Abstract
PURPOSE
The relapse rate after primary retroperitoneal lymph node dissection (RPLND) for patients with pathologic stage (PS) IIA nonseminomatous germ cell tumors (NSGCTs) is 10%-20% but increases to ≥ 50% for PS IIB disease. We report our experience with 2 cycles of adjuvant etoposide plus cisplatin (EP×2) after therapeutic primary RPLND.
PATIENTS AND METHODS
All patients with PS II NSGCT seen at Memorial Sloan Kettering Cancer Center from March 1989 to April 2016 and who were planned to receive EP×2 were included. Each cycle consisted of cisplatin 20 mg/m2 and etoposide 100 mg/m2 on days 1 through 5 at 21-day intervals. Demographic characteristics, histopathologic features, therapeutic and survival outcomes were recorded.
RESULTS
Of 156 patients, 30 (19%) had pathologic N1, 122 (78%) had pathologic N2 (pN2), and 4 (3%) had pathologic N3 (pN3) disease. The median number of involved lymph nodes was 3 (range, 1-37 nodes), and the median size of the largest involved node was 2.0 cm (range, 0.4-7.0 cm); extranodal extension was present in 69 patients (45%). Embryonal carcinoma was the most frequent RPLND histology, present in 143 patients (92%). One hundred fifty patients (96%) received EP×2, five received EP×1 and one received EP×4. With a median follow-up of 9 years, 2 patients (1.3%; 1 patient each with pN2 and pN3 disease) experienced relapse; both patients remain continuously disease free at more than 5 and 22 years after salvage chemotherapy. Three patients died, all unrelated to NSGCT, yielding 10-year disease-specific, relapse-free, and overall survival rates of 100%, 98%, and 99%, respectively.
CONCLUSION
Adjuvant EP×2 for PS II NSGCT is highly effective, has acceptable toxicity, and incurs less drug cost than 2 cycles of bleomycin, etoposide, and cisplatin. Inclusion of bleomycin in this setting is not necessary.
INTRODUCTION
Primary retroperitoneal lymph node dissection (RPLND) remains a standard treatment option for patients with marker-negative clinical stage (CS) I and low-volume CS II nonseminomatous germ cell tumors (NSGCTs).1 For patients with proven regional lymph node metastases at RPLND (pathologic stage [PS] II NSGCT), the following 2 management options exist: adjuvant chemotherapy and active surveillance. Provided patient compliance is optimal, patients with pathologic N1 (pN1) disease (defined as ≤ 5 nodes positive, all nodes ≤ 2 cm, and no extranodal extension) have a relapse rate of 10%-20%, and surveillance is usually favored.2 Conversely, because up to 50% or more of patients with pathologic N2 (pN2) disease (defined as > 5 nodes involved, any node ≥ 2 cm but ≤ 5 cm, or extranodal extension) will experience relapse on observation alone,3 management with 2 cycles of adjuvant chemotherapy is often preferred.4 Patients with pN1 disease who are unlikely or unable to adhere to a surveillance protocol should also receive adjuvant chemotherapy. Currently, the accepted adjuvant chemotherapy regimens consist of 2 cycles of either bleomycin, etoposide, and cisplatin (BEP×2) or etoposide plus cisplatin (EP×2).5-7 In reported series, with median follow-up times of 7 and 8 years, respectively, the disease-specific survival was 100%.
Although 2 cycles of adjuvant cisplatin-based chemotherapy reduce the risk of relapse to approximately 1%, questions regarding patient selection and the necessity of bleomycin remain unanswered. Considering the excellent disease-specific survival regardless of adjuvant strategy, minimization of toxicity while maintaining optimal efficacy is critical.8 We report our experience with the efficacy and tolerability of adjuvant EP×2.
PATIENTS AND METHODS
From March 1989 to April 2016, 156 patients with NSGCT were treated with adjuvant etoposide and cisplatin (EP) at Memorial Sloan Kettering Cancer Center (MSKCC) for PS II NSGCT after primary RPLND. All patients had normal or normalizing serum tumor markers and no radiographic evidence of metastases after RPLND. Nodal pathology was classified according to the American Joint Committee on Cancer (AJCC) staging system.9
The boundaries of a bilateral or modified bilateral RPLND, including the nodal dissection templates for right- and left-sided testis tumors, have been previously described.7 Nerve-sparing technique with prospective dissection and preservation of postganglionic sympathetic fibers, the hypogastric plexus, and both sympathetic chains was performed in most patients.3 The adjuvant EP regimen has been previously described.6 In brief, 2 cycles of etoposide 100 mg/m2 plus cisplatin 20 mg/m2 per day were administered on days 1-5 of each cycle accompanied by standard hydration and antiemetic regimens. The second cycle was planned to begin on day 22. If the total WBC was < 2,500/μL on day 22, cycle 2 was delayed for a maximum of 1 week.10 Patient characteristics (age at chemotherapy, AJCC staging before RPLND, baseline serum tumor markers before starting chemotherapy, and histopathology at orchiectomy) were captured for all patients. RPLND details included date, the total number of nodes, the number of positive nodes, and size of largest lymph node(s). All reported histologic subtypes at RPLND were recorded, including the predominant subtype and the presence of extranodal extension. The total number of chemotherapy cycles received and number of dose delays were recorded.
Disease-specific, relapse-free, and overall survival outcomes were calculated using the Kaplan-Meier method11; the survival outcomes for the 87 previously reported patients were updated.7 Relapse-free survival was defined as the time from the start of chemotherapy to the date of relapse. Patients who were alive and continuously disease free were censored at the date of their last follow-up. The site and method of detection (radiologic, biochemical, or both) of relapse were determined, and the salvage regimen and response to salvage therapy were recorded. Regimen drug cost was estimated using Centers for Medicare and Medicaid Services Average Sales Price (ASP) Drug Pricing.12 The institutional review board approved this retrospective analysis.
RESULTS
Patient Characteristics and Treatment
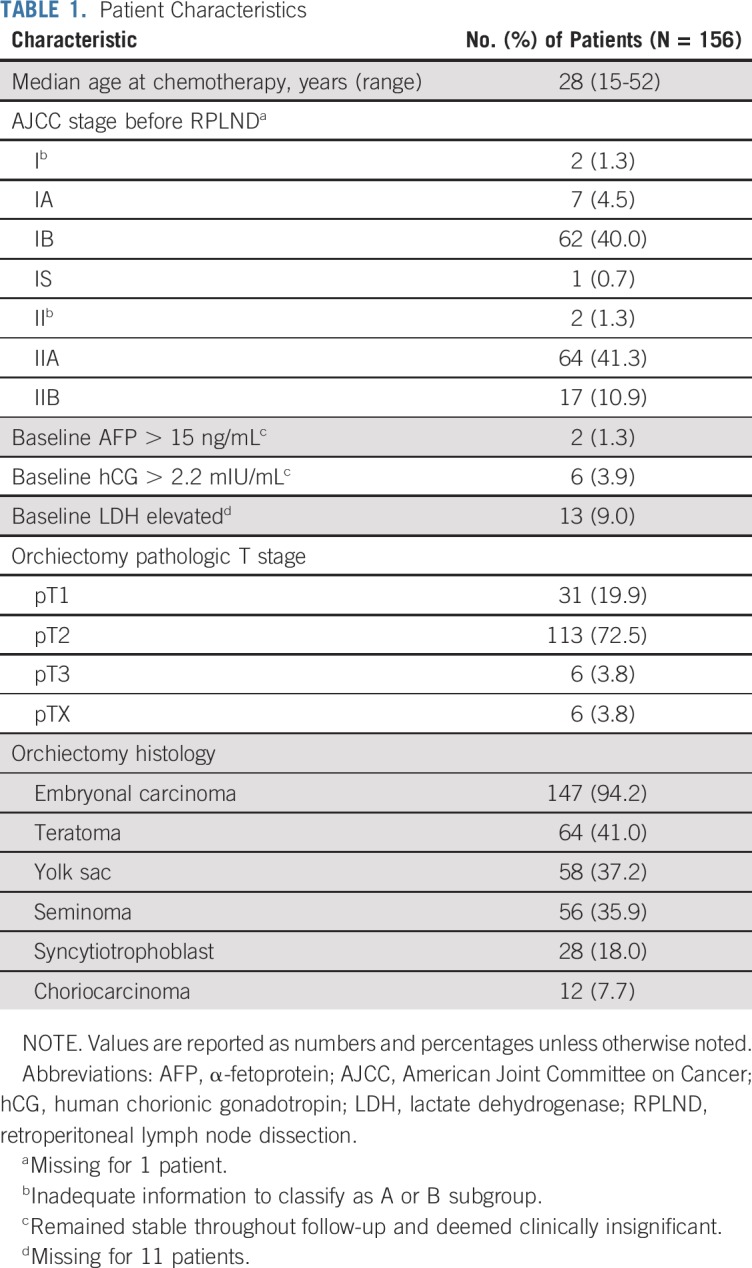
The median age was 28 years (Table 1). Before chemotherapy, minimal baseline elevations in α-fetoprotein (AFP; > 15 ng/mL) and human chorionic gonadotropin (hCG; > 2.2 mIU/mL) were seen in 2 patients (1%) and 6 patients (4%), respectively. One of these patients was treated with EP×4 for an increase in AFP during his first cycle (see later discussion in Adjuvant Chemotherapy). One patient with elevated baseline hCG (3.5 mIU/mL) before chemotherapy experienced relapse after a complete response to EP×2 (see later discussion in Relapse-Free and Overall Survival). Marker values of the remaining 4 patients remained stable throughout follow-up (3+, 10+, 15+, and 21+ years) and were deemed clinically insignificant. Seventy-two percent and 20% of patients had pathologic T2 and pathologic T1 disease at orchiectomy, respectively. In the orchiectomy specimen, embryonal carcinoma was the most common histologic component, observed in 94% of patients, followed by teratoma (41%). Information about intratubular germ cell neoplasia in the orchiectomy specimen was available for 152 patients, and was present in 128 (84%). The median time between orchiectomy and primary RPLND was 39 days. While awaiting RPLND, disease was upstaged from stage I to stage II based on postorchiectomy imaging in 19 patients (12%). Before RPLND, the majority of patients had either CS IB (40%) or CS IIA (41%) disease (Table 1).
TABLE 1.
Patient Characteristics
RPLND
Most patients underwent either a full (67%) or modified (14%) bilateral RPLND (Table 2). Almost 95% of patients had their RPLND performed at MSKCC. All patients who underwent ipsilateral RPLND at MSKCC did so before 1999; bilateral RPLND was performed exclusively thereafter. Among 124 patients with total nodal yield available from the histopathology report, the median number of lymph nodes resected was 44 (range, 6-129 nodes). The median number of positive lymph nodes present was 3 (range, 1-37 nodes), and the median size of the largest positive node was 2.0 cm (range, 0.4-7.0 cm). Embryonal carcinoma was the most frequent histology (n = 143, 92%) observed in the RPLND specimens and was also the predominant histologic component in 115 (90%) of the 128 patients for whom this information was available. The most common pathologic lymph node stage was pN2, present in 122 patients (78%).
TABLE 2.
RPLND Characteristics

Adjuvant Chemotherapy
The median time from RPLND to chemotherapy was 26 days (range, 1-73 days). Six patients (4%) did not receive EP×2. Five patients (3%) received 1 cycle of EP as a result of small bowel obstruction, Clostridium difficile infection, and poor wound healing after adhesiolysis (n = 1); acute renal failure (n = 1); ascites (n = 2); and patient refusal (n = 1). One patient who had mildly elevated hCG and AFP before chemotherapy received EP×4 as a result of an increasing AFP after his first cycle, which was felt to be consistent with marker surge in the setting of good-risk micrometastatic disease. This patient is free of disease at more than 19 years from the start of chemotherapy.
Relapse-Free and Overall Survival
The median follow-up time for survivors was 9 years (range, 2 months to 26 years), with distribution illustrated in Appendix Figure A1 (online only); among the original 87 patients, the median follow-up time was 15 years. There were 3 deaths among the 156 patients, all of which were unrelated to NSGCT or treatment, yielding 10-year disease-specific, relapse-free, and overall survival rates of 100%, 99%, and 98%, respectively (Figs 1 and 2). One patient died at age 34 years in a motor vehicle accident; 1 patient died at age 47 years secondary to complications of metastatic colon cancer; and 1 patient died of unknown causes but was disease free from NSGCT at 11.5 years from the start of chemotherapy. Two patients experienced relapse, both within 6 months of starting adjuvant chemotherapy. The first patient, who had a mildly elevated hCG of 3.5 mIU/mL before receiving adjuvant EP×2, experienced relapse after 143 days, with an increasing hCG and new adenopathy in the upper retroperitoneum and mediastinum. The second patient, who had embryonal carcinoma within a 5.5-cm nodal mass (pathologic N3 [pN3]) at RPLND, experienced relapse after 120 days with increasing AFP and hCG and a new solitary liver metastasis. Both patients achieved a complete response to salvage chemotherapy with 4 cycles of paclitaxel, ifosfamide, and cisplatin (TIP)13 and are disease free at more than 5 and 22 years, respectively, from the start of TIP.
FIG 1.
Overall survival.
FIG 2.
Relapse-free survival.
Toxicity
Of 150 patients who received EP×2, 140 patients were evaluable for dose delay (Table 3). Dose delays (≥ 7 days) occurred in 54 patients (36%) and were predominantly a result of neutropenia, occurring in 46 patients (31%; Table 3). Of the 46 patients with dose delays as a result of neutropenia, 41 (86%) had WBC nadir values available, with a median WBC nadir of 1,800/μL (range, 700-3,600/μL). Only seven (5%) of 150 patients were hospitalized for neutropenic fever; 5 of these patients had a 7-day delay in the administration of cycle 2.
TABLE 3.
Chemotherapy Treatment

Drug Cost
Using publicly available drug cost information, the drug costs, based on ASP in July 2019, for a 1.8 m2 male are $180.04 for EP×2 and $511.68 for BEP×2 (Appendix Table A1, online only).12 Bleomycin costs were determined from unit costs for a total of 30 units received weekly for 6 weeks.
DISCUSSION
This series represents the largest group of patients who received adjuvant chemotherapy for PS II NSGCT and reaffirms the efficacy of EP×2. At a median follow-up time of 9 years, disease-specific survival was 100% and 10-year relapse-free survival was 98.7%. The 1.3% relapse rate is the same as, or less than, the relapse rate in other adjuvant trials.1,3,4,13,14 Two patients experienced relapse, both within 6 months of completing EP, and both achieved durable relapse-free survival with salvage TIP chemotherapy. One patient with CS IIB disease had pN3 disease before receiving adjuvant chemotherapy. Today, this patient would have received EP×4 or BEP×3 rather than a primary RPLND.4 The Indiana University experience in 86 patients with adjuvant BEP×2 revealed that 49 patients (57%) had PS IIA disease and 37 patients (43%) had PS IIB disease (Table 4).5 These proportions of stage IIA and stage IIB disease contrast with those in our series, in which 81% of patients had pN2 or pN3 disease. With almost double the number of patients treated and more than triple the number of patients with pN2 or pN3 disease, EP×2 demonstrated survival outcomes that are the same as BEP×2. Because EP×2 and BEP×2 are equally effective, the major contemporary questions are the optimal management of CS IB NSGCT (RPLND v surveillance v adjuvant BEP) and CS IIA NSGCT and the comparative clinical toxicity and financial cost of adjuvant BEP×2 or EP×2 in patients with PS II NSGCT.
TABLE 4.
Adjuvant Chemotherapy in Patients With Pathologic Stage II NSGCT
The debate regarding surveillance, BEP×1, and RPLND for CS IB NSGCT (T2-4 primary tumor but no other evidence of disease) has been extensively reviewed.14,15 At MSKCC, we prefer a nerve-sparing RPLND to maximally limit chemotherapy exposure.16 The management of marker-negative CS IIA disease has not been as heavily debated. Because between 25% and 40% of CS IIA patients are found to have PS I disease at RPLND3,17 and do not need chemotherapy, we prefer RPLND, thereby avoiding chemotherapy in most patients proven to have PS I disease. A primary RPLND should be limited to patients with normal markers or markers that have normalized; patients with CS IS or CS IIA disease and an elevated (marker half-life taken into consideration) or increasing AFP and/or hCG should receive primary chemotherapy.1
One limitation of this study is that all RPLNDs were performed at MSKCC by highly experienced surgeons, potentially decreasing the risk of relapse. Nodal dissections performed at lower volume centers, with less experienced surgeons, could be less complete. Once the primary RPLND is performed, most patients with pN1 disease (relapse rate of approximately 10%-20%) should be observed, unless compliance or other factors suggest that the benefit of adjuvant chemotherapy exceeds the risk of unnecessary chemotherapy. Patients with pN2 disease are usually offered adjuvant chemotherapy because surveillance followed by relapse would require a greater amount of chemotherapy and, therefore, a greater risk of short- and long-term toxicity. Although the presence of extranodal extension did not affect the recurrence risk in one retrospective analysis,18 most studies (and the AJCC staging system) have included this criterion, and we continue to do so at MSKCC.
Toxicity is 1 of 2 major issues in the choice of chemotherapy regimen once pN2 disease is established. Although myelosuppression is observed with both regimens, febrile neutropenia occurred in 5% of patients receiving EP×2 and 12% of patients who received BEP×25 (Table 4). In one small study, BEP×2 was associated with more frequent grade 2-3 leukopenia than EP×2.19 Febrile reactions may occur within 48 hours in up to 50% of patients treated with intravenous bleomycin.20 Bleomycin-induced pulmonary toxicity and Raynaud phenomenon21 are other well-recognized adverse effects of BEP. Although clinical pulmonary and vascular toxicity are rare when treatment is limited to 2 cycles, a recent meta-analysis of 25 trials examined the pulmonary toxicity of regimens containing or not containing bleomycin to treat metastatic disease and showed that bleomycin administration was significantly associated (P = .012) with all-grade pulmonary toxicity when compared with no bleomycin administration.22 The authors noted that an insufficient sample size prevented an assessment of a linear relationship between doses of bleomycin and toxicity. In a United Kingdom–based prospective evaluation of BEP×2 in the setting of high-risk stage I NSGCT, no symptomatic respiratory dysfunction was reported. However, of 16 patients with pretreatment pulmonary function testing (PFT) and repeat PFT at least 9 months after treatment, 15 patients showed a significant mean decrease (15%; range, 2%-36%; P = .002) in transfer factor coefficient (KCO).23 The MRC TE17 trial studied the use of 2 cycles of bleomycin, vincristine, and cisplatin in high-risk NSGCT, with pretreatment and posttreatment PFT at 12 and 24 months. Similarly, although there was no clear evidence of clinically relevant changes, there was a significant mean reduction in KCO (5%; P = .03).24 The risk of pulmonary or vascular sequelae is essentially eliminated with EP×2.
The cost of drug therapy is an increasingly recognized and important consideration. Because the doses of etoposide and cisplatin in BEP and EP are the same, the drug cost difference between the 2 regimens is attributable to bleomycin administration. Using publicly available drug cost information, bleomycin in BEP×2 increases the total drug cost by $331.64 per patient (Appendix Table A1).12 This additional cost of bleomycin does not include the additional facility fee charges and possible professional fees for the weekly bleomycin visit. In addition, the more frequent episodes of febrile neutropenia associated with BEP would also add financial cost to the clinical risk described earlier.
BEP×2 and EP×2 are unlikely to be directly compared in a sufficiently powered study to determine the optimal adjuvant chemotherapy regimen for PS II NSGCT. Because bleomycin administration adds cost and potential toxicity, we believe EP×2 should be considered the preferred treatment regimen in patients with pN2 NSGCT after RPLND.
ACKNOWLEDGMENT
We thank Leonard Saltz, MD, Chair, Pharmacy and Therapeutics Committee, and Amelia Chan, RPh, Pharmacy, for their information and comments.
APPENDIX
FIG A1.
Distribution of follow-up time for patients with pathologic stage II nonseminomatous germ cell tumors treated with adjuvant etoposide plus cisplatin.
TABLE A1.
Estimated Difference in Cost for BEP×2 and EP×212
PRIOR PRESENTATION
Presented in part at the 2018 American Society of Clinical Oncology Genitourinary Cancers Symposium, San Francisco, CA, February 8-10, 2018, and in part at the 54th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018.
SUPPORT
Supported by National Institutes of Health (NIH) P30 Cancer Center Support Grant No. P30 CA008748, NIH Award No. R25CA020449, and the Tifford Fund.
AUTHOR CONTRIBUTIONS
Conception and design: Deaglan J. McHugh, Samuel A. Funt, Sujata Patil, Robert J. Motzer, Darren R. Feldman
Financial support: Darren R. Feldman
Administrative support: George J. Bosl, Darren R. Feldman
Provision of study materials or patients: Victor E. Reuter, Robert J. Motzer, George J. Bosl, Darren R. Feldman
Collection and assembly of data: Deaglan J. McHugh, Samuel A. Funt, Deborah Silber, Devon O’Donnell, Stephanie Tsai, Victor E. Reuter, Brett S. Carver, Robert J. Motzer, Darren R. Feldman
Data analysis and interpretation: Deaglan J. McHugh, Samuel A. Funt, Deborah Silber, Andrea Knezevic, Sujata Patil, Stephanie Tsai, Joel Sheinfeld, Brett S. Carver, Robert J. Motzer, Dean F. Bajorin, George J. Bosl, Darren R. Feldman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Adjuvant Chemotherapy With Etoposide Plus Cisplatin for Patients With Pathologic Stage II Nonseminomatous Germ Cell Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Deaglan J. McHugh
Consulting or Advisory Role: Progenics
Samuel A. Funt
Stock and Other Ownership Interests: Kite Pharma, Urogen Pharma (I), Hubble (I), Second Science, Allogene Therapeutics, Neogene Therapeutics (I), Kronos Bio (I), Vida Ventures (I)
Consulting or Advisory Role: AstraZeneca/MedImmune (Inst)
Research Funding: Genentech (Inst), AstraZeneca (Inst), Decibel Therapeutics (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, AstraZeneca/MedImmune
Victor E. Reuter
Consulting or Advisory Role: Cepheid
Uncompensated Relationships: PaigeAI
Robert J. Motzer
Consulting or Advisory Role: Pfizer, Novartis, Eisai, Exelixis, Merck, Genentech, Incyte, Eli Lilly
Research Funding: Pfizer (Inst), Bristol-Myers Squibb (Inst), Eisai (Inst), Novartis (Inst), Genentech (Inst)
Dean F. Bajorin
Honoraria: Merck Sharp & Dohme
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Genentech, Merck, Roche, Eli Lilly, Fidia Farmaceutici S.p.A., Urogen Pharma, Pfizer, EMD Serono
Research Funding: Novartis (Inst), Genentech (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Seattle Genetics/Astellas (Inst)
Travel, Accommodations, Expenses: Genentech, Merck, Bristol-Myers Squibb, Eli Lilly, Urogen Pharma
Darren R. Feldman
Research Funding: Novartis, Seattle Genetics, Decibel Therapeutics (Inst), Astellas Pharma
Other Relationship: UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1.Stephenson AJ, Bosl GJ, Motzer RJ, et al. Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: Impact of patient selection factors on outcome. J Clin Oncol. 2005;23:2781–2788. doi: 10.1200/JCO.2005.07.132. [DOI] [PubMed] [Google Scholar]
- 2.Rabbani F, Sheinfeld J, Farivar-Mohseni H, et al. Low-volume nodal metastases detected at retroperitoneal lymphadenectomy for testicular cancer: Pattern and prognostic factors for relapse. J Clin Oncol. 2001;19:2020–2025. doi: 10.1200/JCO.2001.19.7.2020. [DOI] [PubMed] [Google Scholar]
- 3.Eggener SE, Carver BS, Sharp DS, et al. Incidence of disease outside modified retroperitoneal lymph node dissection templates in clinical stage I or IIA nonseminomatous germ cell testicular cancer. J Urol. 2007;177:937–943. doi: 10.1016/j.juro.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 4. National Comprehensive Cancer Network: NCCN clinical practice guidelines for the treatment of testicular cancer (version 1.2019). https://www.nccn.org/professionals/physician_gls/PDF/testicular.pdf.
- 5.Behnia M, Foster R, Einhorn LH, et al. Adjuvant bleomycin, etoposide and cisplatin in pathological stage II non-seminomatous testicular cancer: The Indiana University experience. Eur J Cancer. 2000;36:472–475. doi: 10.1016/s0959-8049(99)00316-0. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Sheinfeld J, Mazumdar M, et al. Etoposide and cisplatin adjuvant therapy for patients with pathologic stage II germ cell tumors. J Clin Oncol. 1995;13:2700–2704. doi: 10.1200/JCO.1995.13.11.2700. [DOI] [PubMed] [Google Scholar]
- 7.Kondagunta GV, Sheinfeld J, Mazumdar M, et al. Relapse-free and overall survival in patients with pathologic stage II nonseminomatous germ cell cancer treated with etoposide and cisplatin adjuvant chemotherapy. J Clin Oncol. 2004;22:464–467. doi: 10.1200/JCO.2004.07.178. [DOI] [PubMed] [Google Scholar]
- 8.Haugnes HS, Bosl GJ, Boer H, et al. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J Clin Oncol. 2012;30:3752–3763. doi: 10.1200/JCO.2012.43.4431. [DOI] [PubMed] [Google Scholar]
- 9. American Cancer Society: Testicular cancer stages. https://www.cancer.org/cancer/testicular-cancer/detection-diagnosis-staging/staging.html.
- 10.Motzer RJ, Geller NL, Bosl GJ. The effect of a 7-day delay in chemotherapy cycles on complete response and event-free survival in good-risk disseminated germ cell tumor patients. Cancer. 1990;66:857–861. doi: 10.1002/1097-0142(19900901)66:5<857::aid-cncr2820660508>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 12. Centers for Medicare and Medicaid Services: 2019 ASP drug pricing files. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2019ASPFiles.html.
- 13.Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. 2005;23:6549–6555. doi: 10.1200/JCO.2005.19.638. [DOI] [PubMed] [Google Scholar]
- 14.de Wit R, Bosl GJ. Optimal management of clinical stage I testis cancer: One size does not fit all. J Clin Oncol. 2013;31:3477–3479. doi: 10.1200/JCO.2013.51.0479. [DOI] [PubMed] [Google Scholar]
- 15.Oldenburg J, Aparicio J, Beyer J, et al. Personalizing, not patronizing: The case for patient autonomy by unbiased presentation of management options in stage I testicular cancer. Ann Oncol. 2015;26:833–838. doi: 10.1093/annonc/mdu514. [DOI] [PubMed] [Google Scholar]
- 16.Feldman DR. Treatment options for stage I nonseminoma. J Clin Oncol. 2014;32:3797–3800. doi: 10.1200/JCO.2014.56.8006. [DOI] [PubMed] [Google Scholar]
- 17.Donohue JP, Thornhill JA, Foster RS, et al. The role of retroperitoneal lymphadenectomy in clinical stage B testis cancer: The Indiana University experience (1965 to 1989) J Urol. 1995;153:85–89. doi: 10.1097/00005392-199501000-00030. [DOI] [PubMed] [Google Scholar]
- 18.Beck SD, Cheng L, Bihrle R, et al. Does the presence of extranodal extension in pathological stage B1 nonseminomatous germ cell tumor necessitate adjuvant chemotherapy? J Urol. 2007;177:944–946. doi: 10.1016/j.juro.2006.10.068. [DOI] [PubMed] [Google Scholar]
- 19.Mezvrishvili Z, Managadze L. Is bleomycin necessary in adjuvant chemotherapy of clinical stage I non-seminomatous testicular cancer? Georgian Med News. 2006;134:25–28. [PubMed] [Google Scholar]
- 20. US Pharmacopeial Convention: USP DI, Volume 1: Drug Information for the Health Care Professional. Greenwood Village, CO, Thomson/MICROMEDEX, 2006. [Google Scholar]
- 21.de Wit R, Stoter G, Kaye SB, et al. Importance of bleomycin in combination chemotherapy for good-prognosis testicular nonseminoma: A randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. J Clin Oncol. 1997;15:1837–1843. doi: 10.1200/JCO.1997.15.5.1837. [DOI] [PubMed] [Google Scholar]
- 22.Necchi A, Miceli R, Oualla K, et al. Effect of bleomycin administration on the development of pulmonary toxicity in patients with metastatic germ cell tumors receiving first-line chemotherapy: A meta-analysis of randomized studies. Clin Genitourin Cancer. 2017;15:213–220.e5. doi: 10.1016/j.clgc.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Cullen MH, Stenning SP, Parkinson MC, et al. Short-course adjuvant chemotherapy in high-risk stage I nonseminomatous germ cell tumors of the testis: A Medical Research Council report. J Clin Oncol. 1996;14:1106–1113. doi: 10.1200/JCO.1996.14.4.1106. [DOI] [PubMed] [Google Scholar]
- 24.Dearnaley DP, Fossa SD, Kaye SB, et al. Adjuvant bleomycin, vincristine and cisplatin (BOP) for high-risk stage I non-seminomatous germ cell tumours: A prospective trial (MRC TE17) Br J Cancer. 2005;92:2107–2113. doi: 10.1038/sj.bjc.6602624. [DOI] [PMC free article] [PubMed] [Google Scholar]







