Abstract
Vitamin D may affect cognitive performance, but previous studies are either short term or observational. We conducted a randomized controlled trial of vitamin D supplementation on domain-specific cognitive measures in postmenopausal women. Overweight/obese women with serum 25-hydroxyvitamin D (25OHD) levels less than 30 ng/mL were recruited. Vitamin D3 supplementation (600, 2,000, or 4,000 IU/d) was randomly assigned in a double-blinded manner for 1 year. Serum 25-hydroxyvitamin D, osteocalcin (total and undercarboxylated), amyloid beta, parathyroid hormone, and estradiol were analyzed before and after supplementation. Cognitive tests were administered after treatment. The women (58 ± 6 years; body mass index, 30.0 ± 3.5 kg/m2) had a baseline serum 25-hydroxyvitamin D level of 22.6 ± 5.8 ng/mL that increased to 30.2 ± 5.6, 36.0 ± 4.9, and 40.8 ± 7.0 ng/mL in the 600, 2,000, and 4,000 IU/d groups, respectively (p < .001). Participants taking 2,000 IU/d compared to other doses performed better in learning and memory tests (p < .05), yet the 4,000 IU/d group had a slower reaction time compared to the 600 IU/d group. Multiple regression indicated that serum undercarboxylated osteocalcin predicted tasks associated with reaction time and executive function, whereas body mass index and parathyroid hormone negatively predicted reaction time and executive function (p ≤ .01). These data suggest that vitamin D has differential effects on domain-specific cognitive measures and that a higher dose may negatively affect reaction time.
Keywords: Memory, Osteocalcin, Postmenopausal women, Reaction time
Cognitive impairment and dementia are significant public health problems, especially with aging (1). Observational evidence shows that vitamin D plays a role in cognition and the normal functioning of the central nervous system (2–6). Specifically, 25-hydroxyvitamin D (25OHD) deficiency of less than 12 ng/mL is associated with cognitive impairments (2–4), although other studies suggest a threshold of less than 20 ng/mL (5,6). The presence of 1-alpha-hydroxylase, the enzyme responsible for the activation of vitamin D, as well as the presence of the vitamin D receptor in the brain, supports a role for vitamin D as a neuroprotective hormone (7,8). Also, an elevated circulating parathyroid hormone (PTH) associated with 25OHD deficiency may be an additional factor contributing to a decline in cognition (9). Alterations in these various processes are seen in aging, making vitamin D an attractive therapeutic or preventative intervention (10).
Cognitive markers can assist in predicting changes in the disease process. There is evidence that amyloid beta (Aβ) peptide aggregation can damage the neuronal microenvironment in the brain and lead to cognitive decline (11). Vitamin D appears to act on Aβ by attenuating its accumulation and increasing blood–brain barrier clearance to reduce amyloid-induced cytotoxicity and apoptosis in neurons (12,13). Also, the brain is sensitive to estrogens, and circulating levels affect cognitive tasks, especially learning and memory (14). In this trial, all women were postmenopausal, not on hormone replacement therapy, and therefore in an estrogen-insufficient state. Osteocalcin (OC) is produced by osteoblasts during bone formation in carboxylated OC or undercarboxylated osteocalcin (ucOC) forms and is released into the circulation. More recently, OC has been shown to have endocrine functions and to protect neurons from apoptosis as shown in murine studies (15). In some patient populations, cognitive performance has been shown to be inversely associated with low serum 25OHD levels (16). As vitamin D affects OC gene transcription (17,18), improving vitamin D status may affect biomarkers that reflect neurodegenerative changes and impaired cognition.
Cognitive outcomes in vitamin D supplementation trials have been unclear, and this may be due to previous studies using cross-sectional designs or use of a single short-term dose. The prevalence of cognitive decline in older persons is a major public health concern with limited preventative treatments. To our knowledge, there are no previous controlled trials examining different doses of vitamin D supplementation on biochemical markers and cognitive function domains. We conducted a double-blind randomized controlled trial to assess whether higher intakes of vitamin D3 than the recommended intake (600 IU/d) (19) affect domain-specific measures of cognitive function and whether change in cognition is associated with serum concentrations of hormones and Aβ markers.
Methods
Participants
Healthy, postmenopausal women (50–70 years old; body mass index [BMI] 25–40 kg/m2) were recruited to participate in a 1-year-long randomized, placebo-controlled, double-blind three-dose vitamin D trial (20). Participants were excluded from the study if they were less than 2 years postmenopausal, had serum 25OHD level of at least 30 ng/mL, or experienced more than 5% weight gain or loss in the 3 months prior to recruitment. Participants were also ineligible if they were taking hormone replacement therapy or using medications known to influence bone metabolism. The details of the recruitment criteria and study design were previously described (20). Participants signed an informed consent approved by the institutional review board at Rutgers University, New Brunswick, New Jersey. This trial was registered at clinicaltrials.gov as NCT01631292. The protocol met the ethical standards in accordance with the Helsinki Declaration.
Study Design
The recruitment and baseline measurements were conducted during the winter months to minimize the effect of sunlight on serum 25OHD levels. Enrolled participants underwent a 1-month stabilization period during which they were standardized to a daily nutritional regimen, including a multivitamin and mineral (Nature Made Multi 50+; Pharmavite) with individualized Ca intake (200 mg Ca/tablet; Citracal, Bayer HealthCare) totaling 600 IU/d of vitamin D3 and 1.2 g Ca/d . This regimen was maintained throughout the duration of the study. Following stabilization, participants were randomized to 600, 2,000, or 4,000 IU/d of total vitamin D3, which included an estimated dietary intake of 200 IU/d and a vitamin D3 capsule (Bio-Tech Pharmacal). Participants were asked to consume vitamin D3 capsules or placebo on five consecutive weekdays and to consume with their largest meal of the day. Participants, investigators, and outcome assessors were blinded to treatment allocation procedures and groups throughout the study (20). Adherence to treatment protocol was assessed by pill count after the first month and bimonthly thereafter when distribution of new tablets was administered. Women in all groups received an individualized diet and a standard weight loss, behavior modification program with healthy lifestyle counseling. Women were offered weekly sessions during the first 6 weeks and monthly thereafter with a registered dietitian during this 1-year trial and were encouraged to achieve modest weight loss. Monthly physical activity level was recorded to estimate baseline levels and change after the intervention (20).
Biochemical Measurements
Fasting serum samples collected at baseline and 1 year were analyzed in batch analysis. Serum 25OHD was measured by radioimmunoassay (DiaSorin; interassay and intraassay coefficient of variation < 15%). Performance of the 25OHD assay was issued a proficiency certificate by the vitamin D External Quality Assessment Scheme. OC and ucOC were analyzed using a radioimmunoassay in the laboratory of Dr. C. Gundberg (Yale School of Medicine, New Haven, Connecticut) (21). Serum was analyzed for intact PTH (immunoradioassay; Scantibodies) and ultrasensitive estradiol (radioimmunoassay; DSL; coefficient of variation ≤ 7%). Serum Aβ40 and Aβ42 levels were determined using human Aβ enzyme-linked immunosorbent assay kits (MyBioSource; coefficient of variation ≤ 8%) according to manufacturer’s instructions.
Cognitive Assessment
Cognitive testing was conducted by using a standard battery of tests (Cambridge Neurological Test Automated Battery [CANTAB]; Cambridge Cognition) at the end of the vitamin D intervention. A trained research assistant tested each participant separately in a quiet room. Testing time was limited to 1 hour to minimize participant fatigue. The battery of tests assessed the following: (i) Executive Functioning: Stockings of Cambridge (SOC) and Intra/Extra-Dimensional Set Shift (IED), (ii) Learning/Memory: Paired Associates Learning (PAL), and (iii) Attention: Reaction Time (RTI). Specifically, SOC measures working memory capacity and spatial planning and motor control, whereas IED measures rule acquisition and flexibility of attention. PAL measures visual and verbal recognition memory and new learning, and the RTI tests measure response speed and visual sustained attention. The tasks were chosen based on their limited capacity to generate ceiling effects (a distinct upper limit for potential responses) (22) and their ability to activate areas of the brain affected by vitamin D status such as the cerebral cortex and the hippocampus regions of the temporal lobe (7,23,24). The integrated software records the responses on each test and generates results as both raw scores and z scores, which indicates the participant’s level of cognitive performance relative to an age-matched group of healthy participants in the CANTAB normative database. Adjusted values indicate correction for failure of test at an earlier stage of testing. The National Adult Reading Test was used to estimate general cognitive ability prior to intervention. It tests the ability to pronounce a set of phonologically irregular words. Owing to the relative preservation of verbal abilities, it is used as an estimate of premorbid mental ability (25).
Safety
Nonserious adverse events were recorded monthly throughout the study and included pain in legs, swelling in legs, pain or heaviness in chest, headaches, dizziness, nausea, fatigue, muscle weakness, muscle aches, abnormal urinary frequency, and abdominal pain. Also, spot urinary calcium was measured before and after treatment, as reported previously (20).
Statistical Analysis
Differences between groups at baseline were assessed by one-way analysis of variance. The interaction effect between treatment and time on serum biochemical concentrations were analyzed by two-way analysis of variance. When the interaction was significant, post hoc analysis using Bonferroni correction for multiple comparisons was performed. The influence of treatment on cognitive outcomes at 12 months was analyzed using analysis of variance adjusting for any covariates that differed at baseline and variables with non-normal distribution were log transformed. Pearson correlation coefficients were used to evaluate relationships between changes in independent and outcome variables. Multiple regression was used to identify factors (serum 25OHD, Aβ peptides, ucOC, PTH, and estradiol) associated with cognition, and it was adjusted for key demographic variables (age, years of education, BMI, and the National Adult Reading Test scores) known to affect cognitive tasks.
The effect size was determined using previous studies assessing cognitive function in healthy men and women (26,27) using a power of 0.90 and α of .05 and allowing for one covariate. To be able to detect a similar difference between the three levels of vitamin D3 intake for spatial and pattern recognition and memory, a sample size of 8 per group was calculated. In another study examining executive function (SOC), it was found that 9 per group would be necessary to determine significant differences between groups (28). A significance level of p < .05 was adopted for all tests and analyses were performed with SPSS, version 24.
Results
Participant Characteristics
One hundred thirty-eight women were assessed for eligibility in this study; 69 met the inclusion criteria and were recruited. Seven women withdrew before randomization because of time commitment or lack of interest in the study. Fifty-five women were randomized to one of the three vitamin D3 groups, and because of dropouts and lost data in the software program, 42 successfully completed the study (Supplementary Figure 1). The mean age of the participants was 58 ± 6 years, weight was 80 ± 12 kg, and BMI was 30 ± 3 kg/m2. The baseline characteristics of participants in each treatment group are given in Table 1. Women were primarily Caucasian and 7% African American, 7% Asian, and 2% Hispanic. There were no significant differences at baseline between groups for age, BMI, years since menopause, serum 25OHD, OC, estradiol, Aβ42, Aβ40, or Aβ42/Aβ40 ratio or any other demographic factors (Table 2). Baseline weight (80.4 ± 11.6 kg) differed between groups and was used as a covariate in the analysis. Serum 25OHD level at screening was 22.7 ± 5.8 ng/mL, and 29% of participants had levels less than 20 ng/mL. After stabilization, baseline 25OHD level was 27.4 ± 4.8 ng/mL, and 7% of women had a serum 25OHD level of less than 20 ng/mL. No serious adverse events occurred and the frequency of nonserious events (cumulative) was not significantly different between groups during the study.
Table 1.
Baseline Characteristics of the Study Population
| Variables | 600 IU/d | 2,000 IU/d | 4,000 IU/d | p |
|---|---|---|---|---|
| Age (y) | 58.0 ± 6.8 | 58.5 ± 5.3 | 57.2 ± 5.9 | .856 |
| BMI (kg/m2) | 29.2 ± 2.6 | 31.5 ± 4.3 | 29.0 ± 3.0 | .096 |
| YSM (y) | 7.6 ± 6.6 | 8.9 ± 8.4 | 5.4 ± 2.5 | .413 |
| Psychotropic meds/person* | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.3 ± 0.6 | .408 |
| Drinks/week (n) | 1.2 ± 0.9 | 2.8 ± 3.5 | 2.0 ± 3.2 | .287 |
| Physical activity level | 2.1 ± 0.8 | 1.9 ± 0.8 | 2.0 ± 0.8 | .876 |
| Education (y) | 16.0 ± 0.0 | 15.7 ± 1.1 | 15.3 ± 2.8 | .553 |
| NART score (correct/61) | 50 ± 5 | 49 ± 6 | 46 ± 7 | .171 |
Notes: Values represent means ± SD (n = 42). BMI = body mass index; NART = National Adult Reading Test; YSM = years since menopause.
*Psychotropic medications (antidepressants/antianxiety): 600 (n = 1), 2,000 (n = 1), 4,000 (n = 2).
Table 2.
BMI and Serum Markers Before and After 1 Year of Vitamin D3 Supplementation
| 600 IU/d | 2,000 IU/d | 4,000 IU/d | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Change | Baseline | Final | Change | Baseline | Final | Change | Group | Time | Group × Time | |
| BMI (kg/m2) | 29.2 ± 2.6 | 28.6 ± 3.3 | −0.6 ± 1.6 | 31.5 ± 4.3 | 30.7 ± 4.3 | −0.8 ± 1.6 | 29.0 ± 3.0 | 28.2 ± 3.1 | −0.7 ± 1.8 | .115 | .004 | .940 |
| 25OHD (ng/mL) | 26.7 ± 5.0 | 30.2 ± 5.6 | 3.5 ± 4.4a,b | 28.2 ± 5.3 | 36.0 ± 4.9 | 7.8 ± 7.3a | 27.4 ± 4.2 | 40.8 ± 7.0 | 13.4 ± 6.6b | .006 | .001 | < .001 |
| PTH (ng/mL) | 36.3 ± 9.5 | 33.5 ± 8.4 | −2.8 ± 4.6 | 38.8 ± 16.6 | 34.8 ± 8.5 | −4.0 ± 14.7 | 38.7 ± 16.2 | 31.0 ± 8.0 | −7.7 ± 12.8 | .846 | .010 | .527 |
| Estradiol (pmol/L) | 11.9 ± 4.7 | 11.2 ± 3.8 | −0.7 ± 2.5 | 16.2 ± 6.1 | 13.9 ± 5.8 | −1.3 ± 6.0 | 16.1 ± 6.0 | 14.5 ± 4.5 | −2.8 ± 5.9 | .092 | .048 | .761 |
| OC (ng/mL) | 6.4 ± 2.5 | 6.7 ± 2.4 | 0.3 ± 3.1 | 5.7 ± 1.6 | 6.0 ± 2.4 | 0.3 ± 2.5 | 5.6 ± 2.5 | 7.9 ± 2.6 | 2.5 ± 2.9 | .409 | .027 | .118 |
| ucOC (ng/mL) | 2.2 ± 0.9 | 2.3 ± 0.7 | 0.02 ± 0.9 | 1.8 ± 0.8 | 2.3 ± 1.2 | 0.5 ± 1.3 | 1.9 ± 0.7 | 2.3 ± 1.1 | 0.36 ± 1.22 | .926 | .104 | .485 |
| Aβ42 (pg/mL) | 14.0 ± 31.9 | 10.1 ± 20.4 | −3.9 ± 12.9 | 12.0 ± 33.8 | 13.5 ± 38.2 | 1.5 ± 4.4 | 6.7 ± 8.0 | 7.0 ± 6.5 | −0.2 ± 1.9 | .893 | .688 | .023 |
| Aβ40 (pg/mL) | 12.3 ± 14.3 | 16.6 ± 23.7 | 4.3 ± 17.9 | 21.1 ± 24.0 | 25.6 ± 28.4 | 3.9 ± 10.7 | 42.5 ± 25.0 | 55.2 ± 11.4 | 12.7 ± 24.7 | .002 | .047 | .219 |
| Aβ42/Aβ40 | 0.9 ± 1.6 | 1.3 ± 2.9 | 0.3 ± 2.01 | 1.1 ± 3.6 | 0.9 ± 3.1 | −0.2 ± 0.5 | 0.2 ± 0.3 | 0.1 ± 0.1 | −0.1 ± 0.2 | .541 | .838 | .555 |
Notes: Values represent means ± SD (n = 42). Baseline levels were taken after 4 weeks of stabilization with 600 IU/d vitamin D intake in all participants. Screening level of 25OHD before daily multivitamin was 22.6 ± 5.8 ng/mL and did not differ significantly between groups. Different superscript letters in the same row indicate significant differences between groups, p < .05. Bold values represent significance, p < .05. Aβ = amyloid beta; BMI = body mass index; 25OHD = 25-hydroxyvitamin D; OC = osteocalcin; PTH = parathyroid hormone.
Vitamin D Supplementation and Effect of Intervention
After 12 months of lifestyle intervention, all groups showed a similar decrease in weight (−3.2 ± 4.5%; p < .001) and BMI, and no significant change in physical activity level. No participant had a 25OHD level below 20 ng/mL following intervention at any of the doses (Table 2). Compared to baseline concentrations, serum 25OHD level increased by 14 ± 17%, 32 ± 31%, and 50 ± 25% in the 600, 2,000 and 4,000 IU/d groups, respectively (p < .01). Serum intact PTH levels decreased over time (p = .01) but did not differ between groups. As expected with weight loss, serum OC concentrations increased over time (p = .01) and estradiol decreased (p < .05), but neither differed significantly between groups. Concentrations of serum Aβ42 and Aβ40, and Aβ42/Aβ40 ratios were not significantly different at baseline or between groups after 12 months of treatment. However, serum Aβ40 concentrations increased over time in all treatment groups (p < .05). We found a trend (p < .08) for serum Aβ42 to increase more in the higher intake groups compared to the recommended, 600 IU/d. In an additional analysis to examine if the recommended intake (600 IU/d) differed from higher vitamin D intakes (2,000 and 4,000 IU/d), there was a trend (p < .08) for a greater increase in serum Aβ42 concentration in the higher treatment groups.
Vitamin D Supplementation and Cognition
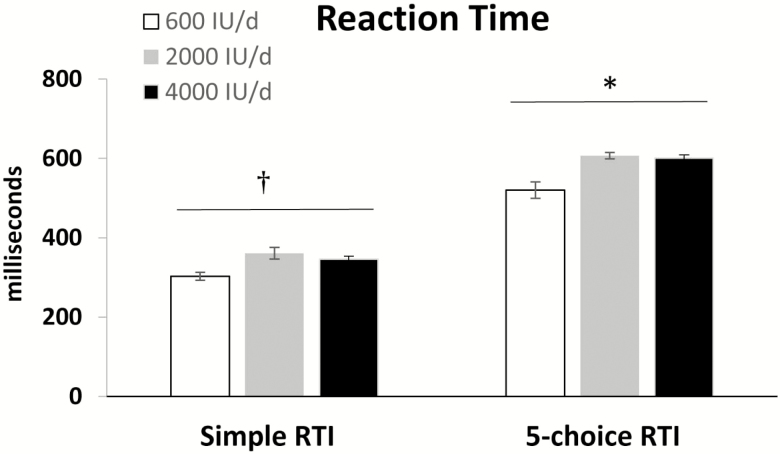
The CANTAB test results indicated that the 2,000 IU/d group, when compared to other groups, performed better in PAL test parameters (p < .05; Table 3). The IED test required more trials to complete all attempted stages in the 4,000 IU/d compared to 600 IU/d group (p < .05). In addition, RTI was slower in the 4,000 IU/d compared to 600 IU/d group for the 5-choice test (p < .01) and showed a trend for the simple RTI (Figure 1). Five-choice movement time also indicated a trend to be slower in the 4,000 IU/d group (p < .06), whereas simple movement time was not significantly different between groups (data not shown). For all cognitive variables measured, participants performed within the “average” population range (ie, z scores between −1 and 1), as expected in this healthy older population. In addition, simple and more complex (5-choice) RTIs and movement time (5-choice) were slower in the higher vitamin D groups (2,000 and 4,000 IU/d) than the 600 IU/d group (p < .01). There were no other significant differences for the other cognitive function domains between groups.
Table 3.
Cognitive Measures After Vitamin D3 Supplementation
| Domain and Test Parameter | 600 IU/d | 2,000 IU/d | 4,000 IU/d | p |
|---|---|---|---|---|
| Memory and learning | ||||
| Paired Associates Learning (PAL) | ||||
| Total errors (adjusted) | 27.7 ± 20.0a | 12.7 ± 11.2b | 27.1 ± 17.1ab | .004 |
| Total trials (adjusted) | 15.0 ± 3.7a | 19.1 ± 4.4b | 14.9 ± 3.4a | .022 |
| Stages completed on first trial | 5.0 ± 1.0a | 5.8 ± 0.8b | 4.9 ± 0.8a | .017 |
| Executive Function | ||||
| Stockings of Cambridge (SOC) | ||||
| Problems solved in minimum moves | 7.3 ± 1.8 | 7.3 ± 2.3 | 7.8 ± 1.7 | .724 |
| Initial thinking time (two moves) (ms) | 3,066 ± 2,106 | 2,348 ± 1,333 | 1,300 ± 930 | .405 |
| Intra/Extra-Dimensional Set Shift (IED) | ||||
| Total errors (adjusted) | 15.3 ± 12.8 | 12.0 ± 5.5 | 22.3 ± 17.4 | .114 |
| Total trials (adjusted) | 71.2 ± 7.9a | 72.3 ± 10.9ab | 88.3 ± 29.9b | .033 |
Notes: Values represent means ± SD (n = 42); lower errors for PAL or IED and total trials attempted (IED) and initial thinking time (SOC) indicate better performance. Different superscript letters in the same row indicate significant differences between groups, p < .05. Bold values represent significance, p < .05.
Figure 1.
Simple and 5-choice reaction time (RTI) in response to vitamin D supplementation in obese/overweight older women. Differs between groups by one-way analysis of variance (ANOVA), †p < .06, *p < .01.
Pearson Correlation and Multiple Regression
Pearson correlation indicated no significant relationships between changes in body BMI, weight, hormones, serum Aβ40 and Aβ42 concentrations, or Aβ42/Aβ40 ratio from baseline to 12 months among treatment groups. However, changes in serum Aβ40 concentration correlated with ucOC changes (r = .340, p < .05). After the intervention, 25OHD correlated with total OC and estradiol (r > .330; p <.05). Also, there was a trend for PTH to inversely correlate with 25OHD (r = −.170), Aβ42 (r = −.356, p < .05), and SOC (problems solved in minimum moves; r = −.297, p < .06). The relationship between total OC and IED total trials and errors was r = .286 (p < .07) and r =.320 (p < .05), respectively. Serum ucOC correlated with IED total trials and IED errors (r > .457, p < .005).
Multiple linear regression models were constructed to determine if serum markers predicted cognitive measures at 12 months after adjustment for demographic variables (Table 4). Serum ucOC was the only marker to predict RTI and IED (p < .05). Higher serum PTH predicted fewer problems solved in the SOC executive function test (p < .01). BMI and estradiol showed trends toward explaining some variance of cognitive task performance (Table 4).
Table 4.
Regression of Biochemical and Demographic Factors on Cognitive Outcomes in Overweight/Obese Older Women
| Simple Reaction Time | Five-Choice Reaction Time | SOC—Problems Solved in Minimum Moves | IED Errors Adjusted | IED Total Trials Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
R
2 = 0. 252 F = 1.74; p = .146 |
R
2 = 0. 289 F = 1.74; p = .137 |
R
2 = 0. 287 F = 1.73; p = .140 |
R
2 = 0. 546 F = 5.16; p = .001 |
R
2 = 0. 461 F = 3.66; p = .006 |
|||||
| β | p | β | p | β | p | β | p | β | p | |
| 25OHD | .252 | .163 | ||||||||
| ucOC | .348 | .047 | .343 | .045 | −.272 | .108 | .617 | <.001 | .608 | <.001 |
| PTH | .238 | .152 | .258 | .112 | −.457 | .007 | .211 | .141 | ||
| Estradiol | −.247 | .198 | −.257 | .119 | −.252 | .113 | ||||
| Aβ40 | .234 | .117 | ||||||||
| Aβ42 | ||||||||||
| Model 2 |
R
2 = 0. 422 F = 1.97; p = .079 |
R
2 = 0. 316 F = 1.09; p = .404 |
R
2 = 0. 332 F = 1.18; p = .349 |
R
2 = 0. 595 F = 3.48; p = .004 |
R
2 = 0. 603 F = 3.60; p = .004 |
|||||
| β | p | β | p | β | p | β | p | β | p | |
| 25OHD | ||||||||||
| ucOC | .426 | .026 | .356 | .081 | −.324 | .114 | .587 | <.001 | .567 | <.001 |
| PTH | .252 | .177 | −.500 | .011 | ||||||
| Estradiol | −.343 | .047 | −.372 | .023 | ||||||
| Aβ40 | .230 | .132 | .311 | .033 | ||||||
| Aβ42 | ||||||||||
| Age | .277 | .116 | .216 | .189 | ||||||
| Education | .242 | .143 | ||||||||
| NART | .231 | .095 | ||||||||
| BMI | .304 | .108 | −.304 | .076 | −.399 | .015 |
Notes: The models include the following serum final values for 25OHD, ucOC, PTH, estradiol, Aβ40 and Aβ42 in Model 1, and is adjusted for demographics (age, education, NART, and BMI) for each cognitive task in the Model 2. n = 42. Values for individual variables are shown only when they reach p < .20 for inclusion in the model, and bolded when p < .09. Aβ = amyloid beta; BMI = body mass index; IED = Intra/Extra-Dimensional Set Shift; NART=National Adult Reading Test; OC = osteocalcin; PTH = parathyroid hormone; RTI = reaction time; SOC = Stockings of Cambridge; ucOC = undercarboxylated OC.
Discussion
There is a strong body of evidence indicating an association between low levels of 25OHD and reduced cognitive performance including memory and executive function (4,5,28). A systematic review and meta-analysis found that hypovitaminosis D increases the risk of cognitive decline and dementia (29). Low 25OHD levels have also been linked to an increased incidence of neurodegenerative disorders, which are characterized by Aβ peptide accumulation in the brain (30), and vitamin D has been shown to enhance Aβ efflux into circulation (13). In addition, vitamin D stimulates OC gene transcription and may increase circulating levels (17,18), and low OC is associated with poor cognitive performance, possibly due to increased neuronal apoptosis (15,31). It is not known whether biochemical markers altered by vitamin D supplementation can affect cognitive function domains. In this 1-year-long double-blind randomized controlled trial, we found that higher doses of vitamin D had both positive and negative effects on cognitive function and that ucOC was the most consistent predictor of different cognitive performance.
Few randomized, controlled, double-blind trials have examined the effect of vitamin D supplementation on both cognitive and biochemical changes in a healthy population. In a post hoc analysis of a randomized controlled trial testing the effect of vitamin D and calcium supplementation on cognitive outcomes in older women, treatment assignment did not attenuate cognitive decline or the onset of mild cognitive impairment and dementia during a follow-up of 7.8 years (32). However, baseline 25OHD level was approximately 20 ng/mL and supplementation of vitamin D was only 400 IU/d. This level of vitamin D intake was lower than our control group of 600 IU/d and could explain their negative findings. In another randomized controlled vitamin D trial (4,000 or 400 IU/d for 18 weeks) in healthy individuals, the higher dose improved memory, but there was no effect on other cognitive domains (33). These effects on memory after a shorter vitamin D intervention are consistent with the improved memory and learning observed in this study after 1 year in older women. Also, observational data suggest that higher serum 25OHD levels positively affect executive function (34), but in this study, participants performed worse in IED task, a measure of executive function. The absence of a dose–response effect in this study may reflect findings in observational studies, suggesting a U-shaped curve for both vitamin D intake and serum levels on nonskeletal outcomes (35,36).
The active form of vitamin D, 1α,25-dihydroxyvitamin D3, has been reported to enhance brain-to-blood human Aβ40 efflux transport across the blood–brain barrier in mouse brain (13). Thus, vitamin D supplementation may similarly promote clearance of Aβ and explain the rise in the Aβ40 isoform over time as all women in this study demonstrated a rise in serum 25OHD levels. In addition, only the 600 IU/d group showed a decline in serum Aβ42 concentration as compared to the higher vitamin D groups. Furthermore, we examined the Aβ42/Aβ40 ratio because it has been associated with onset of cognitive decline (37,38), but we found no relationship with cognitive outcomes. However, we did find that greater circulating Aβ40 tended to predict higher IED errors and trials, especially when corrected for age, education, and BMI. Because all circulating Aβ does not originate only in the brain, but rather in other organs too (39), this is considered one source of variability using Aβ markers to predict cognitive function domains and could explain its inability to predict outcomes in this study. Also, if age is a major factor affecting vitamin D modulation of amyloid burden (40,41), this may have limited an ability to observe differences in this relatively young population.
OC can protect neurons from apoptosis (15), and low OC or ucOC is associated with poor cognitive performance in some patient populations, such as in the obese (16,31). These data indicate ucOC predicts poorer performance on interdimensional shifts and RTI. It is possible that vitamin D supplementation given to all study groups affected OC gene transcription and accounted for the rise in OC and ucOC after the intervention. Our findings also support evidence that higher serum PTH is a predictor of impaired executive function. We did not find that estradiol was a predictor of memory and learning, possibly because levels were low in all postmenopausal women (42). And although estradiol decreased slightly over time in all participants, the decline can be attributed to the small amount of weight loss and adipose tissue over time. Others have found that BMI negatively affects cognitive outcomes (43), consistent with findings in this data set, showing more IED errors and trials with higher BMI. Because ucOC was a predictor of tasks in a few different cognitive function domains and regulates anxiety and cognition in mice (15), inclusion of this biomarker should be considered in future studies.
Some strengths of this study are that the women were not on estrogen therapy which is known to affect working memory. In addition, all women received a vitamin and mineral tablet, and therefore, a deficiency in another micronutrient would not have influenced the results. Another study strength includes participant recruitment that was conducted in the same season to avoid seasonal influence on 25OHD concentrations. A limitation is the small sample size, reducing the chances of detecting significant differences and the 23% loss to follow-up predominantly due to software failure. However, most trials anticipate a loss to follow-up of 20%–25% and this is typical of diet interventions (44). In addition, because of the relatively intense protocol conditions (16 sessions), compliance was good, and to reduce variability, batch analysis was conducted for biochemical analysis. Test and retest reliability is a concern in the cognitive field because tests invoke a learning effect (45). This was avoided in this study by only testing after the intervention, but there was no baseline testing. Because women were well matched between groups, this should have reduced differences in cognition at baseline. On another topic, one limitation of these findings is that these cognitive tests may not represent clinical significance. For example, we did not include standard clinical measures used to assess neuropsychological function such as the Wechsler Memory Scale, but there is modest agreement with CANTAB (46). In addition, this CANTAB battery has been used widely for research in neurocognitive disorders and was selected for its relative insensitivity to cultural differences. The stimuli are visuospatial and are not subject to cultural influences that often affect verbally based tests. Finally, because most women had serum 25OHD levels of more than 20 ng/mL at baseline, values may have been insufficiently low to cause a pronounced effect on cognition (19), but the results can address future studies in the area of prevention.
In conclusion, the 2,000 IU/d dose of vitamin D showed positive effects on visual and working memory and learning, and the 4,000 IU/d vitamin D dose was associated with slower RTI. Of note, higher vitamin D intakes in controlled trials increase falls risk (36,47,48) and slower RTIs affect gait and falls risk (49,50), but whether vitamin D affects RTI to influence gait or falling is not known. In addition, future trials should consider controlling for gene polymorphisms, such as the apolipoprotein E4 allele, that affect cognitive impairment and gait (51). These findings indicate both positive and negative cognitive outcomes to vitamin D, which may explain some of the inconsistent findings with vitamin D examining more global cognitive exams (ie, Mini-Mental State Examination). A future multiple-dose vitamin D study measuring cognitive domains and rates of falls with falls-related injuries, as a priori set point, is needed to specify whether RTI is related to rates of falls and injuries in at-risk populations.
Funding
This work was supported by the National Institutes of Health (NIA-AG12161) and a Busch Biomedical Award (2010695157) to S.A.S.
Supplementary Material
Acknowledgments
We thank Robert Zurfluh, MS, RD, and the other clinical staff for their invaluable clinical assistance and Julia Amariti, BS, and Brandon Alderman, PhD, for their careful review of the final manuscript. We are also grateful for the commitment of the volunteers.
Conflict of interest statement
None reported.
References
- 1. Llewellyn DJ, Lang IA, Langa KM, Melzer D. Vitamin D and cognitive impairment in the elderly U.S. population. J Gerontol A Biol Sci Med Sci. 2011;66A(1):59–65. doi:10.1093/gerona/glq185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goodwill AM, Szoeke C. A systematic review and meta-analysis of the effect of low vitamin D on cognition. J Am Geriatr Soc. 2017;65:2161–2168. doi:10.1111/jgs.15012 [DOI] [PubMed] [Google Scholar]
- 3. Goodwill AM, Campbell S, Simpson S Jr, et al. Vitamin D status is associated with executive function a decade later: data from the Women’s Healthy Ageing Project. Maturitas. 2018;107:56–62. doi:10.1016/j.maturitas.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 4. Miller JW, Harvey DJ, Beckett LA, et al. Vitamin D status and rates of cognitive decline in a multiethnic cohort of older adults. JAMA Neurol. 2015;72:1295–1303. doi:10.1001/jamaneurol.2015.2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buell JS, Scott TM, Dawson-Hughes B, et al. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci. 2009;64:888–895. doi:10.1093/gerona/glp032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–1141. doi:10.1001/archinternmed.2010.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi:10.1210/jcem.86.2.7220 [DOI] [PubMed] [Google Scholar]
- 8. Cui X, Gooch H, Petty A, McGrath JJ, Eyles D. Vitamin D and the brain: genomic and non-genomic actions. Mol Cell Endocrinol. 2017;453:131–143. doi:10.1016/j.mce.2017.05.035 [DOI] [PubMed] [Google Scholar]
- 9. Lourida I, Thompson-Coon J, Dickens CM, et al. Parathyroid hormone, cognitive function and dementia: a systematic review. PLoS One. 2015;10(5):e0127574. doi:10.1371/journal.pone.0127574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gezen-Ak D, Yılmazer S, Dursun E. Why vitamin D in Alzheimer’s disease? The hypothesis. J Alzheimer’s Dis. 2014;40(2):257–269. doi:10.3233/JAD-13c1970 [DOI] [PubMed] [Google Scholar]
- 11. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi:10.1101/cshperspect.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mizwicki MT, Menegaz D, Zhang J, et al. Genomic and nongenomic signaling induced by 1α,25(OH)2-vitamin D3 promotes the recovery of amyloid-β phagocytosis by Alzheimer’s disease macrophages. J Alzheimers Dis. 2012;29:51–62. doi:10.3233/JAD-2012-110560 [DOI] [PubMed] [Google Scholar]
- 13. Ito S, Ohtsuki S, Nezu Y, Koitabashi Y, Murata S, Terasaki T. 1α,25-Dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-β peptide(1-40) from mouse brain across the blood-brain barrier. Fluids Barriers CNS. 2011;8:20. doi:10.1186/2045-8118-8-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luine V, Serrano P, Frankfurt M. Rapid effects on memory consolidation and spine morphology by estradiol in female and male rodents. Horm Behav. 2018;104:111–118. doi:10.1016/j.yhbeh.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oury F, Khrimian L, Denny CA, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228–241. doi:10.1016/j.cell.2013.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fang H, Xu XY, Xu RZ, Zhen YF, Xu G, Li YK. Decreased serum undercarboxylated osteocalcin is associated with cognitive impairment in male patients with type 2 diabetes. J Diabetes Complications. 2018;32:56–60. doi:10.1016/j.jdiacomp.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 17. Kerner SA, Scott RA, Pike JW. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989;86(12):4455–4459. doi:10.1073/pnas.86.12.4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Staal A, Van Wijnen AJ, Desai RK, et al. Antagonistic effects of transforming growth factor-beta on vitamin D3 enhancement of osteocalcin and osteopontin transcription: reduced interactions of vitamin D receptor/retinoid X receptor complexes with vitamin E response elements. Endocrinology. 1996;137:2001–2011. doi:10.1210/endo.137.5.8612541 [DOI] [PubMed] [Google Scholar]
- 19. Ross AC, Manson JE, Abrams SA, et al. The 2011 Report on Dietary Reference Intakes for Calcium and Vitamin D from the Institute of Medicine. J Clin Endocrinol Metab. 2011;111:524–7. doi:10.1016/j.jada.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pop LC, Sukumar D, Schneider SH, et al. Three doses of vitamin D, bone mineral density, and geometry in older women during modest weight control in a 1-year randomized controlled trial. Osteoporos Int. 2017;28:377–388. doi:10.1007/s00198-016-3735-z [DOI] [PubMed] [Google Scholar]
- 21. Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–3266. doi:10.1210/jcem.83.9.5126 [DOI] [PubMed] [Google Scholar]
- 22. Wild K, Howieson D, Webbe F, Seelye A, Kaye J. Status of computerized cognitive testing in aging: a systematic review. Alzheimers Dement. 2008;4:428–437. doi:10.1016/j.jalz.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi:10.1016/j.jchemneu.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 24. Diesel B, Radermacher J, Bureik M, et al. Vitamin D(3) metabolism in human glioblastoma multiforme: functionality of CYP27B1 splice variants, metabolism of calcidiol, and effect of calcitriol. Clin Cancer Res. 2005;11:5370–5380. doi:10.1158/1078-0432.CCR-04-1968 [DOI] [PubMed] [Google Scholar]
- 25. Crawford JR, Deary IJ, Starr J, Whalley LJ. The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66-year interval. Psychol Med. 2001;31:451–458. doi:10.1017/S0033291701003634 [DOI] [PubMed] [Google Scholar]
- 26. Harmer CJ, McTavish SF, Clark L, Goodwin GM, Cowen PJ. Tyrosine depletion attenuates dopamine function in healthy volunteers. Psychopharmacology (Berl). 2001;154(1):105–111. doi:10.1007/s002130000613 [DOI] [PubMed] [Google Scholar]
- 27. File SE, Jarrett N, Fluck E, Duffy R, Casey K, Wiseman H. Eating soya improves human memory. Psychopharmacology (Berl). 2001;157:430–436. doi:10.1007/s002130100845 [DOI] [PubMed] [Google Scholar]
- 28. Lee DM, Tajar A, Ulubaev A, et al. ; EMAS Study Group Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80:722–729. doi:10.1136/jnnp.2008.165720 [DOI] [PubMed] [Google Scholar]
- 29. Balion C, Griffith LE, Strifler L, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79:1397–1405. doi:10.1212/WNL.0b013e31826c197f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buell JS, Dawson-Hughes B, Scott TM, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74:18–26. doi:10.1212/WNL.0b013e3181beecb7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Puig J, Blasco G, Daunis-i-Estadella J, et al. Lower serum osteocalcin concentrations are associated with brain microstructural changes and worse cognitive performance. Clin Endocrinol (Oxf). 2016;84:756–763. doi:10.1111/cen.12954 [DOI] [PubMed] [Google Scholar]
- 32. Rossom RC, Espeland MA, Manson JE, et al. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J Am Geriatr Soc. 2012;60:2197–2205. doi:10.1111/jgs.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pettersen JA. Does high dose vitamin D supplementation enhance cognition?: a randomized trial in healthy adults. Exp Gerontol. 2017;90:90–97. doi:10.1016/j.exger.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 34. Pettersen JA. Vitamin D and executive functioning: are higher levels better? J Clin Exp Neuropsychol. 2016;38:467–477. doi:10.1080/13803395.2015.1125452 [DOI] [PubMed] [Google Scholar]
- 35. Shapses S, Rosen CJ. Case 1-1 1. Optimal vitamin D Levels in health and disease: current understanding based on IOM guidelines. Transl Endocrinol Metab. 2011:13–42. doi:10.1210/TEAM.9781879225848.ch1 [Google Scholar]
- 36. Smith LM, Gallagher JC, Suiter C. Medium doses of daily vitamin D decrease falls and higher doses of daily vitamin D3 increase falls: a randomized clinical trial. J Steroid Biochem Mol Biol. 2017;173:317–322. doi:10.1016/j.jsbmb.2017.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Graff-Radford NR, Crook JE, Lucas J, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi:10.1001/archneur.64.3.354 [DOI] [PubMed] [Google Scholar]
- 38. Locascio JJ, Fukumoto H, Yap L, et al. Plasma amyloid beta-protein and C-reactive protein in relation to the rate of progression of Alzheimer disease. Arch Neurol. 2008;65:776–785. doi:10.1001/archneur.65.6.776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marcinkiewicz M, Seidah NG. Coordinated expression of beta-amyloid precursor protein and the putative beta-secretase BACE and alpha-secretase ADAM10 in mouse and human brain. J Neurochem. 2000;75(5):2133–2143. doi:10.1046/j.1471-4159.2000.0752133.x [DOI] [PubMed] [Google Scholar]
- 40. Mayeux R, Honig LS, Tang MX, et al. Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61:1185–1190. doi:10.7916/D8J11FQH [DOI] [PubMed] [Google Scholar]
- 41. Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60:958–964. doi:10.1001/archneur.60.7.958 [DOI] [PubMed] [Google Scholar]
- 42. Herrera AY, Hodis HN, Mack WJ, Mather M. Estradiol therapy after menopause mitigates effects of stress on cortisol and working memory. J Clin Endocrinol Metab. 2017;102:4457–4466. doi:10.1210/jc.2017-00825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sabia S, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutr. 2009;89:601–607. doi:10.3945/ajcn.2008.26482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–157. doi:10.7326/0003-4819-153-3-201008030-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lowe C, Rabbitt P. Test/re-test reliability of the CANTAB and ISPOCD neuropsychological batteries: Theoretical and practical issues. Neuropsychologia. 1998;36:915–923. doi:10.1016/S0028-3932(98)00036-0 [DOI] [PubMed] [Google Scholar]
- 46. Smith PJ, Need AC, Cirulli ET, Chiba-Falek O, Attix DK. A comparison of the Cambridge Automated Neuropsychological Test Battery (CANTAB) with “traditional” neuropsychological testing instruments. J Clin Exp Neuropsychol. 2013;35:319–328. doi:10.1080/13803395.2013.771618 [DOI] [PubMed] [Google Scholar]
- 47. Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Estimating vitamin D status and the choice of supplementation dose-reply. JAMA Intern Med. 2016;176:865–866. doi:10.1001/jamainternmed.2016.1629 [DOI] [PubMed] [Google Scholar]
- 48. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi:10.1001/jama.2010.594 [DOI] [PubMed] [Google Scholar]
- 49. Bunce D, Haynes BI, Lord SR, et al. Intraindividual stepping reaction time variability predicts falls in older adults with mild cognitive impairment. J Gerontol A Biol Sci Med Sci. 2017;72:832–837. doi:10.1093/gerona/glw164 [DOI] [PubMed] [Google Scholar]
- 50. Woolley SM, Czaja SJ, Drury CG. An assessment of falls in elderly men and women. J Gerontol A Biol Sci Med Sci. 1997;52:M80–M87. doi:10.1093/gerona/52A.2.M80 [DOI] [PubMed] [Google Scholar]
- 51. Sakurai R, Montero-Odasso M. Apolipoprotein E4 Allele and Gait performance in mild cognitive impairment: results from the Gait and Brain Study. J Gerontol A Biol Sci Med Sci. 2017;72:1676–1682. doi:10.1093/gerona/glx075 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.