Abstract
Omega-3 polyunsaturated fatty acids (n3-PUFA) are well recognized for their potent triglyceride-lowering effects, but the potential influence of these bioactive lipids on other biological processes, particularly in the context of healthy aging, remains unknown. With the goal of gaining new insight into some less well-characterized biological effects of n3-PUFAs in healthy older adults, we performed metabolomics of fasting peripheral blood plasma collected from 12 young adults and 12 older adults before and after an open-label intervention of n3-PUFA (3.9 g/day, 2.7 g eicosapentaenoic [EPA], 1.2 g docosahexaenoic [DHA]). Proton nuclear magnetic resonance (1H-NMR) based lipoprotein subclass analysis revealed the expected reduction in total triglyceride (TG), but also demonstrated that n3-PUFA supplementation reduced very low-density lipoprotein (VLDL) particle number, modestly increased high-density lipoprotein (HDL) cholesterol, and shifted the composition of HDL subclasses. Further metabolite profiling by 1H-NMR and mass spectrometry revealed pronounced changes in phospholipids, cholesterol esters, diglycerides, and triglycerides following n3-PUFA supplementation. Furthermore, significant changes in hydroxyproline, kynurenine, and 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid (CMPF) following n3-PUFA supplementation provide further insight into some less well-recognized biological effects of n3-PUFA supplementation, including possible effects on protein metabolism, the kynurenine pathway, and glucose metabolism.
Keywords: n3-PUFA, EPA, DHA, Metabolomics, Lipoproteins
Life expectancy in the United States is estimated to be approximately 79 years (1), and there is growing interest in narrowing the gap between healthy life expectancy (ie, healthspan) and death. In 2016, the leading causes of death included numerous age-associated diseases such as heart disease, cancer, stroke, Alzheimer’s disease, and diabetes (1). Of these, ischemic heart disease and stroke account for the vast majority of deaths (2). In the quest to extend healthspan, numerous strategies (eg, exercise, nutrition, pharmacology) have emerged in attempt to forestall age-related chronic disease. Dietary intake of omega-3 polyunsaturated fatty acids (n-3 PUFA) is one such strategy that has been extensively investigated. The longer-chain fatty acids, eicosapentaenoic (EPA) and docosahexaenoic (DHA), are recognized primarily for their triglyceride-lowering (3,4) and anti-inflammatory effects (5,6) in humans. These properties have sparked interest in the therapeutic potential of n3-PUFAs in prevention and treatment of heart disease (7,8), diabetes (9,10), retinopathy (11), cancer (12), and age-related muscle wasting (13–15). Despite some promising preclinical data and individual trials in humans, meta analyses lead to more ambiguous conclusions with some finding modest benefit (16) while others cast some doubt on any real beneficial effects of dietary n-3 PUFAs on cardiovascular disease, cancer, or diabetes (17,18). In the absence of undeniable evidence that omega-3 fatty acids offer cardiovascular disease protection, the fact remains that n3-PUFAs are bioactive lipids with many physiological effects that go beyond simply lowering circulating triglycerides. Both EPA and DHA incorporate into cell membranes, and their unique chemical and physical properties promote lipid raft formation (19) and allow them to enter into the binding pockets of proteins (20). Our understanding of the biological effects of dietary omega-3 fatty acids is incomplete, particularly in the context of aging. Here, we performed metabolomics of peripheral blood to provide a window into some of the less well-characterized biological effects of n3-PUFAs in healthy older adults.
Methods
Participants and Study Design
Fasting peripheral blood plasma was obtained from a previously published study of 12 young (18–35 years) and older (65–85 years) men and women (13). All individuals provided written informed consent as approved by the Mayo Foundation Institutional Review Board and consistent with principles outlined in the Declaration of Helsinki. All participants were in good health and carefully screened to exclude individuals with diabetes, heart disease, uncontrolled thyroid disorders, renal disease, anemia, liver disease, pregnancy, breastfeeding, smoking, substance abuse, or individuals who were attempting to gain or lose weight. Following the screening visit, all participants were provided with 3 days of standardized, weight-maintenance meals that were prepared in our metabolic kitchen. The caloric content of the meals was estimated on an individual basis to achieve energy balance based on the Harris Benedict equation. Macronutrient distribution of the diet was 20% protein, 50% carbohydrate, and 30% fat. Participants reported to the metabolic kitchen each day for breakfast, to ensure weight stability, and to pick up prepared meals for the remainder of the day. If necessary, adjustments to the caloric content of the days meals were made by the dietician based on body mass measured that day. On the evening of the third day of the diet, participants were admitted to the Mayo Clinic Clinical Research and Trials Unit at 1700 hours and consumed nothing but water after 2200 hours. A fasting blood sample was collected at 0800 hours the following morning and immediately processed and stored at −80°C until analysis. Older participants were studied again following a 4-month open-label intervention of n3-PUFA (3.9 g/day, 2.7 g EPA, 1.2 g DHA, NCT02103842). The 4-month duration was selected to allow sufficient time for robust incorporation of EPA and DHA into cell membranes (measured by RBC EPA and DHA levels) and adipose tissue lipid pools (measured by fasting FFA lipid profiles) as we previously published (13). The young participants were studied as a comparison group and did not undergo any intervention. Peripheral blood plasma metabolomics was performed using multiple analytical platforms including proton nuclear magnetic resonance (1H-NMR), gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS) for small metabolite profiling and quantitation as well as lipoprotein subclass analysis.
1H-NMR Lipoprotein Subclass Analysis
Lipoprotein subclass analysis was performed using the B.I.-LISA lipoprotein platform (Bruker, Rheinstaten, Germany). Samples were prepared using Bruker standard operating procedures for plasma/serum: The samples were thawed on ice for 30 minutes. Four hundred microliters of plasma were transferred into 1.5 mL Eppendorf tubes containing 400 μL of Bruker plasma buffer. The mixture was gently shaken for 1 minute. Six hundred microliters of the mixed sample were then transferred into a 5 mm Sample Jet rack tube. The NMR spectra were acquired on a Bruker 600 MHz Avance III HD spectrometer equipped with a BBI room temperature probe head and SampleJet auto sampler (Bruker Biospin, Billerica, MA). 1H-NMR spectra were recorded using 1D NOESY pulse sequence with presaturation (noesygppr1d), collecting 32 scans. After the measurement the recorded spectra were transferred to Bruker Data Analysis server for fully automated remote analysis, and the results were sent back via private file transfer program.
1H -NMR Small Metabolite Profiling
Plasma samples were thawed on ice at 4°C for 30–60 minutes. Two hundred-microliter aliquots were transferred into 1.5 mL Eppendorf tubes and 600 μL of cold MeOH was added. The mixture was vortexed for 20 seconds and then centrifuged at 13,300 rpm for 15 minutes. The supernatants (~760 μL) were transferred into 1.5 mL Eppendorf tubes and dried in a centrifugal vacuum evaporator for 12 hours. In each dried sample, 500 μL of 0.1 M phosphate buffer (K2HPO4/KH2PO4, pH 7.4) and 50 μL of 1 mM TSP-d4 in deuterium oxide (D2O) were added. The mixture was vortexed for 20 seconds and the solution was then transferred to a 5 mm SampleJet rack tube. All chemicals were purchased from Sigma–Aldrich (St. Louis, MO). The NMR spectra were acquired on a Bruker 600 MHz Avance III HD spectrometer equipped with a BBI room temperature probehead and SampleJet auto sampler 1H-NMR spectra were recorded using 1D NOESY pulse sequence with presaturation (noesygppr1d), collecting 128 scans with 64k data points, 14 ppm spectral with calibrated 90 degree pulse (~11 ms), 3.90-second acquisition time, and 5-second relaxation delay. Metabolites were identified and quantified using Chenomx NMR suite 8.2 software, by fitting the spectral lines of library compounds into the recorded NMR spectrum of plasma samples. The quantification was based on peak area of TSP-d4 signal. The metabolite concentrations were exported as µM in NMR sample and recalculated as µM in plasma.
LC/MS-Biocrates p400 Kit
Metabolites were extracted using the Absolute IDQ p400 Kit (BIOCRATES Life Sciences AG, Innsbruck, Austria). Plasma samples (10 μL) were pipetted onto a 96-well (FIA plate) Biocrates kit. The samples were dried at room temperature (RT) for 30 minutes. Fifty microliters of 5% phenylisothiocyanate (PITC) reagent were added to each well, and the plate was incubated at RT for 20 minutes and then the samples were dried under nitrogen stream for 60 minutes. Three hundred microliters of 5 mM ammonium acetate in methanol were added, and the plate was shaken at 450 rpm at RT for 30 minutes and then centrifuged for 2 minutes at 500 g. One hundred and fifty microliters from each well were transferred to a 96-deep well LC plate and 150 μL of water was added to each well. To the FIA plate, 250 μL of FIA solvent were added. Both plates were then shaken at RT for 5 minutes.
The ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) utilized a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer coupled with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. Five-microliter sample was injected onto a BIOCRATES UHPLC Absolute IDQ column for analysis. The mobile phases for the LC plate were solvent A (milli-Q water containing 0.2% formic acid) and solvent B (acetonitrile containing 0.2% formic acid) The gradient used to separate the metabolites was: 0–0.25 minutes 0% B, 1.5 minutes: 12% B; 2.7 minutes: 17.5% B; 4 minutes: 50% B; 4.50 minutes: 95% B; 5.25 minutes: 0% B. Evaluation of the samples was carried out using the MetIDQ (BIOCRATES) software. The column oven temperature was set to 500°C. MS spectra were acquired in the positive mode. Molar concentrations were calculated with Xcalibur 4.0 Software. For the FIA plate, 20 µL injection volume directly onto the MS was used at a flow of 30 µL/min, with a mobile phase of H2O/ACN (1:1) containing 0.2% formic acid. The flow rate used was: 0–1.4 minutes: 5 µL/min; 1.6 minutes: 200 µL/min; 2.80 minutes: 200 µL/min; and 3.00 minutes: 5 µL/min. Concentrations were calculated using the Analyst/MetIDQ (BIOCRATES, Biocrates Life Sciences AG, Innsbruck, Austria) software.
Untargeted Metabolite Profiling by Mass Spectrometry
To aid in elucidation of an unknown compound identified by 1H-NMR, we employed two complementary analytical platforms (LC-MS and GC-MS) for untargeted metabolite profiling in plasma from a subset of four older adults before and after n3-PUFA supplementation. LC-MS untargeted profiling was performed using an established method (21). Plasma samples were deproteinized using cold methanol (1:4 ratio), and metabolites were extracted. 13C6-phenylalanine was added as an internal standard to all samples before extraction to compute extraction efficiency. Extracted metabolite supernatants were divided into four aliquots and analyzed on a UPLC-Q-TOF system (Agilent Technologies 6550 Q-TOF). Metabolites were separated, independently, using a hydrophilic interaction column (HILIC) and a reversed-phase C18 column. For each column, profiling data was acquired in positive and negative electrospray ionization modes (in separate runs), resulting in a total of four runs per sample. Mass Profiler (Agilent, Santa Clara, CA) software was used to read the chromatograms from the Agilent instrument native files, filter the noise, and detect peaks. Intensity of the peaks was normalized using the total ion current seen per sample. Detected peaks were identified by matching their precursor masses and retention times against the NIST LC-MS (version 11) and METLIN metabolite databases.
Plasma samples were also profiled using a GC-MS platform using the Fiehn laboratory protocol (22). In brief, metabolites were analyzed as their methyl oxime, trimethylsilyl derivatives (MOX-TMS) under electron impact conditions. Full scan data were processed using Fiehn GC/MS metabolomics RTL library. AMDIS software was used to process the experimental mass spectra and retention time indices (MSRI), remove noise peaks, detect components, and deconvolute spectra. Processed peaks were matched against the reference GC-MS library for identification. Intensities of the identified metabolites that were within ±0.1 minutes of the library reference retention time (RT) were reported.
Statistical Analysis
Values are presented as means and standard error. To evaluate the effect of age on metabolite and lipoprotein concentrations, unpaired t tests were used for comparison between young adults and older adults (preintervention). Due to the large number of metabolite comparisons between groups, the Benjamini–Hochberg Procedure was used to maintain the type I error rate at 5%. To evaluate the effects of omega-3 fatty acid supplementation in older adults, paired t tests were used for comparison of metabolite values before and after the intervention. The Benjamini–Hochberg Procedure was used to adjust p-values to minimize the false discovery rate.
Results
Participant Characteristics
Physical descriptors and clinical characteristics of the participants have been described previously (13). Body mass index was marginally yet nonsignificantly elevated in older (26.3 ± 2.8 kg/m2) compared to young (24.3 ± 2.7 kg/m2). Fasting blood glucose was significantly higher in older (91.6 ± 8.6 mg/dL) compared to young (84.3 ± 7.2 mg/dL), although still within normal limits.
Blood Lipids and Lipoprotein Subclass Analyses
The NMR-based measurement of lipoprotein class-specific concentrations of cholesterol and triglycerides revealed subtle differences between young and old adults, but more substantial changes with n3-PUFA supplementation (Tables 1–3). Prior to supplementation, older adults exhibited increased number of small, dense low-density lipoprotein (LDL) particles (LDL-6) and increased phospholipid and Apo-B concentrations in this LDL subclass (Table 1). No other parameters met the FDR cutoffs for significance in young versus older adults. In contrast to the subtle differences between young and old, n3-PUFA supplementation resulted in more profound changes in the lipoprotein profile of older adults with 53 of the 117 features being significantly changed following the intervention (Tables 1–3). As expected, total triglycerides were reduced with n3-PUFAs, as were the triglyceride levels in very low-density lipoprotein (VLDL) and IDL lipoprotein fractions. The triglyceride concentrations in the LDL fraction were significantly increased following n3-PUFA supplementation, attributed to higher triglyceride (TG) levels in the smaller, denser LDL-4, LDL-5, and LDL-6 particles. In contrast to the small, dense LDL particles, the larger LDL-1 particles showed decreased TG content after supplementation. The TG content of high-density lipoprotein (HDL) main fraction did not change significantly with n3-PUFAs, but there was a significant decrease in TG content of the small, dense HDL-4 subfraction and a reciprocal increase in TG content of the larger HDL-1 and HDL-2 subfractions.
Table 1.
Lipoprotein Main Parameters, Particle Number, and Main Fractions
| Young N = 12 |
Old N = 12 |
Old n-3 N = 12 |
Young vs Old | Old n-3 vs Old | |||
|---|---|---|---|---|---|---|---|
| Ratio | Adj p | Ratio | Adj p | ||||
| Main Parameters | |||||||
| Triglycerides | 85.5 ± 8 | 88.0 ± 6.4 | 72.7 ± 4.8 | 0.97 | .668 | 0.83 | .774 |
| Cholesterol | 176 ± 9 | 192 ± 9 | 190 ± 10 | 0.92 | .902 | 0.99 | .002 |
| LDL Cholesterol | 97.9 ± 7.7 | 104 ± 7 | 105 ± 8 | 0.94 | .866 | 1.01 | .842 |
| HDL Cholesterol | 54 ± 4 | 58.5 ± 2.7 | 64.5 ± 3.6 | 0.92 | .720 | 1.10 | .041 |
| Apo-A1 | 136 ± 6 | 143 ± 4 | 141 ± 5 | 0.95 | .759 | 0.99 | .513 |
| Apo-A2 | 31.6 ± 1.8 | 27.6 ± 0.7 | 24.5 ± 0.8 | 1.14 | .403 | 0.89 | .003 |
| Apo-B100 | 70.8 ± 3.8 | 80.7 ± 4.6 | 80.1 ± 4.8 | 0.88 | .457 | 0.99 | .825 |
| Particle Number | |||||||
| Apo-B | 1290 ± 70 | 1467 ± 83.3 | 1457 ± 87 | 0.88 | .457 | 0.99 | .825 |
| VLDL | 112 ± 14 | 120 ± 12 | 96.2 ± 9.4 | 0.93 | .882 | 0.80 | .009 |
| IDL | 58.3 ± 7.7 | 64.0 ± 10.4 | 56.7 ± 9.0 | 0.91 | .882 | 0.89 | .312 |
| LDL | 1094 ± 71 | 1240 ± 75.4 | 1256 ± 84.7 | 0.88 | .591 | 1.01 | .757 |
| LDL-1 | 230 ± 12 | 283 ± 21 | 266 ± 26 | 0.81 | .403 | 0.94 | .186 |
| LDL-2 | 203 ± 20 | 209 ± 18 | 192 ± 18 | 0.97 | .909 | 0.92 | .312 |
| LDL-3 | 186 ± 19 | 179 ± 20 | 164 ± 16 | 1.04 | .906 | 0.92 | .264 |
| LDL-4 | 161 ± 19 | 135 ± 19 | 115 ± 20 | 1.19 | .759 | 0.85 | .264 |
| LDL-5 | 117 ± 11 | 121 ± 16 | 142 ± 21 | 0.97 | .909 | 1.17 | .249 |
| LDL-6 | 179 ± 13 | 259 ± 15 | 319 ± 20 | 0.69 | .029 | 1.23 | .017 |
| Lipoprotein Main Fractions | |||||||
| Triglycerides, VLDL | 47.9 ± 5.6 | 43.0 ± 4.4 | 34.6 ± 2.9 | 1.11 | .830 | 0.80 | .009 |
| Triglycerides, IDL | 6.41 ± 1.03 | 6.31 ± 0.87 | 3.17 ± 0.65 | 1.02 | .950 | 0.50 | <.001 |
| Triglycerides, LDL | 13.6 ± 1.3 | 17.0 ± 1.5 | 19.1 ± 1.4 | 0.80 | .444 | 1.12 | .020 |
| Triglycerides, HDL | 8.74 ± 1.08 | 9.88 ± 1.02 | 10.6 ± 1.1 | 0.88 | .806 | 1.07 | .129 |
| Cholesterol, VLDL | 13.9 ± 1.6 | 13.6 ± 1.5 | 9.54 ± 1.30 | 1.02 | .930 | 0.70 | .002 |
| Cholesterol, IDL | 6.65 ± 0.96 | 7.20 ± 1.46 | 6.01 ± 1.20 | 0.92 | .902 | 0.83 | .264 |
| Cholesterol, LDL | 97.9 ± 7.7 | 104 ± 7 | 105 ± 8 | 0.94 | .866 | 1.01 | .842 |
| Cholesterol, HDL | 53.5 ± 3.5 | 58.5 ± 2.7 | 64.5 ± 3.6 | 0.91 | .720 | 1.10 | .041 |
| Free Cholesterol, VLDL | 6.28 ± 0.71 | 6.75 ± 0.66 | 5.16 ± 0.53 | 0.93 | .882 | 0.76 | .003 |
| Free Cholesterol, IDL | 2.19 ± 0.30 | 2.25 ± 0.42 | 1.60 ± 0.34 | 0.97 | .934 | 0.71 | .051 |
| Free Cholesterol, LDL | 26.9 ± 2.1 | 29.4 ± 2.0 | 29.9 ± 2.0 | 0.91 | .806 | 1.02 | .637 |
| Free Cholesterol, HDL | 10.2 ± 0.8 | 12.1 ± 0.8 | 15.0 ± 0.9 | 0.84 | .457 | 1.24 | .002 |
| Phospholipids, VLDL | 14.5 ± 1.6 | 13.7 ± 1.3 | 11.1 ± 1.0 | 1.06 | .882 | 0.81 | .016 |
| Phospholipids, IDL | 4.42 ± 0.36 | 4.28 ± 0.53 | 3.58 ± 0.56 | 1.03 | .909 | 0.84 | .076 |
| Phospholipids, LDL | 57.2 ± 3.6 | 61.2 ± 3.7 | 61.0 ± 3.9 | 0.93 | .806 | 1.00 | .928 |
| Phospholipids, HDL | 79.5 ± 4.3 | 83.4 ± 3.8 | 85 ± 4.6 | 0.95 | .830 | 1.02 | .513 |
| Apo-A1, HDL | 137 ± 6 | 143 ± 5 | 142 ± 6 | 0.96 | .806 | 0.99 | .788 |
| Apo-A2, HDL | 32.0 ± 1.7 | 28.4 ± 0.6 | 25.5 ± 0.7 | 1.13 | .435 | 0.90 | .003 |
| Apo-B, VLDL | 6.2 ± 0.8 | 6.6 ± 0.6 | 5.3 ± 0.5 | 0.94 | .882 | 0.80 | .009 |
| Apo-B, IDL | 3.21 ± 0.42 | 3.52 ± 0.57 | 3.12 ± 0.50 | 0.91 | .882 | 0.89 | .312 |
| Apo-B, LDL | 60.2 ± 3.9 | 68.2 ± 4.1 | 69.1 ± 4.7 | 0.88 | .591 | 1.01 | .757 |
Note: Data are shown as mean ± SEM with p-values adjusted for multiple comparisons using the Benjamini–Hochberg Adjustment. HDL = High-density lipoprotein; LDL = Low-density lipoprotein; VLDL = Very low-density lipoprotein.
Table 2.
Lipoprotein VLDL and LDL Subfractions
| Young N = 12 |
Old N = 12 |
Old n-3 N = 12 |
Young vs Old | Old n-3 vs Old | |||
|---|---|---|---|---|---|---|---|
| Ratio | Adj p | Ratio | Adj p | ||||
| VLDL Subfractions | |||||||
| Triglycerides, VLDL-1 | 22.8 ± 3.3 | 18.0 ± 2.4 | 13.1 ± 1.7 | 1.27 | .684 | 0.73 | .014 |
| Triglycerides, VLDL-2 | 6.12 ± 1.26 | 4.63 ± 0.70 | 5.22 ± 0.41 | 1.32 | .742 | 1.12 | .296 |
| Triglycerides, VLDL-3 | 5.12 ± 1.27 | 3.96 ± 0.71 | 3.72 ± 0.54 | 1.29 | .806 | 0.94 | .757 |
| Triglycerides, VLDL-4 | 5.85 ± 1.09 | 6.68 ± 0.87 | 5.88 ± 0.67 | 0.88 | .868 | 0.88 | .312 |
| Triglycerides, VLDL-5 | 2.98 ± 0.31 | 3.61 ± 0.20 | 3.49 ± 0.23 | 0.82 | .457 | 0.97 | .589 |
| Cholesterol, VLDL-1 | 5.96 ± 0.58 | 4.47 ± 0.42 | 2.86 ± 0.40 | 1.33 | .830 | 0.64 | .003 |
| Cholesterol, VLDL-2 | 1.56 ± 0.30 | 1.32 ± 0.17 | 1.01 ± 0.13 | 1.18 | .830 | 0.76 | .044 |
| Cholesterol, VLDL-3 | 1.74 ± 0.42 | 1.13 ± 0.26 | 0.56 ± 0.19 | 1.54 | .668 | 0.49 | .036 |
| Cholesterol, VLDL-4 | 2.97 ± 0.57 | 3.56 ± 0.64 | 2.71 ± 0.51 | 0.83 | 0.830 | 0.76 | .100 |
| Cholesterol, VLDL-5 | 2.07 ± 0.21 | 2.55 ± 0.16 | 1.92 ± 0.18 | 0.81 | .444 | 0.75 | .003 |
| Free Cholesterol, VLDL-1 | 1.92 ± 0.22 | 1.60 ± 0.21 | 0.85 ± 0.15 | 1.2 | .742 | 0.53 | .001 |
| Free Cholesterol, VLDL-2 | 0.80 ± 0.15 | 0.59 ± 0.09 | 0.39 ± 0.06 | 1.36 | .684 | 0.66 | .001 |
| Free Cholesterol, VLDL-3 | 0.80 ± 0.18 | 0.60 ± 0.12 | 0.35 ± 0.09 | 1.36 | .799 | 0.58 | .029 |
| Free Cholesterol, VLDL-4 | 1.37 ± 0.30 | 1.39 ± 0.29 | 1.01 ± 0.25 | 0.99 | .964 | 0.73 | .097 |
| Free Cholesterol, VLDL-5 | 0.81 ± 0.12 | 1.03 ± 0.11 | 0.57 ± 0.10 | 0.79 | .659 | 0.55 | .001 |
| Phospholipids, VLDL-1 | 4.28 ± 0.51 | 3.11 ± 0.36 | 2.22 ± 0.26 | 1.38 | .444 | 0.71 | .007 |
| Phospholipids, VLDL-2 | 1.95 ± 0.31 | 1.38 ± 0.18 | 1.31 ± 0.11 | 1.41 | .497 | 0.95 | .637 |
| Phospholipids, VLDL-3 | 2.28 ± 0.40 | 1.66 ± 0.28 | 1.36 ± 0.21 | 1.37 | .668 | 0.82 | .286 |
| Phospholipids, VLDL-4 | 3.31 ± 0.51 | 3.61 ± 0.47 | 3.06 ± 0.39 | 0.92 | .882 | 0.85 | .186 |
| Phospholipids, VLDL-5 | 2.23 ± 0.23 | 2.59 ± 0.16 | 2.10 ± 0.19 | 0.86 | .659 | 0.81 | .010 |
| LDL Subfractions | |||||||
| Triglycerides, LDL-1 | 5.17 ± 0.57 | 7.15 ± 0.65 | 6.19 ± 0.63 | 0.72 | .403 | 0.87 | .009 |
| Triglycerides, LDL-2 | 2.32 ± 0.17 | 2.74 ± 0.24 | 2.92 ± 0.28 | 0.85 | .591 | 1.07 | .279 |
| Triglycerides, LDL-3 | 2.24 ± 0.18 | 2.89 ± 0.21 | 2.98 ± 0.21 | 0.77 | .403 | 1.03 | .426 |
| Triglycerides, LDL-4 | 1.57 ± 0.27 | 1.71 ± 0.26 | 2.22 ± 0.25 | 0.92 | .882 | 1.30 | .029 |
| Triglycerides, LDL-5 | 1.19 ± 0.17 | 1.59 ± 0.20 | 2.18 ± 0.21 | 0.75 | .527 | 1.37 | .022 |
| Triglycerides, LDL-6 | 1.82 ± 0.18 | 2.60 ± 0.17 | 4.13 ± 0.18 | 0.70 | .100 | 1.59 | <.001 |
| Cholesterol, LDL-1 | 24.7 ± 1.5 | 29.5 ± 2.3 | 27.2 ± 2.7 | 0.84 | .444 | 0.92 | .111 |
| Cholesterol, LDL-2 | 20.1 ± 2.4 | 20.5 ± 2.0 | 19.2 ± 2.0 | 0.98 | .934 | 0.94 | .501 |
| Cholesterol, LDL-3 | 17.9 ± 2.1 | 16.7 ± 2.0 | 14.8 ± 1.7 | 1.07 | .882 | 0.89 | .186 |
| Cholesterol, LDL-4 | 14.5 ± 1.7 | 12.2 ± 1.7 | 11.0 ± 1.8 | 1.19 | .759 | 0.90 | .426 |
| Cholesterol, LDL-5 | 9.60 ± 0.91 | 9.43 ± 1.27 | 10.9 ± 1.8 | 1.02 | .934 | 1.16 | .296 |
| Cholesterol, LDL-6 | 11.4 ± 1.0 | 16.5 ± 1.0 | 21.7 ± 1.4 | 0.69 | .046 | 1.31 | .009 |
| Free Cholesterol, LDL-1 | 6.77 ± 0.46 | 8.37 ± 0.64 | 7.79 ± 0.77 | 0.81 | .403 | 0.93 | .175 |
| Free Cholesterol, LDL-2 | 5.71 ± 0.65 | 5.91 ± 0.59 | 6.07 ± 0.64 | 0.97 | .909 | 1.03 | .788 |
| Free Cholesterol, LDL-3 | 5.02 ± 0.55 | 5.32 ± 0.56 | 5.56 ± 0.48 | 0.94 | .882 | 1.04 | .449 |
| Free Cholesterol, LDL-4 | 4.01 ± 0.44 | 3.81 ± 0.43 | 4.26 ± 0.42 | 1.05 | .902 | 1.12 | .155 |
| Free Cholesterol, LDL-5 | 2.54 ± 0.27 | 2.70 ± 0.28 | 3.38 ± 0.41 | 0.94 | .882 | 1.25 | .049 |
| Free Cholesterol, LDL-6 | 2.70 ± 0.27 | 3.77 ± 0.24 | 5.04 ± 0.35 | 0.72 | .135 | 1.34 | .004 |
| Phospholipids, LDL-1 | 14.0 ± 0.7 | 16.7 ± 1.2 | 15.6 ± 1.4 | 0.84 | .435 | 0.93 | .104 |
| Phospholipids, LDL-2 | 11.3 ± 1.2 | 11.5 ± 1.0 | 11.0 ± 1.0 | 0.98 | .909 | 0.96 | .537 |
| Phospholipids, LDL-3 | 10.1 ± 1.0 | 9.56 ± 1.01 | 8.70 ± 0.82 | 1.06 | .882 | 0.91 | .202 |
| Phospholipids, LDL-4 | 8.25 ± 0.89 | 7.11 ± 0.85 | 6.50 ± 0.91 | 1.16 | .785 | 0.91 | .428 |
| Phospholipids, LDL-5 | 5.68 ± 0.47 | 5.48 ± 0.62 | 6.29 ± 0.91 | 1.04 | .906 | 1.15 | .287 |
| Phospholipids, LDL-6 | 7.31 ± 0.50 | 9.79 ± 0.46 | 12.4 ± 0.7 | 0.75 | .046 | 1.27 | .006 |
| Apo-B, LDL-1 | 12.7 ± 0.7 | 15.6 ± 1.2 | 14.6 ± 1.4 | 0.81 | .403 | 0.94 | .186 |
| Apo-B, LDL-2 | 11.2 ± 1.1 | 11.5 ± 1.0 | 10.6 ± 1.0 | 0.97 | .909 | 0.92 | .312 |
| Apo-B, LDL-3 | 10.2 ± 1.0 | 9.83 ± 1.09 | 9.03 ± 0.89 | 1.04 | .906 | 0.92 | .264 |
| Apo-B, LDL-4 | 8.83 ± 1.05 | 7.41 ± 1.03 | 6.31 ± 1.10 | 1.19 | .759 | 0.85 | .264 |
| Apo-B, LDL-5 | 6.46 ± 0.60 | 6.65 ± 0.86 | 7.81 ± 1.14 | 0.97 | .909 | 1.17 | .249 |
| Apo-B, LDL-6 | 9.86 ± 0.71 | 14.3 ± 0.8 | 17.5 ± 1.1 | 0.69 | .029 | 1.22 | .017 |
Note: Data are shown as mean ± SEM with p-values adjusted for multiple comparisons using the Benjamini–Hochberg Adjustment. LDL = Low-density lipoprotein; VLDL = Very low-density lipoprotein.
Table 3.
Lipoprotein HDL Subfraction
| Young N = 12 |
Old N = 12 |
Old n-3 N = 12 |
Young vs Old | Old n-3 vs Old | |||
|---|---|---|---|---|---|---|---|
| Ratio | Adj p | Ratio | Adj p | ||||
| HDL Subfractions | |||||||
| Triglycerides, HDL-1 | 2.24 ± 0.38 | 3.54 ± 0.52 | 4.81 ± 0.74 | 0.63 | .403 | 1.36 | .004 |
| Triglycerides, HDL-2 | 1.55 ± 0.24 | 1.67 ± 0.21 | 2.06 ± 0.25 | 0.93 | .882 | 1.23 | .005 |
| Triglycerides, HDL-3 | 2.06 ± 0.28 | 1.97 ± 0.20 | 1.94 ± 0.17 | 1.05 | .906 | 0.98 | .825 |
| Triglycerides, HDL-4 | 3.10 ± 0.36 | 2.92 ± 0.21 | 2.07 ± 0.20 | 1.06 | .882 | 0.71 | .001 |
| Cholesterol, HDL-1 | 13.3 ± 2.0 | 19.5 ± 1.9 | 27.2 ± 3.1 | 0.68 | .403 | 1.39 | .004 |
| Cholesterol, HDL-2 | 8.47 ± 0.80 | 8.98 ± 0.64 | 11.2 ± 0.9 | 0.94 | .882 | 1.25 | .003 |
| Cholesterol, HDL-3 | 11.1 ± 0.7 | 10.5 ± 0.5 | 10.5 ± 0.5 | 1.06 | .837 | 1.00 | .921 |
| Cholesterol, HDL-4 | 19.7 ± 1.1 | 18.7 ± 0.7 | 14.1 ± 1.0 | 1.05 | .806 | 0.75 | <.001 |
| Free Cholesterol, HDL-1 | 2.81 ± 0.47 | 3.52 ± 0.50 | 5.49 ± 0.64 | 0.80 | .742 | 1.56 | .001 |
| Free Cholesterol, HDL-2 | 1.77 ± 0.19 | 1.54 ± 0.18 | 1.94 ± 0.18 | 1.15 | .799 | 1.26 | .002 |
| Free Cholesterol, HDL-3 | 1.75 ± 0.20 | 1.50 ± 0.14 | 1.38 ± 0.10 | 1.17 | .742 | 0.92 | .186 |
| Free Cholesterol, HDL-4 | 2.99 ± 0.31 | 2.71 ± 0.10 | 1.90 ± 0.21 | 1.10 | .806 | 0.70 | .002 |
| Phospholipids, HDL-1 | 17.5 ± 2.3 | 23.6 ± 2.5 | 31.6 ± 3.7 | 0.74 | .742 | 1.34 | .001 |
| Phospholipids, HDL-2 | 14.0 ± 1.2 | 14.4 ± 1.0 | 16.5 ± 1.2 | 0.97 | .906 | 1.15 | .007 |
| Phospholipids, HDL-3 | 18.5 ± 1.2 | 17.3 ± 0.7 | 15.8 ± 0.7 | 1.07 | .806 | 0.91 | .001 |
| Phospholipids, HDL-4 | 29.0 ± 1.4 | 27.0 ± 0.6 | 19.6 ± 1.3 | 1.07 | .666 | 0.73 | <.001 |
| Apo-A1, HDL-1 | 18.9 ± 3.4 | 27.7 ± 3.5 | 39.7 ± 5.2 | 0.68 | .444 | 1.43 | .004 |
| Apo-A1, HDL-2 | 18.6 ± 1.3 | 19.4 ± 1.0 | 19.7 ± 1.2 | 0.96 | .882 | 1.01 | .646 |
| Apo-A1, HDL-3 | 29.0 ± 1.8 | 27.8 ± 1.0 | 26.1 ± 0.9 | 1.04 | .882 | 0.94 | .009 |
| Apo-A1, HDL-4 | 70.6 ± 3.5 | 68.8 ± 1.5 | 55.0 ± 2.7 | 1.03 | .882 | 0.80 | <.001 |
| Apo-A2, HDL-1 | 2.13 ± 0.34 | 2.42 ± 0.36 | 3.18 ± 0.48 | 0.88 | .882 | 1.31 | .006 |
| Apo-A2, HDL-2 | 3.62 ± 0.32 | 3.16 ± 0.27 | 3.30 ± 0.31 | 1.14 | .742 | 1.04 | .473 |
| Apo-A2, HDL-3 | 6.93 ± 0.53 | 5.63 ± 0.33 | 5.34 ± 0.26 | 1.23 | .403 | 0.95 | .186 |
| Apo-A2, HDL-4 | 18.8 ± 1.1 | 16.3 ± 0.6 | 11.2 ± 1.0 | 1.15 | .403 | 0.69 | <.001 |
Note: Data are shown as mean ± SEM with p-values adjusted for multiple comparisons using the Benjamini–Hochberg Adjustment. HDL = High-density lipoprotein.
Total cholesterol was unchanged with age or n3-PUFAs, but n3-PUFAs decreased total VLDL cholesterol (esterified and free), decreased IDL cholesterol (free only), increased HDL cholesterol (esterified and free) with no changes in IDL (esterified) or LDL cholesterol fractions (Table 1). Within the VLDL subfractions, cholesterol was reduced across all five VLDL subfractions following the n3-PUFAs (Table 2). Across the six LDL subfractions, there was an increase in cholesterol in the small, dense LDL-5 (free only) and LDL-6 fractions (free and esterified) after the intervention (Table 2). There were no significant effects of n3-PUFAs on cholesterol content of the larger LDL fractions (LDL1-4). The free and esterified cholesterol content in HDL fractions was increased in the less dense fractions (HDL-1, HDL-2) and decreased in the denser fractions (HDL-3, HDL-4) (Table 3). Similar patterns were observed for phospholipids, Apo-A1, and Apo-A2, which were increased following n3-PUFA supplementation in the less dense HDL fractions (HDL-1, HDL-2) and decreased in the more dense fractions (HDL-3, HDL-4) (Table 3).
Aside from the aforementioned shift in the triglyceride content of LDL subfractions, there were negligible changes following n3-PUFA supplementation in the cholesterol, phospholipid, or Apo-B content of any of the LDL subfractions with the exception of the smallest, most dense LDL-6 (Table 2). Following the intervention, LDL-6 particles increased significantly in triglyceride content, cholesterol (free and esterified), phospholipids, and Apo-B. There was also a notable increase in LDL-6 particle number in older adults that was further increased following n3-PUFA supplementation (Table 2).
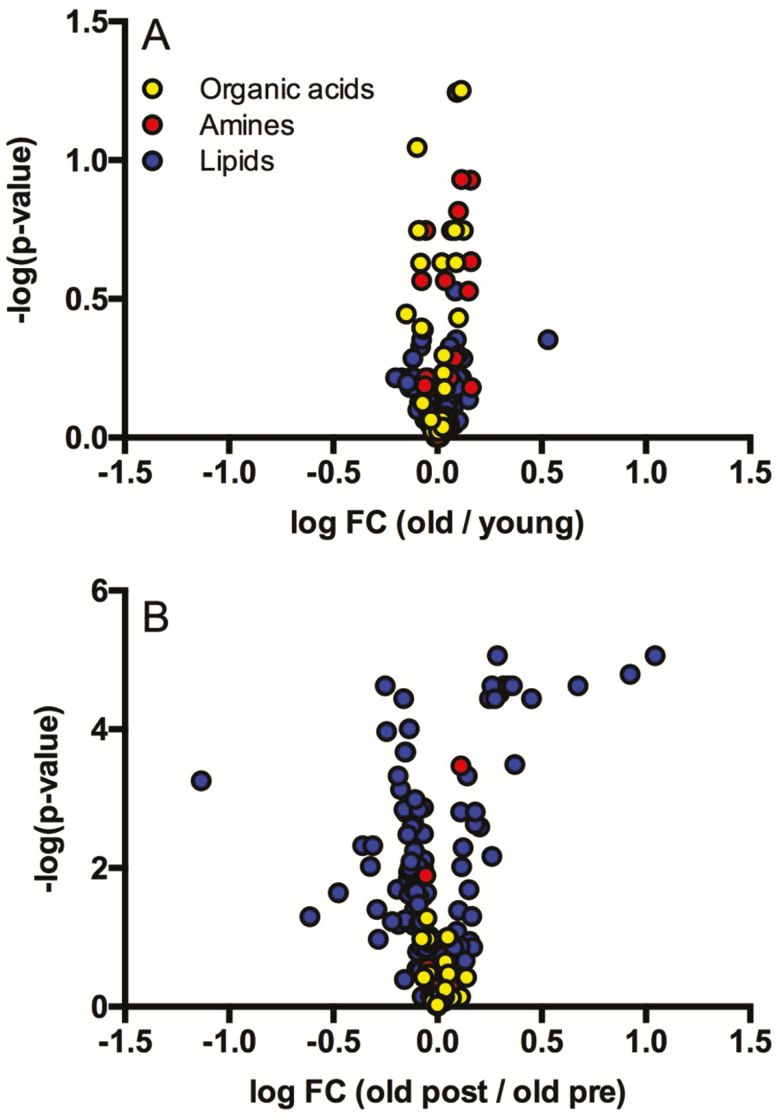
We used a quantitative analytical approach involving NMR and mass spectrometry platforms to measure the concentrations of >250 plasma metabolites in healthy young and older adults (Figure 1A, Supplementary Tables 1–5). The analytes detected included acylcarnitines, organic acids, amino acids, biogenic amines, glycerophospholipids, sphingolipids, cholesterol esters, diglycerides, and triglycerides. Of these metabolites, only urea reached significance (elevated in older adults) after adjusting for false discovery in the comparison of young and older adults before n3-PUFA supplementation (Supplementary Table S1). There were several noteworthy metabolites that were on average at least 25% higher in plasma from older adults compared to young, including citrate, kynurenine, 2-oxoglutarate, cis-aconitate, glycerol, lactate, ornithine, myo-inositol, and 1-methylhistidine (Supplementary Tables S1 and S2) but did not reach significance based on FDR corrected p-values. Following n3-PUFA supplementation, there were modest changes in amino acids including significant increases in hydroxyproline and decreases in kynurenine (Figure 1B, Supplementary Tables S1 and S2). Notable metabolites that exhibited marginal but nonsignificant effects of n3-PUFA supplementation include lactate, pyruvate, 3-hydroxybutyrate, alanine, phenylalanine, and tryptophan. The most pronounced changes in response to n3-PUFA supplementation were observed in various lipid classes, including phospholipids, cholesterol esters, diglycerides, and triglycerides, which demonstrated an overall pattern (Figure 1B, Supplementary Tables S3–S5) where the majority of these lipid species were decreased following n3-PUFA supplementation with the exception of the individual species containing long chain polyunsaturated acyl chains, which increased in response to n3-PUFA.
Figure 1.
Quantitative plasma metabolomics. Quantitative metabolomics was performed in plasma using 1H-NMR and MS analytical platforms. Volcano plots compare metabolite abundance in old preintervention with young (panel A) and old postintervention with old preintervention (panel B).
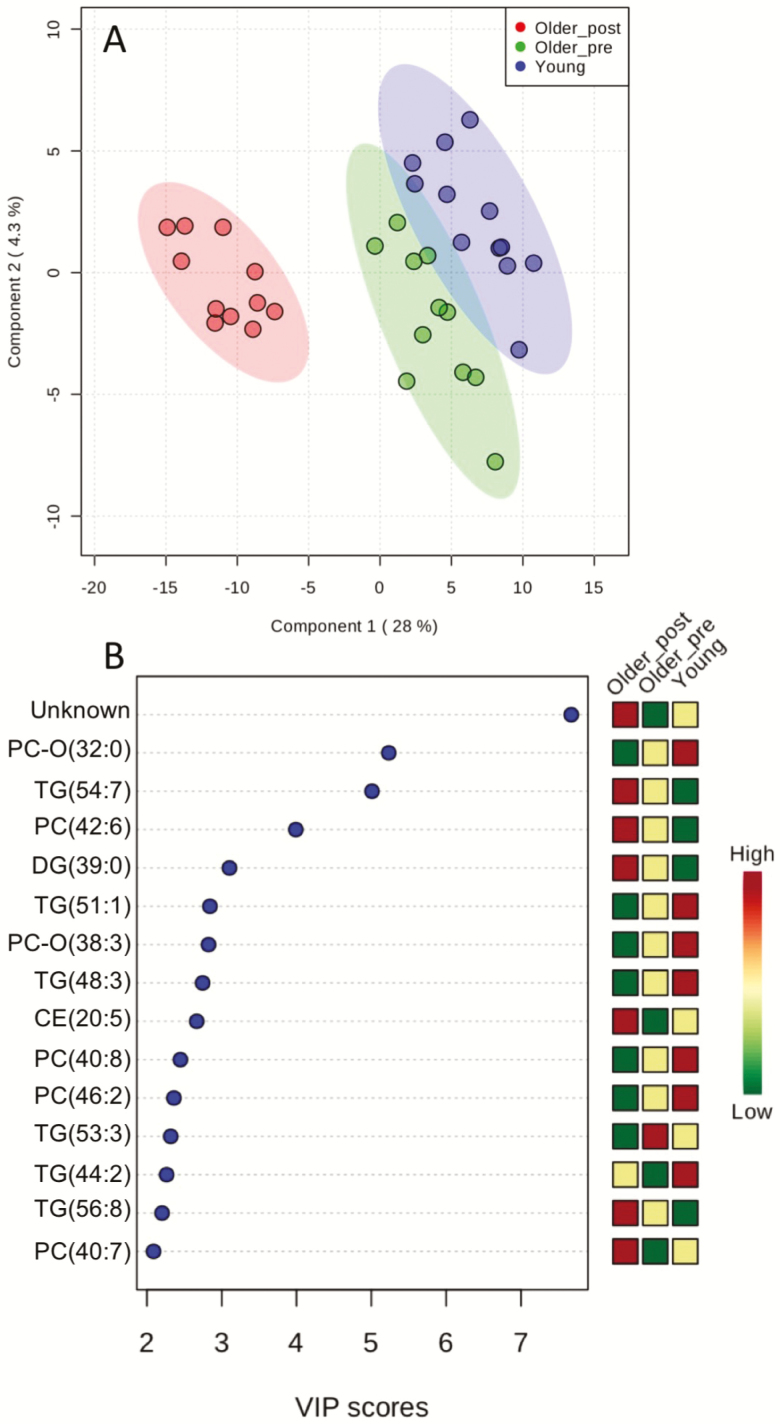
A merged data set containing metabolite concentrations measured by NMR and MS was further analyzed by partial least squares discriminant analysis (PLS-DA) to differentiate the plasma of young adults and older adults before and after n3-PUFA supplementation. The scores plot of component 1 versus component 2 (Figure 2A) shows modest separation of young and old baseline plasma samples, but obvious clustering and separation of samples from older adults before versus after n3-PUFA supplementation. The variable importance in projection (VIP) scores indicates that 14 of the top 15 metabolites contributing to the PLS model were lipids (6 phospholipids, 6 triglycerides, 1 diglyceride, and 1 cholesterol ester; Figure 2B). Notably, the metabolite feature that emerged as the most important feature contributing to the model was an unknown metabolite detected by NMR profiling. The signals originating from this unidentified component were absent from the NMR spectra of young and older adults at baseline, but clearly visible after n3-PUFA supplementation (Supplementary Figures S1 and S2). Specifically, the signals at δ 0.85 (t, J = 7.4 Hz, 3H,), δ 1.56 (sextet, J = 7.4, Hz 2H), δ 2.50 (t, J = 7.4 Hz, 2H), and δ 1.96 ppm (s, 3H) could be attributed to propyl and methyl group of the unknown component (Supplementary Figure S3). When the spectrum of older adults at baseline was subtracted from its counterpart after the supplementation with highest content of the unknown, the spectral difference revealed two additional signals at δ2.46, (d, J = 7.8 Hz, 2H,) δ3.02, (d, J = 7.8 Hz, 2H) that could be assigned to two mutually coupled CH2 groups (Supplementary Figures S4 and S5). Yet this 1H-NMR information had no match in Chenomx and HMDB databases and was not sufficient for structure elucidation. In an attempt to resolve the identity of this unknown metabolite, we performed additional discovery-based untargeted metabolomics using LC-MS and GC-MS in a subset of four individuals who exhibited the most robust changes in this particular unknown metabolite. A notable difference was observed in the LC-MS chromatogram recorded using a C18 column in negative detection mode. A strong peak at 7.99 minutes appeared in plasma samples from older adults following n3-PUFA, which was absent from baseline samples (Supplementary Figure S6). The peak exhibited [M-H]− at m/z 239.0930, corresponding to molecular formula C12H16O5. The METLIN library search identified component as 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid (Supplementary Figure S7). In C18-positive mode, the peak at the same RT was observed, showing [M+Na]+ at m/z 263.0892 and several other adducts, all in accordance with C12H16O5 formula (Supplementary Figure S8). Again the first library search hit was 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid (CMPF) (Supplementary Figure S9). For further confirmation of the identity of the unknown metabolite, additional untargeted metabolomics was performed using an optimized GC-MS assay using the Fiehn method (Agilent G1676AA) with MOX/MSTFA derivatization. The major difference between baseline and n3-PUFA supplementation was a component at 18.28 minutes, with molecular ion at m/z 384 and base peak at m/z 266 (Supplementary Figure S10). Fiehn and NIST libraries search showed no positive match, but the present EI MS spectrum was in accordance with the structure of CMPF-2TMS and available literature (23). The observed NMR signals originating from the unknown compound were, indeed, in good agreement with published data, taking into account that the different solvents were used (24). Thus, by leveraging several complementary analytical platforms, we were able to determine that the abundant unknown compound observed in plasma of older adults following n3-PUFA supplementation is (CMPF), a major metabolite of furan fatty acids.
Figure 2.
Partial Least Squares – Discriminant Analysis (PLS-DA). PLS-DA was performed using combined NMR and MS data sets. (A) Scores plot between the selected components with the explained variances are shown in brackets for each component. (B) Important features identified by PLS-DA. The colored boxes on the right show the relative concentrations of each metabolite in each group.
Discussion
There is considerable evidence that n3-PUFAs reduce circulating lipids (25,26), but fewer studies have examined the effects of n3-PUFAs on serum lipoproteins with attention to particle size, number, and composition, which provide a better window into cardiovascular risk. These studies have been historically done in overweight, obese, or hyperlipidemic individuals (27–29) whereas we targeted healthy, nonobese, nonhypertriglyceridemic older adults. The main effects of n3-PUFA supplementation in this population were a reduction in total TG, a reduction in VLDL particle number, a modest increase in HDL cholesterol, a shift in the composition of HDL subclasses, and no significant effects on LDL subclasses other than a significant increase in small, dense LDL particles.
Our finding that n3-PUFAs lowered total circulating TG without changing total cholesterol or LDL cholesterol is consistent with prior reports in other populations (25,28), but the current study demonstrates that this holds true in healthy older adults with normal triglycerides. Although total Apo-B100 levels did not change with n3-PUFAs, there was a significant decrease in VLDL Apo-B levels, suggesting that the TG lowering effects that we observed in healthy older adults could be at least partially mediated by reduction in VLDL apo B production as shown by others (30–32) rather than increased catabolism of Apo B-containing LDL or IDL, which remained unchanged following n3-PUFAs in the current study.
The effects of n3-PUFAs on HDL are less consistent in the literature with some studies reporting that HDL is increased (33,34), decreased (25), or unchanged (28,29,35). We find a modest but significant increase in total HDL levels following n3-PUFA supplementation in healthy older adults. While this finding may suggest some cardiovascular benefit of n3-PUFAs beyond lowering TGs, we further examined this possibility by examining the composition of the various HDL subclasses, which exhibit considerable heterogeneity in size and composition. Of particular interest, we observed that the smaller, dense HDL particles contained more cholesterol following n3-PUFA supplementation whereas the larger, less dense particles contained less cholesterol. This observation is consistent with the notion that n3-PUFAs may improve HDL function as more cholesterol is scavenged by the small, dense LDLs and more cholesterol is offloaded to the liver for hepatobiliary secretion by the large, dense HDL particles (ie, greater cholesterol efflux capacity). This finding is consistent with prior studies that showed that n3-PUFA consumption is associated with increased large, less dense HDL and decreased small, dense HDL subspecies (34,36,37). Although we did not measure HDL function in terms of reverse cholesterol transport, antioxidant, and anti-inflammatory activities, there is a growing body of literature supporting a beneficial role for n3-PUFAs in HDL function (38). It is possible that the observed reduction in some cholesterol esters (eg, CE(18:3)) may reflect such influence on HDL function, although this possibility requires further investigation.
The NMR lipoprotein subclass analysis altogether suggests potentially antiatherogenic effects of n3-PUFAs in healthy, nonhypertyglyceridemic older adults based on observed decreased VLDL particle number, decreased TG, increased HDL, and shifted composition of HDL subclasses suggestive of improved HDL function. It was therefore surprising to observe a significant increase in the number of small, dense LDL particles, which are recognized for their atherogenic properties because of the ease at which they penetrate vessel walls, delayed clearance from plasma, affinity for proteoglycans, and susceptibility to oxidation (39,40). This finding is in contrast to previous studies that report lower levels of small, dense LDL particles following n3-PUFA administration (41–43). However, these studies enrolled patients with hypertriglyceridemia whereas studies in patients with normal TGs, including ours, showed that n3-PUFAs increase small, dense LDLs (27) and increased LDL apo-B concentrations (44). In terms of cardiovascular disease risk factors, the participants in this study were healthy, relatively lean older adults, without evidence of hypertension, diabetes, or hypertriglyceridemia (13). Nevertheless, n3-PUFA administration improved several established parameters that define cardiovascular risk, including lowering systolic blood pressure, decreasing total cholesterol, and lowering fasting insulin (13).
Beyond their influence on circulating lipid profiles, other biological effects of dietary n3-PUFAs have not been characterized in detail. There was an early flurry of excitement about potential metabolic benefits of n3-PUFAs in glucose metabolism and insulin sensitivity, although the promising findings from preclinical models have been difficult to reproduce in humans (10). Emerging evidence suggests that n3-PUFAs may have beneficial influence on mitochondrial physiology (13,45) and muscle protein metabolism (13,15), although the mechanisms have not been fully unveiled. In an effort to help characterize some less well-recognized effects of n3-PUFA supplementation in healthy older adults, we performed untargeted metabolite profiling of peripheral blood by NMR and MS platforms. Consistent with known anabolic effects of n3-PUFAs (13,15), we observed significant increases in hydroxyproline following supplementation in older adults. Hydroxyproline is produced by hydroxylation of proline during protein synthesis (46). Indeed, we previously reported significant increases in muscle protein synthesis following n3-PUFA supplementation in these individuals (13). We also observed significant reductions in circulating kynurenine levels in older adults following n3-PUFA supplementation. The kynurenine pathway of tryptophan degradation is increased in response to inflammation (47), and activation of this pathway is associated with depression (48). A previous study demonstrated that fish oil supplementation attenuated the activation of the kynurenine pathway upon administration of lipopolysaccharide as a model of sickness behavior (49). Another recent paper describes how n3-PUFAs protect against hippocampal neurogenesis in response to IL-1β by modulating the kynurenine pathway (50). Here, we show that n3-PUFAs significantly reduce circulating kynurenine levels in healthy older adults who exhibited modest elevations in kynurenine compared to young as well as increased circulating inflammatory markers such as interleukin 6 (13). The possibility that n3-PUFAs may confer benefits to individuals with age-related neurodegenerative diseases by modulating the kynurenine pathway will require further investigation.
Substantial efforts were made to identify the major unknown compound observed by 1H-NMR profiling because this compound was the strongest contributor to the PLS-DA separation of plasma samples before and after n3-PUFA supplementation. We identified this compound as 3‐carboxy‐4‐methyl‐5‐propyl‐2‐furanpropionic acid (CMPF) through a combination of NMR, LC-MS, and GC-MS metabolite profiling. CMPF is a furan fatty acid that is strongly associated with dietary fish intake (51). The precedent literature is mixed regarding the biological effects of CMPF. Plasma levels of CMPF were found to be elevated in humans with gestational diabetes and type 2 diabetes (52). The authors go on to show that CMPF is detrimental to islet function through a series of experiments where CMPF impaired glucose-stimulated insulin secretion when administered to mice or to cultured islets (52). In the current study, we observe plasma concentrations of CMPF approximately 50 μM, which is substantially lower than reported CMPF levels in diabetic patients (52). Furthermore, we found that fasting plasma insulin and HOMA-IR were significantly decreased following n3-PUFA supplementation in these subjects (13), suggesting that insulin sensitivity was improved by n3-PUFA supplementation despite increasing CMPF levels. In agreement with this, another study showed that the increase in CMPF levels following fish consumption was not associated with impaired glucose metabolism (53). The source of elevated CMPF in diabetic patients is currently unknown, but early evidence suggests that the milder elevation in CMPF resulting from n3-PUFA supplementation is not detrimental to glucose metabolism.
In conclusion, the current study shows that n3-PUFA supplementation in healthy older adults with normal triglycerides results in significant reduction in total TG, reduction in VLDL particle number, modestly increased HDL cholesterol, and a shift in the composition of HDL subclasses. Furthermore, n3-PUFA supplementation decreased levels of many diacylglycerols, phospholipids, and triacylglycerols, which are associated with metabolic and cardiovascular disease (54,55). It is important to point out that the current study design cannot address the possible temporal or seasonal changes in circulating lipids or metabolites. Another caveat of the study design is that controlling diet for 3 days prior to blood sampling may obscure potential metabolite differences between young and old related to dietary intake. Although plasma metabolomics revealed subtle differences between healthy young and older adults, n3-PUFA supplementation in older adults was accompanied by pronounced changes in phospholipids, cholesterol esters, diglycerides, and triglycerides. Furthermore, significant changes in hydroxyproline, kynurenine, and CMPF following n3-PUFA supplementation provide further insight into some less well-recognized biological effects of n3-PUFA supplementation.
Supplementary Material
Acknowledgments
We are grateful for the expert technical assistance from Roberta Soderberg, Katherine Klaus, Dawn Morse, and the excellent clinical support and services of the CRTU, the Dan Abraham Healthy Living Center, and the Mayo Clinic Metabolomics Core Laboratory.
Funding
This work was supported by the Clinical and Translational Science Award KL2 TR-000136, and the CTSA Grant Number UL TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (NIH). Additional funding was provided by the Strickland Career Development Award and U24DK100469 from the National Institute of Diabetes and Digestive and Kidney Diseases, which originates from the NIH Director’s Common Fund. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
None reported.
References
- 1. Kochanek KD, Murphy S, Xu J, Arias E.. Mortality in the United States, 2016. NCHS Data Brief. Atlanta, GA: CDC/NCHS. 2017:1–8. PMID: 29319473. [PubMed] [Google Scholar]
- 2. Global health Estimates. 2016: Deaths by Cause, Age, Sex, by Country and Region, 2000–2016. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 3. Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi:10.1016/S0002-9343(01)01114-7 [DOI] [PubMed] [Google Scholar]
- 4. Harris WS, Ginsberg HN, Arunakul N, et al. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4:385–391. [PubMed] [Google Scholar]
- 5. Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi:10.1016/j.cell.2010.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spencer M, Finlin BS, Unal R, et al. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. 2013;62:1709–1717. doi:10.2337/db12-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marik PE, Varon J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin Cardiol. 2009;32:365–372. doi:10.1002/clc.20604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med. 2011;364:2439–2450. doi:10.1056/NEJMra1008153 [DOI] [PubMed] [Google Scholar]
- 9. Lalia AZ, Johnson ML, Jensen MD, Hames KC, Port JD, Lanza IR. Effects of dietary n-3 fatty acids on hepatic and peripheral insulin sensitivity in insulin-resistant humans. Diabetes Care. 2015;38:1228–1237. doi:10.2337/dc14-3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lalia AZ, Lanza IR. Insulin-sensitizing effects of omega-3 fatty acids: lost in translation? Nutrients. 2016;8. doi:10.3390/nu8060329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi:10.1038/nm1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi:10.1093/ajcn/79.6.935 [DOI] [PubMed] [Google Scholar]
- 13. Lalia AZ, Dasari S, Robinson MM, et al. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging (Albany NY). 2017;9:1096–1129. doi:10.18632/aging.101210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodacki CL, Rodacki AL, Pereira G, et al. Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr. 2012;95:428–436. doi:10.3945/ajcn.111.021915 [DOI] [PubMed] [Google Scholar]
- 15. Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93:402–412. doi:10.3945/ajcn.110.005611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Del Gobbo LC, Imamura F, Aslibekyan S, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: Pooling Project of 19 cohort studies. JAMA Intern Med. 2016;176:1155–1166. doi:10.1001/jamainternmed.2016.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hooper L, Thompson RL, Harrison RA, et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–760. doi:10.1136/bmj.38755.366331.2F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu JH, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107(Suppl 2):S214–S227. doi:10.1017/S0007114512001602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi:10.1126/science.1174621 [DOI] [PubMed] [Google Scholar]
- 20. Gawrisch K, Eldho NV, Holte LL. The structure of DHA in phospholipid membranes. Lipids. 2003;38:445–452. doi:10.1007/s11745-003-1082-0 [DOI] [PubMed] [Google Scholar]
- 21. Dutta T, Chai HS, Ward LE, et al. Concordance of changes in metabolic pathways based on plasma metabolomics and skeletal muscle transcriptomics in type 1 diabetes. Diabetes. 2012;61:1004–1016. doi:10.2337/db11-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kind T, Wohlgemuth G, Lee DY, et al. FiehnLib: mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal Chem. 2009;81:10038–10048. doi:10.1021/ac9019522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sassa T , Matsuno H, Niwa M, et al. Measurement of furancarboxylic acid, a candidate for uremic toxin, in human serum, hair, and sweat, and analysis of pharmacological actions in vitro. Arch Toxicol. 2000; 73:649–654. [DOI] [PubMed] [Google Scholar]
- 24. Nagy E, Liu Y, Prentice KJ, et al. Synthesis and characterization of urofuranoic acids: in vivo metabolism of 2-(2-carboxyethyl)-4-methyl-5-propylfuran-3-carboxylic acid (CMPF) and effects on in vitro insulin secretion. J Med Chem. 2017;60:1860–1875. doi:10.1021/acs.jmedchem.6b01668 [DOI] [PubMed] [Google Scholar]
- 25. Phillipson BE, Rothrock DW, Connor WE, Harris WS, Illingworth DR. Reduction of plasma lipids, lipoproteins, and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N Engl J Med. 1985;312:1210–1216. doi:10.1056/NEJM198505093121902 [DOI] [PubMed] [Google Scholar]
- 26. Harris WS. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res. 1989;30:785–807. [PubMed] [Google Scholar]
- 27. Mostad IL, Bjerve KS, Lydersen S, Grill V. Effects of marine n-3 fatty acid supplementation on lipoprotein subclasses measured by nuclear magnetic resonance in subjects with type II diabetes. Eur J Clin Nutr. 2008;62:419–429. doi:10.1038/sj.ejcn.1602703 [DOI] [PubMed] [Google Scholar]
- 28. Padro T, Vilahur G, Sánchez-Hernández J, et al. Lipidomic changes of LDL in overweight and moderately hypercholesterolemic subjects taking phytosterol- and omega-3-supplemented milk. J Lipid Res. 2015;56:1043–1056. doi:10.1194/jlr.P052217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mori TA, Burke V, Puddey IB, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–1094. doi:10.1093/ajcn/71.5.1085 [DOI] [PubMed] [Google Scholar]
- 30. Nestel PJ, Connor WE, Reardon MF, Connor S, Wong S, Boston R. Suppression by diets rich in fish oil of very low density lipoprotein production in man. J Clin Invest. 1984;74:82–89. doi:10.1172/JCI111422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan DC, Watts GF, Mori TA, Barrett PH, Redgrave TG, Beilin LJ. Randomized controlled trial of the effect of n-3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am J Clin Nutr. 2003;77:300–307. doi:10.1093/ajcn/77.2.300 [DOI] [PubMed] [Google Scholar]
- 32. Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65(5 Suppl):1645S–1654S. doi:10.1093/ajcn/65.5.1645S [DOI] [PubMed] [Google Scholar]
- 33. Suzukawa M, Abbey M, Howe PR, Nestel PJ. Effects of fish oil fatty acids on low density lipoprotein size, oxidizability, and uptake by macrophages. J Lipid Res. 1995;36:473–484. [PubMed] [Google Scholar]
- 34. Thomas TR, Smith BK, Donahue OM, Altena TS, James-Kracke M, Sun GY. Effects of omega-3 fatty acid supplementation and exercise on low-density lipoprotein and high-density lipoprotein subfractions. Metabolism. 2004;53:749–754. doi:10.1016/j.metabol.2003.12.019 [DOI] [PubMed] [Google Scholar]
- 35. Sanchez-Muniz FJ, Bastida S, Viejo JM, Terpstra AH. Small supplements of N-3 fatty acids change serum low density lipoprotein composition by decreasing phospholid and apolipoprotein B concentrations in young adult women. Eur J Nutr. 1999;38:20–27. doi:10.1007/s003940050042 [DOI] [PubMed] [Google Scholar]
- 36. Calabresi L, Villa B, Canavesi M, et al. An omega-3 polyunsaturated fatty acid concentrate increases plasma high-density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metabolism. 2004;53:153–158. doi:10.1016/j.metabol.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 37. Bogl LH, Maranghi M, Rissanen A, Kaprio J, Taskinen MR, Pietiläinen KH. Dietary omega-3 polyunsaturated fatty acid intake is related to a protective high-density lipoprotein subspecies profile independent of genetic effects: a monozygotic twin pair study. Atherosclerosis. 2011;219:880–886. doi:10.1016/j.atherosclerosis.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 38. Pizzini A, Lunger L, Demetz E, et al. The role of omega-3 fatty acids in reverse cholesterol transport: a review. Nutrients. 2017;9. doi:10.3390/nu9101099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamarche B, Tchernof A, Moorjani S, et al. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Québec Cardiovascular Study. Circulation. 1997;95:69–75. [DOI] [PubMed] [Google Scholar]
- 40. Sancho-Rodríguez N, Avilés-Plaza FV, Granero-Fernández E, et al. Observational study of lipid profile and LDL particle size in patients with metabolic syndrome. Lipids Health Dis. 2011;10:162. doi:10.1186/1476-511X-10-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilkinson P, Leach C, Ah-Sing EE, et al. Influence of alpha-linolenic acid and fish-oil on markers of cardiovascular risk in subjects with an atherogenic lipoprotein phenotype. Atherosclerosis. 2005;181:115–124. doi:10.1016/j.atherosclerosis.2004.12.029 [DOI] [PubMed] [Google Scholar]
- 42. Minihane AM, Khan S, Leigh-Firbank EC, et al. ApoE polymorphism and fish oil supplementation in subjects with an atherogenic lipoprotein phenotype. Arterioscler Thromb Vasc Biol. 2000;20:1990–1997. [DOI] [PubMed] [Google Scholar]
- 43. Baumstark MW, Frey I, Berg A, Keul J. Influence of n-3 fatty acids from fish oils on concentration of high- and low-density lipoprotein subfractions and their lipid and apolipoprotein composition. Clin Biochem. 1992;25:338–340. doi:10.1016/0009-9120(92)80012-6 [DOI] [PubMed] [Google Scholar]
- 44. Sullivan DR, Sanders TA, Trayner IM, Thompson GR. Paradoxical elevation of LDL apoprotein B levels in hypertriglyceridaemic patients and normal subjects ingesting fish oil. Atherosclerosis. 1986;61:129–134. doi:10.1016/0021-9150(86)90072-9 [DOI] [PubMed] [Google Scholar]
- 45. Herbst EA, Paglialunga S, Gerling C, et al. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol. 2014;592:1341–1352. doi:10.1113/jphysiol.2013.267336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu G, Bazer FW, Burghardt RC, et al. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids. 2011;40:1053–1063. doi:10.1007/s00726-010-0715-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dantzer R. Role of the Kynurenine metabolism pathway in inflammation-induced depression: preclinical approaches. Curr Top Behav Neurosci. 2017;31:117–138. doi:10.1007/7854_2016_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi:10.1038/sj.mp.4002006 [DOI] [PubMed] [Google Scholar]
- 49. de Gomes MG, Souza LC, Goes AR, et al. Fish oil ameliorates sickness behavior induced by lipopolysaccharide in aged mice through the modulation of kynurenine pathway. J Nutr Biochem. 2018;58:37–48. doi:10.1016/j.jnutbio.2018.05.002 [DOI] [PubMed] [Google Scholar]
- 50. Borsini A, Alboni S, Horowitz MA, et al. Rescue of IL-1β-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav Immun. 2017;65:230–238. doi:10.1016/j.bbi.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hanhineva K, Lankinen MA, Pedret A, et al. Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J Nutr. 2015;145:7–17. doi:10.3945/jn.114.196840 [DOI] [PubMed] [Google Scholar]
- 52. Prentice KJ, Luu L, Allister EM, et al. The furan fatty acid metabolite CMPF is elevated in diabetes and induces β cell dysfunction. Cell Metab. 2014;19:653–666. doi:10.1016/j.cmet.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 53. Lankinen MA, Hanhineva K, Kolehmainen M, et al. CMPF does not associate with impaired glucose metabolism in individuals with features of metabolic syndrome. PLoS One. 2015;10:e0124379. doi:10.1371/journal.pone.0124379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jové M, Naudí A, Portero-Otin M, et al. Plasma lipidomics discloses metabolic syndrome with a specific HDL phenotype. FASEB J. 2014;28:5163–5171. doi:10.1096/fj.14-253187 [DOI] [PubMed] [Google Scholar]
- 55. Li X, Fang P, Li Y, et al. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler Thromb Vasc Biol. 2016;36:1090–1100. doi:10.1161/ATVBAHA.115.306964 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.