Abstract
Objective:
In-hospital pediatric sepsis mortality has decreased substantially, but long-term mortality and morbidity among children initially surviving sepsis, is unknown. Accordingly, the Life After Pediatric Sepsis Evaluation (LAPSE) investigation was conducted to describe the trajectory of mortality and health-related quality of life (HRQL) morbidity for children encountering community-acquired septic shock.
Design:
Prospective, cohort-outcome study, conducted 2013-2017.
Setting:
Twelve academic pediatric intensive care units (PICUs) in the United States.
Patients:
Critically ill children, 1 month-18 years, with community-acquired septic shock requiring vasoactive-inotropic support.
Interventions:
Demographic, infection, illness severity, organ dysfunction, and resource utilization data were collected daily during PICU admission. Serial parent proxy-report HRQL assessments were obtained at baseline, 7 days, and 1, 3, 6, and 12 months following PICU admission utilizing the Pediatric Quality of Life Inventory™ or Stein-Jessop Functional Status Scale.
Measurements and Main Results:
Among 389 children enrolled, mean age was 7.4±5.8 years; 46% were female; 18% were immunocompromised; and 51% demonstrated chronic comorbidities. Baseline Pediatric Overall Performance Category was normal in 38%. Median [Q1-Q3] Pediatric Risk of Mortality and Pediatric Logistic Organ Dysfunction scores at PICU admission were 11.0 [6.0-17.0] and 9.0 [6.0-11.0]; durations of vasoactive-inotropic and mechanical ventilation support were 3.0 [2.0-6.0] and 8.0 [5.0-14.0] days; and durations of PICU and hospital stay were 9.4 [5.6-15.4] and 15.7 [9.2-26.0] days. At 1, 3, 6, and 12 months following PICU admission for the septic shock event, 8%, 11%, 12% and 13% of patients had died, while 50%, 37%, 30%, and 35% of surviving patients had not regained their baseline HRQL.
Conclusions:
This investigation provides the first longitudinal description of long-term mortality and clinically relevant, HRQL morbidity, among children encountering community-acquired septic shock. Although in-hospital mortality was 9%, 35% of survivors demonstrated significant, HRQL deterioration from baseline that persisted at least one year following hospitalization for septic shock.
Keywords: septic shock, mortality, health-related quality of life, functional status, children, long-term outcomes
BACKGROUND
Sepsis remains a major cause of childhood mortality worldwide [1, 2]. In developed nations, the mortality risk associated with pediatric septic shock has decreased from 50-60% in the 1950-1960s to 5-10% currently [3–5]. Although this statistic represents a paramount achievement for pediatric critical care medicine, it ignores the increasing morbidity burden of children surviving sepsis [6]. With recognition that critical illness begins and ends outside of the intensive care unit and hospital [7], clinicians and researchers alike are increasingly interested in long-term, patient-centered, clinically meaningful outcomes for critically ill patients. Previous investigations have reported increased risk for chronic disability, hospital readmission and late mortality for children surviving sepsis, but these studies were frequently limited by small sample size, lack of baseline status quantification, and/or variable time intervals for follow-up evaluation(s) [8–13].
Accordingly, Specific Aim 1 of the Life After Pediatric Sepsis Evaluation (LAPSE) (R01HD073362) prospective, cohort-outcome investigation was conducted to characterize critically ill children with contemporary, community-acquired septic shock and to describe the trajectory of mortality and health-related quality of life (HRQL) morbidity among those who survived. LAPSE investigators hypothesized that critically ill children with septic shock would demonstrate long-term mortality and significant HRQL morbidity during the year following admission for the sepsis event. A companion article examines critical illness variables associated with long-term death or persistent, serious HRQL disability among children initially surviving septic shock [14].
METHODS
Performance Sites, Study Conduct, Study Participants, Definitions
Twelve United States academic pediatric intensive care unit (PICU) research teams (eFigure 1) enrolled critically ill children with septic shock into the investigation. Details of performance sites, principal investigators, co-principal investigators, research coordinators, and allied research personnel are summarized in the Acknowledgements. Research coordinators continuously screened PICU admissions for potential study participants. Complete inclusion and exclusion criteria are provided in eText 1. Responsible attending physicians directed hemodynamic resuscitation, antimicrobial administration, mechanical ventilation, renal replacement therapy, extracorporeal life support, blood product transfusion, and nutritional management that were not mandated by the study protocol. Institutional review boards approved the study for each performance site, and parents provided written permission prior to patient participation. Developmentally appropriate patients were requested to provide assent for their own continued study participation following PICU discharge.
All patient participants exhibited septic shock that was operationally defined as: documented or suspected infection with onset within 48 hours of hospital admission; and presence of ≥ 2 systemic inflammatory response syndrome criteria [15], including abnormal leukocyte count/differential and/or abnormal body temperature; and requirement for fluid resuscitation and vasoactive-inotropic support that was initiated within 72 hours of hospital admission and within 48 hours of PICU admission. At enrollment, research coordinators collected data related to demographics, infectious disease, chronic comorbid conditions, [16] (eText 2), and immunodeficiency (eText 3). Patients were assessed for initial illness severity using Pediatric Risk of Mortality (PRISM-IV) [17] and Pediatric Logistic Organ Dysfunction (PELOD-2) score around PICU admission [18]. Durations of vasoactive-inotropic support, mechanical ventilation, and PICU and hospital stay were recorded.
Patients were serially assessed by research staff for functional status utilizing Pediatric Cerebral Performance Category (PCPC) and Pediatric Overall Performance Category (POPC) [19] and the Functional Status Scale [20] at study entry (reflecting baseline pre-sepsis status during the month prior to PICU admission), study Day 7, and study Day 28 or Hospital Discharge, whichever occurred first. Similarly, participating families completed serial parent-proxy assessments of their child’s HRQL utilizing the Pediatric Quality of Life Inventory, 4.0 Generic Core Scales (PedsQL™) [21, 22] or PedsQL™ Infant Scales [23] or the Stein-Jessop Functional Status Scale (short form, 14 item, double element, FSII-R) [24]. Parents selected the HRQL instrument that they believed provided the most meaningful assessment for their child. Health-related quality of life was assessed at study entry (reflecting baseline pre-sepsis status), study Day 7 and 1, 3, 6, and 12 months following PICU admission for the septic shock event. Both surveys employ a 0-100 point scale. For consistency, magnitude of HRQL morbidity is reported in multiples 4.5 points, a minimal clinically important difference (MCID) established for PedsQL™ [21], but not FSII-R. Detailed methodology for serial assessments of functional status and HRQL is provided in eText 4.
Data Collection, Organization and Analysis
Clinical data related to PICU admission were entered into an electronic data capture system provided by the data coordinating center at the University of Utah (OpenClinica, LLC, Waltham, MA). Data managers monitored data quality throughout the study. Parent-proxy HRQL assessments were entered into a separate database managed by the Seattle Children’s Research Institute (DatStat, Inc., Seattle, WA).
Interval or continuous variables are reported as medians, and IQRs, while variables collected categorically are summarized with counts and percentages. Sample size calculations for this investigation are detailed in eText 5. Bar graphs provide longitudinal summaries for PedsQL™ and FSII-R, as well as PCPC/POPC and FSS at each study time point for which data were collected. Differences in long-term outcomes, focused on change from baseline status, were measured using standard statistical tests such as the Wilcoxon rank-sum test, Fisher’s exact test, and the Cochran-Armitage test for trend. To account for potential bias due to loss of follow-up, longitudinal HRQL data were also analyzed utilizing multiple imputation techniques as discussed in eText 6.
Analyses were performed using SAS 9.4 (SAS Institute; Cary, NC) and R version 3.4.4 (The R Foundation for Statistical Computing). P-values are based on a two-sided alternative with p-values <0.05 considered significant. Results are reported according to STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) Guidelines for cohort studies [25].
RESULTS
The study flow diagram, Figure 1, and eTable 1 summarize exclusions and the number of patients with complete change from baseline survey information available for each study time point. From 1/1/2014–6/30/2017, 838 patients were screened, 632 were eligible, 570 (90%) were approached, 392 (62% of those eligible) were enrolled, and 389 provided complete baseline clinical data. Cumulative study enrollment and hospital survival are summarized graphically in eFigure 2. Of the enrolled patients with complete baseline data, 35/389 (9%) died during hospitalization and an additional 16/389 (4%) subjects died during the one-year follow-up.
Figure 1.
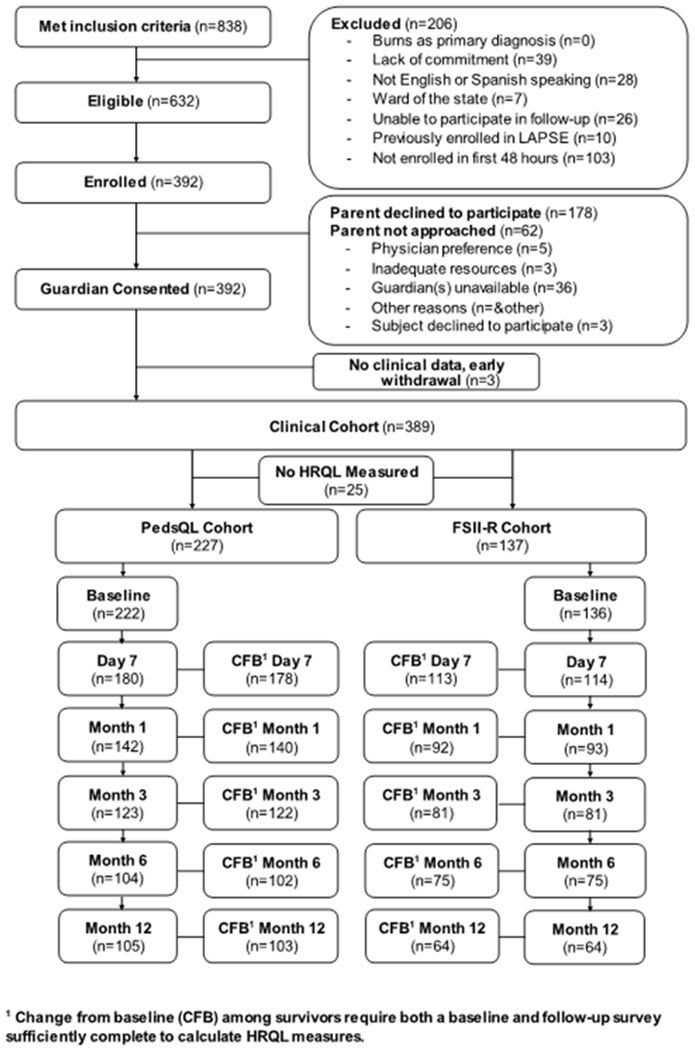
LAPSE Consort Diagram
Patient Characteristics
Table 1 summarizes demographics, illness severity, and resource utilization for the study cohort. Separate summaries are provided for all patients and for those completing a baseline HRQL survey, as only the latter group (n = 358) was utilized for subsequent change from baseline analyses. With broad overall age distribution, proportion of male patients exceeded females by 4%. Children with chronic conditions comprised 51% of the cohort; 18% were immunocompromised. Durations of vasoactive-inotropic infusion and mechanical ventilation support (median [Q1-Q3]) were 3.0 [2.0-6.0] and 8.0 [5.0-14.0] days; and durations of PICU and hospital stay were 9.4 [5.6-15.4] and 15.7 [9.2-26.0] days. Additional data indicated that patients’ health insurance status (n=351) was primarily private insurance (44%) or Medicaid (55%). Prior to hospitalization, 95% of patients resided at their home. At Month 1 follow-up, 146/235 (62%) of patients were residing at home, while 88/235 (37%) remained hospitalized. Among all enrolled patients, 89/389 (23%) were hospitalized for more than 28 days. Cohort infection characteristics are summarized in eTable 2 and eTable 3. Bacterial infection, viral infection, combined bacterial and viral infection, and no infection were documented in 49%, 45%, 20%, and 32% among all enrolled patients respectively.
TABLE 1.
Study Cohort Demographics, Initial Illness Severity, Resource Utilization
| Patient Characteristic | Overall Study Cohort (N = 389) | Completed Baseline HRQL (N = 358) |
|---|---|---|
| Age | ||
| 0-12 months | 67 (17.2%) | 63 (17.6%) |
| 13-24 months | 40 (10.3%) | 39 (10.9%) |
| 2-4 years | 70 (18.0%) | 63 (17.6%) |
| 5-7 years | 43 (11.1%) | 38 (10.6%) |
| 8-12 years | 72 (18.5%) | 66 (18.4%) |
| 13-17 years | 97 (24.9%) | 89 (24.9%) |
| Female | 178 (45.8%) | 163 (45.5%) |
| Hispanic or Latino | 88 (22.6%) | 78 (21.8%) |
| Race | ||
| White | 234 (60.2%) | 220 (61.5%) |
| Black or African American | 80 (20.6%) | 70 (19.6%) |
| Multiracial | 13 (3.3%) | 13 (3.6%) |
| Other | 31 (8.0%) | 27 (7.5%) |
| Unknown or not reported | 31 (8.0%) | 28 (7.8%) |
| Weight at PICU admission (kg) | 389, 22 [11,42] | 358, 22 [11,42] |
| Height at PICU admission (cm) | 348, 114 [79,145] | 321, 114 [79,144] |
| Subject immunocompromised | 68 (17.5%) | 63 (17.6%) |
| Medical complexity algorithm category | ||
| Missing PHIS | 1 (0.3%) | 1 (0.3%) |
| No chronic comorbid conditions | 189 (48.6%) | 170 (47.5%) |
| Chronic comorbid conditions (non-complex) | 20 (5.1%) | 19 (5.3%) |
| Chronic comorbid conditions (complex) | 179 (46.0%) | 168 (46.9%) |
| PRISM1 | 11.0 [6.0, 17.0] | 11.0 [6.0, 17.0] |
| PELOD, First day2 | 9.0 [6.0, 11.0] | 8.0 [6.0, 11.0] |
| Hospital duration of stay (days) | 15.7 [9.2, 26.0] | 16.0 [9.4, 25.6] |
| PICU duration of stay (days) | 9.4 [5.6, 15.4] | 9.3 [5.6, 15.1] |
| Vasoactive-inotropic support duration (days) | 368, 3.0 [2.0,6.0] | 338, 3.0 [2.0,6.0] |
| Mechanical ventilation duration (days) | 380, 8.0 [5.0,14.0] | 349, 8.0 [5.0,14.0] |
PRISM data were collected during a modified period of 2 hours prior to PICU admission through 4 hours post PICU admission.
First day is defined as day of admission if admission time is before 12:00 pm or following day if admission is after 12:00 pm.
Abbreviations: HRQL, health-related quality of life survey; PICU, pediatric intensive care unit; PHIS, Pediatric Health Information System; PMCA, Pediatric Medical Complexity Algorithm category, determined using ICD 9/10 diagnosis codes up to three years prior the index sepsis admission. If no diagnostic codes were identified, the subjects were assumed to have no chronic comorbid conditions. PHIS, Pediatric Health Information System; PRISM, Pediatric Risk of Mortality, version IV; PELOD-2, Pediatric Logistic Organ Dysfunction score, version 2;
Data are presented as n (%) or n, median [Q1, Q3].
Functional Status Changes
Figure 2 and eTable 4 summarize graphically and numerically changes in PCPC and POPC scores over time. At Baseline only 38% of patients exhibited normal POPC. Median Baseline POPC score was 2.0 [1.0, 3.0], while median change in POPC was 0.0 [0.0, 1.0] comparing Baseline and Day 28/Hospital Discharge. Assuming normality, a mean change in POPC of 0.6 was observed with 95% CI [0.5 to 0.7] indicating a significant deterioration in functional status (p<0.001). At Day 28/Hospital Discharge, 85/384 (22%) of children exhibited poor gross functional status (POPC ≥ 3 and an increase of ≥ 1 from baseline).
Figure 2.
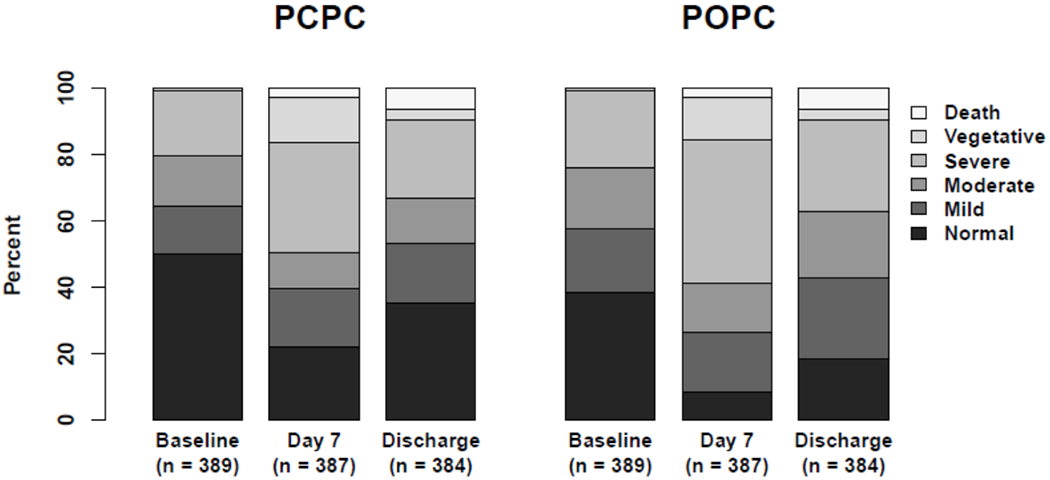
Distributions of Pediatric Cerebral Performance Category and Pediatric Overall Performance Category Scores Over Time.
Abbreviations: PCPC, Pediatric Cerebral Performance Category; POPC, Pediatric Overall Performance Category; Baseline, status in the month preceding PICU admission for the sepsis event; Discharge, assessment at Day 28 or Hospital Discharge, which ever occurred first.
Figure 3 and eTable 5 summarize graphically and numerically changes in FSS scores over time. Baseline FSS (n=389) was 6.0 [6.0, 12.0], with normal FSS status recorded (% of patients) for the following dimensions: communication (65.3%), feeding (64.5%), mental status (74.3%), motor function (63.8%), respiratory status (78.7%), and sensory function (79.7%). At Day 28/Hospital Discharge (n = 359), total FSS increased to 9.0 [6.0-15.0], p<0.001, again documenting a significant deterioration of functional status. eTable 5 summarizes itemized data for all FSS dimensions and substantiates a deterioration in communication, feeding, mental status, motor function, respiratory and sensory function, during hospitalization for pediatric septic shock.
Figure 3.
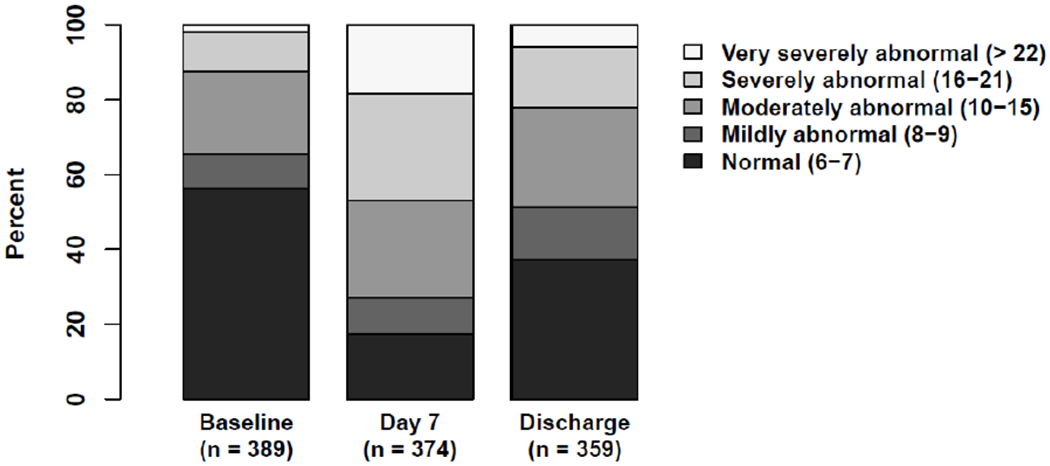
Distribution of Functional Status Scale (FSS) Categories Over Time
Baseline, status in the month preceding PICU admission for the sepsis event; Discharge, assessment at Day 28 or Hospital Discharge, which ever occurred first.
Health Related Quality of Life Changes
Less than 10% of subjects provided assent for self-report of HRQL. Accordingly, parent proxy-report is described for all HRQL data. Most families, 227/364 (62%), selected the PedsQL™ instruments for HRQL assessments. Baseline and Month 1 absolute PedsQL™ scores were 78.3 [65.9-92.4] (n=222) and 64.7 [47.8-81.9] (n=142) respectively. Median change for PedsQL™, comparing Baseline and Month 1, was −11.0 [−29.2-5.55], p<0.001, reflecting a significant deterioration in HRQL. Assuming normality, an estimated mean change of −12.2 with 95% CI [−16.3 to −8.08] was observed. This confidence interval reflects at least a MCID deterioration in PedsQL™ at Month 1.
Figure 4A and eTable 6 display graphical and numerical percentages of patients failing to return to their baseline PedsQL™ over the one-year follow-up, utilizing multiples of 4.5 points, the MCID for this HRQL instrument. Additional plots of PedsQL™ longitudinal data are presented in eFigure 5. At 1, 3, 6, and 12 month assessments, 56%, 41%, 32% and 38% of patients assessed with PedsQL™ remained at least 4.5 points (1xMCID) below their baseline HRQL, while 40%, 16%, 15% and 17% remained at least 18.0 points (4xMCID) below their baseline HRQL.
Figure 4.
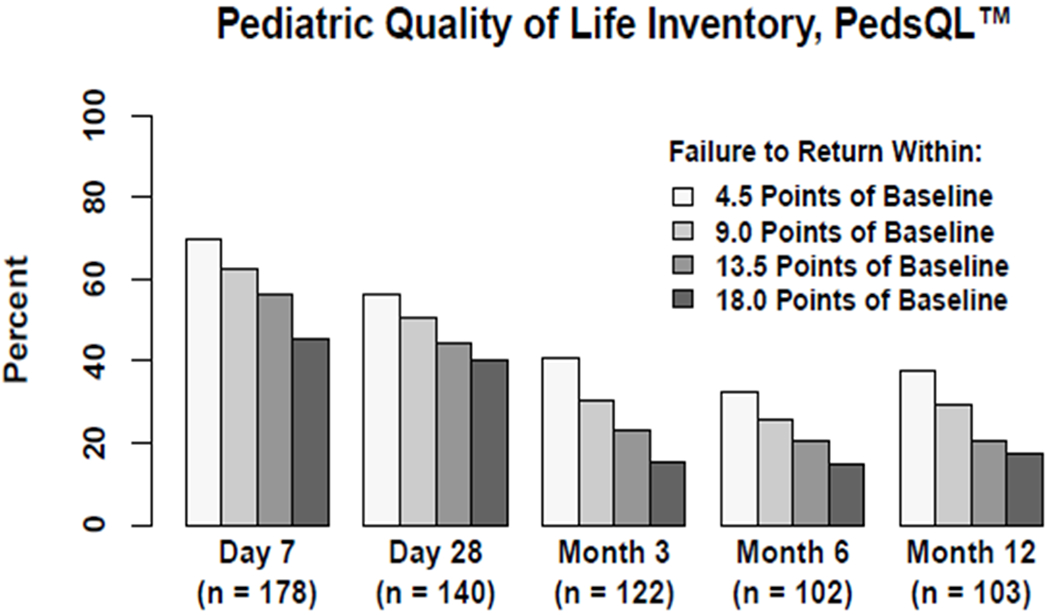
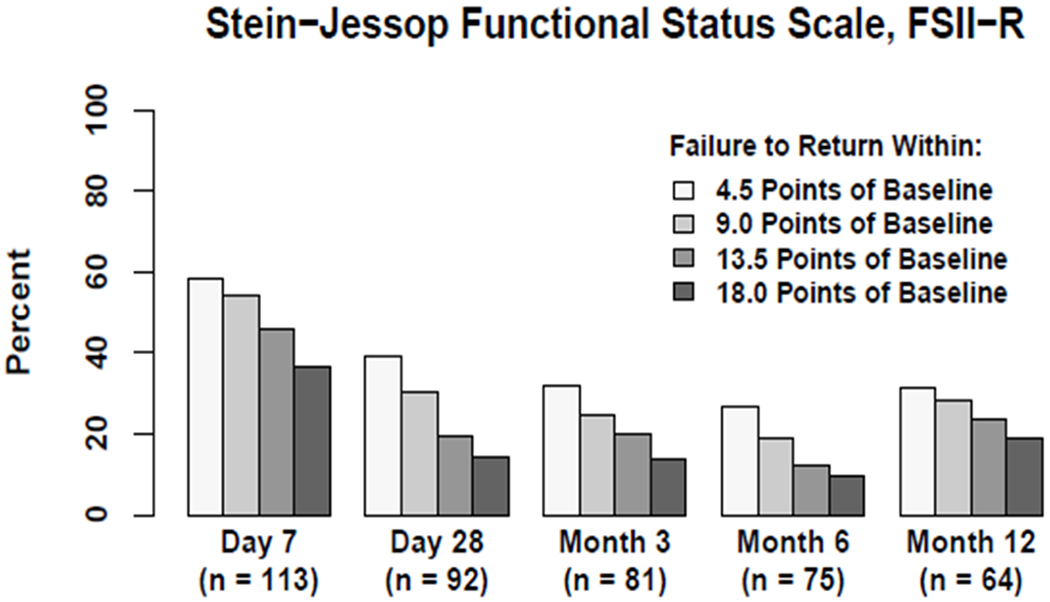
Longitudinal Assessment of Failure to Return to Baseline Health-related Quality of Life Utilizing the PedsQL™ (Figure 4A) and FS-IIR (Figure 4B) Instruments.
Magnitude of HRQL deterioration is depicted in in multiples of 4.5 points, namely 4.5, 9.0, 13.5, and 18.0 points
Some families, 137/364 (38%), selected the FSII-R instrument because they considered it more meaningful and relevant to their children, who typically manifested significant developmental delay. Baseline and Month 1 absolute FSII-R scores were 71.4 [60.7-83.4] (n=136) and 73.1 [60.7-82.1] (n=93) respectively. Median change for FSII-R, comparing baseline and Month 1, was 0.0 [−10.7-10.7], p=0.93. Assuming normality, an estimated mean change of −1.1 with 95% CI [−5.3 to 3.0] was observed. Figure 4B and eTable 7 display graphical and numerical percentages of patients failing to return to their baseline FSII-R over the one-year follow-up, again utilizing multiples of 4.5 points for this HRQL instrument. Additional plots of FSII-R longitudinal data are presented in eFigure 6. At 1, 3, 6, and 12 month assessments, 39%, 32%, 27% and 31% of patients assessed with FSII-R remained at least 4.5 points below their baseline HRQL, while 14%, 14%, 9% and 19% remained at least 18.0 points below their baseline HRQL.
In total, at 1, 3, 6, and 12 months following PICU admission for the septic shock event, 8%, 11%, 12% and 13% of patients had died, and among survivors with adequate change from baseline data, 50%, 37%, 30%, and 35% of surviving patients had not regained their baseline HRQL. Patients assessed with the FSII-R instrument, as a whole, seemed to demonstrate less deterioration from and faster recovery to baseline HRQL. Patients assessed with either HRQL instrument appeared to exhibit a plateau in HRQL recovery, Months 3-12 (Figure 4).
Patients Lost to Follow-up
Inspection of Figure 1 and eTable 1 reveals significant loss to follow-up for the study cohort, despite parental incentives for continued participation. eTable 8 documents that patients who were lost to follow up at the Month 3 assessment exhibited similar initial illness severity as per initial PRISM-IV and PELOD-2 scores, as well as duration of vasoactive-inotropic and mechanical ventilation support and PICU and hospital stay, as compared to patients who completed the Month 3 survey. However, patients lost to follow-up accrued higher summation of daily PELOD-2 scores and more frequently required renal replacement therapy and extracorporeal life support. Data analysis employing multiple imputation techniques, to account for patients lost to follow-up, suggests an even greater burden of long-term HRQL morbidity among children surviving septic shock (eFigures 7 and 8). Specifically, imputation tended to predict lower values for missing HRQL measures, consistent with the notion that patients without completed surveys had at least some measures of higher illness severity, and higher illness severity was associated with higher risk for adverse HRQL outcomes.
DISCUSSION
This investigation reports several significant, novel findings: First, in-hospital and one year mortality from community-acquired septic shock were 9% and 13%, but among the survivors, 35% had not yet regained their baseline HRQL one year later. Second, for the cohort as a whole, HRQL improved between one and three months, but further improvement was not apparent over the ensuing 9 months. Accordingly, a 3 month follow-up may represent a pivitol check point relative to monitoring and intervening for post intensive care syndrome among children surviving septic shock [26, 27]. Third, 51% of the patients enrolled demonstrated significant chronic comorbid conditions as assessed by the Pediatric Medical Complexity Algorithm, with impaired baseline functional status per POPC and FSS, even though such children comprise only 3% of the overall pediatric population [28]. This preponderance of critically ill children with chronic conditions is consistent with national PICU data [29]. While the median Baseline score for patients assessed with PedsQL™ was similar to published norms (30) for generally healthy children, the median Baseline score for patients assessed with FSII-R was significantly lower than normative data for chronically ill children (24). These observations confirm that children with chronic conditions bear a disproportionate burden of critical illness, including sepsis. Fourth, patients assessed with FSII-R unexpectedly exhibited less decline from and seemingly faster resolution towards baseline HRQL compared to patients assessed with PedsQL™. This finding may relate to differential views of what constitues HRQL and HRQL recovery among parents of children with severe developmental disability [30]. Alternatively this finding may reflect greater sensitivity to change for the PedsQL™ instrument. Fifth, fortunately, the majority of children recovered to or exceeded their baseline HRQL one year following septic shock. Improved HRQL following acute illness has been previously reported [13] and may relate to actual improvement in HRQL status (e.g. successful chemotherapy, organ transplantation, curative surgery), potential parental recall bias of lower HRQL at baseline, or the acute illness as a life transforming event [13].
Collectively, this study demonstrates substantial HRQL morbidity among many critically ill children surviving septic shock. Previous investigations have reported increased risk for chronic disability, hospital readmission and late mortality for children surviving sepsis, but these studies, largely retrospective in design, were variably limited by small sample size, lack of baseline HRQL ascertainment, and/or variable time intervals for follow-up evaluation(s) [8–13]. The LAPSE investigation enrolled a moderate sized, prospectively-derived cohort, from twelve tertiary PICUs across the United States. Accordingly the results are likely to be generalizable among pediatric academic centers, but perhaps not for community hospital PICUs. Children enrolled into LAPSE were assessed for chronic, comorbid conditions [16], and underwent baseline functional status and HRQL evaluations. Establishing a baseline for subsequent HRQL comparisons is feasible and provides the best approach for differentiating the effects of chronic conditions versus acute illness on long-term HRQL morbidity.
This study has several limitations. First, subjects lost to follow-up was problematic, especially because these events were likely not random. Although HRQL and functional status are clearly important outcome measures [31], results from this study highlight the logistical challenges when such outcomes are utilized for long-term endpoints. A variety of strategies [http://www.improveLTO.com/cohort-retention-tools/] have been suggested and evaluated [32] to maximize subject research participation retention including financial incentives for participants, that were employed in the current investigation. Second, this investigation focused on community-acquired pediatric sepsis. It is likely that patients with hospital-acquired sepsis demonstrate a greater burden of acute and chronic comorbidities that may impact short and long-term outcomes. Third, some would argue that HRQL or functional status should be based on patient-reported data [33, 34] [https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf] utilizing a single instrument. However, the practicality of patient-reported data for critically ill children is limited, as they are frequently very young, routinely sedated, and often developmentally delayed. Moreover, as the consumers of pediatric healthcare, families are uniquely positioned to provide their perspectives of pediatric HRQL [35]. In addition to PedsQL™, LAPSE investigators used FSII-R as a measure of HRQL. Although the name FSII-R suggests this instrument is primarily a functional status measure, in fact, it is generally regarded as a validated measure of general health status for children of all ages [36, 37], and some parents preferred this tool to describe their child’s situation. Accordingly, in order to maintain involvement of families with children with severe developmental disability and record meaningful parent-proxy information, we offered use of either tool. Ultimately, this decision was likely a strengh of the investigation in terms of inclusion and diversity in enrollment and continued study engagement. Trajectories of failure to recover HRQL following pediatric septic shock, presented separately for the two instruments in Figures 4A, 4B and eFigures 7, 8 appear remarkably similar. Fourth, recall bias, as was used to establish baseline HRQL for this investigation, is a limitation inherent in any retrospective evaluation. However, parents were informed of the importance of accuracy for this baseline assessessment, at a time when they were not overtly stressed, typically following resuscitation and stabilization of their child.
CONCLUSIONS
This investigation provides the first longitudinal description of long-term mortality and clinically relevant, enduring HRQL morbidity, among children encountering community-acquired septic shock. Pediatric septic shock is not only life-threatening in terms of onging risk for mortality, but also life-altering among children surviving their septic shock. All cause 28 day mortality alone no longer articulates the entire story of pediatric septic shock outcomes. In addition to ensuring survival from septic shock, optimizing a child’s long-term HRQL is an equally important goal for pediatric septic shock critical care.
Supplementary Material
ACKNOWLEDGMENTS
The LAPSE Investigators thank all subjects and families for participating in the LAPSE investigation.
Following is a summary of LAPSE Performance Sites, Principal Investigators (PI), Co-investigators (CI), Research Coordinators (RC), and Allied Research Personnel.
Children’s Hospital of Michigan, Detroit, MI: Kathleen L. Meert, PI; Sabrina Heidemann, CI; Ann Pawluszka, RC; Melanie Lulic, RC.
Children’s Hospital of Philadelphia, Philadelphia, PA: Robert A Berg, PI; Athena Zuppa, CI; Carolann Twelves, RC; Mary Ann DiLiberto, RC.
Children’s National Medical Center, Washington, DC: Murray Pollack, PI; David Wessel, PI; John Berger, CI; Elyse Tomanio, RC; Diane Hession, RC; Ashley Wolfe, RC.
Children’s Hospital of Colorado, Denver, CO: Peter Mourani, PI; Todd Carpenter, CI; Diane Ladell, RC; Yamila Sierra, RC; Alle Rutebemberwa, RC.
Nationwide Children’s Hospital, Columbus, OH: Mark Hall, PI; Andy Yates, CI; Lisa Steele, RC; Maggie Flowers, RC; Josey Hensley, RC.
Mattel Children’s Hospital, University of California Los Angeles, Los Angeles, CA: Anil Sapru, PI; Rick Harrison, CI, Neda Ashtari, RC; Anna Ratiu, RC.
Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, PA: Joe Carcillo, PI; Michael Bell, CI; Leighann Koch, RC; Alan Abraham, RC.
Benioff Children’s Hospital, University of California, San Francisco, San Francisco, CA: Patrick McQuillen, PI; Anne McKenzie, RC; Yensy Zetino, RC.
Children’s Hospital of Los Angeles, Los Angeles, CA: Christopher Newth, PI; Jeni Kwok, RC; Amy Yamakawa, RC.
CS Mott Children’s Hospital, University of Michigan, Ann Arbor, MI: Michael Quasney, PI; Thomas Shanley, CI; CJ Jayachandran, RC.
Cincinnati Children’s Hospital, Cincinnati, OH: Ranjit Chima PI; Hector Wong, CI; Kelli Krallman, RC; Erin Stoneman, RC; Laura Benken, RC; Toni Yunger, RC.
St Louis Children’s Hospital, Washington University, St Louis, MO: Alan Doctor, PI; Micki Eaton, RC.
Seattle Children’s Hospital, Seattle Children’s Research Institute (LAPSE Follow-up Center), University of Washington, Seattle, WA: Jerry J Zimmerman, PI; Catherine Chen, RC; Erin Sullivan, RC; Courtney Merritt, RC; Deana Rich, RC; Julie McGalliard; Wren Haaland; Kathryn Whitlock, Derek Salud.
University of Utah (LAPSE Data Coordinating Center), Salt Lake City, UT: J Michael Dean, PI; Richard Holubkov, CI; Whit Coleman, RC; Samuel Sorenson, RC; Ron Reeder; Russell Banks; Angie Webster; Jeri Burr; Stephanie Bisping; Teresa Liu; Emily Stock, Kristi Flick.
Texas A&M University, College Station, TX: James Varni
No performance site investigators disclose financial interests, activities, relationships, or affiliations that could be construed as real or potential conflicts of interest related to the manuscript or the related investigation. Dr. Varni holds the copyright and the trademark for the PedsQL™ and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory™. Dr Varni provided consultation on original study design and final manuscript edits, but played no role in data acquisition or analysis.
This investigation was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, R01HD073362, and was supported, in part, by the following cooperative agreements: UG1HD050096, UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171, UG1HD083166, UG1HD083170, U10HD050012, U10HD063106, and U01HD049934.
Copyright form disclosure: Dr. Zimmerman’s institution received funding from National Institutes of Child Health and Human Development (NICHD) and Immunexpress, and he received funding from Elsevier Publishing (royalties) and the Society of Critical Care Medicine (travel reimbursements). Drs. Zimmerman, Banks, Berg, Zuppa, Newth, Wessel, Pollack, Meert, Hall, Sapru, Carcillo, McQuillen, Mourani, Wong, Chima, Holubkov, Coleman, Sorenson, Varni, Whitlock, Dean, and Reeder received support for article research from the National Institutes of Health. Dr. Banks’s institution received funding from NICHD/CPCCRN, and he disclosed government work. Dr. Berg, Zuppa, Newth, Wessel, Pollack, Meert, Hall, Sapru, Carcillo, Mourani, Wong, Holubkov, Varni, Whitlock, Dean, and Reeder’s institution received funding from the NIH. Dr. Newth received funding from Philips Research North America and Hamilton Medical AG. Dr. McQuillen’s institution received funding from the NICHD UG1 HD083166. Dr. Holubkov received funding as a DSMB member from Pfizer, Medimmune, and Revance, and funding from biostatistical consulting for Physicians Committee for Responsible Medicine and DURECT Corporation. Dr. Coleman’s institution received funding from Seattle Children’s. Dr. Sorenson’s institution received funding from Seattle Children’s Research Institute. Dr. Varni disclosed that he holds the copyright and the trademark for the PedsQL and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory. Dr. McGalliard disclosed work for hire. The authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
No reprints will be ordered for this article.
Limited aspects of this manuscript have previously been presented in abstract form at the 2018 Society of Critical Care Medicine Annual Congress
REFERENCES
- 1.Weiss SL, Fitzgerald JC, Pappachan J, et al. : Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kissoon N, Uyeki TM: Sepsis and the Global Burden of Disease in Children. JAMA Pediatr 2016; 170:107–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartman ME, Linde-Zwirble WT, Angus DC, et al. : Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med 2013; 14:686–693 [DOI] [PubMed] [Google Scholar]
- 4.Ruth A, McCracken CE, Fortenberry JD, et al. : Pediatric severe sepsis: current trends and outcomes from the Pediatric Health Information Systems database. Pediatr Crit Care Med 2014; 15:828–838 [DOI] [PubMed] [Google Scholar]
- 5.Balamuth F, Weiss SL, Neuman MI, et al. : Pediatric severe sepsis in U.S. children’s hospitals. Pediatr Crit Care Med 2014; 15:798–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon DW, Clark RS, Watson RR: No pain, no gain in pediatric sepsis?. Pediatr Crit Care Med 2014; 15:264–266 [DOI] [PubMed] [Google Scholar]
- 7.Angus DC, Carlet J, Brussels Roundtable Participants: Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med 2003; 29:368–377 [DOI] [PubMed] [Google Scholar]
- 8.Buysse CM, Raat H, Hazelzet JA, et al. : Surviving meningococcal septic shock: health consequences and quality of life in children and their parents up to 2 years after pediatric intensive care unit discharge. Crit Care Med 2008; 36:596–602 [DOI] [PubMed] [Google Scholar]
- 9.Czaja AS, Zimmerman JJ, Nathens AB: Readmission and late mortality after pediatric severe sepsis. Pediatrics 2009; 123:849–857 [DOI] [PubMed] [Google Scholar]
- 10.Bronner MB, Knoester H, Sol JJ, et al. : An explorative study on quality of life and psychological and cognitive function in pediatric survivors of septic shock. Pediatr Crit Care Med 2009; 10:636–642 [DOI] [PubMed] [Google Scholar]
- 11.Edmond K, Dieye Y, Griffiths UK, et al. : Prospective cohort study of disabling sequelae and quality of life in children with bacterial meningitis in urban Senegal. Pediatr Infect Dis J 2010; 29:1023–1029 [DOI] [PubMed] [Google Scholar]
- 12.Als LC, Nadel S, Cooper M, et al. : Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med 2013; 41:1094–1103 [DOI] [PubMed] [Google Scholar]
- 13.Killien EY, Farris RWD, Watson RS, et al. : Health-related quality of life among survivors of pediatric sepsis. Pediatr Crit Care Med 2019; 20:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman JJ, Banks R, Berg RA, et al. : Critical illness variables associated with long-term mortality and/or persistent, serious health-related quality of life morbidity following community acquired septic shock. Pediatr Crit Care Med 2019; xx:zzz–zzz [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein B, Giroir B, Randolph A: International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8 [DOI] [PubMed] [Google Scholar]
- 16.Simon TD, Cawthon ML, Stanford S, et al. : Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics 2014; 133:e1647–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollack MM, Holubkov R, Funai T, et al. : The Pediatric Risk of Mortality Score: Update 2015. Pediatr Crit Care Med 2016; 17:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leteurtre S, Duhamel A, Salleron J, et al. : PELOD-2: an update of the Pediatric Logistic Organ Dysfunction Score. Crit Care Med 2013; 41:1761–1773 [DOI] [PubMed] [Google Scholar]
- 19.Fiser DH, Long N, Roberson PK, et al. : Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000; 28:2616–2620 [DOI] [PubMed] [Google Scholar]
- 20.Pollack MM, Holubkov R, Glass P, et al. : Functional Status Scale: new pediatric outcome measure. Pediatrics 2009; 124:e18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varni JW, Burwinkle TM, Seid M, et al. : The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr 2003; 3:329–341 [DOI] [PubMed] [Google Scholar]
- 22.Aspesberro F, Fesinmeyer MD, Zhou C, et al. : Construct Validity and Responsiveness of the Pediatric Quality of Life Inventory 4.0 Generic Core Scales and Infant Scales in the PICU. Pediatr Crit Care Med 2016; 17:e272–279 [DOI] [PubMed] [Google Scholar]
- 23.Varni JW, Limbers CA, Neighbors K, et al. : The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res 2011; 20:45–55 [DOI] [PubMed] [Google Scholar]
- 24.Stein RE, Jessop DJ: Functional status II(R). A measure of child health status. Med Care 1990; 28:1041–1055 [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, et al. : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61:344–349 [DOI] [PubMed] [Google Scholar]
- 26.Watson RS, Choong K, Colville G, et al. : Life after Critical Illness in Children-Toward an Understanding of Pediatric Post-intensive Care Syndrome. J Pediatr 2018; 198:16–24 [DOI] [PubMed] [Google Scholar]
- 27.Anderson-Shaw L: Surviving Severe Sepsis: Is That Enough? Crit Care Med 2016; 44:1603–1604 [DOI] [PubMed] [Google Scholar]
- 28.Cohen E, Kuo DZ, Agrawal R, et al. : Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics 2011; 127:529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards JD, Houtrow AJ, Vasilevskis EE, et al. : Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay. Crit Care Med 2012; 40:2196–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rennick JE, Childerhose JE: Redefining success in the PICU: new patient populations shift targets of care. Pediatrics 2015; 135:e289–291 [DOI] [PubMed] [Google Scholar]
- 31.Merritt C, Menon K, Agus MSD, et al. : Beyond survival: Pediatric critical care interventional trial outcome measure preferences of families and healthcare professionals. Pediatr Crit Care Med 2018; 19:e105–e111 [DOI] [PubMed] [Google Scholar]
- 32.Treweek S, Pitkethly M, Cook J, et al. : Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev 2018; 2:MR000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United States Department of Health and Human Services: Patient-Reported Outcome Measures. Office of Communications, Division of Drug Information, Center for Drug Evaluation and Research. Food and Drug Administration, Silver Spring, MD, 2009 [Google Scholar]
- 34.Sprangers MA, Aaronson NK: The role of health care providers and significant others in evaluating the quality of life of patients with chronic disease: a review. J Clin Epidemiol 1992; 45:743–760 [DOI] [PubMed] [Google Scholar]
- 35.Varni JW, Limbers CA, Burwinkle TM: Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes 2007; 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keenan HT, Runyan DK, Nocera M: Longitudinal follow-up of families and young children with traumatic brain injury. Pediatrics 2006; 117:1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Da Costa D, Bann CM, Hansen NI, et al. : Validation of the Functional Status II questionnaire in the assessment of extremely-low-birthweight infants. Dev Med Child Neurol 2009; 51:536–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


