Abstract
Adequate calcium intake is important for the prevention of bone loss and osteoporosis. For some populations such as those of Southeast Asia where calcium intake is very low, supplements represent a suitable dietary source of calcium. The objective of this study was to compare the relative oral bioavailability of calcium from calcium glucoheptonate, a highly soluble calcium salt containing 8.2% of elemental calcium, to that of calcium carbonate. A single‐dose, randomized‐sequence, open‐label, two‐period crossover study, with a 7‐day washout period, was conducted in 24 Indonesian healthy adult volunteers. After a 12‐hour (overnight) fast, subjects received either two oral ampoules of 250 mg/10 mL of calcium glucoheptonate each or one effervescent tablet of calcium carbonate containing 500 mg of elemental calcium. The relative oral bioavailability of calcium from calcium glucoheptonate as compared to calcium carbonate was 92% within 6 hours and 89% within 12 hours after study drug administration. The 90% confidence intervals for the mean test/reference ratios of the maximum plasma concentration and the area under the concentration‐time curve at 12 hours post‐administration were 77.09%–120.31% and 60.58%–122.30%, respectively. Five subjects experienced a total of eight adverse events which were all mild and transient; no serious adverse events or deaths were reported. These results indicate that calcium glucoheptonate is associated with a high relative bioavailability of calcium compared to calcium carbonate, and is well‐tolerated. Calcium glucoheptonate might thus be a potential choice for calcium supplementation in Southeast Asian populations.
Keywords: bioavailability, calcium carbonate, calcium glucoheptonate, pharmacokinetics, Southeast Asia
Abbreviations
- AE
adverse event
- AUC0‐∞
area under the concentration‐time curve from 0 to infinity
- AUC0–t
area under the concentration‐time curve from 0 to time of the last measurable concentration
- CI
confidence interval
- Cmax
maximum plasma concentration
- CV%
coefficient of variation
- F
relative bioavailability
- ICP‐OES
inductively coupled plasma‐optical emission spectrometry
- LLOQ
lower limit of quantification
- SD
standard deviation
- t1/2
elimination half‐life
- Tmax
time to maximum plasma concentration
1. INTRODUCTION
Calcium is the most abundant stored nutrient in the human body, and is involved in several physiological processes, such as bone development, vascular contraction, vasodilation, muscle functions, nerve transmission, intracellular signaling, and hormonal secretion. 1 , 2 Calcium homeostasis, which refers to the maintenance of a serum calcium concentration between 8.5 and 10.5 mg/dL (2.1 to 2.6 mmol/L), is controlled by the parathyroid hormone, calcitriol and calcitonin. 3 , 4 Perturbations of calcium homeostasis can be life‐threatening, with hypercalcemia resulting in lethargy, kidney stones, constipation, loss of appetite and confusion, and hypocalcemia resulting in osteoporosis, seizures and arythmias. 5 Hence, balanced dietary calcium intake plays a critical role for the maintenance of calcium homeostasis and the integrity of the skeleton. 6
Recommendations for daily calcium intake have been issued by governmental and non‐governmental organizations in several countries. 1 , 4 Adequate calcium intakes currently vary from 500 to 1200 mg/day in adults, depending on age and gender. 4 While calcium intake comes from dietary sources such as dairy products, certain vegetables, and fortified foods, many people do not achieve the recommended intake from diet alone. 7 A recent systematic review, which compiled available data on average national dietary calcium intake around the globe, found that most Southeast Asian countries have low average dietary calcium intake (<400 mg/day). 8 In Indonesia, Southeast Asia's most populous country, average calcium intake was reported to be 342 mg/day. 8 Hence, for some populations such as those of Southeast Asia, supplements represent a suitable dietary source of calcium. 9
Several forms of calcium supplements are available on the market today, mainly differing in bioavailability and elemental calcium content. 7 The most commonly used oral calcium preparations include calcium carbonate, calcium citrate, and, to a lesser extent, calcium lactate and calcium gluconate. 10 Calcium carbonate is inexpensive, widely available, and provides relatively high elemental calcium content (40%), consequently requiring fewer tablets than other forms of calcium. 10 , 11 However, calcium carbonate is the most constipating supplement, has a very low solubility in water, and should be taken with meals, since gastric acidity is required for sufficient absorption. 10 , 12 Calcium citrate is the most easily absorbed calcium supplement, and it may be taken with or without meals, since its absorption is not dependent on gastric acidity. 10 However, as compared with calcium carbonate, calcium citrate is expensive, and provides less elemental calcium (21%), which translates into a reduced compliance due to the need to take more tablets or capsules to make the dose equivalent to that of calcium carbonate. 10 , 11 There currently remains a paucity of data on whether clinically important differences exist between calcium supplement formulations with respect to skeletal benefits and potential side effects. 10
Calcium glucoheptonate is a highly soluble calcium salt containing 8.2% of elemental calcium. 13 The high solubility and tolerability of calcium glucoheptonate suggest that it might be an adequate source of calcium for dietary supplementation in Southeast Asian populations. 14 However, no information is available regarding the bioavailability of calcium from calcium glucoheptonate. Hence, the aim of this study was to compare the relative oral bioavailability of calcium from a single dose of calcium glucoheptonate (ie, test drug) with that of calcium carbonate (ie, reference drug) in Indonesian healthy adults. Calcium carbonate was chosen as the reference drug due to its widespread use as a calcium supplement, particularly in Southeast Asia where this study was conducted, and the accumulating knowledge on its bioavailability over the last 20 years. The results of this study show that the plasma concentration‐time profile of calcium glucoheptonate is comparable with that of calcium carbonate.
2. MATERIALS AND METHODS
2.1. Study design
This was a phase I, single‐center, single‐dose, randomized‐sequence, open‐label, two‐period crossover study conducted in Jakarta, Indonesia, between April 23 and May 21, 2018. Each subject participated in two treatment periods, where he/she served as his/her own control and was assigned a supplement order using a block randomization scheme. The treatment periods were separated by a washout period of 7 days to avoid any possible carryover effects from one study period to the other. The present study was performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. The protocol of this study was approved by the independent ethics committee of the National Institute of Health Research and Development (Badan Penelitian dan Pengembangan Kesehatan, Ministry of Health), and by the National Agency of Drug and Food Control of the Republic of Indonesia.
2.2. Participants
Indonesian healthy adults aged between 18 and 55 years and of either sex were enrolled in the study. A clinical screening procedure including a personal interview, a physical examination, and laboratory tests which included hematology, blood biochemistry, urine analysis and serologic tests for hepatitis B and C and HIV antibodies was undertaken before eligible subjects were selected. In addition, a pregnancy test was performed for female subjects of childbearing potential, and an electrocardiogram was performed for all subjects.
Exclusion criteria included: heavy smoking (>10 cigarettes per day); abnormal electrocardiogram, vital signs, or laboratory test results; positive test for hepatitis B or C virus, or HIV; history of significant use of alcohol or recreational drugs within one year of screening; history of allergic reactions to food or medications; or use of a prescription or an over‐the‐counter medication within 7 days of screening. Subjects were also excluded from the study if they were pregnant or breastfeeding; had a current or a past history of a relevant medical condition which might influence the bioavailability or the pharmacokinetics of the study drugs (eg, calcium or vitamin D malabsorption, hypercalcemia, bone diseases, diabetes mellitus, hypo‐ or hyperparathyroidism, etc); had donated ≥300 mL of blood in the 3 months before the study; or participated in clinical trials during the previous 3 months. Moreover, women of childbearing potential not willing to use contraceptive methods during the study were excluded.
All participants had been informed about the details of this study, including its potential risks and benefits, and provided written informed consent before the commencement of screening tests and other study‐related procedures. They were free to withdraw from the study at any given time.
2.3. Study drug administration
The volunteers were randomized in a 1:1 ratio into two treatment groups. One group received a single dose of the test drug (batch no. 16003NC‐f1B; manufacturing date, September 2016; expiration date, September 2018) in period 1 and the reference drug (batch no. J1485; manufacturing date, September 2016; expiration date, August 2018) in period 2; the other group received the reference drug in period 1 and the test drug in period 2. In each treatment period, at 07:00 am after an overnight fast of 12 hours, subjects were administered, under supervision by study investigators, two oral ampoules of 250 mg/10 mL of calcium glucoheptonate (3056.16 mg) each (Calcium Corbiere Plus®, Sanofi Vietnam Shareholding Company) or one effervescent tablet of 300 mg of calcium carbonate and 2940 mg of calcium lactate gluconate (Calcium Sandoz®, Novartis; one tablet containing 500 mg of elemental calcium). We opted to use an effervescent formulation of calcium carbonate, because it is recommended in comparative studies of bioavailability to use an aqueous solution as the reference dosage form, since it eliminates all factors concerned in the dissolution process. 15
Both the test and reference products were administered with 240 mL of water in a seated position. For calcium carbonate administration, the effervescent tablet was first dissolved in water and the solution was then drunk immediately after complete dissolution of the tablet. For calcium glucoheptonate administration, after breaking the ampoule open, the solution was withdrawn into the glass of water and then drunk. Each ampoule was immediately disposed after use. No food or beverages were allowed for four hours after ingestion of the doses. Subjects were asked to remain in an upright position (standing or sitting) for 1 hour after study drug administration. The volunteers were instructed to abstain from the use of any medication for at least 1 week before and during the study, and from alcohol, tobacco, milk, and foods or beverages containing xanthine derivatives within 24 hours of each treatment period.
2.4. Tolerability
Participants were under continuous medical supervision at the study site throughout the trial. Vital signs (blood pressure, heart rate, respiratory rate, and body temperature) were monitored at the screening visit, at 24, 12 hours and immediately before study drug administration, and at 1, 3, 5, 12, 24, 36, and 48 hours after each study drug administration. Participants were asked to report any adverse event (AE) to the investigators at any time during the study, including the 7‐day washout period.
Tolerability was assessed by investigators based on subject interviews, spontaneous reporting, vital signs, physical examination, and laboratory test results (hematology, blood biochemistry, urine analysis) before and after study completion. AEs were considered serious if they were life‐threatening or led to death, disability, hospitalization, or medical intervention to prevent permanent impairment or damage. All AEs were recorded in case report forms by an investigator.
2.5. Blood sample collection
For the assessment of pharmacokinetic parameters, 6‐mL whole blood samples were drawn into tubes containing EDTA immediately before drug administration, and at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 10 and 12 hours after administration. Blood samples were collected by direct venipuncture. All blood samples were centrifuged at 1613 rcf or G‐force for 10 minutes (corresponding to 3000 rpm for a rotational radius of 16 cm). Plasma samples were separated promptly by centrifugation and stored frozen (–20°C) until assay.
Plasma concentrations of calcium were determined using inductively coupled plasma‐optical emission spectrometry (ICP‐OES). ICP‐OES was validated in terms of specificity, selectivity, precision, accuracy, carry‐over, lower limit of quantification (LLOQ), calibration curve, stability, and dilution integrity. Accuracy and precision were both validated within a single run (within‐run) and between different runs (between‐run). The LLOQ for calcium was determined to be 0.1 mg/L.
2.6. Pharmacokinetic analysis
The bioavailability of an orally administered exogenous compound is defined as the fraction of the dose that reaches the systemic circulation. For an endogenous substance such as calcium, measurement of the absolute bioavailability (fractional absorption) of an oral dose requires the use of isotopic methods, but for assessing relative oral bioavailability, the pharmacokinetic method is much more convenient. 12
The maximum plasma concentration (C max) and the time taken to reach C max (T max) were determined directly from the visual inspection of individual plasma concentration–time curves of calcium carbonate and calcium glucoheptonate. The area under the concentration‐time curve from time zero to the time of the last measurable concentration (AUC0–t) was calculated according to the linear trapezoidal rule from 0 to 6 hours and from 0 to 12 hours after drug administration. We were not able to calculate the area under the concentration‐time curve from 0 to infinity (AUC0‐∞) and the elimination half‐life (t 1/2), because the slope of the terminal logarithm‐linear portion of the concentration‐time curve could not be determined.
The relative bioavailability (F) of the test product was calculated as follows: F = AUC0‒ t (test)/AUC0‒ t (reference) × 100%. The 90% confidence intervals (CIs) of the test/reference ratios for the log‐transformed values of C max and AUC0–t were also determined. According to guidelines established by the United States Food and Drug Administration (2002) 16 and the Association of Southeast Asian Nations (2015), 17 for AUC0‐t and C max, 90% CIs for the test/reference ratio should be contained within the predetermined acceptance interval of 80% to 125%.
2.7. Statistical analysis
Sample size determination was based on the intrasubject coefficient of variation (CV%), derived from the 90% CIs of AUC0– t as reported by Nowak et al (2008) 18 in a study which aimed to compare the oral bioavailability of calcium from tablets containing calcium fumarate to that of calcium gluconate. The derived intrasubject CV%, which was determined to be 14%, yielded a sample size of 24 subjects for a statistical power of ≥80% to detect a 5% difference in the pharmacokinetic parameters between the two products, while accounting for a 10% dropout rate.
The statistical comparison of individual pharmacokinetic parameters (AUC0‐t and C max) of the two study preparations was performed by a two‐way ANOVA. The ANOVA was performed on AUC0‐t and C max after log transformation, using a linear mixed effects model to evaluate the effects of product, period, sequence, and subject (within sequence). All pharmacokinetic parameters were summarized by descriptive statistics such as arithmetic means with standard deviations, geometric means, medians and ranges, and intrasubject CV%, so that the results could be interpreted in relation to both a normal distribution and a log‐normal distribution. All statistical tests were two‐sided and were performed at a 0.05 significance level.
3. RESULTS
3.1. Study population
Twenty‐four Indonesian healthy adult volunteers (16 men and eight women) were enrolled and completed the study. The demographic and baseline characteristics of the study participants are shown in Table 1. Of the 24 enrolled subjects, four (16.7%) were classified as light smokers (<10 cigarettes per day). In all subjects, serum levels of calcium and of vitamin D varied at baseline within the normal references ranges of 8.5‐10.5 mg/dL and >20 ng/mL, respectively.
TABLE 1.
Demographic and baseline characteristics of the 24 healthy volunteers
| Baseline characteristic | Study population (N = 24) |
|---|---|
| Age, years | 35.5 ± 8.7 (19‐51) |
| Male/female, n (%) | 16 (66.7)/8 (33.3) |
| Weight, kg | 53.6 ± 5.7 (45‐65) |
| Height, cm | 161.3 ± 8.0 (143‐172) |
| Body mass index, kg/cm2 | 20.6 ± 2.0 (18.1‐24.8) |
| Systolic blood pressure, mmHg | 110.4 ± 11.2 (90‐130) |
| Diastolic blood pressure, mmHg | 74.2 ± 7.8 (60‐90) |
| Heart rate, beats per minute | 72.6 ± 9.4 (55‐88) |
| Body temperature, °C | 36.3 ± 0.2 (35.9‐36.8) |
| Respiratory rate, breaths per minute | 18.4 ± 2.8 (16‐24) |
| Serum calcium levels, mg/dL | 9.7 ± 0.3 (9.0‐10.4) |
| Serum vitamin D levels, ng/mL | 30.7 ± 7.8 (22.4‐53.3) |
All variables, except gender, are expressed as mean ± standard deviation (range). Percentages are calculated as n/N.
Four types of protocol deviations were recorded during the study: (a) blood sampling time deviations occurred in 10 subjects; (b) six subjects left the study site (accompanied by a study team member) for one hour on the day of study drug administration in periods 1 and 2 to attend religious prayer service; (c) three subjects fasted for five hours after study drug administration instead of four; and (d) as previously mentioned, AUC0‐∞ and t 1/2 were not calculated in this study. However, all protocol deviations were judged as unlikely to have affected the results and conclusions of the study.
3.2. Tolerability
No deaths or serious AEs were reported during this study. Five subjects experienced a total of eight AEs during the study (Table 2). Of the eight post‐administration AEs, three were deemed to be probably related to the test treatment (tympanites, myalgia, and abdominal discomfort). There were no AEs that were considered associated with the administration of the reference treatment. All reported AEs were mild and disappeared within one day. The results of the physical examination, laboratory tests, and vital signs measurements remained normal throughout the study and confirmed the absence of clinically relevant changes in the participants’ state of health. None of the volunteers withdrew from the study because of AEs.
TABLE 2.
Adverse events occuring after administration of calcium glucoheptonate (test) or calcium carbonate (reference) in 24 Indonesian healthy adult volunteers
| Subject No. | Adverse event | Relation to study drug (test or reference) | Action | Severity |
|---|---|---|---|---|
| 4 | Tympanites | Probably related (test) | Observation | Mild |
| 6 | Dizziness | Unlikely related (reference) | Observation | Mild |
| 6 | Headache | Unlikely related (reference) | Administration of one tablet of paracetamol 500 mg | Mild |
| 6 | Myalgia | Probably related (test) | Observation | Mild |
| 9 | Dizziness | Unlikely related (test) | Observation | Mild |
| 11 | Abdominal discomfort | Probably related (test) | Observation | Mild |
| 20 | Fever | Unlikely related (test) | Administration of one tablet of paracetamol 500 mg | Mild |
| 20 | Myalgia | Unlikely related (test) | Observation | Mild |
3.3. Pharmacokinetic findings
The mean plasma concentration‐time profiles following oral administration of a single dose of calcium glucoheptonate and calcium carbonate were comparable (Figure 1). The main pharmacokinetic parameters (C max, T max, and AUC0– t) determined at 6 and 12 hours after oral administration of calcium carbonate and calcium glucoheptonate are summarized in Table 3.
FIGURE 1.
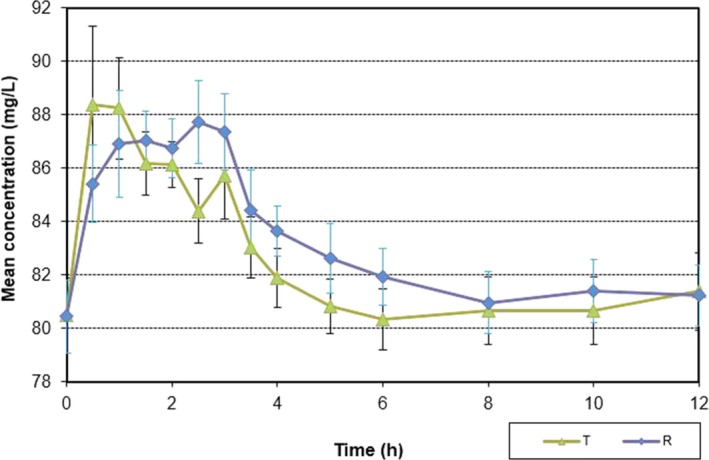
Mean ± standard deviation plasma concentration‐time profiles in 24 Indonesian healthy subjects after single‐dose administration of the test product (T) calcium glucoheptonate and the reference product (R) calcium carbonate under fasting conditions
TABLE 3.
Pharmacokinetic parameters at 6 and 12 hours after oral administration of calcium glucoheptonate and calcium carbonate
| Parameter | Test (calcium glucoheptonate) | Reference (calcium carbonate) | ||
|---|---|---|---|---|
| 6 hours | 12 hours | 6 hours | 12 hours | |
| C max, mg/L | ||||
| Mean ± SD | 16.07 ± 13.44 | 15.67 ± 13.30 | 14.64 ± 7.47 | 14.76 ± 7.49 |
| Median (range) | 11.48 (3.85‐68.21) | 11.54 (3.85‐68.21) | 12.93 (4.88‐36.73) | 13.58 (4.88‐36.73) |
| Geometric mean | 12.98 | 12.58 | 12.96 | 13.07 |
| T max, hours | ||||
| Mean ± SD | 1.80 ± 1.29 | 2.56 ± 2.91 | 2.23 ± 0.94 | 2.54 ± 1.85 |
| Median (range) | 1.50 (0.50‐6.00) | 1.50 (0.50‐12.00) | 2.50 (0.50‐4.00) | 2.50 (0.50‐10.00) |
| Geometric mean | 1.45 | 1.69 | 1.97 | 2.09 |
| AUC0– t, mg*h/L | ||||
| Mean ± SD | 28.27 ± 22.32 | 41.50 ± 40.89 | 30.65 ± 21.22 | 46.64 ± 37.84 |
| Median (range) | 20.58 (2.07‐91.98) | 26.22 (5.49‐150.09) | 26.05 (4.69‐77.75) | 32.54 (4.69‐121.26) |
| Geometric mean | 21.01 | 26.70 | 23.28 | 31.02 |
Abbreviations: AUC0– t, area under the plasma concentration‐time curve; C max, maximum plasma concentration; SD, standard deviation; T max, time at which C max is observed.
The 90% CIs of the ratios (test/reference) for the log‐transformed values of C max and AUC0–t at 6 and 12 hours post‐administration are shown in Table 4. At 6 hours after administration of either calcium carbonate or calcium glucoheptonate, the 90% CIs were 78.88%–124.83% and 66.42%–125.22% for the log‐transformed values of C max and AUC0–t, respectively. At 12 hours after study drug administration, the 90% CIs were 77.09%–120.31% and 60.58%–122.30%, respectively.
TABLE 4.
90% confidence interval (CI) ratios of natural logarithm‐transformed pharmacokinetic parameters of calcium glucoheptonate (test drug)/calcium carbonate (reference drug)
| Parameter | Geometric mean ratio – % | 90% CIs of the geometric mean ratio (%) | Intra‐subject coefficient of variation (%) | |||
|---|---|---|---|---|---|---|
| 6 hours | 12 hours | 6 hours | 12 hours | 6 hours | 12 hours | |
| C max, mg/L | 99.23 | 96.30 | 78.88‐124.83 | 77.09‐120.31 | 45.19 | 44.90 |
| AUC0– t, mg*h/L | 91.20 | 86.07 | 66.42‐125.22 | 60.58‐122.30 | 62.43 | 70.86 |
Abbreviations: AUC0– t, area under the plasma concentration‐time curve; C max, maximum plasma concentration.
The relative oral bioavailability (F) of calcium from calcium glucoheptonate as compared to calcium carbonate was 92% within 6 hours and 89% within 12 hours after study drug administration.
4. DISCUSSION
In the present study, the plasma concentration‐time profile of calcium glucoheptonate appeared to be well‐characterized. The higher mean C max values obtained with calcium glucoheptonate at 6 and 12 hours post‐administration in comparison with calcium carbonate indicate higher serum calcium levels achieved in a relatively shorter period of time. Similarly, compared with calcium carbonate, the lower AUC0–t values associated with calcium glucoheptonate administration translate into a shorter drug retention period which eventually leads to a reduced long‐term toxicity.
Calcium absorption from calcium supplements has been shown to be affected by several variables including the size of the dose and the presence or absence of a coadministered meal. 12 , 19 The enhancing effect of a coadministered meal on calcium absorption might be a composite of prolonged gastric emptying from a meal source, as well as interactions between food macromolecules and calcium particles in ways that enhance the presentation of calcium to the absorptive surface. 20 Hence, to minimize the likelihood of food‐drug interactions and overcome the influence of the coadministration of food on calcium absorption, we conducted this study with subjects under fasting conditions. In addition, it has been reported that doses over 500 mg per day can diminish the efficiency of calcium absorption from supplements. 19 , 21 Accordingly, 500‐mg single doses of calcium carbonate and calcium glucoheptonate (and no more) were used in this study to optimize calcium absorption.
To the best of our knowledge, our study is the first to evaluate the bioavailability of calcium from calcium glucoheptonate. Even though calcium glucoheptonate was associated with a very good relative calcium absorption compared to calcium carbonate (92% at 6 hours and 89% at 12 hours post‐administration), we found that the 90% CIs of the treatment ratios of the log‐transformed values of C max and AUC0–t were outside the recommended range of 80% to 125%. Differences in excipients, pharmaceutical formulations (effervescent tablets versus oral ampoules), or manufacturing processes may be responsible for these differences in product performance. 20 , 22 Indeed, it has been shown that pharmaceutical formulation can make a very large difference in bioavailability, and that the absorbability of some agents in tablet form significantly differs when taken in other oral forms such as powders, syrups, or ampoules. 20 , 23 In addition, the marked intrasubject variability that was found in our study might also explain the differences in drug exposure. 24 As traditional bioavailability studies, based on the 80%–125% limit, cannot evaluate the differences in drug exposure levels in terms of C max and AUC0–t in individuals, population bioequivalence and individual bioequivalence approaches might be of great value for assessing the total variability in C max and AUC0–t in the population, and intrasubject variability for the test and reference products and the subject‐by‐formulation interaction, respectively. 24
Both calcium carbonate and calcium glucoheptonate were well‐tolerated in this study. All calcium supplements are generally well‐tolerated; however, some patients complain of gastrointestinal symptoms, including constipation, gas, flatulence, and bloating. 11 In our study, only one volunteer who received calcium glucoheptonate experienced a gastrointestinal AE that was deemed probably related to the study treatment. Moreover, there were two reports of dizziness in two different subjects, one treated with calcium carbonate and one with calcium glucoheptonate. There was also one report of headache in a subject who received calcium carbonate. These symptoms of dizziness and headache have been reported in previous bioavailability studies evaluating calcium supplements. 25
Calcium supplements are recommended for individuals who cannot achieve the recommended daily calcium intake. In Southeast Asia, dietary calcium consumption has been found to be alarmingly low. 8 Of great concern was the finding from a 2003 cross‐sectional study that up to 96% of pregnant women in Central Java, Indonesia had inadequate calcium intake. 26 Given that suboptimal dietary calcium intake can increase the risk of osteoporosis and of subsequent fractures, 8 as well as the risk of preeclampsia in pregnant women, 27 it is important to encourage calcium supplementation in Southeast Asian countries where the incidence of osteoporotic fractures has been growing at alarming rates. 28 The present study found a high relative bioavailability of calcium from calcium glucoheptonate, which might be probably related to the ionizing ability of calcium glucoheptonate, as well as its high solubility. 14 , 29 Taking into account these results, the good tolerability of calcium glucoheptonate, and its widespread availability across different Southeast Asian countries such as Indonesia and Vietnam, calcium glucoheptonate might be a potential choice for calcium supplementation in this population at increasing risk for both diminished bone density and osteoporotic fractures. The results of this study might serve as a reference for future controlled studies of calcium glucoheptonate in a larger patient population.
There are however a number of limitations to this study. First, we were unable to analyze calcium content in urine or measure fractional intestinal calcium absorption isotopically because commercial preparations cannot be labeled with calcium isotopes. Second, since our study was limited to healthy subjects who were administered a single dose, the pharmacokinetic characteristics of calcium glucoheptonate might differ in target populations such as postmenopausal women and adolescents. Nevertheless, because calcium absorption is significantly influenced by age and hormonal state among other factors, we chose to recruit only healthy volunteers for this study to minimize individual‐specific variability in the pharmacokinetics of the study drugs. 21 Third, our study had a relatively small sample size. However, the sample size was sufficient to allow adequate testing of relative bioavailability despite the limitation of high intrasubject variability. Finally, since the slope of the terminal logarithm‐linear portion of the concentration‐time curve could not be determined, we were unable to calculate AUC0‐∞ and t1/2. Despite these limitations, our study also had many strengths, including its randomized‐sequence controlled design and the absence of missing data.
In summary, this single‐dose pharmacokinetic study conducted in fasting, Indonesian healthy adult volunteers showed that calcium glucoheptonate was well‐tolerated, and was associated with a high relative bioavailability of calcium compared to calcium carbonate. This suggests a potential use of calcium glucoheptonate as a calcium supplement in Southeast Asia.
DISCLOSURES
This work was funded by Sanofi. Etienne Pouteau, Surajit Dutta, and Phuc HB Nguyen are current employees of Sanofi. The other authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Wiria MSS, Etienne Pouteau, Olivia Valencia, Surajit Dutta, and Phuc HB Nguyen contributed to the conception and design of the study. Wiria MSS conducted the study experiments. Wiria MSS, Hung Manh Tran, Phuc HB Nguyen, Olivia Valencia, Surajit Dutta, and Etienne Pouteau performed data analysis. Wiria MSS, Hung Manh Tran, Phuc HB Nguyen, Olivia Valencia, Surajit Dutta, and Etienne Pouteau contributed to the writing of the manuscript. All authors approved the final version of the manuscript.
5. ETHIC STATEMENT
The study was performed according to the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. The protocol of this study was approved by the independent ethics committee of the National Institute of Health Research and Development (Badan Penelitian dan Pengembangan Kesehatan, Ministry of Health, Jakarta, Indonesia), and by the National Agency of Drug and Food Control of the Republic of Indonesia. Informed consent was obtained from all study participants.
ACKNOWLEDGEMENTS
The authors would like to thank Thomas Rohban and Magalie El Hajj (Partner 4 Health, France) for medical writing support that was funded by Sanofi in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3 ).
Wiria MSS, Tran HM, Nguyen PHB, Valencia O, Dutta S, Pouteau E. Relative bioavailability and pharmacokinetic comparison of calcium glucoheptonate with calcium carbonate. Pharmacol Res Perspect. 2020;e00589 10.1002/prp2.589
DATA AVAILABILITY STATEMENT
The authors confirm that all data supporting the findings of this study are available within the article.
REFERENCES
- 1. Beto JA. The role of calcium in human aging. Clin Nutr Res. 2015;4:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Erfanian A, Rasti B, Manap Y. Comparing the calcium bioavailability from two types of nano‐sized enriched milk using in‐vivo assay. Food Chem. 2017;214:606‐613. [DOI] [PubMed] [Google Scholar]
- 3. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium ; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56070/. Accessed March 05, 2020. [PubMed] [Google Scholar]
- 4. Li K, Wang XF, Li DY, et al. The good, the bad, and the ugly of calcium supplementation: a review of calcium intake on human health. Clin Interv Aging. 2018;13:2443‐2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Catalano A, Chilà D, Bellone F, et al. Incidence of hypocalcemia and hypercalcemia in hospitalized patients: Is it changing? J Clin Transl Endocrinol. 2018;13:9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pu F, Chen N, Xue S. Calcium intake, calcium homeostasis and health. Food Sci Hum Wellness. 2016;5:8‐16. [Google Scholar]
- 7. Wasilewski GB, Vervloet MG, Schurgers LJ. The bone‐vasculature axis: Calcium supplementation and the role of vitamin K. Front Cardiovasc Med. 2019;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balk EM, Adam GP, Langberg VN, et al. Global dietary calcium intake among adults: a systematic review. Osteoporos Int. 2017;28:3315‐3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cormick G, Belizán JM. Calcium Intake and Health. Nutrients. 2019;11:1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauer DC. Clinical practice. Calcium supplements and fracture prevention. N Engl J Med. 2013;369:1537‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin Pract. 2007;22:286‐296. [DOI] [PubMed] [Google Scholar]
- 12. Hanzlik RP, Fowler SC, Fisher DH. Relative bioavailability of calcium from calcium formate, calcium citrate, and calcium carbonate. J Pharmacol Exp Ther. 2005;313:1217‐1222. [DOI] [PubMed] [Google Scholar]
- 13. Trailokya A, Srivastava A, Bhole M, Zalte N. Calcium and Calcium Salts. J Assoc Physicians India. 2017;65:100‐103. [PubMed] [Google Scholar]
- 14. Drop LJ, Cullen DJ. Comparative effects of calcium chloride and calcium gluceptate. Br J Anaesth. 1980;52:501‐505. [DOI] [PubMed] [Google Scholar]
- 15. Breimer DD, Winten MA. Pharmacokinetics and relative bioavailability of cyclobarbital calcium in man after oral administration. Eur J Clin Pharmacol. 1976;9:443‐450. [DOI] [PubMed] [Google Scholar]
- 16. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research . Guidance for Industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. 2002. Available at: https://www.fda.gov/files/drugs/published/Guidance-for-Industry-Bioavailability-and-Bioequivalence-Studies-for-Orally-Administered-Drug-Products---General-Considerations.PDF. Accessed August 08, 2019.
- 17. Association of Southeast Asian Nations . ASEAN Guideline for the Conduct of Bioequivalence Studies. 2015. Available at: https://asean.org/wp-content/uploads/2012/10/BE_Guideline_FinalMarch2015_endorsed_22PPWG.pdf. Accessed August 11, 2019.
- 18. Nowak MG, Szulc‐Musioł B, Ryszka F. Pharmacokinetics of calcium from calcium supplements in healthy volunteers. Pak J Pharm Sci. 2008;21:109‐112. [PubMed] [Google Scholar]
- 19. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes . Calcium ‐ dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington (DC): National Academies Press (US); 1997. https://www.ncbi.nlm.nih.gov/books/NBK109827/. Accessed August 08, 2019. [PubMed] [Google Scholar]
- 20. Heaney RP. Factors influencing the measurement of bioavailability, taking calcium as a model. J Nutr. 2001;131:1344S‐1348S. [DOI] [PubMed] [Google Scholar]
- 21. Pereira GAP, Genaro PS, Pinheiro MM, Szejnfeld VL, Martini LA. Dietary calcium ‐ strategies to optimize intake. Rev Bras Reumatol Engl Ed. 2009;49:172‐180. [Google Scholar]
- 22. Belotto KC, Raposo NR, Ferreira AS, Gattaz WF. Relative bioavailability of two oral formulations of risperidone 2 mg: a single‐dose, randomized‐sequence, open‐label, two‐period crossover comparison in healthy Brazilian volunteers. Clin Ther. 2010;32:2106‐2115. [DOI] [PubMed] [Google Scholar]
- 23. Heaney RP, Dowell MS, Bierman J, Hale CA, Bendich A. Absorbability and cost effectiveness in calcium supplementation. J Am Coll Nutr. 2001;20:239‐246. [DOI] [PubMed] [Google Scholar]
- 24. Yu Y, Teerenstra S, Neef C, Burger D, Maliepaard M. A comparison of the intrasubject variation in drug exposure between generic and brand‐name drugs: a retrospective analysis of replicate design trials. Br J Clin Pharmacol. 2016;81:667‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Bua P, Capodice J. A comparative study of calcium absorption following a single serving administration of calcium carbonate powder versus calcium citrate tablets in healthy premenopausal women. Food Nutr Res. 2014;58:23229 10.3402/fnr.v58.23229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hartini TN, Winkvist A, Lindholm L, et al. Nutrient intake and iron status of urban poor and rural poor without access to rice fields are affected by the emerging economic crisis: the case of pregnant Indonesian women. Eur J Clin Nutr. 2003;57:654‐666. [DOI] [PubMed] [Google Scholar]
- 27. Kumar A, Kaur S. Calcium: a nutrient in pregnancy. J Obstet Gynaecol India. 2017;67:313‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheung CL, Ang SB, Chadha M, et al. An updated hip fracture projection in Asia: the Asian Federation of Osteoporosis Societies study. Osteoporos Sarcopenia. 2018;4:16‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Center for Biotechnology Information . PubChem Database. Calcium gluceptate, CID=109291. 2019. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Calcium-gluceptate#section=FDA-Orange-Book. Accessed August 08, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data supporting the findings of this study are available within the article.


