppresses host immune responses, predisposing infected animals to secondary infections (Sawatsky et al., 2012).
In 1993/1994, a virulent CDV epidemic caused the death of an estimated 30% of the African lion Panthera leo population in the Serengeti National Park (NP) (Roelke‐Parker et al., 1996). In hyenas, noticeable “hotspots of infection” (i.e. clinical disease and increased mortality) were primarily observed in cubs stationed at communal dens (Haas et al., 1996). CDV strains that infected lions and hyenas during this epidemic were genetically distinct from those infecting canids and encoded unique mutations that most likely increased the virulence of the 1993/1994 epidemic for non‐canids (Nikolin et al., 2017).
In our study population, low‐ranking females and low‐ranking males spend a larger proportion of each year travelling long distances between the clan territory and distant areas containing high densities of migratory prey: a foraging tactic termed commuting (Hofer & East, 1993b). High‐ranking females (Hofer & East, 2003) and males (East & Hofer, 1991) are thus present more often in the clan territory than low‐ranking ones and have high intimate contact with clan members, including cubs at the communal den (East, Hofer, & Wickler, 1993). Furthermore, the mean rate at which cubs, subadults, adult females and immigrant adult males received friendly oral contacts as well as bites increases with their social status (East et al., 2001). Similarly, in another population, high‐ranking females are also more gregarious than low‐ranking ones, forming more bonds and receiving more frequent social support (Engh, Siebert, Greenberg, & Holekamp, 2005; Ilany, Booms, & Holekamp, 2015; Smith, Memenis, & Holekamp, 2007). The importance of elevated contact rates among high‐ranking clan members for the transmission of infectious pathogens is illustrated by significantly higher seroprevalence to virus infections among high‐ranking than low‐ranking hyenas in our study population (East et al., 2001) as well as in another one (Harrison et al., 2004). High‐ranking hyenas within the separate linear dominance hierarchies among adult females (plus their offspring) (Hofer & East, 2003) and among adult reproductively active males (East & Hofer, 2001) have high rates of contact with clan members, thereby facilitating “hyena‐to‐hyena” CDV transmission through contact with virus shedding individuals. The exposure hypothesis therefore predicts that high‐ranking hyenas, females in particular, are more likely to get infected with CDV than low‐ranking ones (Figure 1).
Figure 1.
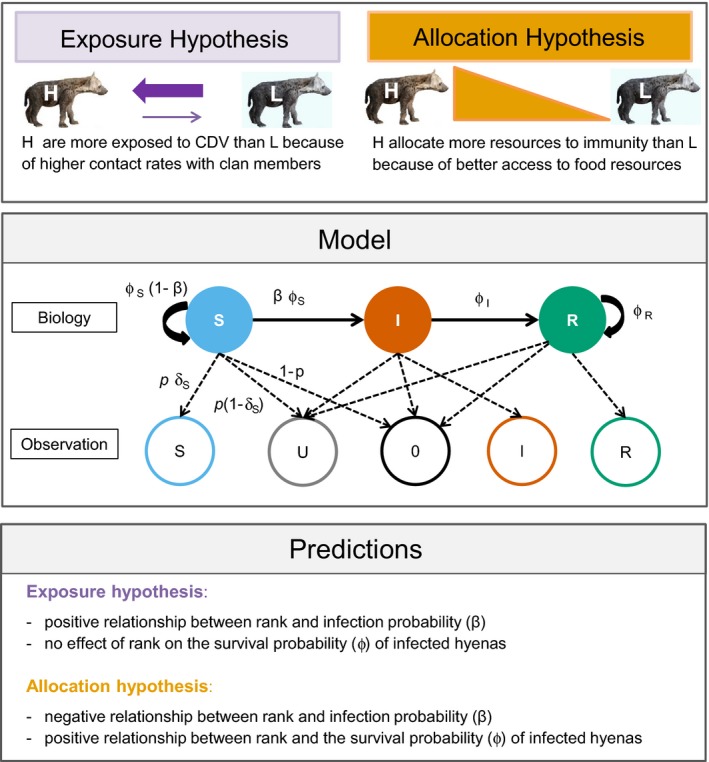
Schematic representation of hypotheses, predictions and study design. Top: The two hypotheses (“H”: high‐ranking, “L”: low‐ranking). Centre: Processes underlying model construction, with infection process at the top and observation process at the bottom: Infection states (solid circles, S [blue]: “susceptible”, I [orange]: “infected”, R [green]: “recovered”) and transitions between states (solid black arrows) as a function of the probability of surviving in a given state (ϕi, with i specific for S, I and R) and the probability of staying susceptible (1 − β) or becoming infected (β). Infection states are linked (dashed black arrows) to four events (left to right): detected individual is assigned S (empty blue circle), detected individual is assigned U (empty grey circle), individual not detected (0, empty black circle), detected individual assigned I (empty red circle) and detected individual assigned R (empty green circle). With p the detection probability, δj the probability of assigning an infection state (j being specific for S, I and R), p δj the probability of assigning a detected individual to an infection state, p (1 − δj ) the probability of detecting an individual and assigning it U and 1 − p j the probability of not detecting an individual—only shown for S for simplicity. Bottom: The predictions of both hypotheses in terms of probability of infection (β) and survival (ϕ)
On the other hand, there is strong evidence that a high social position within the sex‐specific linear dominance hierarchies has major benefits for hyenas in terms of their access to resources and fitness (Hofer & East, 2003; Holekamp, Smale, & Szykman, 1996; Höner et al., 2010), allostatic load (“stress”) (Goymann et al., 2001) and allocation of resources to immune processes (East et al., 2015; Flies, Mansfield, Flies, Grant, & Holekamp, 2016). Thus, the allocation hypothesis predicts that high‐ranking hyenas are less likely to get infected with CDV and more likely to survive the infection than low‐ranking hyenas (Figure 1). To our knowledge, our study is the first to disentangle the relative importance of individual exposure and resource allocation to immune processes in a wildlife population.
2. MATERIALS AND METHODS
2.1. Study design and data collection
We continuously monitored three hyena clans located at the centre of the Serengeti NP (East et al., 2015; Hofer & East, 1993b) between 1990 and 2010. Data were collected from all clan members and individuals were identified by their unique spot patterns, scars and other characteristics such as ear notches. Clans contained philopatric females, which breed throughout the year (Hofer & East, 1995), and their offspring, plus breeding males that are mostly immigrants (East & Hofer, 2001). Females and their offspring were socially dominant over immigrant males (Hofer & East, 2003). Females and natal males were first detected and aged within their first few weeks of life as previously detailed (e.g. Hofer & East, 1993a, 2003). Sex was assessed at c. 3 months of age using the dimorphic glans morphology of the erect phallus following Frank, Glickman, and Licht (1991). Weaning occurs at 12–20 months of age (Hofer & East, 1995; Holekamp et al., 1996).
For health monitoring and disease diagnosis, we recorded the start and end of clinical signs of CDV. For virus screening, we collected saliva, faeces and blood from known individuals and, opportunistically, tissue samples from dead individuals we encountered that had died of natural causes or had been hit by vehicles.
All procedures were performed in accordance with the requirements of the Leibniz Institute for Zoo and Wildlife Research Ethics Committee on Animal Welfare (permit number: 2014‐09‐03).
2.2. Definition of demographic, social and infection states
To meet the assumptions of capture–mark–recapture (CMR) models, only systematic observations of study clan members at communal and birth dens were included. For this purpose, multiple observations of individuals during a given year were synthesized into a single yearly summary: any clan member that was observed at its clan communal and/or birth dens at least once throughout the year was set as “detected” that year. This dataset includes information on the detection (presence) or non‐detection (putative absence, possibly death, or in the case of males, emigration) of any clan member for every year between 1990 and 2010. When an individual was detected in a given year (thereafter a “detection year”), it was assigned to a specific demographic, social and infection state, accounting for uncertainty in the assignment of the infection state (see below “2.2.3”). Females and males were treated separately because the behavioural mechanism by which they acquire and maintain their social states differed. In adult female hyenas, the key mechanism is behavioural support provided by coalition partners during social interactions (East et al., 2009; Hofer & East, 2003). In contrast, immigrant males queue for social status, thus their social status increases with clan tenure (East & Hofer, 2001).
2.2.1. Demographic states
Females (N = 625) were classified as cubs (C), subadults (SA), breeders (B) or non‐breeders (NB). Age was determined from dates of witnessed births or to an accuracy of 1 week using pelage characteristics, body size, the degree to which ears were extended and the degree of coordination and mobility (e.g. East, Burke, Wilhelm, Greig, & Hofer, 2003; Hofer, Benhaiem, Golla, & East, 2016; Hofer & East, 2003). Female cubs were younger than 1 year. Subadult females were aged between 1 and 2 years. Female breeders gave birth to a litter during a given year, as documented by a freshly ruptured clitoris caused by parturitions (Hofer & East, 1993b) and/or subsequent lactation, whereas non‐breeders did not. The vast majority of female breeders were lactating females; hence, this state represented the elevated energetic cost of lactation (Hofer et al., 2016). Males (N = 816) were classified as cubs (C), pre‐breeders (PB) or breeders (B). Male cubs were younger than 1 year. Male pre‐breeders were older than 1 year, still members of their natal clan and had not yet started reproducing. Both pre‐breeder males and subadult females spent most of their time away from communal dens. Male breeders showed reproductive behaviour (East & Hofer, 2001) towards female clan members or were verified by DNA microsatellite profiling to have fathered at least one cub (Höner et al., 2012). Breeders were predominately immigrant males, a minority of cases (12%) were reproductively active natal males. As males do not participate in parental care, we did not distinguish between male breeder and non‐breeder states.
2.2.2. Social states
Females and males were classified as either high‐ranking (H) or low‐ranking (L), based on their positions in the (strictly linear) adult female and adult male dominance hierarchies, respectively. We recorded submissive behaviours during dyadic adult female–female and adult male–male interactions (e.g. East et al., 2003; Hofer & East, 2003) and constructed strictly linear dominance hierarchies for each clan. Interactions were recorded ad libitum during frequent observation periods of c. 3 hr duration at both dawn and dusk, mostly at clan dens and during all‐night observations. Dominance hierarchies were adjusted after each loss or recruitment of adults and when dyadic interaction data revealed than an individual had increased or fallen in rank. To permit the comparison of the ranks held by individuals within hierarchies containing different numbers of animals within and across clans and years, we computed for each rank held by an individual during its lifetime a standardized rank. This measure places the ranks within a given hierarchy evenly between the highest (standardized rank: +1) and the lowest (standardized rank: −1) rank (East et al., 2003; Goymann et al., 2001). For breeder and non‐breeder females, and breeder and pre‐breeders males older than 2 years, the social states were high‐ranking (H: average standardized rank ranging from 0.01 to +1) and low‐ranking (L: average standardized rank ranging from −1 to 0). If different social states were observed for an individual within a year, we assigned the most frequently observed state (i.e. H or L) during that year for that individual. The yearly proportions of H and L states among females and among males were not exactly 50%–50% in each clan because (1) we calculated standardized ranks for each stable period (i.e. when no losses or recruitment of adults to the hierarchy occurred and the ranks held by individuals did not change) and each year was constituted by a variable number of such periods and because (2) we assigned the social state of the genetic mother to female and male cubs, female subadults and male pre‐breeders younger than 2 years for non‐adopted offspring, and that of the surrogate mother for adopted offspring (as offspring typically acquire a rank immediately below that of the female that reared them, Hofer & East, 2003; East et al., 2009).
2.2.3. Infection states
Infection states in both females and males were assigned using three diagnostic procedures: (1) RT‐PCR screening for the presence or absence of CDV RNA in samples (see above); results were classified as “viropositive” or “vironegative”, respectively, (2) CDV antibody titres in sera; results were classified “seropositive” when serum contained a significant antibody titre against CDV and “seronegative” when not, and (3) the observation of clinical signs associated with CDV infection in hyenas, and the secondary infections it causes in this species (Haas et al., 1996, also see Supporting Information, section 2.c), hereafter termed “clinical signs.” Individuals were assigned as:
Susceptible (S): individuals with a seronegative result, unless clinical signs and/or a viropositive result were observed during the same year. Cubs with a vironegative result were also considered susceptible unless clinical signs and/or another viropositive result were observed during the same year. We assumed that we would not have missed any clinical sign in cubs since they are under particularly detailed observations at communal dens.
Infected (I): individuals with clinical signs and/or a viropositive result. This state encompassed both the non‐contagious and contagious stages of CDV infection.
Recovered (R): individuals with a seropositive result without clinical signs and/or a viropositive result during the same year.
Unknown (U): individuals lacking both RT‐PCR screening or serological results, and in which clinical signs were not observed.
As for all morbiliviruses, individuals that survive CDV infection acquire lifelong immunity (e.g. Appel & Summers, 1995; Beineke, Puff, Seehusen, & Baumgärtner, 2009; Beineke et al., 2015; Deem et al., 2000; Garenne, Leroy, Beau, & Sene, 1991; Haas et al., 1996; Harrison et al., 2004; Sawatsky et al., 2012; Tatsuo et al., 2001). For this reason, CDV infection occurs only once in an individual's life. By applying this fact, any individual classified as (1) “susceptible” in a given year was classified as “susceptible” during all previous years when the individual was detected; (2) “infected” in a given year was classified as “susceptible” during all previous years, and “recovered” during all subsequent years when the individual was detected; (3) “recovered” in a given year was classified as “recovered” during all subsequent years when the individual was detected. Sample sizes in terms of number of individuals and number of states for (1) each infection state and for (2) each combination of demographic, social and infection state are provided in Table S1 and Table S2, respectively.
2.3. Multi‐event capture–mark–recapture model
We used a multi‐event CMR (MECMR) model (Pradel, 2005) fitted in E‐SURGE 1.9.0. (Choquet & Nogue, 2011) to estimate survival and state transition probabilities (the “biological processes”). This model permits the estimation of such parameters whilst simultaneously accounting for potential methodological biases. These include gaps between monitoring periods, left‐censored data when individuals were observed first as adults at the beginning of the study, and right‐censored data when individuals were still alive but not necessarily detected at the end of the study (the “observation processes”; see Schaub, Gimenez, Schmidt, & Pradel, 2004; Lebreton, Nichols, Barker, Pradel, & Spendelow, 2009; Gimenez et al., 2012 and Figure 1 for a graphical representation of the biological and observation processes). This model also accounts for the potentially imperfect detection of male hyenas that have temporarily or permanently dispersed, females raising their offspring outside communal dens, or females staying with the migratory prey herds for periods exceeding 1 year (M.L. East, personal obs.). Individuals detected (“captured”) in a given year were assigned an (S, I or R) infection state if data were available or an unknown infection state if data were unavailable (Conn & Cooch, 2009). More classical models would normally discard individuals with unknown infection states, but this would most likely result in biases (as shown in e.g. Desprez, McMahon, Hindell, Harcourt, & Gimenez, 2013). We assumed known infection states were assigned correctly, i.e. we deliberately ignored potential errors in the assignment of infection states (Chambert et al., 2012; Conn & Cooch, 2009). The biological processes included survival (ϕ) and transition probabilities. As the lack of detection of males might result from death or emigration, we measured apparent survival (see the Supporting Information section 2f for details). The infection probability β was the probability for a susceptible individual to become infected, r was the probability of staying in the same social state and the breeding probability ψ was the probability that subadult, breeder and non‐breeder females became breeder females, and pre‐breeder males became breeder males. We report Maximum likelihood estimates (MLE) with associated SE.
We fitted two sets of candidate models separately for females and males. The biological processes were the product of four squared matrices representing transitions between demographic states, social states, infection states and survival.
2.3.1. Transitions between demographic states
The matrix Demo (Equation (1)) considers the transitions of females to three demographic states; subadults (SA), breeders (B) or non‐breeders (NB):
| (1) |
with Ψ the transition probability to the B state accessible from SA, B and NB females and with 1 − Ψ its complement. Each entry in Demo is the probability of transition from a “starting” demographic state (four rows corresponding to the demographic states C, SA, B, NB on the left side of the matrix) to the “following” demographic state (four columns corresponding to the demographic states C, SA, B, NB, not shown for simplicity). Here for example, surviving cubs (C) (“starting state”) have a transition probability to the subadult state (SA) (“following state”) that is equal to 1. The equivalent matrix for males is presented in the Supporting Information, section 2.f. Please note that Ψ is a symbolic notation here. This parameter could vary between states depending on the model being tested.
2.3.2. Transitions between social states
The matrix Social (Equation (2)) considers the transitions of females or males to two social states, High social state (H) or Low social state (L), as individuals can either remain or change their social state:
| (2) |
with r the probability of staying in the same social state and (1 − r) its complement. Each entry in Social is the probability of transition from a “starting” social state (two rows corresponding to the social states L and H) to a “following social state (two columns corresponding to the social states L and H, not shown for simplicity). Please note that r is a symbolic notation here. This parameter could vary between states depending on the model being tested.
2.3.3. Transitions between infection states
The matrix Infection (Equation (3)) considers the transitions of females or males to three infection states, susceptible (S), infected (I) and recovered (R):
| (3) |
with β the infection probability (i.e. the probability of transition from a susceptible to an infected state) and 1 − β its complement. Each entry in Infection is the probability of transition from a “starting” infection state category (three rows corresponding to the infection states S, I, R) to a “following” infection state (three columns corresponding the infection states S, I, R, not shown for simplicity). Please note that β is a symbolic notation here. This parameter could vary between states depending on the model being tested.
2.3.4. Survival
The matrix Survival (Equation (4)) accounts for the annual survival probabilities of females, shows annual apparent survival probabilities and is:
| (4) |
with ϕ the survival probability. Each entry in Survival is the probability of surviving from a “starting” demographic state (four rows corresponding to the demographic states C, SA, B, NB on the left side of the matrix). Dd represents the transition to the “dead” state. The equivalent matrix for males is presented in the Supporting Information section 2.f. Please note that ϕ is a symbolic notation here. This parameter could vary between states depending on the model being tested.
To represent all possible transitions between demographic, social, and infection states of surviving individuals, we then combined these matrices in a way that is fully described in the Supporting Information.
2.3.5. Goodness‐of‐fit
Prior to model selection, we performed a goodness‐of‐fit test to (1) determine whether our data met the assumptions of CMR models and (2) validate our model (Grosbois et al., 2008). The results on the model fit are presented in the Supporting Information, see section 2.f.
2.3.6. Model selection
We fitted two sets of candidate models separately for females and males. It is often recommended to parameterize the observation process (here: detection and assignment), before the biological one (e.g. Culina, Lachish, Pradel, Choquet, & Sheldon, 2013). Starting with a constant‐only model, we sequentially parameterized the: (1) assignment of infection states, (2) detection, (3) survival, (4) infection, (5) social transitions and (6) breeding transitions, testing for effects of social, demographic and infection states on those processes. To test our two main hypotheses and disentangle the importance of exposure and allocation in affecting the outcome of CDV infection, we tested for the effect of social states on infection probability and on the survival of individuals in different infection states, confronting our results with the predictions derived from each hypothesis as summarized in Figure 1.
For the observation processes, we chose models with the lowest value of the quasi‐Akaike information criterion corrected for small sample size (QAICc, Hannan & Quinn, 1979). QAICc was used rather than the common AIC to correct for potentially autocorrelated and overdispersed data (Hannan & Quinn, 1979). For the biological processes, best models were those with the largest number of parameters within a range of two QAICc units of difference from the model with the lowest QAICc value, in order to avoid dismissing potentially important biological predictors of interest. We considered that fully identifiable models differing by <2 QAICc units from the model with the lowest QAICc might have substantial empirical support for explaining variation in the response variable (Burnham & Anderson, 2003) and report these also in Table 1.
Table 1.
The best fully identifiable models which predict variation in survival (ϕ), infection (β), breeding transitions (Ψ) and social transitions (r) for females and malesa
| Dataset | Process | Effects | NP | Dev | QAICc | ΔQAICc |
|---|---|---|---|---|---|---|
| ♀ | ϕ | cub (social × infection), subadult (social × infection)b, breeder, non‐breeder | 26 | 10,422.9 | 10,476.4 | 429.0 |
| cub (social × infection), subadult (social × infection)c, breeder (social), non‐breeder | 28 | 10,419.1 | 10,476.9 | 429.4 | ||
| cub (social × infection), subadult (social × infection)c, breeder, non‐breeder | 27 | 10,421.7 | 10,477.3 | 429.9 | ||
| cub (social × infection), subadult (social × infection)c, breeder, non‐breeder (social) | 28 | 10,421.2 | 10,477.7 | 430.3 | ||
| β | cub (social), subadult & breeder & non‐breeder (social) | 32 | 10,343.8 | 10,410.1 | 363.7 | |
| cub (social), subadult & breeder & non‐breeder | 31 | 10,346.1 | 10,410.3 | 362.8 | ||
| Ψ | cub, subadult (social), breeder (social), non‐breeder (social) | 37 | 9,973.8 | 10,051.0 | 3.5 | |
| cub, subadult, breeder, non‐breeder | 34 | 9,981.6 | 10,052.3 | 4.8 | ||
| r | social | 38 | 9,968.1 | 10,047.4 | 0 | |
| ♂ | ϕ | cub(social × infection), pre‐breeder (social × infection)d, breeder (social) | 24 | 9,341.3 | 9,389.8 | 51.3 |
| β | cub(social), pre‐breeder, breeder | 28 | 9,305.5 | 9,362.2 | 0 | |
| Ψ | constant | 28 | 9,305.5 | 9,362.2 | 0 | |
| r | constant | 28 | 9,305.5 | 9,362.2 | 0 |
The rows marked in grey show the selected models. The effects of states are shown in the following sequence: demography, social, infection. The ampersand (“&”) indicates that demographic, social or infection states were pooled. Round brackets (“()”) after a demographic state indicate that there was an effect of social states, infection states, or an interaction (symbol ×) between social and infection states. A raised number after round brackets (“()n”) indicates the footnote which explains details of how some social or infection states were pooled. For example, the model formulation for the first grey row was: among cubs an interaction between social and infection states, among subadults an interaction between social and infection states but infected and recovered subadults pooled among high‐ranking and among low‐ranking females as described in footnotec, among breeders an effect of social states, among non‐breeders no effect of social or infection states. The number of identifiable parameters is indicated by the abbreviation NP. Dev denotes the deviance, QAICc the quasi‐Akaike Information Criterion corrected for small sample size and overdispersed data
Best models were those with a value for the quasi‐Akaike Information Criterion corrected for small sample size (QAICc) differing from the model with the lowest value (ΔQAICc) by values of <2.
subadult high‐ranking susceptible; subadult low‐ranking susceptible; subadult high and low‐ranking infected and recovered
subadult high‐ranking susceptible; subadult low‐ranking susceptible; subadult high‐ranking infected and recovered; subadult low‐ranking infected and recovered
pre‐breeder high and low‐ranking susceptible; pre‐breeder high‐ranking infected and recovered; pre‐breeder low‐ranking infected and recovered.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3. RESULTS
During the study period, hyenas were identified in 90% of encounter occasions. The sex, demographic state and social state were assigned to 82% of individuals. The remaining individuals (not detected at communal dens or with at least one unclear or unknown demographic or social state during their encounter history) were excluded. Our final datasets were composed of 625 females and 816 males. For 43% of females and 44% of males, information on their infection state was available on at least one occasion during their encounter history. Amongst females, 18.9% were cubs, 14.9% subadults, 34.6% non‐breeders and 31.6% breeders; amongst males 22.2% were cubs, 18.2% pre‐breeders and 59.6% breeders. High‐ranking females and males were slightly more commonly observed than low‐ranking ones (1,542 high‐ranking vs. 1,306 low‐ranking states for females; 1,463 high‐ranking vs. 1,167 low‐ranking states for males). Fifty‐four females were infected with CDV between 1991 and 1997 (8.6% of females). Thirty‐eight males were infected between 1991 and 1997 (4.7% of males).
3.1. Disease course
The proportion of infected individuals peaked during the 1993/1994 CDV epidemic and declined rapidly thereafter (Figure 2). After 1997, no infected clan members were observed. Since then, the proportion of susceptible individuals substantially increased and the proportion of recovered individuals declined.
Figure 2.
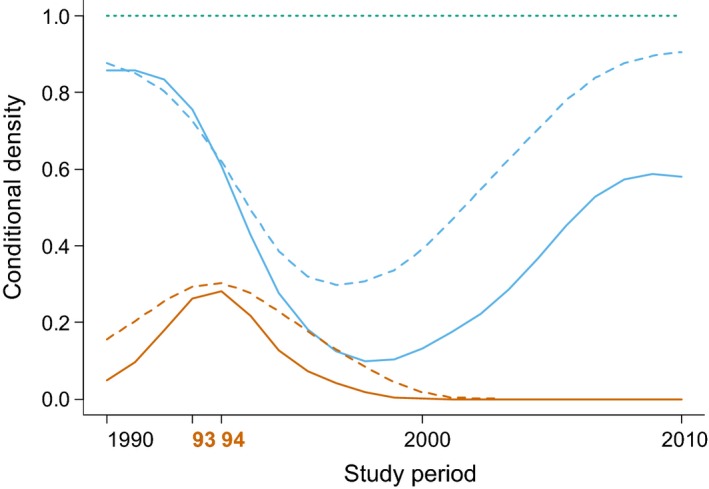
Conditional probability distribution of the three infection states “susceptible”, “infected” and “recovered” during the study period 1990–2010. These probability densities are obtained with the cdplot function in R which computes a smoothing kernel density function. Solid lines: females, dashed lines: males. The probability densities of “infected” are represented by the area below the orange lines, those of “susceptible” cover the area between the orange and the blue lines and those of “recovered” cover the area between the blue lines and 1, the green dotted horizontal line. The virulent canine distemper virus (CDV) epidemic (1993–1994) is indicated in orange on the x‐axis. An interpolation factor was used to smooth the lines between data points
3.2. Females
3.2.1. Effect of social status on infection probability
Social status influenced the infection probability of cubs and of older individuals (pooled subadults, breeders and non‐breeders) in opposite directions (Table 1). Infection probability was lower for high‐ranking than low‐ranking cubs (Table 2, Figure 3a) and higher for high‐ranking subadults, breeder and non‐breeder females than low‐ranking ones (Table 2).
Table 2.
Maximum likelihood estimates (MLE) (±SE) of annual probabilities of surviving (ϕ), becoming infected (β), breeding (Ψ) and remaining within the same social state (r), according to the best models, respectively, for females and males (shown in Table 1). H: high‐ranking, L: low‐ranking
| Process | Effects | MLE ± SE | |
|---|---|---|---|
| ♀ | ♂ | ||
| ϕ | H susceptible cubs [C.H.S] | 0.87 ± 0.05 | 0.86 ± 0.06 |
| L susceptible cubs [C.L.S] | 0.80 ± 0.08 | 0.78 ± 0.10 | |
| H infected cubs [C.H.I] | 0.73 ± 0.05 | 0.68 ± 0.07 | |
| L infected cubs [C.L.I] | 0.56 ± 0.07 | 0.50 ± 0.10 | |
| H susceptible subadults [SA.H.S] | 0.93 ± 0.06 | –a | |
| L susceptible subadults [SA.L.S] | 0.84 ± 0.17 | –a | |
| H infected and recovered subadults [SA.(I&R)] | 0.66 ± 0.03 | –a | |
| L infected and recovered subadults [SA.(I&R)] | 0.60 ± 0.04 | –a | |
| H and L susceptible pre‐breeders [PB.S] | – | 0.87 ± 0.08 | |
| H infected and recovered pre‐breeders [PB.(I&R)] | – | 0.64 ± 0.04 | |
| L infected and recovered pre‐breeders [PB.(I&R)] | – | 0.58 ± 0.04 | |
| H and L non‐breeders [NB] | 0.83 ± 0.01 | –a | |
| H breeders [B.H] | 0.95 ± 0.01 | 0.70 ± 0.02 | |
| L breeders [B.L] | 0.92 ± 0.01 | 0.82 ± 0.01 | |
| β | H cubs [C.H] | 0.59 ± 0.09 | 0.67 ± 0.09 |
| L cubs [C.L] | 0.83 ± 0.07 | 0.91 ± 0.08 | |
| H subadults, breeders and non‐breeders [SA.H & B.H & NB.H] | 0.36 ± 0.06 | – | |
| L subadults, breeders and non‐breeders [SA.L & B.L & NB.L] | 0.23 ± 0.06 | – | |
| H and L pre‐breeders [PB] | – | 0.80 ± 0.10 | |
| H and L breeders [B] | – | 0.45 ± 0.09 | |
| Ψ | H subadults [SA.H →B.H] | 0.04 ± 0.02 | – |
| L subadults [SA.L →B.L] | 0.01 ± 0.01 | – | |
| H non‐breeders [NB.H →B.H] | 0.68 ± 0.02 | – | |
| L non‐breeders [NB.L →B.L] | 0.60 ± 0.03 | – | |
| H breeders [B.H →B.H] | 0.49 ± 0.02 | – | |
| L breeders [B.L →B.L] | 0.45 ± 0.03 | – | |
| H and L pre‐breeders [PB (H&L) → B(H&L)] | – | 0.40 ± 0.02 | |
| r | H remaining H | 0.94 ± 0.01 | – |
| L remaining L | 0.97 ± 0.01 | – | |
| L and H remaining within their social state | – | 0.92 ± 0.01 | |
In males, subadults are included in the pre‐breeder category.
In males, once males became reproductively active, they were assumed to have a continuous interest in reproduction, as they do not contribute to the rearing of the young and reproduction in Serengeti hyenas is year‐round, without any obvious seasonality (Hofer & East, 1995).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 3.
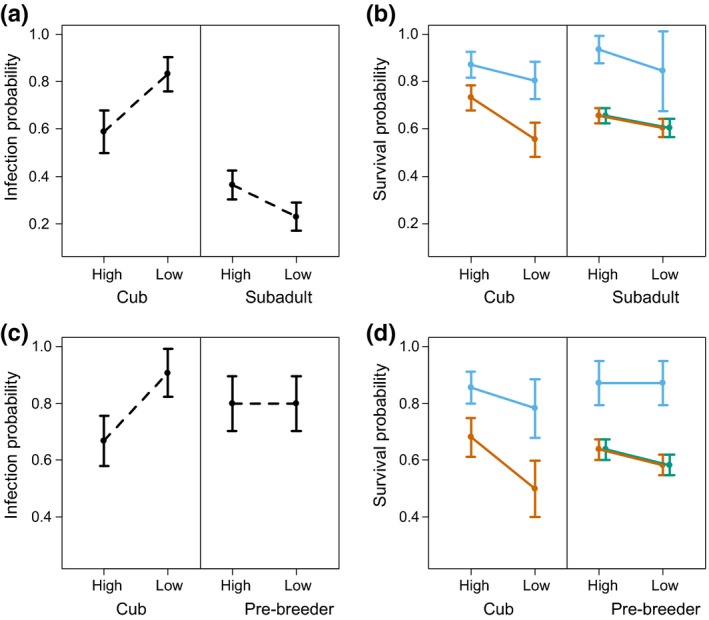
Maximum likelihood estimate (MLE) (±SE) probabilities of annual infection with canine distemper virus (CDV) ((a) and (c), dashed lines) and survival ((b) and (d), solid lines) of Serengeti spotted hyenas as a function of demographic, social and infection states as detected by the best‐ranked model. (a) and (b) High and low‐ranking female cubs and subadults; (c) and (d) High and low‐ranking male cubs and pre‐breeders. Infection states were susceptible (pale blue), infected (orange) and recovered (green)
3.2.2. Effect of social status on survival of susceptible and infected females
Social status affected the survival probability of susceptible and infected cubs (Table 1), with susceptible high‐ranking female cubs surviving better than susceptible low‐ranking ones and infected high‐ranking female cubs surviving better than infected low‐ranking ones (Table 2, Figure 3b). Similarly, susceptible high‐ranking subadults had a higher survival than susceptible low‐ranking ones, and pooled infected and recovered high‐ranking subadults, a slightly higher survival than pooled infected and recovered low‐ranking subadults (Table 2, Figure 3b). Both high‐ranking and low‐ranking susceptible subadults had a substantially higher survival than pooled infected and recovered ones (Table 2, Figure 3b). Breeder survival was higher than non‐breeder survival (Table 2). Among breeders, the survival of high‐ranking females was higher than that of low‐ranking females (Table 2).
3.3. Males
3.3.1. Effect of social status on infection probability
Social status influenced the infection probability amongst cubs (Table 1). High‐ranking cubs had a lower infection probability than low‐ranking ones, as for females (Table 2, Figure 3c).
3.3.2. Effect of social status on survival of susceptible and infected
As for females, susceptible high‐ranking male cubs survived better than susceptible low‐ranking ones (Tables 1 and 2) and infected high‐ranking male cubs survived better than infected low‐ranking ones (Table 2, Figure 3d). Susceptible pre‐breeders survived better than pooled infected and recovered ones and within this pooled group, high‐ranking pre‐breeders survived better than low‐ranking ones (Table 2, Figure 3d). High‐ranking breeders had a lower survival than low‐ranking ones (Table 2).
4. DISCUSSION
The impact of the 1993/1994 CDV epidemic on hyenas in the Serengeti NP was substantial, particularly among young animals. Maternal social status had a substantial impact on the probability of cub infection and its outcome. High‐born cubs were less likely to be infected and incurred a smaller reduction in their survival once infected (female cubs 16%, male cubs 20%) than low‐born cubs (female cubs 31%, male cubs 36%, Table 2, Figure 3b,d). These findings are consistent with the idea that high‐born cubs allocate substantially more resources to immune processes than low‐born ones because their milk intake is significantly higher (Hofer & East, 1993b, 2003; Hofer et al., 2016). This suggests that the rank‐related ability to allocate resources to immune processes is more important in determining the outcome of infection in dependent hyena cubs than differences in CDV exposure. Our results are consistent with other studies on hyenas that provide evidence that social status affects the allocation of resources to immune processes and the outcome of infection. During lactation, low‐ranking female hyenas have significantly higher helminth egg burdens and are more likely to have concurrent protozoan parasite infections than high‐ranking females (East et al., 2015). Similarly, nutritionally disadvantaged hyenas suffered higher mortality during an outbreak of a pathogenic bacterium (Höner et al., 2012). Higher serum concentrations of the immunoglobulin IgM in high‐ranking females may reflect a greater allocation of resources to immune processes (Flies et al., 2016). Rank‐related differences in the expression of immune genes have been reported in a non‐human primate (Tung et al., 2012).
High‐ranking hyena cubs may also have a greater ability than low‐ranking cubs to invest in the repair of cells and tissues damaged by CDV and immune processes to combat secondary infections that typically accompany CDV infection (Beineke et al., 2009), which would have aided their recovery. Even so, we cannot exclude the possibility that high‐ and low‐ranking cubs differ in their immune responses because of differences in their allelic composition of immune genes. The cost of mate‐choice is higher for low‐ranking than high‐ranking females (East et al., 2003) and hence a genetic component may in part explain the more severe outcome of CDV infection low‐ranking cubs.
In contrast to female cubs, high‐ranking female subadults were more likely to be infected with CDV than low‐ranking ones (consistent with the exposure hypothesis) but once infected their survival was better than that of low‐ranking ones (Figure 3b, Table 2) (consistent with the allocation hypothesis). Interestingly, the reduction in survival was similar between social states (high‐born 30%, low‐born 28%; Table 2). As weaning usually takes place during the second year of life, the demographic class of subadults comprised both unweaned and weaned life stages. Variation in the contribution of milk and solid food to the diet of subadults may explain why differences in the survival of high‐ranking and low‐ranking infected and recovered female subadults were less clear‐cut than for cubs, whose survival completely depends on maternal milk (Hofer & East, 1993b). Among pre‐breeder males, there was no effect of social status and no difference in the survival of infected high‐ranking and low‐ranking individuals. The reduction in survival for pre‐breeder males was substantial and of a similar magnitude to that in subadult females (high‐born 27%, low‐born 33%, Table 2, Figure 3d).
We found clear evidence of a delayed detrimental effect on the survival of subadult females and pre‐breeding males that recovered from infection, suggesting a longer term fitness cost of CDV infection in terms of reduced survival (Figure 3c, d, Table 2). CDV substantially depletes lymphoid organs and lymphocytes, reduces responses of immunomodulatory cytokines and upregulates inflammatory cytokines. Direct virus‐mediated damage and pro‐inflammatory cytokine‐induced damage to the central nervous system can also occur (Beineke et al., 2009). The CDV strain that infected hyenas in the 1993/1994 epidemic encoded one novel amino acid in the V‐protein (Nikolin et al., 2017). The V‐protein is known to disrupt host interferon signalling (von Messling, Svitek, & Cattaneo, 2006; Röthlisberger et al., 2010), hence rendering the outcome of infection particularly severe. As repairing CDV‐damaged tissues and mounting immune responses to infection are costly in terms of body resources, hosts that do not meet these costs are prone to secondary infections that then fuel this “vicious circle” (Beineke et al., 2009; Beldomenico & Begon, 2010). The hitherto unsuspected, delayed and detrimental effect of CDV infection in female subadult and male pre‐breeder hyenas most likely resulted from the severe pathologies caused by a non‐canid adapted CDV strain.
Our results confirm previous observations (Haas et al., 1996) that mostly young hyenas succumbed to clinical CDV, as was the case for infection of hyenas with Alphacoronavirus (Goller, Fickel, Hofer, Beier, & East, 2013), Hepatozoon (East et al., 2008) and Dipylidium helminths (East, Kurze, Wilhelm, Benhaiem, & Hofer, 2013) in our study population. Although adults exposed to CDV before the 1993/1994 epidemic presumably had protective antibody titres, the lack of clinical canine distemper in adult hyenas during this epidemic was probably due to other components of the immune system. Hyenas, like the domestic cat Felis catus (Beineke et al., 2015), can have CDV replicating in leucocytes without developing clinical disease (Nikolin et al., 2017). Since in long‐lived species, adult survival is a critical fitness component, natural selection may also favour the development of stronger adult immune defences to minimize the impact of infections (Boots, Best, Miller, & White, 2009).
The outcome of CDV infection in terms of reduced survival was more severe among males than among females, which is also supported by our results on infection probabilities (Table 2). These suggest that immune responses may be more efficacious in female than in male hyenas, as found in many mammals, birds and reptiles (reviewed in Klein & Flanagan, 2016). Rank‐related differences in social contact among females probably explain why high‐ranking females are more likely to become infected than low‐ranking ones (Table 2). This result is consistent with the findings of a previous study in the same hyena population where the higher contact rates of high‐ranking adult females significantly increased their probability of exposure to rabies (East et al., 2001). Interestingly, more frequent (asymptomatic) exposure to CDV among high‐ranking adult females would induce protective CDV titres and may have benefitted their cubs through the transfer of these antibodies during lactation, an idea consistent with our finding that high‐ranking cubs had a lower infection probability than low‐ranking ones. The effect of social status among males was not identifiable. Interestingly, males had a higher infection probability than females despite the lower probability of assigning an infected state to males (see Supporting Information, section 3.b). Because our MECMR model allowed us to account for such uncertainty, we consider this a robust result.
Our results suggest that CDV transmission mostly occurred between virus shedding cubs at communal dens and susceptible clan members visiting these social centres. Hence, communal dens would have promoted a high level of exposure among clan members during the epidemic. Similarly, the transmission of airborne viruses in clusters of susceptible individuals with intense social contacts, such as in schools and kindergartens, is known to drive epidemics of measles (Garenne et al., 1991; Paunio et al., 1998) and the H1N1 strain of avian influenza (Cauchemez et al., 2011). The impact of these viruses on the fitness of susceptible (non‐vaccinated) juveniles was not quantified. Our study does quantify the lethal impact of an infectious juvenile viral disease in a fission–fusion society, similar to that in humans, and with transmission hotspots (communal dens) similar to schools and kindergartens. Hence, our results may provide useful insights into infectious juvenile diseases in humans as well as those in other mammalian species with a similar social structure.
There has been considerable and valuable research on the relationships between sociality and disease based on network analyses. This research demonstrates that social contact rates (e.g. grooming) could influence disease transmission or burden and vice‐versa (e.g. Bansal, Read, Pourbohloul, & Meyers, 2010; Chen et al., 2014; Duboscq et al., 2016). Social networks are increasingly integrating disease dynamics in their analyses (e.g. Springer, Kappeler, & Nunn, 2017; Vazquez‐Prokopec et al., 2013; Volz & Meyers, 2007) but to our knowledge no network‐based study has ever considered dynamic processes in both the pathogen and the host. The main challenge of social network analyses most likely lies in the difficulty of describing feedback dynamics between host social networks and disease‐related fitness costs in the host (Van Segbroeck, Santos, & Pacheco, 2010; Volz & Meyers, 2007). As an alternative to social network analysis, other studies, including ours, use surrogate variables for contact rates. We used classes of social status, others used the number of animals encountered simultaneously in the same area (Cross et al., 2004), breeding status (Genton et al., 2015) or indirect measures of socio‐economic status in many human societies (Sapolsky, 2005).
Many studies, including detailed clinical surveys on the infection histories of patients, as determined by repeated virus screening and serological analyses, focus on allocation of resources to immune processes and heterogeneity on the outcome of infection, yet neglect to consider the potential contribution of variation in host exposure. Therefore, such studies do not disentangle the effect of variation in exposure in terms of contact rates with infected individuals from immunocompetence (Kappeler et al., 2015). Nevertheless, some empirical studies have used statistical inference to disentangle the effects of exposure from those of immune responses to exposure in the propagation of infectious disease (Civitello & Rohr, 2014) or describe potential methodological approaches to achieve this (Rhodes, Halloran, & Longini, 1996). These methodological approaches may be applicable to experimental studies but not to non‐invasive studies on free‐ranging species that include uncertainty in the infection status of the host. Models that account for unavoidable uncertainty in the infection state of individuals either lump these two processes (i.e. exposure and allocation) or consider only one of these two processes (e.g. Chambert et al., 2012; Choquet, Carrié, Chambert, & Boulinier, 2013).
In our study, the detection probability of individuals was on average very high and did not vary with infection states. Thus, we are not dealing with any heterogeneity in state‐specific detection probabilities (such as a lower detection of infected animals as compared to susceptible ones, which can bias estimations; cf. Jennelle, Cooch, Conroy, & Senar, 2007). However, one potential limitation of our study, and possibly of other studies based on non‐invasive or opportunistic sample collections, is the risk of misclassification of infection states due to diagnostic inaccuracy and imperfect state observation. Buzdugan, Vergne, Grosbois, Delahay, and Drewe (2017) proposed the first approach to estimate probabilities associated with an individual's infection state at any given point, depending on prior and current knowledge about that individual's health status. Although this approach is promising, it is not applicable to studies such as ours that do not routinely run multiple diagnostic tests in series or parallel. We chose to model uncertainty on the assignment of infection states; others chose to model the risk of not correctly assigning those states (Buzdugan et al., 2017). To our knowledge, no study has ever modelled both types of uncertainty simultaneously; probably because this would require extensive data on health status.
Our modelling approach permits the separation of the influence of variation in virus exposure and resource allocation to immune processes on CDV infection probability. With our approach, it is also possible to test for an effect of social status on both the likelihood of pathogen exposure and contracting the disease. Furthermore, we were able to test for an interaction effect between social and infection states on survival. We therefore could quantify the outcome of CDV infection in different demographic and social classes and demonstrate that demographic and social states mediated the positive relationship between CDV infection and mortality. Finally, the parameters estimated by this approach can easily be combined with matrix models to provide a highly powerful tool to examine the long‐term consequences of epidemics at the population level (Oli et al., 2006).
AUTHORS' CONTRIBUTIONS
S.K.S, M.L.E and J.D.L designed the initial research; M.L.E., H.H., X.A.O.C. and S.B. performed the field and/or laboratory work; L.M. and S.B constructed the models under the supervision of O.G., S.K.S, J.D.L. and H.H., and S.B., L.M., M.L.E., H.H. and S.K.S wrote the paper. All authors contributed critically to the drafts and gave final approval for publication.
Supporting information
ACKNOWLEDGEMENTS
We are grateful to the Commission for Science and Technology of Tanzania (COSTECH), the Tanzania Wildlife Research Institute (TAWIRI) and Tanzania National Parks (TANAPA) for their support of our research. We thank the Deutsche Forschungsgemeinschaft (grants EA 5/3‐1, KR 4266/2‐1, DFG‐Grako 1121), the Leibniz‐Institute for Zoo and Wildlife Research, the Fritz‐Thyssen‐Stiftung, the Stifterverband der deutschen Wissenschaft and the Max‐Planck‐Gesellschaft for financial assistance. We thank Rémi Choquet, Sarah Cubaynes, Matthias Franz and Sylvain Gandon for discussions; and Annie Francis, Thomas Shabani, Malvina Andris, Nelly Boyer, Robert Fyumagwa, Traudi Golla, Katja Goller, Nicole Gusset‐Burgener, Richard Hoare, Karin Hönig, Mark Jago, Stephan Karl, Berit Kostka, Michelle Lindson, Sonja Metzger, Melody Roelke‐Parker, Dagmar Thierer, Agnes Türk, Harald Wiik and Kerstin Wilhelm for assistance. We thank J. Duboscq and three anonymous reviewers for their helpful suggestions.
Marescot L, Benhaiem S, Gimenez O, et al. Social status mediates the fitness costs of infection with canine distemper virus in Serengeti spotted hyenas. Funct Ecol. 2018;32:1237–1250. 10.1111/1365-2435.13059
DATA ACCESSIBILITY
The data are archived in figshare: https://doi.org/10.6084/m9.figshare.5840970 (Benhaiem et al., 2018).
REFERENCES
- Appel, M. J. , & Summers, B. A. (1995). Pathogenicity of morbilliviruses for terrestrial carnivores. Veterinary Microbiology, 44, 187–191. 10.1016/0378-1135(95)00011-X [DOI] [PubMed] [Google Scholar]
- Bansal, S. , Read, J. , Pourbohloul, B. , & Meyers, L. A. (2010). The dynamic nature of contact networks in infectious disease epidemiology. Journal of Biological Dynamics, 4, 478–489. 10.1080/17513758.2010.503376 [DOI] [PubMed] [Google Scholar]
- Beineke, A. , Baumgärtner, W. , & Wohlsein, P. (2015). Cross‐species transmission of canine distemper virus—an update. One Health, 1, 49–59. 10.1016/j.onehlt.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beineke, A. , Puff, C. , Seehusen, F. , & Baumgärtner, W. (2009). Pathogenesis and immunopathology of systemic and nervous canine distemper. Veterinary Immunology and Immunopathology, 127, 1–18. 10.1016/j.vetimm.2008.09.023 [DOI] [PubMed] [Google Scholar]
- Beldomenico, P. M. , & Begon, M. (2010). Disease spread, susceptibility and infection intensity: Vicious circles? Trends in Ecology & Evolution, 25, 21–27. 10.1016/j.tree.2009.06.015 [DOI] [PubMed] [Google Scholar]
- Benhaiem, S. , Marescot, L. , Gimenez, O. , Hofer, H. , Lebreton, J‐D , Olarte‐Castillo, X. A. , … East, M. L. (2018). Capture‐Mark‐Recapture (CMR) datasets of Serengeti spotted hyenas infected with CDV figshare. 10.6084/m9.figshare.5840970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boots, M. , Best, A. , Miller, M. R. , & White, A. (2009). The role of ecological feedbacks in the evolution of host defence: What does theory tell us? Philosophical Transactions of the Royal Society of London B: Biological Sciences, 364, 27–36. 10.1098/rstb.2008.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2003). Model selection and multimodel inference: A practical information‐theoretic approach. Berlin, Germany; New York, NY, Springer Science & Business Media. [Google Scholar]
- Buzdugan, S. N. , Vergne, T. , Grosbois, V. , Delahay, R. J. , & Drewe, J. A. (2017). Inference of the infection status of individuals using longitudinal testing data from cryptic populations: Towards a probabilistic approach to diagnosis. Scientific Reports, 7, 1111 10.1038/s41598-017-00806-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchemez, S. , Bhattarai, A. , Marchbanks, T. L. , Fagan, R. P. , Ostroff, S. , Ferguson, N. M. , … Angulo, F. J. (2011). Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proceedings of the National Academy of Sciences of the United States of America, 108, 2825–2830. 10.1073/pnas.1008895108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli, S. A. , & Chaudhry, H. S. (2012). Social status, glucocorticoids, immune function, and health: Can animal studies help us understand human socioeconomic‐status‐related health disparities? Hormones and Behavior, 62, 295–313. 10.1016/j.yhbeh.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Chambert, T. , Staszewski, V. , Lobato, E. , Choquet, R. , Carrie, C. , McCoy, K. D. , … Boulinier, T. (2012). Exposure of black‐legged kittiwakes to Lyme disease spirochetes: Dynamics of the immune status of adult hosts and effects on their survival. Journal of Animal Ecology, 81, 986–995. 10.1111/j.1365-2656.2012.01979.x [DOI] [PubMed] [Google Scholar]
- Chen, S. , White, B. J. , Sanderson, M. W. , Amrine, D. E. , Ilany, A. , & Lanzas, C. (2014). Highly dynamic animal contact network and implications on disease transmission. Scientific Reports, 4, 4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet, R. , Carrié, C. , Chambert, T. , & Boulinier, T. (2013). Estimating transitions between states using measurements with imperfect detection: Application to serological data. Ecology, 94, 2160–2165. 10.1890/12-1849.1 [DOI] [PubMed] [Google Scholar]
- Choquet, R. , & Nogue, E. (2011) E‐SURGE 1.8 user's manual. CEFE, UMR 5175, Montpellier, France: [Google Scholar]
- Civitello, D. J. , & Rohr, J. R. (2014). Disentangling the effects of exposure and susceptibility on transmission of the zoonotic parasite Schistosoma mansoni . Journal of Animal Ecology, 83, 1379–1386. 10.1111/1365-2656.12222 [DOI] [PubMed] [Google Scholar]
- Clutton‐Brock, T. H. , & Huchard, E. (2013). Social competition and selection in males and females. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 368, 20130074 10.1098/rstb.2013.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn, P. B. , & Cooch, E. G. (2009). Multistate capture–recapture analysis under imperfect state observation: An application to disease models. Journal of Applied Ecology, 46, 486–492. 10.1111/j.1365-2664.2008.01597.x [DOI] [Google Scholar]
- Cross, P. C. , Lloyd‐Smith, J. O. , Bowers, J. A. , Hay, C. T. , Hofmeyr, M. , & Getz, W. M. (2004). Integrating association data and disease dynamics in a social ungulate: Bovine tuberculosis in African buffalo in the Kruger National Park. Annales Zoologici Fennici, 41, 879–892. [Google Scholar]
- Culina, A. , Lachish, S. , Pradel, R. , Choquet, R. , & Sheldon, B. C. (2013). A multievent approach to estimating pair fidelity and heterogeneity in state transitions. Ecology and Evolution, 3, 4326–4338. 10.1002/ece3.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem, S. L. , Spelman, L. H. , Yates, R. A. , & Montali, R. J. (2000). Canine distemper in terrestrial carnivores: A review. Journal of Zoo and Wildlife Medicine, 31, 441–451. [DOI] [PubMed] [Google Scholar]
- Desprez, M. , McMahon, C. R. , Hindell, M. A. , Harcourt, R. , & Gimenez, O. (2013). Known unknowns in an imperfect world: Incorporating uncertainty in recruitment estimates using multi‐event capture–recapture models. Ecology and Evolution, 3, 4658–4668. 10.1002/ece3.846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboscq, J. , Romano, V. , Sueur, C. , & MacIntosh, A. J. J. (2016). Network centrality and seasonality interact to predict lice load in a social primate. Scientific Reports, 6, 22095 10.1038/srep22095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- East, M. L. , Burke, T. , Wilhelm, K. , Greig, C. , & Hofer, H. (2003). Sexual conflicts in spotted hyenas: Male and female mating tactics and their reproductive outcome with respect to age, social status and tenure. Proceedings of the Royal Sociely of London Series B: Biological Sciences, 270, 1247–1254. 10.1098/rspb.2003.2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- East, M. L. , & Hofer, H. (1991). Loud calling in a female dominated mammalian society: II. Behavioural context and function of whooping of spotted hyaenas, Crocuta crocuta . Animal Behaviour, 42, 651–669. 10.1016/S0003-3472(05)80247-7 [DOI] [Google Scholar]
- East, M. L. , & Hofer, H. (2001). Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behavioral Ecology, 12, 558–568. 10.1093/beheco/12.5.558 [DOI] [Google Scholar]
- East, M. L. , Hofer, H. , Cox, J. H. , Wulle, U. , Wiik, H. , & Pitra, C. (2001). Regular exposure to rabies virus and lack of symptomatic disease in Serengeti spotted hyenas. Proceedings of the National Academy of Sciences of the United States of America, 98, 15026–15031. 10.1073/pnas.261411898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- East, M. L. , Hofer, H. , & Wickler, W. (1993). The erect ‘penis’ is a flag of submission in a female‐dominated society: Greetings in Serengeti spotted hyenas. Behavioral Ecology and Sociobiology, 33, 355–370. [Google Scholar]
- East, M. L. , Höner, O. P. , Wachter, B. , Wilhelm, K. , Burke, T. , & Hofer, H. (2009). Maternal effects on offspring social status in spotted hyenas. Behavioral Ecology, 20, 478–483. [Google Scholar]
- East, M. L. , Kurze, C. , Wilhelm, K. , Benhaiem, S. , & Hofer, H. (2013). Factors influencing Dipylidium sp. infection in a free‐ranging social carnivore, the spotted hyaena (Crocuta crocuta). International Journal for Parasitology: Parasites and Wildlife, 2, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East, M. L. , Otto, E. , Helms, J. , Thierer, D. , Cable, J. , & Hofer, H. (2015). Does lactation lead to resource allocation trade‐offs in the spotted hyaena? Behavioral Ecology and Sociobiology, 69, 805–814. 10.1007/s00265-015-1897-x [DOI] [Google Scholar]
- East, M. L. , Wibbelt, G. , Lieckfeldt, D. , Ludwig, A. , Goller, K. , Wilhelm, K. , … Hofer, H. (2008). A Hepatozoon species genetically distinct from H. canis infecting spotted hyenas in the Serengeti ecosystem, Tanzania. Journal of Wildlife Diseases, 44, 45–52. 10.7589/0090-3558-44.1.45 [DOI] [PubMed] [Google Scholar]
- Engh, A. L. , Siebert, E. R. , Greenberg, D. A. , & Holekamp, K. E. (2005). Patterns of alliance formation and postconflict aggression indicate spotted hyaenas recognize third‐party relationships. Animal Behaviour, 69, 209–217. 10.1016/j.anbehav.2004.04.013 [DOI] [Google Scholar]
- Flies, A. S. , Mansfield, L. S. , Flies, E. J. , Grant, C. K. , & Holekamp, K. E. (2016). Socioecological predictors of immune defenses in wild spotted hyenas. Functional Ecology, 30, 1549–1557. 10.1111/1365-2435.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, L. G. , Glickman, S. E. , & Licht, P. (1991). Fatal sibling aggression, precocial development, and androgens in neonatal spotted hyenas. Science, 252, 702–704. 10.1126/science.2024122 [DOI] [PubMed] [Google Scholar]
- Garenne, M. , Leroy, O. , Beau, J.‐P. , & Sene, I. (1991). Child mortality after high‐titre measles vaccines: Prospective study in Senegal. The Lancet, 338, 903–907. 10.1016/0140-6736(91)91771-L [DOI] [PubMed] [Google Scholar]
- Genton, C. , Pierre, A. , Cristescu, R. , Lévréro, F. , Gatti, S. , Pierre, J. S. , … Le Gouar, P. (2015). How Ebola impacts social dynamics in gorillas: A multistate modelling approach. Journal of Animal Ecology, 84, 166–176. 10.1111/1365-2656.12268 [DOI] [PubMed] [Google Scholar]
- Gervasi, S. S. , Civitello, D. J. , Kilvitis, H. J. , & Martin, L. B. (2015). The context of host competence: A role for plasticity in host–parasite dynamics. Trends in Parasitology, 31, 419–425. 10.1016/j.pt.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez, O. , Lebreton, J.‐D. , Gaillard, J.‐M. , Choquet, R. , & Pradel, R. (2012). Estimating demographic parameters using hidden process dynamic models. Theoretical Population Biology, 82, 307–316. 10.1016/j.tpb.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Goller, V. K. , Fickel, J. , Hofer, H. , Beier, S. , & East, M. L. (2013). Coronavirus genotype and infection prevalence in wild carnivores in the Serengeti National Park, Tanzania. Achives of Virology, 158, 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann, W. , East, M. L. , Wachter, B. , Höner, O. P. , Mostl, E. , Van't Hof, T. J. , & Hofer, H. (2001). Social, state‐dependent and environmental modulation of faecal corticosteroid levels in free‐ranging female spotted hyenas. Proceedings of the Royal Sociely of London Series B: Biological Sciences, 268, 2453–2459. 10.1098/rspb.2001.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann, W. , & Wingfield, J. C. (2004). Allostatic load, social status and stress hormones: The costs of social status matter. Animal Behaviour, 67, 591–602. 10.1016/j.anbehav.2003.08.007 [DOI] [Google Scholar]
- Grosbois, V. , Gimenez, O. , Gaillard, J. M. , Pradel, R. , Barbraud, C. , Clobert, J. , … Weimerskirch, H. (2008). Assessing the impact of climate variation on survival in vertebrate populations. Biological Reviews, 83, 357–399. 10.1111/j.1469-185X.2008.00047.x [DOI] [PubMed] [Google Scholar]
- Haas, L. , Hofer, H. , East, M. , Wohlsein, P. , Liess, B. , & Barrett, T. (1996). Canine distemper virus infection in Serengeti spotted hyaenas. Veterinary Microbiology, 49, 147–152. 10.1016/0378-1135(95)00180-8 [DOI] [PubMed] [Google Scholar]
- Hannan, E. J. , & Quinn, B. G. (1979). The determination of the order of an autoregression. Journal of the Royal Statistical Society Series B, 41, 190–195. [Google Scholar]
- Harrison, T. M. , Mazet, J. K. , Holekamp, K. E. , Dubovi, E. , Engh, A. L. , Nelson, K. , … Munson, L. (2004). Antibodies to canine and feline viruses in spotted hyenas (Crocuta crocuta) in the Masai Mara National Reserve. Journal of Wildlife Diseases, 40, 1–10. 10.7589/0090-3558-40.1.1 [DOI] [PubMed] [Google Scholar]
- Hawley, D. M. , & Altizer, S. M. (2011). Disease ecology meets ecological immunology: Understanding the links between organismal immunity and infection dynamics in natural populations. Functional Ecology, 25, 48–60. 10.1111/j.1365-2435.2010.01753.x [DOI] [Google Scholar]
- Hofer, H. , Benhaiem, S. , Golla, W. , & East, M. L. (2016). Trade‐offs in lactation and milk intake by competing siblings in a fluctuating environment. Behavioral Ecology, 27, 1567–1578. 10.1093/beheco/arw078 [DOI] [Google Scholar]
- Hofer, H. , & East, M. L. (1993a). The commuting system of Serengeti spotted hyaenas – How a predator copes with migratory prey. I Social organization. Animal Behaviour, 46, 547–557. 10.1006/anbe.1993.1222 [DOI] [Google Scholar]
- Hofer, H. , & East, M. L. (1993b). The commuting system of Serengeti spotted hyaenas – How a predator copes with migratory prey. II Attendance and maternal care. Animal Behaviour, 46, 575–589. 10.1006/anbe.1993.1224 [DOI] [Google Scholar]
- Hofer, H. , & East, M. L. (1995). Population dynamics, population size, and the commuting system of Serengeti spotted hyenas In Sinclair A. & Arcese P. (Eds.), Serengeti II: dynamics, management, and conservation of an ecosystem (pp. 332–363). Chicago, IL: University of Chigago Press. [Google Scholar]
- Hofer, H. , & East, M. L. (2003). Behavioral processes and costs of co‐existence in female spotted hyenas: A life history perspective. Evolutionary Ecology, 17, 315–331. 10.1023/A:1027352517231 [DOI] [Google Scholar]
- Holekamp, K. E. , Smale, L. , & Szykman, M. (1996). Rank and reproduction in the female spotted hyaena. Journal of Reproduction and Fertility, 108, 229–237. 10.1530/jrf.0.1080229 [DOI] [PubMed] [Google Scholar]
- Höner, O. P. , Wachter, B. , Goller, K. V. , Hofer, H. , Runyoro, V. , Thierer, D. , … East, M. L. (2012). The impact of a pathogenic bacterium on a social carnivore population. Journal of Animal Ecology, 81, 36–46. 10.1111/j.1365-2656.2011.01873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höner, O.P. , Wachter, B. , Hofer, H. , Wilhelm, K. , Thierer, D. , Trillmich, F. , … East, M.L. (2010). The fitness of dispersing spotted hyaena sons is influenced by maternal social status. Nature Communications 1, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilany, A. , Booms, A. S. , & Holekamp, K. E. (2015). Topological effects of network structure on long‐term social network dynamics in a wild mammal. Ecology Letters, 18, 687–695. 10.1111/ele.12447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennelle, C. S. , Cooch, E. G. , Conroy, M. J. , & Senar, J. C. (2007). State‐specific detection probabilities and disease prevalence. Ecological Applications, 17, 154–167. 10.1890/1051-0761(2007)017[0154:SDPADP]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kappeler, P. M. , Cremer, S. , & Nunn, C. L. (2015). Sociality and health: Impacts of sociality on disease susceptibility and transmission in animal and human societies. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 370, 20140116 10.1098/rstb.2014.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, S. L. , & Flanagan, K. L. (2016). Sex differences in immune responses. Nature Reviews Immunology, 16, 626–638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- Lebreton, J. D. , Nichols, J. D. , Barker, R. J. , Pradel, R. , & Spendelow, J. A. (2009). Chapter 3 modeling individual animal histories with multistate capture–recapture models. Advances in Ecological Research, pp. 87–173. Academic Press. 10.1016/S0065-2504(09)00403-6 [DOI] [Google Scholar]
- MacIntosh, A. J. J. , Jacobs, A. , Garcia, C. , Shimizu, K. , Mouri, K. , Huffman, M. A. , & Hernandez, A. D. (2012). Monkeys in the middle: Parasite transmission through the social network of a wild primate. PLoS ONE, 7, e51144 10.1371/journal.pone.0051144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock, B. T. , Nichols, J. D. , Bailey, L. L. , MacKenzie, D. I. , Kendall, W. , & Franklin, A. B. (2010). Seeking a second opinion: Uncertainty in disease ecology. Ecology Letters, 13, 659–674. 10.1111/j.1461-0248.2010.01472.x [DOI] [PubMed] [Google Scholar]
- Nikolin, V. N. , Olarte‐Castillo, X. A. , Osterrieder, N. , Hofer, H. , Dubovi, E. , Mazzoni, C. J. , … East, M. L. (2017). Canine distemper virus in the Serengeti ecosystem: Molecular adaptation to different carnivore species. Molecular Ecology, 26, 2111–2130. 10.1111/mec.13902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn, C. L. , Jordán, F. , McCabe, C. M. , Verdolin, J. L. , & Fewell, J. H. (2015). Infectious disease and group size: More than just a numbers game. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 370, 20140111 10.1098/rstb.2014.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oli, M. K. , Venkataraman, M. , Klein, P. A. , Wendland, L. D. , & Brown, M. B. (2006). Population dynamics of infectious diseases: A discrete time model. Ecological Modelling, 198, 183–194. 10.1016/j.ecolmodel.2006.04.007 [DOI] [Google Scholar]
- Paunio, M. , Peltola, H. , Valle, M. , Davidkin, I. , Virtanen, M. , & Heinonen, O. P. (1998). Explosive school‐based measles outbreak: Intense exposure may have resulted in high risk, even among revaccinees. American Journal of Epidemiology, 148, 1103–1110. 10.1093/oxfordjournals.aje.a009588 [DOI] [PubMed] [Google Scholar]
- Pradel, R. (2005). Multievent: An extension of multistate capture–recapture models to uncertain states. Biometrics, 61, 442–447. 10.1111/j.1541-0420.2005.00318.x [DOI] [PubMed] [Google Scholar]
- Rhodes, P. H. , Halloran, M. E. , & Longini Jr., I. M. (1996). Counting process models for infectious disease data: Distinguishing exposure to infection from susceptibility. Journal of the Royal Statistical Society Series B, 58, 751–762. [Google Scholar]
- Roelke‐Parker, M. E. , Munson, L. , Packer, C. , Kock, R. , Cleaveland, S. , Carpenter, M. , … Appel, M. J. (1996). A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature, 379, 441–445. 10.1038/379441a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röthlisberger, A. , Wiener, D. , Schweizer, M. , Peterhans, E. , Zurbriggen, A. , & Plattet, P. (2010). Two domains of the V protein of virulent canine distemper virus selectively inhibit STAT1 and STAT2 nuclear import. Journal of Virology, 84, 6328–6343. 10.1128/JVI.01878-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky, R. M. (2005). The influence of social hierarchy on primate health. Science, 308, 648–652. 10.1126/science.1106477 [DOI] [PubMed] [Google Scholar]
- Sawatsky, B. , Wong, X. X. , Hinkelmann, S. , Cattaneo, R. , & von Messling, V. (2012). Canine distemper virus epithelial cell infection is required for clinical disease but not for immunosuppression. Journal of Virology, 86, 3658–3666. 10.1128/JVI.06414-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub, M. , Gimenez, O. , Schmidt, B. R. , & Pradel, R. (2004). Estimating survival and temporary emigration in the multistate capture‐recapture framework. Ecology, 85, 2107–2113. 10.1890/03-3110 [DOI] [Google Scholar]
- Schmid‐Hempel, P. (2003). Variation in immune defence as a question of evolutionary ecology. Proceedings of the Royal Sociely of London Series B: Biological Sciences, 270, 357–366. 10.1098/rspb.2002.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth, R. M. (1977). A model of social grooming among adult female monkeys. Journal of Theoretical Biology, 65, 671–698. 10.1016/0022-5193(77)90015-7 [DOI] [PubMed] [Google Scholar]
- Smith, J. E. , Memenis, S. K. , & Holekamp, K. E. (2007). Rank‐related partner choice in the fission–fusion society of the spotted hyena (Crocuta crocuta). Behavioral Ecology and Sociobiology, 61, 753–765. 10.1007/s00265-006-0305-y [DOI] [Google Scholar]
- Springer, A. , Kappeler, P. M. , & Nunn, C. L. (2017). Dynamic vs. static social networks in models of parasite transmission: Predicting Cryptosporidium spread in wild lemurs. Journal of Animal Ecology, 86, 419–433. 10.1111/1365-2656.12617 [DOI] [PubMed] [Google Scholar]
- Tatsuo, H. , Ono, N. , & Yanagi, Y. (2001). Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. Journal of Virology, 75, 5842–5850. 10.1128/JVI.75.13.5842-5850.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung, J. , Barreiro, L. B. , Johnson, Z. P. , Hansen, K. D. , Michopoulos, V. , Toufexis, D. , … Gilad, Y. (2012). Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proceedings of the National Academy of Sciences of the United States of America, 109, 6490–6495. 10.1073/pnas.1202734109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Segbroeck, S. , Santos, F. C. , & Pacheco, J. M. (2010). Adaptive contact networks change effective disease infectiousness and dynamics. PLOS Computational Biology, 6, e1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Messling, V. , Svitek, N. , & Cattaneo, R. (2006). Receptor (SLAM [CD150]) recognition and the V protein sustain swift lymphocyte‐based invasion of mucosal tissue and lymphatic organs by a morbillivirus. Journal of Virology, 80, 6084–6092. 10.1128/jvi.00357-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWaal, K. L. , & Ezenwa, V. O. (2016). Heterogeneity in pathogen transmission: Mechanisms and methodology. Functional Ecology, 30, 1601–1622. [Google Scholar]
- Vazquez‐Prokopec, G. M. , Bisanzio, D. , Stoddard, S. T. , Paz‐Soldan, V. , Morrison, A. C. , Elder, J. P. , … Kitron, U. (2013). Using GPS technology to quantify human mobility, dynamic contacts and infectious disease dynamics in a resource‐poor urban environment. PLoS ONE, 8, e58802 10.1371/journal.pone.0058802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz, E. , & Meyers, L. A. (2007). Susceptible–infected–recovered epidemics in dynamic contact networks. Proceedings of the Royal Society of London B: Biological Sciences, 274, 2925–2934. 10.1098/rspb.2007.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are archived in figshare: https://doi.org/10.6084/m9.figshare.5840970 (Benhaiem et al., 2018).


