Abstract
Objectives:
Septic shock, defined as sepsis with hypotension not responding to fluid resuscitation or requiring vasopressor support, results in the worst outcomes in sepsis patients. This subtype of the patient is often difficult to detect. The shock index (SI) has demonstrated the potential for predicting hemodynamic compromise and collapse and predicting patient outcomes in multiple medical and surgical settings. In our study, we assessed the utility of the SI as a hemodynamic screening tool to identify patients likely to fail to respond to fluids and ultimately to be diagnosed with septic shock.
Methodology:
A single-center cross-sectional analysis of patients presenting with hypotension and septicemia over 1 year. The study was conducted using the electronic medical records of the emergency department patients presenting to King Saud University Medical City. The charts were reviewed from 2 May 2015 to 24 April 2016 using the local medical registry. The study was approved by the hospital institutional review board (IRB). Data extraction was performed using a standardized form.
Results:
The area under the curve was 0.77 (P < 0.001) for the prediction of hemodynamic collapse. An initial SI ≥0.875 had a sensitivity of 81% and a specificity of 72% for the identification of patients in whom fluid resuscitation would fail.
Conclusions:
Based on our findings, we found that the SI was a reliable screening tool for the identification of hypotensive patients with sepsis who would ultimately be diagnosed with septic shock.
Keywords: Emergency department, fluid responsiveness, intensive care, sepsis, septic shock, shock index, vasopressor, vital indices
Introduction
Circulatory shock is defined as a state of decreased perfusion and oxygenation to distant tissues. There are multiple types of circulatory shock depending on its underlying cause (i.e., hypovolemic, cardiogenic, distributive, or obstructive). Early recognition is one of the most important steps in the management of circulatory shock and is associated with improving the resulting mortality rate.[1] Septic shock is the most common manifestation of shock and severe sepsis represents the 10 most common cause of mortality in the United States.[2] Screening tools have been developed to identify patients with sepsis, most notably the systemic inflammatory response syndrome (SIRS) and quick sequential organ failure assessment (qSOFA), both of which are used to screen for sepsis cases but also have prognostic roles.[3] Prognostic tools in sepsis have also been developed using multivariable models, and they are likely to have complex applicability at the bedside and during initial resuscitation.[4]
The shock index (SI) is one of the most commonly used perfusion indices because it is easily calculated at the bedside. It is defined as the heart rate (HR) over the systolic blood pressure (SBP) (SI = HR/SBP), with a normal range from 0.5 to 0.7 bpm/mmHg.[5] The SI has been studied extensively in acute hypovolemia, hemorrhage, and trauma.
In two prospective observational studies with blood bank donors, the SI was shown to have better diagnostic value than traditional vital signs alone in the detection of acute hypovolemia.[6] In a population-based cohort study that was performed using the “TraumaRegister DGU”, the SI was shown to be as good as the base deficit (BD) for the recognition of hypovolemic shock patients and their hemostatic resuscitation requirements.[7] Sepsis and septic shock are common presentations in emergency departments (EDs). The morbidity and mortality rates of sepsis are high, with early recognition and prompt management being crucial to decreasing these rates.[8]
In severe sepsis and septic shock patients, the SI calculated 2 h after resuscitation can predict mortality. An SI ≤1 has a negative predictive value (NPV) of 88% for the improvement of hemodynamics after volume expansion in septic shock patients.[9,10]
Sustained elevation of the SI after fluid resuscitation has been demonstrated to sensitively predict hemodynamic collapse in patients presenting at the ED with severe sepsis, as well as organ dysfunction.[11] The aim of this study was to evaluate whether an initial SI/modified SI cutoff can be used to estimate hemodynamic collapse (vasopressor requirement) in patients presenting at the ED with hypotensive septicemia. We also assessed secondary patient characteristics associated with fluid resuscitation nonresponsiveness.
Methodology
Study settings and design
The cross-sectional study was conducted using the electronic medical records of ED patients presenting to King Saud University Medical City, a local academic hospital with an annual census of 160000 patients, in Riyadh, Saudi Arabia. The charts were reviewed from 2 May 2015 to 24 April 2016 using the local medical registry. The study was approved by the hospital IRB. Data extraction was performed using a standardized form.
Study population and measurements
A total of 69305 patient electronic records were screened and reviewed. The inclusion criteria were adult patients ≥15 years of age who presented to the ED with SBP <100 mmHg and had a positive blood culture result during their visit. Hemodynamic collapse was defined as requiring vasopressor support during the ED stay.
The individual patient characteristics recorded were age, sex, SBP, initial diastolic blood pressure (DBP), heart rate (from ED triage vital signs), admission quarter of the year, and whether vasopressor therapy was initiated (yes/no). Then, the mean arterial pressure (MAP), SI, and modified shock index (MSI) were calculated. Microsoft Office Excel™ was used for the data collection and calculation.
MAP = [(1/3)*SBP] + [(2/3)*DBP)]
SI = HR/SBP
MSI = HR/MAP
Out of the total 69305 patient charts screened, 3929 (5.67%) met the inclusion criteria and had an ED triage SBP less than or equal to 100 mmHg. Of those, 98 (2.49%) patients had a positive blood culture during the same visit.
Data analysis
Means and standard deviations are used to describe continuous covariates and the frequency and percentages are used for categorical and binary variables. The Chi-squared (χ2) test of independence was used to assess the correlation between categorically measured factors and the independent groups. A t-test was used to assess categorically measured factors for statistically significant differences in the mean patient SI scores. One-way analysis of variance was also used to assess the patients' categorical variables with more than two groups for statistically significant differences in their mean SI scores.
Multivariate logistic binary regression analysis was employed to assess combined and individual associations of patient demographics and hemodynamic measures with the odds of the patient being fluid responsive, with the effect size expressed as the odds ratio. The area under the curve (AUC) for the receiver operating characteristic (ROC) curve was used to assess the overall specificity and sensitivity of the logistic regression model, and the AUC ROC was employed to examine the sensitivity and specificity of the SI for the prediction of the patient's fluid responsiveness when analyzed alone. SPSS IMB V.20 was used throughout the analysis and the alpha significance level was set to 0.05 throughout the analysis.
Results
Patient demographics
Descriptive analysis
Table 1. The subjects' demographic and admission characteristics are shown. Most of the subjects (51%) were female. Their mean age was 57.4 (17.9) years. The patients' admission months were grouped into four quarters of the year to help account for possible seasonal variations in the patients' severity of illness.
Table 1.
Admitted subjects’ demographic and admission characteristics
| Characteristic | Frequency | Percentage |
|---|---|---|
| Sex | ||
| Female | 50 | 51 |
| Male | 48 | 49 |
| Age (years), mean (SD) | 57.40 (17.9) | |
| Age group | ||
| 15-31 years | 12 | 12.2 |
| 31-47 years | 14 | 14.3 |
| 48-63 years | 38 | 38.8 |
| >63 years | 34 | 34.7 |
| Admission quarter of the year | ||
| First | 29 | 29.6 |
| Second | 23 | 23.5 |
| Third | 23 | 23.5 |
| Fourth | 23 | 23.5 |
Hemodynamic parameter findings
Descriptive analysis
Table 2. The measured mean admission Systolic blood pressure (SYSBP) for patients upon arrival to the ED was 88.01 (12.1) mmHg, the mean DBP for subjects was 52.1 (12.3) mmHg. The overall calculated MAP was 64.1 (10.9) mmHg. The mean HR was equal to 95.1 (22.7) beats/min. The mean calculated SI for all the subjects was 1.11 (0.4) points, with those patients presenting with an SI >0.87 accounting for most of the patients (75.5%). This cut off SI value of 0.87 or more was determined to have the highest sensitivity and specificity for predicting the patient's lack of responsiveness to fluid resuscitation. The mean MSI score for subjects was 1.54 (0.51) points.
Table 2.
Admitted subjects’ hemodynamic measurements and characteristics upon admission
| Characteristic | Mean (SD) | Min, Max | (Q1, Q3) |
|---|---|---|---|
| Admission systolic blood pressure (SYSBP), mmHg | 88.01 (12.1) | 42, 100 | (81, 96.3) |
| Admission diastolic blood pressure (DIASBP), mmHg | 52.1 (12.3) | 25, 90 | 43.8, 60) |
| Mean arterial blood pressure (MAP), mmHg | 64.1 (10.9) | 31, 91 | (58, 72) |
| Pulse rate | 95.1 (22.7) | 30, 158 | (80, 108) |
| Shock index (SI) | 1.11 (0.4) | 0.33, 2.67 | (0.9, 1.3) |
| Shock index (SI)<=0.87 | 24 (24.5%) | ||
| Shock index >0.87 | 74 (75.5%) | ||
| Modified shock index (MSI) | 1.54 (0.51) | 0.44, 3.7 | (1.21, 1.8) |
| Vasopressors needed/fluid refractory | |||
| Yes, n (%) | 33 (33.7%) | - | - |
| No, n (%) | 65 (66.3%) | - | - |
The percentage of patients who were fluid responsive was 66.3%, with 33.7%, requiring the administration of inotropic/vasopressor support.
Relationship between patients' hemodynamic characteristics and fluid responsiveness
Bivariate analysis
Table 3. The bivariate analysis tested for statistically significant associations between hemodynamic characteristics and fluid responsiveness and the results are shown in Table 3. The mean age (years) was found to be associated with fluid responsiveness (t = 3.4, P =0.001), with those responsive to fluid therapy being younger (mean age 53.2, SD [18.6]) than those who were nonresponsive to fluid therapy (mean age 65.6, SD [13.7]). Furthermore, the analysis of the categorized patient age groups suggested that patients 15–31 years old were the most likely to be fluid responsive, with patients older than 63 years old being the least likely to be responsive [Figure 1].
Table 3.
The bivariate analysis of subjects’ demographic and admission characteristics stratified by fluid resuscitation responsiveness (n=98)
| Characteristic | Fluid responsive | Test statistic | P | |
|---|---|---|---|---|
| No n=33 | Yes n=31 | |||
| Sex | ||||
| Female | 16 (48.5%) | 34 (52.3%) | χ2 (1)=0.13 | 0.721 |
| Male | 17 (51.5%) | 31 (47.7%) | ||
| Age (years), mean (SD) | 65.6 (13.7) | 53.21 (18.6) | t(96)=3.4 | 0.001 |
| Age group | ||||
| 15-31 years | 0 | 12 (18.5%) | χ2 (3)=13.63 | 0.003 |
| 31-47 years | 3 (9.1%) | 11 (16.9%) | LR | |
| 48-63 years | 14 (42.4%) | 24 (36.9%) | ||
| >63 years | 16 (48.5%) | 18 (27.7%) | ||
| Admission quarter of the year | ||||
| First | 13 (39.4%) | 16 (24.6%) | χ2 (3)=2.81 | 0.421 |
| Second | 6 (18.2%) | 17 (26.2%) | ||
| Third | 8 (24.2%) | 15 (23.1%) | ||
| Fourth | 6 (18.2%) | 17 (23.1%) | ||
| Admission systolic blood pressure (SYSBP), mmHg | 81.73 (15.5) | 91.2 (8.4) | t(41.7)=3.3 | 0.002 |
| Admission diastolic blood pressure (DIASBP), mmHg | 48.4 (13) | 53.9 (11.5) | t(96)=2.2 | 0.034 |
| Mean arterial blood pressure (MAP), mmHg | 59.6 (12.6) | 66.4 (9.2) | t(49.9)=2.8 | 0.008 |
| Pulse rate | 99.1 (22.6) | 93.1 (22.6) | t(96)=1.24 | 0.217 |
| Shock index (SI) | 1.28 (0.5) | 1.03 (0.3) | t(41.96)=2.8 | 0.007 |
| Modified shock index (MSI) | 1.77 (0.7) | 1.42 (0.4) | t(41.4)=2.80 | 0.008 |
Figure 1.
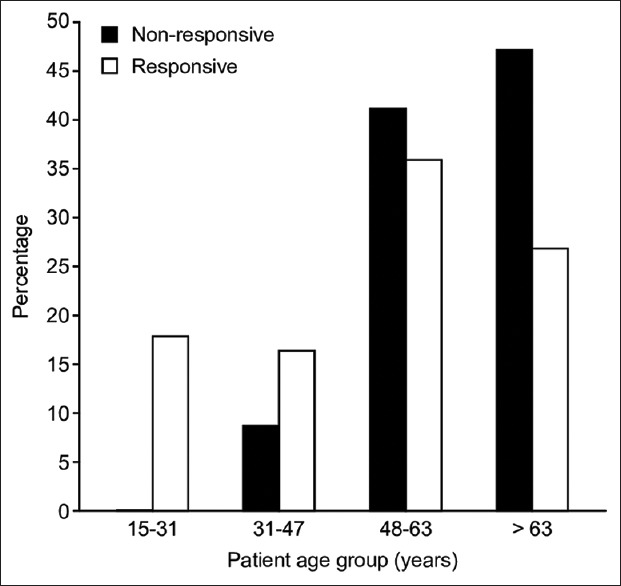
The bivariate association between people's age groups and their responsiveness to fluid resuscitation in percentages
Both the admission mean SBP and DBP were associated with fluid responsiveness (t = 3.3, P =0.002 and t = 2.2, P =0.034), with patients with higher SBP and patients with higher DBP more likely to respond to fluids and not require vasopressors than those with lower values. As expected, the MAP, which is calculated from the SBP and DBP, was significantly higher (mean 66.4, SD [9.2]) in fluid-responsive patients than in nonresponders (M = 59.6, SD [12.6]), (t = 2.8, P =.008).
The calculated SI values were associated with fluid responsiveness at admission (t = 2.8, P =0.007), with lower SI values predicting responsiveness (mean of 1.03, SD[0.3]). The MSI values were associated with fluid responsiveness, with lower MSI values predicting responsiveness (t = 2.8, P =0.008).
The patient admission time (quarter of the year) and admission HR were both found to not be significantly associated with responsiveness.
Multivariate analysis
Figure 2: Logistic regression analysis was performed, and a ROC curve was generated to test the overall significance of the model based on the variables included [Table 4]; that SBP, DBP, and MAP were excluded due to their relationship with the SI. The model was statistically significant (χ2(5) = 24.02, P < .001). The model was determined to be accurate, as evidenced by the nonsignificant Hosmer–Lemeshow test result (χ2 (8) =10.24, P = 0.249). The AUC of 0.77 (P <.001) indicated the model's very good sensitivity and specificity for the prediction of patient fluid responsiveness.
Figure 2.
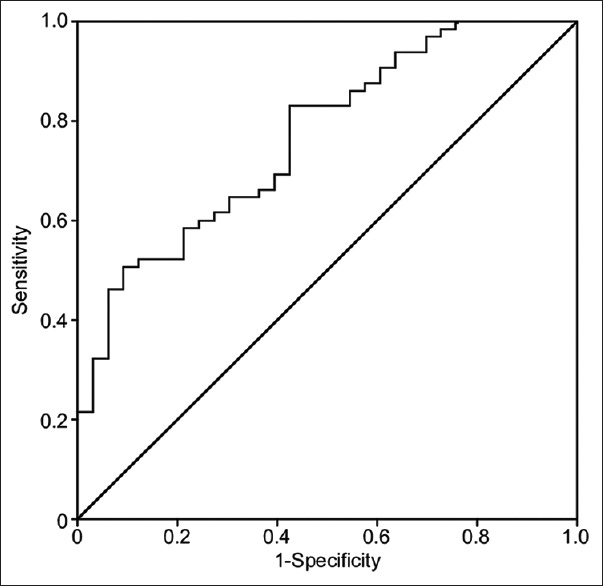
Multivariate binary logistic regression analysis. The regression analysis model was statistically significant, χ2(5)=24.02, P< 0.001, indicating that at least one of the tested patients predictor variables, or more, had a statistically significant multivariate association with their odds of responding to fluid therapy. The AUC-ROC was statistically significant, AUC = 0.77, P< 0.001, indicating the overall great specificity and sensitivity
Table 4.
Multivariate logistic regression analysis explaining the combined and individual associations between patient’s demographic characteristics and hemodynamic shock index factors and their odds of responding to fluid resuscitation during hemodynamic shock resuscitation, n=98
| Characteristic | B | SE | Wald | Adjusted odds ratio | 95% CI for OR | P | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Shock index score | −3.644 | 1.529 | 5.684 | 0.026 | 0.001 | 0.523 | 0.017 |
| Sex=male | −.398 | 0.501 | 0.631 | 0.672 | 0.252 | 1.793 | 0.427 |
| Age (years) | −.041 | 0.017 | 5.579 | 0.960 | 0.928 | 0.993 | 0.018 |
| Pulse rate score | 0.030 | 0.021 | 2.009 | 1.030 | 0.989 | 1.074 | 0.156 |
| Quarter of the year | 0.186 | 0.219 | 0.720 | 1.204 | 0.784 | 1.851 | 0.396 |
| Constant | 4.122 | 1.828 | 5.084 | 61.673 | 0.024 | ||
The model demonstrated that the SI converged significantly on the odds of having responded to fluid replacement therapy when considering the rest of the predictor variables as equal (P =0.017). As such, for each additional point increase in the patients' measured SI, their odds of responding to fluid replacement therapy at admission decreased by ((1–0.026) × 100) = 97.4%, suggesting that the higher the measured SI, the less likely the patient was to be fluid responsive.
The model similarly demonstrated that patient age correlated significantly and negatively with the odds of fluid responsiveness (P =0.018). As the patients' age increased, their chances of being fluid responsive decreased by ([1–0.928]×100) = 7.2%.
We also investigated fluid responsiveness while considering the age group (x-axis) and SI (y-axis) in a dichotomized fashion, using an SI of 0.875 as the cutoff. Based on the same logistic model [Figure 3], it was evident that for patients in the age group ≥15 and ≤47 years, the adjusted propensity for responding to fluid therapy tended to be lower for both SI cutoff groups; however, the reduction was steeper for those with SI >0.875 in the age group of 47 years old or less compared to those whose SI was <0.875 points.
Figure 3.
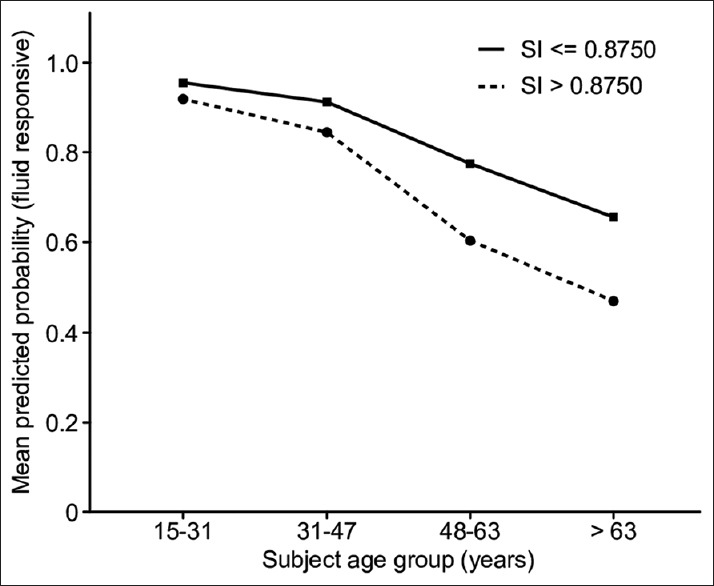
The adjusted association between patient age, SI above threshold of 0.87 and the probability of responsiveness to fluid resuscitation among patients with septic shock
Similar to the bivariate analysis, the multivariate analysis did not demonstrate significance for the patient's sex (P =0.427), HR (P =0.156), or admission time (season) of the year. Notably, the HR, although not statistically significant, demonstrated a positive association with being fluid responsive (OR 1.03).
Shock index as an independent factor
The AUC for the SI when analyzed using the ROC curve for the prediction of the need for vasopressors in septicemic hypovolemic patients in the ED was not statistically significant (AUC = 0.66), indicating that the score was not sufficiently specific or sensitive enough to predict a patient's need for vasopressors. A cutoff value of the SI equal to or above 0.875 was found to provide the highest specificity and sensitivity when examining the coordinates of the curve (sensitivity 0.81, specificity 0.723) [Figure 4].
Figure 4.
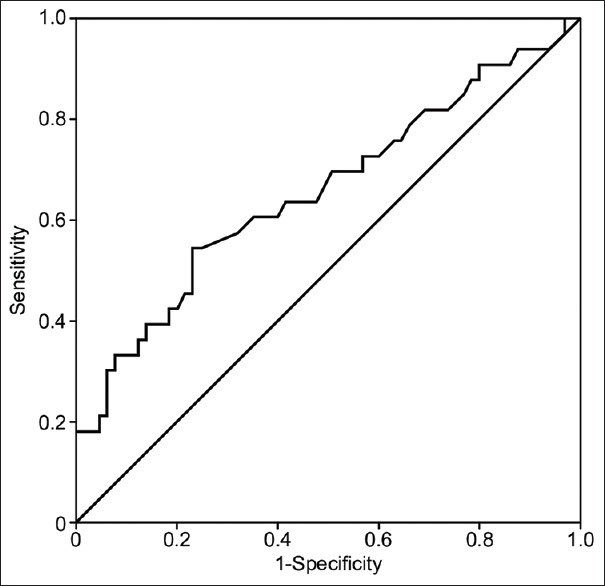
The AUC for the SI when analyzed using the ROC for specificity and sensitivity predicting the outcome of vasopressors requirement among shock patients in the emergency room was not statistically substantial, AUC = 0.66, denoting the score is not sufficiently specific and sensitive to predict the patients requirement for vasopressors during hypovolemic shock conditions
Discussion
The SI has been used as a risk stratification tool for detecting changes in hemodynamic parameters before the onset of systemic hypotension and cardiorespiratory collapse.[12] It has been extensively researched in the context of trauma. In a systematic review published in 2014, an SI score greater than or equal to 0.9 was the most sensitive cutoff value for the prediction of critical bleeding in trauma patients. For prehospital personnel, an SI score ≥1 was recommended because of its greater simplicity and higher specificity. However, lowering the SI threshold to ≥0.8 increases the sensitivity for the detection of bleeding and the need for hemostasis interventions.[13,14]
The SI is also predictive of mortality in poly-trauma patients, with an SI score ≥0.9 predictive of mortality rates in such patients. In geriatric trauma patients, an SI score ≥1 was associated with higher morbidity and mortality rates and identified patients who would benefit from a transfer to a level 1 trauma center. Moreover, an increasing trend in SI scores was shown to be more reliable at predicting outcomes in geriatric trauma patients than a single reading.[15,16,17,18]
In a prospective study of 9860 trauma patients, the MSI, which is defined as the HR over the MAP (MSI = HR/MAP), predicted the mortality rate better than the SI score and traditional vital signs alone.[19]
Prehospital SI scores for trauma patients correlate with hospital resource usage and mortality rates. An SI score ≥1 after a 1 L of crystalloid resuscitation is predictive of the need for oxygen carrier resuscitation.[20,21]
The efficacy of the SI has also been demonstrated in other subgroups of patients, including aortic dissection patients. The SI and the false/true lumen ratio on computed tomography angiography were equally good in the assessment of the transportation risk.[22]
In gastrointestinal bleeding patients, the preembolization SI score correlates with extravasation on angiography.[23]
The SI has been studied in obstetric and gynecological emergencies. In a study on postpartum hemorrhage patients who had lost ≥1500 mL of blood, the SI score correlated with their outcomes: an SI <0.9 was reassuring, and an SI ≥1.7 required urgent interventions.[24] In another study performed in Nigeria on the prediction of ruptured ectopic pregnancy, the SI was better than traditional vital signs alone. In another study, the SI was a part of a new predictive scoring system for ruptured ectopic pregnancy, which had a high NPV and a high degree of sensitivity.[25,26]
In pulmonary embolism (PE) patients, both the SI and the simplified pulmonary embolism severity index (sPESI) can accurately predict the mortality risk. However, the sPESI is more reliable for the prediction of a lower risk, thereby identifying patients who can be treated in outpatient settings. For the prediction of severe PEs, a clinical scoring model was suggested by Bircan et al.[27] which is composed of an electrocardiogram, the SI, and arterial blood gas analysis, and adding the SI to the scoring model increased its specificity. In another study, an SI ≥0.7 was associated with an increased mortality rate in PE patients.[27,28,29]
In ST-segment elevation myocardial infarction patients, the SI correlates with the mortality risk. An SI ≥0.7 is a predictor of early mortality and major adverse cardiovascular events. The MSI was suggested to be more accurate than the SI for predicting the 7-day mortality rate and major adverse cardiovascular event rate.[30,31,32]
In a recently published prospective cohort study, extremes of the SI in acute stroke patients were predictive of worse outcomes and a higher early mortality rate, with the lowest quantile SI predicting the 3-day mortality rate (odds ratio: 2.45).[33]
In a cohort of medical ED patients, the triage SI, MSI, and SIA (SIA=Age (years) × SI) were all equally good and were superior to blood pressure (BP) alone for the prediction of the mortality rate in nontrauma level 2 emergency severity index patients.[34]
In community-acquired pneumonia (CAP) patients, the SI is able to predict the mortality rate in admitted patients.[35] However, adding SI to the well-validated and most commonly used CAP severity index (CURB-65) by changing the hemodynamic component of the score from BP to the SI or SIA did not improve its predictive value for the mortality rate. There were no demonstrable differences among the CURB-65, confusion, urea, respiratory rate, and SI (CURSI) or CURASI (CURSI with adjusted SI).[36,37]
In inpatient settings, a five-point scoring system “NaURSE” (Na+, urea, respiratory rate, and SI) was derived to predict the in-hospital mortality rate for elderly patients.
In terminal cancer patients, an SI score ≥1 and a decreased level of consciousness on admission predicted a mean survival time of less than 1 week. An SI score of 0.85 or greater was associated with unplanned intensive care unit transfer (OR 3.0).[38,39,40]
With regard to postintubation hypotension, a preintubation SI score ≥0.9 was found to be predictive of postintubation hypotension in apparently hemodynamically stable patients.[41]
Although the SI is readily available, easily calculable, and accurate, it has some limitations (i.e., extremes of age, some chronic illnesses, and medications). It has been noted that the normal SI values differ among age groups and between sexes, which is rarely considered when it is studied.[42]
To the best of our knowledge, this is the first study to assess the triage SI as a screening tool for the estimation of hemodynamic collapse and fluid nonresponsiveness. This study adds to the literature on the SI in that it tests the accuracy of the SI as an initial screening tool for the identification of hypotensive septicemic patients in the ED. This finding differs from those in other studies in that it is not based on assessments made after patients had received fluid resuscitation.
Although he SI proved to be linearly nonaccurate as an overall predictor of the need for vasopressors in our study, when used with a higher cutoff value of 0.875, it demonstrated a more robust screening ability. When combined with older age (>47), it proved even more accurate.
The SI has also shown more promise as a broader screening tool for all patients presenting to the ED with normal vital signs[43] and as a good prognostic tool comparable to elevated lactate in the severe sepsis cohort.[44]
Limitations
This study is limited by the retrospective data extraction process and a relatively small sample size. Additionally, patients were selected from a registry that was not all-inclusive, in which many clinical interventions and diagnostic data were not available. In addition, the inherent weakness of the cross-sectional design means that causality cannot be determined, only associations.
We could not determine whether patients had received appropriate fluid resuscitation and whether the administration of fluids adhered to the current guideline recommendation of 30 ml/kg, which may have affected the appropriateness of vasopressor initiation.[45] Due to the inherent nature of our database, we were not able to screen for sepsis (SIRS plus presumed infection or elevated sequential organ failure assessment score).[45,46] Rather, we used septicemia as a surrogate marker, and it is necessary to understand its limitations in terms of missing septic patients, attaining false positive blood cultures, with some cultures possibly being contaminated, and falsely identifying hypotension arising from another cause.[47]
The retrospective data extraction was limited by many factors inherent to the process, including possible errors in the medical records.
Conclusions
The SI appears to be an accurate screening modality for assessing hemodynamic collapse in hypotensive septicemic patients when used with a cutoff value of 0.875, especially in patients older than 47 years of age in this cohort. Future studies should focus on validating these findings. In addition, the SI was tested as a general screening tool for hemodynamic collapse in all hypotensive patients presenting to the ED.
Ethical approval
The study was approved by King Saud University, College of Medicine IRB (No. E-16-1878).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensiv Care Med. 2014;40:1795–815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Askim A, Moser F, Gustad LT, Stene H, Gundersen M, Asvold BO, et al. Poor performance of quick-SOFA (qSOFA) score in predicting severe sepsis and mortality-A prospective study of patients admitted with infection to the emergency department. Scand J Trauma Resusc Emerg Med. 2017;25:56. doi: 10.1186/s13049-017-0399-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborn TM, Phillips G, Lemeshow S, Townsend S, Schorr CA, Levy MM, et al. Sepsis severity score: An internationally derived scoring system from the surviving sepsis campaign database. Crit Care Med. 2014;42:1969–76. doi: 10.1097/CCM.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 5.Koch E, Lovett S, Nghiem T, Riggs RA, Rech MA. Shock index in the emergency department: Utility and limitations. Open Access Emerg Med. 2019;11:179–99. doi: 10.2147/OAEM.S178358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durukan P, Ikizceli I, Akdur O, Ozkan S, Sözüer EM, Avşaroǧullari L, et al. Use of the shock index to diagnose acute hypovolemia. Turk J Med Sci. 2009;39:833–5. [Google Scholar]
- 7.Mutschler M, Nienaber U, Münzberg M, Wölfl C, Schoechl H, Paffrath T, et al. The shock index revisited-A fast guide to transfusion requirement? A retrospective analysis on 21,853 patients derived from the TraumaRegister DGU. Crit Care. 2013;17:4. doi: 10.1186/cc12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045–53. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 9.Yussof SJ, Zakaria MI, Mohamed FL, Bujang MA, Lakshmanan S, Asaari AH. Value of shock index in prognosticating the short-term outcome of death for patients presenting with severe sepsis and septic shock in the emergency department. Med J Malaysia. 2012;67:406–11. [PubMed] [Google Scholar]
- 10.Lanspa MJ, Brown SM, Hirshberg EL, Jones JP, Grissom CK. Central venous pressure and shock index predict lack of hemodynamic response to volume expansion in septic shock: A prospective, observational study. J Crit Care. 2012;27:609–15. doi: 10.1016/j.jcrc.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wira CR, Francis MW, Bhat S, Ehrman R, Conner D, Siegel M. The shock index as a predictor of vasopressor use in emergency department patients with severe sepsis. West J Emerg Med. 2014;15:60–6. doi: 10.5811/westjem.2013.7.18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rady MY. The role of central venous oximetry, lactic acid concentration and shock index in the evaluation of clinical shock: A review. Resuscitation. 1992;24:55–60. doi: 10.1016/0300-9572(92)90173-a. [DOI] [PubMed] [Google Scholar]
- 13.Olaussen A, Blackburn T, Mitra B, Fitzgerald M. Review article: Shock index for prediction of critical bleeding post-trauma: A systematic review. Emerg Med Australas. 2014;26:223–8. doi: 10.1111/1742-6723.12232. [DOI] [PubMed] [Google Scholar]
- 14.DeMuro JP, Simmons S, Jax J, Gianelli SM. Application of the shock index to the prediction of need for hemostasis intervention. Am J Emerg Med. 2013;31:1260–3. doi: 10.1016/j.ajem.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Montoya KF, Charry J, Calle-Toro J, Niñez LR, Poveda G. Shock index as a mortality predictor in patients with acute polytrauma. J Acute Dis. 2015;4:202–4. [Google Scholar]
- 16.Cannon CM, Braxton CC, Kling-Smith M, Mahnken JD, Carlton E, Moncure M. Utility of the shock index in predicting mortality in traumatically injured patients. J Trauma. 2009;67:1426–30. doi: 10.1097/TA.0b013e3181bbf728. [DOI] [PubMed] [Google Scholar]
- 17.Pandit V, Rhee P, Hashmi A, Kulvatunyou N, Tang A, Khalil M, et al. Shock index predicts mortality in geriatric trauma patients: An analysis of the national trauma data bank. J Trauma Acute Care Surg. 2014;76:1111–5. doi: 10.1097/TA.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 18.McNab A, Burns B, Bhullar I, Chesire D, Kerwin A. An analysis of shock index as a correlate for outcomes in trauma by age group. Surgery. 2013;154:384–7. doi: 10.1016/j.surg.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Ali S, Agarwal A, Srivastava RN. Correlation of shock index and modified shock index with the outcome of adult trauma patients: A prospective study of 9860 patients. North American J Med Sci. 2014;6:450–2. doi: 10.4103/1947-2714.141632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNab A, Burns B, Bhullar I, Chesire D, Kerwin A. A prehospital shock index for trauma correlates with measures of hospital resource use and mortality. Surgery. 2012;152:473–6. doi: 10.1016/j.surg.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Mitra B, Fitzgerald M, Chan J. The utility of a shock index >/= 1 as an indication for pre-hospital oxygen carrier administration in major trauma. Injury. 2014;45:61–5. doi: 10.1016/j.injury.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Guo ZJ, Lin Q, Zi XR, Xu Q, Liu HT, Lu JY, et al. Correlation of computed tomography angiography parameters and shock index to assess the transportation risk in aortic dissection patients. Radiol Med. 2015;120:386–92. doi: 10.1007/s11547-014-0463-3. [DOI] [PubMed] [Google Scholar]
- 23.Nakasone Y, Ikeda O, Yamashita Y, Kudoh K, Shigematsu Y, Harada K. Shock index correlates with extravasation on angiographs of gastrointestinal hemorrhage: A logistics regression analysis. Cardiovasc Intervent Radiol. 2007;30:861–5. doi: 10.1007/s00270-007-9131-5. [DOI] [PubMed] [Google Scholar]
- 24.Nathan HL, El Ayadi A, Hezelgrave NL, Seed P, Butrick E, Miller S, et al. Shock index: An effective predictor of outcome in postpartum haemorrhage? BJOG. 2015;122:268–75. doi: 10.1111/1471-0528.13206. [DOI] [PubMed] [Google Scholar]
- 25.Kahyaoglu S, Turgay I, Gocmen M, Sut N, Batioglu S. A new predictive scoring system including shock index for unruptured tubal pregnancy patients. Eur J Obstet Gynecol Reprod Biol. 2006;126:99–103. doi: 10.1016/j.ejogrb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Onah HE, Oguanuo TC, Mgbor SO. An evaluation of the shock index in predicting ruptured ectopic pregnancy. J Obstet Gynaecol. 2006;26:445–7. doi: 10.1080/01443610600747314. [DOI] [PubMed] [Google Scholar]
- 27.Bircan A, Karadeniz N, Ozden A, Cakir M, Varol E, Oyar O, et al. A simple clinical model composed of ECG, shock index, and arterial blood gas analysis for predicting severe pulmonary embolism. Clin Appl Thromb Hemost. 2011;17:188–96. doi: 10.1177/1076029609351877. [DOI] [PubMed] [Google Scholar]
- 28.Kilic T, Ermis H, Gulbas G, Kaya O, Aytemur ZA, Inceoglu F, et al. Prognostic role of the simplified pulmonary embolism severity index and shock index in pulmonary embolism. Pol Arch Med Wewn. 2014;124:678–87. doi: 10.20452/pamw.2552. [DOI] [PubMed] [Google Scholar]
- 29.Toosi MS, Merlino JD, Leeper KV. Prognostic value of the shock index along with transthoracic echocardiography in risk stratification of patients with acute pulmonary embolism. Am J Cardiol. 2008;101:700–5. doi: 10.1016/j.amjcard.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 30.Bilkova D, Motovska Z, Widimsky P, Dvorak J, Lisa L, Budesinsky T. Shock index: A simple clinical parameter for quick mortality risk assessment in acute myocardial infarction. Can J Cardiol. 2011;27:739–42. doi: 10.1016/j.cjca.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Huang B, Yang Y, Zhu J, Liang Y, Tan H, Yu L, et al. Usefulness of the admission shock index for predicting short-term outcomes in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2014;114:1315–21. doi: 10.1016/j.amjcard.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 32.Shangguan Q, Xu JS, Su H, Li JX, Wang WY, Hong K, et al. Modified shock index is a predictor for 7-day outcomes in patients with STEMI. Am J Emerg Med. 2015;33:1072–5. doi: 10.1016/j.ajem.2015.04.066. [DOI] [PubMed] [Google Scholar]
- 33.McCall SJ, Musgrave SD, Potter JF, Hale R, Clark AB, Mamas MA, et al. The shock index predicts acute mortality outcomes in stroke. Int J Cardiol. 2015;182:523–7. doi: 10.1016/j.ijcard.2014.12.175. [DOI] [PubMed] [Google Scholar]
- 34.Torabi M, Mirafzal A, Rastegari A, Sadeghkhani N. Association of triage time shock index, modified shock index, and age shock index with mortality in emergency severity index level 2 patients. Am J Emerg Med. 2016;34:63–8. doi: 10.1016/j.ajem.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Sankaran P, Kamath AV, Tariq SM, Ruffell H, Smith AC, Prentice P, et al. Are shock index and adjusted shock index useful in predicting mortality and length of stay in community-acquired pneumonia? Eur J Intern Med. 2011;22:282–5. doi: 10.1016/j.ejim.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Curtain JP, Sankaran P, Kamath AV, Myint PK. The usefulness of confusion, urea, respiratory rate, and shock index or adjusted shock index criteria in predicting combined mortality and/or ICU admission compared to CURB-65 in community-acquired pneumonia. Biomed Res Int. 2013;1:1–6. doi: 10.1155/2013/590407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nullmann H, Pflug MA, Wesemann T, Heppner HJ, Pientka L, Thiem U. External validation of the CURSI criteria (confusion, urea, respiratory rate and shock index) in adults hospitalised for community-acquired pneumonia. BMC Infect Dis. 2014;14:39. doi: 10.1186/1471-2334-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson AH, Kidd AC, Skinner J, Musonda P, Pai Y, Lunt CJ, et al. Asimple 5-point scoring system, NaURSE (Na+, urea, respiratory rate and shock index in the elderly), predicts in-hospital mortality in oldest old. Age Ageing. 2014;43:352–7. doi: 10.1093/ageing/afu002. [DOI] [PubMed] [Google Scholar]
- 39.Sato K, Yokoi H, Tsuneto S. Shock index and decreased level of consciousness as terminal cancer patients' survival time predictors: A retrospective cohort study. J Pain Symptom Manage. 2016;51:220–31e222. doi: 10.1016/j.jpainsymman.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Keller AS, Kirkland LL, Rajasekaran SY, Cha S, Rady MY, Huddleston JM. Unplanned transfers to the intensive care unit: The role of the shock index. J Hosp Med. 2010;5:460–5. doi: 10.1002/jhm.779. [DOI] [PubMed] [Google Scholar]
- 41.Trivedi S, Demirci O, Arteaga G, Kashyap R, Smischney NJ. Evaluation of preintubation shock index and modified shock index as predictors of postintubation hypotension and other short-term outcomes. J Crit Care. 2015;30:861e1–7. doi: 10.1016/j.jcrc.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Rappaport LD, Deakyne S, Carcillo JA, McFann K, Sills MR. Age- and sex-specific normal values for shock index in national health and nutrition examination survey 1999-2008 for ages 8 years and older. Am J Emerg Med. 2013;31:838–42. doi: 10.1016/j.ajem.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Rady MY, Smithline HA, Blake H, Nowak R, Rivers E. A comparison of the shock index and conventional vital signs to identify acute, critical illness in the emergency department. Ann Emerg Med. 1994;24:685–90. doi: 10.1016/s0196-0644(94)70279-9. [DOI] [PubMed] [Google Scholar]
- 44.Berger T, Green J, Horeczko T, Hagar Y, Garg N, Suarez A, et al. Shock index and early recognition of sepsis in the emergency department: Pilot study. West J Emerg Med. 2013;14:168–74. doi: 10.5811/westjem.2012.8.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 46.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long B, Koyfman A. Best clinical practice: Blood culture utility in the emergency department. J Emerg Med. 2016;51:529–39. doi: 10.1016/j.jemermed.2016.07.003. [DOI] [PubMed] [Google Scholar]


