Abstract
The research and development of pharmaceutical intervention is insufficient for the frail older adults, especially in preclinical stage for the frail individuals with osteoporosis. Garlic exerts an antiosteoporotic effect and its vital component allicin could protect organisms against aging. The present study aimed to investigate the effect of long-term intragastric administration of allicin (low dose of 4 mg·kg−1·d−1; middle dose of 8 mg·kg−1·d−1; high dose of 16 mg·kg−1·d−1) on frailty with osteoporosis in aging male Fischer 344 rats. Frailty was assessed with a 27-item frailty index based on quantifying health-related deficits in adult male rats varied from 13 to 21 months and in control rats from 6 to 9 months. Osteoporosis was appraised by bone mineral density detected by dual-energy X-ray absorptiometry, biomechanical properties measured by a three-point bending test, and bone metabolic analysis using ELISA. Allicin could attenuate frailty index scores by reducing the accumulation of health deficits in aging male Fischer 344 rats. Meanwhile, allicin could protect against senile osteoporosis, and the underlying mechanism may involve in increasing low bone turnover through elevation of both bone formation and bone resorption, and subsequently lead to increase of bone mineral density, contributing to reversing deleterious bone biomechanical features associated with aging. The present study reveals firstly that long-term oral administration with allicin attenuated frailty with osteoporosis during the process of aging, which provides a preclinical evidence for intervention of frailty.
Keywords: Pharmaceutical intervention, Frailty index, Antiosteoporosis effect
Recently, concerns are growing about frailty in aging process, which is characterized by the special state of decreased physical reserves and increased vulnerability to stress, and the subsequent adverse health outcomes and mortality (1). In bone tissues, frailty has a close relationship with osteoporosis owing to the decrease of bone mineral density (BMD) and bone strength, contributing to skeletal function limitation and high risk of fracture (2). Generally, the frailer an individual is, the greater risk of osteoporotic fractures there may be (3). Therefore, preventing osteoporosis may help to reduce the risk of aging-related frailty (4).
In recent years, there has been an increasing focus on animal models of frailty based on these clinical assessment tools. A 27-item frailty index (FI) based on accumulation of health deficits was developed in old Fischer 344 (F344) rats, a commonly used tool for aging-related diseases, especially osteoporosis (5–7). It is generally known that aging rats often suffer from osteoporosis (8), so the old F344 rats can be used to assess the effect of interventions on frailty with osteoporosis.
Several pharmaceuticals have been put through trials focusing either on the decreased levels of catabolic hormones or on bulk improvement in muscle and skeletal tissue, but most of these drugs failed to improve frailty in the older adults (9). In recent years, studies of other pharmaceutical agents have paved the way for the development of therapeutic strategy for frailty (10,11). l-Carnitine supplementation exhibited a beneficial effect on frail status in 26 prefrail older adults (12), but it needs to be consolidated by more studies in more populations. Angiotensin-converting enzyme inhibitors such as enalapril could exert a long-term effect on the FI in middle-aged and old mice, suggesting that the intervention may delay the onset of frailty (13). Vitamin D has been recommended only for frail individuals deficient in vitamin D (14). Hitherto, pharmaceutical intervention studies have been rare in the frail older adults, especially in those with osteoporosis.
Garlic has been demonstrated to prevent from osteoporosis, which involves in modulation of proinflammatory cytokine activity, and allicin was taken as its anti-inflammatory component (15,16). Our previous evidence highlighted the anti-apoptotic effect of allicin (17), which could protect bone formation mediated by osteoblasts from oxidative stress, a vital factor of aging (18). Furthermore, allicin could affect the cytokine framework and the secretion of M-CSF and RNAKL from osteoblasts, contributing to inhibition of osteoclastogenesis (19). Moreover, vitamin D3 was chosen as a positive control in this study because there was no appropriate drug, and because vitamin D3 is commonly used as an anabolic agent for prevention and treatment of osteoporosis clinically, and exhibited therapeutic potential of sarcopenia simultaneously (20). In addition, BMD of men shows an obvious reduction only in trabecular area with aging, whereas BMD of women exhibits a decrease in both trabecular area and cortical area with aging, which is also affected by menopause to a large extent (21). Based on these evidences, this study aimed to explore the effect of long-term intragastric administration of allicin on frailty in male aging F344 rats with osteoporosis.
Materials and Methods
Animal Experiments
Seventy-eight male F344 rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The middle-aged rats (13 months, n = 70) were randomly assigned to the following five groups (14 rats per group): O (old-aged control), standard chow diet and distilled water; OA4, allicin 4 mg·kg−1·d−1 (low dose); OA8, allicin 8 mg·kg−1·d−1 (middle dose); OA16, allicin 16 mg·kg−1·d−1 (high dose); OV, vitamin D3 0.045 µg·kg−1·d−1, as described in our previous publication (17,22). This age was selected because it is an optimal age at which age-related risk factors may still be modifiable (23) and because it is suitable for pharmaceutical intervention in a long-term study from middle age (13 months) to old age (21 months). Another group of young rats (n = 8) were fed with standard chow diet and distilled water from 6 to 9 months and served as young adult controls.
The experiments were ethically approved by the Laboratory Animal Administration and Ethics Committee of Xiamen University and housed in Xiamen Medical College according to the recommendations in the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All rats were maintained on a 12-hour light–dark cycle at 22°C, in individual cages with ad libitum access to food and water. Middle-aged rats were administered intragastrically with either allicin or vitamin D3 (Longsheng Keyi Co., Ltd., Beijing, China) between 9 am and 10 am once a day for 8 months. Body weights of all rats were measured twice weekly as bases for calculation the amount of drugs. To assess the changes in food intake, an equal amount of food (20 g/d per rat) was given to each rat at 10 am. Food pellets were stored in mesh hoppers within each cage to prevent spillage. After 24 hours, rats were removed from their cages to assess the food intake. The amount of remaining food, including any on the bottom of the cages was recorded. Intake can be calculated as the weight (in grams) of food provided minus food recovered (24).
Clinical FI Assessment for Rats
A 27-item FI was used in the present model as described in previous literature to acquire clinical FI (5). In brief, values for all deficits were summed up and divided by the total number of deficits measured to yield an FI score theoretically between 0 and 1. Middle-aged rats were assessed for frailty bimonthly from 13 to 21 (old) months of age during the experiment. Young rats were appraised separately at 6 and 9 months, as comparison (control) of old rats using the same assessment criteria and treatment.
Sample Collection and Applications for Osteoporosis-Related Analysis
Young and old male F344 rats (9 months, n = 8; 21 months, n = 39, seven to nine rats per group, the rest rats having died natural deaths) were sacrificed by cardiac puncture under anesthesia with intraperitoneal injection of sodium pentobarbital (1.5 mg/kg). Serum was collected by centrifugation for bone metabolic markers assays. Right tibiae, right femurs, and the fifth lumbar vertebrae were collected and wrapped with gauze soaked in prechilled saline solution to preserve at −20°C for evaluation of BMD and bone biomechanical features.
BMD Assessment
BMD is the gold standard for diagnosing osteoporosis and monitoring changes in bone mass. Meanwhile, BMD of lumbar spine and leg bone can be used to predict osteoporotic fracture risk because these types of bones are prone to fracture clinically. BMD of each ex vivo specimen was measured by dual-energy X-ray absorptiometry with Hologic DXA equipment (Hologic QDR-4500A) using a small animal software as previously described (25). In brief, right tibiae, right femurs, and the fifth lumbar vertebrae were thawed at room temperature and detected with a scan speed of 10 mm/s and a scan resolution of 0.5 mm × 0.5 mm. Then the femurs were wrapped and preserved at −20°C again for further evaluation of biomechanical parameters.
Three-Point Bending Test
Mechanical strength of a long bone was measured by a three-point bending test using the testing machinery (MTS, Eden Prairie, MN) as described in our previous publication (26). In brief, the frozen right femurs were thawed at room temperature and tested with a 1-mm indenter at a speed of 2 mm/min with a 15-mm span. Maximum load and elastic load were obtained by calculation according to the load-deformation curve.
Bone Metabolic Markers Assay
Serum levels of cross-linked carboxy-terminal telopeptide of type I collagen (CTX-I, a marker of bone resorption) and procollagen type 1 amino-terminal propeptide (P1NP, a marker of bone formation) were measured using commercial kits (Longsheng Keyi Co., Ltd.) by ELISA according to instructions.
Statistical Analysis
Data were reported as the mean ± SD or the mean ± SEM, and p < .05 was defined as the threshold of significance. Data were analyzed with the SPSS v20.0 statistical software package (IBM, Chicago, IL). Samples were considered normally distributed if p > .05 with Kolmogorov–Smirnov test. One-way analysis of variance was used to separately analyze differences of dependent variables between groups. Heterogeneity of variance was accepted if p > .05, and Least Significant Difference method was used to perform appropriate multiple comparisons among groups. Otherwise, the Tukey's post hoc test was adopted. Differences in FI scores between different groups at a particular time point were compared with chi-square analysis.
Results
Effects of Allicin on Frailty With Aging
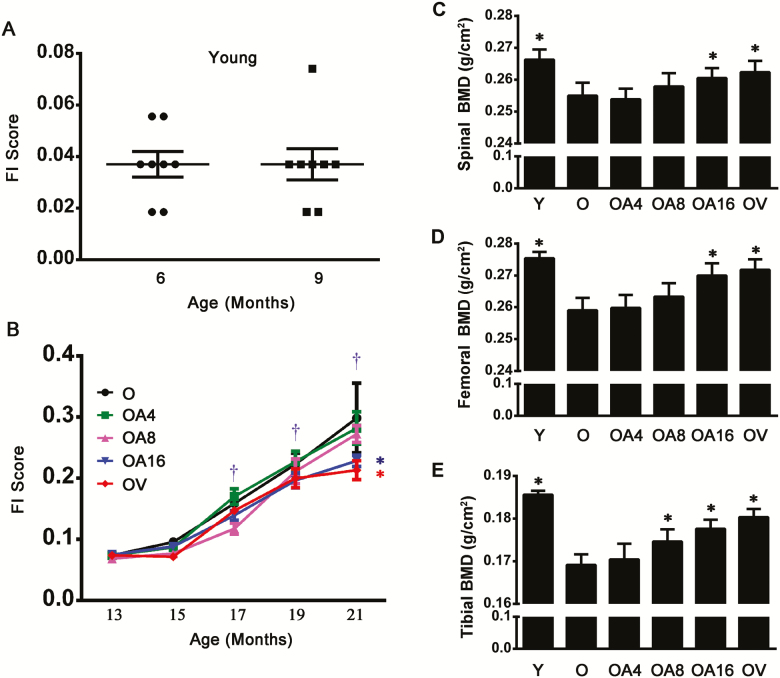
Mean FI scores showed no change in young rats from 6 to 9 months (Figure 1A), but an increase in rats of O group (old-aged control) along with age from 17 to 21 months compared with themselves with age of 13 months (Figure 1B). We next explored whether allicin attenuated FI scores when the treatment was started in aging life. After rats at the age of 13 months were treated with allicin for 8 months, their FI scores were inhibited compared with the old control at the same age. Although FI scores increased with age in the control old rats, this consequence related to age was markedly attenuated by allicin treatment for 8 months, and allicin treatment was as effective as the treatment with vitamin D3 (Figure 1B). These findings suggest that there is an increasing trend of the development of frailty with aging, and long-term treatment with allicin could suppress FI scores in old rats even when it is started in later life.
Figure 1.
Allicin treatment delayed frailty and reversed the decreased BMD in old rats. Mean frailty index (FI) scores were evaluated in young F344 rats (A) and in old individuals treated either with allicin at dose of 4 mg·kg−1·d−1 (OA4, low dose), 8 mg·kg−1·d−1 (OA8, middle dose), and 16 mg·kg−1·d−1 (OA16, high dose), or with vitamin D3 0.045 µg·kg−1·d−1 (OV), or without treatment as old control rats (B). Data are presented as the mean ± SEM (n = 8–14). The levels of BMD for spine (C), femur (D), and tibia (E) were measured by dual-energy X-ray absorptiometry. Data are presented as the mean ± SD (n = 7–9). †p < .05, versus 13 mo (in the Old group); *p < .05, versus the Old group (21 mo). O = Old; OA4 = Old + Low-dose allicin; OA8 = Old + Middle-dose allicin; OA16 = Old + High-dose allicin; OV = Old + Vitamin D3.
Effects of Allicin on BMD of Different Types of Bone
We first measured BMD of vertebra, femur, and tibia. In all old rat control, an obvious decrease of values of BMD lied in vertebra, femur, and tibia. However, there was no significant alteration of BMD in old male rats at 21 months after the start of treatment with low-dose allicin. Interestingly, a significant difference was observed in these three types of bones of old rats exposed either to high-dose allicin or to vitamin D3 compared with old rat control. Especially, middle-dose allicin exerted the same protective effect on tibial BMD as high-dose allicin did. Collectively, our present evidence suggests that the protective efficacy of allicin on senile bone tissue shows a dose-dependent trend in increase of BMD in old male rats (Figure 1C–E).
Effects of Allicin on Bone Strength of Hind Limb
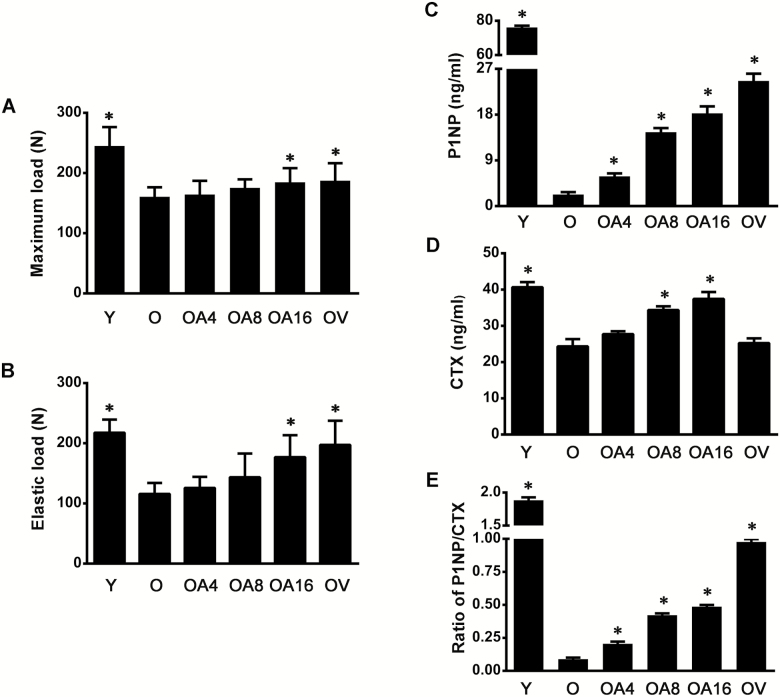
Next, we determined biomechanical parameters related to bone strength, including elastic load responsible for the nonlinearity of the external load-deformation, and maximum load, which presents fracture toughness or resistance against breaking of skeleton. In old-aged male rats, both elastic load and maximum load of femurs were significantly lower than those in young individuals. Similar to results of BMD, the protective efficacy of allicin on biomechanical parameters in old-aged rats tended to be increasing in a dose-dependent manner, but there were no statistical differences at the low- and middle-dose levels. Nevertheless, high-dose allicin, as well as vitamin D3, reversed the further decline of bone strength related to aging (Figure 2A and B). Totally, these evidences have revealed that allicin could attenuate decrease in bone strength along with increase of age.
Figure 2.
Allicin rescued the impairment of bone strength and promoted the low bone turnover in old rats. Maximum load (A) and elastic load (B) of femur were determined by three-point bending assay. The concentrations of P1NP (C), CTX (D), and P1NP/CTX (E) in serum were detected by ELISA. Data are presented as the mean ± SD (n = 7–9). *p < .05, versus the Old group (21 mo). O = Old; OA4 = Old + Low-dose allicin; OA8 = Old + Middle-dose allicin; OA16 = Old + High-dose allicin; OV = Old + Vitamin D3.
Effect of Allicin on Low Bone Turnover Related to Aging
Levels of both P1NP and CTX were significantly reduced in serum of old-aged rats than those in young adults. Especially, the alternation of P1NP in old rats exhibited an approximate 28-fold decline compared with that in young adults. Consequently, the ratio of P1NP/CTX decreased significantly in old-aged rats. Interestingly, the level of P1NP and the ratio of P1NP/CTX were increasing in a dose-dependent manner in old rats exposed to allicin for 8 months. Moreover, serum CTX was elevated in rats of middle- and high-dose groups (Figure 2C–E). Taken together, these findings provide an evidence that allicin could exert an inhibitory effect on low bone turnover in the process of bone metabolism during aging.
Discussion
Well-documented evidence confirms that frailty is associated with osteoporosis in older individuals. Development of pharmaceuticals is indispensable for intervention of frailty with osteoporosis, but has been rare up to now. Therefore, we expected to explore a natural product allicin from garlic, as a promising candidate to serve this purpose. In this study, we first examined the long-term beneficial effect of this pharmaceutical agent at different doses on the FI score in male F344 rats from middle age to old age. Moreover, we found that long-term treatment with allicin alleviated bone loss, poor bone quality, and low bone turnover in these rats.
Recently, the frailty phenotype model (27) and the accumulative deficit mode for calculation of FI score (28) were used as assessment tools to research the characteristics of frailty in animal models, including mice and rats, according to the most commonly used clinical frailty assessments in humans (5,23,29). The rat FI provides a continuous measurement for pharmaceutical interventions on various alterations of frailty in different stages of aging, rather than a cutoff point for observation of the phenotype. Thus, we chose the accumulative deficit model to evaluate the inhibitory influence of long-term treatment with allicin at low dose, middle dose, and high dose on frailty with osteoporosis in male F344 rats from middle age to old age. The data showed a noticeable reduction in frailty of old-aged male rats treated with allicin when compared with age-matched control rats. Although allicin showed a distinct trend in reduction of frailty in middle-aged male rats, this treatment did not start to attenuate significantly mean FI scores until the stage of old age. Thus, allicin may delay the onset of frailty. Allicin as a possible senolytic agent exerted a protective effect on cellular senescence (30), especially on osteoblasts (18). Our study provided direct evidence that allicin may hamper the overall accumulation on a wide range of health-related deficits in old male rats, especially frailty related to aging skeletal tissue. In brief, we can use the FI tool to investigate the efficacy of drugs to delay or reverse frailty with osteoporosis due to skeletal aging.
Generally, frailty is associated with lower BMD and poorer bone health; thus, interventions focusing on inhibition of frailty might be a promising way to restore BMD (31,32). Our data showed that the BMD value declined in lumbar vertebra and leg bone of the old male rats, especially in tibia, implying BMD heterogeneity in diverse skeletal tissues. We also found that the skeletal deterioration with aging could be reversed by allicin at high dose in the two types of skeletons and at middle dose only in the tibia. This may reflect a tendency that different skeletal tissues are sensitive in various degrees to pharmaceutical intervention in different individuals. Our data have suggested that allicin may be beneficial for ameliorating the deleterious properties related to frailty in bone tissues. Under the condition of same level of BMD, the bone quality is largely determined by bone strength, especially in the older individual. Maximum load has been used as an indicator of cortical bone strength, approximately 70% of which contributes to BMD (33). Elastic load is another basic material property independent of geometry and represents the ability of bone to resist a deformation. Here, the two parameters in frail individuals suggested a more brittle state of bone in old male individual, which could be reversed after long-term treatment of allicin. Interestingly, our evidence indicates that allicin can upregulate directly the levels of P1NP and CTX, contributing to elevation of bone turnover, which may be useful for intervention of frailty owing to the correlation between frailty and low BMD (2). In addition, reduced bone formation and low bone turnover in men are associated mainly with aging (34). Future studies should attempt to investigate the underlying molecular mechanisms of allicin for improving frail bones in male individuals.
The present study provides preclinical evidences for the potential use of allicin to attenuate frailty with osteoporosis. There are still several questions to be elucidated. The optimal time to start treatment and the optimal dosages are still not definite. The interrelationship of frailty with deleterious features of senile bones may shed light on what types of items and how many items might be effective and necessary for the research and development of pharmaceutical intervention for frailty with osteoporosis. The frailty started to be attenuated at 21 months in old male rats treated with allicin, and the observation suggests that allicin is still beneficial for intervention of frailty. It would be interesting to determine whether allicin could reverse higher degrees of frailty in future studies, by starting treatment once higher FI scores are observed in male individuals with aging. In addition, it would be another interesting topic to stop treatment and observe whether the beneficial effects still persist beyond the treatment period, and how long this secondary effect will be last. Admittedly, there are some limitations to this study. Although our results are statistically significant, they should be confirmed with female animals, larger sample numbers and other species, such as mouse. Our study design did not allow us to determine whether allicin treatment could extend life span because the rats were closely studies terminally as tissue was collected for use in other experiments mentioned above. It would be worthy to determine whether allicin treatment affected life span and health span in future studies.
Conclusion
The present study revealed that long-term allicin treatment attenuated frailty with osteoporosis from middle age to old age in male individuals. This article highlights the importance of tools and protocols using F344 male rats with aging and significant items of design for studying pharmaceutical intervention of frailty, and provides preclinical evidence that allicin may delay the onset of frailty with osteoporosis starting in middle age of life.
Funding
This work was supported by the National Natural Science Foundation of China (81673814, 81874394); the Science and Technology Planning Project of Guangdong Province of China (2016A020215148); the Medical Scientific Research Foundation of Guangdong Province of China (A2016293); the Key Laboratory of Functional and Clinical Translational Medicine, Fujian Province University (JNYLC1812); and the “Group-Type” Special Support Project for Education Talents in Universities (Southern Medical University G619080438, Guangdong Medical University 4SG19045G).
Conflict of Interest
None reported.
References
- 1. Todorovic ST, Smiljanic KR, Ruzdijic SD, Djordjevic ANM, Kanazir SD. Effects of different dietary protocols on general activity and frailty of male Wistar rats during aging. J Gerontol A Biol Sci Med Sci. 2018;73:1036–1044. doi:10.1093/gerona/gly015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cook MJ, Oldroyd A, Pye SR, et al. ; EMAS Study Group Frailty and bone health in European men. Age Ageing. 2017;46:635–641. doi:10.1093/ageing/afw205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li G, Papaioannou A, Thabane L, et al. Modifying the phenotypic frailty model in predicting risk of major osteoporotic fracture in the elderly. J Am Med Dir Assoc. 2017;18:414–419. doi:10.1016/j.jamda.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 4. Yoshimura N. Aging-related frailty and sarcopenia. Frailty, sarcopenia and osteoporosis: a 4-year observation of the second and third ROAD study surveys. Clin Calcium. 2018;28:1209–1214. doi:CliCa180912091214 [PubMed] [Google Scholar]
- 5. Yorke A, Kane AE, Hancock Friesen CL, Howlett SE, O'Blenes S. Development of a rat clinical frailty index. J Gerontol A Biol Sci Med Sci. 2017;72:897–903. doi:10.1093/gerona/glw339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim H, Kim C, Kim DU, Chung HC, Hwang JK. Inhibitory effects of boesenbergia pandurata on age-related periodontal inflammation and alveolar bone loss in Fischer 344 rats. J Microbiol Biotechnol. 2018;28:357–366. doi:10.4014/jmb.1711.11034 [DOI] [PubMed] [Google Scholar]
- 7. Banu J, Wang L, Kalu DN. Age-related changes in bone mineral content and density in intact male F344 rats. Bone. 2002;30:125–130. doi:10.1016/s8756-3282(01)00636-6 [DOI] [PubMed] [Google Scholar]
- 8. Turner RT, Maran A, Lotinun S, et al. Animal models for osteoporosis. Rev Endocr Metab Disord. 2001;2:117–127.doi:10.1023/A:1010067326811 [DOI] [PubMed] [Google Scholar]
- 9. Campbell S, Szoeke C.. Pharmacological treatment of frailty in the elderly. J Pharm Pract Res. 2009;39:147–151. doi:10.1002/j.2055–2335.2009.tb00440.x [Google Scholar]
- 10. Jeffery CA, Shum DW, Hubbard RE. Emerging drug therapies for frailty. Maturitas. 2013;74:21–25. doi:10.1016/j.maturitas.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 11. Bendayan M, Bibas L, Levi M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part II. Ongoing and unpublished randomized trials. Prog Cardiovasc Dis. 2014;57:144–151. doi:10.1016/j.pcad.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 12. Badrasawi M, Shahar S, Zahara AM, Nor Fadilah R, Singh DK. Efficacy of l-carnitine supplementation on frailty status and its biomarkers, nutritional status, and physical and cognitive function among prefrail older adults: a double-blind, randomized, placebo-controlled clinical trial. Clin Interv Aging. 2016;11:1675–1686. doi:10.2147/CIA.S113287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keller K, Kane A, Heinze-Milne S, Grandy SA, Howlett SE. Chronic treatment with the ACE inhibitor enalapril attenuates the development of frailty and differentially modifies pro-and anti-inflammatory cytokines in aging male and female C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2018;74:1149–1157. doi:10.1093/gerona/gly219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dent E, Lien C, Lim WS, et al. The Asia-Pacific clinical practice guidelines for the management of frailty. J Am Med Dir Assoc. 2017;18:564–575. doi:10.1016/j.jamda.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 15. Mozaffari-Khosravi H, Hesabgar HA, Owlia MB, Hadinedoushan H, Barzegar K, Fllahzadeh MH. The effect of garlic tablet on pro-inflammatory cytokines in postmenopausal osteoporotic women: a randomized controlled clinical trial. J Diet Suppl. 2012;9:262–271. doi:10.3109/19390211.2012.726703 [DOI] [PubMed] [Google Scholar]
- 16. Qian YQ, Feng ZH, Li XB, et al. Downregulating PI3K/Akt/NF-κB signaling with allicin for ameliorating the progression of osteoarthritis: in vitro and vivo studies. Food Funct. 2018;9:4865–4875. doi:10.1039/c8fo01095a [DOI] [PubMed] [Google Scholar]
- 17. Liu Y, Qi H, Wang Y, et al. Allicin protects against myocardial apoptosis and fibrosis in streptozotocin-induced diabetic rats. Phytomedicine. 2012;19:693–698. doi:10.1016/j.phymed.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 18. Ding G, Zhao J, Jiang D. Allicin inhibits oxidative stress-induced mitochondrial dysfunction and apoptosis by promoting PI3K/AKT and CREB/ERK signaling in osteoblast cells. Exp Ther Med. 2016;11:2553–2560. doi:10.3892/etm.2016.3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guan X, Zhou Y, Chen H, Wang C, Wang H. Antibacterial, anti-inflammatory, and anti-osteoclastogenesis roles of allicin in periodontitis. Int J Clin Exp Med. 2018;11:6721–6730. [Google Scholar]
- 20. Wagatsuma A, Sakuma K. Vitamin D signaling in myogenesis: potential for treatment of sarcopenia. Biomed Res Int. 2014;2014:121254. doi:10.1155/2014/121254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruff CB, Hayes WC. Sex differences in age-related remodeling of the femur and tibia. J Orthop Res. 1988;6:886–896. doi:10.1002/jor.1100060613 [DOI] [PubMed] [Google Scholar]
- 22. Zhou M, Li J, Wu J, et al. Preventive effects of Polygonum multiflorum on glucocorticoid-induced osteoporosis in rats. Exp Ther Med. 2017;14:2445–2460. doi:10.3892/etm.2017.4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller MG, Thangthaeng N, Shukitt-Hale B. A clinically relevant frailty index for aging rats. J Gerontol A Biol Sci Med Sci. 2017;72:892–896. doi:10.1093/gerona/glw338 [DOI] [PubMed] [Google Scholar]
- 24. Babaei P, Damirchi A, Hoseini R. The interaction effects of aerobic exercise training and vitamin D supplementation on plasma lipid profiles and insulin resistance in ovariectomized rats. J Exerc Nutr Biochem. 2015;19:173–182. doi:10.5717/jenb.2015.15070703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liao C, Li T, Liu Y, et al. Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis. PLoS One. 2012;7:e34647. doi:10.1371/journal.pone.0034647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang Y, Su Y, Wang D, et al. Tanshinol rescues the impaired bone formation elicited by glucocorticoid involved in KLF15 pathway. Oxid Med Cell Longev. 2016;2016:1092746. doi:10.1155/2016/1092746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 28. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi:10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kane AE, Huizer-Pajkos A, Mach J, et al. A comparison of two mouse frailty assessment tools. J Gerontol A Biol Sci Med Sci. 2017;72:904–909. doi:10.1093/gerona/glx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malavolta M, Bracci M, Santarelli L, et al. Inducers of senescence, toxic compounds, and senolytics: the multiple faces of Nrf2-activating phytochemicals in cancer adjuvant therapy. Mediators Inflamm. 2018;2018:4159013. doi:10.1155/2018/4159013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chao CT, Huang JW, Chan DC; Cohort of Geriatric Nephrology in NTUH (COGENT) Study Group Frail phenotype might herald bone health worsening among end-stage renal disease patients. PeerJ. 2017;5:e3542. doi:10.7717/peerj.3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoneki K, Kitagawa J, Hoshi K, et al. Association between frailty and bone loss in patients undergoing maintenance hemodialysis. J Bone Miner Metab. 2019;37:81–89. doi:10.1007/s00774-017-0898-4 [DOI] [PubMed] [Google Scholar]
- 33. Zhang L, Liu Y, Wang D, et al. Bone biomechanical and histomorphometrical investment in type 2 diabetic Goto-Kakizaki rats. Acta Diabetol. 2009;46:119–126. doi:10.1007/s00592-008-0068-1 [DOI] [PubMed] [Google Scholar]
- 34. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364–376. doi:10.1016/S0140-6736(18)32112-3 [DOI] [PubMed] [Google Scholar]