Abstract
Aramchol, an oral stearoyl-coenzyme-A-desaturase-1 (SCD1) inhibitor, has been shown to reduce hepatic-fat content in patients with primary nonalcoholic-fatty-liver-disease (NAFLD), however, its effect in patients with HIV-associated NAFLD is unknown. The ARRIVE trial was a double-blind, randomized, investigator-initiated, placebo-controlled trial to test the efficacy of 12 weeks of treatment with aramchol versus placebo in HIV-associated NAFLD. Fifty patients with HIV-associated NAFLD, defined by MRI-proton-density-fat-fraction (PDFF) ≥5%, were randomized to receive either aramchol 600 mg daily (n=25) or placebo (n=25) for 12 weeks. The primary endpoint was a change in hepatic-fat as measured by MRI-PDFF in co-localized regions-of-interest. Secondary endpoints included changes in liver-stiffness using MR-elastography (MRE) and vibration-controlled-transient-elastography (VCTE) and exploratory endpoints included changes in total body fat and muscle depots on DXA, whole-body and cardiac MRI. The mean (±SD) of age and BMI were 48.2±10.3 years and 30.7±4.6kg/m2 respectively. There was no difference in the reduction in mean MRI-PDFF between the aramchol group at −1.3% (baseline-MRI-PDFF:15.6% vs end-of-treatment MRI-PDFF:14.4%, p=0.24) versus placebo at −1.4% (baseline-MRI-PDFF:13.3% vs end-of-treatment MRI-PDFF:11.9%, p=0.26), respectively. There was no difference in the relative decline in mean MRI-PDFF between aramchol group and placebo (6.8% versus 1.1%, p=0.68). There were no differences in MRE and VCTE derived liver-stiffness, and whole body (fat and muscle) composition analysis by MRI or DXA. Compared to baseline, end-of-treatment aminotransferases were lower in the aramchol group but not in the placebo arm. There were no significant adverse events.
Conclusion:
Aramchol, over a 12-week period, did not reduce hepatic-fat or change body fat and muscle composition by utilizing novel MRI-based assessment in patients with HIV-associated NAFLD. (clinicaltrials.gov ID:NCT02684591)
Keywords: NASH, steatosis, MRI-PDFF
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is one of the most prevalent etiologies of chronic liver disease worldwide and an increasingly common cause of cirrhosis and hepatocellular carcinoma (1, 2). NAFLD can be broadly classified into primary NAFLD that is typically seen in the presence of metabolic syndrome and secondary NAFLD that may be associated with other causes such as medications inducing steatosis, viral hepatitis, inborn errors of metabolism, or human immunodeficiency virus (HIV) infection.
Among patients with HIV infection, liver disease is one of the leading causes of death (3). NAFLD affects 20–40% of people with HIV(4) and is associated with increased histologic severity compared to HIV negative controls (5). While many aspects of the pathogenesis of HIV-associated NAFLD likely overlap with primary NAFLD, unique factors may also exist in patients with HIV-associated NAFLD. Increased disease severity can be seen at lower BMI and may be related to a greater amount of visceral adipose tissue (6), antiviral therapy (7) direct viral effects and increased permeability of the gut epithelium (8). Despite the ongoing development of therapeutic treatments for NAFLD (9–11) patients with HIV-associated NAFLD are largely excluded from those trials and currently have no approved options available for treatment.
Aramchol, also known as arachidyl amido cholanoic acid, is a fatty acid and a bile acid conjugate (FABAC) that was created by conjugating 2 natural components, cholic acid and arachidic acid, through a stable amide bond. Aramchol inhibits stearoyl-coenzyme A desaturase 1 (SCD1), a key enzyme in fatty acid synthesis. SCD1 is an endoplasmic reticulum enzyme that catalyzes the rate-limiting step in the biosynthesis of monounsaturated fatty acids from saturated fatty acids. Inhibiting SCD1 decreases synthesis and increases beta-oxidation of fatty acids, causing decreased storage of fatty acids. In 2003, Gilat and colleagues showed that (12) aramchol significantly reduced hepatic fat content in animals (rats, hamsters, and mice) with a high-fat diet model. In 2014, Safadi and colleagues (13) demonstrated that aramchol significantly reduced hepatic fat content, measured by magnetic resonance sprectroscopy (MRS), in a randomized trial of 60 Israeli NAFLD patients (without HIV) after 12 weeks of 300mg aramchol orally daily. A Phase I study of aramchol, found a good safety profile with dosing up to 900 mg daily and an international, multicenter trial is currently being conducted in patients with biopsy-proven NASH, however, aramchol has not yet been studied in HIV-associated NAFLD.
Thus, we hypothesized that aramchol 600 mg orally daily would be superior to placebo in improving hepatic fat on MR imaging in patients with HIV-associated NAFLD. Utilizing a double-blind randomized controlled trial of patients with HIV-associated NAFLD, we tested the efficacy of aramchol versus placebo on improvement in MRI proton density fat fraction (MRI-PDFF) over 12 weeks. Secondary aims were to assess the effect of aramchol on serum aminotransferases and liver stiffness and exploratory aims included longitudinal assessment of body composition using advanced MRI and dual energy X-ray absorptiometry (DXA).
MATERIAL AND METHODS
Study Design and Population
This ARRIVE trial was a double-blind, randomized, investigator-initiated, placebo-controlled trial to test the efficacy of 12 weeks of treatment with 600 mg of oral aramchol daily versus placebo in the treatment of HIV-associated NAFLD. The study was designed and conducted according to CONSORT Guidelines and registered at clinicaltrials.gov (registration number NCT02684591) (CONSORT Checklist: Supplemental Materials). Patients were enrolled between March 1, 2016 and January 1, 2018 and the study was conducted at the University of California at San Diego (UCSD) NAFLD Research Center (14–18). The ARRIVE trial patient population was derived from primary care clinics, subspecialty clinics including HIV-treatment and liver disease clinics and through institutional review board approved advertisements. The protocol was Health Insurance Portability and Accountability Act (HIPAA) compliant and was approved by the UCSD Institutional Review Board. Informed written consent was obtained from each participant before study enrollment.
Inclusion and Exclusion Criteria
Inclusion Criteria: All patients were aged 18 years or older with a history of stable HIV defined by an unchanged antiretroviral therapy (ART) regimen for at least 12 weeks prior to study screening and had the presence of NAFLD defined by ≥ 5% steatosis by MRI-PDFF. Patients also had to have at least one or more of the following risk factors for more severe fatty liver disease; hypertriglyceridemia (>150 mg/dL), dyslipidemia (low density lipoprotein (LDL) > 160 mg/dL or high density lipoprotein (HDL) < 40 mg/dL), serum ALT above the upper limit of normal (>19 U/L for women and >30 U/L for men), BMI >25 kg/m2, hyperuricemia, prediabetes or diabetes defined by American Diabetes Association criteria.
Subjects were excluded if they had evidence of other forms of liver disease including the presence of serum hepatitis B surface antigen, hepatitis C viral RNA, positive autoimmune serologies with biopsy consistent with autoimmune hepatitis, cholestatic liver disease, hemochromatosis by 3+ or 4+ stainable iron on biopsy and homozygosity/heterozygosity on genetic analysis, low ceruloplasmin levels with biopsy suggestive of Wilson’s disease, or low alpha‐1‐antitrypsin levels with biopsy suggestive of alpha‐1‐antitrypsin disease. Further exclusion criteria included alcohol intake of more than 30 g/day in the previous 10 years or greater than 10 g/day in the previous year, evidence of cirrhosis based on clinical assessment or imaging, active illicit drug use, pregnancy, evidence of hepatocellular carcinoma, ingestion of drugs known to cause hepatic steatosis, ingestion of drugs known to improve NASH such as vitamin E or pioglitazone, or inability to undergo MRI. Patients on medications with known drug-drug interactions with aramchol or hypersensitivity to aramchol or associated medications were excluded. Patients with significant other systemic illness or poorly controlled diabetes defined by a hemoglobin A1c > 9% were excluded.
Baseline Assessments
Patients were screened in the UCSD NAFLD research center clinic with history, physical examination, review of outside medical records (including HIV status) and routine blood tests. Alcohol history was assessed with the AUDIT and Skinner lifetime alcohol consumption inventories. All patients were asked to stop any medications being used for their liver disease, including herbal medications. Only those meeting all inclusion criteria and avoiding all exclusion criteria were invited to participate in the study. Those who met all eligibility criteria and had no exclusion criteria underwent more thorough evaluation with laboratory testing including homeostatic model assessment for insulin resistance (HOMA-IR), adipose insulin resistance (Adipo-IR), liver MRI, cardiac MRI, vibration controlled transient elastography (VCTE) with controlled attenuation parameter (CAP), DXA for full body fat assessment, a commercial MRI protocol for body composition analysis (19) and an oral glucose tolerance test (OGTT). CD4 cell count and HIV viral loads were measured at baseline and lipodystrophy was assessed by physical examination for facial, temporal, upper or lower extremity lipoatrophy and visceral or dorsocervical fat accumulation.
Definition of HIV Associated NAFLD
Subjects were defined to have HIV-associated NAFLD based on MRI-PDFF > 5% after exclusion of other causes of liver disease, excessive alcohol use or use of medications that cause hepatic steatosis (see exclusion criteria above for details).
Randomization and Allocation Concealment
Subjects were randomized to either an aramchol or a placebo group in blocks of four in a 1:1 ratio by the investigational drug services at the University of California at San Diego using computer‐generated numbers. Patients were randomized to receive 600 mg daily of aramchol (including 200 mg tablet and 400 mg tablet) versus identical placebo orally for a total of 12 weeks. Medication diaries and a count of residual tablets monitored patient compliance at scheduled visits. Independent investigational drug services pharmacists dispensed either active or placebo treatment pills, which were identical in appearance. Pills were prepackaged in identical bottles, labeled according to the computer‐generated randomization numbers, and delivered to the research clinic. The allocation sequence was concealed from the research coordinators and all investigators including hepatologists and radiologists. Radiology investigators were blinded to clinical data. Treatment allocation was unblinded only after the completion of all study procedures in the entire study including all post-treatment MRI studies on all patients. Data analysis were performed by an experienced statistician using a pre-specified analysis plan.
Study Visits
Patients returned to the research clinic at weeks 4, 8, and 12 after randomization. At these clinic visits, routine blood tests were obtained, body weight and vital signs were recorded, and the number of pills was counted to document compliance. A physical exam and careful history of liver‐related symptoms as well as possible side effects of aramchol were also obtained at each visit. At the completion of 12 weeks of therapy, patients underwent MRI-PDFF, MRE, cardiac MRI, VCTE with CAP, OGTT, DXA and MRI for body compositional analysis and repeat blood work.
Primary and Secondary Endpoints
The primary endpoint was a change in liver fat as measured by MRI‐PDFF in co-localized regions of interest (ROI) within each of the nine liver segments as performed previously.(20–22) Secondary endpoints included change in aminotransferases and liver stiffness and exploratory endpoints included body compositional analysis.
Advanced Liver MRI Phenotyping
MRI was performed at the UCSD MR3T Research Laboratory using the 3T research scanner (GE Signa EXCITE HDxt; GE Healthcare, Waukesha, WI) with all participants in the supine position. MRI-PDFF was used to measure hepatic steatosis and MRE was used to measure hepatic stiffness. The details of the MRI protocol have been previously described.(15, 23)
MRI-PDFF for Fat Quantification:
MRI-PDFF utilizes a low-flip angle, gradient echo sequence to acquire multiple echoes in which fat and water protons are in and out of phase. A custom algorithm created by the Liver Imaging Group at UCSD was used to create MR imaging-PDFF maps. MRI-PDFF measurements are reliable and independent of field strength, scanner manufacturer, and patient characteristics including BMI, age and sex.(23–25) Co-localized ROIs were used to asses changes in liver fat over time with one co-localized ROI placed in each of nine liver segments, which allows for increased efficiency and higher precision and accuracy in assessing changes over time.(14, 20, 21)
MR Elastography:
MRE is the most accurate biomarker for the quantitative assessment of liver stiffness as a surrogate for hepatic fibrosis.(18, 26, 27) A passive driver was fitted around the body over the liver and connected to an acoustic active driver that delivered continuous vibrations at 60 Hz to produce shear waves in the liver, which were processed to generate elastograms depicting liver stiffness. Four slices were assessed, and co-localized ROIs were manually specified.
Ultrasound-Based Assessment
Transient elastography was performed by an experienced operator using the FibroScan 502 Touch model (M Probe, XL Probe; Echosens, Paris, France) as previously described.(28) The liver stiffness measurement was obtained after at least a three hour fast and included a minimum of 10 measurements. All patients were initially scanned with the M probe and when indicated by the initial assessment rescanned with the XL probe. An unreliable liver stiffness measurement was defined as a ratio of successful to total acquisitions of < 60% and/or < 10 valid measurements and/or IQR/median > 30%.(29) The CAP value was measured simultaneously and quantifies the attenuation of ultrasound waves in dB/m to provide a measure that correlates with liver steatosis.(30)
Body Composition Analysis with MRI and DXA
A commercially available sequence to estimate abdominal adipose tissue and thigh muscle volume was implemented and obtained in approximately six minutes by imaging the base of the skull to the knees using MRI without contrast was performed (AMRA Medical AB, Linkoping, Sweden). This accurate and repeatable method(19, 31) measured visceral adipose tissue (VAT), abdominal subcutaneous adipose tissue (ASAT), and four thigh muscle volumes. Multiple indexes(32) were calculated as follows: visceral fat ratio = VAT / (VAT + ASAT) * 100, muscle ratio = weight/total thigh volume; fat storage = (VAT + ASAT)/height2; visceral fat index = VAT/height2; fat ratio = (VAT + ASAT) / (VAT + ASAT + total thigh volume) * 100. In addition, full body DXA scan was performed at baseline and week 12. Finally, epicardial fat volume was calculated on cardiac MRI as the region of high signal intensity external to the myoepicardium to the pericardium.
Rationale for Total Body MRI
Pharmacologic agents in NAFLD targeting lipid metabolism can have off-target effects, and assessment of body composition including visceral adiposity is of particular importance in patients with HIV who are at risk for lipodystrophy. Frequently, therapeutic trials in NAFLD incorporate advanced liver imaging with MRI.(10, 33) Body composition analysis with MRI allows for accurate and reproducible assessment of extrahepatic muscle and fat in three dimensions and without ionizing radiation.
Sample Size Assessment and Statistical analysis
Our primary endpoint was the absolute difference in change in MRI-PDFF between placebo and aramchol arms. Based upon previous studies we expected a 5% greater improvement in MRI-PDFF in the aramchol group compared to the placebo arm. This requires a sample size of 22 patients in each arm to have a power 90% (or higher) with an α of 0.05. These estimates were also based upon our recent trial using MRI-PDFF as an accurate and reproducible marker of hepatic steatosis.(34) Therefore, we planned to randomize 25 patients to each arm to have adequate power to detect a difference in placebo and experimental groups accounting for a 10% drop rate, consistent with prior trials using MRI based endpoints.(14, 20). The chi-squared test was used for comparisons between categorical variables and a paired t test was used to compare mean differences between continuous variables in the aramchol versus placebo groups. The primary endpoint of this study was improvement in hepatic steatosis by liver MRI and the difference in improvement between aramchol and placebo was tested using a two-sample t-test.
RESULTS
Between March 1, 2016 and January 1, 2018, 50 patients with HIV-associated NAFLD were randomized to either aramchol or placebo. Of the 25 patients into each arm, 24 patients in the treatment arm and 22 patients in the placebo arm completed 12 weeks of intervention with pre and post treatment MRI-PDFF (CONSORT Diagram: Supplemental Figure 1). The study population included 92% men and was predominantly non-white (66%) with the majority of patients of Hispanic ethnicity (66%). Baseline characteristics of the two groups are shown in Table 1.
Table 1.
Baseline Demographic, Biochemical, and Histologic Characteristics of Subjects
| N | Aramchol (n=25) | Placebo (n=25) | P-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 50 | 46.6 ± 11.4 | 49.7 ± 9.0 | 0.3002 |
| Male patients | 50 | 22 (88%) | 24 (96%) | 0.6092 |
| White (vs. non-White) | 50 | 11 (44%) | 6 (24%) | 0.1355 |
| Hispanic (vs. non) | 50 | 14 (56%) | 19 (76%) | 0.1355 |
| Clinical | ||||
| Weight (kg) | 50 | 94.7 ± 16.8 | 88.9 ± 16.6 | 0.2196 |
| Height (m) | 49 | 172.4 ± 8.5 | 171.4 ± 7.0 | 0.6601 |
| BMI (kg/m2) | 49 | 31.4 ± 5.0 | 30.1 ± 4.2 | 0.3086 |
| BMI ≥ 30 | 49 | 14 (58.3%) | 9 (36.0 %) | 0.1174 |
| Diabetes | 50 | 2 (8%) | 8 (32%) | 0.0339 |
| Biochemical profile | ||||
| ALT (IU/L) | 50 | 58.0 (55.0) | 43.0 (26.0) | 0.0080 |
| AST (IU/L) | 50 | 42.0 (29.0) | 29.0 (23.0) | 0.0624 |
| AST:ALT | 50 | 0.6 (0.2) | 0.7 (0.2) | 0.0397 |
| Alk Phos (U/L) | 50 | 77.0 (32.0) | 85.0 (40.0) | 0.9536 |
| Total bilirubin (mg/dL) | 50 | 0.6 (0.3) | 0.5 (0.3) | 0.3222 |
| Direct bilirubin (mg/dL) | 47 | 0.2 (0.0) | 0.2 (0.0) | 0.2811 |
| Albumin (g/dL) | 49 | 4.5 (0.4) | 4.5 (0.3) | 0.4240 |
| Protime (s) | 50 | 11.3 (0.9) | 11.3 (0.9) | 0.6903 |
| Triglycerides (mg/dL) | 50 | 166.0 (67.0) | 184.0 (124.0) | 0.3986 |
| Total cholesterol (mg/dL) | 50 | 177.0 (58.0) | 171.0 (36.0) | 0.9690 |
| LDL (mg/dL) | 48 | 109.0 (46.0) | 95.0 (31.0) | 0.4090 |
| FFA (mmol/L) | 49 | 0.5 (0.3) | 0.7 (0.3) | 0.3318 |
| Glucose (mg/dL) | 50 | 98.0 (17.0) | 108.0 (33.0) | 0.2401 |
| Insulin (μU/mL) | 50 | 30.0 (17.0) | 23.0 (25.0) | 0.9831 |
| HOMA-IR | 50 | 7.2 (4.0) | 6.3 (7.3) | 0.7859 |
| Hgb A1C (%) | 48 | 5.5 (0.6) | 5.6 (0.7) | 0.5278 |
| HIV-related | ||||
| CD4 count (cells/mm3) | 48 | 696.0 (351.0) | 680.0 (351.0) | 0.9671 |
| Viral load (copies/ml) | 46 | 0 (0) | 0 (0) | 0.4018 |
| Time since HIV diagnosis (years) | 46 | 14.5 (14) | 18.0 (17) | 0.4343 |
| Imaging | ||||
| MRI PDFF | 50 | 16.2 (10.1) | 12.1 (10.3) | 0.5094 |
| MRE (kPa) | 46 | 2.5 (0.8) | 2.3 (0.7) | 0.4221 |
| VCTE LSM (kPa) | ||||
| Median | 49 | 6.1 (3.0) | 5.5 (3.5) | 0.4831 |
| IQR | 49 | 1.2 (0.7) | 0.7 (1.0) | 0.2211 |
| IQR/M | 49 | 15.0 (12.0) | 11.0 (8.0) | 0.1961 |
| Success rate <60%, n(%) | 48 | 2 (8%) | 4 (17.4%) | 0.9232 |
| Unreliable liver stiffness, n(%) | 50 | 0 | 1(4%) | 1.0000 |
| Controlled attenuation parameter (dB/m) | ||||
| Median | 48 | 334.0 (42.0) | 319.0 (105.0) | 0.6423 |
| IQR | 48 | 33.0 (21.0) | 30.0 (26.0) | 0.7883 |
| Probe size, n(%) | 0.5712 | |||
| Medium | 23 | 11 (44%) | 12 (52.2%) | |
| XL | 25 | 14 (56%) | 11 (47.8%) | |
| Adipose Indices | ||||
| Total Body % Fat | 43 | 31.7 (9.5) | 31.8 (6.8) | 0.4952 |
| Fat Mass/Height2 (kg/m2) | 43 | 9.8 (5.6) | 9.0 (2.7) | 0.1885 |
| Android/Gynoid Ratio | 43 | 1.4 (0.3) | 1.4 (0.5) | 0.2278 |
| % Fat Trunk/% Fat Legs | 43 | 1.3 (0.3) | 1.5 (0.9) | 0.0659 |
| Trunk/Limb Fat Mass Ratio | 43 | 1.6 (0.5) | 1.9 (0.7) | 0.0445 |
| Est. VAT Mass (g) | 43 | 956 (418) | 1051 (299) | 0.6262 |
| Est. VAT Volume (cm3) | 43 | 1033 (452) | 1137 (323) | 0.6262 |
| Est. VAT Area (cm2) | 43 | 198 (87) | 218 (62) | 0.6348 |
| Lean Indices | ||||
| Lean/Height2 (kg/m2) | 43 | 19.7 (3.8) | 19.4 (2.4) | 0.3805 |
| Appen. Lean/Height2 (kg/m2) | 43 | 8.7 (2.1) | 8.1 (1.7) | 0.3122 |
| Metabolic Factors, n(%) | ||||
| Waist >102cm men, > 88cm women | 49 | 15 (62%) | 15 (60%) | 0.8575 |
| Triglycerides ≥ 150 mg/dL | 50 | 14 (56%) | 16 (64%) | 0.5637 |
| HDL < 40mg/dL men, < 50 mg/dl women | 48 | 14 (58%) | 8 (33%) | 0.0822 |
| SBP ≥ 130 and/or DBP ≥ 85 | 50 | 10 (40%) | 12 (48%) | 0.5688 |
| FPG ≥ 100 mg/dL | 50 | 10 (40%) | 16 (64%) | 0.0894 |
| Metabolic Syndrome | 50 | 12 (48%) | 11 (44%) | 0.7766 |
| MRI Body Composition Indices | ||||
| Pericardial Volume (mm3) | 32 | 174700 (117770) | 134100 (63059) | 0.2582 |
| VAT (l) | 44 | 6.3 (2.5) | 6.6 (2.7) | 0.8142 |
| ASAT (l) | 43 | 7.9 (4.6) | 6.8 (5.2) | 0.4963 |
| VAT + ASAT (l) | 43 | 14.3 (4.3) | 14.6 (5.1) | 0.7246 |
| Visceral fat ratio (%) | 43 | 43.4 (15.6) | 46.4 (19.3) | 0.5040 |
| Thigh volume (l) | 40 | 12.8 (3.2) | 11.6 (2.6) | 0.0216 |
| Muscle ratio (kg/l) | 40 | 7.3 (1.0) | 7.5 (2.1) | 0.3918 |
| Fat storage (l/m2) | 42 | 5.0 (1.5) | 4.9 (1.5) | 0.9699 |
| Visceral Fat Index (l/m2) | 43 | 2.1 (0.8) | 2.3 (0.7) | 0.5031 |
| Fat ratio (%) | 39 | 53.3 (8.8) | 53.6 (11.2) | 0.7890 |
BMI, Body Mass Index; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; Hgb A1C, hemoglobin A1C; LDL, Low-Density Lipoprotein; HDL, High-Density Lipoprotein; FFA, Free Fatty Acids; CRP, C-Reactive Protein; ALK Phos, Alkaline Phosphatase; GGT, Gamma-Glutamyl Transferase; HOMA, homeostatic model assessment; LSM, Liver Stiffness Measurement; VAT, Visceral Adipose Tissue; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; ASAT, abdominal subcutaneous adipose tissue.
Muscle ratio = weight/total thigh volume; Fat storage = (VAT + ASAT)/height2; Visceral Fat Index = VAT/height2; Fat ratio = (VAT + ASAT) / (VAT + ASAT + total thigh volume) * 100
Data presented as mean ± sd, median(iqr) or n(%) as appropriate.
T-test performed on continuous variables presented as mean ± sd, Wilcoxon-Mann-Whitney performed on all other continuous/ordinal variables. Chi-square or Fisher’s exact test as appropriate on all categorical variables.
P-values in bold denote statistical significance < 0.05.
Primary Endpoint: Effect of Aramchol on Liver Fat as Assessed by MRI-PDFF
Aramchol was not significantly better than placebo at reducing liver fat content as measured by mean ± standard deviation (SD) MRI-PDFF change in aramchol and placebo arms −1.3% ± 5.9 and −1.4% ± 5.1, p=0.93 (Table 2) (Supplemental Figure 2). The mean percent change in MRI-PDFF did not differ in the aramchol and placebo groups (Figure 1). Similarly, in sub-analysis restricted to patients with MRI-PDFF ≥ 8% at baseline (N=38) aramchol was not significantly better than placebo at reducing liver fat content measured by mean ± SD MRI-PDFF change (−1.66% ± 5.5 and −3.01% ± 5.3, p=0.46). Advanced MRI imaging of representative patients are shown in Figures 2 and 3.
Table 2.
Aramchol Versus Placebo: Longitudinal Full Liver Fat Mapping Using Magnetic Resonance Imaging Proton Density Fat-Fraction (MRI PDFF)
| Aramchol (n=24) | Placebo (n=22) | Difference | |||||
|---|---|---|---|---|---|---|---|
| Liver Segments | Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | P-Value |
| 1 | 15.4 (6.9) | 14.3 (7.9) | 0.2989 | 13.3 (5.8) | 11.6 (5.0) | 0.1890 | 0.7589 |
| 2 | 14.1 (6.3) | 13.3 (7.3) | 0.4123 | 11.9 (6.2) | 10.2 (5.0) | 0.1779 | 0.5409 |
| 3 | 14.8 (6.5) | 13.7 (7.7) | 0.3371 | 13.2 (6.6) | 11.7 (6.1) | 0.2744 | 0.8000 |
| 4a | 15.7 (6.8) | 14.3 (7.6) | 0.1993 | 13.0 (6.1) | 11.5 (5.0) | 0.2361 | 0.9609 |
| 4b | 15.8 (6.9) | 14.3 (7.8) | 0.2118 | 13.3 (6.4) | 11.9 (5.5) | 0.3042 | 0.9486 |
| 5 | 15.6 (7.1) | 14.6 (8.3) | 0.3967 | 13.0 (5.5) | 11.9 (6.1) | 0.4579 | 0.9407 |
| 6 | 15.5 (7.1) | 14.3 (8.0) | 0.2709 | 13.5 (6.5) | 11.3 (6.3) | 0.1236 | 0.6302 |
| 7 | 16.5 (7.8) | 15.2 (8.5) | 0.2360 | 14.3 (6.3) | 13.1 (6.6) | 0.4051 | 0.9281 |
| 8 | 16.6 (7.7) | 15.6 (8.5) | 0.3820 | 14.4 (6.2) | 13.3 (6.9) | 0.4348 | 0.9756 |
| MRI PDFF average | 15.6 (6.9) | 14.4 (7.8) | 0.2400 | 13.3 (5.9) | 11.9 (5.6) | 0.2648 | 0.9308 |
| MRE average (kPa) | 2.6 (0.6) | 2.5 (0.5) | 0.4777 | 2.4 (0.5) | 2.5 (0.6) | 0.7151 | 0.4459 |
| VCTE (kPa) | |||||||
| Median | 6.7 (2.3) | 7.4 (3.3) | 0.2582 | 6.3 (2.8) | 5.2 (1.4) | 0.0321 | 0.0307 |
| IQR | 1.0 (0.5) | 1.3 (0.8) | 0.1319 | 0.9 (0.6) | 0.8 (0.5) | 0.2175 | 0.0542 |
| IQR/M | 15.6 (8.4) | 16.3 (6.2) | 0.7102 | 14.4 (6.8) | 13.6 (6.9) | 0.7256 | 0.6068 |
| Controlled attenuation parameter (dB/m) | |||||||
| Median | 330.3 (41.2) | 313.5 (59.4) | 0.1912 | 316.4 (70.9) | 312.5 (54.8) | 0.8193 | 0.5287 |
| IQR | 32.8 (14.9) | 35.0 (20.5) | 0.6577 | 33.3 (18.8) | 35.9 (16.9) | 0.6823 | 0.9615 |
Data are expressed as means (sd) or mean difference with p-values in parentheses. Associated p-values are from t test. P-values in bold denote statistical significance < 0.05.
MRI-PDFF, Magnetic Resonance Imaging Proton Density Fat Fraction;
MRI PDFFs measured in all nine liver segments are used to calculate segmental and overall fat fraction averages at baseline and posttreatment between Drug and placebo group.
Only reliable VCTE values used
Figure 1:
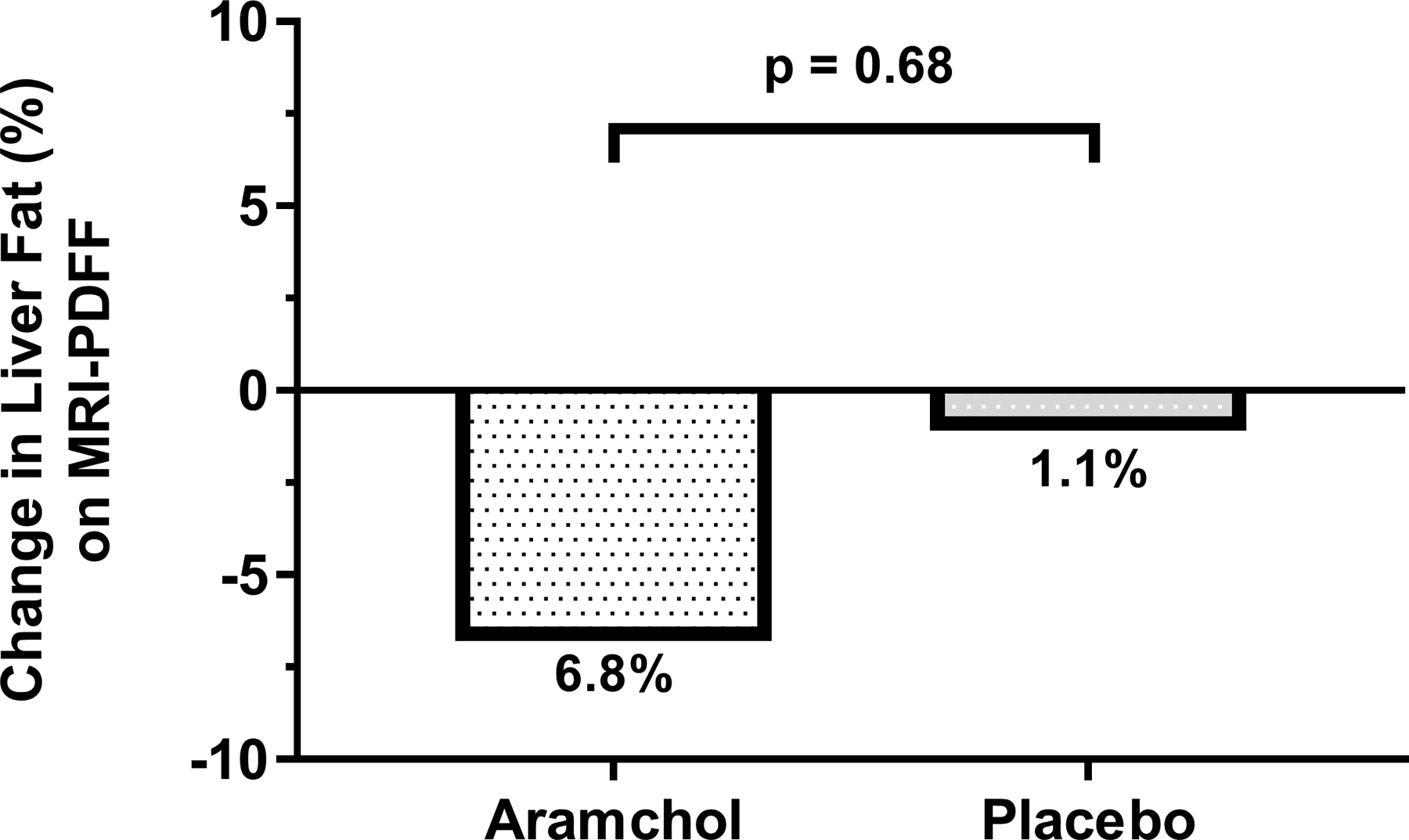
Percent mean change in liver fat relative to baseline as assessed by MRI-PDFF by treatment group. The difference between the aramchol group and placebo group was not statistically significant (p=0.68)
Figure 2:
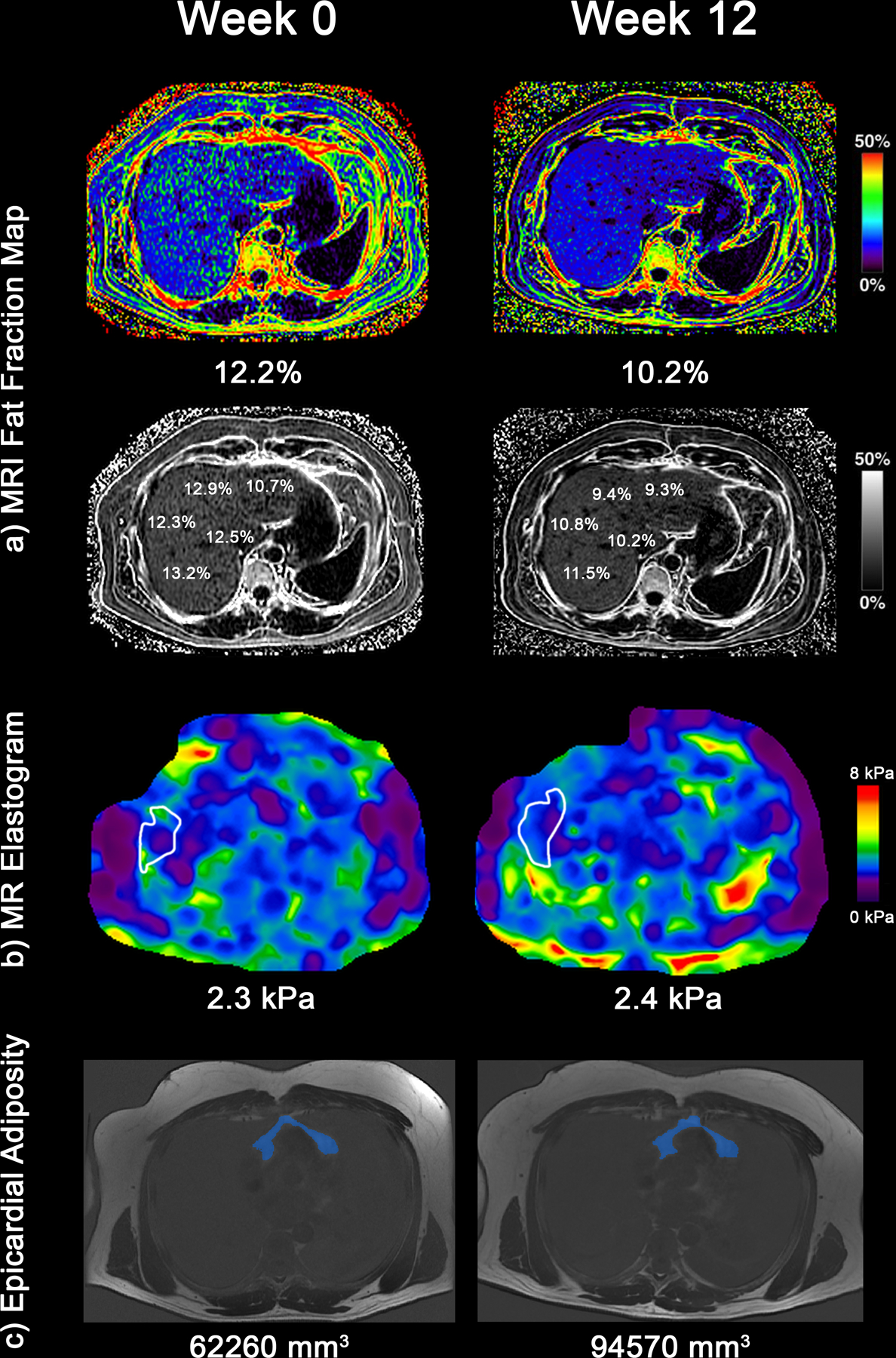
In a representative patient (a) MRI-PDFF fat mapping of the liver. The patient’s average liver fat fraction decreased from 12.2% (Week 0) to 10.2% (Week 12) (b) MRE elastograms depicting liver stiffness throughout the entire liver with average liver stiffness increasing from 2.3 kPa to 2.4 kPa (c) Epicardial fat volume on MRI increased from 62,260 mm3 at week 0 to 94,570 mm3 at week 12.
Figure 3:
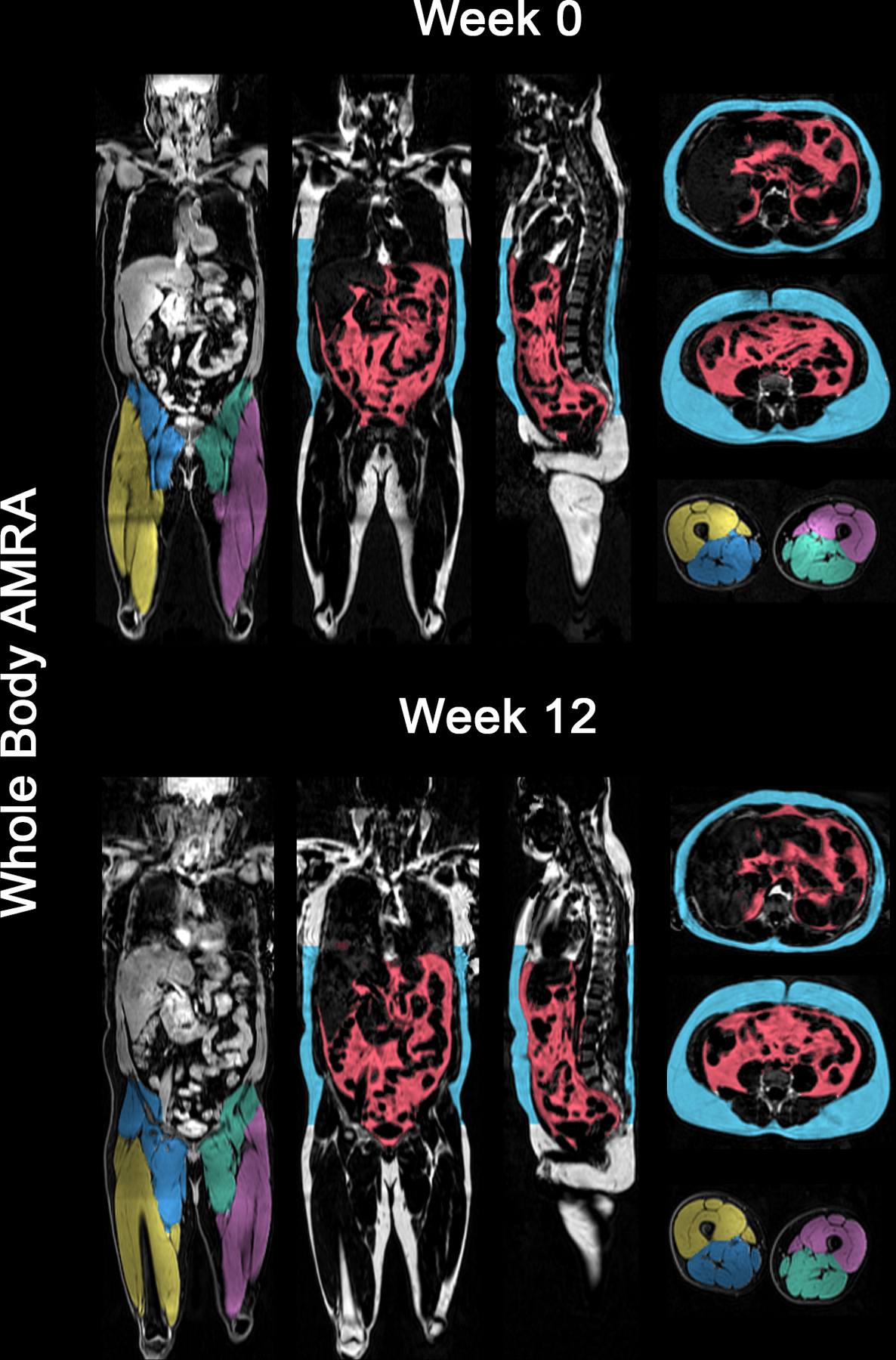
Advanced whole-body composition analysis with MRI in a representative patient. Changes from Week 0 to Week 12 in visceral adipose tissue, abdominal subcutaneous adipose tissue, were 4.38 to 4.78 L and 7.67 to 7.88 L respectively. Week 0 to Week 12 thigh muscle volume changes for left posterior, right posterior, left anterior and right anterior were 3.84 to 3.89 L, 3.95 to 3.98 L, 2.23 to 2.32 L and 2.45 to 2.56 L respectively.
Effect of Aramchol on Liver Stiffness by MRE and VCTE
The mean ± SD liver stiffness by MRE in the aramchol and placebo groups at baseline were similar 2.6 ± 0.6 kPa and 2.4 ± 0.6 kPa respectively. At the end of study the liver stiffness by MRE in the aramchol and placebo groups 2.5 ± 0.5 kPa and 2.5 ± 0.6 kPa respectively, and aramchol was not associated with an improvement in liver stiffness by MRE compared to placebo, p=0.45 (Table 2).
Liver stiffness was also assessed by VCTE and was similar between the aramchol and placebo groups at baseline 6.7 ± 2.3 kPa and 6.3 ± 5.2 kPa respectively. Change in liver stiffness measurement by VCTE was not significantly different between the aramchol and placebo groups (Table 2).
Effect of Aramchol on Anthropometric and Biochemical Measurements
There were no significant differences in change in BMI, aminotransferases, HOMA-IR or CD4 count between the aramchol and placebo groups. Median total cholesterol increased in the aramchol group (18 mg/dL) compared to a decrease in placebo (−2.0 mg/dL), which was statistically significant, p=0.02. Within the aramchol group, ALT, AST and AST:ALT ratio improved at end of treatment compared to baseline. Within the placebo group, fasting free fatty acids (FFA) and glucose improved from baseline to end of treatment (Table 3).
Table 3.
Changes in Anthropometric and Biochemical Variable Between the Aramchol- Versus Placebo-Treated Patients
| Aramchol (n=22) | Placebo (n=21) | Difference | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | P-Value | |
| Weight (kg) | 89.3 (11.9) | 90.0 (8.2) | 0.6486 | 87.3 (18.8) | 85.4 (20.5) | 0.4683 | 0.3685 |
| BMI (kg/m2) | 30.4 (4.3) | 30.1 (4.3) | 0.2919 | 29.1 (3.1) | 29.3 (4.0) | 0.0831 | 0.0595 |
| ALT (IU/L) | 58.0 (53.0) | 51.0 (37.0) | 0.0002 | 43.0 (26.0) | 36.0 (44.0) | 0.1211 | 0.1051 |
| AST (IU/L) | 40.5 (29.0) | 37.0 (15.0) | 0.0189 | 28.5 (25.0) | 25.5 (16.0) | 0.1572 | 0.5180 |
| AST:ALT | 0.6 (0.2) | 0.7 (0.3) | 0.0002 | 0.7 (0.2) | 0.7 (0.3) | 0.4392 | 0.1625 |
| Alk Phos (U/L) | 77.0 (31.0) | 80.5 (32.0) | 0.5437 | 79.5 (42.0) | 76.5 (41.0) | 0.1478 | 0.2003 |
| Total bilirubin (mg/dL) | 0.6 (0.3) | 0.5 (0.3) | 0.4484 | 0.5 (0.3) | 0.4 (0.4) | 0.1172 | 0.5262 |
| Direct bilirubin (mg/dL) | 0.2 (0.0) | 0.2 (0.0) | 1.0000 | 0.2 (0.0) | 0.2 (0.0) | 0.5000 | 0.3101 |
| Albumin (g/dL) | 4.5 (0.4) | 4.5 (0.2) | 0.2366 | 4.5 (0.2) | 4.5 (0.3) | 0.1928 | 0.0830 |
| Protime (s) | 11.2 (0.7) | 11.3 (0.9) | 1.0000 | 11.4 (0.9) | 11.2 (1.1) | 0.8138 | 0.8785 |
| Triglycerides (mg/dL) | 166.0 (79.0) | 180.0 (184.0) | 0.0797 | 187.0 (165.0) | 159.0 (147.0) | 0.2764 | 0.0907 |
| Total cholesterol (mg/dL) | 177.0 (58.0) | 187.0 (181.0) | 0.0029 | 179.0 (54.0) | 169.0 (37.0) | 0.7683 | 0.0176 |
| LDL (mg/dL) | 110.0 (45.0) | 113.0 (42.0) | 0.0450 | 96.0 (27.0) | 102.0 (37.5) | 0.8910 | 0.2352 |
| FFA (mmol/L) | 0.6 (0.2) | 0.5 (0.3) | 0.4894 | 0.7 (0.2) | 0.5 (0.2) | 0.0006 | 0.0559 |
| Glucose (mg/dL) | 98.0 (17.0) | 101.0 (19.0) | 0.8017 | 107.5 (36.0) | 98.5 (24.0) | 0.0357 | 0.2218 |
| Insulin (μU/mL) | 29.5 (17.0) | 25.5 (19.0) | 0.9919 | 24.5 (25.0) | 23.0 (19.0) | 0.2798 | 0.2448 |
| HOMA-IR | 7.2 (4.6) | 6.3 (4.7) | 0.7756 | 6.2 (8.2) | 5.6 (5.9) | 0.1756 | 0.2177 |
| Hgb A1C (%) | 5.4 (0.4) | 5.4 (0.4) | 0.7646 | 5.6 (0.7) | 5.6 (0.7) | 0.2188 | 0.1266 |
| CD4 count (cells/mm3) | 644.0 (354.0) | 640.0 (469.0) | 0.5412 | 680.0 (383.0) | 633.0 (371.0) | 0.7500 | 0.4813 |
Data are expressed as median (IQR) with p-values from Wilcoxon signed rank test or mean difference with P-value in parentheses. P-values in bold denote statistical significance < 0.05.
BMI, Body Mass Index; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; Hgb A1c, hemoglobin A1c; LDL, Low-Density Lipoprotein; HDL, High-Density Lipoprotein; FFA, Free Fatty Acids; Alk Phos, Alkaline Phosphatase; GGT, Gamma-Glutamyl Transferase; HOMA, homeostatic model assessment; CRP, C-Reactive Protein.
Change in Body Composition by DXA and Novel Whole-Body MRI Measures Including Visceral Adipose and Epicardial Fat Volume
DXA assessment of total % body fat were similar at baseline and at the end of study. The mean ± SD trunk/limb fat mass ratio was higher in the placebo group than the aramchol group at baseline 1.9 ±0.7 and 1.6 ± 0.5 respectively, p=0.04 (Table 1). Other measures of the distribution of fat including Android/Gynoid ratio, % Fat Trunk/% Fat legs, did not differ between groups at baseline and the differences in change over the study period between aramchol and placebo were not significant (Table 4a).
Table 4a.
Changes in Adipose and Lean Indices Between the Aramchol- Versus Placebo-Treated Patients on DXA
| Aramchol (n=20) | Placebo (n=15) | Difference | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | P-Value | |
| Adipose Indices | |||||||
| Total Body % Fat | 31.4 (9.2) | 31.8 (9.4) | 0.4121 | 31.8 (7.0) | 32.4 (7.7) | 0.3029 | 0.5687 |
| Fat Mass/Height2 (kg/m2) | 9.8 (4.9) | 10.1 (4.5) | 0.3577 | 8.4 (3.4) | 9.3 (2.7) | 0.6097 | 0.9751 |
| Android/Gynoid Ratio | 1.4 (0.3) | 1.3 (0.2) | 0.2794 | 1.4 (0.7) | 1.4 (0.8) | 0.7609 | 0.1349 |
| % Fat Trunk/% Fat Legs | 1.3 (0.3) | 1.3 (0.2) | 0.4918 | 1.6 (0.9) | 1.4 (0.7) | 0.0007 | 0.1948 |
| Trunk/Limb Fat Mass Ratio | 1.6 (0.4) | 1.6 (0.4) | 0.5706 | 2.0 (0.8) | 1.8 (0.8) | 0.0317 | 0.7873 |
| Est. VAT Mass (g) | 957 (501) | 982 (537) | 0.8983 | 971 (408) | 996 (543) | 0.1070 | 0.2619 |
| Est. VAT Volume (cm3) | 1034 (541) | 1061 (583) | 0.8983 | 1050 (441) | 1076 (588) | 0.1070 | 0.2637 |
| Est. VAT Area (cm2) | 198 (104) | 204 (112) | 0.8909 | 201 (85) | 207 (112) | 0.1037 | 0.2626 |
| Lean Indices | |||||||
| Lean/Height2 (kg/m2) | 19.9 (3.3) | 19.8 (2.7) | 0.9317 | 19.4 (2.2) | 19.0 (3.5) | 0.0276 | 0.2608 |
| Appen. Lean/Height2 (kg/m2) | 8.8 (2.0) | 8.7 (1.6) | 0.5705 | 8.1 (1.4) | 7.9 (1.9) | 0.0145 | 0.1939 |
Data are expressed as median (IQR) with p-values from Wilcoxon signed rank test or mean difference with P-value from t-test in parentheses. P-values in bold denote statistical significance < 0.05.
Epicardial fat volume in mm3 was similar in both groups at baseline and increased in both groups over the study period, however, the difference over the study period between aramchol and placebo was not significant.
Further characterization of body composition using MRI confirmed VAT, ASAT and the ratio of visceral to total adiposity were similar between groups at baseline. Assessment of mean ± SD muscle mass with thigh volume was higher in the treatment group than placebo at baseline 12.8 ± 3.2 L and 11.6 ± 2.6 L respectively, p=0.02. There were no significant differences in changes in fat distribution or muscle indices between the aramchol and placebo groups (Table 4b).
Table 4b.
Changes in whole body fat depots and muscle volume by MRI in Aramchol- Versus Placebo-Treated Patients
| Aramchol (n=19) | Placebo (n=20) | Difference | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Posttreatment | P-Value | Baseline | Posttreatment | P-Value | P-Value | |
| VAT (l) | 6.1 (2.8) | 5.6 (3.0) | 0.5533 | 6.7 (2.7) | 6.4 (2.3) | 0.6280 | 0.6118 |
| ASAT (l) | 7.9 (4.7) | 8.2 (5.9) | 0.9530 | 8.1 (4.3) | 8.1 (3.9) | 0.6507 | 0.4959 |
| VAT + ASAT (l) | 14.6 (4.3) | 14.3 (4.9) | 0.9323 | 14.9 (4.2) | 15.3 (4.1) | 0.7906 | 0.4634 |
| Visceral fat ratio (%) | 42.1 (18.9) | 40.9 (13.5) | 0.9323 | 44.1 (15.6) | 44.0 (15.5) | 0.5678 | 0.9403 |
| Thigh volume (l) | 12.6 (1.8) | 12.5 (1.5) | 0.4037 | 11.8 (2.9) | 11.7 (2.9) | 0.8063 | 0.4447 |
| Muscle ratio (kg/l) | 7.2 (1.2) | 7.1 (1.1) | 0.3894 | 7.7 (2.3) | 7.7 (1.9) | 0.8176 | 0.3661 |
| Fat storage (l/m2) | 5.0 (1.3) | 5.0 (1.6) | 0.8900 | 5.0 (1.8) | 5.0 (1.2) | 0.3529 | 0.8737 |
| Visceral Fat Index (l/m2) | 2.0 (0.7) | 1.9 (0.9) | 0.6685 | 2.3 (0.6) | 2.1 (0.6) | 0.2121 | 0.7221 |
| Fat ratio (%) | 53.2 (7.3) | 52.9 (8.1) | 0.8904 | 54.4 (11.9) | 55.0 (9.5) | 0.9661 | 0.3584 |
| Pericardial Volume (mm3) | 183300 (103100) | 202800 (75900) | 0.2500 | 136500 (72019) | 171050 (176500) | 0.0134 | 0.1633 |
Data are expressed as median (IQR) with p-values from Wilcoxon signed rank test or mean difference with P-value from t-test in parentheses. N’s are “best case” P-values in bold denote statistical significance < 0.05.
Sensitivity Analysis in Patients with Obesity
Fourteen obese patients in the aramchol group and nine obese placebo patients were assessed in subgroup analysis. ALT was higher in the drug group at baseline 76 IU/L vs 43 IU/L, p=0.01 (Supplemental Table 1a). Mean ALT improved in the aramchol group to 61 IU/L at follow up, p=0.01 (Supplemental Table 1b). The difference in improvement in ALT between the aramchol and placebo groups was not statistically significant, p=0.47. Changes in MRI-PDFF, MRE and CAP were not different between groups, however, liver stiffness measured by VCTE improved more in the placebo (−1.1 kPa) group than the treatment group (+0.3 kPa), p=0.03.
Adverse Events
No patients in the treatment arm discontinued treatment due to an adverse event. One patient in the placebo arm discontinued treatment due to an asymptomatic increase in creatinine phosphokinase level. There were no serious adverse events or deaths. All adverse events were mild, Grade 1, and occurred with similar frequency in the placebo 48% and treatment 40% groups (Supplemental Table 2).
DISCUSSION
In this randomized, double-blind, placebo controlled trial, aramchol was not superior to placebo at reducing hepatic fat measured by MRI-PDFF, improving aminotransferases or liver stiffness by MRE or VCTE. Treatment with aramchol was associated with lower ALT at end of treatment particularly among obese patients with HIV-associated NAFLD. Aramchol was well tolerated with no significant adverse events and a similar safety profile to placebo.
The major innovation applied in the design of this clinical trial was incorporation of advanced MRI based measures of body composition including total visceral adipose tissue volume, total abdominal subcutaneous adipose tissue volume, thigh muscle volume as well as epicardial fat volume assessment before and after treatment in a NAFLD trial. This trial has three-fold innovation in longitudinal body composition analysis in NAFLD. First, this trial utilized MRI-PDFF and MRE, to non-invasively assess longitudinal changes in liver fat content and liver stiffness in co-localized regions. These modalities are the most accurate non-invasive tools for the assessment of liver fat(34) and liver stiffness(35) in primary NAFLD and this study is the first to incorporate them in a trial of HIV-associated NAFLD. Second, this trial evaluated body composition changes with whole-body MRI for the first time in a NAFLD trial. The distribution of obesity, which is not captured by BMI, is associated with cardiovascular risk, metabolic disease activity and the development of NAFLD (36, 37). Clinical trials of NAFLD should consider the potential impact of treatment on other body fat compartments. This is of particular importance in HIV where peripheral fat atrophy and visceral fat accumulation occur at lower BMI and might be independent of ART(38). We utilized multiple modalities to assess fat and muscle compartments throughout the body. Specifically, we utilized the well-established DXA, which is widely available and inexpensive but limited by its two-dimensional assessment of volumes. As more treatment trials of NAFLD incorporate MRI based imaging (39, 40), we successfully incorporated the addition of body composition analysis using an MRI based brief commercial protocol. Third, we assessed epicardial fat volume on magnetic resonance imaging, which may be an independent risk factor for endothelial dysfunction(41) and cardiovascular disease(42), which are the leading cause of death in patients with NAFLD. This trial demonstrates the feasibility of incorporating these measurements into a clinical trial of NAFLD and sets the stage for incorporation of these advanced measures and how to assess them before and after treatment in longitudinal studies in NAFLD.
NAFLD is increasingly common among persons with HIV and may be associated with increased disease severity (5, 43). However, unique factors affecting patients with HIV including changes in the intestinal microbiome (44), and metabolic changes associated with chronic infection and antiretroviral therapy (45) may contribute to a differential response to therapy from patients with primary NAFLD. This is the first clinical trial of pharmacologic intervention for HIV-associated NAFLD. While pre-clinical and clinical studies of aramchol in patients with primary NAFLD demonstrated promising results, including improvement in hepatic steatosis, this study showed no benefit on hepatic fat fraction by MRI-PDFF at 12 weeks.
While we performed in-depth, longitudinal, non-invasive assessments of liver and body composition we acknowledge certain limitations to this study. The study did not include liver histology, which may have limited the ability to detect an improvement in disease activity, particularly, related to the improvement in ALT seen in the aramchol group. However, currently, early phase studies of NAFLD have utilized highly accurate MRI based imaging to accurately characterize changes in hepatic fat content and liver stiffness. Furthermore, MRI-PDFF may be more accurate than liver histology for detecting quantitative changes in hepatic steatosis and the putative mechanism of aramchol suggested a strong effect on liver fat content, which was recently demonstrated to be associated with histologic disease progression (34, 46). Furthermore, the trial period was short, which may have limited detection of changes in liver stiffness, however, changes in the primary endpoint, hepatic fat content, are dynamic and had been demonstrated in a previous trial of aramchol for the treatment of primary NAFLD (13). Finally, while our sample size estimate was reasonable based on a previous phase 2a study of patients with primary NAFLD(13), our negative results could be secondary to a potential type II error, particularly if the effect size of aramchol is more modest than estimated. Importantly, this work will help inform sample size estimates for future studies in HIV-associated NAFLD.
In conclusion, aramchol was not superior to placebo in reducing liver fat in patients with HIV-associated NAFLD. Aramchol was well tolerated and patients in the treatment group had a significant reduction in ALT. There were no off-target effects of aramchol on body composition measured by advanced, whole-body MRI. This trial also demonstrates that noninvasive assessment of body composition is feasible in NAFLD trials.
Supplementary Material
Acknowledgements:
MRI based body compositional analysis provided by AMRA Medical
Funding: Supported by an investigator initiated study grant to R.L by Galmed. Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, RL serves as co-PIs on the grant R01-DK106419. VA is supported by the AASLD Foundation Clinical and Translational Research Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- SCD1
stearoyl-coenzyme-A-desaturase-1
- NAFLD
Non-alcoholic fatty liver disease
- HIV
human immunodeficiency virus
- MRI-PDFF
magnetic resonance imaging-proton density fat fraction
- MRE
magnetic resonance elastography
- VCTE
vibration controlled transient elastography
- MRI
magnetic resonance imaging
- DXA
Dual-Energy X-ray Absorptiometry
- MRS
magnetic resonance sprectroscopy
- NASH
nonalcoholic steatohepatitis
- OGTT
oral glucose tolerance test
- ROI
region of interest
- HOMA-IR
homeostatic model assessment for insulin resistance
- ADIPO-IR
adipose insulin resistance
- FFA
free fatty acids
- BMI
body mass index
- UCSD
University of San Diego
Footnotes
Conflict of interests: Rohit Loomba: Dr. Loomba serves as a consultant or advisory board member for Bird Rock Bio, Celgene, Enanta, GRI Bio, Madrigal, Metacrine, NGM, Receptos, Sanofi, Arrowhead Research, Galmed, NGM, GIR, Inc. and Metacrine, Inc. In addition, his institution has received grant support from Allergan, BMS, BI, Daiichi-Sankyo Inc., Eli-Lilly, Galectin, Galmed, GE, Genfit, Intercept, Janssen Inc, Madrigal, Merck, NGM, Pfizer, Prometheus, Siemens, and Sirius. He is also co-founder of Liponexus Inc.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686–690. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006;166:1632–1641. [DOI] [PubMed] [Google Scholar]
- 4.Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. Aids 2017;31:1621–1632. [DOI] [PubMed] [Google Scholar]
- 5.Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther 2015;41:368–378. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed SS, Aghdassi E, Salit IE, Avand G, Sherman M, Guindi M, Heathcote JE, et al. HIV-positive patients with nonalcoholic fatty liver disease have a lower body mass index and are more physically active than HIV-negative patients. J Acquir Immune Defic Syndr 2007;45:432–438. [DOI] [PubMed] [Google Scholar]
- 7.Price JC, Seaberg EC, Latanich R, Budoff MJ, Kingsley LA, Palella FJ Jr., Witt MD, et al. Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol 2014;109:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, Wanke CA, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis 2015;211:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman SL, NY ISoMaMSDoLDMS, Ratziu V, France PSHaPaMCUP, Harrison SA, TX PCRSA, Abdelmalek MF, et al. A randomized, placebo‐controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016;150:1147–1159.e1145. [DOI] [PubMed] [Google Scholar]
- 12.Konikoff FM, Leikin-Frenkel A, Goldiner I, Michowitz M, Brezowski E, Harats D, Gilat T. Biliary and systemic effects of fatty acid bile acid conjugates. Eur J Gastroenterol Hepatol 2003;15:649–655. [DOI] [PubMed] [Google Scholar]
- 13.Safadi R, Konikoff FM, Mahamid M, Zelber-Sagi S, Halpern M, Gilat T, Oren R, et al. The Fatty Acid-Bile Acid Conjugate Aramchol Reduces Liver Fat Content in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2014. [DOI] [PubMed] [Google Scholar]
- 14.Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012;56:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel NS, Peterson MR, Brenner DA, Heba E, Sirlin C, Loomba R. Association between novel MRI-estimated pancreatic fat and liver histology-determined steatosis and fibrosis in non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2013;37:630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarrinpar A, Gupta S, Maurya MR, Subramaniam S, Loomba R. Serum microRNAs explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: a prospective study. Gut 2016;65:1546–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, Nguyen P, et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology 2015;149:1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology 2017;152:598–607 e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Middleton MS, Haufe W, Hooker J, Borga M, Dahlqvist Leinhard O, Romu T, Tunon P, et al. Quantifying Abdominal Adipose Tissue and Thigh Muscle Volume and Hepatic Proton Density Fat Fraction: Repeatability and Accuracy of an MR Imaging-based, Semiautomated Analysis Method. Radiology 2017;283:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, Soaft L, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015;61:1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol 2016;65:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012;56:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 2012;36:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013;267:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging 2011;34:729–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology 2016;150:626–637.e627. [DOI] [PubMed] [Google Scholar]
- 27.Hsu E, Feghali-Bostwick CA. Insulin-like growth factor-II is increased in systemic sclerosis-associated pulmonary fibrosis and contributes to the fibrotic process via Jun N-terminal kinase- and phosphatidylinositol-3 kinase-dependent pathways. American Journal of Pathology 2008;172:1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003;29:1705–1713. [DOI] [PubMed] [Google Scholar]
- 29.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008;48:835–847. [DOI] [PubMed] [Google Scholar]
- 30.Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, Ajmera V, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 2018;67:1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borga M, Thomas EL, Romu T, Rosander J, Fitzpatrick J, Dahlqvist Leinhard O, Bell JD. Validation of a fast method for quantification of intra-abdominal and subcutaneous adipose tissue for large-scale human studies. NMR Biomed 2015;28:1747–1753. [DOI] [PubMed] [Google Scholar]
- 32.Linge J, Borga M, West J, Tuthill T, Miller MR, Dumitriu A, Thomas EL, et al. Body Composition Profiling in the UK Biobank Imaging Study. Obesity (Silver Spring) 2018;26:1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loomba R, Kayali Z, Noureddin M, Ruane P, Lawitz EJ, Bennett M, Wang L, et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018;155:1463–1473.e1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, Le MD, et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Granillo GA, Reynoso E, Capunay C, Carpio J, Carrascosa P. Pericardial and visceral, but not total body fat, are related to global coronary and extra-coronary atherosclerotic plaque burden. Int J Cardiol 2018;260:204–210. [DOI] [PubMed] [Google Scholar]
- 37.Kim D, Chung GE, Kwak MS, Kim YJ, Yoon JH. Effect of longitudinal changes of body fat on the incidence and regression of nonalcoholic fatty liver disease. Dig Liver Dis 2018;50:389–395. [DOI] [PubMed] [Google Scholar]
- 38.Grant PM, Kitch D, McComsey GA, Collier AC, Bartali B, Koletar SL, Erlandson KM, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. Aids 2016;30:2805–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caussy C, Reeder SB, Sirlin CB, Loomba R. Non-invasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loomba R Role of imaging-based biomarkers in NAFLD: Recent advances in clinical application and future research directions. J Hepatol 2018;68:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 42.Mahabadi AA, Berg MH, Lehmann N, Kalsch H, Bauer M, Kara K, Dragano N, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: the Heinz Nixdorf Recall Study. J Am Coll Cardiol 2013;61:1388–1395. [DOI] [PubMed] [Google Scholar]
- 43.Morse CG, McLaughlin M, Matthews L, Proschan M, Thomas F, Gharib AM, Abu-Asab M, et al. Nonalcoholic Steatohepatitis and Hepatic Fibrosis in HIV-1-Monoinfected Adults With Elevated Aminotransferase Levels on Antiretroviral Therapy. Clin Infect Dis 2015;60:1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen KK, Pedersen M, Troseid M, Gaardbo JC, Lund TT, Thomsen C, Gerstoft J, et al. Microbial translocation in HIV infection is associated with dyslipidemia, insulin resistance, and risk of myocardial infarction. J Acquir Immune Defic Syndr 2013;64:425–433. [DOI] [PubMed] [Google Scholar]
- 45.Noor MA, Seneviratne T, Aweeka FT, Lo JC, Schwarz JM, Mulligan K, Schambelan M, et al. Indinavir acutely inhibits insulin-stimulated glucose disposal in humans: a randomized, placebo-controlled study. Aids 2002;16:F1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ajmera V, Park CC, Caussy C, Singh S, Hernandez C, Bettencourt R, Hooker J, et al. Magnetic Resonance Imaging Proton Density Fat Fraction Associates With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


