Abstract
RATIONALE:
Diacetyl (DA; 2,3-butanedione) is a chemical found commonly in foods and e-cigarettes. When inhaled, DA causes epithelial injury, though the mechanism of repair remain poorly understood. The objective of this study was to evaluate airway basal cell repair after DA vapor exposure.
METHODS:
Primary human bronchial epithelial cells were exposed to DA or PBS for 1 hour. Lactate dehydrogenase, cleaved caspase 3/7 and trans-epithelial electrical resistance were measured prior to and following exposure. Exposed cultures were analyzed for the airway basal cell markers keratin 5 and p63 as well as ubiquitin and proteasome activity. Cultures were also treated with a proteasome inhibitor (MG132).
RESULTS:
DA vapor exposure caused a transient decrease in trans-epithelial electrical resistance in all DA-exposed cultures. Supernatant lactate dehydrogenase and cleaved caspase 3/7 increased significantly at the highest DA concentration but not at lower DA concentrations. Increased keratin 5 ubiquitination occurred after DA exposure but resolved by day 3. Damage to airway basal cells persisted at day 3 in the presence of MG132.
CONCLUSIONS:
Diacetyl exposure results in airway basal cell injury with keratin 5 ubiquitination and decreased p63 expression. The ubiquitin-proteasome-pathway partially mediates airway basal cell repair after acute DA exposure.
Keywords: diacetyl, airway epithelium, keratin 5, ubiquitin proteasome, epithelial repair, flavoring-induced lung disease
Graphical abstract
1. INTRODUCTION
Diacetyl (DA; 2,3-butanedione) is a flavoring chemical added or naturally occurring in a variety of foods and alcoholic beverages. In the past decade, DA use as a flavoring additive has risen dramatically in foods and e-cigarettes (1, 22). Though previously classified as safe for consumption by the FDA, DA inhalation is associated with debilitating airways disease in humans known as bronchiolitis obliterans (BO) (3, 15). Despite its common use and limited regulation, the exact mechanism of how exposure to DA vapor causes flavoring-induced lung disease remains poorly understood.
Inhalation exposure to DA vapor can result in severe and debilitating lung disease, known as flavoring-related lung disease. The sentinel report of a cohort of workers employed by a microwave popcorn factory in Missouri, US, occurred in May 2000 (15, 24). Workers presented with shortness of breath and cough. Lung function testing demonstrated severe decreases in forced expiratory volume in 1 second (FEV1) without response to bronchodilator medication, supportive of severe, fixed obstructive lung disease. Further investigation by the National Institute for Occupational Safety and Health (NIOSH) identified 10-fold greater rate of decline in lung function of exposed workers compared to non-smoking controls (15). NIOSH extended their evaluation to five additional microwave popcorn plants total greater than 700 workers evaluated for symptoms and lung function. DA-exposed workers, and more specifically mixers, with greater than one year of work had more chest symptoms and poorer lung function as measured by FEV1 than mixers who worked for less than one year (10). Collectively, these investigations highlight the risk of severe respiratory impairment and debilitating, irreversible lung disease associated with DA inhalation exposure.
Following the sentinel report of BO in popcorn factory workers exposed to DA vapors (10, 15, 24), multiple preclinical in vivo models of DA vapor exposure developed. Acutely, rats exposed to DA vapors for 6 hours at greater than 100ppm develop airway epithelial injury (7, 8). Subacutely, rats exposed to multiple days of DA vapors develop fibrotic, intrapulmonary airway lesions (19). Additionally, when allowed to recover for two weeks after DA exposures, fibrotic airway lesions persist in exposed rats (19). Collectively, these in vivo rat models of DA exposure provide evidence of direct airway epithelial injury as well as persistent airway remodeling recapitulating some of the human pathology.
To complement these in vivo models of DA vapor exposures, in vitro DA vapor exposure models have also developed (5, 6, 11, 21). One of the in vitro culture systems developed for studying DA vapor exposures utilized primary human bronchial epithelial cells differentiated at air-liquid interface (ALI) (11). When exposed to one-hour of 25 mM DA (~1000ppm) vapors for repetitive exposures, a significant rise in supernatant lactate dehydrogenase (LDH) occurred (5, 11). Zaccone et al. also evaluated airway epithelial function and morphometry in an air-liquid interface culture after a six-hour exposure to 25 ppm DA (27). A significant reduction in sodium transport occurred at 18 hours after DA exposure, but without a significant change in trans-epithelial electrical resistance (TEER). At this lower concentration, single day exposure, the airway epithelium also retained its ability to metabolize DA to its less reactive metabolites via dicarbonyl/L-xylulose reductase (DCXR). At higher DA concentrations (100-360ppm) for six hours, the airway epithelial layer detached, resulting in cellular death (27). Thus, at lower DA concentrations or for shorter exposure periods, the airway epithelium retains its ability to recover from chemical exposure, but for longer or higher concentration exposures, DA causes epithelial cell death. Thus, after a single DA exposure, the airway epithelium retains its ability to recover from chemical exposure, but after repeated DA exposures, airway epithelial cell death occurs. We hypothesize that the airway epithelium retains its regenerative capacity following a single, lower concentration DA vapor exposure through airway basal cell repair. The primary purpose of this study was to evaluate for airway basal cell injury and repair following a single one-hour DA vapor exposure in primary human airway epithelial cultures exposed to clinically relevant concentrations of DA vapors.
2. MATERIALS AND METHODS
2.1. Chemical
Diacetyl (2,3-butanedione, 98.5% purity) was purchased from Sigma-Aldrich (St. Louis, MO). The proteasome inhibitor MG132 (>98% purity) was purchased from Tocris (Pittsburgh, PA).
2.2. Primary Human Airway Epithelial Cultures
Human EpiAirway™ (AIR-100) generated from primary human bronchial epithelial cells from healthy, non-smoking donor (TBE-20), were purchased from MatTek, Corporation (Ashland, MA). All tissues were well differentiated at air-liquid interface (ALI) on a microporous (9 mm internal diameter) membrane in plastic inserts prior to exposure. Upon receipt from MatTek, cultures were placed into 1 ml of culture medium (MatTek, Ashland, MA) in 6-well culture plates for at least 24 hours to equilibrate prior to exposure.
2.3. In Vitro Diacetyl (DA) Vapor Cup Exposure
Immediately prior to exposure, DA was diluted in phosphate-buffered solution (PBS) to final concentrations of 12, 25, and 50 mM. PBS vehicle was used a negative control. Concentrations were chosen from previous published estimations of vapor concentrations within the vapor cup exposure at 37°C, and relevant to peak levels of DA in factories with artificial butter flavoring (5, 6, 11, 15). The majority of experiments were conducted at DA exposure concentrations of 25 and 50 mM. These concentrations were chosen to model the intermittent, high-concentration exposure or mixers in buttery factories. Previous publications have calculated the expected DA vapor concentrations to be ~1100 and ~2200 ppm for 25 mM and 50 mM DA exposures (5, 11). Using equivalent DA concentrations allows for direct comparison to prior publications on in vitro DA vapor cup exposures as well as can be used to contrast results of single and multiple DA vapor exposures (5, 6, 11).
ALI tissue cultures were exposed to DA-derived vapors for one-hour using vapor cups as described previously (11). Briefly, 50 microliters (μl) DA or PBS was pipetted onto a 6 mm. antibiotic sensitivity disk (BD BBL™; Franklin Lakes, NJ) placed within a 1.5ml Eppendorf tube top. The vapor cup was inverted over the tissue culture, sealed tightly onto the plastic well insert, and placed into the 5% CO2 incubator for 1 hour. After 1 hour, the Eppendorf tube top was removed, and the tissue cultures were returned to the incubator prior to analysis at 1, 3 and 5 days after DA exposure. A single, one-hour DA vapor exposure was used (over repeated exposure) to characterize the concentration and temporal response of airway epithelial basal cells to an acute DA vapor exposure. Ten separate DA exposures occurred with different human samples for each exposure. All DA exposures were performed with four replicates per exposure and repeated at least twice with the same donor for each experiment.
2.4. Lactate Dehydrogenase (LDH) Activity
Lactate dehydrogenase (LDH) activity (Thermo Scientific Pierce; Rockford, IL) was measured in apical washes of the cellular supernatant as a surrogate marker of cellular injury after exposure. The apical surface of the ALI tissues was gently rinsed with 0.4ml PBS prior to exposure and at 23 hours after exposure (Day 1). Apical rinses were centrifuged prior to LDH activity testing to remove mucus/debris. LDH activity was expressed as fold change over PBS (vehicle) control.
2.5. Caspase-3/7 Activity
As a marker of cellular apoptosis, supernatant caspase-3/7 (Casp-3/7) activity was measured in apical washes of the cellular supernatant. The apical surface of the ALI tissues was gently rinsed with 0.4ml PBS prior to exposure and at 23 hours after exposure (Day 1). Apical rinses were centrifuged prior to testing to remove mucus/debris. After centrifugation, Casp-3/7 activity was generated by following the commercially available protocol as described (Promega, Madison, WI). Luminescent activity was expressed as fold change over PBS (vehicle) control.
2.6. Trans-epithelial Electrical Resistance (TEER)
Trans-epithelial electrical resistance (TEER) was measured in all tissue cultures prior to and at 1 day after exposure using silver chloride electrodes (EVOM2, World Precision Instruments, Sarasota, FL). Electrodes were connected to the volt-ohmmeter and were equilibrated in balanced PBS solution (MatTek) for 15 minutes before use. 0.4 ml of warm PBS solution (MatTek) was added to the apical surface of ALI cultures. TEER was measured by placing the longer electrode into the basal media, and the shorter electrode into the apical transwell insert. Two measurements were taken from each insert. ALI cultures were not used for exposure if the TEER measurement was ≤ 300 Ohms*cm2 (25, 27)
2.7. Histologic Analyses of Airway Cultures
On Day 1 after exposure, tissue cultures were fixed in 10% neutral buffered formalin (NBF) overnight at 4 °C followed by cold PBS wash. Tissue cultures were excised from transwell insert, placed in a Kim wipe and enclosed within a tissue embedding cassette. Tissue cultures were dehydrated in 80% ethanol, embedded in paraffin, sectioned (5 μm), and mounted on silane-coated glass slides. Sections were stained with hematoxylin and eosin (H&E).
2.8. Immunofluorescent Staining for Basal and Ciliated Epithelial Cell Markers
Embedded sections of airway epithelium were stained for common airway epithelial cell markers, including keratin 5 (Krt5; 1:2000, Biolegend, Dedham, MA), ΔN isoform of transcription factor p63 (ΔNp63; 1:200, Biolegend, Deham, MA), Ki-67 staining (1:200, Abcam, Cambridge, MA), and acetylated tubulin (AT, 1:1000, Millipore Sigma, St. Louis, MO). Rabbit and Mouse IgG (1:1000, Agilent, Santa Clara, CA) were used as negative controls. Briefly, sections were deparaffinized with xylene, followed by dehydration in graded alcohol and heated in antigen retrieval solution (Vector, Burlingame, CA). Sections were washed in PBS buffer and blocked with 10% normal BSA in PBS for 1h at room temperature. Slides were then rinsed and incubated overnight at 4 °C with respective primary antibody. After PBS rinse, the slides were counterstained with an AlexaFluor secondary immunofluorescent antibody (1:1000; ThermoFisher Scientific, Rockford, Illinois, USA) and mounted with DAPI Fluoromount-G (Southern Biotechnology, Birmingham, Alabama).
2.9. Western blot analyses for Krt5, ΔNp63, β-actin, and GAPDH
Airway cultures were homogenized in RIPA lysis buffer (Abcam; Cambridge, MA) supplemented with a protease inhibitor cocktail (Roche; Indianapolis, IN). Following centrifugation at 12,000 rpm for 20 minutes at 4 °C, soluble supernatant fractions were collected for total protein and western blot analysis. Total protein concentrations were determined by BCA assay kit (Thermo Scientific, Waltham, MA). Ten micrograms (μg) total protein were resolved in pre-casted 4-15% gradient Tris-Glycine gel (Bio-Rad, Hercules, CA), and immunoblotted for Krt5 (1:5000; Biolegend), ΔNp63 (1:1000; Biolegend), and K48-ubiquitin (1:1000, R&D Systems). Beta-actin and GAPDH served as loading controls and for densitometry analysis normalization. Gels were transferred to 0.1 μm nitrocellulose membrane (GE Healthcare). HRP and SuperSignal West Pico chemiluminescent substrates (Thermo Scientific) were used to detect protein signal intensity.
2.10. Immunoprecipitation of Krt5 from Airway Epithelial Homogenates
To isolate Krt5 from total airway homogenates, tissue culture lysates were intubated with 50 μl magnetic beads (Miltenyi Biotec) and 2 μl Krt5 antibody (1mg/ml) overnight. Solution was then passed through a magnetic column. The column was washed multiple times using RIPA buffer (Abcam) and eluted using SDS PAGE (Bio-Rad). The immunoprecipitated protein was quantified via Western blot analysis as detailed above. Co-localization of polyubiquitin-C with Krt5 was performed by performing immunoblotting for Ubiquitin C antibody (1:1000, Invitrogen) on immunoprecipitated Krt5 protein. Airway cultures incubated with 100mM MG132 for 24 hours was used as a positive control.
2.11. Proteasome Activity of Total Airway Epithelial Cellular Homogenates
To measure 20S, chymotrypsin-like (CT-L), trypsin-like (T-L), and caspase-like (C-L) proteasome activities in airway cultures, cultures were lysed and collected in 50 mM HEPES (pH 7.5), 5 mM EDTA, 150 mM NaCl and 1% Triton X-100 supplemented with 2 mM ATP buffered solution. Proteasome activities were determined using the Chemicon 20S Proteasome Activity Assay (Millipore) and Proteasome-Glo 3 Substrate System, respectively, according to the manufacturer’s instructions. The luminescent signal was quantified in a SpectraMax M5 plate reader (Molecular Devices, San Jose, CA). Enzymatic activity in DA-exposed samples was expressed relative to the activity of PBS controls.
2.12. Proteasome Inhibition of Exposed Airway Epithelial Samples with MG 132
To address whether proteasome activity is involved with keratin 5 DA-induced ubiquitination and degradation, primary human airway cultures were co-incubated with proteasome inhibitor MG132 (100nM; Tocris, >98% purity). 100nM MG132 concentration is published previously as a non-cytotoxic concentration in in vitro cell cultures of normal human bronchial epithelial cells (16). MG132 was diluted in the basolateral maintenance media (MatTek) of the airway cultures and replaced every other day after DA exposure.
2.13. Statistical Analysis
For normally distributed data, results of quantitative measures were expressed as means ± standard deviation (SD) and analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc analysis. When data were not normally distributed, results were expressed as medians with min/max error bars and analyzed using a Kruskal-Wallis test. All data were analyzed using with Prism 7.0 software (GraphPad, La Jolla, CA).
3. RESULTS
3.1. Rise in Lactate Dehydrogenase (LDH) and Cleaved Caspase 3/7 Activity after a Single One-Hour DA Vapor Exposure
Lactate dehydrogenase (LDH) and cleaved caspase 3/7 (Casp-3/7) activity in airway supernatants were used as surrogate markers of cell death and apoptosis after DA exposure, respectively. Twenty-four hours after exposure, airway supernatant LDH increased significantly in 50mM DA-exposed cultures compared to PBS, 12 and 25 mM DA-exposed culture (Figure 1A; ANOVA with Tukey’s; n=4/group; ** p<0.01). Airway supernatant Casp-3/7 also increased significantly in 50mM DA-exposed cultures compared to PBS, 12 and 25 mM DA-exposed cultures (Figure 1B; ANOVA; n=4/group; **** p<0.0001). Both LDH and Casp-3/7 did not differ in 12 and 25 mM DA-exposed cultures from PBS controls. These results suggest that cell death in human airway epithelial cultures did not occur until 50 mM DA exposure for one hour and is partially mediated through apoptosis.
Figure 1.
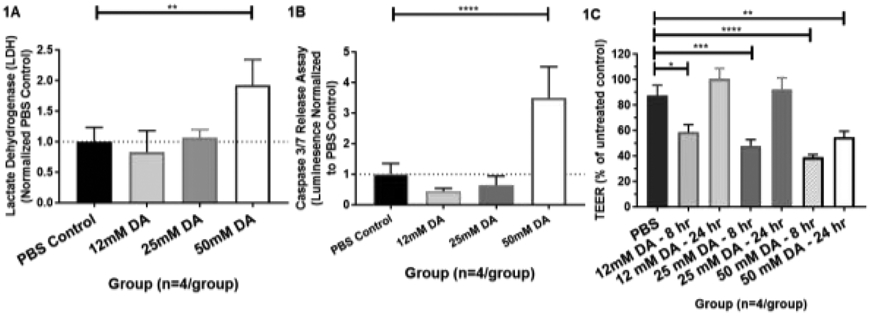
(A) Supernatant lactate dehydrogenase (LDH) at 24 hours post exposure. Diacetyl (DA) concentrations included 12, 25, and 50 mM or PBS control. Supernatant LDH differed significantly from PBS control at 50mM DA exposure (ANOVA; n=4/group; **p<0.01) (B) Supernatant caspase 3/7 release assay at 24 hours post exposure. Diacetyl (DA) concentrations included 12, 25, and 50 mM or PBS control. Supernatant caspase 3/7 release differed significantly at 50mM DA exposure from PBS control (ANOVA; n=4/group; ****p<0.0001). (C) Trans-epithelial electrical resistance (TEER) at 8 and 24 hours post-DA exposure for 12, 25, and 50 mM DA concentrations. TEER differed significantly at 8 hours from PBS controls in all DA concentrations (ANOVA with Tukey’s; n=4/group; 12 mM DA, *p<0.05; 25 mM DA, ***p<0.001; 50 mM DA, ****p<0.0001) and at 24 hours at 50 mM DA (ANOVA; n=4/group; **p<0.01).
3.2. Transient Decrease of Trans-Epithelial Electrical Resistance (TEER)
Trans-epithelial electrical resistance (TEER) was used as a marker of airway epithelial cellular permeability before and after DA exposures. In all DA-exposed cultures, TEER measurements decreased significantly at 8 hours post-exposure compared to PBS exposed controls (Figure 1C; ANOVA; n=4/group; * p<0.05). TEER remained significantly decreased at 24 hours in 50mM DA-exposed cultures (ANOVA with Tukey’s; n=4/group; ** p<0.01), but did not differ significantly at lower DA concentrations (12 and 25 mM) from PBS controls. Thus, all airway epithelial cultures at the tested DA concentrations demonstrated a transient decrease in airway epithelial permeability within 8 hours of DA exposure, but only cells exposed to 50 mM DA demonstrated persistent cellular permeability at 24 hours.
3.3. H&E of Primary Human Airway Epithelial Cells Exposed to DA
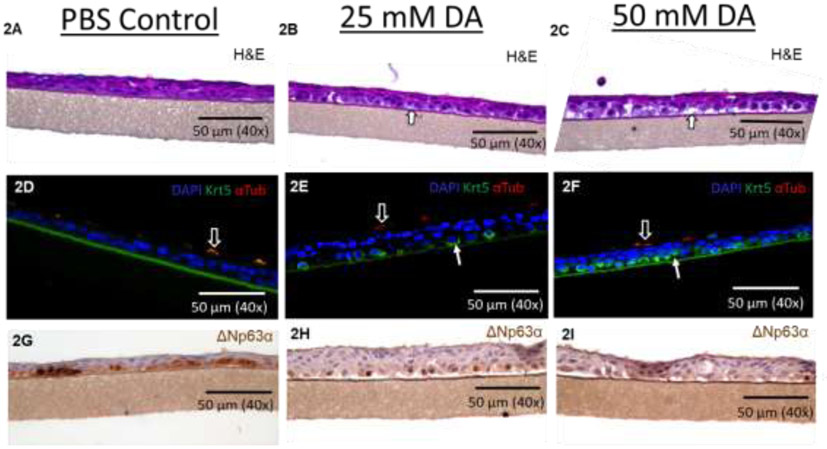
Human airway epithelial cultures were fixed at 24 hours post-exposure to evaluate for histologic evidence of airway injury. Airway epithelial cultures exposed to PBS demonstrated no morphologic changes by histology (Figure 2A). Conversely, morphologic changes to the airway epithelium demonstrated intra- and intercellular clearing with cytoplasmic hypopigmentation in the basal and suprabasal layers (Figure 2B and 2C; white arrows).
Figure 2.
Representative images of hematoxylin and eosin (H&E)-stained sections of airway epithelial cultures at 24 hours after exposure to PBS (A), 25 mM DA (B) and 50 mM DA (C). Representative images of immunofluorescent-stained sections for keratin 5 (Krt5; green; solid arrow), ciliated cell (α-tubulin; red; outlined arrow), and nuclear (DAPI; blue) at 24 hours following exposure to PBS (D), 25 mM DA (E) and 50 mM DA (F). Of note, autofluorescence of culture membrane occurred with red and green fluorescent antibodies in PBS controls (D). Representative images of delta N p63 (ΔNp63)-stained sections in airway cultures at 24 hours after exposure to PBS (G), 25 mM DA (H) and 50 mM DA (I).
3.4. Antibody Staining for Airway Epithelial Cell Markers
Considering DA exposure primarily affected the airway basal cell layer, staining for common airway basal cell markers, specifically Krt5 and delta N p63 (ΔNp63) was performed. Acetylated tubulin, a common marker for airway ciliated cells, was also performed for comparison. Compared to PBS controls, more prominent Krt5 staining was apparent in 25 mM and 50mM DA-exposed culture sections (Figure 2D, 2E, and 2F; green; solid arrows). Staining for ΔNp63 decreased in 50mM DA-exposed cultures (Figure 2G, 2H, and 2I; brown) compared to PBS controls. Ciliated cell staining with acetylated tubulin did not differ between PBS controls and DA-exposed cultures (Figure 2D, 2E, and 2F; red; hollow arrows). Considering increased expression of Krt5 may be due to increased basal cell proliferation, Ki67+ staining was also performed in PBS and DA-exposed cultures. The number of Ki67+ cells per 1000 μm of culture basement membrane did not differ significantly between 25mM DA exposed sections (Supplemental Figure A.1; n=8/group; t-test, p=0.20).
3.5. Quantification of Airway Basal Cell Markers - Krt5 and ΔNp63
To quantitate changes seen on histology, western blot analyses for Krt5, ΔNp63, and β-actin (internal control) were performed. Krt5 expression (58 kDa) normalized for β-actin did not differ significantly in 25mM and 50mM DA-exposed cultures compared PBS controls (Figure 3A; n=4/group; ANOVA, p>0.05). Surprisingly, a significant increase in Krt5 expression was seen at higher molecular weights in both 25 and 50 mM DA-exposed cultures (Figure 3A; n=4/group; ANOVA, **p=0.01). Quantification of Krt5 expression at 116 kDa (double Krt5 molecular weight) normalized for β-actin differed significantly at 24 hours in 25 mM DA (Figure 3A; ; n=4/group; ANOVA, ***p<0.001) and 50 mM DA (Figure 3A; n=4/group; ANOVA with Tukey’s, **p<0.01) cultures compared to PBS controls. ΔNp63α decreased significantly in 50 mM DA-exposed cultures compared to PBS controls (Figure 3B; n=4/group; ANOVA, *p=0.016).
Figure 3.
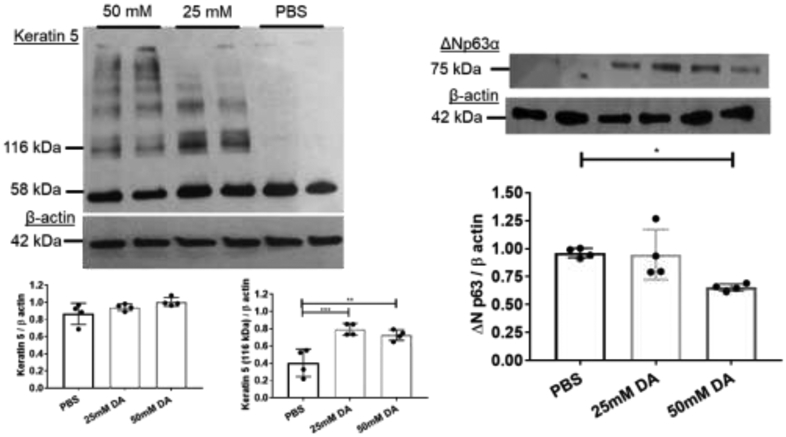
Representative western blot analyses for (A) Keratin 5 and (B) ΔNp63 expression in airway cellular homogenates at 24 hours post-exposure in DA (25 and 50 mM) and PBS controls. Beta-actin used as a loading control (42 kDa). Quantification of Krt5 expression at 58 kDa and 116 kDa normalized to β-actin for PBS, 25 mM, and 50 mM DA (n=4/group; lower left graph). Normalized Krt5 expression at 116kDa differed significantly from PBS control at 25 mM DA (ANOVA with Tukey’s; n=4/group ***p<0.001; lower right graph) and 50 mM DA (ANOVA with Tukey’s; n=4/group; **p<0.01). (B) Normalized ΔNp63α expression decreased significantly from PBS control at 50 mM DA (ANOVA with Tukey’s; n=4/group; *p=0.016).
3.6. Time-Dependent Degradation of Keratin 5 After DA Exposure
Next, we assessed for changes to Krt5 and ΔNp63 expression at 1, 3, and 5 days after 25mM DA exposure. Increased expression of higher molecular weight Krt5 (116 kDa) occurred in airway homogenates exposed to 25mM DA vapor compared to PBS controls at Day 1 after DA exposure (left four lanes; Figure 4A). At Day 3 and Day 5 post-exposure, higher molecular weight Krt5 expression was not significantly increased in 25mM DA-exposed cultures compared to PBS controls (Figure 4A; n=4/group; ANOVA with Tukey’s, p>0.05). In contrast, Krt5 (58kDa) decreased at Day 3 and 5 after DA exposure in 25mM DA-exposed cultures compared to PBS controls (Figure 4A; n=4/group; ANOVA, **p=0.002). ΔNp63 expression also decreased at Day 3 and 5 after DA exposure in 25mM DA-exposed cultures compared to PBS controls (Figure 4B; n=4/group; ANOVA, *p=0.011).
Figure 4.
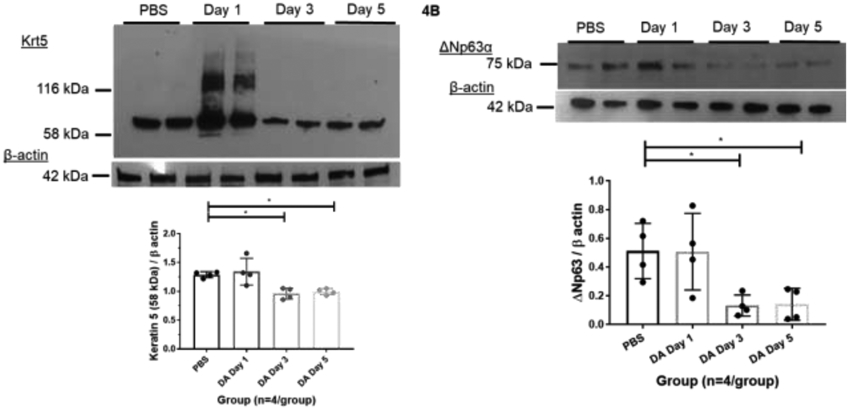
Representative western blot of (A) keratin 5 (Krt5) and (B) ΔNp63α expression in cellular homogenates exposed to 25mM DA for 1 hour at Day 1, 3 and 5 after exposure compared to PBS controls (n=4/group). Beta-actin used as a loading control (42 kDa). Normalized Krt5 expression at 58 kDa differed significantly from PBS control at Day 3 (ANOVA with Tukey’s; n=4/group; *p=0.019) and Day 5 (ANOVA with Tukey’s; n=4/group; **p=0.035). (B) Normalized ΔNp63α expression decreased significantly from PBS control at Day 3 (ANOVA with Tukey’s; n=4/group; *p=0.045) and Day 5 (ANOVA with Tukey’s; n=4/group; *p=0.048).
3.7. Increased K48-ubiquitination and Co-localization of Ubiquitin C with Krt5 After Exposure
One potential reason for the shift in Krt5 molecular weight seen after DA vapor exposure is keratin ubiquitination due to protein damage (6, 23). Considering that cytoskeletal keratin ubiquitination requires proteasome degradation for proper recycling (23), we performed staining for K48-linked ubiquitination in exposed human airway epithelial culture homogenates after DA exposure. Airway epithelial cultures exposed to 25mM DA vapor demonstrated increased staining for K48-linked ubiquitination at Day 1 after DA exposure compared to PBS control samples (Figure 5A; middle two lanes). At three and five days after DA exposure (when Krt5 damage had resolved), K48-linked ubiquitination expression in 25mM DA-exposed cultures was similar to PBS controls (Figure 5A; far right four lanes).
Figure 5.
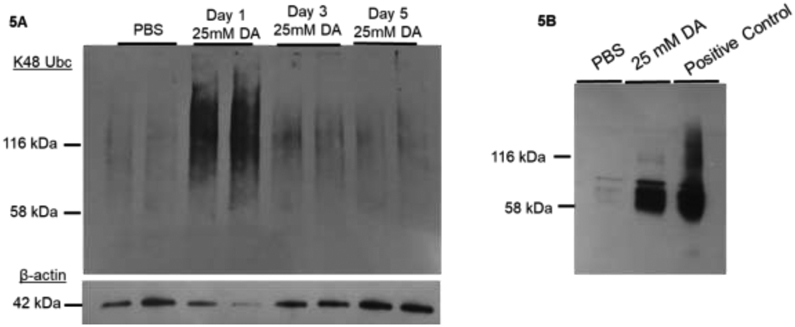
(A) Representative western blot image of K48-linked ubiquitin in airway cellular homogenates exposed to PBS or 25mM DA. Beta-actin used as a loading control (42 kDa). Increased staining for K48-linked ubiquitin occurred at Day 1 following 25mM DA exposure compared to PBS controls (middle left two lanes). By Day 3 and 5 after 25mM DA exposure, K48-linked ubiquitin normalized to PBS control levels. (B) Representative western blot image of ubiquitin C in cellular homogenates immunoprecipitated (IP) for keratin 5 (Krt5) after exposure to PBS or 25mM DA. Airway cultures incubated with 100mM MG132 for 24 hours were used as a positive control. Increased staining for ubiquitin C in 25mM DA-exposed Krt5 IP compared to PBS controls.
Next, we performed immunoprecipitation (IP) for Krt5 from human airway epithelial cultures following exposure to 25mM DA or PBS to assess for co-localization of poly-ubiquitination with Krt5. Airway cultures treated with a high concentration of MG132 (100mM) but not exposed to DA was used as the positive control (Figure 5B; far right lane). At Day 1, Krt5 IP demonstrated increased ubiquitin C expression in 25mM DA exposed airway epithelial cultures compared PBS controls samples (Figure 5B).
3.8. Proteasome Activity Associated Temporally with Keratin 5 Damage Resolution
When K48-linked ubiquitination occurs, proteasome degradation ensues (9). Thus, we assessed for proteasome 20S activity following DA exposure in primary human airway epithelial cellular homogenates. No significant change in proteasome 20S activity was seen in DA-exposed cultures compared to PBS control samples at Day 1 after DA exposure (Figure 6A; ANOVA with Tukey’s; p>0.05; n=8/group). By Day 3, proteasome 20S activity increased significantly in DA-exposed controls compared to PBS controls (Figure 6A; ANOVA with Tukey’s; **p<0.01; n=8/group). Increased proteasome 20S activity correlated temporally (at Day 3) with resolution of higher molecular weight Krt5 expression after DA exposure.
Figure 6.
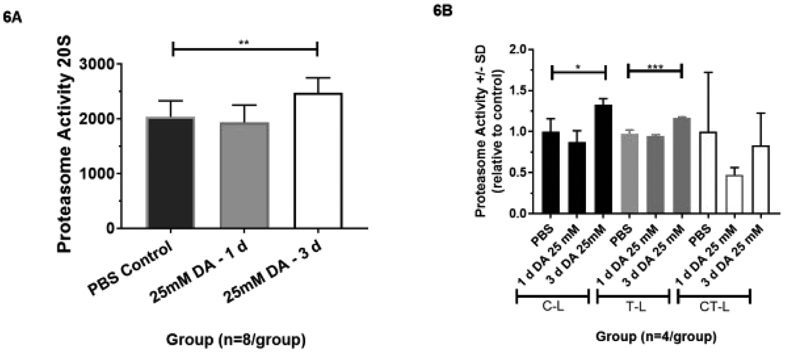
(A) Proteasome 20S activity in 25mM DA or PBS vapor-exposed airway epithelial cellular homogenates at 1 and 3 days post-exposure. Proteasome 20S activity increased significantly in 25mM DA exposed cellular homogenates at Day 3 from PBS control (ANOVA with Tukey’s; **p<0.01, n=8/group). (B) Proteasome activity by sub-type (caspase-like (C-L), trypsin-like (T-L), chymotrypsin-like (CT-L)) in PBS or 25 mM DA exposed samples at 1 and 3 days post-exposure. C-L and T-L activity increased significant in human airway epithelial samples at Day 3 after 25 mM DA exposure compared to PBS and Day 1 DA-exposed samples (ANOVA; n=4/group; *p<0.05 and ***p<0.001, respectively). Conversely, no significant increase in CT-L activity occurred at Day 1 or Day 3 after exposure compared to PBS controls (ANOVA; n=4/group; p<0.05).
Individual proteolytic site activities, including caspase-like (C-L), trypsin-like (T-L), and chymotrypsin-like (CT-L), were also assessed in cellular homogenates at Days 1 and 3 after DA exposure. Again, no significant change in C-L, T-L, or CT-L activity occurred in DA-exposed cultures compared to PBS controls at Day 1 after exposure (Figure 6B; n=4/group; ANOVA with Tukey’s, p>0.05). At Day 3 post-exposure, a significant increase in C-L and T-L activity occurred in DA-exposed cultures compared to PBS controls (Figure 6B; ANOVA with Tukey’s; *p<0.05 and ***p<0.001, n=4/group, respectively). No significant increase in CT-L activity in DA-exposed cultures compared to PBS controls occurred at Day 3 after DA exposure (Figure 6B; n=4/group; ANOVA with Tukey’s, p>0.05). Variance of CT-L activity was greater than that of C-L and T-L activities, most likely due to difference in substrate content, cleavage site preference, and cell type specificity (12).
3.9. Proteasome Inhibition with MG132 Causes Persistence of Keratin 5 Injury
To further validate Krt5 protein degradation is mediated through the ubiquitin-proteasome pathway after DA exposure, human primary airway epithelial cells were incubated with the reversible proteasome inhibitor MG132 (100nM) daily for 3 days. Prior to DA exposure, we verified proteasome 20S activity being significantly inhibited in PBS controls incubated with MG132 compared to PBS controls without MG132 (Supplemental Figure A.2; Welch’s t-test; n=4/group; **p<0.0011). Similar to prior 25mM DA exposures, increased Krt5 expression at higher molecular weight (116 kDa) occurred at Day 1 after DA exposure with and without MG132 co-incubation (Figure 7A; middle 8 lanes; n=4/group; ANOVA, ****p<0.0001). In contrast, higher molecular weight Krt5 expression persisted at Day 3 after DA exposure when co-incubated with MG132, but resolved in the absence of MG132 (Figure 7A; far right eight lanes). Higher molecular weight Krt5 expression differed significant in 25mM DA-exposed cultures + MG132 compared to 25mM DA-exposed cultures alone (Figure 7B; n=4/group; ANOVA with Tukey’s, *p=0.04). Co-incubation with MG132 also increased Krt5 expression at 58 kDa at Day 3 after 25mM DA exposure compared to Day 3 25mM DA without MG 132 (Figure 7B; n=4/group; ANOVA with Tukey’s, ***p=0.0002). Collectively, proteasome inhibition with MG132 suggest impaired clearance of Krt5 following DA vapor exposure.
Figure 7.
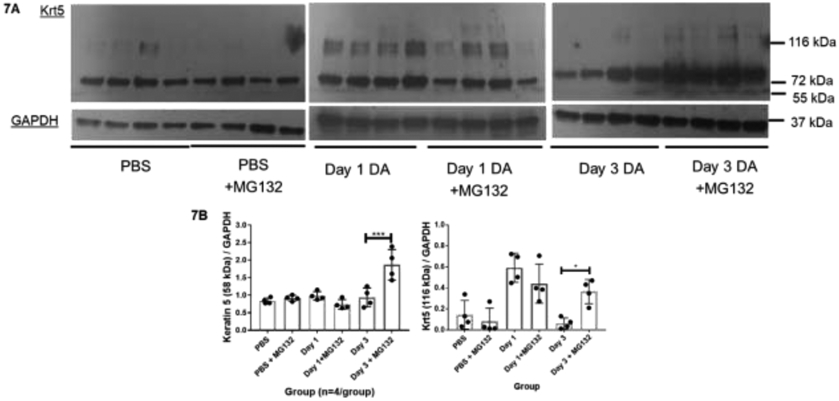
(A) Representative western blot images of Keratin 5 in PBS or 25mM DA-exposed airway epithelial cells co-incubated with proteasome inhibitor MG132 (100nM) or PBS for 1 or 3 days after exposure. GAPDH expression used as a loading control. (B) Quantification of Keratin 5 (58 kDa; leff) and higher molecular weight Krt5 (116 kDa; right) normalized to GAPDH under each condition (n=4/group). Increased expression of Krt5 (58kDa) in cellular homogenates at Day 3 after exposure to 25mM DA exposure + MG132 (100nM) compared to Day 3 25mM DA without MG132 (ANOVA; n=4/group, ***p=0.0002). When treated with MG132 100nM, normalized Krt5 at 116kDa persisted in cellular homogenates at 3 days after DA 25mM exposure with increased expression compared to Day 3 25mM DA without MG132 (ANOVA with Tukey’s; n=4/group *p=0.043).
4. DISCUSSION
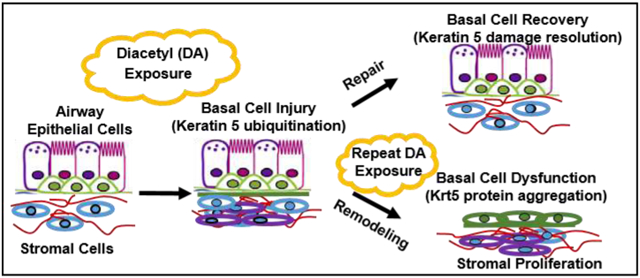
A single, one-hour diacetyl (DA) vapor exposure caused significant airway basal cell injury with ubiquitination of keratin 5 (Krt5) and decreased ΔNp63. At DA exposure concentrations below 50mM DA for one hour, the airway epithelium recovered without significant cellular death or persistent loss of cellular integrity by trans-epithelial electrical resistance. Independent of cell death or loss of barrier integrity, DA exposure resulted in histologic evidence of basal cell injury with decreased ΔNp63 expression and Krt5 ubiquitination. When monitored for up to five days after exposure, ubiquitin-proteasome system activation occurred, resulting in the subsequent reduction of total Krt5 and ΔNp63 expression. Additionally, proteasome inhibition resulted in the persistence of DA-induced damage to basal cell keratin 5.
Unique to this work, changes to the airway basal cells occur prior to a significant rise in lactate dehydrogenase after a single DA exposure. Common airway basal cell markers, specifically cytoplasmic keratin 5 and transcription factor ΔNp63α, were used for identification of DA-induced basal cell injury after exposure. Using immunoprecipitation, poly-ubiquitination co-localized with Krt5 following DA exposure (Figure 5B). In prior experiments using repeated DA exposures, other investigators have also identified damage to airway keratins (6). One potential mechanism for this damage to airway keratins is non-enzymatic protein adduction of DA to arginine (18). Arginine is found abundantly on keratin intermediate filament (2). Protein adduction occurs through a Michael reaction, where DA (an electrophile) reacts non-enzymatically with arginine (a nucleophile). We hypothesize the increased susceptibility of airway basal cells to DA vapor exposure is secondary to the high reactivity of DA with the common and abundant nucleophilic base arginine on keratin intermediate filaments. Our work emphasizes the specific susceptibility of airway basal cells to DA vapor exposure.
In addition to Krt5 ubiquitination, transcription factor ΔNp63α expression decreased after a single 50mM DA exposure. One of the primary functions of p63 is maintenance of epidermal stratification (13, 14, 26). When p63 expression is downregulated, p53 is activated for terminal differentiation, preventing further proliferation (26). Two potential long-term effects of decreased ΔNp63 expression after DA exposure are suppressed proliferative capacity and/or early terminal differentiation in airway basal cells. Consistent with the prior, we did not find a significant increase in Ki67+ staining in airway cultures 24 hours after DA vapor exposure (Supplemental Figure A.1). Foster et al. identified previously increased involucrin expression, a marker of squamous metaplasia and decreased cilia expression after repeated DA exposure (6). Collectively, these results of decreased ΔNp63α with increased involucrin expression are most consistent with early terminal differentiation of the airway basal cells after DA exposure.
The primary purpose of our experiments was to evaluated airway basal cell repair, and not epithelial injury alone, after DA exposure. To characterize the temporal resolution after DA exposure, we evaluated airway epithelial homogenates for changes in Krt5 and ΔNp63α expression at various time points (1, 3 and 5 days) after a single 25 mM DA exposure. Twenty-five millimolar DA was chosen as cells did not undergo cell death or a persistent loss of barrier integrity at this concentration (Figure 1). Three days after exposure, Krt5 damage resolved in airway cellular homogenates (Figure 4). Damage resolution occurred temporally (at day 3) with a subsequent decrease in Krt5 and ΔNp63α expression (Figure 4). When proteasome function was inhibited with MG132, Krt5 damage persisted in the airway epithelial cells (Figure 7). Proteasome-mediated degradation of epithelial intermediate filaments is the primary damage repair response to stress in the lung, as seen previously with shear stress or hypoxia (20, 23). Under continual shear stress, ubiquitin co-localizes with keratin 8 and 18 (9). With the addition of proteasome inhibition, damaged keratins accumulate and form aggresomes (17). Similar to shear stress, keratin injury following chemical DA vapor exposure resulted in keratin 5 ubiquitination. To the best of our knowledge, this is the first study to identify keratin 5 ubiquitination and proteasome degradation mediating changes to Krt5 expression after diacetyl vapor exposure.
There are some limitations to the current study requiring further investigation. First, all of the work occurred in vitro. Future in vivo studies are required to evaluate the implications of decreased ΔNp63 expression and/or Krt5 ubiquitination after DA exposure. Second, changes to the airway epithelium were characterized after a single DA exposure. The calculated parts-per-million (ppm) concentrations for the DA exposures ranged from 500 to 2000 ppm (5, 11). These concentrations are similar in magnitude to peak DA concentrations measured in popcorn plant factories (4, 10) as well as those concentrations used in previous in vitro airway epithelial culture experiments (15). Future experiments are required to assess whether increased keratin ubiquitination contributes to decreased p63 expression or additional basal cell dysfunction after DA exposure. Lastly, we did not assess for other non-proteasome causes of DA-induced epithelial injury. Other non-proteasome signaling cascades, such as K63-linked ubiquitination, may be activated after DA exposure (8).
In conclusion, a single one-hour DA vapor exposure causes significant airway basal cell injury with keratin 5 ubiquitination and decreased delta Np63 expression. With sufficient time, keratin 5 ubiquitination resolves, and is mediated through proteasome degradation. Future studies are required to evaluate the persistent effects of keratin 5 damage on airway basal cell function after repeated in vitro and in vivo DA vapor exposures.
Supplementary Material
HIGHLIGHTS.
Acute diacetyl vapor exposure causes significant airway basal cell injury with keratin 5 ubiquitination and decreased ΔNp63 expression
Airway epithelial repair after diacetyl vapor exposure occurs through ubiquitination and subsequent proteasome degradation of keratin 5 protein damage.
Inhibition of the ubiquitin-proteasome pathway after diacetyl vapor exposure results in impaired clearance of keratin 5 ubiquitinated proteins
ACKNOWLEDGEMENTS
The authors thank the University of Rochester’s Inhalation Exposure Facility, specifically Director Alison Elder, PhD, David Chalupa, MS, and Robert Gelein, MS, for their continued support with diacetyl inhalation exposures. Authors also thank Antony Leonard, MS, for his assistance with immunoprecipitation and Jon Oldach and Anna Maione with MatTek, Corporation for support on the project.
FUNDING
This work was supported in part by the David H. Smith Fund (UR), WNY Center for Research on Flavored Tobacco Products (CRoFT) #U54 CA228110, and a grant from the National Institute of Environmental Health Sciences #P30 ES001247.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, and Christiani DC. Flavoring Chemicals in E-Cigarettes: Diacetyl, 2,3-Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit-, Candy-, and Cocktail-Flavored E-Cigarettes. Environ Health Perspect 124: 733–739, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders MW. Diacetyl and related flavorant alpha-Diketones: Biotransformation, cellular interactions, and respiratory-tract toxicity. Toxicology 388: 21–29, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Bailey RL, Cox-Ganser JM, Duling MG, LeBouf RF, Martin SB Jr., Bledsoe TA, Green BJ, and Kreiss K. Respiratory morbidity in a coffee processing workplace with sentinel obliterative bronchiolitis cases. Am J Ind Med 58: 1235–1245, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boylstein R, Piacitelli C, Grote A, Kanwal R, Kullman G, and Kreiss K. Diacetyl emissions and airborne dust from butter flavorings used in microwave popcorn production. J Occup Environ Hyg 3: 530–535, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Brass DM, Gwinn WM, Valente AM, Kelly FL, Brinkley CD, Nagler AE, Moseley MA, Morgan DL, Palmer SM, and Foster MW. The Diacetyl-Exposed Human Airway Epithelial Secretome: New Insights into Flavoring-Induced Airways Disease. Am J Respir Cell Mol Biol 56: 784–795, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster MW, Gwinn WM, Kelly FL, Brass DM, Valente AM, Moseley MA, Thompson JW, Morgan DL, and Palmer SM. Proteomic Analysis of Primary Human Airway Epithelial Cells Exposed to the Respiratory Toxicant Diacetyl. J Proteome Res 16: 538–549, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubbs AF, Battelli LA, Goldsmith WT, Porter DW, Frazer D, Friend S, Schwegler-Berry D, Mercer RR, Reynolds JS, Grote A, Castranova V, Kullman G, Fedan JS, Dowdy J, and Jones WG. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol Appl Pharm 185: 128–135, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Hubbs AF, Fluharty KL, Edwards RJ, Barnabei JL, Grantham JT, Palmer SM, Kelly F, Sargent LM, Reynolds SH, Mercer RR, Goravanahally MP, Kashon ML, Honaker Jc, Jackson MC, Cumpston AM, Goldsmith WT, McKinney W, Fedan JS, Battelli LA, Munro T, Bucklew-Moyers W, McKinstry K, Schwegler-Berry D, Friend S, Knepp AK, Smith SL, and Sriram K. Accumulation of Ubiquitin and Sequestosome-1 Implicate Protein Damage in Diacetyl-Induced Cytotoxicity. Am J Pathol 186: 2887–2908, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaitovich A, Mehta S, Na N, Ciechanover A, Goldman RD, and Ridge KM. Ubiquitin-proteasome-mediated degradation of keratin intermediate filaments in mechanically stimulated A549 cells. J Biol Chem 283: 25348–25355, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanwal R, Kullman G, Piacitelli C, Boylstein R, Sahakian N, Martin S, Fedan K, and Kreiss K. Evaluation of flavorings-related lung disease risk at six microwave popcorn plants. J Occup Environ Med 48: 149–157, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Kelly FL, Sun J, Fischer BM, Voynow JA, Kummarapurugu AB, Zhang HL, Nugent JL, Beasley RF, Martinu T, Gwinn WM, Morgan DL, and Palmer SM. Diacetyl induces amphiregulin shedding in pulmonary epithelial cells and in experimental bronchiolitis obliterans. Am J Respir Cell Mol Biol 51: 568–574, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kisselev AF, Callard A, and Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem 281: 8582–8590, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Koster MI, Kim S, Mills AA, DeMayo FJ, and Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 18: 126–131, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koster MI, and Roop DR. The role of p63 in development and differentiation of the epidermis. J Dermatol Sci 34: 3–9, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, and Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med 347: 330–338, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Krunkosky TM, Martin LD, Fischer BM, Voynow JA, and Adler KB. Effects of TNFalpha on expression of ICAM-1 in human airway epithelial cells in vitro: oxidant-mediated pathways and transcription factors. Free Radio Biol Med 35: 1158–1167, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Loeb KR, and Haas AL. Conjugates of ubiquitin cross-reactive protein distribute in a cytoskeletal pattern. Mol Cell Biol 14: 8408–8419, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathews JM, Watson SL, Snyder RW, Burgess JP, and Morgan DL. Reaction of the butter flavorant diacetyl (2,3-butanedione) with N-alpha-acetylarginine: a model for epitope formation with pulmonary proteins in the etiology of obliterative bronchiolitis. J Agrio Food Chem 58: 12761–12768, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan DL, Jokinen MP, Johnson CL, Price HC, Gwinn WM, Bousquet RW, and Flake GP. Chemical Reactivity and Respiratory Toxicity of the alpha-Diketone Flavoring Agents: 2,3-Butanedione, 2,3-Pentanedione, and 2,3-Hexanedione. Toxiool Pathol 44: 763–783, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Na N, Chandel NS, Litvan J, and Ridge KM. Mitochondrial reactive oxygen species are required for hypoxia-induced degradation of keratin intermediate filaments. Faseb J 24: 799–809, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park HR, O'Sullivan M, Vallarino J, Shumyatcher M, Himes BE, Park JA, Christiani DC, Allen J, and Lu Q. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci Rep 9: 1400, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce JS, Abelmann A, Spicer LJ, Adams RE, and Finley BL. Diacetyl and 2,3-pentanedione exposures associated with cigarette smoking: implications for risk assessment of food and flavoring workers. Crit Rev Toxicol 44: 420–435, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Rogel MR, Jaitovich A, and Ridge KM. The role of the ubiquitin proteasome pathway in keratin intermediate filament protein degradation. Proc Am Thorac Soc 7: 71–76, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simoes E, Phillips P, Maley R, Kreiss K, Malone J, and Kanwal R. Fixed obstructive lung disease in workers at a microwave popcorn factory - Missouri 2000-2002 (Reprinted from MMWR, vol 51, pg 345-347, 2002). Jama-J Am Med Assoc 287: 2939–2940, 2002. [Google Scholar]
- 25.Wang Q, Bhattacharya S, Mereness JA, Anderson C, Lillis JA, Misra RS, Romas S, Huyck H, Howell A, Bandyopadhyay G, Donlon K, Myers JR, Ashton J, Pryhuber GS, and Mariani TJ. A novel in vitro model of primary human pediatric lung epithelial cells. Pediatr Res 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, and McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 2: 305–316, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Zaccone EJ, Goldsmith WT, Shimko MJ, Wells JR, Schwegler-Berry D, Willard PA, Case SL, Thompson JA, and Fedan JS. Diacetyl and 2,3-pentanedione exposure of human cultured airway epithelial cells: Ion transport effects and metabolism of butter flavoring agents. Toxicol Appl Pharmacol 289: 542–549, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.