Systemic hypertension has traditionally been attributed to excessive vasoconstriction and remodeling of resistance vessels, however stiffening of central arteries, particularly the aorta, has become increasingly recognized as having a major role in the genesis of hypertension and associated end-organ damage.1 The primary function of large arteries is to store elastic energy as they distend during systole and to use this energy to propel blood forward as they recoil during diastole. Because of this, healthy central arteries decrease the workload on the heart and maintain flow to critical organs such as the heart, brain, and kidney in diastole.2 In diverse conditions, including aging, diabetes, obesity, hypertension, and cigarette smoking, the aorta stiffens and its capacitance, or Windkessel function, is reduced. In both humans and experimental models, aortic stiffening is associated with a striking deposition of collagen and fibronectin in perivascular tissues, particularly in the adventitia. Importantly, T cells and T cell-derived cytokines seem to play an important role in this process via mechanisms that are only incompletely understood.
In this issue of Circulation Research, Nosalski et al provide new insight into the mechanisms of aortic stiffening in hypertension.3 Using RNA array to identify differences in aortic microRNAs (miRs), they identified miR-214 as the only significantly upregulated miR in vessels of mice with experimental hypertension, and that the predominant site of this upregulation was the perivascular tissues. Subsequent studies showed that miR-214 was not changed in adipose cells but that it was significantly increased in peripheral blood mononuclear cells and in the spleen. Because inflammatory cells populate the perivascular fat in hypertension,4 the authors reasoned that the increase in miR-214 was due to infiltration of inflammatory cells into this compartment. Indeed, they confirmed that miR-214 was markedly increased in T lymphocytes of hypertensive mice, and not in other immune cells. The authors subsequently showed mice lacking miR-214 did not develop perivascular fibrosis upon ang II infusion. Elegant studies of vascular mechanics confirmed that the shift in stress/strain relationship that occurs upon ang II infusion was absent in miR-214−/− mice. Likewise, they showed that mice lacking the recombinase activating gene (RAG1−/− mice), which are deficient in both T and B cells, did not develop an increase in aortic fibrosis during ang II infusion and that fibrosis was restored upon adoptive transfer of normal T cells to these animals, but not by adoptive transfer of T cells from miR-214 deficient mice. Subsequent studies identified an important role of miR-214 in modulating T cell chemotaxis and expression of several chemokine receptors in hypertension. Finally, the investigators showed that hypertensive humans have much higher circulating levels of miR-214 than normotensive subjects, and that these levels correlate with pulse wave velocity in the hypertensive but not normotensive subjects.
MicroRNAs are small RNAs approximately 18–24 nucleotides in length that have been conserved through evolution. Initially, microRNA transcripts are transcribed by RNA polymerase II as 500–10,000 nucleotide pri-microRNAs.5 The primary transcripts may contain protein coding genes and often contain multiple microRNAs. In the nucleus, these transcripts for a stem loop structure that is further processed to form an approximately 70 nucleotide pre-microRNA. This is then exported from the nucleus to the cytoplasm where it is processed by Dicer to form the mature microRNA. MicroRNAs are then loaded into the Argonaute-microRNA-target complex. Within the 3’ untranslated region of the target mRNA, a Watson-Crick paired duplex forms with the 5’ 2–8 nucleotide “seed” sequence of the microRNA. It is this seed sequence that determines specificity of microRNA target genes. Complex formation then leads to the binding of members of the RNA-induced-silencing-complex (miRISC) that functions to suppress target genes by inducing mRNA degradation or translational repression.
miR-214 is transcribed in a cluster with miR-199a-2. The miR-214 locus is on the reverse strand from the host gene, the non-coding RNA dynamin 3 opposite strand. During embryogenesis, miR-214 levels decrease beginning at embryonic day 15.5 but can be markedly upregulated thereafter in response to pathological conditions such as myocardial ischemia and cardiac hypertrophy.6 Prior studies have shown that miR-214 targets the cardiac sodium-calcium exchanger-1 involved in calcium handling, cyclophilin D, a regulator of oxidative damage induced cell death, and glutathione-S-transferases in liver tissues.6, 7 Subsequent studies have revealed miR-214 can be induced by reactive oxygen species. In House Ear Institute-Organ of Corti 1 cells, treatment with the lipid peroxide tertbutyl hydroperoxide induced miR-214 expression.8
The function of miR-214 defined by Nosalski et al mirrors previous studies which showed its important role in cardiac and renal fibrosis. While the authors hypothesize an indirect effect of miR-214 in regulation of tissue growth factor-β, no specific target gene was identified. TargetScan analysis indicates that there are approximately 207 conserved predicted mRNA targets of miR-214 in humans. Recent studies into microRNA function have revealed their important role as regulators of signaling hubs and networks.9 This is facilitated by fine-tuning of potentially hundreds of target transcripts. Additionally, individual microRNA’s work in concert with other unique microRNAs given the potentially numerous microRNA binding sites within a single 3’-untranslated region. It is therefore unlikely that a single target drives the overarching function of miR-214. Future studies of miR-214 modulated networks within the T-cell compartment will likely reveal numerous target genes that contribute to the functions described in the present study.
Using RNA profiling, Nosalski et al showed that T cells of hypertensive mice express increased levels of IL-17A, interferon γ and the chemokine ligand CCL5. These changes were greatly diminished in T cells from miR-214−/− mice. The increase IL-17A is particularly notable. IL-17A is produced by CD4+ T cells and γ/δ T cells, and we and others have shown that it plays an essential role in hypertension. Mice lacking IL-17A are partly protected against the development of hypertension in response to ang II and do not develop aortic stiffening upon ang II infusion.10 Exposure of aortic fibroblasts in culture to IL-17A dramatically stimulates production of collagens 3a1 and 5a1,11 analogous to the finding of Nosalski et al. Thus, reduced production of IL-17A by T cells in the miR-214−/− mice likely contributes to their absence of collagen accumulation and protection against aortic stiffening.
Infiltration of immune cells into tissues requires two general phenomena. One is an increase in the local levels of chemokines and adhesion molecules that promote endothelial rolling, adhesion and ultimately transmigration. Several features of hypertension, including altered mechanical forces and oxidative stress enhance endothelial expression of endothelial homing molecules including the chemokine ligand CCL5 and the intracellular adhesion molecule 1. The second is the presence of receptors for these chemokines and adhesion molecules on the surface of immune cells. Nosalski et al show that several of these, including CCR1, CCR2, CCR4, CCR5, CCR6 and CXCR3 are increased in T cells of wild-type mice given ang II, but not in the miR-214−/− mice. This was reflected by an impairment in chemotactic capacity of T cells from miR-214−/− mice. They also show that mice lacking miR-214 exhibit reduced vascular oxidative stress in response to ang II infusion, which in turn might affect endothelial adhesiveness.
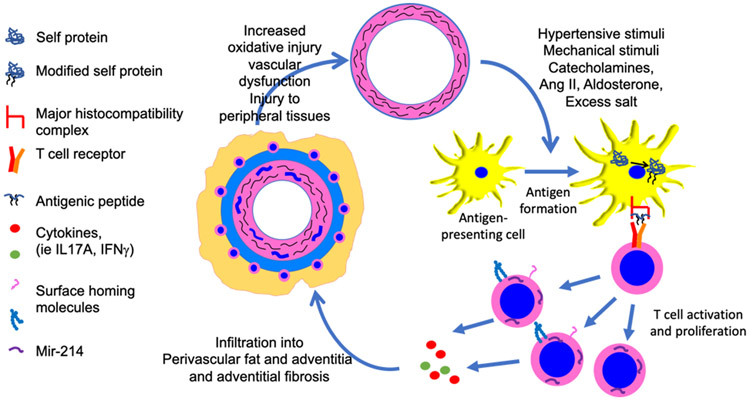
T cell activation occurs when the T cell receptor complex encounters a peptide antigen presented in the context of a major histocompatibility complex. Experimentally, one can mimic this by exposure of T cells to an antibody to CD3. The ultimate phenotype of the T cell depends on additional signals received from the local milieu. Interestingly, Nosalski et al showed that anti-CD3 treatment induced miR-214 in T cells ex vivo. Taken together with other studies, it is likely that neo-antigens formed early in hypertension, perhaps derived from the vessel wall, lead to T cell activation and production of miR-214, which in turn orchestrates a program of gene expression that further promotes vascular oxidative stress, endothelial dysfunction and perivascular fibrosis (Figure 1), likely in a feed forward fashion. We have previously shown that likely neoantigens in hypertension are isolevuglandin-modified proteins.12 Isolevuglandins are formed upon non-enzymatic oxidation of arachidonic acid, and rapidly form covalent bonds with lysines on proteins. In keeping with the scenario shown in Figure 1, we have shown that vascular oxidative stress can cause formation of these and their accumulation in dendritic cells, which in turn activate T cells. A consequence of aortic stiffening is enhanced forward pulse wave propagation and damage in peripheral tissues. As suggested by Nosalski et al, lowering blood pressure could reduce vascular oxidative stress, which would then reduce T cell activation and induction of miR-214, and thus break the pathway depicted in the Figure. Future studies are needed to further define these vascular/immune cell interactions.
Figure 1:
Paradigm supported by findings of Nosalski et al. Various hypertensive stimuli, including altered mechanical factors, oxidative stress, catecholamines, ang II and excess sodium intake induce formation of neoantigens, which are probably modified self-proteins. These are likely formed in vessels and acquired by antigen-presenting cells that in turn stimulate activation and proliferation of T cells. Nosalski et al show that miR-214 is expressed in activated T cells and that it orchestrates T cell chemotaxis and infiltration into perivascular adipose tissue. Cytokines produced by activated T cells, including IL-17A promote local fibrosis and vascular stiffening.
Acknowledgments
Sources of Funding: This manuscript was supported by NIH F32 HL144050 to DMP and National Institutes of Health Grants R35HL140016 and Program Project Grant P01HL129941 to DGH.
Abbreviations
- RNA
ribonucleic acid
- miRs
microRNAs
- miR-214
microRNA 214
- pri-microRNA
primary microRNA transcripts
- RAG
recombinase activating gene
- 3’-UTR
3’ untranslated region
- IL
interleukin
- CD
cluster of differentiation
- CCL
CC type chemokine ligand
- CCR
CC type chemokine receptor
- DGCR8
DiGeorge syndrome chromosomal region 8
Footnotes
Disclosures: The authors have no disclosures
LITERATURE CITED
- 1.Adji A, O’Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens. 2011;24:5–17 [DOI] [PubMed] [Google Scholar]
- 2.Safar ME. Arterial aging--hemodynamic changes and therapeutic options. Nat Rev Cardiol. 2010;7:442–449 [DOI] [PubMed] [Google Scholar]
- 3.Nosalski R, Siedlinski M, Denby L, McGinnigle E, Nowak M, Nguyen Dinh Cat A, Medina-Ruiz L, Cantini M, Skiba D, Wilk G, et al. T cell-derived miRNA-214 mediates perivascular fibrosis in hypertension Circulation Research. 2020; In press - this issue Circ Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D, Sagan A, Wu J, Vinh A, Marvar PJ, et al. Role of chemokine rantes in the regulation of perivascular inflammation, t-cell accumulation, and vascular dysfunction in hypertension. FASEB J. 2016;30:1987–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla GC, Singh J, Barik S. MicroRNAs: Processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92 [PMC free article] [PubMed] [Google Scholar]
- 6.Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, Humphries KM, Hill JA, Bassel-Duby R, Sadek HA, et al. microRNA-214 protects the mouse heart from ischemic injury by controlling Ca(2)(+) overload and cell death. J Clin Invest. 2012;122:1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu SC, Mato JM, Espinosa-Diez C, Lamas S. microRNA-mediated regulation of glutathione and methionine metabolism and its relevance for liver disease. Free Radic Biol Med. 2016;100:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Liu Y, Han N, Chen X, Yu W, Zhang W, Zou F. Profiles of oxidative stress-related microRNA and mRNA expression in auditory cells. Brain Res. 2010;1346:14–25 [DOI] [PubMed] [Google Scholar]
- 9.Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17:719–732 [DOI] [PubMed] [Google Scholar]
- 10.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin ii-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res. 2014;114:616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, et al. DC isoketal-modified proteins activate t cells and promote hypertension. J Clin Invest. 2014;124:4642–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]