Abstract
Immunological memory provides long-term protection against pathogen re-infection and is the foundation for successful vaccination. We have previously shown an antigen-specific recall response in nurse sharks almost one year after primary exposure. Herein, we extend the time between prime and successful recall to >8 years, the longest period for which immunological memory has been shown in any non-mammalian vertebrate. We confirm that that antigen binding is mediated by monomeric IgM and IgNAR, but not pentameric IgM, in both the primary and recall phases. Our inability to find target-binding clones in recombinant VNAR expression libraries suggests that, at least in this instance, antigen-specific memory cells comprise a small fraction of the IgNAR-positive B cells in epigonal and spleen. Further, that the few memory cells present can generate a robust antigen-specific IgNAR titer following re-stimulation. Our results continue to challenge the long-held, but erroneous, belief that the shark adaptive immune system is ‘primitive’ when compared to that of mammals.
Keywords: cartilaginous fish, shark, antibody, IgM, IgNAR, memory
1. Introduction
Immunological memory provides long-term protection against pathogen re-infection (Taub et al., 2008) and is one of the hallmarks of vertebrate adaptive immunity. In mammals, naïve B cells are activated upon primary antigenic exposure and some activated lymphocytes undergo iterative rounds of diversification and selection in germinal centers (GCs) found in the follicles of secondary lymphoid tissues. Exiting the GC, some B cells differentiate into long-lived plasma cells and others into memory cells. The latter exist in a quiescent state until secondary exposure to antigen when they rapidly differentiate into immunoglobulin (Ig) secreting plasma cells (Kurosaki et al., 2015). The resultant secondary response is generally characterized by faster antibody production relative to the primary response, increased serum antibody titers, and a switch of Ig isotype (Janeway, 2005). Classical mammalian memory B cells have undergone somatic hypermutation followed by affinity maturation via selection on antigen displayed on follicular dendritic cells (FDCs) in the GC (Victora and Nussenzweig, 2012; Palm and Henry, 2019).
Cartilaginous fishes (sharks, skates and rays, and chimeras) are the oldest extant phylogenetic group to possess an adaptive immune system with many of the basic cellular and molecular components present in mammals, including B cells that express Ig in either a transmembrane form on naïve B cells or a secreted form in plasma cells, T cells that express either α/β or δ/γ T cell receptors, and classical and nonclassical major histocompatibility complex (MHC) molecules (Flajnik, 2014). While cartilaginous fishes lack lymph nodes and organized mucosal lymphoid tissue, they do have a secondary lymphoid organ, the spleen, with organized lymphoid follicles. It is thought that the activation of lymphocytes and the generation of antigen-specific immunity is initiated in these splenic follicles. To date, the true equivalent of mammalian GCs, including FDC, have not been identified in cartilaginous fishes or any other ectothermic vertebrate (Zapata and Amemiya, 2000). Nevertheless, there is clear evidence that B cells in sharks and other ectothermic vertebrates can hypermutate their Ig genes and can affinity maturate their antigen-specific antibody responses (Dooley and Flajnik, 2005; Dooley et al., 2006).
In the absence of bone marrow, cartilaginous fish have two tissues that act as sites of lymphopoiesis and potential reservoirs for antibody-secreting B cells, the epigonal organ associated with the gonads and the Leydig organ associated with the esophagus (Zapata and Amemiya, 2000; Miracle et al., 2001; Rumfelt et al., 2002; Castro et al., 2013; Luer et al., 2014). Different species may have both or only one of these bone marrow-equivalents, e.g. the nurse shark only has the epigonal organ.
Based upon experiments performed in the 1960s (reviewed in Dooley and Flajnik, 2006) it was long believed that immunological memory was ‘weak or totally lacking’ in cartilaginous fishes. However, even at the time these studies were performed, concerns were raised about the experimental design – specifically, that antigen-specific antibody from the primary response was still evident when the animals were stimulated to examine memory, and that this may have confounded the experimental result (Sigel and Clem, 1966 and disscussion notes therein). Further, sharks were shown to have IgM at high serum concentrations (>20 mg/mL) but were presumed to possess no other Ig isotypes (Marchalonis and Edelman, 1965). Subsequent work in nurse sharks (Ginglymostoma cirratum) showed that their humoral response is dominated by two Ig isotypes; the first, IgM, is found in both a pentameric (pIgM) and monomeric (mIgM) form. These IgM forms do not interconvert and, although present in roughly equal amounts in the blood stream, only mIgM can move from the vasculature into the tissues (Small et al., 1970). Indeed, it has been proposed that pIgM and mIgM are produced by distinct shark B cell lineages, with pIgM being produced from a B1-like lineage in a T cell-independent manner, but mIgM being produced by a T-dependent B2-like lineage (Dooley and Flajnik, 2005). The second isotype, IgNAR, is a heavy chain homodimeric isotype which does not associate with light chains (Clem et al., 1967; Greenberg et al., 1995). The single-domain nature of the IgNAR variable region (VNAR) (Roux et al., 1998; Stanfield et al., 2004), presence of only a few IgNAR genes in nurse sharks (thus allowing accurate identification of somatic mutations), and ease of production of recombinant VNARs in bacteria has greatly facilitated Ig repertoire and binding-affinity analyses in sharks (Diaz et al., 2002; Dooley et al., 2003; Dooley et al., 2006; Stanfield et al., 2007).
Armed with a more comprehensive knowledge of shark Igs, better experimental tools, and optimized immunization-challenge protocols, we previously demonstrated that sharks are indeed capable of robust, antigen-specific antibody responses with associated low-level affinity maturation, albeit on slower timescales than responses in endotherms. We also proved immunological memory in this lineage, showing antigen-specific antibody recall responses approx. 10 months after induction of a primary response (Dooley and Flajnik, 2005). Herein, we probe the duration of the memory response in the nurse shark. Our results continue to challenge the long-held, but erroneous, belief that the shark adaptive immune system is ‘primitive’ when compared to that of mammals.
2. Materials and Methods
2.1. Animal maintenance, immunization, and blood sampling
A wild-caught, adult nurse shark (Ginglymostoma cirratum) ‘Orange’ was maintained at approx. 28°C in artificial seawater in indoor tanks at the Institute of Marine & Environmental Technology, Baltimore, USA, during the experimental time course (as detailed in the results section). The animal was anesthetized with MS-222 (0.16 g/L seawater) before conducting any procedure. Bleeds were drawn from the caudal vein into a syringe containing 1/10 blood volume of porcine heparin reconstituted to 1000 U/ml in shark-modified PBS (mammalian PBS supplemented with 15 ml 5 M NaCl and 100 ml 3.5 M urea per L), then centrifuged at 1000 rpm for 10 min to isolate blood plasma for subsequent tests. Once a memory response had been shown the animal was sacrificed, tissue samples were taken and stored at −80°C. All procedures were conducted in accordance with approved University of Maryland School of Medicine IACUC protocols.
2.2. Antigen-binding ELISAs
Plasma samples were tested for the presence of antigen-specific Ig by ELISA as previously detailed (Dooley and Flajnik, 2005) but with the following amendments; antigen-coated microtiter plates were blocked with 5% milk powder in PBS (MPBS), and the monoclonal antibody (mAb) GA8 was used to detect IgNAR binding.
2.3. Nurse shark plasma fractionation
Plasma from the pre-memory boost and post-memory boost samples (700 μl of each) were fractionated by passage over a high-prep 16/60 Sephacryl S300 high resolution size exclusion column (GE healthcare life sciences Ltd.), as previously described (Dooley and Flajnik, 2005). Samples from each fraction were run non-reducing on 4–12% NuPAGE gels in MOPS buffer and bands visualized with Coomassie blue; as expected, pentameric IgM was limited to early fractions (#5–7; supplemental figure 1), while monomeric IgM and IgNAR eluted in later fractions (#10–13). Separate pIgM and mIgM/IgNAR pools were made by recombining equal volumes of the relevant fractions. These were tested by binding ELISA, performed as detailed above but using undiluted pools in the top well then serially diluting 3-fold down the plate.
Generation of VNAR bacterial expression libraries for analysis of the anti-HEL IgNAR memory repertoire
Spleen and epigonal tissue samples were homogenized in phenol solution and total RNA prepared from each as per standard protocols. Oligo-dT-primed cDNA was prepared and used as the template for PCR amplification of IgNAR variable regions (VNARs) with the handled primers NARFr4-Rev1 (5’-ATA ATC AAG CTT GCG GCC GCA TTC ACA GTC ACG ACA GTG CCA CCT C-3’) and NARFr4-Rev2 (5’-ATA ATC AAG CTT GCG GCCGCA TTC ACA GTC ACG GCA GTG CCA TCT C-3’) mixed in an equal ratio, and NARFr1-For (5’-ATA ATA AGG AAT TCCATG GCT CGA GTG GAC CAA ACA CCG-3’). The PCR products (~400bp) were cleaned, digested with the restriction enzymes NcoI and NotI at sites introduced in the primer handles, and cloned into the bacterial expression vector pIMS100 (Hayhurst and Harris, 1999); upon induction with IPTG this vector produces soluble VNARs tagged with a human Ig kappa constant domain and 6His tail. The resultant libraries were transformed into heat-shock competent E. coli TG1 and grown on LB agar containing 2% glucose (G2) and 100 μg/ml ampicillin (A100). The VNAR inserts of 40 clones (20 from each library) were sequenced to ensure the expression libraries had a high percentage of functional clones and good sequence diversity. Individual colonies were picked and used to inoculate 500 μl LB/G2/A100 in the wells of a 96 deep-well plate; 2 wells on each plate were inoculated with the anti-hen egg white lysozyme (HEL) VNARs HEL-5A7 and PBLA8 to act as positive controls and 2 wells left un-inoculated to act as negative controls. The plates were sealed with breathable film and grown overnight at 37°C, shaking at 250 rpm. The following day 100 μl was taken from each well and added to the wells of a fresh deep-well plate containing 100 μl LB/G2/A100 with 30% sterile glycerol and frozen at −80°C as glycerol stocks. The original plates were centrifuged at 1000 rpm for 10 min and the supernatant removed. Cell pellets were resuspended in 400 μl LB/A100 (no glucose) and grown at 25°C, shaking at 250rpm. After 30 min the plates were removed, 100 μl LB/A100 containing 0.5 mM IPTG added to each well (giving a final concentration of 0.1 mM IPTG). The plates were then returned to incubator for an additional 4 h to allow VNAR expression. At the end of this period the plates were centrifuged at 1000 rpm for 10 min at 4°C. Following removal of the supernatant cell pellets were resuspended in 150 μl ice-cold fractionation buffer (100 ml of 200 mM Tris-HCl pH 7.5 containing 20% sucrose and 1 ml 100 mM EDTA) and incubated on ice with gentle shaking for 15 min. Following the addition of 150 μl ice-cold MilliQ water to each well the plate was incubated for a further 15 min. Plates were then centrifuged at 1000 rpm for 10 min at 4°C and the supernatants removed for binding analysis.
For this ELISA plates were coated, blocked, washed, and developed as detailed above however 50 μl of culture supernatant mixed with an equal volume of 5% MPBS was added per well in place of shark plasma and mouse anti-human kappa light chain antibody (Sigma Aldrich; K4337) diluted 1:1000 in 5% MPBS used to detect VNAR binding.
3. Results and discussion
In January 2010 the nurse shark ‘Orange’ was primed with a cocktail of antigens that included 30 μg hen egg-white lysozyme (HEL), emulsified in CFA and administered subcutaneously into the lateral fin. The animal was subsequently boosted three times with soluble antigen (no adjuvant) containing 30 μg HEL, given intravenously (IV) at 4-week intervals. Small bleeds were taken 2 weeks after each immunization to test for an antigen-specific (primary) antibody response. Blood plasma from each bleed was tested by ELISA and, in this instance, a robust HEL-specific IgM titer was observed after the third boost (May 2010; bleed 4) with no increase in binding to the control wells that were both coated and blocked with 5% MPBS (Fig 1A). In contrast, only a very small increase in HEL-specific IgNAR was observed when comparing bleed 4 titers to the pre-bleed sample (Fig 1B). At this point the study was halted and the animal maintained for 8 years without further exposure to HEL.
Figure 1:
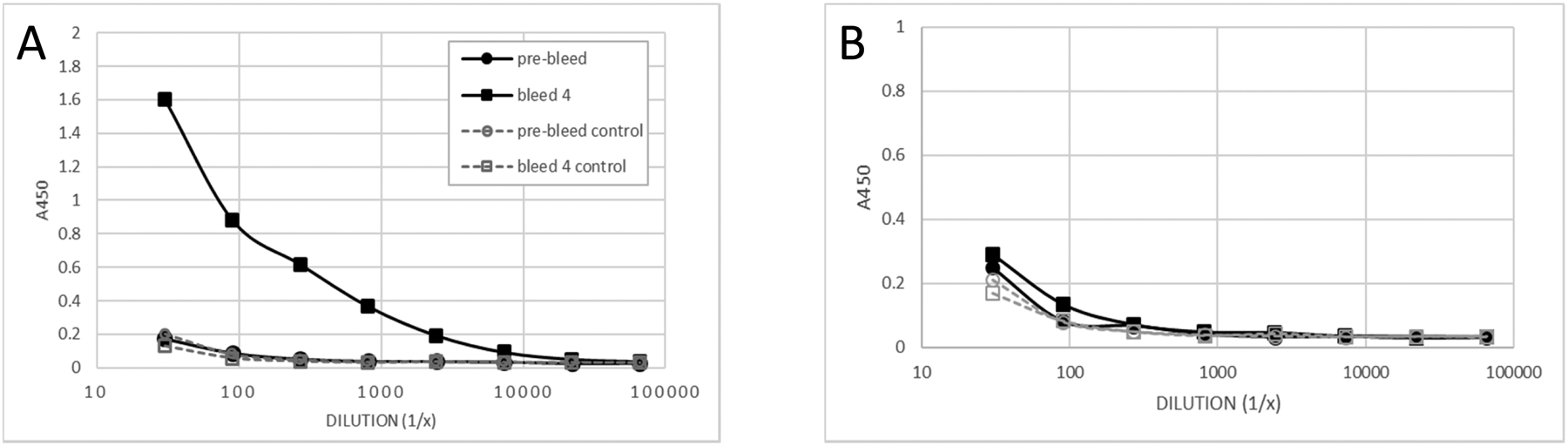
Analysis of blood plasma taken from nurse shark ‘Orange’ before immunization (pre-bleed) and following induction of a primary response (bleed 4) demonstrates (A) a robust HEL-specific IgM response and (B) low level IgNAR response by antigen-binding ELISA. Results shown are duplicate averages. Control wells were both coated and blocked with 5% MPBS.
In July 2018 a small blood sample was taken, the animal boosted once with 250 μg soluble HEL (dose adjusted for increased animal size) administered IV and a second blood sample taken 5 weeks later. Again, pre- and post-boost plasma samples were analyzed for HEL-specific antibody by ELISA. As expected, given the long interval since the primary response, the pre-boost sample showed no HEL-specific IgM (Fig 2A) or IgNAR (Fig 2B). In contrast, both isotypes demonstrated dramatic increases in HEL binding post-boost. We have previously established that even multiple immunizations with soluble antigen, administered IV without adjuvant, cannot generate primary immune responses in nurse sharks (Dooley and Flajnik; unpublished data). As in our previous study (Dooley and Flajnik, 2005), binding analysis of size-exclusion chromatography (SEC)-fractionated plasma showed that the increase in HEL-specific IgM was solely due to an increase in mIgM binding (Fig 2C) with no contribution from pIgM (Fig 2D). Thus, we are confident the response observed is a true memory response and demonstrates that sharks are capable of immunologic recall over periods of 8 years or more.
Figure 2:
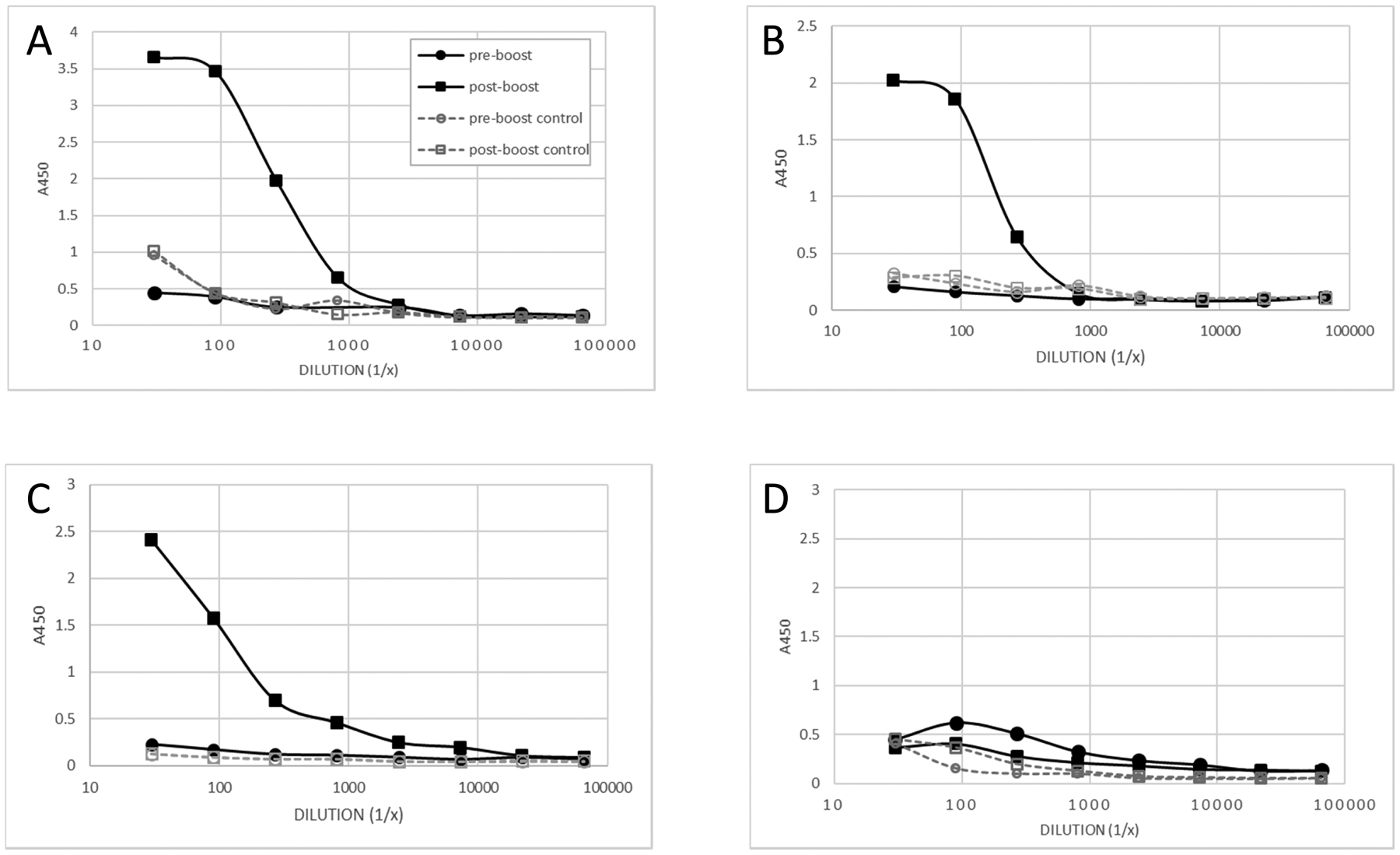
Analysis of blood plasma taken from nurse shark ‘Orange’ pre-boost and 5 weeks after boosting (post-boost) with unadjuvanted, soluble HEL, 8 years after the primary response, demonstrates robust, HEL-specific (A) IgM and (B) IgNAR memory responses by antigen-binding ELISA. Relative binding of (C) monomeric IgM and (D) pentameric IgM obtained via S300 size exclusion chromatography of pre- and post-boost plasma. Results shown are duplicate averages. Control wells were both coated and blocked with 5% MPBS.
As mentioned above, the unique attributes of IgNAR (i.e. single-domain binding region, accurate assignment of mutations, and the routine production of recombinant VNAR proteins in bacteria) have aided the exploration of Ig repertoire and antigen binding affinities in shark (Diaz et al., 2002; Dooley et al., 2003; Dooley et al., 2006; Stanfield et al., 2007). Thus, attempting to better understand the composition of the IgNAR memory repertoire (e.g. pool diversity, antigen-binding affinity, mutational load, etc.) we harvested the epigonal, the bone marrow-equivalent in nurse sharks, and spleen from the animal one week after memory response establishment. We generated VNAR bacterial expression libraries from both tissues; clone sequencing showed both libraries contained clones of high functionality and diversity (all 40 clones sequenced encoded a functional VNAR with no duplicate sequences). Individual clones were picked into 96-well culture plates for soluble monoclonal VNAR protein production and each tested for HEL binding by ELISA; the clones HEL-5A7 and PBLA8, which bind HEL with high (nM) affinity (Dooley et al., 2003; Dooley et al., 2006), were used as positive controls, and un-inoculated wells as negative controls. While our positive controls performed exactly as expected, none of the 1,100 monoclonal VNARs screened (550 clones from epigonal plus 550 from spleen) bound HEL with an absorbance above that of the negative controls. Given that similar screens, performed following generation of primary responses with equivalent titers towards HEL and multiple other targets, yield between 5–25% antigen-positive clones (Dooley; unpublished data), our inability to find HEL-specific memory clones was unexpected. We are not yet sure if such a low frequency of antigen-positive clones (<0.2%) is typical of the nurse shark IgNAR memory response. Certainly, recent studies suggest that in mammals only a small proportion of the GC-experienced memory cells generated during the primary response can be productively recalled (Mesin et al., 2020). Alternately, the low frequency of antigen-specific VNARs in the recall phase could be a consequence of the extremely low anti-HEL IgNAR titers induced in the primary phase in this instance. Again, data from mouse studies indicate that the number of GC-experienced memory cells engaged in the recall phase is proportional to the number generated in the primary response (Mesin et al., 2020 and references therein). We will need to screen post-recall VNAR libraries generated from tissues of animals with robust primary titers to determine which of these scenarios is correct. In either case, the high IgNAR titers observed in the memory phase here demonstrate that, as in mammals (Weisel et al., 2010; Weisel and Shlomchik, 2017), even a small pool of antigen-specific memory cells are capable of generating robust antibody titers upon re-stimulation. It is also clear that in future we will need to employ an enrichment strategy, such as panning of phage display libraries (Dooley et al., 2003), if we are to retrieve enough clones to interrogate the antigen-specific memory cell repertoire.
4. Conclusions
Our work offers conclusive proof that cartilaginous fishes are capable of long-term immunological memory. Indeed, the nurse shark response shares all the defining features of mammalian T-cell dependent memory responses; specifically that (i) the recall response is more rapid than the primary response (Dooley and Flajnik, 2005); (ii) is dominated by high affinity, monomeric antibodies (IgG in mammals; mIgM and IgNAR in sharks) (Dooley and Flajnik, 2005; Dooley et al., 2006); and (iii) is long-lived and self-sustaining. How and where memory cells are maintained in the cartilaginous fish lineage, and whether the frequency and diversity of antigen-specific memory B cells is equivalent to those observed in mammalian responses, remains to be determined. Nevertheless, it is apparent that the immune system of cartilaginous fishes is far from ‘primitive’ and implies that the adaptive immune system had already achieved a high level of sophistication in the common ancestor of all jawed vertebrates.
Supplementary Material
Supplemental figure 1: SDS-PAGE analysis of fractions eluted after size-exclusion chromatography (SEC) of the post-boost plasma. Samples from collected fractions were boiled in non-reducing Laemmli sample buffer and run on a 4–15% NuPAGE gel in MOPS buffer. The gel was stained with Coomassie blue for protein visualization. An identical profile was observed for the pre-boost sample (not shown).
Highlights:
A primary antibody response was raised in a nurse shark via immunization.
The shark was rested for 8 years without further exposure to the antigen.
Re-stimulation with soluble antigen induced a robust and specific antibody recall response.
Antigen binding is mediated by monomeric IgM and IgNAR, not pentameric IgM, in the recall phase.
This is the longest memory period recorded for any non-mammalian vertebrate to date.
Acknowledgements:
Our thanks to Martin Flajnik for providing the shark samples used in this work; shark maintenance and reagents were supported by NIH grant R01 AI140326-27 awarded to MF. We thank the School of Biological Sciences, University of Aberdeen, for the travel bursary awarded to OE that helped fund his trip to Baltimore to perform this study. HM was a trainee under NIH Institutional Training Grant T32 AI095190 when this work was undertaken.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Castro CD, Ohta Y, Dooley H, Flajnik MF, 2013. Noncoordinate expression of J-chain and Blimp-1 define nurse shark plasma cell populations during ontogeny. Eur J Immunol 43, 3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem LW, De Boutaud F, Sigel MM, 1967. Phylogeny of Immunoglobulin Structure and Function: II. Immunoglobulins of the Nurse Shark. The Journal of Immunology 99, 1226–1235. [PubMed] [Google Scholar]
- Diaz M, Stanfield RL, Greenberg AS, Flajnik MF, 2002. Structural analysis, selection, and ontogeny of the shark new antigen receptor (IgNAR): identification of a new locus preferentially expressed in early development. Immunogenetics 54, 501–512. [DOI] [PubMed] [Google Scholar]
- Dooley H, Flajnik MF, 2005. Shark immunity bites back: affinity maturation and memory response in the nurse shark, Ginglymostoma cirratum. Eur. J. Immunol 35, 936–945. [DOI] [PubMed] [Google Scholar]
- Dooley H, Flajnik MF, 2006. Antibody repertoire development in cartilaginous fish. Dev. Comp Immunol 30, 43–56. [DOI] [PubMed] [Google Scholar]
- Dooley H, Flajnik MF, Porter AJ, 2003. Selection and characterization of naturally occurring single-domain (IgNAR) antibody fragments from immunized sharks by phage display. Mol. Immunol 40, 25–33. [DOI] [PubMed] [Google Scholar]
- Dooley H, Stanfield RL, Brady RA, Flajnik MF, 2006. First molecular and biochemical analysis of in vivo affinity maturation in an ectothermic vertebrate. Proc. Natl. Acad. Sci. U. S. A 103, 1846–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, 2014. Re-evaluation of the immunological Big Bang. Curr Biol 24, R1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg AS, Avila D, Hughes M, Hughes A, 1995. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. nature 374, 168. [DOI] [PubMed] [Google Scholar]
- Hayhurst A, Harris WJ, 1999. Escherichia coli skp chaperone coexpression improves solubility and phage display of single-chain antibody fragments. Protein Expr Purif 15, 336–343. [DOI] [PubMed] [Google Scholar]
- Janeway C, 2005. Immunobiology : the immune system in health and disease, 6th ed ed. Garland Science, New York ; London. [Google Scholar]
- Kurosaki T, Kometani K, Ise W, 2015. Memory B cells. Nature Reviews Immunology 15, 149. [DOI] [PubMed] [Google Scholar]
- Luer C, Walsh CJ, Bodine AB, 2014. Sites of immune cell production in elasmobranch fishes: lymphomyeloid tissues and organs, in: Smith SL, Sim RB, Flajnik M (Eds.), Immunobiology of the shark. CRC press, Boca Raton, FL. USA, pp. 79–88. [Google Scholar]
- Marchalonis J, Edelman GM, 1965. Phylogenetic origins of antibody structure. I. Multichain structure of immunoglobulins in the smooth dogfish (Mustelus canis). J. Exp. Med 122, 601–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesin L, Schiepers A, Ersching J, Barbulescu A, Cavazzoni CB, Angelini A, Okada T, Kurosaki T, Victora GD, 2020. Restricted Clonality and Limited Germinal Center Reentry Characterize Memory B Cell Reactivation by Boosting. Cell 180, 92–106 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AL, Anderson MK, Litman RT, Walsh CJ, Luer CA, Rothenberg EV, Litman GW, 2001. Complex expression patterns of lymphocyte-specific genes during the development of cartilaginous fish implicate unique lymphoid tissues in generating an immune repertoire. Int. Immunol 13, 567–580. [DOI] [PubMed] [Google Scholar]
- Palm A-KE, Henry C, 2019. Remembrance of things past: long-term B cell memory after infection and vaccination. Frontiers in immunology 10, 1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux KH, Greenberg AS, Greene L, Strelets L, Avila D, McKinney EC, Flajnik MF, 1998. Structural analysis of the nurse shark (new) antigen receptor (NAR): molecular convergence of NAR and unusual mammalian immunoglobulins. Proc. Natl. Acad. Sci. U. S. A 95, 11804–11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumfelt LL, McKinney EC, Taylor E, Flajnik MF, 2002. The development of primary and secondary lymphoid tissues in the nurse shark Ginglymostoma cirratum: B-cell zones precede dendritic cell immigration and T-cell zone formation during ontogeny of the spleen. Scand. J. Immunol 56, 130–148. [DOI] [PubMed] [Google Scholar]
- Sigel MM, Clem LW, 1966. Immunologic anamnesis in elasmobranchs Phylogeny of Immunity. Univ. of Florida Press, Gainesville, 190–198. [Google Scholar]
- Small PA Jr., Klapper DG, Clem LW, 1970. Half-lives, body distribution and lack of interconversion of serum 19S and 7S IgM of sharks. J. Immunol 105, 29–37. [PubMed] [Google Scholar]
- Stanfield RL, Dooley H, Flajnik MF, Wilson IA, 2004. Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science 305, 1770–1773. [DOI] [PubMed] [Google Scholar]
- Stanfield RL, Dooley H, Verdino P, Flajnik MF, Wilson IA, 2007. Maturation of shark single-domain (IgNAR) antibodies: evidence for induced-fit binding. J. Mol. Biol 367, 358–372. [DOI] [PubMed] [Google Scholar]
- Taub DD, Ershler WB, Janowski M, Artz A, Key ML, McKelvey J, Muller D, Moss B, Ferrucci L, Duffey PL, Longo DL, 2008. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med 121, 1058–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC, 2012. Germinal centers. Annual Review of Immunology 30, 429–457. [DOI] [PubMed] [Google Scholar]
- Weisel F, Shlomchik M, 2017. Memory B Cells of Mice and Humans. Annu Rev Immunol 35, 255–284. [DOI] [PubMed] [Google Scholar]
- Weisel FJ, Appelt UK, Schneider AM, Horlitz JU, van Rooijen N, Korner H, Mach M, Winkler TH, 2010. Unique requirements for reactivation of virus-specific memory B lymphocytes. J Immunol 185, 4011–4021. [DOI] [PubMed] [Google Scholar]
- Zapata A, Amemiya CT, 2000. Phylogeny of lower vertebrates and their immunological structures. Curr. Top. Microbiol. Immunol 248, 67–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: SDS-PAGE analysis of fractions eluted after size-exclusion chromatography (SEC) of the post-boost plasma. Samples from collected fractions were boiled in non-reducing Laemmli sample buffer and run on a 4–15% NuPAGE gel in MOPS buffer. The gel was stained with Coomassie blue for protein visualization. An identical profile was observed for the pre-boost sample (not shown).


