Abstract
Background
Older adults deemed to be at a high risk of falling will often display visual search behaviors likely to impair movement planning when negotiating environmental hazards. It has been proposed that these behaviors may be underpinned by fall-related anxiety. Thus, the aim of this study was to explore the effects of fall-related anxiety on visual search and stepping behaviors during adaptive gait.
Methods
Forty-four community-dwelling older adults (mean age = 74.61; standard deviation = 6.83) walked along a path and stepped into two raised targets. All participants completed walks at ground level, whereas participants deemed to be at a low risk of falling (n = 24) also completed walks under conditions designed to induce fall-related anxiety (walkway elevated 0.6 m). Participants’ movement kinematics and gaze behavior were measured.
Results
During ground trials, “high-risk” participants visually prioritized the immediate walkway areas 1–2 steps ahead, at the expense of previewing future stepping constraints. This reduced planning appeared to negatively affect safety, with greater stepping errors observed for future constraints. When completing walks on the elevated walkway, “low-risk” participants similarly prioritized immediate walkway areas, at the expense of planning future stepping actions. These behaviors were associated with greater attention directed toward consciously processing walking movements.
Conclusions
These findings provide evidence of a link between heightened fall-related anxiety and “high-risk” visual search behaviors associated with greater stepping errors. This information enhances our understanding of why high-risk older adults are less able to safely navigate environmental constraints.
Keywords: Fear of falling, Eye tracking, Gait, Attention, Falls
Falls in older adults most commonly occur during walking (1,2), with trips, slips, and misplaced steps accounting for the majority of incidences (1). Safely navigating complex environments requires a walker to sample visual information in a manner that allows for planning future actions (3,4). For example, a task as simple as walking through a residential common area will present various constraints that need to be navigated, such as stepping around a chair leg or avoiding an oncoming walker. During such situations, it is imperative that visual information is sampled in a “feedforward” manner, allowing sufficient time for the walker to make proactive adjustments to safely avoid the environmental constraint (3). Despite the importance of feedforward planning, older adults deemed to be at a high risk of falling often display visual search behaviors likely to reduce their ability to perceive, and subsequently plan the adaptations needed to negotiate, environmental constraints (4,5). For example, Young and colleagues (4) found that, when approaching a series of stepping constraints, low-risk older adults displayed proactive patterns of visual exploration; fixating, and transferring their gaze between, subsequent stepping constraints. In contrast, their high-risk counterparts directed their gaze predominantly toward the proximal constraint and displayed reduced visual previewing of subsequent obstacles. During complex tasks requiring the navigation of multiple environmental hazards, such reduced previewing/planning will likely impair the walker’s ability to navigate future constraints (6). As such, there is a clear need to identify the underlying factors contributing to these altered patterns of visual search.
Researchers have proposed increased fall-related anxiety, or fear of falling, as one potential mechanism underpinning these “high-risk” behaviors (6). For example, as the high-risk older adults studied by Young and colleagues (4) also reported significantly greater levels of state anxiety compared to their low-risk counterparts, the authors attributed the observed reductions in visual previewing to heightened fear of falling. Although these findings highlight an association between fear of falling and altered gaze behavior when walking, they fail to establish a causal link; a process that necessitates both the direct experimental manipulation of fall-related anxiety and the measurement of visual search.
An extensive body of research has, however, described the dramatic changes in walking behaviors adopted by older adults during conditions of experimentally induced fear of falling. For example, when walking along a walkway elevated 0.6 m above the laboratory floor, older adults will adopt more cautious gait, displaying slower gait speed, shorter steps, and spending longer in double-limb support (7). Although this body of work demonstrates that older adults fearful of falling will alter how they walk on a flat path, it remains unknown how experimentally induced fall-related anxiety will influence older adults’ visuomotor control of locomotion during more complex, adaptive gait tasks.
Therefore, this study aimed to evaluate a possible causal link between fall-related anxiety and altered patterns of visual search in older adults when navigating a series of stepping constraints. Specifically, we explored whether the patterns of visual search behaviors previously observed in high-risk older adults (4,5,8) could be induced in a group of low-risk older adults during conditions designed to elicit fall-related anxiety.
Methods
Participants
Forty-four community-dwelling older adults (aged > 60; female/male: 30/14; mean ± SD age: 74.61 ± 6.83) were recruited from local community groups. Previous related research has reported effect sizes between 0.57 and 1.38 for key, comparable variables (9). Consequently, a power analysis determined that 19 participants per-group (low- and high-risk) would be required to obtain 80% power. Participants were deemed to be at a high risk of falling if they had experienced two or more falls within the past 12 months (N = 10), or if they presented two or more of the following risk factors (N = 10): one fall within the past 12 months; slow walking speed, determined by a Timed up and Go (TuG) score more than 12 seconds (10), or; low strength (11–13), determined by dominant handgrip strength less than 22 kgf for females and 32 kgf for males (12,14). Handgrip strength was used to provide further sensitivity to the classification algorithm; however, every individual classified as “high-risk” had either recently fallen and/or exhibited slow gait, independent of low grip strength. Consequently, 24 participants were classified as “low-risk” and 20 participants were classified as “high-risk.” Before participation, participants also completed the Falls-Efficacy Scale International (FES-I (15)). Demographic information for each group is reported in Table 1.
Table 1.
Participant Characteristics
| Measure: mean (± SD) | Low-risk group (n = 24) | High-risk group (n = 20) |
|---|---|---|
| Age** | 72.04 (± 5.74) | 77.70 (± 6.88) |
| Gender (males) | 9 (37.50%) | 5 (25.00%) |
| Number of fallers (past 12 months) | 1/24 | 12/20 |
| TU&G (seconds)*** | 9.33 (± 1.29) | 13.22 (± 2.88) |
| Grip strength (kgf)** | 29.20 (± 10.75) | 20.76 (± 4.29) |
| MiniCog | 4.25 (± 0.79) | 4.25 (± 0.79) |
| FES-I*** | 19.83 (± 3.10) | 26.25 (± 5.32) |
**p < .01; ***p < .001.
Participants were free from any neurological, cardiovascular, or musculoskeletal impairment that prohibited them from walking 10 m without a walking aid. Participants were excluded if they demonstrated major cognitive impairment (MiniCog score of < 3 (16)), or if they were currently prescribed anxiety or dizziness medication. All participants were free from significant deficits in either visual acuity (20/40 vision or better) or contrast sensitivity. Individuals who required the use of glasses during daily locomotion were screened for compatibility with the eye-tracking equipment and invited to participate if it was possible to calibrate the eye-tracker over their glasses. Five participants (three low-risk and two high-risk) wore glasses during testing. Of these, one high-risk and one low-risk wore bifocal glasses, while the remainder wore single lens glasses for distant vision. Participants requiring the use of glasses during daily locomotion completed tests of visual acuity and contrast sensitivity and while wearing their glasses. Institutional ethical approval was obtained from the local ethics committee and the research was carried out in accordance with the principles laid down by the Declaration of Helsinki. All participants provided written informed consent before participation.
Protocol
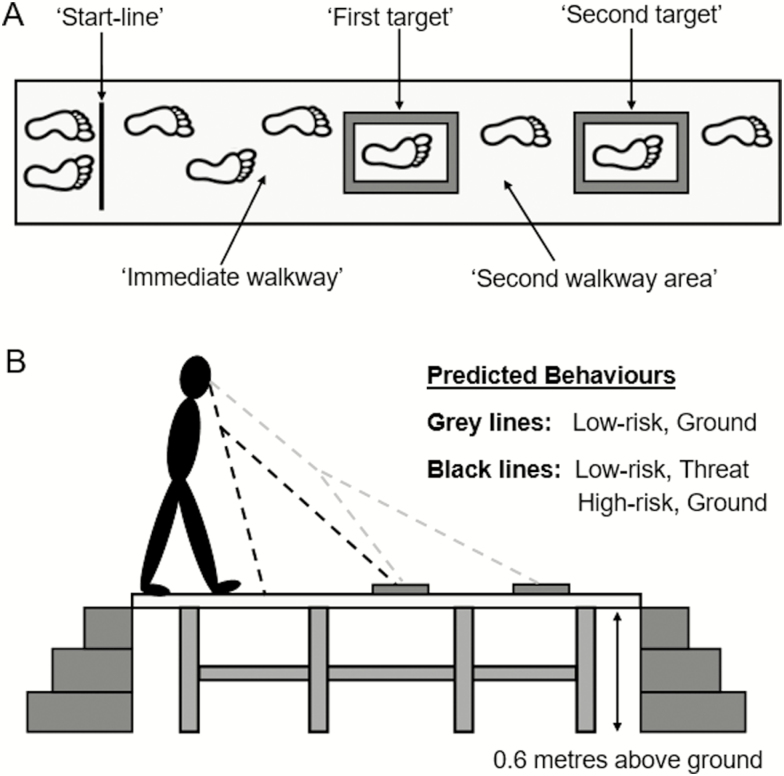
On arrival, participants were fitted with reflective markers placed on the heel, mid-foot, and toe of both feet, and then with a Mobile Eye-XG portable eye-tracking system (ASL, Bedford, MA). As per Ellmers and Young (9), the experimental task involved walking at a comfortable, self-determined pace along a wooden walkway (width of 40 cm and length of 3.4 m) and stepping into two foam rectangular targets (Figure 1A). Targets could appear in two possible locations (midpoint of first target: either 1.5 m or 1.4 m from the walkway start-line; midpoint of second target: either 2.5 m or 2.4 m from the start-line). Target locations were rearranged after every third trial to reduce familiarization. The foam targets had raised borders to impose a degree of postural threat (foam border width and height = 4 cm), and the inside target area was 19 cm × 41.5 cm (width and length, respectively; see Figure 1A). Participants were instructed to step into the targets as centrally as possible, with whichever foot they wished. Before the start of each trial, participants stood at a “start-line” with their eyes closed to prevent them from visually previewing the walkway. Following an auditory “go” tone, participants opened their eyes and commenced walking. Before data collection, participants completed three familiarization trials.
Figure 1.
(A) Schematic diagram of the walkway and precision stepping task. The foam targets had a border width and height of 4 cm (ie, the foam border was 4 cm wide and raised 4 cm from the walkway). The arrows denote the different areas of interest for which the walkway was separated into for the gaze analysis. (B) Schematic diagram of the raised walkway during threat. The black dashed lines represent the “restricted” visual previewing/planning predicted in both the low-risk participants during threat and the high-risk participants during ground trials. In contrast, the gray dashed lines represent the “proactive” visual search predicted in low-risk participants during ground trials.
Low-risk participants completed the protocol under two conditions: (i) ground (walkway at ground level), and (ii) threat (walkway elevated 0.6 m above the laboratory floor; see Figure 1B). Participants completed one 5-trial block of walks for each condition, and the presentation order of these conditions was counterbalanced across participants. In contrast, high-risk participants completed a single block of five walks at ground. All trials were completed in the absence of a safety harness. To enhance safety, two experimenters were present at all times, and participants were reminded of their right to withdraw from the study.
State Psychological Measures
To assess concern about falling, participants reported state levels of both balance confidence and fear of falling (17). Before each block, participants rated their confidence to maintain balance and avoid a fall. Scores ranged from 0% (not at all confident) to 100% (completely confident). After each block, participants rated their fear of falling (averaged across the previous five trials) on a scale ranging from 0% (not at all fearful) to 100% (completely fearful). After each block participants also rated, on an 11-point Likert scale (1 = never, 11 = always), the degree to which they were thinking about, or paying attention to: Movement processes; Threats to balance; Worries or disturbing thoughts; Self-regulatory strategies; and Task-irrelevant information. These categories, and the descriptions provided to participants, were based on previous research (9). The descriptions provided to participants for each category can be found in Supplementary Table 1. To explore the impact of fall-related anxiety on processing efficiency (the amount of mental effort required to maintain effective task performance), participants also completed the Rating Scale of Mental Effort (18). This involved participants rating the level of mental effort required to complete the previous five trials, on a single continuum scale ranging from 0 to 150.
Motor Performance
The following motor performance variables were calculated: (i) time to complete the walking trial(s); (ii) stance duration preceding the first and second target; and (iii) stepping error (mm) in both anterior–posterior (AP) and mediolateral (ML) directions for the first and second target. Kinematic data were collected at 100 Hz using a Vicon motion capture system (Oxford Metrics, England) and passed through a low-pass Butterworth filter with a cutoff frequency of 5 Hz (4,9). Time to complete the walking trial was calculated as the time between the “go” tone and heel contact of the final step on the walkway. “Stance durations” were defined as the duration between heel contact and toe-off of the foot performing the target step. Stepping error was calculated by subtracting the coordinate of the mid-foot marker from the coordinate of the center of the target, in AP and ML directions, respectively (4,9). Data were analyzed using custom algorithms in MATLAB, version 7.11 (MathWorks, Natick, MA). Kinematic data were assigned a randomized code to allow for blinded analysis, and variables were averaged across conditions. Because of technical limitations, one high-risk participant was excluded from kinematic data analyses.
Gaze Behavior
Visual fixations were defined as a gaze that endured on a single location for 100 ms or longer (19). Fixation locations were classified as one of the four areas of interest (Figure 1A): (i) immediate walkway (the walkway area before the first target); (ii) the first target; (iii) second walkway area (the walkway between the first and second target); and (iv) the second target. These areas of interest were used to determine the duration spent fixating each location during the approach to the first target (until heel contact into this target). Fixation duration data were normalized to individual trial length by presenting data as the percentage of time spent fixating each area of interest. As a further measure of visual previewing, the number of fixations made toward the second target (until heel contact into the first target) were also calculated. The location of the first fixation was also assessed. To determine this variable, each area of interest was allocated a number from 1 to 4 (immediate walkway = 1; first target = 2; second walkway area = 3; second target = 4), with a lower number indicating that the first fixation occurred toward more proximal walkway areas. Finally, the number of gaze transfers between the four areas of interest per-second (before heel contact in the first target) were also calculated to indicate the extent of visual exploration.
Variables were averaged across each condition. Trials where the point-of-gaze crosshair disappeared for the duration of four frames or more were discarded (9). Participants with a trial-discard rate higher than 40% were excluded from all eye-tracking analyses. This procedure resulted in one high-risk participant’s data being excluded. A total of 81 trials were analyzed for high-risk participants at ground (M = 4.50 trials per participant), whereas for low-risk participants, 110 trials were analyzed at ground (M = 4.35 trials per participant) and 108 analyzed for threat (M = 4.55 trials per participant). Although attempts were made to blind the assessor to experimental conditions, this was not possible given between-condition differences in the environmental scene.
Statistical Analysis
Between-group ground comparisons
Separate independent samples t-tests were used to compare high- and low-risk participants for all aforementioned variables during ground trials. Where data were nonnormally distributed, separate Mann–Whitney U-tests were used instead. For all statistical comparisons, effect sizes are reported as Cohen’s d; unless the assumption of normality is violated, whereby effect sizes are reported as r = Z/√N. Separate partial correlations (controlling for age, TuG, and MiniCog scores) were used to compare the relationships between any state-psychological measures for which a statistically significant between-group difference was observed, and all statistically significant (between-group difference) motor performance and gaze behavior variables. Where data were nonnormally distributed, analyses were conducted using Spearman’s correlation.
Within-subject (low-risk) ground–threat comparisons
Separate paired-samples t-tests were used to explore within-subject ground–threat changes in all variables. Where data were non-normally distributed, separate Wilcoxon tests were used instead. One low-risk participant did not wish to complete threat trials, resulting in within-subject analyses being conducted on the remaining 23 low-risk participants.
Results
Please see Supplementary Table 2 for mean values (and standard error of the mean) for all assessed variables.
Between-Group Ground Comparisons
State psychological measures
During the walking task, high-risk participants reported significantly lower balance confidence (U = 114.50, p = .001, r = .46), significantly higher fear of falling (U = 76.50, p < .001, r = .64), and significantly greater mental effort (U = 86.50, p < .001, r = .56), compared with low-risk participants. High-risk participants also reported directing significantly greater attention toward movement processes (U = 63.50, p < .001, r = .64), threats to balance (U = 85.00, p < .001, r = .58), worries or disturbing thoughts (U = 131.00, p < .001, r = .50), and self-regulatory strategies (U = 151.50, p = .011, r = .35). There was a lack of significant between-group difference in attention directed toward task-irrelevant thoughts (U = 216.00, p = .17, r = .14).
Motor performance measures
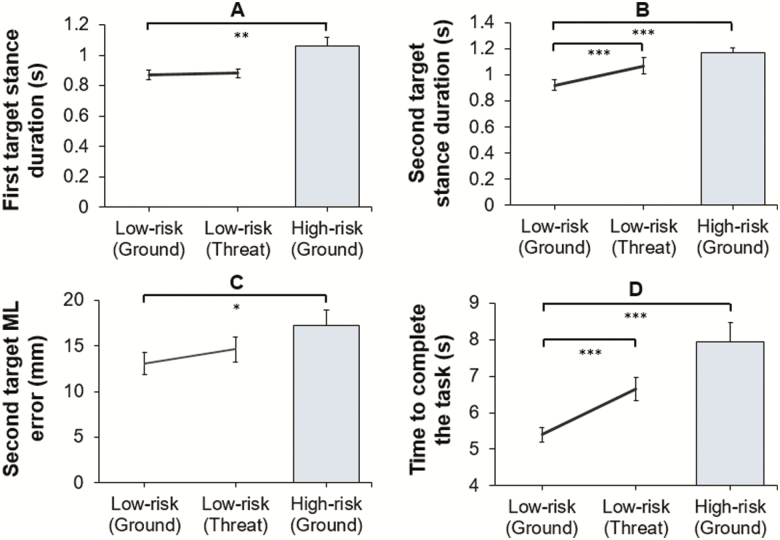
Compared with low-risk participants, high-risk participants took significantly longer to complete the walking task (t(41) = –4.92, p < .001, d = 1.44). They also exhibited significantly longer stance durations preceding both the first (U = 108.00, p = .003, r = .45) and second target (t(41) = –4.54, p < .001, d = 1.39). High-risk participants also had significantly greater ML stepping errors when stepping into the second target (U = 150.00, p = .028, r = .29; Figure 2C). There was, however, no significant between-group difference for either AP (U = 180.00, p = .12, r = .18) or ML (U = 188.00, p = .17, r = 0.15) stepping errors into the first target, or AP stepping errors into the second target (U = 223.00, p = .45, r = .02). Motor performance data are presented in Figure 2.
Figure 2.
Comparisons of low- and high-risk participants at ground (between-group analysis), and low-risk participants at ground and threat (within-subject change), for stance durations preceding the first (A) and second target (B), ML stepping error into the second target (C) and time to complete the task (D), *p < .05, **p < .01, ***p < .001 (mean ± standard error of the mean).
Gaze behavior measures
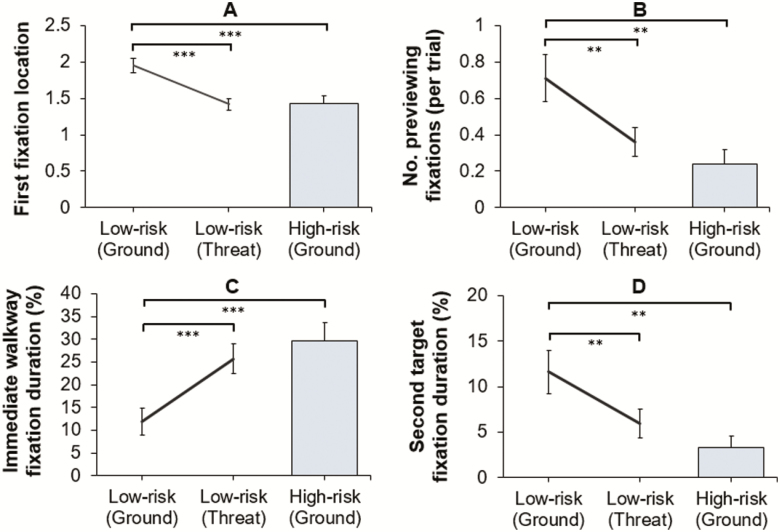
Compared with their low-risk counterparts, high-risk participants’ first fixations were located toward more proximal walkway areas (U = 89.50, p < .001, r = .52). High-risk participants also spent a significantly greater percentage of time fixating the immediate walkway (U = 77.00, p < .001, r = .56), and a significantly smaller percentage of time fixating the second target (U = 103.00, p = .001, r = .47). High-risk participants also exhibited significantly fewer previewing fixations toward the second target (U = 106.50, p = .001, r = .46; Figure 3B)—with 52.63% of high-risk participants failing to make a single previewing fixation toward the second target (compared to only 13.04% of low-risk participants). High-risk participants also transferred their gaze between the different areas of the walkway significantly less (U = 137.00, p = .013, r = .34). There was no significant between-group difference in time spent fixating either the first target (t(41) = 1.75, p = .09, d = 0.54) or the second walkway area (U = 201.00, p = .51, r = .10). Gaze data are presented in Figure 3.
Figure 3.
Comparisons of low- and high-risk participants at ground (between-group analysis), and low-risk participants at ground and threat (within-subject change), for first fixation location (with lower values indicating fixations toward more proximal areas; A), number of previewing fixations toward the second target (average per trial; B), and duration of fixations (as a %) toward the immediate walkway (C) and second target (D), **p < .01, ***p < .001 (mean ± standard error of the mean).
Correlational analyses
Only significant correlations are reported in this section. Please see Supplementary Tables 3 and 4 for a complete list of r-values for all analyzed correlations. All correlations are reported while controlling for age, TuG, and MiniCog scores.
Regarding motor performance variables, greater trial completion times were associated with greater fear of falling (r = .294, p = .033), mental effort (r = .385, p = .014), and attention directed toward both movement processes (r = .479, p = .001) and threats to balance (r = .353, p = .013). Greater stance durations preceding the first target were associated with lower balance confidence (r = –.335, p = .017) and greater attention toward movement processes (r = .264, p = .050), whereas greater stance durations preceding the second target were associated with greater mental effort (r = .342, p = .031) and attention toward movement processes (r = .461, p = .001).
Regarding gaze behavior, greater attention directed toward threats to balance was associated with first fixations occurring toward more proximal walkway areas (r = –.308, p = .027). Longer times fixating the immediate walkway were associated with both lower balance confidence (r = –.351, p = .013) and greater attention toward movement processes (r = .386, p = .007). Both a lower duration and frequency of fixations on the second target were associated with greater attention toward both movement processes (r = –.331, p = .019 and r = –.284, p = .038, respectively) and self-regulatory strategies (r = –.332, p = .036 and r = –.376, p = .016, respectively).
Within-Subject (Low-Risk) Ground–Threat Changes
State psychological measures
During threat, participants reported significant reductions in balance confidence (Z = −3.43, p < .001, r = .72), and significant increases in both fear of falling (Z = −3.60, p < .001, r = .75) and mental effort (Z = −3.41, p < .001, r = .71). They also reported directing significantly greater attention toward movement processes (Z = −2.75, p = .003, r = .57), threats to balance (Z = −2.36, p = .009, r = .49), worries/disturbing thoughts (Z = −2.03, p = .021, r = .42), and self-regulatory strategies (Z = −2.79, p = .003, r = .58). There was a lack of significant change in attention directed toward task-irrelevant information (Z = −0.73, p = .23, r = .15).
Motor performance measures
Participants took significantly longer to complete the walking task during threat (t(22) = –5.57, p < .001, d = 0.99). Although there was no significant ground–threat change in stance durations preceding the first target (t(22) = –0.44, p = .34, d = 0.08), stance durations preceding the second target were significantly longer during threat (Z = −3.50, p < .001, r = .74). Stepping errors in all directions for both the first target (AP: Z = –.06, p = .48, r = .01; ML: Z = −1.28, p = .10, r = .27) and the second target (AP: Z = −1.40, p = .08, r = .29; ML: Z = −1.10, p = .14, r = .23) remained unchanged. Motor performance data are presented in Figure 2.
Gaze behavior measures
Participants’ first fixations were located toward more proximal walkway areas during threat (Z = −4.02, p < .001, r = .84). Participants also spent a significantly greater percentage of time fixating the immediate walkway (Z = −3.74, p < .001, r = .78), and a significantly smaller percentage of time fixating both the first target (t(22) = 3.53, p = .002, d = 0.57) and second target (Z = −2.95, p = .002, r = .62). During threat, participants also exhibited significantly fewer previewing fixations toward the second target (Z = −3.06, p = .001, r = .64; Figure 3B)—with 39.13% of participants failing to make a single previewing fixation toward the second target during threat trials (compared with only 13.04% during ground). Both the number of gaze transfers (Z = −1.13, p = .13, r = .24) and the percentage of time spent fixating the second walkway area (Z = −1.01, p = .31, r = .21) remained unchanged. Gaze data are presented in Figure 3.
Discussion
The aim of this study was to evaluate a possible link between fall-related anxiety and visual search behaviors reported previously in high-risk older adults during adaptive location (4,5,8). We observed significant between-group differences (based on fall-risk) in visual search behaviors during trials at ground level. Specifically, high-risk participants directed their gaze initially toward more proximal areas of the walkway (ie, a gaze bias for immediate threats to balance (9)). Following these initial fixations, they continued to visually prioritize proximal areas of the walking path (immediate walkway), at the expense of previewing future stepping constraints (second target; Figure 3). These behaviors likely represent a compensatory mechanism serving to reduce the immediate risk of producing a misplaced step. In some situations—particularly those without the need for extensive feedforward planning—such behaviors may enhance safety. However, in the present research, this restricted feedforward planning appeared to reduce safety—with high-risk participants exhibiting significantly greater ML stepping errors for the second target. Given the lack of significant between-group difference in either AP or ML stepping error for the first target (which accompanied the lack of significant between-group difference in time spent fixating this constraint), we argue that the increased stepping errors for the second target are unlikely to simply reflect a general inability to produce an accurate step. Rather, we suggest that these unsafe stepping behaviors are the likely consequence of a suboptimal visual planning strategy.
As with previous research (4), high-risk participants reported greater state fear of falling, and lower balance confidence, when completing the adaptive gait task at ground level. When walking at height, low-risk participants too reported reduced state balance confidence and greater fear of falling. They also adopted patterns of gaze behavior largely reminiscent of those observed in high-risk participants during ground trials (Figure 3). Although we acknowledge that factors other than anxiety will have likely contributed to and/or exacerbated these behaviors in the high-risk group, these results nonetheless highlight a link between heightened fall-related anxiety and “high-risk” behaviors reported both previously (4) and herein.
The gaze behaviors observed in both high-risk participants at ground, and low-risk participants during threat, are also comparable to those reported previously in young adults during conditions inducing the conscious processing of stepping movements (9). Interestingly, both high-risk participants at ground level, and low-risk participants walking at height reported directing greater attention toward consciously processing walking movements. Researchers have previously proposed that individuals anxious about falling will consciously process individual stepping movements—visually prioritizing areas of the walking environment needed to do so (eg, looking 1–2 steps ahead to ensure accurate placement of individual steps (9)). The correlational analyses conducted in the present study provide preliminary support for such an assumption. When controlling for age, cognitive, and physical functioning, we found significant positive associations between self-reported conscious movement processing and the time spent fixating the proximal walkway, during ground level walks. During threat trials, low-risk participants also reported heightened conscious movement processing, providing further support for the assumption that these gaze behaviors might be a consequence of anxious individuals prioritizing the visual information needed to consciously process individual stepping movements.
High-risk participants exhibited longer stance durations preceding both stepping targets, with correlational analyses associating these behaviors with heightened conscious movement processing (when controlling for, among other variables, gait speed). This latter finding is consistent with previous research (20). We suggest that high-risk participants likely prolonged the stance phase of these steps in an attempt to (consciously) maximize stepping accuracy into the target. Nonetheless, significantly greater stepping errors were observed in high-risk participants when stepping into the unpreviewed second target. This indicates that despite prolonged stance phases preceding the second target (indicating increased preparation/preprogramming of the following target step (21)), high-risk participants might require visual information before this phase in the gait cycle to step accurately. Alternatively, it is possible that high-risk participants merely required longer stance durations than those exhibited to acquire the relevant visual information.
In contrast, anxious low-risk participants appeared able to successfully adapt and refine stepping actions to compensate for restrictions in visual previewing and feedforward planning. Specifically, our results indicate these individuals were able to successfully obtain the visual information needed for successful negotiation by increasing the stance durations preceding the unpreviewed target (Figure 2B). Although both groups increased stance durations preceding the unpreviewed second target, these increases were proportionately larger in low-risk participants. For example, during threat trials, low-risk participants’ stance durations preceding the second target were on average over 20% longer than those preceding the first target. In contrast, stance durations between the previewed first target, and the unpreviewed second, differed by only 10% in high-risk participants. Perhaps high-risk participants might have similarly been able to compensate for reductions in feedforward planning by further increasing the duration of these stance phases. If so, then this raises the interesting question as to why these individuals were unable to determine that they had acquired insufficient visual information to plan and prepare the subsequent precision step.
It is, however, noteworthy that while low-risk participants were able to counteract such restricted visual search patterns during the present task through compensatory adaptive strategies, this came at a cost to movement efficiency (eg, increased stance durations). It is, therefore, likely that failing to preview upcoming constraints in a feedforward manner will nonetheless lead to negative behavioral consequences in this population during more complex locomotive tasks (eg, tasks requiring rapid, accurate, and possibly reactive stepping movements).
One limitation of this study is the variables used to categorize participants as either high or low risk. For example, although TuG is a commonly used screening tool for fall-risk in both research and clinical settings (10,22), a more thorough assessment of functional balance would have nonetheless allowed for greater sensitivity when determining an individual’s physical risk of falling. Relatedly, although low handgrip strength has been associated with increased fall-risk(13), this assessment nonetheless remains less of an established risk-factor than either previous falls or walking speed. However, as grip strength was used to categorize participants in conjunction with these other well-established risk-factors, we do not consider this to be a major weakness of the study. Finally, although the current results describe associations between self-reported conscious movement processing and numerous anxiety-related gaze behaviors, such analyses provide only weak evidence of a causal relationship. Future research should, therefore, look to experimentally manipulate levels of conscious movement processing in older adults, independent of anxiety.
Conclusion
In conclusion, our findings highlight a link between fall-related anxiety and “high-risk” visual search behaviors. Specifically, our results indicate that older adults anxious about falling (either high-risk participants walking at ground level, or low-risk participants walking at height) will display an initial gaze bias for immediate threats to their balance, prioritizing initial fixations toward proximal walkway areas. To overcome these heightened threats, it appears that anxious older adults will then attempt to consciously process individual steps—visually prioritizing the walkway areas needed for such conscious processing (eg, the walkway 1–2 steps ahead). However, the current results also highlight that such behaviors may paradoxically reduce stepping safety by virtue of restricting the visual information obtained about subsequent stepping constraints—thus impairing an individual’s ability to perceive and negotiate upcoming environmental hazards. Thus, although these behaviors likely represent a compensatory mechanism serving to reduce the immediate risk of falling, they may subsequently increase future risk. This information enhances our understanding of why high-risk older adults are less able to safely navigate environmental constraints and suggests that strategies targeting fall-related anxiety may be an effective strategy for reducing unsafe stepping behaviors in older adults.
Supplementary Material
Acknowledgments
T.J.E. conceived the study. All authors designed the study. T.J.E. and A.J.C. collected the data. T.J.E. analyzed the data. T.J.E. and W.R.Y. interpreted the data. All authors wrote the manuscript.
Conflict of Interest
None declared.
References
- 1. Berg WP, Alessio HM, Mills EM, Tong C. Circumstances and consequences of falls in independent community-dwelling older adults. Age Ageing. 1997;26:261–268. doi:10.1093/ageing/26.4.261 [DOI] [PubMed] [Google Scholar]
- 2. Robinovitch SN, Feldman F, Yang Y, et al.. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet. 2013;381:47–54. doi:10.1016/S0140-6736(12)61263-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matthis JS, Fajen BR. Visual control of foot placement when walking over complex terrain. J Exp Psychol Hum Percept Perform. 2014;40:106–115. doi:10.1037/a0033101 [DOI] [PubMed] [Google Scholar]
- 4. Young WR, Wing AM, Hollands MA. Influences of state anxiety on gaze behavior and stepping accuracy in older adults during adaptive locomotion. J Gerontol B Psychol Sci Soc Sci. 2012;67:43–51. doi:10.1093/geronb/gbr074 [DOI] [PubMed] [Google Scholar]
- 5. Chapman GJ, Hollands MA. Evidence for a link between changes to gaze behaviour and risk of falling in older adults during adaptive locomotion. Gait Posture. 2006;24:288–294. doi:10.1016/j.gaitpost.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 6. Young WR, Mark Williams A. How fear of falling can increase fall-risk in older adults: applying psychological theory to practical observations. Gait Posture. 2015;41:7–12. doi:10.1016/j.gaitpost.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 7. Delbaere K, Sturnieks DL, Crombez G, Lord SR. Concern about falls elicits changes in gait parameters in conditions of postural threat in older people. J Gerontol A Biol Sci Med Sci. 2009;64:237–242. doi:10.1093/gerona/gln014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chapman GJ, Hollands MA. Evidence that older adult fallers prioritise the planning of future stepping actions over the accurate execution of ongoing steps during complex locomotor tasks. Gait Posture. 2007;26:59–67. doi:10.1016/j.gaitpost.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 9. Ellmers TJ, Young WR. The influence of anxiety and attentional focus on visual search during adaptive gait. J Exp Psychol Hum Percept Perform. 2019;45:697–714. doi:10.1037/xhp0000615 [DOI] [PubMed] [Google Scholar]
- 10. Lusardi MM, Fritz S, Middleton A, et al.. Determining risk of falls in community dwelling older adults: a systematic review and meta-analysis using posttest probability. J Geriatr Phys Ther. 2017;40:1–36. doi:10.1519/JPT.0000000000000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Landi F, Liperoti R, Russo A, et al.. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652–658. doi:10.1016/j.clnu.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, Huang P, Dou Q, et al. Falls among older adults with sarcopenia dwelling in nursing home or community: a meta-analysis. Clin Nutr.In Press. doi:10.1016/j.clnu.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 13. Scott D, Hayes A, Sanders KM, Aitken D, Ebeling PR, Jones G. Operational definitions of sarcopenia and their associations with 5-year changes in falls risk in community-dwelling middle-aged and older adults. Osteoporos Int. 2014;25:187–193. doi:10.1007/s00198-013-2431-5 [DOI] [PubMed] [Google Scholar]
- 14. Bahat G, Tufan A, Tufan F, Kilic C, Akpinar TS, Kose M, et al. Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr. 2016;35:1557–1563. doi:10.1016/j.clnu.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 15. Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing. 2005;34:614–619. doi:10.1093/ageing/afi196 [DOI] [PubMed] [Google Scholar]
- 16. Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451–1454. doi:10.1046/j.1532-5415.2003.51465.x [DOI] [PubMed] [Google Scholar]
- 17. Zaback M, Cleworth TW, Carpenter MG, Adkin AL. Personality traits and individual differences predict threat-induced changes in postural control. Hum Mov Sci. 2015;40:393–409. doi:10.1016/j.humov.2015.01.015 [DOI] [PubMed] [Google Scholar]
- 18. Zijlstra FRH. Efficiency in Work Behaviour: A Design Approach for Modern Tools. TU Delft, Delft University of Technology. Delft, Netherlands: Delft University Press; 1993. [Google Scholar]
- 19. Patla AE, Vickers JN. Where and when do we look as we approach and step over an obstacle in the travel path? Neuroreport. 1997;8:3661–3665. doi:10.1097/00001756-199712010-00002 [DOI] [PubMed] [Google Scholar]
- 20. Uiga L, Capio CM, Ryu D, et al. The role of movement-specific reinvestment in visuomotor control of walking by older adults. Journals Gerontol Ser B. In Press. doi:10.1093/geronb/gby078 [DOI] [PubMed] [Google Scholar]
- 21. Lyon IN, Day BL. Control of frontal plane body motion in human stepping. Exp Brain Res. 1997;115(2):345–356. doi:10.1007/PL00005703 [DOI] [PubMed] [Google Scholar]
- 22. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society Clinical Practice Guideline for Prevention of Falls in Older Persons. J Am Geriatr Soc. 2011;59:148–157. doi:10.1111/j.1532-5415.2010.03234.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.