ABSTRACT
Stable, long-term interactions between fungi and algae or cyanobacteria, collectively known as lichens, have repeatedly evolved complex architectures with little resemblance to their component parts. Lacking any central scaffold, the shapes they assume are casts of secreted polymers that cement cells into place, determine the angle of phototropic exposure and regulate water relations. A growing body of evidence suggests that many lichen extracellular polymer matrices harbor unicellular, non-photosynthesizing organisms (UNPOs) not traditionally recognized as lichen symbionts. Understanding organismal input and uptake in this layer is key to interpreting the role UNPOs play in lichen biology. Here, we review both polysaccharide composition determined from whole, pulverized lichens and UNPOs reported from lichens to date. Most reported polysaccharides are thought to be structural cell wall components. The composition of the extracellular matrix is not definitively known. Several lines of evidence suggest some acidic polysaccharides have evaded detection in routine analysis of neutral sugars and may be involved in the extracellular matrix. UNPOs reported from lichens include diverse bacteria and yeasts for which secreted polysaccharides play important biological roles. We conclude by proposing testable hypotheses on the role that symbiont give-and-take in this layer could play in determining or modifying lichen symbiotic outcomes.
Keywords: alga, bacteria, EPS, exopolysaccharide, fungus, glucan, mannan, Rhizobiales, yeast, uronic acid
Polysaccharide composition of lichens is reviewed with an emphasis on extracellular matrix and potential input and uptake by organismal components of the matrix.
ABBREVIATIONS USED
- EIM
extracellular interaction matrix
- EPS
extracellular polymeric substances
- UNPO
unicellular non-photosynthesizing organism
INTRODUCTION
Most forms of multicellular, multidomain symbiosis recognized today involve large, structurally defining hosts dependent on microbial partners (Moran 2007; McFall-Ngai et al. 2013), such as a fruit fly and a Wolbachia bacterium (Jaenike et al. 2010). Some symbioses, by contrast, possess body plans that are achieved only when two or more essentially microscopic symbionts combine to build a macroscopic, 3D structure. In isolation, these symbionts look nothing like the final product. This is the case with the symbiotic architectures arising from stable interactions between one or more multicellular fungi and one or more unicellular microbes, including photosynthesizing single-celled algae or cyanobacteria. Such architectures are collectively referred to as lichens.
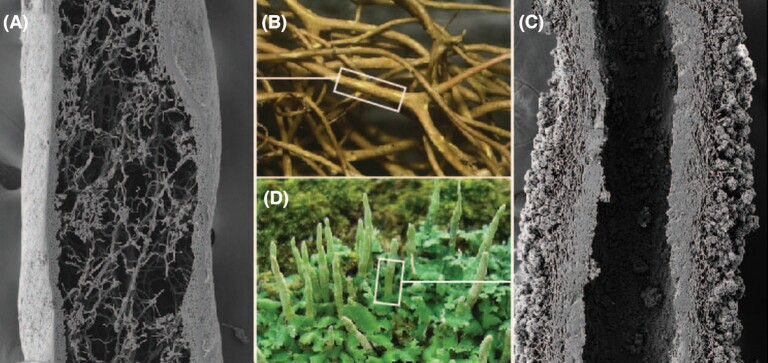
Unlike majority-partner symbionts that provide a backbone or scaffold, the components of lichen symbioses do not undergo cell fate differentiation and subsequent tissue development. Although the dominant fungi in lichens are multicellular, every cell is totipotent (Money 2002). The complex three-dimensionality and structural integrity characteristic of many lichens instead results from the component parts gluing each other into place with extracellular polymers as the assemblage grows. In some lichens, the zone in which this happens is external (Fig. 1A and B), while in others it is internal to the resulting thallus (Fig. 1C and D). The zone consists of hyphal cells and hydrophilic, extracellular polymeric substances (EPS). When dry, it constitutes a dense, flexible to brittle matrix of shrunken EPS and compressed hyphal cells. When hydrated, the zone expands to many times its desiccated volume. In many lichens, this zone also contains embedded bacteria and basidiomycete yeasts not visible from the outside. All of the component organisms, not least the dominant lichen fungus, both assimilate from and secrete into this medium. It is this combination of properties—a well-developed EPS matrix and multiorganismal composition—that has led us to begin treating this zone, the structure-defining feature of lichen thalli, as a biofilm (Spribille 2018; Tuovinen et al. 2019). In the past, the area defined by this matrix has been called cortex (in cases where it is external to the overall thallus, as mentioned above) or stereome (if internal), or been referred to as ‘gelatinous matrix’ (Ahmadjian 1993), ‘conglutinate zone’ (Honegger 1991) or ‘hydrophilic mucilages’ (Honegger 1998). As the zone mediates all extracellular communication between its inhabitants, and does not just consist of polymeric substances, a term more inclusive than EPS is required. For clarity, we will refer to the zone as defined above, excluding its component cells and cell walls, as the extracellular interaction matrix (EIM).
Figure 1.
External and internal polysaccharide-dense zones in two common lichen symbioses. (A, B)Bryoria fremontii and (C, D)Cladonia ochrochlora, showing scanning electron micrograph of lengthwise section through thallus. Field photos by Jason Hollinger, used with permission.
Only a fraction of all described lichen symbioses have evolved structures that form 3D thalli separate from their substrates, and these are usually called macrolichens. In such lichens, EIMs form first and foremost a kind of exoskeleton, cementing together the filamentous hyphae of the dominant fungus and endowing the structure with rigidity. As strongly hydrophilic media, EIMs play an outsized role in thallus water relations, their properties determining both the point at which surface tension is broken and water is taken up from the environment (Valladares 1994), and how much is retained, and for how long (Esseen et al. 2015). In this role, they are often counteracted by other, hydrophobic parts of the lichen thallus in which the dominant fungal partner secretes surface hydrophobins (Scherrer et al. 2002; Trembley et al. 2002), creating complex thallus-internal water regulation (Dyer 2002). In many lichens, the EIM also serves as a surface-maximized receptacle for mineral nutrients, and a deployment zone for secondary metabolites. Finally, if they are anything like EPS matrices in other biofilms, EIMs must serve as the main media by which intercell recognition and signaling take place.
EIMs, with emphasis on their extracellularity, have not been the focus of much study. Instead, their properties have been considered intrinsic to the lichen cortex as a kind of named organ of the lichen as botanical specimen, rather than microbiological interaction. Often, the EIM is dried, ground to powder and analyzed along with the rest of the lichen and results reported as properties of the whole. This makes it difficult, but perhaps not impossible, to infer specific properties from existing analyses.
The purpose of this review is twofold: (i) to assess what is known of the polysaccharide composition and diversity of EIMs on the level of broad-scale fungal-photosymbiont combinations and (ii) to summarize what unicellular non-photosynthesizing organisms (UNPOs) may occur in them and what, if anything, we know about their use and production of polysaccharides. We will focus here on macrolichens.
POLYSACCHARIDES IN CELL WALLS AND THE EXTRACELLULAR MATRIX
Microscopic evidence of exopolysaccharides as lichen glue
The existence of a differentiated layer of hydrophilic fungal cells that are rigid when dry, but swell and become flexible upon contact with water, was already discussed by Goebel (1926). Bednar and Juniper (1964) later observed that fungal cell walls of Xanthoria parietina differed from the surrounding, extracellular matrix under a transmission electron microscope (TEM). The extracellular material was microfibrillar in structure, and based on their size and staining properties, the authors suggested these fibrils could be polysaccharides. Jacobs and Ahmadjian (1969) further visualized microfibrils in the extracellular matrix in several other lichen symbioses. Notably, they observed bacteria in the microfibrillar matrix, and suggested these microorganisms could have a role in the physiology of the symbiosis. After pioneering the use of SEM to study the algal–fungal interface, Peveling (1970) published a series of images examining what she called ‘Kittsubstanz’, or cementing substance, filling the space between hyphae. Hale (1973) went further and distinguished a thin external veneer, often with apparent ‘pores’ and heterogeneous across the lichen thallus, which he termed the epicortex. Originally reported only from symbioses involving the fungal subclass Lecanoromycetidae (family Parmeliaceae), it has since also been reported from Umbilicariomycetidae (Lasallia; Valladares 1994).
The ‘filler’-like nature of macrolichen EIMs was beautifully demonstrated in a series of experiments by Greenhalgh and coworkers. After experimenting with a series of laboratory and commercial detergents, Anglesea et al. (1982, 1983a,b) and Greenhalgh and Whitfield (1987) removed this layer and exposed the intricate branching of underlying hyphal cells. Their ‘maceration’ technique was later emulated in studies of other lichens (Honegger and Haisch 2001). Also, Greenhalgh and Whitfield (1987) observed the distinct appearances of the hyphal cell walls and the EPS, and estimated that the volume of the matrix was 5–8 times larger compared to the cell walls. According to their observations, the extracellular matrix in the outer parts of the cortex develops first. Anglesea et al. (1982) speculated that the substance could be proteinaceous in nature as it was dissolved by commercial laundry detergent containing proteolytic enzymes.
Modenesi and Vanzo (1986) expressed skepticism that this layer was made of protein, and performed a series of histological stains (toluidine blue, alcian blue, periodic acid–Schiff) to determine the properties of the EIM. They concluded that it was a strongly acidic (probably sulfated) polysaccharide and speculated it could be hyaluronic acid based on its dissolution by hyaluronidases; subsequently the same authors revised their assessment to the much broader category of polyuronic acids (Giordani et al. 2003). Several subsequent studies have employed histological stains to highlight acidic polysaccharides. The most detailed is perhaps Giordani et al. (2003), who studied the relationship of EIM chemistry to the formation of calcium oxalate deposits on lichen surfaces. They observed metachromatic staining, i.e. distinguishable differences in anionic density between cell walls and EIM, in 15 out of 17 species studied. Other examples that have highlighted the sharply different staining behavior of the EIM include Tretiach et al. (2005), Barbosa et al. (2009a,b) and Zanetti et al. (2015), and an example is provided in Fig. 2.
Figure 2.
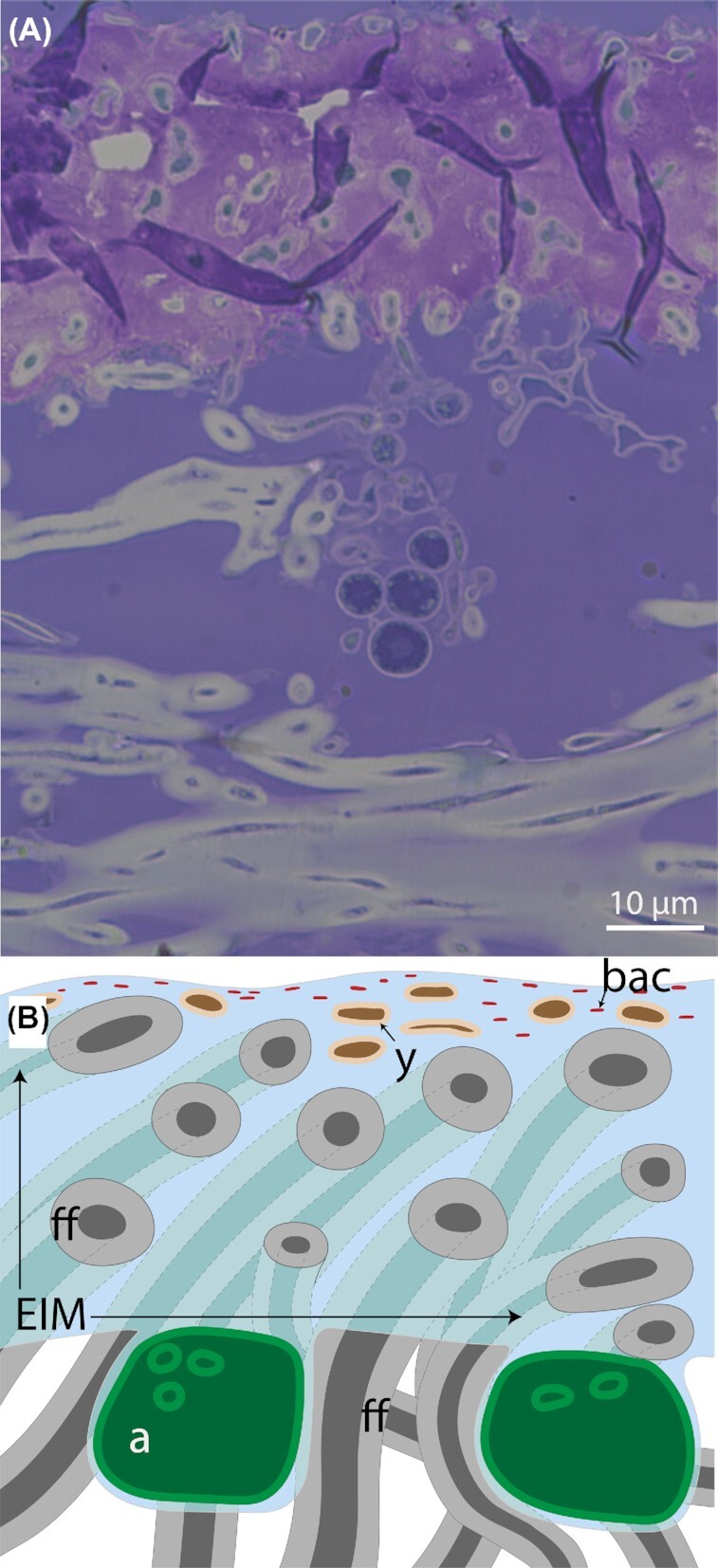
(A) Metachromatic staining in a sample of the lichen Letharia vulpina, using Richardson's stain. Sample fixed in resin and sectioned with diamond blade on microtome. Even use of new blades results in tearing of the pink-stained EIM, likely because of differential hardness of the EIM and the fungal cell walls embedded within it. (B) Schematic representation of a section through the EIM and into the hydrophobic interior of a lichen, showing typical location of bacteria (bac), basidiomycete yeasts (y) and the filamentous fungus (ff).
Dominant polysaccharides in ground, powdered whole lichens
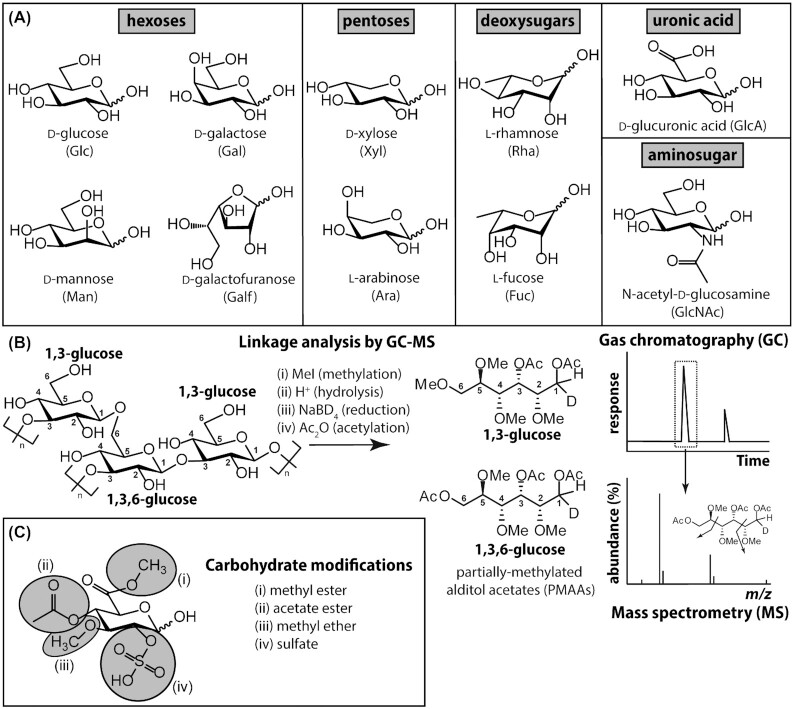
To test whether the presence of a zone of acidic sugars is corroborated by studies of lichen polysaccharides, we reviewed >60 papers published between 1900 and the present. Only a small subset of all lichen symbioses have been studied for their polysaccharide composition; most derive from the visible and dominant macrolichen symbioses, especially those formed by members of the class Lecanoromycetes, subclass Lecanoromycetidae (Parmeliaceae, Cladoniaceae, Teloschistaceae), the dominant group of extant lichen-forming fungi. The large majority of studies report only neutral polysaccharides with varying ratios of Glc, Gal and Man (typical monosaccharide constituents of lichen polysaccharides and a depiction of the major analytical techniques by which their relative abundances may be deduced are shown in Fig. 3). The main structures that have been found are β- and α-glucans and α-mannans. β-glucans are represented by β-(1 →3), (1 →4)-glucans (lichenan) and β-(1→3)-glucans (laminarin), while α-glucans are represented by α-(1 →3), (1 →4)-glucan (isolichenan), and α-(1 →3), (1 →4)-glucan (nigeran), considered a subgroup of isolichenans differing in their linkage ratios (Olafsdottir and Ingólfsdottir 2001). Overall, these glucans are similar in their structure to core cell wall polysaccharides in other fungi (Gow et al.2017). An additional, mixed linkage α-(1→4), (1→6)-glucan has been found in Teloschistes (Reis et al. 2002). Branched β-(1 →3), (1 →6)-glucans, originally thought to be typical of Basidiomycetes (in lichens in Dictyonemaglabratum: Iacomini et al.1987, as Cora pavonia), have also been reported from Lecanoromycetidae (Collema: Prado et al. 1999; Teloschistes: Reis et al.2002; Thamnolia: Olafsdottir et al. 2003), as well as from the cultured Physcia fungus (Cordeiro et al. 2012). Outside of the Lecanoromycetidae, studies have focused on Umbilicariomycetidae, in which the dominant glucan is a β-(1→6)-glucan called pustulan (Kjølberg and Kvernheim 1984; Pereyra et al. 2003; also present in Lecanoromycetidae, Cladonia: Iacomini et al. 1985). As expected, different cell wall components have been found in the few basidiomycete-based lichen symbioses that have been studied. In Dictyonema glabratum, a linear α-(1→3)-glucan (pseudonigeran) occurs (Carbonero et al. 2002a). A comprehensive review of these and other polysaccharides is provided by Olafsdottir and Ingólfsdottir (2001) and Stocker-Wörgötter et al. (2013).
Figure 3.
Common monosaccharide building blocks of lichen polysaccharides. (A) Although hundreds of different monosaccharides have been found in nature, only a fraction of them have been identified in lichen polysaccharides. These monosaccharides may be classified as neutral hexoses or pentoses (six and five carbon sugars, respectively), deoxyhexoses [in which the carbon 6 (C6) hydroxy group is removed], hexosamines or aminosugars (bearing an acetylated amino group at C2) and uronic acids (in which C6 bears a carboxylate moiety). Note that for simplicity, throughout the text the absolute configurations (D or L) are omitted from the full monosaccharide name or abbreviation; likewise, a pyranose (i.e. six-membered) ring configuration is assumed for all monosaccharides with the exception of galactofuranose (Galf). Refer to Varki et al. (2015, 2017) for common monosaccharide and polysaccharide conventions. (B) The monosaccharide constituents of lichen polysaccharides, and details concerning the regiochemisty of how they are glycosidically linked, may be deduced using GC-MS-based linkage (or methylation) analysis, shown here for a hypothetical β-glucan fragment. In brief, unsubstituted hydroxy groups are methylated (i) prior to complete polysaccharide hydrolysis under strongly acidic conditions (ii). Subsequent reduction using sodium borodeuteride (iii) tags the reducing end of each monosaccharide and acetylation with acetic anhydride (iv) caps hydroxyl groups that had been protected from methylation by virtue of the fact that they were glycosidically linked. The partially methylated alditol acetates (PMAAs) so created are resolvable by GC and may be identified, using appropriate standards, based on their predictable fragmentation by MS. Note that this technique is unable to assign anomeric (i.e. α or β) configurations. (C) Many lichen-derived monosaccharides bear biochemically modified hydroxy groups, represented here for a fictitious glucuronic acid. Most of these modifications (i, ii and iv) are acid labile and undetected by linkage analysis, unless substantially modified protocols are used.
The two most abundant monosaccharides consistently reported from lichen thalli, in addition to Glc, are Gal and Man, and these are usually reconstructed as components of heteromannans. In the Lecanoromycetes, these are usually built around an α-(1→6)-mannan main chain (Cladoniaceae: Iacomini et al. 1985; Woranovicz et al.1997; Ramalinaceae: Cordeiro et al. 2003a; Parmeliaceae: Carbonero et al. 2005a; Teixeira et al. 1994; Physciaceae: Pereira et al. 2010; Cordeiro et al. 2012). A common expression of this includes galactoglucomannans with variable ratios of Man, Glc and Gal units (Cladonia: Woranovicz et al.1999; Carbonero et al. 2002b; Pseudocyphellaria: Cordeiro et al. 2005a). Other heteroglycans involving Man, Glc and Gal with which these sometimes occur are galactomannoglucans, in which Galf and Man side chains are arranged at O-2,6 on β-(1→3)-glucan main chains (Cladonia: Woranovicz-Barreira et al.1999; Carbonero et al. 2002b). An α,β-(1→2), (1→6) galactomannan including Manf structural units has been reported from Evernia (Mićović et al. 1969). In an arthoniomycete–Trentepohliales lichen symbiosis, a fungal-derived α-(1→4)-mannan main chain has been reported (Roccella: Carbonero et al. 2005b). In the basidiomycete–cyanobacterial lichen symbiosis Dictyonema, both an α-(1→3)-xylomannan (Iacomini et al.1987) and a β-(1→6)-mannan (Carbonero et al. 2002a) have been reported. The side chain insertion and linkage patterns of heteromannans are diverse and distinctive at the level of species and genera (Gorin and Iacomini 1985; Cordeiro et al. 2003a) or above (Leal et al. 2010). Prior to molecular phylogenetics, polysaccharide linkages were even cited as a potential aid in taxonomy (reviewed by Teixeira et al. 1995). Indeed, the presence of unique α-Galf residues on α-(1→6)-mannans in Lichina was taken as support for the recognition of a taxonomic class, Lichinomycetes (Prieto et al.2008).
Fungal polysaccharides rare or missing from ground, powdered, whole lichens
Perhaps the most notable absence from whole lichen polysaccharide studies is chitin. Though expected by textbook definition to occur in nearly all fungi, chitin requires targeted sampling of amino sugars for detection and quantification. In one pulse-chase experiment where it was visualized using tritiated hydrogen labeling of a GlcNAc precursor, it was found to be an abundant cell wall component (Galun et al. 1976). Most other reports have likewise been from targeted studies (Solberg 1970; Schlarmann et al. 1990; Dahlman et al.2002; Meichik and Vorob'ev 2012). To our knowledge, it has been detected as part of a broader survey of polysaccharides only twice (Honegger and Bartnicki-Garcia 1991; Pereyra et al. 2003).
Joining amino sugars on the roster of rare or infrequently reported monosaccharides is an array of neutral sugars found in low frequency. Xyl has been detected in the basidiomycete–cyanobacterial symbiosis Dictyonema (Iacomini et al.1987; Elifio et al. 2000; Carbonero et al. 2002a); in lecanoromycete-Trebouxiales symbioses (Kjølberg and Kvernheim 1989: Umbilicaria; Olafsdottir et al. 1999: as terminal Xyl in thamnolan, a rhamnogalactofuranan of Thamnolia vermicularis); and finally in lecanoromycete-cyanobacterial symbioses (Prado et al. 1999: Sticta; Omarsdottir et al. 2005: Peltigera). Other monomers reported only rarely, and in low quantity, are Ara (Kjølberg and Kvernheim 1989; Corradi da Silva et al. 1993; Prado et al. 1999; Cordeiro et al.2005a; Omarsdottir et al. 2005, 2006), Rha (Kjølberg and Kvernheim 1989; Olafsdottir et al. 1999; Prado et al. 1999; Omarsdottir et al. 2005, 2006; Wang et al. 2018) and Fuc (Kjølberg and Kvernheim 1989; Omarsdottir et al. 2005). Some of the infrequently detected monosaccharides may be associated with glycolipids and glycoproteins. Glycolipids have rarely been studied (Ramalina: Machado et al. 1994; Dictyonema: Sassaki et al. 2001, 2005; older references reviewed by Dembitsky 1992) and glycoproteins a few times (Lobaria orientalis: Takahashi et al. 1974; Sticta: Corradi da Silva et al. 1993; Dictyonema: Elifio et al. 2000). Both classes of glycoconjugates are probably ubiquitous in lichen symbioses, but may be overlooked unless they are specifically the targets of sampling.
Acidic sugars are excellent candidates for forming the layers stained by Modenesi and Vanzo (1986) and others (as described above), but have been spottily reported in polysaccharide analyses. Uronic acids were already mentioned as a component of lichen samples by Buston and Chambers (1933). All of the uronic acid that has been identified to date has been GlcA (an early report of GalA from Evernia by Mićović et al. 1969 was shown to be GlcA by Teixeira et al. 1994). Uronic acids have been tentatively identified from lecanoromycete–Trebouxiales symbioses, especially involving the fungal family Parmeliaceae (Evernia, Parmotrema, Usnea: Teixeira et al. 1992, 1994, 1995; Cetraria: Hranisavljević-Jakovljević et al. 1980), as well as in lecanoromycete–cyanobacterial symbioses (Prado et al. 1999; Jensen et al. 2010). To the extent this has been analyzed, GlcA has been reported as a residue of α-mannans, with the exception of Hranisavljević-Jakovljević et al. (1980), who reported it from a β-(1→3)-glucan. Tuominen (1967) used carbazole in an ecophysiological study to quantify uronic acids in 12 lichen species, mostly in Parmeliaceae-based symbioses. He found uronic acids in all lichen symbioses studied, including the Cladonia–Asterochloris symbiosis in which they have never been found in neutral polysaccharide analyses.
An indication of the extent to which acidic sugars may evade detection in studies of neutral polysaccharides comes from the genus Umbilicaria. The latter (including Lasallia) has been extensively studied for its content of pustulan, a neutral homopolysaccharide, without reports of uronic acids (Nishikawa et al. 1970; Kjølberg and Kvernheim 1984; Carbonero et al. 2006). However, Kjølberg and Kvernheim (1989) detected uronic acids as well as sulfates in two Umbilicaria species they had studied earlier, by incorporating Me2SO-based extraction coupled with ion exchange chromatography. Similarly, Wang et al. (2015) detected uronic acids in Umbilicaria esculenta using the m-hydroxydiphenyl method.
Acidic sugars are so seldom detected that when they are found in quantity, they are assumed to be of non-fungal origin. Jensen et al. (2010) described a complex heteropolysaccharide called colleman from the lecanoromycete–cyanobacterial symbiosis Collema flaccidum, with 2-O-methylated β-Man linked 1→4 with α-Ara, α-Gal, β-GlcA and β-Xyl in four different configurations (Jensen et al. 2010). Though they concede that 2-O-methylated β-Man and α-Ara residues are also not known from cyanobacteria, the elevated presence of Xyl and GlcA, which they hold to be rare in lichens, led the authors to conclude that the glycan must be cyanobacterial in origin. They acknowledge that, as is widely the case in capsular and released polysaccharides in cyanobacteria, analytical methods may lead to underdetection of methyl- and amino sugars (de Philippis et al. 2001). Similarly, the occurrence of β-(1→4)-xylans in Sticta (Corradi da Silva et al. 1993) and Dictyonema (Carbonero et al. 2002a) has been ascribed to the presence of cyanobacteria in those symbioses. That being said, Xyl has also been detected in symbioses that lack cyanobacteria (see above).
Missing photosymbiont polysaccharides
Targeted structural studies of the polysaccharides from isolated photosymbionts have revealed polysaccharides not detected in whole lichen material (reviewed by Stocker-Wörgötter et al. 2013). Trebouxia, one of the most common eukaryote photosymbionts, yielded amylose and a β-xylan not found in whole lichen samples (Ramalina: Cordeiro et al. 2003b); in the same algal genus, the same workers later isolated a β-(1→5)-galactofuranan that likewise could not be found in the lichen (Cordeiro et al. 2005b, 2008). Also in Trebouxia and in association with the same genus of fungi (Ramalina), Casano et al. (2015) isolated a β-galactofuranan from cell walls and separately examined EPS attributes, recording up to 10.9% uronic acids in the EPS. A xylorhamnogalactofuranan with a β-(1→3)-Galf main chain and non-reducing Xyl residues was isolated from Asterochloris cell walls by Cordeiro et al. (2007) but again not from the lichen Cladonia from which it derived. Similarly, Myrmecia algae isolated from the lichen Lobaria produced a rhamnogalactofuranan with a β-(1→3)-Galf main chain, which has not been detected in the whole Lobaria lichen (Cordeiro et al. 2013). Coccomyxa yielded a β-(1→6)-mannogalactan with partially O-methylated α-Man residues that could not be detected in the whole lichen (Peltigera: Cordeiro et al. 2010). The same algal genus in association with the lichen Solorina has been shown to contain cellulose and a 4-linked mannan (Centeno et al. 2016). The authors note that a similar mannan had been previously detected in Roccella (Carbonero et al.2005b), but this arthoniomycete-derived lichen contains a different photobiont, Trentepohlia, which is not closely related to Coccomyxa. In cyanobacterial EPS, Ruthes et al. (2010) found a linear β-(1→4)-xylan and a complex polysaccharide formed of β-linked Ara and Xyl units, neither of which had been detected in whole lichen samples. The failure to detect β-(1→4)-xylans and other compounds has been explained as possibly being due to low relative abundance or insufficiently strong alkaline extraction conditions in whole lichen analyses (Cordeiro et al. 2003b), or genuine absence when the alga is in symbiosis (Cordeiro et al. 2010).
Fewer polysaccharides have been reported from the cell walls of lichen-associated Trebouxia species than in the better-studied Chlorella algae, which also belong to Trebouxiophyceae (order Chlorellales). Polysaccharides of Chlorella cell walls can be divided into two fractions (Takeda and Hirokawa 1978). The first, rigid wall polysaccharides, are not alkali soluble; in different strains, they consist of either glucosamine or Glc and Man (Takeda 1991). Kapaun and Reisser (1995) suggested that the glucosamine polymer found in Chlorella cell wall might have a chitin-like structure. Since these polymers are also not soluble in 2 M trifluoroacetic acid (TFA), they need strong acid treatment in order to be hydrolyzed (stepwise hydrolysis with 72–74% H2SO4 following 6 M HCl is described by Takeda 1991). The second class of cell wall polysaccharides, dubbed ‘matrix’, is soluble in alkali and TFA. Matrix composition is variable; in different strains it includes Man, Gal, Rha, Fuc, Ara, Glc and Xyl (Takeda and Hirokawa 1978; Takeda 1991; Kapaun et al. 1992). The concentration of uronic acid in cell walls is high and amounts to 10–25% of cell wall dry weight (Kapaun et al. 1992; Cheng et al. 2011). Treatments of Chlorella cell walls with cellulases produced little change in the cells, leading to speculation that they contain little or no cellulose (Gerken et al. 2013), though it has been reported in other studies (Rodrigues and da Silva Bon 2011).
Localizing known polysaccharides
Which polysaccharides, then, are most likely to account for those that fill extracellular space in the EIM? The localization of most polysaccharides in lichens has not been well established (Olafsdottir and Ingólfsdottir 2001), but some studies have been undertaken. Galun et al. (1976) used fluorescein-conjugated lectin staining and isotope-labeled pulse-chase experiments for chitin, α-linked polysaccharides, Fuc and Man. The visualizations of Galun et al. (1976), performed on axenically cultured fungal symbionts, confirmed the presence of chitin and α-linked polysaccharides in the inner fungal cell wall, consistent with their localization in non-lichen-forming fungi. Honegger and Haisch (2001) used an antibody to localize β-(1 →3), (1 →4)-glucan in two lichens and succeeded only in Cetraria islandica. Most of the β-(1 →3), (1 →4)-glucan they detected was in the outer cell wall. The claim of Honegger and Haisch (2001) that β-(1 →3), (1 →4)-glucan accounts for the extracellular matrix is however not supported by their images, in which the molecule appears to be localized in hyphal cell walls both inside and outside of the EIM, but the EIM itself is stained at most in thin arcs (see Fig. 3B in Honegger and Haisch 2001 ), reminiscent of deposited cell wall material. This would be consistent with TEM observations of cell wall shedding in another lichen, Ramalina menziesii (Sanders and Ascaso 1995) but does not explain the bulk composition of the EIM.
Are some polysaccharides systematically missed in whole lichen analysis?
Does the low detection rate of chitin, known algal polysaccharides and acidic sugars really reflect the absence of these substances in lichen symbioses? Two different factors may have led to the underreporting of polymeric substances in the past. First, polysaccharide inventories have emphasized abundant elements, which tend to be α- and β-glucans and α-mannans. The near-absence of algal products, despite the abundance of algae in powdered whole lichen samples, raises questions of methodology and biology: were they not detected because they are not synthesized in statu symbiotico? Or are they hidden among low-abundance heterogeneous fractions that have been set aside for further analysis (e.g. uronic acid-containing fractions in Prado et al. 1999)? Selective harvesting of algae from within lichens, such as through cell sorting, might help to address this question. Similarly, selective characterization of certain lichen parts, e.g. the extracellular matrix, without grinding and powdering the whole lichen, may yield different results than bulk analysis. Second, we suspect that the types of analytical protocols used may lead to underdetection of acidic sugars. For example, glycosidic linkages adjacent to uronic acids (e.g. GlcA or GalA) are difficult to hydrolyze under acidic conditions, and hence these monosaccharides are not detected as alditol acetates (i.e. by linkage analysis) unless their carboxyl groups are pre-reduced (Pettolino et al. 2012). The few studies in lichens that have detected uronic acids have incorporated additional, parallel analyses, such as treatment of polysaccharide fractions with Cetavlon, use of paper or ion exchange chromatography, or use of carbazole staining. Meanwhile, the sulfate moieties within polysaccharides may be erroneously interpreted as a monosaccharide substituents unless permethylated samples are first de-O-sulfonated under conditions that do not cleave glycosidic linkages, and then subjected to a second round of permethylation, typically with isotopically labeled methyl iodide (Jiao et al. 2011). Polysaccharide extraction conditions/solvents may also have a significant impact on subsequent monosaccharide profiles (Sun et al. 2018). Finally, the choice of acid used to hydrolyze polysaccharides may bias analytical results, for example, cellulose and chitin may be recalcitrant to hydrolysis when TFA-catalyzed procedures are used (Pettolino et al. 2012).
The identification of the polysaccharide EIM will ultimately be possible through targeted sampling of this layer in combination with visualization techniques such as those that employ fluorophore-conjugated lectins and antibodies. We can imagine several scenarios that would reconcile the disparate data points we have:
Among the polysaccharides that have been described to date, heteromannans constitute a good candidate for providing ascomycete-derived capsule material. Whether these account for some of the acidic polysaccharides detected in histological studies cannot be determined with certainty, as they have not been visualized and attributes used to determine whether they would stain have not been consistently characterized in polysaccharide studies.
Specific undescribed glycoproteins and glycolipids dominate this layer. This theory would seem to be favored by the highly efficient and selective removal (e.g. by Anglesea et al. 1982) of the EIM by Ariel laundry detergent (Proctor & Gamble, Cincinnati, OH, USA), the full composition of which is proprietary, but which contains at least proteases, lipases and α-amylases (https://www.pg.com/productsafety/). Glycoconjugates are major components of biofilms (Zippel and Neu 2011) and can be difficult to detect using lectin probes if sulfated. This explanation is not necessarily mutually exclusive of (i).
Additional compounds are involved but have been missed due to analytical methods missing acidic or modified sugars.
ORGANISMAL INPUT AND UPTAKE FROM EIMs
The EIMs of most lichen symbioses are dominated, in terms of cellular mass, by hyphal tips or protrusions from the dominant fungal symbiont (Fig. 2). The interdigitation of fungal hyphae in EIMs has been demonstrated through the selective removal of the EIM (Anglesea et al. 1982, 1983a,b; Greenhalgh and Whitfield 1987). Fungi are a likely candidate for contributing the bulk of secreted polysaccharides, and much of this review has focused on that contribution. Green algal symbionts, by contrast, tend to be located out of direct contact with EIMs, in the hydrophobin-lined internal chambers of lichen thalli. Exceptions to this include cyanobacterial symbionts, which are typically embedded in EIM matrices of their own. Recently, UNPOs such as bacteria and yeasts have been detected in lichen symbioses, and most of those that have been visualized have been found embedded within EIMs. The potential polysaccharide input and uptake from this UNPO component has not been reviewed to date.
Bacteria
Speculation over the possible role of symbionts other than the fungus and alga in forming lichens goes back nearly one hundred years. As early as 1926, the Italian microbiologist Maria Cengia Sambo cultured bacteria from lichen thalli, assigning them to the diazotroph genus Azotobacter (Cengia Sambo 1926). Cengia Sambo postulated that Azotobacter formed an essential third symbiont that fixed nitrogen in a three-way interdependence with the fungus and alga. Henkel and Yuzhakova (1936) and Iskina (1938) independently reported Azotobacter in a wide range of lichen symbioses, which led them too to assert the tripartite nature of lichen symbiosis. Other Soviet scientists endeavored to replicate these results, with mixed success (Lenova and Blum 1983). Krasil'nikov (1949), while not being able to isolate Azotobacter from lichens, found a large variety of so-called ‘oligonitrophilic’ bacteria isolated on a nitrogen-free medium. He assigned most of them to the genera Pseudomonas and Bacterium. Later, Panosyan and Nikogosyan (1966) suggested that these potentially diazotrophic bacteria might play an essential role in lichen symbiosis. Krasil'nikov (1949) estimated that between 5 and 50 million bacterial cells are present in one gram of lichen material, with the vast majority inside a thallus and not on its surface.
More recently, a combination of culturing and molecular techniques, culminating in amplicon sequencing studies, has confirmed highly structured and diverse bacterial communities associated with specific macrolichen symbioses (Aschenbrenner et al. 2016; Biosca et al. 2016; Suzuki et al. 2016; Pankratov et al. 2017). Those most intensely studied for their bacteria are lecanoromycete–Trebouxiales pairings in which the dominant lecanoromycete is Cladonia, Umbilicaria or members of Parmeliaceae, and lecanoromycete-dominated symbioses that include cyanobacteria, especially Lobaria pulmonaria. Some trends in bacterial composition are emerging. Alpha-proteobacteria are abundant in nearly all lichens studied to date (Table 1). A mostly lichen-specific clade of Rhizobiales, LAR1, has been described based on molecular data (Hodkinson and Lutzoni 2009). Some members of this clade were subsequently cultured (Jiang et al. 2017) and two strains were recently formally named (Lichenihabitans: Noh et al. 2019; Lichenibacter: Pankratov et al.2019). The existence of a lichen-specific clade of Acetobacteraceae has also been suggested (Cardinale et al. 2008). Hodkinson et al. (2012), using amplicon sequencing, showed that patterns of bacterial diversity and abundance were different between lecanoromycete–Trebouxiales symbioses and symbioses involving cyanobacteria. These trends may reflect nitrogen availability associated with the presence of masses of nitrogen-fixing cyanobacteria, although alternatively the structuring may be related to thallus pH. Gauslaa and Holien (1998) found that parmelioid lichens (corresponding to the symbioses that contain uronic acids, mentioned earlier) have a lower thallus pH than the surrounding bark, with values between 3.5 and 4.1 (bark pH 3.9 to 4.2, in each individual measurement higher than the lichen). Lichens with cyanobacteria, by contrast, had a pH between 4.4 and 4.8, higher than the bark on which they occur (pH 4.1 to 4.4). Whether or not the bacteria occur here because they prefer these habitats or whether they help build them (or both) is unknown.
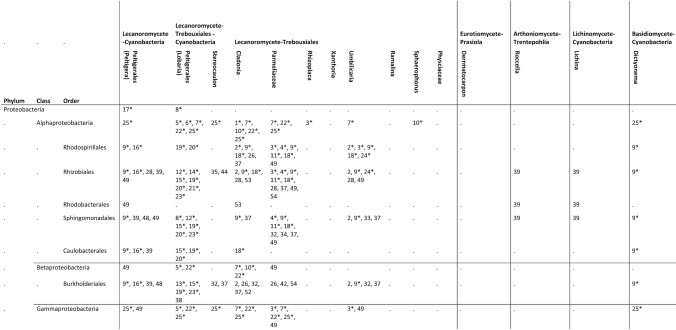
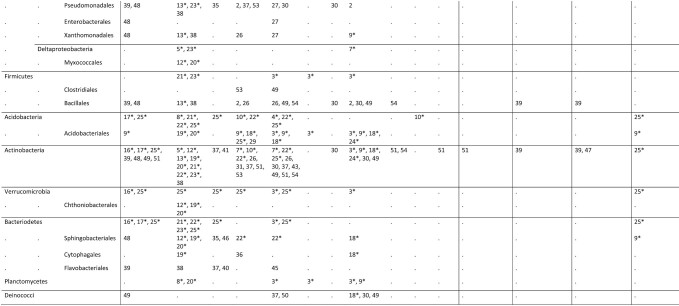
Table 1.
Overview of modern reports of Eubacteria from macrolichen symbioses to the level of order. For quantitative studies, only reports in which a bacterial group is represented by >5% of amplicons or reads are included. Quantitative studies are denoted by *.
|
|
Quantitative studies [1–25]: 1: Cardinale et al. 2008; 2: Grube et al. 2009; 3: Bates et al. 2011; 4: Mushegian et al. 2011; 5: Schneider et al. 2011; 6: Cardinale et al. 2012a; 7: Cardinale et al. 2012b; 8: Grube et al. 2012; 9: Hodkinson et al. 2012; 10: Pankratov 2012; 11: Printzen et al. 2012; 12: Aschenbrenner et al. 2014; 13: Cernava et al. 2015b; 14: Erlacher et al. 2015; 15: Grube et al. 2015; 16: Sigurbjörnsdóttir et al. 2015; 17: Garg et al. 2016; 18: Park et al. 2016; 19: Aschenbrenner et al. 2017; 20: Cernava et al. 2017; 21: Eymann et al. 2017; 22: Pankratov 2018; 23: Cernava et al. 2019; 24: Tzovaras et al. 2019; 25: Sierra et al. 2019; Qualitative studies (culture or PCR) [26–54]: 26: Cardinale et al. 2006; 27: Liba et al. 2006; 28: Hodkinson and Lutzoni 2009; 29: Pankratov and Dedysh 2010: 30: Selbmann et al. 2010; 31: Cardinale et al. 2011; 32: Kim et al. 2012; 33: Lee et al. 2012a; 34: Lee et al. 2012b; 35: Kim et al. 2013; 36: Lee et al. 2013; 37: Lee et al. 2014; 38: Cernava et al. 2015a; 39: Parrot et al. 2015; 40: Ahn et al. 2016; 41: Han et al. 2016a; 42: Han et al. 2016b; 43: Han et al. 2016c; 44: Kang et al. 2016; 45: Oh et al. 2016a; 46: Oh et al. 2016b; 47: Parrot et al. 2016; 48: Sigurbjörnsdóttir and Vilhelmsson 2016; 49: Jiang et al. 2017; 50: Kim et al. 2017; 51: Liu et al. 2017; 52: Yang et al. 2017; 53: Almendras et al. 2018; 54: Kim et al. 2019
The location and abundance of bacteria within lichen thalli has been visualized by fluorescent in situ hybridization combined with confocal laser scanning microscopy since Cardinale et al. (2008) applied the method in the reindeer lichen Cladonia arbuscula. Although bacteria have been localized as free-living cells adhering to fungal hyphae outside of biofilms (Cardinale et al. 2008), the greatest numbers of bacteria appear embedded in EIMs (Cardinale et al. 2008, 2012a; Grube et al. 2009, 2015; Aschenbrenner et al. 2014; Erlacher et al. 2015). Estimates of the numbers of bacteria, e.g. in Cladonia, averaged 6.27 ± 4.37 × 107 g–1, with single estimates of 2.1 ± 0.38 × 108 bacteria g–1 (Cardinale et al. 2008), similar to or greater than the estimates of Krasil'nikov (1949).
The only lichen-associated bacteria for which carbohydrate use and production characteristics are available based on direct experimental evidence are Granulicella (Acidobacteria; Pankratov and Dedysh 2010) and Lichenibacter (Pankratov et al.2019). Pankratov and Dedysh (2010) described five cultured species of Granulicella, including one (G. paludicola) from Cladonia lichens, that grew optimally at pH 3.8–4.5, hydrolyzed pectin and xylan as well as, notably, laminarin and lichenan, and produced an amorphous EPS matrix, putatively composed of polysaccharides. However, the exact nature of Granulicella-derived EPS matrix is unknown and it has yet to be demonstrated in whole lichens. In Lichenibacter, bacterial cells were only found to utilize starch and xylan (Pankratov et al. 2019).
Less is known of the carbohydrate use and EPS production of other lichen-associated bacteria. However, the EPS-producing capacity of some can be inferred by the presence of genes for nitrogen fixation; the ability to fix atmospheric N is dependent on creating at least a partially anaerobic biofilm environment, which is made possible by EPS components such as polysaccharides. The nifH gene, which is involved in N fixation, is known from several lichen-associated alpha-proteobacteria (Grube et al. 2009; Almendras et al. 2018), gamma-proteobacteria (Liba et al. 2006), Firmicutes (Grube et al. 2009; Almendras et al. 2018) and Actinobacteria (Almendras et al. 2018). Prominent among the first group are Rhizobiales, with the lichen-associated LAR1 clade thought to carry the nifH gene (Hodkinson and Lutzoni 2009). While LAR1 culture attributes have not been characterized in detail, other symbiotic Rhizobiales deploy several exopolysaccharides in initiation of root nodule symbiosis (Fraysse et al. 2003). The best-known Rhizobiales exopolysaccharides are succinoglycan and a pyruvulated α-(1→3)-galactoglucan; the latter can also occur with the substitution of GlcA for single glucose monomers (Skorupska et al. 2006). Root nodules mature as organs once Rhizobiales EPS is sensed by the plant, the infection thread formed and colonization for symbiotic nitrogen fixation has started. Root nodulation and nitrogen fixation are under quorum sensing control, a cell–cell density sensing mechanism that enables bacteria such as rhizobia to coordinate gene expression through the exchange of signals in biofilm matrix (Rosemeyer et al. 1998; Daniels et al. 2002). This is important because the biofilm lifestyle for bacteria is encased in polysaccharide, creating their 3D architecture and the medium for communication.
EPS production in bacteria is commonly inversely regulated with motility and colonization as the EPS embeds the cell to a surface. As the cells continue to grow, microniches form within the biofilm due to localized heterogeneity in metabolites (Battin et al. 2007; Limoli, Jones and Wozniak 2015). Importantly, this allows oxygen gradients to be established so that aerobic (e.g. respiration) and anaerobic (e.g. nitrogen fixation) processes can occur within the same community. This type of cellular differentiation is distinct from nitrogen-fixing cyanobacteria, where cell differentiation of heterocyst allows nitrogen fixation to occur adjacent to photosynthesizing cells generating oxygen (Risser and Callahan 2009). Besides the role EPS plays in creating anaerobic niches, it has recently been found to play a role in the differentiation of nitrogen-fixing bacteroid cells in rhizobia (Hawkins et al. 2017) and protection against low pH and metals (Kopycinska et al. 2018).
Basidiomycetous yeasts
Although Lenova and Blum (1983) already reviewed the presence of yeasts in lichens, the widespread occurrence of basidiomycetous yeasts in certain groups of macrolichens has only recently been recognized. Spribille et al. (2016) documented high constancy of unculturable Cyphobasidiales yeasts embedded in the EIM of parmelioid lichens in particular. They also detected these yeasts in isolated samples of other lecanoromycete-based symbioses. Since then, Černajova and Škaloud (2019) have detected culturable strains of a separate lineage of Cystobasidiomycetes, which they named Lichenozyma, from Cladonia symbioses, and Tuovinen et al. (2019) detected high global constancy of Tremella yeasts in the lecanoromycete–trebouxia symbiosis Letharia vulpina. It is possible that Tremella yeasts broadly correspond to earlier reports of Cryptococcus (Lenova and Blum 1983), as this genus is nested within Tremella in recent phylogenetic analyses (Liu et al. 2015).
While little is known of the carbohydrate use and production by Cystobasidiomycetes aside from the composition of their cell walls (Takashima et al. 2000), much is known about members of the Tremella clade. This is largely owing to the existence of a well-studied deadly pathogen (Cryptococcus neoformans) and one species of economic interest (Tremella fuciformis) in this clade of Tremellales. Both produce copious exopolysaccharide capsule material in the form of glucuronoxylomannans (GXMs; Gorin and Barreto-Bergter 1983; De Baets and Vandamme 2001; Martinez and Casadevall 2015). In cases where fungi are dimorphic, such as T. fuciformis, GXMs have been shown to be produced by both the teleomorph and the anamorph (Kakuta et al. 1979). Recent studies of C. neoformans have further revealed the deposition of GXMs far from the actual fungal cells, so-called exo-GXM (Denham et al. 2018). No GXM or monosaccharide profile consistent with GXM has yet been reported from lichens, but given the frequent ensconcement of Tremella yeasts in lichens, we consider it likely that GXM will occur. Suggestively, the symbioses from which uronic acids have been detected (Tuominen 1967; Teixeira et al. 1995) include those that harbor both tremelloid and cystobasidiomycete yeasts.
WHERE TO FROM HERE?
Polysaccharide studies from whole lichen thalli have provided troves of information on structural cell wall components for the symbiont that provides the bulk of cellular biomass in lichens, the filamentous fungus. However, multiple lines of evidence, including the low detection rate for fungal chitin, the near-absence of detection of algal polysaccharides and the spotty detection of methylated and sulfated residues, leave little doubt that the surveys to date are not exhaustive. These observations, coupled with the paucity of detailed studies on localization of most detected compounds, mean it is not currently possible to paint an accurate picture of the composition of EIMs. It follows from this that it is also not currently feasible to rule in or out, based on direct evidence, whether EIMs are materially altered by UNPOs. That being said, the phylogenetic neighborhoods of detected UNPOs suggest that it is a reasonable hypothesis that they utilize and produce carbohydrates in situ in lichen EIMs.
Narrowing the scope of questions regarding lichen EIMs holds promise for obtaining a clearer understanding of the biology at play. First, targeting polysaccharides by focusing on purified extracts or even narrower fractions of EIMs, as well as widening the analytical toolbox to target methylated and sulfated polysaccharides, would provide much more specific data on natural matrices without getting swamped by bulk cell wall signal. Second, ascertaining carbohydrate use and production by UNPOs in culture, such as has been done for Granulicella (Pankratov and Dedysh 2010), can provide clues to symbiont functioning. For UNPOs that cannot be cultured, it may be possible to predict carbohydrate activity via genomes assembled from deeply sequenced metagenomes, facilitated by international databases of carbohydrate active enzymes (Cantarel et al. 2009). A third line of research for interpreting UNPO occurrence is the development of statistical quantification protocols to determine abundance and frequency of UNPOs in different symbioses.
It is too early to say where research on the role of UNPOs in lichen symbioses will lead. Two attributes of the system make the symbiotic give-and-take difficult to pin down. First, the goods and services exchanged may be among the most structurally diverse and difficult biopolymers to study in all of organic chemistry; second, the symbionts are difficult to detect in vivo and even more difficult to obtain in vitro. As a result, we are not yet at the point where we can clearly identify functionalities that allow us to place newly detected symbionts on the spectrum from mutualism to parasitism. This research goes at the heart of the question of what is fundamentally required to build individual lichen symbioses. At present, the assumptions for what is required to synthesize a natural, whole thallus of a given symbiosis are still largely untested, but the evidence accumulated so far points to three hypothetical possibilities (Fig. 4). Models for ‘what it takes to make a lichen’ must account for the possibility that some or all symbioses may be capable of forming a fully developed thallus based on fungus and photosymbiont alone, under the right conditions (Fig. 4A); that others may require one or more additional UNPOs to form the thallus ‘base plan’ (Fig. 4B); and that some or all may form fully developed thalli using one of these two ‘base plans’, but be capable of taking ‘add-on’ UNPO symbionts that may add an optional veneer changing the interaction of the whole symbiosis with its environment (Fig. 4C). By asking new questions about the EIM and treating it as a discrete entity, we will be able to generate data to test these hypotheses and probe the mechanisms that led to the evolution and sustenance of 3D biofilms.
Figure 4.
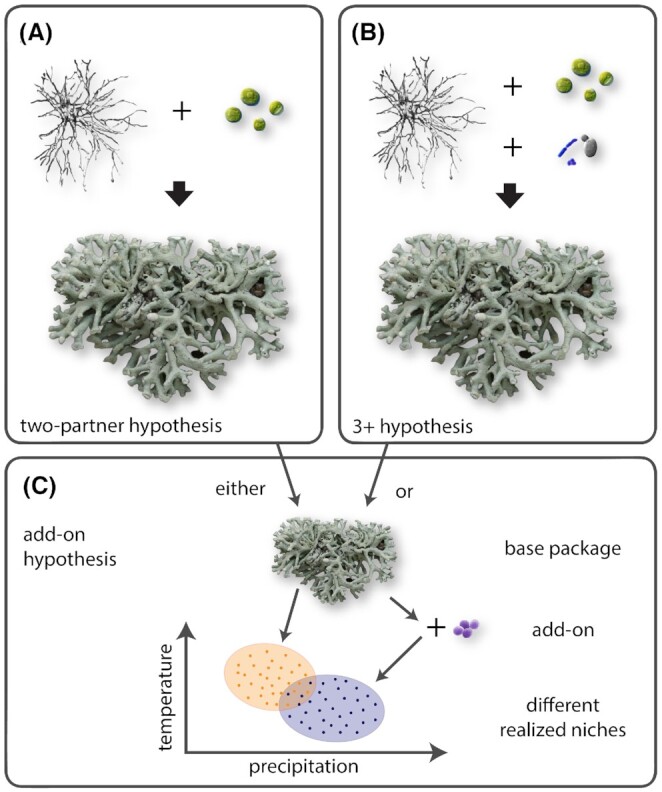
Theoretical models of lichen symbioses. Note that because lichen symbioses are highly polyphyletic along the evolutionary trees of each of their symbionts, and no a priori constraints exist for number of symbionts, no single model is likely to fit for all symbioses. (A) The traditional ‘two-partner’ hypothesis still widely espoused in lichen research holds that a fully formed, complete lichen thallus is achieved by fungus and photosymbiont alone; (B) the ‘3+ hypothesis’ proposes that a fully formed, complete lichen thallus will not be achieved without the fungus and photosymbiont as well as one or more additional UNPOs; and (C) the ‘add-on’ hypothesis proposes that either A or B could be used to achieve a ‘base package’, but that other UNPOs may facultatively join to form an additional, new, stable lichen symbiosis, sufficiently influencing the symbiotic outcome such that the realized niche and perhaps phenotype are altered. Photo credit Tim Wheeler.
FUNDING
Work by TS, GT and SG was supported by a National Science and Engineering Research Council of Canada (NSERC) Discovery grant to TS as well as a Tier II Canada Research Chair. RC acknowledges support from NSERC, and WZ acknowledges funding from NSERC and the Canada Foundation for Innovation. We are grateful to Arlene Oatway (Microscopy Core Facility, University of Alberta) for assistance with microscopy. We thank Carmen Allen, Erin Carr, Yngvar Gauslaa, Paulo Modenesi and Göran Thor for commenting on earlier versions of this manuscript.
Conflict of interest None declared.
REFERENCES
- Ahmadjian V. The Lichen Symbiosis. New York: John Wiley & Sons, 1993. [Google Scholar]
- Ahn D-H, Han S-R, Oh T-Jet al.. Complete genome sequence of ionizing radiation-resistant Hymenobacter sp. strain PAMC26628 isolated from an Arctic lichen. J Biotechnol. 2016;223:50–1. [DOI] [PubMed] [Google Scholar]
- Almendras K, García J, Carú Met al.. Nitrogen-fixing bacteria associated with Peltigera Cyanolichens and Cladonia Chlorolichens. Molecules. 2018;23:3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglesea D, Greenhalgh GN, Veltkamp CJ. The cortex of branch tips in Usnea subfloridana. T Brit Mycol Soc. 1983b;81:438–44. [Google Scholar]
- Anglesea D, Greenhalgh GN, Veltkamp CJ. The structure of the thallus tip in Usnea subfloridana. Lichenologist. 1983a;15:73–80. [Google Scholar]
- Anglesea D, Veltkamp C, Greenhalgh GNet al.. The upper cortex of Parmelia saxatilis and other lichen thalli. Lichenologist. 1982;14:29–38. [Google Scholar]
- Aschenbrenner IA, Cardinale M, Berg Get al.. Microbial cargo: do bacteria on symbiotic propagules reinforce the microbiome of lichens? Env Microbiol. 2014;16:3743–52. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner IA, Cernava T, Berg Get al.. Understanding microbial multi-species symbioses. Front Microbiol. 2016;7:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner IA, Cernava T, Erlacher Aet al.. Differential sharing and distinct co-occurrence networks among spatially close bacterial microbiota of bark, mosses and lichens. Mol Ecol. 2017;26:2826–38. [DOI] [PubMed] [Google Scholar]
- Barbosa SB, Machado SR, Marcelli MP. Thallus structure and isidium development in two Parmeliaceae species (lichenized Ascomycota). Micron. 2009b;40:536–42. [DOI] [PubMed] [Google Scholar]
- Barbosa SB, Marcelli MP, Machado SR. Evaluation of different protocols for anatomical studies in Parmeliaceae (lichenized Ascomycota). Micron. 2009a;40:218–25. [DOI] [PubMed] [Google Scholar]
- Bates ST, Cropsey GWG, Caporaso JGet al.. Bacterial communities associated with the lichen symbiosis. Appl Environ Microb. 2011;77:1309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battin TJ, Sloan WT, Kjelleberg Set al.. Microbial landscapes: new paths to biofilm research. Nat Rev Microbiol. 2007;5:76–81. [DOI] [PubMed] [Google Scholar]
- Bednar TW, Juniper BE. Microfibrillar structure in the fungal portions of the lichen Xanthoria parietina (L.) Th. Fr. Exp Cell Res. 1964;36:680–3. [DOI] [PubMed] [Google Scholar]
- Biosca EG, Flores R, Santander RDet al.. Innovative approaches using novel lichen enriched media to improve isolation and culturability of lichen associated bacteria. PLoS One. 2016;11:e0160328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buston HW, Chambers VH. Some cell-wall constituents of Cetraria islandica (“Iceland Moss”). Biochem J. 1933;27:1691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarel BL, Coutinho PM, Rancurel Cet al.. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 2009;37:D233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonero ER, Cordeiro LM, Mellinger CGet al.. Galactomannans with novel structures from the lichen Roccella decipiens Darb. Carbohyd Res. 2005b;340:1699–705. [DOI] [PubMed] [Google Scholar]
- Carbonero ER, Montai AV, Mellinger CGet al.. Glucans of lichenized fungi: significance for taxonomy of the genera Parmotrema and Rimelia. Phytochemistry. 2005a;66:929–34. [DOI] [PubMed] [Google Scholar]
- Carbonero ER, Montai AV, Woranovicz-Barreira SMet al.. Polysaccharides of lichenized fungi of three Cladina spp.: significance as chemotypes. Phytochemistry. 2002b;61:681–6. [DOI] [PubMed] [Google Scholar]
- Carbonero ER, Sassaki GL, Gorin PAet al.. A (1→ 6)-linked β-mannopyrananan, pseudonigeran, and a (1→ 4)-linked β-xylan, isolated from the lichenised basidiomycete Dictyonema glabratum. FEMS Microbiol Lett. 2002a;206:175–8. [DOI] [PubMed] [Google Scholar]
- Carbonero ER, Smiderle FR, Gracher AHPet al.. Structure of two glucans and a galactofuranomannan from the lichen Umbilicaria mammulata. Carbohyd Polym. 2006;63:13–8. [Google Scholar]
- Cardinale M, Castro JV Jr, Müller Het al.. In situ analysis of the bacterial community associated with the reindeer lichen Cladonia arbuscula reveals predominance of Alphaproteobacteria. FEMS Microbiol Ecol. 2008;66:63–71. [DOI] [PubMed] [Google Scholar]
- Cardinale M, Grube M, Berg G. Frondihabitans cladoniiphilus sp. nov., an actinobacterium of the family Microbacteriaceae isolated from lichen, and emended description of the genus Frondihabitans. Intl J Syst Mol Microbiol. 2011;61:3033–8. [DOI] [PubMed] [Google Scholar]
- Cardinale M, Grube M, Castro Jr JVet al.. Bacterial taxa associated with the lung lichen Lobaria pulmonaria are differentially shaped by geography and habitat. FEMS Microbiol Lett. 2012a;329:111–5. [DOI] [PubMed] [Google Scholar]
- Cardinale M, Puglia AM, Grube M. Molecular analysis of lichen-associated bacterial communities. FEMS Microbiol Ecol. 2006;57:484–95. [DOI] [PubMed] [Google Scholar]
- Cardinale M, Steinová J, Rabensteiner Jet al.. Age, sun and substrate: triggers of bacterial communities in lichens. Env Microbiol Rep. 2012b;4:23–8. [DOI] [PubMed] [Google Scholar]
- Casano LM, Braga MR, Álvarez Ret al.. Differences in the cell walls and extracellular polymers of the two Trebouxia microalgae coexisting in the lichen Ramalina farinacea are consistent with their distinct capacity to immobilize extracellular Pb. Plant Sci. 2015;236:195–204. [DOI] [PubMed] [Google Scholar]
- Cengia Sambo M. Ancora della polysimbiosi nei licheni ad alghe cianoficee. 1. Batteri simbionti. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano. 1926;64:191–5. [Google Scholar]
- Centeno DC, Hell AF, Braga MRet al.. Contrasting strategies used by lichen microalgae to cope with desiccation–rehydration stress revealed by metabolite profiling and cell wall analysis. Env Microbiol. 2016;18:1546–60. [DOI] [PubMed] [Google Scholar]
- Černajová I, Škaloud P. The first survey of Cystobasidiomycete yeasts in the lichen genus Cladonia; with the description of Lichenozyma pisutiana gen. nov., sp. nov. Fungal Biol. 2019;123:625–37. [DOI] [PubMed] [Google Scholar]
- Cernava T, Aschenbrenner IA, Grube Met al.. A novel assay for the detection of bioactive volatiles evaluated by screening of lichen-associated bacteria. Front Microbiol. 2015a;6:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernava T, Aschenbrenner IA, Soh Jet al.. Plasticity of a holobiont: desiccation induces fasting-like metabolism within the lichen microbiota. ISME J. 2019;13:547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernava T, Erlacher A, Aschenbrenner IAet al.. Deciphering functional diversification within the lichen microbiota by meta-omics. Microbiome. 2017;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernava T, Müller H, Aschenbrenner IAet al.. Analyzing the antagonistic potential of the lichen microbiome against pathogens by bridging metagenomic with culture studies. Front Microbiol. 2015b;6:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YS, Zheng Y, Labavitch JMet al.. The impact of cell wall carbohydrate composition on the chitosan flocculation of Chlorella. Process Biochem. 2011;46:1927–33. [Google Scholar]
- Cordeiro LM, Carbonero ER, Sassaki GLet al.. A fungus-type β-galactofuranan in the cultivated Trebouxia photobiont of the lichen Ramalina gracilis. FEMS Microbiol Lett. 2005b;244:193–8. [DOI] [PubMed] [Google Scholar]
- Cordeiro LM, de Fátima Reinhardt V, Iacomini Met al.. Glucomannan and branched (1→ 3)(1→ 6) β-glucan from the aposymbiotically grown Physcia kalbii mycobiont. Phytochemistry. 2012;84:88–93. [DOI] [PubMed] [Google Scholar]
- Cordeiro LM, de Oliveira SM, Buchi DFet al.. Galactofuranose-rich heteropolysaccharide from Trebouxia sp., photobiont of the lichen Ramalina gracilis and its effect on macrophage activation. Int J Biol Macromol. 2008;42:436–40. [DOI] [PubMed] [Google Scholar]
- Cordeiro LM, Montai AV, Gorin PAet al.. Polysaccharide production by the chlorolichen Pseudocyphellaria clathrata. Bryologist. 2005a;108:118–23. [Google Scholar]
- Cordeiro LM, Reis RA, Tischler CAet al.. Linear β-mannose-containing polysaccharide, β-xylan, and amylose from the cultured photobiont Trebouxia sp. of the ascolichen Ramalina celastri. FEMS Microbiology Letters. 2003a;220:89–94. [DOI] [PubMed] [Google Scholar]
- Cordeiro LM, Sassaki GL, Gorin PAet al.. O-Methylated mannogalactan from the microalga Coccomyxa mucigena, symbiotic partner of the lichenized fungus Peltigera aphthosa. Phytochemistry. 2010;71:1162–7. [DOI] [PubMed] [Google Scholar]
- Cordeiro LM, Sassaki GL, Iacomini M. First report on polysaccharides of Asterochloris and their potential role in the lichen symbiosis. Int J Biol Macromol. 2007;41:193–7. [DOI] [PubMed] [Google Scholar]
- Cordeiro LM, Stocker-Wörgötter E, Gorin PAet al.. Comparative studies of the polysaccharides from species of the genus Ramalina—lichenized fungi—of three distinct habitats. Phytochemistry. 2003b;63:967–75. [DOI] [PubMed] [Google Scholar]
- Cordeiro LMC, Beilke F, Reinhardt VFet al.. Rhamnogalactofuranan from the microalga Myrmecia biatorellae, symbiotic partner of Lobaria linita. Phytochemistry. 2013;94:254–9. [DOI] [PubMed] [Google Scholar]
- Corradi da Silva ML, Iacomini M, Jablonski Eet al.. Carbohydrate, glycopeptide and protein composition of the lichen Sticta sp. and effect of storage. Phytochemistry. 1993;33:547–52. [DOI] [PubMed] [Google Scholar]
- Dahlman L, Zetherström M, Sundberg Bet al.. Measuring ergosterol and chitin in lichens. In: Kranner IC, Beckett R, Varma A (eds). Protocols in Lichenology—Culturing, Biochemistry, Physiology and use in Biomonitoring. Berlin, Heidelberg: Springer, 2002, 348–62. [Google Scholar]
- Daniels R, De Vos DE, Desair Jet al.. The cin quorum sensing locus of Rhizobium etli CNPAF512 affects growth and symbiotic nitrogen fixation. J Biol Chem. 2002;277:462–8. [DOI] [PubMed] [Google Scholar]
- De Baets S, Vandamme EJ. Extracellular Tremella polysaccharides: structure, properties and applications. Biotechnol Lett. 2001;23:1361–6. [Google Scholar]
- Dembitsky VM. Lipids of lichens. Prog Lipid Res. 1992;31:373–97. [DOI] [PubMed] [Google Scholar]
- Denham ST, Verma S, Reynolds RCet al.. Regulated release of cryptococcal polysaccharide drives virulence and suppresses immune cell infiltration into the central nervous system. Infect Immun. 2018;86:e00662–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Philippis R, Sili C, Paperi Ret al.. Exopolysaccharide-producing cyanobacteria and their possible exploitation: a review. J Appl Phycol. 2001;13:293–9. [Google Scholar]
- Dyer P. Hydrophobins in the lichen symbiosis. New Phytol. 2002;154:1–4. [Google Scholar]
- Elifio SL, Corradi da Silva ML, Iacomini Met al.. A lectin from the lichenized basidiomycete Dictyonema glabratum. New Phytol. 2000;148:327–34. [Google Scholar]
- Erlacher A, Cernava T, Cardinale Met al.. Rhizobiales as functional and endosymbiontic members in the lichen symbiosis of Lobaria pulmonaria L. Front Microbiol. 2015;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseen PA, Olsson T, Coxson Det al.. Morphology influences water storage in hair lichens from boreal forest canopies. Fungal Ecol. 2015;18:26–35. [Google Scholar]
- Eymann C, Wegner U, Bernhardt Jet al.. Symbiotic interplay of fungi, algae, and bacteria within the lung lichen Lobaria pulmonaria L. Hoffm. as assessed by state-of-the-art metaproteomics. J Proteome Res. 2017;16:2160–73. [DOI] [PubMed] [Google Scholar]
- Fraysse N, Couderc F, Poinsot V. Surface polysaccharide involvement in establishing the rhizobium-legume symbiosis. Eur J Biochem. 2003;270:1365–80. [DOI] [PubMed] [Google Scholar]
- Galun M, Braun A, Frehnsdorff Aet al.. Hyphal walls of isolated lichen fungi. Autoradiographic localization of precursor incorporation and binding of fluorescein-conjugated lectins. Arch Microbiol. 1976;108:9–16. [DOI] [PubMed] [Google Scholar]
- Garg N, Zeng Y, Edlund Aet al.. Spatial molecular architecture of the microbial community of a Peltigera lichen. mSystems. 2016;1:e00139–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauslaa Y, Holien H. Acidity of boreal Picea abies-canopy lichens and their substratum, modified by local soils and airborne acidic depositions. Flora. 1998;193:249–57. [Google Scholar]
- Gerken HG, Donohoe B, Knoshaug EP. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta. 2013;237:239–53. [DOI] [PubMed] [Google Scholar]
- Giordani P, Modenesi P, Tretiach M. Determinant factors for the formation of the calcium oxalate minerals, weddellite and whewellite, on the surface of foliose lichens. Lichenologist. 2003;35:255–70. [Google Scholar]
- Goebel K v VII. Ein Beitrag zur Biologie der Flechten. Annales Jardin Botanique de Buitenzorg. 1926;36:1–83. [Google Scholar]
- Gorin PAJ, Barreto-Bergter E. The chemistry of polysaccharides of fungi and lichens. In: Aspinall GO (ed). The Polysaccharides. Vol. 2. New York: Academic Press, 1983, 365–409. [Google Scholar]
- Gorin PAJ, Iacomini M. Structural diversity of D-galacto-D-mannan components isolated from lichens having ascomycetous mycosymbionts. Carbohyd Res. 1985;142:253–67. [Google Scholar]
- Gow NAR, Latge J-P, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiology Spectrum. 2017;5:FUNK–0035-2016. [DOI] [PubMed] [Google Scholar]
- Greenhalgh GN, Whitfield A. Thallus tip structure and matrix development in Bryoria fuscescens. Lichenologist. 1987;19:295–305. [Google Scholar]
- Grube M, Cardinale M, Castro JV Jret al.. Species-specific structural and functional diversity of bacterial communities in lichen symbioses. ISME J. 2009;3:1105–15. [DOI] [PubMed] [Google Scholar]
- Grube M, Cernava T, Soh Jet al.. Exploring functional contexts of symbiotic sustain [sic] within lichen-associated bacteria by comparative omics. ISME J. 2015;9:412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, Köberl LS, Berg Cet al.. Host–parasite interaction and microbiome response: effects of fungal infections on the bacterial community of the Alpine lichen Solorina crocea. FEMS Microbiol Ecol. 2012;82:472–81. [DOI] [PubMed] [Google Scholar]
- Hale ME. Fine structure of the cortex in the lichen family Parmeliaceae viewed with the scanning electron microscope. Smithsonian Contr Bot. 1973;10:1–92. [Google Scholar]
- Han S-R, Kim K-H, Ahn D-Het al.. Complete genome sequence of carotenoid-producing Microbacterium sp. strain PAMC28756 isolated from an Antarctic lichen. J Biotechnol. 2016a;226:18–9. [DOI] [PubMed] [Google Scholar]
- Han S-R, Yu S-C, Ahn D-Het al.. Complete genome sequence of Burkholderia sp. strain PAMC28687, a potential octopine-utilizing bacterium isolated from Antarctica lichen. J Biotechnol. 2016b;226:16–7. [DOI] [PubMed] [Google Scholar]
- Han S-R, Yu S-C, Kang Set al.. Complete genome sequence of Frondihabitanssp. strain PAMC28766, a novel carotenoid-producing and radiation-resistant strain isolated from an Antarctic lichen. J Biotechnol. 2016c;226:20–1. [DOI] [PubMed] [Google Scholar]
- Hawkins JP, Geddes BA, Oresnik IJ. Succinoglycan production contributes to acidic pH tolerance in Sinorhizobium meliloti Rm1021. Mol Plant Microbe In. 2017;30:1009–19. [DOI] [PubMed] [Google Scholar]
- Henkel PA, Yuzhakova LA. Azotfiksiruyuschie bakterii v lishaynikah [nitrogen-fixing bacteria in lichens]. Izv Biol Inst Permsk Gos Univ. 1936;10:9–10. [Google Scholar]
- Hodkinson BP, Gottel NR, Schadt CWet al.. Photoautotrophic symbiont and geography are major factors affecting highly structured and diverse bacterial communities in the lichen microbiome. Env Microbiol. 2012;14:147–61. [DOI] [PubMed] [Google Scholar]
- Hodkinson BP, Lutzoni F. A microbiotic survey of lichen-associated bacteria reveals a new lineage from the Rhizobiales. Symbiosis. 2009;49:163–80. [Google Scholar]
- Honegger R, Bartnicki-Garcia B. Cell wall structure and composition of cultured mycobionts from the lichens Cladonia macrophylla, Cladonia caespiticia, and Physcia stellaris (Lecanorales, Ascomycota). Mycol Res. 1991;95:905–14. [Google Scholar]
- Honegger R, Haisch A. Immunocytochemical location of the (1→ 3)(1→ 4)-β-glucan lichenin in the lichen-forming ascomycete Cetraria islandica (Icelandic moss). New Phytol. 2001;150:739–46. [Google Scholar]
- Honegger R. Functional aspects of the lichen symbiosis. Annu Rev Plant Biol. 1991;42:553–78. [Google Scholar]
- Honegger R. The lichen symbiosis—what is so spectacular about it? Lichenologist. 1998;30:193–212. [Google Scholar]
- Hranisavljević-Jakovljević M, Miljković-Stojanović J, Dimitrijević Ret al.. An alkali-soluble polysaccharide from the oak lichen Cetraria islandica (L.) Ach. Carbohyd Res. 1980;80:291–5. [Google Scholar]
- Iacomini M, Schneider CL, Gorin PA. Comparative studies on the polysaccharides of Cladonia alpestris (Reindeer moss), Cladonia confusa, and Cladonia amaurocraea. Carbohyd Res. 1985;142:237–51. [Google Scholar]
- Iacomini M, Zanin SM, Fontana JDet al.. Isolation and characterization of β-D-glucan, heteropolysaccharide, and trehalose components of the basidiomycetous lichen Cora pavonia. Carbohyd Res. 1987:168:55–65. [Google Scholar]
- Iskina RE. K voprosu ob azotfiksiruyushchikh bakteriyakh v lishaynikakh (On the question of nitrogen-fixing bacteria in lichens). Izvestiya Permskogo Nauchno-Issledovatel'skogo Instituta. 1938;11:133–40. [Google Scholar]
- Jacobs JB, Ahmadjian V. The ultrastructure of lichens. I. A general survey. J Phycol. 1969;5:227–40. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Unckless R, Cockburn SNet al.. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science. 2010;329:212–5. [DOI] [PubMed] [Google Scholar]
- Jensen JSRE, Petersen BO, Veselinovic Tet al.. Structural characterisation of a new O-methylated heteroglycan, colleman, from the cyanolichen Collema flaccidum. Carbohyd Polym. 2010;80:799–807. [Google Scholar]
- Jiang D-F, Wang H-Y, Si H-Let al.. Isolation and culture of lichen bacteriobionts. Lichenologist. 2017;49:175–81. [Google Scholar]
- Jiao G, Yu G, Zhang Jet al.. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9:196–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuta M, Sone Y, Umeda Tet al.. Comparative structural studies on acidic heteropolysaccharides isolated from “Shirokikurage,” fruit body of Tremella fuciformis Berk, and the growing culture of its yeast-like cells. Agric Biol Chem. 1979;43:1659–68. [Google Scholar]
- Kang S, Han S-R, Oh T-Jet al.. Complete genome sequence of thiosulfate-oxidizing Bosea sp. strain PAMC26642 isolated from an Arctic lichen. J Biotechnol. 2016;223:38–9. [DOI] [PubMed] [Google Scholar]
- Kapaun E, Loos E, Reisser W. Cell wall composition of virus-sensitive symbiotic Chlorella species. Phytochemistry. 1992;31:3103–4. [Google Scholar]
- Kapaun E, Reisser W. A chitin-like glycan in the cell wall of a Chlorella sp. (Chlorococcales, Chlorophyceae). Planta. 1995;197:577–82. [Google Scholar]
- Kim B, Han S-R, Lamichhane Jet al.. Draft genome analysis of antimicrobial Streptomyces isolated from Himalayan lichen. J Microbiol Biotechnol. 2019;29:1144–54. [DOI] [PubMed] [Google Scholar]
- Kim J, Kwon KK, Kim BKet al.. Genome Sequence of Deinococcus marmoris PAMC 26562 isolated from Antarctic lichen. Genome Announc. 2017;5:e00013–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-K, Park H, Oh T-J. Antibacterial properties associated with microorganisms isolated from Arctic lichens. Korean J Microbiol Biotechnol. 2012;40:380–8. [Google Scholar]
- Kim M-K, Park H, Oh T-J. Antimicrobial properties of the bacterial associates of the Arctic lichen Stereocaulon sp. African J Microbiol Res. 2013;7:3651–7. [Google Scholar]
- Kjølberg O, Kvernheim AL. Studies on the polysaccharides of lichens. II. The structure of water-soluble polysaccharides in Umbilicaria pustulata (L.). Acta Chem Scand. 1984;38:735–9. [PubMed] [Google Scholar]
- Kjølberg O, Kvernheim AL. Studies on the polysaccharides of lichens. III. The structure of alkali-soluble polysaccharides in Umbilicaria pustulata (L.) Hoffm. and Umbilicaria spodochroa (Ach.) Hoffm. Acta Chem Scand. 1989;43:280–5. [Google Scholar]
- Kopycinska M, Lipa P, Ciesla Jet al.. Extracellular polysaccharide protects Rhizobium leguminosarum cells against zinc stress in vitro and during symbiosis with clover. Env Microbiol Rep. 2018;10:355–68. [DOI] [PubMed] [Google Scholar]
- Krasil'nikov NA. Mikroflora lishaynikov [microflora of lichens]. Mikrobiologiya. 1949;18:224–32. [Google Scholar]
- Leal JA, Prieto A, Bernabé Met al.. An assessment of fungal wall heteromannans as a phylogenetically informative character in ascomycetes. FEMS Microbiol Rev. 2010;34:986–1014. [DOI] [PubMed] [Google Scholar]
- Lee D-H, Hur JS, Kahng H-Y. Sphingobacterium cladoniae sp. nov., isolated from lichen, Cladonia sp., and emended description of Sphingobacterium siyangense. Intl J Syst Evol Microbiol. 2013;63:755–60. [DOI] [PubMed] [Google Scholar]
- Lee H, Shin SC, Lee Jet al.. Genome sequence of Sphingomonas sp. strain PAMC 26621, an arctic-lichen-associated bacterium isolated from a Cetraria sp. J Bacteriol. 2012b;194:3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Shin SC, Kim SJet al.. Draft genome sequence of a Sphingomonas sp., an endosymbiotic bacterium isolated from an Arctic lichen Umbilicaria sp. J Bacteriol. 2012a;194:3010–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Kim EH, Lee HKet al.. Biodiversity and physiological characteristics of Antarctic and Arctic lichens-associated bacteria. World J Microbiol Biotechnol. 2014;30:2711–21. [DOI] [PubMed] [Google Scholar]
- Lenova LI, Blum OB. K voprosu o tret'em komponente lishaynikov [On the question of the third component of lichens]. Bot Zhurn. 1983;68:21–8. [Google Scholar]
- Liba CM, Ferrara FIS, Manfio GPet al.. Nitrogen-fixing chemo-organotrophic bacteria isolated from cyanobacteria-deprived lichens and their ability to solubilize phosphate and to release amino acids and phytohormones. J Appl Microbiol. 2006;101:1076–86. [DOI] [PubMed] [Google Scholar]
- Limoli DH, Jones CJ, Wozniak DJ. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectrum. 2015;3:MB–0011-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Jiang Y, Wang Xet al.. Diversity, antimicrobial activity, and biosynthetic potential of cultivable Actinomycetes associated with lichen symbiosis. Microb Ecol. 2017;74:570–84. [DOI] [PubMed] [Google Scholar]
- Liu X-Z, Wang Q-M, Göker Met al.. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol. 2015;81:85–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado MJ, Gorin PA, Torri Get al.. The occurrence of glycolipids in the lichen Ramalina celastri. Braz J Med Biol Res. 1994;27:523–6. [PubMed] [Google Scholar]
- Martinez LR, Casadevall A. Biofilm formation by Cryptococcus neoformans. Microbiol Spectrum. 2015;3:MB–0006-2014. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai M, Hadfield MG, Bosch TCet al.. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA. 2013;110:3229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meichik NR, Vorob'ev DV. Chitin-glucan complex in cell walls of the Peltigera aphthosa lichen. Applied Biochem Micro. 2012;48:307–11. [PubMed] [Google Scholar]
- Mićović VM, Hranisavljević-Jakovljević M, Miljković-Stojanović J. Structural study of polysaccharides from the oak lichen Evernia prunastri (L) Ach.: Part I. An alkali-soluble galatomannan. Carbohyd Res. 1969;10:525–33. [Google Scholar]
- Modenesi P, Vanzo C. The cortical surfaces in Parmelia saxatilis and P. caperata: a histochemical approach. Lichenologist. 1986;18:329–38. [Google Scholar]
- Money NP. Mushroom stem cells. Bioessays. 2002;24:949–52. [DOI] [PubMed] [Google Scholar]
- Moran NA. Symbiosis as an adaptive process and source of phenotypic complexity. Proc Natl Acad Sci USA. 2007;104:8627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushegian AA, Peterson CN, Baker CCMet al.. Bacterial diversity across individual lichens. Appl Environ Microb. 2011;77:4249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa Y, Takeda T, Shibata Set al.. Polysaccharides of lichens and fungi. IV. Antitumour active O-acetylated pustulan-type glucans from the lichens of Umbilicaria species. Chem Pharm Bull. 1970;18:1431–4. [DOI] [PubMed] [Google Scholar]
- Noh H-J, Baek K, Hwang CYet al.. Lichenihabitans psoromatis gen. nov., sp. nov., a member of a novel lineage (Lichenihabitantaceae fam. nov.) within the order of Rhizobiales isolated from Antarctic lichen. Int J Syst Mol Microbiol. 2019;69:003695. [DOI] [PubMed] [Google Scholar]
- Oh T-J, Han S-R, Ahn D-Het al.. Complete genome sequence of Hymenobacter sp. strain PAMC26554, an ionizing radiation-resistant bacterium isolated from an Antarctic lichen. J Biotechnol. 2016a;227:19–20. [DOI] [PubMed] [Google Scholar]
- Oh T-J, Han S-R, Kang Set al.. Complete genome sequence of the xylan-degrading Mucilaginibacter sp. strain PAMC26640 isolated from an Arctic lichen. J Biotechnol. 2016b;227:23–24. [DOI] [PubMed] [Google Scholar]
- Olafsdottir ES, Ingólfsdottir K. Polysaccharides from lichens: structural characteristics and biological activity. Planta Med. 2001;67:199–208. [DOI] [PubMed] [Google Scholar]
- Olafsdottir ES, Omarsdottir S, Smestad Paulsen Bet al.. Rhamnopyranosylgalactofuranan, a new immunologically active polysaccharide from Thamnolia subuliformis. Phytomedicine. 1999;6:273–9. [DOI] [PubMed] [Google Scholar]
- Olafsdottir ES, Omarsdottir S, Smestad Paulsen Bet al.. Immunologically active O6-branched (1→3)-β-glucan from the lichen Thamnolia vermicularis var. subuliformis. Phytomedicine. 2003;10:318–24. [DOI] [PubMed] [Google Scholar]
- Omarsdottir S, Freysdottir J, Barsett Het al.. Effects of lichen heteroglycans on proliferation and IL-10 secretion by rat spleen cells and IL-10 and TNF-α secretion by rat peritoneal macrophages in vitro. Phytomedicine. 2005;12:461–7. [DOI] [PubMed] [Google Scholar]
- Omarsdottir S, Petersen BO, Barsett Het al.. Structural characterisation of a highly branched galactomannan from the lichen Peltigera canina by methylation analysis and NMR-spectroscopy. Carbohyd Polym. 2006;63:54–60. [Google Scholar]
- Pankratov TA, Dedysh SN. Granulicella paludicola gen. nov., sp. nov., Granulicella pectinivorans sp. nov., Granulicella aggregans sp. nov. and Granulicella rosea sp. nov., acidophilic, polymer-degrading acidobacteria from Sphagnum peat bogs. Intl J Syst Evol Microbiol. 2010;60:2951–9. [DOI] [PubMed] [Google Scholar]
- Pankratov TA, Grouzdev DS, Patutina EOet al.. Lichenibacterium ramalinae gen. nov, sp. nov., Lichenibacterium minor sp. nov., the first endophytic, beta-carotene producing bacterial representatives from lichen thalli and the proposal of the new family Lichenibacteriaceae within the order Rhizobiales. Antonie Van Leeuwenhoek, 2019; 10.1007/s10482-019-01357-6. [DOI] [PubMed] [Google Scholar]
- Pankratov TA, Kachalkin AV, Korchikov ESet al.. Microbial communities of lichens. Microbiology. 2017;86:293–309. [Google Scholar]
- Pankratov TA. Acidobacteria in microbial communities of the bog and tundra lichens. Microbiology. 2012;81:51–8. [PubMed] [Google Scholar]
- Pankratov TA. Bacterial complexes of Khibiny Mountains lichens revealed in Cladonia uncialis, C. portentosa, Alectoria ochroleuca, and Nephroma arcticum. Microbiology. 2018;87:79–88. [Google Scholar]
- Panosyan AK, Nikogosyan VG. K voprosu o nalichii azotfiksatorov v lishaynikah [On the question of the presence of diazotrophs in lichens]. Biol Zhurn Armen. 1966;19:3–11. [Google Scholar]
- Park CH, Kim KM, Kim O-Set al.. Bacterial communities in Antarctic lichens. Antarct Sci. 2016;28:455–61. [Google Scholar]
- Parrot D, Antony-Babu S, Intertaglia Let al.. Littoral lichens as a novel source of potentially bioactive Actinobacteria. Sci Rep. 2015;5:15839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrot D, Legrave N, Intertaglia Let al.. Cyaneodimycin, a bioactive compound isolated from the culture of Streptomyces cyaneofuscatus associated with Lichina confinis. Eur J Org Chem. 2016;3977–82. [Google Scholar]
- Pereira MI, Ruthes AC, Carbonero ERet al.. Chemical structure and selected biological properties of a glucomannan from the lichenized fungus Heterodermia obscurata. Phytochemistry. 2010;71:2132–9. [DOI] [PubMed] [Google Scholar]
- Pereyra MT, Prieto A, Bernabé Met al.. Studies of new polysaccharides from Lasallia pustulata (L.) Hoffm. Lichenologist. 2003;35:177–85. [Google Scholar]
- Pettolino FA, Walsh C, Fincher GBet al.. Determining the polysaccharide composition of plant cell walls. Nat Protoc. 2012;7:1509–607. [DOI] [PubMed] [Google Scholar]
- Peveling E. Die Darstellung der Oberflächenstrukturen von Flechten mit dem Raster-Elektronenmikroskop. Deutsche Bot Ges NF. 1970;4:89–101. [Google Scholar]
- Prado SRT, Gorin PAJ, Stuelp PMet al.. An unusual juxtaposition of polysaccharides components of Collema leptosporum. Carbohydr Polym. 1999;40:271–6. [Google Scholar]
- Prieto A, Leal JA, Bernabé Met al.. A polysaccharide from Lichina pygmaea and L. confinis supports the recognition of Lichinomycetes. Mycol Res. 2008;112:381–388. [DOI] [PubMed] [Google Scholar]
- Printzen C, Fernández-Mendoza F, Muggia Let al.. Alphaproteobacterial communities in geographically distant populations of the lichen Cetraria aculeata. FEMS Microbiol Ecol. 2012;82:316–25. [DOI] [PubMed] [Google Scholar]
- Reis RA, Tischer CA, Gorin PAet al.. A new pullulan and a branched (1→3)-,(1→6)-linked β-glucan from the lichenised ascomycete Teloschistes flavicans. FEMS Microbiol Lett. 2002;210:1–5. [DOI] [PubMed] [Google Scholar]
- Risser DD, Callahan SM. Genetic and cytological evidence that heterocyst patterning is regulated by inhibitor gradients that promote activator decay. Proc Natl Acad Sci USA. 2009;106:19884–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MA, Da Silva Bon EP. Evaluation of Chlorella (Chlorophyta) as source of fermentable sugars via cell wall enzymatic hydrolysis. Enzyme Res. 2011;2011:405603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemeyer V, Michiels J, Verreth Cet al.. luxI- and luxR-homologous genes of Rhizobium etli CNPAF512 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris. J Bacteriology. 1998;180:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthes AC, Komura DL, Carbonero ERet al.. Structural characterization of the uncommon polysaccharides obtained from Peltigera canina photobiont Nostoc muscorum. Carbohyd Polym. 2010;81:29–34. [Google Scholar]
- Sanders WB, Ascaso C. Reiterative production and deformation of cell walls in expanding thallus nets of the lichen Ramalina menziesii (Lecanorales, Ascomycetes). Am J Bot. 1995;82:1358–66. [Google Scholar]
- Sassaki GL, Gorin PA, Reis RAet al.. Carbohydrate, glycolipid, and lipid components from the photobiont (Scytonema sp.) of the lichen Dictyonema glabratum. Carbohydrate Res. 2005;340:1808–17. [DOI] [PubMed] [Google Scholar]
- Sassaki GL, Gorin PA, Tischer CAet al.. Sulfonoglycolipids from the lichenized basidiomycete Dictyonema glabratum: isolation, NMR and ESI-MS approaches. Glycobiology. 2001;11:345–51. [DOI] [PubMed] [Google Scholar]
- Scherrer S, Haisch A, Honegger Ret al.. Characterization and expression of XPH1, the hydrophobin gene of the lichen‐forming ascomycete Xanthoria parietina. New Phytologist. 2002;154:175–84. [Google Scholar]
- Schlarmann G, Peveling E, Tenberge K. The occurrence of chitin in the cell walls of ascomycetes mycobionts. Biblioth Lichenol. 1990;38:395–409. [Google Scholar]
- Schneider T, Schmid E, Castro Jr JVet al.. Structure and function of the symbiosis partners of the lung lichen (Lobaria pulmonaria L. Hoffm.) analyzed by metaproteomics. Proteomics. 2011;11:2752–6. [DOI] [PubMed] [Google Scholar]
- Selbmann L, Zucconi L, Ruisi Set al.. Culturable bacteria associated with Antarctic lichens: affiliation and psychrotolerance. Polar Biol. 2010;33:71–83. [Google Scholar]
- Sierra MA, Danko DC, Sandoval TAet al.. The microbiomes of seven lichen genera reveal host specificity, a reduced core community and potential as source of antimicrobials. BioRxiv. 2019; 10.1101/789032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurbjörnsdóttir MA, Andrésson ÓS, Vilhemsson O. Analysis of the Peltigera membranacea metagenome indicates that lichen-associated bacteria are involved in phosphate solubilization. Microbiology. 2015;161:989–96. [DOI] [PubMed] [Google Scholar]
- Sigurbjörnsdóttir MA, Vilhelmsson O. Selective isolation of potentially phosphate-mobilizing, biosurfactant-producing and biodegradative bacteria associated with a sub-Arctic, terricolous lichen, Peltigera membranacea. FEMS Microbiol Ecol. 2016;92:fiw090. [DOI] [PubMed] [Google Scholar]
- Skorupska A, Janczarek M, Marczak Met al.. Rhizobial exopolysaccharides: genetic control and symbiotic functions. Microb Cell Fact. 2006;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg YJ. Studies on the chemistry of lichens. IX: Quantitative determination of monosaccharides and amino acids in hydrolysates of several Norwegian lichen species. Lichenologist. 1970;4:283–8. [Google Scholar]
- Spribille T, Tuovinen V, Resl Pet al.. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science. 2016;353:488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spribille T. Relative symbiont input and the lichen symbiotic outcome. Curr Opin Plant Bio. 2018;44:57–63. [DOI] [PubMed] [Google Scholar]
- Stocker-Wörgötter E, Cordeiro LMC, Iacomini M. Accumulation of potential pharmaceutically relevant lichen metabolites in lichens and cultured lichen symbionts. Stud Nat Prod Chem. 2013;39:337–80. [Google Scholar]
- Sun Y, Hou S, Song Set al.. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int J Biol Macromol. 2018;112:985–95. [DOI] [PubMed] [Google Scholar]
- Suzuki MT, Parrot D, Berg Get al.. Lichens as natural sources of biotechnologically relevant bacteria. Appl Microbiol Biot. 2016;100:583–95. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Takeda T, Shibata Set al.. Polysaccharides of lichens and fungi. VI. Antitimour active polysaccharides of lichens of Stictaceae. Chem Pharm Bull. 1974;22:404–8. [Google Scholar]
- Takashima M, Hamamoto M, Nakase T. Taxonomic significance of fucose in the class Urediniomycetes: distribution of fucose in cell wall and phylogeny of urediniomycetous yeasts. Syst Appl Microbiol. 2000;23:63–70. [DOI] [PubMed] [Google Scholar]
- Takeda H, Hirokawa T. Studies on the cell wall of Chlorella I. Quantitative changes in cell wall polysaccharides during the cell cycle of Chlorella ellipsoidea. Plant Cell Physiol. 1978;19:591–8. [Google Scholar]
- Takeda H. Sugar composition of the cell wall and the taxonomy of Chlorella (Chlorophyceae). J Phycol. 1991;27:224–32. [Google Scholar]
- Teixeira AZ, Iacomini M, Gorin PA. An unusual glucomannan from Tornabenia intricata. Phytochemistry. 1992;31:3467–70. [DOI] [PubMed] [Google Scholar]
- Teixeira AZ, Iacomini M, Gorin PA. Chemotypes of mannose-containing polysaccharides of lichen mycobionts: a possible aid in classification and identification. Carbohyd Res. 1995;266:309–14. [Google Scholar]
- Teixeira AZ, Iacomini M, McCune Bet al.. Heteropolysaccharides of the lichen Evernia prunastri. Carbohyd Res. 1994;264:63–71. [DOI] [PubMed] [Google Scholar]
- Trembley ML, Ringli C, Honegger R. Differential expression of hydrophobins DGH1, DGH2 and DGH3 and immunolocalization of DGH1 in strata of the lichenized basidiocarp of Dictyonema glabratum. New Phytol. 2002;154:185–95. [Google Scholar]
- Tretiach M, Crisafulli P, Pittao Eet al.. Isidia ontogeny and its effect on the CO2 gas exchanges of the epiphytic lichen Pseudevernia furfuracea (L.) Zopf. Lichenologist. 2005;37:445–62. [Google Scholar]
- Tuominen Y. Studies on the strontium uptake of the Cladonia alpestris thallus. Ann Bot Fenn. 1967;4:1–28. [Google Scholar]
- Tuovinen V, Ekman S, Thor Get al.. Two basidiomycete fungi in the cortex of wolf lichens. Curr Biol. 2019;29:476–83. [DOI] [PubMed] [Google Scholar]
- Tzovaras BG, Segers FHID, Bicker Aet al.. What is in a lichen? A metagenomic approach to reconstruct the holo-genome of Umbilicaria pustulata. BioRxiv. 2019; 10.1101/810986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares F. Texture and hygroscopic features of the upper surface of the thallus in the lichen family Umbilicariaceae. Ann Bot 1994;73:493–500. [Google Scholar]
- Varki A, Cummings RD, Aebi Met al.. Symbol nomenclature for graphical representation of glycans. Glycobiology. 2015;25:1323–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Esko JDet al.. Essentials of Glycobiology, 3rd edn. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press, 2017. [PubMed] [Google Scholar]
- Wang JH, Du YQ, Sun HJet al.. Extraction and preliminary characterization of polysaccharide from Umbilicaria esculenta cultivated in Huangshan Mountain. Biotechnol Biotec Eq. 2015;29:714–22. [Google Scholar]
- Wang Y, Shu X, Chen Yet al.. Enrichment, purification and in vitro antioxidant activities of polysaccharides from Umbilicaria esculenta macrolichen. Biochem Engineering J. 2018;130:10–20. [Google Scholar]
- Woranovicz-Barreira SM, Gorin PAJ, Sassaki PLet al.. Galactomannoglucans of lichenized fungi of Cladonia spp.: significance as chemotypes. FEMS Microbiology Letters. 1999;181:313–7. [DOI] [PubMed] [Google Scholar]
- Woranovicz SM, Gorin PAJ, Marcelli MPet al.. Structural studies on the galactomannans of lichens of the genus Cladonia. Lichenologist. 1997;29:471–81. [Google Scholar]
- Woranovicz SM, Pinto BM, Gorin PAJet al.. Novel structures in galactoglucomannans of the lichens Cladonia substellata and Cladonia ibitipocae: significance as chemotypes. Phytochemistry. 1999;51:395–402. [DOI] [PubMed] [Google Scholar]
- Yang JA, Hong SG, Oh H-M. Genome sequence of Caballeronia sordidicola strain PAMC 26577 isolated from Cladonia sp., an Arctic lichen species. Korean J Microbiol. 2017;53:141–3. [Google Scholar]
- Zanetti CA, Marcelli MP, Jungbluth P. Comparative anatomy of Canoparmelia and Crespoa species (Parmeliaceae, lichenized fungi). Bryologist. 2015;118:184–95. [Google Scholar]
- Zippel B, Neu TR. Characterization of glycoconjugates of extracellular polymeric substances in tufa-associated biofilms by using fluorescence lectin-binding analysis. Appl Environ Microb. 2011;77:505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]