Abstract
Newborn Holstein (n = 48) and Jersey (n = 30) calves were studied to compare absorption of immunoglobulin G (IgG) from maternal colostrum (n = 39) or colostrum replacement containing an Ig concentrate derived from bovine serum (n = 39). Calves were also fed milk replacer with (n = 38) or without (n = 40) animal plasma (20% of crude protein) to 29 d of age to determine effect of plasma protein on IgG status, health, and growth. Calves were fed maternal colostrum or colostrum replacement at 1.5 and 13.5 h of age and provided a total of 250 or 249 and 180 or 186 g of IgG for Holsteins and Jerseys fed maternal colostrum or colostrum replacement, respectively. Milk replacer (12.5% DM) was fed at 31% of metabolic birth weight (2 feedings/d). Plasma was sampled at 0 h, 24 h, and weekly to determine IgG by turbidimetric immunoassay. At blood collection, calves were weighed and measured to determine growth. Health scores, fecal scores, and grain intake were measured daily. Plasma IgG at 24 h did not differ between calves fed maternal colostrum (13.78 ± 0.39 g/L) and colostrum replacement (13.96 ± 0.38 g/L). Average daily gain, withers height, hip height, body length, heart girth, health, and incidence of diarrhea were not different between treatment groups. Calves fed maternal colostrum used feed more efficiently than calves fed colostrum replacement. Plasma IgG and performance were not affected by the addition of animal plasma to milk replacer. The colostrum replacement used in this study provided adequate IgG for newborn calves. Animal plasma was an acceptable source of protein but did not enhance growth or immunity under the conditions of this study.
Key words: IgG, colostrum replacement, animal plasma, calf milk replacer
Abbreviations key: AEA, apparent efficiency of absorption; CR, colostrum replacer; FPT, failure of passive transfer; MC, maternal colostrum; PUN, plasma urea nitrogen; CMR, calf milk replacer
Introduction
Timely, adequate colostrum intake is the most important management factor affecting morbidity and mortality in preweaned calves (Wells et al., 1996). Absorption of intact immunoglobulins begins to decline immediately after birth and ends completely by about 24 h (Stott et al., 1979). Inadequate absorption of colostral immunoglobulins, or failure of passive transfer (FPT), is common and increases morbidity and mortality risk (NAHMS, 1993). Many diseases, including Johne's, can be passed to calves via maternal colostrum (MC), and producers are advised to discard or pasteurize MC from infected cows (Stabel, 2001). This practice can reduce the supply of available MC and may force producers to use colostrum containing less IgG.
Colostrum supplement products, which typically contain 25 to 45 g/L IgG, can be added to MC to increase IgG mass (Davenport et al., 2000). Supplements made from dried colostrum (Zaremba et al., 1993) or dried whey (Garry et al., 1996; Mee et al., 1996) did not improve absorption of IgG over MC. Supplements based on purified blood proteins have improved IgG absorption in some reports (Todd et al., 1993; Arthington et al., 2000b), but not in other reports (Arthington et al., 2000a; Quigley et al., 2000a). Supplementation does not eliminate MC as a disease transmission vehicle; however, replacement of MC may be able to prevent disease. Quigley et al. (1998b, 2002) reported colostrum replacer (CR) products provided adequate IgG to calves. Any CR must contain sufficient absorbable IgG to effectively mimic colostrum in establishing passive immunity. Although immunoglobulins contained in CR may not provide protection to farm-specific pathogens, CR have potential advantages in consistent composition and quality. An effective CR would provide a viable alternative for producers facing limited or contaminated colostrum supplies.
Blood proteins also have potential as milk replacer protein sources (Morrill et al., 1995; Quigley and Bernard, 1996). Spray-dried plasma protein is soluble, has an AA profile similar to skim milk, and was used at 95% of casein utilization in rats (Duarte et al., 1999). Plasma proteins may provide immunoglobulins that could exert local protection in the intestine and improve animal health and performance (Drew, 1994). Since IgG absorbed from MC is largely returned to the gastrointestinal tract (Besser et al., 1988), it is possible plasma IgG could reduce clearance of IgG from blood.
The objectives of the present study were to compare 24-h IgG concentrations in calves fed pooled MC or CR (Acquire, APC, Inc., Ankeny, IA) and to compare plasma IgG status and apparent IgG clearance rate in calves fed calf milk replacer (CMR) with or without animal plasma.
Materials and Methods
Animal and Feeding Practices
Calves from the Virginia Tech Dairy Cattle Center were blocked by gender and breed and were assigned to each block independently. Seventy-eight calves born between June 5 and December 3, 2000, were assigned to one of 4 treatments: CR + milk replacer with animal plasma contributing 20% of CP, CR + milk replacer without animal plasma, MC + milk replacer with animal plasma, MC + milk replacer without animal plasma (control). Table 1 describes treatment, breed, and gender groups.
Table 1.
Number of calves in each treatment, breed, and gender group.
| Milk replacer |
Total | ||
|---|---|---|---|
| No plasma | Plasma | ||
| IgG source | |||
| Maternal colostrum | 20 | 19 | 39 |
| Colostrum replacement | 20 | 19 | 39 |
| Total | 40 | 38 | 78 |
| Breed-gender | Female | Male | Total |
| Holstein | 23 | 25 | 48 |
| Jersey | 11 | 19 | 30 |
| Total | 34 | 44 | 78 |
Calvings were supervised, and any calf whose birth was not observed was not included. Within 15 min of birth, calves were moved to a calf facility, identified, and weighed. They were vaccinated for bovine rhinotracheitis, parainfluenza 3 (Pfizer, Exton, PA), rotavirus, and corona virus (Pfizer) injected with vitamins A, D, and E (Phoenix Pharmaceuticals, St. Joseph, MO) and selenium (Schering Plough, Kenilworth, NJ), and navels were dipped with 7% iodine tincture. At birth, 24 h, and weekly for 4 wk, BW, withers height, hip height, body length (point of shoulder to caudal projection of pin bone), and heart girth were measured. Calves were housed individually in 1.22- × 1.83-m stalls.
Calves were fed CR or pooled MC within 1.5 h of birth and 12 h later. All calves were fed by nipple bottle, although an esophageal feeder was used when calves refused to suckle. Amounts of MC and CR were adjusted to provide IgG equal to a standard dose of CR (9.83 g of IgG/kg of metabolic BW). Using average BW of calves at the Virginia Tech Dairy Cattle Center, it was determined that Jerseys should be fed 75% of standard dose.
Maternal colostrum was collected, pooled, and refrozen into aliquots prior to the start of the study. First and second milking MC that registered green on a colostrometer (Biogenics, Mapleton, OR) was pooled. Samples of MC were collected for IgG analysis prior to freezing. Amounts of MC fed were adjusted based on IgG concentration, as determined by radial immunodiffusion (Triple J Farms, Redmond, WA). Holstein and Jersey calves fed MC received 1.42 and 1.06 L at each feeding. Holstein and Jersey calves fed CR received 454 and 340.5 g of powder dissolved in 1.89 and 1.42 L of water (38°C). The CR was manufactured as described by Quigley et al. (2001). Nutrient composition of feeds is in Table 2 .
Table 2.
Nutrient composition of feeds on a DM basis.
| Item | Colostrum1 | Colostrum replacement | Milk replacer |
Starter grain | |
|---|---|---|---|---|---|
| No plasma | Plasma | ||||
| DM (%) | 24.0 | 96.02 | 96.81 | 97.3 | 88.88 |
| CP (%) | 58.3 | 44.35 | 22.02 | 22.81 | 20.44 |
| Milk protein (% of CP) | … | … | 100 | 80 | … |
| Plasma protein (% of CP) | … | … | 0 | 20 | … |
| Fat (%) | 27.9 | 3.44 | 21.27 | 21.08 | 4.67 |
| Ash (%) | 4.63 | 4.69 | 7.46 | 8.59 | 6.09 |
| ADF (%) | … | … | … | … | 4.74 |
| Ca (mg/100 g) | 1083.3 | 373 | 1090 | 1510 | 1020 |
| P (mg/100 g) | … | 619 | 983 | 1240 | 709 |
| K (mg/100 g) | 583.3 | 142 | 1210 | 1170 | 999 |
| Mg (mg/100 g) | 166.7 | 80.6 | 130 | 178 | 213 |
Colostrum nutrient composition was not analyzed; average values (Foley and Otterby, 1978) are reported for comparison only.
Calves were fed CMR with or without animal plasma from nipple pails twice daily at 0800 and 1630 h beginning 24 h after birth. The CMR was reconstituted to 12.5% DM and fed at 31% of metabolic birth weight for 29 d. To determine feeding rate, energy requirement of calves gaining 227 g/d, and energy content of average milk replacer were calculated (Davis and Drackley, 1998).
Beginning on d 1, calves were offered 250 g/d starter grain containing decoquinate (Deccox, Alpharma, Fort Lee, NJ). Starter intake was measured daily by weighing refusals. Starter was increased by 50 g if daily orts were less than 25 g. Water was available free choice beginning 1 to 2 d after birth.
Sampling and Laboratory Methods
Prefeeding blood samples were drawn at 1 h (0 d), 24 h (1 d), 8 d, 15 d, 22 d, and 29 d. Packed cell volume was measured at 1 and 24 h to estimate changes in plasma volume. Blood samples were collected by jugular venipuncture into evacuated glass tubes containing potassium EDTA. Blood was immediately centrifuged at 1745 × g (15 m at 4°C) and stored at −20°C. At the end of the trial, one plasma aliquot was sent to APC, Inc. for IgG (Etzel et al., 1997) and total protein analyses (Smith et al., 1985). Remaining plasma aliquot was analyzed at Virginia Tech for urea N (Chaney and Marbach, 1962; Weatherburn, 1967).
Calves were monitored during each feeding, and health problems and treatments were recorded. Fecal scores were evaluated once daily using a 3-point scale (0 = firm, 1 = loose, and 2 = watery). Clinical health was scored based on ability to stand and presence or absence of suckle reflex. The score had a maximum of 2 points, 1 for each category (does not stand = 0, stands = 1 plus suckle reflex absent = 0, suckle reflex present = 1).
Statistical Analysis
Data were analyzed as a split-plot design with repeated measures on the subplot (time) using SAS mixed procedure (Littell et al., 1996). The model was:
where:
- S
source of IgG, colostrum or replacement (i = 1, 2); fixed effect
- M
milk replacer, no plasma or plasma (j = 1, 2); fixed effect
- B
breed, Holstein or Jersey (k = 1, 2); fixed effect
- G
gender, female or male (l = 1, 2); fixed effect
- R
replication, colostrum pool (m = 1, 2); random effect
- A
calf (n = 1...20); (total of 78 calves) random effect
- T
time, d or wk, depending on variable tested, if d (o = 1, 8, 15, 22, 29), if wk (o = 1...4), fixed effect
- ɛ
residual
Replication and calf were random; all other effects were fixed. Tests of effect of IgG source, milk replacer, breed, gender, and replication were conducted using calf within the interaction of source by replacer, breed, gender, and replication as the error term. Significance was declared at P < 0.05. When interactions of main effects were significant, the slice option was used to clarify which effects were significant. When the variable tested was an overall change, effect of time (day or week) was removed from the model. Health and fecal scores were tested by performing χ2 tests in SAS using the frequency procedure.
Results and Discussion
Plasma IgG at 24 h
Differences in birth BW or age at first feeding were not detected (Table 3 ). Plasma IgG was undetectable in all 0-h samples, which verified calves did not nurse their dams. Plasma IgG increased to 13.76 ± 0.66 g/L in MC-fed calves and 13.84 ± 0.65 g/L in CR-fed calves at 24 h. Differences were not detected between treatments in 24-h IgG or apparent efficiency of absorption (AEA). The AEA was 19.2 ± 0.8 and 20.3 ± 0.8% for MC- and CR-fed calves (Table 4 ).
Table 3.
Least square means of calf birth weight, age at feedings, and Ig intake.
| Variable | Least square means |
SE | |||
|---|---|---|---|---|---|
| Treatment1 | |||||
| CN | CPL | RN | RPL | ||
| Calves, initial number | 20 | 19 | 20 | 19 | … |
| Calves, final number | 19 | 16 | 20 | 19 | … |
| Birth weight (kg) | 34.0 | 35.5 | 35.9 | 36.4 | 2.0 |
| Age at feeding (h) | |||||
| First feeding | 1.2 | 1.1 | 1.0 | 0.9 | 0.2 |
| Second feeding | 13.2 | 13.1 | 13.0 | 12.9 | 0.2 |
| IgG intake (g) | |||||
| Holsteins | 250.6 | 250.6 | 250.0 | 250.0 | … |
| Jerseys | 182.5 | 180.2 | 180.0 | 180.0 | … |
CN = Pooled maternal colostrum at birth, no plasma in milk replacer; CPL = Pooled maternal colostrum at birth, plasma in milk replacer; RN = colostrum replacement at birth, no plasma in milk replacer; RPL = colostrum replacement at birth, plasma in milk replacer.
Table 4.
Least square means of plasma protein, IgG, and urea nitrogen (PUN) in calves fed pooled maternal colostrum (C) or replacement (R) and milk replacer with (PL) or without (N) animal plasma at birth and 24 h.
| Variable | Least square means |
SE | Differences of means1 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Colostrum |
Replacement |
N minus PL |
MC minus R |
||||||
| N | PL | N | PL | MC | R | N | P | ||
| 0-h IgG (g/L) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 24-h IgG (g/L) | 13.74 | 13.77 | 14.05 | 13.63 | 0.74 | −0.03 | 0.42 | −0.31 | 0.15 |
| AEA of IgG2 (%) | 18.73 | 19.65 | 20.28 | 20.35 | 0.89 | −0.92 | −0.06 | −1.55 | −0.69 |
| 0-h Total protein (g/dl) | 4.312 | 4.323 | 4.107 | 4.282 | 0.105 | −0.012 | −0.175 | 0.205 | 0.042 |
| 24-h Total protein (g/dl) | 6.063 | 5.946 | 5.416 | 5.505 | 0.128 | 0.116 | −0.088 | 0.646** | 0.442** |
| 0-h PUN (mg/dl) | 13.858 | 14.569 | 15.329 | 15.776 | 0.842 | −0.711 | −0.446 | −1.471 | −1.206 |
| 24-h PUN (mg/dl) | 12.291 | 12.900 | 10.214 | 9.840 | 0.756 | −0.609 | 0.374 | 2.077* | 3.060** |
Significance determined by slicing interaction of IgG source and milk replacer.
Apparent efficiency of absorption: (plasma IgG at 24 h × BW at birth, kg × 0.091)/IgG intake (g).
P < 0.05.
P < 0.01.
Calves fed CR had greater plasma IgG concentrations at 24 h than previously reported (5.0 to 8.3 g/L) for bovine serum-based products (Arthington et al., 2000a, 2000b; Quigley et al., 2000a). In previous research, CR provided 90 g of IgG. In the present experiment, calves received 250 (Holsteins) or 180 (Jerseys) g of IgG. The IgG concentrations were similar to those reported in calves fed the same dose of this CR (Quigley et al., 2002). The AEA was in the low end of the range of previously reported values for MC (30 to 35%) and CR (30%) (Quigley et al., 2002), but similar to a recent experiment conducted at Virginia Tech using an earlier formulation of the same product (Quigley et al., 2000a). There was concern that volume of fluid may have been confounded with treatment as calves fed CR received a greater volume of liquid than those receiving MC. However, the mass of IgG fed was similar regardless of treatment, and Stott and Fellah (1983) found that mass of IgG fed was more important than concentration of IgG fed.
Jersey calves had higher 24-h IgG concentrations than Holsteins (16.47 ± 0.71 and 11.12 ± 0.60 g/L), and absorbed IgG with 21.9 ± 0.9% efficiency compared with 17.0 ± 0.7% for Holsteins (Table 5 ). Jersey calves maintained greater plasma IgG concentrations than Holsteins from d 1 through d 15.
Table 5.
Least square means of plasma protein, IgG, and urea nitrogen (PUN) in Holstein (H) and Jersey (J), male (M) and female (F) calves at birth and 24 h of age.
| Variable | Least square means |
SE | Differences of means1 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Holstein |
Jersey |
F minus M |
H minus J |
||||||
| F | M | F | M | H | J | F | M | ||
| 0-h IgG (g/L) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 24-h IgG (g/L) | 9.90 | 12.35 | 16.75 | 16.19 | 0.92 | −2.44* | 0.56 | −6.85** | −3.84** |
| AEA of IgG2 (%) | 15.48 | 19.74 | 21.54 | 22.25 | 1.10 | −4.27** | −0.71 | −6.07** | −2.51† |
| 0-h Total protein (g/dL) | 4.284 | 4.440 | 4.196 | 4.104 | 0.130 | −0.155 | 0.091 | 0.089 | 0.335* |
| 24-h Total protein (g/dL) | 5.203 | 5.723 | 6.032 | 5.972 | 0.160 | −0.520** | 0.060 | −0.829** | −0.249† |
| 0-h PUN (mg/dL) | 13.949 | 14.388 | 16.008 | 15.187 | 1.069 | −0.439 | 0.821 | −2.059 | −0.799 |
| 24-h PUN (mg/dL) | 9.902 | 10.408 | 12.526 | 12.408 | 0.940 | −0.506 | 0.118 | −2.624* | −2.000* |
Significance determined by slicing interaction of breed and gender.
Apparent efficiency of absorption: (plasma IgG at 24 h × BW at birth, kg × 0.091)/IgG intake (g).
P < 0.10.
P < 0.05.
P < 0.01.
No differences in IgG concentration or AEA were found between male and female Jerseys, but Holstein males absorbed IgG more efficiently and attained higher IgG concentrations at 24 h than females (Table 5). Breed differences in IgG absorption have been reported previously and may result from differences in body size and plasma volume (Quigley et al., 1998a).
Plasma IgG concentrations less than 10 g/L were observed in 15 calves (19.2%), and 24-h IgG concentrations ranged from 0.01 to 21.09 g/L (Table 6 ). Percentage of calves with FPT was within the range of normally observed values but is high given tightly controlled experimental conditions. Average age of calves at first feeding was 1.05 h, and only 5 calves were more than 2-h-old at first feeding. All calves received MC or CR containing greater than 66 g/L IgG. Calves were fed following best management practices, yet still experienced a high rate of FPT. Reasons for the high frequency of FPT in female Holstein calves were not apparent, as all calves were treated similarly during the trial.
Table 6.
Failure of passive transfer incidence (percent) as determined by plasma IgG concentration at 24 h.
| n | % Calves <10 g/L | Range of IgG (g/L) |
||
|---|---|---|---|---|
| min | max | |||
| Overall | 78 | 19.2 | 0.01 | 21.09 |
| Treatment | ||||
| Colostrum | 39 | 20.5 | 6.80 | 19.95 |
| Replacement | 39 | 17.9 | 0.01 | 21.09 |
| Breed | ||||
| Jersey | 30 | 0.0 | 10.80 | 21.09 |
| Holstein | 48 | 31.3 | 0.01 | 17.85 |
| Gender | ||||
| Male | 44 | 11.4 | 7.95 | 20.39 |
| Female | 34 | 29.4 | 0.01 | 21.09 |
Apparent Clearance of IgG
Concentrations of IgG declined in a quadratic manner from d 1 to 29. IgG concentrations in calves fed CR were higher than in MC-fed calves on d 8 and 15 (Figure 1 ). Differences in IgG concentrations between calves fed plasma in CMR and calves fed control CMR were not detected. To estimate clearance rate, change in IgG from day × to day y was divided by IgG on d x (e.g., [(d 1 − d 8)/d 1] × 100). Apparent clearance rates were calculated for each 7-d period (Figure 2 ). Calves fed MC had an apparent clearance rate of 3.5% per day in wk 1, which agrees well with estimates determined by clearance of 125I-labeled IgG1 (Besser et al., 1988). Rate of clearance in wk 2 (1.98%) was also similar to calculations of Besser et al. (1988). Based on these estimates, calves either cleared less IgG or produced more IgG as age increased. Since endogenous IgG production can begin at 3 d (Devery et al., 1979), it is likely that endogenous production increased with age, resulting in an apparent decrease in clearance rate in wk 3 and a gain in IgG over wk 4.
Figure 1.
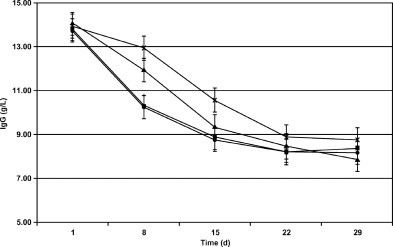
Plasma IgG concentrations (g/L) on d 1 to d 29 in calves fed maternal colostrum at birth and milk replacer without animal plasma for 1 mo (●), maternal colostrum at birth and milk replacer with animal plasma for 1 mo (■), colostrum replacement at birth and milk replacer without animal plasma for 1 mo (▴), or colostrum replacement at birth and milk replacer with animal plasma for 1 mo (×). Significant source × time interaction; differences detected d 8 and 15. Significant linear and quadratic contrasts detected.
Figure 2.
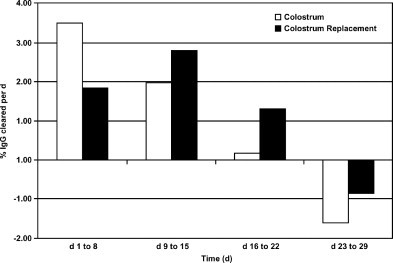
Calculated estimates of apparent plasma IgG clearance rates (% of total plasma IgG cleared daily) from d 1 to 29 of age in calves fed colostrum (open bars) or colostrum replacement (solid bars).
These estimates are probably appropriate for calves fed MC, but may not apply to calves fed CR. Ratio of IgG1 to IgG2 in serum is approximately 1:1 (Mayer et al., 2002), but in colostrum it is skewed toward IgG1 at 95:5 (Butler, 1983). In calves fed serum-derived CR, half of the passively acquired IgG was likely IgG2, and clearance of IgG acquired from CR may follow a different pattern. Calves fed CR appeared to clear IgG less rapidly. This may be explained by Mayer et al. (2002), who found that the Fc receptor in the neonate is specific for IgG1 and is responsible for resecretion of circulating IgG back into the lumen of the intestine. It would follow that if there were more IgG1 in the blood of calves receiving MC it would be cleared from the blood more rapidly, resulting in lower IgG levels in the calf with increasing age. Alternatively, increased endogenous IgG production may have occurred. Previous work has shown that hypogammaglobulinemic calves begin synthesizing IgG earlier than calves with adequate IgG (Logan et al., 1974).
Growth, Feed Efficiency, and Health
Body weights were not different between treatment groups at birth (Table 3) and from d 8 through 29. Body weight on d 1 was greater in CR calves; however, CR calves received more liquid in the first 24 h than MC calves due to the high IgG content of MC. Based on the assumption that added weight was fluid, not true body mass increase; d 1 weights were replaced with d 0 weights for contrast tests and calculations of average daily gain. Body weight increased quadratically with age.
From d 0 to 8, calves fed CR tended to gain less BW than calves fed MC (P < 0.06; −120.7 ± 7.8 and 51.6 ± 7.8 g/d). By d 15, differences between treatments were undetectable. Overall BW gain, average daily gain, withers height, hip height, body length, and heart girth did not differ by treatment. Calves fed CMR containing animal plasma grew at the same rate as calves fed control CMR. Other researchers feeding animal plasma reported similar results; however, gains in the current experiment were lower than those reported previously (Morrill et al., 1995; Quigley and Bernard, 1996). Growth measurements by breed and gender are in Table 7 .
Table 7.
Least square means of growth and intake parameters in Holstein (H) and Jersey (J), male (M) and female (F) calves.
| Variable | Least square means |
SE | Differences of means1 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Holstein |
Jersey |
F minus M |
H minus J |
||||||
| F | M | F | M | H | J | F | M | ||
| BW gain d 0—29 (kg) | 6.420 | 8.591 | 3.789 | 2.045 | 1.429 | −2.172* | 1.744 | 2.631† | 6.546** |
| ADG2 d 0—29 (kg/d) | 0.222 | 0.309 | 0.136 | 0.066 | 0.056 | −0.087* | 0.070 | 0.087† | 0.243** |
| Withers height3 (cm) | 4.24 | 3.74 | 2.54 | 2.86 | 0.63 | 0.50 | −0.32 | 1.70* | 0.88 |
| Hip height3 (cm) | 4.74 | 4.23 | 2.25 | 3.42 | 0.81 | 0.51 | −1.18] | 2.49* | 0.81 |
| Body length3 (cm) | 4.58 | 4.28 | 2.49 | 2.65 | 1.09 | 0.30 | −0.16 | 2.10 | 1.64 |
| Heart girth3 (cm) | 5.52 | 5.69 | 4.45 | 2.77 | 0.57 | −0.17 | 1.68† | 1.08 | 2.92** |
| Total DMI (kg) | 18.466 | 18.463 | 11.415 | 10.574 | 1.234 | 0.004 | 0.841 | 7.052** | 7.889** |
| Feed efficiency4 | 0.338 | 0.445 | 0.309 | 0.179 | 0.087 | −0.107* | 0.130* | 0.029 | 0.266** |
Significance determined by slicing interaction of breed and gender.
ADG = Average daily gain.
Gain, d 1 to 29.
d 0 to 29; calculation: (kg BW gain)/(kg DMI).
P < 0.10.
P < 0.05.
P < 0.01.
Starter intake increased in a linear and quadratic manner from d 1 to 29, and no differences were detected between treatments (Table 8 ). Calves fed MC were more feed efficient than CR-fed calves overall (0.394 ± 0.082 vs. 0.241 ± 0.083 kg of gain/kg of DMI). However, when gains were analyzed by week, calves fed MC were more feed efficient only in wk 1 (Figure 3 ). Holsteins converted feed into BW gain more efficiently than Jerseys (0.391 ± 0.081 compared with 0.244 ± 0.084 kg of gain/kg of DMI). Observed feed efficiencies fall within the range of values (0.330 to 0.680 kg of gain/kg of DMI) reported by other researchers (Lammers et al., 1998; Quigley et al., 2000b).
Table 8.
Least square means of DMI and feed efficiency in calves fed pooled maternal colostrum (MC) or replacement (R) and milk replacer with (PL) or without (N) animal plasma.
| Variable | Least square means |
SE | Differences of means1 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ColoSstrum |
Replacement |
N minus PL |
MC minus R |
||||||
| N | PL | N | PL | MC | R | N | P | ||
| Total DMI (kg) | 15.668 | 14.996 | 13.667 | 14.586 | 1.239 | 0.671 | −0.918 | 2.000 | 0.411 |
| Milk replacer DMI (kg) | 10.681 | 11.036 | 9.782 | 10.925 | 0.584 | −0.355 | −1.143 | 0.899 | 0.112 |
| Starter DMI (kg) | 4.951 | 3.940 | 3.851 | 3.705 | 0.772 | 1.011 | 0.145 | 1.100 | 0.234 |
| Feed efficiency2 | 0.432 | 0.357 | 0.224 | 0.258 | 0.087 | 0.075 | −0.034 | 0.208** | 0.099† |
Significance determined by slicing interaction of IgG source and milk replacer.
d 0 to 29; calculation: (kg BW gain)/(kg DMI).
P < 0.10.
P < 0.01.
Figure 3.
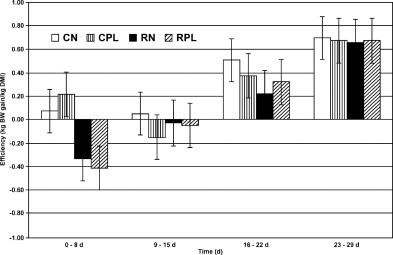
Feed efficiency (kg BW gain/kg DMI) by week in calves fed maternal colostrum at birth and milk replacer without animal plasma for 1 mo (CN), maternal colostrum at birth and milk replacer with animal plasma for 1 mo (CPL), colostrum replacement at birth and milk replacer without animal plasma for 1 mo (RN), or colostrum replacement at birth and milk replacer with animal plasma for 1 mo (RPL). Calves fed colostrum were more efficient than calves fed replacement from d 0 to 8.
Plasma Urea Nitrogen
No differences in plasma urea nitrogen (PUN) were found between treatment groups at birth or from d 8 to 29; however, calves fed MC had higher concentrations of PUN on d 1 than calves fed CR (12.60 ± 0.78 and 10.03 ± 0.77 mg/dL). Calves fed CR received a greater volume of liquid in the first 24 h and probably receive less protein than calves fed MC. Colostrum was not analyzed for protein, but contains about 15% protein (Foley and Otterby, 1978) as compared with the CR used in this study, which contained about 10% protein. Calves fed CR appeared to experience a greater increase in plasma volume as evidenced by reduced packed cell volume from d 0 to 1 than MC-fed calves; packed cell volume decreased by 4.39 ± 0.75 and 2.63 ± 0.76 percentage points from d 0 to 1 for CR and MC-fed calves, respectively. Therefore, PUN concentrations may be diluted in CR calves on d 1.
From d 1 to 22, Jerseys had higher PUN concentrations than Holsteins. Jersey calves fed CR experienced greater plasma volume expansion than Holstein calves fed CR (5.72 ± 0.97% and 3.07 ± 0.82%), but Jerseys have lower plasma volume than Holsteins (Quigley et al., 1998a). Jersey calves had greater total plasma protein than Holstein calves on d 1, 8, and 22, which could also occur due to lower plasma volume. Alternatively, greater total plasma protein, as a result of greater absorption of protein from initial feedings of MC or CR, could lead to increased PUN concentrations as the excess protein is metabolized and cleared from the body.
Health
Distribution of fecal scores was similar for all 4 treatments. Distribution of scores was different for breed-gender groups, but biological significance of these differences was difficult to explain. Four calves died during the course of the experiment. Incidences of death and health problems were not different between treatment or breed-gender groups.
Conclusions
Calves fed an equal amount of IgG from MC or a CR attained equivalent plasma IgG concentrations at 24 h of age. Apparent clearance rate of passively acquired IgG was faster in calves fed MC than in calves fed CR. Calves fed MC had greater feed efficiency than calves fed CR. No other differences in growth or health performance were attributable to source of IgG in the first 24 h. However, while CR may provide adequate amounts of IgG, the viability and specificity of antibodies, as well as the ratio of IgG1 to IgG2, are potential concerns for the efficacy of these products. If IgG molecules are not viable and specific for pathogens in the local environment, then amount provided is irrelevant. Effects of intense processing required to isolate and concentrate IgG from animal proteins on activity of IgG also need to be determined. Furthermore, the ratio of IgG1 to IgG2 in colostrum is likely skewed in favor of IgG1 for a physiological reason. The impact of changing this ratio needs to be evaluated more closely. If calves receiving CR are stimulated to produce IgG1, then the product could be very valuable in providing enough initial IgG1 to effectively protect the calf until endogenous production begins. At the same time, IgG2 would be present in excess of normal concentrations and may offer additional benefits. On the other hand, increased concentrations of IgG2 may inhibit IgG1 production and lead to reduced immunity as IgG is cleared from the blood. However, since no differences were observed in morbidity or mortality in this trial, it may be assumed that CR offered equivalent protection as MC under the conditions of this study.
No differences from controls were observed in the growth or health of calves fed milk replacer containing animal plasma, which indicates it is an acceptable replacement for up to 20% of milk protein in milk replacers. Further investigation is required to determine whether animal plasma provides health benefits to calves in addition to meeting nutritional requirements.
Acknowledgments
The authors would like to thank Stacy Wampler, Scott Bascom, and Curtis Caldwell for their help in feeding and in caring for calves. Support for this project provided by the John Lee Pratt Foundation and APC, Inc.
Supplementary data
References
- Arthington J.D., Cattell M.B., Quigley J.D., III Effect of dietary IgG source (colostrum, serum, or milk-derived supplement) on the efficiency of Ig absorption in newborn Holstein calves. J. Dairy Sci. 2000;83:1463–1467. doi: 10.3168/jds.S0022-0302(00)75018-1. [DOI] [PubMed] [Google Scholar]
- Arthington J.D., Cattell M.B., Quigley J.D., III, McCoy G.C., Hurley W.L. Passive immunoglobulin transfer in newborn calves fed colostrum or spray-dried serum protein alone or as a supplement to colostrum of varying quality. J. Dairy Sci. 2000;83:2834–2838. doi: 10.3168/jds.S0022-0302(00)75183-6. [DOI] [PubMed] [Google Scholar]
- Besser T.E., McGuire T.C., Gay C.C., Pritchett L.C. Transfer of functional immunoglobulin G (IgG) antibody into the gastrointestinal tract accounts for IgG clearance in calves. J. Virol. 1988;62:2234–2237. doi: 10.1128/jvi.62.7.2234-2237.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J.E. Bovine immunoglobulins: An augmented review. Vet. Immunol. Immunopathol. 1983;4:43–152. doi: 10.1016/0165-2427(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Chaney A.L., Marbach E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962;8:130–132. [PubMed] [Google Scholar]
- Davenport D.F., Quigley III J.D., Martin J.E., Holt J.A., Arthington J.D. Addition of casein or whey protein to colostrum or a colostrum supplement product on absorption of IgG in neonatal calves. J. Dairy Sci. 2000;83:2813–2819. doi: 10.3168/jds.S0022-0302(00)75180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C.L., Drackley J.K. 1st ed. Iowa State University Press; Ames: 1998. The Development, Nutrition, and Management of the Young Calf. [Google Scholar]
- Devery J.E., Davis C.L., Larson B.L. Endogenous production of immunoglobulin IgG1 in newborn calves. J. Dairy Sci. 1979;62:1814–1818. doi: 10.3168/jds.s0022-0302(79)83504-3. [DOI] [PubMed] [Google Scholar]
- Drew M.D. Effects of immunoglobulin fortification of milk replacers on the performance of calves challenged with Escherichia coli. J. Dairy Sci. 1994;77(Suppl. 1):298. (Abstr.) [Google Scholar]
- Duarte R.T., Carvalho Simões M.C., Sgarbieri V.C. Bovine blood components: fractionation, composition, and nutritive value. J. Agric. Food Chem. 1999;47:231–236. doi: 10.1021/jf9806255. [DOI] [PubMed] [Google Scholar]
- Etzel L.R., Strohbehn R.E., McVicker J.K. Development of an automated turbidimetric immunoassay for quantification of bovine serum immunoglobulin G. Am. J. Vet. Res. 1997;58:1201–1205. [PubMed] [Google Scholar]
- Foley J.A., Otterby D.E. Availability, storage, treatment, composition, and feeding value of surplus colostrum: a review. J. Dairy Sci. 1978;61:1033–1060. [Google Scholar]
- Garry F.B., Adams R., Cattell M.B., Dinsmore R.P. Comparison of passive immunoglobulin transfer to dairy calves fed colostrum or commercially available colostral-supplement products. JAVMA. 1996;208:107–110. [PubMed] [Google Scholar]
- Lammers B.P., Heinrichs A.J., Aydin A. The effect of whey protein concentrate or dried skim milk in milk replacer on calf performance and blood metabolites. J. Dairy Sci. 1998;81:1940–1945. doi: 10.3168/jds.S0022-0302(98)75767-4. [DOI] [PubMed] [Google Scholar]
- Littell R.C., Milliken G.A., Stroup W.W., Wolfinger R.D. 1st ed. SAS Inst., Inc; Cary, NC: 1996. SAS System for Mixed Models. [Google Scholar]
- Logan E.F., McBeath D.G., Lowman B.G. Quantitative studies on serum immunoglobulin levels in suckled calves from birth to five weeks. Vet. Rec. 1974;94:367–370. doi: 10.1136/vr.94.16.367. [DOI] [PubMed] [Google Scholar]
- Mayer B., Zolnai A., Frenyó L.V., Jancsik V., Szentirmay Z., Hammarstrom L., Kacskovics I. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology. 2002;107:288–296. doi: 10.1046/j.1365-2567.2002.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee J.F., O’Farrell K.J., Reitsma P., Mehra R. Effect of a whey protein concentrate used as a colostrum substitute or supplement on calf immunity, weight gain, and health. J. Dairy Sci. 1996;79:886–894. doi: 10.3168/jds.S0022-0302(96)76437-8. [DOI] [PubMed] [Google Scholar]
- Morrill J.L., Morrill J.M., Feyerherm A.M., Laster J.F. Plasma proteins and a probiotic as ingredients in milk replacer. J. Dairy Sci. 1995;75:902–907. doi: 10.3168/jds.S0022-0302(95)76704-2. [DOI] [PubMed] [Google Scholar]
- NAHMS . USDA: APHIS:VS; Fort Collins, CO: 1993. Dairy herd management practices focusing on preweaned heifers. [Google Scholar]
- Quigley J.D., III, Bernard J.K. Milk replacers with or without animal plasma for dairy calves. J. Dairy Sci. 1996;79:1881–1884. doi: 10.3168/jds.S0022-0302(96)76556-6. [DOI] [PubMed] [Google Scholar]
- Quigley J.D., III, Drewry J.J., Martin K.R. Estimation of plasma volume in Holstein and Jersey calves. J. Dairy Sci. 1998;81:1308–1312. doi: 10.3168/jds.S0022-0302(98)75693-0. [DOI] [PubMed] [Google Scholar]
- Quigley J.D., III, Fike D.L., Egerton M.N., Drewry J.J., Arthington J.D. Effects of a colostrum replacement product derived from serum on immunoglobulin G absorption by calves. J. Dairy Sci. 1998;81:1936–1939. doi: 10.3168/jds.S0022-0302(98)75766-2. [DOI] [PubMed] [Google Scholar]
- Quigley J.D., III, French P., James R.E. Effect of pH on absorption of immunoglobulin G in neonatal calves. J. Dairy Sci. 2000;83:1853–1855. doi: 10.3168/jds.S0022-0302(00)75056-9. [DOI] [PubMed] [Google Scholar]
- Quigley J.D., III, Jaynes C.A., Miller M.L., Schanus E., Chester-Jones H., Marx G.D., Allen D.M. Effects of hydrolyzed spray dried red blood cells in milk replacer on calf intake, body weight gain, and efficiency. J. Dairy Sci. 2000;83:788–794. doi: 10.3168/jds.s0022-0302(00)74941-1. [DOI] [PubMed] [Google Scholar]
- Quigley J.D., III, Kost C.J., Wolfe T.M. Absorption of protein and IgG in calves fed a colostrum supplement or replacer. J. Dairy Sci. 2002;85:1243–1248. doi: 10.3168/jds.S0022-0302(02)74188-X. [DOI] [PubMed] [Google Scholar]
- Quigley J.D., Strohbehn R.E., Kost C.J., O’Brien M.M. Formulation of colostrum supplements, colostrum replacers and acquisition of passive immunity in neonatal calves. J. Dairy Sci. 2001;84:2059–2065. doi: 10.3168/jds.S0022-0302(01)74650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimmoto E.K., Goeke N.M., Olson B.J., Klenk D.G. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stabel J.R. On-farm batch pasteurization destroys Mycobacterium paratuberculosis in waste milk. J. Dairy Sci. 2001;84:524–527. doi: 10.3168/jds.S0022-0302(01)74503-1. [DOI] [PubMed] [Google Scholar]
- Stott G.H., Fellah A. Colostral immunoglobulin absorption linearly related to concentration for calves. J. Dairy Sci. 1983;66:1319–1328. doi: 10.3168/jds.S0022-0302(83)81941-9. [DOI] [PubMed] [Google Scholar]
- Stott G.H., Marx D.B., Menefee B.E., Nightengale G.T. Colostral immunoglobulin transfer in calves I. period of absorption. J. Dairy Sci. 1979;62:1632–1638. doi: 10.3168/jds.S0022-0302(79)83472-4. [DOI] [PubMed] [Google Scholar]
- Todd A.G., Whyte P.B.D., Carroll P.D. A comparison of serum immunoglobulin concentrations in neo-natal calves fed substitute colostrums. Aust. Vet. J. 1993;70:154–155. doi: 10.1111/j.1751-0813.1993.tb06113.x. [DOI] [PubMed] [Google Scholar]
- Weatherburn M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967;39:971–974. [Google Scholar]
- Wells S.J., Dargatz D.A., Ott S.L. Factors associated with mortality to 21 days of life in dairy heifers in the United States. Prevent. Vet. Med. 1996;29:9–19. [Google Scholar]
- Zaremba W., Guterbock W.M., Holmberg C.A. Efficacy of a dried colostrum powder in the prevention of disease in neonatal Holstein calves. J. Dairy Sci. 1993;76:831–836. doi: 10.3168/jds.S0022-0302(93)77408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


