Abstract
Background
Hypofractionated stereotactic radiotherapy (HFSRT) is indicated for large brain metastases (BM) or proximity to critical organs (brainstem, chiasm, optic nerves, hippocampus). The primary aim of this study was to assess factors influencing BM local control after HFSRT.
Then the effect of surgery plus HFSRT was compared with exclusive HFSRT on oncologic outcomes, including overall survival.
Materials and methods
Retrospective study conducted in Léon Bérard Cancer Center, included patients over 18 years-old with BM, secondary to a tumor proven by histology and treated by HFSRT alone or after surgery. Three different dose-fractionation schedules were compared: 27 Gy (3 × 9 Gy), 30 Gy (5 × 6 Gy) and 35 Gy (5 × 7 Gy), prescribed on isodose 80%. Primary endpoint were local control (LC). Secondary endpoints were overall survival (OS) and radionecrosis (RN) rate.
Results
A total of 389 patients and 400 BM with regular MRI follow-up were analyzed. There was no statistical difference between the different dose-fractionations. On multivariate analysis, surgery (p = 0.049) and size (< 2.5 cm) (p = 0.01) were independent factors improving LC. The 12 months LC was 87.02% in the group Surgery plus HFSRT group vs 73.53% at 12 months in the group HFSRT. OS was 61.43% at 12 months in the group Surgery plus HFSRT group vs 50.13% at 12 months in the group HFSRT (p < 0.0085). Prior surgery (OR = 1.86; p = 0.0028) and sex (OR = 1.4; p = 0.0139) control of primary tumor (OR = 0.671, p = 0.0069) and KPS < 70 (OR = 0.769, p = 0.0094) were independently predictive of OS. The RN rate was 5% and all patients concerned were symptomatic.
Conclusions
This study suggests that HFSRT is an efficient and well-tolerated treatment. The optimal dose-fractionation remains difficult to determine. Smaller size and surgery are correlated to LC. These results evidence the importance of surgery for larger BM (> 2.5 cm) with a poorer prognosis. Multidisciplinary committees and prospective studies are necessary to validate these observations.
Keywords: Hypofractionated stereotactic radiotherapy, Brain metastases, Radionecrosis
Background
Brain metastases (BM) occur in up to 20–40% of all cancer patients [1]. Their incidence increases with the development of performant imaging and the advances of systemic therapies providing a longer survival [2]. Neurologic symptoms associated to BM can significantly impact quality of life [3]. Local treatment arsenal for BM includes neurosurgery, whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS) and more recently hypofractionated stereotactic radiotherapy (HFSRT) [4]. SRS is largely reported in literature. The use of SRS is however correlated to a high risk of radionecrosis [5]. Large lesions distant from critical structures are preferably treated by surgery [6]. In case of surgical contraindication HFSRT may be an option [7]. HFSRT provides radiobiological advantages compared to SRS when BM are larger or closer to critical structures such as brainstem or chiasm [8, 9]. HFSRT offers the ability to deliver higher dose in terms of radiobiological equivalent dose on large BM, and to reduce toxicity of normal brain tissue [9]. HFSRT probably improves the therapeutic ratio and increases tumor control probability as suggested by an in silico study [8]. However, one of HFSRT relevant challenges is to assess the optimal dose and fractionation regimen associated to local control of BM, according to size and volume [9].
The objectives of this study were to investigate prognostic factors influencing BM local control (LC), overall survival (OS) and radionecrosis (RN) development, in patients treated by HFSRT in the clinical practice of a single institution over 8 years. Thus, the effect of surgery plus HFSRT was compared with exclusive HFSRT on oncologic outcomes, including overall survival.
Materials and methods
Study population
This is a retrospective and monocentric study conducted at the Léon Bérard Cancer Center in Lyon. Eligible patients were over 18 years of age, treated by exclusive intracranial HFSRT or surgery with post-operative HFSRT. All patients had a histological proof of primary tumor by biopsy. Patients who underwent prior WBRT or surgery were also included in outcome analysis. Exclusion criteria were the presence of primary intracranial tumors (e.g. meningioma or glioblastoma), and other stereotactic irradiation. This study was ethically approved by the Léon Bérard Cancer Center institutional board.
Data collection
The main clinical data were extracted from computerized patients’ records were: age, sex, Karnofsky performance status (KPS), primary tumor histology, extracranial disease control. Prognostic scores Recursive Partitioning Analysis (RPA) [10], modified Recursive Partitioning Analysis (mRPA) [11, 12], Graded Prognostic Assessment (GPA) [13], Diagnostic-Specific Graded Prognostic Assessment (DS-GPA) were calculated for each patient [13] . Neurological symptoms prior BM treatment (motor or sensitive dysfunction, intracranial hypertension, and aphasia) were also reported from retrospective data [3]. The assessment of neurological symptoms was only qualitative. We also took into account the systemic treatments delivered concomitantly or within 1 month of radiotherapy, as well as whether or not the extracerebral disease was controlled. The systemic treatments included chemotherapy, targeted therapy and immune-checkpoint inhibitors [14] .The fact that the extracerebral disease is not controlled may be related either to a bifocal recurrence or to an initial metastatic tumour.
Regarding BM characteristics, reported informations were maximal diameter, number and location. BM diagnosis was performed either on a cerebral MRI, biopsy or operative piece histology. Percentage of meningial spreading was also assessed for brain metastases treated by surgery and HFSRT [15] .
Hypofractionated stereotactic radiotherapy (HFSRT)
For each patient, surgery or HFSRT indication was discussed at a multidisciplinary staff meeting. HFSRT was delivered by Cyberknife™ (Accuray Inc., Sunnyvale, CA). The non-injected simulation CT-scanner was performed in supine position.
A thermoformed frame mask system was used. MRI images (1 mm slice thickness) were registered with planning CT scan (1 mm slice thickness) on Multiplan (Accuray) workstation. Gross Tumor Volume (GTV) corresponded to contrast-enhancing lesion on T1 sequences of brain fusion MRI. If an adjuvant HFSRT was performed, GTV represented the surgical cavity. In both cases, a 2 mm margin was systematically added to GTV to generate the planning target volume (PTV). When BM was closer to critical structures, margin could be reduced to 0–1 mm and organs at risk (OAR) was excluded from PTV.
The main risk-delineated organs were brainstem, optic nerves, chiasm and hippocampus. Dose and fractionation regimens were chosen at the discretion of the radiotherapist, depending on the BM size, OAR proximity (brainstem, corpus callosum, optic nerves and chiasm), and prior WBRT or surgery. For the smaller lesions, 3 fraction protocols are frequently used. Concerning larger lesions or lesions located near OAR or in functional sites, 5 fraction schedules are used. The prescription isodose was 80%. Treatment plan validation was done according to PTV coverage (up to 95%) and respect of maximal dose received by OAR according Timmerman recommendations [16].
Brain metastases or post-operative cavities were irradiated on alternate day, every other day.
Follow-up after HFSRT
The follow-up was based on a clinical neurological examination and on a brain MRI every 3 months 1st year and every 6 months the following years, according to ANOCEF recommendations [17].
Study endpoints
Primary endpoint was local control defined by stability, partial or complete response of the lesion after HFSRT. Secondary endpoints were overall survival (OS) and rate of radionecrosis (RN). OS corresponded to the interval between the date of HFSRT and date of last follow-up or death. The definition of RN was more extensive and heterogeneous. The RN diagnosis was performed on MRI surveillance or histology [18] . Further images as spectrometric MRI could be requested, if there was uncertainty between RN or local failure diagnosis [19, 20]. MRI were reviewed by an experimented radiotherapist. Stability of BM size over several months was in favor of RN.
Statistical analysis
Categorical variables were presented as numbers (percentage) and continuous variables were described as median and standard deviation (minimum and maximum values).
LC and RN analyses were performed using a per-lesion basis. Evaluation of OS was conducted on per-patient basis. Kaplan Meier curves were generated to estimate both endpoints. Log-Rank test was used to assess predictive factors on survival outcomes.
For OS and LC estimates, living patients were censored at the last follow-up and for others, at the most recent visit or death.
Cox univariate and multivariate regression were performed to determine independent predictive factors of local control and OS. Co-variables with p-value less than 0.2 on univariate analysis, were introduced in the statistical model. Variables with more than 20% missing data were not included in the multivariate model. All tests were two sided and P values were considered significant when less than 0.05. Analyzes were performed using the SAS 9.4 statistical software.
Results
Patient population
Between January 2011 and January 2018, a total of 427 patients were treated by HFSRT at Léon Bérard Cancer Center. Median age was 62 years-old (18–87). There were 188 men and 239 women. WBRT was performed before HFSRT on 39 patients (9%).
Patients characteristics are presented in Table 1. Patients were divided into two groups Surgery plus HFSRT and HFSRT. Both groups were similar in terms of age, sex, GPA, DS-GPA, systemic treatment and control of primary tumor. Significant differences between both groups were based on extracranial control, neurologic symptoms, RPA and mRPA.
Table 1.
Characteristics of patients
| Surgery + HFSRT N = 99 |
HFSRT N = 328 |
pValue | |
|---|---|---|---|
| Age | 0.1511 | ||
| Median [min - max] | 59.0 [13.2–87.0] | 60.8 [8.3–85.9] | |
| Sex | 0.4587 | ||
| male | 52 (52.5%) | 185 (56.7%) | |
| Female | 47 (47.5%) | 143 (43.3%) | |
| KPS | 0.25 | ||
| 100–90 | 38 (38%) | 96 (30%) | |
| 80–70 | 46 (46%) | 169 (52%) | |
| < 70 | 15 (15%) | 60 (18%) | |
| RPA | <.0001 | ||
| 1 | 29 (29.3%) | 35 (10.7%) | |
| 2 | 55 (55.6%) | 233 (71.0%) | |
| 3 | 15 (15.2%) | 60 (18.3%) | |
| m RPA | < 0.001 | ||
| 1 + 2a | 55 (56%) | 89 (27%) | |
| 2b | 17 (17%) | 90 (28%) | |
| 2c + 3 | 27 (27%) | 146 (45%) | |
| GPA | 0.0868 | ||
| < = 3 | 98 (99.0%) | 311 (94.8%) | |
| > 3 | 1 (1.0%) | 17 (5.2%) | |
| DS-GPA | 0.2485 | ||
| < = 3 | 78 (87.6%) | 265 (91.7%) | |
| > 3 | 11 (12.4%) | 24 (8.3%) | |
| Not applicable | 10 | 39 | |
| Survival at the last follow-up | 0.0227 | ||
| Alive | 63 (63.6%) | 166 (50.6%) | |
| Dead | 36 (36.4%) | 162 (49.4%) | |
| Extracranial control | 0.0001 | ||
| Yes | 49 (49.5%) | 116 (34.4%) | |
| No | 41 (41.4%) | 182 (55.5%) | |
| NC | 9 (9.1%) | 30 (9.1%) | |
| Neurologic symptoms | <.0001 | ||
| No | 77 (77.8%) | 133 (52.0%) | |
| Yes | 22 (22.2%) | 123 (48.0%) | |
| NC | 0 | 72 | |
| Systemic treatement | 0.3197 | ||
| No | 56 (58.3%) | 197 (64.0%) | |
| Yes | 40 (41.7%) | 111 (36.0%) | |
| Control of primary tumor | 0.6333 | ||
| Yes | 49 (50.5%) | 148 (47.7%) | |
| No | 50 (49.5%) | 180 (52.3%) |
Brain metastasis
A total of 535 BM was identified in the study and 400 BM (389 patients) were followed with an MRI. Median size was 2.3 cm (0.6–6.5 cm). The main histology and location was lung cancer (55%), followed breast cancer (12%), clear cell renal carcinoma (10%) and melanoma (5.2%). On 535 BM, 88 (20%) had surgical resection before HFSRT. Among BM treated by surgery and HFSRT, 13 cases of meningial spreading were detected on MRI or cytology. BM were split into radio resistant group (clear cell carcinoma cancer, sarcoma and melanoma) and radiosensitive group (other histological types), counting 120 and 414 BM respectively.
In the group of Surgery plus HFSRT (Table 2), 71% BM were larger than 2.5 cm vs 36.7% in comparison with the group HFSRT (p < .0001). More than half of post-operative cavities received a dose of 30 Gy in 5 fraction or 35 in 5 fractions. In the group HFSRT, a dose of 27 Gy in 3 fractions was applied on 47.1% (n = 202). Concerning prior systemic treatment, there was no significant differences between both groups (Table 1).
Table 2.
Characteristic of treated brain metastasis
| Surgery + HFSRT N = 105 |
HFSRT N = 429 |
pvalue | |
|---|---|---|---|
| Size | <.0001 | ||
| < 2.5 cm | 29 (29.0%) | 246 (63.2%) | |
| 2.5–4 cm | 47 (47.0%) | 123 (31.6%) | |
| > 4 cm | 24 (24.0%) | 20 (5.1%) | |
| 5 | 40 | ||
| Total dose and fractionation (gy) | <.0001 | ||
| 27 | 12 (11.4%) | 202 (47.1%) | |
| 30 | 54 (51.4%) | 108 (25.2%) | |
| 35 | 39 (37.1%) | 119 (27.7%) | |
| WBRT | 0.0329 | ||
| Yes | 3 (2.9%) | 35 (8.1%) | |
| No | 102 (97.1%) | 394 (91.9%) | |
| Tumor Histology | 0.5626 | ||
| Breast | 13 (12.4%) | 53 (12.4%) | |
| Kidney | 7 (6.7%) | 40 (9.3%) | |
| Lung | 64 (61.0%) | 231 (53.8%) | |
| Melanoma | 3 (2.9%) | 25 (5.8%) | |
| Other | 18 (17.1%) | 80 (18.6%) | |
| Location | 0.0614 | ||
| Brainstem | 0 (0.0%) | 15 (3.5%) | |
| Infratentorial | 32 (30.5%) | 100 (23.3%) | |
| Supratentorial | 73 (69.5%) | 314 (73.2%) | |
| radiosensible | 0.4984 | ||
| Yes | 84 (80.0%) | 330 (76.9%) | |
| No | 21 (20.0%) | 99 (23.1%) |
Local control
Analyses were performed on 400 BM with available follow-up images. Patients excluded from analyses of LC because of inadequate imaging follow up were not statistically different from patients included in analyses.The median follow-up is 40 months (1–60 months). If we consider the whole group, local control at 6 months, 1-year and 2-years was 88%, 76. 5 and 63.9% respectively. In the group of patients treated with Surgery +HFSRT, the local control was 89.58% at 6 months, 87.02% at 12 months 77.53% at 24 months vs 88.17% at 6 months, 73.75% at 12 months, 59.93% at 24 months in the groupe of patients treated with exclusive HFSRT (Fig. 1). LC was improved when size was less than to 2.5 cm (p = 0.0164) (Fig. 2). On univariate analyses and multivariate, maximal BM size (< 2.5 cm) and prior surgery were the only predictive factor of LC (Table 4). Systemic treatment and fractionation schedules were not statically associated with LC (Table 4).
Fig. 1.
Kaplan Meier curve of local control
Fig. 2.
Kaplan Meier curve of local control in function brain metastases size
Table 4.
Univariate and multivariate analysis on OS
| Univariate analyses | Multvariate analyses | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | HR | IC95% | p | HR | IC95% | p |
| Age | N = 389 | 0.0891 | 0.4633 | ||||
| < 70 vs ≥ 70 | 1.402 | [0.69–1.525] | 1.473 | [0.574–1.353] | |||
| Sex | N = 387 | 0.0024 | 0.0139 | ||||
| Female vs Male | 1.578 | [1.175–2.12] | 1.578 | [1.08–2.00] | |||
| KPS | N = 389 | 0.0094 | 0.0094 | ||||
| v < 70 vs ≥70 | 0.622 | [0.435–0.89] | 0.769 | [0.522–1.133] | |||
| RPA | N = 389 | 0.0301 | 0.4 | ||||
| II-III vs I | 1.756 | [1.08–2.854] | 0.799 | [0.472; 1.35] | |||
| m RPA | N = 389 | 1.700 | 0.001 | 1.74 | [1.12–2.69] | 0.014 | |
| II-III vs I | [1.1–2.854] | ||||||
| GPA | N = 389 | 0.0342 | 0.1411 | ||||
| < 3 vs ≥3 | 0.642 | [0.427–0.968] | 0.712 | [0.45–1.12] | |||
| DS-GPA | N = 345 | 0.3525 | |||||
| < 3 vs ≥3 | 0.842 | [0.587–1.209] | |||||
| Surgery | N = 389 | 0.00111 | 0.0028 | ||||
| Yes vs No | 1.623 | [1.117–2.357] | 1.86 | [1.24–2.79] | |||
| Symptoms | N = 322 | 0.696 | |||||
| No vs Yes | 0.956 | [0.697–1.312] | |||||
| Single BM | N = 389 | 0.696 | |||||
| Yes vs No | 1.074 | [0.752–1533] | |||||
| Radiosensitivity | N = 389 | 0.2592 | |||||
| Yes vs No | 0.83 | [0.601–1.147] | |||||
| Control of primary tumor | N = 373 | 0.0036 | 0,0087 | ||||
| Yes vs No | 0.645 | [0.480–0.867] | 0.671 | [0.497–0.904] | |||
| Systemic treatment | N = 370 | 0.6987 | |||||
| Yes vs No | 0.942 | [0.694–1.277] | |||||
| Group | N = 389 | 0.0092 | 0,0089 | ||||
| Surgery + HFSRT vs HFSRT | 0.601 | [0.419–0.884] | 0.410 | [0.419–0.884] | |||
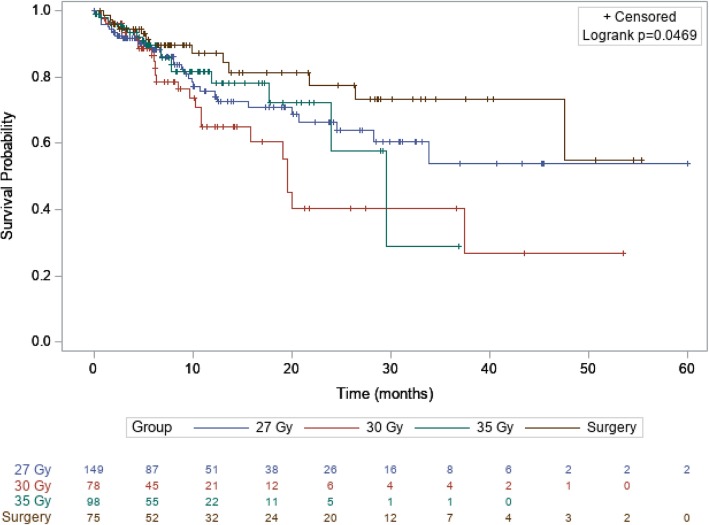
For further analyses, BM were divided into 4 groups of treatment modality. The group of patients treated by surgery plus adjuvant HFSRT was compared to 3 other groups of BM treated by 3x9Gy, 5x6Gy, and 5x7Gy schedules (Fig. 3). Surgery had a significant impact on LC (p = 0.0469), whereas dose-fraction regimen had no impact (Table 3).
Fig. 3.
Kaplan Meier curve of local control according to surgery and dose-fraction regimens. The group surgery represents all patients treated by surgery followed by HFSRT (27, 30 or 35 Gy)
Table 3.
Univariate and multivariate analyses on local control (LC)
| Univariate analyses | Multivariate analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | N | HR | IC95% | p | HR | IC95% | p | |
| Size | N = 378 | 0.0178 | 0,0072 | |||||
| > =2.5 cm vs < 2.5 cm | 1.699 | [1.096–2.634] | 1.909 | [1.191–3.060] | ||||
| Fractionation | N = 400 | 0.3902 | 0,2072 | |||||
| 30 Gy in 5 vs 27 Gy in 3 | 1.331 | [0.823–2.151] | 0.917 | [0.508–1.655] | ||||
| 35 Gy in 5 vs 27 Gy in 3 | 0.955 | [0.553–1.649] | 1.453 | [0.853–2.477] | ||||
| Control of primary tumor | N = 383 | 0.0598 | ||||||
| Yes vs No | 0.661 | [0.429–1.017] | ||||||
| Systemic treatment | N = 382 | 0.9556 | ||||||
| Yes vs No | 0.987 | [0.630–1.546] | ||||||
| Surgery | N = 400 | 0.0490 | 0,0044 | |||||
| Yes vs no | 1.816 | [1.002–3.289] | 2.506 | [1.333–4.712] | ||||
| Group | N = 400 | 0.0490 | ||||||
| Surgery + HFSRT vs HFSRT | 0.551 | [0.304–0.998] | 0.437 | [0.235–0.812] | 0,0088 | |||
Overall survival
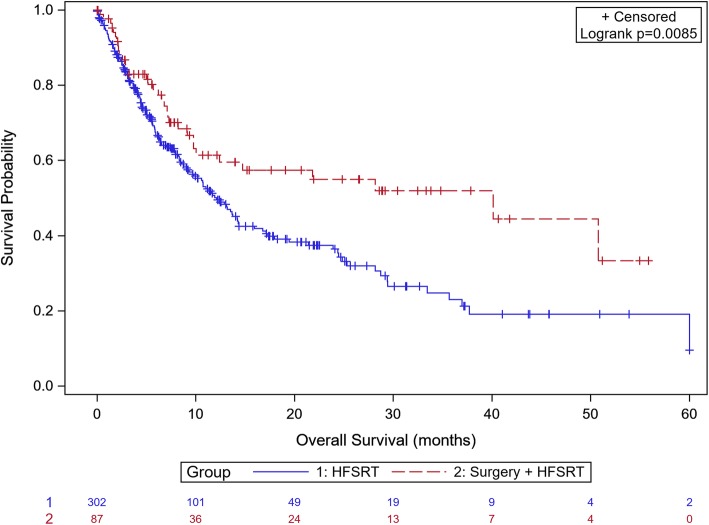
With a median follow-up of 40 months (1–60 months) median overall survival was 13.17 months (10.68–17.22). One-year OS was 52.7%. Overall survival at 12 months was 61.43% in the surgery +HFSRT group vs 50.13% in the HFSRT group (p < 0.0085) (Fig. 4).
Fig. 4.
Kaplan Meier curve of overall survival according to surgery
On Cox univariate model, clinical parameters such as sex, RPA, mRPA, GPA, DS-GPA, control of extra cerebral tumor, prior surgery and KPS were significantly associated with overall survival, whereas age, DS-GPA, radio sensitivity were not (Table 4). In Cox Multivariate model, prior surgery (OR = 1.86; p = 0.0028), sex (OR = 1.4; p = 0.0139), control of extra cerebral tumor (OR = 0.671, p = 0.0069) and KPS < 70 (OR = 0.769, p = 0.0094) were independently predictive for OS (Table 4).
Late toxicity: radionecrosis
With a median follow-up of 16.4 months (5.6–71.8 months), a total of 20 cases (5%) of RN were reported above 400 BM with regular MRI follow-up. All patients presented neurologic symptoms. For RN diagnosis, follow-up MRI was mandatory (at least 2 consecutive MRI with RN diagnosis). Follow-up MRI performed were multi-modal with T1, T2, FLAIR and perfusion sequences. Most of RN occurred on BM originated from lung cancer (n = 10; 50%), followed by breast cancer (n = 6; 30%), melanoma (n = 2; 10%), digestive tract (n = 1; 5%), head and neck cancer (n = 1; 5%). Median size of BM was 2.3 cm (1–4 cm). RN occurred on 7 post-operative cavities (35%). Two patients needed surgery because of neurologic symptoms.
Discussion
This study aimed to assess the efficiency and safety of HFSRT with or without surgery. In our institution, HFSRT and SRS were used upfront WBRT, even if multiple BM were observed (> 3) to reduce late neurocognitive toxicity. This practice was supported by EORTC study comparing WBRT vs surveillance after surgery or SRS [21]. Their data demonstrated that median overall survival was not significantly different between both groups (10.7 months vs 10.9 months; p = 0.89). More recently a second analysis was performed and showed similar results: Churilla et al. compared limited BM 1 to 3 vs extended BM (> 3) and concluded that number of BM treated by SRS was not associated to overall outcome [22].
Patients with multiple BM were more likely to have extracranial progression. Therefore, we found an estimated overall 1 year survival of 52.7%, which is lower than what it was found in some studies [23] previously reported previous studies.
For each patient RPA, mRPA, GPA, and DS-GPA prognostic scores were calculated and reported. On univariate analyses, RPA mRPA and GPA were significantly associated with overall survival. On multivariate analysis, only mRPA was significantly associated to overall survival. According literature, mRPA is more discriminant than RPA [11]. The group 2 from RPA score is heterogenous [12]. Thus, many studies suggested that a subclassification of group 2 is necessary to predict more accurately overall survival [12]. Our finding might be in agreement with those observations.
However our study might be limited by the fact that nomograms were not systematically used before HFSRT indication. Moreover, even if these scores systems have been validated, they were developed based on patients treated by WBRT and not by SRS or HFSRT alone [13, 24]. Each score refers to different predictive factors such as age, BM number, histology of primitive tumor and performance status score [22, 25]. Nieder et al. suggested that all these criteria can not be reported in some clinical situations [26]. Other publications showed that cumulated volume size and surgery should be integrated in nomogram [7, 27]. Therefore, it is impossible to take into account all those factors highlighting the importance of a multidisciplinary staff meeting.
In the present series local control was 88, 76.5 and 63.9% at 6 months, 1 year and 2 years, respectively. This is in agreement with literature showing that HFSRT is an efficient treatment for BM (Table 5). Those studies are mainly retrospective and included small cohorts. However, their data reported a satisfying local control at 1 year (76 and 93%). The only prospective study based on HFSRT included 51 patients and local control was 76% at 1 year [28].
Table 5.
Literature review of hypofractionated stereotactic studies
| Publication date |
Nb pts/BM |
Median Volume (cc) or size (mm) | Regimen | Isodose (%) | WBRT (%) | LC at 12 m (%) | Median OS at 12 m (%) |
RN (%) | Prognostic factors |
|---|---|---|---|---|---|---|---|---|---|
| Matsuyama et al. 2005–2009 | 299/573 | 8.6 mm (2.8–47.4) | Variable regimen | NC | 10 | 94 | 57,8 | 2 | Size |
| LIiang-hua et al. 2001–2011 | 171/354 | 14.3 cc (0.16–86) | 4 × 8 Gy | 80–90 | 68 | 68 | 51 | 6 | WBRT for OS |
| Martens et al. 2006–2010 | 75/108 | 1 cc (0.1–29.2) |
6Gyx5 6-7x5gy 7-10 × 4 Gy |
NC | 52 | 52 | 35 | 1 | Volume |
| Giubilei et al. 2001–2006 | 30/44 | 4.8 cc (0.4–24.3) |
3 × 6 Gy 4 × 8 Gy |
Isocenter | 100 | 86 | 36 | 0 | NC |
| Marchetti et al. 2001–2005 | 65/81 | 8 cc (0.3–48.2) | 3 × 8 Gy | 80 | 44 | 58 | 25 | 1 | NC |
| Fahrig et al. 2000–2005 | 150/228 | 30 mm |
5 × 6-7Gy 10x4Gy 7 × 5 Gy |
NC | 34 | NC | 66 | NC | Dose volume |
| Jiang et al. 2003–2009 | 40/NC |
30 mm (3.1–5.5) 17,5 cc (6–64.6) |
4 × 10 gy | 90 | 25 | 94.2 | 55.3 | 2.5 | Histology KPS |
| Scorsettiet al. 2004–2007 | 78/113 | 3.3 cc (0.1–28) |
6x4Gy 7x5Gy 1 × 20 Gy |
80 | 10 | 69 | NC | NC | RPA |
| Nagai et al. 2009–2013 | 54/128 | 1.9 cc (0.1–18) | 4 × 7 Gy | 80 | NC | 91 | 52 | 0 | NC |
| Fokas et al. 2012 | 214/214 | 30 mm |
7 × 5 Gy 4x10Gy |
Isocenter | 0 | 90 | 31 | NC | RPA |
| Ernst et al. 2003–2005 | 51/72 | 3 cc |
5 × 7 Gy 5 × 6 Gy |
NC | NC | NC | NC | NC | V4 < 20 cc |
| Inoue et al. 2010–2014 | 88/92 | 10 -74 cc |
3 × 9 Gy 3x10Gy |
57 | 0 | 90 | NC | 0 |
V14 RN |
LC local control, V14 Irradiated volume receiving 14 Gy, RN Radionecrosis, RPA Recursive Patitioning Analysis, m months, Vol Volume, NC not communicated, pts. patients, Nb Number
Our study analyzed dose-fractionation prescribed on the same isodose (80%). Intent was to adapt fractionation regimen to clinical situations. Higher dose-fractionation 5 × 7 Gy corresponded to a biological equivalent dose with α/β = 10 (BED10 Gy) of 59.9 Gy, was delivered on larger BM (> 2.5 cm). Schedules 3 × 9 Gy and 5 × 6 Gy corresponding to lower BED10 Gy of 51.3 Gy and 48 Gy respectively, were more often applied on post-operative cavity and smaller lesions [29]. In Table 2, we can see that physician’s adapted volume fractionation, number of lesions prior to WBRT or surgery. In regard of literature, rare studies assessed the indication of HFSRT for smaller BM (< 2.5 cm) [29, 30]. Studies that analyzed HFSRT outcomes were mainly focused on larger BM [7]. In our study, we also included smaller BM treated by HFSRT at the proximity of eloquent structure. Although, dose and fractionation schedule prescriptions were influenced by clinical parameters, none of the HFSRT fractionation schedules emerged as an optimal treatment leading to a significantly improvement of local control no matter BM size.
In our study BM size was the main prognosis factor influencing local control. Local control was better when BM were smaller < 2.5 cm. Our results are consistent with literature showing that size is a robust prognosis factor of local control upon SRS and HFSRT [8, 27]. However, definition of large BM is heterogeneous among studies, making the comparison difficult [6].
Our data demonstrated that larger BM (> 2.5 cm) had worse local control whatever the dose and fractionation. In Léon Bérard Cancer Center, a dose escalation was performed up to 35 Gy on larger tumors. Despite this dose escalation, this suggests that larger BM still have poorer local control compared to smaller lesions. Adaptation of fractionation to tumor volume failed to compensate the bad prognostic induced by tumor volume. Some authors suggested the use of a further fractionated treatment. Determining optimal dose is a controversial debate [31]. Partly this can be explained by fundamental radiobiology [29]. The larger the BM is, the more important the hypoxic fraction is, leading to radio-resistance [30]. Another explanation may be that dose-fractionation used in brain cannot reach higher BED10 Gy due to dose constraints [16]. In extracranial stereotactic body radiotherapy, it is well established that dose escalation is strongly linked to cell death and tumor decrease when BED10 Gy is higher [23, 32, 33].
Late toxicity (radio necrosis) was limited to 5% in this large cohort. All patients with diagnosed radionecrosis were symptomatic. Radionecrosis diagnosis was performed on MRI follow-up most of the time. Minitti and al. performed analyses on a large cohort including 289 patients [34]. A group treated by SRS was compared to a group treated by HFSRT. Nineteen percent of patients in the SRS group vs 9% in the HFSRT group presented a radionecrosis. In our study this rate was lower. The incidence of radionecrosis depends on the definition. Asymptomatic RN are not reported in this study. Nonetheless, Zindler et al. demonstrated that HFSRT reduces RN rate [8] . Indeed, the higher the fraction number, the more OAR are protected from late toxicity. A 24 studies meta-analyse conducted by lerhar et al. also demonstrated that HFSRT offers lower rate of radio-necrosis with an improved LC [35].
Surgery is a prognostic factor correlated to local control and overall survival on multivariate analysis. This finding suggests that surgery should be proposed for BM larger than 2.5 cm, when this feasible without morbidity. Previous studies compared post-operative SRS versus SRS alone. Local control after surgery alone is estimated to 50% [4]. A recent prospective phase III study compared follow-up or SRS after surgery [36]. The local free recurrence at 1-year was 43% in the group treated by surgery vs 72% the group treated by surgery plus SRS. Lamba et al. found that surgery plus SRS was associated with an improved LC and overall survival in comparison to SRS alone [37].
To our knowledge, this is one of largest studies comparing HFSRT and surgery plus HFSRT. In the EORTC study comparing SRS or surgery with or without WBRT, including 289 patients, local control and global survival were comparable between both groups. This suggested that SRS is as efficient as surgery. For patients not eligible to surgery due to multiple or deep BM, optimal dose-fractionation should be assessed by randomized prospective studies. Association with systemic agents should be further investigated [38–40].
Conclusion
Our study suggests that HFSRT is an efficient and safe treatment. Lesion size and surgery are correlated to a robust local control. Surgery may provide a better outcome in term of overall survival. This study confirms the importance of surgery in BM management. Further studies are required to assess the interest of dose escalation in BM management.
Acknowledgements
We thank Isabel Gregoire, PhD Medical Writer CGFL, and Gilles Créhange for carefully reading the manuscript.
Abbreviations
- ANOCEF
Association des Neuro-Oncologues d’Expression Française
- BED10 Gy
Biological equivalent dose with α/β = 10
- BM
Brain metastases
- DS-GPA
Diagnostic-Specific Graded Prognostic Assessment
- GPA
Graded Prognostic Assessment
- GTV
Gross Tumor Volume
- HFSRT
Hypofractionated stereotactic radiotherapy
- KPS
Karnofsky performance status
- LC
Local control
- OAR
Organs at risk
- OS
Overall survival
- PTV
Planning target volume
- RN
Radionecrosis
- RPA
Recursive Partitioning Analysis
- SRS
Stereotactic radiosurgery
- WBRT
Whole brain radiotherapy
Authors’ contributions
The study was carried out by Laurence Mengue and was supervised by Marie-Pierre SUNYACH. All authors reviewed the manuscript before submission. The author(s) read and approved the final manuscript.
Funding
No funding to declare.
Availability of data and materials
Datasets generated for this study are available upon request to the corresponding author.
Ethics approval and consent to participate
This study was ethically approved by the Léon Bérard Cancer Center institutional board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am janv. 2011;22(1):1–6. doi: 10.1016/j.nec.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, et al. Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO) Neuro-Oncol. 2017;19(2):162–174. doi: 10.1093/neuonc/now241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noh T, Walbert T. Brain metastasis: clinical manifestations, symptom management, and palliative care. Handb Clin Neurol. 2018;149:75–88. doi: 10.1016/B978-0-12-811161-1.00006-2. [DOI] [PubMed] [Google Scholar]
- 4.Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, et al. Updates in the management of brain metastases. Neuro-Oncol. 2016;18(8):1043–1065. doi: 10.1093/neuonc/now127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 6.Masucci GL. Hypofractionated radiation therapy for large brain metastases. Front Oncol. 2018;8:379. doi: 10.3389/fonc.2018.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed KA, Sarangkasiri S, Chinnaiyan P, Sahebjam S, Yu H-HM, Etame AB, et al. Outcomes following Hypofractionated stereotactic radiotherapy in the Management of Brain Metastases. Am J Clin Oncol. 2016;39(4):379–383. doi: 10.1097/COC.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 8.Zindler JD. Improving patient selection and outcome of stereotactic radiosurgery as a single treatment modality for brain metastases. 2017; University of Maastricht; Disponible sur: 10.26481/dis.20171025jz.
- 9.Kumar AMS, Miller J, Hoffer SA, Mansur DB, Coffey M, Lo SS, et al. Postoperative hypofractionated stereotactic brain radiation (HSRT) for resected brain metastases: improved local control with higher BED10. J Neurooncol. 2018;139(2):449–454. doi: 10.1007/s11060-018-2885-6. [DOI] [PubMed] [Google Scholar]
- 10.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol. 1997;37(4):745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Kawabe T, Higuchi Y, Sato Y, Nariai T, Watanabe S, et al. Validity of prognostic grading indices for brain metastasis patients undergoing repeat radiosurgery. World Neurosurg. 2014;82(6):1242–1249. doi: 10.1016/j.wneu.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Sato Y, Serizawa T, Kawabe T, Higuchi Y, Nagano O, et al. Subclassification of recursive partitioning analysis Class II patients with brain metastases treated radiosurgically. Int J Radiat Oncol Biol Phys. 2012;83(5):1399–1405. doi: 10.1016/j.ijrobp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQM. Systemic therapy of brain metastases: non–small cell lung cancer, breast cancer, and melanoma. Neuro-Oncol. 2017;19(1):i1–24. doi: 10.1093/neuonc/now197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atalar B, Modlin LA, Choi CYH, Adler JR, Gibbs IC, Chang SD, et al. Risk of Leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol. 2013;87(4):713–718. doi: 10.1016/j.ijrobp.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Timmerman R, Bastasch M, Saha D, Abdulrahman R, Hittson W, Story M. Stereotactic body radiation therapy: Normal tissue and tumor control effects with large dose per fraction. IMRT IGRT SBRT. 2011;43:382–394. doi: 10.1159/000322494. [DOI] [PubMed] [Google Scholar]
- 17.Le Rhun É, Dhermain F, Noël G, Reyns N, Carpentier A, Mandonnet E, et al. ANOCEF guidelines for the management of brain metastases. Cancer Radiother J Soc Francaise Radiother Oncol. 2015;19(1):66–71. doi: 10.1016/j.canrad.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Minniti G, Esposito V, Clarke E, Scaringi C, Bozzao A, Falco T, et al. Fractionated stereotactic radiosurgery for patients with skull base metastases from systemic cancer involving the anterior visual pathway. Radiat Oncol. 2014;9(1):110. doi: 10.1186/1748-717X-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Rhun E, Dhermain F, Vogin G, Reyns N, Metellus P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Rev Neurother. 2016;16(8):903–914. doi: 10.1080/14737175.2016.1184572. [DOI] [PubMed] [Google Scholar]
- 20.Minniti G, Clarke E, Lanzetta G, Osti M, Trasimeni G, Bozzao A, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6(1):48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952–26001 Study. J Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Churilla TM, Handorf E, Collette S, Collette L, Dong Y, Aizer AA, et al. Whole brain radiotherapy after stereotactic radiosurgery or surgical resection among patients with one to three brain metastases and favorable prognoses: a secondary analysis of EORTC 22952–26001. Ann Oncol Off J Eur Soc Med Oncol. 2017;28(10):2588–2594. doi: 10.1093/annonc/mdx332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahrig A, Ganslandt O, Lambrecht U, Grabenbauer G, Kleinert G, Sauer R, et al. Hypofractionated stereotactic radiotherapy for brain metastases. Strahlenther Onkol. 2007;183(11):625–630. doi: 10.1007/s00066-007-1714-1. [DOI] [PubMed] [Google Scholar]
- 24.Sperduto PW, Wang M, Robins HI, Schell MC, Werner-Wasik M, Komaki R, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with Temozolomide or Erlotinib for non-small cell lung Cancer and 1 to 3 brain metastases: radiation therapy oncology group 0320. Int J Radiat Oncol. 2013;85(5):1312–1318. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieder C. What approach will Lead to cure of Glioblastoma Multiforme?: in regard to Barani et al. (Int J Radiat Oncol biol Phys 2007;68:324–333) and Jones and Sanghera (Int J Radiat Oncol biol Phys 2007;68:441–448) Int J Radiat Oncol. 2007;69(2):640–641. doi: 10.1016/j.ijrobp.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Nieder C, Marienhagen K, Geinitz H, Molls M. Validation of the graded prognostic assessment index for patients with brain metastases. Acta Oncol. 2009;48(3):457–459. doi: 10.1080/02841860802342390. [DOI] [PubMed] [Google Scholar]
- 27.Baschnagel AM, Meyer KD, Chen PY, Krauss DJ, Olson RE, Pieper DR, et al. Tumor volume as a predictor of survival and local control in patients with brain metastases treated with gamma knife surgery. J Neurosurg. 2013;119(5):1139–1144. doi: 10.3171/2013.7.JNS13431. [DOI] [PubMed] [Google Scholar]
- 28.Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol. 2006;81(1):18–24. doi: 10.1016/j.radonc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Kirkpatrick JP, Soltys SG, Lo SS, Beal K, Shrieve DC, Brown PD. The radiosurgery fractionation quandary: single fraction or hypofractionation? Neuro-Oncol. 2017;19(suppl_2):ii38–ii49. doi: 10.1093/neuonc/now301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacelli R, Mansi L, Hall E, Amato J. Giaccia: Radiobiology for the radiologist, 6th edn. Eur J Nucl Med Mol Imaging. 2007;34(6):965–966. [Google Scholar]
- 31.Inoue HK, Sato H, Suzuki Y, Saitoh J, Noda S, Seto K, et al. Optimal hypofractionated conformal radiotherapy for large brain metastases in patients with high risk factors: a single-institutional prospective study. Radiat Oncol Lond Engl. 2014;9:231. doi: 10.1186/s13014-014-0231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giubilei C, Ingrosso G, D’Andrea M, Benassi M, Santoni R. Hypofractionated stereotactic radiotherapy in combination with whole brain radiotherapy for brain metastases. J Neurooncol. 2008;91(2):207–212. doi: 10.1007/s11060-008-9700-8. [DOI] [PubMed] [Google Scholar]
- 33.Marchetti M, Milanesi I, Falcone C, De Santis M, Fumagalli L, Brait L, et al. Hypofractionated stereotactic radiotherapy for oligometastases in the brain: a single-institution experience. Neurol Sci. 2011;32(3):393–399. doi: 10.1007/s10072-010-0473-4. [DOI] [PubMed] [Google Scholar]
- 34.Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol. 2016;95(4):1142–1148. doi: 10.1016/j.ijrobp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Lehrer EJ, Peterson JL, Zaorsky NG, Brown PD, Sahgal A, Chiang VL, et al. Single versus Multifraction Stereotactic Radiosurgery for Large Brain Metastases: An International Meta-analysis of 24 Trials. Int J Radiat Oncol Biol Phys. 2019;103(3):618–630. doi: 10.1016/j.ijrobp.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 36.Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-Centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040–1048. doi: 10.1016/S1470-2045(17)30414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamba N, Cagney DN, Brigell RH, Martin AM, Besse LA, Catalano PJ, et al. Neurosurgical resection and stereotactic radiation versus stereotactic radiation alone in patients with a single or solitary brain metastasis. World Neurosurg. 2019;122:e1557–e1561. doi: 10.1016/j.wneu.2018.11.100. [DOI] [PubMed] [Google Scholar]
- 38.Lesueur P, Lequesne J, Barraux V, Kao W, Geffrelot J, Grellard J-M, et al. Radiosurgery or hypofractionated stereotactic radiotherapy for brain metastases from radioresistant primaries (melanoma and renal cancer) Radiat Oncol Lond Engl. 2018;13(1):138. doi: 10.1186/s13014-018-1083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scorsetti M, Facoetti A, Navarria P, Bignardi M, De Santis M, Ninone SA, et al. Hypofractionated stereotactic radiotherapy and radiosurgery for the treatment of patients with radioresistant brain metastases. Anticancer Res. 2009;29(10):4259–4263. [PubMed] [Google Scholar]
- 40.Fokas E, Henzel M, Surber G, Kleinert G, Hamm K, Engenhart-Cabillic R. Stereotactic radiosurgery and fractionated stereotactic radiotherapy: comparison of efficacy and toxicity in 260 patients with brain metastases. J Neurooncol. 2012;109(1):91–98. doi: 10.1007/s11060-012-0868-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets generated for this study are available upon request to the corresponding author.