Abstract
PURPOSE
Clinical stage I (CSI) nonseminoma (NS) is a disease limited to the testis without metastases. One treatment strategy after orchiectomy is adjuvant chemotherapy. Little is known about the outcome of patients who experience relapse after such treatment.
PATIENTS AND METHODS
Data from 51 patients with CSI NS who experienced a relapse after adjuvant bleomycin, etoposide, and cisplatin (BEP) from 18 centers/11 countries were collected and retrospectively analyzed. Primary outcomes were overall and progression-free survivals calculated from day 1 of treatment at first relapse. Secondary outcomes were time to, stage at, and treatment of relapse and rate of subsequent relapses.
RESULTS
Median time to relapse was 13 months, with the earliest relapse 2 months after start of adjuvant treatment and the latest after 25 years. With a median follow-up of 96 months, the 5-year PFS was 67% (95% CI, 54% to 82%) and the 5-year OS was 81% (95% CI, 70% to 94%). Overall, 19 (37%) of 51 relapses occurred later than 2 years. Late relapses were associated with a significantly higher risk of death from NS (hazard ratio, 1.10 per year; P = .01). Treatment upon relapse was diverse: the majority of patients received a combination of chemotherapy and surgery. Twenty-nine percent of patients experienced a subsequent relapse. At last follow-up, 41 patients (80%) were alive and disease-free, eight (16%) had died of progressive disease, and one patient (2%) each had died from therapy-related or other causes.
CONCLUSION
Outcomes of patients with relapse after adjuvant BEP seem better compared with patients who experience relapse after treatment of metastatic disease but worse compared with those who have de-novo metastatic disease. We found a substantial rate of late and subsequent relapses. There seem to be three patterns of relapse with different outcomes: pure teratoma, early viable NS relapse (< 2 years), and late viable NS relapse (> 2 years).
INTRODUCTION
Clinical stage I (CSI) nonseminoma (NS) is defined as disease limited to the testis without evidence of metastases. After inguinal orchiectomy, there are different management possibilities for these patients: active surveillance, retroperitoneal lymph node dissection, and adjuvant chemotherapy with bleomycin, etoposide, cisplatin (BEP).1 The risk of relapse in patients without adjuvant treatment after orchiectomy is approximately 15% for patients without lymphovascular invasion (LVI; low risk) and up to 50% for patients in whom LVI is present (high risk). Adjuvant chemotherapy with one or two cycles of BEP reduces the risk of relapse by approximately 90%, to about 1% to 3%.2-7 In some countries/centers, active surveillance is the preferred management option for disease in the majority of patients, independent of presence of LVI.8 In other countries, adjuvant chemotherapy often is preferred in patients with high-risk, LVI-positive disease, whereas active surveillance frequently is used for patients with low risk.1 Data about patients who experience relapse despite adjuvant chemotherapy with BEP are rare. So far, to our knowledge, no study has investigated the timing, location, treatment patterns, and respective outcomes of such relapses. There is uncertainty about whether the prognosis of patients who experience relapse after adjuvant BEP is affected by their previous adjuvant treatment and whether certain characteristics predict a worse outcome. Because of the rarity of the situation, it is almost impossible to conduct a prospective trial in this patient population. Therefore, we performed an international retrospective data collection to assess disease characteristics at time of relapse, mode of detection, treatments given, and patient outcomes.
PATIENTS AND METHODS
Using the network of the Global Germ-Cell Cancer Group, we contacted 30 centers worldwide to explore whether they had treated patients with a relapse after one or two cycles of adjuvant BEP for CSI NS. Eighteen centers had treated such patients, but the others could not identify suitable patient cases, underlining the rarity of this clinical situation.
Approval of the local ethics committee was obtained before the retrospective data collection. Patient information was collocated using predefined structured questionnaires. The protocol and case report forms are available in the Data Supplement (online only).
We collected data on patient characteristics at the time of treatment with adjuvant BEP as well as initial histology, time to, detection of, and location of relapse. In addition, data about treatment modalities at relapse and outcome were gathered. If applicable, data about subsequent relapses, including treatment, were obtained, as well as cause of death if a patient had died. Data were collected and anonymized locally at each center and then transferred and entered into a joint database in St Gallen, Switzerland.
Patients
Inclusion criteria were male sex, age ≥ 18 years, NS germ-cell cancer (GCC) in initial histology, and CSI at initial diagnosis. Additional conditions for inclusion were (1) orchiectomy for NS, (2) one or two cycles of adjuvant BEP, and (3) clinical or radiologic confirmation of recurrent testicular cancer.
Criteria for exclusion were (1) other malignancies requiring cytotoxic therapy during time of follow-up, (2) pure seminoma histology at initial diagnosis, (3) treatment with > two cycles of adjuvant BEP, (4) any other kind of adjuvant chemotherapy than BEP, and (5) contralateral testicular cancer at time of relapse—because this criterion would have made a clear distinction impossible between metastases from a contralateral cancer versus a true relapse from the incident primary tumor.
Disease stage was reported according to the Union Internationale Contre le Cancer (UICC) classification.9 For allocation to risk categories, the International Germ Cell Cancer Collaborative Group (IGCCCG) prognostic classification10 was used.
Statistical Analysis
Data of 51 patients who had received adjuvant treatment with BEP between October 1987 and July 2017 and who had experienced relapse were retrospectively analyzed. Primary outcomes were overall survival (OS) and progression-free survival (PFS). Time to event endpoints was analyzed by the Kaplan-Meier method. Calculation of time to first relapse started with day 1 of adjuvant chemotherapy. PFS and OS started with day 1 of treatment at first relapse; PFS ended with progression of disease or death as a result of any cause; OS ended with death. Censoring was done at the date of last contact. For 11 patients, the exact date of initiation of treatment of relapse was missing. For these patients, date of relapse plus 28 days (median difference in patients with both dates available) was taken as the starting date. Follow-up time was calculated with the reverse Kaplan-Meier method, which is calculated as the Kaplan-Meier estimate of the survival function, but death censors the unknown observational time of an individual. Medians and quantiles of Kaplan-Meier estimated potential follow-ups are presented. Cox univariable models were used to explore the prognostic value of covariables. The proportional hazards assumption was checked, for example by testing the scaled Schoenfeld residuals.
RESULTS
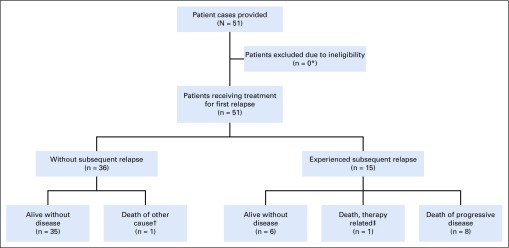
Information on 51 patients with relapse after adjuvant treatment with BEP for CSI NS was collected and analyzed. No patient cases needed to be excluded. The median follow-up was 96 months (interquartile range [IQR], 44-130 months). A patient flow diagram is provided in Figure 1.
FIG 1.
Patient flow diagram. (*) One patient of the cohort received chemotherapy with temozolomide and a combination of procarbazine, lomustine, and vincristine (PCV) for progressing oligodendroglioma 5 years and 10 months after his NS relapse; because his first relapse was pure teratoma and was treated with surgery alone, because of the long GCC-free interval until initiation of chemotherapy for his second malignancy, and because none of the other patients with pure teratoma relapses experienced a subsequent relapse, it was decided not to exclude this patient from data analysis. (†) Death from oligodendroglioma. (‡) Patient died of sepsis, bleeding, and respiratory insufficiency during high-dose chemotherapy with carboplatin/etoposide.
Characteristics at Baseline, Mode of Detection, and Stage at Relapse
The median age at orchiectomy was 30.5 years (IQR, 25-38 years), and adjuvant chemotherapy was started at a median of 40 days (min, 10 days; max, 88 days) after surgery. Table 1 lists patient characteristics at baseline and time of relapse.
TABLE 1.
Patient Characteristics at Baseline and at Relapse
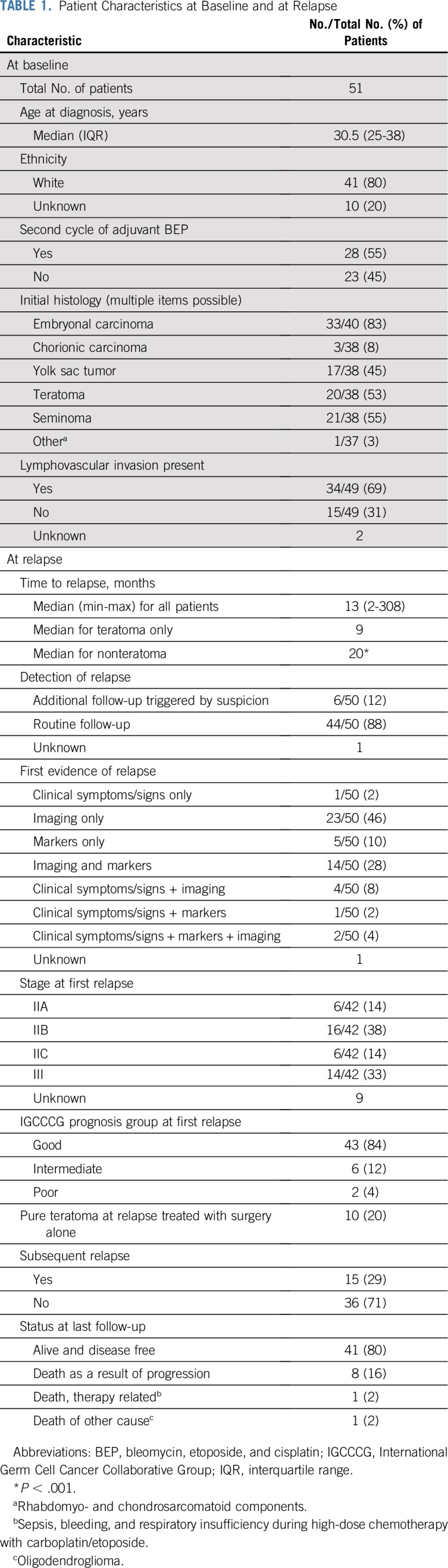
Twenty-eight (55%) of 51 patients received two cycles of adjuvant BEP; 23 (45%) of 51 were treated with one cycle in the adjuvant setting. The majority of tumors had components of embryonal carcinoma (33 [83%] of 40 patients for whom this information was available). In approximately two thirds of the patient cases (69%), LVI was present at baseline.
The median time to relapse was 13 months (IQR, 9-42 months). The earliest relapse was documented 2 months after start of adjuvant treatment, and the latest relapse was reported after 25 years. Overall, 63% of relapses occurred during the first 2 years; 8% were documented between year 2 and 3; and 29% of the patients experienced relapse > 3 years after adjuvant treatment. The median time to relapse for patients who experienced relapse with teratoma only was significantly shorter than for patients who experienced relapse with nonteratoma (9 v 20 months; P < .001).
Most of the relapses (88%) were found in routine follow-up. Only a minority of 12% were detected by additional visits triggered by suspicion of relapse.
First evidence of relapse was found by imaging alone in the majority of patient cases (46%). Approximately a quarter of the relapses (28%) were detected by imaging and elevation of tumor markers. Only a minority of patients presented with a combination of symptoms/clinical signs and/or marker elevation.
The majority of patients presented with stage IIB (38%) or stage III (33%) disease at relapse. Fourteen percent had stage IIA or IIC disease at relapse; for nine patients, stage at relapse was unknown. Using the IGCCCG classification—keeping in mind that it was developed for de-novo metastatic disease—the majority of patients were classified in the IGCCCG good prognosis group at time of relapse (84%), 12% were considered intermediate prognosis, and 4% were considered poor prognosis.
At relapse, three patients (6%) showed histologic dedifferentiation (eg, sarcomatoid components; Data Supplement) in their surgical specimens. Ten patients (20%) had pure teratoma.
Treatments
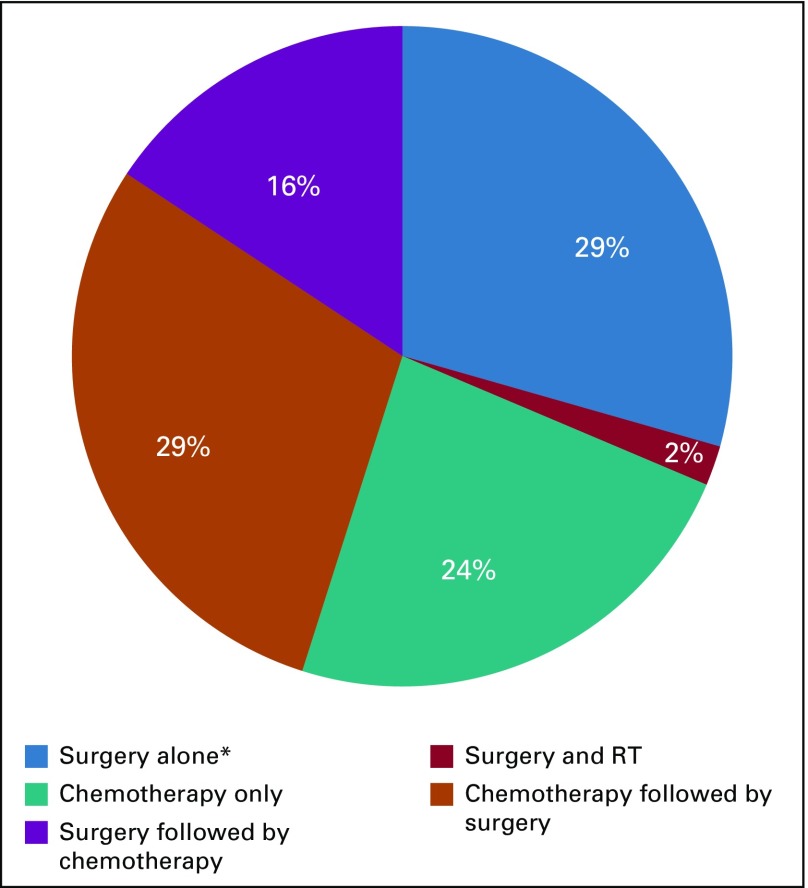
Treatments used at first relapse are presented in Figure 2 and the Data Supplement and show a remarkable diversity. The majority of patients received a combination of chemotherapy and surgery. Twenty-nine percent received chemotherapy first followed by surgery, and 16% underwent primary resection followed by different regimens of chemotherapy after vital NS had been documented (Data Supplement). Twenty-four percent of patients received chemotherapy alone, and 29% underwent surgery as single-treatment modality. Two thirds of these patients who underwent surgery alone had pure teratoma.
FIG 2.
Treatment modalities used for treatment of first relapse. RT, radiotherapy; (*) 67% of these patients had teratoma-only relapses.
Surgery was performed in the retroperitoneum alone (82%) or in the retroperitoneum as well as at other localizations, such as lung or mediastinum (Data Supplement). Only one patient (2%) received radiotherapy in combination with surgery as treatment of first relapse.
Concerning primary chemotherapy, most patients received treatment with BEP; etoposide, ifosfamide, and cisplatin; or paclitaxel, ifosfamide, and cisplatin (Data Supplement). Only two patients were treated with etoposide plus cisplatin, and one patient received high-dose chemotherapy upfront as treatment of first relapse. Of those patients receiving chemotherapy as part of the treatment of first relapse, according to the IGCCCG prognosis group, 16 (46%) of 35 received what would be a standard chemotherapy regimen for de-novo metastatic disease (eg, three cycles BEP or four cycles of etoposide plus cisplatin for good prognosis or four cycles BEP or etoposide, ifosfamide, and cisplatin for intermediate and poor prognosis, respectively), and 17 (49%) of the 35 patients received a more intense/salvage regimen upfront. We observed no significant difference in terms of outcome whether patients had received a standard or salvage regimen (data not shown).
One patient received a combination of carboplatin and paclitaxel on the initial assumption of an adenocarcinoma of unknown primary. After histology review, the diagnosis was corrected to recurrent GCC, and he went on to receive BEP after surgery. One patient was treated with a regimen that was less intense than the standard. All chemotherapy treatments given to each individual patient and cumulative cycles of cisplatin-based regimens are provided in the Data Supplement.
Subsequent Relapses and Survival
Fifteen (29%) of 51 patients experienced a subsequent relapse. The median time from first to subsequent relapse was 9 months (min, 1.4 months; max, 33.5 months). Of the patients with subsequent relapses, three had primary progression as a response to treatment of first relapse. None of these three patients achieved cure from their disease. Nine of the 15 patients with subsequent relapses died: eight died as a result of progression of NS, and one died as a result of sepsis, bleeding, and respiratory insufficiency during high-dose chemotherapy (Fig 1).
At last follow-up, 41 patients (80% of the entire study population) were alive and disease-free. Ten patients (20%) had died, eight (16%) as a result of progressing NS. One patient (2%) died of another malignancy during follow-up (oligodendroglioma), and one death (2%) was considered treatment related.
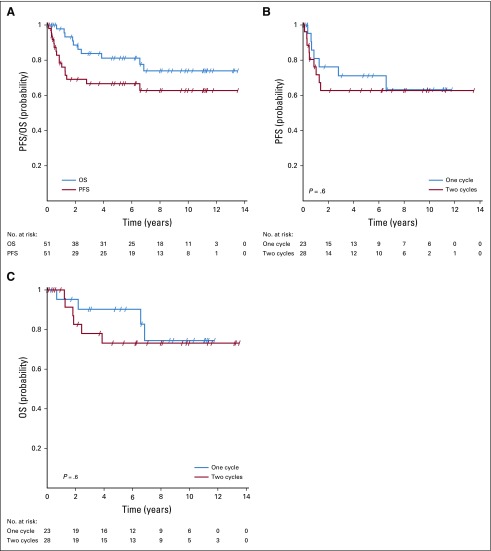
The 5-year PFS for the total population in this study was 67% (95% CI, 54% to 82%), and the 5-year OS was 81% (95% CI, 70% to 94%; Fig 3A). The 5-year cancer-specific survival was 83% (95% CI, 73% to 95%).
FIG 3.
Survival of patients. (A) Progression-free (PFS) and overall survival (OS) for all patients. (B) PFS in relation to number of adjuvant treatment cycles. (C) OS in relation to number of adjuvant treatment cycles.
We observed no significant difference for PFS and OS between patients receiving one versus two cycles of adjuvant BEP (Figs 3B and 3C). Of the patients with pure teratoma at relapse treated with surgery alone, none experienced a subsequent relapse, and none died of GCC.
With 10 relapses (20%) occurring more than 5 years after adjuvant treatment, we found a substantial rate of very late relapses. Univariable Cox analyses for the whole study population showed that late relapses were associated with an increased risk for subsequent progression (PFS: hazard ratio [HR], 1.13 per year; 95% CI, 1.05 to 1.23; P = .002) and death (HR, 1.10; 95% CI, 1.02 to 1.18; P = .01).
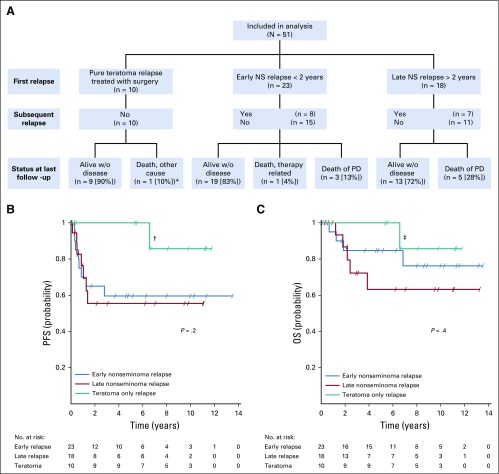
Patterns of Relapse in Relation to Outcome
Considering the different treatments and outcomes between patients experiencing relapse with pure teratoma compared with patients experiencing relapse early or late with vital undifferentiated cancer, we performed additional survival analyses by dividing the population in this study into three groups: (1) pure teratoma relapses treated with surgery alone, (2) early NS relapses < 2 years after adjuvant treatment, and (3) late NS relapses > 2 years after adjuvant BEP. The rate of death as a result of NS was 0% in group 1, 13% in group 2, and 28% in group 3 (Fig 4A). PFS and OS for the three groups are shown in Figures 4B and 4C. The single PFS/OS event for group 1 was attributed to death as a result of oligodendroglioma. There seems to be a distinction between the three groups in terms of OS. However, this did not reach statistical significance.
FIG 4.
Outcome in relation to pattern of relapse. (A) Subsequent relapses and outcomes according to the three groups: teratoma-only relapse, early nonseminoma relapse < 2 years after adjuvant treatment, and late nonseminoma relapse > 2 years after adjuvant treatment; (*) oligodendroglioma; one teratoma relapse also occurred > 2 years after adjuvant treatment. NS, nonseminoma; PD, progressive disease; w/o, without. (B) Progression-free survival (PFS) probability in relation to group; (†) PFS event allocated to death from oligodendroglioma. (C) Overall survival (OS) in relation to group; (‡) death from oligodendroglioma.
Using relapse time as a continuous variable and including only the non-teratoma relapses, this comparison again reached significance, with a HR for death of 1.09 per year (95% CI, 1.01 to 1.17; P = .03). These results suggest that the effect observed for the whole study population is driven not only by early, prognostically favorable teratoma relapses.
DISCUSSION
We report the first, to our knowledge, retrospective analysis focusing exclusively on patients with CSI NS who were treated with adjuvant BEP and who had an unequivocal relapse. The median time to relapse in this analysis was long, at 13 months, and approximately one third of patients experienced relapse > 3 years after adjuvant treatment.
Comparing our results with surveillance studies, adjuvant chemotherapy with BEP not only reduces but also seems to postpone relapses. For example, Kollmannsberger et al11 observed a median time to relapse of only 6 months for patients with CSI NS managed with active surveillance. A more recent single-center surveillance study observed a similar median time to relapse of 7 months.12 Interestingly, in the data we reported for relapses after adjuvant carboplatin treatment of CSI seminoma, the time to relapse also seemed postponed.13
Late relapses in this study seemed to be associated with a higher risk of death as a result of disease for the whole study population, and there is some suggestion that this is also true when looking at the nonteratoma relapses only. This observation is in line with other studies14-22 that also describe late relapses as a predictor of poor outcome.
Despite the fact that most of the relapses in our analysis occurred in an early stage and with good prognosis features, we documented a substantial rate of subsequent relapses (29%). In comparison, in the aforementioned surveillance study,11 only 6% of patients experienced subsequent relapses. However, LVI was present in only 16% in that series, which suggests a selection of favorable patient cases.
In general, our outcome data seem to compare unfavorably with datasets of patients with chemotherapy-naive metastatic NS in current times. In the IGCCCG population,10 patients had a 5-year PFS of 75% and a 5-year OS of 80% compared with a 5-year PFS of 67% and a 5-year OS of 81% in our cohort. A Spanish study23 also found better outcomes for patients with chemotherapy-naive metastatic NS, documenting a 3-year OS rate of 96.8% for patients with IGCCCG good prognosis and rates of 88.6% and 72% for intermediate and poor prognoses, respectively.
A negative selection of patients may explain the worse outcome in this study. First, adjuvant chemotherapy usually is given to patients who are judged to be at higher risk of relapse. Approximately two thirds of patients in this analysis had LVI at initial diagnosis, and 83% had components of embryonal carcinoma24 in initial histology. Second, the focus of this study was exclusively on patients experiencing relapse after adjuvant BEP, so we might have selected patients with biologically more aggressive disease or who have developed a form of chemotherapy resistance. Both hypotheses could explain the less favorable outcome compared with patients who had de-novo metastatic disease. However, the outcome of patients in this study still compares favorably to patients experiencing relapse after prior chemotherapy for metastatic NS.25-27
This study has limitations. We have a lack of full annotation of the interval between initial computed tomography staging and start of adjuvant treatment. Therefore, it cannot be ruled out that some patients had experienced progression to clinical stage II before the start of adjuvant BEP. Moreover, the sample size is small, in particular for the three suggested subgroups; also, the analysis was retrospective, and we solely focused on patients with a relapse after adjuvant BEP. Therefore, we cannot comment on the general population of patients with CSI NS the sample in this study was drawn from.
However, to our knowledge, we performed the first systematic analysis of patients experiencing relapse after adjuvant BEP. Prospective evaluation of a larger number of relapses is likely not possible because of the rarity of this disease setting. Thus, this study may provide some clinical guidance for this rare situation.
Because of the substantial rate of late and subsequent relapses in this study, we recommend that patients and their doctors be advised of that risk. This information should be provided when discussing adjuvant chemotherapy with patients. Because the overall risk of relapse after adjuvant BEP, fortunately, is low, we think that an imaging-based follow-up longer than mandated in current guidelines should not be recommended. However, it is important that the patients and their physicians are aware of the potential of a late relapse when new symptoms occur, especially because most centers finish follow-up for patients with GCC after 5 years. One patient in this study was treated primarily for adenocarcinoma before diagnosis was corrected to recurrent GCC, so it is important that the differential diagnosis of a late relapse of NS is always considered, that biopsies are taken, and that expert pathology review is sought. Moreover, the potential of malignant somatic transformation should be considered. Analysis for isochromosome 12 [i(12p)] might be helpful to confirm differential diagnosis of recurrent GCC.28
Considering the remarkable diversity of treatment strategies observed in this study, giving a clear treatment recommendation for patients with CSI NS who experience relapse after adjuvant BEP is difficult. Treatment at GCC expert centers29 is strongly encouraged. Patients with a high likelihood of teratoma (negative markers, suggestive on imaging) should undergo surgery first. If indeed pure teratoma is found, the likelihood of cure with surgery alone is high.
According to existing guidelines, patients with late relapses > 2 years might also be candidates for upfront surgery. They subsequently should be treated according to the histology found.
All other patients with a relapse after adjuvant BEP for CSI NS should be treated primarily with chemotherapy according to IGCCCG stage, keeping in mind that they may have a slightly worse prognosis and higher risk of additional relapse compared with patients who have de-novo metastatic disease. Bearing in mind the cumulative dose of bleomycin, including adjuvant treatment, and the potential of lung toxicity, giving three to four cycles of a three-drug regimen with replacement of bleomycin by another drug, such as ifosfamide, might be considered for these patients. Also, lowering the threshold for postchemotherapy retroperitoneal lymph node dissection should be considered, even if complete response after such chemotherapy is achieved.
In conclusion, this study was not intended to and cannot solve the ongoing debate on the optimal choice of management for CSI NS. However, it adds information to the rare clinical situation of relapses after adjuvant BEP for CSI NS for which no comprehensive data were available so far. We think that this new knowledge should be included in the discussion with patients, carefully weighing all advantages and disadvantages of the different management strategies for CSI NS.
ACKNOWLEDGMENT
We thank Laiana Schneider and Jolanda Niedermann for their support in administrative work and Laszlo Pecze for statistical support. We also thank the following investigators for contributions:Aristotle Bamias, National & Kapodistrian University of Athens, Alexandra Hospital, Athens, Greece; Anna Fingerhut, Universitätsklinikum Düsseldorf, Klinik für Urologie, Germany; Armin Gerger, Abteilung für Onkologie, Universitätsklinik für Innere Medizin, Medizinische Universität Graz, Austria; Pia Paffenholz, Department of Urology, Uro-Oncology, Robot-Assisted and Specialized Urologic Surgery, University Hospital Cologne, Germany; Christoph Seidel, Universitätsklinikum Hamburg-Eppendorf, Germany; Christopher Sweeney, Dana-Farber Cancer Institute, Boston, MA; and Peter Wilson, St Bartholomew’s Hospital, London, United Kingdom.
PRIOR PRESENTATION
Presented in part at the 2019 ASCO Genitourinary Cancers Symposium, San Francisco, CA, February 14-16, 2019.
SUPPORT
Supported in part by the SAKK (Swiss Group for Clinical Cancer Research)/Astellas GU Oncology Award 2016 and by the Swiss Cancer League.
AUTHOR CONTRIBUTIONS
Conception and design: Stefanie Fischer, Silke Gillessen
Collection and assembly of data: Stefanie Fischer, Torgrim Tandstad, Gabriella Cohn-Cedermark, Constance Thibault, Bruno Vincenzi, Dirk Klingbiel, Costantine Albany, Andrea Necchi, Angelika Terbuch, Anja Lorch, Jorge Aparicio, Axel Heidenreich, Matthew Wheater, Carl W. Langberg, Olof Ståhl, Christian Daniel Fankhauser, Anis A. Hamid, Konstantinos A. Koutsoukos, Jonathan Shamash, Jeff White, Carsten Bokemeyer, Jörg Beyer, Silke Gillessen
Data analysis and interpretation: Stefanie Fischer, Torgrim Tandstad, Gabriella Cohn-Cedermark, Bruno Vincenzi, Dirk Klingbiel, Costantine Albany, Angelika Terbuch, Jorge Aparicio, Marcus Hentrich, Olof Ståhl, Anis A. Hamid, Carsten Bokemeyer, Jörg Beyer, Silke Gillessen
Provision of study material or patients: Torgrim Tandstad, Gabriella Cohn-Cedermark, Bruno Vincenzi, Jorge Aparicio, Marcus Hentrich, Matthew Wheater, Olof Ståhl, Konstantinos Koutsoukos, Jeff White, Carsten Bokemeyer, Jörg Beyer
Administrative support: Stefanie Fischer
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Outcome of Men With Relapses After Adjuvant Bleomycin, Etoposide, and Cisplatin for Clinical Stage I Nonseminoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stefanie Fischer
Research Funding: Astellas Pharma
Travel, Accommodations, Expenses: Bayer, Astellas Pharma
Constance Thibault
Honoraria: Astellas Pharma, Janssen-Cilag, Sanofi, Pfizer, Amgen
Consulting or Advisory Role: Janssen-Cilag, Pfizer, Sanofi Pasteur, Ipsen, AstraZeneca, MedImmune
Research Funding: Sanofi Pasteur (Inst), AstraZeneca (Inst), MedImmune (Inst)
Travel, Accommodations, Expenses: Astellas Scientific and Medical Affairs, Pfizer, Janssen-Cilag, Pfizer, Astellas Pharma, Roche, Genentech, AstraZeneca, MedImmune
Bruno Vincenzi
Speakers’ Bureau: PharmaMar
Dirk Klingbiel
Employment: Roche
Stock and Other Ownership Interests: F. Hoffmann-La Roche AG
Travel, Accommodations, Expenses: Roche
Costantine Albany
Stock and Other Ownership Interests: Advaxis
Honoraria: Sanofi
Consulting or Advisory Role: Seattle Genetics, AstraZeneca, MedImmune
Speakers’ Bureau: Sanofi
Research Funding: Astex Pharmaceuticals (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Lilly (Inst), Bayer (Inst)
Travel, Accommodations, Expenses: Sanofi
Andrea Necchi
Employment: Bayer (I)
Stock and Other Ownership Interests: Bayer (I)
Honoraria: Roche, Merck, AstraZeneca, Janssen, Foundation Medicine
Consulting or Advisory Role: Merck Sharp & Dohme, Roche, Bayer, AstraZeneca, Clovis Oncology, Janssen, Incyte, Seattle Genetics, Astellas, Bristol-Myers Squibb, Rainier Therapeutics
Research Funding: Merck Sharp & Dohme (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Roche, Merck Sharp & Dohme, AstraZeneca, Janssen, Rainier Therapeutics
Other Relationship: Bayer (I)
Anja Lorch
Honoraria: Astellas Scientific and Medical Affairs, Ipsen, MSD Oncology
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, AstraZeneca, Roche, MSD Oncology, Janssen Oncology
Travel, Accommodations, Expenses: Novartis, Ipsen, AstraZeneca
Jorge Aparicio
Consulting or Advisory Role: Servier, Merck, Bayer, Amgen, Sirtex Medical, Sanofi, Roche, Celgene
Axel Heidenreich
Honoraria: Amgen, Astellas Pharma, Bayer, Ferring, Ipsen, Janssen-Cilag, Sanofi, Takeda
Consulting or Advisory Role: Astellas Pharma, Bayer, Janssen-Cilag, Clovis Oncology
Speakers' Bureau: Amgen, Astellas Pharma, Bayer, Ipsen, Johnson & Johnson, Sanofi, Takeda, Pfizer
Research Funding: Astellas Pharma, Bayer, Sanofi
Marcus Hentrich
Consulting or Advisory Role: Amgen, Janssen-Cilag, Bristol-Myers Squibb, Sanofi, AbbVie, Hexal, Jazz Pharmaceuticals
Speakers' Bureau: Amgen, Janssen-Cilag, Sanofi, Takeda, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Amgen, Celgene, Janssen-Cilag, Novartis, Sanofi, Takeda
Matthew Wheater
Honoraria: Bristol-Myers Squibb, Novartis, Pfizer, Bristol-Myers Squibb, Delcath Systems, MSD, Pierre Fabre
Consulting or Advisory Role: Novartis, Healthcare at Home, Bristol-Myers Squibb
Research Funding: Roche (Inst), GlaxoSmithKline (Inst), Novartis (Inst), MSD (Inst), AVEO (Inst)
Travel, Accommodations, Expenses: MSD, Bristol-Myers Squibb
Carl W. Langberg
Consulting or Advisory Role: Bristol-Meyers Squibb
Research Funding: Merck Sharp & Dohme (Inst)
Olof Ståhl
Honoraria: Bayer
Anis A. Hamid
Honoraria: Bayer
Consulting or Advisory Role: MSD
Konstantinos A. Koutsoukos
Honoraria: Roche Hellas, Ipsen Greece, Bristol-Myers Squibb, MSD Oncology, Pfizer, Novartis
Consulting or Advisory Role: Pierre Fabre, Roche, Ipsen, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Ipsen, MSD Oncology, Pfizer, Bristol-Myers Squibb
Jonathan Shamash
Speakers’ Bureau: Pfizer, EMD Serono
Carsten Bokemeyer
Honoraria: Merck KGaA, Sanofi, Roche, Bayer, Bristol-Myers Squibb, Servier, Pfizer, AstraZeneca
Consulting or Advisory Role: Lilly, ImClone, Merck Serono, Sanofi, Mundipharma, Bayer Scering Pharma, Hexal, Merck Sharp & Dohme, GSO, AOK Health Insurance
Research Funding: AbbVie (Inst), ADC Therapeutics (Inst), Agile Therapeutics (Inst), Alexion Pharmaceuticals (Inst), Amgen (Inst), Apellis Pharmaceuticals (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Bayer (Inst), BerGenBio (Inst), BGB (Inst), Blueprint Medicines (Inst), Bristol-Myers Squibb (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Daiichi Sankyo (Inst), Eisai (Inst), GIEHO (Inst), Gilead Sciences (Inst), Glycotope GmbH (Inst), GlaxoSmithKline (Inst), Incyte (Inst), IO Biotech (Inst), Isofol Medical (Inst), Janssen-Cilag (Inst), Karyopharm Therapeutics (Inst), Lilly (Inst), Millennium (Inst), MSD (Inst), Nektar (Inst), Novartis (Inst), Rafael Pharmaceuticals (Inst), Roche (Inst), Springworks Therapeutics (Inst), Taiho Pharmaceutical (Inst)
Travel, Accommodations, Expenses: Merck Serono, Sanofi, Pfizer, Bristol-Myers Squibb
Jörg Beyer
Honoraria: Roche, Janssen Oncology, AstraZeneca
Silke Gillessen
Honoraria: Janssen
Consulting or Advisory Role: Astellas Pharma (Inst), Curevac (Inst), Novartis (Inst), Active Biotech (Inst), Bristol-Myers Squibb (Inst), Ferring (Inst), MaxiVax, Advanced Accelerator Applications, Roche, Janssen (Inst), Innocrin Pharma (Inst), Sanofi, Bayer (Inst), Orion Pharma GmbH, Clovis Oncology (Inst), Menarini Silicon Biosystems (Inst)
Patents, Royalties, Other Intellectual Property: Method for biomarker (WO 3752009138392 A1)
Other Relationship: Nektar, ProteoMediX
No other potential conflicts of interest were reported.
REFERENCES
- 1.Beyer J, Albers P, Altena R, et al. Maintaining success, reducing treatment burden, focusing on survivorship: Highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Ann Oncol. 2013;24:878–888. doi: 10.1093/annonc/mds579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tandstad T, Dahl O, Cohn-Cedermark G, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: The SWENOTECA management program. J Clin Oncol. 2009;27:2122–2128. doi: 10.1200/JCO.2008.18.8953. [DOI] [PubMed] [Google Scholar]
- 3.Tandstad T, Ståhl O, Håkansson U, et al. One course of adjuvant BEP in clinical stage I nonseminoma: Mature and expanded results from the SWENOTECA group. Ann Oncol. 2014;25:2167–2172. doi: 10.1093/annonc/mdu375. [DOI] [PubMed] [Google Scholar]
- 4.Albers P, Siener R, Krege S, et al. Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group. J Clin Oncol. 2008;26:2966–2972. doi: 10.1200/JCO.2007.12.0899. [DOI] [PubMed] [Google Scholar]
- 5.Westermann DH, Schefer H, Thalmann GN, et al. Long-term follow-up results of 1 cycle of adjuvant bleomycin, etoposide, and cisplatin chemotherapy for high-risk clinical stage I nonseminomatous germ cell tumors of the testis. J Urol. 2008;179:163–166. doi: 10.1016/j.juro.2007.08.172. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert DC, Norman AR, Nicholl J, et al. Treating stage I nonseminomatous germ cell tumours with a single cycle of chemotherapy. BJU Int. 2006;98:67–69. doi: 10.1111/j.1464-410X.2006.06188.x. [DOI] [PubMed] [Google Scholar]
- 7.Vidal AD, Thalmann GN, Karamitopoulou-Diamantis E, et al. Long-term outcome of patients with clinical stage I high-risk nonseminomatous germ-cell tumors 15 years after one adjuvant cycle of bleomycin, etoposide, and cisplatin chemotherapy. Ann Oncol. 2015;26:374–377. doi: 10.1093/annonc/mdu518. [DOI] [PubMed] [Google Scholar]
- 8.Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31:3490–3493. doi: 10.1200/JCO.2012.47.6010. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Compton CC. TNM seventh edition: What's new, what's changed—Communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–5339. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 10.International Germ Cell Cancer Collaborative Group International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 11.Kollmannsberger C, Tandstad T, Bedard PL, et al. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol. 2015;33:51–57. doi: 10.1200/JCO.2014.56.2116. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton RJ, Nayan M, Anson-Cartwright L, et al. Treatment of relapse of clinical stage I nonseminomatous germ cell tumors on surveillance. J Clin Oncol. 2019;37:1919–1926. doi: 10.1200/JCO.18.01250. [DOI] [PubMed] [Google Scholar]
- 13.Fischer S, Tandstad T, Wheater M, et al. Outcome of men with relapse after adjuvant carboplatin for clinical stage I seminoma. J Clin Oncol. 2017;35:194–200. doi: 10.1200/JCO.2016.69.0958. [DOI] [PubMed] [Google Scholar]
- 14.Sharp DS, Carver BS, Eggener SE, et al. Clinical outcome and predictors of survival in late relapse of germ cell tumor. J Clin Oncol. 2008;26:5524–5529. doi: 10.1200/JCO.2007.15.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baniel J, Foster RS, Gonin R, et al. Late relapse of testicular cancer. J Clin Oncol. 1995;13:1170–1176. doi: 10.1200/JCO.1995.13.5.1170. [DOI] [PubMed] [Google Scholar]
- 16.Gerl A, Clemm C, Schmeller N, et al. Late relapse of germ cell tumors after cisplatin-based chemotherapy. Ann Oncol. 1997;8:41–47. doi: 10.1023/a:1008253323854. [DOI] [PubMed] [Google Scholar]
- 17.Shahidi M, Norman AR, Dearnaley DP, et al. Late recurrence in 1,263 men with testicular germ cell tumors: Multivariate analysis of risk factors and implications for management. Cancer. 2002;95:520–530. doi: 10.1002/cncr.10691. [DOI] [PubMed] [Google Scholar]
- 18.George DW, Foster RS, Hromas RA, et al. Update on late relapse of germ cell tumor: A clinical and molecular analysis. J Clin Oncol. 2003;21:113–122. doi: 10.1200/JCO.2003.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Dieckmann KP, Albers P, Classen J, et al. Late relapse of testicular germ cell neoplasms: A descriptive analysis of 122 cases. J Urol. 2005;173:824–829. doi: 10.1097/01.ju.0000154013.96349.36. [DOI] [PubMed] [Google Scholar]
- 20.Oldenburg J, Alfsen GC, Waehre H, et al. Late recurrences of germ cell malignancies: A population-based experience over three decades. Br J Cancer. 2006;94:820–827. doi: 10.1038/sj.bjc.6603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronnen EA, Kondagunta GV, Bacik J, et al. Incidence of late-relapse germ cell tumor and outcome to salvage chemotherapy. J Clin Oncol. 2005;23:6999–7004. doi: 10.1200/JCO.2005.21.956. [DOI] [PubMed] [Google Scholar]
- 22.Oldenburg J, Martin JM, Fosså SD. Late relapses of germ cell malignancies: Incidence, management, and prognosis. J Clin Oncol. 2006;24:5503–5511. doi: 10.1200/JCO.2006.08.1836. [DOI] [PubMed] [Google Scholar]
- 23.Germà-Lluch JR, Garcia del Muro X, Maroto P, et al. Clinical pattern and therapeutic results achieved in 1490 patients with germ-cell tumours of the testis: The experience of the Spanish Germ-Cell Cancer Group (GG) Eur Urol. 2002;42:553–562. doi: 10.1016/s0302-2838(02)00439-6. discussion 562-563. [DOI] [PubMed] [Google Scholar]
- 24.Heidenreich A, Sesterhenn IA, Mostofi FK, et al. Prognostic risk factors that identify patients with clinical stage I nonseminomatous germ cell tumors at low risk and high risk for metastasis. Cancer. 1998;83:1002–1011. [PubMed] [Google Scholar]
- 25.Lorch A, Beyer J, Bascoul-Mollevi C, et al. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol. 2010;28:4906–4911. doi: 10.1200/JCO.2009.26.8128. [DOI] [PubMed] [Google Scholar]
- 26.Lorch A, Bascoul-Mollevi C, Kramar A, et al. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: Evidence from a large international database. J Clin Oncol. 2011;29:2178–2184. doi: 10.1200/JCO.2010.32.6678. [DOI] [PubMed] [Google Scholar]
- 27.Lorch A, Kleinhans A, Kramar A, et al. Sequential versus single high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: Long-term results of a prospective randomized trial. J Clin Oncol. 2012;30:800–805. doi: 10.1200/JCO.2011.38.6391. [DOI] [PubMed] [Google Scholar]
- 28.Atkin NB, Baker MC. Specific chromosome change, i(12p), in testicular tumours? Lancet. 1982;2:1349. doi: 10.1016/s0140-6736(82)91557-4. [DOI] [PubMed] [Google Scholar]
- 29.Tandstad T, Kollmannsberger CK, Roth BJ, et al. Practice makes perfect: The rest of the story in testicular cancer as a model curable neoplasm. J Clin Oncol. 2017;35:3525–3528. doi: 10.1200/JCO.2017.73.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]