Abstract
RNA modifications can be added or removed by a variety of enzymes that catalyse the necessary reactions, and these modifications play roles in essential molecular mechanisms. The prevalent modifications on mRNA include N6-methyladenosine (m6A), N1-methyladenosine (m1A), 5-methylcytosine (m5C), 5-hydroxymethylcytosine (hm5C), pseudouridine (Ψ), inosine (I), uridine (U) and ribosemethylation (2’-O-Me). Most of these modifications contribute to pre-mRNA splicing, nuclear export, transcript stability and translation initiation in eukaryotic cells. By participating in various physiological processes, RNA modifications also have regulatory roles in the pathogenesis of tumour and non-tumour diseases. We discussed the physiological roles of RNA modifications and associated these roles with disease pathogenesis. Functioning as the bridge between transcription and translation, RNA modifications are vital for the progression of numerous diseases and can even regulate the fate of cancer cells.
Keywords: RNA modifications, m6A, m1A, m5C, diseases
Introduction
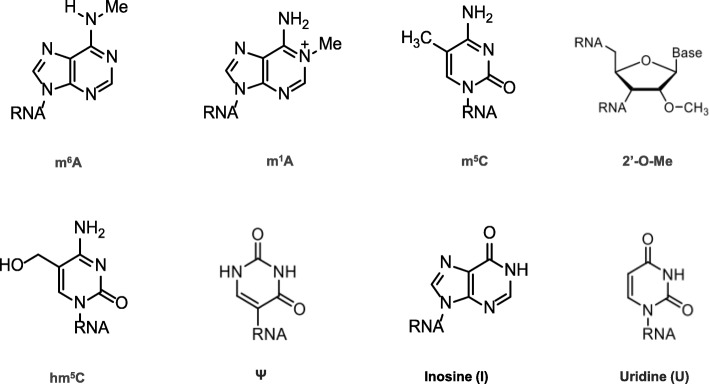
In the 1950s, the first RNA nucleoside modification was identified [1]; since then, researchers have focused on updating the understanding of RNA modifications. At the very beginning, the 5’cap and the poly(A) tail, which represent cap and tail modifications, respectively, were discovered. However, with the limitations of technology, modifications of eukaryotic mRNA ends were considered the only post-transcriptional alterations to mRNA for a while. Fortunately, this situation did not last for a long time. Internal mRNA modifications have been investigated in succession in the last 50 years. The revealed mRNA modifications included but were not limited to N6-methyladenosine (m6A), N1-methyladenosine (m1A), 5-methylcytosine (m5C), 5-hydroxymethylcytosine (hm5C), pseudouridine (Ψ), inosine (I), uridine (U) and ribose-methylation (2’-O-Me) [2–4] (Figs. 1 and 2). m6A is the most abundant modification and was therefore thoroughly investigated [5].
Fig. 1.
Chemical structures of mRNA modifications. Chemical structures in eukaryotic mRNA including m6A, m1A, m5C, hm5C, Ψ, I, U and 2’-O-Me
Fig. 2.
Locations of chemical modifications in mRNA. Chemical RNA modifications are shown in mRNA with their approximate distribution in transcripts. m6A with a widespread distribution prefers to be located in the consensus motif in the 3’UTRs as well as the 5’UTRs, which closely correlate with translation. Although m1A-containing mRNA is 10 times less common than m6A-containing mRNA, m1A is discovered in every segment of mRNA, including the 5’UTRs, CDS and 3’UTRs and mostly in highly structured 5’UTRs. Analogous to m1A, m5C can occur in coding and non-coding regions of mRNA, especially in GC-rich regions. Nevertheless, m5C within different positions regulates transcription differently. Tet-family enzymes prefer to oxidize m5C modifications in coding regions, so hm5C has a greater possibility of being present in CDS. Subsequently, Ψ is demonstrated to have a diversified location, whereas I is present at a large number of sites in the CDS, and U accumulates in 3’UTRs. 2’-O-Me focuses on decorating specific regions of mRNA that encode given amino acids. Additionally, as reversible modifications, most have their own readers, writers and erasers
Analogous to mRNA modification, we also identified many modifications on transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs), such as queuosine (Q) [6]. Eukaryotic tRNAs contain, on average, over 10 modifications per molecule. From elementary isomerization or methylation to complicated modifications of ring structures, the number of tRNA modifications is the largest and has the widest chemical variety. Moreover, there are over 200 modifications on human rRNAs. Thus, their less complicated nature and greater abundance led to more investigations of tRNAs and rRNAs, even beyond mRNAs. Early studies have demonstrated that this variety of modifications leads to extra cellular functions for diverse RNA species [7].
The regulatory role of RNA modifications
Modifications on different RNAs were found to regulate various cellular processes. Researchers demonstrated that these modifications can initiate translation, stabilize transcripts, splice pre-mRNA, facilitate nuclear export, etc. [8–12]. With respect to RNA modifications and technological advances in high-throughput sequencing and mass spectrometry, the mechanisms of different cellular processes influenced by RNA modifications are underexplored, including the less ubiquitous modifications on rare RNA species. tRNAs, which have the greatest number of types of different chemical modifications, regulate molecular mechanisms by selecting and protecting the reading framework, promoting tRNA decoding capability as well as changing codon-anti-codon connections [13–17]. Moreover, the functions of 2’-O-Me, Ψ and m5C, which are abundant in rRNA, have been investigated in detail. Without any doubt, mRNA modifications play roles in modulating molecular mechanisms. Subsequently, RNA modifications contribute to tumorigenesis by regulating cell survival, differentiation, migration and drug resistance [18].
m6A RNA modification
Introduction to m6A RNA modification
m6A accounts for approximately 0.2~0.6% of total adenosines in mammalian RNA [2, 5]. General m6A modifications occur in mammals, plants, bacteria and even other types of eukaryotic RNA [19–22]. In addition to their widespread distribution, there is no less than 1-2 methylated adenosines in every single mRNA [23]. Studies have reported that m6A is located in the 3’ untranslated region (3’UTR), predominantly in a consensus motif, GGm6ACU [24–26]. Recently, m6A was also found in the 5’ untranslated region (5’UTR), a region that closely correlates with translation. It has been reported that methylated adenosine in the 5’UTR of mRNA can support cap-independent translation commencement and can increase translation [27, 28].
As a reversible mRNA modification, m6A has its own writers, readers and erasers. Methyltransferase-like 3 (METTL3) was the first demonstrated m6A writer [29]. In addition to METTL3, other proteins possessing methyltransferase (MTase) capability were recently identified, including methyltransferase-like 14 (METTL14), Wilms tumour 1-associated protein (WTAP), RNA-binding motif protein 15 (RBM15), KIAA 1429 and zinc finger CCCH-type containing 13 (ZC3H13) [30–33]. By binding to mRNA, readers, such as members of the YT521-B homology (YTH) domain family of proteins (YTHDF1, YTHDF2, YTHDF3, YTHDC1 and YTHDC2) and heterogeneous nuclear ribonucleoprotein (HNRNP) proteins (HNRNPA2B1 and HNRNPC) can execute the physiological functions of the modification [8, 10, 12, 34–38]. Additionally, eukaryotic initiation factor 3 (eIF3), insulin-like growth factor 2 mRNA-binding proteins (IGF2BP1, IGF2BP2 and IGF2BP3), fragile X mental retardation 1 (FMR1) and leucine-rich pentatricopeptide repeat-containing (LRPPRC) all can read m6A modifications [39, 40]. Both fat mass and obesity-associated protein (FTO) and alkB homologue 5 (ALKBH5) are erasers of m6A modifications [11, 41, 42].
Regulatory role of m6A RNA modification in molecular functions
Accumulation of pre-mRNA and diminution of mature mRNA in cyclo-leucine-treated avian sarcoma virus-infected cells and neplanocin A (NPC)-treated SV40 RNA demonstrate that m6A is essential in pre-mRNA splicing [43, 44]. Both cyclo-leucine and NPC are inhibitors of methylation that can be used to investigate m6A [45, 46]. Subsequently, MTases and demethylases might be involved in regulating RNA splicing. By changing RNA structure and regulating the combination of RNA and reader proteins, HNRNPC can modulate the splicing of m6A-containing mRNAs [10]. More recently, by relying on the RGG region in the low-complication region of HNRNPG, a reader was reported to cooperate with modified pre-mRNA and the phosphorylated C-terminal domain of RNA polymerase II to modulate splicing [47]. Moreover, FTO is vital to mRNA splicing because it prefers to bind to introns of nascent mRNA [48]. Another splicing-related eraser is ALKBH5. Immunofluorescence analysis revealed that ALKBH5 was tightly related to splicing factors [11].
Writers, readers and erasers can all regulate mRNA export. By modulating the clock genes Per2 and Arntl, METTL3 regulates the export of mature mRNA [49]. By interacting with SRSF3 and regulating the combination of SRSF3 and NXF1 on RNA, YTHDC1 mediates the export of modified mRNA [50]. Subsequently, knockdown of ALKBH5 leads to acceleration of mRNA export, suggesting that m6A is essential to regulating mRNA export [11].
AU-rich element (ARE), iron-responsive element (IRE) and cytoplasmic polyadenylation element (CPE) represent functional domains and are responsible for mRNA decay in 3’UTRs [51]. Coincidentally, m6A accumulates in 3’UTRs. Thus, the neighbouring sites of m6A and Hu antigen R (HuR), which is supposed to bind ARE to increase the stability of mRNA, lead to weak HuR function and mRNA instability [52]. However, ELAV1/HuR, a potential m6A-binding protein, can stabilize transcripts with the cooperation of the ARE domain [53]. Subsequently, it was reported that the stability of mRNA was decreased slightly in cells lacking ALKBH5 [11].
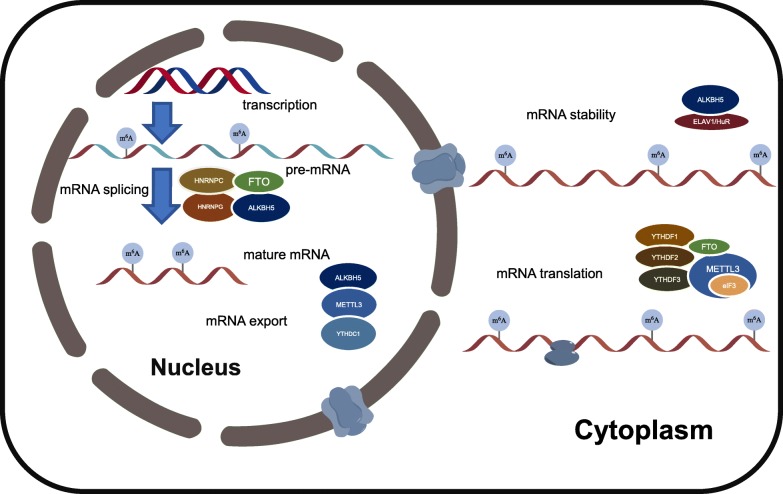
The YTH domain family of proteins has a conserved m6A-binding pocket so that these proteins can tightly bind to m6A in a consensus sequence and directly transcribe the molecule [12, 26, 34–38]. Specifically, YTHDF2 accelerates mRNA decay by transferring RNA from the translatable pool to processing bodies [12]. Under heat shock conditions, dysfunction of FTO in 5’UTRs, which is regulated by YTHDF2, contributes to the promotion of cap-independent translation [28]. Moreover, YTHDF1 can increase the efficiency of translation by binding m6A [37]. Subsequently, YTHDF3 can regulate translation by both interacting with ribosomal proteins with bound YTHDF1 and by decaying the translation-related mRNA region with bound YTHDF2 [54, 55]. However, METTL3 can regulate translation flexibly because it can either recruit eIF3 to the initiation complex directly to increase translation or can inhibit translation efficiency [56, 57]. The translation efficiency is increased when METTL3 is knocked out in mouse embryonic stem cells (mESCs) and embryoid bodies (EBs) [57] (Fig. 3).
Fig. 3.
m6A RNA modification regulates physiological processes in cell. m6A RNA modification in mRNA plays an essential role in cellular processes, including mRNA splicing, mRNA export, mRNA stability and mRNA translation. Both readers (HNRNPC and HNRNPG) and erasers (FTO and ALKBH5) can modulate the splicing of mRNA. After splicing and combination, pre-mRNA evolves into mature mRNA. Regulated by ALKBH5, METTL3 and YTHDC1, mature mRNA is exported from the nucleus to the cytoplasm. Once exported to the cytoplasm, both ALKBH5 and ELAV1/HuR can maintain mRNA stability. Finally, numerous enzymes contribute to the process of translation. YTHDF1, YTHDF2, YTHDF3, FTO and METTL3 together with eIF3 can regulate translation with different mechanisms individually
m1A RNA modification
Introduction of m1A RNA modification
m6A has been reported to occur in DNA from a minor cluster of microorganisms and in RNA from an extensive range of organisms, and additionally, m1A was identified in the 1960s [58]. Rather than accumulating in mRNA, m1A is predominant in tRNA and rRNA, but we recently determined that it also exists in mRNA [59, 60]. However, m1A-containing mRNA is 10 times less common than m6A-containing mRNA [61, 62]. In tRNA and rRNA, m1A conserves the tertiary structure and affects translation [63, 64]. In mRNA, m1A has been discovered in every mRNA segment, including the coding sequence (CDS), 5’UTR and 3’UTR, although it is mostly found in the highly structured 5’UTR [62]. As a result, the location of the m1A methylated atom determines the function and mechanism of this kind of modification.
Because the distribution of m1A is imbalanced, the large number of m1A modifications on tRNA results in more tRNA m1A MTases than writers on mRNA. However, TRMT6/61A recognized a T-loop-like structure with a GUUCRA tRNA-like motif in mRNAs and decorated it with the m1A modification, TRMT61B installed m1A in mt-mRNA transcripts, and TRMT10C methylated the 1374 position of ND5 mt-mRNA [65, 66]. All of these phenomena contribute to tRNA m1A MTases and can function as mRNA writers. By binding to m1A-bearing RNA, YTHDF1, YTHDF2, YTHDF3 and YTHDC1 act as readers [67]. Subsequently, similar to ALKBH5 functioning as an eraser for m6A, ALKBH1 and ALKBH3 were able to demethylate m1A mRNA modifications [62, 68].
Regulatory role of the m1A RNA modification in molecular functions
It has been reported that m1A methylation occurs in highly structured or GC-rich regions of 5’UTRs (which is also the most frequent location) and may modify the predicted secondary structure, which hints at the potential of m1A to alter mRNA structural stability [61, 62].Moreover, m1A methylation can not only increase translation by decreasing the binding of the releasing factor but also prevent effective translation of m1A-containing CDS in mt-mRNA [26, 65]. Ultimately, it has been reported that the protein level is higher when a transcript carries the m1A modification around the initiation codon [69].
m5C RNA modification
Introduction of the m5C RNA modification
m5C is a long-standing DNA modification that is essential for gene expression and epigenetic regulation [70, 71]. However, it can also be found in RNA. Although the m5C RNA modification can appear in both coding and non-coding regions, it has been reported to accumulate in the UTRs of mRNA and especially prefers to be located in GC-rich regions [72]. Since a number of studies have investigated the function of m5C in specific mRNAs, we concluded that m5C modifications in different locations (5’UTRs, 3’UTRs, coding regions) exert different transcriptional regulation activities [73].
It was revealed that m5C RNA modifications are catalysed by the NOL1/NOP2/SUN domain (NSUN) family of proteins (NSUN1, NSUN2, NSUN3, NSUN4, NSUN5, NSUN6 and NSUN7) as well as the DNA methyltransferase (DNMT) homologue DNMT2 [74–76]. However, among such diversified writers, only NSUN2 can install m5C on mRNA because rest of these proteins are writers of tRNAs and rRNAs. Subsequently, Aly/REF export factor (ALYREF), a specific mRNA m5C-binding protein that can read modifications, was identified as a reader of m5C [77]. According to liquid chromatography-tandem mass spectrometry analysis, YBX1 was defined as the other m5C reader that can maintain the stability of target mRNA [78]. Knowledge is limited about the protein factors responsible for removing modifications (Table 1).
Table 1.
Writers, readers and erasers of the predominant mRNA modifications
| RNA modification | Writers | Readers | Erasers |
|---|---|---|---|
| m6A | METTL3; METTL14; WTAP; RBM15; ZC3H13 | YTH domain family of proteins (YTHDF1, YTHDF2, YTHDF3, YTHDC1 and YTHDC2); HNRNP (HNRNPA2B1 and HNRNPC); eIF3; IGF2BP (IGF2BP1, IGF2BP2, and IGF2BP3); FMR1; LRPPRC | FTO; ALKBH5 |
| m1A | TRMT6/61A; TRMT61B; TRMT10C | YTHDF1; YTHDF2; YTHDF3; YTHDC1 | ALKBH1; ALKBH3 |
| m5C | NSUN2; DNMT2 | ALYREF; YBX1 | N.A. |
Regulatory role of the m5C RNA modification in molecular functions
ALYREF, the reader of m5C, can adjust the export of transcripts by recognizing a unique RNA-binding motif [77]. Subsequently, NSUN2 adds m5C to both p27 mRNA at cytosine C64 in the 5’UTR and p21 mRNA in the 3’UTR [79, 80]. Deleting NSUN2 in human diploid fibroblasts (HDFs) can induce the elevation of p27, and overexpressing NSUN2 results in contrasting outcomes [79]. These results suggest that the m5C catalysed by NSUN2 in the 5’UTRs can limit the translation of p27. However, the m5C modifications added by NSUN2 to the 3’UTRs of p21 mRNA coordinate with the m6A modifications added by METTL3/METTL14 together to enhance the expression of p21 [80]. With regard to m5C modification in mRNA coding regions, it was revealed that in both bacterial whole-cell extracts and HeLa cell extracts, m5C could diminish translation significantly [27, 81]. Moreover, we demonstrated that when the m5C modification was present on interleukin-17A (IL-17A) mRNA, this modification could promote the translation of IL-17A [82]. The results of the above investigations revealed that the m5C RNA modification affects the expression of proteins by regulating both translation efficiency and transcript export (Table 2).
Table 2.
mRNA modifications regulate the physiological process from transcription to translation
| Modifications | Process | Enzymes involved | Description | Ref | |
|---|---|---|---|---|---|
| m6A RNA modification | mRNA splicing | HNRNPC | HNRNPC modulates the splicing of mRNAs by changing RNA structure and regulating the combination of RNA and reader | [10] | |
| HNRNPG | HNRNPG cooperates with modified pre-mRNA and the phosphorylated C-terminal domain of RNA polymerase II to regulate splicing | [47] | |||
| FTO | FTO prefers to bind to introns of nascent mRNA | [48] | |||
| ALKBH5 | ALKBH5 relates to splicing factors tightly according to the analysis of immunofluorescence | [11] | |||
| mRNA export | METTL3 | METTL3 regulates the export of mature mRNA by modulating clock genes Per2 and Arntl | [49] | ||
| YTHDC1 | YTHDC1 mediates the export of decorated mRNA by interacting with SRSF3 and regulating the combination of SRSF3 an NXF1 on RNA | [50] | |||
| ALKBH5 | Knockdown of ALKBH5 leads to acceleration in mRNA export | [11] | |||
| mRNA stability | ALKBH5 | The stability of mRNA was decreased slightly in RNA lacking ALKBH5 | [11] | ||
| N.A. | Neighbouring sites of m6A and HuR weaken the function of HuR and increase the instability of mRNA | [52] | |||
| N.A. | ELAV1/HuR, which is one of m6A-binding proteins and stabilizes transcripts with the cooperation of the ARE domain | [53] | |||
| mRNA translation | YTHDF2 | YTHDF2 regulates translation by transferring the bound RNA from the translatable pool to processing bodies to promote mRNA decay | [12] | ||
| YTHDF2 induces the dysfunction of FTO in the 5'UTRs and contribute to promoting cap-independent translation | [28] | ||||
| YTHDF1 | YTHDF1 increases the efficiency of translation by binding to m6A | [37] | |||
| YTHDF3 | YTHDF3 interacts with ribosomal proteins along with YTHDF1 to regulate translation | [54] | |||
| YTHDF3 decays of convinced translation related region in mRNA together with YTHDF2 | [55] | ||||
| METTL3 | When knocking out METTL3 in mESCs and Ebs, the translation efficiency is increased | [57] | |||
| METTL3 recruits eIF3 to the initiation complex directly and enhance translation level | [56] | ||||
| m1A RNA modification | mRNA stability | N.A. | m1A in highly structured or GC-rich regions of 5'UTRs alters mRNA structural stability by modifying the predicted secondary structure | [61, 62] | |
| mRNA translation | N.A. | m1A upregulated translation by depressing binding of releasing factor | [26] | ||
| N.A. | m1A prevents effective translation of CDS in mt-mRNA | [65] | |||
| N.A. | The protein level would be superior when the transcript was modified by m1A at/around the initiation codon | [69] | |||
| m5C RNA modification | mRNA export | ALYREF | ALYREF adjusts the export of transcripts by recognizing the unique RNA-binding motif | [77] | |
| mRNA translation | NSUN2 | Deleting NSUN2 in HDFs can induce the elevation of p27, and overexpressing NSUN2 induces the opposite outcome | [79] | ||
| m5C catalysed by NSUN2 in 3'UTRs of p21 mRNA coordinates with m6A methylated by METTL3/METTL14 together to enhance p21 expression | [80] | ||||
| N.A. | Translation diminishes significantly in both bacterial whole-cell extracts and HeLa cell extracts when m5C modifies the coding regions of mRNA | [27, 81] | |||
| N.A. | m5C found on IL-17A mRNA can promote the translation of IL-17A | [82] | |||
| Other | hm5C | mRNA translation | N.A. | hm5C associates with translation activation in Drosophila | [69] |
| Ψ | mRNA splicing | N.A. | Ψ, which is near the 3' splice site in the polypyrimidine tract, prevents pre-mRNA splicing by regulating U2AF | [83] | |
| mRNA stability | N.A. | The higher expression of heat shock-induced Pus7-dependent pseudouridylated transcripts in wild-type yeast than in Pus7-knockdown yeast indicates that Ψ has the capability to maintain stability of RNA | [84] | ||
| mRNA translation | N.A. | Compared to U modifications located at similar sequences, Ψ-containing mRNA indicates an increase in translation levels of approximately 25% | [84] | ||
| N.A. | Ψ doubles the expression of an unmodified transcript | [85] | |||
| N.A. | When a separate Ψ modifies the special position of codon "UUU", mRNA translation can be limited | [81] | |||
| I | mRNA structure | N.A. | I fastens pairs of nucleotides to influence the native secondary structure of mRNA | [86] | |
| mRNA translation | N.A. | Guanosine, adenosine and uracil are the products decoded from I by the translation machinery | [87] | ||
| U | Protein expression | N.A. | Protein level alterations accompany C-to-U editing of RNA | [88] | |
| 2'-O-Me | Viral RNA infection | N.A. | 2'-O-Me-modified viral RNA disrupts native host antiviral responses by escaping suppression mediated by IFIT | [89] | |
| mRNA translation | N.A. | 2'-O-Me modifies specific regions of mRNA that are translated to glutamate, lysine and glutamine, hinting that 2'-O-Me has the potential to affect translation efficiency | [90] | ||
Other RNA modifications
hm5C
m5C can be oxidized into hm5C via the function of the Tet-family enzymes [91–93]. Moreover, hMeRIP-seq showed that Tet-family enzymes prefer to oxidize m5C modifications in coding regions; these results indicate that hm5C is highly likely to be located in the introns and exons of coding transcripts. However, in contrast to m5C methylation in the coding regions of mRNA, which plays a negative role in translation, hm5C tends to associate with translation activation in Drosophila [69].
Ψ
As hm5C is analogous to the oxidization of m5C, Ψ is produced by the isomerization of U. Ψ is the most abundant RNA modification and prefers to accumulate in tRNA and rRNA; however, it has also been reported to be present on mRNA and snRNA [94, 95]. Interestingly, the number of Ψ sites in mRNA ranges from 96 to 2084 in humans [84, 96–98].
However, by regulating U2 auxiliary factor (U2AF), Ψ, which is near the 3’ splice site in the polypyrimidine tract, prevents pre-mRNA splicing [83]. Expression of heat shock-induced Pus7-dependent pseudouridylated transcripts is higher in wild-type yeast than in Pus7-knockdown yeast and indicates that Ψ has the capability to maintain RNA stability [84]. Nevertheless, modifications were examined at similar sequences, and compared to U-containing mRNA, Ψ-containing mRNA experienced an increase in translation by approximately 25% [84]. Such modifications could double the expression of translation when compared to blank control transcript without any modification [85]. Although Ψ can promote translation and enhance the lifespan of RNA, it has negative effects on protein expression [85]. It has been reported that Ψ-containing mRNA exhibits a 30% decrease in protein expression. Specifically, bacterial mRNA translation can be limited when a separate Ψ modification is present at a given position of codon “UUU”, especially at the third codon position [81]. Moreover, both in vitro and in vivo, the Ψ modification might change the nonsense codons into sense codons [99, 100]. Above all, some of these investigations were conducted by Ψ in artificial mRNA, and the function of Ψ in biological mRNA has yet to be elucidated.
I and U
Catalysed by adenosine or cytidine deaminating enzymes, RNA editing is a kind of programmed alteration [101]. However, rather than permanent DNA mutations or reversible RNA modifications, RNA editing has its own limited lifespan and results in more permanent modification [102].
Adenosine-to-inosine RNA editing (A-to-I editing), also called I, is catalysed by adenosine deaminases acting on RNA (ADARs) [101, 103, 104]. Recently, 1741 I sites have been reported in CD regions of transcripts from RNA-seq data of different human tissues [105]. Moreover, it has been reported that ADAR1 and ADAR2 act only on double-stranded regions, which limits the areas of mRNA that I can modify [106]. I can fasten pairs of nucleotides; thus, this widespread modification in metazoan mRNA can influence the native secondary structure of mRNA [86]. An in vitro translation system was implemented to scientifically test the decoding of I, revealing that guanosine, adenosine and uracil are the products decoded from I by translation machinery [87].
However, with regard to cytidine-to-uridine RNA editing (C-to-U editing), also called U, it has been reported that U accumulates in 3’UTRs, and over 70 new sites have been discovered by transcriptome-wide research [88, 107]. Subsequently, after exploring several intestinal mRNAs, it was revealed that the protein level is altered by C-to-U editing of RNA [88]. However, there is little research on the relationship between the expression of transcripts and U. The biological influence of U has yet to be investigated.
2’-O-Me
Unlike how I and U are modifications on a base, 2’-O-Me is methylation of ribose at the 2’ position [59]. It was revealed that by escaping the suppression mediated by IFN-induced proteins with tetratricopeptide repeats (IFIT), 2’-O-Me-modifiedviral RNA disrupts native host antiviral responses [89]. Surprisingly, 2’-O-Me focuses on modifying specific regions of mRNA where the encoded amino acids are immobilized; these amino acids include glutamate, lysine and glutamine [90]. This phenomenon hints at the hypothesis that 2’-O-Me has the potential to affect translation efficiency, which has previously been demonstrated in bacterial mRNA [81].
Regulatory roles of RNA modifications in pathogenesis
Aberrant m6A RNA modifications in diseases
In acute myeloid leukaemia (AML), FTO decreases m6A abundance on ASB2 and RARA mRNA in several certain subtypes of AML, including t(11q23)/MLL rearrangements, t(15;17)/PML-RARA, FLT3-ITD, and/or NPM1 mutations [41, 108]. Moreover, by constraining YTHDF2-mediated decay, FTO decreases m6A frequency on MYC mRNA [109], METTL3 promotes translation of BCL2 and PTEN mRNA by upregulating the m6A levels and supports expression of SP1 by binding to the unique region with the help of the transcription factor CEBPZ [110, 111], and METTL14 enhances mRNA expression of MYB and MYC [112]. All pathological pathways contribute to carcinogenesis in AML. According to the datasets from The Cancer Genome Atlas, nearly 10.5% of AML patients carry copy number variations (CNVs) of ALKBH5, which predicts poor prognosis and p53 mutations [113].
In gastric cancer (GC), METTL3 can cause m6A to accumulate on HDGF mRNA, which indicates proliferation and poor prognosis and enhances the stability of zinc finger MYM-type containing 1 (ZMYM1) mRNA so that it accelerates epithelial-mesenchymal transition (EMT) and metastasis [114, 115]. However, METTL3 can also reduce m6A on SEC62 with the help of MiR-4429 [116]. In hepatocellular carcinoma (HCC), METTL3 enhances the degradation of m6A-containing SOCS2 mRNA together with YTHDF2 [117]. Additionally, YTHDF2 supresses ERK/MAPK signalling cascades and cell proliferation by destabilizing the EGFR mRNA [118]. Regarding clinical diagnosis, downregulated METTL14 is detected in HCC patients, and the level of expression in metastatic HCC is further decreased [119]. In pancreatic cancer, m6A and METTL3 protein and mRNA levels were much higher in tumour specimens than in para-cancerous specimens [120]. Meanwhile, upregulation of YTHDF2 destabilizes YAP mRNA by initiating the AKT/GSK3β/cyclin D1 pathway, which promotes proliferation and inhibits the migration of pancreatic cancer [121].
In lung cancer, METTL3 enhances the translation of EGFR and TAZ mRNA [56]. Furthermore, SUMOylated METTL3 promotes non-small-cell lung cancer (NSCLC) by diminishing the amount of m6A [122]. Moreover, YTHDF2 enhances the translation of 6-phosphogluconate dehydrogenase (6PGD) mRNA by binding to a given region in lung cancer cells [123]. Additionally, FTO is overexpressed in human NSCLC tissues and stimulates lung cancer by stabilizing and increasing the expression of ubiquitin-specific protease 7 (USP7) [124]. In lung squamous cell carcinoma (LUSC), overexpressed FTO accelerates oncogene MZF1 expression by diminishing m6A and stabilizing mRNA as well [125, 126].
For the nervous system, decreased levels of METTL3 or METTL14 determine the diminution of m6A on ADAM19 mRNA, which promotes protein expression [127, 128]. Conversely, increased levels of ALKBH5 lead to decreased levels of m6A on FOXM1 mRNA and enhance protein expression [129]. Consequently, a high level of ALKBH5 predicts poor prognosis [130]. However, both pathways can contribute to glioblastoma. Subsequently, overexpressed METTL3 recruits HuR to modified SOX2 mRNA and enhances radio-resistance. Playing an oncogenic role in glioblastoma, METTL3 hints at poor prognosis and a potential therapeutic strategy as well [131].
In prostate cancer, reduced YTHDF2 elevates m6A contents dramatically, which suppresses proliferation and migration [132]. In bladder cancer, increased METTL3 predicts poor survival because with the help of pri-miR221/222, upregulated METTL3 results in downregulated PTEN and tumorigenesis of cancer [133].
Aberrant m6A modification can also lead to carcinomas in the reproductive system. It has been reported that m6A on KLF4 and NANOG can be suppressed by the cooperation of ZNF217 and ALKBH5, especially in a HIF-dependent manner, so that it enhances the stability of mRNA and contributes to breast cancer in a hypoxic microenvironment [134, 135]. Increased METTL3 leads to enhancement of m6A on hepatitis B X-interacting protein (HBXIP) and proliferation of breast cancer stem cells (BCSCs) [136]. Moreover, elevated FTO leads to downregulated methylation and degradation of BNIP3. It is suggested that FTO enhances the colony formation and metastasis of breast cancer [137]; Nevertheless, in cervical squamous cell carcinoma (CSCC), high expression of FTO and low levels of β-catenin lead to chemoradiotherapy resistance, which hints that FTO is a potential target to increase the chemoradiotherapy sensitivity of CSCC [138]. In endometrial cancer, either mutated METTL14 or reduced METTL3 limits the expression of m6A. However, limited m6A activates the AKT signalling pathway and stimulates proliferation and tumorigenicity by decreasing the negative AKT regulator PHLPP2 and increasing the positive AKT regulator mTORC2 [139].
Besides the regular cancers with high incidence referenced above, aberrant m6A modifications also play roles in sensory organs. The fate of ocular melanoma can be modulated by m6A modifications. With the help of YTHDF1, the translation of methylated HINT2 mRNA, a tumour suppressor of ocular melanoma, was significantly accelerated, meaning m6A modification obviously inhibits the progression of ocular melanoma. Moreover, investigation of ocular melanoma samples indicated that decreased m6A levels were highly associated with poor prognosis [140].
Aberrant m1A RNA modification in diseases
Physiological functions lead to pathological impacts on diverse diseases. In ovarian and breast cancers, demethylation of m1A by ALKBH3 induces increased modified CSF-1 mRNA, which contains m1A in the 5’UTR near the translation initiation site. Hence, accumulated ALKBH3 means improved CSF-1 mRNA expression and invasion of cancer cells [141]. Subsequently, ALKBH3, considered the eraser of m1A, tightly correlates with the mTOR pathway in gastrointestinal cancer and is attributed to the limited expression of ErbB2 and AKT1S1 after ALKBH3 knockdown; the downstream genes of m1A are associated with cell proliferation according to Gene Ontology analysis [142]. Additionally, silencing of ALKBH3 arrests the cell cycle at the G1 phase and contributes to the progression, angiogenesis and invasion of urothelial carcinomas by modulating NADPH oxidase-2-reactive oxygen species (NOX-2-ROX) and TNF-like weak inducer of apoptosis (TWEAK)/Fibroblast growth factor-inducible 14 (Fn14)-VEGF signals [143]. As a classical chemical modification of mRNA, the pathological pathways of m1A need to be elucidated.
Aberrant m5C RNA modification in diseases
Since m5C bridges transcription and translation, we propose a hypothesis that m5C can also regulate the pathological mechanisms of various diseases. For instance, diminishing NSUN2 leads to decreased levels of translation and an increased tumour initiating population in skin cancer [144]. In breast cancer, NSUN2 is reported to be upregulated as well at the mRNA and protein levels [145]. For patients with urothelial carcinoma of the bladder (UCB), m5C-modified 3’UTR in HDGF mRNA can be recognized by YBX1 and activate the oncogene of UCB [78]. m5C can also be regarded as a cancer biomarker because the amount of m5C RNA modification is increased in circulating tumour cells from patients with lung cancer [146].
Aberrant hm5C, Ψ, I, U and 2’-O-Me RNA modifications in diseases
Although the amounts of hm5C, Ψ, I, U and 2’-O-Me RNA modifications on mRNA are much lower than the three predominant types of modifications, their roles do not change and are vital to human disease. First, Ψ can function as a biomarker for prostate cancer because certain nucleolar RNAs (H/ACA snoRNAs) and the dyskerin (DKC1) protein can upregulate the transformation of U to Ψ and contribute to the advancement to cancer [147]. Regarded as the gene encoding the Ψ synthase, the mutation of DKC1 causes downregulated Ψ and X-linked dyskeratosis congenita (X-DC) [148]. The risk for cancer development is higher in patients with X-DC than those without gene mutation [149]. Besides, H/ACA snoRNAs are limited in acute leukaemia, lymphoma and multiple myeloma [150–152].
Subsequently, edited AZIN1 stimulates a serine to glycine (S/G) conversion in HCC and leads to proliferation and poor prognosis [153, 154]. In HCC and in cervical cancer, increased editing of BLCAP activates the AKT/mTOR signalling pathway or STAT3, which can increase cell proliferation and limit apoptosis [155–158]. In breast cancer, editing of DHFR transcripts at the 3’UTR by ADAR1 stabilizes the mRNA and enhances cell growth. Surprisingly, methotrexate, a chemotherapy agent, prevents cancer cell division by targeting DHFR. It is suggests that downregulated ADAR1 can contribute to methotrexate treatment [159]. In gastric cancer, ADAR2 edits the CDS of PODXL, which induces a histidine to arginine conversion. The relationship between reduced ADAR2 and increased malignancy hints that transcript editing is essential to prevent cancer progression [160]. Additionally, adenosine deaminase RNA-specific B1 (ADARB1), a special type of ADAR, is expressed at low levels in H358 and A549 lung adenocarcinoma (LUAD) cells, which suggests that I might be a potential target in diagnostic and prognostic progression for patients with LUAD [161].
Finally, uridine phosphorylase 1 (UPP1) is another enzyme that can reversibly catalyse the phosphorolysis of uridine to uracil [162, 163]. It has been reported that expression of UPP1 significantly depends on lymph node metastasis and tumour stage and size in patients with thyroid carcinoma [164] (Table 3, Fig. 4).
Table 3.
Aberrant mRNA modifications in diseases
| Modification | Disease | Enzyme | Target | Description | Ref |
|---|---|---|---|---|---|
| m6A | AML | FTO | ASB2/ RARA | FTO decreases m6A abundance on ASB2 and RARA mRNA in certain subtypes of AML and diminishes the amount of protein | [41, 108] |
| MYC | FTO decreases m6A frequency on MYC mRNA by limiting YTHDF2-mediated RNA decay | [109] | |||
| METTL3 | BCL2/ PTEN | METTL3 promotes the translation of BCL2 and PTEN mRNA by upregulating m6A levels | [110] | ||
| SP1 | METTL3 supports the expression of SP1 by binding to the unique region with the help of the transcription factor CEBPZ | [111] | |||
| METTL 14 | MYB/ MYC | METTL14 enhances the expression of MYB and MYC mRNA in AML | [112] | ||
| ALKBH5 | N.A. | Approximately 10.5% of AML patients carry CNVs of ALKBH5, which predicts poor prognosis and p53 mutations | [113] | ||
| Gastric cancer | METTL3 | HDGF | METTL3 causes m6A to accumulate on HDGF mRNA, which indicates proliferation and poor prognosis of gastric cancer | [114] | |
| ZMYM1 | METTL3 enhances the stability of ZMYM1 mRNA to accelerate EMT and metastasis | [115] | |||
| SEC62 | METTL3 reduces m6A on SEC62 with the help with MiR-4429 | [116] | |||
| Hepatic carcinoma | METTL3 | SOCS2 | METTL3 works with YTHDF2 together to enhance the degradation of SOCS2 m6A-containing mRNA, which leads to HCC | [117] | |
| YTHDF2 | EGFR | YTHDF2 suppresses ERK/MAPK signalling cascades and cell proliferation via destabilizing the EGFR mRNA | [118] | ||
| METTL14 | N.A. | The expression of METTL14 is decreased in HCC, especially in metastatic HCC | [119] | ||
| Pancreatic cancer | METTL3 | N.A. | METTL3 protein, m6A abundance and mRNA levels are much higher in tumour specimens than in para-cancerous specimens | [120] | |
| YTHDF2 | YAP | Increased YTHDF2 promotes proliferation and suppresses migration of pancreatic cancer by destabilizing YAP mRNA | [121] | ||
| Lung cancer | METTL3 | EGFR/ TAZ | METTL3 enhances the translation of EGFR and TAZ mRNA in lung cancer | [56] | |
| SUMOylated METTL3 | N.A. | SUMOylated METTL3 promotes NSCLC by diminishing the amount of m6A | [122] | ||
| YTHDF2 | 6PGD | YTHDF2 enhances 6PGD mRNA translation by binding to m6A sites uniquely in lung cancer cells | [123] | ||
| FTO | USP7 | FTO stabilizes and increases the expression of USP7 by reducing m6A content | [124] | ||
| FTO | MZF1 | Overexpressed FTO accelerates oncogene MZF1 expression by diminishing m6A and stabilizing MZF1 in LUSC | [125, 126] | ||
| Glioblastoma | METTL3/ METTL14 | ADAM19 | Decreased METTL3 or METTL14 determines the diminution of m6A on ADAM19 mRNA, which promotes the expression of protein and contributes to glioblastoma | [127, 128] | |
| ALKBH5 | FOXM1 | Increased levels of ALKBH5 lead to decreased levels of m6A on FOXM1 mRNA and enhance protein translation, which predicts poor prognosis | [129, 130] | ||
| METTL3 | SOX2 | Elevated METTL3 stabilizes SOX2 mRNA and enhances radio-resistance of glioblastoma | [131] | ||
| Prostate cancer | YTHDF2 | N.A. | Downregulated YTHDF2 suppresses the proliferation and migration of prostate cancer by elevating m6A contents | [132] | |
| Bladder cancer | METTL3 | PTEN | With the help of pri-miR221/222, upregulated METTL3 leads to downregulated PTEN and tumorigenesis of cancer | [133] | |
| Breast cancer | ALKBH5 | KLF4/ NANOG | m6A on KLF4 and NANOG can be suppressed by the cooperation of ZNF17 and ALKBH5 to promote protein expression and contribute to breast cancer | [134, 135] | |
| METTL3 | HBXIP | Enhanced levels of m6A on HBXIP are attributed to increased METTL3 and promote the proliferation of breast cancer stem cells | [136] | ||
| FTO | BNIP3 | Elevated FTO leads to decreased expression of BNIP3 and metastasis of breast cancer | [137] | ||
| Cervical cancer | FTO | β-catenin | High expression of FTO and low levels of β-catenin lead to chemoradiotherapy resistance in cervical squamous cell carcinoma | [138] | |
| Endometrial cancer | METTL14/METTL3 | N.A. | Either mutated METTL14 or reduced METTL3 activates the AKT signalling pathway and stimulates proliferation and tumorigenicity by limiting the expression of m6A | [139] | |
| Ocular melanoma | YTHDF1 | HINT2 | YTHDF1 promotes the translation of methylated HINT2 mRNA and inhibits the progression of ocular melanoma | [140] | |
| m1A | Ovarian/Breast cancer | ALKBH3 | CSF-1 | Accumulated ALKBH3 indicates improved CSF-1 mRNA expression and invasion of cancer cells | [141] |
| Gastrointestinal cancer | ALKBH3 | ErbB2/ AKT1S1 | Aberrant m1A modifications regulate gastrointestinal cancer by modulating the mTOR pathway associated with cell proliferation | [142] | |
| Urothelial carcinoma | ALKBH3 | N.A. | ALKBH3 promotes the progression, angiogenesis and invasion of urothelial carcinomas via NOX-2-ROS and TWEAK/Fn14-VEGF signals | [143] | |
| m5C | Skin cancer | NSUN2 | N.A. | Inactivating NSUN2 prevents protein translation and stimulates the tumour-initiating population of skin cancer | [144] |
| Breast cancer | NSUN2 | N.A. | NSUN2 is reported to be upregulated at the mRNA and protein levels | [145] | |
| Urothelial carcinoma | YBX1 | HDGF | m5C modified 3'UTR in HDGF mRNA can be recognized by YBX1 and activate the advancement of UCB | [78] | |
| Lung cancer | N.A. | N.A. | M5C RNA modification is upregulated in circulating tumour cells from patients with lung cancer | [146] | |
| Ψ | Prostate cancer | DKC1 | N.A. | Certain nucleolar RNAs (H/ACA snoRNAs) and DKC1 that transfer U to Ψ contribute to the progression of cancer | [147] |
| Haematological malignancies | N.A. | N.A. | H/ACA snoRNAs are limited in acute leukaemia, lymphoma and multiple myeloma | [150–152] | |
| I | Hepatocellular carcinoma | ADAR1 | AZIN1 | Edited AZIN1 stimulates S/G conversion and induces proliferation and poor prognosis in hepatocellular carcinoma | [153, 154] |
| ADAR1 | BLCAP | Increased editing of BLCAP accelerates cell proliferation by activating the Akt/mTOR signalling pathway or STAT3 | [158] | ||
| Cervical cancer | ADAR1 | BLCAP | Increased editing of BLCAP accelerates cell proliferation by activating the Akt/mTOR signalling pathway or STAT3 | [157] | |
| Breast cancer | ADAR1 | DHFR | Editing of DHFR by ADAR1 stabilizes mRNA and accelerates cell growth | [159] | |
| Gastric cancer | ADAR2 | PODXL | Downregulated ADAR2 reduces the decoration on PODXL and increases the malignancy of gastric cancer | [160] | |
| Lung adenocarcinoma | ADARB1 | N.A. | ADARB1 has low expression in H358 and A549 lung adenocarcinoma cells | [161] | |
| U | Thyroid carcinoma | UPP1 | N.A. | It is reported that the expression of UPP1 significantly depends on lymph node metastasis, tumour stage and size | [164] |
Fig. 4.
Regulatory roles of RNA modifications in pathogenesis. Applying physiology to pathology, RNA modifications redefine the bridge between transcription and translation and regulate disease pathogenesis. In AML, METTL3 and METTL14 enhance the expression of m6A modifications as well as the BCL2, PTEN, SP1, MYB and MYC genes, which lead to tumour progression. Simultaneously, FTO decreases m6A abundance on ASB2 and RARA mRNA. In digestive system tumours, aberrant METTL3 leads to aberrant expression of HDGF, ZMYM1, SEC62 and SOCS2, which can regulate cancer cells in the stomach, liver and pancreas, respectively. In lung cancer, METTL3 enhances the translation of EGFR and TAZ, whereas SUMOylated METTL3 promotes NSCLC; aberrant YTHDF2 enhances the expression of 6PGD in lung cancer, and overexpressed FTO stabilizes and accelerates the expression of USF7 and MZF1 as well. In glioblastoma, METTL3, METTL14 and ALKBH5 promote the expression of ADAM19 and FOXM1 and predict poor prognosis. In prostate cancer, aberrant YTHDF2 suppresses proliferation and migration. In bladder cancer, METTL3 reduces the expression of PTEN and tumorigenesis of cancer. In the reproductive system, METTL3 and FTO contribute to the aberrant expression of KLF4, NANOG, HBXIP, BNIP3 and β-catenin, which induce proliferation of breast cancer and chemoradiotherapy resistance of cervical cancer separately. In sensory organs, YTHDF1 accelerates the translation of methylated HINT2 and inhibits the progression of ocular melanoma. Aberrant eraser ALKBH3 reduces m1A modifications, leads to aberrant expression of CSF-1, ErbB2 and AKT1S1, and induces the progression of ovarian cancer, breast cancer, gastrointestinal cancer and urothelial cancer. In UCB, YBX1 recognizes m5C-modified HDGF mRNA and leads to tumour advancement. Upregulated USUN2 is detected in breast cancer. Ultimately, aberrant ADAR1 edits AZIN1, BLCAP, and DHFR separately, which leads to hepatocellular carcinoma, cervical cancer and breast cancer. Additionally, together with Ψ, I and U, DKC1, ADAR1 and UPP1 can function as biomarkers to indicate prostate cancer progression, LUAD presentation and thyroid carcinoma status
Clinical prospects of RNA modifications
RNA modifications and enzyme complexes exhibit upregulated and downregulated levels of expression in cancers, which means RNA modifications can serve as biomarkers to diagnose diseases in a manner that is helpful and precise. For example, upregulated YTHDF2 is found in pancreatic cancer, increased m5C is detected in lung cancer and accumulated Ψ contributes to the advancement of prostate cancer [121, 146, 147]. However, other biomarkers need to be elucidated. Besides biomarkers to diagnose cancers, RNA modifications are also biomarkers to predict patient prognosis. Since they stimulate or inhibit the progression of cancer, RNA modifications have therapeutic potential. 3-deazaadenosine (DAA) interrupts METTL3/14 and inhibits the decoration of m6A by obstructing SAH hydrolase [165], SPI1 is considered a potential target for AML because of inhibition of METTL14 [112], and meclofenamic acid (MA), a non-steroidal anti-inflammatory drug, silences FTO by competing for binding sites [166]. Novel targets for treatment of cancer require further investigation.
Conclusion
In summary, chemical modifications in mRNA are vital for many processes of cell life, such as pre-mRNA splicing, nuclear export, transcript stability and translation initiation. Importantly, RNA modifications play a critical role in driving cell fate in cancer. The importance of the relationship between RNA modification and various diseases cannot be overly emphasized. In this review, we redefined the bridge between transcription and translation and applied it to physiological and pathological processes. To date, we have demonstrated 2 roles of mRNA modifications in transcription. Generally, one type is mRNA modifications that can change the structure of transcripts, and the other is mRNA modifications that can regulate transcription by joining hands with a complex of enzymes, such as METTL3 or NSUN2. Considering that modifications can regulate the fate of diverse diseases, such modifications have the potential to be utilized in targeted therapy. Surely, RNA modifications as well as the related diseases mentioned above are a fraction of those affecting human beings in nature. Thus, these modifications need to be elucidated in the following few years.
Acknowledgements
Not applicable
Abbreviations
- 2’-O-Me
Ribose-methylation
- 3’UTR
3’ untranslated region
- 5’UTR
5’ untranslated region
- 6PGD
6-phosphogluconate dehydrogenase
- Ψ
Pseudouridine
- ADAM19
A disintegrin and metallopeptidase domain 19
- ADAR
Adenosine deaminases acting on RNA
- ADARB1
Adenosine deaminase RNA-specific B1
- AKT
AKT serine/threonine kinase
- AKT1S1
AKT1 substrate 1
- ALKBH5
α-ketoglutarate-dependent dioxygenase alkB homolog 5
- ALYREF
Aly/REF export factor
- AML
Acute myeloid leukaemia
- Arntl
Aryl hydrocarbon receptor nuclear translocator-like
- ARE
AU-rich element
- ASB2
Ankyrin repeat and SOCS box-containing 2
- A-to-I editing
Adenosine-to-inosine RNA editing
- AZIN1
Antizyme inhibitor 1
- BCL2
B cell leukaemia
- BCSC
Breast cancer stem cell
- BLCAP
Bladder cancer-associated protein
- BNIP3
BCL2 interacting protein 3
- CDS
Coding sequence
- CEBPZ
CCAAT enhancer binding protein zeta
- CNV
Copy number variation
- CPE
Cytoplasmic polyadenylation element
- CSCC
Cervical squamous cell carcinoma
- CSF-1
Colony stimulating factor 1
- C-to-U editing
Cytidine-to-uridine RNA editing
- DAA
3-deazaadenosine
- DHFR
Dihydrofolate reductase
- DKC1
Dyskerin pseudouridine synthase 1
- DNMT
DNA methyltransferase homologue
- EB
Embryoid body
- EGFR
Epidermal growth factor receptor
- eIF3
Eukaryotic initiation factor 3
- ELAV1
ELAV-like RNA-binding protein 1
- EMT
Epithelial-mesenchymal transition
- Fasn
Fatty acid synthase
- FLT3
Fms-related tyrosine kinase 3
- FMR1
Fragile X mental retardation 1
- Fn14
Fibroblast growth factor-inducible 14
- FOXM1
Forkhead box M1
- FTO
Fat mass and obesity-associated protein
- GC
Gastric cancer
- HBXIP
Hepatitis B X-interacting protein
- HCC
Hepatocellular carcinoma
- HDF
Human diploid fibroblast
- HDGF
Hepatitis B X-interacting protein
- HIF
Hypoxia inducible factor
- hm5C
5-hydroxymethylcytosine
- HNRNP
Heterogeneous nuclear ribonucleoprotein
- HuR
Hu antigen R
- IFIT
IFN-induced proteins with tetratricopeptide repeats
- IGF2BP
Insulin-like growth factor 2 mRNA-binding protein
- IL-17A
Interleukin-17A
- IRE
Iron-responsive element
- KLF4
Kruppel like factor 4
- LRPPRC
Leucine-rich pentatricopeptide repeat-containing
- LUAD
Lung adenocarcinoma
- LUSC
Lung squamous cell carcinoma
- m1A
N1-methyladenosine
- m6A
N6-methyladenosine
- m5C
5-methylcytosine
- MA
Meclofenamic acid
- MAPK
Mitogen-activated protein kinase
- mESC
Mouse embryonic stem cell
- METTL3
Methyltransferase-like 3
- METTL14
Methyltransferase-like14
- MiR-4429
MicroRNA 4429
- MLL
Mixed lineage leukaemia
- mRNA
Message RNA
- mt-mRNA
Mitochondrial mRNA
- mTOR
Mammalian target of rapamycin
- MYB
Myeloblastosis oncogene
- MYC
Myelocytomatosis oncogene
- MZF1
Myeloid zinc finger 1
- NANOG
Nanog homeobox
- ND5
-
NADH
ubiquinone oxidoreductase core subunit 5
- NOX-2-ROX
NADPH oxidase-2-reactive oxygen species
- NPC
Neplanocin A
- NPM1
Nucleophosmin 1
- NSCLC
Non-small-cell lung cancer
- NSUN
NOL1/NOP2/SUN domain
- NXF1
Nuclear RNA export factor 1
- Per2
Period circadian regulator 2
- PHLPP2
PH domain and leucine rich repeat protein phosphatase 2
- PML
Promyelocytic leukaemia
- PODXL
Podocalyxin like
- PTEN
Phosphatase and tensin homologue
- Q
Queuosine
- RA
Rheumatoid arthritis
- RARA
Retinoic acid receptor alpha
- RBM15
RNA-binding motif protein 15
- rRNA
Ribosomal RNA
- SAH
S- adenosylhomocysteine
- SEC62
SEC62 homologue, preprotein translocation factor
- snRNA
Small nuclearRNA
- SOCS2
Suppressor of cytokine signalling 2
- SOX2
SRY-box transcription factor 2
- SP1
Sp1 transcription factor
- SPI1
Spi-1 proto-oncogene
- SRSF3
Serine and arginine rich splicing factor 3
- STAT3
Signal transducer and activator of transcription 3
- SUMO
Small ubiquitin-like modifier
- T2DM
Type 2 diabetes mellitus
- TAZ
Tafazzin
- TRMT
tRNA methyltransferase
- tRNA
Transfer RNA
- TWEAK
TNF-like weak inducer of apoptosis
- U2AF
U2 auxiliary factor
- UCB
Urothelial carcinoma of the bladder
- UPP1
Uridine phosphorylase 1
- USP7
ubiquitin-specific protease 7
- VEGF
Vascular endothelial growth factor
- WTAP
Wilms tumour 1-associated protein
- X-DC
X-linked dyskeratosis congenita
- YAP
Yes-associated protein
- YBX1
Y-Box binding protein 1
- YTH
YT521-B homology
- ZC3H13
Zinc finger CCCH-type containing 13
- ZMYM1
Zinc finger MYM-type containing 1
- ZNF217
Zinc finger protein 217
Authors’ contributions
RJ and XF provided direction and guidance throughout the preparation of this manuscript. HS collected and interpreted studies and was a major contributor to the writing and editing of the manuscript. PC reviewed and made significant revisions to the manuscript. All authors read and approved the final manuscript.
Funding
The authors thank the National Natural Science Foundation of China (grants 81570884 and 81770961), the SMC ChenXing Yong Scholar Program (2016, Class A) and the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (20152223), the Innovation Fund for Translational Medicine (15ZH1005), and the Ph.D. Programs Foundation of Shanghai JiaoTong University School of Medicine (BXJ201834). The funders had no role in the design of the study; in the collection, analysis and interpretation of the data; or in the writing of the manuscript.
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hanhan Shi and Peiwei Chai contributed equally to this work.
Contributor Information
Renbing Jia, Email: renbingjia@sjtu.edu.cn.
Xianqun Fan, Email: fanxq@sjtu.edu.cn.
References
- 1.Davis FF, Allen FW. Ribonucleic acids from yeast which contain a fifth nucleotide. J Biol Chem. 1957;227:907–915. [PubMed] [Google Scholar]
- 2.Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5' terminus of HeLa cell messenger RNA. Cell. 1975;4:379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- 3.Cohn WE. Pseudouridine, a carbon-carbon linked ribonucleoside in ribonucleic acids: isolation, structure, and chemical characteristics. J Biol Chem. 1960;235:1488–1498. [PubMed] [Google Scholar]
- 4.Dubin DT, Taylor RH. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975;2:1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juhling F, Morl M, Hartmann RK, Sprinzl M, Stadler PF, Putz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vagbo CB, Shi Y, Wang WL, Song SH, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjork GR, Hagervall TG. Transfer RNA modification: presence, synthesis, and function. EcoSal Plus. 2014;6. [DOI] [PubMed]
- 14.Rezgui VA, Tyagi K, Ranjan N, Konevega AL, Mittelstaet J, Rodnina MV, Peter M, Pedrioli PG. tRNA tKUUU, tQUUG, and tEUUC wobble position modifications fine-tune protein translation by promoting ribosome A-site binding. Proc Natl Acad Sci U S A. 2013;110:12289–12294. doi: 10.1073/pnas.1300781110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tukenmez H, Xu H, Esberg A, Bystrom AS. The role of wobble uridine modifications in +1 translational frameshifting in eukaryotes. Nucleic Acids Res. 2015;43:9489–9499. doi: 10.1093/nar/gkv832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hori H. Methylated nucleosides in tRNA and tRNA methyltransferases. Front Genet. 2014;5:144. doi: 10.3389/fgene.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustilo EM, Vendeix FA, Agris PF. tRNA’s modifications bring order to gene expression. Curr Opin Microbiol. 2008;11:134–140. doi: 10.1016/j.mib.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaunay S, Frye M. RNA modifications regulating cell fate in cancer. Nat Cell Biol. 2019;21:552–559. doi: 10.1038/s41556-019-0319-0. [DOI] [PubMed] [Google Scholar]
- 19.Krug RM, Morgan MA, Shatkin AJ. Influenza viral mRNA contains internal N6-methyladenosine and 5'-terminal 7-methylguanosine in cap structures. J Virol. 1976;20:45–53. doi: 10.1128/JVI.20.1.45-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sommer S, Salditt-Georgieff M, Bachenheimer S, Darnell JE, Furuichi Y, Morgan M, Shatkin AJ. The methylation of adenovirus-specific nuclear and cytoplasmic RNA. Nucleic Acids Res. 1976;3:749–765. doi: 10.1093/nar/3.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy TD, Lane BG. Wheat embryo ribonucleates. XIII. Methyl-substituted nucleoside constituents and 5'-terminal dinucleotide sequences in bulk poly(AR)-rich RNA from imbibing wheat embryos. Can J Biochem. 1979;57:927–931. doi: 10.1139/o79-112. [DOI] [PubMed] [Google Scholar]
- 22.Deng X, Chen K, Luo GZ, Weng X, Ji Q, Zhou T, He C. Widespread occurrence of N6-methyladenosine in bacterial mRNA. Nucleic Acids Res. 2015;43:6557–6567. doi: 10.1093/nar/gkv596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams JM, Cory S. Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature. 1975;255:28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- 24.Kane SE, Beemon K. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol Cell Biol. 1985;5:2298–2306. doi: 10.1128/MCB.5.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 27.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 5' UTR m(6)A promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature. 2015;526:591–594. doi: 10.1038/nature15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5' sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visvanathan A, Somasundaram K. mRNA traffic control reviewed: N6-Methyladenosine (m(6) A) takes the driver’s seat. Bioessays. 2018;40. [DOI] [PubMed]
- 33.Wen J, Lv R, Ma H, Shen H, He C, Wang J, Jiao F, Liu H, Yang P, Tan L, et al. Zc3h13 regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69:1028–1038. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo S, Tong L. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc Natl Acad Sci U S A. 2014;111:13834–13839. doi: 10.1073/pnas.1412742111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y, Lu Z, He C, Min J. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10:927–929. doi: 10.1038/nchembio.1654. [DOI] [PubMed] [Google Scholar]
- 36.Zhu T, Roundtree IA, Wang P, Wang X, Wang L, Sun C, Tian Y, Li J, He C, Xu Y. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res. 2014;24:1493–1496. doi: 10.1038/cr.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, Mesquita A, Liu C, Yuan CL, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arguello AE, DeLiberto AN, Kleiner RE. RNA chemical proteomics reveals the N(6)-Methyladenosine (m(6)A)-regulated protein-RNA interactome. J Am Chem Soc. 2017;139:17249–17252. doi: 10.1021/jacs.7b09213. [DOI] [PubMed] [Google Scholar]
- 41.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoltzfus CM, Dane RW. Accumulation of spliced avian retrovirus mRNA is inhibited in S-adenosylmethionine-depleted chicken embryo fibroblasts. J Virol. 1982;42:918–931. doi: 10.1128/JVI.42.3.918-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finkel D, Groner Y. Methylations of adenosine residues (m6A) in pre-mRNA are important for formation of late simian virus 40 mRNAs. Virology. 1983;131:409–425. doi: 10.1016/0042-6822(83)90508-1. [DOI] [PubMed] [Google Scholar]
- 45.Caboche M, Bachellerie JP. RNA methylation and control of eukaryotic RNA biosynthesis. Effects of cycloleucine, a specific inhibitor of methylation, on ribosomal RNA maturation. Eur J Biochem. 1977;74:19–29. doi: 10.1111/j.1432-1033.1977.tb11362.x. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi M, Yaginuma S, Yoshioka H, Nakatsu K. Studies on neplanocin A, new antitumor antibiotic. II. Structure determination. J Antibiot (Tokyo) 1981;34:675–680. doi: 10.7164/antibiotics.34.675. [DOI] [PubMed] [Google Scholar]
- 47.Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Z, Pan JN, He C, Parisien M, Pan T. Regulation of Co-transcriptional Pre-mRNA splicing by m(6)A through the low-complexity protein hnRNPG. Mol Cell. 2019;76:70–81. doi: 10.1016/j.molcel.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, Vanacova S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3'-end processing. Nucleic Acids Res. 2017;45:11356–11370. doi: 10.1093/nar/gkx778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I, Okamura H. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 50.Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, et al. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 2017;6. [DOI] [PMC free article] [PubMed]
- 51.Wickens M, Bernstein DS, Kimble J, Parker R. A PUF family portrait: 3'UTR regulation as a way of life. Trends Genet. 2002;18:150–157. doi: 10.1016/S0168-9525(01)02616-6. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li A, Chen YS, Ping XL, Yang X, Xiao W, Yang Y, Sun HY, Zhu Q, Baidya P, Wang X, et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27:315–328. doi: 10.1038/cr.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin S, Choe J, Du P, Triboulet R, Gregory RI. The m(6)A methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell. 2016;62:335–345. doi: 10.1016/j.molcel.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS, et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 58.Dunn DB. The occurrence of 1-methyladenine in ribonucleic acid. Biochim Biophys Acta. 1961;46:198–200. doi: 10.1016/0006-3002(61)90668-0. [DOI] [PubMed] [Google Scholar]
- 59.Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al. MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozanick S, Krecic A, Andersland J, Anderson JT. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. RNA. 2005;11:1281–1290. doi: 10.1261/rna.5040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dominissini D, Nachtergaele S, Moshitch-Moshkovitz S, Peer E, Kol N, Ben-Haim MS, Dai Q, Di Segni A, Salmon-Divon M, Clark WC, et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Xiong X, Wang K, Wang L, Shu X, Ma S, Yi C. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol. 2016;12:311–316. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 63.El Yacoubi B, Bailly M, de Crecy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- 64.Sharma S, Watzinger P, Kotter P, Entian KD. Identification of a novel methyltransferase, Bmt2, responsible for the N-1-methyl-adenosine base modification of 25S rRNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2013;41:5428–5443. doi: 10.1093/nar/gkt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Xiong X, Zhang M, Wang K, Chen Y, Zhou J, Mao Y, Lv J, Yi D, Chen XW, et al. Base-resolution mapping reveals distinct m(1)A methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell. 2017;68:993–1005. doi: 10.1016/j.molcel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, Bar-Yaacov D, Erlacher M, Rossmanith W, Stern-Ginossar N, Schwartz S. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–255. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 67.Dai X, Wang T, Gonzalez G, Wang Y. Identification of YTH domain-containing proteins as the readers for N1-methyladenosine in RNA. Anal Chem. 2018;90:6380–6384. doi: 10.1021/acs.analchem.8b01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu F, Clark W, Luo G, Wang X, Fu Y, Wei J, Wang X, Hao Z, Dai Q, Zheng G, et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell. 2016;167:1897. doi: 10.1016/j.cell.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 69.Delatte B, Wang F, Ngoc LV, Collignon E, Bonvin E, Deplus R, Calonne E, Hassabi B, Putmans P, Awe S, et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science. 2016;351:282–285. doi: 10.1126/science.aac5253. [DOI] [PubMed] [Google Scholar]
- 70.Doerfler W. DNA methylation--a regulatory signal in eukaryotic gene expression. J Gen Virol. 1981;57:1–20. doi: 10.1099/0022-1317-57-1-1. [DOI] [PubMed] [Google Scholar]
- 71.Adams RL, Burdon RH. DNA methylation in eukaryotes. CRC Crit Rev Biochem. 1982;13:349–384. doi: 10.3109/10409238209108714. [DOI] [PubMed] [Google Scholar]
- 72.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xing J, Yi J, Cai X, Tang H, Liu Z, Zhang X, Martindale JL, Yang X, Jiang B, Gorospe M, Wang W. NSun2 promotes cell growth via elevating cyclin-dependent kinase 1 translation. Mol Cell Biol. 2015;35:4043–4052. doi: 10.1128/MCB.00742-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reid R, Greene PJ, Santi DV. Exposition of a family of RNA m(5)C methyltransferases from searching genomic and proteomic sequences. Nucleic Acids Res. 1999;27:3138–3145. doi: 10.1093/nar/27.15.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, Zhang X, Golic KG, Jacobsen SE, Bestor TH. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 76.Schaefer M, Pollex T, Hanna K, Tuorto F, Meusburger M, Helm M, Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–1595. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang X, Yang Y, Sun BF, Chen YS, Xu JW, Lai WY, Li A, Wang X, Bhattarai DP, Xiao W, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X, Li A, Sun BF, Yang Y, Han YN, Yuan X, Chen RX, Wei WS, Liu Y, Gao CC, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat Cell Biol. 2019;21:978–990. doi: 10.1038/s41556-019-0361-y. [DOI] [PubMed] [Google Scholar]
- 79.Tang H, Fan X, Xing J, Liu Z, Jiang B, Dou Y, Gorospe M, Wang W. NSun2 delays replicative senescence by repressing p27 (KIP1) translation and elevating CDK1 translation. Aging (Albany NY) 2015;7:1143–1158. doi: 10.18632/aging.100860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Q, Li X, Tang H, Jiang B, Dou Y, Gorospe M, Wang W. NSUN2-mediated m5C methylation and METTL3/METTL14-mediated m6A methylation cooperatively enhance p21 translation. J Cell Biochem. 2017;118:2587–2598. doi: 10.1002/jcb.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoernes TP, Clementi N, Faserl K, Glasner H, Breuker K, Lindner H, Huttenhofer A, Erlacher MD. Nucleotide modifications within bacterial messenger RNAs regulate their translation and are able to rewire the genetic code. Nucleic Acids Res. 2016;44:852–862. doi: 10.1093/nar/gkv1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo Y, Feng J, Xu Q, Wang W, Wang X. NSun2 deficiency protects endothelium from inflammation via mRNA methylation of ICAM-1. Circ Res. 2016;118:944–956. doi: 10.1161/CIRCRESAHA.115.307674. [DOI] [PubMed] [Google Scholar]
- 83.Chen C, Zhao X, Kierzek R, Yu YT. A flexible RNA backbone within the polypyrimidine tract is required for U2AF65 binding and pre-mRNA splicing in vivo. Mol Cell Biol. 2010;30:4108–4119. doi: 10.1128/MCB.00531-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, Leon-Ricardo BX, Engreitz JM, Guttman M, Satija R, Lander ES, et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kariko K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nigita G, Veneziano D, Ferro A. A-to-I RNA editing: current knowledge sources and computational approaches with special emphasis on non-coding RNA molecules. Front Bioeng Biotechnol. 2015;3:37. doi: 10.3389/fbioe.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Licht K, Hartl M, Amman F, Anrather D, Janisiw MP, Jantsch MF. Inosine induces context-dependent recoding and translational stalling. Nucleic Acids Res. 2019;47:3–14. doi: 10.1093/nar/gky1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blanc V, Park E, Schaefer S, Miller M, Lin Y, Kennedy S, Billing AM, Ben Hamidane H, Graumann J, Mortazavi A, et al. Genome-wide identification and functional analysis of Apobec-1-mediated C-to-U RNA editing in mouse small intestine and liver. Genome Biol. 2014;15:R79. doi: 10.1186/gb-2014-15-6-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin TY, Schneller S, Zust R, Dong H, et al. 2'-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dai Q, Moshitch-Moshkovitz S, Han D, Kol N, Amariglio N, Rechavi G, Dominissini D, He C. Nm-seq maps 2'-O-methylation sites in human mRNA with base precision. Nat Methods. 2017;14:695–698. doi: 10.1038/nmeth.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huber SM, van Delft P, Mendil L, Bachman M, Smollett K, Werner F, Miska EA, Balasubramanian S. Formation and abundance of 5-hydroxymethylcytosine in RNA. Chembiochem. 2015;16:752–755. doi: 10.1002/cbic.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu L, Guerrero CR, Zhong N, Amato NJ, Liu Y, Liu S, Cai Q, Ji D, Jin SG, Niedernhofer LJ, et al. Tet-mediated formation of 5-hydroxymethylcytosine in RNA. J Am Chem Soc. 2014;136:11582–11585. doi: 10.1021/ja505305z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang HY, Xiong J, Qi BL, Feng YQ, Yuan BF. The existence of 5-hydroxymethylcytosine and 5-formylcytosine in both DNA and RNA in mammals. Chem Commun (Camb) 2016;52:737–740. doi: 10.1039/C5CC07354E. [DOI] [PubMed] [Google Scholar]
- 94.Horowitz S, Horowitz A, Nilsen TW, Munns TW, Rottman FM. Mapping of N6-methyladenosine residues in bovine prolactin mRNA. Proc Natl Acad Sci U S A. 1984;81:5667–5671. doi: 10.1073/pnas.81.18.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kane SE, Beemon K. Inhibition of methylation at two internal N6-methyladenosine sites caused by GAC to GAU mutations. J Biol Chem. 1987;262:3422–3427. [PubMed] [Google Scholar]
- 96.Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143–146. doi: 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X, Zhu P, Ma S, Song J, Bai J, Sun F, Yi C. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol. 2015;11:592–597. doi: 10.1038/nchembio.1836. [DOI] [PubMed] [Google Scholar]
- 98.Lovejoy AF, Riordan DP, Brown PO. Transcriptome-wide mapping of pseudouridines: pseudouridine synthases modify specific mRNAs in S. cerevisiae. PLoS One. 2014;9:e110799. doi: 10.1371/journal.pone.0110799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernandez IS, Ng CL, Kelley AC, Wu G, Yu YT, Ramakrishnan V. Unusual base pairing during the decoding of a stop codon by the ribosome. Nature. 2013;500:107–110. doi: 10.1038/nature12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karijolich J, Yu YT. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature. 2011;474:395–398. doi: 10.1038/nature10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishikura K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat Rev Mol Cell Biol. 2016;17:83–96. doi: 10.1038/nrm.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Licht K, Jantsch MF. Rapid and dynamic transcriptome regulation by RNA editing and RNA modifications. J Cell Biol. 2016;213:15–22. doi: 10.1083/jcb.201511041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tajaddod M, Jantsch MF, Licht K. The dynamic epitranscriptome: A to I editing modulates genetic information. Chromosoma. 2016;125:51–63. doi: 10.1007/s00412-015-0526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Picardi E, Manzari C, Mastropasqua F, Aiello I, D'Erchia AM, Pesole G. Profiling RNA editing in human tissues: towards the inosinome Atlas. Sci Rep. 2015;5:14941. doi: 10.1038/srep14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lehmann KA, Bass BL. Double-stranded RNA adenosine deaminases ADAR1 and ADAR2 have overlapping specificities. Biochemistry. 2000;39:12875–12884. doi: 10.1021/bi001383g. [DOI] [PubMed] [Google Scholar]
- 107.Rosenberg BR, Hamilton CE, Mwangi MM, Dewell S, Papavasiliou FN. Transcriptome-wide sequencing reveals numerous APOBEC1 mRNA-editing targets in transcript 3' UTRs. Nat Struct Mol Biol. 2011;18:230–236. doi: 10.1038/nsmb.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, Huang H, Nachtergaele S, Dong L, Hu C, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer cell. 2017;31:127–141. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, Deng X, Wang Y, Weng X, Hu C, et al. R-2HG exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell. 2018;172:90–105. doi: 10.1016/j.cell.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M, et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–1376. doi: 10.1038/nm.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millan-Zambrano G, Robson SC, Aspris D, Migliori V, Bannister AJ, Han N, et al. Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature. 2017;552:126–131. doi: 10.1038/nature24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, Shi H, Skibbe J, Shen C, Hu C, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell. 2018;22:191–205. doi: 10.1016/j.stem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kwok CT, Marshall AD, Rasko JE, Wong JJ. Genetic alterations of m(6)A regulators predict poorer survival in acute myeloid leukemia. J Hematol Oncol. 2017;10:39. doi: 10.1186/s13045-017-0410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, Jiang Z, Zhang Y, Xu G, Zhang J, et al. METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2019. [DOI] [PubMed]
- 115.Yue B, Song C, Yang L, Cui R, Cheng X, Zhang Z, Zhao G. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer. 2019;18:142. doi: 10.1186/s12943-019-1065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He H, Wu W, Sun Z, Chai L. MiR-4429 prevented gastric cancer progression through targeting METTL3 to inhibit m(6)A-caused stabilization of SEC62. Biochem Biophys Res Commun. 2019;517:581–587. doi: 10.1016/j.bbrc.2019.07.058. [DOI] [PubMed] [Google Scholar]
- 117.Chen M, Wei L, Law CT, Tsang FH, Shen J, Cheng CL, Tsang LH, Ho DW, Chiu DK, Lee JM, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67:2254–2270. doi: 10.1002/hep.29683. [DOI] [PubMed] [Google Scholar]
- 118.Zhong L, Liao D, Zhang M, Zeng C, Li X, Zhang R, Ma H, Kang T. YTHDF2 suppresses cell proliferation and growth via destabilizing the EGFR mRNA in hepatocellular carcinoma. Cancer Lett. 2019;442:252–261. doi: 10.1016/j.canlet.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Ma JZ, Yang F, Zhou CC, Liu F, Yuan JH, Wang F, Wang TT, Xu QG, Zhou WP, Sun SH. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65:529–543. doi: 10.1002/hep.28885. [DOI] [PubMed] [Google Scholar]
- 120.Xia T, Wu X, Cao M, Zhang P, Shi G, Zhang J, Lu Z, Wu P, Cai B, Miao Y, Jiang K. The RNA m6A methyltransferase METTL3 promotes pancreatic cancer cell proliferation and invasion. Pathol Res Pract. 2019;215:152666. doi: 10.1016/j.prp.2019.152666. [DOI] [PubMed] [Google Scholar]
- 121.Chen J, Sun Y, Xu X, Wang D, He J, Zhou H, Lu Y, Zeng J, Du F, Gong A, Xu M. YTH domain family 2 orchestrates epithelial-mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle. 2017;16:2259–2271. doi: 10.1080/15384101.2017.1380125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Du Y, Hou G, Zhang H, Dou J, He J, Guo Y, Li L, Chen R, Wang Y, Deng R, et al. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018;46:5195–5208. doi: 10.1093/nar/gky156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sheng H, Li Z, Su S, Sun W, Zhang X, Li L, Li J, Liu S, Lu B, Zhang S, Shan C. YTH domain family 2 promotes lung cancer cell growth by facilitating 6-phosphogluconate dehydrogenase mRNA translation. Carcinogenesis. 2019. [DOI] [PubMed]
- 124.Li J, Han Y, Zhang H, Qian Z, Jia W, Gao Y, Zheng H, Li B. The m6A demethylase FTO promotes the growth of lung cancer cells by regulating the m6A level of USP7 mRNA. Biochem Biophys Res Commun. 2019;512:479–485. doi: 10.1016/j.bbrc.2019.03.093. [DOI] [PubMed] [Google Scholar]
- 125.Liu J, Ren D, Du Z, Wang H, Zhang H. Jin Y: m(6)A demethylase FTO facilitates tumor progression in lung squamous cell carcinoma by regulating MZF1 expression. Biochem Biophys Res Commun. 2018;502:456–464. doi: 10.1016/j.bbrc.2018.05.175. [DOI] [PubMed] [Google Scholar]
- 126.Cayir A, Barrow TM, Guo L, Byun HM. Exposure to environmental toxicants reduces global N6-methyladenosine RNA methylation and alters expression of RNA methylation modulator genes. Environ Res. 2019;175:228–234. doi: 10.1016/j.envres.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 127.Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG, et al. m(6)A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 2017;18:2622–2634. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Visvanathan A, Patil V, Abdulla S, Hoheisel JD, Somasundaram K. N(6)-methyladenosine landscape of glioma stem-like cells: METTL3 is essential for the expression of actively transcribed genes and sustenance of the oncogenic signaling. Genes (Basel) 2019;10:141. doi: 10.3390/genes10020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bogler O, et al. m(6)A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer cell. 2017;31:591–606. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dixit D, Xie Q, Rich JN, Zhao JC. Messenger RNA methylation regulates glioblastoma tumorigenesis. Cancer cell. 2017;31:474–475. doi: 10.1016/j.ccell.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V, Somasundaram K. Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2018;37:522–533. doi: 10.1038/onc.2017.351. [DOI] [PubMed] [Google Scholar]
- 132.Li J, Meng S, Xu M, Wang S, He L, Xu X, Wang X, Xie L. Downregulation of N(6)-methyladenosine binding YTHDF2 protein mediated by miR-493-3p suppresses prostate cancer by elevating N(6)-methyladenosine levels. Oncotarget. 2018;9:3752–3764. doi: 10.18632/oncotarget.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC, Yuan WB, Lu JC, Zhou ZJ, Lu Q, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18:110. doi: 10.1186/s12943-019-1036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang C, Zhi WI, Lu H, Samanta D, Chen I, Gabrielson E, Semenza GL. Hypoxia-inducible factors regulate pluripotency factor expression by ZNF217- and ALKBH5-mediated modulation of RNA methylation in breast cancer cells. Oncotarget. 2016;7:64527–64542. doi: 10.18632/oncotarget.11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, He X, Semenza GL. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A. 2016;113:E2047–E2056. doi: 10.1073/pnas.1602883113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, Liu Y, Zhang X, Zhang W, Ye L. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018;415:11–19. doi: 10.1016/j.canlet.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 137.Niu Y, Lin Z, Wan A, Chen H, Liang H, Sun L, Wang Y, Li X, Xiong XF, Wei B, et al. RNA N6-methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019;18:46. doi: 10.1186/s12943-019-1004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhou S, Bai ZL, Xia D, Zhao ZJ, Zhao R, Wang YY, Zhe H. FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting beta-catenin through mRNA demethylation. Mol Carcinog. 2018;57:590–597. doi: 10.1002/mc.22782. [DOI] [PubMed] [Google Scholar]
- 139.Liu J, Eckert MA, Harada BT, Liu SM, Lu Z, Yu K, Tienda SM, Chryplewicz A, Zhu AC, Yang Y, et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20:1074–1083. doi: 10.1038/s41556-018-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jia R, Chai P, Wang S, Sun B, Xu Y, Yang Y, Ge S, Jia R, Yang YG, Fan X. m(6)A modification suppresses ocular melanoma through modulating HINT2 mRNA translation. Mol Cancer. 2019;18:161. doi: 10.1186/s12943-019-1088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woo HH, Chambers SK. Human ALKBH3-induced m(1)A demethylation increases the CSF-1 mRNA stability in breast and ovarian cancer cells. Biochim Biophys Acta Gene Regul Mech. 2019;1862:35–46. doi: 10.1016/j.bbagrm.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 142.Zhao Y, Zhao Q, Kaboli PJ, Shen J, Li M, Wu X, Yin J, Zhang H, Wu Y, Lin L, et al. m1A regulated genes modulate PI3K/AKT/mTOR and ErbB pathways in gastrointestinal cancer. Transl Oncol. 2019;12:1323–1333. doi: 10.1016/j.tranon.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shimada K, Fujii T, Tsujikawa K, Anai S, Fujimoto K, Konishi N. ALKBH3 contributes to survival and angiogenesis of human urothelial carcinoma cells through NADPH oxidase and tweak/Fn14/VEGF signals. Clin Cancer Res. 2012;18:5247–5255. doi: 10.1158/1078-0432.CCR-12-0955. [DOI] [PubMed] [Google Scholar]
- 144.Blanco S, Bandiera R, Popis M, Hussain S, Lombard P, Aleksic J, Sajini A, Tanna H, Cortes-Garrido R, Gkatza N, et al. Stem cell function and stress response are controlled by protein synthesis. Nature. 2016;534:335–340. doi: 10.1038/nature18282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frye M, Dragoni I, Chin SF, Spiteri I, Kurowski A, Provenzano E, Green A, Ellis IO, Grimmer D, Teschendorff A, et al. Genomic gain of 5p15 leads to over-expression of Misu (NSUN2) in breast cancer. Cancer Lett. 2010;289:71–80. doi: 10.1016/j.canlet.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 146.Huang W, Qi CB, Lv SW, Xie M, Feng YQ, Huang WH, Yuan BF. Determination of DNA and RNA methylation in circulating tumor cells by mass spectrometry. Anal Chem. 2016;88:1378–1384. doi: 10.1021/acs.analchem.5b03962. [DOI] [PubMed] [Google Scholar]
- 147.Stockert JA, Gupta A, Herzog B, Yadav SS, Tewari AK, Yadav KK. Predictive value of pseudouridine in prostate cancer. Am J Clin Exp Urol. 2019;7:262–272. [PMC free article] [PubMed] [Google Scholar]
- 148.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 149.Penzo M, Casoli L, Ceccarelli C, Trere D, Ludovini V, Crino L, Montanaro L. DKC1 gene mutations in human sporadic cancer. Histol Histopathol. 2013;28:365–372. doi: 10.14670/HH-28.365. [DOI] [PubMed] [Google Scholar]
- 150.Valleron W, Laprevotte E, Gautier EF, Quelen C, Demur C, Delabesse E, Agirre X, Prosper F, Kiss T, Brousset P. Specific small nucleolar RNA expression profiles in acute leukemia. Leukemia. 2012;26:2052–2060. doi: 10.1038/leu.2012.111. [DOI] [PubMed] [Google Scholar]
- 151.Valleron W, Ysebaert L, Berquet L, Fataccioli V, Quelen C, Martin A, Parrens M, Lamant L, de Leval L, Gisselbrecht C, et al. Small nucleolar RNA expression profiling identifies potential prognostic markers in peripheral T-cell lymphoma. Blood. 2012;120:3997–4005. doi: 10.1182/blood-2012-06-438135. [DOI] [PubMed] [Google Scholar]
- 152.Ronchetti D, Todoerti K, Tuana G, Agnelli L, Mosca L, Lionetti M, Fabris S, Colapietro P, Miozzo M, Ferrarini M, et al. The expression pattern of small nucleolar and small Cajal body-specific RNAs characterizes distinct molecular subtypes of multiple myeloma. Blood Cancer J. 2012;2:e96. doi: 10.1038/bcj.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Qin YR, Qiao JJ, Chan TH, Zhu YH, Li FF, Liu H, Fei J, Li Y, Guan XY, Chen L. Adenosine-to-inosine RNA editing mediated by ADARs in esophageal squamous cell carcinoma. Cancer Res. 2014;74:840–851. doi: 10.1158/0008-5472.CAN-13-2545. [DOI] [PubMed] [Google Scholar]
- 154.Chen L, Li Y, Lin CH, Chan TH, Chow RK, Song Y, Liu M, Yuan YF, Fu L, Kong KL, et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat Med. 2013;19:209–216. doi: 10.1038/nm.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yao J, Duan L, Fan M, Yuan J, Wu X. Overexpression of BLCAP induces S phase arrest and apoptosis independent of p53 and NF-kappaB in human tongue carcinoma : BLCAP overexpression induces S phase arrest and apoptosis. Mol Cell Biochem. 2007;297:81–92. doi: 10.1007/s11010-006-9332-2. [DOI] [PubMed] [Google Scholar]
- 156.Fan DG, Zhao F, Ding Y, Wu MM, Fan QY, Shimizu K, Dohjima T, Nozawa S, Wakahara K, Ohno T, et al. BLCAP induces apoptosis in human Ewing’s sarcoma cells. Exp Biol Med (Maywood) 2011;236:1030–1035. doi: 10.1258/ebm.2011.010315. [DOI] [PubMed] [Google Scholar]
- 157.Chen Y, Wang H, Lin W, Shuai P. ADAR1 overexpression is associated with cervical cancer progression and angiogenesis. Diagn Pathol. 2017;12:12. doi: 10.1186/s13000-017-0600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu X, Wan S, Ou Y, Zhou B, Zhu J, Yi X, Guan Y, Jia W, Liu X, Wang Q, et al. RNA over-editing of BLCAP contributes to hepatocarcinogenesis identified by whole-genome and transcriptome sequencing. Cancer Lett. 2015;357:510–519. doi: 10.1016/j.canlet.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 159.Nakano M, Fukami T, Gotoh S, Nakajima M. A-to-I RNA editing up-regulates human dihydrofolate reductase in breast cancer. J Biol Chem. 2017;292:4873–4884. doi: 10.1074/jbc.M117.775684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chan TH, Qamra A, Tan KT, Guo J, Yang H, Qi L, Lin JS, Ng VH, Song Y, Hong H, et al. ADAR-mediated RNA editing predicts progression and prognosis of gastric cancer. Gastroenterology. 2016;151:637–650. doi: 10.1053/j.gastro.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang X, Xu Z, Ren X, Chen X, Wei J, Lin W, Li Z, Ou C, Gong Z, Yan Y. Function of low ADARB1 expression in lung adenocarcinoma. PLoS One. 2019;14:e0222298. doi: 10.1371/journal.pone.0222298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cao D, Pizzorno G. Uridine phosophorylase: an important enzyme in pyrimidine metabolism and fluoropyrimidine activation. Drugs Today (Barc) 2004;40:431–443. doi: 10.1358/dot.2004.40.5.850491. [DOI] [PubMed] [Google Scholar]
- 163.Cao D, Russell RL, Zhang D, Leffert JJ, Pizzorno G. Uridine phosphorylase (-/-) murine embryonic stem cells clarify the key role of this enzyme in the regulation of the pyrimidine salvage pathway and in the activation of fluoropyrimidines. Cancer Res. 2002;62:2313–2317. [PubMed] [Google Scholar]
- 164.Guan Y, Bhandari A, Zhang X, Wang O. Uridine phosphorylase 1 associates to biological and clinical significance in thyroid carcinoma cell lines. J Cell Mol Med. 2019;23(11):7438–7448. doi: 10.1111/jcmm.14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bader JP, Brown NR, Chiang PK, Cantoni GL. 3-Deazaadenosine, an inhibitor of adenosylhomocysteine hydrolase, inhibits reproduction of Rous sarcoma virus and transformation of chick embryo cells. Virology. 1978;89:494–505. doi: 10.1016/0042-6822(78)90191-5. [DOI] [PubMed] [Google Scholar]
- 166.Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, Gan J, Jiang H, Jia GF, Luo C, Yang CG. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43:373–384. doi: 10.1093/nar/gku1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable