Abstract
Climate‐related environmental changes have increasingly been linked to emerging infectious diseases in wildlife. The Arctic is facing a major ecological transition that is expected to substantially affect animal and human health. Changes in phenology or environmental conditions that result from climate warming may promote novel species assemblages as host and pathogen ranges expand to previously unoccupied areas. Recent evidence from the Arctic and subarctic suggests an increase in the spread and prevalence of some wildlife diseases, but baseline data necessary to detect and verify such changes are still lacking. Wild birds are undergoing rapid shifts in distribution and have been implicated in the spread of wildlife and zoonotic diseases. Here, we review evidence of current and projected changes in the abundance and distribution of avian diseases and outline strategies for future research. We discuss relevant climatic and environmental factors, emerging host–pathogen contact zones, the relationship between host condition and immune function, and potential wildlife and human health outcomes in northern regions.
Current discussions about infectious diseases in wildlife underscore the potential for climate warming to influence the distribution, frequency, and virulence of certain pathogens. Changes in environmental factors, including temperature and precipitation, have been linked to the geographic spread of many emerging or endemic diseases (Harvell et al. 2002; Altizer et al. 2013). Although other anthropogenic and landscape‐level changes may play an equally important role in disease dynamics in many parts of the world (Lafferty 2009), the Arctic provides an ideal setting in which to study the effects of climate warming on host–pathogen systems, given the region's rapid rate of ecological change, relatively low levels of biodiversity, and the limited anthropogenic influence relative to tropical and temperate regions (Hoberg et al. 2008; Kutz et al. 2013). Over the past century, temperature increases in the Arctic have been twice the global average and future warming is expected to occur more quickly and at a greater magnitude at high latitudes (Parry et al. 2007). Arctic ecosystems face pronounced loss of sea ice, permafrost degradation, changes in hydrology, wetland drying, and related alterations in nutrient cycling (Hinzman et al. 2013); consequently, plant and animal communities are changing in terms of both composition and distribution (Tape et al. 2006; Post et al. 2009).
In a nutshell:
Climate warming is affecting the distribution and spread of some infectious diseases
In the Arctic, where temperatures are increasing at twice the global rate, evidence suggests that changes in pathogen communities are already underway
As a result, wildlife hosts face increasing exposure to novel or expanding diseases
Birds play a key role in the spread of disease due to their long‐distance movements and ability to serve as reservoirs, but baseline information is lacking for northern regions
We present strategies for addressing information gaps about disease in Arctic birds, and highlight important links to wildlife and human health
Disruptions to fundamental ecological processes, such as those resulting from shifts in the timing and extent of sea‐ice cover, may result in increased contact between historically segregated species or populations (Post et al. 2013), promoting opportunities for pathogen transmission and spillover (when a novel host becomes infected by a reservoir population). Environmental pressures associated with climate warming may also affect the timing and duration of migration in some species, leading to different patterns of migratory overlap (Patterson and Guerin 2013). In addition, altered phenology due to seasonal shifts in local conditions will affect both host and pathogen life cycles (Altizer et al. 2013). Pathogens in northern regions are expected to respond quickly to these new challenges and opportunities, potentially exposing naïve hosts to novel diseases (Bradley et al. 2005; Burek et al. 2008; Hueffer et al. 2011). The emergence and establishment of parasitic nematodes in ungulates in the Canadian Arctic exemplifies the rapidly changing disease dynamics in this region (Kutz et al. 2013).
Projections of climate‐driven changes in host and pathogen ranges in northern ecosystems have led to calls for additional studies and predictive modeling of Arctic host–parasite systems (Kutz et al. 2009; Davidson et al. 2011), investigation of the fitness consequences associated with known pathogens (Merino and Møller 2010), and broad‐scale wildlife disease surveillance (Burek et al. 2008; Hoberg et al. 2008; Revich et al. 2012). Although preliminary efforts have been initiated to address these needs, research on wildlife disease in the North has largely been restricted to mammals of commercial or subsistence value. Here, we emphasize the importance of extending disease investigations to include wild birds.
Because they commonly occur in mixed species aggregations and are often found in close proximity to humans and domestic animals, birds play a key role in disease ecology. Many birds are highly migratory and travel thousands of kilometers between wintering and breeding areas, providing direct links between pathogen populations on different continents. Thus, by serving as reservoirs or facilitating the transfer of pathogens through short‐ and long‐distance movements, wild birds have been implicated in the spread of wildlife diseases and zoonotic diseases (Friend et al. 2001; Altizer et al. 2011). A high proportion of the global populations of many bird species rely on Arctic and boreal breeding habitats, so the study of avian health in this region has important implications for avian conservation. Waterfowl and other wild birds also serve as a food resource for local circumpolar human residents.
Pathogens in the Arctic
The avoidance of disease has been proposed as one explanation for the energetically costly annual migrations of wild birds from tropical or temperate wintering areas to Arctic breeding grounds (Piersma 1997; Altizer et al. 2011). Within a theoretical model of life‐history tradeoffs, the relatively low abundance of pathogens or parasites at high latitudes provides an immunological release that allows animals to allocate more energy to reproduction (Piersma 1997; Buehler et al. 2010). In turn, offspring encounter fewer immune challenges and may have greater chances of survival. It is an oversimplification to assume the Arctic is free from disease; however, cold temperatures, photoperiod extremes, and geographic isolation have restricted pathogen diversity and require specialized adaptations if disease organisms are to persist in local hosts (Hueffer et al. 2011; Hoberg et al. 2012). For example, previous studies concluded that avian blood parasites (hematozoa) were absent or occurred only rarely in Arctic tundra ecosystems, a pattern attributed to a lack of suitable vectors and to unfavorable environmental conditions for transmission (Bennett et al. 1992). Other parasites exhibit distinctive physiological strategies, such as freeze tolerance, that allow them to survive in Arctic settings. For instance, Trichinella nativa, an intestinal roundworm responsible for trichinosis in humans and non‐human animals, overwinters at extremely cold temperatures (Hueffer et al. 2011). The parasitic fauna of the Arctic is closely tied to its unique genetic history of episodic linkages across the Bering Land Bridge and represents a long period of host–parasite coevolution (Hoberg et al. 2012; Kutz et al. 2014). Historical fluctuations in climate may also provide some context for understanding the evolutionary processes related to disease under a warming scenario, although environmental changes are currently occurring on a much shorter timescale (Hoberg et al. 2008).
Given the rapid rate of environmental change in the Arctic, the introduction or spread of novel pathogens could have major effects on previously unexposed hosts (Burek et al. 2008; Hueffer et al. 2011). Some of the most marked declines in wild populations were the result of infectious diseases that spread rapidly through naïve populations (Harvell et al. 2002; Frick et al. 2010; LaPointe et al. 2012). A wide range of pathogens have been implicated in such disease outbreaks, but they share one feature in common: after introduction or emergence, each experienced range expansion that resulted in high mortality of susceptible hosts. A similar scenario could emerge among Arctic birds and other wildlife exposed to new disease threats in areas undergoing climate‐driven environmental changes.
Preliminary evidence from the Arctic suggests that shifts in the distribution and abundance of avian pathogens are already underway (Larsson et al. 2007; Harriman and Alisauskas 2010; Descamps et al. 2012; Loiseau et al. 2012). However, a lack of baseline information has hindered our ability to detect and verify such changes. The limited number of avian disease studies that have been conducted in the Arctic and subarctic have targeted specific pathogens – such as avian influenza virus – or focused on diagnosis of isolated morbidity and mortality events. In contrast, there has been minimal general disease surveillance, due in part to the extreme environmental conditions, logistical constraints, and remoteness of these northern regions. As a result, much of our understanding of disease in Arctic and subarctic birds has been extrapolated from useful but limited local datasets, which cannot be generalized across broad geographic areas.
Due to the lack of background information on diseases in northern birds, it is often challenging to determine whether initial detection of a pathogen represents true emergence, whether it warrants concern, and whether it is pathogenic for a given host. A new geographic or host record is noteworthy but does not necessarily represent an emerging infectious disease (Hoberg et al. 2008). For instance, the first Arctic record of the Borrelia spirochetes that cause Lyme disease was documented in seabirds in Norway, but the implications of this for human and wildlife health remain unclear (Larsson et al. 2007). Similarly, a recent study reported evidence of Plasmodium transmission in Alaska and proposed that climate warming will lead to exposure of naïve birds to new parasites (Loiseau et al. 2012). Yet without long‐term datasets, relationships between climatic variables and disease occurrence are ambiguous and difficult to quantify.
Mechanisms of change: new hosts and new pathogens
Future changes in wildlife disease occurrence in northern ecosystems will likely be facilitated by shifts in host phenology, distribution, and abundance (Altizer et al. 2013). For both resident and migratory bird species, seasonal activity in the Arctic can be strongly influenced by local conditions, such as extent and depth of spring snow cover and timing of ice breakup (eg Grabowski et al. 2013). Shifts in breeding or migration phenology could alter the temporal overlap of hosts and pathogens and increase the potential for contact between hosts. Many bird species are experiencing range expansions due to climate warming, resulting in introduction of species to previously unoccupied areas (eg Gibson and Kessel 1992; Benson et al. 2000). As additional habitat becomes available, through shifts in vegetation cover and longer snow‐free periods, some populations of waterfowl are adapting by breeding in higher latitude areas and occasionally at greater densities (eg Flint et al. 2008). Larger numbers of other normally migratory species such as mallard (Figure 1; Anas platyrhynchos) and brant (Branta bernicla nigricans) are remaining in northern locales over winter (Ward et al. 2009; National Audubon Society 2012). In addition to creating concentrated aggregations of birds, year‐round residence may prevent the culling of diseased animals that naturally occurs on account of the energetic demands of migration (Bradley and Altizer 2005; Altizer et al. 2011; Patterson and Guerin 2013).
Figure 1.

Researchers capture mallards (Anas platyrhynchos) in Fairbanks, Alaska, as part of a study of avian influenza virus persistence. Recently, local populations of some normally migratory waterfowl species have remained resident throughout the year in northern regions.
Several recent studies provide evidence that migratory birds serve as effective long‐distance vectors of wildlife and zoonotic pathogens to the Arctic, highlighting the potential for changes in movement patterns to affect disease transmission. In 2011, Coxiella burnetii, the causative agent of Q fever in humans, was detected in northern fur seals (Callorhinus ursinus) and in environmental samples from St Paul Island in the Bering Sea for the first time. Genetic sequencing identified both terrestrial and marine strains of the organism and suggested that seabirds may play an important role in its regional distribution (Duncan et al. 2013). Similarly, in Svalbard, barnacle geese (Branta leucopsis) and pink‐footed geese (Anser brachyrhynchus) have been identified as likely vectors for transmission of Toxoplasma gondii – the coccidian parasite responsible for toxoplasmosis – to Arctic foxes (Vulpes lagopus), polar bears (Ursus maritimus), seals, and other marine mammals (Sandström et al. 2013). Borrelia‐infected ticks (the agents of Lyme disease in humans) were found on sea‐birds in the North Atlantic (Larsson et al. 2007) and on songbirds in northern Canada (Scott et al. 2012), suggesting possible long‐distance transport via avian migrants.
Even relatively small shifts in geographic distribution may create opportunities for pathogens to spread between hosts that were previously separated by an ecological or geographical divide. Along the Arctic coastline, increasing overlap between marine and terrestrial habitats due to loss of sea ice is leading to novel assemblages of bird and mammal species (Post et al. 2013). Polar bears, for example, are being seen more frequently on land during the peak of the summer breeding season for migratory birds. Consequently, the bears have more interactions with birds, sometimes preying on adults, chicks, and eggs (eg Iles et al. 2013) and sharing foraging habitat and food resources (Figure 2).
Figure 2.

Polar bears (Ursus maritimus) and glaucous gulls (Larus hyperboreus) feed on a bowhead whale (Balaena mysticetus) carcass on the Arctic coast. Cross‐species interactions such as these may facilitate disease transfer between mammals and birds and between marine and terrestrial wildlife habitats.
Changes in dietary patterns that result either directly or indirectly from climate warming have the potential to alter exposure to parasites and other pathogens, as has been observed among Arctic‐nesting seabirds (Hoberg et al. 2013; Post et al. 2013). Limited foraging opportunities and increased anthropogenic activities may lead to dense aggregations of animals at concentrated food sources. For instance, resident and migratory birds, polar bears, brown bears (Ursus arctos), and red (Vulpes vulpes) and Arctic foxes congregate at garbage dumps and at bowhead whale (Balaena mysticetus) carcasses remaining after subsistence hunts in northern communities (Figure 2). Birds provide a key link between marine and terrestrial environments and may facilitate cross‐species transmission of pathogens, such as avian influenza viruses that spread among coastal congregations of wildlife, sometimes causing illness or mortality in alternate hosts (Krauss et al. 2010; Anthony et al. 2012).
Underlying changes in vector populations are likely to contribute to the emergence or expansion of infectious diseases in the Arctic. As climate warming alters plant communities (eg through encroachment of shrubs into tundra habitats), conditions may become more suitable for arthropod vectors, including mosquitoes, ticks, and biting flies. Milder winters and a longer growing season are thought to have been responsible for increases in the range and abundance of ticks in Sweden (Jaenson et al. 2012). Northward expansion of ixodid ticks has also been observed in parts of Russia and northern Canada (Kutz et al. 2009; Revich et al. 2012). In the Czech Republic, tick‐borne encephalitis has spread to higher altitudes, a shift that is linked to climate‐related changes in the distribution of the passerine birds that serve as hosts (Danielova et al. 2010). Timing of emergence of insects, including vector species, may be influenced by warming and could influence pathogen life cycles and subsequent disease exposure (Altizer et al. 2013). Transmission of some pathogens is strongly associated with temperature, suggesting that vectors already present in local environments may spread disease more efficiently in areas experiencing climate warming. For example, mosquitoes (Culex spp) known to be effective vectors for West Nile virus currently extend into northern Canada and Alaska. Spread of the virus to these regions is thought to be limited by temperature (Roth et al. 2010), so a major warming trend could promote northward expansion (Parkinson and Butler 2005).
Changes in disease occurrence may also result from the release of environmental constraints on pathogens or parasites themselves. Higher temperatures and a longer growing season can lead to shifts in geographic distribution or accelerated larval development among some parasites (Harvell et al. 2002; Hoberg et al. 2008). Such changes have been well‐documented among parasites of northern ungulates, resulting in range expansion and a shortened developmental cycle for an important lung parasite of muskoxen (Ovibos moschatus; Kutz et al. 2009, 2013). Similarly, a study of red grouse (Lagopus lagopus scoticus) in Scotland determined that temperature and precipitation strongly influenced the rate of development and timing of transmission of parasitic nematodes (Cattadori et al. 2005). In subarctic Fennoscandia, the emergence of epidemic disease caused by mosquito‐borne filaroid nematodes has been associated with higher temperatures (Laaksonen et al. 2010). Systematic studies have not yet been conducted in Arctic birds, but similar trends may be emerging, especially among avian species that remain resident throughout the year (Figure 3). In Nunavut, Canada, newly detected parasitic fleas on Ross's geese (Chen rossii) and lesser snow geese (Chen caerulescens) have been associated with reduced reproductive success, and flea numbers are expected to increase with future climate warming (Harriman and Alisauskas 2010).
Figure 3.
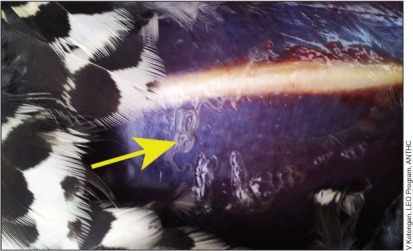
Spruce grouse (Falcipennis canadensis) with subcutaneous nematode worms (arrow) in breast tissue, reported by local hunters in northwestern Alaska in 2012. Filaroid nematodes have not previously been documented in grouse from this region.
With additional demands for resource development and infrastructure in the Arctic and the potential for new overland and marine transport corridors (Kumpula et al. 2011; Smith and Stephenson 2013), the exposure of wildlife to zoonotic pathogens will likely also increase. Pathogen pollution via runoff into marine or freshwater habitats, contact with domestic animals, and the potential for introduction of invasive species all present heightened disease risks associated with increasing anthropogenic activities (Burek et al. 2008; Jenkins et al. 2013).
Research priorities
Given the considerable knowledge gaps about avian disease in the North, how should future research objectives be determined? Basic detection of pathogens will be a necessary component of many initial studies in the Arctic; however, attempting to collect and analyze samples from all species across this vast and remote region is clearly impractical. An effective strategy will need to identify the pathogens, hosts, and geographic areas most likely to respond to future warming in order to develop predictions for wildlife and human health outcomes (Hoberg et al. 2008). Here we outline research topics and priorities for future research based on a conceptual model of climate‐related impacts on avian disease dynamics in the Arctic (Figure 4).
Figure 4.
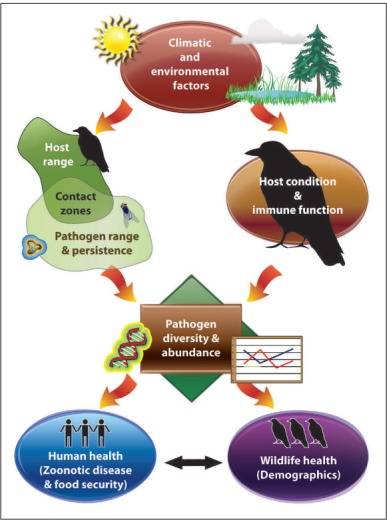
Conceptual model depicting climate‐related impacts on avian disease dynamics in the Arctic. Changes in climatic and environmental factors may lead to changes in host and pathogen range, affect host condition and immune function, and ultimately influence the diversity and abundance of pathogens. These changes will influence both wildlife and human health through exposure to disease and availability of subsistence food resources.
Climatic and environmental factors
Initial research efforts should target organisms known to be sensitive to environmental features that may be influenced by climate change. Parasites with a free‐living stage and vector‐borne pathogens are especially responsive to temperature fluctuations and therefore provide useful models for the study of climate–pathogen interactions. The prevalence and distribution of avian blood parasites (Figure 5) – for which blackflies, biting midges, and mosquitoes serve as vectors – are projected to expand in response to warmer temperatures and vegetation changes in Arctic tundra areas (Loiseau et al. 2012; Altizer et al. 2013). Some helminth parasites respond favorably to a milder climate and could infect bird populations in northern regions where environmental conditions have previously been unsuitable. Conversely, in other cases, warming may actually impede parasite development (Altizer et al. 2013; Kutz et al. 2014). Targeted sampling across physical and ecological gradients such as latitude, elevation, and habitat features will help to expose these complexities and identify unique ecological drivers of disease dynamics. Quantification of variables –including air, soil, and water temperatures; precipitation; vegetation structure; and hydrology – can be used to determine critical thresholds for pathogen survival and bird phenology that can then be incorporated into predictive models of future distribution for specific pathogens.
Figure 5.
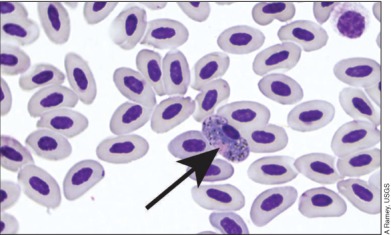
Avian blood parasite of the genus Haemoproteus (arrow). Hematozoan parasites and their vectors are sensitive to diurnal temperatures and are expected to increase their range and abundance in response to climate warming in the Arctic.
Host and pathogen contact zones
An important consideration in selecting geographic areas for sampling is the potential for new or increased contact between pathogens and potential hosts. Ecological transition zones that are undergoing rapid rates of change present new frontiers in Arctic wildlife disease research. For example, the coastline of the Arctic Ocean attracts a unique species assemblage and is changing as a result of declining sea ice, high rates of coastal erosion, tidal inundation, and rapidly increasing graminoid production (Hinzman et al. 2013; Post et al. 2013). Animals congregate along this narrow coastal margin, creating opportunities for pathogen exchange between birds and mammals and between marine and terrestrial environments (reviewed in Post et al. 2013). Another key ecological transition zone, the tundra–boreal interface, is experiencing a regime shift that has important implications for disease transmission. Rising temperatures, shrub encroachment, northward expansion of the tree line, and changes in hydrology may all influence vector populations in this region (Hoberg et al. 2013). Surveillance for diseases at their known or presumed northern limits will help to establish current patterns of distribution and detect future changes. For pathogens that are expected to edge northward slowly rather than being introduced via large migratory movements (eg West Nile virus; Roth et al. 2010), efforts should target subarctic regions that share connectivity with temperate zones.
Migratory crossroads
Regions that harbor species of birds arriving from divergent nonbreeding areas – known as migratory crossroads – are vital for tracking the arrival of foreign diseases as well as for understanding pathogen evolution (Figure 6). Research on avian influenza viruses has demonstrated considerable levels of pathogen exchange at large breeding and staging areas for waterfowl and shorebirds (eg in the extensive wetland complexes of western Alaska; Reeves et al. 2013). Similarly, a genetically diverse assortment of coronaviruses from migratory and resident species detected in the Beringia region between Russia and Alaska highlights the importance of wild birds as reservoirs and the potential for transmission of pathogens at mixing areas, followed by movement across large distances (Muradrasoli et al. 2010). Summer breeding and stopover sites will be subject to changes in bird density and species composition due to altered migration patterns associated with climate warming, creating additional opportunities for pathogen exchange and dispersal. Such changes may not only have impacts on the northern breeding grounds but could also affect disease dynamics in temperate and tropical wintering areas.
Figure 6.
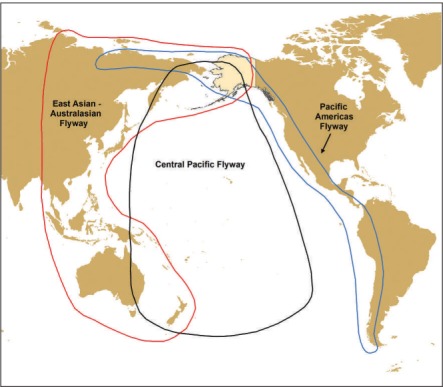
Map showing the bird flyways of the Pacific Basin, with Alaska highlighted as an example of a key migratory crossroads. Pathogen exchange occurs at breeding and staging areas, where shorebirds and waterfowl from divergent wintering areas overlap. Changes in migration patterns due to climate warming may influence infectious disease dynamics at these sites.
Factors influencing immune function
When a pathogen and potential host come into contact, the host's susceptibility to infection is influenced by a wide range of factors. Immunologically naïve hosts, such as resident Arctic birds lacking previous experience with recently introduced pathogens, may be especially vulnerable. Endemic island populations provide clear examples of rapid change associated with introduced diseases (eg the emergence of avian malaria in Hawaiian forest birds; LaPointe et al. 2012). In the Canadian Arctic, the invasion and establishment of two parasitic nematodes on Victoria Island may affect the viability of local muskox and caribou populations (Kutz et al. 2013). Similarly, outbreaks of avian cholera among common eiders (Somateria mollissima) in the eastern Canadian Arctic have had notable demographic impacts and may threaten the existence of this breeding colony (Descamps et al. 2012).
Physiological stressors such as poor nutrition and reduced body condition compromise immune function and therefore make individuals more susceptible to infection (Acevedo‐Whitehouse and Duffus 2009). Birds may be especially vulnerable during the breeding season because of the substantial physiological demands of reproduction and, for many species, migration. Birds that breed in northern environments face various challenges associated with climate warming, including reduced foraging opportunities, changes in quality or timing of food availability, migration hazards associated with unstable weather patterns, and alterations to breeding or wintering habitats (Merino and Møller 2010). Ambient temperature can also directly influence host immunity (Altizer et al. 2013). Environmental contaminants are becoming more widespread in the Arctic, and wildlife exposure to some compounds has been shown to affect immune function (Acevedo‐Whitehouse and Duffus 2009). These and other factors may influence an animal's ability to respond to disease and should be considered when evaluating the cumulative effects of climate change on wildlife health.
Understanding the immune capacity of individuals and populations would lead to much more comprehensive predictions on the effects of emerging diseases in wildlife. Although there has been growing interest in integrating the fields of disease ecology and immunology, many methodological challenges remain (Hawley and Altizer 2011). Further development of immunological assays for non‐model organisms, measures of gene expression, and other techniques that provide a reliable index of immune response will be needed to identify vulnerable populations and species and to establish links between life‐history characteristics, environmental conditions, and host immunity (Hawley and Altizer 2011).
Wildlife and human health outcomes
Examples from recent wildlife disease outbreaks provide compelling evidence that infectious diseases influence population dynamics. However, the effects of a pathogen often vary across species and populations, potentially causing widespread mortality in one instance and little or no demographic impact in another. Therefore, measurement of the specific fitness consequences of pathogens on their wildlife hosts is an important area for future research. Well‐studied populations with known demographic parameters (eg age, reproductive history, movement patterns) may provide useful model systems for tracking subtle changes in individual health status that negatively affect reproduction and survival.
The outcomes of disease outbreaks on wild bird populations have important implications not only for avian conservation but also for other wildlife and for humans who rely on these resources. Human–wildlife interactions are common in the Arctic and the occurrence of zoonotic diseases in harvested birds, mammals, or fish may pose a threat to human health or present risks to food security (Parkinson and Butler 2005; Jenkins et al. 2013; Hueffer et al. 2013). Zoonotic pathogens – including Toxoplasma, Trichinella, Echinococcus, and Giardia –that have been detected in Arctic or subarctic wildlife species affect a wide range of organisms (Kutz et al. 2008; Hueffer et al. 2013; Jenkins et al. 2013; Sandström et al. 2013; Schurer et al. 2014). Birds have been implicated in the spread of many infectious diseases to other wildlife and to humans, but more research is needed to identify the mechanisms and scale of pathogen transport. Future studies could help to address such information gaps by sampling across migratory flyways and in birds and mammals that overlap spatially.
Progress through collaboration
Because sampling opportunities for individual studies are generally limited, the most effective disease surveillance will depend on a coordinated effort between agencies, research groups, and local residents. Incorporating pathogen screening into existing avian monitoring programs offers an efficient means of collecting important baseline information. In the future, longitudinal sampling that includes archiving of blood or other tissues will help in detecting changes in animal health related to climate‐driven ecological shifts; retrospective analyses of historical samples, where available, can be used to identify changes that have already occurred. Given the prevalence of subsistence hunting and strong human connections to the environment in many northern communities, local residents are also an important source of information and can often provide early detection of changes in the health of wildlife species (Figure 3; Hoberg et al. 2008, 2013; Kutz et al. 2009). Including hunter‐harvested animals in research efforts can also help to maximize sample collection and complement community‐based education programs about potential threats to traditional subsistence foods.
Conclusions
The Arctic is undergoing drastic ecological changes that are expected to have widespread effects on wildlife and human health. Effective long‐term management of Arctic wildlife will require a better understanding of disease dynamics in this region, an arena in which birds are likely to play a key role. We urge researchers to recognize the importance of establishing baselines, instituting both general surveillance programs and more targeted approaches along identified risk pathways, and creating collaborative networks to track persisting and emerging threats.
Acknowledgements
This work was supported by the Ecosystem and Environmental Health Mission areas of the US Geological Survey. We thank A Reeves for helpful feedback on an earlier version of the manuscript, as well as M Brubaker and V Kotongan for providing the image used in Figure 3. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
References
- Acevedo-Whitehouse K and Duffus AL. 2009. Effects of environmental change on wildlife health. Philos T R Soc B 364: 3429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S, Bartel R, and Han BA. 2011. Animal migration and infectious disease risk. Science 331: 296–302. [DOI] [PubMed] [Google Scholar]
- Altizer S, Ostfeld RS, Johnson PT , et al 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341: 514–19. [DOI] [PubMed] [Google Scholar]
- Anthony SJ, St Leger JA, Pugliares K , et al 2012. Emergence of fatal avian influenza in New England harbor seals. mBio 3: e00166–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett GF, Montgomerie R, and Seutin G. 1992. Scarcity of haematozoa in birds breeding on the Arctic tundra of North America. Condor 94: 289–92. [Google Scholar]
- Benson A, Pogson TH, and Doyle TJ. 2000. Updated geographic distribution of eight passerine species in central Alaska. West Birds 31: 100–05. [Google Scholar]
- Bradley CA and Altizer S. 2005. Parasites hinder monarch butterfly flight: implications for disease spread in migratory hosts. Ecol Lett 8: 290–300. [Google Scholar]
- Bradley MJ, Kutz SJ, Jenkins E, and O'Hara TM. 2005. The potential impact of climate change on infectious diseases of Arctic fauna. Int J Circumpol Heal 64: 468–77. [DOI] [PubMed] [Google Scholar]
- Buehler DM, Tieleman BI, and Piersma T. 2010. How do migratory species stay healthy over the annual cycle? A conceptual model for immune function and for resistance to disease. Integr Comp Biol 50: 346–57. [DOI] [PubMed] [Google Scholar]
- Burek KA, Gulland FM, and O'Hara TM. 2008. Effects of climate change on Arctic marine mammal health. Ecol Appl 18: 126–34. [DOI] [PubMed] [Google Scholar]
- Cattadori IM, Haydon DT, and Hudson PJ. 2005. Parasites and climate synchronize red grouse populations. Nature 433: 737–41. [DOI] [PubMed] [Google Scholar]
- Danielova V, Daniel M, Schwarzova L , et al 2010. Integration of a tick-borne encephalitis virus and Borrelia burgdorferi sensu lato into mountain ecosystems, following a shift in the altitudinal limit of distribution of their vector, Ixodes ricinus (Krkonose mountains, Czech Republic). Vector-Borne Zoonot 10: 223–30. [DOI] [PubMed] [Google Scholar]
- Davidson R, Simard M, Kutz SJ , et al 2011. Arctic parasitology: why should we care? Trends Parasitol 27: 239–45. [DOI] [PubMed] [Google Scholar]
- Descamps S, Jenouvrier S, Gilchrist HG, and Forbes MR. 2012. Avian cholera, a threat to the viability of an Arctic seabird colony? PLoS ONE 7: e29659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C, Savage K, Williams M , et al 2013. Multiple strains of Coxiella burnetii are present in the environment of St Paul Island, Alaska. Transbound Emerg Dis 60: 345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint P, Mallek E, King R , et al 2008. Changes in abundance and spatial distribution of geese molting near Teshekpuk Lake, Alaska: interspecific competition or ecological change? Polar Biol 31: 549–56. [Google Scholar]
- Frick WF, Pollock JF, Hicks AC , et al 2010. An emerging disease causes regional population collapse of a common North American bat species. Science 329: 679–82. [DOI] [PubMed] [Google Scholar]
- Friend M, McLean RG, and Dein FJ. 2001. Disease emergence in birds: challenges for the twenty-first century. Auk 118: 290–303. [Google Scholar]
- Gibson DD and Kessel B. 1992. Seventy-four new avian taxa documented in Alaska 1976–1991. Condor 94: 454–67. [Google Scholar]
- Grabowski MM, Doyle FI, Reid DG , et al 2013. Do Arctic-nesting birds respond to earlier snowmelt? A multi-species study in north Yukon, Canada. Polar Biol 36: 1097–105. [Google Scholar]
- Harriman VB and Alisauskas RT. 2010. Of fleas and geese: the impact of an increasing nest ectoparasite on reproductive success. J Avian Biol 41: 573–79. [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR , et al 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296: 2158–62. [DOI] [PubMed] [Google Scholar]
- Hawley DM and Altizer SM. 2011. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct Ecol 25: 48–60. [Google Scholar]
- Hinzman LD, Deal CJ, McGuire AD , et al 2013. Trajectory of the Arctic as an integrated system. Ecol Appl 23: 1837–68. [DOI] [PubMed] [Google Scholar]
- Hoberg EP, Galbreath KE, Cook JA , et al 2012. Northern host-parasite assemblages: history and biogeography on the borderlands of episodic climate and environmental transition. Adv Parasit 79: 1–97. [DOI] [PubMed] [Google Scholar]
- Hoberg EP, Kutz SJ, Cook JA , et al 2013. Parasites. In: Meltofte H. (Ed). Arctic biodiversity assessment: status and trends in Arctic biodiversity. Akureyri, Iceland: Conservation of Arctic Flora and Fauna. [Google Scholar]
- Hoberg EP, Polley L, Jenkins EJ , et al 2008. Integrated approaches and empirical models for investigation of parasitic diseases in northern wildlife. Emerg Infect Dis 14: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueffer K, O'Hara TM, and Follmann EH. 2011. Adaptation of mammalian host-pathogen interactions in a changing Arctic environment. Acta Vet Scand 53: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueffer K, Parkinson AJ, Gerlach R, and Berner J. 2013. Zoonotic infections in Alaska: disease prevalence, potential impact of climate change and recommended actions for earlier disease detection, research, prevention and control. Int J Circumpol Health 72: 19562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles DT, Peterson SL, Gormezano LJ , et al 2013. Terrestrial predation by polar bears: not just a wild goose chase. Polar Biol 36: 1373–79. [Google Scholar]
- Jaenson T, Jaenson D, Eisen L , et al 2012. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasite Vector 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins EJ, Castrodale LJ, de Rosemond S , et al 2013. Tradition and transition: parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv Parasitol 82: 33–204. [DOI] [PubMed] [Google Scholar]
- Krauss S, Stallknecht DE, Negovetich NJ , et al 2010. Coincident ruddy turnstone migration and horseshoe crab spawning creates an ecological “hot spot” for influenza viruses. P R Soc B 277: 3373–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpula T, Pajunen A, Kaarlejärvi E , et al 2011. Land use and land cover change in Arctic Russia: ecological and social implications of industrial development. Glob Environ Chang 21: 550–62. [Google Scholar]
- Kutz SJ, Checkley S, Verocai GG , et al 2013. Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Glob Change Biol 19: 3254–62. [DOI] [PubMed] [Google Scholar]
- Kutz SJ, Hoberg EP, Molnár PK , et al 2014. A walk on the tundra: host–parasite interactions in an extreme environment. Int J Parasitol: Parasites Wildl 3: 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz SJ, Jenkins EJ, Veitch AM , et al 2009. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host–parasite interactions. Vet Parasitol 163: 217–28. [DOI] [PubMed] [Google Scholar]
- Kutz S, Thompson RA, Polley L , et al 2008. Giardia assemblage A: human genotype in muskoxen in the Canadian Arctic. Parasite Vector 1: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksonen S, Pusenius J, Kumpula J , et al 2010. Climate change promotes the emergence of serious disease outbreaks of filarioid nematodes. EcoHealth 7: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty KD. 2009. The ecology of climate change and infectious diseases. Ecology 90: 888–900. [DOI] [PubMed] [Google Scholar]
- LaPointe DA, Atkinson CT, and Samuel MD. 2012. Ecology and conservation biology of avian malaria. Ann NY Acad Sci 1249: 211–26. [DOI] [PubMed] [Google Scholar]
- Larsson C, Comstedt P, Olsen B, and Bergstrom S. 2007. First record of Lyme disease Borrelia in the Arctic. Vector-Borne Zoonot 7: 453–56. [DOI] [PubMed] [Google Scholar]
- Loiseau C, Harrigan RJ, Cornel AJ , et al 2012. First evidence and predictions of Plasmodium transmission in Alaskan bird populations. PLoS ONE 7: e44729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino S and Møller AP. 2010. Host–parasite interactions and climate change. In: Møller AP, Fiedler W, and Berthold P. (Eds). Effects of climate change on birds. New York, NY: Oxford University Press. [Google Scholar]
- Muradrasoli S, Bálint Á, Wahlgren J , et al 2010. Prevalence and phylogeny of coronaviruses in wild birds from the Bering Strait area (Beringia). PLoS ONE 5: e13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Audubon Society . 2012. The Christmas bird count historical results. http://netapp.audubon.org/cbcobservation. Viewed 15 Jan 2013. [Google Scholar]
- Parkinson AJ and Butler JC. 2005. Potential impacts of climate change on infectious diseases in the Arctic. Int J Circumpol Health 64: 478–86. [DOI] [PubMed] [Google Scholar]
- Parry ML, Canziani OF, Palutikof JP , et al 2007. Climate change 2007: impacts, adaptation, and vulnerability. Intergovernmental Panel on Climate Change. London, UK: Cambridge University Press. [Google Scholar]
- Patterson C and Guerin M. 2013. The effects of climate change on avian migratory patterns and the dispersal of commercial poultry diseases in Canada – Part I. World Poul Sci J 69: 17–26. [Google Scholar]
- Piersma T. 1997. Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos 80: 623–31. [Google Scholar]
- Post E, Bhatt US, Bitz CM , et al 2013. Ecological consequences of sea-ice decline. Science 341: 519–24. [DOI] [PubMed] [Google Scholar]
- Post E, Forchhammer MC, Bret-Harte MS , et al 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325: 1355–58. [DOI] [PubMed] [Google Scholar]
- Reeves AB, Pearce JM, Ramey AM , et al 2013. Genomic analysis of avian influenza viruses from waterfowl in western Alaska, USA. J Wildl Dis 49: 600–10. [DOI] [PubMed] [Google Scholar]
- Revich B, Tokarevich N, and Parkinson AJ. 2012. Climate change and zoonotic infections in the Russian Arctic. Int J Circumpolar Health 71; doi:10.3402/ijch.v71i0.18792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D, Henry B, Mak S , et al 2010. West Nile virus range expansion into British Columbia. Emerg Infect Dis 16: 1251–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström CAM, Buma AGJ, Hoye BJ , et al 2013. Latitudinal variability in the seroprevalence of antibodies against Toxoplasma gondii in non-migrant and Arctic migratory geese. Vet Parasitol 194: 9–15. [DOI] [PubMed] [Google Scholar]
- Schurer JM, Gesy KM, Elkin BT, and Jenkins EJ. 2014. Echinococcus multilocularis and Echinococcus canadensis in wolves from western Canada. Parasitology 141: 159–63. [DOI] [PubMed] [Google Scholar]
- Scott JD, Anderson JF, and Durden LA. 2012. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J Parasitol 98: 49–59. [DOI] [PubMed] [Google Scholar]
- Smith LC and Stephenson SR. 2013. New trans-Arctic shipping routes navigable by midcentury. P Natl Acad Sci USA 110: E1191–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tape K, Sturm M, and Racine C. 2006. The evidence for shrub expansion in northern Alaska and the Pan-Arctic. Glob Change Biol 12: 686–702. [Google Scholar]
- Ward DH, Dau CP, Tibbitts TL , et al 2009. Change in abundance of Pacific brant wintering in Alaska: evidence of a climate warming effect? Arctic 62: 301–11. [Google Scholar]


