Abstract
Introduction
This 8-week multisite, randomized controlled trial of snus examined the differential effects of instructions on (1) snus use, (2) smoking and smoking-related measures, and (3) exposure to tobacco-related constituents.
Method
US adult daily cigarette smokers (n = 150; 43.3% female; Medianage = 43.5) were recruited from Minneapolis, Minnesota; Columbus and Coshocton, Ohio; and Buffalo, New York. Following a 1-week sampling phase of snus, participants who used at least 7 pouches were randomized to either (1) partial substitution (PS; “use snus as you like with your cigarettes”), (2) complete substitution (CS; “avoid cigarettes”), or (3) usual brand cigarettes (UB). Analyses included between-group analyses (eg, PS vs. CS) using Wilcoxon rank sum test of cigarettes per day and snus pouches per day, and a linear mixed model (biomarkers).
Results
Compared to the PS and UB groups, smokers assigned to CS reported greater reductions in cigarettes per day (ps < .001), using more snus pouches per day (p = .02), and more smoke-free days (CS median = 14.5, PS and UB medians = 0, p < .001). In addition, results demonstrated reductions in carbon monoxide (p < .001), total nicotine equivalents (p = .02), and four out of five measured volatile organic compounds (ps < .01) over time among the CS group. Exposure to N′-nitrosonornicotine increased by trial end only among the PS group (p < .04). Phenanthrene tetraol increased among all groups by trial end (p = .02) with no difference between groups.
Conclusions
Instructions to completely switch from cigarettes to snus resulted in the greatest reduction in cigarettes and exposure to harmful constituents.
Implications
Directly instructing smokers to switch completely to snus, rather than using ad libitum (with no instructions to avoid cigarettes), is necessary for reductions in smoking and subsequent exposure to harmful constituents.
Introduction
Snus, a smokeless tobacco product with purportedly lower levels of tobacco-specific nitrosamines, results in substantially lower exposure to harmful constituents compared to cigarettes. Thus, switching from cigarettes to snus completely could reduce smoking-related death and disease.1–4 For example, Sweden observed a significant reduction in tobacco-related disease over the past several decades as more smokers switched to snus.2 A recent review of Swedish cohorts found that many smokers who switched to snus have similar risks of cancer and cardiovascular disease as smokers who quit tobacco altogether.5,6 Given the introduction of snus in the United States, it is important to examine potential ways to optimize any beneficial effects and minimize any negative impacts when smokers are considering snus as an alternative nicotine product.
Instructions for use will likely influence the extent of snus uptake, smoking behaviors, and potentially subsequent health effects. In research examining switching from cigarettes to snus, instructions for use have varied from partial to complete substitution, and from prescribed minimum product use to ad libitum use (use as you like).7 Results from these studies suggest that smokers can successfully reduce smoking with snus; however, complete substitution is rare, particularly when smokers are not instructed to stop smoking cigarettes.7–9 However, no study to the best of our knowledge has randomized participants to and directly compared the effects of instructions for use on smokers’ exposure to harmful constituents. Such data are important for informing regulatory decisions.
This study measured the effects of instructions for complete versus partial substitution of snus for cigarettes, on (1) snus use, (2) smoking and smoking-related factors, and (3) level of exposure to nicotine- and tobacco-related harmful constituents. In addition, patterns of cigarette and snus use over time were examined.
Methods
Participants
Smokers were recruited from Minneapolis, Minnesota; Columbus and Coshocton, Ohio, and Buffalo, New York between May 2013 and August 2016. Internet and local media advertisements read: “Smokers who want to try a new oral tobacco product are needed for a research study that may reduce their exposure to harmful tobacco smoke.” Interested smokers who called the respective study site, were informed about the study, and were initially screened for eligibility over the telephone. Eligibility criteria included (1) at least 18 years of age, (2) smoking at least 5 cigarettes/day (CPD) for the past year, (3) no regular use of other nicotine/tobacco products (eg, ≤9 days/month), (4) good physical and mental health (eg, no unstable or untreated medical or psychiatric conditions), (5) not planning to quit smoking in the next 3 months, and (6) no chronic conditions affecting results of biochemical analyses (eg, liver disease). Participants were excluded if they were or had (1) a serious quit attempt in the past 3 months, (2) current or recent (<3 months) alcohol or drug abuse problems, (3) currently using nicotine replacement or other cessation methods, or (4) pregnant, planning to become pregnant, or breastfeeding. Each site’s institutional review board approved this study (Clinicaltrials.gov #NCT01867242).
Design
The groups in this study were combined from two studies (study A and B) with similar designs, one of which also examined groups of e-cigarette use (study B) not included in this study. The only differences between the two study designs were the instructions for use and amount of monetary compensation (described later).
Orientation, Screening, and Sampling Phase (Week −3)
Potentially eligible participants were invited to an orientation visit during which they completed informed consent and further screening for medical and tobacco use history. Demographic and self-report measures of smoking-related variables were completed. Vitals and carbon monoxide (CO) were assessed, and pregnancy tests were conducted on women of childbearing potential. Smoking status was confirmed with exhaled CO at least 10 ppm (tested in the clinic); if CO was less than 10 ppm, then NicAlert test = level 6.
Next, eligible participants began the sampling phase. Participants chose two of three snus flavors to smell—Winterchill, Frost, or Robust—in blinded tins for 30 seconds. Participants sampled the product for a timed 5-minute period. After each sampling, they completed several questionnaires about the product (not reported here). Participants drank water and ate a saltine cracker to cleanse their palate between samplings.
Participants chose their preferred flavor and were provided four tins containing 15 pouches each to sample over the next week. Participants were told “Some people like snus and use a lot, others do not like it and don’t use it. Use the product as you wish over the next week. Most people get the maximum effect if they keep the pouch in their mouth for at least 30 minutes.” They were also instructed on how to complete daily automated phone calls regarding the previous day’s tobacco use and scheduled for their second appointment 1 week (±3 days) later.
Sampling Phase, Week −2
After 1 week, participants returned to the clinic with snus tins and unused snus pouches. Tobacco use over the past week was assessed and participants completed self-report questionnaires. Participants who used at least seven snus pouches (based on potential use of one pouch per day) and continued to smoke were eligible to enter the clinical trial. These criteria were withheld from participants to ensure an unbiased willingness to use snus.
Clinical Trial Phase
Following the sampling week, participants attended a total of 8 visits over 10 weeks including 2 baseline weeks (weeks −1 and 0). During the baseline weeks, they smoked as usual, provided first morning urine samples, and completed daily phone diaries of tobacco use.
At week 0, participants were randomized to 1 of the 5 conditions for 8 weeks: (1) smoking usual brand cigarette control (UB); (2) complete substitution—ad libitum snus use (ie, “stop smoking cigarettes and use only snus; use the snus whenever you like; use enough snus to satisfy your cravings for cigarettes”); (3) complete substitution—specific instructions for snus use (ie, those smoking ≤20 CPD were instructed to use ≥8 snus pouches per day [SPD], and ≥20 CPD were instructed to use ≥12 SPD); (4) partial substitution—ad libitum snus use (ie, “use snus whenever you like instead of a cigarette; smoke as many or as few cigarettes as you want”); and (5) partial substitution—specific instructions (similar snus dosing as complete substitution—specific instructions group). Mid-study, conditions (3) and (5) were eliminated to increase recruitment numbers. For data analyses, instructions for use and study (A or B) were entered as covariates and groups were combined based on substitution instructions (complete vs. partial substitution). This article reports on three groups: UB, partial substitution (PS), and complete substitution (CS).
At each following visit, daily phone diaries were reviewed, CO was measured, all tins and unused snus were collected and counted, and participants completed self-report measures. At each visit, all groups engaged in sessions in which compliance to product use instructions were discussed. For those in the CS groups who were unable to completely switch, participants problem-solved ways to foster complete switching. At week 8, all subjects were strongly encouraged to stop using all tobacco products and coached on setting a quit date.
Compensation
In study A, participants could earn up to $585. Participants received compensation for transportation ($5 per visit), clinic visits ($40 including a follow-up visit), daily diary completion (up to $150), protocol compliance ($290; including avoiding cigarettes for those in the CS groups), and two follow-up phone calls ($10). In study B, total compensation increased to $750. Specifically, participants received $25 per clinic visit and an additional “bonus” $25 for urine samples, protocol compliance (eg, avoiding cigarettes for those in the CS groups), and daily diary completion.
Products
Participants chose from Winterchill, Frost Large, and Robust flavored Camel Snus (Reynolds American Inc, Winston-Salem, NC) with 2.5–2.6 mg free nicotine per pouch, according to our analyses. Participants indicating the dose was too strong were switched to a small pouch Frost or Mellow, which contains 1.5-mg nicotine per pouch. All snus were provided free to participants.
Measures
Demographics and Tobacco Use
Demographic and tobacco use variables were collected for eligibility and potential moderators. Participants reported cigarette, snus, and other nicotine-containing product use via daily automated phone calls. The following tobacco use variables were assessed at clinic visits: CPD and SPD, and nicotine dependence via the Fagerström Test for Nicotine Dependence (FTND).10 FTND total scores were used (range 0–10) with higher scores indicating greater dependence. The 20-item Center for Epidemiological Studies-Depression (CES-D) scale11 was completed at baseline and week 8 to assess eligibility and monitor depressive symptoms.
Additional Measures Not Included
Additional measures assessing tobacco-related variables, evaluation of snus, psychiatric and medical variables, and perceived health risks were completed, but not reported here. At each visit, participants’ blood pressure, heart rate, and oxygen saturation were measured.
Biomarker Analyses
Biomarkers included (1) urinary total nicotine equivalents (total nicotine + total cotinine + total 3′hydroxycotinine; TNEs),12 (2) exhaled CO, (3) urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and its glucuronides (total NNAL) and N′-nitrosonornicotine (NNN), (4) urinary phenanthrene tetraol (PheT; a proxy for carcinogenic polycyclic aromatic hydrocarbons), and (5) urinary metabolites of volatile organic compounds (VOCs)—2-cyanoethylmercapturic acid (CEMA) for acrylonitrile, 3-hydroxypropylmercapturic acid (3-HPMA) for acrolein, 3-hydroxy-1-methylpropylmercapturic acid (HMPMA) for crotonaldehyde, 2-hydroxypropylmercapturic acid (2-HPMA) for propylene oxide, and N-acetyl-S-(2-carbamoylmethyl)-L-cysteine (AAMA) for acrylamide. These biomarkers come from an empirically informed panel of biomarkers for examining tobacco carcinogen and toxicant uptake for the purposes of tobacco product evaluation and cancer prevention.13,14 See Supplementary Table 1 for a description of these biomarkers and example health effects.
Participants provided exhaled CO using a Bedfont Smokerlyzer. TNE, tobacco-specific nitrosamines, and mercapturic acids were analyzed using liquid chromatography–mass spectrometry.15–19 PheT was analyzed by gas chromatography–tandem mass spectrometry.16 Biomarker analysis was conducted as described in our previous work for NNAL,16 NNN,23 PheT,16 3-HPMA,17 HMPMA,17 CEMA,17 2-HPMA,20 and AAMA.20 Validation procedures from previously published work were used for each biomarker (TNE, creatinine21; NNAL, PheT, 3-HPMA, HMPMA, and CEMA22; NNN23; 2-HPMA18). Urinary creatinine concentrations were analyzed using a colorimetric microplate assay (CRE34-K01; Eagle Bioscience, Amherst, NH). All biomarker analyses were adjusted for creatinine to account for urine dilution variability between participants.
CO was collected weekly. Urinary TNEs were analyzed at baseline (week -1, 0) and weeks 4 and 8. All other biomarkers were analyzed at week 0, 4, and 8. TNEs at week −1 and 0 were averaged to create a baseline TNE measurement.
Data Analysis
Baseline demographics were summarized using median, range, frequency, and percent. Biomarkers below the limit of quantitation were imputed as 50% the limit of quantitation (samples below limit of quantitation = 15/371 (4%) for NNN, 3/495 (0.6%) for NNAL, and 0/396 (0%) across all MA biomarkers). No other data imputation procedures were conducted. All biomarkers were log-transformed and reported as geometric means. Chi-square and Wilcoxon rank sum tests were used to compare baseline demographic and tobacco use history variables between groups. All analyses were performed according to the intent-to-treat principle.
Poisson regression with repeated measures using generalized estimating equations was used to evaluate CPD and SPD between weeks from baseline until week 8. These endpoints were modeled via the logarithmic link function. The optimal variance–covariance structure was autoregressive for CPD and independent for SPD determined by the quasi-likelihood under independence model criterion.24 A linear mixed model was used to compare study groups and timepoints when analyzing the biomarkers.25 To model the within-subject effect, the optimal variance–covariance matrix was selected for each biomarker based on the Akaike and Bayesian information criteria. The following analytic approach was used for all the repeated measures analyses. First step: unadjusted model including the group indicator, week, and their interaction. If the interaction p value was greater than .1, the interaction term was dropped. Second step: adjusted model that included a preselected set of baseline covariates in addition to the group and week. If the interaction p value was less than .1, the three study groups were analyzed separately with an adjusted model including the week and the preselected covariates (baseline sex, race [white/nonwhite], age, employment [part/full time vs. other], FTND, CES-D, TNE, ad libitum/instructions, study A or B, and use of other combusted tobacco. Using a stepdown selection procedure to obtain the most parsimonious model, only significant covariates (p value < .05) were retained. Group and week indicators always remained in the model. The coefficients from the regression models are exponentiated to represent the estimated ratio (95% CI) of CPD, SPD, and biomarkers in their original scale for every one unit per level increase in the covariates. Linear mixed models and generalized estimating equation models treat occasional missing observations or missed visit as missing at random. The frequent dropouts in this study were compared between groups in a separate analysis using a chi-square test.
Between-group analyses (PS vs. CS) of CPD and SPD at each week were conducted using Wilcoxon rank sum test. Paired t tests were conducted to determine when patterns of use stabilized by examining mean change scores in CPD and SPD from week to week. Days with no cigarette smoking were summarized by study group as the median percent of smoke-free days over the entire study period, the frequency of smoke-free weeks, and the percent of smokers that had at least one smoke-free day. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). Final analyses were considered statistically significant with p less than .05.
Results
Participant Characteristics
Of the 1806 individuals who were phone screened (792 of these participants responded to a study advertisement that also included e-cigarette groups not reported here), 435 consented, and 150 were eligible to be randomized to the clinical trial. The most common reasons for ineligibility were nonmedical reasons (n = 85; eg, other tobacco use), medically ineligible (n = 51), lost to follow-up during baseline (n = 49), insufficient snus use during the sampling phase (n = 33), and personal reasons (n = 14; eg, too busy). Only three participants withdrew from the study due to reporting disliking the product during sampling. Fifty participants were randomized to e-cigarette conditions not reported here.
Table 1 shows baseline demographic information and tobacco use history of randomized participants across groups. Participants were primarily white (68.0%), with 43.3% female and a median age of 43.5 years. Nicotine dependence differed between groups at baseline; participants in the CS group (FTND median = 3.0) were more dependent on tobacco than the other two groups. Most participants chose Winterchill or Frost-flavored snus (69.2%–78.1%). There were no significant differences in dropout rates between groups following randomization (dropouts: CS, n = 24, 50%; PS, n = 16, 30.2%; UB, n = 8, 36.4%; p > .05). Most dropouts occurred by week 4 (week 1 [n = 15, 31.3%], week 2 [n = 8, 16.7%], week 3 [n = 7, 14.6%], week 4 [n = 7, 14.6%], week 6 [n = 3, 6.3%], and week 8 [n = 8, 16.7%]).
Table 1.
Demographics Across Use Groups
| Variable | Total (N = 150) | Complete substitution (N = 64) | Partial substitution (N = 60) | Usual brand (N = 26) | p valuea |
|---|---|---|---|---|---|
| Study site, N (%) | |||||
| UMN | 45 (30.0) | 17 (26.6) | 20 (33.3) | 8 (30.8) | |
| OSU/Coshocton Clinic | 84 (56.0) | 37 (57.8) | 33 (55.0) | 14 (53.9) | .92 |
| Roswell | 21 (14.0) | 10 (15.6) | 7 (11.7) | 4 (15.4) | |
| Age, median (min/max) | 43.5 (18/83) | 42.5 (18/83) | 42.0 (18/64) | 47.0 (23/68) | .38 |
| Sex, Female, N (%) | 65 (43.3) | 28 (43.8) | 24 (40.0) | 13 (50.0) | .69 |
| Race, N ( %) | |||||
| White | 102 (68.0) | 44 (68.8) | 43 (71.7) | 15 (57.7) | |
| Black | 43 (28.7) | 16 (25.0) | 16 (26.7) | 11 (42.3) | .30b |
| Other | 5 (3.3) | 4 (6.3) | 1 (1.7) | 0 (0.0) | |
| Education, N (%) | |||||
| Eighth grade or less | 1 (0.7) | 1 (1.6) | 0 (0.0) | 0 (0.0) | |
| Some high school | 13 (8.7) | 7 (10.9) | 5 (8.3) | 1 (3.9) | |
| High school | 44 (29.3) | 17 (26.6) | 20 (33.3) | 7 (26.9) | — |
| Some college | 70 (46.7) | 26 (40.6) | 28 (46.7) | 16 (61.5) | |
| College grad | 17 (11.3) | 10 (15.6) | 6 (10.0) | 1 (3.9) | |
| Graduate/professional | 5 (3.3) | 3 (4.7) | 1 (1.7) | 1 (3.9) | |
| Education, N (%) | |||||
| High school/less | 58 (38.7) | 25 (39.1) | 25 (41.7) | 8 (30.8) | .63 |
| Some college/more | 92 (61.3) | 39 (60.9) | 35 (58.3) | 18 (69.2) | |
| Income, N (%) | |||||
| Less than $30,000 | 97 (64.7) | 42 (65.6) | 39 (65.0) | 16 (61.5) | .93 |
| More than $30,000 | 53 (35.3) | 22 (34.4) | 21 (35.0) | 10 (38.5) | |
| Current Employment, full/part-time, N (%) | 55 (36.7) | 26 (40.6) | 17 (28.3) | 12 (46.2) | .20 |
| FTND total score, median (min/max) | 3.0 (0/7) | 3.0 (2/6) | 3.0 (0/7) | 3.0 (1/6) | .02c |
| CES-D (depression), median (min/max) | 6.0 (0/34) | 8.0 (0/34) | 6.0 (0/19) | 6.0 (0/24) | .07 |
| Flavor, Winterchill/Frost, N (%) | 104 (75.9) | 50 (78.1) | 45 (75.0) | 9/13 (69.2) | .77 |
| Baseline cigarettes/day, median (range) | 14.0 (4.3/34.4) | 11.7 (6.0/39.9) | 12.1 (5.6/31.5) | .77 | |
| Baseline TNE nmol/mg creatinine, median (range) | 58.3 (18.5/383.1) | 55.9 (0.04/307.3) | 65.7 (5.4/152.4) | .52 | |
| Dropout, N (%) | 48 (32.0%) | 24 (50.0%) | 16 (30.2%) | 8 (36.4%) | .12 |
CES-D = Center for Epidemiologic Studies Depression scale; FTND = Fagerström Test for Nicotine Dependence; OSU = The Ohio State University; TNE = total nicotine equivalents; UMN = University of Minnesota.
aThe p values were derived from the chi-square test or the Wilcoxon rank sum test.
bThis p value compares whites and blacks only.
cParticipants assigned to Complete Substitution had higher FTND scores than the other groups.
Tobacco Use
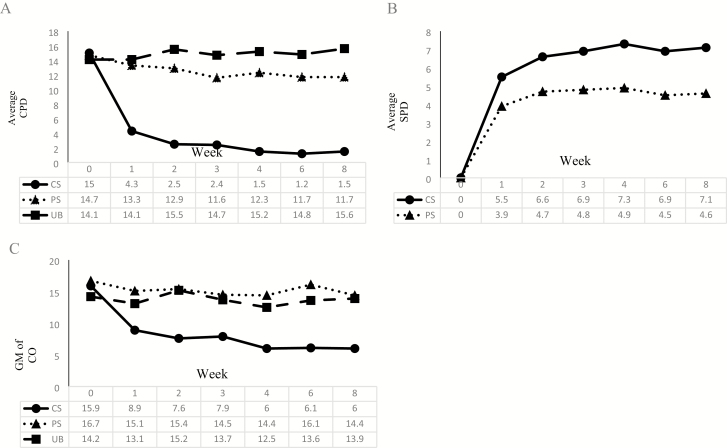
Average CPD and SPD between groups and across weeks are shown in Figure 1. For CPD, a significant interaction emerged between week and study group (p < .001). Thus, the three groups were analyzed separately. The CS group reported significant reductions in CPD at each week compared to week 0 (CPD week 1:0 = 0.31, week 2:0 = 0.22, week 3:0 = 0.23, week 4:0 = 0.16, week 6:0 = 0.10, week 8:0 = 0.12; ps < .001); however, many smokers did not avoid cigarettes completely despite being incentivized and instructed to do so. The PS group reported a smaller but significant reduction in CPD at each week (except week 4) compared to week 0 (CPD week 1:0 = 0.92, p = .004; week 2:0 = 0.90, p = .03; week 3:0 = 0.90, p = .04; week 6:0 = 0.88, p = .005; week 8:0 = 0.86, p = .002). The UB group’s CPD remained consistent throughout the trial, except for weeks 1 and 3, during which they reported a slight reduction compared to week 0 (CPD week 1:0 = 0.92, p = .02; week 3:0 = 0.91, p = .02).
Figure 1.
(A) Average CPD, (B) average SPD, and (C) exhaled CO by tobacco use group. CO = carbon monoxide; CPD = cigarettes per day; CS = complete substitution; GM = geometric mean; SPD = snus per day; PS = partial substitution; UB = usual brand.
No significant interaction emerged between week and study group for SPD. Over the 8-week study, the CS group used, on average, 36% more SPD than the PS group (SPD CS:PS ratio = 1.36, p = .02). For all snus groups, average SPD were significantly lower at week 1 (SPD week 1:8 = 0.78, p = .002) than week 8, but increased at week 2 to a similar amount used at week 8 (SPD week 2:8 = 0.99, p = .84), remaining consistent throughout the trial (ps > .05).
Between-week differences of SPD and CPD patterns among PS and CS groups are shown in Supplementary Tables 2 and 3. Stabilization of SPD occurred within 2 weeks among the PS and CS groups evidenced by significant increases in SPD from week 1 to 2 (PS Meanchange = 0.88 SPD, p = .001; CS Meanchange = 1.03 SPD, p = .009). Week-to-week changes in SPD were nonsignificant after week 2 (except for a slight drop at week 6 that eventually rebounded). Likewise, stabilization of CPD occurred within 2 weeks among CS group evidenced by significant decreases in CPD from week 0 to 1 (Meanchange = −10.89 CPD, p < .001), week 1–2 (Meanchange = −1.30 CPD, p = .008), and subsequent nonsignificant between week changes. However, stabilization of CPD occurred within the first week among the PS group evidenced by significant decreases in CPD from week 0 to 1 (Meanchange = −1.23 CPD, p = .002), and nonsignificant changes from subsequent week to week. These patterns sustained when analyses were repeated among only participants who completed the entire trial.
Smoke-Free Days
Smoke-free days throughout the trial are shown in Supplementary Table 4. Over the 8-week study (~56 days), smokers in the CS group reported more smoke-free days (median = 14.5, range 0–61 days) than those in the PS and UB groups (PS and UB medians = 0, χ2 (2, N = 150) = 52.8, p < .001).
When examining weeks with 100% smoke-free days, 80 weeks were identified, with 77 among the CS group and 3 among the PS group (all from one person). More smokers reported at least one smoke-free day, with the greatest number among the CS group (n = 34/48, 70.8%), followed by the PS group (n = 5/53, 9.4%), and the UB group (n = 2/22, 9.1%, p < .001; assuming those who dropped returned to smoking).
Supplementary Table 4 shows smoke-free weeks verified by a CO reading of less than or equal to 6 ppm among participants in the CS group. Among those who self-reported a smoke-free week, 84.4% were CO-verified (all weeks range = 66.7%–100%).
Biomarkers
CO levels by group are shown in Figure 1. Table 2 shows geometric means and medians for the other biomarkers.
Table 2.
Biomarkers Summary Statistics by Week and Study Group
| Biomarker | Week | Study Group | N | GM (95% CI) | Median (Range) | p valuea |
|---|---|---|---|---|---|---|
| GM TNE nmol/mgb | Baseline | UB | 22 | 61.5 (44.8 to 84.5) | 65.7 (5.4/152.4) | |
| PS | 53 | 48.3 (34.5 to 67.8) | 55.9 (0.04/307.3) | .52 | ||
| CS | 48 | 57.5 (48.9 to 67.6) | 58.3 (18.5/383.1) | |||
| 4 | UB | 15 | 52.3 (34.5 to 79.3) | 55.1 (7.6/132.8) | ||
| PS | 42 | 61.9 (47.3 to 81.0) | 64.2 (1.6/257.7) | .09 | ||
| CS | 26 | 42.2 (31.1 to 57.3) | 44.8 (8.8/125.5) | |||
| 8 | UB | 16 | 63.4 (42.7 to 94.0) | 62.4 (8.0/167.8) | ||
| PS | 39 | 65.0 (53.1 to 79.4) | 68.5 (13.4/196.1) | .30 | ||
| CS | 24 | 46.7 (32.5 to 67.0) | 54.7 (4.7/178.6) | |||
| GM NNAL pmol/mg creatinine | Baseline | UB | 22 | 1.14 (0.65 to 2.00) | 1.25 (0.02/9.48) | |
| PS | 53 | 1.06 (0.80 to 1.41) | 1.14 (0.02/7.91) | .51 | ||
| CS | 47 | 1.31 (1.03 to 1.66) | 1.52 (0.07/5.28) | |||
| 4 | UB | 15 | 1.29 (0.78 to 2.15) | 1.17 (0.17/6.81) | ||
| PS | 42 | 1.29 (1.02 to 1.64) | 1.35 (0.20/8.73) | .94 | ||
| CS | 26 | 1.15 (0.85 to 1.57) | 1.39 (0.20/3.42) | |||
| 8 | UB | 16 | 1.38 (0.91 to 2.08) | 1.22 (0.22/5.66) | ||
| PS | 39 | 1.27 (1.01 to 1.61) | 1.14 (0.31/4.85) | .56 | ||
| CS | 24 | 1.43 (1.07 to 1.91) | 1.47 (0.33/4.30) | |||
| GM NNN pmol/mg creatinine | Baseline | UB | 22 | 0.022 (0.012 to 0.038) | 0.030 (0.002/0.178) | |
| PS | 52 | 0.026 (0.017 to 0.039) | 0.024 (0.001/1.527) | .89 | ||
| CS | 45 | 0.027 (0.018 to 0.039) | 0.027 (0.002/0.570) | |||
| 4 | UB | 15 | 0.017 (0.008 to 0.036) | 0.016 (0.002/0.291) | ||
| PS | 41 | 0.044 (0.028 to 0.068) | 0.046 (0.002/4.258) | .02 | ||
| CS | 26 | 0.020 (0.012 to 0.036) | 0.020 (0.002/0.187) | |||
| 8 | UB | 16 | 0.023 (0.013 to 0.040) | 0.030 (0.004/0.096) | ||
| PS | 38 | 0.048 (0.029 to 0.080) | 0.041 (0.003/11.187) | .18 | ||
| CS | 24 | 0.025 (0.014 to 0.045) | 0.027 (0.001/0.325) | |||
| GM PheT pmol/mg creatinine | Baseline | UB | 18 | 2.10 (1.58 to 2.81) | 2.37 (0.63/4.39) | |
| PS | 46 | 2.15 (1.76 to 2.62) | 2.13 (0.46/9.37) | .97 | ||
| CS | 39 | 2.20 (1.81 to 2.67) | 2.23 (0.72/7.16) | |||
| 4 | UB | 15 | 1.79 (1.17 to 2.74) | 2.11 (0.55/5.62) | ||
| PS | 42 | 2.06 (1.66 to 2.55) | 2.30 (0.28/6.57) | .62 | ||
| CS | 26 | 1.76 (1.30 to 2.39) | 1.81 (0.31/9.28) | |||
| 8 | UB | 16 | 2.16 (1.48 to 3.13) | 2.60 (0.58/5.45) | ||
| PS | 38 | 2.58 (2.07 to 3.22) | 2.71 (0.82/11.77) | .83 | ||
| CS | 24 | 2.51 (1.79 to 3.53) | 2.18 (0.75/18.42) | |||
| GM CEMA pmol/mg creatinine | Baseline | UB | 19 | 499.9 (311.9 to 801.2) | 491.3 (46.2/2576.7) | |
| PS | 47 | 453.0 (335.6 to 611.5) | 484.9 (2.9/1963.6) | .99 | ||
| CS | 39 | 478.8 (381.8 to 600.5) | 458.5 (99.8/2440.2) | |||
| 4 | UB | 15 | 463.2 (313.6 to 684.2) | 424.6 (140.9/1950.9) | ||
| PS | 42 | 511.4 (396.8 to 659.0) | 554.8 (30.1/2212.8) | .001 | ||
| CS | 26 | 188.6 (112.5 to 316.0) | 195.7 (13.9/4321.4) | |||
| 8 | UB | 15 | 594.6 (441.9 to 800.0) | 648.3 (225.1/1657.9) | ||
| PS | 38 | 483.8 (365.0 to 657.5) | 671.5 (41.8/2223.9) | .03 | ||
| CS | 24 | 248.1 (142.7 to 431.2) | 282.5 (21.9/2243.4) | |||
| GM 2-HPMA pmol/mg creatinine | Baseline | UB | 19 | 551.6 (386.4 to 787.3) | 613.8 (171.7/2037.3) | |
| PS | 47 | 567.8 (471.2 to 684.2) | 627.3 (153.0/1893.6) | .44 | ||
| CS | 39 | 453.7 (351.2 to 586.1) | 427.4 (73.6/2117.6) | |||
| 4 | UB | 15 | 557.7 (350.7 to 887.1) | 635.9 (171.6/4502.3) | ||
| PS | 42 | 511.4 (383.5 to 682.0) | 492.1 (30.1/3734.9) | .28 | ||
| CS | 26 | 391.4 (265.2 to 577.7) | 438.9 (92.3/6047.9) | |||
| 8 | UB | 15 | 722.7 (465.3 to 1122.5) | 522.9 (158.1/4302.6) | ||
| PS | 38 | 589.2 (466.6 to 744.0) | 609.6 (89.0/2026.2) | .60 | ||
| CS | 24 | 500.5 (347.4 to 721.0) | 580.6 (72.4/2311.6) | |||
| GM 3-HPMA pmol/mg creatinine | Baseline | UB | 19 | 5328.6 (3625.1 to 7832.7) | 3902.3 (1495.4/33160.4) | |
| PS | 47 | 4240.9 (3459.5 to 5198.8) | 4578.5 (815.2/17377.9) | .76 | ||
| CS | 39 | 4482.9 (3650.3 to 5505.4) | 4795.6 (994.4/20286.4) | |||
| 4 | UB | 15 | 4098.5 (3070.5 to 5470.7) | 4135.6 (1710.6/12307.4) | ||
| PS | 42 | 5297.3 (4179.9 to 6713.4) | 4959.8 (562.3/30538.1) | .002 | ||
| CS | 26 | 2381.6 (1584.5 to 3579.8) | 2245.7 (292.3/24270.9) | |||
| 8 | UB | 15 | 4869.4 (3160.5 to 7502.2) | 4798.2 (1091.9/16617.0) | ||
| PS | 38 | 5269.5 (4071.3 to 6820.4) | 5760.5 (615.8/18301.2) | .04 | ||
| CS | 24 | 3151.8 (2207.7 to 4499.5) | 2856.5 (997.0/26281.5) | |||
| GM AAMA pmol/mg creatinine | Baseline | UB | 19 | 540.7 (358.8 to 814.7) | 469.5 (99.4/2599.4) | |
| PS | 46 | 531.0 (419.9 to 671.4) | 519.0 (45.8/3209.4) | .56 | ||
| CS | 39 | 607.8 (509.8 to 724.8) | 619.0 (232.3/1963.6) | |||
| 4 | UB | 14 | 389.1 (280.7 to 539.4) | 378.1 (159.0/1118.5) | ||
| PS | 42 | 624.7 (499.1 to 781.8) | 659.9 (50.5/2814.4) | .001 | ||
| CS | 26 | 337.1 (237.4 to 478.8) | 357.3 (25.8/2679.1) | |||
| 8 | UB | 15 | 553.3 (393.3 to 778.6) | 569.4 (194.8/1495.4) | ||
| PS | 38 | 591.3 (480.4 to 727.8) | 673.9 (145.6/2407.8) | .04 | ||
| CS | 24 | 382.4 (281.7 to 519.0) | 381.4 (88.5/1543.5) | |||
| GM HMPMA pmol/mg creatinine | Baseline | UB | 19 | 4620.3 (3197.5 to 6676.2) | 4086.8 (1618.7/21661.2) | |
| PS | 47 | 3916.8 (3224.2 to 4758.2) | 4326.6 (599.7/12709.7) | .90 | ||
| CS | 39 | 4041.6 (3335.1 to 4897.8) | 3871.2 (1331.9/10702.6) | |||
| 4 | UB | 15 | 3765.3 (2674.5 to 5300.9) | 3735.1 (990.1/9744.7) | ||
| PS | 42 | 4894.1 (3940.1 to 6078.9) | 4814.7 (776.7/22123.5) | .001 | ||
| CS | 26 | 2072.2 (1417.0 to 3030.4) | 1361.7 (520.8/11613.8) | |||
| 8 | UB | 15 | 4365.6 (3013.6 to 6324.2) | 4693.1 (1193.2/10319.0) | ||
| PS | 38 | 4449.0 (3493.9 to 5665.1) | 4689.9 (604.3/12362.2) | .19 | ||
| CS | 24 | 3116.4 (2179.2 to 4456.6) | 2506.0 (650.7/17596.4) |
CEMA = 2-cyanoethylmercapturic acid; CS = complete substitution; GM = geometric mean; HMPMA = 3-hydroxy-1-methylpropylmercapturic acid; HPMA = hydroxypropylmercapturic acid; PS = partial substitution; PheT = phenanthrene tetraol; TNE = total nicotine equivalent; NNN = N′-nitrosonornicotine; UB = usual brand.
aThe p value is derived from the nonparametric Kruskal–Wallis test.
bTNE at baseline is the average of week 91 and 00.
Carbon Monoxide
An interaction between week and study group emerged (p < .001). As a result, the three groups were analyzed separately. The CS group demonstrated significant decreases in CO throughout the trial compared to baseline (ps < .001). Compared to week 0, CO reduced by 45% by week 1 and 64% by week 8. The PS group demonstrated no significant changes in CO until week 8 (CO week 8:0 = 0.84, p = .03) and the UB group demonstrated no significant changes throughout the trial (ps > .05).
The stabilization of CO in the CS group occurred by week 2, as only weeks 0 (CO week 0:8 = 2.76, p < .001) and 1 (CO week 1:8 = 1.51, p = .007) were significantly different from week 8. Among the PS group, stabilization of CO occurred by week 1; only week 0 was significantly different from week 8 (CO week 0:8 = 1.17, p = .046).
Nicotine and Tobacco-Specific Nitrosamine
Significant interactions emerged between study group and week for urinary TNE (p = .02) and NNN (p = .04). Among the CS group, TNE levels decreased significantly from baseline to week 4 (ratio = 0.71, p = .01), but were nonsignificant from baseline to week 8 (ratio = 0.77, p = .06). Levels of TNE among the PS group showed a slight increase from baseline to week 4 that became statistically significant by week 8 (TNE baseline:4 = 1.17, p = .11; TNE baseline:8 = 1.22, p = .047). Levels of TNE among the UB group remained relatively unchanged (ps > .05). Levels of NNN remained the same for the CS (ps > .05) and UB groups (ps > .05) but increased by 75% among the PS group by the end of the trial (NNN week 0:4 = 1.50, p = .07; NNN week 0:8 = 1.75, p = .01). No interactions between week and study group emerged for NNAL (p = .18). Levels of NNAL remained the same across the trial and between study groups (ps > .05).
Phenanthrene Tetraol
No interaction between week and study group emerged (p > .05) for levels of PheT. Overall, there was a nonsignificant decrease in levels of PheT from week 0 to week 4 (PheT week 0:4 = 0.89, p = .06), followed by an increase from week 0 to week 8 (PheT week 0:8 = 1.17, p = .02). There were no differences between groups (ps > .05).
Volatile Organic Compounds
There were significant interactions between study group and week for CEMA (p < .001), 3-HPMA (p = .003), AAMA (p < .001), HMPMA (p = .001), but not 2-HPMA (p > .05). Levels of CEMA, 3-HPMA, AAMA, and HMPMA showed similar interactions patterns. Namely, levels of these biomarkers remained similar to baseline at weeks 4 and 8 among the PS and UB groups (ps > .05), with one exception for AAMA (ie, UB AAMA week 0:4 = 0.67, p = .03), but significantly lower levels of these biomarkers at weeks 4 and 8 among the CS group (ps < .05). Levels of 2-HPMA did not differ throughout the trial, nor between groups (ps > .05).
Discussion
Smokers instructed to completely substitute snus for their cigarettes reported smoking fewer CPD, using more SPD, experiencing more smoke-free days, and demonstrated reductions in some biomarkers of exposure levels (ie, TNE, CEMA, 3-HPMA, AAMA, and HMPMA). Although smokers who were instructed to use snus ad libitum demonstrated some reductions in reported CPD, most of their biomarkers of exposure levels did not differ from baseline and the UB group, and levels of TNE and NNN increased by the trial’s end (suggesting an overall increase in tobacco exposure from snus).
These results indicate potential harm reduction can only be realized if smokers are instructed to stop smoking and completely switch to snus; partial reduction in smoking has minimal effects on biomarkers of exposure. Previous research has shown reductions in VOCs even when participants dual use26; however, this previous study observed larger reductions in CPD than observed in the current study (potentially due to the previous study’s (1) higher CPD eligibility requirements, (2) research staff lit each cigarette for participants in a confined setting, and (3) participants were only able to smoke between 7 am and 11 pm and every 32 minutes).
On the other hand, snus products are not free from risks. Levels of total NNAL did not decrease because of complete switching. Results from previous studies are mixed as to whether switching to snus lowers exposure to NNK, as some studies show reductions in urinary total NNAL26,27 whereas other do not.8 More importantly, smokers who used both cigarettes and snus (PS) demonstrated increases in NNN in this study. Slight increases in PheT were seen in this study, which is unlike previous studies that observed decreases in PheT levels even when smokers continued to use cigarettes.26,28
Patterns of use appeared to stabilize in 2–4 weeks. Snus use and CO largely stabilized by week 2. Similarly, many biomarkers of VOC exposure, with elimination half-lives conducive for a shorter clinical trial,29,30 reached stabilization by week 4. Other biomarker levels continued to change from weeks 4 to 8 (eg, TNE, PheT).
This study has several limitations. First, smokers in the CS group were provided monetary bonuses for avoiding cigarettes, limiting real-world applications; however, this incentive allowed for better estimates of the maximal changes in biomarkers of exposure because of complete switching. Second, we combined two studies for analyses, one of which involved e-cigarettes; however, we statistically controlled for study A and B. Third, dropout rates ranged from 30% to 50%, with the highest rates among the CS group, potentially limiting generalizability. The dropout rates also might indicate that complete substitution with snus may be difficult to achieve for many smokers. A recent review of the literature showed that switching completely from cigarettes to smokeless tobacco is rare (0%–1.4% of adults).31 Furthermore, although many smokers tried snus in efforts to cut back on cigarettes, uptake of snus is still relatively low.32 Fourth, only smokers uninterested in quitting, who used at least seven pouches during the sampling phase, were eligible to enter the clinical trial. Then again, this procedure reflects consumers who are interested in continuing to use snus. Fifth, results of our own constituent analyses of snus products showed reductions in levels of NNN and NNK from 2013 to 2015. However, these reductions would not likely change the direction of the results as both complete and partial substitution groups experienced similar changes and we controlled for study group (A or B).
In summary, completely switching to snus seemingly reduces smokers’ exposure to some harmful constituents (ie, acrolein, crotonaldehyde, acrylonitrile, acrylamide), but not all (NNK, propylene oxide, phenanthrene), whereas partial substitution increases exposure to nicotine and NNN. This finding suggests snus would be a modified risk product only if complete switching occurred. However, the uptake of this product and the success for complete switching may be low and therefore the public health benefit of snus as a modified risk product may be modest.
Funding
Research reported in this publication was supported by grants from National Cancer Institute (U19CA157345 to DKH/PGS), National Center for Advancing Translational Science of the National Institutes of Health (UL1 TR000062), National Cancer Institute (R01 CA180880 to IS), and National Institute of Drug Abuse (T32 DA007097 to EM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Declarations of Interest
RO’C is a member of the FDA Tobacco Products Scientific Advisory Committee. PGS serves or has served as an expert witness in tobacco company litigation on behalf of plaintiffs.
Supplementary Material
References
- 1. Fagerström KO, Schildt EB. Should the European Union lift the ban on snus? Evidence from the Swedish experience. Addiction. 2003;98(9):1191–1195. [DOI] [PubMed] [Google Scholar]
- 2. Foulds J, Ramstrom L, Burke M, Fagerström K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tob Control. 2003;12(4):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levy DT, Mumford EA, Cummings KM, et al. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: Estimates of a panel of experts. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2035–2042. [PubMed] [Google Scholar]
- 4. Bates C, Fagerström K, Jarvis MJ, Kunze M, McNeill A, Ramström L. European Union policy on smokeless tobacco: A statement in favour of evidence based regulation for public health. Tob Control. 2003;12(4):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee PN. The effect on health of switching from cigarettes to snus—a review. Regul Toxicol Pharmacol. 2013;66(1):1–5. [DOI] [PubMed] [Google Scholar]
- 6. Rostron BL, Chang JT, Anic GM, Tanwar M, Chang CM, Corey CG. Smokeless tobacco use and circulatory disease risk: a systematic review and meta-analysis. Open Heart. 2018;5(2):e000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatsukami DK, Hanson K, Briggs A, et al. Clinical trials methods for evaluation of potential reduced exposure products. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3143–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatsukami DK, Severson H, Anderson A, et al. Randomised clinical trial of snus versus medicinal nicotine among smokers interested in product switching. Tob Control. 2016;25(3):267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caldwell B, Burgess C, Crane J. Randomized crossover trial of the acceptability of snus, nicotine gum, and Zonnic therapy for smoking reduction in heavy smokers. Nicotine Tob Res. 2010;12(2):179–183. [DOI] [PubMed] [Google Scholar]
- 10. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 11. Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–287. [DOI] [PubMed] [Google Scholar]
- 12. Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul Toxicol Pharmacol. 2007;47(2):171–183. [DOI] [PubMed] [Google Scholar]
- 13. Hecht SS, Yuan JM, Hatsukami D. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23(6):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Urban M, Kavvadias D, Riedel K, Scherer G, Tricker AR. Urinary mercapturic acids and a hemoglobin adduct for the dosimetry of acrylamide exposure in smokers and nonsmokers. Inhal Toxicol. 2006;18(10):831–839. [DOI] [PubMed] [Google Scholar]
- 15. Murphy SE, Villalta P, Ho SW, von Weymarn LB. Analysis of [3’,3’-d(2)]-nicotine and [3’,3’-d(2)]-cotinine by capillary liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carmella SG, Ming X, Olvera N, Brookmeyer C, Yoder A, Hecht SS. High throughput liquid and gas chromatography-tandem mass spectrometry assays for tobacco-specific nitrosamine and polycyclic aromatic hydrocarbon metabolites associated with lung cancer in smokers. Chem Res Toxicol. 2013;26(8):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carmella SG, Chen M, Zarth A, Hecht SS. High throughput liquid chromatography-tandem mass spectrometry assay for mercapturic acids of acrolein and crotonaldehyde in cigarette smokers’ urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;935(1):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zarth AT, Carmella SG, Le CT, Hecht SS. Effect of cigarette smoking on urinary 2-hydroxypropylmercapturic acid, a metabolite of propylene oxide. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;953–954(1):126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pluym N, Gilch G, Scherer G, Scherer M. Analysis of 18 urinary mercapturic acids by two high-throughput multiplex-LC-MS/MS methods. Anal Bioanal Chem. 2015;407(18):5463–5476. [DOI] [PubMed] [Google Scholar]
- 20. Carmella SG, Chen M, Han S, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22(4):734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphy SE, Park SS, Thompson EF, et al. Nicotine N-glucuronidation relative to N-oxidation and C-oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis. 2014;35(11):2526–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hatsukami DK, Luo X, Jensen JA, et al. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure: a randomized clinical trial. JAMA. 2018;320(9):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kotandeniya D, Carmella SG, Pillsbury ME, Hecht SS. Combined analysis of N’-nitrosonornicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in the urine of cigarette smokers and e-cigarette users. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1007(1):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diggle PJ, Heagerty P, Liang KY, Zeger SL.. Analysis of Longitudinal Data. Vol 2 Oxford, United Kingdom: Oxford University Press; 2002. [Google Scholar]
- 25. Verbeke G, Molenberghs G.. Linear Mixed Models for Longitudinal Data. New York, NY: Springer; 2000. [Google Scholar]
- 26. Sarkar M, Liu J, Koval T, et al. Evaluation of biomarkers of exposure in adult cigarette smokers using Marlboro snus. Nicotine Tob Res. 2010;12(2):105–116. [DOI] [PubMed] [Google Scholar]
- 27. Kotlyar M, Hertsgaard LA, Lindgren BR, et al. Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meier E, Vogel RI, Carmella S, Paiano V, Hecht SS, Hatsukami DK. Polycyclic aromatic hydrocarbon biomarker levels among smokers who switch to oral nicotine. Tobacco Regulatory Science. 2017;3(2):204–209. [Google Scholar]
- 29. Zhong Y, Wang J, Carmella SG, et al. Metabolism of [D10]phenanthrene to tetraols in smokers for potential lung cancer susceptibility assessment: comparison of oral and inhalation routes of administration. J Pharmacol Exp Ther. 2011;338(1):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watzek N, Scherbl D, Feld J, et al. Profiling of mercapturic acids of acrolein and acrylamide in human urine after consumption of potato crisps. Mol Nutr Food Res. 2012;56(12):1825–1837. [DOI] [PubMed] [Google Scholar]
- 31. Tam J, Day HR, Rostron BL, Apelberg BJ. A systematic review of transitions between cigarette and smokeless tobacco product use in the United States. BMC Public Health. 2015;15:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biener L, Roman AM, Mc Inerney SA, et al. Snus use and rejection in the USA. Tob Control. 2016;25(4):386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.