Abstract
Context
Among sports-recovery methods, cold-water immersion (CWI), contrast-water therapy (CWT), and whole-body cryotherapy (WBC) have been applied widely to enhance recovery after strenuous exercise. However, the different timing effects in exercise-induced muscle damage (EIMD) after these recovery protocols remain unknown.
Objective
To compare the effects of CWI, CWT, and WBC on the timing-sequence recovery of EIMD through different indicator responses.
Design
Crossover study.
Setting
Laboratory.
Patients or Other Participants
Twelve male middle- and long-distance runners from the Beijing Sport University (age = 21.00 ± 0.95 years).
Intervention(s)
Participants were treated with different recovery methods (control [CON], CWI, CWT, WBC) immediately postexercise and at 24, 48, and 72 hours postexercise.
Main Outcome Measure(s)
We measured perceived sensation using a visual analog scale (VAS), plasma creatine kinase (CK) activity, plasma C-reactive protein (CRP) activity, and vertical-jump height (VJH) pre-exercise, immediately postexercise, and at 1, 24, 48, 72, and 96 hours postexercise.
Results
For the VAS score and CK activity, WBC exhibited better timing-sequence recovery effects than CON and CWI (P < .05), but the CWT demonstrated better effects than CON (P < .05). The CRP activity was lower after WBC than after the other interventions (P < .05). The VJH was lower after WBC than after CON and CWI (P < .05).
Conclusions
The WBC positively affected VAS, CK, CRP, and VJH associated with EIMD. The CWT and CWI also showed positive effects. However, for the activity and timing-sequence effect, CWT had weaker effects than WBC.
Keywords: water immersion, whole-body cryotherapy, cooling
Key Points
For the treatment of middle- and long-distance runners with exercise-induced muscle damage, whole-body cryotherapy was more effective than cold-water immersion and contrast-water therapy for the activity and recovery timing-sequence effect.
Given that whole-body cryotherapy enhanced muscle recovery and reduced muscle-performance decrements after exercise-induced muscle damage, this method was a useful nonpharmacologic and noninvasive therapy for promoting muscle recovery.
Exercise-induced muscle damage (EIMD) commonly results from performing unaccustomed exercise or exercise with increased intensity or duration.1 The well-documented symptoms of muscle damage include disruption of the intracellular muscle structure, sarcolemma, and extracellular matrix; prolonged impairment of muscle function; delayed-onset muscle soreness; increased muscle soreness; presence of muscle-damage and inflammation markers; stiffness; and swelling.2 A number of causes, including lactic acid, muscle spasm, connective tissue damage, muscle damage, inflammation, and enzyme efflux, have been proposed to explain the pain associated with EIMD.3 Typically, EIMD increases in intensity in the first 24 hours postexercise, peaks from 24 to 48 hours, and subsides within 5 to 7 days. It presents with tenderness to palpation, movement, or both and decreased flexibility and maximal voluntary force production.4 Given that EIMD is a multifaceted integrated process, no completely effective intervention strategy exists for its prevention. The alternative is to treat EIMD symptoms after manifestation. The proposed mechanisms of EIMD have prompted researchers to investigate various treatment strategies aimed at alleviating the symptoms of EIMD, restoring maximal muscle function as rapidly as possible, or reducing the magnitude of the initial injury.5 Middle- and long-distance runners complete training sessions every 1 or 2 days, during which they perform a large proportion of eccentric work that can lead to EIMD. Hence, acute recovery modalities are a vital factor in supporting supplementary training loads or competitions.
Cryotherapy is commonly used to relieve pain, particularly that of inflammatory diseases, injuries, and overuse.6 Cooling the body to accelerate recovery decreases muscle-damage and inflammation markers.6,7 Among the water-immersion recovery modalities, cold-water immersion (CWI) involves submersing a part of or the whole body except the head in a cold-water bath (<15°C) for 10 to 12 minutes.8 Contrast-water therapy (CWT) involves immersing a part of or the whole body, except the head, in alternating cold (15°C for 1 minute) and hot (38°C for 1 minute) water for 12 to 14 minutes for 6 to 7 cycles.8 Whole-body cryotherapy (WBC) exposes the body to extremely cold air (−110°C to −195°C) in a specifically designed room or cryochamber for 3 to 4 minutes.9 All of these cryotherapy models may positively affect human physiological and psychological conditions.6–9 Cold-water immersion,10 CWT,11 and WBC12 reduce muscle soreness and improve muscle-damage and systemic inflammation markers, which are closely associated with EIMD,13 thereby accelerating recovery from muscle damage.9,14 These recovery strategies also beneficially affect sport performance after muscle damage.10–12 However, only a few researchers have compared their effects on EIMD. Therefore, the purpose of our study was to determine the most appropriate recovery modality for middle- and long-distance runners with EIMD by measuring multiple indicators over long tracking times. As a continuation of previous studies, we hypothesized that these 3 cryotherapy modalities would decrease muscle damage in middle- and long-distance runners at different times. We also hypothesized that WBC would be more beneficial for decreasing muscle damage in middle- and long-distance runners.
METHODS
Study Design
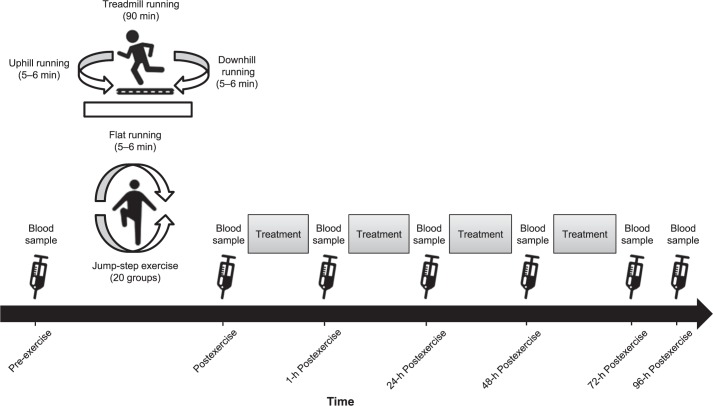
We analyzed the effects of 3 recovery modalities on EIMD after a simulated exercise program using a controlled crossover study design (Figure 1).12,15 In our laboratory over 4 noncontiguous weeks, participants performed identical repetitions of a simulated exercise program that was designed to induce muscle damage. The order of interventions was as follows: control group (CON), CWI, CWT, and WBC. The participants received different recovery modalities (CON, CWI, CWT, and WBC) immediately and at 24, 48, and 72 hours after the simulated exercise program. The EIMD indicators, consisting of perceived sensation on the visual analog scale (VAS), plasma creatine kinase (CK) activity, plasma C-reactive protein (CRP) activity, and vertical-jump height (VJH), were assessed immediately before and after each EIMD exercise program and after each recovery session (1, 24, 48, 72, and 96 hours postexercise). Between trials, a 1-week washout was ensured12,15 to allow complete muscular recovery. To limit and control the development of additional EIMD, participants were prohibited from training during the trials preceding and succeeding data recording.
Figure 1.
Study design for the 4 interventions: control, cold-water immersion, contrast-water therapy, and whole-body cryotherapy.
Participants
Twelve male middle- and long-distance runners from Beijing Sport University participated in the study (Table). Recruits were healthy and performed no regular or temporary training during measurements. We conducted brief interviews with all participants at baseline and the end of the study in which they were asked about their health condition; lifestyle, including diet and sleep; and training intensity. No participant reported having an infectious disease or taking anti-inflammatory or anti-fatigue medications during the study period.
Table.
Baseline Characteristics of Participants (N = 12)
| Variable |
Mean ± SD |
| Age, y | 21.00 ± 0.95 |
| Height, m | 1.77 ± 0.04 |
| Mass, kg | 70.08 ± 7.34 |
| Training in running, sessions/wk | 3.5 ± 1.1 |
| Maximal O2 consumption, mL/kg/min | 52.91 ± 4.60 |
Two weeks before the experiment, the necessary restrictions were explained to the participants. During the experiment, to control the influence of other recovery modalities, they were prohibited from using any nutritional supplements and ingesting alcohol, coffee, and spicy food and were not allowed to perform any other exercise. All participants provided written informed consent, and the study was approved by the Ethical Committee of the China Institute of Sport Science.
Exercise Protocol
For each group, the participants reported to the laboratory 1 day before the experiment for preliminary testing. Maximal oxygen uptake (V˙O2max) was determined during running on a motorized treadmill (H/P/CosmosH Saturn, Traunstein, Germany). The test consisted of a 2-minute warm-up involving running at 7 km/h on a flat surface and an incremental period in which running speed was increased by 1 km/h each minute. After the participant reached 14 km/h, this speed was maintained and the treadmill gradient was increased by 1.0% each minute until volitional exhaustion. The criteria used to determine V˙O2max were as follows: a plateau in oxygen uptake (V˙O2) despite an increase in power output, respiratory exchange ratio greater than 1:1, and heart rate greater than 90% of the predicted maximal heart rate.16 After the preliminary running exercise, all participants were familiarized with the EIMD exercise program so they understood the movement mode, intensity, and duration.
Given that EIMD results from multiple causes, relying on a single exercise mode to simulate the condition presents difficulty.1,3 Therefore, our EIMD exercise program consisted of 2 parts: treadmill running12,15,17 and jump-step exercise.18,19 The EIMD treadmill running time was 90 minutes and consisted of five 18-minute durations.12,15,17 Participants completed a 5-minute warm-up of flat-surface running at a treadmill speed of 6 km/h. The exercise started after the warm-up. The treadmill running speed was based on the percentage of V˙O2max20 measured in the participants.21 Each part contained initial flat-surface (6 minutes), uphill (6 minutes), and downhill (6 minutes) running. The conversion time from uphill to downhill running was 1 minute, during which time participants were not running. The treadmill speed during each stage was unchanged, whereas the gradients of uphill and downhill running varied. The gradients of uphill running were 6.0%, 5.0%, 4.0%, 3.0%, and 2.0% and of downhill running were −8.0%, −7.0%, −6.0%, −5.0%, and −4.0%.12,15 Immediately after treadmill running, the participants performed 20 sets of 40 accentuated eccentric-load drop jumps with a 30-second rest between sets.18,19 All participants completed the EIMD exercise program.
Treatment Protocol
All participants received each recovery modality during the experiment. During CON, participants were seated comfortably in a recliner for 12 minutes at room temperate (24°C). For CWI and CWT, participants submerged their entire bodies in water to a level covering their shoulders and leaving only their heads and necks above the waterline.7,8 For CWI, participants were immersed in 15°C water (model IC-Compact Twin XP, IC-iSprint; iCool Australia Ltd, Burleigh Heads, Australia) for 12 minutes.8,10,13 During CWT, participants were immersed alternately in cold (15°C for 1 minute) and hot (38°C for 1 minute) water over 6 cycles (model IC-Compact Twin XP, IC-iSprint; iCool Australia Ltd) for 12 minutes. They were required to transfer between hot and cold baths in less than 5 seconds to ensure maximal water-exposure duration.11,14 During WBC, participants were exposed to temperatures from −110°C to −140°C for 3 minutes in a head-out cryochamber that used gaseous nitrogen (model Cryomed Space Cabin; Nanjing Highermed Health Technology Co, Nanjing, China). The temperature and duration of WBC exposure were based on the conditions proposed by Costello et al.9 The participants were instructed to wear a bathing suit, surgical mask, earband, triple-layer gloves, dry socks, and sabots with thermal protection to protect the extremities and to dry sweat. The water-immersion and WBC treatments are shown in Figure 2.
Figure 2.
Participant receiving, A–C, water-immersion treatment and, D–F, whole-body cryotherapy treatment.
Measurements
The EIMD caused by muscle soreness can last for approximately 96 hours. We studied EIMD from occurrence to regression at 7 time points to understand the different methods of EIMD recovery and their timing effects. Participants were examined pre-exercise; immediately postexercise; and at 1, 24, 48, 72, and 96 hours postexercise. At each time point, we measured subjective indicators first, collected blood samples second to avoid the potential influence of exercise on the samples, and measured the VJH third. Knee-extensor muscle soreness was assessed using a 10-cm VAS, with scores of no soreness (0 cm) to severe soreness (10 cm). We obtained the blood-plasma sample from a 5-mL sample of whole blood collected into vacutainer tubes through antecubital venipuncture. After the blood sample was extracted, the tubes were mixed by shaking and then placed on ice for 30 seconds before centrifugation at 4°C for 10 minutes at 3000 rpm. The plasma sample was stored in multiple aliquots at −80°C until analysis. Plasma CK was analyzed spectrophotometrically (model AU-5400 analyzer; Olympus Corp, Tokyo, Japan, and model 7170A analyzer; Hitachi Ltd, Tokyo, Japan) using test kits (Xiamen In Tec Products Inc, Xiamen, China). Plasma CRP was determined by enzyme-linked immunosorbent assay with commercially available high-sensitivity enzyme-linked immunosorbent assay kits (Shanghai BlueGene Biotech Corp, Shanghai, China). For the VJH, the best result of 2 trials was analyzed. Jumps were measured using an electronic vertical-jump meter (Beijing Xindong Huateng Sports Equipment Ltd, Beijing, China).
Data Analysis
All data were expressed as means ± standard deviations. A repeated-measures analysis of variance (recovery modality of 7 timing periods) was performed to analyze the effects of the recovery intervention (pre-exercise; immediately postexercise; and at 1, 24, 48, 72, and 96 hours postexercise) on variable indicators. Horizontal comparisons of the intervention methods were conducted at different times to determine within-group effects, whereas the longitudinal comparison was performed at the same times to determine between-groups effects. Statistical analysis was performed using SPSS (version 19; IBM Corp, Armonk, NY). The α level was set at .05 for all statistical analyses.
RESULTS
Visual Analog Scale Score
We observed a time-by-group effect (F18,264 = 9.647, P < .001) for VAS. The VAS score decreased fastest after WBC: the 72-hour postexercise result was close to the baseline result, followed by CWT, which produced a 96-hour postexercise result close to the baseline result (Figure 3). We observed better effects after CWI than after CON.
Figure 3.
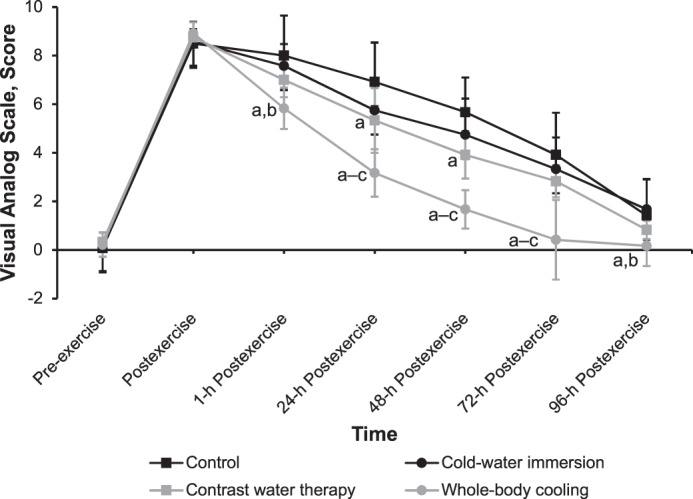
Effects of different cryotherapy models on visual analog scale score at 7 time points. a Different from the control intervention (P < .05). b Different from the cold-water–immersion intervention (P < .05). c Different from the contrast-water–therapy intervention (P < .05).
At 1 and 96 hours postexercise, the VAS score was lower after WBC than after CON and CWI (P < .001). At 24, 48, and 72 hours postexercise, the VAS was lower after WBC than after CON, CWI, and CWT (P < .001); scores at 24 and 48 hours postexercise were lower after CWT than after CON (P < .001).
Creatine Kinase
A time-by-group effect (F15,215 = 1.883, P = .03) for CK was demonstrated. Plasma CK activity decreased fastest after WBC: the 48-hour postexercise result was close to the baseline result, followed by CWT, which resulted in a 96-hour postexercise result close to the baseline result (Figure 4). Similar changes in timing effects were observed after CWI.
Figure 4.
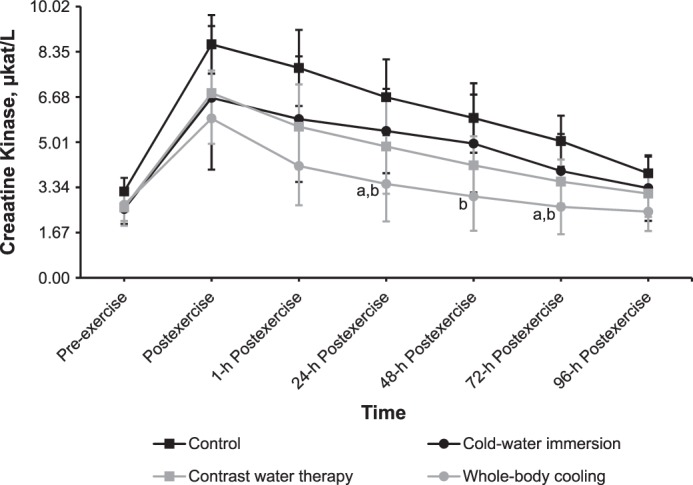
Effects of different cryotherapy models on plasma creatine kinase activity at 7 time points. a Different from the control intervention (P < .05). b Different from the cold-water–immersion intervention (P < .05).
Plasma CK activity at 24 hours and 72 hours postexercise was less after WBC than after CON and CWI (P < .05). In addition, plasma CK activity at 48 hours postexercise was less after WBC than after CWI (P < .001).
C-Reactive Protein
We noted a time-by-group effect (F18,264 = 7.934, P < .001) for CRP. Plasma CRP activity decreased the fastest after WBC, which produced a 48-hour postexercise result close to the baseline result, followed by CWI, which displayed a 96-hour postexercise result close to the baseline result (Figure 5). The CWT yielded unstable CRP results.
Figure 5.
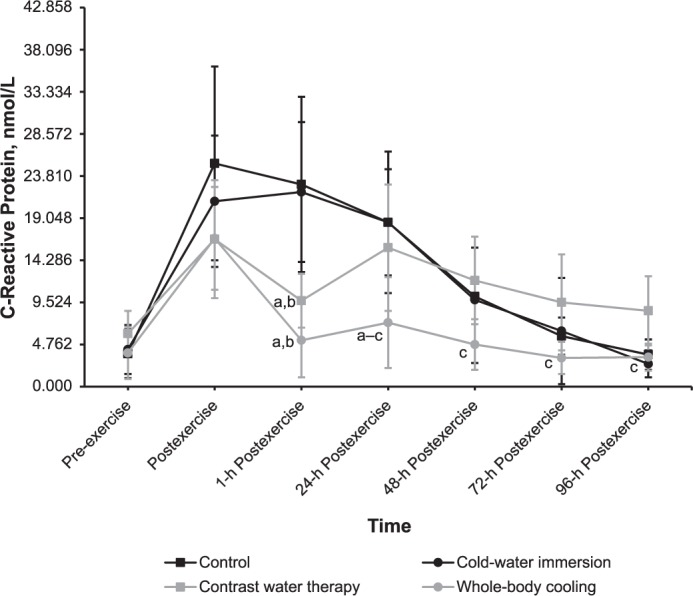
Effects of different cryotherapy models on plasma C-reactive protein activity at 7 time points. a Different from the control intervention (P < .05). b Different from the cold-water–immersion intervention (P < .05). c Different from the contrast-water–therapy intervention (P < .05).
Plasma CRP activity at 1 hour postexercise was less after WBC than after CON and CWI and less after CWT than after CON and CWI (all P values < .001). At 24 hours postexercise, plasma CRP activity was less after WBC than after CON, CWI, and CWT (P < .02). At 48, 72, and 96 hours postexercise, plasma CRP activity was less after WBC than after CWT (P < .01).
Vertical-Jump Height
A time-by-group effect (F18,264 = 3.650, P < .001) for VJH was present. The VJH increased fastest after WBC, which resulted in a 72-hour postexercise measurement close to the baseline measurement, followed by CWT, which produced a 96-hour postexercise measurement close to the baseline measurement (Figure 6). Similar changes in timing effects were observed after CON and CWI.
Figure 6.
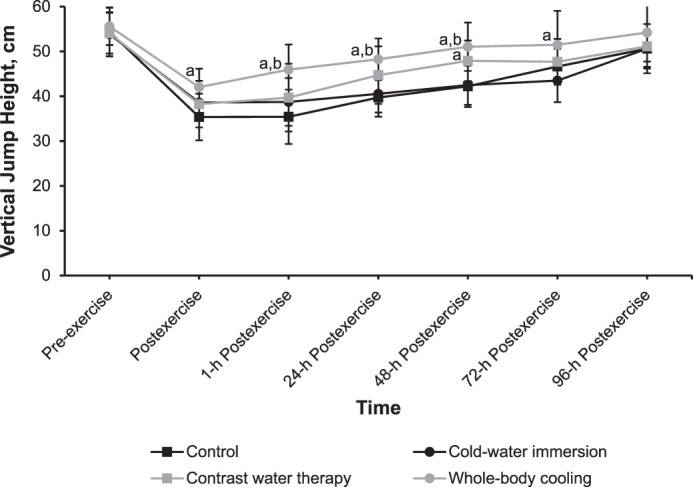
Effects of different cryotherapy models on vertical jump height at 7 time points. a Different from the control intervention (P < .05). b Different from the cold-water–immersion intervention (P < .05).
At 1, 24, and 48 hours postexercise, VJH was greater after WBC than after CON and CWI (P < .05). At 48 hours postexercise, CWT resulted in greater VJH than the CON (P = .03) or CWI (P = .04) condition, whereas at 72 hours postexercise, WBC resulted in greater VJH than the CON condition (P < .001).
DISCUSSION
The EIMD Exercise Protocol
In long-term endurance exercises, such as downhill running and downhill walking, Nosaka et al22 found that the peak CK value occurred at 0 to 12 hours postexercise, suggesting that the participants experienced EIMD. In our study, the CK values of the 4 interventions increased and peaked immediately postexercise. These characteristics are consistent with the criteria for the long-term endurance EIMD model. The highest VAS score was achieved immediately postexercise. In the study by Su et al,23 participants presented with exhaustion, supporting the effects of the EIMD exercise protocol. In summary, the EIMD exercise protocol in our study met these modeling criteria.
Muscle Soreness and Damage
In our investigation, muscle-soreness effects could be determined using the VAS score. By assessing plasma CK activity, we observed muscle-damage–marker recovery. Given the various adaptive capacities of the participants, differences existed among the CK levels in each group postexercise.
In the postexercise timing-sequence recovery, the VAS score and CK level decreased the fastest after WBC (P < .05), followed by CWT. Compared with the other interventions, WBC was more effective in reducing muscle-soreness and muscle-damage markers. Numerous researchers12,24 have shown that WBC decreased participants' muscle soreness. Other authors25 also reported that WBC can improve recovery from EIMD. Therefore, by reducing the CK level, WBC effectively reduced the VAS score. These 2 indicators were consistent in timing-sequence recovery. The difference may be attributed to the different media in the cryotherapy models. The WBC uses a gaseous medium, whereas CWI and CWT use a liquid medium; the different media result in different absorption effects on the human body, causing the body's perceived sensations to vary.6,26 Another reason is that cryotherapy models lower the temperature of the body tissues; this lowered temperature may improve the stability of muscle lysosomal membranes and inhibit the release of active enzymes.6,8
Inflammation Markers
The inflammation-marker recoveries were observed through plasma CRP activity. Different effects of cryotherapy models on CRP activity were seen: CRP activity was lower after WBC than after the other 3 interventions (P < .05). Researchers25,27 have determined that WBC can reduce systemic inflammation markers. The reason for the difference may be the lower temperature in the WBC intervention and the more intense body-stress response, resulting in increased secretion of epinephrine and norepinephrine. This phenomenon enhances the anti-inflammatory system of the body, thereby effectively reducing the number of inflammatory factors.6,25,27 In our study, CRP activity fluctuated after CWT because of the alternate immersions in cold and hot water and the order of the alternate immersions.11 In their analysis of WBC effects on inflammatory rheumatic diseases in humans, Guillot et al28 showed that WBC might downregulate proangiogenic and proinflammatory pathways, such as vascular endothelial growth factor, proinflammatory cytokines, and enzymatic activities.
Muscle Performance
A variety of factors affect VJH, and different cryotherapy models can result in different effects on these variables. The WBC and CWT interventions produced better results, followed by the CWI intervention (P < .05). Similarly, Fonda and Sarabon29 reported that VJH increased after a WBC intervention compared with the CON condition at certain recovery times. This finding may be attributed to the capability of WBC to enhance eccentric muscle-performance recovery30; however, it did not decrease neuromuscular performance during isokinetic exercise without impairing muscular performance.31 The lower limb muscles may stiffen and tighten after CWI,32 affecting the performance of these body parts.33 In CWT, alternate exposure to cold and hot water (cold-water stimulation and hot-water soothing) are performed to avoid violent muscle contractions, thereby preventing stiffness and restoring the muscle strength of the lower limbs.8,11 Possible reasons for these inconsistent results include simulation of the exercise protocol, exposure frequency, participant training status, and inconsistent times between measures in other studies.
Different investigators have used various indicators to evaluate the muscle's athletic ability. Hence, carrying out a unified, standardized analysis is difficult. Given individual differences and various exercise program characteristics, more research must be conducted before we can draw a conclusion. At present, numerous inconsistent results for the effects of WBC and water immersion on muscle damage have been demonstrated. The efficacy and postulated physiological mechanisms remain unclear. Future study and unified experimental conditions are necessary to elucidate the benefits of these interventions on recovery and adaptation to provide practitioners with recommendations for evidence-based practice.
The limitations of this study are as follows. First, 1 week of intervening time and strict control of diet and physical activity reduced the effects of EIMD and the interventions on groups. Because of the body's ability to adapt, a long tracking time will interfere with the EIMD exercise model and affect each intervention. Second, the sample size was extremely small for certain variables. Third, muscle and skin temperatures and heart rate, which may provide useful information on the heat exchange afforded by each recovery procedure, were not evaluated.
To our knowledge, our study is one of the few to demonstrate the effects of CWI, CWT, and WBC on EIMD and track their timing on EIMD recovery, which provides an interesting link with practical application to sport recovery. Although more work has been conducted involving CWI, CWT, and WBC, only a limited number of investigators have focused on complete recovery times in EIMD. More uniform experimental conditions and a large number of participants are necessary in future research.
CONCLUSIONS
Our results suggested that WBC can reduce the perceived muscle soreness of middle- and long-distance runners; reduce the increase in muscle-damage markers, such as plasma CK activity; and inhibit the increase in plasma CRP activity. The recovery of lower limb athletic ability was promoted after the EIMD exercise program.
This study was designed to compare the effects of 4 recovery modalities (CWI, CWT, WBC, CON) during the recovery period (96 hours postexercise) after a simulated EIMD exercise program. As shown in previous work,6,12,24,26,29 WBC positively affects muscle soreness and muscle recovery. In our study, WBC positively affected the VAS score, CK activity, CRP activity, and VJH associated with EIMD. Therefore, for middle- and long-distance runners with EIMD, WBC exerted better recovery effects than CWI, CWT, and CON. Recovery using CWI and CWT also positively affected VAS score, CK activity, CRP activity, and VJH associated with EIMD in middle- and long-distance runners, but the extent of their action and timing-sequence effects were poorer than those of WBC.
ACKNOWLEDGMENTS
This study was supported by grant number 11775059 from the National Natural Science Foundation of China (all authors) and grant numbers 17-18 and 17-19 from the Fundamental Research Foundation of the China Institute of Sport Science (all authors).
We thank all the study participants for their time and effort. We also thank Wenyuan Shang, Weiying Zhang, XingYa Yang, and ZhiGuang Zhao for their assistance with this experiment and the Beijing Institute of Sports Science for technical assistance.
REFERENCES
- 1.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81(suppl 11):S52–S69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 2.Byrne C, Twist C, Eston R. Neuromuscular function after exercise-induced muscle damage: theoretical and applied implications. Sports Med. 2004;34(1):49–69. doi: 10.2165/00007256-200434010-00005. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Med Sci Sports Exerc. 1984;16(6):529–538. [PubMed] [Google Scholar]
- 4.Kanda K, Sugama K, Hayashida H, et al. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc Immunol Rev. 2013;19:72–85. [PubMed] [Google Scholar]
- 5.Howatson G, van Someren KA. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008;38(6):483–503. doi: 10.2165/00007256-200838060-00004. [DOI] [PubMed] [Google Scholar]
- 6.Banfi G, Lombardi G, Colombini A, Melegati G. Whole-body cryotherapy in athletes. Sports Med. 2010;40(6):509–517. doi: 10.2165/11531940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Wilcock IM, Cronin JB, Hing WA. Physiological response to water immersion: a method for sport recovery? Sports Med. 2006;36(9):747–765. doi: 10.2165/00007256-200636090-00003. [DOI] [PubMed] [Google Scholar]
- 8.Versey NG, Halson SL, Dawson BT. Water immersion recovery for athletes: effect on exercise performance and practical recommendations. Sports Med. 2013;43(11):1101–1130. doi: 10.1007/s40279-013-0063-8. [DOI] [PubMed] [Google Scholar]
- 9.Costello JT, Baker PR, Minett GM, Bieuzen F, Stewart IB, Bleakley C. Whole-body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults. Cochrane Database Syst Rev. 2015;(9) doi: 10.1002/14651858.CD010789.pub2. CD010789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonseca LB, Brito CJ, Silva RJ, Silva-Grigoletto ME, da Silva WM, Franchini E. Use of cold-water immersion to reduce muscle damage and delayed-onset muscle soreness and preserve muscle power in jiu-jitsu athletes. J Athl Train. 2016;51(7):540–549. doi: 10.4085/1062-6050-51.9.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaile JM, Gill ND, Blazevich AJ. The effect of contrast water therapy on symptoms of delayed onset muscle soreness. J Strength Cond Res. 2007;21(3):697–702. doi: 10.1519/R-19355.1. [DOI] [PubMed] [Google Scholar]
- 12.Hausswirth C, Louis J, Bieuzen F, et al. Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS One. 2011;6(12):e27749. doi: 10.1371/journal.pone.0027749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ascensão A, Leite M, Rebelo AN, Magalhaes S, Magalhaes J. Effects of cold water immersion on the recovery of physical performance and muscle damage following a one-off soccer match. J Sports Sci. 2011;29(3):217–225. doi: 10.1080/02640414.2010.526132. [DOI] [PubMed] [Google Scholar]
- 14.French DN, Thompson KG, Garland SW, et al. The effects of contrast bathing and compression therapy on muscular performance. Med Sci Sports Exerc. 2008;40(7):1297–1306. doi: 10.1249/MSS.0b013e31816b10d5. [DOI] [PubMed] [Google Scholar]
- 15.Pournot H, Bieuzen F, Louis J, et al. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS One. 2011;6(11):e22748. doi: 10.1371/journal.pone.0022748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howley ET, Bassett DR, Jr, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27(9):1292–1301. [PubMed] [Google Scholar]
- 17.Marcora SM, Bosio A. Effect of exercise-induced muscle damage on endurance running performance in humans. Scand J Med Sci Sports. 2007;17(6):662–671. doi: 10.1111/j.1600-0838.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 18.Miyama M, Nosaka K. Muscle damage and soreness following repeated bouts of consecutive drop jumps. Adv Exerc Sports Physiol. 2004;10(3):63–69. [Google Scholar]
- 19.Miyama M, Nosaka K. Influence of surface on muscle damage and soreness induced by consecutive drop jumps. J Strength Cond Res. 2004;18(2):206–211. doi: 10.1519/R-13353.1. [DOI] [PubMed] [Google Scholar]
- 20.Nie J, George KP, Tong TK, Shi Q. Effect of repeated endurance runs on cardiac biomarkers and function in adolescents. Med Sci Sports Exerc. 2011;43(11):2081–2088. doi: 10.1249/MSS.0b013e31821d4a82. [DOI] [PubMed] [Google Scholar]
- 21.Tian Y, Nie J, Huang C, George KP. The kinetics of highly sensitive cardiac troponin T release after prolonged treadmill exercise in adolescent and adult athletes. J Appl Physiol (1985) 2012;113(3):418–425. doi: 10.1152/japplphysiol.00247.2012. [DOI] [PubMed] [Google Scholar]
- 22.Nosaka K, Newton M, Sacco P. Muscle damage and soreness after endurance exercise of the elbow flexors. Med Sci Sports Exerc. 2002;34(6):920–927. doi: 10.1097/00005768-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Su QS, Tian Y, Zhang JG, Zhang H. Effects of allicin supplementation on plasma markers of exercise-induced muscle damage, IL-6 and antioxidant capacity. Eur J Appl Physiol. 2008;103(3):275–283. doi: 10.1007/s00421-008-0699-5. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira-Junior JB, Bottaro M, Vieira A, Sigueira AF, Vierra CA, Durigan JL. One session of partial-body cryotherapy (−110 °C) improves muscle damage recovery. Scand J Med Sci Sports. 2015;25(5):e524–e530. doi: 10.1111/sms.12353. [DOI] [PubMed] [Google Scholar]
- 25.Banfi G, Dogliotti G, D'Eril GM, et al. Effects of whole-body cryotherapy on serum mediators of inflammation and serum muscle enzymes in athletes. J Therm Biol. 2009;34(2):55–59. [Google Scholar]
- 26.Bouzigon R, Grappe F, Ravier G, Dugue B. Whole- and partial-body cryostimulation/cryotherapy: current technologies and practical applications. J Therm Biol. 2016;61:67–81. doi: 10.1016/j.jtherbio.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Ziemann E, Olek RA, Kujach S, et al. Five-day whole-body cryostimulation, blood inflammatory markers, and performance in high-ranking professional tennis players. J Athl Train. 2012;47(6):664–672. doi: 10.4085/1062-6050-47.6.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guillot X, Tordi N, Mourot L, et al. Cryotherapy in inflammatory rheumatic diseases: a systematic review. Expert Rev Clin Immunol. 2014;10(2):281–294. doi: 10.1586/1744666X.2014.870036. [DOI] [PubMed] [Google Scholar]
- 29.Fonda B, Sarabon N. Effects of whole-body cryotherapy on recovery after hamstring damaging exercise: a crossover study. Scand J Med Sci Sports. 2013;23(5):e270–e278. doi: 10.1111/sms.12074. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira-Junior JB, Bottaro M, Vieira CA, et al. Effects of partial-body cryotherapy (−110°C) on muscle recovery between high-intensity exercise bouts. Int J Sports Med. 2014;35(14):1155–1160. doi: 10.1055/s-0034-1382057. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira-Junior JB, Vieira CA, Soares SR, et al. Effects of a single whole body cryotherapy (−110°C) bout on neuromuscular performance of the elbow flexors during isokinetic exercise. Int J Sports Med. 2014;35(14):1179–1183. doi: 10.1055/s-0034-1374602. [DOI] [PubMed] [Google Scholar]
- 32.Goodall S, Howatson G. The effects of multiple cold water immersions on indices of muscle damage. J Sports Sci Med. 2008;7(2):235–241. [PMC free article] [PubMed] [Google Scholar]
- 33.Jakeman JR, Macrae R, Eston R. A single 10-min bout of cold-water immersion therapy after strenuous plyometric exercise has no beneficial effect on recovery from the symptoms of exercise-induced muscle damage. Ergonomics. 2009;52(4):456–460. doi: 10.1080/00140130802707733. [DOI] [PubMed] [Google Scholar]