Abstract
Dietary restriction (DR) is a well-established intervention to extend lifespan across taxa. Recent studies suggest that DR-driven lifespan extension can be cost-free, calling into question a central tenant of the evolutionary theory of aging. Nevertheless, boosting parental longevity can reduce offspring fitness. Such intergenerational trade-offs are often ignored but can account for the “missing costs” of longevity. Here, we use the nematode Caenorhabditis remanei to test for effects of DR by fasting on fitness of females and their offspring. Females deprived of food for 6 days indeed had increased fecundity, survival, and stress resistance after re-exposure to food compared with their counterparts with constant food access. However, offspring of DR mothers had reduced early and lifetime fecundity, slower growth rate, and smaller body size at sexual maturity. These findings support the direct trade-off between investment in soma and gametes challenging the hypothesis that increased somatic maintenance and impaired reproduction can be decoupled.
Keywords: Dietary restriction, Lifespan extension, Transgenerational effects, Temporary fasting, Reproduction, Trade-offs
Dietary restriction (DR), defined as a reduction in nutrient intake without malnutrition, is known to increase lifespan across the tree of life, from unicellular organisms to worms to rodents to primates (1–4). Moreover, DR is often associated with improved stress resistance (5), reduced rate of aging (6), and even improved reproduction in late life (7). Despite the ubiquity of DR-driven lifespan extension, and considerable multidisciplinary interest in this topic, the evolutionary underpinnings of this effect remain poorly understood. The leading evolutionary hypothesis maintains that DR is an evolved resource allocation strategy (“resource allocation hypothesis”) aimed at increasing survival during the time of temporary famine (8). This hypothesis argues that organisms deliberately re-allocate scarce resources away from reproduction to somatic maintenance. Indeed, DR commonly results in reduced fecundity, and this effect has been reported so often that reduced reproduction is sometimes included in the formal definition of DR (9).
The resource allocation hypothesis has been challenged in recent years by empirical studies showing that the relationship between lifespan extension and reduced reproduction under DR is not obligatory and can be uncoupled. Grandison and colleagues showed that addition of the dietary amino acid, methionine, restores fecundity of Drosophila melanogaster fruit flies under DR to normal levels without affecting their extended longevity. Additionally, DR extends lifespan in sterile or nonreproducing animals (10–13). Experimental evolution studies suggest that longevity and reproduction do not necessarily co-evolve under DR (14,15). These previous results suggest that animals under DR may not live longer because of resource re-allocation from reproduction to survival and leads some to question whether there are “missing costs” of lifespan-reproduction trade-offs. DR effect on fecundity and longevity is perhaps best viewed as a case of phenotypic plasticity allowing the organisms to maximize their fitness across different environments (16). This view is further supported by the fact that genetic pathways that underlie DR response affect a vast array of traits including growth, immune response, and metabolism (16).
Previous studies that investigated trade-offs under DR focused primarily on survival and fecundity of the parents and have not considered important traits such as offspring quality (but see ref. 16). Intergenerational trade-offs between fitness-related traits in parents and their offspring are well known from classic life-history theory (17,18) but are rarely incorporated in the empirical analyses of the fitness consequences of DR. Increased investment into somatic maintenance can improve survival and even post-DR reproduction of organisms compared with nonstarved controls (19). There is also some indication in the literature that DR can result in improved late-life reproduction (13). However, little is known about the quality of the resulting offspring. Life-history theory suggests that increased investment into current survival and reproduction can come at the cost of the propagule size (17,18). A few studies have found positive effects of DR on maternal lifespan and negative impact on fecundity, but positive impacts on offspring size, performance, and fecundity (20). However, these studies have only considered scenarios where offspring environment match that of the parents. Such a response may not be likely in systems where environments or resources fluctuate and/or there is a mismatch between adult and offspring environments.
DR has been shown to robustly increase lifespan in Caenorhabditis nematodes using a broad variety of techniques including through caloric restriction where daily calorie intake is limited (21) and temporary fasting by withholding food completely for a period of time (19,22). Although there are differences, both dietary regimes involve similar genetic pathways (23,24) and have positive effects on lifespan and reproduction (21). Here we used the temporary fasting (TF) approach to investigate the effects of DR on survival, stress resistance, and reproduction of females and their female offspring in the nematode Caenorhabditis remanei. The large majority of research into dietary impacts on lifespan in nematodes has been performed using C. elegans, a closely related species that is gynodioecious (hermaphrodites + males) and reproduces largely through self-fertilization. In contrast, C. remanei has separate sexes and is obligately sexual. DR has similar positive impacts on lifespan in both species (19). We focused our research on C. remanei females and their female offspring for two reasons. First, female fecundity is more readily quantifiable relative to male fecundity. Second, though males can affect population-level processes, female fecundity is often most closely linked to population dynamics in general (18,25).
To test for the “missing costs” of DR, we manipulated diet by either depriving females of a bacterial food source for six consecutive days or constant access to food. Following the treatment, we studied age-specific reproduction, survival, and heat-shock resistance in mothers and their female offspring to investigate intergenerational effects of maternal TF. Building on the previous research and predictions derived from the life-history theory, we hypothesized that TF during early adulthood will positively affect fitness-related traits of aging mothers, but there will be negative intergenerational effects that will reduce fitness of their daughters.
Method
We used wild-type Caenorhabditis remanei SP8 strain worms that were maintained for 50 generations under standard conditions of 20°C and 60% humidity and later stored in a −80°C freezer before use in experiments (referred to as SP8-50). Worms were thawed and maintained at 20°C for an additional 5 generations under standard lab conditions on NGM agar plates containing kanamycin and streptomycin and seeded with E. coli OP50-1 (pUC4K) bacteria as a food source. Plates were kept in a Panasonic MLR-352H Environmental Chamber under standard conditions. Worms were bleached twice, 4 and 8 days after thawing, to kill unwanted microbes and generate worms that mature at approximately the same time for experimental use.
Two days after the second bleaching, L4-female larvae were placed in groups of 10 on standard NGM agar plates with food and allowed to complete sexual maturation overnight. Early the following morning, adult virgin females were split between two treatments of either ad libitum access to food (AL) or without access to any food (temporary fasting, TF). Female worms were maintained for 6 days in their respective diet treatments. The worms were moved to fresh plates approximately every 48 and 24 hours before being placed in assays. We performed a series of assays to investigate the effect of diet treatment on an array of fitness components of treatment females and of their offspring. See Supplementary Material 1 for a diagram of the experimental design.
Round 1: Treatment Female Lifespan and Reproduction
From each food treatment, 50 females were placed individually on standard NGM plates (with antibiotics) seeded with standard food. Each female was placed with two males derived from SP8-50 stock populations. Males provided to females were of the same age (1–3 days post sexual maturity) and two new males were provided every third day after removal of previous males. To assess reproduction and lifespan, females and males were transferred to a new plate every 24 hours. The plate-harboring eggs from the previous 24-hour laying period were kept under standard conditions for an additional ~30 hours to allow offspring to hatch. After this period, larvae were euthanized in an environmental chamber at 39°C for 2 hours. Plates were then immediately placed in a refrigerator at 4°C until larval counts took place. Larval counts were performed within 24 hours after euthanization. After 13 days, reproduction had ceased, and females were transferred to new plates every third day, but still checked daily for lifespan scoring. Females were maintained as above until death. We recorded the date of death for each female. One female in the ad libitum treatment died from dehydration after climbing the plastic side of the petri dish and was excluded from the data.
Heat Stress Resistance
To assess heat stress resistance, we placed 6 plates of 10 females from each treatment (12 plates total) in an environmental chamber at 37.5°C. Starting 2 hours after and every hour up to 7 hours after initial exposure, females were monitored for a knockdown response. At each time point, females were scored and placed in one of the three categories following Herndon and colleagues (26): (i) freely moving or normal movement after being prodded gently by scorer; (ii) abnormal, nonsinusoidal movement or not freely moving (ie, only anterior portion of body moving) after being prodded gently by scorer; and (iii) not moving even after being stimulated by scorer, but with buccal pump moving or completely nonresponsive. Females scored in categories 2 and 3 were combined later for analytical purposes. After the 7-hour heat shock period, plates were stored at 20°C and females reassessed using the same categories for recovery ~ 21 hours after final seventh hour scoring.
Effects on Offspring Reproduction and Lifespan
We collected 1 L4 stage female larvae from the first 24-hour laying period from each female in both treatments. Reproduction and lifespan assays followed the same procedure as the mothers above, but female offspring were immediately placed into reproduction assays after collection to ensure reproduction commenced as soon as they reached sexual maturity. We recorded daily measures of larval output and the date of death for each female offspring. Because of logistical constraints and because heat stress assays are terminal, we had to measure development time, body size, and heat stress using offspring from different females in an additional round of experiments.
Round 2
A second round of experiments was performed and aimed to collect additional offspring traits. As in the first round, groups of 10 females were exposed to 6 days of one of the diet treatments (ad libitum or temporary fasting). Following the diet exposure, all females within each diet were placed in the same groups of 10 on seeded standard NGM plates that had ten 2-day (postsexual maturity) adult males such that there was 1:1 sex ratio. Females were kept on these plates for 2 hours to allow for feeding, mating, and for egg laying to commence. Any eggs laid during this period were discarded. Afterwards, females and males were transferred to a new seeded plate and allowed to lay eggs for 1 hour. This procedure was performed a second time on a new empty, seeded plate. The eggs from the two separate hour-long periods were used to estimate offspring development time and body size at maturity (plate 1), as well as to assess heat stress resistance (plate 2). Treatment females and males were removed and discarded after the two laying periods.
Development Time and Size at Maturity
The offspring from the first plate were allowed to mature on the laying plates. On the third day postegg laying, we monitored plates hourly for sexually mature females. Females were removed from the plates either at the time the vulva showed full maturation (ie, when the two lips of the vulva were visible as bumps) or if they appeared to have mated with males present on the plate. These two events typically occur in close proximity. We recorded the (whole) hour at which each female was collected as sexually mature and subtracted it from the time at which mothers were removed from the lay plate to estimate development time. Plates were monitored until there were no females remaining. Immediately after females were removed from the plates (ie, at sexual maturity), we photographed them using a Lumenera Infinity2-5C digital microscope camera mounted on a Leica M165C stereomicroscope. These photos were then used to measure body area as an estimate of body size. We followed the methods of ref. 27. Briefly, photos were imported to ImageJ (v. 1.50i; https://imagej.nih.gov/ij/). An initial image with a stage micrometer was used to calibrate the length of a pixel at the settings used to take photos of offspring. Images of each offspring were imported and converted to gray scale. The threshold function was used to provide a physical outline of the worm’s body. Any area not highlighted within the body of the worm after this procedure was then colored in manually. The total area was then estimated using the measure function in ImageJ.
Heat Stress Resistance
Virgin females were collected from the second plate on the third day postlaying (as above). We isolated two plates of 10 females from each of the three laying plates in each treatment (for a total of 12 plates). On Day 4 postsexual maturity, females were moved to new plates and placed immediately in to heat stress treatment. We followed the same protocols that were used for the parental generation in Experiment 1 to assess the response of offspring.
Statistical Analysis
Data were analyzed using R 3.4.0 statistical software (28). Age-specific and lifetime reproductive output were analyzed using a multilevel mixed model in the lme4 package (29) with a Poisson error distribution, log-link function. Both age and age2 were included in the model to asses linear and quadratic effects of this factor (see Supplemental Material). Individual (to account for repeated measures) and individual record values (to account for any overdispersion) (30) were included as random effects for age-specific analysis; only line was used as a random effect in reproduction analyses. Heat stress resistance was also analyzed using a multilevel model but using a binomial error distribution and logit link function. We included a random effect of “plate” to account for variation of plates on which females were treated. Development time and body size were analyzed using a general linear model. Lifespan was analyzed using a Cox Proportional hazards model in the survival package (31). A t-test was used to test for difference in age at last reproduction between treatments. See Supplementary Material 2 for statistical report.
Results
Temporary Fasting Increases Female Fitness Components
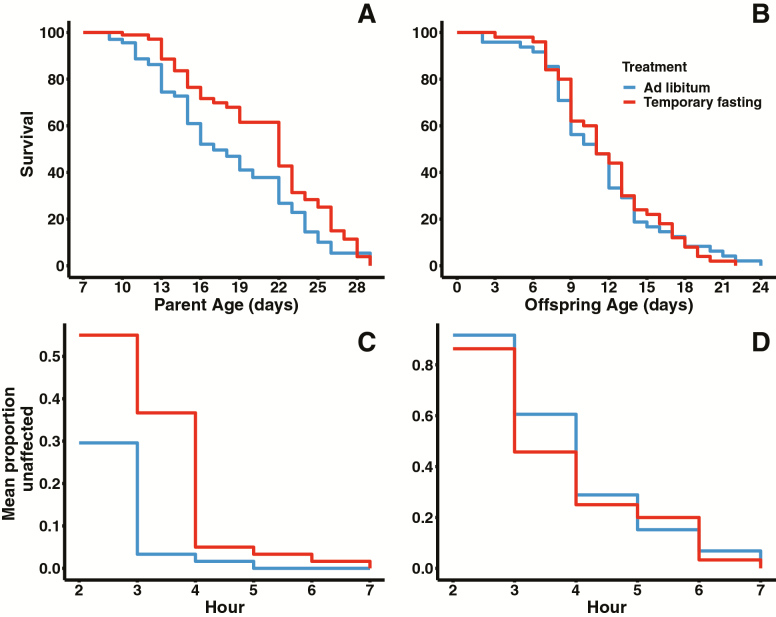
Females in the temporary fasting (TF) treatment produced a significantly higher number of offspring once returned to food and allowed mating (mean ± se; 463.45 ± 20.70, N = 50) relative to females given complete access to food (330. 76 ± 22.73; Wald Z = 19.33, p < .005, N = 49). There was also an interaction between age at last reproduction and diet treatment on offspring production (Z = 3.12, p < .002), suggesting that higher offspring production is partially a result of TF females reproducing to later ages when compared with AL females. Indeed, there is a statistical difference in age at last reproduction between diet treatment (t = 4.58, p = 1.41 × 10−5; TF 14.71 ± 0.34 d; AL 12.50 ± 0.34 d). There was no statistical difference in daily reproductive output between diet treatments (Z = 0.650, p > .52, Figure 1A), though TF females consistently produced a higher median number of offspring per day than AL females accounting for statistical differences in lifetime reproduction (see above). In line with fecundity, lifespan and heat stress resistance differed between treatments. Females that were deprived of bacteria had a significantly longer lifespan (median [95% CI], TF: 17 [15, 22] days; Coxph model, Wald’s Z = 2.497, p < .013) relative to females that had access to food (13.5 [13, 16] d; Figure 2A). Similarly, females deprived of bacteria (N = 60) were more heat stress resistant relative to AL females (N = 59) (Wald’s Z = 3.939, p < .001). This effect was dependent upon the length of exposure (Wald’s z = 2.192, p < .03), with a difference between TF and AL females up to the third hour of exposure (Figure 2C).
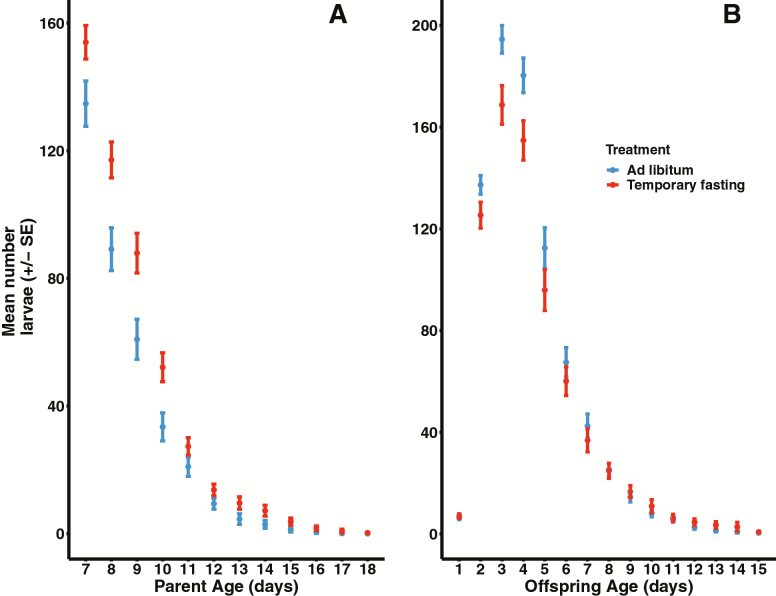
Figure 1.
(A) The influence of diet treatment on C. remanei (SP8 strain) on daily reproductive output after 6 days of either temporary fasting (TF in red; N = 50) or ad libitum (AL in blue; N = 49) access to food. Data were analyzed using mixed models in the lme4 package in R with Poisson error distribution and log-link function with individual as a random effect to account for repeated measures. Daily reproduction between diet treatments is not significant, but there is a difference in lifetime reproductive output with TF female producing more lifetime larvae than AL females. (B) The influence of maternal diet treatment on offspring reproduction (TF in red N = 50; AL in blue N = 48) starting at sexual maturity. Data were analyzed as in (A). Maternal diet treatment impact offspring reproduction with those from TF mothers showing reduced larval output (days 2 to 4) and reduced lifetime total number of larvae relative to offspring from AL mothers. Figure shows mean larval count ± se.
Figure 2.
Differences in survival between TF and AL females (A) and the effect of maternal diet treatments on offspring survival (B) (sample sizes as in Figure 1A and B). Data were analyzed using Cox Proportional Hazard models. TF females lived significantly longer than AL females. There was no difference in survival in offspring. Heat stress resistance of TF and AL females (C) (TF N = 60, AL N = 59) and the impact of maternal diet treatment on heat stress resistance in their offspring (D) (TF N = 58, AL N = 59). Data were analyzed using generalized linear mixed models. TF females showed increased heat stress resistance relative to AL females. There was no difference in heat stress response in offspring.
Temporary Fasting Has Negative or Neutral Effects on Offspring
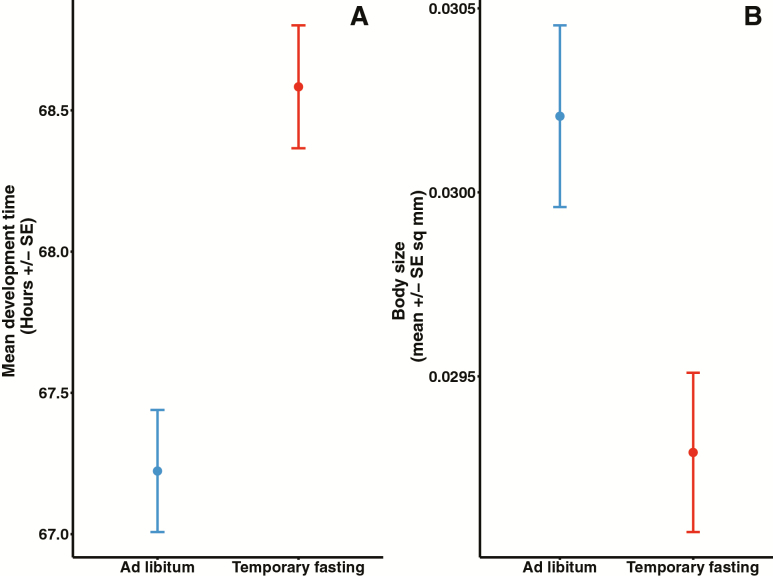
Offspring from mothers deprived of bacteria show reduced daily reproductive output during peak reproduction (D2–D4) relative to offspring from mothers in the AL treatment (Figure 1B; sample size for all test TF N = 50, AF N = 48). There was a significant quadratic effect of age in daily reproduction (Wald’s Z = 16.66, p < .001) and this quadratic effect interacted with diet treatment (Wald’s Z = 3.846, p < .001). In addition, total lifetime reproduction differed between treatments (Wald’s Z = 17.41, p < .0001). Offspring from AL mothers had a higher lifetime fecundity (751.67 ± 32.96) relative to offspring from TF mothers (690.90 ± 34.46). Any difference in age at last reproduction did not account for differences in reproduction (Wald’s Z = 3.868, p > .65). Correspondingly, there was no statistical difference in age at last reproduction between treatments (t = 1.5142, p > .10). Maternal diet treatment did not influence offspring lifespan (Wald’s Z = 0.096, p = .924; median [95% CI], TF: 11 [9, 12]; AL: 11 [9, 13], Figure 2B). There was also no difference in heat stress resistance based on maternal diet (Wald’s Z = −0.892, p = .372, Figure 2D; TF N = 58, AL N = 59). In contrast, maternal diet has a significant effect on both development time and body size (Figure 3A and B; TF N = 132, AF N = 132). Offspring from mothers in the TF treatment took significantly longer to develop (68.6 ± 0.22 h) than offspring from mothers in the AL treatment (67.2 ± 0.22; t = 3.07, p = .002). Similarly, offspring from females in the temporary fasting experiment were significantly smaller (0.0293 ± 0.00022 mm2) than those with mothers in the ad libitum treatment (0.0302 ± 0.00025; t = 2.78, p < .0058).
Figure 3.
The impact of maternal temporary fasting treatment on offspring development time (A) and body size (B) (TF N = 132; AL N = 132). Data were analyzed using linear mixed-effect models. Development time and body size were both negatively affected by maternal TF relative to AL treatment. Figures show mean ± se.
Discussion
Temporary fasting had a strong positive effect across the three fitness-related traits that we measured in females exposed to TF treatment. Consistent with previous research in nematodes (19,21,22) and other animals (6,32), the dietary restriction by temporary fasting (DR by TF) treatment extended mean lifespan in females. Additionally, DR increased stress resistance as TF females also showed increased resistance to high temperature relative to control females, aligning with previously reported links between heat-shock resistance and lifespan in nematodes (33,34). TF females also showed an increase in total lifetime fecundity and reproduced for longer than females that had constant access to food. The differences in reproduction and lifespan were not associated with a change in reproductive schedule as there was no difference in age-specific fecundity. A decline in lifetime and peak reproduction in both dietary treatment groups was observed as a result of our preventing reproduction for the first 6 days (cf. x-axis, Figure 1A and B). It could be argued that lifespan-reproduction patterns might differ if DR was applied earlier and in concert with reproduction. However, a previous study has shown DR starting at the onset of adulthood achieved similar results (19). At face value, these results align with the idea that lifespan can be extended through reduced food intake, without a corresponding cost to reproduction as has previously been suggested (32,35–38).
Despite clear positive effects of DR on maternal survival in different environments and on lifetime reproductive success, none of these benefits were transferred to their female offspring. On the contrary, daughters of TF females show reduced initial reproduction (Figure 1B) and no increase in late-life reproductive output. As such, offspring from TF females show overall lower lifetime fecundity relative to offspring from females with constant access to food. Consistent with, and compounding lower fecundity, offspring from TF mothers were smaller and took on average an hour and a half longer to develop to sexual maturity. We failed to detect any positive effects that might counter-balance these negative effects in offspring. There was neither a difference in survival in normal temperature nor a difference in heat-shock resistance between female offspring of TF and control mothers. Our results stand in contrast to a previous study in C. elegans that showed that DR offspring show increased larval resistance to starvation and produce more offspring relative to control worms (20), suggesting that DR in mothers adaptively primes their offspring. We did not explicitly test for adaptive effects under offspring nutrient stress that mimic maternal food environments, though our results from heat-shock trials provide circumstantial evidence arguing against a generalized adaptive stress priming effect in C. remanei. More research is needed to determine whether there are more specific priming effects under similar nutrient conditions as mothers.
The negative effects in offspring are a result of maternal dietary treatment, as all offspring were reared on full, ad libitum (AL) diet. The effects on offspring can be explained by changes in maternal gamete provisioning (39,40). There are well-known trends across taxa of both a trade-off between offspring number and size, as well as a positive correlation between gamete size and adult size (18,41). Our results of increased fecundity of DR females and smaller adult size of their offspring are consistent with this explanation. This explanation is also congruent with the evidence from C. elegans of DR females showing lower fecundity, but larger investment in gamete size (20). Different species can have different responses to DR in terms of their reproductive strategies; however, different DR regimes can also produce different types of response with respect to allocation decisions between investment in propagule number versus propagule size (42). Nevertheless, both scenarios highlight the importance of intergenerational trade-offs in the evolution of DR response. Our results show that dietary restriction can indeed improve fitness-related traits in aging mothers but at the cost of reduced fitness-related traits in their daughters. Different forms of DR, including temporary fasting, as well as different DR mimetics, are increasingly used in humans both in clinical practice and in research. Future studies should incorporate intergenerational effects of DR across a broad range of taxa and in both sexes to improve our understanding of the potential “missing costs” of the beneficial DR effects.
Data Accessibility
Data can be accessed on Dryad at 10.5061/dryad.z08kprr8k.
Supplementary Material
Acknowledgments
This research was supported by the European Research Council AgingSexDiff and GermlineAgeingSoma and Swedish Research Council to A.A.M. The authors would like to thank Hanne Carlsson for her assistance and two anonymous reviewers for their helpful comments. A.A.M. conceived of the project and contributed to the manuscript. A.A.M. and B.S.M. designed the experiment. M.I.L. executed the experiment and contributed to the manuscript. B.S.M. executed the experiments, analyzed the data, and wrote the manuscript.
Conflict of Interest
The authors declare no conflict of interests as defined by Journal of Gerontology: Biological Sciences.
References
- 1. Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell Metab. 2011;14:154–160. doi: 10.1016/j.cmet.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adler MI, Bonduriansky R. Why do the well-fed appear to die young? BioEssays. 2014;36:439–450. doi:10.1002/bies.201300165 [DOI] [PubMed] [Google Scholar]
- 4. Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hine C, Harputlugil E, Zhang Y, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakagawa S, Lagisz M, Hector KL, Spencer HG. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell. 2012;11:401–409. doi: 10.1111/j.1474-9726.2012.00798.x [DOI] [PubMed] [Google Scholar]
- 7. Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7:622–629. doi: 10.1111/j.1474-9726.2008.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shanley DP, Kirkwood TB. Calorie restriction and aging: a life-history analysis. Evolution. 2000;54:740–750. doi: 10.1111/j.0014-3820.2000.tb00076.x [DOI] [PubMed] [Google Scholar]
- 9. Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mair W, Sgrò CM, Johnson AP, Chapman T, Partridge L. Lifespan extension by dietary restriction in female Drosophila melanogaster is not caused by a reduction in vitellogenesis or ovarian activity. Exp Gerontol. 2004;39:1011–1019. doi: 10.1016/j.exger.2004.03.018 [DOI] [PubMed] [Google Scholar]
- 11. Crawford D, Libina N, Kenyon C. Caenorhabditis elegans integrates food and reproductive signals in lifespan determination. Aging Cell. 2007;6:715–721. doi: 10.1111/j.1474-9726.2007.00327.x [DOI] [PubMed] [Google Scholar]
- 12. Bjedov I, Toivonen JM, Kerr F, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitteldorf J. Can experiments on caloric restriction be reconciled with the disposable soma theory for the evolution of senescence? Evolution. 2001;55:1902–5; discussion 1906. doi: 10.1111/j.0014-3820.2001.tb00841.x [DOI] [PubMed] [Google Scholar]
- 14. Zajitschek F, Zajitschek SR, Canton C, Georgolopoulos G, Friberg U, Maklakov AA. Evolution under dietary restriction increases male reproductive performance without survival cost. Proc Biol Sci. 2016;283:20152726. doi: 10.1098/rspb.2015.2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zajitschek F, Georgolopoulos G, Vourlou A, et al. Evolution under dietary restriction decouples survival from fecundity in Drosophila melanogaster females. Journals Gerontol Ser A. 2018;74:1542–1548. doi:10.1093/gerona/gly070 [DOI] [PubMed] [Google Scholar]
- 16. Regan JC, Froy H, Walling CA, Moatt JP, Nussey DH. Dietary restriction and insulin‐like signalling pathways as adaptive plasticity: A synthesis and re‐evaluation. Funct Ecol. 2019;00:1–22. doi:10.1111/1365-2435.13418 [Google Scholar]
- 17. Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992. ISBN: 0198577419 [Google Scholar]
- 18. Roff D. Evolution of Life Histories: Theory and Analysis. Berlin, Germany: Springer Science & Business Media; 1993. [Google Scholar]
- 19. Sutphin GL, Kaeberlein M. Dietary restriction by bacterial deprivation increases life span in wild-derived nematodes. Exp Gerontol. 2008;43:130–135. doi: 10.1016/j.exger.2007.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hibshman JD, Hung A, Baugh LR. Maternal diet and insulin-like signaling control intergenerational plasticity of progeny size and starvation resistance. PLoS Genet. 2016;12:e1006396. doi: 10.1371/journal.pgen.1006396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Houthoofd K, Johnson TE, Vanfleteren JR. Dietary restriction in the nematode Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2005;60:1125–1131. doi: 10.1093/gerona/60.9.1125 [DOI] [PubMed] [Google Scholar]
- 22. Smith ED, Kaeberlein TL, Lydum BT, et al. Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC Dev Biol. 2008;8:49. doi: 10.1186/1471-213X-8-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uno M, Nishida E. Lifespan-regulating genes in C. elegans. NPJ Aging Mech Dis. 2016;2:16010. doi: 10.1038/npjamd.2016.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harts AMF, Schwanz LE, Kokko H. Demography can favour female-advantageous alleles. Proc R Soc B Biol Sci. 2014;281:20140005. doi:10.1098/rspb.2014.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herndon LA, Schmeissner PJ, Dudaronek JM, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135 [DOI] [PubMed] [Google Scholar]
- 27. Lind MI, Zwoinska MK, Meurling S, Carlsson H, Maklakov AA. Sex-specific tradeoffs with growth and fitness following life-span extension by rapamycin in an outcrossing nematode, caenorhabditis remanei. J Gerontol A Biol Sci Med Sci. 2016;71:882–890. doi: 10.1093/gerona/glv174 [DOI] [PubMed] [Google Scholar]
- 28. R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2017. https://www.r-project.org/. [Google Scholar]
- 29. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4 June 2014. http://arxiv.org/abs/1406.5823. Accessed July 21, 2017.
- 30. Harrison XA. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ. 2014;2:e616. doi: 10.7717/peerj.616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Therneau T, Lumley T.. Package “survival.” 2017. https://cran.r-project.org/package=survival. Accessed July 21, 2017.
- 32. Partridge L, Piper MD, Mair W. Dietary restriction in s. Mech Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023 [DOI] [PubMed] [Google Scholar]
- 33. Zhou KI, Pincus Z, Slack FJ. Longevity and stress in Caenorhabditis elegans. Aging (Albany NY). 2011;3:733–753. doi: 10.18632/aging.100367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lind MI, Chen H, Meurling S, et al. Slow development as an evolutionary cost of long life. Funct Ecol. 2017;31:1252–1261. doi:10.1111/1365-2435.12840 [Google Scholar]
- 35. Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 36. Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980 [DOI] [PubMed] [Google Scholar]
- 37. Flatt T. Survival costs of reproduction in Drosophila. Exp Gerontol. 2011;46:369–375. doi: 10.1016/j.exger.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 38. Antebi A. Regulation of longevity by the reproductive system. Exp Gerontol. 2013;48:596–602. doi: 10.1016/j.exger.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Räsänen K, Kruuk L. Maternal effects and evolution at ecological time‐scales. Funct Ecol. 2007;21:408–421. doi:10.1111/j.1365-2435.2007.01246.x [Google Scholar]
- 40. Mousseau TA, Uller T, Wapstra E, Badyaev AV. Evolution of maternal effects: past and present. Philos Trans R Soc Lond B Biol Sci. 2009;364:1035–1038. doi: 10.1098/rstb.2008.0303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fox CW, Czesak ME. Evolutionary ecology of progeny size in arthropods. Annu Rev Entomol. 2000;45:341–369. doi: 10.1146/annurev.ento.45.1.341 [DOI] [PubMed] [Google Scholar]
- 42. Dušek A, Bartoš L, Sedláček F. Pre-breeding food restriction promotes the optimization of parental investment in house mice, Mus musculus. Olsson IAS, ed. PLoS One. 2017;12:e0173985. doi:10.1371/journal.pone.0173985 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be accessed on Dryad at 10.5061/dryad.z08kprr8k.