Abstract
PURPOSE
The Tamoxifen and Exemestane Trial (TEXT)/Suppression of Ovarian Function Trial (SOFT) showed superior outcomes for premenopausal women with hormone receptor (HR)–positive breast cancer treated with adjuvant exemestane plus ovarian function suppression (OFS) or tamoxifen plus OFS versus tamoxifen alone. We previously reported the magnitude of absolute improvements in freedom from any recurrence across a continuous, composite measure of recurrence risk to tailor decision making. With longer follow-up, we now focus on distant recurrence.
METHODS
The TEXT/SOFT HR-positive/human epidermal growth factor receptor 2 (HER2)–negative analysis population included 4,891 women stratified by predetermined chemotherapy use. Kaplan-Meier estimates of 8-year freedom from distant recurrence were analyzed using subpopulation treatment effect pattern plot (STEPP) methodology across subpopulations defined by the continuous composite measure of recurrence risk. For each patient, the composite risk value was obtained from a Cox model that incorporated age; nodal status; tumor size; grade; and estrogen receptor, progesterone receptor, and Ki-67 labeling index expression levels.
RESULTS
The overall rate of 8-year freedom from distant recurrence was 91.1% and ranged from approximately 100% to 63% across lowest to highest composite risks. TEXT patients who received chemotherapy had an average absolute improvement with exemestane plus OFS versus tamoxifen plus OFS of 5.1%, and STEPP analysis showed improvements from less than 1% to more than 15% from lowest to highest composite risks. SOFT patients who remained premenopausal after chemotherapy had an average 5.2% absolute improvement with exemestane plus OFS versus tamoxifen and reached 10% across composite risks; for tamoxifen plus OFS versus tamoxifen, the maximum improvement was approximately 3.5%. Women who did not receive chemotherapy had a more than 97% rate of 8-year freedom from distant recurrence, and improvements with exemestane plus OFS ranged from 1% to 4%.
CONCLUSION
Premenopausal women with HR-positive/HER2-negative breast cancer and high recurrence risk, as defined by clinicopathologic characteristics, may experience a 10% to 15% absolute improvement in 8-year freedom from distant recurrence with exemestane plus OFS versus tamoxifen plus OFS or tamoxifen alone. The potential benefit of escalating endocrine therapy versus tamoxifen alone is minimal for those at low recurrence risk.
INTRODUCTION
Adjuvant endocrine therapy recommendations for premenopausal women with hormone receptor (HR)–positive breast cancer have changed on the basis of the 5-year results of the Tamoxifen and Exemestane Trial (TEXT) and the Suppression of Ovarian Function Trial (SOFT; ClinicalTrials.gov identifiers: NCT00066703 and NCT00066690, respectively).1,2 Tamoxifen alone remains the standard of care in women at low risk of relapse, as defined by clinical and pathologic features, and by genomic parameters, when available. Ovarian function suppression (OFS) should be proposed to patients at higher risk for recurrence in addition to oral endocrine therapy with either tamoxifen or an aromatase inhibitor.3-6
A secondary analysis of the women enrolled in TEXT and SOFT with HR-positive/human epidermal growth factor receptor 2 (HER2)–negative cancers estimated absolute improvements in 5-year freedom from breast cancer (including invasive locoregional, distant, or contralateral breast cancer) according to recurrence risk defined from clinicopathologic characteristics. The women at high recurrence risk experienced absolute improvements of 10% to 15% with exemestane plus OFS versus tamoxifen plus OFS and versus tamoxifen alone, and improvements were of at least 5% for women at intermediate risk.7 In contrast, improvement was minimal for women at lowest risk. Such estimates of the absolute magnitude of improvement can help clinicians to weigh benefits against adverse effects in treatment decisions with individual patients.
Recent updates of TEXT and SOFT after a median follow-up of 9 and 8 years, respectively, demonstrated significant improvement in disease-free and overall survival with the addition of OFS to tamoxifen in the SOFT population.8 The combined analysis of TEXT and SOFT demonstrated sustained improvements with exemestane plus OFS versus tamoxifen plus OFS in disease-free survival, freedom from breast cancer, and freedom from distant recurrence but not from overall survival.8 Among the patients with HR-positive/HER2-negative tumors, the rate of freedom from distant recurrence was improved by 3.6% with the use of exemestane plus OFS versus tamoxifen and by 3.5% with exemestane plus OFS versus tamoxifen plus OFS.
Distant recurrence in a premenopausal woman has personal, economic, and social costs of greater impact than locoregional recurrence.9 Greater toxicity may be acceptable in prevention of distant recurrence, which generally portends premature death as a result of breast cancer. With extended follow-up, we are now able to refine the estimates of absolute treatment effects in preventing distant recurrence across the spectrum of recurrence risk in the subgroup of women with HR-positive/HER2-negative cancers.
METHODS
Study Designs
The designs and conduct of the trials have been described previously.1,2 In both trials, eligible premenopausal women had invasive early breast cancer assessed as 10% or greater estrogen receptor (ER)– and/or progesterone receptor (PgR)–expressing cells by local determination. The ethics committee at each participating center approved the study protocols, and all patients provided written informed consent for trial participation and mandatory tissue collection for central pathology assessment. Central review of histopathologic features and expression of ER, PgR, HER2, and Ki-67 labeling index (Ki-67) was conducted for 84% of trial patients in the International Breast Cancer Study Group Central Pathology Office.10
In TEXT, all women received OFS by gonadotropin-releasing hormone agonist triptorelin from the start of adjuvant therapy; after at least 6 months of triptorelin, patients could opt for bilateral oophorectomy or ovarian irradiation. Chemotherapy was optional and if administered, was started concurrently with triptorelin. Between November 2003 and March 2011, 2,672 premenopausal women were randomly assigned to 5 years of exemestane plus OFS or tamoxifen plus OFS. Randomization was stratified according to intended use of adjuvant chemotherapy and lymph node status.
SOFT was designed to determine the value of adding OFS to tamoxifen and the role of exemestane plus OFS in two cohorts of premenopausal women: those who remained premenopausal after completion of (neo)adjuvant chemotherapy and those for whom adjuvant tamoxifen without chemotherapy was considered suitable treatment. Between December 2003 and January 2011, 3,066 women were randomly assigned to 5 years of exemestane plus OFS or tamoxifen plus OFS or tamoxifen alone. Randomization was stratified according to prior use of chemotherapy, lymph node status, and intended initial method of OFS (if randomly assigned to OFS).
End Point and Statistical Considerations
The analysis population was previously defined and included 4,891 patients with tumors centrally assessed as HR positive/HER2 negative7 (Appendix Fig A1, online only). Distant recurrence–free interval (DRFI) was defined as the duration of time from random assignment until first appearance of invasive breast cancer recurrence at a distant site; in the absence of a distant recurrence, DRFI was censored at date of last follow-up. The median follow-up was 9 years in TEXT and 8 years in SOFT.8
While following the approach previously described,7 the composite measure of recurrence risk (hereafter referred to as composite risk) was determined for the entire HR-positive/HER2-negative analysis population by fitting a Cox proportional hazards regression model for DRFI, stratified by cohort (defined by trial and chemotherapy use) and treatment assignment. This use of a stratified model provided the same risk scale for the two trials, which do not share a common control group. Prognostic factors included in the model and their groupings were specified a priori, and there was no intention to optimize the model on the basis of model selection procedures (Table 1; Appendix Table A1, online only). For tumor grade and ER and PgR expression, the centrally determined values were used when available, and locally determined values were used otherwise; Ki-67 expression was available only from central assessment. Unknown categories were used to avoid systematic exclusion of patients without centrally assessed tumor features. After estimating the model parameters, the composite risk value was calculated for each trial patient by summing the model parameter estimates corresponding to her observed values of clinicopathologic factors.
TABLE 1.
Clinicopathologic Characteristics of the HR-Positive/HER2-Negative Analysis Population of TEXT and SOFT
Nonparametric sliding window subpopulation treatment effect pattern plot (STEPP) methodology11,12 was used to investigate patterns in absolute treatment effects as measured by Kaplan-Meier estimates of 8-year freedom from distant recurrence (y-axis) across the continuum of composite risk values (x-axis). The STEPP of the overall analysis population had a window size of 500 and was slid by 300 patient values to form each subpopulation. For SOFT and TEXT, the size of windows were 420 and 300, respectively, and slid by 120 and 150 patient values, respectively. With this approach, most composite risk values are represented in more than one subpopulation.
RESULTS
Among the 4,891 patients in the HR-positive/HER2-negative analysis population, the clinicopathologic characteristics across the four trial cohorts reflected the selection of adjuvant chemotherapy use and whether OFS would be received by all patients (TEXT) or by random assignment (SOFT; Table 1; Appendix Table A1). Poor prognostic features were more frequent on average among the cohorts who received chemotherapy. The distribution of age (youngest among patients in SOFT who received chemotherapy before enrollment) reflected the SOFT eligibility criteria that patients remain premenopausal after chemotherapy.
The relationship of each of the seven clinicopathologic characteristics individually with DRFI is shown in Figure 1. The estimated 8-year freedom from distant recurrence rates are shown for each characteristic by treatment group, along with the relative treatment effects, in Figure 2 and Appendix Figures A2 and A3 (online only) and listed in Appendix Tables A2-A6 (online only).
FIG 1.
Kaplan-Meier estimates of distant recurrence (DR)–free interval in the overall hormone receptor–positive/human epidermal growth factor receptor 2–negative analysis population according to seven clinicopathologic characteristics. (A) Age at random assignment, (B) number of positive lymph nodes, (C) tumor size, (D) estrogen receptor (ER) expression, (E) progesterone receptor (PgR) expression, (F) tumor grade, and (G) labeling index Ki-67 (Ki67) expression. Unknown values are omitted.
FIG 2.
Kaplan-Meier estimates of 8-year freedom from distant recurrence in seven clinicopathologic subgroups in the four patient cohorts defined by trial and chemotherapy use according to treatment assignment. The values are listed in Appendix Tables A3 and A6. Unknown values are omitted. ER, estrogen receptor; EXEM, exemestane; Ki-67, Ki-67 labeling index; OFS, ovarian function suppression; PgR, progesterone receptor; SOFT, Suppression of Ovarian Function Trial; TAM, tamoxifen; TEXT, Tamoxifen and Exemestane Trial.
The clinicopathologic characteristic with the greatest contribution to the multivariable model for the composite measure of recurrence risk, relative to the complementary reference category, was four or more positive nodes followed by tumor grade 2 or 3 and younger age (Table 1); one to three positive nodes, tumor size greater than 2 cm, PgR less than 20%, and Ki-67 of 26% or more contributed similarly. ER less than 50% had a very small contribution possibly because only 4% of patients had low ER expression described.10 The distribution of resulting composite risks is illustrated for the overall population in Figure 3 and summarized by each characteristic subgroup in Appendix Table A7 (online only).
FIG 3.
(A) Subpopulation treatment effect pattern plot of 8-year freedom from distant recurrence according to median composite risk in subpopulations and (B) histogram of the composite risk distribution in the overall hormone receptor (HR)–positive/human epidermal growth factor receptor 2 (HER2)–negative analysis population. The horizontal lines above the histogram indicate the ranges of composite risks in subpopulations that are plotted at the subpopulation median value. The overall 8-year freedom from distant recurrence rate of 91.1% also is indicated on the y-axis. The overall median composite risk of 1.42 is indicated by the vertical dashed lines.
Overall, for the 4,891 patients, 433 distant recurrences were reported, and the 8-year freedom from distant recurrence rate was 91.1% (95% CI, 90.2% to 92.0%). When the clinicopathologic features were integrated as a composite measure of recurrence risk (median, 1.42; interquartile range [IQR], 0.81-2.15), the 8-year freedom from distant recurrence rate varied markedly across patient populations, ranging from approximately 100% to 63% among patients in the subpopulations with lowest composite risks to highest composite risks, respectively (Fig 3). As expected, the majority of distant recurrences occurred among patients who received chemotherapy (Fig 4).
FIG 4.
Kaplan-Meier estimates of distant recurrence (DR)–free interval in the four patient cohorts defined by trial and chemotherapy use according to treatment assignment. (A) Tamoxifen and Exemestane Trial (TEXT) chemotherapy, (B) Suppression of Ovarian Function Trial (SOFT) prior chemotherapy, (C) TEXT no chemotherapy, and (D) SOFT no chemotherapy. EXEM, exemestane; OFS, ovarian function suppression; TAM, tamoxifen.
Patients for Whom Chemotherapy Was Part of Adjuvant Therapy
The patients who received chemotherapy, whether in TEXT or SOFT, had similar distributions of composite risk values (Figs 5A and 5B), with a median composite risk of 2.02 (IQR, 1.43-2.71) and 2.00 (IQR, 1.42-2.68), respectively. TEXT patients who received chemotherapy after enrollment had an average 8-year freedom from distant recurrence rate of 87.5% (159 distant recurrences among 1,276 patients after a median follow-up of 9 years). The average absolute improvement in freedom from distant recurrence with exemestane plus OFS versus tamoxifen plus OFS was 5.1% (90.0% v 84.9%; Fig 4A). The STEPP analysis showed a distinct pattern of increasing magnitude of improvement with exemestane plus OFS as composite risk increased and reached more than 15% in the subpopulation with the highest composite risks (Fig 5A).
FIG 5.
Subpopulation treatment effect pattern plots of 8-year freedom from distant recurrence according to median composite risk in subpopulations and histograms of the composite risk distributions for each of the four cohorts in the hormone receptor (HR)–positive/human epidermal growth factor receptor 2 (HER2)–negative analysis population according to treatment assignment. (A) Tamoxifen and Exemestane Trial (TEXT) chemotherapy, (B) Suppression of Ovarian Function Trial (SOFT) prior chemotherapy, (C) TEXT no chemotherapy, and (D) SOFT no chemotherapy. The horizontal lines above each histogram indicate the ranges of composite risks in subpopulations that are plotted at the subpopulation median composite risk value. The vertical dashed lines indicate the median composite risk of 1.42 in the overall HR-positive/HER2-negative analysis population. EXEM, exemestane; OFS, ovarian function suppression; TAM, tamoxifen.
Patients enrolled in SOFT who remained premenopausal after chemotherapy had an 8-year freedom from distant recurrence rate of 82.5% (216 distant recurrences among 1,271 patients after a median follow-up of 8 years). The average absolute improvement with exemestane plus OFS versus tamoxifen was 5.2% (86.2% v 81.0%; Fig 4B). The STEPP analysis suggested that all patients across composite risks benefited from exemestane plus OFS versus tamoxifen, with the maximum absolute improvement of approximately 10% (Fig 5B). The absolute improvement with tamoxifen plus OFS versus tamoxifen alone ranged from 0 to at most approximately 3.5% for the subpopulations with the highest composite risks.
Patients for Whom Endocrine Therapy Alone Was Planned as Adjuvant Therapy
In contrast to the patients who received chemotherapy, those who received only adjuvant endocrine therapy had lower composite risks. In TEXT, the median composite risk was 1.13 (IQR, 0.72-1.56), and in SOFT, the median was 0.81 (IQR, 0.17-1.27; Fig 5).
Among the 991 patients in TEXT who did not receive chemotherapy, 35 experienced a distant recurrence after a median follow-up of 9 years, and the overall 8-year freedom from distant recurrence rate was 97.0%. The overall absolute improvement in 8-year freedom from distant recurrence with exemestane plus OFS versus tamoxifen plus OFS was less than 1% (97.4% v 96.5%; Fig 4C). The STEPP analysis showed absolute improvement with exemestane plus OFS only in the subpopulations with the highest composite risks, for a maximum magnitude of improvement in the range of 2.5% to 4% (Fig 5C).
The 1,353 patients in SOFT who did not receive chemotherapy had the lowest composite risks, 23 patients experienced a distant recurrence after a median follow-up of 8 years. The overall 8-year freedom from distant recurrence rate was 98.5%, and for exemestane plus OFS, tamoxifen plus OFS, and tamoxifen, the rate was 99.3%, 98.3%, and 98.0%, respectively (Fig 4D). The absolute improvement in 8-year freedom from distant recurrence with exemestane plus OFS versus tamoxifen ranged from approximately 1% to 2.5%, and the improvement with tamoxifen plus OFS versus tamoxifen was at most 1% (Fig 5D).
DISCUSSION
After 5 years of adjuvant endocrine therapy, breast cancer recurrences steadily occur up to 20 years. In an Early Breast Cancer Trialists’ Collaborative Group meta-analysis (62,923 patients disease free after 5 years of endocrine therapy), the risk of distant recurrence was strongly correlated with stage (13% and 41% in T1N0 and T2N2 disease, respectively) and tumor grade (10% and 17% for low-grade T1N0 and high-grade disease, respectively).13 In the absence of predictive biomarkers, standard clinicopathologic characteristics continue to provide meaningful prognostic information about risk of distant recurrence and guide treatment decision making. We put these features together into a composite measure of recurrence risk, which allows clinicians to better understand and estimate the magnitude of benefit of escalating adjuvant endocrine therapy in premenopausal women with HR-positive/HER2-negative breast cancer.
The combined analysis of TEXT/SOFT, without regard to tumor HER2 status, showed an average 2.1% absolute improvement in freedom from distant recurrence at 8 years in premenopausal women treated with an aromatase inhibitor versus tamoxifen with OFS.8 This benefit is comparable to that observed for postmenopausal women in the Early Breast Cancer Trialists’ Collaborative Group meta-analysis, wherein 5 years of aromatase inhibitors were associated with a 2% absolute 10-year reduction in distant recurrence compared with 5 years of tamoxifen (16.3% v 14.3%, respectively).14
We focused on the predominant subgroup with HR-positive/HER2-negative cancers. As previously highlighted,15 SOFT/TEXT data suggest differential relative treatment efficacy by HER2 status and show lesser benefit of aromatase inhibitors and greater benefit from adding OFS to tamoxifen for HER2-positive than for HER2-negative cancers. Secondary analyses from the Hormonal Adjuvant Treatment Bone Effects (HOBOE)16 and Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO)17 trials were consistent with this observation. A closer analysis of the HER2-positive subgroup is planned.
Among women with HR-positive/HER2-negative tumors who received chemotherapy (on average, a higher-risk group), an average 5% absolute improvement was achieved by escalating endocrine therapy to exemestane plus OFS in both TEXT and SOFT. In the STEPP analysis, the composite risk for a high-risk scenario (eg, 35 to 39 years of age, grade 3, pT2pN1a, ER and PgR of 50% or greater, Ki-67 26% or greater; composite risk, 3.00; Appendix Fig A4A, online only) was represented in subpopulations that had an absolute improvement greater than the average (range, 7% to 10%). The magnitude of improvement with exemestane plus OFS versus tamoxifen with or without OFS was larger in TEXT than in SOFT high-risk patients, but improvement was consistently observed in both cohorts. In contrast, in low-risk patients (Appendix Fig A4C) who did not receive chemotherapy, the 8-year freedom from distant recurrence was improved on average by approximately 1% in patients who received exemestane plus OFS compared with tamoxifen alone or tamoxifen plus OFS in both SOFT and TEXT. The STEPP analysis suggested that this low-risk scenario (eg, 40 to 44 years of age, grade 2, pT1pN0, ER and PgR of 50% or greater, Ki-67 of 14% to 19%; composite risk, 0.89) was well characterized by the average improvement of approximately 1%. Most intermediate-risk patients in SOFT and TEXT received chemotherapy. In this patient scenario (eg, 40 to 44 years of age, grade 2, pT1pN1a, ER and PgR of 50% or greater, Ki-67 of 20% to 25%; composite risk, 1.78; Appendix Fig A4B), the absolute improvement with exemestane plus OFS was estimated to be less than the average improvement of 5%. Of note, intermediate-risk patients in TEXT who did not receive chemotherapy were estimated to have an improvement with exemestane plus OFS that exceeded 4%; these patients also achieved approximately 10% improvement in freedom from any breast cancer recurrence at 5 years with exemestane plus OFS.7
TEXT and SOFT results may encourage physicians to reconsider the indication for adjuvant chemotherapy in intermediate-risk premenopausal women with HR-positive/HER2-negative breast cancer. The patient selection for chemotherapy differed in SOFT and TEXT. The knowledge that all patients would receive OFS in TEXT, whereas OFS was administered by random assignment in SOFT, possibly prompted the prescription of chemotherapy for some SOFT patients. For instance, more patients younger than 40 years of age (47.8% and 28.4%) and with node-negative disease (41.5% and 31.4%) received chemotherapy in SOFT than in TEXT. This also can be inferred by the higher median composite risk in TEXT patients in the no-chemotherapy cohorts (eg, 21.5% of patients who received endocrine therapy alone in TEXT had one to three positive nodes; Fig 4) compared with 8.6% in SOFT. These different clinical characteristics of women who received adjuvant chemotherapy in SOFT and TEXT might partly explain the slightly different absolute improvements. Premenopausal women represented approximately 30% of patients in the clinical trials that addressed the added benefit of adjuvant chemotherapy over endocrine therapy18-20 in HR-positive/HER2-negative breast cancer. Tamoxifen was the standard of care in premenopausal women when these trials were designed and conducted, and the additional benefit of adjuvant chemotherapy in this age-group cannot be extrapolated to women treated with combined endocrine therapy as in TEXT and SOFT. The Rx for Positive Node, Endocrine Responsive Breast Cancer (RxPONDER) trial (ClinicalTrials.gov identifier: NCT01272037) enrolled patients with one to three positive nodes between 2011 and 2015 who may have received combined endocrine therapy and could provide additional insight for intermediate-risk patients.
Several gene expression assays also have been developed for postmenopausal women21 to estimate better the risk of early (0 to 5 years) and late (5 to 10 years) distant recurrence22-24 of patients with HR-positive early breast cancer and tailor their adjuvant endocrine treatment. None of these tests was developed to select which endocrine therapy is more appropriate according to genomic risk. The prognostic information provided by these assays should not be interpreted automatically as a prediction of treatment benefit.25 Although the risk of late distant recurrence is seemingly similar across age-groups,13 the different algorithms cannot be applied to premenopausal patients without further validation because most of the data derive from postmenopausal women.
The 5% to 15% absolute improvement in freedom from distant recurrence from escalating endocrine therapy to tamoxifen plus OFS or exemestane plus OFS needs to be integrated in the clinical situation of each individual patient. The results in patients at higher risk of recurrence who received chemotherapy are of particular clinical relevance. A retrospective cohort study of 1,616 women showed that the median time to all-cause mortality was significantly longer in women with locoregional recurrence than in those with distant metastases (6.4 v 3.4 years, respectively).26 Moreover, the 10-year survival rate of 101 women with local recurrence was 56% compared with 9% in those with distant recurrence.27 In patients with HER2-negative disease who did not receive adjuvant chemotherapy, the majority with a lower risk of recurrence, after 8 to 9 years median follow-up in SOFT and TEXT, very few distant recurrences (23 and 35, respectively) occurred. In these patients, the benefit of treatment escalation is derived largely from locoregional and contralateral breast cancer reduction.7 Given the impact on patients’ quality of life from escalating endocrine therapy,28 clinicians need to weigh the risk of recurrence and the expected absolute improvement in disease outcomes carefully against the added adverse effects.
With all women beyond the 5-year treatment period, the toxicity profiles of exemestane plus OFS and tamoxifen plus OFS are similar to postmenopausal women, and no new toxicity signal has emerged.8 Nonadherence and early discontinuation of oral adjuvant endocrine therapy is frequent among young women29 and associated with reduced overall survival.30 In TEXT/SOFT, early discontinuation of all assigned endocrine therapy was approximately 20% in each treatment group, which is in line with published data.29 Several demographic and clinical characteristics can help physicians to identify patients at risk for nonpersistence/adherence.31 By better quantifying treatment benefits in the individual patient, our results may allow for a more-tailored risk/benefit discussion and an increase in women’s motivation, particularly those at highest risk of distant recurrence, to follow treatment prescriptions.
Given the potential for late recurrences of HR-positive breast cancer, conclusions about overall survival remain premature. In postmenopausal women, the benefit of aromatase inhibitors versus tamoxifen on breast cancer mortality only emerged at 10 years14 (2.1% absolute gain, 14.2% v 12.1%, respectively).
The current results add to our earlier study7 to assist physicians and patients with estimating the individual risk-based benefit of escalating endocrine therapy. A recent survey showed that practicing US medical oncologists underestimated the absolute improvement in 5-year freedom from breast cancer with the use of aromatase inhibitors plus OFS versus tamoxifen for high-risk patients (physician estimate, 5.9%) compared with our previous study findings of 10% to 15%.7,32 An online tool is in development to assist clinicians with using SOFT/TEXT in their daily practice risk/benefit calculations in premenopausal women with HR-positive/HER2-negative breast cancer.
ACKNOWLEDGMENT
We thank the patients, physicians, nurses, trial coordinators, and pathologists who participated in the TEXT and SOFT clinical trials. The trials were coordinated by the International Breast Cancer Study Group in collaboration with the Breast International Group and its cooperative groups and US National Cancer Institute National Clinical Trials Network cooperative groups. We thank Hui Huang for programming assistance with the online tool.
Appendix
FIG A1.
Flow diagram of the 4,891 patients included in the Suppression of Ovarian Function Trial (SOFT) and the Tamoxifen and Exemestane Trial (TEXT) hormone receptor (HR)–positive/human epidermal growth factor receptor 2 (HER2)–negative analysis population. EXEM, exemastane; ITT, intent to treat; OFS, ovarian function suppression; TAM, tamoxifen.
FIG A2.
Kaplan-Meier estimates of 8-year freedom from distant recurrence in seven clinicopathologic subgroups, according to treatment assignment, separately by trial. The plotted values are provided in Tables A2 and A5. Unknown values are omitted. ER, estrogen receptor; EXEM, exemestane; Ki-67, Ki-67 labeling index; PgR, progesterone receptor; OFS, ovarian function suppression; SOFT, Suppression of Ovarian Function Trial; TAM, tamoxifen; TEXT, Tamoxifen and Exemestane Trial.
FIG A3.
Estimated relative treatment effects on distance recurrence–free interval (DRFI) overall and according to seven clinicopathologic subgroups for the hormone receptor (HR)–positive/human epidermal growth factor receptor (HER2)–negative analysis population. The hazard ratios were estimated from Cox proportional hazards models stratified by cohort. Estimates not provided for unknown groups of < 50 patients. Estimates in subgroups having few events should be interpreted with caution. (A) The Tamoxifen and Exemestane Trial (TEXT) cohorts comparing exemestane (EXE) + ovarian function suppression (OFS) versus tamoxifen (TAM) + OFS. (B) Combined TEXT and Suppression of Ovarian Function Trial (SOFT) cohorts comparing EXE + OFS versus TAM + OFS. (C) SOFT cohorts comparing EXE + OFS versus TAM. (D) SOFT cohorts comparing TAM + OFS versus TAM. ER, estrogen receptor; Ki-67, Ki67 labeling index; PgR, progesterone receptor.
FIG A4.
Subpopulation treatment effect pattern plots (STEPP) of 8-year freedom from distant recurrence (y-axis) according to median composite risk in subpopulations (x-axis) and histograms of the composite risk distributions for each of the four cohorts in the hormone receptor (HR)–positive/human epidermal growth factor receptor 2 (HER2)–negative analysis population according to treatment assignment. The horizontal lines above each histogram indicate the ranges of composite risks in subpopulations that are plotted at the subpopulation median composite risk value in each corresponding STEPP. The red vertical line on the histograms indicates the selected composite risk, and intersects one or more subpopulations in which the composite risk value is represented. The dark orange circles on the STEPPs indicate the corresponding subpopulations in which the selected composite risk is represented. The black vertical dashed lines indicate the median composite risk of 1.42 in the overall HR-positive/HER2-negative analysis population. Three scenarios are presented: (A) A high-risk scenario (composite risk = 3.00; 35-39 years of age, pT2pN1a, grade 3, estrogen receptor [ER] ≥ 50%, progesterone receptor [PgR] ≥ 50% and Ki-67 labeling index [Ki-67] ≥ 26%). In this scenario, there are no circles on the Tamoxifen and Exemestane Trial (TEXT) no chemotherapy and Suppression of Ovarian Function Trial (SOFT) no chemotherapy STEPPs because there were too few patients with the same or similar composite risk values.
TABLE A1.
Clinicopathologic Characteristics of the HR-Positive/HER2-Negative Analysis Population of TEXT and SOFT Overall and According to Cohort Defined by Trial and Chemotherapy Use
TABLE A2.
TEXT: Kaplan-Meier Estimates of 8-Year Freedom From DR in Clinicopathologic Subgroups According to Treatment Assignment
TABLE A3.
TEXT: Kaplan-Meier Estimates of 8-Year Freedom From DR in Clinicopathologic Subgroups According to Chemotherapy Use and Treatment Assignment
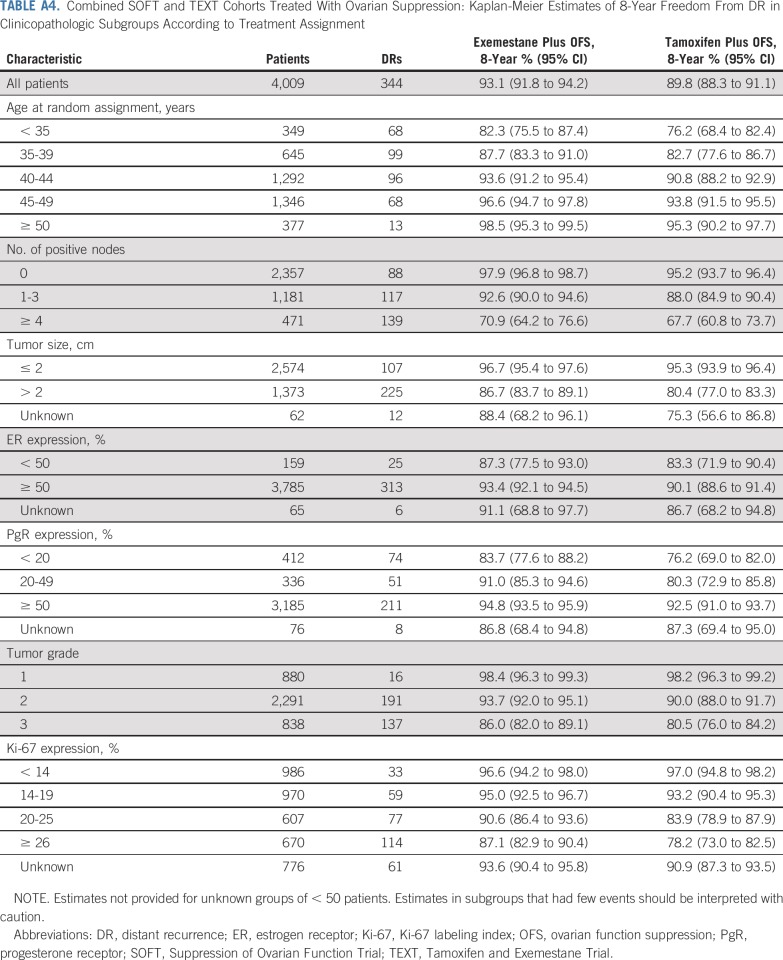
TABLE A4.
Combined SOFT and TEXT Cohorts Treated With Ovarian Suppression: Kaplan-Meier Estimates of 8-Year Freedom From DR in Clinicopathologic Subgroups According to Treatment Assignment
TABLE A5.
SOFT: Kaplan-Meier Estimates of 8-Year Freedom From DR in Clinicopathologic Subgroups According to Treatment Assignment
TABLE A6.
SOFT: Kaplan-Meier Estimates of 8-Year Freedom From DR in Clinicopathologic Subgroups According to Chemotherapy Use and Treatment Assignment
TABLE A7.
Distribution of the Composite Measures of Recurrence Risk in Clinicopathologic Subgroups
Footnotes
Presented at the 2018 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018.
Supported by Pfizer, the International Breast Cancer Study Group, the US National Cancer Institute, and the Breast Cancer Research Foundation (16-185, 17-187, and 18-003) for Tamoxifen and Exemestane Trial and Suppression of Ovarian Function Trial conduct. Pfizer and Ipsen provided the drug supply. Support for central pathology included Susan G. Komen for the Cure Promise Grant KG080081 and the Breast Cancer Research Foundation. Support for the coordinating group, International Breast Cancer Study Group, was from the US National Cancer Institute (CA075362), Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research, Cancer Research Switzerland, Oncosuisse, Cancer League Switzerland, Foundation for Clinical Cancer Research of Eastern Switzerland. Support of cooperative groups was as follows: Breast Cancer Trials Australia & New Zealand (National Health and Medical Research Council grants 351161, 510788, and 1105058); Institute of Cancer Research Clinical Trials and Statistics Unit on behalf of the National Cancer Research Institute Breast Clinical Studies Group United Kingdom (Cancer Research UK grants CRUKE/03/022 and CRUKE/03/023 and grant A15955 National Institute for Health Research Royal Marsden/Institute of Cancer Research Biomedical Research Centre, National Institute for Health Research/Cambridge Biomedical Research Centre); Alliance for Clinical Trials in Oncology (US National Institutes of Health [NIH] grant CA180821); SWOG (US NIH grant CA32102); Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (US NIH grants CA21115 and CA16116); NRG Oncology (US NIH grants U10CA180868, U10CA180822, and UG1CA189867); and Canadian Cancer Trials Group (US NIH grant CA077202 and Canadian Cancer Society Research Institute grants 015469 and 021039).
NCT00066690 and NCT00066703.
See accompanying Oncology Grand Rounds on page 1258
AUTHOR CONTRIBUTIONS
Conception and design: Olivia Pagani, Prudence A. Francis, Gini F. Fleming, Barbara A. Walley, Giuseppe Viale, Alan S. Coates, Aron Goldhirsch, Richard D. Gelber, Meredith M. Regan
Provision of study material or patients: Prudence A. Francis, Barbara A. Walley, Giuseppe Viale, István Láng, Henry L. Gómez, Carlo Tondini, Graziella Pinotti
Collection and assembly of data: Prudence A. Francis, Marco Colleoni, István Láng, Henry L. Gómez, Carlo Tondini, Aron Goldhirsch, Meredith M. Regan
Data analysis and interpretation: Olivia Pagani, Prudence A. Francis, Gini F. Fleming, Barbara A. Walley, Giuseppe Viale, Henry L. Gómez, Carlo Tondini, Graziella Pinotti, Angelo Di Leo, Aron Goldhirsch, Meredith M. Regan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Absolute Improvements in Freedom From Distant Recurrence to Tailor Adjuvant Endocrine Therapies for Premenopausal Women: Results From TEXT and SOFT
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Prudence A. Francis
Honoraria: AstraZeneca, Novartis
Travel, Accommodations, Expenses: Pfizer
Gini F. Fleming
Honoraria: Curio Science
Research Funding: Corcept Therapeutics (Inst), AbbVie (Inst), Genentech (Inst), Tesaro (Inst), Syndax (Inst), Forty Seven (Inst), Iovance Biotherapeutics (Inst), Syros Pharmaceuticals (Inst), Astex Pharmaceuticals (Inst), Merck (Inst), Sanofi (Inst), Sermonix Pharmaceuticals (Inst), Compugen (Inst), Incyte (Inst), Leap Therapeutics (Inst), Hoffmann La Roche (Inst)
Giuseppe Viale
Honoraria: MSD Oncology, Pfizer
Consulting or Advisory Role: Dako, Roche, Genentech, Astellas Pharma, Novartis, Bayer AG
Speakers’ Bureau: Roche, Genentech
Research Funding: Roche, Genentech, Ventana Medical Systems (Inst), Dako (Inst), Agilent Technologies (Inst)
Travel, Accommodations, Expenses: Roche, Celgene
Marco Colleoni
Honoraria: Novartis
Consulting or Advisory Role: Pierre Fabre, Pfizer, OBI Pharma, Puma Biotechnology, Celldex, AstraZeneca
Carlo Tondini
Consulting or Advisory Role: Myriad Genetics
Speakers’ Bureau: Amgen
Travel, Accommodations, Expenses: Roche, Genentech, Novartis, Celgene
Angelo Di Leo
Honoraria: Roche, Novartis, Pfizer, AstraZeneca, Eisai (I), Eli Lilly, Bayer AG, Celgene (I), Amgen
Consulting or Advisory Role: Roche, Novartis, Pfizer, AstraZeneca, Eli Lilly, Celgene (I), Puma Biotechnology, Ipsen, Genentech, Amgen, Seattle Genetics, Genomic Health
Research Funding: Novartis (Inst), Pfizer (Inst), Genomic Health (Inst)
Travel, Accommodations, Expenses: Roche (I), Pfizer, Celgene, Eli Lilly
Richard D. Gelber
Research Funding: AstraZeneca (Inst), Novartis (Inst), Roche (Inst), Celgene (Inst), Merck (Inst), Pfizer (Inst), Ipsen (Inst), Ferring Pharmaceuticals (Inst)
Meredith M. Regan
Consulting or Advisory Role: Ipsen (Inst)
Research Funding: Veridex (Inst), OncoGenex (Inst), Pfizer (Inst), Ipsen (Inst), Novartis (Inst), Merck (Inst), Ferring Pharmaceuticals (Inst), Celgene (Inst), AstraZeneca (Inst), Pierre Fabre (Inst), Bayer AG (Inst), Bristol-Myers Squibb (Inst), Roche (Inst), Astellas Pharma (Inst), Medivation (Inst), Janssen Pharmaceuticals (Inst), Millennium Pharmaceuticals (Inst), Sanofi (Inst), Sotio (Inst), Dendreon (Inst), TerSera (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paluch-Shimon S, Pagani O, Partridge AH, et al. ESO-ESMO 3rd International Consensus Guidelines for Breast Cancer in Young Women (BCY3) Breast. 2017;35:203–217. doi: 10.1016/j.breast.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 4. Curigliano G, Burstein HJ, Winer EP, et al: De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol 28:2153, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression. J Clin Oncol. 2016;34:1689–1701. doi: 10.1200/JCO.2015.65.9573. [DOI] [PubMed] [Google Scholar]
- 6.Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 7.Regan MM, Francis PA, Pagani O, et al. Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative early breast cancer: TEXT and SOFT trials. J Clin Oncol. 2016;34:2221–2231. doi: 10.1200/JCO.2015.64.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122–137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Lascio S, Pagani O. Is it time to address survivorship in advanced breast cancer? A review article. Breast. 2017;31:167–172. doi: 10.1016/j.breast.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Regan MM, Pagani O, Francis PA, et al. Predictive value and clinical utility of centrally assessed ER, PgR, and Ki-67 to select adjuvant endocrine therapy for premenopausal women with hormone receptor-positive, HER2-negative early breast cancer: TEXT and SOFT trials. Breast Cancer Res Treat. 2015;154:275–286. doi: 10.1007/s10549-015-3612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonetti M, Gelber RD. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics. 2004;5:465–481. doi: 10.1093/biostatistics/5.3.465. [DOI] [PubMed] [Google Scholar]
- 12.Lazar AA, Cole BF, Bonetti M, et al. Evaluation of treatment-effect heterogeneity using biomarkers measured on a continuous scale: Subpopulation treatment effect pattern plot. J Clin Oncol. 2010;28:4539–4544. doi: 10.1200/JCO.2009.27.9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan H, Gray R, Braybrooke J, et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 15.Regan MM, Fleming GF, Walley B, et al. Adjuvant systemic treatment of premenopausal women with hormone receptor-positive early breast cancer: Lights and shadows. J Clin Oncol. 2019;37:862–866. doi: 10.1200/JCO.18.02433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrone F, De Laurentiis M, De Placido S, et al. Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur J Cancer. 2019;118:178–186. doi: 10.1016/j.ejca.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Lambertini M, Campbell C, Bines J, et al. Adjuvant anti-HER2 therapy, treatment-related amenorrhea, and survival in premenopausal HER2-positive early breast cancer patients. J Natl Cancer Inst. 2019;111:86–94. doi: 10.1093/jnci/djy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 20.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical practice guideline. J Clin Oncol. 2016;34:1134–1150. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: A prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013;14:1067–1076. doi: 10.1016/S1470-2045(13)70387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowsett M, Sestak I, Lopez-Knowles E, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31:2783–2790. doi: 10.1200/JCO.2012.46.1558. [DOI] [PubMed] [Google Scholar]
- 24.Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:545–553. doi: 10.1001/jamaoncol.2017.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cuzick J: Prognosis vs treatment interaction. JNCI Cancer Spectrum 2:pky006, 2018. [DOI] [PMC free article] [PubMed]
- 26.Lamerato L, Havstad S, Gandhi S, et al. Economic burden associated with breast cancer recurrence: Findings from a retrospective analysis of health system data. Cancer. 2006;106:1875–1882. doi: 10.1002/cncr.21824. [DOI] [PubMed] [Google Scholar]
- 27.Lê MG, Arriagada R, Spielmann M, et al. Prognostic factors for death after an isolated local recurrence in patients with early-stage breast carcinoma. Cancer. 2002;94:2813–2820. doi: 10.1002/cncr.10572. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg SM, Stanton AL, Petrie KJ, et al. Symptoms and symptom attribution among women on endocrine therapy for breast cancer. Oncologist. 2015;20:598–604. doi: 10.1634/theoncologist.2015-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat. 2012;134:459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert LK, Balneaves LG, Howard AF, et al. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: An integrative review. Breast Cancer Res Treat. 2018;167:615–633. doi: 10.1007/s10549-017-4561-5. [DOI] [PubMed] [Google Scholar]
- 32.Patel R, Maxwell S, Yan D, et al. Medical oncologists’ perception of antiestrogen therapy benefit in premenopausal women with hormone receptor-positive early-stage breast cancer. Ann Oncol. 2018;29:772–773. doi: 10.1093/annonc/mdx719. [DOI] [PMC free article] [PubMed] [Google Scholar]