Abstract
Several Phase II and III clinical trials have demonstrated that immunotherapy can induce objective responses in otherwise refractory malignancies in tumors outside the central nervous system. In large part, effector T cells mediate much of the antitumor efficacy in these trials, and potent antitumor T cells can be generated through vaccination, immune checkpoint blockade, adoptive transfer, and genetic manipulation. However, activated T cells must still traffic to, infiltrate, and persist within tumor in order to mediate tumor lysis. These requirements for efficacy pose unique challenges for brain tumor immunotherapy, due to specific anatomical barriers and populations of specialized immune cells within the central nervous system that function to constrain immunity. Both autoimmune and infectious diseases of the central nervous system provide a wealth of information on how T cells can successfully migrate to the central nervous system and then engender sustained immune responses. In this review, we will examine the commonalities in the efferent arm of immunity to the brain for autoimmunity, infection, and tumor immunotherapy to identify key factors underlying potent immune responses.
Keywords: T-lymphocytes, brain neoplasms, immunotherapy, central nervous system, immune privilege, dendritic cells
I. Introduction
More than half a century ago, it was demonstrated that foreign tissue grafts in the brain parenchyma were not rejected by an immune response (Medawar, 1948; Tansley, 1946). This led to the conceptualization of central nervous system (CNS) immune privilege as a shield, preventing the entry and exit of soluble antigen and migrating immune cells to the brain and spinal cord. However, work from the past decades in autoimmune and infectious diseases of the CNS demonstrate that immune privilege is far from absolute. Indeed, immune surveillance of the brain under homeostatic conditions must occur, as individuals with genetic or induced immune suppression possess increased susceptibility to opportunistic infections of the CNS (Klein & Hunter, 2017). It is true that excessive immune-mediated inflammation and swelling of the brain can be deadly, and therefore inflammatory responses must be constrained. However, the recent rediscovery of lymphatics in the meninges of rodents and humans (Absinta, et al., 2017; Aspelund, et al., 2015; Louveau, et al., 2015) indicates that the immune system may function in some sub-sections of the CNS similarly to the periphery. Furthermore, immunity to infections of the brain as well as autoimmunity in multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE) provide critical insights into the nature of how robust immunity can be initiated and potentiated within the CNS.
Recently, immune-mediated therapies against cancer have demonstrated truly remarkable advances against peripheral tumors such as hematological malignancies (Brentjens, et al., 2013; Kantarjian, et al., 2017; Maude, et al., 2014; Schuster, et al., 2017), non-small-cell lung cancer (Antonia, et al., 2017; Hellmann, et al., 2018), melanoma and Merkel cell carcinoma (Hodi, et al., 2010; Nghiem, et al., 2016; Phan, et al., 2003; Schwartzentruber, et al., 2011), as well as tumors with mismatch-repair deficiency (Le, et al., 2015), with objective responses in patients with otherwise refractory late stage malignancy. However, while remarkable cases of patient responders to brain tumor immunotherapy show promise, recent failures in clinical trials (Omuro, et al., 2017; Weller, et al., 2017) (NCT01814813) illustrate that an enhanced understanding of the requirements for effective immunity within the brain are desperately needed. As patients with brain tumors treated with immunotherapy can demonstrate the generation and persistence of peripheral antitumor T cells after treatment (Batich, et al., 2017; Okada, et al., 2011), it is unclear how to translate these systemic immune responses into cytotoxic responses deep within the parenchyma where most brain tumors reside. The truth remains that the physical barriers to T cell migration to the brain and the specialized antigen presenting cells (APCs) of the CNS do present a unique environment for immunotherapy. In the context of CNS infection and autoimmunity, research into the requirements for how immune responses are initiated and potentiated within the brain may additionally help reveal how to initiate and potentiate such immune responses against brain tumors. In this review, through infection, autoimmunity and tumor immunotherapy, we will examine the efferent arm of immunity to the brain and how activated systemic T cells access the CNS to engender robust immunity.
2. The Central Nervous System
In the periphery, a classical adaptive antigen-specific T cell immune response is initiated when a professional APC, such as a dendritic cell (DC), acquires antigen, matures, and migrates via lymphatics to the draining lymph node (dLN). The APC then presents this antigen via cell-surface class II and class I molecules to respectively activate first CD4+ and then CD8+ T cells, usually within the dLNs (K. Murphy, Travers, Walport, & Janeway, 2012). The expression of cytokines, chemokine receptors, and adhesion molecules on these activated T cells then directs them to inflammatory milieus. Furthermore, conditioning by DCs during T cell activation can even alter the expression of these receptors on T cells to direct organ-specific tropism (Sigmundsdottir & Butcher, 2008). However, entry to the CNS is unique due to the physical barriers surrounding it and the defined physiological sites at which immune cells may enter it (Engelhardt, Vajkoczy, & Weller, 2017; Korn & Kallies, 2017). Furthermore, there are populations of resident immune cells particular to the CNS that function as CNS-specific APCs for T cell activation (Herz, Filiano, Smith, Yogev, & Kipnis, 2017). For the purpose of brain tumor immunotherapy, these factors play a vital role in creating an environment in which immune activation can differ from the periphery.
Anatomical Barriers of the CNS
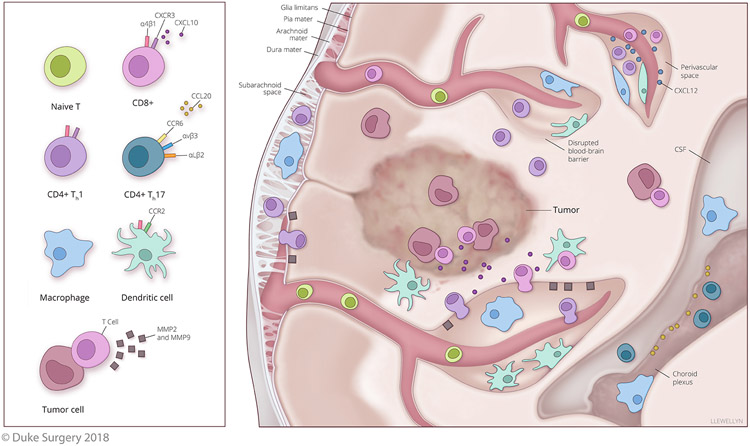
Immune cells within blood vessels can travel to the CNS and transmigrate across these vessels within specific locations: the meninges, the choroid plexus, or the perivascular spaces within the parenchyma (Figure 1). As one of the gateways to CNS entry, the meninges are layers that encapsulate the brain. From outside to inside they are the dura mater, the arachnoid mater, and the pia mater. The outermost layer, the dura mater, contains the newly rediscovered lymphatics of the brain. Under the dura is the arachnoid mater, which serves as a fluid impermeable barrier between the dura and underlying cerebrospinal fluid (CSF) that fills the subarachnoid space (SAS). Thus, the meninges are a CSF drained space and the arachnoid mater serves as a blood CSF barrier (BCSFB). Under the SAS is the pia mater, and underneath that is the sub-pial space and then the glia limitans. The glia limitans is a unique layer of basement membrane and astrocyte end feet surrounding the gray and white matter and serves as the final barrier between the migratory blood cells and the parenchyma. Therefore, while certain immune cells can extravasate through vessels within the meninges, they will still need to pass the glia limitans to reach the parenchyma (Engelhardt, et al., 2017; Klein & Hunter, 2017). Another route of entry is the choroid plexus in the center of the brain, this consists of a group of specialized ependymal cells that generate the majority of the CSF and serve as another BCSFB to the ventricles. To access the choroid plexus CSF, immune cells can extravasate through the cells or the tight junctions connecting the BCSFB. Once immune cells pass the choroid plexus and access the CSF of the ventricles, they can reach other CSF drained sites like the meninges. However, to reach the parenchyma they will also still need to pass the glia limitans. The parenchyma can also be accessed via the arteries and veins within it that are surrounded by the blood brain barrier (BBB). This barrier consists of a layer of specialized vascular endothelium adjacent to the glia limitans and prevents both the diffusion of solutes as well as immune cell entry through both tight junctions and adherens junctions among the endothelial cells. In the white matter of the parenchyma, swellings around post capillary venules engender perivascular spaces filled with brain interstitial fluid (ISF) between the endothelium and the glia limitans (Engelhardt, et al., 2017). Immune cells can leave the blood and enter the perivascular space via traditional diapedesis, but then must pass the glia limitans to reach the parenchyma. The meninges, choroid plexus, and perivascular spaces all represent potential points of parenchymal access for immune cells, but these also represent significant barriers to transmigration of immune cells with specific requirements as expanded upon later in this review.
Figure 1. Proposed Mechanisms of T cell Infiltration of the CNS and the Generation of Antitumor Immunity.
Evidence indicates that activated T cells expressing CXCR3 (potentially recruited via CNS expression of CXCL10) will preferentially traffic to the CNS. Infiltration of the CNS has also been shown to be dependent on receptor expression and T cell subtype. Th1 CD4+ and CD8+ T cells show a dependence on α4β1 integrin for CNS infiltration, whereas Th17 T cells show a dependence on the integrins αVβ3 and αLβ2 as well as the chemokine receptor CCR6 binding to CCL20 for infiltration of the CSF from the choroid plexus. T cells that migrate across endothelial barriers and have accessed the meninges, CSF, and perivascular spaces will still need to bypass the glia limitans to reach the parenchyma. The glia limitans is the last barrier of the BBB and CXCL12 is important for retaining T cells within perivascular spaces and preventing their migration past the glia limitans. To breach this barrier, the scavenging of CXCL12 and the secretion of MMP2 and MMP9 by activated immune cells can permit T cells access to the parenchyma. However, in the context of brain tumors, disruption of the BBB may permit immune cell infiltration of the parenchyma without these steps. In EAE, evidence also suggests that DCs are critical for supporting both T cell infiltration and robust antigen-specific autoimmunity within the CNS. Preclinical models of brain tumor immunotherapy suggest that DCs recruited to the CNS may be instrumental for enhancing both T cell infiltration and retention within brain tumors.
Antigen Presenting Cells of the CNS
It is also worth noting that the immune cells in the meninges, choroid plexus, perivascular spaces, and parenchyma are quite different from both the periphery and from each other due to unique populations of resident macrophages in these locations (Herz, et al., 2017). This is significant as these macrophages serve as APCs to surveilling T cells migrating within the CNS. In mice, both the meninges and perivascular spaces possess tissue resident macrophages that derive from hematopoietic precursors during embryogenesis. In contrast, choroid plexus macrophages have a population derived from hematopoietic precursors but also have a population replenished at a steady state by peripheral bone marrow-derived monocytes (Goldmann, et al., 2016). In the parenchyma, tissue-resident macrophages, termed microglia, arise from yolk sac-derived embryonic precursors which are unrelated - and not replenishment - by bone marrow-derived precursors (Herz, et al., 2017; Korn & Kallies, 2017). The varying antigens that these APCs will be exposed to can also differ as meningeal and choroid plexus macrophages will access the CSF while perivascular macrophages will access the ISF of the parenchyma. While DCs seem absent from the parenchyma, limited and transient numbers of peripherally derived DCs do exist in the meninges, choroid plexus and perivascular spaces and are replenished from migrating bone marrow precursors. Although they reside in the CNS, these monocyte-derived DCs (moDCs) do possess a transcriptional profile similar to splenic DCs, potentially indicative of a similar functional ability (De Laere, Berneman, & Cools, 2018). B cells can also serve as APCs, but are largely absent from the homeostatic CNS. Thus, the meninges, choroid plexus, and perivascular spaces possess macrophages and DCs to provide antigen presentation to T cells for immune surveillance, while the parenchyma – where brain tumors are usually found - possesses a higher level of immune restriction with microglia as the APC.
T Cells in the CNS
During homeostasis, naïve T cells are absent from the entirety of the CNS. However, activated T cells can be found in the meninges, CSF, and perivascular spaces independent of their antigen specificity. Importantly, past the glia limitans within the parenchyma, T cells are notably nonexistent in healthy young individuals, although resident memory T cells in older individuals can be found that are indicative of previous CNS immunity (Korn & Kallies, 2017). While it has been suggested that activated T cells can also access the parenchyma without recognition of cognate antigen in an inflammatory context like EAE (Wekerle, Linington, Lassmann, & Meyermann, 1986), this has not necessarily been observed in other experimental systems such as infectious immunity (Klein & Hunter, 2017). It is generally accepted that to pass the glia limitans, activated T cells in the meninges, choroid plexus or perivascular spaces likely need to be reactivated by cognate antigen presentation by an APC (Engelhardt, et al., 2017).
3. T Cell Infiltration of the Central Nervous System
T Cell Trafficking to the CNS in Infection and Autoimmunity
T cell mediated immune responses are fundamental to both the resolution of infection within the CNS and for the development of pathology in autoimmunity (Engelhardt, et al., 2017; Klein & Hunter, 2017; Korn & Kallies, 2017). However, for both infection and autoimmunity, most immune responses that occur within the CNS are actually initiated in the periphery, requiring the systemic activated T cells to migrate to the brain to find cognate antigen presented by CNS APCs. During CNS infection, the cues for T cell migration are initiated by pathogen-derived pathogen associated molecular patterns. These pathogen associated molecular patterns bind pattern recognition receptors on macrophages, DCs, and other CNS cells resulting in the expression of chemoattractants and adhesion molecules that will then guide activated T cells to roll, adhere, and extravasate from the blood into the meninges, choroid plexus, or perivascular spaces (Klein & Hunter, 2017). During autoimmunity, it has been suggested that activated T cells in EAE access the CNS in a random manner, with retention dependent on recognition of cognate antigen on APCs (Hickey, Hsu, & Kimura, 1991). The expression of proinflammatory cytokines (IFNα, TNFα, IFNγ, GM-CSF) from activated macrophages or T cells in turn induces the expression of the chemokines and adhesion molecules that are necessary to recruit more activated T cells and immune cells to the CNS (C. A. Murphy, Hoek, Wiekowski, Lira, & Sedgwick, 2002; Sporici & Issekutz, 2010).
While there is no known instructive intrinsic program by which T cells are predetermined to travel to the CNS, certain chemokines and chemokine receptors have been shown to be critical for parenchymal infiltration. A recent paper in a rat model of EAE demonstrated that activated T cells specific for myelin basic protein (MBP) had to become licensed in the lung before they could successfully invade the CNS (Odoardi, et al., 2012), suggesting that initial migration is not always random. After lung residency, the MBP-specific T cell blasts upregulated transcriptional expression of chemokine and adhesion receptors and altered their migration from the chemokines CCL19 and CCL21 to CCL5 and CXCL11, chemokines that have also been detected in the CNS of patients with MS (Sporici & Issekutz, 2010). This indicates changes in T cell chemokine receptor usage, as CCL19 and CCL21 are ligands for CCR7, whereas CCL5 binds CCR1, CCR3 and CCR5 and CXCL11 binds CXCR7 and CXCR3 (Karin & Wildbaum, 2015). Importantly, inhibition of CXCR3 signaling impaired the invasion of MBP-specific T cells in this model, suggesting this receptor is critical to parenchymal infiltration from the blood. In mouse models of CNS infection, CXCR3 was required for successful clearance of the pathogens west nile virus and mouse hepatitis virus and CCR5 was necessary for effective immunity against west nile virus (Korn & Kallies, 2017). Interestingly, in a mouse model of EAE, inhibition of CCR5 did not impair disease progression and inhibition of CXCR3 actually induced more significant EAE. This is likely due to the more diffuse T cell infiltration of the parenchyma and decreased immunosuppressive TRegs in this CXCR3-deficient model system (Muller, et al., 2007). Furthermore, EAE involves multiple CD4+ T cell subsets with differing receptor dependencies, so the loss of a single receptor may still permit the entry of a different subset of pathogenic T cells to the CNS (Rothhammer, et al., 2011). In particular, early in EAE, type 17 helper CD4+ T cells (Th17) appear to use CCR6 to bind CCL20 produced by choroid plexus epithelial cells to cross the BCSFB and gain access to the ventricular CSF (Engelhardt, et al., 2016; Reboldi, et al., 2009). Another critical chemokine/receptor pair in EAE is CXCR4 and CXCL12. In mice, pathogenic T cells accumulate in the perivascular spaces and do not access the parenchyma to induce EAE due to an abundance of CXCL12. However, expression of CXCR7 on the endothelial cells, potentially from IL-17 expression by perivascular immune cells, results in the internalization of CXCL12. The reduction in perivascular CXCL12 decreases CXCR4 activation in T cells culminating in T cell entry to the parenchyma (Cruz-Orengo, et al., 2011; McCandless, Wang, Woerner, Harper, & Klein, 2006). Corroborating data shows that CXCL12 is redistributed in active lesions of patients with MS (McCandless, et al., 2008).
In addition to chemokine and chemokine receptor involvement, key integrins are also required for the infiltration of autoimmune T cells past the BBB. Rolling leukocytes with activated chemokine receptors in turn activate integrins, permitting the firm adhesion and extravasation of the cell. In EAE, adoptive transfer of both autoimmune activated type 1 helper CD4+ T cells (Th1) and Th17 CD4+ T cells can induce EAE (Axtell, et al., 2010; Grifka-Walk, Lalor, & Segal, 2013). However, for pathogenic Th1 CD4+ T cells, invasion of the spinal parenchyma was dependent on expression of the α4β1 integrin also known as very late antigen 4. Antibody-mediated blocking of α4β1 integrin did not affect cytokine secretion or antigen recognition, but did affect infiltration of T cells as well as the onset and severity of disease (Baron, Madri, Ruddle, Hashim, & Janeway, 1993; Rothhammer, et al., 2011; Sonar & Lal, 2017). In contrast, autoimmune Th17 CD4+ T cells entered the brain parenchyma and were not dependent on α4β1 integrin, but instead appeared to require the αvβ3 integrin (Du, et al., 2016) as well as the αLβ2 integrin, also known as lymphocyte function-associated antigen 1 (Rothhammer, et al., 2011).
However, in the final step to parenchymal invasion, activated T cells must still pass the glia limitans. In EAE, T cell-derived TNFα and IL-17 induced the secretion of matrix metalloproteinases 2 and 9 (MMP2; MMP9) from both immune and CNS-resident cells which then cleaved the extracellular matrix receptors on the astrocytic foot processes and permit leukocyte invasion of the parenchyma (Agrawal, et al., 2006; Engelhardt, et al., 2016; Sonar & Lal, 2017; Song, et al., 2015). MMP2 and 9 are present in the CSF and lesions of patients with MS (Sonar & Lal, 2017), but the glia limitans is much thicker in humans than rodents and the exact mechanisms of bypassing this barrier in humans are not yet identified. These cumulative data highlight the fact that there are key chemokines, chemokine receptors, adhesion molecules, and enzymes responsible for successful T cell infiltration of the parenchyma, that these molecules can differ depending on T cell subtype, and that many of these molecule’s functions appear to be parallel in mice and humans.
In an effort to reduce unwanted T cell infiltration, impairing the ability of T cells to migrate and extravasate has led to novel therapeutics in MS and other diseases. The antibody natalizumab blocks cellular interaction with α4 integrin and reduces relapse rates as well as the number of new or enlarging lesions in patients with MS (Rudick, Polman, Clifford, Miller, & Steinman, 2013). While natalizumab was temporarily taken off the market due to an increased chance for progressive multifocal leukoencephalopathy from infection of the brain by John Cunningham virus, it was brought back into use with rigorous monitoring of treated patients for viral infection. The αLβ2 integrin was also targeted for blockade, and the antibody efalizumab was given to patients with plaque psoriasis, a disease typified by activated T cell infiltration of the skin. However, rare patients also developed PML, and this drug was removed from the market due to the availability of other effective treatment options (Raab-Westphal, Marshall, & Goodman, 2017). However, these underscore the caveat that preventing T cell infiltration of the CNS can also reduce immunosurveillance leading to opportunistic infection of the brain. The αvβ3 integrin has also been targeted by both antibody as well as the peptide cilengitide (Raab-Westphal, et al., 2017), and while cilengitide reduced the severity of EAE (Du, et al., 2016), these agents are not in use to treat patients with MS. In patients with relapsing-remitting MS, preventing T cell egress from secondary lymphoid organs with the therapeutic fingolimod has reduced the relapse rate (Cohen, et al., 2016). However, long-term studies unfortunately did not show a decrease in progression to disability for patients with primary-progressive MS (Correale, Gaitán, Ysrraelit, & Fiol, 2017). While the cumulative data demonstrate that therapeutic strategies preventing T cell infiltration can ameliorate CNS autoimmunity, careful monitoring is required to ensure this immunosuppression does not result in opportunistic infection.
There is also an emerging role for T cell infiltration in other neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease. While the exact mechanism of T cell function is less clear in these contexts than in MS, T cells have been found in CNS tissue samples of patients with Alzheimer’s or Parkinson’s at sites of disease pathology (Gonzalez & Pacheco, 2014; Sommer, Winner, & Prots, 2017). Parkinson’s is a consequence of dopaminergic neuron loss, and in an animal model of this disease CD4+ T cells were required for neuronal degeneration (Brochard, et al., 2009). Alzheimer’s disease is typified by significant cognitive decline and the formation of amyloid plaques within the CNS. Intriguingly, autoreactive T cells to the β-amyloid peptide are found in patients with this disease (Lanuti, et al., 2012). While it is not clear how these autoreactive T cells may affect clinical severity in patients, in a mouse model of Alzheimer’s the transfer of amyloid-specific Th1 CD4+ T cells were associated with worsening cognitive capacity (Browne, et al., 2013). Significant research remains to clarify how T cells contribute to the pathology of Alzheimer’s and Parkinson’s; however, these data do suggest that T cell infiltration of the CNS may have a broad role in neurodegeneration.
Effector T Cells in Brain Tumors
Brain tumors are generally regarded as immunologically “cold” tumors; the most common malignant brain tumor glioblastoma (GBM) has poor immune infiltration with tumor infiltrating lymphocytes (TILs) absent from roughly half of the examined cases (Reardon, Wucherpfennig, & Chiocca, 2017; Rutledge, et al., 2013). However, spontaneous systemic immune responses to brain tumor antigens have been reported (Johanns, Bowman-Kirigin, Liu, & Dunn, 2017; Schumacher, et al., 2014) and low levels of TIL infiltration can be found in some cases, indicating that peripheral T cells have the capacity to migrate to the CNS and infiltrate tumor. It is known that gliomas can express class I molecules for possible presentation to CD8+ cytotoxic T lymphocytes (CTLs) (Yeung, et al., 2013), and it has been observed that CTLs in human glioma form contacts with tumor cells inducing TCR clustering and granzyme B polarization (Barcia, et al., 2009). There are also associations found between infiltrating lymphocytes and survival in GBM (Johanns, et al., 2017). Patients with primary GBM bearing a higher ratio of CD8+ T cells to immunosuppressive regulatory T cells (TRegs) do possess longer overall survival (Sayour, et al., 2015), suggesting CD8+T cells at the tumor can be cytotoxic and possess an antitumor effect. It is also worth noting that GBMs may be more open to immune infiltration than normal brain tissue due to the well documented disruption of the BBB (Johanns, et al., 2017). However, these tumors are still surrounded by large regions of intact BBB (Sarkaria, et al., 2018), and as individual tumor cells commonly infiltrate the normal brain far from the tumor mass, successful immune-eradication of all tumor cells will still likely require T cells to access the normal parenchyma possessing intact anatomical barriers. Therefore, immunotherapies against brain tumors that leverage the peripheral immune system to generate robust tumor-targeted CD4+ and CD8+ T cells will require that these T cells not only traffic to the CNS, but also bypass the anatomical restrictions of the CNS to be fully efficacious.
T Cell Trafficking to Brain Tumors
In regards to tumor immunotherapy, CXCL10 has emerged as a mediator to enhance T cell infiltration of brain tumors. Importantly, CXCL10 is also a ligand for the CXCR3 receptor that is involved with T cell infiltration of the CNS in EAE and infection as discussed above. Nishimura et al. demonstrated that CXCR3+ Th1 T cells mediated antitumor efficacy against intracranially implanted melanoma in a CXCL10 dependent manner. In this model, DCs transduced with IFNα cDNA were administered intratumorally to induce CXCL10 expression in ovalbumin positive melanoma cells. In addition to intratumoral DC administration, the adoptive transfer of ovalbumin-specific Th1 T cells extended the survival of tumor bearing mice; however, CD8+ T cell infiltration and survival were significantly reduced in the presence of a blocking antibody to CXCL10 (Nishimura, et al., 2006). Furthermore, in the murine glioma model GL261, it was shown that including the clinically approved adjuvant polyinosinicpolycytidylic acid [poly(I:C)] stabilized by lysine and carboxymethylcellulose (poly-ICLC) with a peptide vaccine of glioma associated antigens induced the expression of CXCL10 within the tumor and significantly increased the frequency of TILs (Zhu, et al., 2010). In a model of subcutaneous ovalbumin positive melanoma, inhibition of CXCL10-cleaving dipeptidylpeptidase 4 (DPP4) increased the levels of bioactive intratumoral CXCL10, resulting in increased intratumoral T cell infiltration that when combined with the adoptive transfer of ovalbumin-specific CD8+ T cells enhanced antitumor efficacy (Barreira da Silva, et al., 2015). In humans, CXCL10 also appears to function as an attractant for recruiting effector T cells to the CNS, as patients with CNS autoimmunity from paraneoplastic neurologic disorder possess elevated CXCL10 within the CSF and an increased frequency of CXCR3+ CD4+ and CD8+ T cells within the CSF in comparison to peripheral blood (Roberts, et al., 2015). Though speculative at this point, it is interesting to note that gliomas express both CXCL10 (Maru, et al., 2008) and DPP4 (Stremenova, et al., 2007), suggesting that blocking the DPP4-mediated cleavage of CXCL10 in human glioma may also increase antitumor T cell infiltration.
In another parallel to EAE, expression of α4β1 integrin may also mediate T cell trafficking to the CNS in murine models of brain tumor immunotherapy. In mice bearing intracranial tumor, T cells primed in the tumor-draining cervical LNs strongly upregulated α4β1 integrin, engendering preferential infiltration of these T cells to the CNS (T. Calzascia, et al., 2005). This upregulation of α4β1 integrin and preferential CNS migration was not seen when T cells were primed in the dLNs of mice with identical subcutaneously implanted tumor. Inhibition of α4β1 integrin was also able to block the migration of ovalbumin-specific Th1 CD8+ T cells in mice bearing ovalbumin positive intracranial melanoma (Sasaki, et al., 2007). However, a recent paper demonstrated that in comparison to human brain microvascular endothelial cells, that endothelial cells derived from primary brain tumors lack vascular cell adhesion protein 1 (VCAM1) and possess reduced expression of intracellular adhesion molecule (ICAM1), binding partners of the α4β1 and αLβ2 integrins respectively (Samaha, et al., 2018). T cells were unable to transmigrate in vitro across these endothelial cells derived from primary brain tumors in the presence of the inflammatory cytokine IL-6, whereas migration across brain microvascular endothelial cells under these conditions was permissive. The authors attribute these findings to the loss of ICAM1 and VCAM1 on the tumor-derived endothelium and these data further suggest that another level of immune evasion in brain tumors may stem from a lack of T cell transmigration across endothelial cells in this context. The cumulative data do indicate that expression of CXCL10 within the CNS, and expression of CXCR3 and α4β1 integrin on effector T cells themselves, may be key mechanisms for the successful trafficking and infiltration of the CNS during immunotherapy. However, careful attention must also be given to the expression of corresponding adhesion molecules on endothelium in the brain tumor microenvironment.
4. Antigen Presentation and T Cell Activation in the Central Nervous System
Once a T cell has trafficked to the CNS, it is thought that antigen recognition by the T cell is critical for passing the glia limitans and entering the parenchyma, and that T cell reactivation is likely needed to sustain potent immunity. However, the antigen presenting capacities of APCs within the CNS are not interchangeable. In a mouse model of EAE, Miller et al. demonstrated that in vitro antigen presentation by DCs (CD11b+CD11c+CD45high) induced the highest proliferation of naive CD4+ T cells, that proliferation was reduced when antigen was presented by (CD11b+CD11c−CD45high) macrophages, and that microglia (CD11b+CD11c−CD45low) were unable to activate these T cells (Miller, McMahon, Schreiner, & Bailey, 2007). Importantly, within the parenchyma while there are low levels of class I expression for potential CD8+ T cell interaction; there is almost no class II expression to engage CD4+ T cells (Korn & Kallies, 2017; Massa, Ozato, & McFarlin, 1993). Microglia are the main APC of the parenchyma, they do not express class II homeostatically, and while class II expression can be induced there is currently no evidence that microglia themselves present antigen via class II to CD4+ T cells in vivo (Korn & Kallies, 2017). Furthermore, homeostatic microglia also do not express common costimulatory molecules required for T cell activation, although these can be induced in the context of inflammation (Chastain, Duncan, Rodgers, & Miller, 2011). This is critical as CD4+ T cells that bind class II provide critical signals to reactivated CD8+ T cells that permit maximized immune responses (K. Murphy, et al., 2012). In the context of viral encephalitis, the loss of CNS localized CD4+ T cells resulted in impaired CD8+ T cell function and viral control (Phares, et al., 2012). In EAE there is a tremendous amount of data demonstrating that it is actually the recruitment and infiltration of antigen-presenting conventional DCs and moDCs within the CNS that engender and sustain potent CNS autoimmunity.
CNS Dendritic Cells in Autoimmunity
In MS, the accumulation of DCs in the CNS correlates with the degree of clinical disease (De Laere, et al., 2018). While the overlapping and ever-changing phenotype of DC subpopulations makes it difficult to pinpoint the exact DC subtypes important in EAE, the recruitment of CD11c+ APCs is dependent upon CCR2 expression (Clarkson, et al., 2015), a receptor that binds the chemokines CCL2, CCL7, CCL8, and CCL13. Importantly, CCR2 deficient mice are resistant to the development of EAE (Fife, Huffnagle, Kuziel, & Karpus, 2000; Izikson, Klein, Charo, Weiner, & Luster, 2000; Klein & Hunter, 2017). It has also been shown that it is the expression of the GM-CSF receptor on the CCR2 positive monocyte precursors that licenses these recruited cells to become pathogenic (Croxford, et al., 2015). Recruited DCs can enter the CNS similar to T cells, through the meninges, choroid plexus, and perivascular spaces and subsequently bypass the glia limitans to enter the parenchyma (De Laere, et al., 2018). While CCL2 and CCR2 have been shown to be a critical axis for recruiting DCs in EAE, α4β1 integrin also emerged as a key molecule responsible for permitting DC infiltration of the CNS (Jain, Coisne, Enzmann, Rottapel, & Engelhardt, 2010).
The location of DCs within the CNS is also critical to disease progression. Intracerebral injection of myelin oligodendrocyte glycoprotein (MOG)-loaded DCs in a mouse model of EAE induced the infiltration of MOG-specific CD4+ T cells and a worsening of EAE score whereas systemic injection of DCs did not (Zozulya, et al., 2009). During EAE, CD11c+ conventional DCs and moDCs in meninges and perivascular areas express the T cell chemoattractants CCL5, CXCL9, and CXCL10 and show sustained interactions with pathogenic Th17 CD4+ T cells prior to parenchymal invasion. Furthermore, inducible depletion of these APCs dramatically reduces the infiltration of T cells and the severity of EAE without affecting the peripheral populations of pathogenic T cells (Paterka, et al., 2016); indicating these DCs are required for CNS entry, infiltration and sustained pathogenicity of autoimmune T cells. DCs are also able to induce EAE. DCs derived from a rat with EAE can induce EAE in naïve recipients after intravenous injection, demonstrating the ability of these cells to prime naïve T cells and induce the cascade of events leading to pathogenic T cells that migrate to the CNS and infiltrate the parenchyma (Knight, Mertin, Stackpoole, & Clark, 1983). These data suggest that it is the recruited cDC and moDC populations within the CNS truly drive T cell infiltration and sustained immunity during EAE.
Intratumoral Dendritic Cells in the CNS
The models of autoimmunity within the CNS outlined above suggest that antitumor T cells recruited to the CNS may also require local reactivation with cognate tumor antigen within the meninges, choroid plexus or perivascular space to bypass the glia limitans, infiltrate parenchymal tumor tissue, and mediate sustained antitumor immunity. Additionally, brain tumors are heavily infiltrated with macrophages and microglia, which constitute the largest infiltrating population of immune cells in humans. These macrophages and microglia do not express the key cytokines and costimulatory molecules required for T cell stimulation (Hussain, et al., 2006), furthering the deficit of antigen presentation within the parenchyma. The data from EAE suggest that recruited DCs may be the necessary APC within the CNS to overcome the deficits of CNS antigen presentation and induce the infiltration of activated antitumor T cells and the induction of sustained immunity.
Several groups have examined the role of intratumoral DCs in the immunotherapy of solid tumors both systemically and within the CNS. In a model of murine melanoma, Salmon et al. found that intratumoral CD103+ DCs carried intact antigen to the dLNs and were required for CD8+ T cell cross priming (Salmon, et al., 2016). Furthermore, the administration of intratumoral FMS-like tyrosine kinase 3 ligand (Flt3L) to commit and expand DC precursors and the addition of intratumoral poly(I:C) to mature these DCs dramatically increased the number of infiltrating CD103+ DCs and T cells within the tumor parenchyma. The authors note that inhibition of T cell migration from the dLNs with the drug fingolimod significantly reduced the desired intratumoral T cell infiltration, suggesting that the CD103+ DCs were trafficking from tumor to the dLNs to prime T cells within. As the authors point out though, additional experiments will be required to determine what the direct contribution of these intratumoral DCs at the tumor site might be.
In preclinical models of glioma, Barnard et al. found the antitumor efficacy of an oncolytic herpes simplex virus with intratumoral Flt3L extended the survival of mice bearing intracranial CT-2A gliomas, whereas the parental virus by itself did not (Barnard, et al., 2012). However, in this work the maturation of DCs was not addressed. This may be significant as Salmon et al. found that FLt3L alone induced the accumulation of immature DCs within melanoma, which did not induce CD8+ T cell infiltration and was instead associated with an increased number of immunosuppressive TRegs. Another paper using orthotopic GL261 demonstrated that tumor antigen pulsed DCs injected both subcutaneously and intratumorally extended survival significantly more than either injection alone. Furthermore, intratumorally injected DCs were not detected in the cervical dLNs, implying they mediated their effects at the tumor (Pellegatta, et al., 2010). The authors attribute this to intratumoral DCs increasing the both the frequency of CD8+ TILs and the inflammatory cytokines IFNγ and TNFα within tumor. This is certainly important, but as others have shown that subcutaneously injected DCs can migrate to the CNS of mice with orthotopic brain tumors (Dey, et al., 2015), it is also possible that subcutaneous antigen-loaded DCs could also migrate to the meninges and perivascular spaces and function to reactive tumor-specific T cells to pass the BBB. The exact subtype of DCs within glioma may also critically influence antitumor immunity. Intratumoral plasmacytoid DCs have been shown to both help (Candolfi, et al., 2012) or hinder antitumor efficacy (Dey, et al., 2015), although these results cannot be directly compared as different model systems were used. While tumor antigen loaded DCs can certainly synergize with activated antitumor T cells to enhance immunity (Nishimura, et al., 2006), it is still speculative at this point as to how they function within the CNS.
The work of Calzascia et al. supports the notion that local APCs within brain tumors are critical for the retention of infiltrating CTLs (Thomas Calzascia, et al., 2003). In this study, mice were implanted intracranially with CW3 positive murine gliomas negative for H2-Kd class I and all class II expression. However, CW3-specific CTLs were detected after implantation, indicating that cross-priming of the H2-Kd-binding CW3 antigen to CD8+ T cells must have occurred. While the exact location and phenotype of the APCs were not identified in this work, efficient cross-presentation is usually mediated by DCs. To test the role of local antigen cross-presentation in CTL retention in brain tumors, mice received either implantation of intracranial CW3 positive tumor or implantation with both intracranial CW3 negative tumor and subcutaneous CW3 positive tumor. While both intracranial tumors demonstrated early infiltration by activated CW3-specific T cells, CTLs were only retained in the antigen positive tumor. Therefore, in regards to brain tumor immunotherapy, local DCs may serve several functions: (1) DCs may present tumor antigen to reactivate T cells and enable crossing of the BBB; (2) DCs may enhance the infiltration of activated T cells within the parenchyma and within the tumor; (3) DCs may serve as a source of class II and costimulatory molecules in a parenchymal environment otherwise deficient in these factors; and, (4) DCs may present tumor antigen to T cells within tumor to increase T cell retention. Therefore, immunotherapies that engender activated antitumor T cells should also focus on recruiting and retaining mature DCs within the CNS to enable T cell infiltration sustained antitumor immunity.
5. Conclusions
Many immunotherapies focus on how to engender robust antitumor T cells, and considerable work has gone into defining what types of T cells most successfully mediate tumor lysis (Gattinoni, Klebanoff, & Restifo, 2012). In this review, we have focused on the next step in brain tumor immunotherapy – once you have strong T cells, how do they traffic, infiltrate, and sustain antitumor immunity in the unique environment of the brain? Many parallels exist between the undesirable but potent immunity found in CNS diseases like MS, and the desirable but weak immunity found in brain tumors. We have highlighted key molecules and receptors repetitiously responsible for T cell trafficking to the CNS in infection, autoimmunity, and immunotherapy (Figure 1). While not an exhaustive list, manipulating activated T cells to express CXCR3, CCR6 and the α4β1, αLβ2, αVβ3 integrins, and manipulating CNS tissues to express corresponding chemokines and adhesion molecules (CCL10, CCL20, VCAM-1, ICAM-1, and vitronectin respectively), may increase the migration of T cells to the CNS and the subsequent parenchymal infiltration and potency of the immunotherapy. Furthermore, we suggest that antigen presentation from DCs is likely required locally within the CNS for truly sustained and effective T cell-mediated antitumor immunity. Future studies that tease apart the mechanisms controlling the efferent arm of CNS immunity will enable the next steps in brain tumor immunotherapy - the conversion of systemic antitumor immunity into intratumoral immunity.
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health (NIH) [R01-CA177476 (JHS) and P50-CA190991 (JHS)], and the NIH National Institute of Neurological Disorders and Stroke [R01-NS099463 (JHS), R01-NS085412 (JHS), R01-NS086943 (JHS), and U01-NS090284 (JHS)].
Abbreviations
- APC
Antigen Presenting Cell
- BBB
Blood Brain Barrier
- BCSFB
Blood CSF Barrier
- CNS
Central Nervous System
- CSF
Cerebrospinal Fluid
- CTL
Cytotoxic T lymphocyte
- DC
Dendritic Cell
- DPP4
Dipeptidylpeptidase 4
- dLN
Draining Lymph Node
- EAE
Experimental Autoimmune Encephalomyelitis
- Flt3L
FMS-like tyrosine kinase 3 ligand
- GBM
Glioblastoma
- ISF
Interstitial Fluid
- ICAM1
Intracellular adhesion molecule
- MMP
Matrix metalloproteinases
- moDC
Monocyte-derived Dendritic Cell
- MS
Multiple Sclerosis
- MBP
Myelin basic protein
- MOG
Myelin oligodendrocyte glycoprotein
- poly-ICLC
Polyinosinicpolycytidylic acid [poly(I:C)] stabilized by lysine and carboxymethylcellulose
- TReg
Regulatory T cell
- SAS
Subarachnoid Space
- VCAM1
Vascular cell adhesion protein 1
Footnotes
Conflict of Interest
K.L.C. and L.S.P. report no conflict of interest. J.H.S. has an equity interest in Annias Immunotherapeutics and an equity interest in Istari Oncology.
References
- Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, & Reich DS (2017). Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, & Sorokin LM (2006). Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. The Journal of Experimental Medicine, 203, 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim Y-C, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, & Özgüroğlu M (2017). Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. New England Journal of Medicine, 377, 1919–1929. [DOI] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, & Alitalo K (2015). A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. Journal of Experimental Medicine, 212, 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, & Raman C (2010). T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nature Medicine, 16, 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C Jr., Gomez A, Gallego-Sanchez JM, Perez-Valles A, Castro MG, Lowenstein PR, Barcia C Sr., & Herrero MT (2009). Infiltrating CTLs in human glioblastoma establish immunological synapses with tumorigenic cells. American Journal of Pathology, 175, 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard Z, Wakimoto H, Zaupa C, Patel AP, Klehm J, Martuza RL, Rabkin SD, & Curry WT Jr. (2012). Expression of FMS-like tyrosine kinase 3 ligand by oncolytic herpes simplex virus type I prolongs survival in mice bearing established syngeneic intracranial malignant glioma. Neurosurgery, 71, 741–748; discussion 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JL, Madri JA, Ruddle NH, Hashim G, & Janeway CA Jr. (1993). Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. Journal of Experimental Medicine, 177, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreira da Silva R, Laird ME, Yatim N, Fiette L, Ingersoll MA, & Albert ML (2015). Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nature Immunology, 16, 850–858. [DOI] [PubMed] [Google Scholar]
- Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, Norberg P, Xie W, Herndon JE, 2nd, Healy P, McLendon RE, Friedman AH, Friedman HS, Bigner D, Vlahovic G, Mitchell DA, & Sampson JH (2017). Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clinical Cancer Research, 23, 1898–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, & Sadelain M (2013). CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science Translational Medicine, 5, 177ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay J-M, Duyckaerts C, Flavell RA, Hirsch EC, & Hunot S (2009). Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. The Journal of Clinical Investigation, 119, 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne TC, McQuillan K, McManus RM, O’Reilly J-A, Mills KHG, & Lynch MA (2013). IFN-γ Production by Amyloid β–Specific Th1 Cells Promotes Microglial Activation and Increases Plaque Burden in a Mouse Model of Alzheimer’s Disease. The Journal of Immunology, 190, 2241–2251. [DOI] [PubMed] [Google Scholar]
- Calzascia T, Di Berardino-Besson W, Wilmotte R, Masson F, Tribolet N. d., Dietrich P-Y, & Walker PR (2003). Cutting Edge: Cross-Presentation as a Mechanism for Efficient Recruitment of Tumor-Specific CTL to the Brain. The Journal of Immunology, 171, 2187–2191. [DOI] [PubMed] [Google Scholar]
- Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Ruegg C, Dietrich PY, & Walker PR (2005). Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity, 22, 175–184. [DOI] [PubMed] [Google Scholar]
- Candolfi M, King GD, Yagiz K, Curtin JF, Mineharu Y, Muhammad AK, Foulad D, Kroeger KM, Barnett N, Josien R, Lowenstein PR, & Castro MG (2012). Plasmacytoid dendritic cells in the tumor microenvironment: immune targets for glioma therapeutics. Neoplasia, 14, 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain EM, Duncan DS, Rodgers JM, & Miller SD (2011). The role of antigen presenting cells in multiple sclerosis. Biochimica et Biophysica Acta, 1812, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson BD, Walker A, Harris MG, Rayasam A, Sandor M, & Fabry Z (2015). CCR2-dependent dendritic cell accumulation in the central nervous system during early effector experimental autoimmune encephalomyelitis is essential for effector T cell restimulation in situ and disease progression. Journal of Immunology, 194, 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Khatri B, Barkhof F, Comi G, Hartung HP, Montalban X, Pelletier J, Stites T, Ritter S, von Rosenstiel P, Tomic D, & Kappos L (2016). Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. Journal of Neurology, Neurosurgery and Psychiatry, 87, 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J, Gaitán MI, Ysrraelit MC, & Fiol MP (2017). Progressive multiple sclerosis: from pathogenic mechanisms to treatment. Brain, 140, 527–546. [DOI] [PubMed] [Google Scholar]
- Croxford Andrew L., Lanzinger M, Hartmann Felix J., Schreiner B, Mair F, Pelczar P, Clausen Björn E., Jung S, Greter M, & Becher B (2015). The Cytokine GM-CSF Drives the Inflammatory Signature of CCR2+ Monocytes and Licenses Autoimmunity. Immunity, 43, 502–514. [DOI] [PubMed] [Google Scholar]
- Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, Russell JH, & Klein RS (2011). CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. Journal of Experimental Medicine, 208, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laere M, Berneman ZN, & Cools N (2018). To the Brain and Back: Migratory Paths of Dendritic Cells in Multiple Sclerosis. Journal of Neuropathology and Experimental Neurology, 77, 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey M, Chang AL, Miska J, Wainwright DA, Ahmed AU, Balyasnikova IV, Pytel P, Han Y, Tobias A, Zhang L, Qiao J, & Lesniak MS (2015). Dendritic Cell-Based Vaccines that Utilize Myeloid Rather than Plasmacytoid Cells Offer a Superior Survival Advantage in Malignant Glioma. Journal of Immunology, 195, 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Garg AV, Kosar K, Majumder S, Kugler DG, Mir GH, Maggio M, Henkel M, Lacy-Hulbert A, & McGeachy MJ (2016). Inflammatory Th17 Cells Express Integrin alphavbeta3 for Pathogenic Function. Cell Rep, 16, 1339–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Carare RO, Bechmann I, Flugel A, Laman JD, & Weller RO (2016). Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathologica, 132, 317–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Vajkoczy P, & Weller RO (2017). The movers and shapers in immune privilege of the CNS. Nature Immunology, 18, 123–131. [DOI] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, & Karpus WJ (2000). CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. Journal of Experimental Medicine, 192, 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, & Restifo NP (2012). Paths to stemness: building the ultimate antitumour T cell. Nature Reviews: Cancer, 12, 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordão MJC, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FMV, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, & Prinz M (2016). Origin, fate and dynamics of macrophages at central nervous system interfaces. Nature Immunology, 17, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, & Pacheco R (2014). T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. Journal of Neuroinflammation, 11, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifka-Walk HM, Lalor SJ, & Segal BM (2013). Highly polarized Th17 cells induce EAE via a T-bet independent mechanism. European Journal of Immunology, 43, 2824–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann MD, Ciuleanu T-E, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O’Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, & Paz-Ares L (2018). Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. New England Journal of Medicine, 378, 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Filiano AJ, Smith A, Yogev N, & Kipnis J (2017). Myeloid Cells in the Central Nervous System. Immunity, 46, 943–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, Hsu BL, & Kimura H (1991). T-lymphocyte entry into the central nervous system. Journal of Neuroscience Research, 28, 254–260. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, & Urba WJ (2010). Improved survival with ipilimumab in patients with metastatic melanoma. New England Journal of Medicine, 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SF, Yang D, Suki D, Aldape K, Grimm E, & Heimberger AB (2006). The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncology, 8, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izikson L, Klein RS, Charo IF, Weiner HL, & Luster AD (2000). Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. Journal of Experimental Medicine, 192, 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Coisne C, Enzmann G, Rottapel R, & Engelhardt B (2010). Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. Journal of Immunology, 184, 7196–7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanns TM, Bowman-Kirigin JA, Liu C, & Dunn GP (2017). Targeting Neoantigens in Glioblastoma: An Overview of Cancer Immunogenomics and Translational Implications. Neurosurgery, 64, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A, Dombret H, Foa R, Bassan R, Arslan O, Sanz MA, Bergeron J, Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst HA, Bruggemann M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland C, Zimmerman Z, & Topp MS (2017). Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. New England Journal of Medicine, 376, 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin N, & Wildbaum G (2015). The Role of Chemokines in Shaping the Balance Between CD4(+) T Cell Subsets and Its Therapeutic Implications in Autoimmune and Cancer Diseases. Frontiers in Immunology, 6, 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, & Hunter CA (2017). Protective and Pathological Immunity during Central Nervous System Infections. Immunity, 46, 891–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight SC, Mertin J, Stackpoole A, & Clark J (1983). Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proceedings of the National Academy of Sciences of the United States of America, 80, 6032–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, & Kallies A (2017). T cell responses in the central nervous system. Nature Reviews: Immunology, 17, 179–194. [DOI] [PubMed] [Google Scholar]
- Lanuti P, Ciccocioppo F, Bonanni L, Marchisio M, Lachmann R, Tabet N, Pierdomenico L, Santavenere E, Catinella V, Iacone A, Thomas A, Gambi D, Miscia S, Onofrj M, & Kern F (2012). Amyloid-specific T-cells differentiate Alzheimer's disease from Lewy body dementia. Neurobiology of Aging, 33, 2599–2611. [DOI] [PubMed] [Google Scholar]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, & Diaz LA Jr. (2015). PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New England Journal of Medicine, 372, 2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, & Kipnis J (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru SV, Holloway KA, Flynn G, Lancashire CL, Loughlin AJ, Male DK, & Romero IA (2008). Chemokine production and chemokine receptor expression by human glioma cells: role of CXCL10 in tumour cell proliferation. Journal of Neuroimmunology, 199, 35–45. [DOI] [PubMed] [Google Scholar]
- Massa PT, Ozato K, & McFarlin DE (1993). Cell type-specific regulation of major histocompatibility complex (MHC) class I gene expression in astrocytes, oligodendrocytes, and neurons. Glia, 8, 201–207. [DOI] [PubMed] [Google Scholar]
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, & Grupp SA (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia. New England Journal of Medicine, 371, 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, & Klein RS (2008). Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. American Journal of Pathology, 172, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless EE, Wang Q, Woerner BM, Harper JM, & Klein RS (2006). CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. Journal of Immunology, 177, 8053–8064. [DOI] [PubMed] [Google Scholar]
- Miller SD, McMahon EJ, Schreiner B, & Bailey SL (2007). Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Annals of the New York Academy of Sciences, 1103, 179–191. [DOI] [PubMed] [Google Scholar]
- Muller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, Dreykluft A, Lu B, Gerard C, King NJ, & Campbell IL (2007). CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. Journal of Immunology, 179, 2774–2786. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Hoek RM, Wiekowski MT, Lira SA, & Sedgwick JD (2002). Interactions between hemopoietically derived TNF and central nervous system-resident glial chemokines underlie initiation of autoimmune inflammation in the brain. Journal of Immunology, 169, 7054–7062. [DOI] [PubMed] [Google Scholar]
- Murphy K, Travers P, Walport M, & Janeway C (2012). Janeway’s immunobiology (8th ed.). New York: Garland Science. [Google Scholar]
- Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, & Cheever MA (2016). PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. New England Journal of Medicine, 374, 2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura F, Dusak JE, Eguchi J, Zhu X, Gambotto A, Storkus WJ, & Okada H (2006). Adoptive transfer of type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Research, 66, 4478–4487. [DOI] [PubMed] [Google Scholar]
- Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schlager C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WE, Lottaz C, Nosov M, Brinkmann V, Spang R, Lehrach H, Vingron M, Wekerle H, Flugel-Koch C, & Flugel A (2012). T cells become licensed in the lung to enter the central nervous system. Nature, 488, 675–679. [DOI] [PubMed] [Google Scholar]
- Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, & Lieberman FS (2011). Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. Journal of Clinical Oncology, 29, 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T, Voloschin A, Ramkissoon SH, Ligon KL, Latek R, Zwirtes R, Strauss L, Paliwal P, Harbison CT, Reardon DA, & Sampson JH (2017). Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro-Oncology, nox208–nox208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterka M, Siffrin V, Voss JO, Werr J, Hoppmann N, Gollan R, Belikan P, Bruttger J, Birkenstock J, Jung S, Esplugues E, Yogev N, Flavell RA, Bopp T, & Zipp F (2016). Gatekeeper role of brain antigen-presenting CD11c+ cells in neuroinflammation. EMBO Journal, 35, 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegatta S, Poliani PL, Stucchi E, Corno D, Colombo CA, Orzan F, Ravanini M, & Finocchiaro G (2010). Intra-tumoral dendritic cells increase efficacy of peripheral vaccination by modulation of glioma microenvironment. Neuro-Oncology, 12, 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, & Rosenberg SA (2003). Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proceedings of the National Academy of Sciences of the United States of America, 100, 8372–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phares TW, Stohlman SA, Hwang M, Min B, Hinton DR, & Bergmann CC (2012). CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis. Journal of Virology, 86, 2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab-Westphal S, Marshall JF, & Goodman SL (2017). Integrins as Therapeutic Targets: Successes and Cancers. Cancers, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Wucherpfennig K, & Chiocca EA (2017). Immunotherapy for glioblastoma: on the sidelines or in the game? Discovery Medicine, 24, 201–208. [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, & Sallusto F (2009). C-C chemokine receptor 6–regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nature Immunology, 10, 514. [DOI] [PubMed] [Google Scholar]
- Roberts WK, Blachere NE, Frank MO, Dousmanis A, Ransohoff RM, & Darnell RB (2015). A destructive feedback loop mediated by CXCL10 in central nervous system inflammatory disease. Annals of Neurology, 78, 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Heink S, Petermann F, Srivastava R, Claussen MC, Hemmer B, & Korn T (2011). Th17 lymphocytes traffic to the central nervous system independently of alpha4 integrin expression during EAE. Journal of Experimental Medicine, 208, 2465–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick R, Polman C, Clifford D, Miller D, & Steinman L (2013). Natalizumab: bench to bedside and beyond. JAMA Neurol, 70, 172–182. [DOI] [PubMed] [Google Scholar]
- Rutledge WC, Kong J, Gao J, Gutman DA, Cooper LA, Appin C, Park Y, Scarpace L, Mikkelsen T, Cohen ML, Aldape KD, McLendon RE, Lehman NL, Miller CR, Schniederjan MJ, Brennan CW, Saltz JH, Moreno CS, & Brat DJ (2013). Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clinical Cancer Research, 19, 4951–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova-Acebes M, Khudoynazarova M, Agudo J, Tung N, Chakarov S, Rivera C, Hogstad B, Bosenberg M, Hashimoto D, Gnjatic S, Bhardwaj N, Palucka AK, Brown BD, Brody J, Ginhoux F, & Merad M (2016). Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity, 44, 924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha H, Pignata A, Fousek K, Ren J, Lam FW, Stossi F, Dubrulle J, Salsman VS, Krishnan S, Hong SH, Baker ML, Shree A, Gad AZ, Shum T, Fukumura D, Byrd TT, Mukherjee M, Marrelli SP, Orange JS, Joseph SK, Sorensen PH, Taylor MD, Hegde M, Mamonkin M, Jain RK, El-Naggar S, & Ahmed N (2018). A homing system targets therapeutic T cells to brain cancer. Nature. [DOI] [PMC free article] [PubMed] [Retracted]
- Sarkaria JN, Hu LS, Parney IF, Pafundi DH, Brinkmann DH, Laack NN, Giannini C, Burns TC, Kizilbash SH, Laramy JK, Swanson KR, Kaufmann TJ, Brown PD, Agar NYR, Galanis E, Buckner JC, & Elmquist WF (2018). Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology, 20, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Zhu X, Vasquez C, Nishimura F, Dusak JE, Huang J, Fujita M, Wesa A, Potter DM, Walker PR, Storkus WJ, & Okada H (2007). Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Research, 67, 6451–6458. [DOI] [PubMed] [Google Scholar]
- Sayour EJ, McLendon P, McLendon R, De Leon G, Reynolds R, Kresak J, Sampson JH, & Mitchell DA (2015). Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunology, Immunotherapy, 64, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, Keil M, Balss J, Rauschenbach K, Grabowska AK, Vogler I, Diekmann J, Trautwein N, Eichmuller SB, Okun J, Stevanovic S, Riemer AB, Sahin U, Friese MA, Beckhove P, von Deimling A, Wick W, & Platten M (2014). A vaccine targeting mutant IDH1 induces antitumour immunity. Nature, 512, 324–327. [DOI] [PubMed] [Google Scholar]
- Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, & June CH (2017). Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. New England Journal of Medicine, 377, 2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, & Hwu P (2011). gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. New England Journal of Medicine, 364, 2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsdottir H, & Butcher EC (2008). Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nature Immunology, 9, 981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Winner B, & Prots I (2017). The Trojan horse - neuroinflammatory impact of T cells in neurodegenerative diseases. Molecular Neurodegeneration, 12, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonar SA, & Lal G (2017). Differentiation and Transmigration of CD4 T Cells in Neuroinflammation and Autoimmunity. Frontiers in Immunology, 8, 1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, Faber C, Schafers M, Korner H, Opdenakker G, Hallmann R, & Sorokin L (2015). Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep, 10, 1040–1054. [DOI] [PubMed] [Google Scholar]
- Sporici R, & Issekutz TB (2010). CXCR3 blockade inhibits T-cell migration into the CNS during EAE and prevents development of adoptively transferred, but not actively induced, disease. European Journal of Immunology, 40, 2751–2761. [DOI] [PubMed] [Google Scholar]
- Stremenova J, Krepela E, Mares V, Trim J, Dbaly V, Marek J, Vanickova Z, Lisa V, Yea C, & Sedo A (2007). Expression and enzymatic activity of dipeptidyl peptidase-IV in human astrocytic tumours are associated with tumour grade. International Journal of Oncology, 31, 785–792. [PubMed] [Google Scholar]
- Wekerle H, Linington C, Lassmann H, & Meyermann R (1986). Cellular immune reactivity within the CNS. Trends in Neurosciences, 9, 271–277. [Google Scholar]
- Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, Ashby L, Mechtler L, Goldlust SA, Iwamoto F, Drappatz J, O’Rourke DM, Wong M, Hamilton MG, Finocchiaro G, Perry J, Wick W, Green J, He Y, Turner CD, Yellin MJ, Keler T, Davis TA, Stupp R, Sampson JH, & investigators, A. I. t. (2017). Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncology, 18, 1373–1385. [DOI] [PubMed] [Google Scholar]
- Yeung JT, Hamilton RL, Ohnishi K, Ikeura M, Potter DM, Nikiforova MN, Ferrone S, Jakacki RI, Pollack IF, & Okada H (2013). LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clinical Cancer Research, 19, 1816–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Fallert-Junecko BA, Fujita M, Ueda R, Kohanbash G, Kastenhuber ER, McDonald HA, Liu Y, Kalinski P, Reinhart TA, Salazar AM, & Okada H (2010). Poly-ICLC promotes the infiltration of effector T cells into intracranial gliomas via induction of CXCL10 in IFN-alpha and IFN-gamma dependent manners. Cancer Immunology, Immunotherapy, 59, 1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zozulya AL, Ortler S, Lee J, Weidenfeller C, Sandor M, Wiendl H, & Fabry Z (2009). Intracerebral dendritic cells critically modulate encephalitogenic versus regulatory immune responses in the CNS. Journal of Neuroscience, 29, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]