Abstract
Background
The success of digital replantation is highly dependent on the patency of the repaired vessels after microvascular anastomosis. Antithrombotic agents are frequently used for preventing vascular occlusion. Low molecular weight heparin (LMWH) has been reported to be as effective as unfractionated heparin (UFH) in peripheral vascular surgery, but with fewer adverse effects. Its benefit in microvascular surgery such as digital replantation is unclear. This is an update of the review first published in 2013.
Objectives
To assess if treatment with subcutaneous LMWH improves the salvage rate of the digits in patients with digital replantation after traumatic amputation.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase, AMED and CINAHL databases, and the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers, to 17 March 2020. The authors searched PubMed, China National Knowledge Infrastructure (CNKI) and Chinese Electronic Periodical Services (CEPS) on 17 March 2020 and sought additional trials from reference lists of relevant publications.
Selection criteria
We included randomised or quasi‐randomised controlled trials comparing treatment with LMWH versus any other treatment in participants who received digital replantation following traumatic digital amputation.
Data collection and analysis
Two review authors (PL, CC) independently extracted data and assessed the risk of bias of the included trials using Cochrane's 'Risk of bias' tool. Disagreements were resolved by discussion. We assessed the certainty of evidence using the GRADE approach.
Main results
We included two new randomised trials in this update, bringing the total number of included trials to four. They included a total of 258 participants, with at least 273 digits, from hospitals in China. Three studies compared LMWH versus UFH, and one compared LMWH versus no LMWH. The mean age of participants ranged from 24.5 to 37.6 years. In the studies reporting the sex of participants, there were a total of 145 men and 59 women. The certainty of the evidence was downgraded to low or very low because all studies were at high risk of performance or reporting bias (or both) and there was imprecision in the results due to the small numbers of participants.
The three studies comparing LMWH versus UFH reported the success rate of replantation using different units of analysis (participant or digit), so we were unable to combine data from all three studies (one study reported results for both participants and digits). No evidence of a benefit in success of replantation was seen in the LMWH group when compared with UFH, regardless of whether the outcomes were reported by number of participants (risk ratio (RR) 0.98, 95% confidence interval (CI) 0.87 to 1.10; 130 participants, 2 studies; very low‐certainty evidence); or by number of digits (RR 0.97, 95% CI 0.90 to 1.04; 200 digits, 2 studies; low‐certainty evidence). No studies reported the incidence of compromised microcirculation requiring surgical or non‐surgical therapy, or any systemic/other causes of microvascular insufficiency. There was no evidence of a clear difference between the LMWH and UFH groups in occurrence of arterial occlusion (RR 1.08, 95% CI 0.16 to 7.10; 54 participants, 1 study; very low‐certainty evidence) or venous occlusion (RR 0.81, 95% CI 0.20 to 3.27; 54 participants, 1 study; very low‐certainty evidence). Two studies reported adverse effects. The LMWH and UFH groups showed no evidence of a difference in wound bleeding (RR 0.53, 95% CI 0.23 to 1.23; 130 participants, 2 studies; low‐certainty evidence), haematuria (RR 0.43, 95% CI 0.09 to 2.11; 130 participants, 2 studies; very low‐certainty evidence), ecchymoses (RR 0.82, 95% CI 0.21 to 3.19; 130 participants, 2 studies; very low‐certainty evidence), epistaxis (RR 0.27, 95% CI 0.03 to 2.32; 130 participants, 2 studies; very low‐certainty evidence), gingival bleeding (RR 0.18, 95% CI 0.02 to 1.43; 130 participants, 2 studies; very low‐certainty evidence), and faecal occult blood (RR 0.27, 95% CI 0.03 to 2.31; 130 participants, 2 studies; very low‐certainty evidence). We could not pool data on coagulation abnormalities as varying definitions and tests were used in the three studies.
One study compared LMWH versus no LMWH. The success rate of replantation, when analysed by digits, was reported as 91.2% success in the LMWH group and 82.1% in the control group (RR 1.11, 95% CI 0.93 to 1.33; 73 digits, 1 study; very low‐certainty evidence). Compromised microcirculation requiring surgical re‐exploration, analysed by digits, was 11.8% in the LMWH group and 17.9% in the control group (RR 0.86, 95% CI 0.21 to 3.58; 73 digits, 1 study; very low‐certainty evidence). Compromised microcirculation requiring incision occurred in five out of 34 digits (14.7%) in the LMWH group and eight out of 39 digits (20.5%) in the control group (RR 0.72, 95% CI 0.26 to 1.98; 73 digits; very low‐certainty evidence). Microvascular insufficiency due to arterial occlusion, analysed by digits, was 11.8% in the LMWH group and 17.9% in the control group (RR 0.66, 95% CI 0.21 to 2.05; 73 digits, 1 study; very low‐certainty evidence), and venous occlusion was 14.7% in the LMWH group and 20.5% in the control (RR 0.72, 95% CI 0.26 to 1.98; 73 digits, 1 study; very low‐certainty evidence). The study did not report complications or adverse effects.
Authors' conclusions
There is currently low to very low‐certainty evidence, based on four RCTs, suggesting no evidence of a benefit from LMWH when compared to UFH on the success rates of replantation or affect microvascular insufficiency due to vessel occlusion (analysed by digit or participant). LMWH had similar success rates of replantation; and the incidence rate of venous and arterial microvascular insufficiency showed no evidence of a difference between groups when LMWH was compared to no LMWH (analysed by digit). Similar rates of complications and adverse effects were seen between UFH and LMWH. There was insufficient evidence to draw conclusions on any effect on coagulation when comparing LMWH to UFH or no LMWH. The certainty of the evidence was downgraded due to performance and reporting bias, as well as imprecision in the results. Further adequately powered studies are warranted to provide high‐certainty evidence.
Plain language summary
Low molecular weight heparin for preventing blockage of blood vessels in replanted fingers or toes after amputation injuries
Background
Microvascular surgery refers to any surgery involving small‐sized blood vessels that is performed under the operating microscope, allowing, for example, for the repair of arteries and veins of amputated parts to the body. These vessels are typically 1 mm to 2 mm in diameter. Replantation is the reattachment of a completely detached body part, with fingers and thumbs being the most commonly replanted body parts. This is often referred to as digital replantation. In principle, digital replantation involves not only restoring the blood flow through the arteries and veins but also restoring the bony skeleton of the toes, fingers or thumbs, along with repairing the tendons and nerves, if necessary. Occlusion (blockage) of one or more of the repaired vessels due to the formation of a clot within the blood vessel can result in failure of the replantation. Anticoagulant medications are used to reduce clotting, and they could potentially prevent such a complication. Anticoagulants such as unfractionated heparin (UFH) have therefore been used to prevent clot formation after digital replantation. It is unclear if low molecular weight heparin (LMWH) has similar benefit.
Key results
A randomised controlled trial (RCT) is a clinical study in which people have an equal chance of receiving the treatment or a comparator. This systematic review identified four RCTs comparing LMWH with either UFH or no LMWH. The trials included a total of 258 people with at least 273 replanted fingers or toes (digits). The evidence is up‐to‐date to 17 March 2020. The trials differed in which results they reported and how they reported them, so we could not combine all the information.
Three trials showed no evidence of a benefit of LMWH when compared with UFH in the success rate of replantation, microvascular insufficiency due to vessel occlusion (poor blood flow due to blockages in the small‐sized blood vessels), and side effects such as bleeding. There was no evidence of clear differences between the use of LMWH or no LWMH in the success rate of replantation in one study. Similarly, no evidence of clear differences in the incidence of reduced blood flow (compromised microcirculation) requiring further microvascular surgery (re‐exploration or incision), or causes of microvascular insufficiency due to vessel blockage, were detected in the study which analysed by digits or people.
The limited evidence, which we judged to be of low to very low certainty, suggests there is no clear benefit of LMWH in increasing the success rate of replantation or preventing microvascular occlusion in digital replantation when compared to UFH or no LMWH.
Certainty of the evidence
The overall certainty of evidence is very low due to limitations in study design and reporting. For example, the studies did not take measures to hide from a person which treatment they had, and they reported results that were not stated in the original plan. The sample size of included studies was not large enough to provide precise effect estimates. There is a need for large studies that are designed to reduce these study limitations.
Summary of findings
Background
Description of the condition
Digital replantation is an established microsurgical procedure in traumatic hand surgery, with reported success rates as high as 90% (Levin 2008). The success is highly dependent on maintaining the patency of the repaired blood vessels after satisfactory microvascular anastomosis. The quality of the chosen vessels to anastomose is one of the key factors in determining whether postoperative treatments are needed or not. Microvascular occlusion can result from either venous or arterial thrombosis or, less frequently, a combination of both. Arterial thrombi, which usually present in areas of high or disturbed blood flow or at sites of endothelial damage, are formed primarily of platelet aggregates bound together by thin fibrin strands; thus both platelet activation and the coagulation cascade are important in its thrombogenesis. Venous thrombi, on the other hand, present in areas of stasis and are formed primarily by red blood cells and fibrin, and less so by platelets; thus the coagulation cascade plays a much more prominent role than platelet activation in their aetiology (Conrad 2001). Studies on replantation of the digits and free‐tissue transfer have indicated that the highest risk of critical thrombosis following microvascular anastomosis is in the first three days following surgery. The risk is then reduced but still exists up to two weeks after surgery (Betancourt 1998; Kroll 1996). Venous thrombosis is reported to occur more often than arterial thrombosis. Nevertheless, 90% of arterial thrombi occur within 24 hours post‐replantation while the majority of venous thromboses tend to occur after the first 24 hours (Askari 2006; Kroll 1996; Levin 2008). Some hospitals or surgical units use artery‐only replantation techniques with the use of anticoagulation to maintain venous outflow until it is re‐established naturally. This is particularly the case for more distal amputations (e.g. those distal to the distal interphalangeal joint) or where the damage is too severe for venous anastomosis to be feasible (Lee 2016).
The use of prophylactic antithrombotic agents is the most commonly reported adjunctive strategy for avoiding vascular thrombosis after vascular repair. A variety of anticoagulation protocols are used by microsurgeons. Current protocols include the use of antithrombotic agents such as aspirin, intravenous heparin, low molecular weight heparins (LMWHs) or intravenous dextran, as well as local heparin delivery through direct injection or continuous drip on an open incision (Askari 2006; Barnett 1989; Buckley 2011; Iglesias 1999). However, the optimum dosage and duration of administration of these agents remains complicated by the wide variability of antithrombotic prophylaxis protocols practiced among different surgical units.
Description of the intervention
Unfractionated heparin (UFH) is a glycosaminoglycan polymer of varying lengths and is the most widely used anticoagulant agent for preventing both arterial and venous thrombosis (Stockmans 1997). Heparin maintains the patency of microvascular anastomoses because it inhibits the synthesis of thrombin. This in turn leads to an improved survival rate of digital replantation. However, heparin prophylaxis is limited by an increased risk of haemorrhage from the surgical site, formation of haematoma, heparin‐induced thrombocytopenia (Chong 1989), and requirement for transfusion (Isaacs 1977). Low‐dose regimens are attractive from the standpoint of bleeding complications (Kroll 1995), but they have been reported as less effective compared to full heparinising doses (Hendel 1984). On the contrary, LMWH is a group of antithrombotic agents with different individual properties. LMWH is derived from UFH and has been reported to be as efficacious as heparin in preventing thrombosis. It has enhanced bioavailability as it binds loosely to protein and interacts less with platelets. In comparison to UFH it has fewer adverse effects. The LMWHs have a long half‐life with a very predictable dose‐response relationship, which minimises the need for monitoring. Furthermore, LMWH is associated with reduced risk of bleeding and a lower risk of heparin‐induced osteoporosis. In addition, it has been shown that short‐term use of LMWH is less frequently associated with undesirable heparin‐induced thrombocytopenia (HIT) compared with UFH (Franklin 2003; Warkentin 1995).
How the intervention might work
When the vessel wall is injured, collagen and tissue factor become exposed to the flowing blood, thereby initiating a proteolytic cascade that leads to the formation of a thrombus (Furie 2008). Exposed tissue factor triggers the formation of thrombin. Unlike the formation of thrombi in large‐sized vessels, thrombus formation in small vessels, such as the digital artery, may completely occlude the blood flow to the tissues supplied by the artery. Similarly, thrombus formation in the digital vein impedes proper venous outflow, which will eventually lead to arterial thrombosis. Both UFH and LMWH exert their anticoagulant activity by activating antithrombin (also known as antithrombin III), which in turn inhibits thrombin and activated factor Xa (factor Xa) to prevent both arterial and venous thrombosis (Hirsh 2001; Wolf 1994). Although systemic anticoagulant therapy does not improve the patency rate when sharply divided vessels are repaired (Elcock 1972; Ketchum 1978), a laboratory study showed a dramatic improvement in the patency rate following repair of traumatised vessels using systemic anticoagulant as an adjunct (Cooley 1985). In a retrospective study, it was found that 20% of replantation failures occurred within four hours of discontinuing systemic heparin (Hendel 1984). Although re‐endothelialisation begins immediately after vascular repair, some form of anticoagulant therapy should be continued for at least three to five days, that is until the endothelium regenerates and covers the anastomotic site (Chow 1983; Morecraft 1985; Servant 1976). The LMWHs are produced by either chemical or enzymatic depolymerization of UFH, resulting in the same inhibitory effect on active factor X but with relatively less anti‐IIa (thrombin) activity and thus less effect on the clotting time than UFH (Weitz 1997).
Why it is important to do this review
There are conflicting reports on both the efficacy and adverse effects of anticoagulants in preventing microvascular thrombosis (Niibayashi 2000; Veravuthipakorn 2004; Vretos 1995), and there is little objective evidence to demonstrate their beneficial effects in digital replantation. In addition, many surgeons favour their own particular protocols, that are derived by trial and error, for perioperative anticoagulation. No consensus or standard exists on the use of LMWH as an antithrombotic agent. As such, there is a strong need to review the effects of LMWH in digital replantation. The previous version of this review included two trials (Chen 2001; Li 2012a); thus the data available were limited (Chen 2013). This update includes the most recent available evidence.
Objectives
To assess if treatment with subcutaneous low molecular weight heparin (LMWH) improves the salvage rate of the digits in patients with digital replantation after traumatic amputation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), with or without blinding of participants or outcome assessors. We also included studies which used alternative methods of randomisation such as alternate days of the week, odd or even dates of birth, or hospital number (quasi‐randomised studies). We excluded studies that used historical controls.
Types of participants
We included all patients who suffered from traumatic digital amputation and received salvage microvascular replantation.
Types of interventions
We included studies in which patients with traumatic digital amputation were randomised to receive subcutaneous LMWH versus any other treatment (such as another anticoagulant, placebo or no intervention) after digital replantation in order to prevent microvascular occlusion. We excluded trials that used LMWH for therapeutic purposes in established microvascular occlusion of replanted digits.
Types of outcome measures
Primary outcomes
Success rate of replantation, defined as the survival rate of replanted digits judged by the physician clinically, by means of excluding necrotic digits
Incidence of compromised microcirculation requiring invasive interventions such as re‐exploration, re‐anastomosis, removal of nail, and incision
Secondary outcomes
Microvascular insufficiency due to vessel occlusion (arterial occlusion, venous occlusion, or both)
Microvascular insufficiency due to other/systemic causes (shock, infection, peripheral vascular pathology, hypercoagulopathy, etc.)
Complications and adverse effects related to the interventions (haemorrhage, thrombocytopenia, etc.)
Coagulation abnormalities
Search methods for identification of studies
The searches were not limited by language or publication status.
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials. There were no restrictions on language, publication year or publication status.
Cochrane Vascular Specialised Register, via the Cochrane Register of Studies (CRS‐Web, searched to 17 March 2020)
Cochrane Central Register of Controlled Trials (CENTRAL) Cochrane Register of Studies Online (2020, Issue 2)
MEDLINE (Ovid MEDLINE® Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) (searched from 1 January 2017 to 17 March 2020)
Embase Ovid (searched from 1 January 2017 to 17 March 2020)
CINAHL Ebsco (searched from 1 January 2017 to 17 March 2020)
AMED Ovid (searched from 1 January 2017 to 17 March 2020)
The Information Specialist modelled search strategies for other databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials (as described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). Search strategies for major databases are provided in Appendix 1.
The Information Specialist searched the following trials registries on 17 March 2020.
The World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch)
ClinicalTrials.gov (clinicaltrials.gov)
The review authors also searched PubMed, using the search strategy which is detailed in Appendix 2, as well as China National Knowledge Infrastructure (CNKI) and Chinese Electronic Periodical Services (CEPS), using Chinese synonyms modified from the PubMed search strategy (Appendix 3; Appendix 4). The search of PubMed, CNKI and CEPS were updated on 17 March 2020.
Searching other resources
We examined the reference lists of relevant review articles and all included trials to identify further studies.
Data collection and analysis
Selection of studies
Two review authors (PL, CC) independently assessed all titles and abstracts of potentially eligible trials identified by the searches for inclusion in this review. We obtained the potentially relevant papers and assessed their full texts for inclusion eligibility. We resolved disagreements by discussion.
Data extraction and management
Two review authors (PL, CC) independently extracted data and checked them for accuracy. If relevant data could not be extracted, we tried to contact the primary authors of any articles and requested and included the additional unpublished data, when available. We resolved any discrepancies by discussion and consensus.
We used a standard data extraction form to capture the following information.
Characteristics of the study, including design, method of randomisation, allocation concealment, withdrawals or dropouts, and funding source
Study participants, including mechanism of amputation and level of amputation
Intervention (dosage and route of LMWH administration)
Comparison intervention (placebo or other anticoagulant medication)
Outcome measures, including replantation success rate (survival rate of digital replantation), incidence of microvascular insufficiency demanding invasive interventions, cause of microvascular occlusion, and complications
Assessment of risk of bias in included studies
Two review authors (PL, CC) separately assessed the risk of bias of included studies using the following key domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other sources of bias. For each of these domains we assigned a judgement of low, high, or unclear risk of bias, using Cochrane's 'Risk of bias' tool (Higgins 2011). We resolved any disagreements by discussion. This information is presented in the Characteristics of included studies table.
Measures of treatment effect
For dichotomous outcomes, we expressed data based on the number of events and the number of participants in the intervention and comparison groups. We used these to calculate the risk ratio (RR) and 95% confidence intervals (CIs).
For continuous outcomes, we planned to express continuous data as mean differences (MDs) with 95% CIs. Where different outcome scales were reported, we planned to express continuous data as standardised mean differences (SMDs) with 95% CIs.
Unit of analysis issues
We expected to find only studies that randomised by individual patients. We planned to apply separate analyses if studies which randomised by digits were found, but this was not necessary.
Dealing with missing data
We contacted the original study authors to request missing information whenever possible. We used an intention‐to‐treat (ITT) approach. We reported on the levels of missing data and assessed this as a source of potential bias.
Assessment of heterogeneity
We assessed that the studies were clinically similar enough to allow pooling of data using meta‐analysis by considering their methodology. We examined between‐study heterogeneity by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. We interpreted an I2 value greater than 50% as indicating possible heterogeneity. If statistical heterogeneity was indicated in relation to the study quality, participants, intervention regimens or outcome measurements we planned to apply a subgroup analysis to explore this.
Assessment of reporting biases
We planned to use funnel plots to investigate the presence of publication bias in the included studies when at least 10 relevant trials for a primary outcome were available, or if any systematic differences existed between small and large studies. Insufficient studies were included in this update to allow for the construction of a funnel plot.
Data synthesis
We performed meta‐analyses using Review Manager 5 software (Review Manager 2014). The decision to meta‐analyse data was based on whether the included trials were similar enough in terms of participants, settings, intervention, comparison and outcome measures to allow meaningful conclusions. For estimates of the RR we used the Mantel‐Haenszel method. For continuous variables, we planned to use the inverse variance method. We used a fixed‐effect model for the meta‐analyses. Had we identified cases where there was heterogeneity, we would have used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses according to the mechanism of digital amputation (for example guillotine, crush, avulsion, or degloving) and the level of digital amputation, when data were available. We also planned to perform a comparison of effects of different heparin doses if sufficient numbers of studies, or trials using different dosage regimens, were identified. However, we were unable to conduct these planned subgroup analyses due to lack of relevant data.
Sensitivity analysis
We originally planned to conduct a sensitivity analysis by excluding studies judged to have a high risk of bias in any domain. However, we were unable to perform the planned sensitivity analysis because all included studies had a high risk of bias for at least one domain.
Summary of findings and assessment of the certainty of the evidence
As a post‐protocol addition, we have presented the main findings of this review update in 'Summary of findings' tables. In accordance with GRADE principles, as described by Higgins 2011 and Atkins 2004, we considered the certainty of evidence, magnitude of effect of interventions examined, and the sum of available data for the outcomes of this review. We presented the findings for the comparisons of LMWH versus unfractionated heparin (UFH) (Table 1), and LMWH versus no LMWH (Table 2). We used the GRADEpro GDT website (gradepro.org) to prepare the 'Summary of findings' tables. We used the GRADE system to describe the certainty of the evidence and the strength of recommendations (GRADE 2013; Guyatt 2011). We have expressed the certainty of evidence on a four‐point adjectival scale ranging from 'high' to 'very low'. We downgraded the certainty if there was unexplained, clinically important heterogeneity, the study methodology was at risk of bias, the evidence was indirect, there was important uncertainty around the estimate of effect, or if there was evidence for publication bias. Therefore, it was possible to grade the evidence at a very low certainty if several of these concerns were present.
Summary of findings for the main comparison. Low molecular weight heparin versus unfractionated heparin for prevention of microvascular occlusion in digital replantation.
| LMWH versus UFH for prevention of microvascular occlusion in digital replantation | ||||||
| Patient or population: patients undergoing digital replantation after traumatic amputation Setting: hospital Intervention: LMWH Comparison: UFH | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with UFH | Risk with LMWH | |||||
|
Success rate of replantation1 (analysed by participant, follow‐up unclear2) |
Study population | RR 0.98 (0.87 to 1.10) | 130 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW3 | ||
| 910 per 1000 | 892 per 1000 (792 to 1000) | |||||
|
Success rate of replantation1 (analysed by digit, follow‐up unclear2) |
Study population | RR 0.97 (0.90 to 1.04) | 200 (2 RCTs) | ⊕⊕⊝⊝ LOW 4 | ||
| 950 per 1000 | 922 per 1000 (855 to 989) | |||||
|
Incidence of compromised microcirculation requiring invasive intervention (re‐exploration) (analysed by participant/digit, follow‐up unclear2) |
See comment | Outcome not reported by Chen 2001, Li 2012a or Xu 2013 | ||||
|
Incidence of compromised microcirculation requiring invasive intervention (incision) (analysed by participant/digit, follow‐up unclear2) |
See comment | Outcome not reported by Chen 2001, Li 2012a or Xu 2013 | ||||
|
Microvascular insufficiency due to vessel occlusion ‐ arterial occlusion (analysed by participant/digit, follow‐up unclear2) |
Study population | RR 1.08 (0.16 to 7.10) | 54 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5 | ||
| 71 per 1000 | 77 per 1000 (11 to 507) | |||||
|
Microvascular insufficiency due to vessel occlusion ‐ venous occlusion (analysed by participant, follow‐up unclear2) |
Study population | RR 0.81 (0.20 to 3.27) | 54 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 5 | ||
| 107 per 1000 | 116 per 1000 (26 to 522) | |||||
|
Complication and adverse reactions ‐ wound bleeding (analysed by participant, follow‐up unclear2) |
Study population | RR 0.53 (0.23 to 1.23) | 130 (2 RCTs) | ⊕⊕⊝⊝ LOW 6 | ||
| 209 per 1000 | 111 per 1000 (48 to 257) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low molecular weight heparin; RR: risk ratio; UFH: unfractionated heparin | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Success rate of replantation was study‐based on physician judgement, with no exact definition. 2 Follow‐up is defined as unclear as there were no available data about duration to follow‐up in Chen 2001 and Li 2012a. Xu 2013 mentioned follow up at 14 days post surgery. 3 We downgraded the level of certainty of evidence from high to very low based on the following reasons: there was a high risk of performance bias and reporting bias; and only two trials with small numbers of participants provided detailed data for this comparison (Chen 2001; Xu 2013). 4 We downgraded the level of certainty of evidence from high to low based on the following reasons: there was a high risk of performance bias; and only two trials with small numbers of participants provided detailed data for this comparison (Li 2012a; Xu 2013). 5 We downgraded the level of certainty of evidence from high to very low based on the following reasons: there was a high risk of performance bias and reporting bias; and only one trial with a small number of participants provided detailed data for this comparison (Chen 2001). 6 We downgraded the level of certainty of evidence from high to low based on the following reasons: there was a high risk of performance bias; and only two trials with small numbers of participants provided detailed data for this comparison (Chen 2001; Xu 2013).
Summary of findings 2. Low molecular weight heparin (LMWH) versus no LMWH for prevention of microvascular occlusion in digital replantation.
| LMWH versus no LMWH for prevention of microvascular occlusion in digital replantation | ||||||
| Patient or population: patients undergoing digital replantation after traumatic amputation Setting: hospital Intervention: LMWH Comparison: control (no LMWH) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control (no LMWH) | Risk with LMWH | |||||
|
Success rate of replantation1 (analysed by participant) |
See comment | Outcome not reported by Hu 2013 | ||||
|
Success rate of replantation1 (analysed by digit, follow‐up unclear2) |
Study population | RR 1.11 (0.93 to 1.33) | 73 (1 RCT) | ⊕⊝⊝⊝ VERY LOW3 | ||
| 821 per 1000 | 911 per 1000 (763 to 1000) | |||||
|
Incidence of compromised microcirculation requiring invasive intervention (re‐exploration) (analysed by digit, follow‐up unclear2) |
Study population | RR 0.86 (0.21 to 3.58) | 73 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 | ||
| 103 per 1000 | 88 per 1000 (22 to 367) | |||||
|
Incidence of compromised microcirculation requiring invasive intervention (incision) (analysed by digit, follow‐up unclear3) |
Study population | RR 0.72 (0.26 to 1.98) | 73 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 | ||
| 205 per 1000 | 148 per 1000 (53 to 406) | |||||
|
Microvascular insufficiency due to vessel occlusion ‐ arterial occlusion (analysed by digit, follow‐up unclear2) |
Study population | RR 0.66 (0.21 to 2.05) | 73 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 | ||
| 179 per 1000 | 118 per 1000 (38 to 368) | |||||
|
Microvascular insufficiency due to vessel occlusion ‐ venous occlusion (analysed by digit, follow‐up 7 day post surgery2) |
Study population | RR 0.72 (0.26 to 1.98) | 73 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 3 | ||
| 205 per 1000 | 148 per 1000 (53 to 406) | |||||
|
Complication and adverse reactions ‐ wound bleeding (analysed by participant, follow‐up unclear2) |
See comment | Outcome not reported by Hu 2013 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; LMWH: low molecular weight heparin; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The success rate of replantation in Hu 2013 was study‐based on physician judgement, with no exact definition. 2 Follow‐up is judged as unclear as there were no available data on duration to follow‐up in Hu 2013. 3 We downgraded the level of certainty of evidence from high to very low based on the following reasons: there was a high risk of performance and reporting bias; and only one trial with a small number of participants provided detailed data for this comparison (Hu 2013).
Results
Description of studies
Results of the search
Our updated searches identified 628 additional records, of which 597 remained after duplicates were removed. We excluded 412 references based on titles and abstracts being irrelevant. Of the remaining 13 articles, we excluded 11 (see Characteristics of excluded studies). We included two new trials in this update (Hu 2013; Xu 2013).
See Figure 1 for a PRISMA flow diagram illustrating the study selection process.
1.
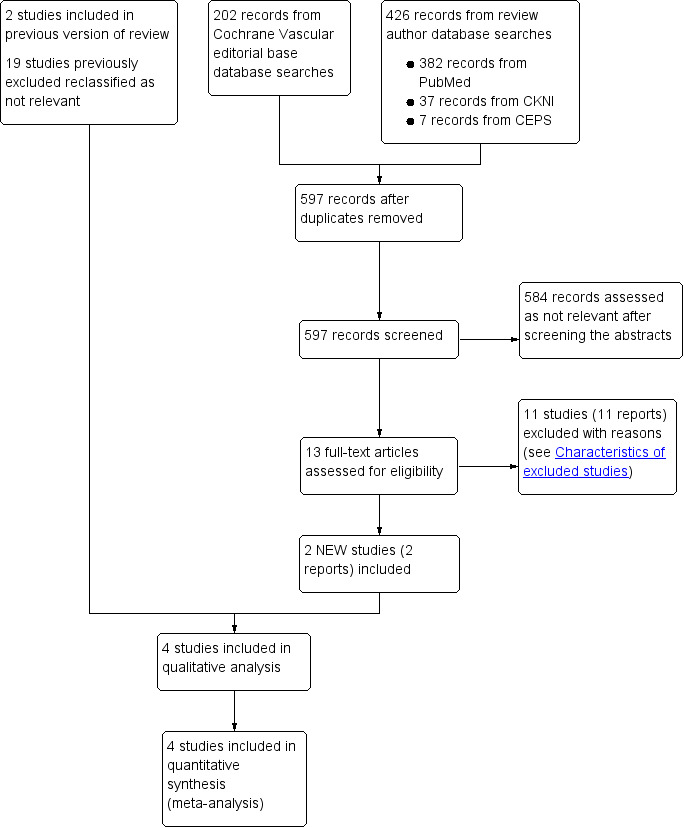
Study flow diagram.
Included studies
Two additional studies met the inclusion criteria for this review (Hu 2013; Xu 2013). This brings the total number of studies included in the quantitative and qualitative analyses to four (Chen 2001; Hu 2013; Li 2012a; Xu 2013). The studies involved a total of 258 participants and at least 273 digits.
Three RCTs compared LMWH with UFH (Chen 2001; Li 2012a; Xu 2013), and one RCT compared LMWH with no LMWH (Hu 2013). The types and dosages of LMWH used in three trials were not reported, and only the volumetric dosage was provided (Hu 2013; Li 2012a; Xu 2013). For full details, see Characteristics of included studies.
Design
All four included studies were RCTs.
Sample size
Chen 2001 enrolled 54 participants and did not reported the number of digits. Li 2012a enrolled 60 participants with 69 either complete or incomplete amputated digits. Xu 2013 enrolled 76 participants with 131 amputated digits. Hu 2013 enrolled 68 participants with 73 amputated digits.
Setting
All four included studies were set in hospitals in China.
Study participants
Chen 2001 enrolled 54 participants and the mean age was 25.8 years in the treatment group and 26.6 years in the control group. In Li 2012a 60 participants were enrolled, including 46 males and 14 females; the participants were aged between 17 and 43 years, with a mean age of 24.5. Xu 2013 enrolled 76 participants (40 males and 36 females) between 18 and 41 years of age; the mean age was 26 years. In Hu 2013 68 participants were enrolled (59 males and nine females); the mean age was 36.2 years and the age range was 10 to 56 years.
The participants in all four included trials received digital replantation for traumatic amputation. Only one trial reported the types of trauma, which consisted of injury by mechanical press (31 digits), chainsaw (30 digits), and cutting (12 digits) (Hu 2013).
Interventions
Three RCTs compared LMWH with UFH (Chen 2001; Li 2012a; Xu 2013), and one RCT compared LMWH with no LMWH (Hu 2013).
In Chen 2001, 5000 international units (IU) subcutaneous (SC) LMWH (Livaracine) was administered 30 minutes before surgery, followed by 2500 to 5000 IU SC every 12 hours for 7 days in the LMWH group. The UFH group received 2500 IU intravenous (IV) UFH 30 minutes to 1 hour before surgery followed by 1250 IU every 12 hours for 7 to 10 days and low molecular weight dextran 500 mL twice a day.
In Li 2012a, the LMWH group received SC LMWH (exact type and dosage unknown) 0.4 mL every 12 hours for 7 to 10 days. The UFH group received 10,000 IU SC UFH every 12 hours for 7 to 10 days. Both groups also received 60 mg intramuscular (IM) papaverine every 6 hours for 7 to 10 days, low molecular weight dextran 500 mL intravenously every 12 hours for 7 to 10 days, dipyridamole 25 mg oral three times per day for 7 to 10 days, and aspirin 50 mg oral once daily for 7 to 10 days.
In Xu 2013, the LMWH group received LMWH (exact type and dosage unknown) 5000 IU SC every 12 hours for 7 days. The control group received heparin 12,500 IU in 1000 mL normal saline, run 24 drops per minute for 24 hours for 7 days. Both groups also received regular therapy with medicine to reduce or prevent infection, spasm, and platelet agglutination, and bed‐rest.
In Hu 2013, the LMWH group received LMWH (exact type and dosage unknown) 0.4 mL SC every 12 hours, while the control group did not receive LMWH. Both groups also received antibiotics, papaverine, aspirin, dipyridamole, and low molecular weight dextran. When venous circulation crisis (cold and pale skin due to poor blood circulation) occurred, the skin was incised and a dressing soaked in heparin diluted in normal saline was applied every 30 minutes.
Outcomes
All four included trials reported the success rate of replantation. One study analysed the success rate of replantation by participants (Chen 2001), two studies analysed by digits (Hu 2013; Li 2012a), and one study analysed by both participants and digits (Xu 2013).
Of the four included trials, one trial reported incidence of compromised microcirculation requiring re‐exploration and incision (Hu 2013); two trials mentioned microvascular insufficiency due to vessel occlusion (Chen 2001; Hu 2013), and two trials reported complications and adverse events (Chen 2001; Xu 2013). Four trials mentioned coagulation abnormalities, including alterations in antithrombin activity, factor Xa activity, bleeding time, clotting time, activated partial thromboplastin time (aPTT), fibrinogen degradation product (FDP) concentration test, platelet count, and fibrinogen levels (Chen 2001; Hu 2013; Li 2012a; Xu 2013). Li 2012a measured aPTT at days 1 and 7 following replantation surgery, while Hu 2013 calculated the mean aPTT from day 1 to day 7 following replantation operation.
Excluded studies
We excluded 11 studies for this update, resulting in a total of 12 excluded studies (Chen 2015; Chen 2016; Chen 2018; Han 2015; Li 2012b; Li 2016; Liu 2015; Ma 2017; Meng 2013; Shu 2011; Zhao 2017; Zhou 2017). The reasons for exclusion are listed in Characteristics of excluded studies. Nineteen previously excluded studies were reassessed as not relevant because they were not RCTs.
Risk of bias in included studies
Details of the methodological quality are reported in the individual study tables (Characteristics of included studies). We present the summarised risk of bias across all included studies in Figure 2 and the respective 'Risk of bias' item for each included study in Figure 3.
2.
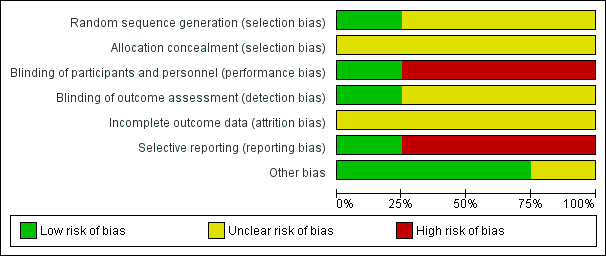
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
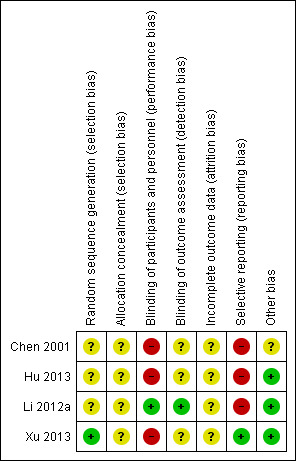
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
One trial used a randomisation table for sequence generation (Xu 2013). The other included trials did not report the methods of random sequence generation. Thus we rated Xu 2013 as having low risk of bias for this domain, and the other trials as having an unclear risk of bias (Chen 2001; Hu 2013; Li 2012a).
The method of allocation concealment was not reported by any of the included trials, and the risk of bias for allocation concealment was rated as unclear for all included studies (Chen 2001; Hu 2013; Li 2012a; Xu 2013).
Blinding
One trial was at low risk of bias for blinding because both the participants and personnel were blinded, and both LMWH and UFH were administered SC every 12 hours for seven to 10 days (Li 2012a). We judged the remaining three trials to have a high risk of performance bias, because the experimental and control interventions were administered via different routes (SC and IV) (Chen 2001; Hu 2013; Xu 2013). We rated Chen 2001, Hu 2013, and Xu 2013 as having unclear risk of detection bias because they did not report any methods for blinding outcome assessment.
Incomplete outcome data
All included trials were classified as having unclear risk of bias for incomplete outcome data due to a lack of descriptions about dropouts or withdrawals. We contacted the authors of Hu 2013 to request data on the methods of randomisation and allocation, the statistical analysis of missing data, and the timing of outcome assessment.
Selective reporting
Three included trials were classified as being at high risk of reporting bias, because the reported outcomes were not pre‐specified (Chen 2001; Hu 2013; Li 2012a). Chen 2001 and Li 2012a reported both efficacy and safety outcomes, but Hu 2013 did not report on safety outcomes. We deemed Xu 2013 to be at low risk of reporting bias, because both the efficacy and safety outcomes were reported and were pre‐specified.
Other potential sources of bias
We judged three of the trials to be at low risk of bias for other sources (Hu 2013; Li 2012a; Xu 2013). These trials did not report which LMWH product was used and the exact dosage, however this would not affect the treatment analysis.
The remaining trial also included amputations of the hand palm and forearm but the number was small (number (n) = 5) and presumed to be balanced between groups (Chen 2001). Low molecular weight dextran was used only in the UFH group; this might have influenced the results so we judged the study to be at unclear risk of other bias.
The details are reported in the 'Risk of bias' tables, under Characteristics of included studies.
Effects of interventions
We were unable to pool the data from the four studies in one meta‐analysis as they compared LMWH to different control arms and analysed by different units. See Table 1 for the comparison LMWH versus UFH for prevention of microvascular occlusion in digital replantation; and Table 2 for the comparison LMWH versus no LMWH for prevention of microvascular occlusion in digital replantation.
Low molecular weight heparin versus unfractionated heparin
Three included trials compared LMWH with UFH and reported the efficacy and adverse effects (Chen 2001; Li 2012a; Xu 2013). However, the trials used different units of analysis to report the success rate of replantation. We performed separate meta‐analyses accordingly. Only Chen 2001 reported the microvascular insufficiency due to vessel occlusion by participants; we also performed subgroup analyses for arterial and venous occlusion. Two trials reported complications and adverse reactions (Chen 2001; Xu 2013). There was a lack of relevant data for the other outcomes planned for this review. For this reason, we were not able to perform subgroup and sensitivity analyses.
Primary outcomes
Success rate of replantation
The trials used different units of analysis to report data on this outcome. Chen 2001 reported the outcome by participants; Li 2012a reported it by digits; and Xu 2013 reported using both participants and digits. Pooling data from the studies showed no evidence if a difference in the success rate with LMWH and UFH, regardless of whether the outcome was assessed by number of participants (RR 0.98, 95% CI 0.87 to 1.10; 2 studies, 130 participants; very low‐certainty evidence), or by number of digits (RR 0.97, 95% CI 0.90 to 1.04; 2 studies, 200 digits; low‐certainty evidence). See Analysis 1.1.
1.1. Analysis.
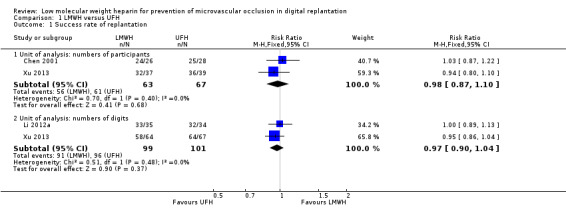
Comparison 1 LMWH versus UFH, Outcome 1 Success rate of replantation.
Incidence of compromised microcirculation requiring invasive interventions such as re‐exploration, re‐anastomosis, removal of nail, incision
This outcome was not mentioned in any of the included RCTs for this comparison.
Secondary outcomes
Microvascular insufficiency due to vessel occlusion
Chen 2001 reported the incidence of postoperative arterial and venous occlusion separately (Analysis 1.2), but the authors did not clarify their definitions for these complications. Arterial occlusion occurred in 2 out of 26 participants (7.7%) in the LMWH group and 2 out of 28 participants (7.1%) in the UFH group (RR 1.08, 95% CI 0.16 to 7.10; 54 participants; very low‐certainty evidence). Compromised venous drainage occurred in 3 out of 26 participants (11.5%) in the LMWH group and 4 out of 28 participants (14.3%) in the UFH group (RR 0.81, 95% CI 0.20 to 3.27; 54 participants; very low‐certainty evidence). The incidence of arterial and venous occlusion showed no evidence of a difference between the LMWH and UFH groups. Li 2012a and Xu 2013 did not report any data relevant to this outcome.
1.2. Analysis.
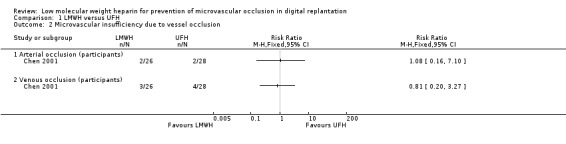
Comparison 1 LMWH versus UFH, Outcome 2 Microvascular insufficiency due to vessel occlusion.
Microvascular insufficiency due to other/systemic causes
This outcome was not mentioned in any of the included RCTs for this comparison.
Complications and adverse effects related to the interventions
Li 2012a did not report any data relevant to this outcome. Chen 2001 and Xu 2013 examined anticoagulation‐related complications and adverse effects, using participants as the unit of analysis (Analysis 1.3). Wound bleeding occurred in 7 participants (11.1%) in the LMWH group and 14 participants (20.9%) in the UFH group (RR 0.53, 95% CI 0.23 to 1.23; 130 participants, 2 studies; very low‐certainty evidence). Haematuria appeared in 2 participants (3.2%) of the LMWH group and 5 participants (7.5%) of the UFH group (RR 0.43, 95% CI 0.09 to 2.11; 130 participants, 2 studies; very low‐certainty evidence). Ecchymoses occurred in 3 participants (4.8%) in the LMWH group and 4 participants (6.0%) in the UFH group (RR 0.82, 95% CI 0.21 to 3.19; 130 participants, 2 studies; very low‐certainty evidence). Epistaxis occurred in 3 (4.5%) participants in the UFH group and 0 participants in the LMWH group (RR 0.27, 95% CI 0.03 to 2.32; 130 participants, 2 studies; very low‐certainty evidence). Gingival bleeding occurred in 5 (7.5%) of participants in the UFH group and 0 participants in the LMWH group (RR 0.18, 95% CI 0.02 to 1.43; 130 participants, 2 studies; very low‐certainty evidence). Faecal occult blood occurred in 3 participants (4.5%) in the UFH group and 0 participants in the LMWH group (RR 0.27, 95% CI 0.03 to 2.31; 130 participants, 2 studies; very low‐certainty evidence).
1.3. Analysis.
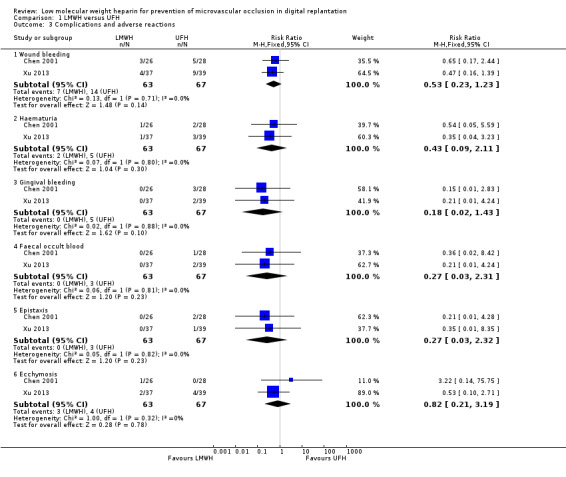
Comparison 1 LMWH versus UFH, Outcome 3 Complications and adverse reactions.
Coagulation abnormalities
We could not perform a meta‐analysis for coagulation abnormality due to the variation of definitions and tests used.
Chen 2001 monitored coagulability changes before anticoagulation and at 1 hour, 3 days, and 7 days after replantation. The study measured the following coagulation‐related outcomes (measured before treatment; after LMWH; and after UFH treatment, respectively): antithrombin activity (0.97; 1.22; 1.56), factor Xa activity (1.02; 0.37; 0.58), bleeding time (2 minutes 24 seconds; 3 minutes 18 seconds; 4 minutes 35 seconds), clotting time (8 minutes 55 seconds; 12 minutes 20 seconds; 25 minutes 32 seconds), activated partial thromboplastin time (aPTT) (41 seconds; 69 seconds; 85 seconds), and fibrinogen degradation product (FDP) concentration test (2.6 mg/L; 11.3 mg/L; 18.8 mg/L). No comparison was made between the LMWH and UFH groups, but the data suggest that coagulability reduced more in the UFH group than in the LMWH group (see Table 3). The study authors reported the mean value of the coagulation test by pooling the data at 1 hour, 3 days, and 7 days after surgery, but standard deviations were not available. Further statistical analysis was therefore not performed.
1. Coagulability measures in the Chen 2001 trial.
| Coagulability measures | Before treatment | After LMWH treatment | After UFH treatment |
| Antithrombin activity | 0.97 | 1.22 | 1.56 |
| Factor Xa activity | 1.02 | 0.37 | 0.58 |
| Bleeding time | 2 minutes 24 seconds | 3 minutes 18 seconds | 4 minutes 35 seconds |
| Clotting time | 8 minutes 55 seconds | 12 minutes 20 seconds | 25 minutes 32 seconds |
| aPTT | 41 seconds | 69 seconds | 85 seconds |
| FDP concentration test | 2.6 mg/L | 11.3 mg/L | 18.8 mg/L |
aPTT: activated partial thromboplastin time FDP: fibrinogen degradation product LMWH: low molecular weight heparin UFH: unfractionated heparin
Li 2012a measured the aPTT and reported no clear differences between the groups on the first postoperative day (LMWH: 28.4 ± 3.3 seconds; UFH: 29.5 ± 3.1 seconds; P > 0.05). However, the difference increased during the second to seventh postoperative days (the aPTT on the seventh postoperative day for LMWH was 30.2 ± 2.1 seconds, and for UFH was 32.5 ± 2.2 seconds; P < 0.05). It must be noted that aPTT changes were expected in the case of UFH, if given at large doses, as raised aPTT levels are a therapeutic target for UFH but not for LMWH. Li 2012a did not directly compare the platelet counts of the LMWH and UFH groups. However, the platelet counts decreased postoperatively in the UFH group (preoperative: 278.4 ± 18.7 x 109/L; postoperative: 194.3 ± 26.5 x 109/L; P < 0.05) but not in the LMWH group (preoperative: 260.8 ± 32.5 x 109/L; postoperative: 252.4 ± 29.1 x 109/L; P > 0.05).
Xu 2013 measured aPTT and platelet counts every day for one week after surgery. The mean aPTT differed between groups (LMWH: 65 ± 2.5 seconds; UFH: 86 ± 2.1 seconds). The UFH group (94 ± 24.1 x 106/L) had lower mean platelet counts than the LMWH group (211 ± 20.4 x 106/L).
The level of aPTT, clotting time, and bleeding time were generally longer in the UFH group than the LMWH group, and the platelet count was generally lower in the UFH group than the LMWH group.
Low molecular weight heparin versus no low molecular weight heparin
One trial compared the efficacy and adverse effects of LMWH with no LMWH (Hu 2013). The trial reported the success rate of replantation, microvascular insufficiency due to vessel occlusion, incidence of compromised microcirculation requiring re‐exploration and incision by digits. The control and LMWH groups also received antibiotics, papaverine, aspirin, dipyridamole, and low molecular weight dextran.
Primary outcomes
Success rate of replantation
The trial analysed by digits. Success rates of replantation were 91.2% in the LMWH group and 82.1% in the control group (RR 1.11, 95% CI 0.93 to 1.33; 73 digits; very low‐certainty evidence). See Analysis 2.1.
2.1. Analysis.
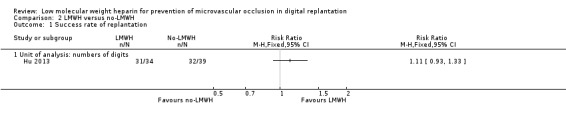
Comparison 2 LMWH versus no‐LMWH, Outcome 1 Success rate of replantation.
Incidence of compromised microcirculation requiring invasive interventions such as re‐exploration, re‐anastomosis, removal of nail, incision
The trial reported that compromised microcirculation requiring surgical re‐exploration occurred in 3 out of 34 digits (8.8%) in the LMWH group and 4 out of 39 digits (10.3%) in the control group (RR 0.86, 95% CI 0.21 to 3.58; 73 digits; very low‐certainty evidence). Compromised microcirculation requiring incision occurred in 5 out of 34 digits (14.7%) in the LMWH group and 8 out of 39 digits (20.5%) in the control group (RR 0.72, 95% CI 0.26 to 1.98; 73 digits; very low‐certainty evidence). See Analysis 2.2.
2.2. Analysis.
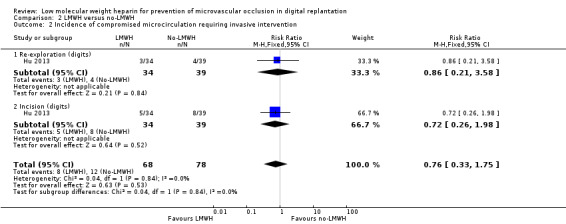
Comparison 2 LMWH versus no‐LMWH, Outcome 2 Incidence of compromised microcirculation requiring invasive intervention.
Secondary outcomes
Microvascular insufficiency due to vessel occlusion
The trial reported that arterial occlusion occurred in 4 out of 34 participants (11.8%) in the LMWH group and 7 out of 39 participants (17.9%) in the control group (RR 0.66, 95% CI 0.21 to 2.05; 73 digits; very low‐certainty evidence). Compromised venous drainage occurred in 5 out of 34 digits (14.7%) in the LMWH group and 8 out of 39 digits (20.5%) in the control group (RR 0.72, 95% CI 0.26 to 1.98; 73 digits; very low‐certainty evidence). See Analysis 2.3.
2.3. Analysis.
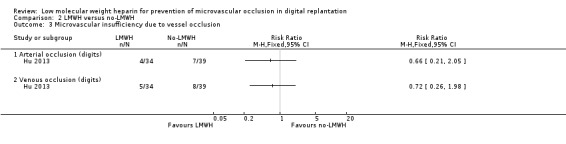
Comparison 2 LMWH versus no‐LMWH, Outcome 3 Microvascular insufficiency due to vessel occlusion.
Microvascular insufficiency due to other/systemic causes
Hu 2013 did not report any data relevant to this outcome.
Complications and adverse effects related to the interventions
Hu 2013 did not report any data relevant to this outcome.
Coagulation abnormalities
Hu 2013 did not report any data relevant to this outcome.
Discussion
Summary of main results
The objective of this review was to compare subcutaneous low molecular weight heparin (LMWH) with other anticoagulants, placebo, or no treatment, to determine the efficacy and safety of LMWH in preventing microvascular occlusion following digital replantation after traumatic amputation.
We found three randomised controlled trials (RCTs) that compared LMWH with unfractionated heparin (UFH) (Chen 2001; Li 2012a; Xu 2013). Outcomes of meta‐analysis on efficacy did not favour LMWH over UFH, irrespective of the unit of analysis being the number of participants or number of digits. We were unable to perform a meta‐analysis for coagulation abnormality due to the variation in definitions used for this outcome. We found no clear differences between use of LMWH and UFH in terms of adverse effects, including haematuria, ecchymoses, epistaxis, gingival bleeding, and faecal occult blood (Chen 2001; Hu 2013). Only Hu 2013 compared LMWH with no LMWH. Evidence was limited by the small number of participants; no clear benefit for the use of LMWH was observed.
Overall completeness and applicability of evidence
We conducted this review to assess whether LMWH treatment improves the salvage rate of digits in patients who had undergone digital replantation after traumatic amputation. In current clinical practice, there is no explicit guideline for the dose and treatment duration of anticoagulant agents after traumatic amputation. Surgeons use anticoagulant agents based on subjective judgement.
The included trials were at high risk of bias for performance or selection bias (or both) and did not provide enough data to investigate all relevant types of injuries and surgical outcomes. Only Hu 2013 described the type of injuries, which included machine cutting injury, saw injury, machine run‐over injury, and mechanical press injury. Other studies did not mention type of injury, but Chen 2001 also included a small number of amputations of the hand palm and forearm. The type of LMWH used in this trial was not reported. The dosage of LMWH was not reported (only the volumetric dosage was provided). We therefore have limited confidence in generalising these results to other situations.
Quality of the evidence
We used the GRADE system to evaluate the certainty of the evidence for outcomes reported in this review. For each comparison and each outcome, we considered the risk of bias, imprecision, inconsistency, indirectness, and publication bias. See Table 1 and Table 2.
Low molecular weight heparin versus unfractionated heparin
All three studies with this comparison reported on the outcomes success rate of replantation, complications, and adverse reactions (Chen 2001; Li 2012a; Xu 2013). LMWH and UFH had similar efficacy on success rate of replantation. Analysis by unit of participants (Chen 2001; Xu 2013) was downgraded to very low‐certainty evidence because of a high risk of performance bias, reporting bias, and small sample size (see Risk of bias in included studies). In Chen 2001, LMWH was administered subcutaneously but UFH was administered intravenously and outcomes of digital amputation (n = 46) were mixed with amputation of the hand palm (n = 3), forearm (n = 2), and toe (n = 3) and cannot be extracted. Low molecular weight dextran was used only in the UFH group, which might have biased the results. The definition of arterial and venous occlusion was not described. In Xu 2013, LMWH and UFH were administrated by different routes and the type and dosage of LMWH used in this trial was not reported. The analysis by unit of digits was downgraded to low‐certainty evidence because of a high risk of performance bias and small sample size (Li 2012a; Xu 2013). LMWH and UFH were both administered subcutaneously in Li 2012a , but not in Xu 2013.
The evidence for the outcome microvascular insufficiency due to vessel occlusion (analysis by unit of participants) was judged to be of very low certainty because of a high risk of performance bias, reporting bias, and imprecision (Chen 2001).
The evidence for the outcome complications and adverse reactions (including wound bleeding) was judged to be of low certainty due to a high risk of performance bias in both trials reporting on this outcome (Chen 2001; Xu 2013).
Low molecular weight heparin versus no low molecular weight heparin
There was one study within this comparison and it provided data on the outcomes success rate of replantation, incidence of compromised microcirculation requiring re‐exploration and incision, and microvascular insufficiency due to vessel occlusion (Hu 2013).
There were similar success rates of replantation in the LMWH group and control group. The analysis was by unit of digits. We deemed the evidence for this outcome to be of very low certainty because of the high risk of performance bias and reporting bias, and the small sample size (Risk of bias in included studies).
The evidence for the outcome incidence of compromised microcirculation requiring re‐exploration and incision (analysis by unit of digits) was deemed to be of very low‐certainty because of the high risk of performance bias and reporting bias, and the small sample size.
The outcomes of microvascular insufficiency due to vessel occlusion analysis (by unit of digits (Hu 2013)) were downgraded to very low‐certainty evidence, because of the high risk of performance bias and reporting bias, and the small sample size.
The outcome of complications and adverse reactions (including wound bleeding) was not reported by Hu 2013.
Potential biases in the review process
We conducted the review according to the published protocol. We tried to avoid bias during the review process by searching a wide range of databases, including two databases in Mandarin (CNKI and CEPS). However, it is possible that some relevant trials published in different languages may not have been identified. Outcomes from replantation of three hand palms and two forearms were included in this review because Chen 2001 pooled these results with the results from digital replantation and they could not be extracted. However, since this trial was a RCT, we assumed these five amputations (5/54) were randomly distributed and were evenly represented in the LMWH and UFH groups. We were unable to establish contact with the trialists to confirm this. We redefined/rephrased two primary outcomes and two secondary outcomes since previous version of the review (Chen 2013). This was due to clinical relevance or to clarify the outcomes further (see Differences between protocol and review). Only RCTs were included in this review. It should be kept in mind that the systematic evaluation of adverse effects of LMWH or other anticoagulants may require other types of studies, such as cohort and case‐control studies (Chi 2009; Cochrane Adverse Effects Methods Group 2012; Loke 2007).
Agreements and disagreements with other studies or reviews
Currently there is little evidence from RCTs to guide peri‐operative anticoagulation in digital replantation. The available evidence consists mainly of retrospective studies (Buckley 2011; Fukui 1989; Fukui 1994; Han 2000; Niibayashi 2000; Noguchi 1999; Oufquir 2006; Poole 1977; Rapoport 1985; Veravuthipakorn 2004; Vretos 1995); comparative studies (Azolov 1983; Furnas 1992; Gao 2007; Maeda 1991; Nikolis 2011; Yang 2008; Yu 2012; Zhang 2002; Zhang 2004); prospective cohort studies (Loisel 2010); or animal studies (Rooks 1994). Most of these studies compared anticoagulants other than LMWH, and many of the authors included major limb replantation or free flap transfer in their studies (Askari 2006; Azolov 1983; Fukui 1994; Khouri 1998; Maeda 1991; Pederson 2008). One RCT compared UFH with no treatment (Shu 2011). In this trial, 24 patients with 42 digital replantations were randomised to the UFH group (50 IU/kg IV bolus) versus 25 patients with 35 digital replantations randomised to the control group. The results showed that single‐dose UFH had no positive effect on microvascular patency or success rate of replantation. It is worth noting that 85.7% of the amputations (36/42 in the UFH group and 30/35 in the control group) were incomplete, and 72.7% (30/42 and 26/35, respectively) of the injury mechanisms were crushing and avulsion. To date, no other systematically reviewed evidence concerning LMWH and digital replantation can be found.
The efficacy of antithrombotic agents for preventing thrombosis had been reviewed systematically for patients who received infrainguinal arterial bypass surgery (Geraghty 2011). Three of the included trials compared LMWH to UFH and did not show a difference in patency (Norgren 2004; Samama 1994; Swedenborg 1996). One of the included trials compared LMWH with placebo, in a population receiving aspirin, and found no improvement in graft patency over the first postoperative year (Jivegard 2005). One trial showed an advantage for LMWH versus aspirin and dipyridamol at one year for patients undergoing operations for limb salvage (Edmondson 1994).The efficacy of LMWH in maintaining the patency of large‐sized vessels is consistent with the findings from our review, when treating much smaller sized vessels such as digital arteries and veins.‐
Authors' conclusions
Implications for practice.
There is currently low to very low‐certainty evidence, based on four RCTS, suggesting no evidence of a benefit from low molecular weight heparin (LMWH) on the success rates of replantation or affect microvascular insufficiency due to vessel occlusion when compared to unfractionated heparin (UFH) (analysed by digit or participant). LMWH had similar success rates of replantation; and the incidence rate of venous and arterial microvascular insufficiency showed no evidence of a difference between groups when LMWH was compared to no LMWH (analysed by digit). Similar rates of complications and adverse effects were seen between UFH and LMWH. There was insufficient evidence to draw conclusions on any effect on coagulation when comparing LMWH to UFH or no LMWH. The certainty of evidence was downgraded due to the presence of performance and reporting bias, as well as imprecision in the results.
Implications for research.
Additional randomised controlled trials (RCTs) with adequate blinding, prespecified outcomes, and with adequate sample size should be conducted to provide evidence regarding the effects of LMWH in this population. Replantation success may also be related to anastomosis technique, quality of the chosen vessels to anastomose, and quality of the tissue around anastomosis. None of these factors were reported by the included trials, however, randomisation may mitigate these confounders. Detailed information of interventions (including dosage, treatment duration, costs, and compromised microcirculation requiring further invasive interventions) and definition of successful replantation should be reported to optimise the treatment regimen and to allow for subgroup analyses. Further studies that include all anticoagulants and their application in replantation or microsurgery are warranted.
Given that digital replantations are uncommon and will likely decline further with improved workplace regulations, conducting RCTs in this area may not be feasible. In absence of RCTs, cohort studies from large databases might provide an alternative to assess the causality between the use of anticoagulant agents and success rate of replantation.
What's new
| Date | Event | Description |
|---|---|---|
| 17 March 2020 | New search has been performed | A new search was conducted and two new studies included. Eleven new studies were excluded. |
| 17 March 2020 | New citation required but conclusions have not changed | A new search was conducted and two new studies included. Eleven new studies were excluded. The text has been updated in keeping with Cochrane policy. A 'Summary of findings' table has been added. The conclusions of the review have not changed. |
Acknowledgements
We acknowledge the Cochrane Vascular editorial base for their assistance in the development of this review and the contributions of the authors of the previous version, including Yi‐Chieh Chen, Fuan Chiang Chan, and Yu‐Wen Wen.
The authors, and the Cochrane Vascular editorial base, are grateful to the following peer reviewers for their time and comments.
Alessandro Thione MD, PhD, Reconstructive and Aesthetic Surgery, Plastic Surgery and Burn Unit, Hospital La Fe ‐ Valencia, Spain Mr Kai Yuen Wong, Department of Plastic Surgery, Oxford University Hospitals NHS Foundation Trust, Oxford, UK Warren C Hammert, MD, University of Rochester, USA Dee Shneiderman, USA
Appendices
Appendix 1. Database searches
| CENTRAL (The Cochrane Library) http://crso.cochrane.org/SearchSimple.php | #1 MESH DESCRIPTOR Hand EXPLODE ALL TREES #2 MESH DESCRIPTOR Finger Injuries EXPLODE ALL TREES #3 hand:TI,AB,KY #4 finger*:TI,AB,KY #5 thumb:TI,AB,KY #6 digit*:TI,AB,KY #7 (((radial or digital or pollicis or ulnar or palmar) near4 arter*)):TI,AB,KY #8 MESH DESCRIPTOR Radial Artery EXPLODE ALL TREES #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10 MESH DESCRIPTOR Amputation EXPLODE ALL TREES #11 amput*:TI,AB,KY #12 avuls*:TI,AB,KY #13 mutilat*:TI,AB,KY #14 MESH DESCRIPTOR Ischemia EXPLODE ALL TREES #15 ischaemi*:TI,AB,KY #16 ischemi*:TI,AB,KY #17 MESH DESCRIPTOR Replantation EXPLODE ALL TREES #18 MESH DESCRIPTOR Reconstructive Surgical Procedures EXPLODE ALL TREES #19 ((replant* or attach*)):TI,AB,KY #20 revascular*:TI,AB,KY #21 salvag*:TI,AB,KY #22 anastomosis:TI,AB,KY #23 ((reconstruct* or re‐construct*)):TI,AB,KY #24 re‐vascula*:TI,AB,KY #25 MESH DESCRIPTOR Microsurgery EXPLODE ALL TREES #26 microsurg*:TI,AB,KY #27 micro‐surg*:TI,AB,KY #28 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 #29 heparin* :TI,AB,KY #30 ((UFH or UH or LMWH )):TI,AB,KY #31 ((Clexane or klexane or lovenox)):TI,AB,KY #32 ((dalteparin or Fragmin or ardeparin)):TI,AB,KY #33 ((nadroparin* or fraxiparin* or enoxaparin)):TI,AB,KY #34 ((normiflo or tinzaparin or logiparin)):TI,AB,KY #35 ((Innohep or certoparin or sandoparin or reviparin)):TI,AB,KY #36 ((clivarin* or danaproid or danaparoid)):TI,AB,KY #37 ((antixarin or ardeparin* or bemiparin*)):TI,AB,KY #38 ((Zibor or cy 222 or embolex or monoembolex)):TI,AB,KY #39 ((parnaparin* or rd 11885 or RD1185)):TI,AB,KY #40 ((tedelparin or Kabi‐2165 or Kabi 2165)):TI,AB,KY #41 ((emt‐966 or emt‐967 or pk‐10 169 or pk‐10169 or pk10169)):TI,AB,KY #42 ((fr‐860 or cy‐216 or cy216)):TI,AB,KY #43 ((wy90493 or "wy 90493")):TI,AB,KY #44 ((parnaparin or fluxum or lohepa or lowhepa)):TI,AB,KY #45 ((seleparin* or tedegliparin or seleparin* or tedegliparin*)):TI,AB,KY #46 (("kb 101" or kb101 or lomoparan or orgaran )):TI,AB,KY #47 ((parnaparin or fluxum or lohepa or lowhepa)):TI,AB,KY #48 ((op 2123 or parvoparin)):TI,AB,KY #49 ((ave 5026)):TI,AB,KY #50 calciparin*:TI,AB,KY #51 MESH DESCRIPTOR Heparin, Low‐Molecular‐Weight EXPLODE ALL TREES #52 #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 #53 #9 AND #28 AND #52 #54 01/01/2012 TO 17/03/2020:CD #55 #53 AND #54 |
24.10.17 ‐ 32 13.12.18 ‐ 18 17.03.20 ‐ 32 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1 exp Hand/ 2 exp Finger Injuries/ 3 hand.ti,ab. 4 finger*.ti,ab. 5 thumb.ti,ab. 6 digit*.ti,ab. 7 ((radial or digital or pollicis or ulnar or palmar) adj4 arter*).ti,ab. 8 exp Radial Artery/ 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10 exp Amputation/ 11 amput*.ti,ab. 12 avuls*.ti,ab. 13 mutilat*.ti,ab. 14 exp Ischemia/ 15 ischaemi*.ti,ab. 16 ischemi*.ti,ab. 17 exp Replantation/ 18 exp Reconstructive Surgical Procedures/ 19 (replant* or attach*).ti,ab. 20 revascular*.ti,ab. 21 salvag*.ti,ab. 22 anastomosis.ti,ab. 23 (reconstruct* or re‐construct*).ti,ab. 24 re‐vascula*.ti,ab. 25 exp Microsurgery/ 26 microsurg*.ti,ab. 27 micro‐surg*.ti,ab. 28 or/10‐27 29 heparin*.ti,ab. 30 (UFH or UH or LMWH).ti,ab. 31 (Clexane or klexane or lovenox).ti,ab. 32 (dalteparin or Fragmin or ardeparin).ti,ab. 33 (nadroparin* or fraxiparin* or enoxaparin).ti,ab. 34 (normiflo or tinzaparin or logiparin).ti,ab. 35 (Innohep or certoparin or sandoparin or reviparin).ti,ab. 36 (clivarin* or danaproid or danaparoid).ti,ab. 37 (antixarin or ardeparin* or bemiparin*).ti,ab. 38 (Zibor or cy 222 or embolex or monoembolex).ti,ab. 39 (parnaparin* or rd 11885 or RD1185).ti,ab. 40 (tedelparin or Kabi‐2165 or Kabi 2165).ti,ab. 41 (emt‐966 or emt‐967 or pk‐10 169 or pk‐10169 or pk10169).ti,ab. 42 (fr‐860 or cy‐216 or cy216).ti,ab. 43 (wy90493 or "wy 90493").ti,ab. 44 (parnaparin or fluxum or lohepa or lowhepa).ti,ab. 45 (seleparin* or tedegliparin or seleparin* or tedegliparin*).ti,ab. 46 ("kb 101" or kb101 or lomoparan or orgaran).ti,ab. 47 (parnaparin or fluxum or lohepa or lowhepa).ti,ab. 48 (op 2123 or parvoparin).ti,ab. 49 ave 5026.ti,ab. 50 calciparin*.ti,ab. 51 exp Heparin, Low‐Molecular‐Weight/ 52 or/29‐51 53 9 and 28 and 52 54 randomized controlled trial.pt. 55 controlled clinical trial.pt. 56 randomized.ab. 57 placebo.ab. 58 drug therapy.fs. 59 randomly.ab. 60 trial.ab. 61 groups.ab. 62 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 63 53 and 62 64 2017*.ed. 65 63 and 64 |
24.10.17 ‐ 3 13.12.18 ‐ 17 17.03.20 ‐ 22 |
| Embase 1974 to present |
1 exp Hand/ 2 exp Finger Injuries/ 3 hand.ti,ab. 4 finger*.ti,ab. 5 thumb.ti,ab. 6 digit*.ti,ab. 7 ((radial or digital or pollicis or ulnar or palmar) adj4 arter*).ti,ab. 8 exp Radial Artery/ 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10 exp Amputation/ 11 amput*.ti,ab. 12 avuls*.ti,ab. 13 mutilat*.ti,ab. 14 exp Ischemia/ 15 ischaemi*.ti,ab. 16 ischemi*.ti,ab. 17 exp Replantation/ 18 exp Reconstructive Surgical Procedures/ 19 (replant* or attach*).ti,ab. 20 revascular*.ti,ab. 21 salvag*.ti,ab. 22 anastomosis.ti,ab. 23 (reconstruct* or re‐construct*).ti,ab. 24 re‐vascula*.ti,ab. 25 exp Microsurgery/ 26 microsurg*.ti,ab. 27 micro‐surg*.ti,ab. 28 or/10‐27 29 heparin*.ti,ab. 30 (UFH or UH or LMWH).ti,ab. 31 (Clexane or klexane or lovenox).ti,ab. 32 (dalteparin or Fragmin or ardeparin).ti,ab. 33 (nadroparin* or fraxiparin* or enoxaparin).ti,ab. 34 (normiflo or tinzaparin or logiparin).ti,ab. 35 (Innohep or certoparin or sandoparin or reviparin).ti,ab. 36 (clivarin* or danaproid or danaparoid).ti,ab. 37 (antixarin or ardeparin* or bemiparin*).ti,ab. 38 (Zibor or cy 222 or embolex or monoembolex).ti,ab. 39 (parnaparin* or rd 11885 or RD1185).ti,ab. 40 (tedelparin or Kabi‐2165 or Kabi 2165).ti,ab. 41 (emt‐966 or emt‐967 or pk‐10 169 or pk‐10169 or pk10169).ti,ab. 42 (fr‐860 or cy‐216 or cy216).ti,ab. 43 (wy90493 or "wy 90493").ti,ab. 44 (parnaparin or fluxum or lohepa or lowhepa).ti,ab. 45 (seleparin* or tedegliparin or seleparin* or tedegliparin*).ti,ab. 46 ("kb 101" or kb101 or lomoparan or orgaran).ti,ab. 47 (parnaparin or fluxum or lohepa or lowhepa).ti,ab. 48 (op 2123 or parvoparin).ti,ab. 49 ave 5026.ti,ab. 50 calciparin*.ti,ab. 51 exp Heparin, Low‐Molecular‐Weight/ 52 or/29‐51 53 9 and 28 and 52 54. randomized controlled trial/ 55 controlled clinical trial/ 56 random$.ti,ab. 57. randomization/ 58. intermethod comparison/ 59. placebo.ti,ab. 60. (compare or compared or comparison).ti. 61. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 62. (open adj label).ti,ab. 63. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 64. double blind procedure/ 65. parallel group$1.ti,ab. 66. (crossover or cross over).ti,ab. 67. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 68. (assigned or allocated).ti,ab. 69. (controlled adj7 (study or design or trial)).ti,ab. 70. (volunteer or volunteers).ti,ab. 71. trial.ti. 72. or/54‐72 |
24.10.17 ‐ 9 13.12.18 ‐ 37 17.03.20 ‐ 41 |
| CINAHL (EBSCOhost) | S61 S9 AND S28 AND S52 AND S59 AND S60 S60 EM 2017 S59 S53 OR S54 OR S55 OR S56 OR S57 OR S58 S58 TX randomly S57 TX "treatment as usual" S56 TX "double‐blind*" S55 TX "single‐blind*" S54 TX trial S53 MH "Clinical Trials" S52 S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 S51 (MH "Heparin, Low‐Molecular‐Weight+") S50 TX calciparin* S49 TX ((ave 5026)) S48 TX ((op 2123 or parvoparin)) S47 TX ((parnaparin or fluxum or lohepa or lowhepa)) S46 TX (("kb 101" or kb101 or lomoparan or orgaran )) S45 TX ((seleparin* or tedegliparin or seleparin* or tedegliparin*)) S44 TX ((parnaparin or fluxum or lohepa or lowhepa)) S43 TX ((wy90493 or "wy 90493")) S42 TX ((fr‐860 or cy‐216 or cy216)) S41 TX ((emt‐966 or emt‐967 or pk‐10 169 or pk‐10169 or pk10169)) S40 TX ((tedelparin or Kabi‐2165 or Kabi 2165)) S39 TX ((parnaparin* or rd 11885 or RD1185)) S38 TX ((Zibor or cy 222 or embolex or monoembolex)) S37 TX ((antixarin or ardeparin* or bemiparin*)) S36 TX ((clivarin* or danaproid or danaparoid)) S35 TX ((Innohep or certoparin or sandoparin or reviparin)) S34 TX ((normiflo or tinzaparin or logiparin)) S33 TX ((nadroparin* or fraxiparin* or enoxaparin)) S32 TX ((dalteparin or Fragmin or ardeparin)) S31 TX ((Clexane or klexane or lovenox)) S30 TX ((UFH or UH or LMWH )) S29 TX heparin* S28 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 S27 TX micro‐surg* S26 TX microsurg* S25 (MH "Microsurgery+") S24 TX re‐vascula* S23 TX (reconstruct* or re‐construct*) S22 TX anastomosis S21 TX salvag* S20 TX revascular* S19 TX (replant* or attach*) S18 (MH "Surgery, Reconstructive+") S17 (MH "Replantation+") S16 TX ischemi* S15 TX ischaemi* S14 (MH "Ischemia+") S13 TX mutilat* S12 TX avuls* S11 TX amput* S10 (MH "Amputation+") S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S8 (MM "Radial Artery") S7 (radial or digital or pollicis or ulnar or palmar) N4 arter*) S6 TX digit* S5 TX thumb S4 TX finger* S3 TX hand S2 (MH "Finger Injuries+") S1 (MH "Hand+") |
24.10.17 ‐ 2 13.12.18 ‐ 4 17.03.20 ‐ 5 |
| ClinicalTrials.gov (www.clinicaltrials.gov) |
hand OR hands OR thumbs OR thumb OR finger OR fingers OR digit OR digits | heparin | 24.10.17 ‐ 6 13.12.18 ‐ 3 17.03.20 ‐ 6 |
| ICTRP | hand OR hands OR thumbs OR thumb OR finger OR fingers OR digit OR digits | heparin | amputation OR replantation OR Microsurgery | 24.10.17 ‐ 1 13.12.18 ‐ 0 17.03.20 ‐ 0 |
| AMED | 1 exp Hand/ 2 exp Finger Injuries/ 3 hand.ti,ab. 4 finger*.ti,ab. 5 thumb.ti,ab. 6 digit*.ti,ab. 7 ((radial or digital or pollicis or ulnar or palmar) adj4 arter*).ti,ab. 8 exp Radial Artery/ 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10 exp Amputation/ 11 amput*.ti,ab. 12 avuls*.ti,ab. 13 mutilat*.ti,ab. 14 exp Ischemia/ 15 ischaemi*.ti,ab. 16 ischemi*.ti,ab. 17 exp Replantation/ 18 exp Reconstructive Surgical Procedures/ 19 (replant* or attach*).ti,ab. 20 revascular*.ti,ab. 21 salvag*.ti,ab. 22 anastomosis.ti,ab. 23 (reconstruct* or re‐construct*).ti,ab. 24 re‐vascula*.ti,ab. 25 exp Microsurgery/ 26 microsurg*.ti,ab. 27 micro‐surg*.ti,ab. 28 or/10‐27 29 heparin*.ti,ab. 30 (UFH or UH or LMWH).ti,ab. 31 (Clexane or klexane or lovenox).ti,ab. 32 (dalteparin or Fragmin or ardeparin).ti,ab. 33 (nadroparin* or fraxiparin* or enoxaparin).ti,ab. 34 (normiflo or tinzaparin or logiparin).ti,ab. 35 (Innohep or certoparin or sandoparin or reviparin).ti,ab. 36 (clivarin* or danaproid or danaparoid).ti,ab. 37 (antixarin or ardeparin* or bemiparin*).ti,ab. 38 (Zibor or cy 222 or embolex or monoembolex).ti,ab. 39 (parnaparin* or rd 11885 or RD1185).ti,ab. 40 (tedelparin or Kabi‐2165 or Kabi 2165).ti,ab. 41 (emt‐966 or emt‐967 or pk‐10 169 or pk‐10169 or pk10169).ti,ab. 42 (fr‐860 or cy‐216 or cy216).ti,ab. 43 (wy90493 or "wy 90493").ti,ab. 44 (parnaparin or fluxum or lohepa or lowhepa).ti,ab. 45 (seleparin* or tedegliparin or seleparin* or tedegliparin*).ti,ab. 46 ("kb 101" or kb101 or lomoparan or orgaran).ti,ab. 47 (parnaparin or fluxum or lohepa or lowhepa).ti,ab. 48 (op 2123 or parvoparin).ti,ab. 49 ave 5026.ti,ab. 50 calciparin*.ti,ab. 51 exp Heparin, Low‐Molecular‐Weight/ 52 or/29‐51 53 9 and 28 and 52 |
24.10.17 ‐ 0 13.12.18 ‐ 0 17.03.20 ‐ 0 |
Appendix 2. Authors' PubMed search strategy
| #1 | hand[MeSH Terms] OR hand[All Fields] OR hands[All Fields] |
| #2 | fingers[MeSH Terms] OR fingers[All Fields] OR finger[All Fields] |
| #3 | thumb[MeSH Terms] OR thumb[All Fields] OR thumbs[All Fields] |
| #4 | digit[All Fields] OR digits[All Fields] OR digital[All Fields] |
| #5 | radial[All Fields] OR digital[All Fields] OR pollicis[All Fields] OR ulnar[All Fields] OR palmar[All Fields] AND Arter* |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 |
| #7 | amput* |
| #8 | avuls* |
| #9 | mutilat* |
| #10 | ischaemia[All Fields] OR ischemia[MeSH Terms] OR ischemia[All Fields] OR ischemic[All Fields] |
| #11 | replantation[MeSH Terms] OR replantation[All Fields] OR replantat* |
| #12 | revascular* |
| #13 | salvage* |
| #14 | microsurgery[MeSH Terms] OR microsurgery[All Fields] OR microsurg* |
| #15 | Reconstruct* |
| #16 | #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 |
| #17 | heparin[MeSH Terms] OR heparin[All Fields] OR heparin* |
| #18 | anticoagulants[MeSH Terms] OR anticoagulants[All Fields] OR anticoagulant[All Fields] OR anticoagulants[Pharmacological Action] OR anticoagula* |
| #19 | antithrombo* |
| #20 | UFH OR UH OR LMWH |
| #21 | nadroparin* OR fraxiparin* OR enoxaparin |
| #22 | Clexane OR klexane OR lovenox |
| #23 | dalteparin OR Fragmin OR ardeparin |
| #24 | normiflo OR tinzaparin OR logiparin |
| #25 | Innohep OR certoparin OR sandoparin OR reviparin |
| #26 | clivarin* OR danaproid OR danaparoid |
| #27 | antixarin OR ardeparin* OR bemiparin* |
| #28 | Zibor OR cy 222 OR embolex OR monoembolex |
| #29 | parnaparin* OR rd 11885 OR RD1185 |
| #30 | tedelparin OR Kabi‐2165 OR Kabi |
| #31 | emt‐966 OR emt‐967 OR pk‐10 169 OR pk‐10169 OR pk10169 |
| #32 | fr‐860 OR cy‐216 OR cy216 |
| #33 | seleparin* OR tedegliparin OR seleparin* OR tedegliparin* |
| #34 | wy90493 OR wy 90493 |
| #35 | kb 101 OR kb101 OR lomoparan OR orgaran |
| #36 | parnaparin OR fluxum OR lohepa OR lowhepa |
| #37 | op 2123 OR parvoparin |
| #38 | ave5026 |
| #39 | calciparin* |
| #40 | #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 |
| #41 | #6 AND #16 AND #40 |
| #1 | hand[MeSH Terms] OR hand[All Fields] OR hands[All Fields] |
| #2 | fingers[MeSH Terms] OR fingers[All Fields] OR finger[All Fields] |
| #3 | thumb[MeSH Terms] OR thumb[All Fields] OR thumbs[All Fields] |
| #4 | digit[All Fields] OR digits[All Fields] OR digital[All Fields] |
| #5 | radial[All Fields] OR digital[All Fields] OR pollicis[All Fields] OR ulnar[All Fields] OR palmar[All Fields] AND Arter* |
| #6 | #1 OR #2 OR #3 OR #4 OR #5 |
| #7 | amput* |
| #8 | avuls* |
| #9 | mutilat* |
| #10 | ischaemia[All Fields] OR ischemia[MeSH Terms] OR ischemia[All Fields] OR ischemic[All Fields] |
| #11 | replantation[MeSH Terms] OR replantation[All Fields] OR replantat* |
| #12 | revascular* |
| #13 | salvage* |
| #14 | microsurgery[MeSH Terms] OR microsurgery[All Fields] OR microsurg* |
| #15 | Reconstruct* |
| #16 | #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 |
| #17 | heparin[MeSH Terms] OR heparin[All Fields] OR heparin* |
| #18 | anticoagulants[MeSH Terms] OR anticoagulants[All Fields] OR anticoagulant[All Fields] OR anticoagulants[Pharmacological Action] OR anticoagula* |
| #19 | antithrombo* |
| #20 | UFH OR UH OR LMWH |
| #21 | nadroparin* OR fraxiparin* OR enoxaparin |
| #22 | Clexane OR klexane OR lovenox |
| #23 | dalteparin OR Fragmin OR ardeparin |
| #24 | normiflo OR tinzaparin OR logiparin |
| #25 | Innohep OR certoparin OR sandoparin OR reviparin |
| #26 | clivarin* OR danaproid OR danaparoid |
| #27 | antixarin OR ardeparin* OR bemiparin* |
| #28 | Zibor OR cy 222 OR embolex OR monoembolex |
| #29 | parnaparin* OR rd 11885 OR RD1185 |
| #30 | tedelparin OR Kabi‐2165 OR Kabi |
| #31 | emt‐966 OR emt‐967 OR pk‐10 169 OR pk‐10169 OR pk10169 |
| #32 | fr‐860 OR cy‐216 OR cy216 |
| #33 | seleparin* OR tedegliparin OR seleparin* OR tedegliparin* |
| #34 | wy90493 OR wy 90493 |
| #35 | kb 101 OR kb101 OR lomoparan OR orgaran |
| #36 | parnaparin OR fluxum OR lohepa OR lowhepa |
| #37 | op 2123 OR parvoparin |
| #38 | ave5026 |
| #39 | calciparin* |
| #40 | #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 |
| #41 | #6 AND #16 AND #40 |
Appendix 3. Authors' CNKI search strategy
(((題名= 低分子肝素 OR 關鍵詞 = 低分子肝素 OR 摘要 = 低分子肝素) OR (題名= 低分子量肝素 OR 關鍵詞 = 低分子量肝素 OR 摘要 = 低分子量肝素)) AND ((題名 =斷指 OR 關鍵詞 =斷指 OR 摘要 =斷指 OR 全文 =斷指) OR (題名 = 再植 OR 關鍵詞 = 再植 OR 摘要 = 再植 OR 全文 = 再植)))
Appendix 4. Authors' CEPS search strategy
(肝素 AND 斷指)=所有欄位
Data and analyses
Comparison 1. LMWH versus UFH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success rate of replantation | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Unit of analysis: numbers of participants | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.87, 1.10] |
| 1.2 Unit of analysis: numbers of digits | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.04] |
| 2 Microvascular insufficiency due to vessel occlusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Arterial occlusion (participants) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Venous occlusion (participants) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Complications and adverse reactions | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Wound bleeding | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.23, 1.23] |
| 3.2 Haematuria | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.09, 2.11] |
| 3.3 Gingival bleeding | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.43] |
| 3.4 Faecal occult blood | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.31] |
| 3.5 Epistaxis | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.32] |
| 3.6 Ecchymosis | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.21, 3.19] |
Comparison 2. LMWH versus no‐LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success rate of replantation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Unit of analysis: numbers of digits | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Incidence of compromised microcirculation requiring invasive intervention | 1 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.33, 1.75] |
| 2.1 Re‐exploration (digits) | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.21, 3.58] |
| 2.2 Incision (digits) | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.26, 1.98] |
| 3 Microvascular insufficiency due to vessel occlusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Arterial occlusion (digits) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Venous occlusion (digits) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2001.
| Methods |
Design: RCT Setting: China Railway Group's Four Innings Second Hospital, Fuyang City, Anhui Province, China Study period: October 1999 to June 2000 Randomisation: method unknown Blinding: nil Intention‐to‐treat/loss to follow‐up: unclear (authors did not report if some patients were randomised but did not complete the trial) |
|
| Participants | 54 participants of replantation (including 39 single digits, 7 multiple digits, 3 hand palms, 2 forearms and 3 toes) were randomised into two groups: group A (LMWH) aged 4.5 to 57 years old, mean age 25.8; group B (UFH) aged 8 to 55 years old; mean age 26.6 years
Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions |
Treatment (group A , n = 26) LMWH (Livaracine, Lee's Pharmaceutical Holdings Ltd, Hong Kong, China) 5000 IU SC 30 minutes before surgery, followed by 2500 to 5000 IU SC every 12 hours for 7 days Control (group B, n = 28) UFH 2500 IU IV 0.5 to 1 hour before surgery, followed by 1250 IU every 12 hours for 7 to 10 days; also received low molecular weight dextran 500 mL twice a day |
|
| Outcomes |
|
|
| Notes | The study authors reported that success rate was calculated by individual patients; the mean value of coagulation test was obtained by pooling the data of 1 hour, 3 days and 7 days after surgery; statistical evaluation was not reported; outcomes of digital amputation (n = 46) were mixed with amputation of hand palm (n = 3), forearm (n = 2) and toe (n = 3) and cannot be extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote p. 53: "...patients were randomised into group A and B... (author's translation)" Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: LMWH was administered SC but UFH was administered IV |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of dropouts or withdrawals |
| Selective reporting (reporting bias) | High risk | Comment: both the efficacy and safety outcomes were reported but efficacy and safety outcomes were not pre‐specified |
| Other bias | Unclear risk | Comment: outcomes of digital amputation (n = 46) were mixed with amputation of hand palm (n = 3), forearm (n = 2) and toe (n = 3) and cannot be extracted. The mean value of coagulation test was obtained by pooling the data of 1h, 3d and 7d; the SD was not available and statistic evaluation was not reported. Low molecular weight dextran was used only in the control group, which might have biased the results. The definition or arterial and venous occlusion was not described. |
Hu 2013.
| Methods |
Design: RCT Setting: Fourth People's Hospital of Yangquan, Yangquan, Shanxi, China Study period: not mentioned Randomisation: method unknown Blinding: no blinding Intention‐to‐treat/loss to follow‐up: unknown (the authors did not report if some participants were randomised but did not complete the trial) |
|
| Participants | 68 participants (59 male, 9 female), aged 10 to 56 years (mean 36.2 years) with 73 replanted digits (62 male, 22 female) The 68 participants (73 digits) were injured by mechanical press (31 digits), chainsaw (30 digits), and cutting (12 digits). There were 35 middle fingers and 38 little fingers. Inclusion/exclusion criteria: not mentioned |
|
| Interventions |
Treatment group (32 participants, 34 digits): LMWH (exact type unknown) 0.4 mL SC every 12 hours Control group (36 participants, 39 digits): no LMWH Both groups also received the following.
|
|
| Outcomes |
|
|
| Notes | Incidence rate was reported by digit. The type of LMWH used in this trial was not reported. The dosage of LMWH was not reported; only the volumetric dosage was provided. This study reports management of venous congestion by incision for bleeding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote p. 2275: “... randomly divided 68 participants with 73 digitals into 2 groups (author’s translation …. ”
Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: method of blinding not described, but treatment and control group used different therapy |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of dropouts or withdrawals |
| Selective reporting (reporting bias) | High risk | Comment: there were no safety outcomes evaluated and efficacy outcomes were not pre‐specified |
| Other bias | Low risk | Comment: the type of LMWH used in this trial was not reported. The dosage of LMWH was not reported; only the volumetric dosage was provided. This would not affect the treatment analysis. |
Li 2012a.
| Methods |
Design: RCT Setting: Integrative Medicine Hospital of Guangdong Province, Foshan City, China Study period: since 2006 Randomisation: method unknown Blinding: double blind Intention‐to‐treat/loss to follow‐up: unclear (the authors did not report if some patients were randomised but did not complete the trial) |
|
| Participants | 60 participants (46 male, 14 female) aged 17 to 43 years (mean 24.5 years) with 69 complete or incomplete amputated digits
Inclusion criteria: amputation of digit(s) between the level of metacarpophalangeal joint and distal phalangeal base, non‐salvageable without vascular anastomosis Exclusion criteria: not stated |
|
| Interventions |
Treatment (30 participants with 35 replanted digits): LMWH (exact type unknown) 0.4 mL SC every 12 hours for 7 to 10 days Control (30 participants with 34 replanted digits): UFH 10,000 IU subcutaneous every 12 hours for 7 to 10 days Both groups also received:
|
|
| Outcomes |
|
|
| Notes | Success rate was reported by digit The type of LMWH used in this trial was not reported. The dosage of LMWH was not reported; only the volumetric dosage was provided. This would not affect the treatment analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote p. 205: "...participants were randomised into two groups... (author's translation)" Comment: method not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote p. 205: "...double blind principle was followed... (author's translation)" Comment: the outcome is not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote p. 205: "...double blinding principle was followed... (author's translation)" Comment: outcome measurement is not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of dropouts or withdrawals |
| Selective reporting (reporting bias) | High risk | Comment: both the efficacy and safety outcomes were reported but efficacy outcomes were not pre‐specified |
| Other bias | Low risk | Comment: the type of LMWH used in this trial was not reported. The dosage of LMWH was not reported; only the volumetric dosage was provided. This would not affect the treatment analysis. |
Xu 2013.
| Methods |
Design: RCT Setting: Chinese People's Liberation Army No.272 Hospital, Tianjin, China Study period: March 2009 to May 2013 Randomisation: randomisation table Blinding: double blind Intention‐to‐treat/loss to follow‐up: unknown (the authors did not report if some patients were randomised but did not complete the trial) |
|
| Participants | 76 participants (40 male, 36 female) with 131 digits, aged 18 to 41 years old (mean 26 years old) Inclusion/exclusion criteria: not mentioned |
|
| Interventions |
Treatment group (37 participants, 64 digits): LMWH (exact type unknown) 5000 IU SC every 12 hours for 7 days Control group (39 participants, 67 digits): heparin 12,500 IU in 1000 mL normal saline run 24 drops per minutes for 24 hours for 7 days Both groups also received regular therapy with medicine to reduce or prevent infection, spasm, and platelet agglutination, and bed‐rest |
|
| Outcomes |
|
|
| Notes | Incidence rate was reported by digit and participant The type of LMWH used in this trial was not reported. The dosage of LMWH was not reported; only the volumetric dosage was provided This would not affect the treatment analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote p. 327: “... this study was double blinded and randomly by randomisation table to divided into 2 groups (author’s translation) …. ”
Comment: randomised by randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote p. 327: “... this study was double blinded and randomly by randomisation table to divided into 2 groups (author’s translation) …. ”
Comment: treatment and control group were administrated by different route (IV for 24 hours and SC), and there were no descriptions of blinding methods in content |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no description of dropouts or withdrawals |
| Selective reporting (reporting bias) | Low risk | Comment: efficacy and safety were both reported and outcomes were pre‐specified |
| Other bias | Low risk | Comment: the type of LMWH used in this trial was not reported. The dosage of LMWH was not reported; only the volumetric dosage was provided. This would not affect the treatment analysis. |
aPTT: activated partial thromboplastin time FDP: fibrinogen degradation product IM: intramuscular IU: international unit IV: intravenous LMWH: low molecular weight heparin RCT: randomised controlled trial SC: subcutaneous UFH: unfractionated heparin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2015 | The comparisons did not focus on LMWH (article in Chinese). 62 cases of replantation of amputated fingers were divided randomly into an observation group and a control group, with 31 cases in each group. The observation group was treated with LMWH sodium 4500 IU once a day combined with Xueshuantong injection 150 mg in 5% glucose injection 200 mL IV drip twice a day for 3 days. The control group was treated with LMWH sodium 4500 IU once a day for 3 days |
| Chen 2016 | The comparisons did not focus on LMWH (article in Chinese). 50 cases of replantation of amputated fingers were divided randomly into an observation group and a control group, with 25 cases in each group. The observation group was treated with papaverine 30 mg IM and heparin sodium 2083 IU every 6 hours IM for 7 days. The control group was treated with LMWH sodium 0.4 mL IM once daily and felodipine 5 mg once daily for 7 days |
| Chen 2018 | The participants had abruption of artery but did not have fingers amputated. |
| Han 2015 | The comparisons did not focus on LMWH (article in Chinese). 64 cases of replantation of amputated fingers were divided randomly into an observation group and a control group, with 32 cases in each group. The observation group was treated with LMWH and Xuesaitong injection for 7 days. The control group was treated with LMWH for 7 days. |
| Li 2012b | The comparisons did not focus on LMWH (article in Chinese). 412 cases of replantation of amputated fingers were divided randomly into an observation group (198 digits) and a control group (214 digits). The observation group was treated with LMWH 0.4 mL SC every 12 hours and with felodipine 5 mg oral once daily for 7 days. The control group was treated with LMWH 4500 IU once daily, all treated for 3 days. |
| Li 2016 | The participants included patients undergoing free flap trauma repair and patients undergoing replantation. The study authors did not present outcome data stratified by patient groups (article in Chinese). This study was conducted from March 2014 to September 2015. 72 cases of traumatic vascular anastomosis, including free flap trauma repair and replantation patients, were divided randomly into observation group and control group with 36 cases in each group. The observation group was treated with oral Huoxue Rongshuan decoction (one dose per day, two times). The control group was treated with SC injection of low molecular heparin calcium. We contacted the study authors for separate data on the digital replantation participants, but we did not receive a reply. |
| Liu 2015 | The participants were flap transplantation patients, not digital replantation patients (article in Chinese). This study was conducted from October 2011 to October 2013. 70 cases of flap transplantation were divided randomly into an observation group and a control group with 35 cases in each group. The observation group was treated with Huoxue Rongshuan Decoction. The control group were treated with SC injection of LMWH calcium once daily for 7 days after the operation. |
| Ma 2017 | The participants included unstable angina, acute myocardial infarction, DVT or diabetic nephropathy patients, not digital replantation patients (article in Chinese). 110 cases of unstable angina, acute myocardial infarction, DVT or diabetic nephropathy patients were divided randomly into an observation group and a control group, with 55 cases in each group. The observation group was treated with regular therapy combined with LMWH. The control group was treated with regular therapy. |
| Meng 2013 | Randomised controlled trial. 30 patients with replantation of severed fingers who were selected as the observation group, and another 30 healthy adults were chosen to be the control group. The indexes of blood rheology of the enrolled patients were observed and compared before and after the operation. The participants included digital replantation patients and healthy adults, and the outcome only the blood rheology information (article in Chinese). |
| Shu 2011 | Randomised controlled trial. 49 patients were randomised to receive single‐dose IV UFH (50 IU/kg) (24 participants with 42 digits), or no treatment (25 participants with 35 digits) (article in Chinese). |
| Zhao 2017 | The comparisons did not focus on LMWH (article in Chinese). 60 cases of replantation of amputated fingers were divided randomly into an observation group and control group, with 30 cases in each group. The observation group was treated with SC injection of heparin in the blood‐congested area and received a small incision at the blood‐congested area. The control group were treated with periderm SC injection of heparin and a small incision at the blood‐congested area. |
| Zhou 2017 | The participants were free flap transplantation patients, not digital replantation patients (article in Chinese). 54 cases of free flap transplantation were divided randomly into low calcium group (27 cases) and heparin sodium group (27 cases). |
DVT: deep vein thrombosis IV: intravenous IM: intramuscular LMWH: low molecular weight heparin SC: subcutaneous UFH: unfractionated heparin
Differences between protocol and review
Difference between protocol and review (2013)
Outcomes from replantation of three hand palms and two forearms were included in this review because Chen 2001 pooled these results with the results from digital replantation and could not be extracted. However, since this trial was a RCT, we assumed these five amputations were randomly distributed evenly in the LMWH and UFH groups. We were unable to establish contact with the trial authors to confirm this.
One secondary outcome (coagulability assessed by antithrombin activity, factor Xa activity, bleeding time, clotting time, aPTT or FDP concentration test) that was not prespecified in the protocol was included in the review as it was judged to be clinically important.
Difference between first version (2013) and review update (2020)
We have redefined the first primary outcome from 'the patency rate of microvascular anastomosis' to 'the survival rate of replanted digits judged by the physician clinically, by means of excluding necrotic digits', to more accurately reflect the description in the included studies.
We have redefined the second primary outcome from 'The incidence of compromised microcirculation requiring surgical re‐exploration or re‐anastomosis, or both', to 'Incidence of compromised microcirculation requiring invasive interventions such as re‐exploration, re‐anastomosis, removal of nail, and incision', to be of greater clinical relevance.
We have rephrased the two secondary outcomes. One is 'Direct cause of microvascular insufficiency (arterial occlusion, venous occlusion, or both' to 'Microvascular insufficiency due to vessel occlusion (arterial occlusion, venous occlusion, or both)', and 'Indirect cause of microvascular insufficiency (shock, infection, peripheral vascular pathology, hypercoagulopathy etc.)' to 'Microvascular insufficiency due to other/systemic causes (shock, infection, peripheral vascular pathology, hypercoagulopathy, etc.)', in order to clarify this outcome.
We have included 'Summary of findings' tables. We reclassified 19 previously excluded studies as not relevant as they were not RCTs.
Contributions of authors
PTL: selected trials, assessed trial quality, extracted the data, interpreted the data, conducted statistical analyses, and drafted the final review SHW: selected trials, assessed trial quality, extracted the data, interpreted the data, and commented on the final review CCC: selected trials, assessed trial quality, extracted the data, interpreted the data, conducted statistical analyses, and drafted the final review
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
PTL: none known SHW: none known CCC: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Chen 2001 {published data only}
- Chen P, Zhang GH, Zhang XK. The Livaracine digital replantation. Anhui Medical 2001;22(5):53‐4. [Google Scholar]
Hu 2013 {published data only}
- Hu RM. The application of low molecular weight heparin after digital replantation. The Medical Forum 2013;17:2275. [Google Scholar]
Li 2012a {published data only}
- Li JX, Li CH, Song HJ. The application of low molecular heparin and heparin after digital replantations (author's translation). Chinese Community Doctors 2012;8:205‐6. [Google Scholar]
Xu 2013 {published data only}
- Xu GO, Zhang JH. Efficacy between heparin and low molecular weight heparin after finger replantation. Seek Medical and Ask the Medicine 2013;11(10):327‐8. [Google Scholar]
References to studies excluded from this review
Chen 2015 {published data only}
- Chen SC, Feng ZT. Curative effect observation of Xueshuantong combined with low molecular weight heparin in prevention of thrombosis after replantation of amputated finger. Chinese Journal of Pharmacoepidemiology 2015;3:148‐51. [Google Scholar]
Chen 2016 {published data only}
- Chen BS, Hou J. Efficacy of low molecular weight heparin combined with felodipine in prevention of vascular crisis after finger replantation. Women's Health Research 2016;21:153‐4. [Google Scholar]
Chen 2018 {published data only}
- Chen X, Chen Z, Zhou J, Xu Y. Unilateral digital arterial ligation combined with low molecular weight heparins in severed finger without venous anastomosis. Experimental and Therapeutic Medicine 2018;16:342‐6. [DOI: 10.3892/etm.2018.6174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2015 {published data only}
- Han ZM, Ding YH. Influence of auxiliary application of Xuesaitong injection on blood coagulation indices and thromboembolic complications in patients after finger replantation. China Pharmacist 2015;12:2087‐9. [Google Scholar]
Li 2012b {published data only}
- Li Y, Wei P, Sun T, Li L, Zhou DY, Chen H, et al. Efficacy of low molecular weight heparin combined with felodipine on vascular crisis prevention after finger replantation. Chinese Journal of New Drugs and Clinical Remedies 2012;31(10):607‐10. [Google Scholar]
Li 2016 {published data only}
- Li P, Liu Y, Sun F, Wang T, Su JL, Li Y. Clinical study of huoxue rongshuan decoction on preventing vascular crisis after skin flap transplantation for trauma. Chinese Archives of Traditional Chinese Medicine 2016;33(2):2984‐7. [Google Scholar]
Liu 2015 {published data only}
- Liu Y, Li Y, Yan W, Wang T, Sun F, Su QL, et al. Clinical study of huoxue rongshuan decoction on preventing vascular crisis after skin flap transplantation for trauma. Chinese Archives of Traditional Chinese Medicine 2015;12:2984‐7. [Google Scholar]
Ma 2017 {published data only}
- Ma ZX. Clinical application of low molecular weight heparin. Capital Medicine 2017;6:48‐9. [Google Scholar]
Meng 2013 {published data only}
- Meng X, Yue Q, Yue WJ, Li W, Meng QG. Clinical study on hemorheological effect of low molecular weight heparin calcium on small vascular injury. Progress in Modern Biomedicine 2013;22:4284‐7. [Google Scholar]
Shu 2011 {published data only}
- Shu T, Li SZ, Miao Z. The efficacy and risk of intravenous heparin injection in digital replantation. Journal of Guangxi Medical University 2011;28(5):731‐3. [Google Scholar]
Zhao 2017 {published data only}
- Zhao CY, Wei LP, Wang D, Wan HX. Analysis on application effect of two different application methods of low molecular heparin in rescuing vein crisis after replantation of severed finger. Attend to Practice and Research 2017;5:9‐11. [Google Scholar]
Zhou 2017 {published data only}
- Zhou LZ, Wu YQ, Xiao RY, Huang CH, Lin WM, Teng JM. Observation on the effect of low molecular weight heparin calcium in free flap transplantation. China Medicine and Pharmacy 2017;13:56‐8. [Google Scholar]
Additional references
Askari 2006
- Askari M, Fisher C, Weniger FG, Bidic S, Lee WP. Anticoagulation therapy in microsurgery: a review. Journal of Hand Surgery 2006;31(5):836‐46. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azolov 1983
- Azolov VV, Ladygin MS. Comparative characteristics of the effect of drugs on the microcirculation in reconstructive operations on the hand. Ortopediia Travmatologiia I Protezirovanie 1983;6:47‐8. [PubMed] [Google Scholar]
Barnett 1989
- Barnett GR, Taylor GI, Mutimer KL. The "chemical leech": intra‐replant subcutaneous heparin as an alternative to venous anastomosis. Report of three cases. British Journal of Plastic Surgery 1989;42(5):556‐8. [DOI] [PubMed] [Google Scholar]
Betancourt 1998
- Betancourt FM, Mah ET, McCabe SJ. Timing of critical thrombosis after replantation surgery of the digits. Journal of Reconstructive Microsurgery 1998;14(5):313‐6. [DOI] [PubMed] [Google Scholar]
Buckley 2011
- Buckley T, Hammert WC. Anticoagulation following digital replantation. Journal of Hand Surgery 2011;36(8):1374‐6. [DOI] [PubMed] [Google Scholar]
Chi 2009
- Chi CC, Lee CW, Wojnarowska F, Kirtschig G. Safety of topical corticosteroids in pregnancy. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007346.pub2] [DOI] [PubMed] [Google Scholar]
Chong 1989
- Chong BH, Fawaz I, Chesterman CN, Berndt MC. Heparin‐induced thrombocytopenia: mechanism of interaction of the heparin‐dependent antibody with platelets. British Journal of Haematology 1989;73(2):235‐40. [DOI] [PubMed] [Google Scholar]
Chow 1983
- Chow SP. The histopathology of microvascular anastomosis: a study of the incidence of various tissue changes. Microsurgery 1983;4(1):5‐9. [DOI] [PubMed] [Google Scholar]
Cochrane Adverse Effects Methods Group 2012
- Cochrane Adverse Effects Methods Group. methods.cochrane.org/adverseeffects/ (accessed 19 October 2012).
Conrad 2001
- Conrad MH, Adams WP Jr. Pharmacologic optimization of microsurgery in the new millennium. Plastic and Reconstructive Surgery 2001;108(7):2088‐96. [DOI] [PubMed] [Google Scholar]
Cooley 1985
- Cooley BC, Hansen FC. Microvascular repair following local crush and avulsion vascular injury. Microsurgery 1985;6(1):46‐8. [DOI] [PubMed] [Google Scholar]
Edmondson 1994
- Edmondson RA, Cohen AT, Das SK, Wagner MB, Kakkar VV. Low molecular weight heparin versus aspirin and dipyridamole after femoropopliteal bypass grafting. Lancet 1994;344(8927):914‐8. [DOI] [PubMed] [Google Scholar]
Elcock 1972
- Elcock HW, Fredrickson JM. The effect of heparin on thrombosis at microvenous anastomotic sites. Archives of Otolaryngology 1972;95(1):68‐71. [DOI] [PubMed] [Google Scholar]
Franklin 2003
- Franklin RD, Kutteh WH. Effects of unfractionated and low molecular weight heparin on antiphospholipid antibody binding in vitro. Obstetrics and Gynecology 2003;101(3):455‐62. [DOI] [PubMed] [Google Scholar]
Fukui 1989
- Fukui A, Maeda M, Sempuku T, Tamai S, Mizumoto S, Inada Y. Continuous local intra‐arterial infusion of anticoagulants for digit replantation and treatment of damaged arteries. Journal of Reconstructive Microsurgery 1989;5(2):127‐36. [DOI] [PubMed] [Google Scholar]
Fukui 1994
- Fukui A, Tamai S. Present status of replantation in Japan. Microsurgery 1994;15(12):842‐7. [DOI] [PubMed] [Google Scholar]
Furie 2008
- Furie B, Furie BC. Mechanisms of thrombus formation. The New England Journal of Medicine 2008;359(8):938‐49. [DOI] [PubMed] [Google Scholar]
Furnas 1992
- Furnas HJ, Lineaweaver W, Buncke HJ. Blood loss associated with anticoagulation in patients with replanted digits. Journal of Hand Surgery 1992;17(2):226‐9. [DOI] [PubMed] [Google Scholar]
Gao 2007
- Gao W. Clexane (enoxaparin) for prevention of vascular insufficiency after digital replantation. Medical Journal of Chinese People's Health 2007;19(10):854‐5. [Google Scholar]
Geraghty 2011
- Geraghty AJ, Welch K. Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD000536.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brozek J, Guyatt G, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
Han 2000
- Han SK, Lee BI, Kim WK. Topical and systemic anticoagulation in the treatment of absent or compromised venous outflow in replanted fingertips. Journal of Hand Surgery 2000;25(4):659‐67. [DOI] [PubMed] [Google Scholar]
Hendel 1984
- Hendel PM. Pharmacologic agents in microvascular surgery. In: Buncke HJ, Furnas DW editor(s). Symposium on clinical frontiers in reconstructive microsurgery. St. Louis: CV Mosby, 1984:427. [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook.
Hirsh 2001
- Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R, et al. Heparin and low‐molecular‐weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001;119(1 Suppl):64S‐94S. [DOI] [PubMed] [Google Scholar]
Iglesias 1999
- Iglesias M, Butrón P. Local subcutaneous heparin as treatment for venous insufficiency in replanted digits. Plastic and Reconstructive Surgery 1999;103(6):1719‐24. [DOI] [PubMed] [Google Scholar]
Isaacs 1977
- Isaacs IJ. The vascular complications of digital replantation. Australian and New Zealand Journal of Surgery 1977;47(3):292‐9. [DOI] [PubMed] [Google Scholar]
Jivegard 2005
- Jivegard L, Drott C, Gelin J, Groth O, Hensater M, Jensen N, et al. Effects of three months of low molecular weight heparin (dalteparin) treatment after bypass surgery for lower limb ischemia ‐ a randomised placebo‐controlled double blind multicenter trial. European Journal of Vascular and Endovascular Surgery 2005;29(2):190‐8. [DOI] [PubMed] [Google Scholar]
Ketchum 1978
- Ketchum LD. Pharmacological alterations in the clotting mechanism: use in microvascular surgery. Journal of Hand Surgery 1978;3(5):407‐15. [DOI] [PubMed] [Google Scholar]
Khouri 1998
- Khouri RK, Cooley BC, Kunselman AR, Landis JR, Yeramian P, Ingram D, et al. A prospective study of microvascular free‐flap surgery and outcome. Plastic Reconstructive Surgery 1998;102(3):711‐21. [DOI] [PubMed] [Google Scholar]
Kroll 1995
- Kroll SS, Miller MJ, Reece GP, Baldwin BJ, Robb GL, Bengtson BP, et al. Anticoagulants and hematomas in free flap surgery. Plastic and Reconstructive Surgery 1995;96(3):643‐7. [DOI] [PubMed] [Google Scholar]
Kroll 1996
- Kroll SS, Schusterman MA, Reece GP, Miller MJ, Evans GR, Robb GL, et al. Timing of pedicle thrombosis and flap loss after free‐tissue transfer. Plastic and Reconstructive Surgery 1996;98(7):1230‐3. [DOI] [PubMed] [Google Scholar]
Lee 2016
- Lee JY, Kim HS, Heo ST, Kwon H, Jung SN. Controlled continuous systemic heparinization increases success rate of artery‐only anastomosis replantation in single distal digit amputation: a retrospective cohort study. Medicine 2016;95(26):e3979. [DOI: 10.1097/MD.0000000000003979] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from training.cochrane.org/handbook.
Levin 2008
- Levin LS, Cooper EO. Clinical use of anticoagulants following replantation surgery. Journal of Hand Surgery 2008;33(8):1437‐9. [DOI] [PubMed] [Google Scholar]
Loisel 2010
- Loisel F, Pauchot J, Gasse N, Meresse T, Rochet S, Tropet Y, et al. Addition of antithrombosis in situ in the case of digital replantation: preliminary prospective study of 13 cases. Chirurgie de la Main 2010;29(5):326‐31. [DOI] [PubMed] [Google Scholar]
Loke 2007
- Loke YK, Price D, Herxheimer A, and the Cochrane Adverse Effects Methods Group. Systematic reviews of adverse effects: framework for a structured approach. BMC Medical Research Methodology 2007;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maeda 1991
- Maeda M, Fukui A, Tamai S, Mizumoto S, Inada Y. Continuous local intra‐arterial infusion of antithrombotic agents for replantation (comparison with intravenous infusion). British Journal of Plastic Surgery 1991;44(7):520‐5. [DOI] [PubMed] [Google Scholar]
Morecraft 1985
- Morecraft R, Blair WF, Chang L. Histopathology of micro‐venous repair. Microsurgery 1985;6(4):219‐28. [DOI] [PubMed] [Google Scholar]
Niibayashi 2000
- Niibayashi H, Tamura K, Fujiwara M, Ikeda N. Survival factors in digital replantation: significance of postoperative anaemia. Journal of Hand Surgery 2000;25(5):512‐5. [DOI] [PubMed] [Google Scholar]
Nikolis 2011
- Nikolis A, Tahiri Y, St‐Supery V, Harris PG, Landes G, Lessard L, et al. Intravenous heparin use in digital replantation and revascularization: the Quebec Provincial Replantation program experience. Microsurgery 2011;31(6):421‐7;31(6):421‐7. [DOI] [PubMed] [Google Scholar]
Noguchi 1999
- Noguchi M, Matsusaki H, Yamamoto H. Intravenous bolus infusion of heparin for circulatory insufficiency after finger replantation. Journal of Reconstructive Microsurgery 1999;15(4):245‐53. [DOI] [PubMed] [Google Scholar]
Norgren 2004
- Norgren L, Swedish EnoxaVasc Study Group. Can low molecular weight heparin replace unfractionated heparin during peripheral arterial reconstruction? An open label prospective randomized controlled trial. Journal of Vascular Surgery 2004;39(5):977‐84. [DOI] [PubMed] [Google Scholar]
Oufquir 2006
- Oufquir A, Bakhach J, Panconi B, Guimberteau JC, Baudet J. Salvage of digit replantations by direct arterial antithrombotic infusion [Sauvetage des revascularisations digitales par administration intra‐artérielle de fibrinolytiques]. Annales De Chirurgie Plastique Esthetique 2006;51(6):471‐81. [DOI] [PubMed] [Google Scholar]
Pederson 2008
- Pederson WC. Clinical use of anticoagulants following free tissue transfer surgery. Journal of Hand Surgery 2008;33(8):1435‐6. [DOI] [PubMed] [Google Scholar]
Poole 1977
- Poole MD, Bowen JE. Two unusual bleedings during anticoagulation following digital replantation. British Journal of Plastic Surgery 1977;30(4):267‐8. [DOI] [PubMed] [Google Scholar]
Rapoport 1985
- Rapoport S, Glickman MG, Salomon JC, Cuono CB. Aggressive postoperative pharmacotherapy for vascular compromise of replanted digits. American Journal of Roentgenology, Radium Therapy and Nuclear Medicine 1985;144(5):1065‐6. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rooks 1994
- Rooks MD, Rodriguez JJ, Blechner M, Zusmanis K, Hutton W. Comparative study of intraarterial and intravenous anticoagulants in microvascular anastomoses. Microsurgery 1994;15(2):123‐9. [DOI] [PubMed] [Google Scholar]
Samama 1994
- Samama CM. Low molecular weight heparin (enoxaparin) versus unfractionated heparin during and after arterial reconstructive surgery; a multicenter randomized study. Thrombosis and Haemostasis 1994;69(6):546 (abstract 24). [Google Scholar]
Servant 1976
- Servant JM, Ikuta Y, Harada Y. A scanning electron microscope study of microvascular anastomoses. Plastic and Reconstructive Surgery 1976;57(3):329‐34. [DOI] [PubMed] [Google Scholar]
Stockmans 1997
- Stockmans F, Stassen JM, Vermylen J, Hoylaerts MF, Nystrom A. A technique to investigate microvascular mural thrombus formation in arteries and veins: II. Effects of aspirin, heparin, r‐hirudin and G‐4120. Annals of Plastic Surgery 1997;38(1):63‐8. [DOI] [PubMed] [Google Scholar]
Swedenborg 1996
- Swedenborg J, Nydahl S, Egberg N. Low molecular mass heparin instead of unfractionated heparin during infrainguinal bypass surgery. European Journal of Vascular and Endovascular Surgery 1996;11(1):59‐64. [DOI] [PubMed] [Google Scholar]
Veravuthipakorn 2004
- Veravuthipakorn L, Veravuthipakorn A. Microsurgical free flap and replantation without antithrombotic agents. Journal of the Medical Association of Thailand 2004;87(6):665‐9. [PubMed] [Google Scholar]
Vretos 1995
- Vretos KA, Tsavissis AG. Antithrombotic and antiinflammatory drugs for protection of microvascular anastomosis. Acta Orthopaedica Scandinavica. Supplementum 1995;264:48‐9. [DOI] [PubMed] [Google Scholar]
Warkentin 1995
- Warkentin TE, Levine MN, Hirsh J, Horsewood P, Roberts RS, Gent M, et al. Heparin‐induced thrombocytopenia in patients treated with low‐molecular‐weight heparin or unfractionated heparin. The New England Journal of Medicine 1995;332(20):1330‐5. [DOI] [PubMed] [Google Scholar]
Weitz 1997
- Weitz JI. Low‐molecular‐weight heparins. The New England Journal of Medicine 1997;337(10):688‐98. [DOI] [PubMed] [Google Scholar]
Wolf 1994
- Wolf H. Low‐molecular‐weight heparin. Medical Clinics of North America 1994;78(3):733‐43. [DOI] [PubMed] [Google Scholar]
Yang 2008
- Yang H, Ma QL. Clinical application of fasudil in the treatment of replantation of amputated finger. Qilu Pharmaceutical Affairs 2008;27(9):562‐3. [Google Scholar]
Yu 2012
- Yu XM, LIN RS, Peng HH. Clinical observation of lidocaine, heparin and dexamethasone in preventing vascular insufficiency after digital replantation (author's translation). Modern Practical Medicine 2012;24(4):458‐60. [Google Scholar]
Zhang 2002
- Zhang HY, Li R, Pu XH. Clinical observation of low molecular weight heparin's effect of preventing thrombosis after digital replantation. Chinese Journal of Laboratory Diagnosis 2002;6(4):239‐40. [Google Scholar]
Zhang 2004
- Zhang C, Feng GP, Cheng B, Sun Q. Application of low molecular weight heparin in atypical digital replantation (author's translation). Chinese Journal of Postgraduates of Medicine 2004;5:50‐1. [Google Scholar]
References to other published versions of this review
Chen 2012
- Chen YC, Chan FC, Wen YW. Low molecular weight heparin for prevention of microvascular occlusion in digital replantation. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009894] [DOI] [PubMed] [Google Scholar]
Chen 2013
- Chen YC, Chi CC, Chan FC, Wen YW. Low molecular weight heparin for prevention of microvascular occlusion in digital replantation. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009894.pub2] [DOI] [PubMed] [Google Scholar]


