Abstract
The development of diagnostics and medical devices has historically been concentrated in high-income countries, despite a significant need to expand healthcare services to low- and middle-income countries (LMIC). Poor quality healthcare extends beyond LMIC to underserved communities in developed countries. This paper reviews diseases and conditions that have not received much attention in the past despite imposing a significant burden on healthcare systems in these circumstances. We review the underlying mechanism of action of these conditions and current technology in use for diagnosis or surgical intervention. We aim to identify areas for technological development and review policy considerations that will enable real-world adoption. Specifically, this review focuses on diseases prevalent in sub-Saharan Africa and south Asia: melioidosis, infant and maternal mortality, schistosomiasis, and heavy metal and pesticide poisoning. Our aim with this review is to identify problems facing the world that require the attention of the medical device community and provide recommendations for research directions for groups interested in this field.
Keywords: medical devices, disadvantaged nations, device development, underrepresented diseases
1 Methods
A formal search of peer-reviewed articles pertaining to diseases affecting low- and middle-income countries (LMIC) was performed using Google Scholar and PubMed in order to compile this qualitative systematic review. Keywords used to identify diseases and technologies included, but were not limited to, the following terms: medical devices, low-cost design, global health, low-income countries, low- and middle-income countries, point-of-care diagnostics, neglected tropical diseases (NTD), neonatal mortality. The names of specific health challenges in conjunction with other keywords were also used in the search. Identified conditions were compared based upon the number of affected people, disability adjusted life years, and current research carried out to address the condition. The diseases chosen for this review are melioidosis and neglected tropical diseases such as schistosomiasis. These two diseases combined affect over 500,000 people in south Asia and sub-Saharan Africa [1]. This is a fraction of the number of people affected by mosquito-borne diseases [2], but still presents a significant healthcare burden on developing economies. In contrast to, for example, malaria or HIV, these diseases have not received notable attention nor the development of technological solutions toward their treatment. One of the key aims of this review is to expose this unmet need. A section of this review focuses on neonatal mortality, which accounts for over 4 × 106 deaths worldwide and is a significant economic burden due to the number of productive years of life lost [3]. Finally, a section on policy and implementation is provided to aid researchers in identifying useful areas of study.
2 Melioidosis
Melioidosis (Whitmore's disease) is an infection caused by the bacterium Burkholderia pseudomallei and is prevalent in south and east Asia and northern Australia. In major regions where the disease is endemic, the annual incidence rates are up to 50 cases per 100,000 people [4]. It has been classified as a category B bioterrorism agent by the United States and recent reports indicate that the endemic areas have expanded to southern China, Hong Kong, Taiwan, and parts of the Americas [5].
Although healthcare facilities have seen significant improvements over the past few years, reports suggest that diagnosis of melioidosis is only possible in large regional referral centers. This could lead to a delay in diagnosis and treatment, and an underestimate of the number of people infected with the disease [6]. Diagnosis of melioidosis can be complicated by the presenting symptoms and their similarity to tuberculosis: abscesses in the lungs, liver, spleen, and brain stem encephalitis. The bacterium typically infects the host by inoculation onto the skin, by inhalation, or by ingestion. Inoculation onto the skin can cause subcutaneous abscesses, inhalation can cause pneumonia, and the bacterium can reach internal organs such as the spleen, liver, and the lungs through the blood. The most common presentation of melioidosis is pneumonia, with over 50% of cases either occurring through lung abscess or due to septicemic spread. An additional challenge with melioidosis is that the bacteria, B. Pseudo-mallei, is a difficult organism to kill, with studies showing that it is capable of surviving in triple distilled water for years [7]. The disease primarily occurs during the rainy season [8] and people who are in direct contact with wet soil are most vulnerable to the disease. Predispositions such as diabetes mellitus, cirrhosis, and alcoholism, or those who are immunosuppressed increase the risk of contracting the bacterium [9]. Over 50% of patients diagnosed with melioidosis already have diabetes mellitus as a predisposition [10]. This presents a major problem for south Asian countries as the number of patients living with diabetes as an underlying condition is projected to increase by 70% between 2010 and 2030 [11]. An additional risk factor is the age group for contracting diabetes mellitus in these countries is 40–60, with reports showing that incidence of melioidosis peaking in this age group, and most countries of this region exhibiting aging demographics.
The recommended treatment of melioidosis involves the intravenous injection of the antibiotic ceftazidime for at least 10 days [12]. The therapeutic response to the drug is slow and physicians tend to prematurely switch antibiotics in a considerable number of cases, with the assumption that the bacterium has developed antibiotic resistance. The mortality for a 2-week administration of the drug is 18% [10]. Although there have been developments over the past 30 years with multiple clinical trials [13–15] in treating melioidosis, the cost of the antibiotics is still high (from US$70–$150 a day [16]), imposing a significant economic burden on developing countries. Simpson et al. [17] comment on the price of drug regimens used to treat the disease and cite lack of financial support from pharmaceutical companies for diseases prevalent in rural areas as one of the challenges in developing treatment and diagnostic solutions. Melioidosis has been described as “the great imitator” of other tropical diseases such as malaria, typhoid fever, leptospirosis, and tuberculosis [18]. The varying clinical manifestations of the disease and the multiple affected organs often lead to an incorrect diagnosis. Delayed identification results in the administration of antibiotics that are usually unsuitable to treat melioidosis, which has led to high mortality rates as indicated by multiple studies [18,19]. It is critical that rapid diagnostic solutions are developed to reduce mortality rates. The most widely used method to detect B. Pseudo-mallei is culturing in a modified Ashdown medium with colistin [20], but even this technique may yield inaccurate results, especially in nonendemic areas where the clinical suspicion is low. In cases where there is a positive culture, it may be difficult for commercial systems to differentiate between B. Pseudo-mallei and phenotypically similar species such as Burkholderia thailandensis [21]. Recent reports highlight not only the limitations of bacterial gene amplification and detection but also the development of new screening and amplification techniques [22]. Since the sequencing of the complete genome of B. Pseudo-mallei [23], several quantitative polymerase chain reaction (PCR) assays have been developed to identify the species [24]. However, it is still a challenge to identify primers that can differentiate B. Pseudo-mallei from closely related species.
Clinical laboratories have recently started adopting matrix-assisted laser desorption/ionization mass spectrometry systems for the identification of bacterium [25–28], with modest clinical success alongside reports that suggest the databases used mass spectrometry require expansion to accurately identify B. Pseudo-mallei. This technique shows great potential to be used as a rapid identification technique but has the limitation of requiring skilled personnel, expensive equipment, and the infrastructure to support them. Low-cost molecular profiling and analysis has been a challenge, and advancements in this field would have a significant beneficial impact on healthcare outcomes in endemic areas. Surface acoustic wave-based atomization has been proposed to simplify the sample extraction and filtration process [29]. High-frequency surface acoustic wave-based atomization was used for the formation of micrometer scale droplets of cell lysate, and the purification step was achieved by loading the sample on a porous membrane placed in the direction of propagation of the surface acoustic waves.
Since the main cause of melioidosis spread is through soil during the rainy season, analyzing soil for the presence of B. Pseudo-mallei would prevent infection and hospitalization. Environmental samples are difficult to purify via PCR due to the typical presence of a diverse species of bacteria and fungi. Development of better enrichment and purification techniques would enable an increase in the number of relevant DNA templates for amplification to occur. An alternative to sampling environmental specimens is the analysis of relevant clinical samples—blood and sputum [30,31]. The sensitivity of PCR-based techniques is high for sputum and pus, but, by contrast, the sensitivity for blood samples is quite low [32,33].
There may be as many as 165,000 cases of melioidosis every year with an estimated 89,000 deaths [34]. The most commonly used technique for identification, bacterial culture, is 100% specific but has low sensitivity. Slow or incorrect diagnosis results in a significant burden on healthcare systems and can be fatal. Matrix assisted laser desorption/ionization-time of flight mass spectrometry is increasingly being used as a rapid and accurate technique to identify isolates [35,36], though significant challenges to its adoption remain but likely can be addressed by further research in this field.
3 Heavy Metal and Organophosphate Contamination of Water
This section details the risks associated with poisoning due to metals, such as lead and cadmium, and associated detection techniques. Additionally, we consider the effects and detection of environmental pollutants arising from intensive agricultural and industrial practices. Evidence of the toxicity of lead has been documented in studies conducted as early as 1914 [37] and studies demonstrating the adverse effects of lead on attention, childhood development, and neurological disorders were established in the 1970s [38]. Lead poisoning has been shown to impair cognitive ability and social function [39].
The toxic properties of lead are partially due to its ability to compete with calcium at a cellular level, for neuronal signaling [40] and in inhibiting calcium influx into cells [41]. The neurological effects are due to its inhibitory impact on stimulated neurotransmitter release [42,43]. At high doses, lead can inhibit myelin formation and compromises the integrity of the blood brain barrier. Children are more sensitive to lead than adults due to increased hand-to-mouth activity and increased absorption in the gut. The acceptable thresholds for lead in blood have drastically changed since the 1960s in the United States, from 60 μg/dl to essentially recognizing that there is no safe level of lead exposure, with amounts under 5 μg/dl having been shown to adversely impact learning [44]. Significant reduction in lead exposure in the United States has been achieved by eliminating lead in gasoline and paint, but lead contamination of water remains a problem without a commensurate level of attention [45]. The decision in 2014 by the city of Flint to change its primary source of water saw lead levels in water rise to as much as 1000 μg/dl in some cases [46], with lasting healthcare implications. The only medical treatment for lead exposure is chelation [47], but chelating drugs may not be available in LMIC and in lower income regions of even wealthy countries. In any case, they are not very useful to treat chronic exposure.
Another heavy metal that has adverse health effects is cadmium, commonly used in the electronics industry in batteries and circuit boards. Chronic exposure to cadmium results in accumulation in the kidneys that eventually leads to renal damage [48]. Cadmium can also be absorbed by plants more readily than other heavy metals, presenting another mode of uptake in regions contaminated with industrial waste. The number of people worldwide affected by heavy metal poisoning is difficult to estimate due to the varying sources of poisoning but, given that incidents like the one in Flint that occurred in a developed country with safeguards in place to prevent lead poisoning, there is a need to estimate atmospheric and water-based heavy metal exposure risks across the world.
The daily per-capita supply of calories has increased by over 30% over the last 50 years [49], and as this demand increases, there has been a push toward maximizing agricultural productivity. Increasing agricultural yield has been possible, in part, by the increased use of pesticides. The food and agriculture organization of the United Nations defines pesticides as growth regulators and includes substances that can prevent harm to crop during or after harvest [50]. Exposure to pesticides may occur through direct means through occupational exposure or via indirect means through drinking water, dust and food. The World Health Organization (WHO) estimates that 3 × 106 severe pesticide poisonings occur annually and that at least 300,000 people die as a result of exposure, with 99% of these cases being from LMICs [51]. The effects of pesticide exposure are wide ranging: chronic diseases such as cancer and developmental disorders [52], immunosuppression, hormone disruption, diminished intelligence, and reproductive abnormalities [53]. Of the various classes of pesticides in use today, organophosphate pesticides have been shown to cause substantial morbidity and mortality [54] but still account for over 25% of fertilizer produced [55,56]. Organophosphates are readily absorbed through the skin, and the gastrointestinal and respiratory tracts. The mechanism of action is by inhibition of acetylcholnesterase, which promotes the production of the neurotransmitter acetylcholine [57]. Overstimulation of nerves due to excess acetylcholine results in autonomic dysfunction, involuntary motor movements, and respiratory depression. Long-term exposure has been shown to result in carcinogenicity [58]. Many farmers in developing countries are exposed to pesticides due to unsafe storage and handling practices, a lack of protective equipment during spraying, or chronic exposure to contaminated soil and water. Although there have been attempts to implement safe handling practices [59] and reduce pesticide use, there is a need to develop protective equipment that can be deployed in developing countries to prevent exposure alongside devices that can detect the presence of harmful pesticides in the local environment.
There is furthermore a need to monitor the presence of heavy metals and organophosphates in water supplies and in soil, particularly in regions of significant industrial or agricultural output. Paper-based microfluidics was first reported by George Whitesides' group [60] and has the advantage of being inexpensive, readily available, and can wick biological fluids without active pumping. A recent point-of-care paper diagnostic assay for the detection of lead and mercury in water was developed by Lewis et al. [61]. The device consists of hydrophobic regions that define where the fluid travels through capillary action. The assay is designed in such a way that the time taken for the fluid to travel from one part of the chip to another is indicative of the concentration of the analyte. This is accomplished by the use of glucose oxidase or streptavidin, which inhibit flow depending on analyte concentration. It has not been determined if time-based assays provide quantitative results for a range of users. More sensitive tests to detect the presence of lead in drinking water have been developed, with one group reporting a fourfold increase in sensitivity over conventional lateral flow assays [62]. The increased sensitivity was achieved by conjugating gold nanoparticles to a lead-specific monoclonal antibody (Anti-Pb(II)-ITCBE). Organophosphate detection schemes have been considered due to their historical use as chemical warfare agents [63], though there has been a lack of development in the detection of trace amounts of organophosphates, despite knowledge they pose a significant health risk.
4 Neonatal Mortality
As of 2005, about 4 × 106 babies die in the neonatal period (the first 4 weeks of life) and a similar number of babies are stillborn [64]. A majority of these deaths are in low-income and middle-income countries in Africa and southeast Asia [65] as illustrated in Fig. 1. Some estimates show that 38% of all deaths in children under the age of five occur within the first month of life [65]. Assessment of the cause of death is difficult due to a lack of data collection in LMICs, but estimates indicate that complications arising from asphyxia, severe infections, and preterm births are the main causes of death [64]. The WHO defines birth asphyxia as the clinical description of a newborn that fails to initiate or maintain regular breathing at birth [68]. This term applies to a clinical condition and is not a specific cause of death. Advanced resuscitation is needed for less than 1% of babies at birth and babies that typically require advanced resuscitation by intubation typically require a neonatal intensive care unit to recover [69,70]. These are typically not available in regional hospitals in LMICs. Basic resuscitation, however, which only requires bag- and mask-type ventilators, is easy to implement for cases where intrapartum breathing assistance is required. Published guidelines vary on when to provide resuscitation [71], but the WHO recommends resuscitation in cases where the baby does not cry, breathe at all, or is gasping for 30 s [68]. It is crucial that these guidelines become widely known, as there is potential for adverse effects such as upper airway damage if respiratory support is provided to healthy babies [72]. Existing devices in use in low-resource settings include mucus extractors with one-way valves and rubber bulb suction devices. The key downside of these devices is that they present an infection hazard for both the neonate and the healthcare provider [73], alongside the potential risk of hypothermia in prolonged resuscitation of a neonate [74,75], and asphyxia. However, asphyxia may be overcome with expanded care and improved healthcare provider training, but it is crucial that clear protocols for resuscitation be established first.
Fig. 1.
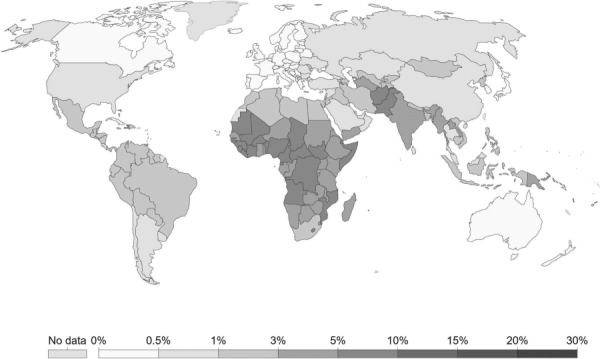
Infant mortality rates in 2017 [66,67]. Neonatal mortality is double the global average in sub-Saharan Africa.
Congenital abnormalities [64] likewise receive less attention than other medical conditions, especially given that they account for 7% of all neonatal deaths. One of the most common abnormalities in neonates is spina bifida, which results from the failure of fusion of the caudal neural tube. A large percentage of cases can be prevented by folic acid supplementation as long as the condition is diagnosed [76]. The underlying cause of the condition is not known but it has been established that women with pregestational diabetes or a high body mass index are at an increased risk of having a child with spina bifida. The protein α-fetoprotein (AFP) and ultrasound can each be used to identify fetuses that have the condition [77]. Point-of-care testing of AFP would enable screening and potential early detection of spina bifida and allow the patient to seek further medical care in a primary setting. The typical values in maternal serum are under 100 ng/ml until 13–18 weeks of gestation [78]. A quantum-dot-based sensor for the detection of AFP has been reported by Yang et al. [79], with sensitivities to 1 ng/ml. This test, however, relies on comparing the relative fluorescence intensities of the sample with a control, and can be difficult to interpret in the absence of optics and skilled personnel. The WHO compendium of medical devices and technologies for low-resource settings lists two ultrasound scanners, which are priced at US$24,000 and US$7,500 [80], still a significant cost in comparison to the annual budget of many hospitals in low-resource settings [81].
Ultrasound is otherwise commonly used to diagnose congenital structural defects during pregnancy. One such condition typically diagnosed using fetal ultrasound is hydrocephalus, a condition where fluid accumulates in the ventricles within the brain. The treatment for this condition involves the implant of a shunt that drains away excess cerebrospinal fluid into the abdominal cavity. The problem with this procedure irrespective of the setting—whether in an LMIC or a high-income country—is that implanted shunts fail due to an obstruction caused by glial and inflammatory cells [82]. A comparison between an inexpensive shunt developed and used in Africa and one currently in use in the United States to treat the condition showed that there was no significant difference in clinical outcomes [83]. The failure rate in the first 2 years for implanted ventriculoperitoneal shunts remains high, at over 40% [84], indicating that there is a need to evaluate failure due to occlusion in greater detail.
5 Neglected Tropical Diseases
Neglected tropical diseases are a group of thirteen bacterial and parasitic infections that affect around 2.7 × 109 people worldwide [85] as illustrated in Fig. 2. They are highly prevalent in sub-Saharan Africa and south Asia, where over 70% of the population lives on less than $2 a day [87]. These diseases make it difficult for people affected by them to rise out of poverty as their effects can be chronic or, in some cases, fatal. Schistosomiasis is such an NTD, affecting 192 × 106 people in sub-Saharan Africa alone, accounting for 93% of the world's cases, and associated with increased human immunodeficiency virus transmission [88]. About 75% of the population of sub-Saharan Africa lives near bodies of water that have been contaminated with schistosomiasis hosts [89], with those living near dam reservoirs at higher risk [90]. The population most affected by schistosomiasis is children and young adults.
Fig. 2.
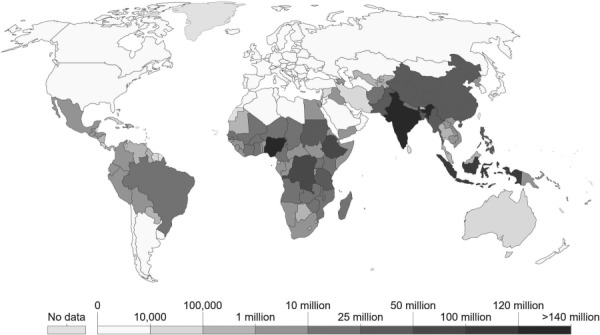
Number of people requiring interventions for NTD in 2015. These data are a subset of the number of people at risk for NTDs [86].
Schistosomiasis can be caused by two strains of bacterium, one of which affects the urinary tract and the second infecting the intestine. Schistosoma haematobium is one of the leading causes for urinary tract infections, and around 150,000 deaths annually [91]. Schistosoma mansoni causes bowel ulceration and an estimated 130,000 deaths [92]. Adult male and female worms can live in the human host for an average of 10 years and the disease spreads by the transmission of eggs to an intermediate snail host typically found in water bodies. The worms feed on glucose present in blood and erythrocytes and excrete waste into the blood stream. Biomarkers present in waste may serve as a target for detecting schistosomiasis. The primary treatment for the disease is the drug praziquantel, which is only effective against mature worms [93]. Prolonged treatment for 3–6 weeks is required to completely cure the disease. Although this drug is being employed in the field to treat schistosomiasis, the mechanism of action is still unknown and side effects include abdominal pain and passage of blood in the stool [94]. The current diagnostic standard for schistosomiasis is the presence of viable eggs in urine or fecal matter, but the test has a low sensitivity [95]. The three recommended detection techniques by the WHO are microscopy, a urine-based dipstick assay [96], and the Kato–Katz fecal examination [97]. The Kato–Katz method was developed in the 1970s and uses a cardboard cutout over a glass slide and microscopy to easily count the number of eggs in a sample and quantify the number of eggs per unit weight but this technique, like the others recommended by the WHO, lack sensitivity.
A point-of-care lateral flow assay to detect the presence of circulating cathodic antigen was developed [98], addressing the sensitivity limitations of the Kato–Katz technique, but only for Schistosoma mansoni. It did no better than the Kato–Katz technique when used for stool samples. Recent reports on diagnosis of the disease have called for better diagnostic tools [99], one of which is PET (positron emission tomography) imaging. This group made use of glucose intake of the worms in vivo to detect and quantify their presence using a PET scanner. With groups starting to develop handheld PET scanners [100], this might present a novel research direction for groups looking to develop diagnostic technologies. PCR-based techniques have also been developed to analyze cell-free parasite deoxyribonucleic acid in human plasma [101], and the development of microfluidic PCR-based systems [102] for schistosomiasis could lead to improved detection.
Control measures against the disease include preventative therapy using praziquantel [103], issued by schools screening and treating children for this and other diseases. An additional screening technique that has been attempted in the past is to monitor and eliminate snails that serve as intermediate hosts. The WHO in 2012 pledged to control the morbidity caused by the disease by 2020 and eliminate it as a public health problem by 2025, leading to an increased effort in diagnosing and educating the communities most affected by the disease. This has stimulated increased funding and opportunities for novel interventions.
6 Policy and Implementation
There have been significant improvements in healthcare outcomes over the past few decades due to a combination of efforts by public and private organizations, and technological advancements in diagnostics and surgical devices. Despite this, there are some limitations to adoption and implementation of technology.
In some cases, the underlying disease or condition may not have a device or diagnostic solution. This is common in the case of neglected tropical diseases that are a significant health burden but have seen insufficient investment from the private sector. A point-of-care monoclonal antibody-based dipstick for urinary schistosomiasis was developed by a Ghanian group in 2004 [104] but has not been commercialized [105].
The technology may exist, but other problems such as distribution or energy required to power the devices may not exist [106]. Oxytocin is used to reduce the risk of postpartum hemorrhage but requires refrigeration, unavailable throughout the day in resource-limited settings. Advances in packaging that could extend the shelf life of drugs and vaccines would expand access to available care in these cases.
In the case of diagnostics, a more sensitive and specific test may exist but there could be reluctance to adopt it or mistrust in the results it produces due to past misdiagnosis with old technology. Rapid diagnostic tests for malaria have been shown to have superior sensitivity and specificity than the current gold standard for malaria detection, microscopy [107]. But a study found that doctors prescribed antimalarial drugs—even in the case of a negative test result [108,109]. Skilled healthcare is crucial to improve surgical outcomes. For example, access to skilled surgeons for delivery is as low as 40% in sub-Saharan Africa and less than 30% in south Asia [110].
Designing medical devices for LMIC's presents unique challenges not seen in developed markets. The WHO estimates that 70% of medical equipment coming from developed countries does not work in hospitals in developing countries [68,111] due to lack of trained personnel, limitations with infrastructure, and the lack of spare parts or support for equipment.
It is evident from these examples that research and development of novel diagnostic tools and devices alone is insufficient for adoption. In the context of the conditions presented in this paper, there is a need to develop an effective way to screen for schistosomiasis and melioidosis. The spread of melioidosis starts through soil in the rainy season and the presence of schistosomiasis can easily be detected in fecal matter. A technique to purify samples coupled with existing point-of-care diagnostics would stop the spread of the disease at the source. This can be extended to detect the presence of environmental contaminants such as organophosphates and heavy metals. An increase in sensitivity can be achieved if adequate processing is done prior to point-of-care diagnosis. The UN's fourth millennium development goal called for a two-third reduction in neonatal mortality by 2015. Although there has been significant progress toward this goal, there are still 4 × 106 neonatal deaths every year, predominantly in south Asia and Africa. Developing medical devices specifically for neonatal care and not having to repurpose devices made for adults would enable easier surgical procedures. Better detection schemes for diseases such as spina bifida or hydrocephalus using low-cost ultrasound transducers would prepare doctors better for surgery. It is critical to rethink what low-cost means for such applications, especially considering the annual budget of hospitals in LMIC's. Frugal technologies that can be used repeatedly can withstand environmental conditions and can be used easily by healthcare workers in target countries should be prioritized.
References
- [1]. McManus, D. P. , Dunne, D. W. , Sacko, M. , Utzinger, J. , Vennervald, B. J. , and Zhou, X.-N. , 2018, “ Schistosomiasis (Primer),” Nat. Rev.: Dis. Primers, 4(1), p. 13. 10.1038/s41572-018-0013-8 [DOI] [PubMed] [Google Scholar]
- [2]. Trampuz, A. , Jereb, M. , Muzlovic, I. , and Prabhu, R. M. , 2003, “ Clinical Review: Severe Malaria,” Crit. Care, 7(4), pp. 315–323. 10.1186/cc2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Adam, T. , Lim, S. S. , Mehta, S. , Bhutta, Z. A. , Fogstad, H. , Mathai, M. , Zupan, J. , and Darmstadt, G. L. , 2005, “ Cost Effectiveness Analysis of Strategies for Maternal and Neonatal Health in Developing Countries,” BMJ, 331(7525), p. 1107. 10.1136/bmj.331.7525.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Wiersinga, W. J. , Currie, B. J. , and Peacock, S. J. , 2012, “ Melioidosis,” New Engl. J. Med., 367(11), pp. 1035–1044. 10.1056/NEJMra1204699 [DOI] [PubMed] [Google Scholar]
- [5]. Currie, B. J. , Dance, D. A. , and Cheng, A. C. , 2008, “ The Global Distribution of Burkholderia Pseudomallei and Melioidosis: An Update,” Trans. R. Soc. Trop. Med. Hygiene, 102(Suppl. 1), pp. S1–S4. 10.1016/S0035-9203(08)70002-6 [DOI] [PubMed] [Google Scholar]
- [6]. Dance, D. , 1991, “ Melioidosis: The Tip of the Iceberg?,” Clin. Microbiol. Rev., 4(1), pp. 52–60. 10.1128/CMR.4.1.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Wuthiekanun, V. , Smith, M. D. , and White, N. J. , 1995, “ Survival of Burkholderia Pseudomallei in the Absence of Nutrients,” Trans. R. Soc. Trop. Med. Hyg., 89(5), pp. 491–491. 10.1016/0035-9203(95)90080-2 [DOI] [PubMed] [Google Scholar]
- [8]. Cheng, A. C. , and Currie, B. J. , 2005, “ Melioidosis: Epidemiology, Pathophysiology, and Management,” Clin. Microbiol. Rev., 18(2), pp. 383–416. 10.1128/CMR.18.2.383-416.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Suputtamongkol, Y. , Chaowagul, W. , Chetchotisakd, P. , Lertpatanasuwun, N. , Intaranongpai, S. , Ruchutrakool, T. , Budhsarawong, D. , Mootsikapun, P. , Wuthiekanun, V. , Teerawatasook, N. , and Lulitanond, A. , 1999, “ Risk Factors for Melioidosis and Bacteremic Melioidosis,” Clin. Infect. Dis., 29(2), pp. 408–413. 10.1086/520223 [DOI] [PubMed] [Google Scholar]
- [10]. White, N. , 2003, “ Melioidosis,” Lancet, 361(9370), pp. 1715–1722. 10.1016/S0140-6736(03)13374-0 [DOI] [PubMed] [Google Scholar]
- [11]. Wild, S. , Roglic, G. , Green, A. , Sicree, R. , and King, H. , 2004, “ Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030,” Diabetes Care, 27(5), pp. 1047–1053. 10.2337/diacare.27.5.1047 [DOI] [PubMed] [Google Scholar]
- [12]. Chetchotisakd, P. , Porramatikul, S. , Mootsikapun, P. , Anunnatsiri, S. , and Thinkhamrop, B. , 2001, “ Randomized, Double-Blind, Controlled Study of Cefoperazone-Sulbactam Plus Cotrimoxazole Versus Ceftazidime Plus Cotrimoxazole for the Treatment of Severe Melioidosis,” Clin. Infect. Dis., 33(1), pp. 29–34. 10.1086/320878 [DOI] [PubMed] [Google Scholar]
- [13]. White, N. , Chaowagul, W. , Wuthiekanun, V. , Dance, D. , Wattanagoon, Y. , and Pitakwatchara, N. , 1989, “ Halving of Mortality of Severe Melioidosis by Ceftazidime,” Lancet, 334(8665), pp. 697–701. 10.1016/S0140-6736(89)90768-X [DOI] [PubMed] [Google Scholar]
- [14]. Sookpranee, M. , Boonma, P. , Susaengrat, W. , Bhuripanyo, K. , and Punyagupta, S. , 1992, “ Multicenter Prospective Randomized Trial Comparing Ceftazidime Plus Co-Trimoxazole With Chloramphenicol Plus Doxycycline and Co-Trimoxazole for Treatment of Severe Melioidosis,” Antimicrob. Agents Chemother., 36(1), pp. 158–162. 10.1128/AAC.36.1.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Suputtamongkol, Y. , Rajchanuwong, A. , Chaowagul, W. , Dance, D. , Smith, M. , Wuthiekanun, V. , Walsh, A. , Pukrittayakamee, S. , and White, N. , 1994, “ Ceftazidime vs. Amoxicillin/Clavulanate in the Treatment of Severe Melioidosis,” Clin. Infect. Dis., 19(5), pp. 846–853. 10.1093/clinids/19.5.846 [DOI] [PubMed] [Google Scholar]
- [16]. Samuel, M. , and Ti, T. , 2002, “ Interventions for Treating Melioidosis,” Cochrane Database Syst. Rev., (4), pp. 1–24. 10.1002/14651858.CD001263 [DOI] [PubMed] [Google Scholar]
- [17]. Simpson, A. J. , Suputtamongkol, Y. , Smith, M. D. , Angus, B. J. , Rajanuwong, A. , Wuthiekanun, V. , Howe, P. A. , Walsh, A. L. , Chaowagul, W. , and White, N. J. , 1999, “ Comparison of Imipenem and Ceftazidime as Therapy for Severe Melioidosis,” Clin. Infect. Dis., 29(2), pp. 381–387. 10.1086/520219 [DOI] [PubMed] [Google Scholar]
- [18]. Leelarasamee, A. , and Bovornkitti, S. , 1989, “ Melioidosis: Review and Update,” Rev. Infect. Dis., 11(3), pp. 413–425. 10.1093/clinids/11.3.413 [DOI] [PubMed] [Google Scholar]
- [19]. Puthucheary, S. , Parasakthi, N. , and Lee, M. , 1992, “ Septicaemic Melioidosis: A Review of 50 Cases From Malaysia,” Trans. R. Soc. Trop. Med. Hyg., 86(6), pp. 683–685. 10.1016/0035-9203(92)90191-E [DOI] [PubMed] [Google Scholar]
- [20]. Hoffmaster, A. R. , AuCoin, D. , Baccam, P. , Baggett, H. C. , Baird, R. , Bhengsri, S. , Blaney, D. D. , Brett, P. J. , Brooks, T. J. , Brown, K. A. , Chantratita, N. , Cheng, A. C. , Dance, D. A. , Decuypere, S. , Defenbaugh, D. , Gee, J. E. , Houghton, R. , Jorakate, P. , Lertmemongkolchai, G. , Limmathurotsakul, D. , Merlin, T. L. , Mukhopadhyay, C. , Norton, R. , Peacock, S. J. , Rolim, D. B. , Simpson, A. J. , Steinmetz, I. , Stoddard, R. A. , Stokes, M. M. , Sue, D. , Tuanyok, A. , Whistler, T. , Wuthiekanun, V. , and Walke, H. T. , 2015, “ Melioidosis Diagnostic Workshop, 2013,” Emerging Infect. Dis., 21(2), pp. 1–9. 10.3201/eid2102.141045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Inglis, T. J. , Merritt, A. , Chidlow, G. , Aravena-Roman, M. , and Harnett, G. , 2005, “ Comparison of Diagnostic Laboratory Methods for Identification of Burkholderia Pseudomallei,” J. Clin. Microbiol., 43(5), pp. 2201–2206. 10.1128/JCM.43.5.2201-2206.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Woo, P. C. , Woo, G. K. , Lau, S. K. , Wong, S. S. , and Yuen, K.-Y. , 2002, “ Single Gene Target Bacterial Identification: GroEL Gene Sequencing for Discriminating Clinical Isolates of Burkholderia Pseudomallei and Burkholderia Thailandensis,” Diagn. Microbiol. Infect. Dis., 44(2), pp. 143–149. 10.1016/S0732-8893(02)00439-X [DOI] [PubMed] [Google Scholar]
- [23]. Holden, M. T. , Titball, R. W. , Peacock, S. J. , Cerdeno-Tarraga, A. M. , Atkins, T. , Crossman, L. C. , Pitt, T. , Churcher, C. , Mungall, K. , Bentley, S. D. , Sebaihia, M. , Thomson, N. R. , Bason, N. , Beacham, I. R. , Brooks, K. , Brown, K. A. , Brown, N. F. , Challis, G. L. , Cherevach, I. , Chillingworth, T. , Cronin, A. , Crossett, B. , Davis, P. , DeShazer, D. , Feltwell, T. , Fraser, A. , Hance, Z. , Hauser, H. , Holroyd, S. , Jagels, K. , Keith, K. E. , Maddison, M. , Moule, S. , Price, C. , Quail, M. A. , Rabbinowitsch, E. , Rutherford, K. , Sanders, M. , Simmonds, M. , Songsivilai, S. , Stevens, K. , Tumapa, S. , Vesaratchavest, M. , Whitehead, S. , Yeats, C. , Barrell, B. G. , Oyston, P. C. , and Parkhill, J. , 2004, “ Genomic Plasticity of the Causative Agent of Melioidosis, Burkholderia Pseudomallei,” Proc. Natl. Acad. Sci., 101(39), pp. 14240–14245. 10.1073/pnas.0403302101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Karger, A. , Stock, R. , Ziller, M. , Elschner, M. C. , Bettin, B. , Melzer, F. , Maier, T. , Kostrzewa, M. , Scholz, H. C. , Neubauer, H. , and Tomaso, H. , 2014, “ PCR-Based Methodologies Used to Detect and Differentiate the Burkholderia Pseudomallei Complex: B. Pseudomallei, B. Mallei, and B. Thailandensis,” Curr. Issues Mol. Biol., 16(2), pp. 23–54.https://www.ncbi.nlm.nih.gov/pubmed/23969318 [PubMed] [Google Scholar]
- [25]. Lau, S. K. , Lam, C. S. , Ngan, A. H. , Chow, W.-N. , Wu, A. K. , Tsang, D. N. , Cindy, W. , Que, T.-L. , Tang, B. S. , and Woo, P. C. , 2016, “ Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry for Rapid Identification of Mold and Yeast Cultures of Penicillium Marneffei,” BMC Microbiol., 16(1), p. 36. 10.1186/s12866-016-0656-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Cunningham, S. A. , and Patel, R. , 2013, “ Importance of Using Bruker's Security-Relevant Library for Biotyper Identification of Burkholderia Pseudomallei, Brucella Species, and Francisella Tularensis,” J. Clin. Microbiol., 51(5), pp. 1639–1640. 10.1128/JCM.00267-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Karger, A. , Stock, R. , Ziller, M. , Elschner, M. C. , Bettin, B. , Melzer, F. , Maier, T. , Kostrzewa, M. , Scholz, H. C. , Neubauer, H. , and Tomaso, H. , 2012, “ Rapid Identification of Burkholderia mallei and Burkholderia Pseudomallei by Intact Cell Matrix-Assisted Laser Desorption/Ionisation Mass Spectrometric Typing,” BMC Microbiol., 12(1), p. 229. 10.1186/1471-2180-12-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Inglis, T. J. , Healy, P. E. , Fremlin, L. J. , and Golledge, C. L. , 2012, “ Use of Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry Analysis for Rapid Confirmation of Burkholderia Pseudomallei in Septicemic Melioidosis,” Am. J. Trop. Med. Hyg., 86(6), pp. 1039–1042. 10.4269/ajtmh.2012.11-0454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Bllaci, L. , Kjellstrom, S. , Eliasson, L. , Friend, J. R. , Yeo, L. Y. , and Nilsson, S. , 2013, “ Fast Surface Acoustic Wave-Matrix-Assisted Laser Desorption Ionization Mass Spectrometry of Cell Response From Islets of Langerhans,” Anal. Chem., 85(5), pp. 2623–2629. 10.1021/ac3019125 [DOI] [PubMed] [Google Scholar]
- [30]. Lew, A. , and Desmarchelier, P. , 1994, “ Detection of Pseudomonas Pseudomallei by PCR and Hybridization,” J. Clin. Microbiol., 32(5), pp. 1326–1332. 10.1128/JCM.32.5.1326-1332.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Gal, D. , Mayo, M. , Spencer, E. , Cheng, A. C. , and Currie, B. J. , 2005, “ Application of a Polymerase Chain Reaction to Detect Burkholderia Pseudomallei in Clinical Specimens From Patients With Suspected Melioidosis,” Am. J. Trop. Med. Hyg., 73(6), pp. 1162–1164. 10.4269/ajtmh.2005.73.1162 [DOI] [PubMed] [Google Scholar]
- [32]. Shaw, T. , Tellapragada, C. , Vandana, K. , AuCoin, D. P. , and Mukhopadhyay, C. , 2018, “ Performance Evaluation of Active Melioidosis Detect-Lateral Flow Assay (AMD-LFA) for Diagnosis of Melioidosis in Endemic Settings With Limited Resources,” PLoS One, 13(3), p. e0194595. 10.1371/journal.pone.0194595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Houghton, R. L. , Reed, D. E. , Hubbard, M. A. , Dillon, M. J. , Chen, H. , Currie, B. J. , Mayo, M. , Sarovich, D. S. , Theobald, V. , Limmathurotsakul, D. , Wongsuvan, G. , Chantratita, N. , Peacock, S. J. , Hoffmaster, A. R. , Duval, B. , Brett, P. J. , Burtnick, M. N. , and Aucoin, D. P. , 2014, “ Development of a Prototype Lateral Flow Immunoassay (LFI) for the Rapid Diagnosis of Melioidosis,” PLoS Neglected Trop. Dis., 8(3), p. e2727. 10.1371/journal.pntd.0002727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Limmathurotsakul, D. , Golding, N. , Dance, D. A. , Messina, J. P. , Pigott, D. M. , Moyes, C. L. , Rolim, D. B. , Bertherat, E. , Day, N. P. , Peacock, S. J. , and Hay, S. I. , 2016, “ Predicted Global Distribution of Burkholderia Pseudomallei and Burden of Melioidosis,” Nat. Microbiol., 1(1), p. 15008. 10.1038/nmicrobiol.2015.8 [DOI] [PubMed] [Google Scholar]
- [35]. Singhal, N. , Kumar, M. , Kanaujia, P. K. , and Virdi, J. S. , 2015, “ MALDI-TOF Mass Spectrometry: An Emerging Technology for Microbial Identification and Diagnosis,” Front. Microbiol., 6, p. 791. 10.3389/fmicb.2015.00791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Carbonnelle, E. , Mesquita, C. , Bille, E. , Day, N. , Dauphin, B. , Beretti, J.-L. , Ferroni, A. , Gutmann, L. , and Nassif, X. , 2011, “ MALDI-TOF Mass Spectrometry Tools for Bacterial Identification in Clinical Microbiology Laboratory,” Clin. Biochem., 44(1), pp. 104–109. 10.1016/j.clinbiochem.2010.06.017 [DOI] [PubMed] [Google Scholar]
- [37]. Blackfan, K. , 1917, “ Lead Poisoning in Children With Especial Reference to Lead as a Cause of Convulsions,” Am. J. Med. Sci. (1827–1924), 153(6), pp. 877–887. 10.1097/00000441-191706000-00010 [DOI] [Google Scholar]
- [38]. Repko, J. D. , Jones, P. , Garcia, L., Jr. , and Corum, C. , 1977, “ Effects of Inorganic Lead on Behavioral and Neurologic Function. Final Report,” University of Louisville, Louisville, KY, Report.
- [39]. Krigman, M. R. , 1978, “ Neuropathology of Heavy Metal Intoxication,” Environ. Health Perspect., 26, pp. 117–120. 10.1289/ehp.7826117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Markovac, J. , and Goldstein, G. W. , 1988, “ Picomolar Concentrations of Lead Stimulate Brain Protein Kinase C,” Nature, 334(6177), pp. 71–73. 10.1038/334071a0 [DOI] [PubMed] [Google Scholar]
- [41]. Simons, T. , 1993, “ Lead-Calcium Interactions in Cellular Lead Toxicity,” Neurotoxicology, 14(2–3), pp. 77–85.https://link.springer.com/chapter/10.1007/978-3-642-71806-9_24 [PubMed] [Google Scholar]
- [42]. Bushnell, P. , and Bowman, R. , 1979, “ Effects of Chronic Lead Ingestion on Social Development in Infant Rhesus Monkeys,” Neurobehav. Toxicol., 1(3), pp. 207–219.https://www.ncbi.nlm.nih.gov/pubmed/551314 [PubMed] [Google Scholar]
- [43]. Rice, D. , 1992, “ Behavioral Impairments Produced by Developmental Lead Exposure: Evidence From Primate Research,” Hum. Lead Exposure, pp. 138–152. [Google Scholar]
- [44]. Seppäläinen, A. M. , Tola, S. , Hernberg, S. , and Kock, B. , 1975, “ Subclinical Neuropathy at “Safe” Levels of Lead Exposure,” Arch. Environ. Health, 30(4), pp. 180–183. 10.1080/00039896.1975.10666672 [DOI] [PubMed] [Google Scholar]
- [45]. Bellinger, D. C. , 2016, “ Lead Contamination in Flint—An Abject Failure to Protect Public Health,” New Engl. J. Med., 374(12), pp. 1101–1103. 10.1056/NEJMp1601013 [DOI] [PubMed] [Google Scholar]
- [46].Edwards, M., Pruden, A., and Falkinham, J., 2019, “ Flint Water Study,” accessed July 24, 2019, www.FlintWaterStudy.org
- [47]. Gracia, R. C. , and Snodgrass, W. R. , 2007, “ Lead Toxicity and Chelation Therapy,” Am. J. Health-Syst. Pharm., 64(1), pp. 45–53. 10.2146/ajhp060175 [DOI] [PubMed] [Google Scholar]
- [48]. Johri, N. , Jacquillet, G. , and Unwin, R. , 2010, “ Heavy Metal Poisoning: The Effects of Cadmium on the Kidney,” Biometals, 23(5), pp. 783–792. 10.1007/s10534-010-9328-y [DOI] [PubMed] [Google Scholar]
- [49].Food and Agriculture Organization of the United Nations, “ Per-Capita Food Consumption,” United Nations, Rome, Italy, accessed July 24, 2019, http://www.fao.org/faostat/en/#data/FBS
- [50].World Health Organization and United Nations Environment Programme, 2019, “ Public Health Impact of Pesticides Used in Agriculture,” World Health Organization, accessed July 24, 2019, https://apps.who.int/iris/handle/10665/39772
- [51]. Kesavachandran, C. N. , Fareed, M. , Pathak, M. K. , Bihari, V. , Mathur, N. , and Srivastava, A. K. , 2009, “ Adverse Health Effects of Pesticides in Agrarian Populations of Developing Countries,” Reviews of Environmental Contamination and Toxicology, Vol. 200, Springer, Boston, MA, pp. 33–52.https://link.springer.com/chapter/10.1007/978-1-4419-0028-9_2 [DOI] [PubMed] [Google Scholar]
- [52]. Śpiewak, R. , 2001, “ Pesticides as a Cause of Occupational Skin Diseases in Farmers,” Ann. Agric. Environ. Med., 8(1), pp. 1–5.https://www.ncbi.nlm.nih.gov/pubmed/11426918 [PubMed] [Google Scholar]
- [53]. McConnell, R. , Pacheco, F. , Wahlberg, K. , Klein, W. , Malespin, O. , Magnotti, R. , Åkerblom, M. , and Murray, D. , 1999, “ Subclinical Health Effects of Environmental Pesticide Contamination in a Developing Country: Cholinesterase Depression in Children,” Environ. Res., 81(2), pp. 87–91. 10.1006/enrs.1999.3958 [DOI] [PubMed] [Google Scholar]
- [54]. Rosenstock, L. , Keifer, M. , Daniell, W. E. , McConnell, R. , and Claypoole, K. , 1991, “ Chronic Central Nervous System Effects of Acute Organophosphate Pesticide Intoxication,” Lancet, 338(8761), pp. 223–227. 10.1016/0140-6736(91)90356-T [DOI] [PubMed] [Google Scholar]
- [55].Food and Agriculture Organization of the United Nations, 2019, “ Total Fertilizer Production,” United Nations, Rome, Italy, accessed July 24, 2019, http://www.fao.org/faostat/en/#data/RA
- [56]. Roser, M. , and Ritchie, H. , 2019, “ Fertilizer and Pesticides,” Our World in Data, Oxford, UK, accessed July 24, 2019, https://ourworldindata.org/fertilizer-and-pesticides
- [57]. Sanchez-Santed, F. , Colomina, M. T. , and Hernandez, E. H. , 2016, “ Organophosphate Pesticide Exposure and Neurodegeneration,” Cortex, 74, pp. 417–426. 10.1016/j.cortex.2015.10.003 [DOI] [PubMed] [Google Scholar]
- [58]. Alavanja, M. C. , Hoppin, J. A. , and Kamel, F. , 2004, “ Health Effects of Chronic Pesticide Exposure: Cancer and Neurotoxicity,” Annu. Rev. Public Health, 25(1), pp. 155–197. 10.1146/annurev.publhealth.25.101802.123020 [DOI] [PubMed] [Google Scholar]
- [59]. Indra, D. , Bellamy, R. , and Shyamsundar, P. , 2007, “ Facing Hazards at Work-Agricultural Workers and Pesticide Exposure in Kuttanad, Kerala,” South Asian Network Develop. Environ. Econ., 19(7), pp. 1–4.http://www.indiaenvironmentportal.org.in/files/policy_brief_19.pdf [Google Scholar]
- [60]. Martinez, A. W. , Phillips, S. T. , Whitesides, G. M. , and Carrilho, E. , 2009, “ Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices,” Anal. Chem., 82(1), pp. 3–10. 10.1021/ac9013989 [DOI] [PubMed] [Google Scholar]
- [61]. Lewis, G. G. , Robbins, J. S. , and Phillips, S. T. , 2014, “ A Prototype Point-of-Use Assay for Measuring Heavy Metal Contamination in Water Using Time as a Quantitative Readout,” Chem. Commun., 50(40), pp. 5352–5354. 10.1039/C3CC47698G [DOI] [PubMed] [Google Scholar]
- [62]. Kuang, H. , Xing, C. , Hao, C. , Liu, L. , Wang, L. , and Xu, C. , 2013, “ Rapid and Highly Sensitive Detection of Lead Ions in Drinking Water Based on a Strip Immunosensor,” Sensors, 13(4), pp. 4214–4224. 10.3390/s130404214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Polhuijs, M. , Langenberg, J. P. , and Benschop, H. P. , 1997, “ New Method for Retrospective Detection of Exposure to Organophosphorus Anticholinesterases: Application to Alleged Sarin Victims of Japanese Terrorists,” Toxicol. Appl. Pharmacol., 146(1), pp. 156–161. 10.1006/taap.1997.8243 [DOI] [PubMed] [Google Scholar]
- [64]. Lawn, J. E. , Cousens, S. , and Zupan, J. , and Lancet Neonatal Survival Steering Team 2005, “ 4 Million Neonatal Deaths: When? Where? Why?,” Lancet, 365(9462), pp. 891–900. 10.1016/S0140-6736(05)71048-5 [DOI] [PubMed] [Google Scholar]
- [65]. Zupan, J. , and Aahman, E. , 2005, “ Perinatal Mortality for the Year 2000: Estimates Developed by WHO,” World Health Organization, Geneva, pp. 129–33.
- [66]. Roser, M. , 2019, “ Child and Infant Mortality,” Our World in Data, Oxford, UK, accessed July 24, 2019, https://ourworldindata.org/child-mortality
- [67].UN Inter-Agency Group for Child Mortality Estimation, 2018, “ Infant Mortality in 2017,” World Bank Group, Washington, DC, accessed July 24, 2019, http://data.worldbank.org/data-catalog/world-development-indicators
- [68].World Health Organization, 1997, “ Guidelines for Health Care Equipment Donations,” World Health Organization, Geneva, Switzerland, Report No. WHO/ARA/97.3.https://www.who.int/medical_devices/publications/en/Donation_Guidelines.pdf
- [69]. Zhu, X. , Fang, H. , Zeng, S. , Li, Y. , Lin, H. , and Shi, S. , 1997, “ The Impact of the Neonatal Resuscitation Program Guidelines (NRPG) on the Neonatal Mortality in a Hospital in Zhuhai, China,” Singapore Med. J., 38(11), pp. 485–487.https://www.ncbi.nlm.nih.gov/pubmed/9550910 [PubMed] [Google Scholar]
- [70]. Deorari, A. , Paul, V. , Singh, M. , Vidyasagar, D. , and Network, M. C. , and The Medical Colleges Network, 2001, “ Impact of Education and Training on Neonatal Resuscitation Practices in 14 Teaching Hospitals in India,” Ann. Trop. Paediatr., 21(1), pp. 29–33. 10.1080/02724930123814 [DOI] [PubMed] [Google Scholar]
- [71]. Kattwinkel, J. , Niermeyer, S. , Nadkarni, V. , Tibballs, J. , Phillips, B. , Zideman, D. , Van Reempts, P. , and Osmond, M. , 1999, “ Resuscitation of the Newly Born Infant: An Advisory Statement From the Pediatric Working Group of the International Liaison Committee on Resuscitation,” Resuscitation, 40(2), pp. 71–88. 10.1016/S0300-9572(99)00012-X [DOI] [PubMed] [Google Scholar]
- [72]. Velaphi, S. , and Vidyasagar, D. , 2008, “ The Pros and Cons of Suctioning at the Perineum (Intrapartum) and Post-Delivery With and Without Meconium,” Semin. Fetal Neonat. Med., 13(6), pp. 375–382. 10.1016/j.siny.2008.04.001 [DOI] [PubMed] [Google Scholar]
- [73]. Wall, S. N. , Lee, A. C. , Niermeyer, S. , English, M. , Keenan, W. J. , Carlo, W. , Bhutta, Z. A. , Bang, A. , Narayanan, I. , Ariawan, I. , and Lawn, J. E. , 2009, “ Neonatal Resuscitation in Low-Resource Settings: What, Who, and How to Overcome Challenges to Scale Up?,” Int. J. Gynecol. Obstetr., 107(Suppl), pp. S47–S64. 10.1016/j.ijgo.2009.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Costeloe, K. , Hennessy, E. , Gibson, A. T. , Marlow, N. , and Wilkinson, A. R. , 2000, “ The EPICure Study: Outcomes to Discharge From Hospital for Infants Born at the Threshold of Viability,” Pediatrics, 106(4), pp. 659–671. 10.1542/peds.106.4.659 [DOI] [PubMed] [Google Scholar]
- [75]. Besch, N. J. , Perlstein, P. H. , Edwards, N. K. , Keenan, W. J. , and Sutherland, J. M. , 1971, “ The Transparent Baby Bag: A Shield Against Heat Loss,” New Engl. J. Med., 284(3), pp. 121–124. 10.1056/NEJM197101212840302 [DOI] [PubMed] [Google Scholar]
- [76]. Mitchell, L. E. , Adzick, N. S. , Melchionne, J. , Pasquariello, P. S. , Sutton, L. N. , and Whitehead, A. S. , 2004, “ Spina Bifida,” Lancet, 364(9448), pp. 1885–1895. 10.1016/S0140-6736(04)17445-X [DOI] [PubMed] [Google Scholar]
- [77]. Drugan, A. , Weissman, A. , and Evans, M. I. , 2001, “ Screening for Neural Tube Defects,” Clin. Perinatol., 28(2), pp. 279–287. 10.1016/S0095-5108(05)70083-X [DOI] [PubMed] [Google Scholar]
- [78]. Harris, R. , Jennison, R. , Barson, A. , Laurence, K. , Ruoslahti, E. , and Seppälä, M. , 1974, “ Comparison of Amniotic-Fluid and Maternal Serum Alpha-Fetoprotein Levels in the Early Antenatal Diagnosis of Spina Bifida and Anencephaly,” Lancet, 303(7855), pp. 429–433. 10.1016/S0140-6736(74)92384-8 [DOI] [PubMed] [Google Scholar]
- [79]. Yang, Q. , Gong, X. , Song, T. , Yang, J. , Zhu, S. , Li, Y. , Cui, Y. , Li, Y. , Zhang, B. , and Chang, J. , 2011, “ Quantum Dot-Based Immunochromatography Test Strip for Rapid, Quantitative and Sensitive Detection of Alpha Fetoprotein,” Biosens. Bioelectron., 30(1), pp. 145–150. 10.1016/j.bios.2011.09.002 [DOI] [PubMed] [Google Scholar]
- [80].WHO Compendium of Innovative Health Technologies for Low-Resource Settings, 2019, “ WHO Medical Devices,” World Health Organization, Geneva, Switzerland, accessed July 24, 2019, https://apps.who.int/iris/bitstream/handle/10665/274893/9789241514699-eng.pdf?ua=1
- [81]. Barasa, E. W. , Cleary, S. , Molyneux, S. , and English, M. , 2017, “ Setting Healthcare Priorities: A Description and Evaluation of the Budgeting and Planning Process in County Hospitals in Kenya,” Health Policy Plann., 32(3), pp. 329–337. 10.1093/heapol/czw132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Blegvad, C. , Skjolding, A. , Broholm, H. , Laursen, H. , and Juhler, M. , 2013, “ Pathophysiology of Shunt Dysfunction in Shunt Treated Hydrocephalus,” Acta Neurochir., 155(9), pp. 1763–1772. 10.1007/s00701-013-1729-6 [DOI] [PubMed] [Google Scholar]
- [83]. Warf, B. C. , 2005, “ Comparison of 1-Year Outcomes for the Chhabra and Codman-Hakim Micro Precision Shunt Systems in Uganda: A Prospective Study in 195 Children,” J. Neurosurg., 102(Suppl. 4), pp. 358–362. 10.3171/ped.2005.102.4.0358 [DOI] [PubMed] [Google Scholar]
- [84]. Drake, J. M. , Kestle, J. R. , Milner, R. , Cinalli, G. , Boop, F. , Piatt, J. , Haines, S. , Schiff, S. J. , Cochrane, D. D. , Steinbok, P. , and MacNeil, N. , 1998, “ Randomized Trial of Cerebrospinal Fluid Shunt Valve Design in Pediatric Hydrocephalus,” Neurosurgery, 43(2), pp. 294–303. 10.1097/00006123-199808000-00068 [DOI] [PubMed] [Google Scholar]
- [85]. Hotez, P. J. , Molyneux, D. H. , Fenwick, A. , Kumaresan, J. , Sachs, S. E. , Sachs, J. D. , and Savioli, L. , 2007, “ Control of Neglected Tropical Diseases,” New Engl. J. Med., 357(10), pp. 1018–1027. 10.1056/NEJMra064142 [DOI] [PubMed] [Google Scholar]
- [86].World Health Organization, 2019, “ Neglected Tropical Diseases,” World Health Organization, Geneva, Switzerland, accessed July 24, 2019, https://www.who.int/neglected_diseases/data/ntddatabase/en/
- [87]. Chen, S. , and Ravallion, M. , 2010, “ The Developing World is Poorer Than We Thought, but No Less Successful in the Fight Against Poverty,” Q. J. Econ., 125(4), pp. 1577–1625. 10.1162/qjec.2010.125.4.1577 [DOI] [Google Scholar]
- [88]. Kjetland, E. F. , Ndhlovu, P. D. , Gomo, E. , Mduluza, T. , Midzi, N. , Gwanzura, L. , Mason, P. R. , Sandvik, L. , Friis, H. , and Gundersen, S. G. , 2006, “ Association Between Genital Schistosomiasis and HIV in Rural Zimbabwean Women,” AIDS, 20(4), pp. 593–600. 10.1097/01.aids.0000210614.45212.0a [DOI] [PubMed] [Google Scholar]
- [89]. Ekpo, U. F. , Mafiana, C. F. , Adeofun, C. O. , Solarin, A. R. , and Idowu, A. B. , 2008, “ Geographical Information System and Predictive Risk Maps of Urinary Schistosomiasis in Ogun State, Nigeria,” BMC Infect. Dis., 8(1), p. 74. 10.1186/1471-2334-8-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Steinmann, P. , Keiser, J. , Bos, R. , Tanner, M. , and Utzinger, J. , 2006, “ Schistosomiasis and Water Resources Development: Systematic Review, Meta-Analysis, and Estimates of People at Risk,” Lancet Infect. Dis., 6(7), pp. 411–425. 10.1016/S1473-3099(06)70521-7 [DOI] [PubMed] [Google Scholar]
- [91]. Parkin, D. M. , 2008, “ The Global Burden of Urinary Bladder Cancer,” Scand. J. Urol. Nephrol., 42(Suppl. 218), pp. 12–20. 10.1080/03008880802285032 [DOI] [PubMed] [Google Scholar]
- [92]. van der Werf, M. J. , de Vlas, S. J. , Brooker, S. , Looman, C. W. , Nagelkerke, N. J. , Habbema, J. D. F. , and Engels, D. , 2003, “ Quantification of Clinical Morbidity Associated With Schistosome Infection in Sub-Saharan Africa,” Acta Trop., 86(2–3), pp. 125–139. 10.1016/S0001-706X(03)00029-9 [DOI] [PubMed] [Google Scholar]
- [93]. Doenhoff, M. J. , Cioli, D. , and Utzinger, J. , 2008, “ Praziquantel: Mechanisms of Action, Resistance and New Derivatives for Schistosomiasis,” Curr. Opin. Infect. Dis., 21(6), pp. 659–667. 10.1097/QCO.0b013e328318978f [DOI] [PubMed] [Google Scholar]
- [94]. King, C. H. , Olbrych, S. K. , Soon, M. , Singer, M. E. , Carter, J. , and Colley, D. G. , 2011, “ Utility of Repeated Praziquantel Dosing in the Treatment of Schistosomiasis in High-Risk Communities in Africa: A Systematic Review,” PLoS Neglected Trop. Dis., 5(9), p. e1321. 10.1371/journal.pntd.0001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. De Vlas, S. , Engels, D. , Rabello, A. , Oostburg, B. , van Lieshout, L. , Polderman, A. , Van Oortmarssen, G. , Habbema, J. , and Gryseels, B. , 1997, “ Validation of a Chart to Estimate True Schistosoma Mansoni Prevalences From Simple Egg Counts,” Parasitology, 114(2), pp. 113–121. 10.1017/S0031182096008207 [DOI] [PubMed] [Google Scholar]
- [96].W. E. C. on the Control of Schistosomiasis, 1993, “ The Control of Schistosomiasis. Second Report of the WHO Expert Committee,” WHO Technical Report Series 830, World Health Organization, Geneva, Switzerland, Nov. 8–15, Report No. 830.https://apps.who.int/iris/handle/10665/37115 [PubMed]
- [97].Katz, N., Chaves, A., and Pellegrino, J., 1972, “ A Simple Device for Quantitative Stool Thick-Smear Technique in Schistosomiasis Mansoni,” Rev. Inst. Med. Trop. São Paulo, 14(6), pp. 397–400.https://www.ncbi.nlm.nih.gov/pubmed/4675644 [PubMed] [Google Scholar]
- [98]. Colley, D. G. , Binder, S. , Campbell, C. , King, C. H. , Tchuem Tchuenté, L.-A. , N'Goran, E. K. , Erko, B. , Karanja, D. M. S. , Kabatereine, N. B. , van Lieshout, L. , and Rathbun, S. , 2013, “ A Five-Country Evaluation of a Point-of-Care Circulating Cathodic Antigen Urine Assay for the Prevalence of Schistosoma Mansoni,” Am. J. Trop. Med. Hyg., 88(3), pp. 426–432. 10.4269/ajtmh.12-0639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Colley, D. G. , Bustinduy, A. L. , Secor, W. E. , and King, C. H. , 2014, “ Human Schistosomiasis,” Lancet, 383(9936), pp. 2253–2264. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100]. Strong, V. E. , Galanis, C. J. , Riedl, C. C. , Longo, V. A. , Daghighian, F. , Humm, J. L. , Larson, S. M. , and Fong, Y. , 2009, “ Portable PET Probes Are a Novel Tool for Intraoperative Localization of Tumor Deposits,” Ann. Surg. Innovation Res., 3(1), p. 2. 10.1186/1750-1164-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Wichmann, D. , Panning, M. , Quack, T. , Kramme, S. , Burchard, G.-D. , Grevelding, C. , and Drosten, C. , 2009, “ Diagnosing Schistosomiasis by Detection of Cell-Free Parasite DNA in Human Plasma,” PLoS Neglected Trop. Dis., 3(4), p. e422. 10.1371/journal.pntd.0000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Chen, J. J. , Shen, C. M. , and Ko, Y. W. , 2013, “ Analytical Study of a Microfluidic DNA Amplification Chip Using Water Cooling Effect,” Biomed. Microdev., 15(2), pp. 261–278. 10.1007/s10544-012-9728-6 [DOI] [PubMed] [Google Scholar]
- [103]. Who, E. , 1985, “ The Control of Schistosomiasis: Report of a WHO Expert Committee,” WHO Technical Report Series, World Health Organization, Geneva, Switzerland, Nov. 8–13, Report No. 728.https://apps.who.int/iris/handle/10665/39529 [PubMed]
- [104]. Bosompem, K. M. , Owusu, O. , Okanla, E. , and Kojima, S. , 2004, “ Applicability of a Monoclonal Antibody-Based Dipstick in Diagnosis of Urinary Schistosomiasis in the Central Region of Ghana,” Trop. Med. Int. Health, 9(9), pp. 991–996. 10.1111/j.1365-3156.2004.01289.x [DOI] [PubMed] [Google Scholar]
- [105]. Simiyu, K. , Daar, A. S. , and Singer, P. A. , 2010, “ Stagnant Health Technologies in Africa,” Science, 330(6010), pp. 1483–1484. 10.1126/science.1195401 [DOI] [PubMed] [Google Scholar]
- [106]. Sarvestani, A. S. , and Sienko, K. H. , 2018, “ Medical Device Landscape for Communicable and Noncommunicable Diseases in Low-Income Countries,” Globalization Health, 14(1), p. 65. 10.1186/s12992-018-0355-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Murray, C. K. , Bell, D. , Gasser, R. A. , and Wongsrichanalai, C. , 2003, “ Rapid Diagnostic Testing for Malaria,” Trop. Med. Int. Health, 8(10), pp. 876–883. 10.1046/j.1365-3156.2003.01115.x [DOI] [PubMed] [Google Scholar]
- [108]. Yager, P. , Domingo, G. J. , and Gerdes, J. , 2008, “ Point-of-Care Diagnostics for Global Health,” Annu. Rev. Biomed. Eng., 10(1), pp. 107–144. 10.1146/annurev.bioeng.10.061807.160524 [DOI] [PubMed] [Google Scholar]
- [109]. Reyburn, H. , Mbakilwa, H. , Mwangi, R. , Mwerinde, O. , Olomi, R. , Drakeley, C. , and Whitty, C. J. , 2007, “ Rapid Diagnostic Tests Compared With Malaria Microscopy for Guiding Outpatient Treatment of Febrile Illness in Tanzania: Randomised Trial,” BMJ, 334(7590), p. 403. 10.1136/bmj.39073.496829.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110]. Malkin, R. A. , 2007, “ Barriers for Medical Devices for the Developing World,” Expert Rev. Med. Devices, 4(6), pp. 759–763. 10.1586/17434440.4.6.759 [DOI] [PubMed] [Google Scholar]
- [111]. Howitt, P. , Darzi, A. , Yang, G. Z. , Ashrafian, H. , Atun, R. , Barlow, J. , Blakemore, A. , Bull, A. M. , Car, J. , Conteh, L. , Cooke, G. S. , Ford, N. , Gregson, S. A. , Kerr, K. , King, D. , Kulendran, M. , Malkin, R. A. , Majeed, A. , Matlin, S. , Merrifield, R. , Penfold, H. A. , Reid, S. D. , Smith, P. C. , Stevens, M. M. , Templeton, M. R. , Vincent, C. , and Wilson, E. , 2012, “ Technologies for Global Health,” Lancet, 380(9840), pp. 507–535. 10.1016/S0140-6736(12)61127-1 [DOI] [PubMed] [Google Scholar]


