Summary
The antimalarials chloroquine and hydroxychloroquine have been used for the treatment of inflammatory diseases for more than 60 years. Even today new indications evolve due to the complex mode of action of these compounds. Due to the fear of side effects, especially irreversible retinopathy, their use is often limited. These side‐effects, however, are a consequence of excessive daily dosages. An effective, safe therapy needs correct dosing, i. e. adherence to maximal daily dosages of 3.5(–4) mg chloroquine or 6(–6.5) mg hydroxychloroquine per kilogram ideal body weight. If the actual body weight is lower than the ideal body weight, this actual weight is used for the calculation of the dosage. Observing these limits allows a rather safe therapy of the diseases like lupus erythematosus, REM syndrome, porphyria cutanea tarda (2 × 125 mg chloroquine/week), cutaneous sarcoidosis and dermatomyositis. If standard therapies fail, then antimalarials can be tried to treat Sjögren syndrome, granu‐loma annulare or erosive lichen planus. If therapy fails, either can be combined with quinacrine to increase their effectiveness. Chloroquine and hydroxychloroquine are indispensable and well‐tolerated essential drugs in dermatology and especially suited as part of a combination scheme, for example with corticosteroids, as they act synergistically and reduce side‐effects.
Keywords: chloroquine, hydroxychloroquine, quinacrine, lupus erythematosus
Introduction
Antimalarial drugs are chemotherapy agents that are used in the treatment of malaria. These include drugs such as chloroquine, mefloquine, and halofantrine. This review focuses on chloroquine, hydroxychloroquine, and quinacrine, also known as Mepacrine® or Atabrine®. Quinacrine is a synthetic drug that was developed in 1930 and became the main antimalarial agent used by the Allies during the World War II. Chloroquine was developed in 1934 to replace quinine. Hydroxychloroquine is derived from chloroquine beta hydroxylation. It was first synthesized during the 1950s. Despite a few positive reports on successful use of older antimalarial drugs in the treatment of chronic discoid lupus erythematosus (DLE), the usefulness of antimalarials was not more widely acknowledged until 1951 when a treatment series using quinacrine was published (17/18 of treated DLE patients improved). Since then these substances have also been successfully used to treat other non‐infectious, inflammatory diseases. Their use is restricted, however, to malaria, rheumatoid arthritis, and systemic lupus erythematosus. Yet in recent decades, in dermatology in particular, antimalarial agents have been used successfully to treat a number of other diseases, and new uses continue to be found even today.
The majority of studies on these drugs were performed decades ago, and thus current clinical studies using modern quality‐based criteria are largely unavailable. Due to lacking interest in the evidence of their effectiveness and lacking financial incentives, it is unlikely that such studies will be performed any time soon. Current knowledge is thus often based only on open studies, patient series, and case reports. In the present review these tried and tested – and safe (if used correctly) – drugs as well as their indications, will be presented. The following information applies equally to adults, adolescents, and children.
Chemistry and mechanism of action
Chloroquine (7‐chloro‐4‐[4’‐diethylamino‐1’‐methyl‐butylamino]‐quinoline [MG 319,89; chloroquine diphosphate MG 515,9; (CAS No. 50–63‐5 (diphosphate)], CQ) (Figure 1) in its pure for, is a white, crystalline, bitter‐tasting powder. Hydroxychloroquine(HCQ) is a derivative of chloroquine (CAS No. 118–42‐3) (Figure 1). The two substances do not differ with regard to their mechanism of action, pharmacokinetics, toxicology, side effects, and indications. All of the following information applies to both drugs. The recommended dosages are based on clinically relevant information for each of the salts, i.e., chloroquine diphosphate and hydroxychloroquine sulfate, and not on the respective bases.
Figure 1.
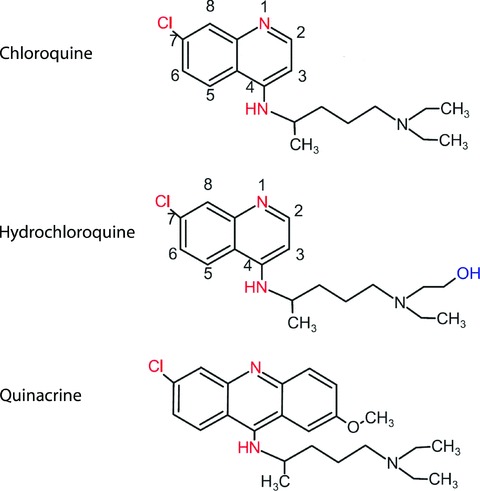
Structure of the 4‐aminoquinoline chloroquine and hydroxychloroquine as well as the acri‐dine dye quinacrine.
The complex mechanism of action of chloroquine/hydroxychloroquine (CQ/HCQ) has been discussed in more detail elsewhere [1, 2]. The drugs’ effects are better explained by various factors than by a single one alone (Table 1). CQ/HCQ have im‐munomodulatory, anti‐inflammatory, and antiproliferative properties; they alleviate UV‐induced inflammation, inhibit thrombocyte aggregation, enhance glucose tolerance, and cause increased porphyrin excretion. These are the effects that makes these substances useful for therapy.
Table 1.
| Effect | Mechanism | Results/Details |
|---|---|---|
| Immunomodulatory | Inhibits autoantigen processing | Diminished class II antigen presentation |
| Reduced stimulation of autoreactive CD4+ T cells | ||
| Reduced cytokine production: of IL1, 2, 6,TNF‐α by macrophages and IL1, 2, 5 by T lymphocytes | ||
| Sequestration of membrane particles | Diminished surface receptors ∼ 50 %; and thus diminished response to mitogenic stimuli | |
| Binding to DNA and thus competitive inhibition of anti‐DNA antibodies | ||
| Anti‐inflammatory | Inhibition of phospholipase A2 and C | Diminished arachidonic acid release and prostaglandin synthesis; reduced bradykinin effect |
| Inhibition of formation of IL1beta, TNF alpha | mRNA and protein level | |
| Inhibition of mast cells | Diminished leukotriene synthesis and histamine release | |
| Inhibition of Toll‐like receptor 9 signal pathway | Diminished antigen presentation and immune stimulation | |
| Antiproliferative | Interaction with protein synthesis | Inhibition of DNA/RNA biosynthesis and polymerase |
| UV absorption | Increased UV filtration? (questionable relevance) | After spectral shift in relation to accumulation in melanin and increase epidermalconcentrations |
| Inhibition of UV‐induced inflammatory reactions | Possibly due to interaction with UV‐B induced C‐jun transcription | |
| Anti‐infectious | Antimicrobial effects on HIV, SARS, coronavirus, influenza viruses | |
| Coagulation | Inhibition of thrombocyte aggregation: diminished CD41a and CD61 expression | Without prolonging time to coagulation |
| Metabolic | Reduced cholesterol, triglyceride, LDL levels | |
| Complex formation with porphyrins | Increased excretion | |
| Reduced hydroxylation vitamin D | Reduction in 1,25‐dihydroxyvitamin D3 | |
| Misc. | Increased pain threshold | Also in healthy individuals |
An important effect results from the “lysosomotropic” properties of CQ/HCQ which will be discussed in greater detail. CQ/HCQ are amphiphilic molecules (lipophilic ring structure, hydrophilic side chain, Figure 1, 2). They thus tend to deposit in interphases, for instance, in phospholipid membranes. As weakly basic molecules, they can pass through the membranes and are protonated in the acidic milieu of the cytoplasm. As a positively loaded molecule, CQ/HCQ lose their ability to pass through the membrane and thus accumulate there (factor 100–1 000), primarily in macrophages and lysosomes. In the Plasmodium parasites that cause malaria, intralysosomal proteases are thus inhibited, leading to heme accumulation which is toxic for the parasites. In human leukocyte lysosomes, the accumulation of antigens (low‐affinity peptides) on the “major histocompatibility complex” as well as stimulation of Toll‐like receptor 9 is inhibited. The processing of autoantigens in particular is diminished and is thus also decreased in subsequent immunological effects (Table 1). These indirect effects on T cells enable these agents to be well combined with other immunomodulatory drugs. The delayed clinical effects may be explained by the fact that they primarily affect antigen‐presenting cells.
Figure 2.
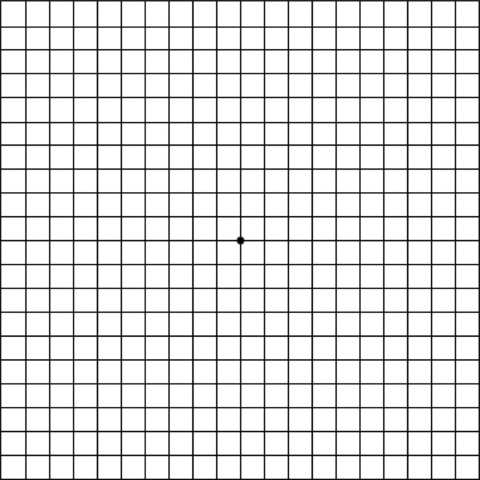
Amsler grid used to detect macular degeneration. The patient focuses with one eye (covering the other) on the dot in the center and looks for any wavy or broken lines. An ophthalmologist should be consulted if there are any abnormalities.
Quinacrine is a yellow acridine dye (CAS numbers: mepacrine = 83–89‐6 quinacrine dihydrochloride = 69–05‐6). It is no longer commercially available, but may be ordered through the pharmaceutical distributor Heinze in Lörrach, Germany, at http://www.pharmavertrieb-heinze.de/ which obtains the drug from England through BCM Specials at http://www.bcm-specials.co.uk/.
Pharmacology
The complex pharmacology of antimalarial drugs has been presented in various reviews [3]. Due to differences between samples, dosing intervals, and modes of administration, the reported results vary considerably.
Resorption
CQ/HCQ are water‐soluble. After being taken orally, they are quickly and almost completely absorbed. The bioavailability is 0.77 ± 0.16 (tablets). If taken together with a high‐fat and protein‐rich meal, CQ/HCQ are better absorbed than on an empty stomach or following a low‐fat, low‐protein meal. Maximum plasma levels are reached within 3–4 hours.
Mepacrine is also quickly and thoroughly absorbed. Maximum plasma levels are achieved in 8–12 hours.
Distribution
50–70 percent of chloroquine is bound to plasma proteins. Because CQ/HCQ accumulate in thrombocytes, granulocytes, and erythrocytes, their concentration in whole blood is 3–10 times higher than in plasma. In many internal organs, the concentration of CQ/HCQ is as much as 1 000 times greater than in the blood. Due to the high affinity of chloroquine with melanin, the highest concentrations are found in the eyes and the skin where the concentration is 100–200 times higher than in plasma; in the epidermis it is 3–7 times higher than in the dermis. The greatest concentrations are found in the spleen, followed by the liver, kidneys, lungs, heart, muscles, and brain. There is virtually no accumulation of chloroquine in adipose tissue.
Due to this accumulation in “deep compartments” the fictitious distribution volume is high: more than 100 l/kg. It takes at least 4 weeks for chloroquine 6 months for hydroxychloroquine to reach plasma‐tissue equilibrium. The same applies to quinacrine which accumulates in the same organs as CQ/HCQ. Equilibrium is achieved after 4 weeks. 80–90 percent is bound to plasma proteins [4].
Excretion
CQ/HCQ are very slowly eliminated. The plasma concentrations drop exponentially. In other words, the concentration decreases rapidly at first and then more gradually. Depending on dosage and exponential decrease, the half‐life of the drug can range from 74 hours to 50 days. To control compliance, plasma levels of HCQ or CQ concentrations in the hair may be measured.
Quinacrine is eliminated slowly, 11 % by the kidneys. After discontinuing the drug, significant urine levels are still detectable for more than 2 months.
40–70 % of CQ is eliminated unaltered in the urine. Renal impairment thus significantly prolongs the half‐life of the drug. CQ itself, and in low levels, HCQ, can cause about a 10 % reduction in creatinine clearance. Especially in older patients, this can slow CQ excretion. Given CQ accumulation, the risk of side effects is higher in such patients and thus the dosage should be reduced accordingly.
25–40 % of CQ is metabolized by the cytochrome P450 in the liver into pharmacologically active metabolites. 5–10 % of CQ is excreted in stool. Drug plasma levels depend less on elimination than on reverse diffusion of the agents from the deep compartments in the plasma. Studies have reported that following a single dose, traces of CQ persisted in the plasma and erythrocytes for 56 days; after a two‐month regime, there were still traces after 6–7 months in the skin but not in the plasma; and after long‐term therapy, it continued to be found in the plasma, erythrocytes, and urine 5 years after the last dose.
Pharmacodynamics
The dose‐effect relationship for CQ is not precisely defined. For HCQ low plasma concentrations (< 1 000 ng/ml) reportedly can exacerbate systemic LE. Higher plasma concentrations have been associated with an increased risk of adverse effects for long‐term therapy as well as with acute intoxication.
Dosage
Adverse effects can be avoided by observing the maximum daily dosages. The most feared complication of antimalarial drugs – irreversible retinopathy – depends neither on treatment duration nor on cumulative overall dose, but rather on whether the maximum daily dosage based on ideal body weight is exceeded. The maximum daily dosage is 3.5(–4) mg/kg of ideal body weight for chloroquine or 6(–6.5) mg/kg ideal body weight for hydroxychloroquine. If the maximum dosages are observed, retinopathy is not a concern, even with for therapy lasting several years [5, 6]. In a study with more than 900 patients (average length of treatment 7 years, cumulative dose of about 608 g), retinopathy only occurred at daily dosages ≥ 4 mg/kg chloroquine and ≥ 6.5 mg/kg hydroxychloroquine. Nevertheless, product information, such as provided with Resochin®, advises that in “adults, cumulative dosages should not exceed 1 g chloroquine per kg of body weight (50–100 g total dose of chloro‐quine).”
For everyday clinical practice, given that CQ/HCQ are not stored in adipose tissue, dosages may be adjusted according to the patient's ideal body weight. For men: (height [in cm]– 100) – 10 %. For women: (height [in cm]– 100) – 15 %. If the patient's actual weight is below the ideal, then the dosage is adjusted according to the actual weight. To avoid retinopathy, the maximum daily dosage of 3.5–(4) mg/kg of chloroquine or 6–(6.5) mg/kg hydroxychloroquine should not be exceeded for longer periods of time. Short‐term administration of higher daily dosages is not a problem. This may be necessary, for instance, giving an initial higher dosage for a few days to rapidly reach therapeutic blood levels (important note: increased side effects, possible misunderstanding) or if prescribing HCQ tablets that cannot be divided (Table 2). For patients with an ideal body weight of 63 kg who are given 1 CQ tablet per day (250 mg) the maximum dosage is thus 4 mg/kg ideal body weight. Patients with an ideal weight of 63–72 kg would be given a dosage of 3.5 to 4 mg/kg body weight of chloroquine. Only at heavier weights is the administration of 1 tablet or more per day unproblematic (Table 2, 3).
Table 2.
Recommendations for maximum daily dosage of hydroxychloroquine (200 mg tablets): 6–6.5 mg/kg ideal body weight ((height – 100) –10 %[men] or –15 %[women]; if this is less than actual weight, the actual weight is used). For the weight range here, lighter patients are given 6.5 mg/kg, and heavier ones 6 mg/kg. For patients with liver or kidney dysfunction, the dosage must be reduced. For long‐term therapy, lower daily dosages should be given if possible (weight = actual or ideal weight; based on [21]). Briefly exceeding the maximum daily dosage is unproblematic if care is taken not to over the longer term. Given for one week, the middle daily doses correspond to the recommended dosages.
| Weight (kg) | Recommended dosages | Cost of day therapy (Red List 2010, fixed fee, in euros) |
|---|---|---|
| 31–35 | 200 mg daily | 0.28 |
| 36–39 | 400 mg per day/week and 200 mg daily for the remaining 6 days of the week | 0.32 |
| 40–43 | 400 mg twice weekly and 200 mg daily for the remaining 5 days of the week | 0.36 |
| 44–48 | 400 mg three times weekly and 200 mg daily for the remaining 4 days of the week | 0.40 |
| 49–52 | 200 mg three times weekly and 400 mg daily for the remaining 4 days of the week | 0.44 |
| 53–56 | 200 mg two days a week and 400 mg daily for the remaining 5 days of the week | 0.49 |
| 57–61 | 200 mg once a week and 400 mg daily for the remaining 6 days of the week | 0.53 |
| > 61 | 400 mg/daily | 0.57 |
Table 3.
Recommendations for maximum daily CQ in clinical practice (tablets): 3.5–4 mg/kg ideal weight ((height –100) –10 %[men] or – 15 %[women]; if this is less than actual weight, then the actual weight is used to calculate dosage). For the weight range here, lighter patients are given 4 mg/kg and heavier ones 3.5 mg/kg. For patients with liver or kidney dysfunction, the dosage should be reduced. For long‐term therapy, lower daily dosages should be given if possible.
| Ideal/actual weight(kg) | Chloroquine (250 mg) | + | Chloroquine junior (81 mg) | Chloroquine (mg) | Cost of day therapy (Red List 2010, fixed fee, in euros) |
|---|---|---|---|---|---|
| 16–18 | 1/4 | – | 61.5 | 0.07 | |
| 20–23 | – | 1 | 81 | 0.43 | |
| 31–35 | 1/2 | – | 125 | 0.14 | |
| 36–41 | 1/4 | + | 1 | 143.5 | 0.28 |
| 42–46 | – | 2 | 162 | 0.85 | |
| 47–53 | 3/4 | – | 187.5 | 0.21 | |
| 54–58 | 1/2 | + | 1 | 206 | 0.57 |
| 58–64 | 1/4 | + | 2 | 224.5 | 0.50 |
| 63–72 | 1 | – | 250 | 0.28 | |
| 78–89 | 1 + 1/4 | – | 312.5 | 0.35 | |
| 83–94 | 1 | + | 1 | 331 | 0.71 |
| 93–107 | 1 + 1/2 | 375 | 0.42 |
Caution should be exercised when treating patients with an actual or ideal body weight of less than 63 kg. This applies especially to smaller or lighter patients, in particular women or shorter but heavier patients. For these patients, even a single tablet at the usual dosage of 250 mg CQ or 400 mg HCQ (= equivalent dosages) given for a prolonged period of time is too much. If kidney or liver function is impaired, the dosage must be further decreased.
Quinacrine is administered orally. To avoid adverse effects, the dosage should not exceed 100 mg/daily (= 1 tablet). Once the optimal effect has been achieved (∼ 3–6 months), the dosage should be reduced every by one tablet a week every 2 months until a maintenance dose of 1–3 tablets/week is reached. If the patient experiences diarrhea or other adverse effects, the daily dose should be reduced to 25–50 mg. At such low doses, it takes longer to achieve a clinical effect [4].
Adverse effects
The adverse effects associated with CQ/HCQ have been discussed in greater detail elsewhere [7]. Table 4 provides a summary of side effects. Specific aspects that are relevant to daily clinical practice will be dealt with in more detail. Certain symptoms should raise suspicion of a drug‐related cause, and tests should be performed to determine whether CQ/HCQ are the source. The incidence of specific side effects is based on the primary indication which is malaria and for which much higher daily dosages are typically given than those recommended here. Drug interactions with chloroquine and hydroxychloroquine are listed in Table 5.
Table 4.
Adverse effects of chloroquine/hydroxychloroquine (based on studies, “Red List”2010; see text).
| Organ | Symptom | Notes |
|---|---|---|
| Unspecific | Nausea, abdominal cramps, bloating, diarrhea, acid reflux, difficulty concentrating, sleep disorders | CQ/HCQ: 1–10 % |
| Eyes | Irreversible retinopathy | Central vision field loss; dependent on observing maximum daily dosage (Tables 3, 5) CQ/HCQ: 0.01–0.1 % |
| Corneal deposits | Reversible after discontinuing therapy CQ: < 1–10 % HCQ: < 0.1–1 % | |
| Accommodation disorders | At higher dosages, reversible < 0.01–0.001 % | |
| Hair | Whitening, hair los | Only in blond/red/light brown hair, reversible after stopping therapy rare < 0.01–0.1 % |
| CNS | Sleep disorders, confusion, dizziness, headache, paresthesia/dysesthesia drowsiness, fatigue, anxiety | < 0.1 %–1 % |
| Psychoses, epileptic seizures | < 0.01 % | |
| Ears | Hearing loss, tinnitus | CQ/HCQ: < 0.1–0.01 % |
| Skin | Hyperpigmentation | |
| Exanthems, exfoliative reactions | ||
| Phototoxic/allergic reactions | ||
| Pruritus | ||
| Hyperpigmentation | Gray discoloration on shins, palate, subungual; reversible | |
| Locomotor system | Myopathies/neuromyopathies | CQ/HCQ: < 0.01 %–0.1 %, reversible |
| Cardiovascular system | Depression of T wave; possible chronic toxicity in conduction disorders | < 0.1–1 %; isolated reports of fatality |
| Drop in blood pressure | CQ: < 0.1–1 % | |
| Cardiomyopathy | CQ: < 0.001 % | |
| Liver/gallbladder | Elevated transaminase | CQ/HCQ: < 0.01–0.1 % |
| Kidney | Phospholipidosis | CQ: < 0.001 % |
| Blood/lymphatic system | Pancytopenia, agranulocytosis, thrombocytopenia | CQ/HCQ: < 0.01–0.1 % |
| Eosinophilic methemoglobinemia | CQ: < 0.001 % | |
| Metabolism | Exacerbation of porphyria |
Table 5.
Drug interactions with chloroquine and hydroxychloroquine (“Red List” 2010).
| No effect | CQ/HCQ reduce bioavailability of | Bioavailability of CQ/HCQ reduced by | Bioavailability of CQ/HCQ enhanced by | CQ/HCQ increase the risk of side effects of | CQ/HCQ increase plasma levels of | Diminished effect of | Increased rate of skin reactions | Increased rate of muscle weakness | |
|---|---|---|---|---|---|---|---|---|---|
| Acetylsalicylic acid | + | ||||||||
| Aminoglycosides | + | ||||||||
| Ammonium chloride | |||||||||
| Ampicillin | + | ||||||||
| Aurothioglucose | + | ||||||||
| Kaolin/pectin | + | ||||||||
| Cholestyramine | + | ||||||||
| Cimetidine | + | ||||||||
| Cyclosporine A | + | ||||||||
| Digoxin | + | ||||||||
| Corticosteroids | + | ||||||||
| Imipramine | + | ||||||||
| Methotrexate | + | ||||||||
| Neostigmine | + | ||||||||
| Penicillamine | + | ||||||||
| Phenylbutazone | + | ||||||||
| Physostigmine | + | ||||||||
| Pyrimethamine/sulfadoxine | + | ||||||||
| Quinacrine | + | ||||||||
| Rabies vaccination | + | ||||||||
| Ranitidine | + | ||||||||
| Ritonavir | + | ||||||||
| Typhus vaccination | + |
Contraindications
CQ/HCQ are contraindicated in patients with retinopathy. Therapy may be attempted (gradually increasing dosage) in patients with hypersensitivity reactions to HCQ or CQ.
Relative contraindications include neuromuscular disorders such as myasthenia gravis or psychoses. In patients with pre‐existing porphyria cutanea tarda, there is a risk of hepatitis at usual dosages of antimalarial drugs. In patients with glucose‐6‐phosphate dehydrogenase deficiency (G6PD deficiency), hemolysis is rare at the dosages advised here [2]. It is the prescribing physician's decision to have G6PD activity assessed prior to beginning antimalarial drug therapy. In any case, if G6PD activity is not assessed, clinical controls for symptoms of potential hemolytic anemia are needed [8].
The German “red list” also contains the following absolute contraindications: diseases of the hematopoietic system, pregnancy and nursing, combination with hepatotoxic agents, combination with monoamine oxidase (MAO) inhibitors. Use is also limited in patients with severe liver or kidney dysfunction or psoriasis. These are further discussed in the text.
Acute overdose
Acute overdose usually occurs in children (accidentally) or as a suicide attempt. A single dose of 750 mg chloroquine, or 3 Resochin® tablets, can be fatal in children (1–4 years). Arrhythmia and cardiac arrest can lead to death. Thus, when prescribing CQ/HCQ, tablets must be kept well out of reach of small children. Given that CQ/HCQ accumulate in the deep compartments, the drugs are virtually impossible to remove from the system. This complications treatment of acute CQ/HCQ intoxication considerably [9].
Adverse effects associated with therapeutic dosages
In general, when individual daily doses (adjusted for ideal body weight) are observed, (Tables 2, 3), CQ/HCQ are well tolerated. Adverse effects (Table 4) can occasionally occur, especially with excessively high daily dosages; these are less common with adequate dosing (Tables 2, 3) [7].
Wallace has suggested that with regard to the risk of side effects, HCQ has certain advantages, although chloroquine is more effective. HCQ is said to have two‐thirds of the effectiveness of CQ, but is only half as toxic [2]. It is not clear, however, which daily dosages were compared.
Unspecific symptoms
If the patient experiences unspecific symptoms (Table 4), treatment may be attempted again after a drug‐free interval and dosage reduction. The side effects listed in the following mandate stopping therapy.
In one study, one out of two patients on quinacrine reported adverse effects at the beginning of therapy (daily dose of 100 mg), most of which were mild and reversible. One in three patients complained of headache, dizziness, or gastrointestinal symptoms (diarrhea, loss of appetite, nausea, abdominal cramps). Symptoms disappeared spontaneously or after reducing the dose. One in five patients had to discontinue treatment. A few patients reported persistent abdominal cramps or diarrhea. Symptoms were effectively treated with antacids or antispasmodics [4].
Ocular symptoms
The challenges of retinopathy, its definition and diagnosis, have been discussed elsewhere [6]. There are still no hard‐and‐fast criteria for diagnosing chloroquine‐associated retinopathy. Diagnosis is based on the subjective assessment of the examining physician. Although retinal damage is highly unlikely when ideal body weight is used to determine the maximum daily dose (see above), an ophthalmological examination (fundoscopy, vision field testing, color vision testing, Amsler grid test) is advised before beginning treatment or within the first few months of therapy as are annual controls – or semi‐annual controls for patients over 65 or with renal or liver insufficiency [8]. The baseline examination is important for detecting central red scotomas which occur in up to 6 % of the healthy population. Scotoma can also occur in patients with systemic lupus, as a result of vasculitis, or in patients with anti‐phospholipid syndrome or corticosteroid‐induced diabetes. Careful surveillance is essential if the suggested dosages are exceeded as well as in older patients and patients on long‐term continuous therapy (> 5 years). Self‐testing with an Amsler grid test (once a month; Figure 2) is recommended for early detection [10].
According to British recommendations, ophthalmological examinations are not mandatory for HCQ if the maximum daily dosages are observed. Clinical controls during regular physician appointments (ask about scintillating scotomas, poor night vision, and difficulty reading or recognizing faces) and annual vision testing are advised. Consultation of an ophthalmologist is only recommended for specific problems or for treatment exceeding five years [11].
CQ deposits in the cornea and anterior stroma (30–70 %, only visible using a slit lamp) do not necessitate stopping treatment. They are less common in HCQ. Vision is not limited, and the condition is reversible after discontinuing the drug.
In a small number of patients (especially those taking higher dosages > 500 mg/day and adolescents), accommodation disorders can occur as a result of direct effects of chloroquine on the smooth muscle of the ciliary body. These symptoms also usually resolve spontaneously if therapy is continued at the same dosage.
Quinacrine does not have any adverse ocular effects.
Skin
Various skin reactions can also occur (hyperpigmentation, maculopapular/lichenoid/urticarial exanthems, exfoliative reactions, DRESS syndrome, hypersensitivity to light).
One study reported hyperpigmentation and reversible yellowish skin discoloration in up to one‐third of patients taking quinacrine. The appearance of the conjunctivae may resemble jaundice, but with normal bilirubin levels. In up to 50 % of patients, there may be blue or black discoloration of the skin and nails.
The pigmentation diminishes when the dose is reduced and disappears once therapy is stopped. Exanthems have been reported in 1.6 % of patients treated with malaria prophylaxis. Symptoms resolve after stopping therapy. In treating systemic lupus, about 5 % of patients have mild, reversible dermatitis. During the Second World War, lichenoid dermatitis reportedly occurred (malaria prophylaxis/therapy) in 1 : 2 000 (daily dose 100 mg/daily) and 1 : 500 (200 mg/daily). In a small number of patients this led to anhidrosis, skin atrophy, alopecia, nail changes, and pigmentary disorders [2].
Psoriasis
Antimalarial drugs are known to occasionally exacerbate existing psoriasis. Psoriasis is not a contraindication for CQ therapy. A systematic analysis of the literature concluded that there is neither evidence for nor against exacerbation of psoriasis in patients taking antimalarial drugs [12]. Their use should be determined on an individual basis.
Heart
At normal dosages, CQ/HCQ have no negative effects on the heart. There are case reports, however, on conduction disorders, cardiomyopathy, and even death.
Nervous system
Low‐dose quinacrine is psychologically stimulating and improves drowsiness. At higher dosages, however, after discontinuing the drug (within 2–3 weeks), reversible symptoms such as restlessness, insomnia, and psychoses can occur.
Blood
Blood count changes such as aplastic anemia, leukopenia, agranulocytosis, and hemolysis are rare and almost always related to glucose‐6‐phophate‐dehydrogenase deficiency. Some authors thus recommend routine measurement of the enzyme, while others believe it is unnecessary since the hemolytic effects of chloroquine are very minimal [2].
During quinacrine therapy, a blood differential should be performed every 2–3 months. If there is a drop in hemoglobin or reticulocytes, treatment must be stopped.
Pregnancy and lactation
According to the manufacturer's information, CQ/HCQ should not be taken by pregnant women, except to treat malaria. There are isolated reports of sensory‐neural hearing loss, blindness, birth defects, and spontaneous abortion. Yet harm to the fetus can also occur if lupus flares up during pregnancy. Thus it should be decided on an individual basis what the risks associated with the underlying disease or alternative therapies are compared with the potential side effects of antimalarial therapy. Several observational studies and literature analyses have reported that HCQ may be safely taken during pregnancy [13]. In one prospective, randomized controlled study, the children of 20 mothers with lupus who were treated with HCQ had higher Apgar scores and no anomalies. In addition, there were no reported lupus flares compared with 3/10 in the control group. Based on published reports, especially on the use of HCQ in pregnant women, the drug appears to be safe and is thus also recommended for use in pregnant women with lupus. Similarly, the “Pharmacovigilance and counselling center for embryonic toxicology “(http://www.embryotox.de) calls the use of CQ/HCQ for antirheumatic therapy “acceptable.” Exposure to CQ/HCQ is thus not a reason to terminate pregnancy, nor does it warrant invasive diagnostic procedures. Ultrasound studies may be performed in women taking longer‐term therapy or higher dosages in order to confirm normal fetal organ development.
CQ/HCQ are passed from the mother to the fetus, and after birth they are also detectable in the urine of infants who are being breastfed by a mother taking the drug. Still, there have been no reports of abnormalities associated with breastfeeding during therapy.
According to “Embryotox”, during malaria prophylaxis or short‐term therapy, mothers may continue breastfeeding as usual. For long‐term or high‐dose therapy, the decision of whether to continue breastfeeding should be made on an individual basis. Based on published reports, HCQ therapy of the mother is considered “acceptable” for healthy, full‐term infants who are closely followed‐up.
Quinacrine is passed from the mother to the fetus through the placenta. The drug should not be administered to pregnant or nursing women, despite reports that it does not interfere with pregnancy.
Laboratory controls
Several studies have found that there are no relevant hematological or hepatotoxic effects of antimalarial drugs if the maximum daily dosage is not exceeded. Since 1999, the American College of Rheumatology has explicitly stated that no laboratory studies are needed before or during therapy. It is advisable, however, to get baseline results for a blood differential and liver values before starting antimalarial therapy in order to rule out any pre‐existing pathological changes. For patients taking quinacrine, the blood differential (very rare: aplastic anemia) should be assessed at the start of therapy, and afterward every 3 to 4 months, and later every 6 months [9].
Confirmed indications
Lupus erythematosus
Cutaneous lupus erythematosus
CQ/HCQ are especially effective against inflammation associated with cutaneous lupus. Several reports have shown that smoking is associated with the development of cutaneous lupus and also that the response rate to therapy is lower in smokers (in one, about 80 % of patients with cutaneous lupus were smokers, and about 50 % of smokers had a “Cutaneous Lupus Erythematosus Disease Area and Severity Index” (CLASI) score of 0–1 compared with 80–90 % of non‐smokers) [14].
Chronic cutaneous lupus erythematosus (DLE)
One study found that only 15 % of discoid skin lesions healed spontaneously; patients who were given antimalarial therapy had a healing rate of more than 85 %. In a double‐blind study on patients with DLE, HCQ was more effective than placebo after 3 months and after 1 year. Verrucous and hypertrophic plaques do not respond well to antimalarial therapy. Because it takes a while to achieve consistent plasma concentrations, it is recommended that therapy be administered for at least two months before switching. Smokers have been shown to have a poorer response to treatment. Quinacrine (100 mg/daily) takes effect after 3–4 weeks and achieves its maximum effectiveness after 6–8 weeks. At lower daily dosages, it takes longer. Monotherapy with quinacrine has been shown to lead to significant or marked improvement in about 73 % of treated patients (literature in [4]). If the therapy appears inadequate after 8 weeks, treatment should be discontinued or combined with another medication such as CQ or HCQ. Combination therapy enhances the effectiveness of therapy compared with monotherapy. In a randomized double‐blind study that lasted 8 weeks (cutaneous lupus), 400 mg HCQ were equally effective as 50 mg acitretin (response rate of about 50 %), but were associated with fewer side effects (literature in [9]).
Lupus panniculitis
In a case series, 23 of 33 patients with lupus panniculitis improved after taking antimalarial drugs. In addition to other positive results from case reports, there are also individual reports on treatment failure and improvement after combination therapy consisting of CQ/HCQ with quinacrine or diltiazem.
Subacute cutaneous lupus erythematosus (SCLE)
In SCLE the reported response rate is 50–75 %. In SCLE as well, quinacrine has a synergistic effect.
Lupus erythematosus tumidus (= intermittent cutaneous LE)
Studies have reported a 90 % success rate of antimalarial drug therapy in patients with intermittent cutaneous lupus [14, 15]. The drug usually takes 4–6 weeks before it begins to take effect.
A possible sub‐group of “lymphocytic infiltration of the skin” is photosensitive lupus erythematosus. In this variant as well, antimalarial drugs have been shown to be effective.
Acute cutaneous LE/systemic lupus erythematosus (ACLE/SLE)
Compared with non‐steroidal anti‐inflammatory agents antimalarial agents have the advantage that they carry a lower risk of cutaneous, liver, or toxic kidney reactions. Compared with immunosuppressants, there is neither the risk of bone marrow suppression nor opportunistic infection. Based on the results of a systematic review, antimalarial drugs can inhibit the exacerbation of longstanding SLE and prolong patient survival [16].
The risk of exacerbation of the disease was 4.6 times higher in patients taking placebo than in those who were given CQ (18 % of those taking CQ vs. 83 % of patients taking placebo). Corticosteroids were not necessary.
A medium level of evidence has been found showing a protective effect against irreversible organ damage, thrombosis, and loss of bone mass. Low evidence levels have been reported for favorable effects on blood lipids, protection against osteonecrosis, a reduction in severe exacerbations, adjuvant effects on remission of lupus‐related nephritis, delayed development of systemic lupus, and a protective effect against developing cancer [16].
Additional symptoms such as fatigue, weakness, arthralgia, myalgia, serositis, and mucous membrane ulcers have also shown improvement in SLE patients. A small case series also reported a synergistic effect of quinacrine in SLE. Thus CQ/HCQ are recommended for basic treatment in the majority of SLE patients [16]. They should never be given alone, however, in patients with cardiac, lung, kidney, hematological, or vascular/central nervous system manifestations.
Rheumatoid arthritis (RA)
Antimalarial drugs lead to improvement of RA in about half of patients, decreasing joint swelling and pain and improving functional parameters after an average of 3–6 months of treatment. In patients with RA, combination therapy with methotrexate, sulfasalazine, and HCQ reportedly led to response rate of 77 % which is significantly better than for monotherapy with methotrexate (33 % response) or combination therapy with sulfasalazine/HCQ (40 % response rate). HCQ also reduces transaminase elevation resulting from methotrexate or acetylsalicylic acid.
REM syndrome
Based on case reports and our own experience, therapy is effective against this rare disorder.
Porphyria cutanea tarda (PCT)
For PCT, CQ/HCQ are given in much lower dosages than usual: twice weekly (!) 125 mg CQ or 2 × 100 mg HCQ. CQ may be given as monotherapy for PCT patients with normal erythrocyte, hemoglobin, and iron levels. In patients with polyglobulia or elevated plasma iron levels, combination therapy, i.e., beginning with bloodletting – 500 ml every 4 weeks – plus CQ 2 × 125 mg/weekly (HCQ 2 × 100 mg/weekly) is recommended. One study reported that after an average of 9 (± 3) months, PCT had resolved in 97 % of treated patients. In a long‐term observational study with 89 PCT patients, the results of biopsy studies showed improvement of liver morphology in 80 %[17]. Side effects rarely occurred at proper dosages. A daily dosage ≥ 250 mg can, on the other hand, trigger a potentially fatal porphyria crisis.
Chronic ulcerative stomatitis
This rare disease occurs at older ages in women. Clinically and histologically, chronic ulcerative stomatitis resembles lichen planus, but with certain immunological characteristics. The disease does not respond to topical or systemic corticosteroids, but it does respond to antimalarial agents. After longer‐term therapy, low‐dose steroids are sometimes required.
Suitable indications
Sarcoidosis
About 35 % of patients with sarcoidosis also have skin involvement. In one randomized prospective double‐blind study in which patients were given therapy for 4 months, CQ was related to a significant improvement after 3 and 6 months; after 12 months there was no longer a difference between groups. This indicates that CQ, similar to corticosteroids, tends to suppress the disease rather than to cure it. In one patient series, skin changes resolved in 12 out of 17 patients allowing other therapies to be discontinued; 3 patients improved slightly, and 2 out of 12 did not respond. In 2 out of 8 patients with lung involvement, the status of the lungs improved. While CQ therapy of cutaneous sarcoidosis is an established method, pulmonary sarcoidosis seems to respond better to steroid therapy.
Indications for administering CQ or HCQ in patients with sarcoidosis are: chronic disfiguring skin lesions, progressive extracutaneous lesions in patients in whom steroid therapy is contraindicated, adjuvant therapy in steroid treatment, continued neuro‐sarcoidosis with steroid failure. Hypercalcemia in sarcoidosis, but not in B‐cell lymphoma, has shown improvement after hydroxychloroquine therapy.
Dermatomyositis
In patients with dermatomyositis whose skin lesions do not heal after systemic steroid therapy, various case series have shown that giving CQ/HCQ alone or in combination with quinacrine can lead to improvement/healing in 40–75 %[18]. This also applies to dermatomyositis in children.
Individual therapy attempts
In the following indications, treatment may be attempted if standard therapies fail.
Sjögren syndrome
Two double‐blind studies reported that laboratory chemical parameters (ESR, significant reduction in IgG and IgA) improved but clinical parameters did not. In one retrospective analysis of 50 patients that spanned 3 years, ocular pain improved (in 55 %) as did dryness of the eyes (in 57 %), cornea integrity (Rose Bengal test improved in 53 %), and the Schirmer test (improved > 2 mm/5 minutes in 50 %), oral pain and dry mouth (57 % and 60 %) as well as salivary flow (in 82 %). On the whole, more than 60 % of treated patients reported improvement. The discrepancies seen in results may be due to the starting point of therapy; therapy is perhaps effective if given during the inflammatory stage but not during the “burned out” stage of disease.
Polymorphous light eruption
For patients with polymorphous light eruption, antimalarial drugs are not the treatment of choice. In two controlled studies, CQ/HCQ led to improved tolerance to sunlight and to a moderate clinical improvement with a significant reduction in the exanthem. The clinical improvement was not very impressive. Treatment is therefore only recommended in patients with severe disease, failure of topical steroids/protective measures against sunlight, or failure or lack of feasibility of UV hardening.
Positive results from case reports (wider application uncertain)
There are a number of case reports on the successful use of antimalarial agents in the treatment of an array of skin diseases. Yet in the absence of larger studies, it is impossible to compare the results or to draw any solid conclusions. The diseases are listed in Table 6. In the following they are discussed more thoroughly.
Table 6.
| Disease | Study type |
|---|---|
| Demonstrated efficacy and first‐line therapy | |
| • Lupus erythematosus (cutaneous and systemic) | Prospective controlled double‐blind study |
| • Rheumatoid arthritis | Prospective controlled double‐blind study |
| • REM syndrome | Case series |
| • Porphyria cutanea tarda | Case series |
| • Chronic ulcerative stomatitis | 2 case reports |
| Suitable indications (strong evidence of effectiveness) | |
| • Sarcoidosis (skin) | Placebo‐controlled clinical study, case series |
| • Skin manifestation of dermatomyositis | Case series |
| • Lymphocytic infiltration | Case series |
| Occasionally indicated (if standard therapies fail) | |
| • Primary Sjögren syndrome | Controlled double‐blind study (only improvement on laboratory chemical tests), case series (clinical improvement) |
| • Polymorphous light eruption | Prospective double‐blind study |
| Positive results – case reports | |
| • Atopic dermatitis | Case series |
| • Eosinophilic fasciitis | Case series |
| • Hereditary bullous epidermolysis | 2 case reports |
| • Granuloma anulare | Case series |
| • Lichen planus mucosae | Case series |
| • Lichen sclerosus et atrophicus | Case reports |
| • Morphea | Case series: combination with penicillin most successful |
| • Necrobiosis lipoidica | Case series |
| • Panniculitis (chronic erythema nodosum, lipoatrophic panniculitis) | Case reports |
| • Solar urticaria | Case reports |
| • Urticarial vasculitis | Case reports |
Atopic dermatitis
In one open study lasting 3–6 months, eczema improved significantly in 46/62 patients who were given CQ, allowing systemic steroid therapy to be discontinued. Eight patients had moderate improvement, and 8 were forced to discontinue therapy due to adverse effects.
Eosinophilic fasciitis
In one case series (52 patients) HCQ was reportedly just as effective as corticosteroids. Yet due to unforeseen spontaneous healing, it was difficult to unequivocally assess the results.
Granuloma anulare
In several case reports, and in one larger case series, CQ/HCQ use in pediatric patients has reportedly led to complete resolution of generalized granuloma anulare. Therapy was continued for up to 2 weeks after clinical resolution. Patients remained symptom‐free for up to 5 years afterward.
Lichen planus
Lichen planus mucosae
In one patient series, oral lichen planus improved in 9/10 patients who were given HCQ. Pain and redness improved after 1–2 months. It took 3–6 months for erosions to heal (healing was reported in 3 out of 6 patients). CQ was also successfully used in lichen planus of the lower lip.
Actinic lichen planus
This form of lichen planus on light‐exposed areas of the skin typically occurs in the Middle East. Based on case reports and in the experience of the authors, it appears that CQ/HCQ therapy is highly effective [1].
Lichen planus of the nail
One case study has reported lichen planus of the nail. The symptoms had manifested 4 years earlier. After a 30‐week‐long regime of CQ, the symptoms disappeared. Ten weeks after stopping therapy the condition returned.
Necrobiosis lipoidica
In one case series, 7 out of 8 patients with necrobiosis lipoidica improved within 6 months [19].
Non‐dermatological indications
Thrombosis prophylaxis, antiphospholipid syndrome
CQ/HCQ inhibit thrombocyte aggregation. In one study, when given to prevent thrombosis following hip replacement surgery, hydroxychloroquine (600–800 mg/daily for 1–2 weeks) significantly reduced the incidence of pulmonary embolism in more than 10 000 patients. In another study, the rate of venous and arterial thromboembolic complications was reduced. Even in a sub‐group of high‐risk SLE patients with phospholipid antibodies, only 4 % who were given HCQ therapy experienced a thromboembolism within 9 years (2/54) while 20 % in the control group did [20]. CQ/HCQ should thus be given as the primary preventive therapy for thromboembolism in patients with SLE [16]. In patients with existing thromboem‐bolic complications anticoagulants are recommended instead.
Effects on blood lipids
Due to an increase in LDL receptors and inhibition of cholesterol synthesis in the liver, total cholesterol, LDL, and triglyceride levels are decreased in SLE patients, especially in those who are on concomitant steroid therapy. Chloroquine reduces blood glucose levels and thus acts against the side effects of corticosteroids [20].
Conclusions for practicing dermatologists:
The use chloroquine/hydroxychloroquine has fallen out of favor due to severe ocular side resulting from incorrect dosing. Risk may be minimized by observing the following principles. The incidence of side effects depends heavily on plasma levels of the drug. These in turn are a result of reverse diffusion of CQ/HCQ from the “deep compartments.” Antimalarial drugs are not stored in adipose tissue. Thus, the key to avoiding retinopathy is to observe the maximum recommended daily dosages of 3.5(–4) mg/kg chloroquine (ideal body weight) and 6(–6.5) mg/kg hydroxychloroquine. This is especially important for lighter patients (especially women) and small but obese patients. If there is delayed elimination due to renal or liver dysfunction, the dosage should be further reduced. Ophthalmological controls during the first few months of therapy are advised, and later in larger intervals. If the above‐mentioned guidelines are followed, CQ/HCQ may even be given over longer periods of time with minimal risk.
Conflict of interest
None.
Correspondence to Prof. Dr. med. Falk R. Ochsendorf Klinik für Dermatologie, Venerologie und Allergologie Klinikum der J.W. Goethe‐Universität Theodor‐Stern‐Kai 7 D‐60590 Frankfurt am Main Tel.: +49‐69‐6301‐6661 Fax: +49‐69‐6301‐81080 E‐mail: ochsendorf@em.uni-frankfurt.de
Fragen zur Zertifizierung durch die DDA
-
1
Chloroquin/Hydroxychloroquin reichern sich in zahlreichen Organen/Strukturen an. In welchen Organen/Strukturen findet keine Anreicherung statt?
-
a)
Epidermis
-
b)
Dermis
-
c)
Melanin
-
d)
Leber
-
e)
Fettgewebe
-
a)
-
2
Über welchen Stoffwechselweg werden CQ/HCQ vor allem ausgeschieden?
-
a)
unverändert im Stuhl
-
b)
Nieren
-
c)
Leber
-
d)
1–3 zu gleichen Teilen
-
e)
2 und 3 zu gleichen Teilen
-
a)
-
3
Ein 37‐jähriger Patient soll mit Chloroquin behandelt werden. Er wiegt 80 kg bei einer Körpergröße von 166 cm. In welchem Bereich sollte die Tagesdosis liegen?
-
a)
210–240 mg
-
b)
231–264 mg
-
c)
280–320 mg
-
d)
360–390 mg
-
e)
396–429 mg
-
a)
-
4
Eine 25‐jährige Frau soll mit Hydroxychloroquin behandelt werden. Sie wiegt 53 kg bei einer Kör‐pergröße von 168 cm. In welchem Bereich sollte die Tagesdosis liegen?
-
a)
185–212 mg
-
b)
203–233 mg
-
c)
318–344 mg
-
d)
348–377 mg
-
e)
380–420 mg
-
a)
-
5
Die Standarddosis von Quinacrin beträgt:
-
a)
25 mg
-
b)
50 mg
-
c)
75 mg
-
d)
100 mg
-
e)
150 mg
-
a)
-
6
Welcher der folgenden Faktoren vermindert die klinische Wirksam‐keit von CQ/HCQ beim kutanen Lupus erythematodes besonders?
-
a)
Einnahme von CQ/HCQ mit einer Mahlzeit
-
b)
Komedikation mit Glucocorticoiden
-
c)
Komedikation mit Methotrexat
-
d)
Rauchen
-
e)
Vorhandensein von anti‐Ro/SSA und anti‐La/SSB‐Antikörpern
-
a)
-
7
Wie lange muss man mindestens warten, bis man den Effekt einer Therapie mit Antimalariamitteln klinisch beurteilen kann?
-
a)
4 Wochen
-
b)
8 Wochen
-
c)
12 Wochen
-
d)
16 Wochen
-
e)
20 Wochen
-
a)
-
8
Welche Dosierung von Chloroquin verwendet man zur Therapie der Porphyria cutanea tarda bei einem 160 cm großen, 80 kg schweren Mann?
-
a)
125 mg/Woche
-
b)
2 × 125 mg/Woche
-
c)
1 ×∼ 200 mg/d
-
d)
1 × 250 mg/d
-
e)
2 × 125 mg/d
-
a)
-
9
Bei welcher der folgenden Erkran‐kungen sind Antimalariamittel am wenigsten wirksam?
-
a)
polymorphe Lichtdermatose
-
b)
Sarkoidose
-
c)
Dermatomyositis
-
d)
REM‐Syndrom
-
e)
chronisch ulzerative Stomatitis
-
a)
-
10
Welches Vorgehen ist bei einem nicht auf eine Monotherapie mit Chloroquin ansprechenden kutanen Lupus erythematodes am sinnvollsten?
-
a)
Erhöhung der Hydroxychloroquin‐Dosis
-
b)
Umstellung auf Chloroquin
-
c)
Umstellung auf Quinacrin
-
d)
Kombination mit Hydroxychloroquin
-
e)
Kombination mit Quinacrin
-
a)
Liebe Leserinnen und Leser,
der Einsendeschluss an die DDA für diese Ausgabe ist der 19. November 2010.
Die richtige Lösung zum Thema „Die photodynamische Therapie in der Dermatologie“in Heft 6 (Juni 2010) ist: 1b, 2c, 3a, 4e, 5a, 6d, 7b, 8a, 9e, 10c.
Bitte verwenden Sie für Ihre Einsendung das aktuelle Formblatt auf der folgenden Seite oder aber geben Sie Ihre Lösung online unter http://jddg.akademie-dda.de ein.
Section Editor Prof. Dr. Jan C. Simon, Leipzig
Footnotes
Chloroquine (CQ), hydroxychloro‐quine (HCQ), and quinacrine are antimalarial drugs that may be used to treat inflammatory diseases.
These drugs are approved for use against malaria, systemic lupus erythematosus, and rheumatoid arthritis.
These agents are also useful in a number of other diseases. Controlled studies are only available for some of these.
The mechanism of action is complex and involves immunomodulatory, anti‐inflammatory, and antiproliferative effects. Thrombocyte aggregation is inhibited, glucose tolerance is enhanced, and porphyrin excretion is increased.
CQ and HCQ are amphiphilic molecules that primarily accumulate in lysosomes.
Quinacrine is difficult to obtain.
CQ/HCQ are taken orally and are almost completely absorbed.
The concentration of CQ/HCQ in the skin is 100–200 times higher than in plasma.
There is virtually no accumulation of CQ/HCQ in adipose tissue.
CQ/HCQ accumulate in “deep compartments” (distribution volumes > 100 l/kg) and especially in melanin.
The half‐life of CQ/HCQ can be as long as 50 days.
In some patients, more than 50 % of CQ/HCQ is eliminated by the kidneys.
Plasma levels of CQ/HCQ primarily depend on reverse diffusion from the deep compartments.
Higher plasma levels have been shown to be related to an increased risk of side effects.
To avoid adverse effects, the maximum dosage should be adjusted according to the ideal body weight of the patient. For chloroquine this is 3.5(–4) mg/kg of ideal body weight and hydroxychloro‐quine at 6(–6.5) mg/kg of ideal body weight.
Care must be taken when calculating the dosage for patients weighing < 63 kg (usually women) or shorter but heavier patients.
Dosage must be further decreased in patients with impaired kidney or liver function.
The standard dosage of quinacrine is 100 mg/daily.
Table 4 gives a summary of related side effects.
An absolute contraindication is retinopathy.
CQ/HCQ must be kept out of reach of small children. Even ingestion of only a few tablets can lead to fatality. Intoxication, which can cause cardiac arrest, is difficult to counteract.
HCQ reportedly has fewer side effects than CQ, but is also less effective.
In patients who experience unspecific symptoms such as nausea or difficulty concentrating, CQ/HCQ may be continued in a low dose after an interval. Certain adverse effects warrant stopping treatment.
Ophthalmological examinations (fundoscopy, vision field testing, color vision testing, Amsler grid test) are recommended before starting therapy or within the first few months of treatment as are annual controls – or semi‐annual controls for patients over 65 or with renal or liver insufficiency.
According to British recommendations, controls are not necessary for patients taking HCQ as long as dosage limits are respected.
CQ/HCQ deposits in the cornea are not critical.
CQ/HCQ can, very rarely, cause various types of rashes.
Quinacrine can cause yellowish discoloration of the skin.
Antimalarial drugs can exacerbate psoriasis.
Conduction disorders are possible, but at therapeutic dosages are very rare.
The hemolytic effects associated with CQ/HCQ are minimal.
The benefits and risks of taking antimalarial drugs during pregnancy should be considered individually.
In pregnant women being treated for lupus, the use of HCQ is considered safe.
A blood differential and liver values should be assessed before beginning CQ/HCQ therapy.
Patients taking quinacrine should undergo regular blood testing.
Smoking is associated with the development of lupus erythematosus and with a poorer response to CQ/HCQ.
In patients with DLE the response rate of inflammatory lesions is more than 80 %.
It can take up to 8 weeks before the maximum effectiveness of CQ/HCQ is reached.
The combination of CQ or HCQ with quinacrine acts synergistically.
CQ/HCQ, given as monotherapy or combination therapy, are effective against lupus panniculitis.
The response rate in patients with SCLE is 50–75 %.
90 % of patients with intermittent cutaneous LE respond to therapy with antimalarial drugs.
In patients with systemic lupus, CQ/HCQ prevent exacerbations and prolong survival.
Antimalarial drugs have a positive effect on many SLE‐associated symptoms and are a part of basic therapy in SLE.
CQ/HCQ, especially when given as combination therapy, can lead to improvement of symptoms associated with rheumatoid arthritis.
CQ/HCQ are indicated in REM syndrome.
In patients with PCT therapy consists of: weekly (!) 2 × 125 mg CQ or 2 × 100 mg HCQ, possibly in combination with bloodletting.
250 mg CQ/daily can trigger a porphyria crisis.
CQ/HCQ are the treatment of choice in chronic ulcerative stomatitis.
There is significantly greater improvement in skin sarcoidosis in patients taking CQ/HCQ compared with those taking placebo.
The skin lesions in patients with dermatomyositis have been shown to improve in more than half of patients treated with CQ/HCQ.
In patients with Sjögren syndrome, laboratory parameters and clinical symptoms may improve, depending on disease stage, after treatment with CQ/HCQ.
CQ/HCQ have a limited effect in patients with polymorphous light eruption and are thus considered only a second‐line option.
Case reports seem to support a positive effect of CQ/HCQ various inflammatory skin diseases (Table 6).
Granuloma anulare appears to respond to CQ/HCQ.
In one study on erosive lichen planus, erosions healed (50 % responders) in up to 6 months.
CQ/HCQ should be considered the primary preventive measure against thromboembolism in patients with SLE or phospholipid antibodies.
CQ/HCQ diminish the effects of corticosteroids on blood lipids and glucose.
References
- 1. Wolf R, Wolf D, Ruocco V. Antimalarials: unapproved uses or indications. Clin Dermatol 2000; 18: 17–35. [DOI] [PubMed] [Google Scholar]
- 2. Kalia S, Dutz JP. New concepts in antimalarial use and mode of action in dermatology. Dermatol Ther 2007; 20: 160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine. Focus on recent advancements. Clin Pharmacokinet 1996; 31: 257–74. [DOI] [PubMed] [Google Scholar]
- 4. Wallace DJ. The use of quinacrine (Atabrine) in rheumatic diseases: a reexamination. Semin Arthritis Rheum 1989; 18: 282–96. [DOI] [PubMed] [Google Scholar]
- 5. Maksymowych W, Russell AS. Antimalarials in rheumatology: efficacy and safety. Semin Arthritis Rheum 1987; 16: 206–21. [DOI] [PubMed] [Google Scholar]
- 6. Ochsendorf FR, Runne U, Goerz G, Zrenner E. Chloroquin‐Retinopathie: durch individuelle Tagesdosis vermeidbar. Dtsch med Wschr 1993; 118: 1895–8. [DOI] [PubMed] [Google Scholar]
- 7. Ochsendorf FR, Runne U. Chloroquin und Hydroxychloroquin: Nebenwirkungsprofil wichtiger Therapeutika. Hautarzt 1991; 42: 140–6. [PubMed] [Google Scholar]
- 8. Kuhn A, Bonsmann G. Kutaner Lupus erythematodes. AWMF Leitlinienregister Nr. 13/060; http://leitlinien.net/; 2008.
- 9. Gunja N, Roberts D, McCoubrie D, Lamberth P, Jan A, Simes DC, Hackett P, Buckley NA. Survival after massive hydroxychloroquine overdose. Anaesth Intensive Care 2009; 37: 130–3. [DOI] [PubMed] [Google Scholar]
- 10. Easterbrook M. Screening for antimalarial toxicity: current concepts. Can J Ophthalmol 2002; 37: 325–8, 331–4. [DOI] [PubMed] [Google Scholar]
- 11. Jones SK. Ocular toxicity and hydroxychloroquine: guidelines for screening. Br J Dermatol 1999; 140: 3–7. [DOI] [PubMed] [Google Scholar]
- 12. Herman SM, Shin MH, Holbrook A, Rosenthal D. The role of antimalarials in the exacerbation of psoriasis: a systematic review. Am J Clin Dermatol 2006; 7: 249–57. [DOI] [PubMed] [Google Scholar]
- 13. Vroom F, De Walle HE, Van De Laar MA, Brouwers JR, De Jong‐van den Berg LT. Disease‐modifying antirheumatic drugs in pregnancy: current status and implications for the future. Drug Saf 2006; 29: 845–63. [DOI] [PubMed] [Google Scholar]
- 14. Kreuter A, Gaifullina R, Tigges C, Kirschke J, Altmeyer P, Gambichler T. Lupus erythematosus tumidus: response to antimalarial treatment in 36 patients with emphasis on smoking. Arch Dermatol 2009; 145: 244–8. [DOI] [PubMed] [Google Scholar]
- 15. Kuhn A, Richter‐Hintz D, Oslislo C, Ruzicka T, Megahed M, Lehmann P. Lupus erythematosus tumidus. A neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol 2000; 136: 1033–41. [DOI] [PubMed] [Google Scholar]
- 16. Ruiz‐Irastorza G, Ramos‐Casals M, Brito‐Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010; 69: 20–8. [DOI] [PubMed] [Google Scholar]
- 17. Wollina U, Kostler E, Koch A, Riedel H, Stolzel U. Does chloroquine therapy of porphyria cutanea tarda influence liver pathology? Int J Dermatol 2009; 48: 1250–3. [DOI] [PubMed] [Google Scholar]
- 18. Ang GC, Werth VP. Combination of antimalarials in the treatment of cutaneous dermatomyositis: a retrospective study. Arch Dermatol 2005; 141: 855–9. [DOI] [PubMed] [Google Scholar]
- 19. Durupt F, Dalle S, Debarbieux S, Balme B, Ronger S, Thomas L. Successful treatment of necrobiosis lipoidica with antimalarial agents. Arch Dermatol 2008; 144: 118–9. [DOI] [PubMed] [Google Scholar]
- 20. Wallace DJ, Linker‐Israeli M, Methger AL, Stecher VM. The relevance of antimalarial therapy with regard to thrombosis, hypercholesterolemia and cytokines in SLE. Lupus. 1993; 2(Suppl 1): S13–S15. [PubMed] [Google Scholar]
- 21. Canadian Consensus Conference on hydroxychloroquine. J Rheumatol 2000; 27: 2919–21. [PubMed] [Google Scholar]


