Abstract
Introduction
Tobacco Heating System (THS) 2.2, a candidate modified-risk tobacco product, aims at offering an alternative to cigarettes for smokers while substantially reducing the exposure to harmful and potentially harmful constituents found in cigarette smoke.
Methods
One hundred and sixty healthy adult US smokers participated in this randomized, three-arm parallel group, controlled clinical study. Subjects were randomized in a 2:1:1 ratio to menthol Tobacco Heating System 2.2 (mTHS), menthol cigarette, or smoking abstinence for 5 days in confinement and 86 subsequent ambulatory days. Endpoints included biomarkers of exposure to harmful and potentially harmful constituents (reported in our co-publication, Part 1) and biomarkers of potential harm (BOPH).
Results
Compliance (protocol and allocated product exposure) was 51% and 18% in the mTHS and smoking abstinence arms, respectively, on day 90. Nonetheless, favorable changes in BOPHs of lipid metabolism (total cholesterol and high- and low-density cholesterol), endothelial dysfunction (soluble intercellular adhesion molecule-1), oxidative stress (8-epi-prostaglandin F2α), and cardiovascular risk factors (eg, high-sensitivity C-reactive protein) were observed in the mTHS group. Favorable effects in other BOPHs, including ones related to platelet activation (11-dehydrothromboxane B2) and metabolic syndrome (glucose), were more pronounced in normal weight subjects.
Conclusions
The results suggest that the reduced exposure demonstrated when switching to mTHS is associated with overall improvements in BOPHs, which are indicative of pathomechanistic pathways underlying the development of smoking-related diseases, with some stronger effects in normal weight subjects.
Implications
Switching to mTHS was associated with favorable changes for some BOPHs indicative of biological pathway alterations (eg, oxidative stress and endothelial dysfunction). The results suggest that switching to mTHS has the potential to reduce the adverse health effects of smoking and ultimately the risk of smoking-related diseases. Switching to mTHS for 90 days led to reductions in a number of biomarkers of exposure in smokers, relative to those who continued smoking cigarettes, which were close to those observed when stopping smoking (reported in our co-publication, Part 1). Initial findings suggest reduced levels of 8-epi-prostaglandin F2α and intercellular adhesion molecule 1, when switching to mTHS for 90 days. These changes are comparable to what is observed upon smoking cessation. In normal weight subjects, additional favorable changes were seen in 11-dehydrothromboxane B2, fibrinogen, homocysteine, hs-CRP, percentage of predicted forced expiratory volume in 1 second, systolic blood pressure, diastolic blood pressure, glucose, high-density lipoprotein, apolipoprotein A1, and triglycerides.
Trial registration
Introduction
Several harm reduction strategies have been proposed to address the health risks of smoking cigarettes. These include the development of novel nicotine or tobacco containing products with the potential to reduce the harm or risk of smoking-related diseases. Products such as heated tobacco products or electronic cigarettes aim at ultimately replacing cigarettes for smokers who would otherwise continue smoking.1–4 The US Food and Drug Administration refers to these products as modified-risk tobacco products (MRTPs). In 2012, the US Food and Drug Administration issued draft guidance for industry on “Modified Risk Tobacco Product Applications” suggesting that “the applicant has to demonstrate that the product, as it is actually used by consumers, will significantly reduce harm and the risk of tobacco-related disease to individual tobacco users.”5 The Tobacco Heating System (THS), developed by Philip Morris International, marketed as IQOS, is part of this new category of products with harm reduction potential.
Preclinical studies have confirmed that THS was associated with favorable changes in biomarkers of lipid metabolism, associated with cardiovascular diseases or respiratory diseases, both in vitro and in vivo.6–14 Clinical studies conducted thus far have mostly aimed at demonstrating reduced exposure upon ad libitum use over a short period of time (5–8 days) under strict control of product distribution in confinement.15–27
A study conducted in Japan for 5 days in a confined setting, followed by an 85-day ambulatory period, showed that switching from menthol cigarettes (mCC) to the menthol Tobacco Heating System 2.2 (mTHS) was associated with significant reductions in biomarkers of exposure to harmful and potentially harmful constituents (HPHCs) as compared with subjects who continued smoking.28 Switching to mTHS was also associated with favorable changes in biomarkers of potential harm (BOPH) in this manuscript. According to the Institute of Medicine, BOPH are defined as the “measurement of an effect due to exposure; these include early biological effects, alterations in morphology, structure, or function, and clinical symptoms consistent with harm.” 29 The BOPHs selected were indicative of oxidative stress, endothelial dysfunction, lipid metabolism, inflammation, and lung function. The changes in the mTHS and smoking abstinence (SA) groups were directionally consistent, comparable in magnitude, and clearly distinct from those seen in the mCC group.30
The BOPHs were selected based on these criteria: (1) to represent several biological pathways and mechanisms associated with smoking-related diseases, (2) to be affected by smoking, and (3) ideally, for the smoking-induced effects to be reversible in the short- to mid-term (eg, within 1 week to 1 year) upon smoking cessation in healthy subjects.31–39 For some of the BOPHs, effects of smoking cessation were reported consistently across multiple studies. For many other BOPHs, the data are sparse, contradictory or inconclusive. Several of the BOPHs included in this study were included as standard cardiovascular risk monitoring markers and were not necessarily expected to change over the duration of this study.
In assessing the risk profile of MRTPs compared to cigarettes, BOPH should cover multiple pathophysiological pathways, provide an early indication of later disease development, and display changes that follow the direction of what is observed when quitting smoking.40–43 In that perspective, it was important to explore early changes in BOPHs related to mTHS.
To obtain data on the US population, this study used a design similar to the study previously conducted in Japan. As the changes in biomarkers of exposure are reported in our co-publication (Part 1),44 the focus of this publication is on BOPHs.
Methods
The study was conducted in accordance with the International Conference on Harmonisation/Good Clinical Practice guidelines and the Declaration of Helsinki45,46 in Dallas, Texas (United States), and Daytona Beach, Florida (United States), between December 2013 and October 2014, after approval by the MidLands Independent Review Board in July 2013, and published on www.ClinicalTrials.gov (NCT01989156). A comprehensive description of the methods is provided in the co-publication (Part 1).44
Design and Procedures
Subjects were randomized on day 0 in a 2:1:1 ratio to the mTHS, mCC, and SA groups for 5 days of randomized exposure in confinement, followed by 86 days in the ambulatory period and a 28-day safety follow-up period. On day 1 and day 0, subjects smoked their own brand of mCC during the baseline assessments. During the confinement period, subjects in the mTHS and mCC groups used mTHS or their own brand of mCC, respectively, exclusively and ad libitum during the designated smoking hours (06:30 am to 11:00 pm). Subjects in the SA group were asked to abstain completely from smoking. On day 6, subjects were discharged from the study site and instructed to continue using their assigned product or to abstain completely from smoking for 86 days. Subjects were required to return to the investigational site for three visits; each visit comprised two consecutive days and an overnight stay (day 30, day 60, and day 90).
During the confinement period, compliance to product allocation was ensured by strict distribution of the product (mTHS or mCC) when requested by the subject. In addition, for subjects in the SA group, compliance was verified chemically throughout the study using an exhaled carbon monoxide (CO) breath test. The cut-off point for the CO breath test value to distinguish smoking from abstinence was 10 ppm.
Participants
Healthy male and female US mCC smokers above 22 years of age and with a body mass index (BMI) greater than 18.5 and less than or equal to 35 kg/m2 were eligible. Subjects with clinically relevant diseases or with a history of alcohol or drug abuse, as well as pregnant or breast-feeding women, were excluded from participating in the study. The full list of exclusion criteria is provided as supplementary material in the co-publication (Part 1).44
Products
The mTHS device used in this study has a maximum heating temperature of 350°C. Tobacco HeatStick yields for menthol (2.62 mg/stick), nicotine (1.21 mg/stick), and glycerine (3.94 mg/stick) were obtained under the Health Canada Intense smoking regimen.47 Reference products were commercially available mCCs of the subject’s preferred brand, which subjects purchased themselves because these were not provided. As mTHS was not commercialized in the United States, tobacco HeatSticks and mTHS devices were provided to the subjects.
Measurements
The BOPHs summarized in Table 1 were selected to assess inflammation, lipid metabolism, lung function, metabolic syndrome, endothelial dysfunction, oxidative stress, platelet activity, and cardiovascular risk factors. Creatinine was measured in 24-hour urine for adjustment of the concentration of all urinary BOPHs. Twenty-four hour urine was collected at baseline of the confinement period and during the two consecutive days of the day 90 ambulatory visit as described in the co-publication (Part 1).44 Blood samples were collected in the evening of the baseline visit during the confinement period and late in the morning of the day 90 ambulatory visit.
Table 1.
Biomarkers of Potential Harm
| BOPH | Domain | Matrix |
|---|---|---|
| WBC count | Inflammation | Blood |
| HDL-cholesterol | Lipid metabolism | Serum |
| LDL-cholesterol | Lipid metabolism | Serum |
| TG | Lipid metabolism | Serum |
| TC | Lipid metabolism | Serum |
| Apo A1 | Lipid metabolism | Serum |
| Apo B | Lipid metabolism | Serum |
| s-ICAM-1 | Endothelial dysfunction | Serum |
| 8-epi-PGF2α | Oxidative stress | Urine |
| 11-DTX-B2 | Platelet activation | Urine |
| Glucose | Metabolic syndrome | Serum |
| HbA1C | Metabolic syndrome | Serum |
| Weight | Metabolic syndrome | — |
| Waist circumference | Metabolic syndrome | — |
| Fibrinogen | Cardiovascular risk factor | Plasma |
| Homocysteine | Cardiovascular risk factor | Serum |
| hs-CRP | Cardiovascular risk factor | Serum |
| Blood pressure | Cardiovascular risk factor | — |
| FEV1 | Lung function | — |
8-epi-PGF2α = 8-epi-prostaglandin F2α; 11-DTX-B2 = 11-dehydro-thromboxane B2; Apo A1 = apolipoprotein A1; Apo B = apolipoprotein B; BOPH = biomarker of potential harm; FEV1 = forced expiratory volume in 1 second; HbA1C = hemoglobin A1C; HDL = high-density lipoprotein; hs-CRP = high-sensitivity C-reactive protein; LDL = low-density lipoprotein; s-ICAM-1= soluble intercellular adhesion molecule-1; TC = total cholesterol; TG = triglycerides; WBC = white blood cells.
Spirometry was performed on day 0 (baseline), day 6, and day 90, in accordance with guidelines published by the American Thoracic Society/European Respiratory Society,48 with predicted values standardized to the National Health and Nutrition Examination Survey III predicted set. All other BOPHs were assessed at baseline and on day 91. A detailed schedule of assessments is provided in Supplementary Table 1.
Statistical Analyses
Blood pressure, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein, triglycerides (TG), apolipoprotein A1 (Apo A1), apolipoprotein B, hemoglobin A1C (HbA1C), white blood cells (WBC), percentage of predicted forced expiratory volume in 1 second (FEV1 %pred), waist circumference, and weight were assumed to be normally distributed and analyzed in the original scale. High-sensitivity C-reactive protein (hs-CRP), homocysteine, glucose, fibrinogen, soluble intercellular adhesion molecule 1 (s-ICAM-1), 8-epi-prostaglandin F2α (8-epi-PGF2α), and 11-dehydrothromboxane B2 (11-DTX-B2) were assumed to be log-normally distributed and log-transformed prior to analysis. Day 90 BOPH values were compared by analysis of covariance (adjusting for the baseline value, sex, and daily average mCC consumption at baseline) to estimate least square (LS) mean differences (mTHS–mCC) or geometric LS mean ratios (mTHS:mCC) and 95% confidence intervals for normally- or log-normally distributed endpoints, respectively.
The analyses were conducted on day 90 in the per-protocol (PP) population. Subjects in the PP population met all eligibility criteria; followed a correct randomization procedure; were correctly sampled for the determination of the primary endpoints; and were compliant to the randomized product allocation, as fully described in the co-publication (Part 1).44 Per-protocol compliance to the allocated group on day 90 was determined for the period between day 60 and 90. Full compliance was defined as exclusive use of the allocated product for subjects randomized to mTHS and mCC groups; strict smoking abstinence in the SA group was based on self-reporting and a CO breath test result of less than 10 ppm.
Exploratory analyses were conducted for factors possibly associated with the observed results. These factors included compliance, measurement variability, medical history, co-medication, and BMI. These analyses were conducted on the analysis population meeting the criteria of the PP population throughout the entire exposure period from baseline to day 90. There was no control of the overall Type I error. All analyses were conducted at the 5% alpha level.
Results
Participants
Of the 659 subjects screened, 495 were screening failures, and 164 were enrolled after a product trial. The most frequent reasons for screen failure were the exclusion of smokers contemplating to quit smoking within the 6 months following screening (about 14% of the screened failures) and eligibility criteria related to the health of subjects.
The study included healthy adult smokers. The choice of healthy smoking subjects, as a population of investigation in the entire clinical program of THS, was undertaken to understand reductions in exposure and related short-term changes in pathomechanistic pathways that mainly contribute to the onset of smoking-related diseases. In addition, the presence of co-morbidities together with the associated concomitant medications could have interfered with the assessments of BOPHs.
The total randomized study population included 80 subjects in the mTHS group, 41 subjects in the mCC group, and 39 subjects in the SA group. The three study groups were comparable in terms of baseline characteristics, provided in Table 2, with 60%, 58.5%, and 61.5% male subjects in the mTHS, mCC, and SA groups, respectively, age distribution with mean ± SD being 39.2 (± 11.7), 33.7 (± 10.2), and 38.8 (± 11.4), and race with frequency of white, black or African American, and other being 61.3%, 28.8% and 8.8% in the mTHS group, 68.3%, 26.8% and 4.9% in the mCC group, and 56.4%, 43.6% and 0% in the SA group.
Table 2.
Baseline Demographic Characteristics by Randomization Group
| Variables | mTHS | mCC | SA | Total |
|---|---|---|---|---|
| N | 80 | 41 | 39 | 160 |
| Age (y) | ||||
| Mean ± SD | 39.2 ± 11.72 | 33.7 ± 10.17 | 38.8 ± 11.42 | 37.7 ± 11.45 |
| Range | 22–66 | 23–60 | 22–58 | 22–66 |
| Sex, n (%) | ||||
| Male | 48 (60.0) | 24 (58.5) | 24 (61.5) | 96 (60.0) |
| Female | 32 (40.0) | 17 (41.5) | 15 (38.5) | 64 (40.0) |
| Race, n (%) | ||||
| White | 49 (61.3) | 28 (68.3) | 22 (56.4) | 99 (61.9) |
| Black or African American | 23 (28.8) | 11 (26.8) | 17 (43.6) | 51 (31.9) |
| Other | 7 (8.8) | 2 (4.9) | 0 | 9 (5.6) |
| Missing | 1 (1.3) | 0 | 0 | 1 (0.6) |
| BMI (kg/m2) | ||||
| Mean ± SD | 27.0 ± 4.11 | 25.8 ± 3.67 | 26.2 ± 3.76 | 26.5 ± 3.93 |
| Range | 19.1–34.9 | 18.6–32.9 | 19.4–34.3 | 18.6–34.9 |
| FTND total score | ||||
| Mean ± SD | 5.6 ± 2.25 | 5.5 ± 1.67 | 5.7 ± 2.14 | 5.6 ± 2.08 |
| Range | 0–10 | 1–9 | 2–9 | 0–10 |
| Daily mCC consumption, n (%) | ||||
| 10–19 | 43 (53.8) | 21 (51.2) | 18 (46.2) | 82 (51.3) |
| >19 | 36 (45.0) | 20 (48.8) | 21 (53.8) | 77 (48.1) |
| Missing | 1 (1.3) | 0 | 0 | 1 (0.6) |
BMI = body mass index; FTND = Fagerström Test for Nicotine Dependence (Revised Version); mCC = menthol cigarette; mTHS = Tobacco Heating System 2.2 Menthol; SA = smoking abstinence.
Compliance
All subjects used their allocated product exclusively during confinement. The compliant population was defined as a subset of the PP population. Subjects who were not compliant with protocol or with allocated product exposure were declared noncompliant, even if the noncompliance was as a result of a protocol deviation by the study site.
The mTHS group and mCC group comprised 80 and 41 subjects, respectively. Five subjects in the mTHS group and six subjects in the mCC group were excluded from the PP population during the confinement period due to major deviations including misrandomization (randomization process was not correctly followed; mistratification with regards to mCC consumption at screening led to a misrandomization). The PP population and compliant population comprised 75 subjects in the mTHS group and 35 subjects in the mCC group.
The SA group comprised 39 subjects, including four misrandomized subjects. Compliance with 100% SA was chemically verified with a CO breath test of less than or equal to 10 ppm (apart from on day 1). Of the 39 subjects in the SA group, six subjects had a CO breath test above 10 ppm and were excluded from the PP set population; all but one value above 10 ppm occurred on day 6. Despite investigations, no plausible reason could be elucidated for a CO breath test value above 10 ppm in the confinement setting where product distribution to subjects was strictly controlled.
Consequently, compliance to the PP requirements in confinement was 94%, 85%, and 62% in the mTHS, mCC, and SA groups, respectively. This compliance to the protocol and adherence to the allocated group dropped by day 90 in the mTHS (51%) and SA (18%) groups, whereas it remained stable in the mCC group (76%) relatively to the other groups. Nicotine equivalent levels were very low in the SA group during the confinement period but increased during the ambulatory period (0.23 mg/g creat on day 5 and 0.82 mg/g creat on day 90 on average), indicating possible noncompliance with the group allocation, exposure to secondhand smoke, or use of nicotine replacement therapy products.
Biomarkers of Exposure
As described in the co-publication (Part 1),44 exposure to HPHCs was markedly lower in the mTHS group compared to the mCC group. Levels of carboxyhemoglobin, 3-hydroxypropylmercapturic acid, monohydroxybutenyl mercapturic acid, and S-phenylmercapturic acid were reduced by 62%, 54%, 87%, and 87%, respectively, in the mTHS group as compared with the mCC group on day 5. The reductions were sustained over the ambulatory period and approached those observed in the SA group. Total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol level assessed on day 90 was reduced by 74%.
Biomarkers of Potential Harm
Favorable changes were observed in the mTHS group for s-ICAM-1, hs-CRP, and 8-epi-PGF2α, with levels similar to those observed in the SA group on day 90 and being 11% (p < .05), 16% (p > .05), and 13% (p < .05) lower, respectively, in the mTHS group than in the mCC group. Further favorable changes were observed on day 90 in total cholesterol, low-density lipoprotein, HDL, Apo A1, and Apo B with mTHS–mCC LS mean differences of −4.0 mg/dL, −3.3 mg/dL, 1.4 mg/dL, 3.1 mg/dL, and −1.6 mg/dL, respectively (p > .05). No notable differences were observed on day 90 in the three study groups for TG, systolic and diastolic blood pressure, fibrinogen, homocysteine, HbA1C, body weight, waist circumference, glucose, WBC, FEV1 %pred, and 11-DTX-B2. Log-transformed, and original-scale BOPHs levels are presented in Table 3 and Table 4, respectively.
Table 3.
Geometric Mean (95% CI) of Biomarkers of Potential Harm—Per-Protocol Population
| n | mTHS | n | mCC | n | SA | Ratioa mTHS:mCC [p value] |
Ratioa mTHS:SA [p value] |
|
|---|---|---|---|---|---|---|---|---|
| Oxidative stress | ||||||||
| 8-Epi-PGF2α (pg/mg creat) | ||||||||
| Baseline | 45 | 244.3 (222.2; 268.5) | 32 | 276.3 (231.0; 330.6) | 9 | 270.8 (178.9; 409.7) | ||
| Day 90 | 47 | 251.6 (227.9; 277.7) | 32 | 317.2 (265.1; 379.4) | 9 | 281.0 (195.5; 404.0) | 86.54 (76.39; 98.05) [.0237] | 94.59 (77.74; 115.10) [.5744] |
| Platelet activation | ||||||||
| 11-DTX-B2 (pg/mg creat) | ||||||||
| Baseline | 45 | 611.9 (532.7; 702.9) | 32 | 575.6 (494.0; 670.8) | 9 | 518.1 (377.9; 710.2) | ||
| Day 90 | 47 | 421.4 (352.7; 503.5) | 32 | 431.1 (345.6; 537.7) | 9 | 372.1 (225.4; 614.4) | 96.44 (75.43; 123.31) [.7701] | 103.88 (70.42; 153.23) [.8461] |
| Endothelial dysfunction | ||||||||
| s-ICAM-1 (ng/mL) | ||||||||
| Baseline | 45 | 263.3 (241.1; 287.5) | 32 | 239.6 (208.1; 275.9) | 9 | 211.1 (159.2; 280.0) | ||
| Day 90 | 47 | 236.7 (217.8; 257.4) | 32 | 249.6 (216.8; 287.5) | 9 | 203.4 (140.9; 293.6) | 89.41 (83.29; 95.97) [.0023] | 99.24 (88.65; 111.09) [.8929] |
| Cardiovascular risk | ||||||||
| Fibrinogen (mg/dL) | ||||||||
| Baseline | 47 | 322.7 (299.9; 347.2) | 32 | 328.9 (304.8; 354.9) | 9 | 298.7 (237.0; 376.5) | ||
| Day 90 | 47 | 303.5 (280.8; 328.0) | 32 | 311.8 (290.0; 335.3) | 9 | 294.2 (236.3; 366.2) | 98.37 (90.92; 106.42) [.6783] | 96.52 (85.18; 109.38) [.5750] |
| Homocysteine (µmol/L) | ||||||||
| Baseline | 47 | 9.927 (8.871; 11.108) | 32 | 9.671 (8.826; 10.596) | 9 | 10.783 (8.014; 14.509) | ||
| Day 90 | 47 | 10.848 (9.615; 12.239) | 32 | 10.457 (9.593; 11.397) | 9 | 11.031 (7.910; 15.383) | 101.84 (92.90; 111.64) [.6936] | 106.86 (92.33; 123.66) [.3690] |
| hs-CRP (mg/L) | ||||||||
| Baseline | 47 | 1.322 (0.926; 1.887) | 32 | 1.028 (0.671; 1.576) | 9 | 0.855 (0.319; 2.290) | ||
| Day 90 | 47 | 1.370 (0.934; 2.009) | 32 | 1.298 (0.864; 1.950) | 9 | 0.937 (0.331; 2.648) | 83.77 (57.67; 121.69) [.3482] | 97.39 (53.83; 176.19) [.9296] |
| Metabolic syndrome | ||||||||
| Glucose (mg/dL) | ||||||||
| Baseline | 47 | 93.3 (91.2; 95.5) | 32 | 94.0 (91.3; 96.9) | 9 | 94.0 (86.0; 102.8) | ||
| Day 90 | 47 | 94.2 (91.6; 96.8) | 32 | 92.3 (89.6; 95.1) | 9 | 95.9 (89.0; 103.4) | 101.79 (98.03; 105.69) [.3502] | 97.54 (91.89; 103.54) [.4087] |
8-Epi-PGF2α = 8-epi-prostaglandin F2α; 11-DTX-B2 = 11-dehydro-thromboxane B2; CI = confidence interval; hs-CRP = high-sensitivity C-reactive protein; mCC = menthol cigarettes; mTHS = menthol Tobacco Heating System 2.2; n = number of subjects analyzed; s-ICAM-1 = soluble intercellular adhesion molecule; SA = smoking abstinence.
aGeometric LS mean ratio from analysis of covariance conducted on log-transformed day 90 values with log-transformed baseline value, study arm, sex, and mCC consumption reported at screening as fixed effect factors.
Table 4.
Arithmetic Mean (95% CI) of Biomarkers of Potential Harm—Per-Protocol Population
| n | mTHS | n | mCC | n | SA | Differencea mTHS–mCC [p value] |
Differencea mTHS–SA [p value] |
|
|---|---|---|---|---|---|---|---|---|
| Lipid metabolism | ||||||||
| LDL Cholesterol (mg/dL) | ||||||||
| Baseline | 47 | 114.9 (104.3; 125.6) | 32 | 122.7 (110.5; 134.9) | 9 | 117.7 (94.8; 140.6) | ||
| Day 90 | 47 | 108.4 (97.9; 118.8) | 32 | 118.1 (106.0; 130.2) | 9 | 115.4 (87.8; 143.1) | −3.3 (−12.0; 5.4) [.4489] | −5.1 (−18.9; 8.6) [.4560] |
| HDL cholesterol (mg/dL) | ||||||||
| Baseline | 47 | 48.7 (45.3; 52.1) | 32 | 55.0 (49.5; 60.5) | 9 | 52.8 (46.3; 59.3) | ||
| Day 90 | 47 | 52.1 (48.6; 55.7) | 32 | 56.3 (50.1; 62.6) | 9 | 54.1 (47.7; 60.5) | 1.4 (−2.3; 5.0) [.4547] | 1.3 (−4.4; 7.1) [.6397] |
| Triglycerides (mg/dL) | ||||||||
| Baseline | 47 | 145.7 (121.6; 169.9) | 32 | 121.6 (106.5; 136.8) | 9 | 106.6 (69.8; 143.3) | ||
| Day 90 | 47 | 146.3 (124.1; 168.4) | 32 | 125.7 (109.4; 142.0) | 9 | 102.1 (72.9; 131.3) | 0.9 (−12.8; 14.6) [.8963] | 11.6 (−10.1; 33.4) [.2891] |
| Total cholesterol (mg/dL) | ||||||||
| Baseline | 47 | 185.7 (173.4; 198.0) | 32 | 195.1 (182.3; 207.9) | 9 | 188.0 (158.2; 217.8) | ||
| Day 90 | 47 | 182.8 (171.0; 194.6) | 32 | 195.0 (181.6; 208.4) | 9 | 185.4 (149.6; 221.3) | −4.0 (−13.3; 5.2) [.3860] | −1.4 (−16.1; 13.2) [.8481] |
| Apolipoprotein A1 (mg/dL) | ||||||||
| Baseline | 47 | 140.2 (134.3; 146.1) | 32 | 147.8 (138.0; 157.6) | 9 | 141.1 (128.3; 153.7) | ||
| Day 90 | 47 | 148.7 (141.9; 155.6) | 32 | 152.6 (142.0; 163.2) | 9 | 146.0 (129.7; 162.3) | 3.1 (−4.6; 10.7) [.4281] |
3.7 (−8.3; 15.7) [.5414] |
| Apolipoprotein B (mg/dL) | ||||||||
| Baseline | 47 | 87.7 (79.5; 95.9) | 32 | 90.8 (83.0; 98.6) | 9 | 82.4 (69.1; 95.8) | ||
| Day 90 | 47 | 84.8 (76.4; 93.2) | 32 | 88.8 (80.3; 97.3) | 9 | 82.6 (63.3; 101.8) | −1.6 (−7.2; 4.1) [.5725] |
−3.2 (−12.1; 5.8) [.4829] |
| Inflammation | ||||||||
| WBC (GI/L) | ||||||||
| Baseline | 47 | 8.321 (7.800; 8.840) | 32 | 8.266 (7.610; 8.920) | 9 | 6.850 (5.190; 8.510) | ||
| Day 90 | 47 | 7.330 (6.840; 7.820) | 32 | 7.172 (6.460; 7.880) | 9 | 5.369 (4.330; 6.410) | 0.17 (−0.47; 0.81) [.5954] | 1.11 (0.07; 2.15) [.0364] |
| Cardiovascular risk | ||||||||
| Systolic blood pressure (mm Hg) | ||||||||
| Baseline | 45 | 118.8 (116.1; 121.5) | 31 | 119.7 (116.1; 123.4) | 6 | 116.8 (103.0; 130.6) | ||
| Day 90 | 47 | 116.8 (113.2; 120.3) | 32 | 117.9 (114.3; 121.5) | 9 | 114.1 (109.6; 118.7) | −0.7 (−4.5; 3.1) [.7101] | 1.7 (−4.3; 7.7) [.5753] |
| Diastolic blood pressure (mm Hg) | ||||||||
| Baseline | 45 | 71.3 (68.9; 73.7) | 31 | 73.5 (70.7; 76.3) | 6 | 71.2 (59.5; 82.9) | ||
| Day 90 | 47 | 70.1 (66.9; 73.3) | 32 | 71.0 (67.7; 74.2) | 9 | 68.6 (63.9; 73.2) | 0.2 (−3.7; 4.0) [.9314] | 2.4 (−3.7; 8.4) [.4337] |
| Metabolic syndrome | ||||||||
| Hemoglobin A1C (%) | ||||||||
| Baseline | 47 | 5.4 (5.3; 5.6) | 32 | 5.3 (5.2; 5.4) | 9 | 5.5 (5.3; 5.7) | ||
| Day 90 | 47 | 5.5 (5.3; 5.6) | 32 | 5.4 (5.3; 5.5) | 9 | 5.3 (5.1; 5.6) | −0.04 (−0.16; 0.09) [.5380] | 0.14 (−0.05; 0.32) [.1407] |
| Body weight (kg) | ||||||||
| Baseline | 47 | 78.94 (74.54; 83.34) | 32 | 77.64 (72.88; 82.40) | 9 | 67.97 (59.37; 76.56) | ||
| Day 90 | 47 | 79.60 (75.12; 84.08) | 32 | 78.39 (73.45; 83.34) | 9 | 68.94 (60.63; 77.25) | −0.12 (−1.35; 1.12) [.8507] | −0.59 (−2.60; 1.42) [.5615] |
| Waist circumference (cm) | ||||||||
| Baseline | 47 | 95.8 (91.4; 100.2) | 32 | 97.2 (89.9; 104.4) | 9 | 81.9 (75.3; 88.5) | ||
| Day 90 | 47 | 95.7 (91.0; 100.4) | 32 | 98.8 (90.4; 107.1) | 9 | 81.7 (75.6; 87.7) | −2.1 (−10.2; 6.1) [.6082] | 9.3 (−3.8; 22.5) [.1611] |
| Lung function | ||||||||
| FEV1 (%pred) | ||||||||
| Baseline | 47 | 92.7 (88.8; 96.7) | 32 | 96.5 (91.2; 101.8) | 9 | 95.7 (87.3; 104.0) | ||
| Day 90 | 47 | 90.0 (85.7; 94.2) | 31 | 92.6 (87.2; 98.0) | 9 | 94.1 (86.3; 101.9) | 0.53 (−2.79; 3.85) [.7499] | −1.46 (−6.63; 3.71) [.5748] |
Apo A1 = apolipoprotein A1; Apo B = apolipoprotein B; CI = confidence interval; FEV1 %pred = percentage of predicted forced expiratory volume in 1 second; HDL = high-density lipoprotein; LDL = low-density lipoprotein; LS = least square; mCC = menthol cigarettes; mTHS = menthol Tobacco Heating System 2.2; SA = smoking abstinence; WBC = white blood cells.
aOriginal scale LS mean difference from analysis of covariance conducted on day 90 values with baseline value, study arm, sex, and cigarette consumption reported at screening as fixed effect factors.
The lack of effects was incongruent with the reductions observed in the biomarkers of exposure and with the results of a Japanese sample28,49; data were therefore explored for factors possibly associated with the observed results. These factors included compliance, measurement variability, medical history, co-medication, and BMI.
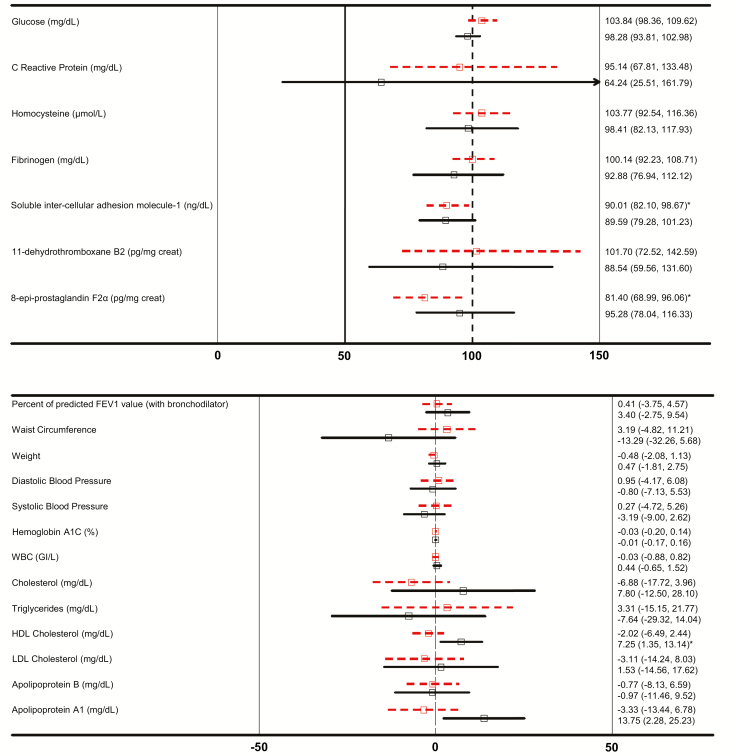
Restricting the analysis to subjects belonging to the PP set throughout the study, did not result in substantial changes between the total sample and the fully compliant subsample. Day 90 differences of, at most, 2.6 percent of the mTHS:mCC LS mean ratios and of four percent points of the mCC group mTHS–mCC LS mean differences were observed between the total sample and the fully compliant subsample. The only exception was for TG, where the LS mean difference was 10 percent (Figure 1).
Figure 1.
Biomarkers of Potential Harm % mTHS:mCC Ratios (Upper Panel) and mTHS–mCC Differences (Lower Panel) with 95% Confidence Intervals on Day 90, for Overweight (Dashed Lines) and Normal Weight (Solid Lines) Subjects (*p < 0.05).
Coefficients of variation were calculated for baseline and day 90 data to explore whether there was any variability in the measurements. In the United States and the Japanese studies, values ranged from 0.04 to less than 0.75 at baseline and on day 90, respectively, for all BOPHs across the three study groups, except for hs-CRP, whose coefficients of variation ranged from 1.75 (mCC group, at baseline) to 2.28 (SA group, on day 90) in the US study, and from 1.06 (mCC group at baseline) to 2.10 (SA group, at baseline) in the Japanese study.
With respect to medical histories and co-medications, no marked effect could be identified between the Japanese and the US subjects.
The BMI was higher at baseline by 4 kg/m2 in the US subjects, as compared with the Japanese subjects, due to weight differences (77.4 and 62.2 kg on average, respectively); 40% and 21% of the US and Japanese subjects were overweight (25 kg/m2 ≤ BMI < 30 kg/m2); 23% and 1% were obese (30 kg/m2 ≤ BMI < 35 kg/m2), respectively. Weight-stratified subsamples were explored without a context of statistical testing.
On day 90, mTHS:mCC ratios for 11-DTX-B2, fibrinogen, and glucose, at 96.4%, 98.4%, and 101.8%, respectively, in the total US sample, were lower, 88.5%, 92.9%, and 98.3%, respectively, in the normal weight subsample. Also, the day 90 mTHS:mCC ratios for hs-CRP and homocysteine of 83.8% and 101.8%, respectively, in the total US sample were lower, at. 64.2% and 98.4%, respectively, in the normal weight subsample (Figure 1). Lower mTHS:mCC ratios suggest a more pronounced favorable effect in the normal weight subsample relative to the overweight subsample. For s-ICAM-1, this was not observed.
Favorable increases in HDL and Apo A1 levels were also more pronounced in normal weight subjects, with mTHS–mCC LS mean differences of 1.4 mg/dL and 3.1 mg/dL, respectively, in the total US sample, compared with 7.3 mg/dL and 13.8 mg/dL, respectively, in the normal weight subsample. Similarly, the mTHS–mCC LS mean differences observed in TG levels, systolic blood pressure, diastolic blood pressure, and FEV1 %pred of 0.9 mg/dL, −0.7 mm Hg, 0.2 mm Hg, and 0.53 %pred respectively, in the total sample were reduced to −7.6 mg/dL, −3.2 mm Hg, −0.8 mm Hg, and 3.4 %pred respectively, in the normal weight subjects. (Figure 1). No differences were identified for HbA1C or WBC.
In contrast, the decrease in 8-epi-PGF2α observed for mTHS relative to mCC was more pronounced on day 90 in overweight (including obese) subjects, with mTHS:mCC ratios of 86.5% in the total US sample and 81.4% in the overweight subsample.
Discussion
mTHS was developed to reduce or minimize the formation of HPHCs by heating tobacco, rather than burning it, while preserving as much as possible the sensory experience (including taste), nicotine delivery profile, and ritual associated with mCC consumption. The present study was designed to demonstrate exposure reduction to selected HPHCs in smokers switching to mTHS and to provide initial insights into the effects on BOPHs indicative of the pathogenesis of smoking-related diseases. The reduction in exposure and changes observed in BOPHs were also benchmarked against SA, considered the gold standard against which to assess a candidate MRTP.
The BOPHs measured in this study covered several pathophysiological mechanisms that are involved in the development of smoking-related diseases. Because some of these BOPH have been reported to change positively31–35,40–43 in the short- to mid-term (eg, within 1 week to 1 year) upon smoking cessation, the exploration of their overall profile of changes, 3 months after switching to mTHS, provides valuable insights into the overall potential of mTHS to reduce the harm and risk of developing smoking-related diseases.
Three months after switching to mTHS, the levels of s-ICAM-1 (endothelial dysfunction), and 8-epi-PGF2α (oxidative stress), were lower relative to mCC, as reported in short-term quitting studies with p < .05.32,50 For 8-epi-PGF2α it should be noted that an increase from baseline was observed, with a more pronounced increase in the mCC arm. The increase in all arms, including SA, could not be elucidated, as it may be related to confounding factors that have not been identified, such as exposure to passive smoking.
In addition, for low-density lipoprotein, HDL, Apo A1, Apo B, and total cholesterol, the mean differences suggested that these BOPHs followed a pattern of change in line with those reported in the literature following smoking cessation but with p > .05.31,38,51,52 In contrast, generally no differences in lung function (FEV1 %pred) were observed across the three study groups, owing to changes in lung function requiring longer investigational periods of 6–12 months in order to be observed.53
Most of the trajectories for BOPHs identified in this study, following switching from mCC to mTHS, were also observed in the Japanese population following 3 months of mTHS use49 with the exceptions of WBC and FEV1 %pred, for which no differences were found in this study. Nevertheless, our overall results are in line with the direction of changes reported following switching to a THS prototype for one year16 and following smoking cessation as reported in the literature. The lower levels of 8-epi-PGF2α and s-ICAM-1 as observed in our study, relative to mCC, for example, are consistently reported in the literature after 3 months of cessation.32,54,55 For hs-CRP, an acute marker of chronic inflammation, it is generally reported that it does not fall immediately following smoking cessation but rather takes many years to normalize.56
We acknowledge that no clinical study is representative of the population, which is why we also conducted actual use studies in a US smoking population.57 In the present study, a quota was used to enforce a minimum representation from each sex and of daily smoking consumption, which was based on balance and not representativeness, so that a stratified analysis of the primary endpoints could also be performed. Although consumer surveys show slightly more females than males using mCC, our study was the opposite. Concerning race, based on our own estimate, the proportion of black subjects in our study was comparable to the US smoking population.
It is important to point out that due to the descriptive nature of our analyses and the short duration of the exposure period, the biological relevance of our results needs to be interpreted with caution, and requires further confirmation and replication by independent researchers. A longer and larger study, which further substantiates these findings, has been conducted in the United States and the results will be available on ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT02396381).
The trajectories of change of some BOPHs, which follow the direction of changes reported in the literature for smoking cessation, suggest that reductions in exposure to HPHCs in complete switchers to mTHS could translate into a positive influence upon some human biological processes involved in disease development.
In the context of a global assessment program to scientifically substantiate the lower risk to health of THS, compared to CC, these results are encouraging. Our a priori premise is that the absence of tobacco combustion results in lower exposure to HPHCs, which would ultimately be associated with reduced risk of harm and disease.
Our published data, together with independent studies, report that THS yields significantly reduced levels of HPHCs compared to CC,58–62 exhibits lower toxicity and biological damage to multiple system organ classes as tested in multiple in vitro assays and animal studies,6–14,63 and leads to reduced exposure to HPHCs in adult smokers who switch from CC to THS.65
We further explored the influence of body weight on a number of BOPHs, such as HDL, s-ICAM-1, Apo A1, apolipoprotein B, TG, 11-DTX-B2, WBC, glucose, HbA1C, 8-epi-PGF2α, homocysteine, systolic and diastolic blood pressure,61 and fibrinogen. When restricting the analyses to normal weight subjects, favorable changes in the mean levels of 11-DTX-B2, fibrinogen, homocysteine, systolic and diastolic blood pressure, glucose levels, TG, and hs-CRP were noted. In addition, mean HDL level increases were more pronounced in the normal weight subsample of the study population.
The observation that BOPH levels can be associated with body mass is concordant with the results described in 2011 by Frost-Pineda et al.65 In their US “total exposure study,” BMI was, more so than smoking, associated with differences observed in levels of BOPH between smokers and non-smokers. Specifically, this was the case for fibrinogen, hs-CRP, systolic and diastolic blood pressure, glucose, TG, 8-epi-PGF2α, and HDL. Increases in BMI were associated with systolic blood pressure and fasting glucose, in epidemiological studies, and inversely associated linearly with reduced levels of HDL and Apo A1.66,67 Similarly, obesity was associated with low plasma HDL levels, abnormal metabolism of HDL particles, and high blood pressure.68 Thus, it is possible that favorable changes in BOPHs were not observed in overweight subjects after switching to mTHS as a consequence of obesity-related fat accumulation and lifestyle factors altering HDL and Apo A1 levels. This is supported by the baseline levels observed in the present study, which were, for HDL and Apo A1, lower in overweight (49.2 and 141.1 mg/dL) subjects than in normal weight (55.0 and 146.3 mg/dL) subjects. From the present findings, however, it cannot be concluded whether the biological consequences of being overweight could affect the BOPHs measured in this study, or whether the influence of being overweight on BOPH measurements is simply masking the effects of exposure reduction upon switching to mTHS or abstinence.
The findings are consistent with the evidence that obesity increases the risk of chronic metabolic disorders and is associated with chronic inflammation, which is known to increase the risks of cardiovascular disease, diabetes, chronic obstructive pulmonary diseases, and many cancers.67,69,70 As the body mass-related findings resulted from an exploratory analysis, out-of-sample replication is required.
Conclusions
These results are promising and need further confirmation by independent researchers in a broader and representative population of US smokers. The data suggest that the reduced exposure demonstrated when switching completely to mTHS may be associated with positive effects on endothelial dysfunction and oxidative stress as reflected by significantly lower levels of sICAM-1 and 8-epi-PGF2α, compared to continued smoking, after 3 months. These findings corroborate those observed in a similar study conducted in Japan28,49 (ClinicalTrials.gov identifier: NCT01970995) and are in line with the overall scientific evidence generated so far to assess the reduced risk to health of THS.
Funding
Philip Morris International is the sole source of funding and sponsor of this project.
Supplementary Material
Acknowledgments
The authors would like to thank William Lewis, Frank Farmer, and Hugh Coleman, study Principal Investigators, and Covance Dallas (United States) and Daytona (United States) sites’ staff as well as Celerion Lincoln (United States) and Celerion Fehraltorf (Switzerland) for laboratory processing of samples. The authors thank Paul Hession for providing medical writing and editorial support. We also gratefully acknowledge all Philip Morris International study team members for their contribution to this study.
Declaration of Interests
All authors are employees of Philip Morris International.
References
References 51–71 are available as supplementary material.
- 1. Royal College of Physicians. Harm Reduction in Nicotine Addiction: Helping People Who Can’T Quit. A Report by the Tobacco Advisory Group of the Royal College of Physicians. London: RCP; 2007. [Google Scholar]
- 2. WHO Study Group, Ashley DL, Burns D, et al. The scientific basis of tobacco product regulation: report of a WHO study group. World Health Organ Tech Rep Ser. 2007(945). [Google Scholar]
- 3. Zeller M, Hatsukami D; Strategic Dialogue on Tobacco Harm Reduction Group The strategic dialogue on tobacco harm reduction: a vision and blueprint for action in the US. Tob Control. 2009;18(4):324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gilmore AB, Britton J, Arnott D, Ashcroft R, Jarvis MJ. The place for harm reduction and product regulation in UK tobacco control policy. J Public Health (Oxf). 2009;31(1):3–10. [DOI] [PubMed] [Google Scholar]
- 5. FDA (Food and Drug Administration). Guidance for industry—Modified risk tobacco product applications—Draft Guidance. Silver Spring, MD: Food and Drug Administration; 2012. [Google Scholar]
- 6. Ansari S, Baumer K, Boué S, et al. Comprehensive systems biology analysis of a 7-month cigarette smoke inhalation study in C57BL/6 mice. Sci Data. 2016;3:150077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Phillips B, Veljkovic E, Boué S, et al. An 8-month systems toxicology inhalation/cessation study in apoe-/- mice to investigate cardiovascular and respiratory exposure effects of a candidate modified risk tobacco product, THS 2.2, compared with conventional cigarettes. Toxicol Sci. 2016;149(2):411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phillips B, Veljkovic E, Peck MJ, et al. A 7-month cigarette smoke inhalation study in C57BL/6 mice demonstrates reduced lung inflammation and emphysema following smoking cessation or aerosol exposure from a prototypic modified risk tobacco product. Food Chem Toxicol. 2015;80:328–345. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez-Suarez I, Martin F, Marescotti D, et al. In Vitro Systems Toxicology Assessment of a candidate modified risk tobacco product shows reduced toxicity compared to that of a conventional cigarette. Chem Res Toxicol. 2016;29(1):3–18. [DOI] [PubMed] [Google Scholar]
- 10. van der Toorn M, Frentzel S, De Leon H, Goedertier D, Peitsch MC, Hoeng J. Aerosol from a candidate modified risk tobacco product has reduced effects on chemotaxis and transendothelial migration compared to combustion of conventional cigarettes. Food Chem Toxicol. 2015;86:81–87. [DOI] [PubMed] [Google Scholar]
- 11. Iskandar AR, Titz B, Sewer A, et al. Systems toxicology meta-analysis of in vitro assessment studies: biological impact of a candidate modified-risk tobacco product aerosol compared with cigarette smoke on human organotypic cultures of the aerodigestive tract. Toxicol Res (Camb). 2017;6(5):631–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Szostak J, Boué S, Talikka M, et al. Aerosol from Tobacco Heating System 2.2 has reduced impact on mouse heart gene expression compared with cigarette smoke. Food Chem Toxicol. 2017;101:157–167. [DOI] [PubMed] [Google Scholar]
- 13. Sewer A, Kogel U, Talikka M, et al. Evaluation of the Tobacco Heating System 2.2 (THS2.2). Part 5: microRNA expression from a 90-day rat inhalation study indicates that exposure to THS 2.2 aerosol causes reduced effects on lung tissue compared with cigarette smoke. Regul Toxicol Pharmacol. 2016;81(suppl 2): S82–S92. http://www.sciencedirect.com/science/article/pii/S0273230016303622. Accessed May 03, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Iskandar AR, Mathis C, Schlage WK, et al. A systems toxicology approach for comparative assessment: biological impact of an aerosol from a candidate modified-risk tobacco product and cigarette smoke on human organotypic bronchial epithelial cultures. Toxicol In Vitro. 2017;39:29–51. [DOI] [PubMed] [Google Scholar]
- 15. Roethig HJ, Zedler BK, Kinser RD, Feng S, Nelson BL, Liang Q. Short-term clinical exposure evaluation of a second-generation electrically heated cigarette smoking system. J Clin Pharmacol. 2007;47(4):518–530. [DOI] [PubMed] [Google Scholar]
- 16. Roethig HJ, Feng S, Liang Q, Liu J, Rees WA, Zedler BK. A 12-month, randomized, controlled study to evaluate exposure and cardiovascular risk factors in adult smokers switching from conventional cigarettes to a second-generation electrically heated cigarette smoking system. J Clin Pharmacol. 2008;48(5):580–591. [DOI] [PubMed] [Google Scholar]
- 17. Unverdorben M, der Bijl A, Potgieter L, Liang Q, Meyer BH, Roethig HJ. Effects of levels of cigarette smoke exposure on symptom-limited spiroergometry. Prev Cardiol. 2007;10(2):83–91. [DOI] [PubMed] [Google Scholar]
- 18. Unverdorben M, van der Bijl A, Potgieter L, et al. Effects of different levels of cigarette smoke exposure on prognostic heart rate and rate–pressure-product parameters. J Cardiovasc Pharmacol Ther. 2008;13(3):175–182. [DOI] [PubMed] [Google Scholar]
- 19. Roethig HJ, Kinser RD, Lau RW, Walk RA, Wang N. Short-term exposure evaluation of adult smokers switching from conventional to first-generation electrically heated cigarettes during controlled smoking. J Clin Pharmacol. 2005;45(2):133–145. [DOI] [PubMed] [Google Scholar]
- 20. Tricker AR, Jang IJ, Martin Leroy C, Lindner D, Dempsey R. Reduced exposure evaluation of an electrically heated cigarette smoking system. Part 4: eight-day randomized clinical trial in Korea. Regul Toxicol Pharmacol. 2012;64(suppl 2):S45–S53. [DOI] [PubMed] [Google Scholar]
- 21. Tricker AR, Kanada S, Takada K, et al. Reduced exposure evaluation of an electrically heated cigarette smoking system. part 6: 6-day randomized clinical trial of a menthol cigarette in Japan. Regul Toxicol Pharmacol. 2012;64(suppl 2):S64–S73. [DOI] [PubMed] [Google Scholar]
- 22. Martin Leroy C, Jarus-Dziedzic K, Ancerewicz J, Lindner D, Kulesza A, Magnette J. Reduced exposure evaluation of an electrically heated cigarette smoking system. Part 7: a one-month, randomized, ambulatory, controlled clinical study in Poland. Regul Toxicol Pharmacol. 2012;64(suppl 2):S74–S84. [DOI] [PubMed] [Google Scholar]
- 23. Frost-Pineda K, Zedler BK, Oliveri D, Feng S, Liang Q, Roethig HJ. Short-term clinical exposure evaluation of a third-generation electrically heated cigarette smoking system (EHCSS) in adult smokers. Regul Toxicol Pharmacol. 2008;52(2):104–110. [DOI] [PubMed] [Google Scholar]
- 24. Munjal S, Koval T, Muhammad R, et al. Heart rate variability increases with reductions in cigarette smoke exposure after 3 days. J Cardiovasc Pharmacol Ther. 2009;14(3):192–198. [DOI] [PubMed] [Google Scholar]
- 25. Unverdorben M, Mostert A, Munjal S, et al. Acute effects of cigarette smoking on pulmonary function. Regul Toxicol Pharmacol. 2010;57(2-3):241–246. [DOI] [PubMed] [Google Scholar]
- 26. Roethig HJ, Koval T, Muhammad-Kah R, Jin Y, Mendes P, Unverdorben M. Short term effects of reduced exposure to cigarette smoke on white blood cells, platelets and red blood cells in adult cigarette smokers. Regul Toxicol Pharmacol. 2010;57(2-3):333–337. [DOI] [PubMed] [Google Scholar]
- 27. Sakaguchi C, Kakehi A, Minami N, Kikuchi A, Futamura Y. Exposure evaluation of adult male Japanese smokers switched to a heated cigarette in a controlled clinical setting. Regul Toxicol Pharmacol. 2014;69(3):338–347. [DOI] [PubMed] [Google Scholar]
- 28. Lüdicke F, Picavet P, Baker G, et al. Effects of Switching to the Tobacco Heating System 2.2 Menthol, smoking abstinence, or continued cigarette smoking on biomarkers of exposure: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (Part 1). Nicotine Tob Res. 2018;20(2):161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. IOM (Institute of Medicine). Clearing the Smoke - Assessing the Science Base for Tobacco Harm Reduction. Washington, DC: The National Academies Press; 2001. http://www.nap.edu/catalog/10029.html. Accessed March 06,2017. [PubMed] [Google Scholar]
- 30. Lüdicke F, Picavet P, Baker G, et al. Effects of Switching to the Menthol Tobacco Heating System 2.2, smoking abstinence, or continued cigarette smoking on clinically relevant risk markers: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (Part 2). Nicotine Tob Res. 2018;20(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eliasson B, Hjalmarson A, Kruse E, Landfeldt B, Westin A. Effect of smoking reduction and cessation on cardiovascular risk factors. Nicotine Tob Res. 2001;3(3):249–255. [DOI] [PubMed] [Google Scholar]
- 32. Palmer RM, Stapleton JA, Sutherland G, Coward PY, Wilson RF, Scott DA. Effect of nicotine replacement and quitting smoking on circulating adhesion molecule profiles (sICAM-1, sCD44v5, sCD44v6). Eur J Clin Invest. 2002;32(11):852–857. [DOI] [PubMed] [Google Scholar]
- 33. Pilz H, Oguogho A, Chehne F, Lupattelli G, Palumbo B, Sinzinger H. Quitting cigarette smoking results in a fast improvement of in vivo oxidation injury (determined via plasma, serum and urinary isoprostane). Thromb Res. 2000;99(3):209–221. [DOI] [PubMed] [Google Scholar]
- 34. Benowitz NL, Fitzgerald GA, Wilson M, Zhang Q. Nicotine effects on eicosanoid formation and hemostatic function: comparison of transdermal nicotine and cigarette smoking. J Am Coll Cardiol. 1993;22(4):1159–1167. [DOI] [PubMed] [Google Scholar]
- 35. Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–2362. [DOI] [PubMed] [Google Scholar]
- 36. King CC, Piper ME, Gepner AD, Fiore MC, Baker TB, Stein JH. Longitudinal impact of smoking and smoking cessation on inflammatory markers of cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2017;37(2):374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song YM, Chang WD, Hsu HY, Chen MD. A short-term smoking cessation may increase the risk of developing metabolic syndrome. Diabetes Metab Syndr. 2015;9(2):135–137. [DOI] [PubMed] [Google Scholar]
- 38. Forey BA, Fry JS, Lee PN, Thornton AJ, Coombs KJ. The effect of quitting smoking on HDL-cholesterol—a review based on within-subject changes. Biomark Res. 2013;1(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161(1):145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scherer G. Suitability of biomarkers of biological effects (BOBEs) for assessing the likelihood of reducing the tobacco related disease risk by new and innovative tobacco products: a literature review. Regul Toxicol Pharmacol. 2018;94:203–233. [DOI] [PubMed] [Google Scholar]
- 41. Peck MJ, Sanders EB, Scherer G, Ludicke F, Weitkunat R. Review of biomarkers to assess the effects of switching from cigarettes to modified risk tobacco products. Biomarkers. 2018;23(3):1–32. [DOI] [PubMed] [Google Scholar]
- 42. Berman ML, Connolly G, Cummings KM, et al. Providing a science base for the evaluation of tobacco products. Tob Regul Sci. 2015;1(1):76–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. IOM (Institute of Medicine). Scientific Standards for Studies on Modified Risk Tobacco Products. Washington, DC: The National Academies Press; 2012. [Google Scholar]
- 44. Haziza C, de La Bourdonnaye G, Donelli A, et al. Reduction in exposure to selected harmful and potentially harmful constituents approaching those observed upon smoking abstinence in smokers switching to the menthol Tobacco Heating System 2.2 for three months (part 1). Nicotine Tob Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. World Medical Association (WMA). Declaration of Helsinki—Ethical principles for medical research involving human subjects. 2008. http://www.wma.net/en/30publications/10policies/b3/17c.pdf. Accessed March 16, 2017. [Google Scholar]
- 46. ICH E6 (R1). Guideline for good clinical practice 1996http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed August 14, 2014.
- 47. Health Canada. Official Method T-212, Determination of Tar and Nicotine in Sidestream Tobacco Smoke. In: Department of Health, ed. Ottawa, Canada: Goverment of Canada; 2016. [Google Scholar]
- 48. Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 49. Ludicke F, Picavet P, Baker G, et al. Effects of switching to the menthol Tobacco Heating System 2.2, smoking abstinence, or continued cigarette smoking on clinically relevant risk markers: a randomized, controlled, open-label, multicenter study in sequential confinement and ambulatory settings (part 2). Nicotine Tob Res. 2018; 20(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.