Abstract
Background:
Intraoperative hypotension is frequently encountered during surgery and it can be associated with adverse outcomes. Blood pressure monitoring is critical during surgery, but there are no universally agreed upon standards for interpreting values of hypotension and no consensus regarding interventions.
Material and Methods:
We performed a retrospective chart review of pediatric patients who underwent idiopathic scoliosis surgery by a single surgeon. We used the arterial line for all measures. Intraoperative hypotension was defined as 20% decrease of the baseline systolic blood pressure (SBP), 30% decrease of baseline SBP, or mean arterial pressure less than 60 mmHg. Use of vasopressor agents was also recorded and correlated with blood pressure definitions.
Results:
Seventy idiopathic scoliosis patients were retrospectively evaluated. There was a significant correlation between the three measures of hypotension. Sixty percent of the patients received vasopressors. There was a significant correlation between a drop of mean arterial pressure to less than 60 mmHg and the use of the ephedrine. We did not find any changes on neuromonitoring measures during the case and there were no intraoperative or one-month postoperative complications.
Conclusions:
Blood pressure is only one of the measures anesthesiologists look to for good perfusion during surgery. Pediatric anesthesiologists and orthopedics agree in trying tight blood pressure control during surgery to decrease blood loss, but what the exact definition of that blood pressure number is, is still unclear. We propose that using mean arterial pressure less than 60 mmHg is perhaps a better definition. We provide recommendations for future studies.
Keywords: Hypotension, intraoperative blood pressure, pediatric anesthesia, pediatric orthopedics, scoliosis surgery, vasopressors
Introduction
The importance of monitoring and controlling blood pressure in the anesthesiology field is clear. Intraoperative hypotension is frequently encountered during surgery and it can be associated with adverse outcomes in patients having surgery. A physiologic state of the human body is to decrease organ perfusion if the arterial blood pressure decreases significantly, with possible deleterious outcomes.[1] Routine monitoring of blood pressure is, therefore, one of the recommended standards of care by the American Society of Anesthesiology. Blood pressure monitoring is critical during surgery but there are no universally agreed upon standards for interpreting values of hypotension intraoperatively and no consensus regarding interventions.[2,3]
Currently, there is no clinically meaningful definition of intraoperative hypotension in children. Pediatric anesthesiologists typically diagnose and treat based on deviations from what they individually consider normal blood pressure values, but there is also no standard for deviation of a normal blood pressure. In 2009, Nafiu et al.[4] sent a survey to 860 pediatric anesthesiologists, of which only 56% responded. Eighty-seven percent of the responders indicated that they used mean arterial pressure (MAP) or systolic blood pressure (SBP) to define intraoperative hypotension. Seventy-two percent indicated that the baseline blood pressure was important for defining hypotension, with 78% indicating that a decrease in SBP of 20% to 30% from baseline values would be considered significant hypotension in healthy pediatric patients. In addition, 55% answered that the type of surgery influences the decision of when to treat hypotension. Hypotensive anesthesia is commonly used to reduce surgical blood loss and improve surgical visualization.[5] In a study published in 2014, a decrease of SBP higher than 20% was often chosen to define intraoperative hypotension in adult patients under general anesthesia.[6]
Corrective scoliosis surgery (spinal fusion) in pediatric patients is typically a very long procedure and is prone to significant blood loss making controlling hypotension an effective technique to reduce intraoperative blood loss. The lack of a consensus definition of pediatric intraoperative hypotension also leads to variability in therapeutic management. Trendelenburg positioning, reducing anesthesia, performing volume challenges, and administering vasopressors are frequently used therapeutic measures. Administration of vasopressors has been incorporated in some intraoperative hypotension definitions.[7,8] Franck et al. performed a systematic review of articles from 2000 to 2006 to determine whether documented intraoperative hypotension, according to the three most common intraoperative hypotension definitions in the literature (MAP less than 60 mmHg, greater than 20% decrease in SBP from preoperative SBP baseline, or greater than 30% decrease in SBP from preoperative SBP), coincided with the use of hypotensive treatment medications.[9] They found that documented intraoperative hypotension according to any of the three definitions was not always associated with administration of antihypotensive medications, and that SBP of greater than 30% of the preoperative SBP baseline was the most sensitive response to the treatment.
The purpose of this study was to examine the differences in three specific definitions of hypotension, including a correlation of hypotension with use of vasopressors and adverse outcomes during a spinal instrumentation and fusion in pediatric scoliosis surgery, using previous definitions of intraoperative hypotension defined in our studies.[9]
Material and Methods
This study was approved by our institutional review board. We performed a retrospective chart review of pediatric patients, ages 12 through 17 years, who underwent corrective scoliosis surgery by the same surgeon between 2013 and 2016. We excluded all patients with neuromuscular scoliosis, anemia, blood disorder, or coagulopathy disorder. We used a single-surgeon cohort of patients with different anesthesiologists, five in total. The anesthesia protocol was the same for all cases, and consisted of using total intravenous anesthesia with propofol and remifentanil. Adverse events were obtained from the electronic medical records (Epic Systems Corporation, Verona WI USA). Adverse events were defined as intraoperative neuromonitoring changes or any postoperative complication from anesthesia or surgery causing end-organ damage or neurologic deficit due to hypotension. Neuromonitoring included somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP). Baseline blood pressure was the first blood pressure reading taken by the noninvasive cuff method when the patient was in the operating room before induction. Systolic, diastolic, and mean blood pressure measurements were obtained from the arterial line. Intraoperative hypotension was defined in three ways: 20% decrease of the baseline SBP; 30% decrease of baseline SBP; or MAP less than 60 mmHg. The total time (minutes) and relative time (percent of procedure time spent in each intraoperative hypotension category) were computed for each intraoperative hypotension category. Use of vasopressor agents (identity and amount) was also recorded. We also recorded blood loss, blood products transfusions, and intravenous fluids. Descriptive statistics were expressed as the mean ± standard deviation. Correlations were done using either the Pearson r or Spearman r depending on whether data were normally distributed. Quantitative variables (e.g., time in hypotension) were compared between the three categories of intraoperative hypotension using analysis of variance with Dunn's multiple comparisons test for post-hoc analyses. All analyses were done using Instat 3.1 (GraphPad Software, San Diego, CA USA).
Results
Seventy patients with idiopathic scoliosis were retrospectively evaluated. Patient demographics [Table 1] were predominantly females (75%), Caucasian (62%), and having a mean age of 14 years. Estimated blood loss was 232 ± 168 ml and total intravenous fluids given were 978 ± 358 ml. There was a statistically significant relationship with the low blood loss (232 ± 168 ml) and MAP less than 60 mmHg (p = 0.004). None of the cases received blood product transfusion. There were no intraoperative or one month after surgery complications. For a little over half of the procedure time, patients had more than a 20% decrease in SPB [Table 2]. There was a significant correlation between the three measures of hypotension. Sixty percent of the patients received phenylephrine, ephedrine, or both. Mean doses received were phenylephrine 167 ± 230 mcg and ephedrine 9.6 ± 6.6 mg. Vasopressor utilization was associated with an MAP less than 60 mmHg, but not the other intraoperative hypotension definitions [Table 3]. There was a significant (r = 0.29) correlation between drop of MAP to less than 60 mmHg and the usage of the vasopressor ephedrine, but not phenylephrine [Figure 1]. Compared with no vasopressors (Mann-Whitney test), the relative risk of receiving vasopressor if MAP is less than 60 mmHg for more than 50 minutes was 1.7 [Table 4].
Table 1.
Demographic and Intraoperative Variables
| Variable | Mean±SD |
|---|---|
| Age, years | 14.1±2.1 |
| Weight, kg | 55.6±11.6 |
| Body mass index | 21.9±4.4 |
| Estimated blood loss, ml | 232±168 |
| Total fluids given, ml | 978±358 |
| Levels fused | 10±1.8 |
| Procedure time, min | 222±41 |
| Anesthesiology time, min | 301±45 |
Table 2.
Comparison of Time in Hypotension
| MAP <60 mmHg | SBP >20% | SBP >30% | ANOVA | |
|---|---|---|---|---|
| Minutes of procedure | 51.8±33.7 | 123.4±68.8 | 38.6±43.7 | P<0.0001* |
| Percent of procedure | 23.1±15 | 56.5±30.3 | 17.5±19.6 | P<0.0001* |
| n=70 | ||||
*Dunn’s Multiple Comparison Test: MAP vs. >20%, P<0.001; >20% vs. >30%, P<0.001; MAP vs. >30%, not significantly different. ANOVA: Analysis of variance; MAP: Mean arterial pressure; SBP: Systolic blood pressure
Table 3.
Correlation Coefficients Between Intraoperative Hypotension Categories and Case Data
| Variable | Comparison | Correlation coefficient (r) |
|---|---|---|
| Percent time of case in hypotension | MAP <60 vs. SBP >20% | r=0.24, P=0.05 |
| MAP <60 vs. SBP >30% | r=0.22. P=0.07 | |
| SBP >20% vs. SBP >30% | r=0.74, P<0.0001 | |
| Any pressor usage | Minutes MAP <60 | r=0.32, P=0.01 |
| Minutes SBP >20% | r=0.06, P=0.64 | |
| Minutes SBP >30% | r=-0.004, P=0.97 | |
| % time of case, MAP <60 | r=0.30, P=0.01 | |
| % time of case, SBP >20% | r=0.003, P=0.98 | |
| % time of case, SBP >30% | r=0.03, P=0.83 | |
| Estimated blood loss | Minutes MAP <60 | r=0.34, P=0.004 |
| Minutes SBP >20% | r=0.11, P=0.36 | |
| Minutes SBP >30% | r=0.12, P=0.32 | |
| Procedure time | r=0.41, P=0.0004 | |
| Total fluids given during case | Minutes MAP <60 | r=0.09, P=0.44 |
| Minutes SBP >20% | r=0.15, P=0.24 | |
| Minutes SBP >30% | r=0.24, P=0.05 |
MAP: Mean arterial pressure; SBP: Systolic blood pressure
Figure 1.
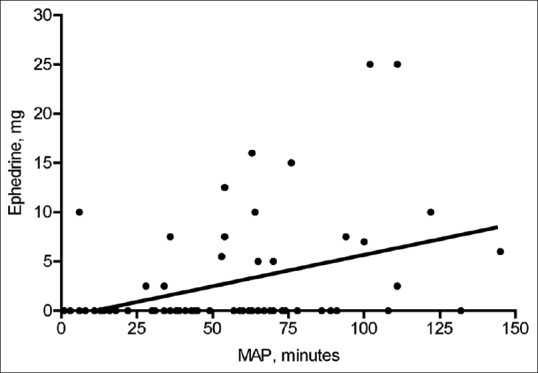
Scatter plot of time spent with mean arterial pressure less than 60 mmHg vs. ephedrine dose. A linear correlation was r = 0.39, P = 0.0008
Table 4.
Minutes of Hypotension in Patients Receiving Vasopressor vs. No Vasopressor
| MAP <60 mmHg | SBP >20% | SBP >30% | ANOVA | |
|---|---|---|---|---|
| Phenylephrine or ephedrine | 59.8±32.6 (42)* | 130±60.8 | 35.3±40.7 | P<0.0001 |
| No vasopressor | 39.6±32.3 (28) | 113±79.4 | 43.4±48.2 | P=0.0007 |
*Dunn’s Multiple Comparison Test: MAP vs. >20%, P<0.001; >20% vs. >30%, P<0.001; MAP vs. >30%, not significantly different. ANOVA: Analysis of variance; MAP: Mean arterial pressure; SBP: Systolic blood pressure
Discussion
In this study, we found significant variability in the correlation, duration, and treatment of hypotension in pediatric idiopathic spinal fusion patients using the three accepted definitions of hypotension. While no single blood pressure reading is sufficient to trigger the treatment of hypotension during surgery in all patients, the type of surgery, precipitating status, physical status, and comorbidities should be considered individually. Data from pediatric and adult trauma victims suggest that SBP is a predictor of mortality.[10] Recent adult data also suggest that intraoperative hypotension may be associated with early and long-term postoperative morbidity and mortality after one year.[11,12] In the current study, there were no adverse outcomes intraoperatively nor upon discharge from hospital or one-month follow up after surgery.
From this study, we concluded that although there is some correlation, the three different definitions of hypotension cannot be used interchangeably. The most sensitive indicator for clinically significant hypotension in this study was the correlation between ephedrine use and MAP of less than 60 mmHg. This is different from the Franck et al.[9] study where the most sensitive definition related to antihypotensive medications was a drop in SBP of more than 30%. The use of MAP less than 60 mmHg could be a better guidance measure of intraoperative blood pressure as it relates to maintenance of perfusion and avoidance of intraoperative and postoperative adverse events. There were no documented changes on SSEP or MEP. The amount of time in hypotension was significant, possibly due to the belief that lower blood pressure is desirable in spine surgery to decrease blood loss and improve visualization. However, we found no correlation between blood loss and hypotension, which may be helpful in advocating for higher blood pressures and avoiding prolonged hypotensive episodes.
Although we collected our study data from an automated capturing system to decrease the likelihood of data collection inaccuracies and used the invasive arterial line to provide a more real-time measure of blood pressures, we still faced several study limitations. First, the baseline blood pressure was difficult to assess due to different factors that can interfere with this measure before surgery including anxiety, pain, and hypovolemia from the required presurgical fasting. In our small study, we took the baseline blood pressure as the first blood pressure reading when the patient was in the operating room before induction. This baseline can also be different due to anxiolytic effects of medication given preoperatively. The current study also only utilized one surgeon to try and control for individual practice variation and also control for anesthesia protocol; however, we did not control the anesthesia team. An expanded, retrospective study involving multiple practitioners will likely find more variability, have unanticipated confounders, and ultimately may obscure a more definitive conclusion. However, a bigger randomized prospective study, with a control group in children undergoing general anesthesia to provide evidence to guide treatment decisions regarding intraoperative hypotension, would not likely be ethical. Further, intraoperative hypotension is also only one of the measures anesthesiologists look to for good perfusion during surgery; therefore, consideration of other parameters, such as urine output, neuromonitoring, and peripheral perfusion, should also be included in future work with the goal of creating a perfusion scoring system that can be used to alert anesthesia providers to an imminent need for pharmacologic intervention and possible avoidance of complications. More pediatric studies are needed since this type of surgery is more common in the last couple of decades compared with more conservative treatments tried in the past.
Conclusions
Blood pressure is only one of the measures anesthesiologists look to for good perfusion during surgery. Pediatric anesthesiologists and orthopedics agree in trying tight blood pressure control during surgery to decrease blood loss, but what the exact definition of that blood pressure number is, is still unclear. We propose that using mean arterial pressure less than 60 mmHg is perhaps a better definition. We provide recommendations for future studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge Dr. Robert Stanton for his support.
References
- 1.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28:310. doi: 10.1016/0022-3468(93)90223-8. [DOI] [PubMed] [Google Scholar]
- 2.Klasen J, Junger A, Hartmann B, Benson M, Jost A, Banzhaf A, et al. Differing incidences of relevant hypotension with combined spinal-epidural anesthesia and spinal anesthesia. Anesth Analg. 2003;96:1491–5. doi: 10.1213/01.ANE.0000057601.90930.18. [DOI] [PubMed] [Google Scholar]
- 3.Eichhorn JH, Cooper JB, Cullen DJ, Maier WR, Philip JH, Seeman RG. Standards for patient monitoring during anesthesia at Harvard medical school. JAMA. 1986;256:1017–20. [PubMed] [Google Scholar]
- 4.Nafiu OO, Voepel-Lewis T, Morris M, Chimbira WT, Malviya S, Reynolds PI, et al. How do pediatric anesthesiologists define intraoperative hypotension? Pediatr Anesth. 2009;19:1048–53. doi: 10.1111/j.1460-9592.2009.03140.x. [DOI] [PubMed] [Google Scholar]
- 5.Oh S, Bang SU, Kang BG. The effect of induced hypotension on the perioperative bleeding and transfusion in the bipolar hemiarthroplasty of hip: Retrospective study for four years. Korean J Anesthesiol. 2013;65(6 Suppl):41–3. doi: 10.4097/kjae.2013.65.6S.S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonjaret L, Lairezo O, Minville V, Geeraerts T. Optimal perioperative management of arterial blood pressure. Integr Blood Press Control. 2014;7:49–59. doi: 10.2147/IBPC.S45292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann B, Junger A, Klasen J, Benson M, Jost A, Banzhaf A, et al. The incidence and risk factors for hypotension after spinal anesthesia induction: An analysis with automated data collection. Anesth Analg. 2002;94:1521–9. doi: 10.1097/00000539-200206000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Röhrig R, Junger A, Hartmann B, Klasen J, Quinzio L, Jost A, et al. The incidence and prediction of automatically detected intraoperative cardiovascular events in noncardiac surgery. Anesth Analg. 2004;98:569–77. doi: 10.1213/01.ane.0000103262.26387.9c. [DOI] [PubMed] [Google Scholar]
- 9.Franck M, Radtke FM, Prahs C, Seeling M, Papkalla N, Wernecke KD, et al. Documented intraoperative hypotension according to the three most common definitions does not match the application of antihypotensive medication. J Int Med Res. 2011;39:846–56. doi: 10.1177/147323001103900318. [DOI] [PubMed] [Google Scholar]
- 10.Reich DL, Hossain S, Krol M, Baez B, Patel P, Bernstein A, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–8. doi: 10.1213/01.ANE.0000175214.38450.91. [DOI] [PubMed] [Google Scholar]
- 11.Monk TG, Saint V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 12.Bijker JB, Persoon S, Peelen LM, Moons KG, Kalkman CJ, Kappelle LJ, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: A nested case-control study. Anesthesiology. 2012;116:658–64. doi: 10.1097/ALN.0b013e3182472320. [DOI] [PubMed] [Google Scholar]


