Abstract
Background
Raine syndrome (RS) is a rare autosomal recessive disorder caused by biallelic loss-of-function mutations of FAM20C. The most common clinical features are microcephaly, exophthalmos, hypoplastic nose and severe midface hypoplasia, leading to choanal atresia. The radiological findings include generalized osteosclerosis and brain calcifications. RS is usually lethal during the neonatal period due to severe respiratory distress. However, there exists a non-lethal RS form, the phenotype of which is extremely heterogeneous. There is paucity of data about clinical course and life expectancy of these patients.
Results
This is the first description of follow-up features of non-lethal RS patients. Moreover, we present three unpublished cases.
There are five Asian and two Arab patients. All were born to consanguineous parents. The most common neonatal comorbidity was respiratory distress secondary to choanal atresia. A variable degree of neurodevelopmental delay was seen in the majority of our cases and seizures and hearing or vision involvement were also frequent. Neurological and orthopedic issues were the most frequent complications seen at follow-up in our group. Persistent hypophosphatemic rickets was the most striking endocrinological manifestation, which was scarcely responsive to therapy with phosphate salts and alfacalcidol. Life expectancy of our patients goes beyond childhood, with the oldest of those described being 18 years old at present.
Conclusions
Manifestations of RS in those surviving the neonatal period are being increasingly recognized. Our study supports previous findings and provides clinical and biochemical observations and data from longer follow up. Finally, we propose multidisciplinary follow up for patients with non-lethal RS.
Keywords: Raine syndrome, Children, Rickets, Osteosclerosis
Background
Raine syndrome (RS) (OMIM #259775) is a rare autosomal recessive disorder with the estimated prevalence of < 1 in 1,000,000 [1]. The first description of the phenotype (congenital sclerosing osteomalacia with cerebral calcification) was reported by White et al. in 1985 [2]. But only in 1992 was the eponym “Raine Syndrome” entered into Online Mendelian Inheritance In Man (OMIM) as lethal osteosclerotic bone dysplasia, after the description of a neonate with osteosclerosis, facial dysmorphism and lethal cranial anomalies by Raine et al. [3, 4]. More than 15 years later, the etiology of RS was discovered by Simpson et al who identified biallelic loss-of-function mutations of FAM20C (Family with sequence similarity 20, member C) gene (OMIM*611061), located on chromosome 7p22.3 [5]. FAM20C is a Golgi casein kinase that phosphorylates secreted phosphoproteins including fibroblast growth factor 23 (FGF23) and regulators of hard tissue formation and bone mineralization [6, 7].
The most classical phenotype was characterized by a combination of microcephaly, exophthalmos, hypoplastic nose, low-set ears, gum hypertrophy, cleft palate/uvula, and severe midface hypoplasia leading to choanal atresia. The radiological findings include generalized osteosclerosis and brain calcification [8].
Most patients with RS die during the neonatal period because of severe respiratory distress due to pulmonary hypoplasia and choanal atresia/stenosis. Despite the initial assumption that RS was always lethal in the neonatal period, some authors reported milder phenotypes associated with survival. The clinical features of non-lethal forms are extremely heterogeneous. Affected children can exhibit craniofacial, thoracic and limb abnormalities, dental and gum alterations, osteosclerosis, hypophosphatemic rickets and neurological disorders such as seizures and developmental delay [9–11].
To the best of our knowledge 47 cases of RS are reported so far and of those, 7 children died in the first two months of life [1, 12–16].
As the clinical features of RS are being increasingly recognized in surviving children and young people, we report clinical manifestations to support recent publications and add follow-up data including management of non-lethal Raine syndrome.
Results
We present the following seven patients diagnosed with non-lethal RS: two pairs of affected siblings and 3 singletons. Three cases have been published before as original case reports ([8, 9, 17]) and they are reviewed here for their prenatal and present characteristics (hereby referred to as cases IV, VI and VII). Two siblings from Pakistan are presented as new cases as they were referred to the Endocrinology Clinic in Italy in 2018 (referred to as cases I and II); another case from Pakistan was referred to us by Dr. Adnan Mirza (referred to as case III).
There were 4 males and 3 females. There are five Asian and two Arab patients.
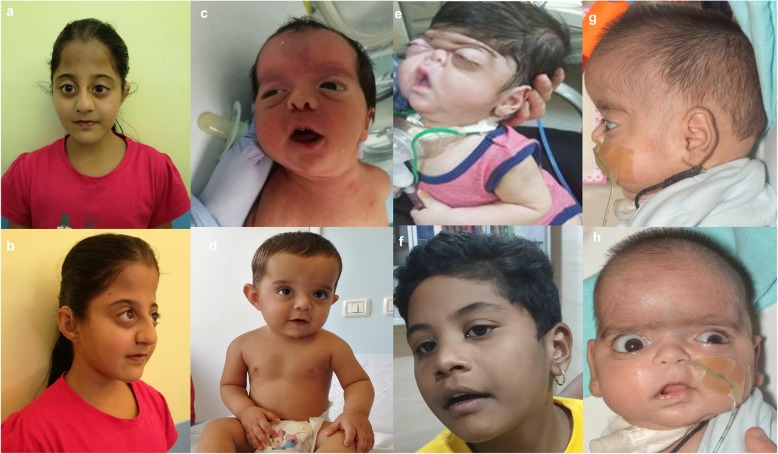
The patients’ photos are presented in Fig. 1. Photos of patients VI and VII are reported elsewhere [9].
Fig. 1.
1a-b: Case I at the age of 14 years. 1c: case II at 3 days of life. 1d: case II at 12 months of life. 1e: case III. 1f: case IV at 6 years of age. 1g: Case V (with permission from reference n° 17)1H: case V (with permission from reference n° 17)
Gestational, prenatal and birth characteristics
All the cases reported were born to consanguineous parents. Case VI and VII had a previously deceased sibling at 7 months of age (no genetic diagnosis was made).
Antenatal investigations revealed one case (case IV) of intra-uterine growth retardation, one of craniofacial dysmorphism (initially judged to be compatible with Crouzon syndrome) in case V; case II showed bilateral periventricular hyperechogenicity associated with anomalous cranial conformation, though in-utero magnetic resonance imaging was normal. The mother of case IV had two previous spontaneous abortions, though no further investigation was performed.
Four out of seven patients were born at term, two infants were late preterm at birth, whilst the gestational age of case I remains unknown (Table 1). Birth weight, according to the World Health Organization (WHO) charts [18], was higher than average in four infants, and lower in two; in one case birth weight was unavailable (Table 1). It was not possible to retrieve data regarding length and head circumference at birth of most patients. Two infants showed normal adaptation to extrauterine life, while two patients suffered from respiratory distress and were diagnosed with choanal atresia. One patient had respiratory distress at birth and choanal stenosis was documented (Table 1).
Table 1.
Summary of antenatal and postnatal features in non-lethal RS individuals
| Pedigree number | Case I | Case II | Case III | Case IV | Case V | Case VI | Case VII |
|---|---|---|---|---|---|---|---|
| Reference | Unpublished | Unpublished | Unpublished | [8] | [17] | [9] | [9] |
| Degree of kinship | Siblings | Siblings | |||||
| Gender | F | M | M | F | M | F | M |
| Ethnicity | Pakistani | Pakistani | Pakistani | Indian | Pakistani | Moroccan | Moroccan |
| Other children with RS in the family | No | One | No, two previous spontaneous abortions | No | No | Noa | Noa |
| Gestational age (wks) | NA | 37 | 37 | 36 | 36 | 40 | 39 |
| Weight at birth (g) /centile (c.le) | NA | 2900/30° | 3500/90° | 2750/60° | 2200/10° | 3500/60° | 3750/80° |
| Length at birth (cm)/ centile (c.le) | NA | 50/50° | 47/3° | NA | NA | NA | NA |
| Neonatal period | NA | Choanal stenosis-respiratory distress | Bilateral choanal atresia-respiratory distress | Normal |
Choanal atresia-respiratory distress - Hypocalcemic seizures |
Normal | Neonatal cerebral hypoxia-ischemia |
c.le percentile; F female, M male NA not available; wks weeks; aOne child deceased at 7 months (the diagnosis was not made)
Finally, case VII suffered from neonatal cerebral hypoxic-ischemia following a complicated delivery. The important antenatal and postnatal complications are listed in Table 1.
Clinical and radiologic features at diagnosis
Age at the time of clinical and molecular diagnosis ranged between 4 months and 17 years (mean 7.1 years). An early diagnosis, before one year of age, was made in three out of seven patients (Table 2). Growth data at diagnosis was available for only three patients (cases I, III and V) and showed short stature in all (defined as height below the third percentile, according to the WHO charts [18]).
Table 2.
Summary of clinical and radiologic features at diagnosis in non-lethal RS individuals
| Pedigree number | Case I* | Case II* | Case III | Case IV | Case V | Case VI° | Case VII° |
|---|---|---|---|---|---|---|---|
| Age at diagnosis | 12 yrs | 5 mo | 4 mo | 6 yrs | 2 mo | 17 yrs | 14 yrs |
| Neurological features | |||||||
| Developmental delay/Seizures | +/− | +/− | +/− | +/+ | −/+ | +/+ | +/− |
| Hearing/vision impairment | +/+ | −/− | −/− | – | −/− | +/− | +/− |
| Major features | |||||||
| Midface hypoplasia/ Microcephaly | −/+ | +/ + | +/+ | +/+ | +/+ | +/− | +/+ |
| Depressed nasal bridge/ Hypoplastic nose | −/− | +/+ | +/+ | +/+ | +/− | +/+ | +/+ |
| Exophthalmos | – | + | + | – | + | + | + |
| Minor features | |||||||
| Low set ears | – | – | + | + | + | + | + |
| Hypertelorism | + | – | + | + | + | + | + |
| Gingival hypertrophy | – | – | + | – | – | + | + |
| Dental malformations | + | – | + | – | – | + | + |
| Limb malformations | + | – | – | + | – | + | + |
| Radiologic features | |||||||
| Bowing of long bones | – | – | – | – | + | – | – |
| Signs of rickets | – | – | – | – | + | – | – |
| Cerebral calcifications | + | + | – | – | + | + | – |
| Osteosclerosis of skull | – | + | + | + | + | – | – |
| Osteosclerosis of long bones | - | + | + | + | + | – | – |
| Endocrinological features | |||||||
| Short stature | + | – | + | – | + | – | – |
| Hypophosphatemia | + | + | + | – | + | + | – |
| Hypocalcemia | – | + | + | – | + | – | – |
| Rickets | – | + | – | – | + | – | – |
| 25 OH Vitamin D deficiency | – | + | – | – | + | – | – |
mo months; yrs years +: present; −: absent; */° siblings
The most common major clinical features observed at diagnosis were: midface hypoplasia and proptosis (6/7) and a depressed nasal bridge (6/7) (see Table 2 for other major clinical features).
The most common minor clinical features at diagnosis were: gingival hypertrophy, low-set ears, hypertelorism, dental abnormalities and limb malformations, of which the most prevalent were clinodactyly of the toes, broad thumbs and flat feet (Table 2).
Other less commonly reported features were: down-slanting palpebral fissures and a bulbous nasal tip (cases II, IV, V, VI), followed by craniofacial disproportion with narrow bifrontal diameter (cases IV, VI) and a flat forehead (case IV), epicanthal folds (case IV), flaring nares (case IV), prominent philtrum (case IV), pointed chin (case IV), dark pigmentation of armpits, genitalia and knuckles (case IV), tented upper lip and frontal bossing (case V), right convergent strabismus (case II), anteverted nostrils (case VI)], high forehead (case I), hypoplasic nasal wings (case III), short columella (case I), inguinal monolateral hernia (case II), maxillary hypoplasia (case III) and severe prognathism (case VII).
Case III also underwent a surgical tracheostomy at day three of life, was mechanically ventilated for two months after birth and discharged from hospital on home oxygen therapy and a budesonide inhaler. He underwent numerous tarsorraphies for severe proptosis.
Tracheostomy was also performed in case V soon after the birth (3rd day of life).
Regarding neurological involvement, six patients showed a variable degree of neurodevelopmental delay; three experienced seizures at the time of diagnosis (Table 2). Mild sensorineural hearing loss was present in case VI and VII. Case I presented both severe bilateral hearing loss and a partial unilateral visual loss due to occlusion of the central retinal artery (Table 2).
The most frequent radiologic findings at diagnosis were cerebral calcifications and osteosclerosis of long bones and skull (Table 2).
The principal clinical and radiologic features at diagnosis are listed in Table 2.
Table 3 describes the genotype of each patient. All parents were tested and found to be heterozygous carriers of the variants described.
Table 3.
Genotype
| Pedigree number and reference | Case I | Case II | Case III | Case IV [8] | Case V [17] | Case V I[9] | Case VII [9] |
|---|---|---|---|---|---|---|---|
| Mutation(s) in FAM20C gene | c.1351G > A (p.Asp451Asn) | c.1351G > A (p.Asp451Asn) |
c.496G > T (p.E166X) |
c1630C > T (p.Arg544Trp) |
c.1135G > A (p.G379R) |
c.676 T > A (p.Trp226Arg) |
c.676 T > A. (p.Trp226Arg) |
| Exon/Intron of gene | Exon 7 | Exon 7 | Exon 1 | Exon 10 | Exon 6 | Exon 2 | Exon 2 |
| Type of mutation | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous | Homozygous |
| Variant interpretation | Pathogenic based on LOVD database | Pathogenic based on LOVD database | Pathogenic based on PVS1 PM2 PP4 | Pathogenic according to ACMG guidelines | Pathogenic according to ACMG guidelines |
Pathogenic based on: SIFT (score0) Polyphen-2 (score 0.993) Mutation Taster (score 0.999) |
Pathogenic based on: SIFT (score0) Polyphen-2 (score 0.993) Mutation Taster (score 0.999) |
| Sequencing methods | Sanger | NGS | NGS | NGS and Sanger | Sanger | NGS | Sanger |
ACMG American College of Medical Genetics and Genomics; LOVD Leiden Open Variation Database; NGS next generation sequencing
Biochemical features and management at diagnosis
With regard to biochemical findings at the time of diagnosis, serum calcium was within the normal range in 4/6 of patients and low in three patients (cases II, III and V). Hypophosphatemia was found in five out of seven patients (cases I, II, III, V, VI) (Table 2). Alkaline phosphatase (ALP) was found to be either normal (cases III and VI) or high (cases I, II and V). Serum parathormone (PTH) level was within normal limits in one patient (case I) and raised in cases II, V and VI; no data were available for the other two cases (III, IV, VII). Vitamin D deficiency was detected in two patients (cases II and V), while in another case the level was adequate (case I). One patient (case V) had low tubular phosphate reabsorption with normal calcium excretion and persistently raised fibroblast growth factor 23 (FGF23), confirming the diagnosis of FGF23 mediated hypophosphataemia. Unfortunately, the level of FGF23 was not available in the other patients. Biochemical features at diagnosis are presented in Table 4.
Table 4.
Biochemical features at diagnosis
| Case I | Case II | Case III | Case IV | Case V | Case VI | Case VII | |
|---|---|---|---|---|---|---|---|
| Calcium |
10.1 mg/dl (nv 8.8–10.8) |
6.4 mg/dl (nv 8.8–10.8) |
1.78 mmol/l (nv 2.20–2.79) |
10 mg/dl (nv 8–11 mg/dl) |
1.92 mmol/L (nv 2.20–2.79) |
2.45 mmol/l (nv 2.2–2.6) |
NA |
| Phosphorus |
2.7 mg/dl (nv 4–7) |
3.9 mg/dl (nv 4–7) |
1.33 mmol/l (nv 1.36–2.26) |
NA |
0.75 mmol/L (nv 1.36–2.26) |
2.2 mg/dl (nv 2.5–4.5) |
NA |
| ALP |
547 U/L (nv 140–400) |
454 U/L (nv 140–400) |
743 IU/L (nv 187–1197) |
NA |
5605 IU/L (nv 187–1197) |
70 IU/L (nv 40–100) |
NA |
| PTH |
51.3 pg/ml (nv 15–65) |
129 pg/ml (nv 15–65) |
NA | NA |
72 pmol/L (nv 1.1–6.9) |
95.3 pg/ml (nv 15–68) |
NA |
| 25OHD |
45.6 ng/ml (nv > 30) |
13.8 ng/ml (nv > 30) | NA | NA |
< 40 nmol/L (nv > 50 nmol/L) |
NA | NA |
| TRP% | NA | NA | NA | NA | 72 | NA | NA |
| FGF23 | NA | NA | NA | NA |
157 RU/ml (nv 0–100) |
NA | NA |
ALP alkaline phosphatase; FGF23 fibroblast growth factor 23; NA not available; NV normal value; PTH parathormone; TRP tubular reabsorption of phosphate; ys years; 25OHD 25-Hydroxy Vitamin D.
Three patients required supplementation of calcium (oral or intravenous), oral phosphate plus oral alfacalcidiol and cholecalciferol alone or in combination (case I, II, V).
Follow-up
It was possible to collect data concerning the follow-up of six out of seven patients (cases I, II, III, V, VI, VII), as it stands in February 2019. Duration of follow up for cases I, II, VI, VII was < 4 years and for case V > 5 years. One child subsequently died at 14 months of age (case III).
Five patients are alive at the time of writing and live with different comorbidities (cases I, II, V, VI and VII). Here we present the phenotypic data collected at the last follow-up visit. Patient ages range between 10 months and 18 years. Follow up frequency and data collected varied between the cases. In particular, the patients were evaluated regularly until respectively the age of eight (case V), eighteen years (case VI) and every six months after diagnosis (case I, II) (case I), at two and eight months of age in case II and at 5, 8 and 10 months in case III.
Endocrinological aspects
Pertaining to growth, severe short stature was observed in case I. She was tested for growth hormone deficiency and IGF1 level at the age of 10 years, but was found to be normal.
The study of calcium-phosphate metabolism was routinely performed during the follow-up according to clinical judgment in three cases (cases I, II, V) and revealed persistent normo-calcemic hypophosphatemia associated with high levels of ALP and PTH (Table 5). Tubular reabsorption of phosphate was low in 2/3 children (case II and case V) and urinary calcium was normal. Radiographs showed features of rickets in 2 patients (cases II and V). Given the presence of hypophosphatemic rickets, cases II and V were prescribed oral phosphate supplementation combined with oral alfacalcidol. In case II, ALP and PTH significantly improved without the development of hypercalciuria; a small and not significant reduction in PTH and ALP level was seen in case V despite increasing doses of both drugs (Table 5). Case I was treated only with phosphate salts for one year and then stopped because no improvement in bone chemistry was seen.
Table 5.
Biochemical features during follow-up
| Case I | Case II | Case V | |||||
|---|---|---|---|---|---|---|---|
| Age | 12 ys 6 mo | 13 ys 7 mo | 14 ys | 4 mo | 1 y 9 mo | 2 mo | 7 ys and 3 mo |
| Pre-therapy | At the end of the therapya | At the end of follow-up | Pre-therapy | At the end of follow-up | Pre-therapy | At the end of follow-up | |
| Calcium |
9.6 mg/dl (nv 8.8–10.8) |
9.8 mg/dl (nv 8.8–10.8) |
9.7 mg/dl (nv 8.8–10.8) |
10 mg/dl (nv 8.8–10.8) |
10.1 mg/dl (nv 8.8–10.8) |
1.92 mmol/L (nv 2.20–2.79) |
2.51 mmol/L (nv 2.20–2.79) |
| Phosphorus |
2.6 mg/dl (nv 4–7) |
2.2 mg/dl (nv 4–7) |
2.6 mg/dl (nv 4–7) |
2.2 mg/dl (nv 4–7) |
2.1 mg/dl (nv 4–7) |
0.75 mmol/L (nv 1.36–2.26) |
0.73 mmol/L (nv 1.36–2.26) |
| ALP |
555 U/L (nv 140–400) |
611 U/L (nv 140–400) |
419 U/L (nv 140–400) |
805 U/L (nv 140–400) |
487 U/L (nv 140–400) |
5605 IU/L (nv 187–1197) |
3874 IU/L (nv 187–1197) |
| PTH |
79 pg/ml (nv 15–65) |
104 g/ml (nv 15–65) |
74 pg/ml (nv 15–65) |
71 pg/ml (nv 15–65) |
64 pg/ml (nv 15–65) |
72 pmol/L (nv 1.1–6.9) |
24 pmol/L (nv 1.1–6.9) |
| 25OHD |
39.2 ng/ml (nv > 30) |
35.2 ng/ml (nv > 30) |
39.2 ng/ml (nv > 30) |
57.9 ng/ml (nv > 30) |
51.2 ng/ml (nv > 30) |
< 40 nmol/L (nv > 50 nmol/L) |
17 nmol/L (nv > 50 nmol/L) |
| TRP% | NA | 66 | NA | 76 | 73 | 72 | NA |
| FGF23 | NA | NA | NA | NA | NA |
157 RU/ml (nv 0–100) |
NA |
|
CaU/CrU (< 3 ys:; nv < 0.5 > 3 ys: nv < 0.39) |
0.22 | 0.09 | 0.12 | 0.026 | 0.10 | Normal | Normal |
| Therapy with oral phospate salt | – | yes | no | – | yes | – | yes |
| Therapy with oral alfacalcidiol | – | no | no | – | yes | – | yes |
| Supplementation with oral cholecalciferol | – | yes | yes | yes | yes | – | yes |
ALP alkaline phosphatase; CaU/CrU urine calcium to creatinine ratio; FGF23 fibroblast growth factor 23; Mo months; NA not available; NV normal value; PTH parathormone; TRP tubular reabsorption of phosphate; 25OHD 25-Hydroxy Vitamin D; ys years; alast measurements before stopping the therapy with phosphate salts
All patients were currently on vitamin D2 or D3 supplementation because of ongoing deficiency.
We report the isolated finding of low TSH and FT4 level without obvious pituitary pathology (case III).
Neurological development
Microcephaly was confirmed in all investigated cases.
Out of the six analyzed cases, five presented moderate-to-severe neuro-developmental delay (Table 2). Two patients (cases V and VII) had reported seizures, one starting at the age of 3 and one at 5 years old. Treatment with sodium valproate was administered in Case V; discontinuation of the drug was unsuccessful due to relapse. Case VII remains without treatment with no further seizure activity.
Craniostenosis was documented in three cases (cases I, II and V), two of which (cases II and V) underwent a ventriculoperitoneal shunt insertion, followed by a surgical intervention of biparietal decompression and bone thinning. Cranioplasty surgery is on the agenda for two patients (cases I and II).
Ear- nose- throat (ENT) aspects
Regarding otorhinolaryngoiatric aspects, a bilateral miringotomy was performed on case V at the age of four years due to glue ear. Furthermore, case III he was diagnosed with auditory neuropathy spectrum disorder. Stable hearing loss was reported in case I.
Choanal stenosis was reported to be still present at the ages of 8 and 12 (case I), while two other siblings were found to have nasal voice (cases VI and VII).
Moreover, one patient displayed occasional mild laryngeal stridor with modestly reduced oxygen-dependency (case III).
Ophthalmological aspects
Case V was diagnosed at six years of age with bilateral lagophtalmos and exposure keratopathy, requiring bilateral tarsorraphy. Furthermore, bilateral partial optic nerve atrophy, myopia and astigmatism were detected.
Ophthalmologic follow-up of case I, after initial diagnosis of partial unilateral visual loss due to occlusion of the central retinal artery, remains stable.
Gastrointestinal and feeding
Difficulties in feeding were reported in one patient due to severe prognathism (case VII). Two patients underwent gastrostomy placement, case III at the age of four months and case V at 18 months (with removal at the age of 4).
Orthopedic aspects
To correct a unilateral lower limb valgus deformity due to the hypophosphataemic rickets, one patient underwent an orthopedic surgical intervention of eight-plate epiphysodesis fixation at the age of seven (case V) with good effect and improvement in mobility.
During follow-up, one patient was diagnosed with bilateral flat feet and dorsal scoliosis (case I), while another with varism of the inferior limbs (case II).
Miscellaneous
In case V, to correct a mild degree hypospadia and ventral foreskin meatus deficiency, a urological procedure of meatomy and circumcision was performed at the age of eight.
Following hemorrhagic complications during surgery, one patient was tested and diagnosed with a mild type I Von Willebrand disease (Case V). Severe odontopathy was documented in two cases (siblings, case I and II).
Discussion
RS is a rare autosomal recessive disorder that tends to present in the offspring of consanguineous couples. It was previously considered a lethal condition, but the literature has demonstrated surviving cases. No clear distinction between lethal and non-lethal RS has been made with regards to genetic and phenotypical expression. However, Hung et al. [16] showed that all the pathogenic variants responsible for lethal versus non-lethal Raine phenotype are situated in close proximity to functional protein regions such as catalytic, dimerization and glycosylation sites. In contrast, polymorphic changes reported in healthy homozygotes are found in the helix furthest away from the catalytic pocket and away from any of the functional regions.
Evidence is also scarce on life expectancy and clinical outcome in non-lethal RS. This is the first description of follow-up features of non-lethal RS patients. Furthermore, we presented three original previously unpublished cases.
The main epidemiological characteristics of our patients at diagnosis do not differ from the previous descriptions reported in the literature; all children were born to consanguinous parents, more frequently of Asian ethnicity. This report, with the addition of three new cases, confirmed that most cases were not Caucasian which is likely to be related to the lower prevalence of consanguinity in this population [1, 19].
The majority of our patients were born at or near term, consistent with the literature [4, 12, 15, 17]. The principal neonatal complication was respiratory distress due to choanal atresia or stenosis. Although the most striking clinical and radiological features at diagnosis did not differ from those previously described (midface hypoplasia, depressed nasal bridge, generalized osteosclerosis and brain calcification), we have reported previously undescribed clinical features, the most common being a bulbous nasal tip, not observed in the cases parents and whose correlation with RS may need further investigation. Chondrodysplasia punctate, previously reported as part of the radiological spectrum of the syndrome, was not observed in our group of patients.
Some degree of neurodevelopmental delay was seen at diagnosis in the majority of our cases. Seizures occurred in 43%, and required treatment in 2/3 of the cases. Abnormalities in hearing were present in 43%. A variable degree of hypophosphatemic rickets was detected in the majority of our group, even though the medical management was distinct from case to case. The difference in management was due to the absence of guidelines specific for this syndrome.
Overall, we present follow-up data of non-lethal RS in the single largest cohort of patients and with the longest follow up duration, the oldest of our patients being 18 years old at present. We, therefore, can support the previous data that life expectancy for these patients goes beyond childhood [19–22]. We found great heterogeneity in terms of the rate of complications emerging during follow-up. Orthopedic and neurological issues were the most frequent in our group: in particular, craniostenosis requiring surgical intervention and limb anomalies that were mostly managed conservatively. A moderate-to-severe neurological delay was present in almost all the patients. However, systematic homogeneous neuropsychiatric evaluations were not performed and we are unable to define prevalent affected areas. Even though epilepsy has been described as a component of RS, only one patient in our group presented with seizures requiring anti-epileptic therapy.
As for endocrinological manifestations, the most striking feature was persistent hypophosphatemic rickets. Three patients were treated with the conventional therapy used for X-linked hypophosphatemic rickets (XLHR), given the absence of a specific guideline tailored for RS [23]. However in all cases ALP did not normalize even using high doses of phosphate salts and alfacalcidol. The reason for this is unclear but may be related to the different pathogenesis of the disease compared with XLHR, as well as the underlying osteosclerosis associated with RS. A possible link between RS and hypophosphatemic rickets has already been postulated [17]. FAM20C is known to encode the human homologue of dentin matrix protein 4 (DMP4) which is highly expressed in odontoblasts and moderately expressed in bone. Although little is known about the effects of DMP4, it is likely to play a role in mineralization, and may be similar to dentin matrix protein 1, a role for which has already been established in the inherited forms of hypophosphataemic rickets [24].
This study has some limitations. First of all, the data were collected retrospectively, and secondly, 50% of the involved centres are medium or low- health resource settings, which may have influenced the number of investigations performed at diagnosis and during follow-up and the low follow-up rate of patients presented. This report, however, summarizes the best available knowledge of this rare disease.
Conclusion
Our study shows that the clinical course of patients affected by non-lethal RS is extremely variable from case to case and confirms that survival is possible beyond childhood. The main complications reported in our group were neurological and orthopedic. Hypophosphatemic rickets, scarcely responsive to therapy and short stature, were the most striking endocrinological manifestations detected in our group.
Based on our experience, we would suggest close monitoring of every patient with a diagnosis of the non-lethal form of RS with: a) anthropometric evaluation; b) neurological evaluation to promptly detect neurodevelopment delay and craniostenosis; c) complete and regular assessment of calcium and phosphate homeostasis; d) orthopedic, otorinolaryngeal and ophthalmologic evaluations at least once at diagnosis and afterwards as clinically required.
Moreover, we aim to continue following the clinical course of our patients in order to widen our knowledge about the disease and to update and improve this proposal.
Further studies including a larger number of patients are needed in order to demonstrate a possible correlation between genetic and phenotypic aspects.
Methods
In September 2018 an initial comprehensive literature search about RS was performed, and revised/updated in 2019 using Pubmed with no restrictions for language, type and year of publication. A total of 19 papers related to paediatric non-lethal RS were found, published between 1996 and 2019.
Subsequently, all the centers identified through the papers were contacted and invited by email to take part in our study. First of all, we approached the corresponding author. In case of no response, other authors were contacted accordingly. Inclusion criteria were the presence of new unpublished materials (eg: data, pictures) and/or at least one follow-up visit of each patient with the non-lethal form of RS, genetically diagnosed. In addition we asked all authors if they had other unpublished cases between the last published paper on this topic and the study invitation. A total of seven authors agreed to participate to our study.
A form including demographical and follow-up data was sent to all participants. For each patient included in the study, all the following parameters at diagnosis were collected: clinical features, imaging, biochemical examinations and treatment, molecular genetics investigations, hospitalizations and surgery. In order to obtain follow-up data we collected anthropometry, biochemical, radiological and clinical features, hospitalizations and surgery from every visit of each patient.
Fasting blood samples (8-h fast) were obtained by venipuncture. A morning urine sample was collected to assess urinary creatinine, calcium and phosphate. Genetic test was performed as detailed in Table 3. FGF23 analysis of case V was reported elsewhere [17].
We used descriptive statistics to present demographic, clinical, and other characteristics of patients.
Data was properly anonymized and sent back to V. Buzzi Hospital (Milan) by February 2019. This study was approved by the institutional review board of ASST-FBF-SACCO. Parents/legal guardians/patients gave consent for publication of clinical photographs.
Acknowledgements
Not applicable.
Abbreviations
- ALP
Alkaline phosphatase
- FGF23
Fibroblast growth factor 23
- PTH
Parathormone
- RS
Raine syndrome
- SD
Standard deviation
- XLHR
X-linked hypophospatemic rickets
- WHO
World Health Organization
Authors’ contributions
CM, GZ, MB, GM wrote the manuscript and researched data. NM, EC, SCE, AM, YS, AG, GSK, PD, FSA, MK researched data and reviewed/edited the manuscript. GVZ reviewed/edited the manuscript. The author(s) read and approved the final manuscipt.
Funding
None.
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Ethics approval and consent to participate
Approved by local Ethical Committee of ASST FBF -SACCO.
Consent for publication
The relevant parents or guardians have given their informed consent for publication of each case.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Faundes V, Castillo-Taucher S, Gonzalez-Hormazabal P, Chandler K, Crosby A, Chioza B. Raine syndrome: An overview. Eur. J. Med. Genet. 2014;57:536–542. doi: 10.1016/j.ejmg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Whyte MP, McAlister WH, Fallon MD, Pierpont ME, Bijanki VN, Duan S, et al. RS (OMIM #259775), Caused By FAM20C Mutation, Is Congenital Sclerosing Osteomalacia With Cerebral Calcification (OMIM 259660) J Bone Min Res. 2017;32:757–769. doi: 10.1002/jbmr.3034. [DOI] [PubMed] [Google Scholar]
- 3.Raine J, Winter RM, Davey A, Tucker SM. Unknown syndrome: microcephaly, hypoplastic nose, exophthalmos, gum hyperplasia, cleft palate, low set ears, and osteosclerosis. J Med Genet. 1989;26:786–788. doi: 10.1136/jmg.26.12.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kan AE, Kozlowski K. New distinct lethal osteosclerotic bone dysplasia (Raine syndrome) Am J Med Genet. 1992;43:860–864. doi: 10.1002/ajmg.1320430522. [DOI] [PubMed] [Google Scholar]
- 5.Simpson MA, Hsu R, Keir LS, Hao J, Sivapalan G, Ernst LM, et al. Mutations in FAM20C Are Associated with Lethal Osteosclerotic Bone Dysplasia (Raine Syndrome), Highlighting a Crucial Molecule in Bone Development. Am J Hum Genet. 2007;1:906–912. doi: 10.1086/522240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinoshita Y, Hori M, Taguchi M, Fukumoto S. Functional analysis of mutant FAM20C in Raine syndrome with FGF23-related hypophosphatemia. Bone. 2014;67:145–151. doi: 10.1016/j.bone.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa XA, Ogura E, Manning G, Irvine KD. The Raine Syndrome Protein FAM20C Is a Golgi Kinase That Phosphorylates Bio-Mineralization Proteins. PLoS One. 2012;7:e42988. doi: 10.1371/journal.pone.0042988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheth J, Bhavsar R, Gandhi A, Sheth F, Pancholi D. A case of Raine syndrome presenting with facial dysmorphy and review of literature. BMC Med Genet. 2018;19:76. doi: 10.1186/s12881-018-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elalaoui SC, Al-Sheqaih N, Ratbi I, Urquhart JE, O'Sullivan J, Bhaskar S, et al. Non lethal Raine syndrome and differential diagnosis. Eur J Med Genet. 2016;59:577–583. doi: 10.1016/j.ejmg.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Mahafza T, El-Shanti H, Omari H. Raine syndrome: report of a case with hand and foot anomalies. Clin Dysmorphol. 2001;10:227–229. doi: 10.1097/00019605-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Acevedo AC, Poulter JA, Alves PG, de Lima CL, Castro LC, Yamaguti PM. Variability of systemic and oro-dental phenotype in two families with non-lethal Raine syndrome with FAM20C mutations. BMC Med Genet. 2015;16:8. doi: 10.1186/s12881-015-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kingston HM, Freeman JS, Hall CM. A new lethal sclerosing bone dysplasia. Skeletal Radiol. 1991;20:117–119. doi: 10.1007/BF00193823. [DOI] [PubMed] [Google Scholar]
- 13.Kochar GS, Choudhary A, Gadodia A, Gupta N, Simpson MA, Crosby AH, et al. Raine syndrome: a clinical, radiographic and genetic investigation of a case from the Indian subcontinent. Clin. Dysmorphol. 2010;3:153–156. doi: 10.1097/MCD.0b013e32833a22c5. [DOI] [PubMed] [Google Scholar]
- 14.Hülskamp G, Wieczorek D, Rieder H, Louwen F, Hörnig-Franz I, Rickert CH. Raine syndrome: report of a family with three affected sibs and further delineation of the syndrome. Clin. Dysmorphol. 2003;12:153–160. doi: 10.1097/01.mcd.0000080413.95344.60. [DOI] [PubMed] [Google Scholar]
- 15.Al-Gazali LI, Jehier K, Nazih B, Abtin F, Haas D, Sadagahatian R. Further delineation of Raine syndrome. Clin. Dysmorphol. 2003;12:89–93. doi: 10.1097/00019605-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Hung CY, Rodriguez M, Roberts A, Bauer M, Mihalek I, Bodamer O. A novel FAM20C mutation causes a rare form of neonatal lethal Raine syndrome. Am J Med Genet Part A. 2019;179A:1866–1871. doi: 10.1002/ajmg.a.61291. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood N, Donnel A, Weber A, Dharmaraj P. Raine syndrome: a review and a report of metabolic bone disease as a new link. Res 2014;1:890. http://dx.doi.org/10.13070/rs.en.1.890.
- 18.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization, 2006.
- 19.Mamedova E, Dimitrova D, Przhiyalkovskaya E, Buryakina S, Vasilyev E, Tiulpakov A, et al. Non-lethal RS in a Middle-Aged Woman Caused by a Novel FAM20C Mutation. Calcif Tissue Int. 2019;105:567–572. doi: 10.1007/s00223-019-00599-w. [DOI] [PubMed] [Google Scholar]
- 20.Rolvien T, Kornak U, Schinke T, Amling M, Oheim R. A novel FAM20C mutation causing hypophosphatemic osteomalacia with osteosclerosis (mild Raine syndrome) in an elderly man with spontaneous osteonecrosis of the knee. Osteoporos Int. 2019;30:685–689. doi: 10.1007/s00198-018-4667-6. [DOI] [PubMed] [Google Scholar]
- 21.Takeyari S, Yamamoto T, Kinoshita Y, Fukumoto S, Glorieux FH, Michigami T, et al. Hypophosphatemic osteomalacia and bone sclerosis caused by a novel homozygous mutation of the FAM20C gene in an elderly man with a mild variant of Raine syndrome. Bone. 2014;67:56–62. doi: 10.1016/j.bone.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Tamai K, Tada K, Takeuchi A, Nakamura M, Marunaka H, Washio Y, et al. Fetal ultrasonographic findings including cerebral hyperechogenicity in a patient with non-lethal form of Raine syndrome. Am J Med Genet A. 2018;176:682–686. doi: 10.1002/ajmg.a.38598. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26:1381–1388. doi: 10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fradin M, Stoetzel C, Muller J, Koob M, Christmann D, Debry C, et al. Osteosclerotic bone dysplasia in siblings with a Fam20C mutation. Clin Genet. 2011;80:177–183. doi: 10.1111/j.1399-0004.2010.01516.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.