Summary
Background
Influenza is an important cause of morbidity and mortality worldwide. Treatment options are scarce, and new drugs with novel mechanisms of action are needed. We aimed to assess the efficacy and safety of nitazoxanide, a thiazolide anti-infective, for treatment of acute uncomplicated influenza.
Methods
We did a double-blind, randomised, placebo-controlled, phase 2b/3 trial in 74 primary care clinics in the USA between Dec 27, 2010, and April 30, 2011. We enrolled participants aged 12–65 years with fever, at least one respiratory symptom, and one constitutional symptom of influenza within 48 h of symptom onset. We randomly assigned participants to receive either nitazoxanide 600 mg, nitazoxanide 300 mg, or placebo twice daily for 5 days, (ratio 1:1:1) and followed them up for 28 days. Randomisation lists were computer generated and done in blocks of three. Sponsor, investigators, study monitors, patients, and laboratory personnel were all masked to treatment allocation in the study. The primary endpoint was the time from first dose to alleviation of symptoms. The primary analysis was by intention-to-treat for participants with influenza infection confirmed by RT-PCR or culture at baseline. This trial is registered with ClinicalTrials.gov, number NCT01227421.
Findings
Of 650 participants screened, 624 (96%) were enrolled. Of these, 212 were randomly assigned to receive placebo twice a day, 201 to receive nitazoxanide 300 mg twice a day, and 211 to receive nitazoxanide 600 mg a day. The median duration of symptoms for participants receiving placebo was 116·7 h (95% CI 108·1–122·1) compared with 95·5 h (84·0–108·0; p=0·0084) for those receiving 600 mg nitazoxanide and 109·1 h (96·1–129·5, p=0·52) for those receiving 300 mg nitazoxanide. Adverse events were similar between the three groups, the most common being headache reported by 24 (11%) of 212 patients enrolled in placebo group, 12 (6%) of 201 patients in the low-dose group, and 17 (8%) of 211 patients in the high-dose group, or diarrhoea, reported by seven (3%) patients in the placebo group, four (2%) patients enrolled in the low-dose group, and 17 (8%) patients in the high-dose group.
Interpretation
Treatment with nitazoxanide 600 mg twice daily for 5 days was associated with a reduction of the duration of symptoms in participants with acute uncomplicated influenza. Further studies are warranted to confirm these findings and to assess efficacy of the drug alone or in combination with existing drugs in seriously ill patients and those at risk of influenza complications.
Funding
Romark Laboratories LC.
Introduction
Influenza, a contagious respiratory illness caused by influenza A, B, or C viruses, is an infection of global concern resulting in about 3–5 million cases of severe disease and 250 000–500 000 deaths annually.1 In the USA, seasonal influenza affects—on average—5–20% of the population per year leading to roughly 200 000 hospital admissions and 3000–49 000 deaths.2, 3 The threat of pandemic influenza caused by emerging viruses such as the avian AH5N1 or more recently AH7N9 is a major concern because of potential effects on human life, economy, national security, and functioning of society. Because of widespread adamantine resistance, the treatment of influenza is at present limited to the neuraminidase inhibitors, oseltamivir and zanamivir. New drugs with novel mechanisms of action that can be used alone or in combination with neuraminidase inhibitors are urgently needed to improve treatment outcomes and mitigate risks of resistance.
Nitazoxanide, a first-in-class thiazolide anti-infective, inhibits replication of a broad range of influenza viruses, including neuraminidase inhibitor-resistant strains, blocking the maturation of viral haemagglutin at the post-translational level.4, 5 In cell culture studies, nitazoxanide acts synergistically with neuraminidase inhibitors.5 Repeated passage of influenza viruses in subinhibitory concentrations of the drug have proven unsuccessful in selecting resistant strains suggesting a high barrier to resistance.5 Nitazoxanide also inhibits replication of respiratory viruses including parainfluenza virus, coronavirus, and respiratory syncytial virus in cell cultures.5
Nitazoxanide (Alinia, Romark Laboratories LC) is licensed in the USA for treatment of diarrhoea caused by Cryptosporidium parvum and Giardia lamblia,6 and has been widely used throughout Latin America for treatment of intestinal parasitic infections. A 300 mg controlled-release tablet was developed to deliver plasma concentrations suitable for treatment of viral respiratory infections. We aimed to assess safety and efficacy of two different doses of nitazoxanide in reducing the duration of symptoms of acute uncomplicated influenza.
Methods
Study design and participants
We designed this phase 2b/3 randomised clinical trial in consultation with US Food and Drug Administration (FDA) Guidance for Industry7 and published clinical trials of oseltamivir and zanamivir.8, 9, 10, 11, 12 Efficacy of nitazoxanide in treatment of acute uncomplicated influenza had to be established before doing studies in seriously ill patients or patients at high risk of complications of influenza. Because of the variability of disease and difficulties defining margins of non-inferiority to existing drugs, we used a placebo to clearly assess the activity of the drug. The 300 mg and 600 mg doses of nitazoxanide used in this study were selected on the basis of past experience with the pharmacokinetics and tolerability.13
We recruited patients from 74 primary care clinics in the USA. The main eligibility criteria were participants aged 12–65 years, oral temperature greater than 38°C (>100·4°F), at least one respiratory symptom (cough, sore throat, nasal discharge, nasal congestion, sneezing) and one constitutional symptom (headache, myalgia, sweats or chills, fatigue), symptom duration of 48 h or less, and confirmation of influenza by a laboratory in the local community (30 mile radius of the site). We excluded because of severity of illness requiring admission to hospital, high risk of influenza-related complications according to Infectious Diseases Society of America guidelines14 or present US Centers for Disease Control and Prevention (CDC) criteria,15 vaccination for seasonal influenza on or after Aug 1, 2010, treatment with any dose of oseltamivir, zanamivir, amantadine, rimantadine, nitazoxanide, or any investigational drug therapy within 30 days before screening, and active respiratory allergies or pre-existing illnesses that could place the participant at an unreasonably increased risk.
The central institutional review board (IRB) or a local IRB at each centre approved the protocol. The trial was done under an investigational new drug application with the FDA and done in accordance with guidelines set by the World Medical Assembly (Declaration of Helsinki, last amendment in Seoul, 2008). Every participant or their guardian gave written informed consent. Participants younger than 18 years of age provided written assent.
Randomisation and masking
Randomisation lists were computer generated. Every participant was randomly assigned (ratio 1:1:1) to receive either two bottles containing nitazoxanide 300 mg tablets (nitazoxanide 600 mg group), one bottle containing nitazoxanide 300 mg tablets plus one bottle containing placebo tablets (nitazoxanide 300 mg group), or two bottles of placebo (placebo group). Nitazoxanide tablets and placebo tablets were packaged in white high-density polyethylene bottles, each containing ten tablets. Participants were instructed to take two tablets, one from each bottle, twice daily with food for 5 days. The randomisation lists and medication packages were prepared by an independent third party who maintained the blinding of the trial until the database was locked. Randomisation was done in blocks of three. Masked study medication (three treatment kits per block) were assigned to every investigator who sequentially assigned treatment numbers to participants as they were enrolled. The randomisation list was masked to study participants such as sponsor, investigators, study monitors, patients, and laboratory personnel.
Procedures
Immediately after obtaining informed consent and verifying eligibility, every patient had a baseline (day 1) physical examination and medical history recorded. We obtained two nasopharyngeal swabs (nylon flocked dry swabs, Copan Diagnostics, Murrieta, CA, USA), and blood and urine samples for laboratory safety testing and calculation of influenza antibody titres. Study drugs were dispensed, and participants were instructed to use a diary twice daily (roughly every 12 h) to record the time and amount of every dose of study drug, symptom severity, concomitant medications, and adverse events. At every diary entry, the participants graded all nine symptoms (runny nose, nasal congestion, sore throat, cough, headache, muscle aches, tiredness or fatigue, feverish, and sweats or chills) on a scale of 0 to 3 as absent (0), mild (1), moderate (2), or severe (3). Participants completed the diaries up to at least study day 7, or until all symptoms were either absent or mild and had remained so for at least 24 h.
A study nurse visited or telephoned every participant daily on study days 2–5 to review symptoms and check for influenza-related complications. Participants returned to the clinic for physical examination and diary review on day 7 and day 28. We obtained blood and urine samples for laboratory safety tests and nasopharyngeal swabs for virology testing on day 7. We obtained a blood sample for measurement of influenza antibody titres on day 28. At ten sites, a nurse visited participants on days 2, 3, 4, and 5 to collect two nasopharyngeal swabs. We selected 24 participants for 12 h assessment of pharmacokinetics immediately after their first dose on the morning of day 2 (12 h after their last dose on day 1). Antiviral drugs for influenza, over-the-counter cough or cold medications, and anti-allergy drugs for respiratory allergies were prohibited during the study. Paracetamol was allowed as necessary for fever (oral temperature ≥38°C). To monitor compliance, participants returned the bottles in which the medication was dispensed along with any unused medication, and pill counts were recorded.
Laboratory safety tests were haematology tests (complete blood count with differential), blood chemistry tests (comprehensive metabolic panel with lipid profile), and urinalysis at baseline and on day 7. North Shore-LIJ Health System Laboratories in collaboration with BARC USA (Lake Success, NY, USA) did virology tests on nasopharyngeal swab, which were placed into 3 mL of viral transport medium and transported refrigerated to a central laboratory within 24 h. The swab samples were eluted, divided into aliquots, and immediately frozen at −70°C. Nasopharyngeal swabs at baseline and on day 7 were cultured for influenza viruses and analysed by RT-PCR with the ProdesseProflu+ (Gen-Probe Prodesse, Waukesha, WI, USA) for influenza A and B and the Luminex xTag RVP v1 assay (Luminex Corporation, Austin, TX, USA) to detect influenza A (non-specific for subtype), influenza A subtypes seasonal H1 and H3, influenza B, respiratory syncytial virus A, respiratory syncytial virus B, adenovirus, human meta-pneumovirus, enterovirus or rhinovirus, parainfluenza viruses 1, 2, 3, and 4, and coronaviruses 229E, OC43, NL63, and HKU1. Samples positive for influenza A were also analysed by real-time RT-PCR for 2009 influenza A H1N1 on the basis of CDC protocol.16 For participants positive for influenza A or B by culture or RT-PCR at baseline, viral titres were measured at baseline and all subsequent collection points. We calculated titres as log10 tissue culture infective dose50 (TCID50)/0·2 mL and as log10 RNA copies with a quantitative RT-PCR method developed and validated by North Shore-LIJ Health System Laboratories. For participants diagnosed with influenza by culture or RT-PCR at baseline, we measured influenza antibody titres by haemagglutination inhibition assay from serum samples obtained at baseline and at day 28 to assess effect of treatment on humoral immune response. Reference antigens used for measurement of antibody titres were H1/A/California/7/2009, H3/A/Perth/16/2009, and B/Brisbane/60/2008. To assess potential for resistance, we tested the viruses cultured from nasopharyngeal swab samples of nitazoxanide-treated participants on day 5 or day 7 and the corresponding virus sample from baseline (day 1) for susceptibility to tizoxanide as previously described.5
Outcomes
The primary endpoint for the clinical trial was time from first dose to alleviation of symptoms based on patient-reported symptom data. This method has been validated by clinical trials of oseltamivir and zanamivir.8, 9, 10, 11, 12 We deemed the symptoms of the participants as alleviated at the beginning of the first 24 h period throughout which each symptom was graded as either absent or mild (0 or 1). In addition to the seven symptoms (cough, nasal obstruction, sore throat, fatigue, headache, myalgia, and feverishness) monitored in the oseltamivir and zanamivir studies, participants enrolled in this study also monitored runny nose and sweats or chills. Secondary endpoints were change in influenza virus titre with time, time to cessation of viral shedding, time to alleviation of individual symptoms, symptom severity, complications of influenza, time to return to normal activity, time lost from work, and influenza antibody response.
Statistical analysis
We analysed data according to a statistical analysis plan developed before the start of the clinical trial. The plan called for two primary efficacy analyses, each comparing the time from first dose to alleviation of symptoms for nitazoxanide 300 mg versus placebo, and nitazoxanide 600 mg versus placebo using a Kaplan-Meier survival analysis, and a Cox proportional hazards test including geographic location as a factor, two-sided α=0·025. We checked the proportional hazards assumption by including interaction terms of all covariates with (log)time and by visual inspection of the graph of the log(-log(survival)) versus the log of time. If hazards were not proportional, we used a Prentice-Wilcoxon test. We did analyses for a population consisting of participants who received at least one dose of study drug and had laboratory-confirmed influenza infection by RT-PCR or culture at baseline. We did sensitivity analyses to assess effects of paracetamol use (repeating the primary analysis including paracetamol use and its interaction with the treatment group) and censored data (assuming participants with censored data had 28 days to alleviation of symptoms).
Sample size calculations assumed a median time to symptom alleviation for placebo group of 4·3 days, a difference in median time to symptom alleviation between placebo and nitazoxanide treatment groups of 1·5 days, and a dropout rate of 5%. With a log-rank test, with α=0·025 (due to two primary efficacy analyses) and a power of 80%, a sample size of 146 randomly assigned and treated participants with laboratory-confirmed influenza per group was calculated.
We also did secondary efficacy analyses of time to alleviation of symptoms for all participants who received study drug irrespective of laboratory evidence of infection and for participants with or without confirmed respiratory virus infections. We did statistical comparisons of changes in viral titre with a mixed model for repeated measures including baseline viral titre, treatment group, and geographic location. The safety population included all participants who received at least one dose of study medication. All analyses were done with SAS software (version 6.12; SAS Institute, Cary, NC, USA).
This trial is registered with ClinicalTrials.gov, number NCT01227421.
Role of the funding source
The sponsor designed the protocol and engaged a contract research organisation to select, initiate, monitor, and close out study sites and collect and analyse data. The sponsor wrote the study report. All authors participated in the interpretation of data, writing of the manuscript, and decision to submit the manuscript for publication. All authors had full access to the data.
Results
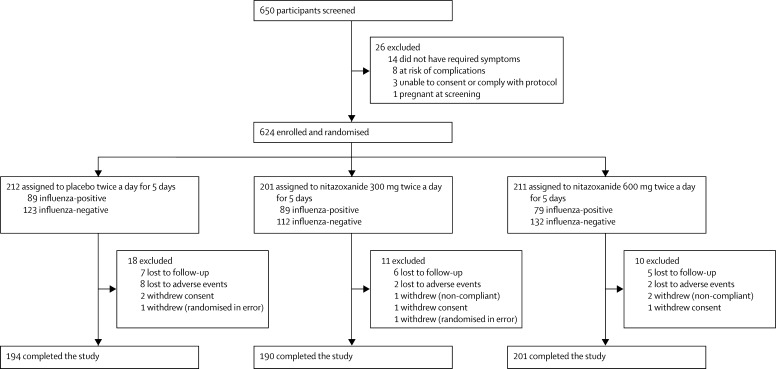
Between Dec 27, 2010, and April 30, 2011, we enrolled 624 participants with influenza-like illness of 650 people screened at 74 centres in the USA (figure 1 ). The study was terminated with about 60% of the planned number of patients infected with influenza because of the end of the influenza season. After randomisation, patients' characteristics were well balanced between groups (table 1 ). 386 (62%) of 624 participants enrolled were diagnosed with a respiratory virus infection at baseline including 257 (41%) with influenza A or B (table 2 ). Demographic and disease-related characteristics of participants were similar for each of the three treatment groups (Table 1, Table 2, Table 3 ).
Figure 1.
Trial profile
Table 1.
Demographic characteristics of treated patients
| Placebo (n=212) | Nitazoxanide 300 mg (n=201) | Nitazoxanide 600 mg (n=211) | |
|---|---|---|---|
| Ethnic origin | |||
| White | 140 (66%) | 120 (60%) | 138 (65%) |
| Black | 40 (19%) | 46 (23%) | 37 (18%) |
| Hispanic | 23 (11%) | 29 (14%) | 29 (14%) |
| Other | 9 (4%) | 6 (3%) | 7 (3%) |
| Sex | |||
| Male | 90 (43%) | 97 (48%) | 82 (39%) |
| Age (years) | |||
| Mean (SD) | 34·3 (13·3) | 32·5 (12·6) | 34·7 (13·2) |
| Range | 12–67 | 12–63 | 12–66 |
| Smoker | |||
| Yes | 37 (18%) | 30 (15%) | 25 (11%) |
| Weight (kg) | |||
| Mean (SD) | 84·7 (25·2) | 84·1 (23·7) | 83·9 (22·8) |
| Range | 40–186 | 44–167 | 39–163 |
| BMI (kg/m2) | |||
| Mean (SD) | 29·8 (7·8) | 28·8 (7·1) | 29·6 (7·9) |
| Range | 17·3–57·2 | 16·6–54·5 | 13·9–59·2 |
Data are n (%) unless otherwise indicated. BMI=body-mass index.
Table 2.
Viruses identified at baseline
| Placebo (n=212) | Nitazoxanide 300 mg (n=201) | Nitazoxanide 600 mg (n=211) | ||
|---|---|---|---|---|
| Influenza A or B | 89 (42%) | 89 (44%) | 79 (37%) | |
| Influenza A | 62 (29%) | 63 (31%) | 56 (27%) | |
| 2009 H1N1 | 37 (17%) | 31 (15%) | 38 (18%) | |
| H3 | 18 (8%) | 20 (10%) | 13 (6%) | |
| Subtype undetermined* | 7 (3%) | 12 (6%) | 5 (2%) | |
| Influenza B | 26 (12%) | 26 (13%) | 22 (10%) | |
| Influenza A and B | 1 (<1%) | 0 | 1 (<1%) | |
| Rhinovirus | 25 (12%) | 34 (17%) | 29 (14%) | |
| All coronavirus | 6 (3%) | 3 (1%) | 6 (3%) | |
| Coronavirus (229E) | 0 | 1 (<1%) | 1 (<1%) | |
| Coronavirus (HKU1) | 1 (<1%) | 1 (<1%) | 0 | |
| Coronavirus (NL63) | 4 (2%) | 1 (<1%) | 2 (1%) | |
| Coronavirus (OC43) | 1 (<1%) | 1 (<1%) | 3 (1%) | |
| All RSV | 6 (3%) | 4 (2%) | 4 (2%) | |
| RSV A | 2 (1%) | 1 (<1%) | 1 (<1%) | |
| RSV B | 3 (1%) | 2 (1%) | 2 (1%) | |
| Subtype undetermined | 1 (<1%) | 1 (<1%) | 1 (<1%) | |
| All parainfluenza | 6 (3%) | 1 (<1%) | 5 (2%) | |
| Parainfluenza (1) | 0 | 0 | 1 (<1%) | |
| Parainfluenza (2) | 2 (1%) | 0 | 0 | |
| Parainfluenza (3) | 3 (1%) | 1 (<1%) | 3 (1%) | |
| Parainfluenza (4) | 1 (<1%) | 0 | 1 (<1%) | |
| hMPV | 5 (2%) | 4 (2%) | 0 | |
| Adenovirus | 1 (<1%) | 1 (<1%) | 0 | |
| No virus identified | 79 (37%) | 69 (34%) | 90 (43%) | |
Data are n (%). RSV=respiratory syncytial virus. hMPV=human metapneumovirus.
Influenza A-positive, but neither H1 nor H3 based upon RT-PCR method (Luminex xTag RVP v1).
Table 3.
Demographic and baseline disease characteristics (confirmed influenza population)
| Placebo (n=89) | Nitazoxanide 300 mg (n=89) | Nitazoxanide 600 mg (n=79) | |
|---|---|---|---|
| Ethnic origin | |||
| White | 67 (75%) | 61 (68%) | 59 (75%) |
| Black | 10 (11%) | 13 (15%) | 11 (14%) |
| Hispanic | 6 (7%) | 11 (12%) | 5 (6%) |
| Other | 6 (7%) | 4 (5%) | 4 (5%) |
| Sex | |||
| Male | 43 (48%) | 40 (45%) | 36 (46%) |
| Age (years) | |||
| Mean (SD) | 32·2 (12·4) | 31·6 (12·9) | 33·2 (14·1) |
| Range | 12–65 | 12–63 | 12–66 |
| Smoker | |||
| Yes | 17 (19%) | 9 (10%) | 9 (11%) |
| Weight (kg) | |||
| Mean (SD) | 82·7 (24·9) | 80·8 (24·6) | 81·8 (23·3) |
| Range | 41–171 | 44–167 | 39–148 |
| BMI (kg/m2) | |||
| Mean (SD) | 28·9 (7·7) | 27·7 (7·0) | 28·1 (7·7) |
| Range | 17·3–55·7 | 16·8–54·5 | 13·9–52–6 |
| Duration of symptoms (h) | |||
| Mean (SD) | 28·1 (11·0) | 28·7 (12·5) | 27·3 (11·5) |
| Range | 5–50 | 3–49 | 4–59 |
| Cause of illness | |||
| Influenza A | 62 (70%) | 63 (71%) | 56 (71%) |
| 2009 H1N1 | 37 (42%) | 31 (35%) | 38 (48%) |
| H3N2 | 18 (20%) | 20 (22%) | 13 (16%) |
| Subtype undetermined | 7 (8%) | 12 (13%) | 5 (6%) |
| Influenza B | 26 (29%) | 26 (29%) | 22 (28%) |
| Influenza A and B | 1 (1%) | 0 | 1 (1%) |
| Oral temperature (°C) | |||
| Mean (SD) | 38·6 (0·4) | 38·6 (0·4) | 38·6 (0·4) |
| Range | 100–104 | 100–103 | 100–103 |
| Symptom severity score, median (95% CI) | |||
| Runny nose | 2 (1–2) | 1 (1–2) | 2 (1–2) |
| Nasal congestion | 2 (2–2) | 2 (2–2) | 2 (1–2) |
| Sore throat | 2 (2–2) | 2 (1–2) | 2 (1–2) |
| Cough | 2 (2–2) | 2 (2–3) | 2·5 (2–3) |
| Headache | 2 (2–2) | 2 (2–3) | 2 (2–2) |
| Myalgia | 2·5 (2–3) | 3 (2–3) | 2 (2–3) |
| Tiredness or fatigue | 3 (2–3) | 3 (2–3) | 3 (2–3) |
| Fever | 2 (1–2) | 3 (2–3) | 2 (2–3) |
| Sweats/chills | 2 (2–2) | 2 (2–3) | 2 (2–3) |
Data are n (%) unless otherwise indicated. BMI=body-mass index.
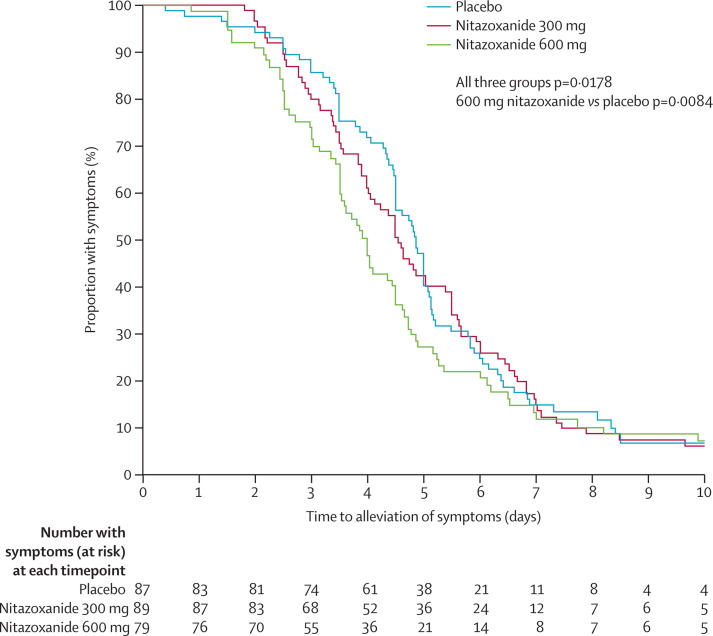
Treatment with nitazoxanide 600 mg twice daily for 5 days significantly decreased the time from first dose to alleviation of symptoms compared with placebo in participants with confirmed influenza (p=0·0084; figure 2 , table 4 ). Time to symptom alleviation also decreased in patients given nitazoxanide 300 mg compared with those given placebo, but the difference was not significant (p=0·52). We used Prentice-Wilcoxon test for these analyses because the proportional hazards assumption required for use of the Cox model was violated (interaction of the 600 mg nitazoxanide treatment group with log(time) was significant [p=0·0294]). At the time of symptom alleviation, no participants were still receiving symptom relief medication (paracetamol).
Figure 2.
Kaplan-Meier plot of time from first dose to alleviation of symptoms for patients with confirmed influenza
Table 4.
Time from first dose to alleviation of symptoms in different populations
|
Placebo |
Nitazoxanide 300 mg |
Nitazoxanide 600 mg |
||||||
|---|---|---|---|---|---|---|---|---|
| n | Median (95% CI) | n | Median (95% CI) | p value* | n | Median (95% CI) | p value* | |
| Confirmed influenza (primary analysis) | 89 | 116·7 (108·1–122·1) | 89 | 109·1 (96·1–129·5) | 0·52 | 79 | 95·5 (84·0–108·0) | 0·0084 |
| Enrolled within 36 h of symptom onset (subset) | 70 | 117·3 (107·7–122·1) | 63 | 108·1 (92·4–120·1) | 0·56 | 61 | 91·0 (84·0–108·0) | 0·047 |
| Influenza A (subset) | 62 | 117·3 (108·0–124·2) | 63 | 109·1 (96·0–120·5) | 0·23 | 56 | 98·0 (91·0–111·4) | 0·042 |
| Influenza B (subset) | 26 | 115·5 (105·1–123·5) | 26 | 109·8 (93·4–144·2) | 0·65 | 22 | 84·0 (79·9–108·0) | 0·096 |
| All treated participants (secondary analysis) | 212 | 108·2 (104·3–119·0) | 201 | 104·9 (95·5–110·3) | 0·32 | 211 | 94·9 (86·1–106·3 | 0·0052 |
| Enrolled within 36 h of symptom onset (subset) | 159 | 107·7 (96·0–116·7) | 153 | 103·1 (93·7–110·3) | 0·47 | 155 | 86·7 (79·3–95·5) | 0·0037 |
| No documented virus (subset) | 79 | 105·7 (91·4–130·9) | 69 | 94·5 (79·1–109·5) | 0·08 | 90 | 88·4 (72·2–106·3) | 0·021 |
p values are comparisons with placebo.
We did sensitivity analyses in the population with confirmed influenza to assess the effects of censored data and use of paracetamol on the primary endpoint. For participants with censored data (dropouts or without symptom alleviation at last diary assessment, n=16), we assumed time to alleviation of symptoms of 28 days. In this analysis, results were similar to the primary efficacy analysis favouring nitazoxanide 600 mg compared with placebo (p=0·0062 [data not shown).
212 (82%) of 257 participants enrolled in the population with confirmed influenza reported taking paracetamol during the study as allowed by the study protocol. Proportions of participants taking paracetamol were similar in the three treatment groups: 73 (82%) of 89 participants in the placebo group, 74 (83%) of 89 in the nitazoxanide 300 mg group, and 65 (82%) of 79 in the nitazoxanide 600 mg group. When we repeated the primary analysis including paracetamol use and its interaction with the treatment group, participants receiving nitazoxanide 600 mg had a shorter time to symptom alleviation than did those receiving placebo (p=0·0085).
In this study, we enrolled participants within 48 h of symptom onset, whereas in studies of neuraminidase inhibitors participants were enrolled within 36 h.8, 9, 11 To assess the effect of this variable, we analysed participants enrolled within 36 h of symptom onset. In these post-hoc analyses, differences (nitazoxanide 600 mg vs placebo) in median time to symptom alleviation were extended to 26·3 h for participants infected with confirmed influenza and 21·0 h for all treated participants (table 4).
Responses for subgroups infected with influenza A or B were similar (table 4). In analyses of 624 participants treated and 238 (38%) participants with no virus identified at baseline, time from first dose to alleviation of symptoms was significantly lower in the 600 mg group than in the placebo group (table 4). We investigated too few participants infected with other individual viral infections to make meaningful analyses.
Another difference between the design of this study and earlier studies of the neuraminidase inhibitors was the scoring of nine symptoms instead of seven.8, 9 Use of two additional symptoms, runny nose and sweats or chills, only affected the time to alleviation of symptoms for six participants (three given placebo, one given nitazoxanide 300 mg, and two given nitazoxanide 600 mg). Analysis based on the seven symptoms used in the oseltamivir studies showed median times to alleviation of symptoms of 115·9 h (95% CI 108–121) for the placebo group, 109·1 h (96–130, p=0·58) for the nitazoxanide 300 mg treatment group, and 95·5 h (84–108, p=0·0074) for the nitazoxanide 600 mg treatment group.
At baseline, cough and nasal congestion were the most persistent symptoms in participants infected with influenza. The median time to alleviation in the placebo group for cough was 104 h (95% CI 84–111) and for nasal congestion was 81 h (67–92). Treatment with nitazoxanide 600 mg was associated with a decrease in the median duration of these symptoms to 84 h (95% CI 60–104) for cough and to 60 h (48–74) for nasal congestion.
For nine symptoms, in participants infected with influenza, mean symptom score-hours (symptom severity score multiplied by hours) summed for every participant from baseline to alleviation of symptoms were 1221 (592 SD) for the placebo group, 1305 (765) for the nitazoxanide 300 mg treatment group, and 1125 (681) for the nitazoxanide 600 mg treatment group, whereas in all treated participants, the score-hours were 1232 (1028) for the placebo group, 1171 (972) for the 300 mg treatment group, and 1045 (661) for the 600 mg treatment group.
Complications of influenza (bronchitis, sinusitis, otitis, pneumonia, and pleurisy) were fairly uncommon in this population. The frequency was not significantly different between treatment groups: 15 events (7%) for placebo, compared with 20 (10%) for the nitazoxanide 300 mg treatment group (p=0·38), and 11 (5%) for the nitazoxanide 600 mg treatment group (p=0·54).
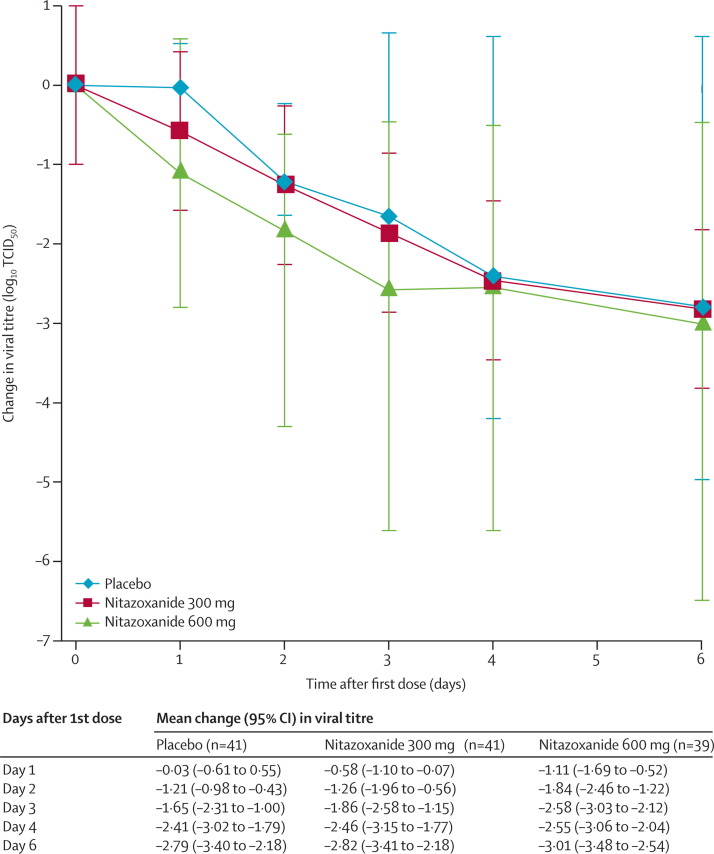
We obtained daily nasopharyngeal swabs at baseline and on days 2–5 for 113 participants (41 in the placebo group, 33 in the nitazoxanide 300 mg treatment group, and 39 in the nitazoxanide 600 mg treatment group). TCID50 viral titres significantly decreased during treatment in the nitazoxanide 600 mg group compared with placebo group (p=0·0006; figure 3 ). Reductions of roughly 1 log10 were apparent within 24 h after initiation of treatment for the nitazoxanide 600 mg group and continued until day 5 when viral shedding stopped for almost all participants in the placebo group. Reductions in TCID50 viral titres were also recorded for the nitazoxanide 300 mg treatment group compared with the placebo group, although these differences were not as large as for the nitazoxanide 600 mg treatment group and were not significant (figure 3). Reductions in viral titres detected by TCID50 were similar to those dectected by RT-PCR except that the magnitude of reductions were somewhat smaller when measured by RT-PCR, which also detects non-infectious virus particles. Median times to cessation of viral shedding were 91·3 h (95% CI 67·5–96·0) for the placebo group, 77·0 h (48·0–95·5) for the nitazoxanide 300 mg treatment group, and 71·8 h (55·0–96·0) for the nitazoxanide 600 mg treatment group. At day 7, only one participant (placebo group) was positive for influenza infection by viral culture. Influenza virus was detected by RT-PCR in samples from day 7 for 23 (39%) of 59 patients in the placebo group, 17 (19%) of 73 patients in the nitazoxanide 300 mg treatment group, and 15 (24%) of 63 patients in the 600 mg treatment group.
Figure 3.
Mean change in TCID50 viral titre from baseline
Analysis of change in TCID50 viral titre for participants with confirmed influenza that we took daily nasopharyngeal swabs from. Statistical comparison with mixed model for repeated measures including baseline viral titre, treatment group, and geographic location: p=0·0006 for the difference between nitazoxanide 600 mg and placebo, p=0·1553 for the difference between nitazoxanide 300 mg and placebo.
We cultured influenza viruses from nasopharyngeal swabs taken on day 5 from 13 participants treated with nitazoxanide. Viruses present in all 13 samples were inhibited by tizoxanide, with effective concentrations EC50 and EC90 similar to those inhibiting viruses present in the corresponding baseline samples (data not shown).
To assess potential effect of treatment on humoral immune response, we measured antibody titres at baseline and day 28 for participants with laboratory-confirmed influenza by RT-PCR or viral culture at baseline. We noted no significant differences in antibody titre change from baseline to day 28 or in the proportions of participants with laboratory-confirmed influenza seroprotected (antibody titre ≥40) or seroconverted (four-fold increase in antibody titre) at day 28 between the treatment groups. Seroconversion rates were 34 (49%) of 70 participants for the placebo group, 36 (58%) of 62 for the nitazoxanide 300 mg treatment group, and 36 (55%) of 65 for the nitazoxanide 600 mg treatment groups (p=0·52).
We analysed plasma concentrations of tizoxanide during the first 12 h after dosing on the morning of day 2 for six participants treated with nitazoxanide 300 mg and ten participants treated with nitazoxanide 600 mg. Mean maximum plasma concentrations of tizoxanide were 2·46 μg/mL (SD 1·36) for the nitazoxanide 300 mg group and 4·60 μg/mL (3·61) for the nitazoxanide 600 mg, and mean trough concentrations were 0·121 μg/mL (0·09) for the nitazoxanide 300 mg treatment group and 0·795 μg/mL (1·97) for the nitazoxanide 600 mg group. Mean area under the curve (AUC0–12 h) for tizoxanide plasma concentrations were 12·6 μg × h/mL (SD 9·2) for the nitazoxanide 300 mg group and 29·1 μg × h/mL (33·8) for the nitazoxanide 600 mg group.
259 participants (93 in the placebo group, 73 in the nitazoxanide 300 mg treatment group, and 93 in the nitazoxanide 600 mg treatment group) had a total of 545 adverse events. No life-threatening events were reported, and only 22 (4%) events were classified as severe (eight [4%] of 212 in the placebo group, six [3%] of 201 in the nitazoxanide 300 mg treatment group, eight [4%] of 211 in the nitazoxanide 600 mg treatment group). The frequency of adverse events was similar between the three treatment groups (table 5 ). Eight (3·8%) of 212 participants in the placebo group discontinued treatment because of an adverse event compared with two (1%) of 201 in the nitazoxanide 300 mg group and two (<1%) of 211 in the nitazoxanide 600 mg group. Laboratory values did not change significantly for any of the treatment groups (data not shown). Chromaturia (yellowish urine) was reported by six (3%) participants in the nitazoxanide 300 mg group and eight (4%) participants in the nitazoxanide 600 mg group compared with none in the placebo group. This event was probably attributed to the colour of nitazoxanide metabolites. Because of the mild nature and relative infrequency of chromaturia (14 [2%] of 624 participants enrolled), the blinding of the study was not affected.
Table 5.
Most common adverse events (≥1% of participants in any treatment group)
| Placebo (n=212) | Nitazoxanide 300 mg (n=201) | Nitazoxanide 600 mg (n=211) | |
|---|---|---|---|
| Diarrhoea | 7 (3%) | 4 (2%) | 17 (8%) |
| Headache | 24 (11%) | 12 (6%) | 17 (8%) |
| Bronchitis | 3 (1%) | 10 (5%) | 7 (3%) |
| Oropharyngeal pain | 7 (3%) | 5 (2%) | 10 (5%) |
| Abdominal pain | 7 (3%) | 4 (2%) | 8 (4%) |
| Vomiting | 2 (1%) | 3 (1%) | 8 (4%) |
| Chromaturia | 0 | 6 (3%) | 8 (4%) |
| Cough | 8 (4%) | 5 (2%) | 8 (4%) |
| Sinusitis | 8 (4%) | 6 (3%) | 3 (1%) |
| Nausea | 6 (3%) | 1 (<1%) | 6 (3%) |
| Pyrexia | 5 (2%) | 4 (2%) | 6 (3%) |
| Rhinorrhoea | 7 (3%) | 5 (2%) | 4 (2%) |
| Liver function tests abnormal | 4 (2%) | 5 (2%) | 5 (2%) |
| Wheezing | 3 (1%) | 2 (1%) | 5 (2%) |
| Nasal congestion | 5 (2%) | 3 (1%) | 5 (2%) |
| Insomnia | 4 (2%) | 0 | 5 (2%) |
| Chills | 0 | 4 (2%) | 1 (<1%) |
| Fatigue | 2 (1%) | 2 (1%) | 0 |
| Otitis media | 0 | 4 (2%) | 1 (<1%) |
| Dyspnoea | 3 (1%) | 2 (1%) | 4 (2%) |
| Ear pain | 3 (1%) | 2 (1%) | 3 (1%) |
| Musculoskeletal stiffness | 6 (3%) | 0 | 3 (1%) |
| Constipation | 0 | 2 (1%) | 0 |
| Dry mouth | 0 | 2 (1%) | 1 (<1%) |
| Nasopharyngitis | 0 | 2 (1%) | 1 (<1%) |
| Blood triglycerides increase | 0 | 2 (1%) | 0 |
| Lipase increase | 2 (1%) | 2 (1%) | 1 (<1%) |
| Poor quality sleep | 0 | 2 (1%) | 0 |
| Respiratory tract congestion | 0 | 2 (1%) | 0 |
| Night sweats | 1 (<1%) | 2 (1%) | 0 |
Data are number of patients (%).
Discussion
Treatment with nitazoxanide given orally 600 mg twice daily for 5 days beginning less than 48 h after symptom onset significantly reduced the time from first dose to alleviation of symptoms in participants aged 12–65 years with acute uncomplicated influenza. The 300 mg dose produced an intermediate response that did not significantly differ to that in the placebo group.
The trial was designed according to regulatory guidance and was consistent with previous trials of the neuraminidase inhibitors, oseltamivir and zanamivir (panel ).8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Reductions in time to alleviation of symptoms (median difference 21·2 h for the 600 mg group) were in the range of those recorded in trials of neuraminadase inhibitors.8, 9, 10, 11, 12, 17, 18, 19 Differences in the size of treatment effect from one trial to another could arise as a result of differences in study design (eg, duration of symptoms at time of enrolment, populations selected for analysis, use of paracetamol) or characteristics of circulating influenza strains (eg, pathogenicity, drug susceptibility). In this study, we enrolled participants within 48 h of symptom onset, whereas most clinical trials of neuraminidase inhibitors enrolled participants within 36 h of symptom onset. Time to symptom alleviation was fastest in the subset enrolled within 36 h of symptom onset into the nitazoxanide 600 mg group.
Panel. Research in context.
Systematic review
We searched PubMed for systematic reviews and meta-analyses published in English language between Jan 1, 2009, and Jan 29, 2014, with the search terms “influenza treatment”, “influenza clinical trial”, “oseltamivir”, “zanamivir”, and “neuraminidase inhibitor”. With the same search terms, we also searched for articles published in English between Jan 1, 1990, and Jan 29, 2014, reporting placebo-controlled clinical trials of drugs for treatment of influenza. We identified nine systematic reviews or meta-analyses within the past 5 years related to the use of the neuraminidase inhibitors, oseltamivir and zanamivir, and we identified several articles reporting randomised placebo-controlled clinical trials reporting a treatment effect of oral oseltamivir, inhaled zanamivir, or intravenous peramivir8, 9, 10, 11, 12, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 (another neuraminidase inhibitor) in reducing the time from first dose to alleviation of symptoms in participants with acute uncomplicated influenza. Articles have also reported beneficial treatment effect of oseltamivir and zanamivir in otherwise healthy children infected with influenza. Evidence to support treatment benefits of neuraminidase inhibitors in elderly and at-risk groups or their effects on rates of admission to hospital and mortality was insufficient.
Interpretation
In our trial, oral administration of nitazoxanide 600 mg twice daily for 5 days reduced the time to alleviation of symptoms of influenza in otherwise healthy adults and adolescents by roughly 1 day. Although many factors can affect the size of treatment effect observed in different clinical trials, the effect recorded in this trial is similar to that previously reported for the neuraminidase inhibitors.8, 9, 10, 11, 12, 17, 18, 27 Data from this trial suggest a treatment benefit of nitazoxanide in participants without influenza or other documented viral infection—a finding that has not been reported for the neuraminidase inhibitors. Importantly, nitazoxanide possesses a novel mechanism of action against influenza viruses. A new drug with a different mechanism of action could ultimately prove beneficial in overcoming resistance to neuraminidase inhibitors or in providing more potent therapy when used in combination with existing drugs. As a first step, it has been important to show effectiveness of nitazoxanide in a randomised placebo controlled trial by use of a method established for licensure of existing drugs. Further studies are warranted to confirm our findings and to assess efficacy of nitazoxanide alone or in combination with existing drugs in seriously ill patients and those at risk of influenza complications.
By contrast with studies of neuraminidase inhibitors, the results from this study show a potential treatment benefit in participants without influenza or other documented viral infection (17·3 h reduction in time to alleviation of symptoms for 600 mg vs placebo). Comparisons of treatment effect of nitazoxanide and neuraminidase inhibitors in participants with confirmed influenza infection or with or without other documented viral infections will be an interesting goal for future trials.
The adverse event profile of nitazoxanide is well known because it has been commercially available since 1996 in global markets for treatment of intestinal infections. This study used a new controlled-release formulation of nitazoxanide designed specifically to deliver drug into the blood and to the respiratory tract and to maintain blood concentrations for 12 h. In the 600 mg dose group, the drug was given at a dose that is 20% higher and for a duration of therapy that is 67% longer than the drug's approved dose for treatment of intestinal infections.6 This increase in dose and duration did not result in any significant change in the known side-effect profile. Frequency or severity of adverse events did not significantly differ between participants in any group of the study.
The findings of this clinical trial need to be confirmed by other studies. Limitations of this study include the number of participants and the fact that they were all enrolled during a single influenza season. The absence of statistical significance for the 300 mg treatment group might reflect an absence of statistical power because only about 60% of the planned number of participants were recruited. Although this trial enrolled participants with influenza AH3N2, ApH1N1, and B, examination of the efficacy and safety of the drug against influenza strains from another season would be beneficial. The drug does not seem to be associated with resistance, as might be expected because of its mechanism of action, but further studies to assess the potential for resistance would be valuable. Furthermore, although this clinical trial has shown a treatment effect in otherwise healthy participants aged 12–65 years, the effect of the drug still needs to be studied in children and patients with serious illness or those at risk of influenza complications.
An international phase 3 clinical trial in participants with acute uncomplicated influenza is ongoing (NCT01610245), which is designed to compare nitazoxanide given orally 600 mg twice daily for 5 days with placebo and to compare the combination of nitazoxanide and oseltamivir with oseltamivir or nitazoxanide given as monotherapy. Another trial in participants admitted to hospital with severe acute respiratory illness (NCT02057757) is in progress. These studies and others should provide additional information related to the value of the drug in the treatment of influenza and other viral respiratory infections.
Acknowledgments
Acknowledgments
The clinical study was done in the USA under the US Food and Drug Administration IND #107 316. We thank Romark Laboratories LC, Tampa, FL, USA, for sponsoring the study; BARC USA, Lake Success, NY, USA, for serving as central laboratory for the study and doing laboratory safety and virology studies; SGS Life Science Services, Gaithersburg, MD, USA, for serving as contract research organisation for the study; and the laboratory of Gabriella Santoro, University of Rome Tor Vergata, Rome, Italy, for doing resistance testing. The international phase 3 clinical trial (NCT01610245) referred to in the Discussion section is funded in whole or in part with Federal funds from the US Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority under contract HHSO100201300004C.
Contributors
J-FR designed the study. JH, AH, MH, HR, and SS recruited participants and collected data. CG supervised the clinical virology work. All authors reviewed and interpreted the data. MB and J-FR drafted the report. All authors approved the final report.
Declaration of interests
JH, AH, MH, HR, SS, CG, and all other clinical investigators (members of the US Nitazoxanide Influenza Clinical Study Group) received honoraria for conducting the trial. J-FR and MB are employed by the study sponsor and J-FR owns an equity interest in the sponsor.
Supplementary Material
References
- 1.WHO Influenza (Seasonal). Fact sheet number 211. March 2009. http://www.who.int/mediacentre/factsheets/fs211/en/index.html (accessed April 29, 2013).
- 2.Thompson WW, Shay DK, Weintraub E. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Estimates of deaths associated with seasonal influenza—United States, 1976–2007. MMWR. 2010;59:1057–1062. [PubMed] [Google Scholar]
- 4.Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem. 2009;43:29798–29808. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belardo G, La Frazia S, Cenciarelli O, Carta S, Rossignol JF, Santoro MG. Nitazoxanide, a novel potential anti-influenza drug, acting in synergism with neuraminidase inhibitors. 49th Infectious Disease Society of America Annual Meeting; Boston, MA, USA; Oct 20–23 2011; [cited 2013 Apr 29] Available from: https://idsa.confex.com/idsa/2011/webprogram/Paper31075.html
- 6.Romark Laboratories, LC. Alinia prescribing information. Tampa, FL; October, 2011.
- 7.US Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) Guidance for Industry, Influenza: Developing Drugs for Treatment and/or Prophylaxis. Silver Spring: 2011. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM091219.pdf (accessed April 29, 2013).
- 8.Treanor JJ, Hayden FG, Vrooman PS. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza. JAMA. 2000;283:1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson KG, Aoki FY, Ostherhaus AD. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomized controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 10.Hayden FG, Osterhaus AD, Treanor JJ. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 11.The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet. 1998;352:1877–1881. [PubMed] [Google Scholar]
- 12.Mäkelä MJ, Pauksens K, Rostila T. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J Infect. 2000;40:42–48. doi: 10.1053/jinf.1999.0602. [DOI] [PubMed] [Google Scholar]
- 13.Stockis A, De Bruyn S, Gengler C, Rosillon D. Nitazoxanide pharmacokinetics and tolerability in man during 7 days of 0.5 g and 1 g bid dosing with food. Int J Clin Pharmacol Ther. 2002;40:221–227. doi: 10.5414/cpp40221. [DOI] [PubMed] [Google Scholar]
- 14.Harper SA, Bradley JS, Englund JA. Seasonal influenza in adults and children– diagnosis, treatment, chemoprophylaxis and institutional outbreak management: Clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1003–1032. doi: 10.1086/604670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR. 2010;59:1–62. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention CDC realtime RTPCR (rRTPCR) protocol for detection and characterization of swine influenza (version 2009) http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay2009_20090430.pdf (accessed April 29, 2013).
- 17.Ebell MH, Call M, Shinholser J. Effectiveness of oseltamivir in adults: a meta-analysis of published and unpublished clinical trials. Fam Pract. 2013;30:125–133. doi: 10.1093/fampra/cms059. [DOI] [PubMed] [Google Scholar]
- 18.Kohno S, Kida H, Mizuguchi M, Shimada J, S-021812 Clinical Study Group Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob Agents Chemother. 2010;54:4568–4574. doi: 10.1128/AAC.00474-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michiels B, Van Puyenbroeck K, Verhoeven V, Vermeire E, Coenen S. The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews. PLoS One. 2013;8:e60348. doi: 10.1371/journal.pone.0060348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K, Shun-Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children (published trials only) Cochrane Database Syst Rev. 2012;4 doi: 10.1002/14651858.CD002744.pub4. CD002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu J, Santesso N, Mustafa R. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2012;156:512–524. doi: 10.7326/0003-4819-156-7-201204030-00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferson T, Jones MA, Doshi P. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2012;1 doi: 10.1002/14651858.CD008965.pub3. CD008965. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Shun-Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database Syst Rev. 2012;1 doi: 10.1002/14651858.CD002744.pub3. CD002744. [DOI] [PubMed] [Google Scholar]
- 24.Jefferson T, Jones M, Doshi P, Del Mar C. Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis. BMJ. 2009;339:b5106. doi: 10.1136/bmj.b5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burch J, Corbett M, Stock C. Prescription of anti-influenza drugs for healthy adults: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:537–545. doi: 10.1016/S1473-3099(09)70199-9. [DOI] [PubMed] [Google Scholar]
- 26.Shun-Shin M, Thompson M, Heneghan C, Perera R, Harnden A, Mant D. Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials. BMJ. 2009;339:b3172. doi: 10.1136/bmj.b3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.