Abstract
Objective.—
To report a case of petrous apicitis that manifested as chronic migraine without aura and to discuss the pathophysiological mechanisms behind this presentation.
Background.—
Petrous apicitis is a rare complication of acute otitis media with varied clinical presentations that stem from the close proximity of the petrous apex to numerous neurovascular structures. Headache is among the common symptoms of petrous apicitis.
Methods.—
A case of new onset headache in the setting of petrous apicitis with symptomatic response to antibiotic therapy was reported. We provided a brief review of peripheral pathophysiological mechanisms of migraine and correlated to mechanism of headache in petrous apicitis.
Results.—
A 65-year-old man with chronic otitis externa/media presented with ongoing headache fulfilling International Classification of Headache Disorders 3rd edition (ICHD-3) criteria for chronic migraine without aura that persisted despite undergoing right mastoidectomy and tympanoplasty with multiple courses of oral antibiotic therapy for his chronic otitis. MRI brain revealed petrous apicitis, otomastoiditis, and clival osteomyelitis. His imaging findings improved and his migraine-like headache completely resolved after treatment with a prolonged course of antibiotics.
Conclusions.—
Petrous apicitis can present as a headache with features of migraine, and in this case in particular, as chronic migraine without aura. The pathophysiological mechanisms that may underlie the generation of migraine-like headache in petrous apicitis may include the activation of nociceptive fibers within the periosteum of the petrous apex and clivus whose cell bodies originate in the trigeminal ganglion and upper cervical dorsal root ganglia. By treating the peripheral pathology, resolution of the headache may be achieved.
Keywords: petrous apicitis, migraine, headache, localization, nociceptive fibers, periosteum
INTRODUCTION
Petrous apicitis is infection and inflammation of the petrous apex of the temporal bone and the air cells it contains. It is a known complication of acute otitis media, as acute otitis media can extend medially into a pneumatized petrous apex. Today, as a result of the widespread availability and use of antibiotics, acute otitis media rarely leads to petrous apicitis. Due to its rarity, the incidence in adults is largely unknown.1 The incidence in infants and children, however, has been reported as 2/100,000.2
Due to the close proximity of the petrous apex to neurovascular structures including the trigeminal ganglion, abducens nerve, and facial nerve, petrous apicitis can lead to neurovascular compromise and corresponding symptoms. The clinical presentation of petrous apicitis can vary. The classic triad known as Gradenigo syndrome includes abducens nerve palsy, symptoms of otitis media, and retro-orbital pain in the distribution of the trigeminal nerve. However, it is rare for petrous apicitis to present as such. Other symptoms can include headache, tinnitus, and facial nerve palsies, among others.3–5 Some cases can be occult and manifest only after surgical procedures such as tympanomastoid surgery have failed to control suppuration.6
Petrous apicitis had traditionally been treated surgically. However, there have been recent reports that have described good outcomes with high-dose broad-spectrum antibiotics. As a result, first-line treatment typically consists of a prolonged course (ie, at least 6 weeks) of appropriate antibiotics, and surgical management is only recommended for those who do not respond quickly to antibiotic therapy or for those who have complications.1,5,7
Here, we describe a case of petrous apicitis that presented as chronic migraine without aura in a man with chronic otitis media/externa who underwent mastoidectomy with tympanoplasty. We also discuss pathophysiological mechanisms that may be responsible for generation of migraine-like headache in petrous apicitis, and how treatment of the peripheral pathology may lead to resolution of the headache.
CASE PRESENTATION
A 65-year-old left-handed man with reportedly no prior history of headache and a past medical history of hypertension, type 2 diabetes, and chronic otitis externa/media with subsequent right mastoidectomy and tympanoplasty presented to the Comprehensive Headache Center at Beth Israel Deaconess Medical Center with complaints of new-onset headache for the past 4–5 months. He described the headache as unilateral, right sided, and located in the temporal region with radiation to the right ear and jaw. The headache was described as throbbing, pulsating, sharp, stabbing, and 6–10/10 in intensity on average. He reported that the headaches occurred on a daily basis, were constant, and were not relieved by opioids. Associated symptoms included photophobia, phonophobia, blurred vision, irritability, and difficulty concentrating. Aggravating factors included standing, walking, physical activity, chewing, talking, neck movement, weather changes, watching television, sunlight, and lack of sleep. Alleviating factors included lying down and rest. By the International Classification of Headache Disorders 3rd edition (ICHD-3) diagnostic criteria,8 his headache was classified as chronic migraine without aura. Initial exam was notable for right temporal tenderness, with neurological exam intact. Blood tests at presentation were notable for a C-reactive protein (CRP) level of 33.2 mg/L. MRI brain with and without contrast demonstrated findings consistent with right petrous apicitis, and treated or ongoing otomastoiditis. MRA neck with and without contrast showed mild narrowing of the high cervical skull base segment of the right internal carotid artery.
The patient had been seeing his primary care physician and otolaryngologist for the treatment of chronic right-sided otitis externa for 4 months prior to his presentation to the headache center. He was treated with acetic acid and hydrocortisone otic drops followed by a week of ofloxacin 0.3% otic drops and a few weeks of ciprofloxacin-dexamethasone otic drops as well as the removal of white keratinous debris from the right external auditory canal. At that time, CT scan of his temporal bone showed nearly completely opacified right mastoids, middle ear, and external auditory canal with non-visualization of the short segment of the tympanic segment mastoid bone. He subsequently completed a 10-day course of amoxicillin 875 mg-clavulanic acid 125 mg twice daily and was referred to an Ear, Nose, and Throat surgeon for persistence of his chronic otitis externa/media. Approximately 2.5 weeks prior to his presentation to the headache center, the patient underwent right-sided post-auricular mastoidectomy with tympanoplasty. Following this surgery, he completed a 2-week course of ciprofloxacin 500 mg twice daily. Given his persistent headaches despite antibiotics in the postoperative period, he was referred to the Headache Center for the evaluation and treatment of primary headache disorder.
Given multiple comorbidities and risk factors, the patient was started on gabapentin in the headache center. He was using butalbital-acetaminophen-caffeine for his headaches and he continued to see his primary care physician and otolaryngologist for his ongoing ear pain and for the concern for petrous apicitis seen on imaging per above. Shortly thereafter, he received a 2-week course of ciprofloxacin 500 mg twice daily. He continued to report daily headache. Upon follow-up at the headache center, approximately 2 months after his initial visit, he underwent an MRI of skull base with and without contrast (Fig. 1A–C), which showed abnormal enhancement without defined mass lesion centered along the right petrous apex and spheno-occipital synchondrosis with extension to the right temporomandibular joint, slightly progressed from prior examination, now with involvement of the left retro pharyngeal musculature, suggesting inflammatory/infectious changes of petrous apicitis; new marrow edema abnormality along the left aspect of the clivus to the left petrous apex. He was subsequently referred to the emergency department several times, and completed two 10-day courses of levofloxacin 750 mg daily. He was then hospitalized for 5 days, during which he received IV vancomycin, cefepime, and metronidazole before leaving against medical advice. Upon discharge, he was continued on levofloxacin 750 mg daily and metronidazole 500 mg every 8 hours. About 2 weeks later, he was seen by infectious disease, who recommended a total 6-week course of the above antibiotics. At this point, he already reported complete resolution of his headaches and ear pain, and his CRP level had normalized. After he completed this course of antibiotics, approximately 4 months after his initial presentation to the headache center, he underwent another MRI of skull base with and without contrast (Fig. 1D–F), which showed stable opacification of the right mastoid air cells, middle ear cavity, and right petrous apex; overall stable to slightly improved inflammatory changes involving the right temporomandibular joint, pharyngeal recess, and posterior nasopharynx; near complete resolution of the previously seen marrow edema in the clivus.
Fig. 1.—
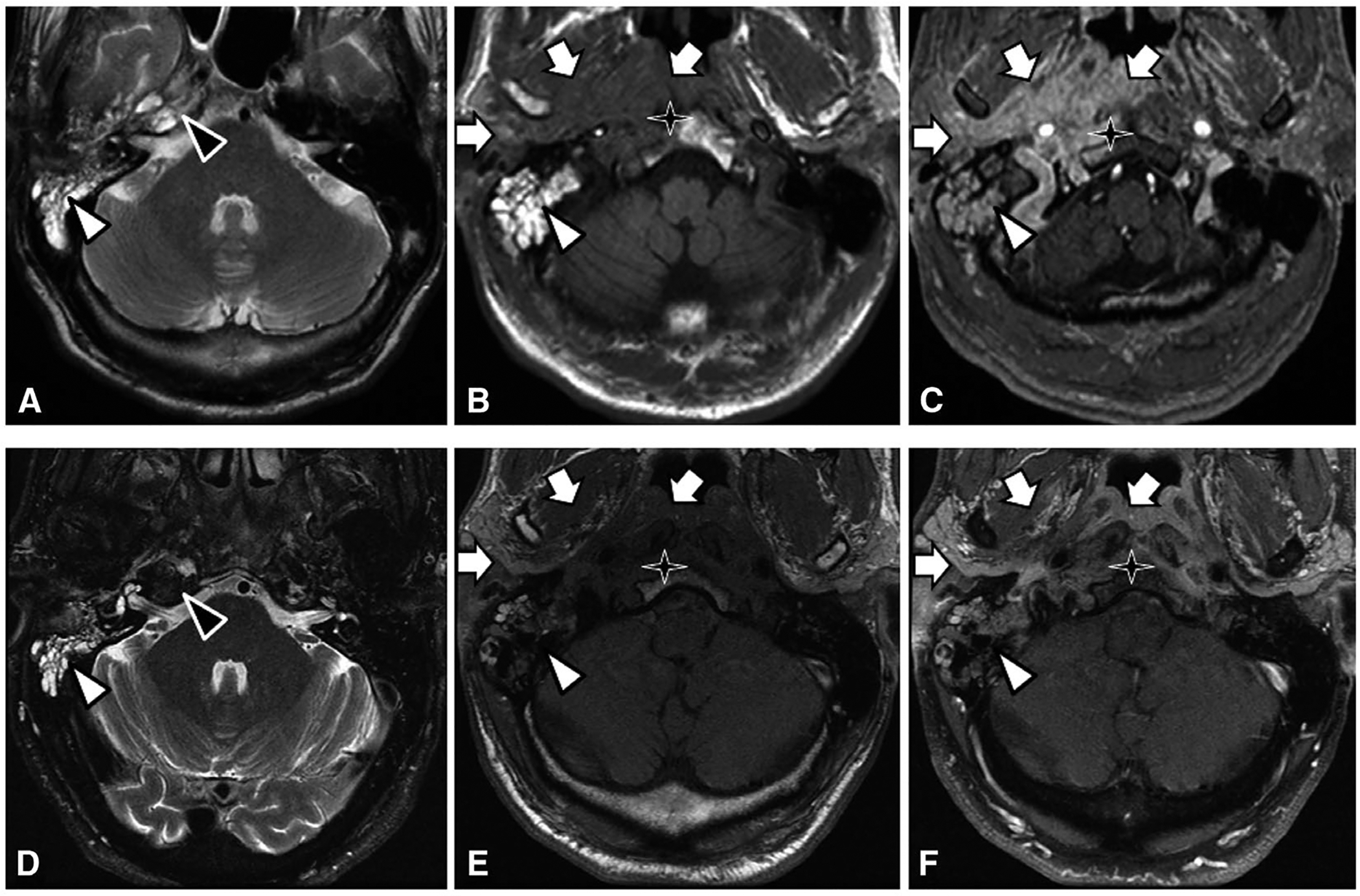
(A-C) Pre-treatment images of right petrous apicitis. T2-weighted image (A) at the level of the petrous apex demonstrating fluid in the pneumatized right petrous apex (black triangle) and mastoid air cells (white triangle). T1 pre-contrast (B) and post-contrast (C) images at the level of the nasopharynx demonstrating abnormal enhancement of the right nasopharynx, parapharyngeal space, and masticator space (white arrows), compatible with infection and inflammation. There is abnormal T1 pre-contrast hypointense marrow signal of the right aspect of the clivus (star), with post-contrast enhancement compatible with osteomyelitis. (D-F) Post-treatment images. T2-weighted image (D) shows resolution of abnormal fluid signal at the right petrous apex. T1 pre- and post-contrast images show near complete resolution of abnormal signal and enhancement of the clivus, right nasopharynx, parapharyngeal space, and masticator space.
DISCUSSION
Petrous apicitis, a known but dwindling complication of acute otitis media, has rarely been shown to present with the classic triad of abducens nerve palsy, symptoms of otitis media, and retro-orbital pain in the distribution of the trigeminal nerve. In reality, presentations of petrous apicitis can vary greatly.3–5 Here, we highlighted a case of petrous apicitis presenting as chronic migraine without aura, based on ICHD-3 diagnostic criteria.8 More specifically, his headaches were right sided in the temporal region with radiation to the right ear and jaw; were throbbing, pulsating, sharp, and stabbing in nature; occurred on a daily basis and were constant throughout the day; and were associated with photophobia, phonophobia, blurred vision, irritability, and difficulty concentrating. It is important to note that the location of the headache in the right temporal region coincides with the location of the peripheral pathology – inflammation and infection of the right petrous apex of the temporal bone. There have been previous case reports of petrous apicitis presenting with headache as one of the main symptoms, some of which exhibited migrainous features. For example, Rho described a case of petrous apicitis in a 12-year-old boy who initially presented with daily severe headaches that had been ongoing for 2 weeks, and were further characterized as pulsatile, located behind the left eye in the forehead and left temporal regions, and associated with nausea.4 Plodpai et al described a case of petrous apicitis in a 63-year-old man presenting as right-sided otorrhea with concomitant constant ipsilateral headache in the right frontal and retro-orbital area radiating to the whole head that had been ongoing for 1 month.5 Finally, Tornabene et al presented a case of a 60-year-old woman with right-sided frontal headaches, facial pain, and diplopia for 7 days who was found to have petrous apicitis.9
To understand how petrous apicitis can lead to migraine headaches, it is first important to touch upon the pathophysiology of migraines. In migraines, activation of the trigeminovascular system – consisting of sensory neurons that originate from the trigeminal ganglion and upper cervical dorsal roots – leads to release of vasoactive neuropeptides including substance P, calcitonin gene-related peptide (CGRP), and neurokinin A. These vasoactive neuropeptides cause neurogenic inflammation, which is thought to be involved in the generation of pain in migraine, and may lead to sensitization.10–12 Sensitization is the process in which neurons become increasingly responsive to nociceptive and non-nociceptive stimulation. Peripheral sensitization affects the primary afferent neurons, while central sensitization affects the second-order neurons in the trigeminal nucleus and higher order neurons in the central nervous system. Sensitization is likely linked to the clinical symptoms of migraine, including the throbbing nature, hyperalgesia, and allodynia. It is also thought to play a role not only within individual migraine attacks, but also in the transformation of episodic migraine (<15 headache days per month) to chronic migraine (≥15 headache days per month with ≥8 headache days per month linked to migraine).8,13–15
In patients with chronic migraine, periosteal inflammation may activate trigeminal nociceptors that reach affected periosteum through suture branches of the occipital nerve, which leads to the clinical presentation of headache and muscle tenderness.16 A similar pathophysiologic mechanism could be implicated in petrous apicitis. In petrous apicitis, there is infection and inflammation of the petrous apex, which is the pyramidal, medial projection of the petrous portion of the temporal bone.17 Like other bones of the calvaria, the temporal bone is heavily innervated by nociceptive fibers whose cell bodies originate in the trigeminal ganglion and upper cervical dorsal root ganglia.18,19 Within the temporal bone, most densely innervated structures include the periosteum, endosteum, bone marrow, and sutures (Fig. 2).18 This network of CGRP and transient receptor potential cation channel subfamily V member 1 (TRPV1)-positive pain fibers, which is well documented in humans, establishes a neural framework for conceptualizing how inflammation of the petrous apex can lead to activation of adjacent nociceptors that are branches of trigeminovascular and cervicovascular neurons.20,21 Such activation, in turn, can initiate a cascade of events that give rise to the perception of migraine headache. Evidence for upregulation of proinflammatory genes and downregulation of anti-inflammatory genes in the periosteum of chronic migraine patients further supports this scenario.16 It is therefore reasonable to believe that treatment of petrous apicitis with a prolonged course of antibiotics would reduce the inflammation of the petrous apex, thus leading to reduced activation of the adjacent nociceptors and trigeminovascular system, decreased release of vasoactive neuropeptides, and improvement or resolution of the migraine-like headache.
Fig. 2.—
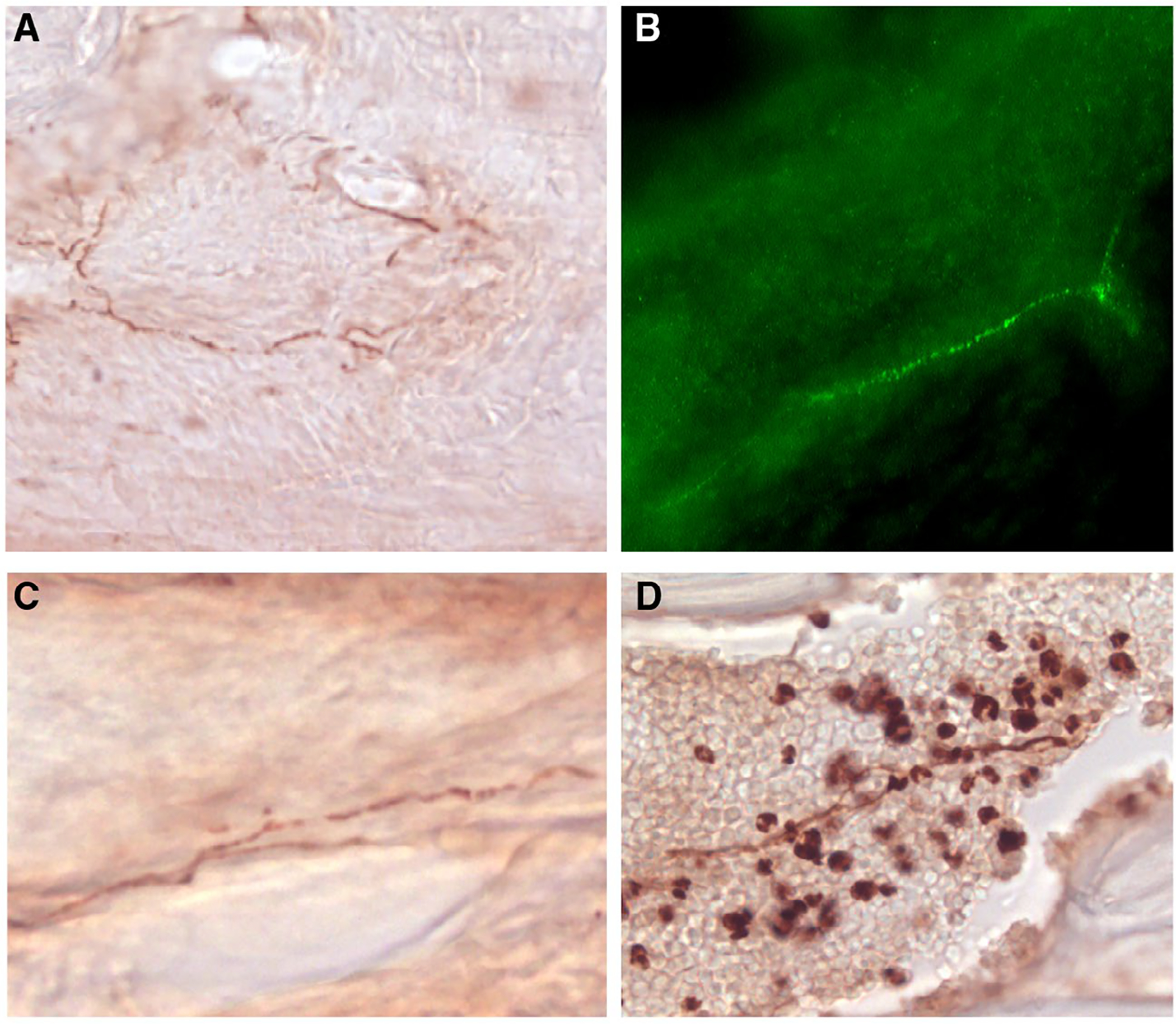
CGRP-positive nerve fibers inside and outside the temporal bone of the adult mouse. (A) Periosteum, (B) endosteum, (C) squamosa suture of the temporal bone, (D) bone marrow.
CONCLUSION
In summary, we have described a case of right- sided petrous apicitis that presented as chronic migraine without aura localized to the right temporal region in a man with chronic otitis media/externa who underwent mastoidectomy with tympanoplasty and who was subsequently treated with a prolonged course of antibiotics with complete resolution of headache. We have also discussed the pathophysiological mechanisms that may be responsible for generation of migraine headache in petrous apicitis, and how treatment of the peripheral pathology may lead to resolution of the headache. Furthermore, this case highlights how persistently localized pain may indicate a peripheral pathology, such as petrous apicitis, on the ipsilateral side.
Acknowledgments:
The patient kindly gave verbal consent for the publication of the case.
Abbreviations:
- CGRP
calcitonin gene-related peptide
- CRP
C-reactive protein
- ICHD-3
International Classification of Headache Disorders 3rd edition
- TRPV1
transient receptor potential cation channel subfamily V member 1
REFERENCES
- 1.Gadre AK, Chole RA. The changing face of petrous apicitis – A 40-year experience. Laryngoscope. 2018;128:195–201. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein NA, Casselbrant ML, Bluestone CD, Kura-Lasky M. Intratemporal complications of acute otitis media in infants and children. Otolaryngol Head Neck Surg. 1998;119:444–454. [DOI] [PubMed] [Google Scholar]
- 3.Gradenigo G Uber die Paralyse des N. Abduzens bei Otitis. Arch Ohrenheilk. 1907;74:149–158. [Google Scholar]
- 4.Rho Y Headache attributed to petrous apicitis without symptoms of acute otitis media. Austin J Clin Neurol. 2015;2:1055–1057. [Google Scholar]
- 5.Plodpai Y, Hirunpat S, Kiddee W. Gradenigo’s syndrome secondary to chronic otitis media on a background of previous radical mastoidectomy: A case report. J Med Case Rep. 2014;8:217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chole RA, Donald PJ. Petrous apicitis: Clinical considerations. Ann Otol Rhinol Laryngol. 1983;92:544–551. [DOI] [PubMed] [Google Scholar]
- 7.Burston BJ, Pretorius PM, Ramsden JD. Gradenigo’s syndrome: Successful conservative treatment in adult and paediatric patients. J Laryngol Otol. 2005;119: 325–329. [DOI] [PubMed] [Google Scholar]
- 8.Headache Classification Committee of the International Headache Society (HIS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 9.Tornabene S, Vilke GM. Gradenigo’s syndrome. J Emerg Med. 2010;38:449–451. [DOI] [PubMed] [Google Scholar]
- 10.Arbab MA, Wiklund L, Svendgaard NA. Origin and distribution of cerebral vascular innervation from superior cervical, trigeminal and spinal ganglia investigated with retrograde and anterograde WGA-HRP tracing in the rat. Neuroscience. 1986; 19:695–708. [DOI] [PubMed] [Google Scholar]
- 11.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–196. [DOI] [PubMed] [Google Scholar]
- 12.Sarchielli P, Alberti A, Floridi A, Gallai V. Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology. 2001;57: 132–134. [DOI] [PubMed] [Google Scholar]
- 13.Burstein R Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89: 107–110. [DOI] [PubMed] [Google Scholar]
- 14.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. [DOI] [PubMed] [Google Scholar]
- 15.Kaube H, Katsarava Z, Przywara S, Drepper J, Ellrich J, Diener HC. Acute migraine headache: Possible sensitization of neurons in the spinal trigeminal nucleus? Neurology. 2002;58:1234–1238. [DOI] [PubMed] [Google Scholar]
- 16.Perry CJ, Blake P, Buettner C, et al. Upregulation of inflammatory gene transcripts in periosteum of chronic migraineurs: Implications for extracranial origin of headache. Ann Neurol. 2016;79:1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman PR, Shah R, Curé JK, Bag AK. Petrous apex lesions: Pictorial review. Am J Roentgenol. 2011; 196:WS26–WS37. [DOI] [PubMed] [Google Scholar]
- 18.Kosaras B, Jakubowski M, Kainz V, Burstein R. Sen sory innervation of the calvarial bones of the mouse. J Comp Neurol. 2009;515:331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noseda R, Melo-Carrillo A, Nir RR, Strassman AM, Burstein R. Non-trigeminal nociceptive innervation of the posterior dura: Implications to occipital headache. J Neurosci. 2019;10:1867–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schueler M, Messlinger K, Dux M, Neuhuber WL, De Col R. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154:1622–1631. [DOI] [PubMed] [Google Scholar]
- 21.Schueler M, Neuhuber WL, De Col R, Messlinger K. Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache. 2014;54:996–1009. [DOI] [PubMed] [Google Scholar]


