Abstract
Background
Although there are known clinical measures that may be associated with risk of future falls in older adults, we are still unable to predict when the fall will happen. Our objective was to determine whether unobtrusive in-home assessment of walking speed can detect a future fall.
Method
In both ISAAC and ORCATECH Living Laboratory studies, a sensor-based monitoring system has been deployed in the homes of older adults. Longitudinal mixed-effects regression models were used to explore trajectories of sensor-based walking speed metrics in those destined to fall versus controls over time. Falls were captured during a 3-year period.
Results
We observed no major differences between those destined to fall (n = 55) and controls (n = 70) at baseline in clinical functional tests. There was a longitudinal decline in median daily walking speed over the 3 months before a fall in those destined to fall when compared with controls, p < .01 (ie, mean walking speed declined 0.1 cm s−1 per week). We also found prefall differences in sensor-based walking speed metrics in individuals who experienced a fall: walking speed variability was lower the month and the week just before the fall compared with 3 months before the fall, both p < .01.
Conclusions
While basic clinical tests were not able to differentiate who will prospectively fall, we found that significant variations in walking speed metrics before a fall were measurable. These results provide evidence of a potential sensor-based risk biomarker of prospective falls in community living older adults.
Keywords: Technology, Digital biomarkers, Pervasive computing
More than one-third of community-living adults over age 65 fall at least once a year (1–3); half of them experience multiple falls (1,2). Approximately 10% of falls result in a major injury such as a fracture (2) and falls are one of the principal causes of death and disability in older persons (1–6). We are able to identify people at particular risk of falls with several clinical tools available to stratify the baseline fall risks (7–13). However, these tools or functional tests are not precise or timely in their predictions, and are subject to significant intra-individual test–retest variability (9,10). It limits their usefulness for detecting subtle but meaningful changes in an individual’s risk over time. Therefore, clinicians may be able to answer the question “Will my patient fall?” (7) but do not know when the fall will occur.
Beyond the identification of high-risk individuals, it is important to realize that fall risk is a continually changing phenomenon. Thus, determining who within the at-risk population may be experiencing a modification to their baseline risk becomes a priority. Warning older adults and their relatives about an insidious but clinically significant worsening in fall risk could be of great value and essential for most effective and timely intervention to reduce the incidence of falls. To achieve this level of dynamic assessment, methods are needed that go beyond episodic sparsely spaced clinic visits. This may be achieved by deploying unobtrusive passive monitoring systems in people’s homes to provide assessment of daily activity in a continuously updated approach (14). Embedded sensors may allow for follow-up of key functional data in one’s own environment and track subtle changes over time (15). Collecting frequent measures also captures intra-individual variability in performance that could be the earliest indicator of an undesirable event such as a fall (14,15).
Walking speed has been consistently demonstrated to be one of the strongest physical parameters predicting adverse outcomes in older adults, such as life expectancy, cognitive and functional well-being, and falls (16–20). Due to its extensive predictive capabilities, it has been proposed to consider walking speed as the “6th Vital Sign” (19). The World Health Organization (WHO) also pointed out the importance of tracking physical activity such as the walking speed of older adults over time (21). Most factors associated with falls can impact walking parameters and although a fall is a sudden event, precipitating factors are likely to induce subtle changes days and weeks before the fall occurs (eg, impaired psychotropic drug elimination, sensory decline). We hypothesized that during the weeks preceding a fall, sensor-based, continuously monitored in-home walking speed would decline and that walking speed day-to-day variability would change.
Method
Objective
To determine whether unobtrusive long-term in-home assessment of walking speed and its variability can detect a future fall.
Study Design and Procedures
This study is a comparative analysis of existing data from two longitudinal cohort studies: the ORCATECH Living Laboratory study and the Intelligent Systems for Assessing Aging Change (ISAAC) study. The ORCATECH Living Laboratory study cohort was originally a pilot study for ISAAC. The study protocols were approved by the Institutional Review Board (Living Laboratory IRB# 2765; ISAAC IRB# 2353). Full details of study enrollment, assessment procedures and technical protocols have previously been described (15,22,23). The data reported here covers the period of continuous in-home sensor activity monitoring from January 2015 through August 2017 in these cohorts.
Participants
Participants were enrolled in one of the two studies: the ORCATECH Living Laboratory study or the ISAAC study. Inclusion criteria were being 60 years and older for the Living Laboratory study and 80 years and older for the ISAAC study, living independently without a formal caregiver, not demented with a Clinical Dementia Rating Score ≤ 0.5 (CDR) (24), a Mini-Mental State Examination score > 24 (MMSE) (25), and with well-controlled chronic diseases and comorbidities or none at all. Exclusion criteria were medical illnesses with the potential to limit physical participation (eg, wheelchair bound) or likely to lead to untimely death over 35 months (eg, late stage heart failure or metastatic cancers). Data collection is reported for the period from January 2015 through August 2017 which includes the subset of participants who were equipped with an upgraded (at the time) passive infrared motion sensor platform to yield uniform data.
Clinical Assessment Procedures
Standard clinical and cognitive measures were administered on a yearly basis. Motor tests included: Tinetti Gait and Balance Instrument (26); Unified Parkinson’s Disease Rating Scale, UPDRS (27); and timed 9-m walk at comfortable pace (28). Health status was documented via the modified Cumulative Illness Rating Scale, CIRS (29). Summary cognitive and behavioral status included the Mini-Mental State Examination, MMSE (25), and Geriatric Depression Scale, GDS (30). Participants respond to weekly scheduled health questionnaires (eg, health events) via their desktop computer, tablet, or smartphone. A question asked participants if they had fallen in the prior week. A fall was defined as “an unexpected event in which the person comes to rest on the ground, floor, or lower level” (31). Participants who did not complete a form for two consecutive weeks were contacted by a research assistant by phone and the falls information for that 2-week period was recorded.
Walking Speed and Day-to-Day Walking Speed Variability Assessment
Continuous activity data were collected using an unobtrusive sensor-based system in the home of each participant. Algorithms were developed to use sensor firings and their timestamps to generate walking speed metrics. The assessment of walking speed and its data validation process are described in detail elsewhere (32,33). Passive infra-red motion sensors (NYCE, NYCE, British Columbia) with a restricted field of view were installed along a hallway ceiling to detect only when a person passed directly under them (32). Data were wirelessly transferred to a research computer placed in the volunteer’s home, were time-stamped, stored in a structured database and then daily uploaded to a central database in the project data center. Walking events <20 or >160 cm s−1 were excluded as outliers (values greater than 2 SD from the mean of all walks). Days with overnight guests were reported by participants online and excluded as a part of a quality control protocol.
Statistical Analysis
Participants who reported a fall during the study period were compared with those who did not report a fall for baseline demographic and clinical characteristics, and baseline in-home sensor-based metrics. Previously published algorithms generated mean daily walking speeds for each participant (34,35). The grand mean of those daily walking speeds, mean number of walks per day and intra-individual variability in speed (standard deviation of mean daily walking speeds) were calculated for the period of interest. Subject characteristics and cognitive data were obtained from the annual clinical evaluation just before the study period. Chi-square test or Fisher’s exact test (for small cell sizes) were used to examine cross-sectional group differences in categorical variables. For each continuous variable, the histogram was visually inspected and a goodness-of-fit test was used to determine the normality of the distribution. Two-sample t-test or Wilcoxon rank-sum test (its nonparametric counterpart) were used to examine group differences in continuous variables. Longitudinal generalized mixed-effects regression models were used to explore trajectories of walking metrics in those destined to fall versus controls the 3 months before a fall or a random 3-month time period for those who didn’t experienced a fall. We also investigated prefall differences in sensor-based walking speed metrics in individuals that experienced a fall: mean daily walking speed, and day-to-day variability in walking speed between 3 months before the fall and the month and the week before the fall. The time frames were determined before the analysis, to reflect real-life health care pathways and to facilitate the future use of such digital biomarkers in health networks. The 3-month period was chosen to correspond to a realistic time that might separate two evaluations in clinical care. Analyses were performed using SAS software 9.4 (Cary, NC). Age and sex were included in all models due to their known association with risk of falls (36).
Results
Subject and Falls Characteristics
Daily walking speed as well as weekly self-reported falls surveys were captured during a 2.7 years period from January 2015 through August 2017 for 125 participants. During this time period 55 participants reported a fall and 70 participants did not. If multiple falls per person were reported the first was chosen for analyses. Baseline characteristics of those destined to fall and controls are presented in Table 1. Controls were more likely to be women than those destined to fall. No differences were seen between those destined to fall and controls in age, history of previous fall or clinical motor measures including stopwatch timed walking speed. There were no differences in sensor-based walking speed or speed variability by gender. Increasing age was negatively correlated with sensor-based walking speed (but not speed variability), r = −.29 and p = .001. Sensor-based walking speed was positively correlated with stopwatch walking speed, r = 0.34, p < .001.
Table 1.
Participant Characteristics at Baseline
| Subject Characteristics | Participants Destined to Fall (n = 55) |
Controls (n = 70) |
p Value |
|---|---|---|---|
| % or Mean (SD) | % or Mean (SD) | ||
| Age (y) | 83.1 (7.5) | 85.3 (8.1) | .09 |
| Gender (% women) | 69 | 86 | .03* |
| Education (y) | 15.8 (2.8) | 15.3 (2.4) | .35 |
| Nonwhite (%) | 15 | 9 | .29 |
| BMI (kg m−2) | 27.9 (4.7) | 26.9 (5.1) | .30 |
| CIRS | 21.1 (2.7) | 20.3 (2.2) | .15 |
| History of fall(s) in past year (%) | 38 | 46 | .35 |
| Clinical motor measures | |||
| UPDRS (motor section) | 0.9 (1.8) | 0.7 (1.2) | .77 |
| Tinetti gait | 2.0 (2.5) | 1.8 (2.3) | .63 |
| Tinetti balance | 5.2 (5.3) | 4.4 (5.1) | .39 |
| Stopwatch walking speed (cm s−1) | 70 (20) | 70 (20) | .34 |
| Sensor-based in-home mean walking speed (cm s−1) | 70 (20) | 64 (19) | .15 |
| Sensor-based in-home walking speed variability (cm s−1) | 11.0 (6.8) | 10 (5) | .16 |
| Mean number of walks per day (n) | 15 (11) | 16 (11) | .54 |
| Psychometric test scores | |||
| MMSE | 28.5 (1.7) | 28.8 (1.5) | .26 |
| MCI (CDR 0.5, %) | 5 | 9 | .73 |
| GDS | 1.0 (1.6) | 1.2 (2.1) | .94 |
Note: SD = standard deviation; BMI = body mass index; CIRS = Cumulative Illness Rating Scale; UPDRS = Unified Parkinson’s Disease Rating Scale; MMS = Mini-Mental State; MCI = mild cognitive impairment; CDR = Clinical Dementia Rating; GDS = Geriatric Depression Scale. CIRS measures level of impairment (0–4) in 14 body/organ systems, higher scores are worse; MMSE ranges from 0 to 30, higher score indicates better cognition; CDR range from 0 (normal cognition) to 3 (severe dementia); GDS ranges from 0 to 15, higher score is worse (more depressive symptoms); Tinetti Gait and Balance tally abnormalities, higher score is worse. Sensor-based baseline data are defined as the mean values for the month beginning 3 months before the fall.
*p < .05.
Mean Daily Walking Speed and Day-to-Day Variability in Walking Speed
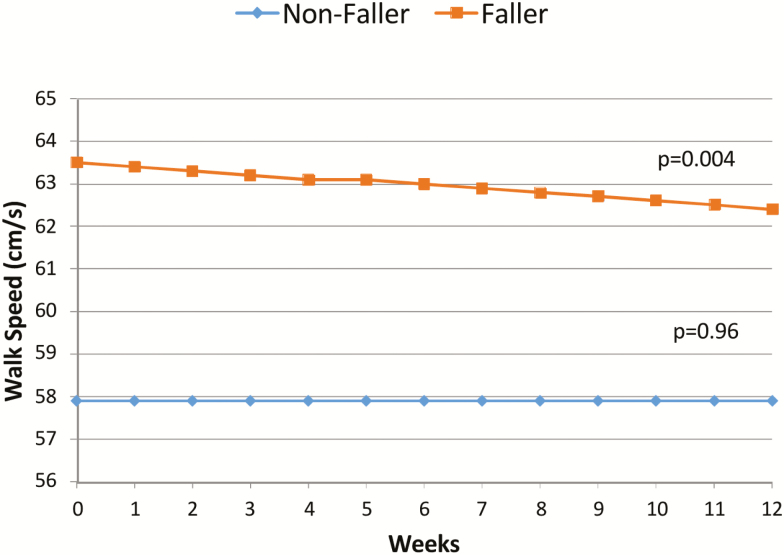
We compared longitudinal daily walking speed metrics for the 3-month time period before a fall (for the fallers, n = 55) or a 3-month control period (for the nonfallers, n = 70). We found a significant decline in mean daily walking speed over the 3 months before a fall in those destined to fall when compared with controls, p < .004. While controls’ mean walking speed did not change, subjects destined to fall presented a decline of 0.1 cm s−1 every week or 1 cm s−1 overall during the 3 months period before falling (Figure 1 and Table 2).
Figure 1.
Longitudinal decline in walking speed before a fall versus controls. The figure presents those destined to fall versus controls longitudinal walking speeds during the 3 months periods. As controls’ walking speed do not change over time, those who will fall experience a decline in walking speed of 0.1 cm s−1 every week. Calculated regression lines are from the mixed-effect model results.
Table 2.
Longitudinal Mixed-Effects Model of Daily Mean Walking Speed Among Those Destined to Fall Versus Controls More Than 3 Months
| Variable | Estimate | Standard Error | Pr > |t| |
|---|---|---|---|
| Time, days | 0.000 | 0.003 | 0.959 |
| Faller vs controls | 5.593 | 3.581 | 0.118 |
| Faller × time | −0.013 | 0.004 | 0.004* |
| Age, years | −0.524 | 0.222 | 0.018* |
| Female vs male | 3.734 | 4.275 | 0.382 |
*p < .05.
We then investigated if there were within-person differences in sensor-based walking speed metrics over time in individuals that experienced a fall: mean daily walking speed, and day-to-day variability in walking speed between a “baseline” period 3 months before their fall, and the month and week just before their fall. We found no significant within-person changes in mean walking speeds, but there was a difference in day-to-day variability such that the walking speed variability at “baseline” was higher than the month and week just before their fall, p < .01 (Table 3). There were no within-person differences in mean number of walks per day between the time periods.
Table 3.
Mean Walking Speed and Day-to-Day Speed Variability Prefall Differences Among Participants Destined to Fall
| In-Home Sensor-Based Measures | 3 Mo Before Fall | 1 Mo Before Fall | p Value | 1 Wk Before fall | p Value |
|---|---|---|---|---|---|
| Mean daily walking speed (cm s−1), mean (SD) | 69.9 (20.2) | 68.5 (20.5) | .12 | 67.7 (22.8) | .37 |
| Day-to-day variability in walking speed, mean (SD) | 11.0 (6.8) | 9.4 (4.8) | <.01* | 8.0 (3.8) | <.01* |
| Mean number of walks per day | 15.5 (11.3) | 15.1 (10.9) | .66 | 15.2 (11.2) | .86 |
* p < .01.
Discussion
In an independently living, largely octogenarian cohort, we identified 55 participants with a fall during the follow-up period. Contrasting with existing literature, there were no significant differences in baseline clinical measures between participants who had a fall and those who did not. Several explanations can be proposed. First, the analysis could be under-powered for these clinical outcomes, performed at discrete points of time. However, the frequent sensor-based measure may be more sensitive; as previously discussed by Dodge and colleagues (37,38), high frequency sensor-based measures could increase sensitivity and decrease sample size needed. These measures were not sensitive to identifying those destined to fall during the overall 2.7 year study period. On the other hand, the continuous in-home sensor-based walking speeds showed a significant decline in median daily walking speed—0.1 cm s−1 every week—over the 3 months before a fall in those destined to fall when compared with those who are not. We also found that in those who did experience a fall the walking speed variability 3 months before a fall was significantly higher than the month just before the fall. These results provide evidence of potential sensor-based fall risk biomarkers (39) of a fall to come in community living older individuals. Only a sensor system, reporting frequently or continuously is able to detect this subtle but significant decline over time. Using unobtrusive continuous gait monitoring may provide a new approach to provide targeted and timely personalized falls evaluation and intervention. From a clinical perspective, long-term longitudinal monitoring could potentially answer the question: among an “at risk” population, which patient should I focus on within an allowed time window?
Despite the difficulties of achieving gait parameters follow-up over time and the potential of technologies to overcome this issue, clinical research in this domain is scarce (40). Stone and colleagues followed 16 older adults over 11 months (41). In-home Kinect-based walking speed provided little intra-individual test–retest variability when compared with monthly traditional assessment; the relationship of this sensor data to fall risk was not assessed. Kearns and colleagues used an ultra-wideband tele-surveillance system to monitor 69 resident’s traveling within the common areas of an assisted living facility over a 1-year period (42). The variability of everyday movements was an independent predictor of a fall. This solution required participants to wear a transponder and authors reported some acceptability issues with the worn technology. Alternatively, some studies have explored monitoring activities with accelerometer-based wearable sensors (40,43–47). Among these studies few used experimental clinical research designs (40), sample sizes were small (including up to 20 participants) and of limited monitoring periods (eg, using short treadmill walks (44), or with monitoring time ranging from a few minutes (43), to hours (45), or days (46,47). Most of these initiatives focused on in depth posture and activities detection rather than walking speed. Finally, a major concern of wearable sensors is their lack of acceptability by older adults. Current technologies require frequent charging and users must remember to wear a device each day.
Beyond traditional fall-risk assessment scores, gait parameter (eg, stride length, step width) variability has demonstrated good fall risk prediction performances using computerized walkway testing set-ups (48). Increased variability in walking function could reflect automaticity regulation impairment leading to falls. Nevertheless, Brach and colleagues found a nonlinear association between step width variability and fall history among community-dwelling older persons. Individuals with either low or high step width variability were more likely to have fallen in the past year (49). Our study focused on day-to-day variability of walking speed rather than step-to-step fluctuations in gait characteristics, but a similar nonlinear evolution could explain our results. More variability could reflect an unstructured activity in early stages of walking impairment; less variability could be a protective routine in more advanced stages. Using ISAAC study data (14), we had previously highlighted the nonlinear trajectory of walking speed variability over time: early MCI presented high baseline walking speed variability, whereas late MCI showed lowest baseline and declining variability. Our analytic sample had a high-fall risk, gait and balance abnormalities (mean age 84 years, low Tinetti scores), and a mean walking speed of 70 ± 20 cm s−1. In comparison, it is slower than that of a large sample of frail older adults evaluated in a Geriatric Day Hospital of Frailty (80 ± 30 cm s−1, n = 1, 108) (50). It is possible that our participant’s increased baseline variability in walking speed may reflect an attempt to compensate for gait impairment. High variability between repeated walking trials has been discussed as a specific sign of frailty onset that increases from the prefrail status (51). Kaye and colleagues previously evaluated in-home measures in 76 older individuals monitored during a 4-week period using the same cohort (32). They found that those using a walking aid walked with greater variability and that a higher UPDRS score, lower Tinetti gait and balance scores and slower walking speed were strongly associated with increased variability in walking speeds. The clear reduction in variability before the fall could then be the marker of functional reserve failure to preserve walking function.
This study had some limitations. We only included participants living alone and our home sensor platform only captures walks occurring inside the home. Nevertheless, this population spends on average 20.5 hours a day in their homes and in-home gait data could be informative about a fall to come wherever it happens, inside or outside (15). However, an advantage of outdoor walking speed analysis is that it may provide data relevant to longer distances and challenging terrain (40). The cohort studied is composed of healthy adults without dementia, living independently. They are well-educated, largely white (88%) and replied regularly online using computer-based forms indicating computer literacy. Thus, this cohort may not be generalizable to other populations. However, although these relatively “early acceptors” of technology may not be representative of the wider current older adult population, it can be anticipated that these findings will be more applicable to the older adult population in the coming decade.
We had previously demonstrated the feasibility of sensor-based long-term walking speed monitoring in a real life setting (32–35). However, the deployment of such solutions in nonresearch contexts, and thus application across large-scale health care systems, is not without challenges. Translation of raw walking speed signals into clinically significant health indicators is dependent on the performance of the sensors, as well as the algorithms used. The algorithm applied in our research is dependent on the type of sensors used and the system architecture (eg, the walking data were derived from a specific set and arrangement of PIR sensors). Thus, solutions using different sensors will have to go through research validation phases both at the sensor level, as well as at the larger clinical outcomes level to show both efficacy as well as population-wide effectiveness of the application. Harmonization of sensor data between studies and larger scale deployment in various populations remains an important issue (52).
Despite these limitations, our study had several strengths including the accurate and high frequency collection of falls incident events over a long period of time (minimizing recall bias) and the ecologically valid, real-world walking parameters provided by unobtrusive sensor-based measurements at home. We used standardized, validated measurements of motor function and definition of falls (53).
Embedded in-home sensors provide previously unattainable metrics. In this case, while basic clinical tests were not able to differentiate who will prospectively fall, we found that a significant decline in mean daily walking speed over the weeks before a fall was measurable and sensitive to indicating subsequent falls. We also found that walking speed variability could also be a sensitive and relevant indicator. Our study is the first providing evidence of a susceptibility/risk “digital biomarker” of a fall to come in community living older individuals and to provide threshold values for the fall events. This work opens up perspectives for real-time measurement using low cost and scalable PIRs sensors solutions. Older adults at high risk of falling, their family and health stakeholders, could find high value in a timely solution to predict a fall before it happens. We plan to explore the possibility of prospective real-time gait monitoring to potentially set up prompt intervention before a fall.
Funding
This work was supported by the following National Institutes of Health (R01AG024059, U2CAG054397, P30AG024978, and P30AG008017).
Author Contributions
J.K., N.M., A.P., Z.B. have involved in study concept and design. Z.B., N.M., and A.P. have involved in the acquisition of data. A.P. and N.M. have involved in the analysis and interpretation of data. A.P., N.M., J.K., and R.C. involved in the drafting of the article. H.D. and J.K. have involved in the critical revision of the article for important intellectual content.
Conflict of Interest
None reported.
References
- 1. Milat AJ, Watson WL, Monger C, et al. Prevalence, circumstances and consequences of falls among community-dwelling older people: results of the 2009 NSW Falls Prevention Baseline Survey. N S W Public Health Bull. 2011;22:43–48. doi:10.1071/NB10065 [DOI] [PubMed] [Google Scholar]
- 2. Tinetti ME, Kumar C. The patient who falls: “it’s always a trade-off”. JAMA. 2010;303:258–266. doi:10.1001/jama.2009.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen TY, Chan A, Andersen-Ranberg K, et al. Prevalence and correlates of falls among centenarians: the results from the Five Country Oldest Old Project (5-COOP). J Gerontol A Biol Sci Med Sci. 2019. doi:10.1093/gerona/glz116 [DOI] [PubMed] [Google Scholar]
- 4. Kannus P, Sievänen H, Palvanen M, Järvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366:1885–1893. doi:10.1016/S0140-6736(05)67604-0 [DOI] [PubMed] [Google Scholar]
- 5. Lohman MC, Sonnega AJ, Nicklett EJ, Estenson L, Leggett AN. Comparing estimates of fall-related mortality incidence among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2018. doi:10.1093/gerona/gly250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Auais M, French S, Alvarado B, Pirkle C, Belanger E, Guralnik J. Fear of falling predicts incidence of functional disability 2 years later: a perspective from an international cohort study. J Gerontol A Biol Sci Med Sci. 2018;73:1212–1215. doi:10.1093/gerona/glx237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297:77–86. doi:10.1001/jama.297.1.77 [DOI] [PubMed] [Google Scholar]
- 8. Welmer AK, Rizzuto D, Laukka EJ, Johnell K, Fratiglioni L. Cognitive and physical function in relation to the risk of injurious falls in older adults: a population-based study. J Gerontol A Biol Sci Med Sci. 2017;72:669–675. doi:10.1093/gerona/glw141 [DOI] [PubMed] [Google Scholar]
- 9. Rockwood K, Awalt E, Carver D, MacKnight C. Feasibility and measurement properties of the functional reach and the timed up and go tests in the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci. 2000;55:M70–M73. doi:10.1093/gerona/55.2.m70 [DOI] [PubMed] [Google Scholar]
- 10. Klenk J, Becker C, Palumbo P, et al. Conceptualizing a dynamic fall risk model including intrinsic risks and exposures. J Am Med Dir Assoc. 2017;18:921–927. doi:10.1016/j.jamda.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 11. Ek S, Rizzuto D, Fratiglioni L, Johnell K, Xu W, Welmer AK. Risk profiles for injurious falls in people over 60: a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2018;73:233–239. doi:10.1093/gerona/glx115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ward RE, Quach L, Welch SA, Leveille SG, Leritz E, Bean JF. Interrelated neuromuscular and clinical risk factors that contribute to falls. J Gerontol A Biol Sci Med Sci. 2019. doi:10.1093/gerona/glz030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahoney JR, Cotton K, Verghese J. Multisensory integration predicts balance and falls in older adults. J Gerontol A Biol Sci Med Sci. 2018. doi:10.1093/gerona/gly245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dodge HH, Mattek NC, Austin D, Hayes TL, Kaye JA. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology. 2012;78:1946–1952. doi:10.1212/WNL.0b013e318259e1de [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaye JA, Maxwell SA, Mattek N, et al. Intelligent systems for assessing aging changes: home-based, unobtrusive, and continuous assessment of aging. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i180–i190. doi:10.1093/geronb/gbq095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menant JC, Schoene D, Sarofim M, Lord SR. Single and dual task tests of gait speed are equivalent in the prediction of falls in older people: a systematic review and meta-analysis. Ageing Res Rev. 2014;16:83–104. doi:10.1016/j.arr.2014.06.001 [DOI] [PubMed] [Google Scholar]
- 17. Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol A Biol Sci Med Sci. 2013;68:39–46. doi:10.1093/gerona/gls174 [DOI] [PubMed] [Google Scholar]
- 18. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32:46–49. doi:10.1519/00139143-200932020-00002 [PubMed] [Google Scholar]
- 20. Quach L, Galica AM, Jones RN, et al. The nonlinear relationship between gait speed and falls: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:1069–1073. doi:10.1111/j.1532-5415.2011.03408.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beard JR, Officer A, de Carvalho IA, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. doi:10.1016/S0140-6736(15)00516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaye J, Reynolds C, Bowman M, et al. Methodology for establishing a community-wide life laboratory for capturing unobtrusive and continuous remote activity and health data. J Vis Exp. 2018;137:e56942. doi:10.3791/56942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piau A, Mattek N, Duncan C, Sharma N, Riley T, Kaye J. The five W’s of falls—weekly online health survey of community-dwelling older adults: analysis of four years prospective follow-up. J Gerontol A Biol Sci Med Sci. 2019. doi:10.1093/gerona/glz114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris JC, Edland S, Clark C, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer’s disease. Neurology. 1993;43:2457–2465. doi:10.1212/wnl.43.12.2457 [DOI] [PubMed] [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 26. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34:119–126. doi:10.1111/j.1532–5415.1986.tb05480.x [DOI] [PubMed] [Google Scholar]
- 27. Fahn S, Elton RL; UPDRS program members Unified Parkinsons Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent developments in Parkinsons disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163. [Google Scholar]
- 28. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi:10.1111/j.1532–5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 29. Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995;43:130–137. doi:10.1111/j.1532-5415.1995.tb06377.x [DOI] [PubMed] [Google Scholar]
- 30. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17:37–49. doi:10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 31. Lamb SE, Jørstad-Stein EC, Hauer K, Becker C; Prevention of Falls Network Europe and Outcomes Consensus Group Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. doi:10.1111/j.1532-5415.2005.53455.x [DOI] [PubMed] [Google Scholar]
- 32. Kaye J, Mattek N, Dodge H, et al. One walk a year to 1000 within a year: continuous in-home unobtrusive gait assessment of older adults. Gait Posture. 2012;35:197–202. doi:10.1016/j.gaitpost.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayes TL, Abendroth F, Adami A, Pavel M, Zitzelberger TA, Kaye JA. Unobtrusive assessment of activity patterns associated with mild cognitive impairment. Alzheimers Dement. 2008;4:395–405. doi:10.1016/j.jalz.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayes TL, Hagler S, Austin D, Kaye J, Pavel M. Unobtrusive assessment of walking speed in the home using inexpensive PIR sensors. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:7248–7251. doi:10.1109/IEMBS.2009.5334746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hagler S, Austin D, Hayes TL, Kaye J, Pavel M. Unobtrusive and ubiquitous in-home monitoring: a methodology for continuous assessment of gait velocity in elders. IEEE Trans Biomed Eng. 2010;57:813–820. doi:10.1109/TBME.2009.2036732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143:1129–1136. doi:10.1093/oxfordjournals.aje.a008690 [DOI] [PubMed] [Google Scholar]
- 37. Dodge H, Kaye J, Zhu J, Kornfield J, Mattek N. Use of intra-individual distributions of daily acquired home-based measures increases RCT sensitivity. Alzheimers Dement. 2014;10(suppl):P204. doi:10.1016/j.jalz.2014.04.255 [Google Scholar]
- 38. Dodge HH, Zhu J, Mattek NC, Austin D, Kornfeld J, Kaye JA. Use of high-frequency in-home monitoring data may reduce sample sizes needed in clinical trials. PLoS One. 2015;10:e0138095. doi:10.1371/journal.pone.0138095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. FDA-NIH; Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource. Silver Spring, MD:Food and Drug Administration, 2016. [PubMed] [Google Scholar]
- 40. Schwenk M, Howe C, Saleh A, et al. Frailty and technology: a systematic review of gait analysis in those with frailty. Gerontology. 2014;60:79–89. doi:10.1159/000354211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stone E, Skubic M, Rantz M, Abbott C, Miller S. Average in-home gait speed: investigation of a new metric for mobility and fall risk assessment of elders. Gait Posture. 2015;41:57–62. doi:10.1016/j.gaitpost.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 42. Kearns WD, Fozard JL, Becker M, et al. Path tortuosity in everyday movements of elderly persons increases fall prediction beyond knowledge of fall history, medication use, and standardized gait and balance assessments. J Am Med Dir Assoc. 2012;13:665.e7–665.e13. doi:10.1016/j.jamda.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 43. Taylor LM, Klenk J, Maney AJ, Kerse N, Macdonald BM, Maddison R. Validation of a body-worn accelerometer to measure activity patterns in octogenarians. Arch Phys Med Rehabil. 2014;95:930–934. doi:10.1016/j.apmr.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 44. Huijben B, van Schooten KS, van Dieën JH, Pijnappels M. The effect of walking speed on quality of gait in older adults. Gait Posture. 2018;65:112–116. doi:10.1016/j.gaitpost.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 45. Hollewand AM, Spijkerman AG, Bilo HJ, Kleefstra N, Kamsma Y, van Hateren KJ. Validity of an accelerometer-based activity monitor system for measuring physical activity in frail older adults. J Aging Phys Act. 2016;24:555–558. doi:10.1123/japa.2014-0290 [DOI] [PubMed] [Google Scholar]
- 46. Culhane KM, Lyons GM, Hilton D, Grace PA, Lyons D. Long-term mobility monitoring of older adults using accelerometers in a clinical environment. Clin Rehabil. 2004;18:335–343. doi:10.1191/0269215504cr734oa [DOI] [PubMed] [Google Scholar]
- 47. Del Din S, Galna B, Godfrey A, et al. Analysis of free-living gait in older adults with and without Parkinson’s disease and with and without a history of falls: identifying generic and disease-specific characteristics. J Gerontol A Biol Sci Med Sci. 2019;74:500–506. doi:10.1093/gerona/glx254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Callisaya ML, Blizzard L, Schmidt MD, et al. Gait, gait variability and the risk of multiple incident falls in older people: a population-based study. Age Ageing. 2011;40:481–487. doi:10.1093/ageing/afr055 [DOI] [PubMed] [Google Scholar]
- 49. Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2:21. doi:10.1186/1743-0003-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tavassoli N, Guyonnet S, Abellan Van Kan G, et al. ; Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability Team Description of 1,108 older patients referred by their physician to the “Geriatric Frailty Clinic (G.F.C) for Assessment of Frailty and Prevention of Disability” at the gerontopole. J Nutr Health Aging. 2014;18:457–464. doi:10.1007/s12603-014-0462-z [DOI] [PubMed] [Google Scholar]
- 51. Montero-Odasso M, Muir SW, Hall M, et al. Gait variability is associated with frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:568–576. doi:10.1093/gerona/glr007 [DOI] [PubMed] [Google Scholar]
- 52. Skubic M, Jimison H, Keller J, Popescu M, Rantz M, Kaye J, Pavel M. A framework for harmonizing sensor data to support embedded health assessment. Presented at 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Aug 26 2014 (pp. 1747–1751). IEEE. doi:10.1109/EMBC.2014.6943946 [DOI] [PubMed] [Google Scholar]
- 53. Hauer K, Lamb SE, Jorstad EC, Todd C, Becker C; PROFANE-Group Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age Ageing. 2006;35:5–10. doi:10.1093/ageing/afi218 [DOI] [PubMed] [Google Scholar]