Abstract
The underlying causes of migraine headache remained enigmatic for most of the 20th century. In 1979, The Lancet published a novel hypothesis proposing an integral role for the neuropeptide-containing trigeminal nerve. This hypothesis led to a transformation in the migraine field and understanding of key concepts surrounding migraine, including the role of neuropeptides and their release from meningeal trigeminal nerve endings in the mechanism of migraine, blockade of neuropeptide release by anti-migraine drugs, and activation and sensitisation of trigeminal afferents by meningeal inflammatory stimuli and upstream role of intense brain activity. The study of neuropeptides provided the first evidence that antisera directed against calcitonin gene-related peptide (CGRP) and substance P could neutralise their actions. Successful therapeutic strategies using humanised monoclonal antibodies directed against CGRP and its receptor followed from these findings. Nowadays, 40 years after the initial proposal, the trigeminovascular system is widely accepted as having a fundamental role in this highly complex neurological disorder and provides a road map for future migraine therapies.
Introduction
Migraine is a highly prevalent and complex disorder characterised by an episodic, severe, often unilateral throbbing or pulsating headache associated with nausea, photophobia, phonophobia, and sometimes auras.1 Headaches are often the most troubling feature and the causes and treatments have been extensively researched.
In 1979, Moskowitz and colleagues2 introduced the trigeminovascular hypothesis of migraine in The Lancet, calling attention to a key role for the trigeminal nerve and its vasoactive neuropeptide-containing axonal projections to the meninges and its blood vessels. The model underscored the potential importance of released neuropeptides and their downstream effects after trigeminal activation. The trigeminal innervation became framed as a final common pathway for upstream headache initiation and a fundamental template for new therapeutic directions.3 Crucial to the hypothesis was emerging knowledge about the importance of vasoactive neuropeptide mediator substance P followed later by two even more potent vasoactive peptides, calcitonin gene-related peptide (CGRP; now a proven therapeutic target) and pituitary adenylate cyclase-activating polypeptide (PACAP).
The hypothesis was prescient because it predated both the 1981 discovery of the sensory innervation to the circle of Willis, and the identification of neuropeptide mediators within the trigeminovascular system (a term used from 1983 to describe the trigeminal–meningeal–CNS relationship).4,5 Over the ensuing decades, experimental studies provided crucial insights into the neurophysiology of migraine-related pain with therapeutic implications (eg, allodynia and peripheral and central sensitisation), coherent central mechanisms of migraine-related pain processing, and promising efforts using neuroimaging to discern the relationship between blood vessel function and migraine. These subsequent findings provided fundamental support to the 1979 trigeminal nerve hypothesis and its contributions to the intellectual underpinnings and subsequent developments regarding migraine theory and therapeutics 40 years later.
In this Personal View, we review the most notable concepts and advances that have emerged from the identification of the role of the trigeminovascular system in migraine with an emphasis on future implications and for treatment of this disorder. These include a brief review of historical developments, as well as other major developments in anatomy, neurophysiology, pharmacology, neurochemistry, human pathophysiology, and drug development, all identified using neuroimaging.
Early findings and further development of the trigeminovascular model
Anatomy
The term trigeminovascular was introduced to encompass the immunohistochemical and neurochemical findings associated with the trigeminal pathway to pial arteries in multiple species, including humans.5,6 Further studies confirmed this new pathway and the well-known trigeminal innervation of the dura mater (table 1).4,7 In cats, upper cervical dorsal root ganglia contribute additional meningeal innervation and together these path ways provide an anatomical substrate for hemicranial pain.31 Within the meninges, the largest density of small diameter unmyelinated C-fibres and thinly myelinated Aδ-fibre axons (of trigeminal origin) are found in blood vessels. Experimental studies in humans showed that electrical or mechanical stimulation of large meningeal blood vessels are associated with headache, whereas areas remote from vessels often are not.32 In mice, dural axons of nociceptors have been observed issuing pial branches that cross the arachnoid space and suture branches that reach the periosteum and possibly some pericranial muscles. These axons establish a direct route of communication between extracranial and intracranial events that can activate nociceptors on both sides of the calvarial bones.33
Table 1:
Major, original discoveries—the trigeminovascular system
| Year | Brief description | |
|---|---|---|
| The trigeminovascular hypothesis2 | 1979 | Proposed a pathophysiological link between migraine and the trigeminal innervation of the meninges, and a potential role for the undiscovered vasoactive neuropeptide transmitters as therapeutic targets |
| Perivascular meningeal axons project from the trigeminal ganglia4 | 1981 | Sensory innervation to the circle of Willis shown by axonal tracing techniques. Labelled cell bodies found in the ipsilateral trigeminal ganglia after horseradish peroxidase was applied to the feline middle cerebral artery |
| The neuropeptide-containing trigeminovascular system is named5 | 1983 | The trigeminovascular system is named and its first neuropeptide identified. The trigeminovascular system is now considered a functional unit on the basis of anatomy, physiology, and pathology of meningeal afferents and their central connections |
| Neuropeptide within the trigeminovasulcar system is released from meninges8 | 1983 | In vitro release of a vasoactive neuropeptide substance P from its trigeminovascular afferents by calcium-dependent mechanisms, suggesting a role as a neuromediator within the meninges |
| CGRP is released from trigeminal ganglion cells9 | 1984 | Immunoreactive CGRP is spontaneously released by cultured trigeminal ganglion cells in a calcium-dependent manner |
| CGRP and substance P coexist in the trigeminal ganglion and nerve fibres around cerebral blood vessels10 | 1985 | The presence of CGRP in cerebrovascular trigeminal innervation provides further versatility and complexity for this sensory afferent system putatively involved in the transmission of intracranial pain |
| Ergot alkaloids inhibit neuropeptide release11 | 1988 | Pharmacological evidence that ergot alkaloids inhibit neuropeptide release within meninges following electrical trigeminal stimulation. A prejunctional inhibitory receptor-driven mechanism was proposed for ergot alkaloids |
| CGRP released upon activation of the trigeminal system in humans12 | 1988 | In vivo human data showing that plasma CGRP levels are increased upon thermal coagulation of the trigeminal ganglion |
| Proof of concept for antibody targeting of neuropeptides13 | 1989 | Antisera directed against CGRP and substance P blocked the peripheral actions of released peptides in neurogenic inflammation |
| Sumatriptan inhibits neuropeptide release14 | 1990 | Pharmacological evidence that sumatriptan inhibits neuropeptide release within meninges following electrical trigeminal stimulation. A prejunctional inhibitory receptor-driven mechanism was proposed for sumatriptan |
| Migraine drugs decrease CGRP release during trigeminal stimulation15 | 1991 | Dihydroergotamine and sumatriptan decreased CGRP blood levels during electrical trigeminal ganglia stimulation |
| Migraine drugs attenuate CGRP levels during attacks16 | 1993 | Elevated CGRP blood levels during spontaneous migraine attacks are attenuated by dihydroergotamine and sumatriptan |
| Brain stem activation in spontaneous human migraine attacks17 | 1995 | During spontaneous migraine attacks, blood flow increased in cingulate, auditory, and visual association cortices (cerebral hemispheres) and in the brainstem. Increased blood flow persisted after headaches, and phonophobia and photophobia were completely relieved by sumatriptan. The findings suggest that migraine is associated with an imbalance in activity between brain stem nuclei and vascular control |
| Neuronal substrate of throbbing is revealed18 | 1996 | Dural stimulation converts peripheral trigeminovascular neurons from mechanically insensitive to mechanically hypersensitive, which explains throbbing and intensification of headache by coughing or bending over |
| Neuronal substrate of scalp tenderness and allodynia is revealed19 | 1998 | Dural stimulation produces long-lasting sensitisation of central trigeminovascular neurons in the spinal trigeminal nucleus |
| The 5-HT1F receptor modulates activity of the trigeminal system20 | 1999 | LY 344864, a selective 5-HT1F receptor agonist attenuates capsaicin provoked early-immediate gene response (c-fos expression) in the spinal trigeminal nucleus. The fact that a 5-HT1F agonist modulates activity within the trigeminovascular system suggests its potential as a drug target |
| Cephalic allodynia is unique to migraine21 | 2000 | Cephalic allodynia is unique to headaches and involves irritation of pain fibres in the dura. This symptom can take years to appear in patients. |
| A link between migraine aura and headache is identified22 | 2002 | Cortical spreading depression activates trigeminovascular afferents and promotes a series of cortical meningeal and brainstem events consistent with evoking headache |
| CGRP triggers migraine in humans23 | 2002 | Intravenous infusion of CGRP triggers migraine attacks without aura in patients with migraine |
| Proof of concept for CGRP-targeted treatment24 | 2004 | Olcegepant, a small molecule CGRP receptor antagonist, shows clinical efficacy in an acute clinical trial with 34 migraine patients |
| PACAP triggers migraine in humans25 | 2009 | Intravenous infusion of PACAP dilates extracerebral arteries and triggers migraine attacks without aura in patients |
| The link between migraine aura and headache is further explored26 | 2010 | Cortical spreading depression leads to long-lasting activation of nociceptors that innervate the meninges |
| Evidence for a meningeal contribution to migraine pain27 | 2011 | CGRP-induced migraine headache is associated with ipsilateral dilation of extracerebral and intracerebral arteries. Constrictions of the extracerebral middle meningeal artery (but not intracerebral arterial constriction) parallels a reduction in headache intensity |
| Simple arterial dilatation is not the cause of migraine pain28 | 2013 | Spontaneous migraine attacks were not accompanied by extracerebral arterial dilatation, or substantial intracerebral dilatation overall. In the few vessels showing enlarged diameters, dilatation persisted even after relief from headache by sumatriptan. These results shifted the focus to peripheral and central pain pathways rather than simple arterial dilatation |
| Anti-CGRP monoclonal antibodies are effective in the prevention of episodic migraine29 | 2014 | This trial showed clinical efficacy and safety, suggesting that anti-CGRP monoclonal antibodies might be a viable therapy for prevention of episodic migraine |
| Meningeal contribution to migraine pain is further explored30 | 2019 | Cilosazol-induced migraine is associated with mild dilation of the middle meningeal artery on the headache side. Hence, dilation of this artery could serve as a surrogate marker for activation of dural perivascular nociceptors, indicating a meningeal site of migraine headache |
5-HT=hydroxytryptamine (serotonin). CGRP=calcitonin gene-related peptide. PACAP=pituitary adenylate cyclase-activating polypeptide
In cats and rodents, trigeminal ganglion neurons projecting to the meninges send central axons that reach trigeminovascular neurons in the spinal trigeminal nucleus, where they converge on neurons that receive additional input from the periorbital skin and pericranial muscles (figure).10,34,35 Axonal projections of 2nd-order trigeminovascular neurons convey pain signals to multiple nuclei in the brainstem, hypothalamus, basal ganglia, and thalamus.37 These projections might mediate autonomic (nausea, vomiting, yawning, lacrimation, urination), affective (anxiety, irritability), and hypothalamic-regulated functions related to keeping homoeostasis (loss of appetite, fatigue).38 Relay trigeminovascular thalamic neurons projecting widely (eg, to the somatosensory, insular, auditory, visual, and olfactory cortices) contribute to the specific nature of migraine pain and the many cortically mediated symptoms in migraine. These include transient symptoms of allodynia, phonophobia, photophobia, and osmophobia.39
Figure: Schematic overview of the trigeminovascular system.
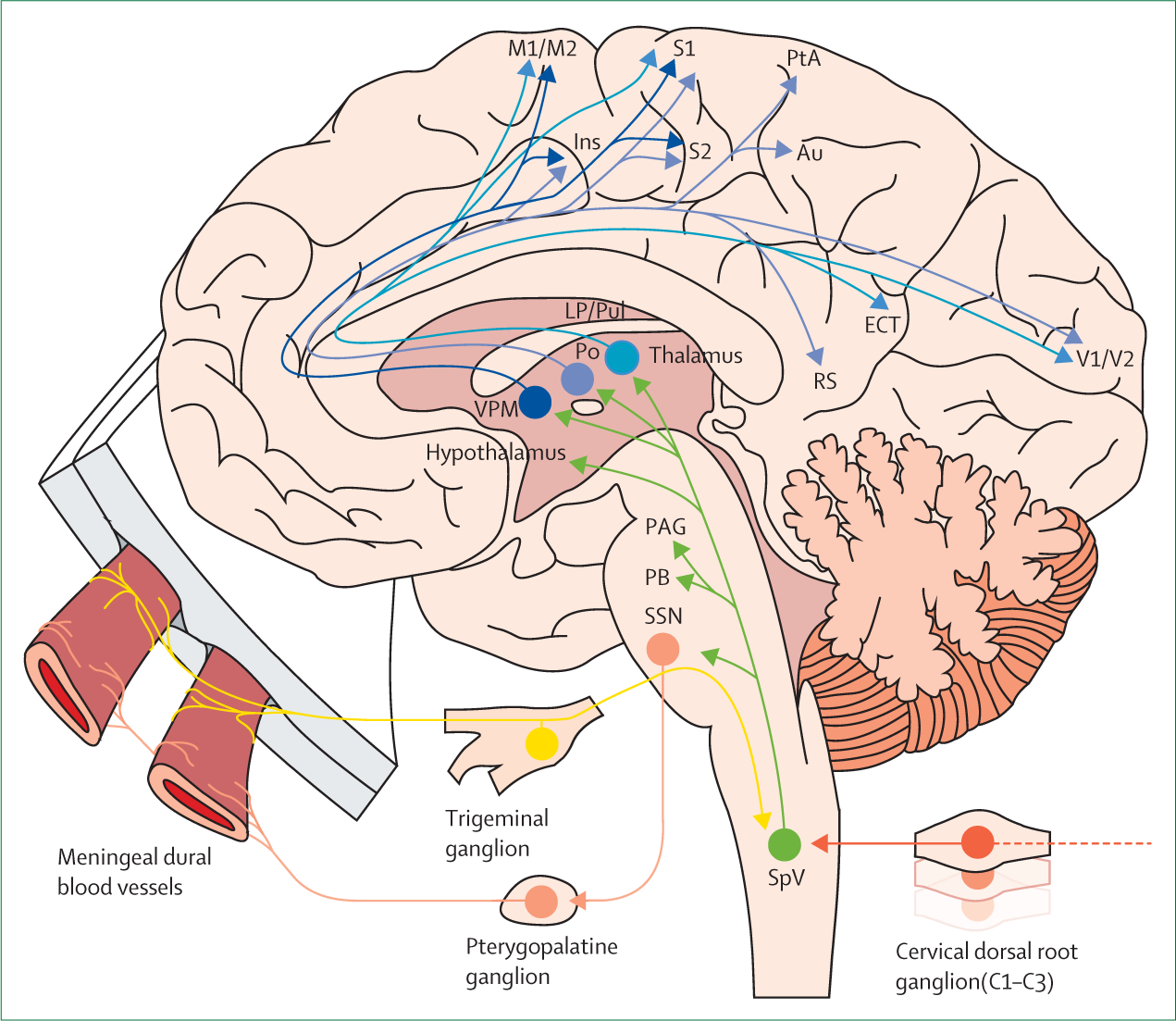
Adapted from Burstein et al.36 Thalamic trigeminovascular neurons project to a wide array of cortical areas that mediate symptoms associated with migraine, such as transient amnesia and cognitive decline, phonophobia, photophobia, and expressive aphasia. Inputs to SpV arise from meningeal dural blood vessels and pial blood vessels (not shown). Green: projections from SpV. Blue: thalamo-cortical projections. Yellow: afferent projections from meningeal blood vessels. Orange: afferent projections from cervical dorsal root ganglions.Peach: efferent projections to meningeal blood vessels. Au=Auditory cortex. ECT= ectorhinal cortex. Ins=insular cortex. LP=lateral posterior thalamic nucleus. M1=primary motor cortex. M2=secondary motor cortex. PAG=periaqueductal gray. PB=parabrachial nucleus. Po=posterior. PtA=parietal association cortex. Pul=pulvinar. RS=retrosplenial cortex. S1=primary somatosensory cortex. S2=secondary somatosensory cortex. SpV=spinal trigeminal nucleus. SSN=superior salivatory nucleus. V1=primary visual cortex. V2=secondary visual cortex. VPM=ventral posteromedial
Because pathways conveying migraine headaches involve both peripheral and CNS components, deciphering this association is complex. Under circumstances such as cortical-spreading depress ion, intense neuroglial activity in grey matter activates signaling cascades that could, in turn, discharge adjacent meningeal trigeminovascular axons. The brain, via spinal trigeminal nucleus inputs and rostral structures, processes and integrates transmitted information to generate migraine headache. Hence, the same organ that processes incoming signals relevant to the generation of headache also depolarises trigeminovascular afferents.
Neurophysiological mechanisms
Migraine aura is the clinical manifestation of cortical spreading depression (CSD).3,40 The aura is characterised by a propagating wave of cellular excitability that is followed by a long period of hyperpolarisation and a consequent headache that is thought to be initiated at least partly by introduction of inflammatory molecules and CGRP to the dura.41
In rodents, CSD initiates delayed and immediate activation of trigeminovascular neurons in the trigeminal ganglion and spinal trigeminal nucleus. Such activation patterns appear similar to the delayed and immediate onset of headache after aura in patients.26,42 These findings support the view that the initiation of headache depends on activation of meningeal nociceptors at the origin of the trigeminovascular pathway. Neuropeptide-induced dural neurogenic inflammation and mast cell degranulation might play a role in the activation or sensitisation of dural nociceptors.43 When activated in the altered molecular environment, peripheral trigeminovascular neurons become sensitised, and in turn, sensitize second and third order trigeminovascular neurons in the spinal trigeminal nucleus and the thalamus.38,44 Intensification of headache when bending over is the manifestation of peripheral sensitisation, whereas cephalic and extracephalic allodynia is the manifestation of sensitisation of trigeminovascular neurons in the spinal trigeminal nucleus and the thalamus.21
Triptans are a class of selective serotonin 5-hydroxytryptamine (5-HT1B) receptor agonists used to treat acute migraine. They disrupt communications between peripheral and central trigeminovascular neurons and are more effective in aborting migraine when given early—before the development of central sensitisation—providing further support to the notion that meningeal nociceptors drive the initial phase of the headache.45 Sumatriptan binds to 5-HT1B receptors in the brain that are associated with known CNS-related adverse events such as dizziness and somnolence, but it is unclear if this CNS binding is relevant for sumatriptan’s therapeutic effect in migraine.46 Further support for disrupted communication is found in studies showing that two peripherally acting drugs, onabotulinumtoxinA and anti-CGRP monoclonal anti bodies (mAbs), effectively prevent migraine in patients by inhibiting the activation and sensitisation of different classes of peripheral meningeal nociceptors. OntabotulinumtoxinA inhibits C fibres, but not Aδ-type meningeal nociceptors.47 Anti-CGRP monoclonal antibodies inhibit thinly myelinated (Aδ) but not unmyelinated (C) meningeal nociceptors.48
Neuropeptides
Three powerful vasodilating peptides are found within trigeminal afferents innervating the meninges (substance P, CGRP, and PACAP). The tachykinin substance P, discovered in 1931, is widely distributed in both the PNS and CNS, including the cranial vasculature, ganglia, and trigeminal sensory afferents.49 Preclinical experiments showed that substance P is widely implicated in pain transmission.50 Substance P resides in small diameter ganglion cells and co-exists to a great extent with CGRP in small unmyelinated fibres.10 Unilateral lesions of the trigeminal ganglia (or sectioning of its meningeal branches) decrease substance P in ipsilateral large cephalic blood vessels.5,51 These findings provided evidence that substance P is released into surrounding tissues from perivascular axons derived from the trigeminal nerve.43 However, as only a minority of trigeminal ganglion cells projecting to the meninges contain substance P, the presence of additional sensory neuromediators within the trigeminovascular system was suspected.7
CGRP, discovered in 1982, was the second neuropeptide to be identified in the trigeminovascular system, with effects in vascular tissues similar to those observed with substance P. CGRP is one of the most potent vasodilators of intracranial blood vessels, elicits a greater vasodilation than substance P, and its depletion leads to a decrease in the diameter of the ipsilateral arterial lumen.10,16 CGRP is found in perivascular trigeminal sensory afferents, and fibres containing CGRP are especially abundant in the walls of the cerebral arteries of the circle of Willis.52 Similar to substance P, CGRP is released by stimulation of meningeal afferents, and calcium - dependent release of CGRP in cultured trigeminal ganglion cells supported its role as an extracellular modulator.9,53 The findings are consistent with in vivo data from a study of nine patients with trigeminal neuralgia and five cats, which showed that plasma CGRP concentrations are increased during thermocoagualation of the trigeminal ganglion in humans and during electrical stimulation of the trigeminal gang lion in cats.12 Electrical stimulation of the trigeminal ganglion releases neurokinin A, substance P, and CGRP simultaneously, suggesting that substance P is not alone in modulating trigeminal pathways.54 Additional data are required to clarify this point; however, the importance of CGRP in migraine and to the human trigeminovascular system was shown by the success of strategies to block the effect of CGRP, whereas a substance P receptor blocker was not effective in clinical trials. Despite the negative outcome, the latter trials were the first to test a bench-to-bedside approach to therapy, did not depend upon a vascular smooth muscle mechanism, and focused on products contained within and released from the trigeminovascular system. The progression of targeting one peptide to the next was then systematically approached by the pharmaceutical industry. Their differing success underscores the need to better understand why selectively blocking one neuromediator and not another effectively treats migraine or why targeting CGRP appears more useful for mitigating headache than it does other visceral or somatic pains.55
PACAP, discovered in 1989, exists in two bioactive forms.56 PACAP is found in trigeminal nerve fibres around cerebral blood vessels.56 Furthermore, it can be found in the trigeminal ganglia, the sphenopalatine, and the trigeminal nucleus caudalis.56 Similar to CGRP, PACAP plasma concentrations increase during electrical stimulation of the trigeminal ganglion and superior sagittal sinus.57,58 However, PACAP concentrations decrease in both plasma and the trigeminal ganglion during dural application of inflammatory substances, perhaps reflecting responses to the nature of different stimuli.56 The clinical importance of PACAP is still primarily hypothesis driven as results of drug trials targeting PACAP and its receptor are pending.
Receptor subtypes
The 5-HT1B/1D/1F receptor subtypes are widespread in the trigeminovascular system. In 1988, a clinical trial59 reported a possible benefit from a novel 5-HT1-ike receptor agonist GR43175 (nowadays known as sumatriptan) for treatment of acute migraine. The same year, pharmacological experiments revealed that ergot alkaloids block neuropeptide release in the meninges following electrical trigeminal stimulation, a finding later replicated for sumatriptan.11,15 Both triptans and ergot alkaloids reduced elevated CGRP plasma concentrations during electrical trigeminal stimulation in rats.15 Taken together, these experimental studies provided the first pharmacological evidence for a prejunctional site of drug activity that coupled serotonin receptor subtypes to inhibition of neuropeptide release, now considered the most coherent therapeutic mechanism for ergots and triptans. These findings directed research away from vascular smooth muscle and towards targeting released trigeminal neuropeptides and their receptors.60
Preclinical discoveries showed that the 5-HT1B/1D subtypes reduce substance P and CGRP release in the trigeminal ganglion and trigeminal nucleus. Furthermore, using other experimental paradigms, 5-HT1 agonists also induce vasoconstriction in intracranial arteries.61 The 5-HT1D receptor subtype plays a possible role in inhibiting CGRP release from trigeminal neurons.62 The 5-HT1F receptor subtype also resides in the trigeminal gang-lion, trigeminal nucleus caudalis, and cerebral vessels; however, unlike the other subtypes, the 5-HT1F receptor subtype does not induce vasoconstriction.63
Substance P binds to the G-protein coupled receptors neurokinin-1 (NK), NK2, and NK3, with highest affinity for NK1 located in the dorsal horn of the spinal cord, the locus coeruleus, and the raphe nucleus.49 Following substance P release, NK1 receptors are activated in the endothelium and cause vasodilation, mast cell degranulation, and plasma protein leakage. NK1 receptor antagonists inhibit substance P-induced vasodilation of pial arteries in vivo.49 However, changes in vascular tone evoked by elec trical stimulation of the trigeminal ganglion are unaffected by NK1 receptor antagonists.64 Hence, receptors and neurotransmitters other than NK1 and substance P are pivotal in evoking neurogenic vasodilation.
The CGRP receptor complex is found in the trigeminal ganglion in all investigated species.65 Although CGRP is expressed in C-fibres, receptor components are found in the thicker Aδ-ibres. Furthermore, receptor components are found in neurons of the trigeminal ganglion. Stimulation of the CGRP receptor increases intracellular cyclic adenosine monophosphate (cAMP) by activating adenylate cyclase.66 CGRP is also a ligand for the amylin receptor.66 The potential role of the amylin receptor in migraine is unknown.
PACAP binds to several G-protein coupled receptors including pituitary adenylate cyclase-activating polypeptide type I receptor (PAC1), vasoactive intestinal polypeptide receptor 1 (VPAC1), and VPAC2, which results in increased intracellular cAMP concentra tions.56 The mRNA of these receptors is found in several structures including the trigeminal ganglia and otic ganglia, and all three receptors are found in cerebral and cranial blood vessels. The VPAC1 and VPAC2 receptors mediate vasodilation and mast cell degranulation, whereas the PAC1 receptor is involved in multiple biological processes.56 Notably, the released contents from mast cell degranulation activate C-fibres innervating the dura mater.67 Furthermore, a PAC1 receptor antagonist attenuates nociception in models of inflammatory and chronic pain, emphasising its role in nociception.68,69 Central activation of the PAC1 receptor appears to mediate the effects of PACAP on central trigeminovascular neurons.70
Neurogenic inflammation
Plasma extravasation and vasodilation are both important components of the neurogenic inflammatory response, and substantial additional evidence suggests a role for other signaling markers of inflammation in migraine.71 Neurogenic inflammation develops because of release of sensory neuropeptides such as substance P and CGRP from innervating fibres, and this release of neuropeptides might also occur in extracranial pain sensitive structures.71,72 Studies focused on the dura mater, a structure that contains vessels outside of the blood–brain barrier, and perivascular nerves and mast cells, showed that chemical and electrical stimulation induces plasma extravasation in the dura mater but not the brain, which remains protected behind the blood–brain barrier.73 Administration of indometacin, acetylsalicylic acid (aspirin), ergotamine tartrate, dihydroergotamine, or triptans blocked neuro genic extra vasation in the dura mater in animal models, as did substance P receptor antagonists.14,74,75 The same studies implicated prejunctional mechanisms and pep tide release inhibition by ergot alkaloids and triptans. Several substance P receptor antagonists blocked plasma protein extravasation in preclinical models.49 However, human clinical trials were ineffective when testing oral and intravenous administration of a substance P receptor antagonist, which indicated that substance P-induced neurogenic inflammation is not sufficient to explain human migraine headache; nevertheless, it could be a useful biomarker indicative of a meningeal inflammatory response.49
However, the CGRP-induced neurogenic vasodilation component of inflammation could be more clinically relevant than is substance P-induced vasodilation. Although neuropeptide release from sensory fibres is getting increasing attention in neuroimmune modulation, research on tissues suggests that the role of neuropeptide release in pain generation remains to be elucidated. Despite these uncertainties, models of neurogenic inflammation provided the data to support pursuing new therapeutic targets (eg, the 5-HT1F receptor subtype) as well as the therapeutic use of monoclonal antibodies.76 Antisera directed against CGRP and substance P blocked the peripheral actions of these peptides, which was a discovery predating that of the efficacy of therapeutic monoclonal antibodies in migraine by 30 years.13
Clinical imaging evidence for trigeminovascular migraine mechanisms
Results from neuroimaging studies have given novel insights into migraine pathophysiology. Although the aura has been notoriously difficult to study, aura-like episodes with corresponding regional blood flow changes consistent with CSD follow carotid puncture.77 Blood flow studies and fMRI studies during spontaneous and evoked visual auras confirm and extend these aura findings77 and reveal spatial and temporal changes in blood oxygen level-dependent signals characteristic of CSD in preclinical models.78,79
Regarding headache, simple vasodilation does not appear to explain the complex phenotype long considered to be the cause of migraine pain. For example, conflicting results were reported using magnetic resonance angiography of the middle meningeal artery, perhaps because of timing variations from attack onset. Results ranged from no dilatation, to ipsilateral dilatation on the pain side, and to dilatation in the early phase followed by bilateral dilatation.27,30,80 By contrast, spontaneous attacks are accompanied by intracranial but not extracranial arterial dilatation,28 and the magnitude of the dilation is minimal. From these studies, it appears unlikely that middle meningeal artery dilation generates migraine pain. Instead, observed changes in vessel diameter could reflect changes in the chemical milieu of the perivascular space and autonomic pain-related reflexes.22
Neuroimaging studies confirm the involvement of trigemi nal structures in migraine. PET studies show increased blood flow in the pons (a surrogate for activation) both during spontaneous attacks and those induced by glyceryl trinitrate.17,81 Lowered basal spinal trigeminal nucleus activity was shown outside of migraine attacks in patients with migraine compared with controls (healthy volunteers who did not have a history of migraine), and this basal level of activity increased at closer timepoints to an episode of migraine.82 Studies in humans also showed increased hypothalamic activity before spontaneous attacks (one patient monitored for 30 consecutive days) and in the premonitory phase of attacks induced by glyceryl trinitrate.83,84 Furthermore, spontaneous migraine attacks were associated with altered functional coupling between the hypothalamus with the spinal trigeminal nucleus the day before and during onset of the attacks.84
Development of drug targets
Putative mechanisms and targets identified in preclinical experiments require translation and at least partial validation in a human model, because migraine could be a uniquely human experience. However, spontaneous attacks are difficult to identify and investigate, especially at their onset. To overcome this challenge, a human model was developed in which migraine attacks were provoked by administering substances to patients with a history of migraine. Attacks, though painful, are fully reversible, making experimentally induced migraine an acceptable model for studying the complex pathophysiological events that occur during a migraine attack.85
In this human model, CGRP, PACAP, or drugs that target downstream signaling cascades following neuropeptide receptor engagement (presumably in proximity to the trigeminovascular pathways) cause typical migraine headaches after infusion. 60–70% of patients experience attacks after infusion of CGRP or PACAP.85 Higher attack rates (>80%) can be observed following administration of phosphodiesterase 3 and 5 inhibitors, implicating the second messengers cAMP and cyclic guanosine mono phosphate. Amplification of both second messengers could suggest a shared target such as modulation of an ion channel (eg, KATP channels).85,86 These studies further emphasise the role of neuropeptides as crucial mediators of migraine and potential drug targets for mechanism-based migraine treatment (table 2).
Table 2:
Drugs used to treat or prevent migraine with putative sites of action
| Year of introduction for clinical use | Acute or preventive drug | Forms of administration | Type of drug | Mechanism of action | Possible sites of action | |
|---|---|---|---|---|---|---|
| Ergotamines* | 1926 | Acute | Intravenous; nasal spray; oral | 5-HT1B,1D,1F receptor agonist | Inhibits peptide release | Prejunctional receptors |
| Triptans | 1991 | Acute | Nasal spray; oral; sublingual; subcutaneous | 5-HT1B,1D/1F receptor agonist | Disrupt communication between peripheral and central trigeminovascular neurons | Prejunctional receptors; presynaptic inhibition at the dorsal horn |
| OnabotulinumtoxinA | 2010 | Preventive | Intramuscular; subcutaneous | Cleaves intracellular SNARE proteins | Cleaves SNAP25 and prevents adhesion of synaptic vesicles to the cell surface membrane, resulting in inhibition of neuropeptides or neurotransmitter release, and insertion of new receptors | Unmyelinated C-class trigeminovascular meningeal nociceptors; unmyelinated C-class cervicovascular extracranial nociceptors |
| Monoclonal antibodies† | 2018 | Preventive | Subcutaneous; intravenous | CGRP-receptor antagonist | Neutralises circulating neuropeptides (or peptide receptor blockade) | Trigeminal ganglion; meningeal nociceptors |
| Ditans‡ | Not yet introduced | Acute | Oral | 5-HT1F receptor agonist | Inhibits peptide release | Central sites and peripheral prejunctional receptors |
| Gepants§ | Not yet introduced | Acute and preventive | Oral | CGRP-receptor antagonist | Peptide receptor blockade | Trigeminal ganglion; meningeal nociceptors; spinal trigeminal nucleus |
5-HT=hydroxytryptamine (serotonin). SNARE=soluble NSF attachment protein receptor. CGRP=calcitonin gene-related peptide. PACAP=pituitary adenylate cyclase-activating peptide.
Ergotamines are non-selective for 5-HT and are active at adrenergic and other receptor sites.
Anti-PACAP38 monoclonal antibodies are in pre-clinical development and will be administered subcutaneously. Anti-PAC1 receptor antibodies are awaiting public results from phase II trials.
The first ditan (lasmiditan) is expected to be approved by the US Food and Drugs Administration in 2019.
The first gepants (rimegepant and ubrogepant) are expected to be approved by the US Food and Drugs Administration in 2019 or 2020.
Since the introduction of the triptans, other drug classes with equivalent clinical efficacy but that do not induce vasoconstriction have been sought for treatment of acute migraine. Candidates include agonists at the 5-HT1F receptor that is expressed on trigeminovascular afferents. One of these agonists, lasmiditan, showed a therapeutic effect similar to sumatriptan in a phase 3 randomised multicenter study with 1856 patients with migraine.87 A high frequency of CNS-related adverse events, such as dizziness and somnolence, suggests that this drug (unlike most triptans) penetrates and possibly targets receptors in the brain. However, these adverse effects are unlikely to hinder a future approval of the drug.
The first drug specifically targeting CGRP was the small molecule CGRP receptor antagonist, olcegepant. Although the drug was never commercialised because it is poorly absorbed via oral administration and had limitations when adminstered intravenously, a proof of concept study with 34 patients with migraine showed that 71% of attacks treated with the highest dose resulted in complete relief of symptoms.24 Nowadays, other gepants (atogepant, rimegepant, and ubrogepant) might be nearing use in clinical practice because phase 3 trials for acute migraine attacks and phase 2 trials for preventive treatment are ongoing. Anti-CGRP mAbs have been approved by both the Food and Drug Administration and European Medical Agency and are highly effective and well tolerated; however, 30–40% of patients do not respond to mAbs.88
The site of action of gepants and mAbs is probably outside of the blood–brain barrier (similar to the ergot alkaloids and triptans) as they do not readily cross it. Possible sites of action include meningeal nociceptors and cells and other targets within the trigeminal ganglion.48,89 Two mAbs against PACAP (ALD1910 [preclinical stage]) and against PAC1 receptor (AMG-301 [phase II trial, NCT03238781]) are being developed and tested.
Conclusion and future research
Fundamental insights and discoveries to understand migraine pathophysiology have led to the emergence of new therapies and targets. However, as in most drug discovery research, the road from bench to bedside has not been straightforward. The time from concept to bedside drug therapy can be more than 30 years, which holds true for therapeutic developments in migraine coming to fruition—2019 will mark the 40-year anniversary of the first publication of the trigeminovascular hypothesis.90
The original trigeminovascular hypothesis successfully anticipated the therapeutic importance of identifying and targeting for therapy neuromediators within a final common pathway transmitting pain signals for headache; offered a more coherent understanding of triptan and ergot action also relevant to the role of released neuropeptides; reinforced the notion that clinically effective drugs do not require blood–brain barrier penetration; provided novel concepts concerning activation and sensitisation of trigeminal afferents by meningeal inflammatory stimuli as well as by intense endogenous brain activity; and emphasised the trigeminal nerve as a target for substances originating within the circulation or released from the brain that trigger headache.
The final common migraine pathway continues as an exciting avenue for discovery and as a vehicle to resolve pressing unanswered questions, such as the exact molecular mechanisms responsible for the initation of migraine attack. Future studies will aim to define the role of candidate mutations or polymorphisms that better inform about the initiating or suppressing mechanisms within the trigeminovascular system that lead to headache. Potential research avenues for clinically useful drugs might be found among ion channels ex pressed on trigeminovascular afferents or in meningeal tissues (eg, transient receptor potential vanilloid family, acid-sensing ion channels, potassium channels). Finally, future studies will investigate other aspects of migraine pathogenesis including the role of inflammation as well as vascular factors—eg, endothelial dysfunction.
Drugs targeting key signaling pathways in the trigeminovascular system will continue to transform clinical practice, thus supporting the development of mechanism-based migraine treatment. The hypothesis published in The Lancet in 1979 changed the research direction and focus at that time and was undoubtedly the first important building block upon which migraine research nowadays is based. With the emergence of new tools and technologies to study pain and neurovascular mechanisms, we anti cipate that the next 40 years will bring keystone discoveries to better understand and treat this enigmatic disorder.
Search strategy and selection criteria.
We identified articles published in English through searches of PubMed, Science Direct, Ovid Medline, Embase, and OVID, with use of the search term “trigeminovascular system”. No publication date restrictions were applied. We also identified papers from the authors’ own files and from references cited in relevant articles. We emphasised original and first to publish research and the references were chosen to reflect the laboratory credited with those original discoveries. Reviews were chosen when space did not permit a more comprehensive treatment of a topic or when limited space did not permit coverage of areas relevant to migraine but not necessarily of immediate importance to developments related to the trigeminovascular system. We generated the final reference list on the basis of articles’ relevance to the topic of this Personal View.
Footnotes
Declaration of interests
MA has received personal fees from Alder BioPharmaceuticals, Allergan, Amgen, Alder, Eli Lilly, Novartis, and Teva. MA also participated in clinical trials as the principal investigator for Alder, Amgen, electroCore, Novartis, and Teva. MA also serves as an associated editor of Cephalalgia, associated editor of the Journal of Headache and Pain, is President-elect of the International Headache Society, and General Secretary of the European Headache Federation. RB has received grant support for his studies on migraine pathophysiology from Teva, Allergan, Dr Reddy, and Trigemina; he also serves as a consultant to Alder Biopharm, Allergan, Amgen, Autonomic Technologies, Avanir, Biohaven, Depomed, Dr Reddy, Electrocore, Johnson and Johnson, Neurolief, Percept, Pernix, Strategic Science and Technologies, Teva, Theranica, and Trigemina. RB and Beth Israel Deaconess Medical Center hold a provisional patent on the use of narrow band green light for the treatment of photophobia in migraine. MAM serves as a consultant for Pear Therapeutics and NeuroTrauma Sciences. JMH, TPD and AM-C declare that they have no competing interests.
References
- 1.GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 954–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moskowitz MA, Reinhard JF, Romero J, Melamed E, Pettibone DJ. Neurotransmitters and the fifth cranial nerve: is there a relation to the headache phase of migraine? Lancet 1979; 2: 883–85. [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol 1984; 16: 157–68. [DOI] [PubMed] [Google Scholar]
- 4.Mayberg M, Langer RS, Zervas NT, Moskowitz MA. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science 1981; 213: 228–30. [DOI] [PubMed] [Google Scholar]
- 5.Liu-Chen LY, Mayberg MR, Moskowitz MA. Immunohistochemical evidence for a substance P-containing trigeminovascular pathway to pial arteries in cats. Brain Res 1983; 268: 162–66. [DOI] [PubMed] [Google Scholar]
- 6.Mayberg MR, Zervas NT, Moskowitz MA. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol 1984; 223: 46–56. [DOI] [PubMed] [Google Scholar]
- 7.Liu-Chen LY, Gillespie SA, Norregaard TV, Moskowitz MA. Co-localization of retrogradely transported wheat germ agglutinin and the putative neurotransmitter substance P within trigeminal ganglion cells projecting to cat middle cerebral artery. J Comp Neurol 1984; 225: 187–92. [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz MA, Brody M, Liu-Chen LY. In vitro release of immunoreactive substance P from putative afferent nerve endings in bovine pia arachnoid. Neuroscience 1983; 9: 809–14. [DOI] [PubMed] [Google Scholar]
- 9.Mason RT, Peterfreund RA, Sawchenko PE, Corrigan AZ, Rivier JE, Vale WW. Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature 308: 653–55. [DOI] [PubMed] [Google Scholar]
- 10.Uddman R, Edvinsson L, Ekman R, Kingman T, McCulloch J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: trigeminal origin and co-existence with substance P. Neurosci Lett 1985; 62: 131–36. [DOI] [PubMed] [Google Scholar]
- 11.Saito K, Marchkowitz S, Moskowitz MA. Ergot alkaloids block neurogenic extravasation in dura mater: proposed action in vascular headaches. Ann Neurol 1988; 24: 732–37. [DOI] [PubMed] [Google Scholar]
- 12.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol 1988; 23: 193–96. [DOI] [PubMed] [Google Scholar]
- 13.Louis SM, Jamieson A, Russell NJ, Dockray GJ. The role of substance P and calcitonin gene-related peptide in neurogenic plasma extravasation and vasodilatation in the rat. Neuroscience 1989; 32: 581–86. [DOI] [PubMed] [Google Scholar]
- 14.Buzzi MG, Moskowitz MA. The antimigraine drug, sumatriptan (GR43175), selectively blocks neurogenic plasma extravasation from blood vessels in dura mater. Br J Pharmacol 1990; 99: 202–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buzzi MG, Carter WB, Shimizu T, Heath H, Moskowitz MA. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology 1991; 30: 1193–200. [DOI] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 1993; 33: 48–56. [DOI] [PubMed] [Google Scholar]
- 17.Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med 1995; 1: 658–60. [DOI] [PubMed] [Google Scholar]
- 18.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996; 384: 560–64. [DOI] [PubMed] [Google Scholar]
- 19.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 1998; 79: 964–82. [DOI] [PubMed] [Google Scholar]
- 20.Mitsikostas DD, Sanchez del Rio M, Moskowitz MA, Waeber C. Both 5-HT1B and 5-HT1F receptors modulate c-fos expression within rat trigeminal nucleus caudalis. Eur J Pharmacol 1999; 369: 271–77. [DOI] [PubMed] [Google Scholar]
- 21.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol 2000; 47: 614–24. [PubMed] [Google Scholar]
- 22.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 2002; 8: 136–142. [DOI] [PubMed] [Google Scholar]
- 23.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia 2002; 22: 54–61. [DOI] [PubMed] [Google Scholar]
- 24.Olesen J, Diener H-C, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med 2004; 350: 1104–10. [DOI] [PubMed] [Google Scholar]
- 25.Schytz HW, Birk S, Wienecke T, Kruuse C, Olesen J, Ashina M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009; 132: 16–25. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci 2010; 30: 8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asghar MS, Hansen AE, Amin FM, et al. Evidence for a vascular factor in migraine. Ann Neurol 2011; 69: 635–45. [DOI] [PubMed] [Google Scholar]
- 28.Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol 2013; 12: 454–61. [DOI] [PubMed] [Google Scholar]
- 29.Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014; 13: 1100–07. [DOI] [PubMed] [Google Scholar]
- 30.Khan S, Mohammad Amin F, Emil Christensen C, et al. Meningeal contribution to migraine pain: a magnetic resonance angiography study. Brain 2019; 142: 93–102. [DOI] [PubMed] [Google Scholar]
- 31.Keller JT, Saunders MC, Beduk A, Jollis JG. Innervation of the posterior fossa dura of the cat. Brain Res Bull 1985; 14: 97–102. [DOI] [PubMed] [Google Scholar]
- 32.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol 2009; 8: 679–90. [DOI] [PubMed] [Google Scholar]
- 33.Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. J Comp Neurol 2009; 515: 331–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Broman J, Edvinsson L. Central projections of sensory innervation of the rat superior sagittal sinus. Neuroscience 2004; 129: 431–37. [DOI] [PubMed] [Google Scholar]
- 35.Davis KD, Dostrovsky JO. Responses of feline trigeminal spinal tract nucleus neurons to stimulation of the middle meningeal artery and sagittal sinus. J Neurophysiol 1988; 59: 648–66. [DOI] [PubMed] [Google Scholar]
- 36.Burstein R, Noseda R, Borsook D. Migraine: multiple processes, complex pathophysiology. J Neurosci 2015; 35: 6619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malick A, Strassman RM, Burstein R. Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J Neurophysiol 2000; 84: 2078–112. [DOI] [PubMed] [Google Scholar]
- 38.Burstein R, Jakubowski M, Garcia-Nicas E, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 2010; 68: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noseda R, Jakubowski M, Kainz V, Borsook D, Burstein R. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 2011; 31: 14204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lauritzen M Pathophysiology of the migraine aura. The spreading depression theory. Brain 1994; 117: 199–210. [DOI] [PubMed] [Google Scholar]
- 41.Karatas H, Erdener SE, Gursoy-Ozdemir Y, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013; 339: 1092–95. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 2011; 69: 855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moskowitz MA, Macfarlane R. Neurovascular and molecular mechanisms in migraine headaches. Cerebrovasc Brain Metab Rev 1993; 5: 159–77. [PubMed] [Google Scholar]
- 44.Burstein R, Falkowsky O, Borsook D, Strassman A. Distinct lateral and medial projections of the spinohypothalamic tract of the rat. J Comp Neurol 1996; 373: 549–74. [DOI] [PubMed] [Google Scholar]
- 45.Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci USA 2004; 101: 4274–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deen M, Hougaard A, Hansen HD, et al. Treatment with sumatriptan during a migraine attack and association with central 5-HT1B receptor binding. JAMA Neurol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burstein R, Zhang X, Levy D, Aoki KR, Brin MF. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: therapeutic implications for migraine and other pains. Cephalalgia 2014; 34: 853–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melo-Carrillo A, Strassman AM, Nir R-R, et al. Fremanezumab-A humanized monoclonal anti-CGRP antibody inhibits thinly myelinated (Aδ) but not unmyelinated (C) meningeal nociceptors. J Neurosci 2017; 37: 10587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.May A, Goadsby PJ. Substance P receptor antagonists in the therapy of migraine. Expert Opin Investig Drugs 2001; 10: 673–78. [DOI] [PubMed] [Google Scholar]
- 50.Rupniak NM, Carlson E, Boyce S, Webb JK, Hill RG. Enantioselective inhibition of the formalin paw late phase by the NK1 receptor antagonist L-733,060 in gerbils. Pain 1996; 67: 189–95. [DOI] [PubMed] [Google Scholar]
- 51.Liu-Chen LY, Han DH, Moskowitz MA. Pia arachnoid contains substance P originating from trigeminal neurons. Neuroscience 1983; 9: 803–08. [DOI] [PubMed] [Google Scholar]
- 52.Ashina H, Schytz HW, Ashina M. CGRP in human models of migraine. Handb Exp Pharmacol 2018; published online June 13. DOI: 10.1007/164_2018_128. [DOI] [PubMed] [Google Scholar]
- 53.Ebersberger A, Averbeck B, Messlinger K, Reeh PW. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 from rat dura mater encephali following electrical and chemical stimulation in vitro. Neuroscience 1999; 89: 901–07. [DOI] [PubMed] [Google Scholar]
- 54.Samsam M, Coveñas R, Ahangari R, Yajeya J, Narváez JA, Tramu G. Simultaneous depletion of neurokinin A, substance P and calcitonin gene-related peptide from the caudal trigeminal nucleus of the rat during electrical stimulation of the trigeminal ganglion. Pain 2000; 84: 389–95. [DOI] [PubMed] [Google Scholar]
- 55.Schou WS, Ashina S, Amin FM, Goadsby PJ, Ashina M. Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain 2017; 18: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vollesen ALH, Amin FM, Ashina M. Targeted pituitary adenylate cyclase-activating peptide therapies for migraine. Neurotherapeutics 2018; 15: 371–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuka B, Helyes Z, Markovics A, et al. Peripheral and central alterations of pituitary adenylate cyclase activating polypeptid-like immunoreactivity in the rat in response to activation of the trigeminovascular system. Peptides 2012; 33: 307–16. [DOI] [PubMed] [Google Scholar]
- 58.Zagami AS, Edvinsson L, Goadsby PJ. Pituitary adenylate cyclase activating polypeptide and migraine. Ann Clin Transl Neurol 2014; 1: 1036–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doenicke A, Brand J, Perrin VL. Possible benefit of GR43175, a novel 5-HT1-like receptor agonist, for the acute treatment of severe migraine. Lancet 1988; 1: 1309–1311. [DOI] [PubMed] [Google Scholar]
- 60.Moskowitz MA. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol Sci 1992; 13: 307–11. [DOI] [PubMed] [Google Scholar]
- 61.van den Broek RW, MaassenVanDenBrink A, de Vries R, et al. Pharmacological analysis of contractile effects of eletriptan and sumatriptan on human isolated blood vessels. Eur J Pharmacol 2000; 407: 165–73. [DOI] [PubMed] [Google Scholar]
- 62.Hou M, Kanje M, Longmore J, Tajti J, Uddman R, Edvinsson L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-elated peptide, substance P and nitric oxide synthase. Brain Res 2001; 909: 112–20. [DOI] [PubMed] [Google Scholar]
- 63.Ramadan NM, Skljarevski V, Phebus LA, Johnson KW. 5-HT1F receptor agonists in acute migraine treatment: a hypothesis. Cephalalgia 2003; 23: 776–85. [DOI] [PubMed] [Google Scholar]
- 64.Beattie DT, Connor HE. The influence of the trigeminal ganglion on carotid blood flow in anaesthetized guinea-pigs. Br J Pharmacol 1994; 112: 262–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eftekhari S, Salvatore CA, Johansson S, Chen T-B, Zeng Z, Edvinsson L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res 2015; 1600: 93–109. [DOI] [PubMed] [Google Scholar]
- 66.Hay DL. CGRP receptor biology: is there more than one receptor? Handb Exp Pharmacol 2018; published online May 25. DOI: 10.1007/164_2018_131. [DOI] [PubMed] [Google Scholar]
- 67.Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain 2007; 130: 166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis-Taber R, Baker S, Lehto SG, et al. Central pituitary adenylate cyclase 1 receptors modulate nociceptive behaviors in both inflammatory and neuropathic pain states. J Pain 2008; 9: 449–56. [DOI] [PubMed] [Google Scholar]
- 69.Ohsawa M, Brailoiu CG, Shiraki M, Dun NJ, Paul K, Tseng LF. Modulation of nociceptive transmission by pituitary adenylate cyclase activating polypeptide in the spinal cord of the mouse. Pain 2002; 100: 27–34. [DOI] [PubMed] [Google Scholar]
- 70.Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: Relevance to migraine. Sci Transl Med 2015; 7: 308ra157. [DOI] [PubMed] [Google Scholar]
- 71.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology 2005; 64 (suppl 2): S9–15. [DOI] [PubMed] [Google Scholar]
- 72.Lundberg JM, Brodin E, Hua X, Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand 1984; 120: 217–27. [DOI] [PubMed] [Google Scholar]
- 73.Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J Neurosci 1987; 7: 4129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated plasma extravasation in dura mater: effect of ergot alkaloids. A possible mechanism of action in vascular headache. Cephalalgia 1988; 8: 83–91. [DOI] [PubMed] [Google Scholar]
- 75.Buzzi MG, Sakas DE, Moskowitz MA. Indomethacin and acetylsalicylic acid block neurogenic plasma protein extravasation in rat dura mater. Eur J Pharmacol 1989; 165: 251–58. [DOI] [PubMed] [Google Scholar]
- 76.Johnson KW, Schaus JM, Durkin MM, et al. 5-HT1F receptor agonists inhibit neurogenic dural inflammation in guinea pigs. Neuroreport 1997; 8: 2237–40. [DOI] [PubMed] [Google Scholar]
- 77.Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol 1981; 9: 344–52. [DOI] [PubMed] [Google Scholar]
- 78.Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA 2001; 98: 4687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arngrim N, Hougaard A, Ahmadi K, et al. Heterogenous migraine aura symptoms correlate with visual cortex functional magnetic resonance imaging responses. Ann Neurol 2017; 82: 925–39. [DOI] [PubMed] [Google Scholar]
- 80.Schoonman GG, van der Grond J, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation--a 3T magnetic resonance angiography study. Brain 2008; 131: 2192–200. [DOI] [PubMed] [Google Scholar]
- 81.Afridi SK, Matharu MS, Lee L, et al. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005; 128: 932–39. [DOI] [PubMed] [Google Scholar]
- 82.Stankewitz A, Aderjan D, Eippert F, May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci 2011; 31: 1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 2014; 137: 232–41. [DOI] [PubMed] [Google Scholar]
- 84.Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016; 139: 1987–93. [DOI] [PubMed] [Google Scholar]
- 85.Ashina M, Hansen JM, Á Dunga BO, Olesen J. Human models of migraine—short-term pain for long-term gain. Nat Rev Neurol 2017; 13: 713–24. [DOI] [PubMed] [Google Scholar]
- 86.Al-Karagholi MA-M, Hansen JM, Severinsen J, Jansen-Olesen I, Ashina M. The KATP channel in migraine pathophysiology: a novel therapeutic target for migraine. J Headache Pain 2017; 18: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuca B, Silberstein SD, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology 2018; 91: e2222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ashina M The most important advances in headache research in 2018. Lancet Neurol 2019; 18: 5–6. [DOI] [PubMed] [Google Scholar]
- 89.Miller S, Liu H, Warfvinge K, et al. Immunohistochemical localization of the calcitonin gene-related peptide binding site in the primate trigeminovascular system using functional antagonist antibodies. Neuroscience 2016; 328: 165–83. [DOI] [PubMed] [Google Scholar]
- 90.Spector JM, Harrison RS, Fishman MC. Fundamental science behind today’s important medicines. Sci Transl Med 2018; 10: DOI:eaaq1787. [DOI] [PubMed] [Google Scholar]


