Abstract
PURPOSE
Primary endocrine therapy for ductal carcinoma in situ (DCIS) as a potential alternative to surgery has been understudied. This trial explored the feasibility of a short-term course of letrozole and sought to determine whether treatment results in measurable radiographic and biologic changes in estrogen receptor (ER)–positive DCIS.
PATIENTS AND METHODS
A phase II single-arm multicenter cooperative-group trial was conducted in postmenopausal patients diagnosed with ER-positive DCIS without invasion. Patients were treated with letrozole 2.5 mg per day for 6 months before surgery. Breast magnetic resonance imaging (MRI) was obtained at baseline, 3 months, and 6 months. The primary end point was change in 6-month MRI enhancement volume compared with baseline.
RESULTS
Overall, 79 patients were enrolled and 70 completed 6 months of letrozole. Of these, 67 patients had MRI data available for each timepoint. Baseline MRI volumes ranged from 0.004 to 26.3 cm3. Median reductions from baseline MRI volume (1.4 cm3) were 0.6 cm3 (61.0%) at 3 months (P < .001) and 0.8 cm3 (71.7%) at 6 months (P < .001). Consistent reductions were seen in median baseline ER H-score (228; median reduction, 15.0; P = .005), progesterone receptor H-score (15; median reduction, 85.0; P < .001), and Ki67 score (12%; median reduction, 6.3%; P = .007). Of the 59 patients who underwent surgery per study protocol, persistent DCIS remained in 50 patients (85%), invasive cancer was detected in six patients (10%), and no residual DCIS or invasive cancer was seen in nine patients (15%).
CONCLUSIONS
In a cohort of postmenopausal women with ER-positive DCIS, preoperative letrozole resulted in significant imaging and biomarker changes. These findings support future trials of extended endocrine therapy as primary nonoperative treatment of some DCIS.
INTRODUCTION
Ductal carcinoma in situ (DCIS) is considered a nonobligate precursor for invasive breast cancer, and its incidence has increased almost eightfold in the United States since the introduction of screening mammography.1 In the United States, DCIS comprises approximately 20% of all mammographically detected breast neoplasms.2 The rationale for treatment of DCIS is prevention of possible invasive progression, but active controversy exists about whether current treatment guidelines may result in overtreatment for some women with low propensity for invasion.
The mainstay of treatment of DCIS has been surgery, either breast-conserving surgery (BCS), in up to 70%, or mastectomy, in more than 30% of patients.3,4 Adjuvant radiation therapy is administered to more than 60% of women who undergo BCS, and greater than one-third of women with estrogen receptor (ER)–positive DCIS also undergo endocrine therapy.5,6 Trials of endocrine therapy have shown clear benefit in the adjuvant, neoadjuvant, and preventive settings. For DCIS, adjuvant endocrine treatment reduces risk of locoregional recurrence, particularly in ER-positive disease.7,8 In invasive cancers, neoadjuvant endocrine therapy has been shown to increase both resectability and breast conservation rate, and it results in up to a 20% rate of clinical complete response.9,10 Primary endocrine therapy also is recommended in the prevention setting for high-risk histologies, such as lobular carcinoma in situ (LCIS) and atypical hyperplasia, to reduce risk of both ipsilateral and contralateral breast events. However, the benefit of endocrine therapy in the absence of surgery for DCIS remains largely unknown.
Thus, we sought to determine the following: (1) whether unresected DCIS responds to short-term preoperative endocrine therapy, as in invasive cancer; (2) whether response could be monitored noninvasively with imaging; and (3) whether some patients with DCIS could achieve a pathologic complete response with a short course of endocrine therapy alone. Our goal was to evaluate the feasibility and assessability of preoperative endocrine therapy for DCIS, to determine whether future studies of endocrine therapy alone for DCIS could be supported.
PATIENTS AND METHODS
Study Design and Treatment Plan
Cancer and Leukemia Group B (CALGB) 40903 is a phase II single-arm study of preoperative endocrine therapy in postmenopausal women diagnosed with ER-positive DCIS (Fig 1). CALGB is now part of the Alliance for Clinical Trials in Oncology. The study was reviewed and supported by the National Institutes of Health, Division of Cancer Prevention, and was approved by the institutional review board at each participating site (ClinicalTrials.gov identifier: NCT01439711). Protocol therapy consisted of 6 months of letrozole administered orally at a dose of 2.5 mg/day. Patients underwent bilateral breast magnetic resonance imaging (MRI) for disease evaluation at baseline, 3 months, and 6 months. Patients found to have radiographic progression at month 3 were scheduled for surgery. Patients with either stable disease or partial response on the 3-month MRI completed 6 months of letrozole and had surgery within 4 weeks of the 6-month MRI. Type of surgery was elected by the patient and her surgeon according to National Comprehensive Cancer Network treatment guidelines for DCIS.11
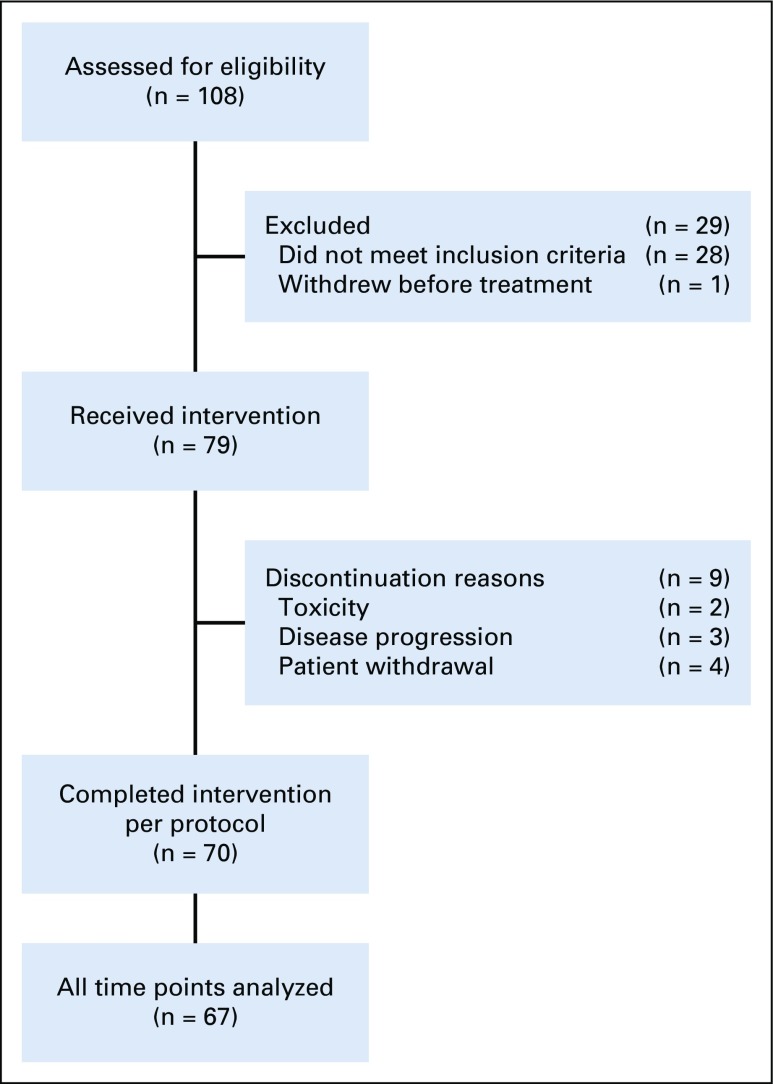
FIG 1.
Study schema for CALGB 40903. The overall cohort included 108 patients. Of those enrolled in the study, 28 patients were deemed ineligible to proceed with the study. The most common reasons for exclusion were as follows: baseline magnetic resonance imaging (MRI) not assessable, missing, or volume = 0 (n = 11), no ductal carcinoma in situ (DCIS) found on baseline mammogram (n = 6), baseline mammogram extent < 10 mm or missing (n = 5), invasive or suspected invasive cancer found on central review of MRI (n = 4), and other reasons (n = 2). Three patients had surgery after 3 months of letrozole treatment because of concerns about possible disease progression on the basis of MRI; two patients withdrew because of adverse effects associated with letrozole treatment; and four withdrew for other reasons.
Study Population and Eligibility Criteria
Key eligibility criteria included postmenopausal women with core biopsy confirmation of ≥ 1% ER-positive and/or progesterone receptor (PR)–positive DCIS (cTis N0 M0) without invasion, with a clip placed at the site of biopsy. Patients were excluded for any prior treatment administered for DCIS. Mammographic extent of calcifications was required to be measurable in at least one dimension with each lesion > 1 cm and < 7 cm, and DCIS was required to be visible on MRI without radiographic suspicion for invasive cancer. Patients with palpable DCIS or ipsilateral axillary adenopathy were excluded. Each participant signed an institutional review board–approved, protocol-specific informed consent document in accordance with federal and institutional guidelines.
End Points
The primary objective was to estimate the change in 6-month MRI tumor volume from baseline. The secondary objective was to determine whether 3-month change in volume correlated with 6-month change. Tumor volume was determined by applying enhancement thresholds to contrast-enhanced MRI data, as described in the Methods. The secondary objectives were to assess change in ER, PR, and Ki67 as well as to evaluate radiographic-pathologic correlation between MRI findings and histopathology. In addition, surgical treatment data and pathologic findings were collected.
Breast Imaging
Patients had two digital bilateral mammograms, one at baseline and one within 4 weeks before surgery. The two standard views (cranio-caudal and medial-lateral oblique) for each breast were acquired along with additional images, as deemed necessary by the breast radiologist. The breast MRI protocol included a T2-weighted sequence, diffusion-weighted imaging sequence, and dynamic contrast-enhanced series using a bilateral, 3-dimensional, fat-suppressed, T1-weighted sequence with 80- to 100-second temporal resolution, performed on a 1.5T or 3.0T whole-body MRI scanner with a dedicated breast coil. Each participating site submitted two case reports performed according to protocol for site qualification before enrolling the first patient. MR images were obtained for each patient at baseline, 3 months, and 6 months. Radiographic response was assessed centrally by investigators at the University of California, San Francisco, breast MRI research laboratory within 2 days of study submission.
Immunohistochemical Markers
Immunohistochemical staining was performed centrally at the University of California, San Francisco, pathology department immunohistochemistry laboratory. A Ventana automated stainer (Ventana Medical Systems, Oro Valley, AZ) was used for ER, PR, and Ki67 stains. Conditions for antigen retrieval, proteolytic processing, antibody titer, and positive and negative controls were previously developed and validated for all antigens. Two study pathologists (Y.-Y,C, and G.K,) scored all slides using a standardized scoring protocol.
Statistical Analysis
Data collection was conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson according to Alliance policies. All final analyses were performed on the study database, which was frozen on March 31, 2018.
Sample size calculations were performed using Power Analysis and Sample Size software version 11.0.10 (PASS; NCSS statistical software, Kaysville, UT). Sample size calculations were based on pilot data, which showed a mean 3-month decrease in tumor volume of 0.87 cm3 (standard deviation, 1.7; unpublished data). On the basis of these preliminary findings, accrual of 96 patients would allow estimation of the mean change to within ± 0.34 cm of the true mean change with 95% power (two-sided test, α = .05). Assuming a 10% dropout rate, the accrual target was 106 patients.
Patient characteristics and surgical-pathologic outcomes were summarized according to treatment (lumpectomy, mastectomy, or no surgery). Differences among treatment groups were evaluated using Fisher’s exact and Kruskal-Wallis tests for categoric and continuous variables, respectively. Change in mammographic and MRI extent of disease from baseline were calculated for each timepoint. Because values were not normally distributed, the median change and median percent change were calculated, and Wilcoxon sign rank tests were used to evaluate the significance of these changes.
Biomarker changes between baseline and surgery were compared using Wilcoxon sign rank tests. Kruskal-Wallis tests were used to evaluate the significance of relationships between baseline biomarker values and pathologic response categories. Correlations between pathologic tumor size and maximum diameters of baseline and 6-month MRI as well as 6-month mammographic extent of disease were evaluated using Spearman correlation coefficients. MRI response at each timepoint was classified as follows: 90% image-complete response (> 90% reduction), 80% imaging complete response (81%-90% reduction), imaging partial response (20%-80% reduction), and imaging sustained disease or progressive disease (< 20% reduction or increase). All statistical tests were two sided and had type I error rates of 5%.
RESULTS
Patient Characteristics
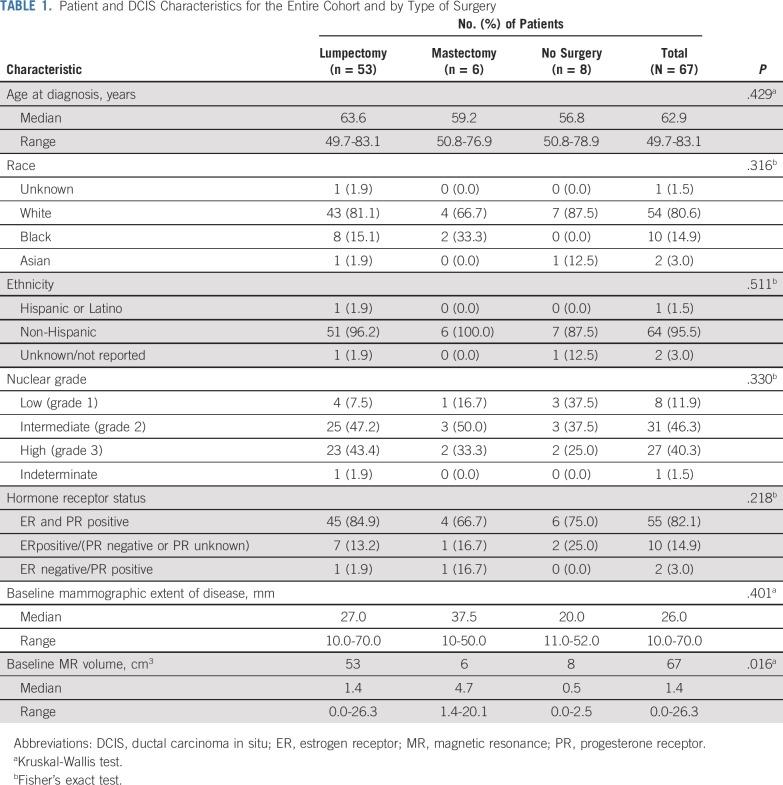
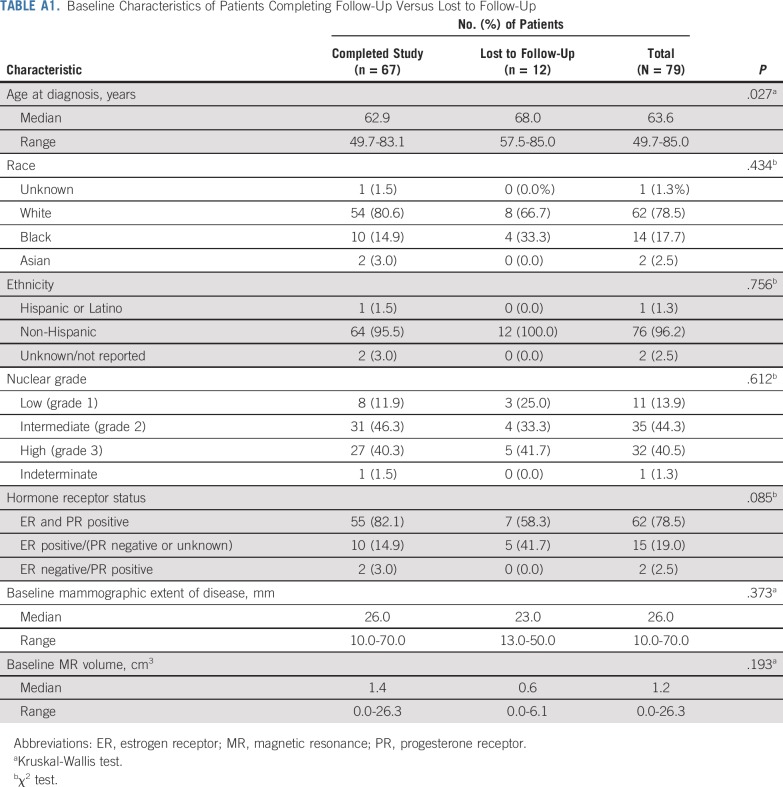
Between August 1, 2012 and February 1, 2016, 108 patients were enrolled. Of those, 29 patients were excluded because they did not meet inclusion criteria (n = 28) or withdrew before treatment (n = 1). The most common reasons for exclusion were as follows: baseline MRI missing, not assessable or volume of 0 mm (n = 11), no DCIS visible on baseline mammogram (n = 6), baseline mammogram extent < 10 mm or missing (n = 5), and invasive or suspected invasive cancer found on subsequent review of baseline MRI (n = 4). Of the 79 patients who started treatment with letrozole, three patients had surgery after 3 months of letrozole treatment because of concerns about possible disease progression on the basis of the MRI, two patients withdrew because of adverse effects associated with letrozole treatment, and four withdrew for other reasons. Patient characteristics were similar between patients who completed treatment and those lost to follow-up (Appendix Table A1, online only. A total of 70 patients completed intervention per protocol, of whom 67 patients had evaluable data for baseline, 3-month and 6-month MRIs; this group constituted the final study cohort (Fig 1). Although the number of evaluable patients in the final cohort was smaller than anticipated, the effect size we observed was larger than expected, allowing detection of significant and clinically relevant changes in tumor size. Patient characteristics of the study participants according to surgery status are listed in Table 1. Characteristics were similar among patients who had surgery (n = 59) and those who did not have surgery (n = 8) and did not differ significantly between treatment groups.
TABLE 1.
Patient and DCIS Characteristics for the Entire Cohort and by Type of Surgery
Radiographic Changes
Mammography.
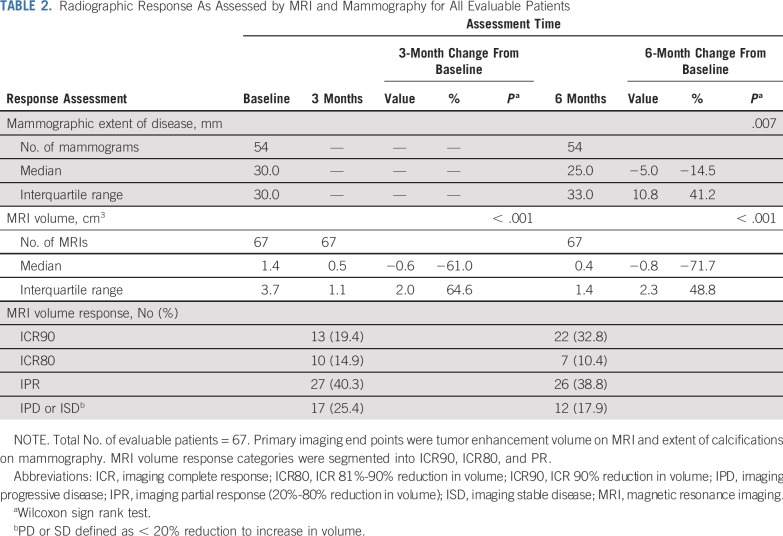
Mammography was obtained at baseline and repeated after 6 months or within 4 weeks before surgical excision. Assessment of mammographic disease was based solely on total extent of calcifications, because any patients with a mass associated with DCIS had been excluded from the trial. Among the 54 patients with both baseline and 6-month mammograms, the median reduction in extent of disease was 5.0 mm (14.5%; interquartile range, 10.8; P = .007; Table 2).
TABLE 2.
Radiographic Response As Assessed by MRI and Mammography for All Evaluable Patients
Breast MRI.
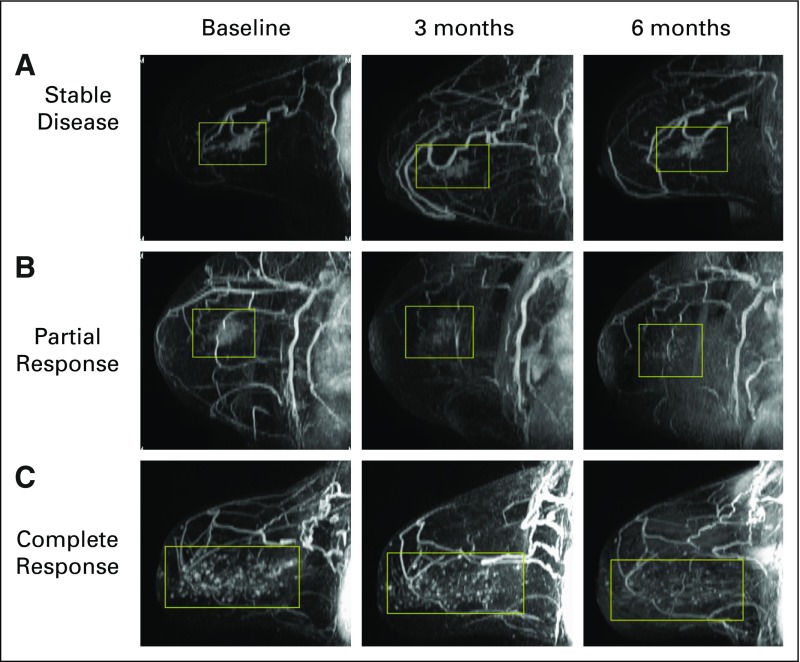
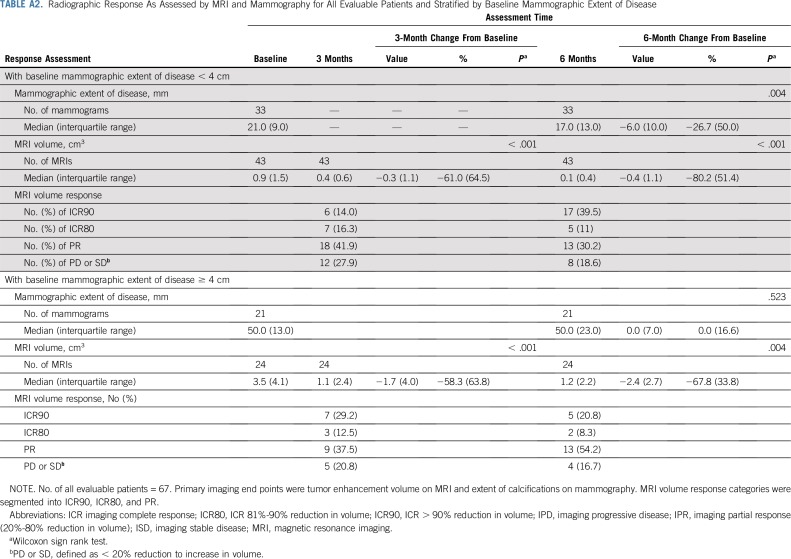
Bilateral breast MRI was obtained at baseline, 3 months, and 6 months according to the imaging protocol. Baseline MRI volumes ranged from 0.004 to 26.3 cm3. The median reduction in tumor volume was 0.6 cm3 (61%) at 3 months (interquartile range, 2.0 cm3; P < .001) and was 0.8 cm3 (71.7%) at 6 months (interquartile range, 2.3 cm3; P < .001). Imaging complete response to treatment (ICR) ranged from stable/progressive disease to radiologic complete response; at the 6-month MRI; 43% of patients had > 80% ICR, 39% had 20%-80% ICR, and 18% had < 20% ICR (Table 2; Fig 2). We noted no significant correlations between radiographic response and age at diagnosis. Moreover, on stratified analysis, these findings remained significant in lesions both < 4cm and ≥ 4cm in extent (Appendix Table 2, online only). To ensure that there was no significant selection bias, we performed a sensitivity analysis that included the four patients for whom 3-month, but not 6-month, MRI measurements were available and found that there remained a significant reduction in MRI volume at 3 months (P < .001).
FIG 2.
Spectrum of magnetic resonance (MR)–based response patterns of hormone receptor–positive ductal carcinoma in situ to preoperative letrozole therapy. Patients exhibited variable patterns at baseline and at 3- and 6-month MR imaging timepoints. The predominant radiographic patterns were (A) no response (17.9% at 6 months), (B) partial response (49.2% at 6 months), or (C) complete response to therapy (32.8% at 6 months).
Surgical and Pathologic Outcomes
Study protocol required that patients undergo definitive surgery at the end of letrozole treatment. Fifty-nine patients had surgery; BCS was achieved in 53 patients (Table 3). Eight patients declined surgery. Notably, the rate of BCS was 86% among patients who had at least 4 cm of calcifications on baseline mammography compared with 92% among patients with < 4 cm of calcifications (P = .63; Appendix Table A3, online only). Eight patients required one re-excision for close/positive margins.
TABLE 3.
Surgical Treatment and Pathologic Findings at Definitive Surgery
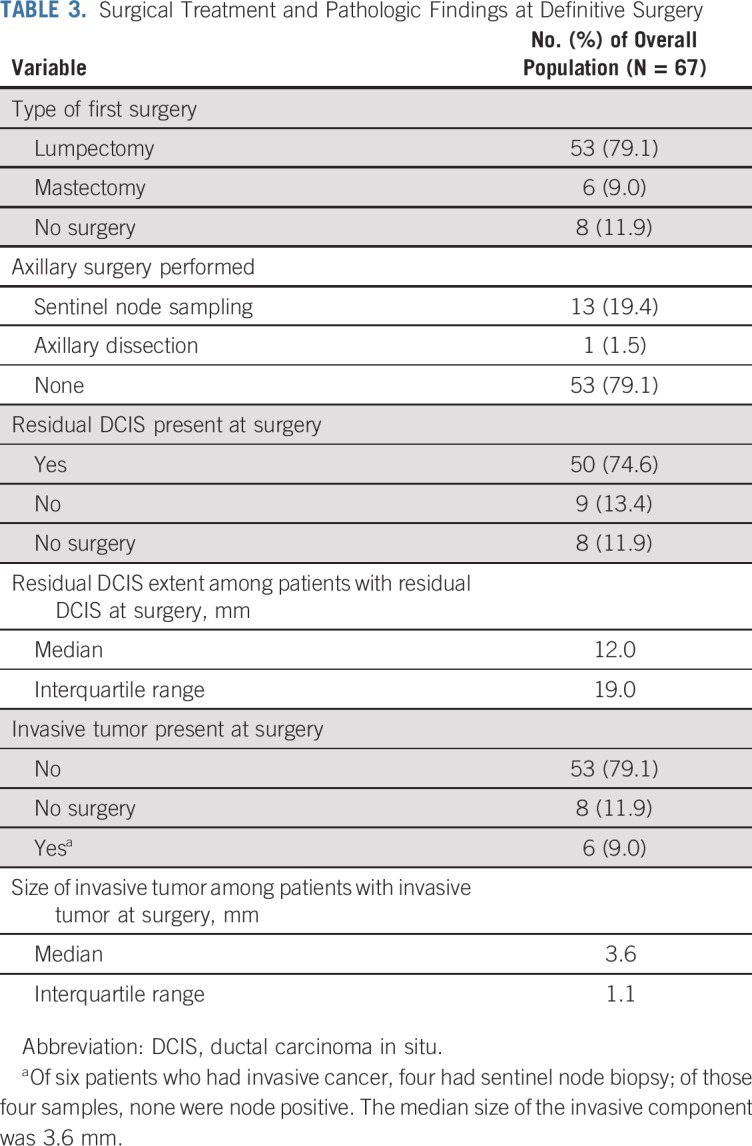
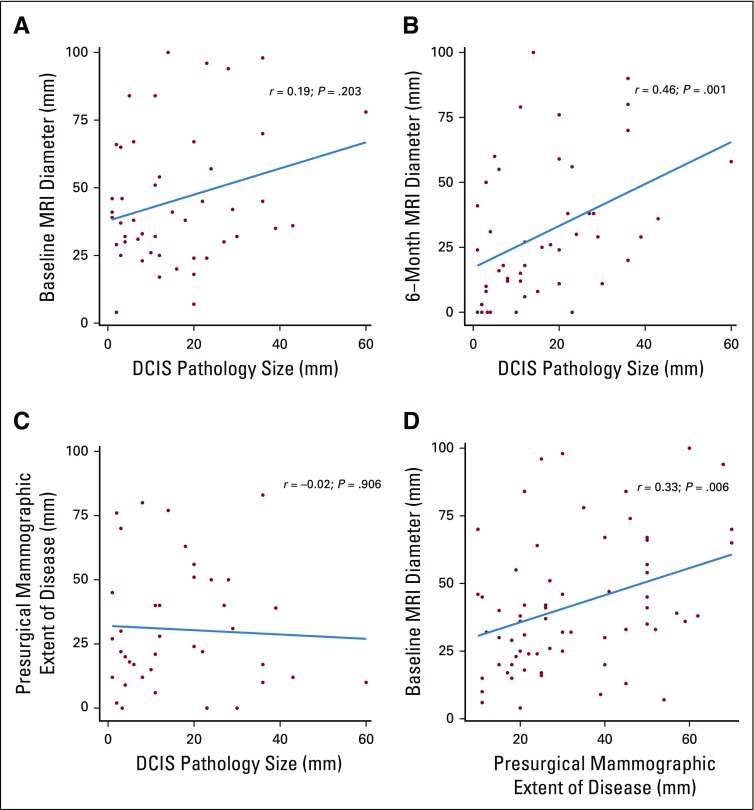
Final pathology revealed residual DCIS in 50 (85%) of 59 patients who had surgery. The median histologic extent of DCIS was 12.0 mm (interquartile range, 19.0 mm) compared with a median reported baseline extent of calcifications of 30.0 mm (interquartile range, 30.0 mm). Upstaging to invasive cancer was seen in six patients (10%): Three had intermediate-grade and three had high-grade cancers; all were node negative. Notably, nine patients (15%) had pathologic complete response, including four with intermediate-grade and two with high-grade DCIS. Baseline extent of calcifications in these patients ranged from 15 to 59 mm. No significant correlation was observed between baseline mammographic maximum extent and pathologic DCIS size (Spearman correlation coefficient, −0.02; P = .906) or between baseline MRI maximum diameter and pathologic DCIS size (Spearman correlation coefficient, 0.19; P = .203; Appendix Figs A1A-A1D, online only). However, significant correlation was observed between 6-month MRI maximum diameter and pathologic DCIS size (Spearman correlation coefficient, 0.46; P = .001).
Biomarkers of Response
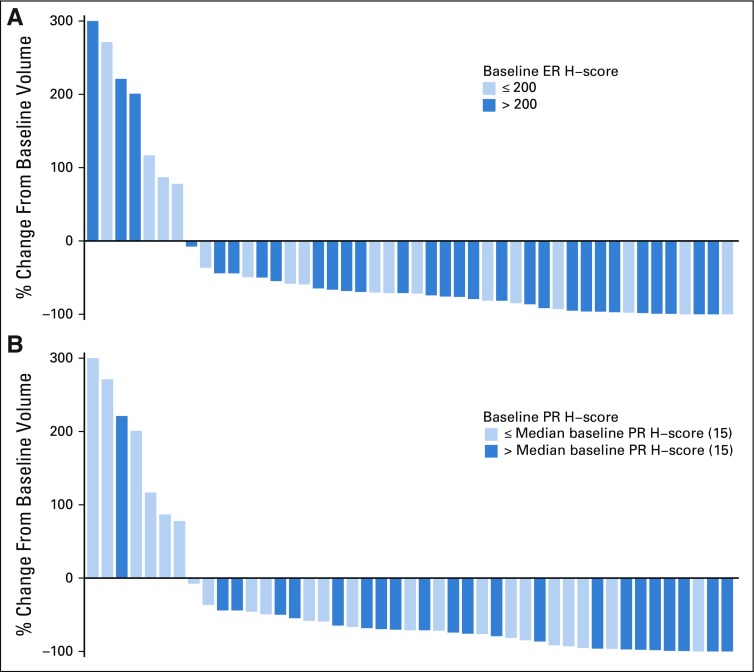
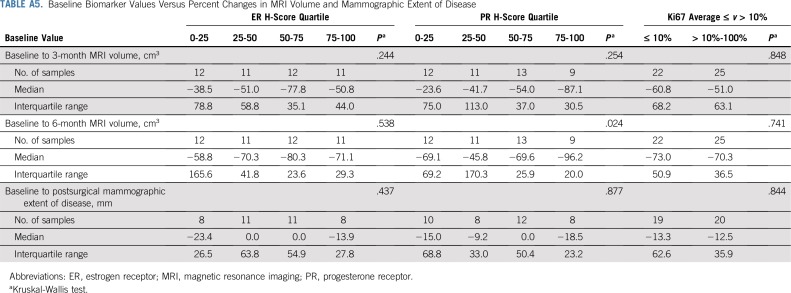
ER, PR, and Ki67 were assessed at baseline and at surgical excision after letrozole treatment. All three markers were significantly reduced with endocrine therapy: The median ER H-score decreased by 15 (P = .005), the median PR H-score decreased by 85 (P < .001), and the median Ki67 score decreased by 6.3% (P = .007). Associations between reduction in DCIS MRI volume and baseline ER and PR H-scores are presented in Figure 3. Baseline ER and Ki67 did not differ significantly between patients with and without pCR (Appendix Table A4, online only). Reductions in MRI volumes did not differ by higher ER H-score quartile or Ki67 score greater than 10% (Appendix Table A5, online only), but higher baseline PR H-score was significantly associated with reduction in MRI volume at 6 months (96% median volume reduction in the highest PR-H score quartile; P = .024; Appendix Table A5).
FIG 3.
Waterfall plot: change in MRI enhancement at 6 months by (A) baseline estrogen receptor (ER) H-score and (B) baseline progesterone receptor (PR) H-score.
DISCUSSION
To our knowledge, this study is the first to evaluate the effect of endocrine therapy for unresected HR-positive DCIS. Significant radiologic and histologic changes were noted after 6 months of therapy. Eighty-five percent of patients had residual DCIS at surgery, possibly reflecting a limitation of the relatively short 6-month duration of letrozole treatment. However, 15% of patients had pathologic complete response, despite a baseline extent of calcifications ranging from 15-59 mm. These results are provocative and support both the relevance and feasibility of future trials of longer-term endocrine treatment alone as treatment of DCIS.
Current guideline recommendations for DCIS include complete surgical resection, often combined with radiation and/or endocrine therapy. This differs markedly from the management of other preinvasive diagnoses, such as atypical hyperplasias and LCIS, for which recommended treatment consists of either core biopsy or surgical sampling followed by surveillance, including the option of endocrine therapy. The benefit of endocrine therapy for women at increased risk for breast cancer, including those with atypia or LCIS, has been established in two large prospective, randomized trials, which showed a 50% reduction in cancer incidence with tamoxifen compared with placebo.12,13 Most recently, IBIS II showed that, for women at high risk on the basis of family history or histology, there is a significant benefit of aromatase inhibitors compared with tamoxifen (hazard ratio, 0.47; 95% CI, 0.32 to 0.68).14 Thus, women with elevated breast cancer risk are routinely recommended to consider endocrine therapy to reduce future risk of breast cancer. However, these studies did not address whether endocrine therapy by itself could provide benefit in unresected DCIS.
In the adjuvant endocrine therapy setting, two randomized trials have demonstrated up to a 30% reduction in new ipsilateral or contralateral breast events with 5 years of tamoxifen compared with placebo in women undergoing BCS.8,15,16 In contrast, neoadjuvant endocrine therapy has only been studied for invasive cancer, in which it has been shown to improve resectability and increase rates of breast conservation.9,10,17,18 The possible benefit of preoperative endocrine treatment of DCIS was first suggested by Boland et al,19 who observed that women diagnosed with DCIS while taking exogenous hormones had a reduction in Ki67 if exogenous hormone therapy was discontinued before surgery.
We built upon these initial observations by treating patients with a preoperative course of aromatase inhibitor. The study design required noninvasive longitudinal radiologic assessments, with MRI selected as the primary end point. Although MRI has been reported to both under- and overestimate extent of DCIS, it has nevertheless been shown to be more highly correlated to pathologic extent than mammography.20-22 Furthermore, breast MRI has been shown to be a useful indicator of response to neoadjuvant therapy in invasive cancer.23-25 We found that the majority of MRI changes were seen early in letrozole treatment, with a significant median reduction in tumor volume of 61% by 3 months. As a secondary end point, mammography also demonstrated a small but significant reduction in greatest extent of DCIS by 6 months, indicating that mammography may also be an effective tool to monitor patients undergoing surveillance.
Similar to neoadjuvant aromatase inhibitor trials for ER-positive invasive cancer, this trial showed biologic evidence of treatment response in DCIS, with ER, PR, and Ki67 significantly reduced after letrozole treatment. We also noted that 10% of patients who underwent surgery after preoperative endocrine therapy had invasive cancer. Although it is possible that invasive progression may have occurred during the 6-month window of letrozole treatment, it is more likely that this represents undersampling of concurrent invasive cancer at baseline and compares favorably with the upstaging rate of 26% reported in a meta-analysis of 7,350 women with a core biopsy diagnosis of DCIS.26 Clearly, this will be an important consideration if nonsurgical treatment of DCIS is to be considered.
As in other neoadjuvant trials, accurate assessment of baseline disease extent is difficult to establish, because pathologic assessment is performed only after preoperative therapy has been administered. However, the median extent of DCIS at excision was 12.0 mm compared with a median baseline extent of calcifications of 26.0 mm, supporting possible reduction of DCIS extent with letrozole. Interestingly, nine patients (15%) had pathologic complete response, including 60% (three of five patients) with low-grade DCIS, despite extent of calcifications that ranged from 15 to 59 mm. Although this finding is not definitive because of the small sample size and short duration of treatment, it nevertheless is provocative and suggests that some women with DCIS may in the future be candidates for primary endocrine therapy alone.
Important caveats should be noted in the interpretation of these data. First, we underscore that, although MRI is a useful research tool, it has known limitations for assessment of DCIS, including variable concordance with pathologic size, which may be even more limiting in the post-treatment setting. We note that more than 25% of baseline MRIs did not meet criteria because of either poor visualization of DCIS or confounding by postbiopsy artifacts, somewhat limiting the generalized utility of this approach. Moreover, as a single-arm study, the baseline radiographic and pathologic assessments served as reference measures, as there was no control group not treated with endocrine therapy. Therefore, definitive evaluation of whether preoperative endocrine therapy could either increase the breast conservation rate for DCIS or obviate the need for surgery altogether must be addressed by future clinical trials.
In conclusion, CALGB 40903, a trial of preoperative letrozole for DCIS, demonstrated both feasibility and assessability of short-term endocrine therapy for ER-positive disease. These results support that the standard accepted approach used for ADH and LCIS—that of close monitoring with the option for endocrine therapy—should be studied more in DCIS as a rational long-term alternative to surgery for low-risk DCIS.
ACKNOWLEDGMENT
We thank the patients and their families as well as the study teams at each study site for their commitment to the trial and to furthering our understanding of the biology and treatment of ductal carcinoma in situ. We thank the funders for their essential role in the generous support of this trial and Novartis for providing study drug. We also thank the following institutional networks that participated in study:
Aurora National Cancer Institute (NCI) Community Oncology Research Program, Milwaukee, WI; Michael Thompson; Bay Area Tumor Institute, Oakland, CA, Lisa Bailey, UG1CA189817; Bethesda North Hospital, Cincinnati, OH, Chin Ho; Cedars-Sinai Medical Center, Los Angeles, CA, Armando Giuliano; Dana-Farber/Partners Cancer Care LAPS, Boston, MA, Harold Burstein, U10CA180867; Delaware/Christiana Care NCI Community Oncology Research Program, Newark, DE, Gregory Masters, UG1CA189819; Duke University, Duke Cancer Institute LAPS, Durham, NC, Jeffrey Crawford, U10CA180857; Heartland Cancer Research NCORP, Decatur, IL, Bryan Faller, UG1CA189830; Mayo Clinic LAPS, Rochester, MN, Steven Alberts, U10CA180790; Medical University of South Carolina Minority Underserved NCORP, Charleston, SC, Scott Lindhorst, UG1CA189848; The Ohio State University Comprehensive Cancer Center LAPS, Columbus, OH, Claire Verschraegen, U10CA180850; Saint Elizabeth Medical Center South, Edgewood, KY, Daniel Flora; Sentara Norfolk General Hospital, Norfolk, VA, Eric Feliberti; Sinai Hospital of Baltimore, Baltimore, MD, Marvin Feldman; Southeast Clinical Oncology Research (SCOR) Consortium NCORP, Winston-Salem, NC, James Atkins, UG1CA189858; Southern Cancer Center PC-Providence, Mobile, AL, Connie Uzel; UCSF Medical Center-Mount Zion, San Francisco, CA, Charalambos Andreadis; UNC Lineberger Comprehensive Cancer Center LAPS, Chapel Hill, NC, Thomas Shea, U10CA180838; University of Iowa/Holden Comprehensive Cancer Center, Iowa City, IA, Umar Farooq; University of Oklahoma Health Sciences Center LAPS, Oklahoma City, OK, Adam Asch, U10CA180798; University of Texas MD Anderson Cancer Center LAPS, Houston, TX, Kelly Hunt, U10CA180858; and University of Texas Southwestern Medical Center LAPS, Dallas, TX, Ann Leitch, U10CA180870.
APPENDIX
TABLE A1.
Baseline Characteristics of Patients Completing Follow-Up Versus Lost to Follow-Up
TABLE A2.
Radiographic Response As Assessed by MRI and Mammography for All Evaluable Patients and Stratified by Baseline Mammographic Extent of Disease
TABLE A3.
Surgery Type According to Baseline Mammographic Extent of Disease
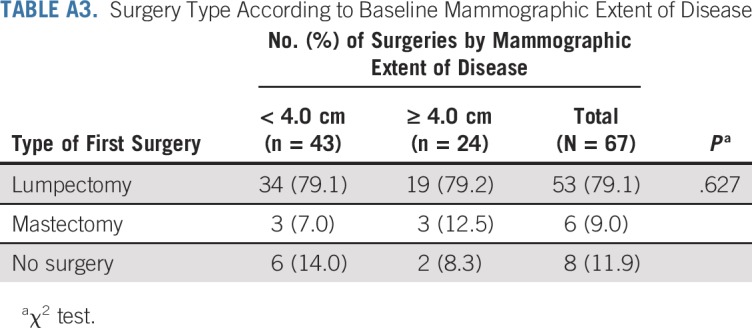
TABLE A4.
Baseline Biomarker Values in Patients With and Without Pathologic Complete Response
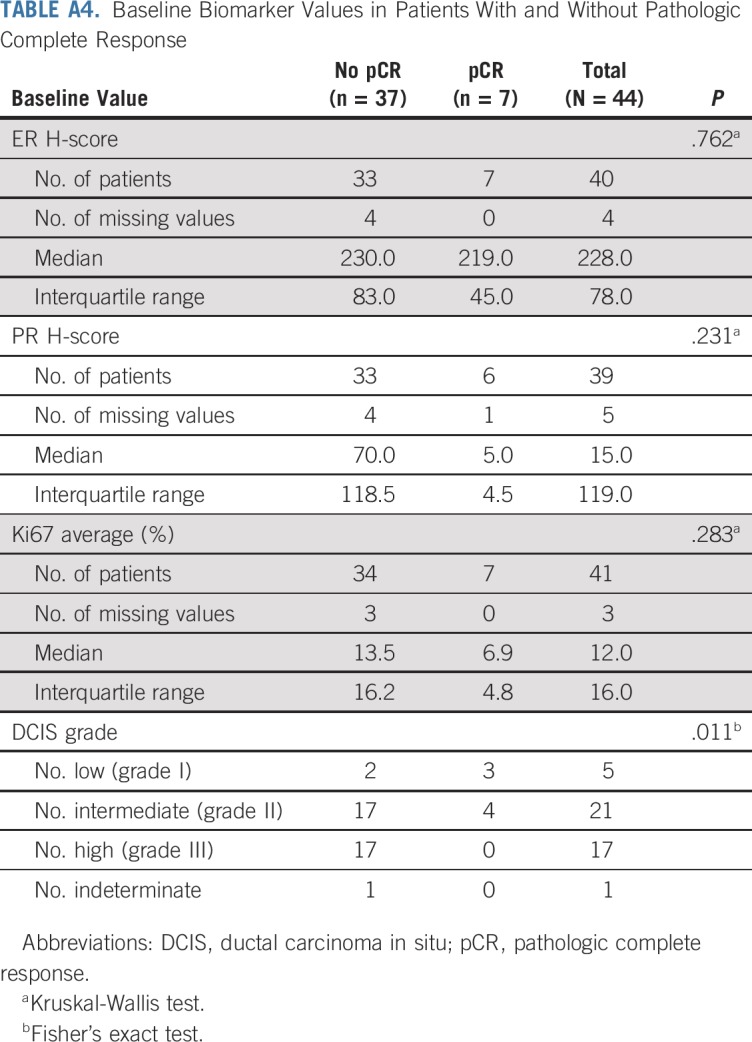
TABLE A5.
Baseline Biomarker Values Versus Percent Changes in MRI Volume and Mammographic Extent of Disease
FIG A1.
Correlation between (A) pathology size and baseline magnetic resonance imaging (MRI) maximum diameter, (B) pathology size and 6-month MRI maximum diameter, (C) 6-month mammogram maximum diameter, and (D) baseline MRI diameter and presurgical mammographic extent of disease. DCIS, ductal carcinoma in situ.
PRIOR PRESENTATION
Presented in part at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 5-9, 2017.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Nos. U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U01CA225427 (to N.H.), U10CA180838, U10CA180857, U10CA180858, and UG1CA189819; and in part by funds from The Breast Cancer Research Foundation (to E.S.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CLINICAL TRIAL INFORMATION
See accompanying Editorial on page 1252
AUTHOR CONTRIBUTIONS
Conception and design: E. Shelley Hwang, Clifford A. Hudis, Eric Winer, David W. Ollila, Laura Esserman, Nola Hylton
Collection and assembly of data: E. Shelley Hwang, Terry Hyslop, Stephanie Duong, Elissa Price, Joseph Guenther, Alan P. Lyss, Diana Dickson-Witmer, Richard Hoefer, Timothy Hardman, Jeffrey Marks, Yunn-Yi Chen, Laura Esserman, Nola Hylton
Data analysis and interpretation: E. Shelley Hwang, Terry Hyslop, Laura H. Hendrix, Stephanie Duong, Isabelle Bedrosian, Elissa Price, Abigail Caudle, Tina Hieken, Joseph Guenther, Clifford A. Hudis, Alan P. Lyss, David W. Ollila, Jeffrey Marks, Yunn-Yi Chen, Gregor Krings, Laura Esserman, Nola Hylton
Provision of study material or patients: Isabelle Bedrosian, Tina Hieken, Joseph Guenther, Diana Dickson-Witmer, Richard Hoefer, Timothy Hardman
Administrative support: Clifford A. Hudis
Financial support: Clifford A. HudisManuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Single-Arm Study of Preoperative Letrozole for Estrogen Receptor–Positive Postmenopausal Ductal Carcinoma In Situ: CALGB 40903 (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Terry Hyslop
Consulting or Advisory Role: AbbVie
Travel, Accommodations, Expenses: AbbVie
Elissa Price
Patents, Royalties, Other Intellectual Property: Husband has patents on devices related to the treatment of cerebral vascular disease (I)
Abigail Caudle
Consulting or Advisory Role: MD Anderson Physician’s Network
Research Funding: EndoMag (Inst)
Travel, Accommodations, Expenses: Endomagnetics
Tina Hieken
Research Funding: Genentech (Inst), Roche (Inst)
Joseph Guenther
Speakers’ Bureau: Castle Biosciences
Clifford A. Hudis
(OPTIONAL) Uncompensated Relationships: Alliance Foundation, Columbia University External Scientific Advisory Board, Memorial Sloan Kettering Cancer Center
(OPTIONAL) Open Payments Link: https://openpaymentsdata.cms.gov/physician/471974/summary
Eric Winer
Stock and Other Ownership Interests: Verastem
Honoraria: Genentech, Roche, Tesaro, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, Genomic Health
Research Funding: Genentech (Inst), Novartis (Inst), Merck (Inst)
David W. Ollila
Stock and Other Ownership Interests: Johnson & Johnson
Gregor Krings
Honoraria: Novartis
Speakers' Bureau: Novartis
Laura Esserman
Consulting or Advisory Role: Blue Cross Blue Shield Association
Research Funding: Merck
Travel, Accommodations, Expenses: Blue Cross Blue Shield Association
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bleyer A, Welch HG. Effect of three decades of screening mammography on breast cancer incidence. N Engl J Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- 2.Allison KH, Abraham LA, Weaver DL, et al. Trends in breast biopsy pathology diagnoses among women undergoing mammography in the United States: A report from the Breast Cancer Surveillance Consortium. Cancer. 2015;121:1369–1378. doi: 10.1002/cncr.29199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward EM, DeSantis CE, Lin CC, et al. Cancer statistics: Breast cancer in situ. CA Cancer J Clin. 2015;65:481–495. doi: 10.3322/caac.21321. [DOI] [PubMed] [Google Scholar]
- 4.Worni M, Akushevich I, Greenup R, et al. Trends in treatment patterns and outcomes for ductal carcinoma in situ. J Natl Cancer Inst. 2015;107:djv263. doi: 10.1093/jnci/djv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagara Y, Freedman RA, Wong SM, et al. Trends in adjuvant therapies after breast-conserving surgery for hormone receptor-positive ductal carcinoma in situ: Findings from the National Cancer Database, 2004-2013. Breast Cancer Res Treat. 2017;166:583–592. doi: 10.1007/s10549-017-4436-9. [DOI] [PubMed] [Google Scholar]
- 6.Virnig BA, Torchia MT, Jarosek SL, et al. Use of Endocrine Therapy Following Diagnosis of Ductal Carcinoma In Situ or Early Invasive Breast Cancer: Data Points # 14. Rockville, MD: Data Points Publication Series; 2011. [PubMed] [Google Scholar]
- 7.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: Long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 9.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol. 2011;29:2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23:5108–5116. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Gradishar WJ, Anderson BO, Balassanian R, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:310–320. doi: 10.6004/jnccn.2018.0012. [DOI] [PubMed] [Google Scholar]
- 12.Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: Extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75. doi: 10.1016/S1470-2045(14)71171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–1048. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 15.Houghton J, George WD, Cuzick J, et al. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ of the breast in the UK, Australia, and New Zealand: Randomised controlled trial. Lancet. 2003;362:95–102. doi: 10.1016/s0140-6736(03)13859-7. [DOI] [PubMed] [Google Scholar]
- 16.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: A study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol. 2001;12:1527–1532. doi: 10.1023/a:1013128213451. [DOI] [PubMed] [Google Scholar]
- 18.Ellis MJ, Coop A, Singh B, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: Evidence from a phase III randomized trial. J Clin Oncol. 2001;19:3808–3816. doi: 10.1200/JCO.2001.19.18.3808. [DOI] [PubMed] [Google Scholar]
- 19.Boland GP, McKeown A, Chan KC, et al. Biological response to hormonal manipulation in oestrogen receptor positive ductal carcinoma in situ of the breast. Br J Cancer. 2003;89:277–283. doi: 10.1038/sj.bjc.6601013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kropcho LC, Steen ST, Chung AP, et al. Preoperative breast MRI in the surgical treatment of ductal carcinoma in situ. Breast J. 2012;18:151–156. doi: 10.1111/j.1524-4741.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- 21.Marcotte-Bloch C, Balu-Maestro C, Chamorey E, et al. MRI for the size assessment of pure ductal carcinoma in situ (DCIS): A prospective study of 33 patients. Eur J Radiol. 2011;77:462–467. doi: 10.1016/j.ejrad.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Wisner DJ, Hwang ES, Chang CB, et al. Features of occult invasion in biopsy-proven DCIS at breast MRI. Breast J. 2013;19:650–658. doi: 10.1111/tbj.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinovich ML, Macaskill P, Irwig L, et al. Agreement between MRI and pathologic breast tumor size after neoadjuvant chemotherapy, and comparison with alternative tests: Individual patient data meta-analysis. BMC Cancer. 2015;15:662. doi: 10.1186/s12885-015-1664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheel JR, Kim E, Partridge SC, et al. MRI, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 trial. AJR Am J Roentgenol. 2018;210:1376–1385. doi: 10.2214/AJR.17.18323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868–877. doi: 10.2214/ajr.184.3.01840868. [DOI] [PubMed] [Google Scholar]
- 26.Brennan ME, Turner RM, Ciatto S, et al. Ductal carcinoma in situ at core-needle biopsy: Meta-analysis of underestimation and predictors of invasive breast cancer. Radiology. 2011;260:119–128. doi: 10.1148/radiol.11102368. [DOI] [PubMed] [Google Scholar]