Summary
Middle East respiratory syndrome coronavirus (MERS-CoV) is a lethal zoonosis that causes death in 35·7% of cases. As of Feb 28, 2018, 2182 cases of MERS-CoV infection (with 779 deaths) in 27 countries were reported to WHO worldwide, with most being reported in Saudi Arabia (1807 cases with 705 deaths). MERS-CoV features prominently in the WHO blueprint list of priority pathogens that threaten global health security. Although primary transmission of MERS-CoV to human beings is linked to exposure to dromedary camels (Camelus dromedarius), the exact mode by which MERS-CoV infection is acquired remains undefined. Up to 50% of MERS-CoV cases in Saudi Arabia have been classified as secondary, occurring from human-to-human transmission through contact with asymptomatic or symptomatic individuals infected with MERS-CoV. Hospital outbreaks of MERS-CoV are a hallmark of MERS-CoV infection. The clinical features associated with MERS-CoV infection are not MERS-specific and are similar to other respiratory tract infections. Thus, the diagnosis of MERS can easily be missed, unless the doctor or health-care worker has a high degree of clinical awareness and the patient undergoes specific testing for MERS-CoV. The largest outbreak of MERS-CoV outside the Arabian Peninsula occurred in South Korea in May, 2015, resulting in 186 cases with 38 deaths. This outbreak was caused by a traveller with undiagnosed MERS-CoV infection who became ill after returning to Seoul from a trip to the Middle East. The traveller visited several health facilities in South Korea, transmitting the virus to many other individuals long before a diagnosis was made. With 10 million pilgrims visiting Saudi Arabia each year from 182 countries, watchful surveillance by public health systems, and a high degree of clinical awareness of the possibility of MERS-CoV infection is essential. In this Review, we provide a comprehensive update and synthesis of the latest available data on the epidemiology, determinants, and risk factors of primary, household, and nosocomial transmission of MERS-CoV, and suggest measures to reduce risk of transmission.
Introduction
In the past 15 years, two new deadly zoonotic coronaviruses with epidemic potential—severe acute respiratory syndrome (SARS)–coronavirus (CoV)1, 2, 3 and Middle East respiratory syndrome (MERS)-CoV4, 5—have emerged, which have focused the attention of global public health authorities. Since the publication of the MERS Seminar in The Lancet in 2015,5 MERS-CoV cases have continued to be reported in communities and hospitals across the Arabian Peninsula, with occasional cases in travellers resulting in non-sustained outbreaks in health-care settings in other continents.4 In this Review, we provide the latest available data on the epidemiology, determinants, and risk factors of primary, household, and nosocomial transmission of MERS-CoV, and highlight measures to reduce the risk of transmission in community and nosocomial settings.
Epidemiology
Nosocomial and household outbreaks are a hallmark of MERS-CoV infection, and account for about 40% of MERS-CoV cases globally. Large health-care associated outbreaks of MERS-CoV have occurred in Saudi Arabia, the United Arab Emirates, and South Korea. From May 20 to July 13, 2015, the largest outbreak of MERS-CoV outside the Arabian Peninsula occurred in South Korea, resulting in 186 confirmed MERS cases with 38 deaths (20·4% mortality rate).4, 6, 7, 8 The outbreak occurred when a South Korean traveller returning from a trip to Qatar, the United Arab Emirates, Saudi Arabia, and Bahrain became ill, and went to several health facilities before finally being diagnosed as having a MERS-CoV infection on May 20, 2015, at the Samsung Medical Center, Seoul, South Korea.6, 8 Human-to-human transmission linked to the individual's admission to these health-care facilities caused 186 people (of whom 25 were health-care workers) to become infected with MERS-CoV within a few weeks. The infection of 181 people was associated with hospitals.8
This outbreak clearly illustrated how MERS-CoV can be transmitted from person to person in the hospitals of a well resourced developed country outside the Middle East. Following this unprecedented outbreak, the tenth meeting of the WHO MERS Emergency Committee9 was convened by the Director General under the International Health Regulations (2005) on Sept 2, 2015. Since then, intermittent sporadic cases, community clusters, and nosocomial outbreaks of MERS-CoV have continued to occur in Saudi Arabia.4 MERS-CoV is on the WHO blueprint list of priority pathogens10 (panel 1 ) since it remains a persistent threat to global health security.
Panel 1. WHO revised list of Blueprint priority diseases, February, 201810.
-
•
Crimean Congo haemorrhagic fever
-
•
Ebola virus disease and Marburg virus disease
-
•
Lassa fever
-
•
Middle East respiratory syndrome coronavirus
-
•
Severe acute respiratory syndrome
-
•
Nipah virus infection and related henipaviral diseases
-
•
Rift valley fever
-
•
Zika virus disease
-
•
Disease X (pathogen unknown)
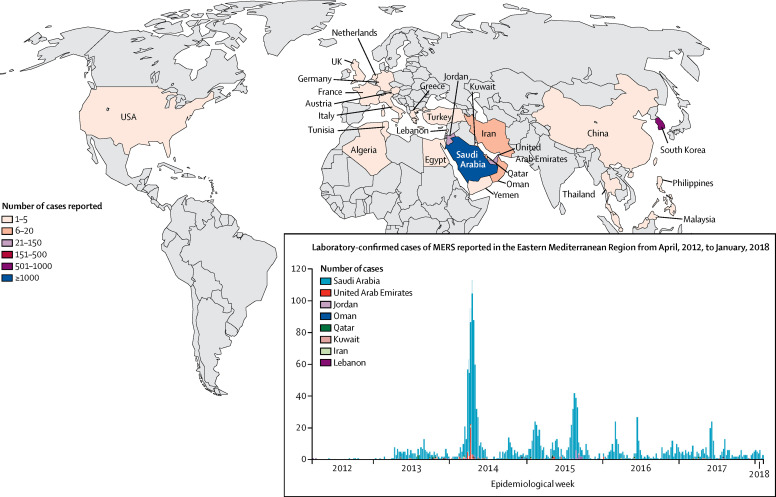
The number of MERS-CoV cases reported to WHO4 has steadily increased since the first isolation of MERS-CoV in September, 2012, from the sputum of a 60-year-old man in Saudi Arabia, who succumbed in June, 2012, to severe pneumonia and multisystem disease.11 Between September, 2012, and Feb 28, 2018, 2182 laboratory-confirmed cases of MERS-CoV infection have been reported in 27 countries (figure ), of which 779 resulted in death (35·7% mortality rate). These countries included Algeria, Austria, Bahrain, China, Egypt, France, Germany, Greece, Iran, Italy, Jordan, Kuwait, Lebanon, Malaysia, Netherlands, Oman, Philippines, Qatar, South Korea, Saudi Arabia, Thailand, Tunisia, Turkey, United Arab Emirates, UK, USA, and Yemen. Cases identified outside of the Middle East are usually travellers who were infected with MERS-CoV in the Middle East. Most MERS-CoV infections in human beings (approximately 82%) have occurred in Saudi Arabia (n=1807), and 705 of these infections have resulted in death (39% mortality rate).4, 12 Up to 50% of human MERS-CoV cases in Saudi Arabia have been classified as primary infections, of which 11% were related to household transmission, 24% to health-care facilities, and 3% were not classifiable.13
Figure.
Global cases of MERS-CoV infection reported to WHO
Reproduced from WHO4 by permission of World Health Organization. MERS CoV=Middle East respiratory syndrome coronavirus.
Source of primary human MERS-CoV infections
Primary MERS-CoV infection refers to human infection acquired in the community. Despite extensive investigations, the specific source and mode of transmission of primary human MERS-CoV infections remains unknown.5, 13 Although bats have been implicated as an original source, so far no definitive data have established an epidemiological link between human MERS-CoV infections and bats. Based on a study of 1100 bat samples, only one gene fragment of MERS-CoV in one bat of the genus Taphozous was found to closely match a human isolate of MERS-CoV.14 Epidemiological, genetic, and exposure linkages between dromedary camels (Camelus dromedarius) and human MERS-CoV infection cases seem to indicate that dromedary camels are the main intermediary animal reservoirs of MERS-CoV (appendix).15, 16 A study done in Qatar in April, 2014, reported that antibodies to MERS-CoV were present in the serum and milk of camels. Evidence for active viral shedding in nasal secretions and faeces was observed in seven of 12 camels, and viral RNA was detected in the milk of five of these seven camels.15 In a systematic, active, surveillance study16 in Egypt, among the 2541 camel serum samples tested, 1808 (71·2%) were seropositive for antibodies to MERS-CoV (as detected by microneutralisation assay), and of the 2825 camel nasal swabs tested, 435 (15·4%) contained MERS-CoV RNA (as detected by RT-PCR). Additionally, MERS-CoV antibodies were also detected in rectal swabs and milk samples of the camels.16 MERS-CoV survives for prolonged periods in camel milk, but a viable virus becomes undetectable after pasteurisation at 63°C for 30 min.17
Risk factors for primary MERS-CoV infection
Some risk factors for primary and household transmission of MERS-CoV have been reported (panel 2 ). A case-controlled study19 in Saudi Arabia found several independent risk factors for increased susceptibility to acquiring primary MERS-CoV infections: direct dromedary exposure in the 2 weeks before illness onset, heart disease, and direct physical contact with dromedary camels during the previous 6 months (panel 2). Compared with seroprevalence of MERS-CoV antibody in the general population in Saudi Arabia (0·2%) in 2012–13, seroprevalence was 15 times higher in camel shepherds (two [2·3%] of 87 shepherds had MERS-CoV antibodies; p=0·0004), and 23 times higher in slaughter-house workers (five of 140 shepherds had MERS-CoV antibodies [3·6%]; p<0·0001).21 Substantial risk factors were identified for primary MERS-CoV infection among camel workers in Qatar:18 involvement in animal training, milking camels, workers with respiratory symptoms requiring overnight stay in hospital, contact with camels' waste, and poor hand hygiene before and after animal tasks.
Panel 2. Risk factors for primary and household transmission of MERS-CoV.
Risk factors for primary MERS-CoV
Substantial risk factors among camel workers in Qatar 18
-
•
Camel training
-
•
Milking camels
-
•
Having respiratory symptoms requiring overnight stay in hospital
-
•
Contact with camel waste
-
•
Poor hand hygiene before and after contact with camels
Independent risk factors of primary MERS-CoV infection in Saudi Arabia 19
-
•
Direct dromedary exposure in the 2 weeks before illness onset (adjusted odds ratio [OR] 7·45; 95% CI 1·57–35·28)
-
•
Diabetes (adjusted OR 6·99; 95% CI 1·89–25·86)
-
•
Heart disease (adjusted OR 6·87; 95% CI 1·81–25·99)
-
•
Currently smoking tobacco (adjusted OR 6·84; 95% CI 1·68–27·94).
-
•
Direct physical contact with dromedaries in the previous 6 months (adjusted OR 14·59; 95% CI 2·38–89·55)
Risk factors for household transmission 20
-
•
Sleeping in an index patient's room (risk ratio [RR] 4·1; 95% CI 1·5–11·2)
-
•
Removing patient's waste: urine, stool, and sputum (RR 3·2; 95% CI 1·2–8·4)
-
•
Touching respiratory secretions from an index patient (RR 4·0; 95% CI 1·6–9·8)
MERS-CoV=Middle East respiratory syndrome coronavirus.
Transmission from camels to human beings
People in close contact with dromedary camels, patients with MERS-CoV infections, and health-care workers caring for patients with MERS-CoV are at an increased risk of acquiring the infection compared with the general population. MERS-CoV transmission from camels to human beings is thought to occur via direct contact with camels through respiratory droplets or saliva, or through consumption of camel products, such as milk or undercooked camel meat.15, 18, 22, 23 Camel to human cross-species transmission of MERS-CoV has been confirmed by viral RNA sequencing of samples obtained from infected dromedary camels, and from symptomatic22, 23 and asymptomatic patients24 after known exposure to the infected camels. A study25 of animal herds associated with patients with MERS-CoV infection in Saudi Arabia found that the nasal swabs of 75 of 584 dromedary camels were positive for MERS-CoV RNA for about 2 weeks, whereas the nasal swabs were negative for MERS-CoV RNA in other animals, including goats, sheep, and cattle. Notably, 70·9% of camels associated with individuals with MERS-CoV infections had MERS-CoV antibodies as determined by ELISA assays, and full genome sequencing identified ten MERS-CoV camel isolates that were identical to the isolates of their corresponding patients.25 Nevertheless, testing of sera from 191 people with various degrees of exposure to an infected dromedary herd found no serological evidence of infection.26 These data suggest that although MERS-CoV infection is widespread among dromedary camels, zoonotic transmission of MERS-CoV from camels to human beings is relatively uncommon, and human disease burden is not directly proportional to potential exposure to camels.26 Furthermore, although dromedary camels are a known host species for MERS-CoV, less than half of the people who have primary MERS-CoV infections have a history of exposure to dromedary camels.4, 5, 13 On the basis of existing MERS-CoV sequence data that examined the phylodynamics between camels and human beings using a structured coalescent model, Dudas and colleagues27 have shown that long-term evolution of MERS-CoV occurs exclusively in camels, whereas human beings serve as a transient and ultimately terminal host. Additionally, the study showed that human outbreaks of MERS-CoV in the Arabian Peninsula were driven by seasonally varying zoonotic transfer of the viruses from camels.27
Non-specific clinical features
Making a diagnosis of MERS requires a high degree of clinical awareness to the possibility of MERS-CoV infection. The clinical features and laboratory and radiological abnormalities associated with MERS-CoV infection are not MERS specific, and are similar to other respiratory tract infections.4, 5, 13, 28, 29, 30, 31, 32, 33, 34 Thus, the diagnosis of MERS-CoV infection can easily be missed when a patient presents to a health-care facility with an acute respiratory illness, meaning the patient places all those people in contact with them (family, patients, and health-care workers) at risk of acquiring a MERS-CoV infection.
Adults who acquire a MERS-CoV infection can develop a spectrum of illness and disease severity, from asymptomatic to mild, moderate, or severe disease.5, 6, 13, 28, 29, 30, 31, 32, 33, 34 The incubation period is between 2 and 14 days. Patients with mild infections can have low-grade fever, a runny nose, dry cough, sore throat, and myalgia. Patients with severe infections have pneumonia that progresses to acute respiratory distress syndrome, multisystem disease, and organ failure. Oh and colleagues35 measured progression of pneumonia in patients with severe infections by scoring the number of lung zones involved on chest radiographs, and showed that pneumonia progressed abruptly after about 7 days of illness, and the severity of the symptoms reached a peak after approximately 14 days. MERS-CoV viral load in lower respiratory tract samples is higher than the viral load in upper respiratory tract samples.35, 36 Additionally, extrapulmonary features including myalgia are common.5, 6, 13, 27, 28, 29, 30, 31, 32, 33, 34 Up to half of all patients with MERS-CoV infections have acute kidney injury, and a third of patients who are critically ill have gastrointestinal symptoms, such as abdominal pain, nausea, vomiting, and diarrhoea,29 and MERS-CoV can be detected in stool specimens.37 Between Dec 2, 2014, and Nov 12, 2016, Ahmed and colleagues38 collected daily information from the Saudi Arabian Ministry of Health on people with MERS-CoV, and recorded 660 confirmed cases of human MERS-CoV infections. The study found that the mortality rate at 3 days was 13·8%, at 30 days was 28·3%, and the overall mortality rate was 29·8%. Patients older than 60 years were more likely to die (45·2% mortality) from their infections than were younger patients (20% mortality).
Risk factors for severe disease and death
Patients with pre-existing medical comorbidities have more severe disease and higher mortality rates than patients who do not have any comorbidities.5, 6, 13, 28, 29, 30, 31, 32, 33 Risk factors for poor outcome (severe disease or death) in people with MERS-CoV infections include advanced age, male sex, comorbid illness (eg, obesity, diabetes, heart disease, lung disease, kidney disease, and immunocompromised states), low serum albumin concentrations, concomitant infections, positive plasma MERS-CoV RNA, professions other than health-care workers, altered mental capacity, high pneumonia severity index score on hospital admission, signs of severe inflammation at initial presentation (ie, elevated interferon-γ inducible protein-10, monocyte chemoattractant protein-1, and interleukin 6 concentrations), and high viral load on days 5–10 after hospital admission with low antibody titres on days 11–16 during the clinical course of disease.33, 38, 39, 40, 41 The MERS-CoV dipeptidyl peptidase 4 receptor has been shown to be upregulated in the lungs of smokers and patients with chronic obstructive pulmonary disease, and this upregulation could explain why patients with comorbid lung diseases are prone to severe illness.42
Person-to-person transmission
MERS-CoV has been identified in clinical specimens such as sputum, endotracheal aspirate, bronchoalveolar lavage, nasal or nasopharyngeal swabs, urine, faeces, blood, and lung tissue.5, 6, 13, 33, 37, 43 When available, it is important to take serial specimens from both the upper and the lower respiratory tract. Although MERS-CoV might also be detected in serum and blood samples, it is uncommonly detected in urine and stool samples. The precise modes of MERS-CoV transmission through direct or indirect contact, including transmission via airborne, droplet, or ingestion, have yet to be defined. MERS-CoV does not seem to be easily transmitted from person to person unless contact with patients infected with MERS-CoV is close.43, 44, 45 Human to human transmission has been described within primary community settings and households,20, 32, 45, 46 but has been more striking within health-care settings.28, 29, 30, 31, 47, 48, 49, 50, 51 Health-care associated outbreaks have occurred in several countries, with the largest outbreaks seen in Saudi Arabia, the United Arab Emirates, and South Korea.28, 29, 30, 31, 47, 48, 49, 50, 51 People in close contact with dromedary camels and health-care workers taking care of patients with MERS-CoV infections are at an increased risk of acquiring the infection, whereas healthy adults might develop a mild form of the illness or even an asymptomatic infection. Although no clear evidence of sustained, person-to-person transmission has been reported, the South Korean outbreak involved within-hospital and hospital-to-hospital transmission.8, 52, 53, 54, 55
Household transmission
Several reports from Saudi Arabia describe transmission between people in the community and in large households and family compounds.20, 22, 32, 46 In 2014, Drosten and colleagues45 investigated 280 households that came into contact with 26 index Saudi Arabian patients infected with MERS-CoV, and did follow-up serological analyses in 44 of these 280 household contacts, to measure the prevalence of silent or subclinical secondary infections after exposure to patients with primary cases of MERS-CoV. The authors identified 12 probable cases of secondary transmission (4%; 95% CI 2–7%). Seven apparently healthy individuals who were household contacts carried MERS-CoV in their upper respiratory tract. This finding, together with the observation that people with asymptomatic MERS-CoV infections had low amounts of MERS-CoV RNA in upper respiratory tract samples during outbreaks in a hospital in Jeddah, Saudi Arabia,48 indicate that MERS-CoV can be transmitted from asymptomatic household or hospital contacts.
Of the 79 relatives that were examined after MERS-CoV infections affected an extended family in Saudi Arabia in 2014, 19 (24%) were positive for MERS-CoV, 11 (58%) were admitted to hospital, and two (11%) died. 11 (58%) of the 19 family members tested positive for MERS-CoV by use of real-time RT-PCR (rtRT-PCR) analysis, but eight (42%) who tested negative for MERS-CoV by use of rtRT-PCR were positive as determined by serology blood tests, with confirmatory immuno-fluorescence assays and microneutralisation.20 Compared with the adult family members who tested negative for MERS-CoV, the relatives who tested positive for MERS–CoV were older and more likely to be men (risk ratio [RR] 4·8; 95% CI 1·6–15·0), and were more likely to have a chronic comorbid illness (2·8; 95% CI 1·4–5·7). Risk factors for household transmission included sleeping in an index patient's room, removing patient's waste, and touching respiratory secretions from an index patient (panel 2). Proximity to the patient and casual contact were not associated with transmission. This study also clearly showed that serological analyses were more sensitive than standard rtRT-PCR for identifying relatives who were infected.20
Nosocomial transmission
Nosocomial outbreaks have been a hallmark of MERS-CoV infections, and account for roughly a third of MERS-CoV cases reported globally.4 MERS-CoV was more stable in aerosol in low temperature (20°C) and low humidity conditions (40% relative humidity) than the influenza virus, and could still be recovered from plastic or steel surfaces after 48 h.17 This property could explain why MERS-CoV is easily transmitted in health-care facilities equipped with central air conditioning, leading to major nosocomial outbreaks and super-spreading events, especially in the presence of aerosol generating procedures (AGPs).
A retrospective study27 of clusters of hospital cases of MERS-CoV infection in Jordan from April, 2012, showed that poor compliance with infection control measures among health-care workers, and the absence of proper isolation rooms were risk factors for nosocomial outbreaks. Another nosocomial outbreak of MERS-CoV infections in Al Hasa, Saudi Arabia, in 2013, involving 23 patients in four different health-care facilities, was caused by poor infection control practices, with the possible contribution of AGPs, including use of continuous positive airway pressure, nebulised medications, and cardiopulmonary resuscitation. After enhancement of infection control measures (panel 3 ), nosocomial outbreaks abated.29
Panel 3. Enhanced infection control measures that were effective in controlling nosocomial outbreaks.
-
•
Hand hygiene, and droplet and contact precautions for febrile patients with a fever before testing these patients for MERS-CoV
-
•
Putting surgical masks on all patients undergoing haemodialysis, and ensuring health-care workers wear N95 filtering facepiece respirators when managing any patient with a confirmed MERS-CoV infection who is undergoing an aerosol-generating procedure
-
•
Patients with suspected MERS-CoV infection admitted to dialysis or intensive care units should be placed in isolation rooms with a portable dialysis machine
-
•
Increasing environmental cleaning, and preventing non-essential staff and visitors from coming into contact with patients infected with MERS-CoV
MERS-CoV=Middle East respiratory syndrome coronavirus.
In 2014, the nosocomial outbreaks in Abu Dhabi, United Arab Emirates,50 were due to the spread of MERS-CoV in health-care settings, predominantly occurring well before MERS-CoV infections were recognised and diagnosed. The rapid spread was attributed to poor compliance among health-care workers with wearing personal protection equipment while interacting with patients and application of AGPs, including intubation, manual ventilation before intubation, nebulised medications, and oxygen therapy. These data underscore the importance of increasing awareness and infection control measures at first points of entry to health-care facilities.56
In Jeddah, Saudi Arabia, in 2014, major nosocomial outbreaks of MERS-CoV infection occurred, involving three health-care facilities in which existing inpatients, clinic patients, and visitors became infected following direct or indirect exposure to the index patients.48 Emergency departments with overcrowding and poor infection control were the main location of exposure, leading to nosocomial outbreaks in hospitals in Saudi Arabia49 and South Korea.7, 8, 57
Two unlinked clusters of MERS-CoV infection14, 58 occurred at the same hospital in Wadi ad-Dawasir, Saudi Arabia, in the Riyadh region in early 2017. The first cluster of ten cases occurred in March, 2017, where the primary case was in the dialysis unit, and the second cluster of five cases occurred in April, 2017. Both clusters involved transmission of the infection to household contacts, health-care workers, and other patients. In June, 2017, an outbreak of 34 MERS-CoV cases occurred in a hospital in Riyadh.58 The primary case was a 47-year-old man admitted to the emergency department for intubation, who at that time was not identified as having a MERS-CoV infection. Before his diagnosis, 220 health-care workers, patients, and visitors came into contact with him. Contact tracing identified an additional 33 people with MERS-CoV infections during this outbreak. 50% of the people who became infected during this outbreak were health-care workers. 11 patients were classified as having severe disease, seven of whom died, and 22 were asymptomatic. One individual from this June, 2017, cluster sought treatment for renal failure and had a renal dialysis at another health-care facility in Riyadh and this led to five additional people being infected with MERS-CoV: three household contacts, one patient contact in the hospital, and one health-care worker. Unrelated to this cluster, in June, 2017, another MERS-CoV cluster of nine cases occurred at a hospital in Riyadh, Saudi Arabia; this outbreak involved a butcher who reported direct contact with dromedary camels and eight health-care workers (four asymptomatic and four with mild disease).58
Health-care workers and transmission
Among health-care workers who treated patients infected with MERS-CoV at the King Faisal Specialist Hospital in Jeddah,59 between March and July, 2014, the percentage of health-care workers who were infected while looking after these patients was 8% (20 of 250 health-care workers), and this prevalence differed by hospital units: 11·7% (15 of 128) for the medical intensive care unit, and 4·1% (five of 122) for the emergency department. Among health-care workers, radiographers had the highest infection risk (29·4%; five of 17), followed by nurses (9·4%; 13 of 138), respiratory therapists (3·2%; one of 31), and physicians (2·4%; one of 41). health-care workers who always covered their nose and mouth with medical masks or N95 masks were less likely to be infected than health-care workers who sometimes or never covered their nose and mouth during AGPs (6·0% [eight of 133] vs 18·8% [six of 32]; RR 0·32; 95% CI 0·12–0·86; p=0·03].59
MERS-CoV viral load and transmission
Analysis of MERS-CoV spreaders (defined as an index patient who was suspected of causing secondary infections of MERS-CoV) in South Korea showed that they could transmit the infection from day 1 to day 11 of their illness (median 7 days, IQR 5–8), and the number of individuals infected by each index patient ranged from one to 84 (median 2, IQR 1–12).54 MERS-CoV viral loads (from sputum, throat swabs, or tracheal aspirates) peaked during the second week of illness, and the lower respiratory tract samples showed higher and more prolonged concentrations of MERS-CoV RNA than upper respiratory tract specimens, as detected by rtRT-PCR.35 Nosocomial transmission in South Korea was independently associated with cycle threshold (odds ratio [OR] 0·84, 95% CI 0·75–0·96) and pre-isolation hospital admission or emergency department visits (OR 6·82, 95% CI 2·06–22·84). Superspreading events (defined as four or more transmissions) were associated with a higher number of pre-isolation contacts (n=777 for superspreading events vs n=78 for other events), more frequent pre-isolation emergency department visits by the index patients (100% vs 35·3%), and more so-called doctor shopping (ie, seeking medical attention in different health-care facilities; 100% vs 47·1%) than events that were not superspreading.52
Severity of illness and transmission
Analysis of data in South Korea suggests that patients who died from MERS-CoV infections were more likely to cause secondary infections than those who survived. Direct human-to-human transmission associated with clinical progression of the fatal cases (eg, lower respiratory tract involvement with higher viral load and need for AGPs), and indirect transmission via environmental contamination (eg, fomites and indoor ventilation systems) are likely to have caused nosocomial amplification of MERS-CoV in South Korea.60 The reproduction number for nosocomial outbreaks in Saudi Arabia and South Korea was estimated to be between two and five.61 The risk factors for nosocomial MERS-CoV outbreaks are summarised in panel 4 .28, 29, 30, 31, 36, 47, 48, 49, 52, 56
Panel 4. Risk factors for nosocomial MERS outbreaks28–31,36,47–49,52,56.
-
•
Exposure of patients, health-care workers, and visitors to contaminated and overcrowded health-care facilities, especially emergency departments, inpatient wards, and dialyses units
-
•
Exposure to patients with symptomatic MERS-CoV infection or health-care workers caring for them
-
•
Poor compliance with MERS-specific infection control guidelines
-
•
Poor compliance with appropriate personal protective equipment when assessing patients with febrile respiratory illnesses
-
•
Application of potential aerosol-generating procedures (eg, resuscitation, continuous positive airways ventilation, and nebulised drugs)
-
•
Inadequate isolation room facilities and distance between beds and dialysis chairs of less than 1 m
-
•
Frequent shifting of health-care seeking behaviour from hospital to hospital, or emergency department and other clinics
-
•
Friends and family members staying as caregivers in overcrowded health-care facilities
MERS=Middle East respiratory syndrome.
Environmental contamination in hospitals
Environmental contamination by MERS-CoV in patients' rooms has been reported in South Korea, with positive MERS-CoV RT-PCR results for cultures from environmental swabs taken from bed sheets, bed rails, intravenous fluid hangers, CT cassettes, and anteroom tables, and viable MERS-CoV viruses could still be isolated from three of four enrolled patients being studied on days 18–25 after symptom onset.53 Another study57 detected the presence of MERS-CoV by RT-PCR in viral cultures from four of seven air samples taken from two patients' rooms, one patient's bathroom, and one common corridor. MERS-CoV was also detected in the viral cultures for 15 of 68 surface swabs. Immunofluorescence assays of the cultures from the air and swab samples revealed the presence of MERS-CoV, and electron microscopy images also revealed intact particles of MERS-CoV in these samples.57 A third study showed low concentrations of MERS-CoV RNA (as shown by RT-PCR results) for environmental swabs taken from bed guardrails and monitors. Even after cleaning the monitors with 70% alcohol based disinfectant, RT-PCR showed low concentrations of MERS-CoV RNA, and the samples only became negative for MERS-CoV after the monitors were wiped with diluted sodium chlorite.62
Transmission of MERS-CoV by asymptomatic individuals
MERS-CoV has been detected in asymptomatic individuals through the screening of health-care workers during nosocomial outbreaks in Saudi Arabia and South Korea. Upper airway samples from health-care workers that were positive for MERS-CoV usually became negative on PCR testing within 2–3 days;23 although prolonged MERS-CoV positivity detected by PCR for up to 35 days has been reported in a health-care worker with subclinical infection.43 Prolonged MERS-CoV-positive PCR results have been noted in some patients with MERS-CoV-negative viral cultures who are recovering from the infection.36, 62, 63 Although prolonged MERS-CoV-positive PCR results could be due to the slow turnover of damaged respiratory epithelium containing non-viable RNA fragments of MERS-CoV in some patients, whether people who are asymptomatic (especially health-care workers) can transmit MERS-CoV to patients who are at high risk of infection, such as patients who are immunocompromised or patients with chronic renal or lung disease is unclear. In a Korean study,64 591 health-care workers who cared for patients with MERS using proper personal protective equipment were screened for MERS-CoV after finishing the patient care. The screening revealed that three (0·5%) health-care workers had acquired asymptomatic MERS-CoV infections. A follow-up study65 of a nurse with an asymptomatic MERS-CoV infection in South Korea showed that she did not transmit the infection to the 82 people they had been in contact with, whereas another study46 speculated that a family cluster of MERS-CoV infections in Saudi Arabia was probably related to exposure to an unrecognised individual with asymptomatic or subclinically mild MERS-CoV in a hospital setting. Four generations of transmission among health-care workers in hospital, shared housing, and home environments were observed in Saudi Arabia after investigation of an outbreak of MERS-CoV caused by an index patient (a nurse with MERS-CoV and pulmonary tuberculosis) who had contact with 73 people. Although the disease was mostly mild, transient, or asymptomatic among the health-care workers, this study51 has shown that health-care workers could transmit MERS-CoV to other health-care workers despite being asymptomatic.
Decreasing risk of transmission
With no effective vaccine available, rapid case identification, isolation, infection prevention and control measures are essential to prevent the spread of MERS-CoV within households, the community, and health-care facilities. Global public health bodies have provided guidelines and recommendations to help achieve these goals (appendix). Diagnosing primary cases of patients with MERS-CoV infection early and accurately is difficult because symptoms and signs of respiratory tract infections are non-specific and are common to all microbial causes of respiratory tract infections. Investment in the development of rapid point-of-care testing for MERS-CoV would be of great value to the global public health community. The upsurge in the number of human MERS-CoV infections over the past few years in health-care facilities in the Middle East and South Korea was attributed to several preventable factors. Low health-care worker awareness of the possibility of MERS-CoV with consequential poor implementation of infection control procedures resulted in nosocomial outbreaks. These outbreaks involved transmission of the infection to existing hospital patients, outpatients, visitors, and health-care workers within health-care facilities. Transmission was associated with overcrowding, absence of isolation room facilities, and environmental contamination, without substantial genetic changes in the transmissibility of the virus.58
Infection prevention and control measures
The SARS epidemic led to important infection prevention and control recommendations for managing patients with suspected CoV infection, and these recommendations are applicable to MERS-CoV.3 A high degree of awareness of the possibility of MERS-CoV infection and early isolation of suspected or confirmed MERS cases with proactive surveillance are crucial to preventing nosocomial outbreaks. To decrease MERS-CoV human-to-human transmission and environmental contamination, AGPs should be avoided in crowded hospital accident and emergency departments and in inpatient medical wards without adequate infection control measures.
Droplet precautions are required for managing patients with confirmed MERS-CoV infection. Wearing a surgical mask within 1–2 m of the patient, and wearing a gown, gloves, mask, and eye protection on entering the patient's room, and removing them upon leaving, are important infection control measures.66 Airborne precautions should be applied for AGPs such as open suctioning or aspiration of the respiratory tract, intubation, bronchoscopy, or cardiopulmonary resuscitation. These precautions include wearing a half-mask air purifying respirator, such as a US National Institute for Occupational Safety and Health approved N95 filtering facepiece respirator, or a European Standard-approved FFP2 or FFP3 filtering facepiece respirator. The respirator should fit properly, and all health-care workers should undergo in-depth training for the proper use, donning and doffing of the respirator, and in performing a user seal check every time the respirator is used.66
To reduce room contamination in the hospital setting, the application of a minimum room ventilation rate of six air changes per h in an existing facility is recommended. A higher ventilation rate of 12 air changes per h is required for new or renovated constructions, especially when caring for patients receiving mechanical ventilation and during AGPs.67 Cleaning environmental surfaces with water and detergent and applying commonly used disinfectants (such as hypochlorite) is an effective and sufficient procedure.66
Unprotected or inadvertent exposure of health-care workers to patients with MERS-CoV should prompt rapid quarantine and testing of the exposed health-care workers. Health-care workers who test positive for MERS-CoV should only be allowed to return to work when at least two upper respiratory tract samples taken at least 24 h apart are negative, spanning the full incubation period of 14 days.51, 68
Education of communities and travellers
In MERS-CoV endemic countries, where MERS-CoV cases are suspected or diagnosed in the community and households, educational awareness of MERS-CoV and MERS prevention measures within the home could reduce further transmission and prevent outbreaks of community clusters. People with comorbidities such as diabetes, kidney disease, chronic lung disease, and cancer, or individuals on immunosuppressive treatments are at high risk of developing severe MERS-CoV disease, thus they should avoid close contact with camels and bats. Patients with chronic renal failure or chronic heart disease can also have lung infiltrates and dyspnoea due to fluid retention from their underlying disease, and the consideration of MERS-CoV infection can be easily missed. Avoiding contact with sick camels and regular handwashing before and after touching camels is advised. People should avoid drinking raw camel milk or camel urine, or eating camel meat that has not been properly cooked. WHO does not advise special screening for MERS-CoV at points of entry after return from the Middle East, nor does it currently recommend the application of any travel or trade restrictions. People with a history of travel from or to the Arabian Peninsula within 10 days of developing symptoms of an acute respiratory infection involving a fever of 38°C or more, or a cough with presentation of radiological pulmonary changes should alert their physician to the possibility of MERS-CoV infection.69
Unanswered questions
It has been over 5 years since the first discovery of MERS-CoV as a lethal human pathogen, and whilst much has been learned, many questions remain unanswered (panel 5 ). Although exposure to dromedary camels and related contact activities are important risk factors for primary MERS-CoV infections, the exact mechanisms of transmission require urgent investigation. Genomic studies indicate that MERS-CoV strains causing infections in human beings are indistinguishable from the virus found in dromedary camels in the Middle East. A surveillance study70 for MERS-CoV in dromedaries in Africa and central Asia which used MERS-CoV spike pseudoparticle neutralisation assays has shown that MERS-CoV antibodies are highly prevalent in camel serum samples from African countries. Using quantitative RT-PCR, positive specimens for MERS-CoV were detected in camel nasal swabs from all the African countries from which samples were collected. By contrast, dromedary serum and swab samples from Kazakhstan in central Asia were shown to be negative for MERS-CoV when using these assays.71 Phylogenetic analysis of the spike gene revealed that MERS-CoVs from Africa formed a cluster closely related to, but distinct from, the viruses in the Arabian Peninsula. These findings indicate that MERS-CoV is actively circulating in dromedary populations in Africa, but the MERS-CoV virus in Africa is phylogenetically distinct from that in the Middle East.69 The finding that MERS-CoV is not universally endemic in dromedaries raises the hypothesis that some species of bats, or some other animal, the environment, or both, could constitute a maintenance community and be the true natural reservoir of MERS-CoV, and that the virus is transmitted to camels and is maintained within camels for varying periods of time.71
Panel 5. Unanswered questions: MERS-CoV determinants, risk factors, and transmission.
-
•
Although camels are known reservoirs, what are the exact animal or environmental sources of MERS-CoV?
-
•
What are the modes of primary transmission to human beings in sporadic cases?
-
•
Why do children have a low prevalence of MERS-CoV infection and disease?
-
•
Do other risk factors for transmission between camels and human beings exist?
-
•
What are the seasonal trends in transmission and disease patterns?
-
•
What is the role of asymptomatic or mild infections in human-to-human transmission in the community and in nosocomial settings?
-
•
What are the drivers of transmission and exact modes of transmission in health-care settings?
-
•
What are the specific exposures that put health-care workers most at risk?
-
•
What roles do health-care workers who have asymptomatic MERS-CoV infections but who are positive for MERS-CoV RNA have in nosocomial transmission and outbreaks?
-
•
To what extent does environmental contamination within hospitals have a role in the nosocomial spread of MERS-CoV?
-
•
What are the MERS-CoV viral shedding patterns in different bodily fluids over the course of the illness
-
•
What are the roles of non-respiratory specimens (eg, faeces, urine, saliva, semen, sweat, blood, and tears) in the transmission of MERS-CoV?
-
•
Why has MERS-CoV persisted for longer than severe acute respiratory syndrome-CoV?
-
•
What is the specific pathogenesis of MERS-CoV infection?
-
•
Why are autopsy and pathological studies scarce, and why does the specific pathology of MERS-CoV remain undefined?
-
•
What are the innate and adaptive protective immune mechanisms involved in MERS-CoV infections?
-
•
Are protective immune mechanisms deleterious for the patient?
-
•
What MERS-CoV mutations and evolutionary changes are taking place over time?
-
•
Do host genetics have a role in susceptibility and pathogenesis of MERS-CoV?
-
•
Do animal models of MERS-CoV accurately reflect human infection?
MERS-CoV=Middle East respiratory syndrome coronavirus.
The role of unpasteurised milk, meat, and secretions (saliva, urine, sweat, and stool) of dromedary camels in the transmission of MERS-CoV requires further study. The risk factors for household transmission include sleeping in the same room as a patient and contact with a patient's secretions. The role of asymptomatic individuals in nosocomial transmission of MERS-CoV infections to patients who are at high risk of infection needs to be clarified. Although AGPs such as intubation, manual ventilation before intubation, tracheotomy, and non-invasive ventilation appear to have played an important part in nosocomial outbreaks of SARS-CoV infection,72 the precise role of these procedures in the transmission of MERS-CoV infection in health-care settings requires further investigation. Additionally, improved compliance with infection prevention and control protocols and adherence to standard precautions (appendix) are required to decrease the risk of human-to-human MERS-CoV transmission and the number of outbreaks in health-care facilities.
Conclusions
MERS-CoV remains an important public health risk, and further international spread through travellers73 can be anticipated in view of the previously observed patterns of nosocomial outbreaks and transmission within health-care facilities in the Middle East and in South Korea.74 With 10 million pilgrims visiting Saudi Arabia each year from 182 countries for the Hajj and Umrah pilgrimages,75 watchful surveillance by public health systems, and a high degree of clinical awareness of the possibility of MERS-CoV infection is essential, especially for countries with inadequate public health systems.76 Nosocomial transmission is often due to a delayed diagnosis of MERS-CoV infection in a patient shedding MERS-CoV in a crowded health-care setting, such as an inpatient ward, emergency department, or renal dialysis unit.28, 29, 30, 31, 36, 47, 48, 49, 52, 56 Early recognition of MERS-CoV infections, improved compliance with internationally recommended infection control protocols, and rapid implementation of infection control measures are required to prevent outbreaks of MERS-CoV associated with health-care facilities.77 The establishment of robust public health infrastructures, effective global consortia to enable rapid and effective detection, definitions and surveillance of new infectious disease threats, and the prioritisation of research, preparedness, response, and control efforts is crucial. 5 years after the first identification of MERS-CoV, many important questions remain. With no specific treatments78 available, and high mortality rates, advancing knowledge on MERS-CoV through collaborative equitable international research and surveillance partnerships remains a priority.79
Search strategy and selection criteria
We searched publications in English on MEDLINE, Embase, and Google Scholar for the period between Jan 1, 2012, and Feb 28, 2018, using the search terms “Middle East respiratory syndrome”, “MERS-CoV”, or “MERS” in combination with the terms “epidemiology”, “transmission”, “infection control”, “outbreak”, “risk factors”, “prevention”, “management”, “hospital”, “household”, “community”, “nosocomial”, “healthcare setting”, or “camels”. We also searched the Centre for Infectious Disease Research and Policy and ProMed websites for news, latest postings, and articles on Middle East respiratory syndrome (MERS) and MERS-coronavirus (CoV). We also searched websites of global and national public health agencies such as WHO, US Centers for Disease Control and Prevention, UK Public Health England, European Centre for Disease Prevention and Control, Saudi Arabia Ministry of Health, and South Korea Centres for Disease Control and Prevention portals. We selected publications relevant to clinical features, MERS-CoV viral load, and shedding, epidemiology, transmission, outbreak management, infection control, and prevention. We also searched the reference lists of articles and reviews identified by this search strategy and selected those we deemed relevant to inform readers of more references and knowledge published on the subject.
Acknowledgments
Acknowledgments
DSH, EIA, ZAM, and AZ are members of the Global Centre for Mass Gatherings Medicine. AZ acknowledges support from the National Institute of Health and Research (NIHR) Biomedical Research Centre at University College London Hospitals (UK) and is in receipt of an NIHR Senior Investigator award.
Contributors
DSH and AZ initiated the idea of the Review, did the literature search, and developed the first draft of the manuscript. All authors contributed equally to subsequent drafts. DSH and AZ finalised the manuscript.
Declaration of interests
All authors declare no other competing interests.
Supplementary Material
References
- 1.Peiris JS, Yuen KY, Osterhaus AD, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 2.WHO Severe acute respiratory syndrome. http://www.who.int/topics/sars/en/ (accessed March 28, 2018).
- 3.Hui DS, Memish ZA, Zumla A. Severe acute respiratory syndrome vs the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20:233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 4.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) http://www.who.int/emergencies/mers-cov/en/ (accessed Jan 16, 2018).
- 5.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JY, Kim YJ, Chung EH. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC Infect Dis. 2017;17:498. doi: 10.1186/s12879-017-2576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majumder MS, Brownstein JS, Finkelstein SN, Larson RC, Bourouiba L. Nosocomial amplification of MERS-coronavirus in South Korea, 2015. Trans R Soc Trop Med Hyg. 2017;111:261–269. doi: 10.1093/trstmh/trx046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korea Centers for Disease Control and Prevention Middle East respiratory syndrome coronavirus outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015;6:269–327. doi: 10.1016/j.phrp.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO WHO statement on the ninth meeting of the IHR Emergency Committee regarding MERS-CoV. June 17, 2015. http://www.who.int/mediacentre/news/statements/2015/ihr-ec-mers/en/ (accessed Oct 15, 2017).
- 10.WHO List of blueprint priority diseases. http://www.who.int/blueprint/priority-diseases/en/ (accessed March 28, 2018).
- 11.Zaki AM, van Boheemen S, Bestebroer TM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 12.Arabi YM, Balkhy HH, Hayden FG. Middle East respiratory syndrome. N Engl J Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saudi Arabia Ministry of Health MERS-CoV daily update. https://www.moh.gov.sa/en/CCC/PressReleases/Pages/statistics-2018-01-14-001.aspx (accessed March 19, 2018).
- 14.Memish ZA, Mishra N, Olival KJ. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reusken CB, Farag EA, Jonges M. Middle East respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Euro Surveill. 2014;19:20829. doi: 10.2807/1560-7917.es2014.19.23.20829. [DOI] [PubMed] [Google Scholar]
- 16.Ali MA, Shehata MM, Gomaa MR. Systematic, active surveillance for Middle East respiratory syndrome coronavirus in camels in Egypt. Emerg Microbes Infect. 2017;6:e1. doi: 10.1038/emi.2016.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Doremalen N, Bushmaker T, Munster VJ. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18:20590. doi: 10.2807/1560-7917.es2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- 18.Sikkema RS, Farag EA, Himatt S. Risk factors for primary Middle East respiratory syndrome coronavirus infection in camel workers in Qatar during 2013–2014: a case-control study. J Infect Dis. 2017;215:1702–1705. doi: 10.1093/infdis/jix174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alraddadi BM, Watson JT, Almarashi A. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arwady MA, Alraddadi B, Basler C. Middle East respiratory syndrome coronavirus transmission in extended family, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22:1395–1402. doi: 10.3201/eid2208.152015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller MA, Meyer B, Corman VM. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015;15:559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azhar EI, El-Kafrawy SA, Farraj SA. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 23.Memish ZA, Cotten M, Meyer B. Human infection with MERS coronavirus after exposure to infected camels, Saudi Arabia, 2013. Emerg Infect Dis. 2014;20:1012–1015. doi: 10.3201/eid2006.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Hammadi ZM, Chu DK, Eltahir YM. Asymptomatic MERS-CoV infection in humans possibly linked to infected dromedaries imported from Oman to United Arab Emirates, May 2015. Emerg Infect Dis. 2015;21:2197–2200. doi: 10.3201/eid2112.151132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasem S, Qasim I, Al-Hufofi A. Cross-sectional study of MERS-CoV-specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. J Infect Public Health. 2017 doi: 10.1016/j.jiph.2017.09.022. published online Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hemida MG, Al-Naeem A, Perera RA, Chin AW, Poon LL, Peiris M. Lack of Middle East respiratory syndrome coronavirus transmission from infected camels. Emerg Infect Dis. 2015;21:699–701. doi: 10.3201/eid2104.141949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dudas G, Carvalho LM, Rambaut A, Bedford T. MERS-CoV spillover at the camel-human interface. ELife. 2018 doi: 10.7554/eLife.31257. published online Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Abdallat MM, Payne DC, Alqasrawi S. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assiri A, McGeer A, Perl TM. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Memish ZA, Zumla AI, Assiri A. Middle East respiratory syndrome coronavirus infections in health care workers. N Engl J Med. 2013;369:884–886. doi: 10.1056/NEJMc1308698. [DOI] [PubMed] [Google Scholar]
- 31.Memish ZA, Al-Tawfiq JA, Assiri A. Hospital-associated Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;369:1761–1762. doi: 10.1056/NEJMc1311004. [DOI] [PubMed] [Google Scholar]
- 32.Memish ZA, Zumla AI, Al-Hakeem RF. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 33.Garbati MA, Fagbo SF, Fang VJ. A comparative study of clinical presentation and risk factors for adverse outcome in patients hospitalised with acute respiratory disease due to MERS coronavirus or other causes. PLoS One. 2016;11:e0165978. doi: 10.1371/journal.pone.0165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohd HA, Memish ZA, Alfaraj SH. Predictors of MERS-CoV infection: a large case control study of patients presenting with ILI at a MERS-CoV referral hospital in Saudi Arabia. Travel Med Infect Dis. 2016;14:464–470. doi: 10.1016/j.tmaid.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh MD, Park WB, Choe PG. Viral load kinetics of MERS coronavirus infection. N Engl J Med. 2016;375:1303–1305. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- 36.Memish ZA, Assiri AM, Al-Tawfiq JA. Middle East respiratory syndrome coronavirus (MERS-CoV) viral shedding in the respiratory tract: an observational analysis with infection control implications. Int J Infect Dis. 2014;29:307–308. doi: 10.1016/j.ijid.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Li C, Zhao G. Human intestinal tract serves an alternative infection route for Middle East respiratory syndrome coronavirus. Sci Adv. 2017;3:eaao4966. doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed AE. The predictors of 3- and 30-day mortality in 660 MERS-CoV patients. BMC Infect Dis. 2017;17:615. doi: 10.1186/s12879-017-2712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivers CM, Majumder MS, Lofgren ET. Risks of death and severe disease in patients with Middle East respiratory syndrome coronavirus, 2012–2015. Am J Epidemiol. 2016;184:460–464. doi: 10.1093/aje/kww013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang YM, Hsu CY, Lai CC. Impact of comorbidity on fatality rate of patients with Middle East respiratory syndrome. Sci Rep. 2017;7:11307. doi: 10.1038/s41598-017-10402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong KH, Choi JP, Hong SH. Predictors of mortality in Middle East respiratory syndrome (MERS) Thorax. 2017 doi: 10.1136/thoraxjnl-2016-209313. published online July 19. [DOI] [PubMed] [Google Scholar]
- 42.Seys LJ, Widagdo W, Verhamme FM. DPP4, the MERS coronavirus receptor, is upregulated in lungs of smokers and COPD patients. Clin Infect Dis. 2018;66:45–53. doi: 10.1093/cid/cix741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JY, Kim YJ, Chung EH. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC Infect Dis. 2017;17:498. doi: 10.1186/s12879-017-2576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Gethamy M, Corman VM, Hussain R. A case of long-term excretion and subclinical infection with Middle East respiratory syndrome coronavirus in a healthcare worker. Clin Infect Dis. 2015;60:973–974. doi: 10.1093/cid/ciu1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drosten C, Meyer B, Müller MA. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371:828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 46.Omrani AS, Matin MA, Haddad Q. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17:e668–e672. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nam HS, Park JW, Ki M. High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. Int J Infect Dis. 2017;58:37–42. doi: 10.1016/j.ijid.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oboho IK, Tomczyk SM, Al-Asmari AM. 2014 MERS-CoV outbreak in Jeddah—a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alenazi TH, Al Arbash H, El-Saed A. Identified transmission dynamics of Middle East respiratory syndrome coronavirus infection during an outbreak: implications of an overcrowded emergency department. Clin Infect Dis. 2017;65:675–679. doi: 10.1093/cid/cix352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al Hosani FI, Pringle K, Al Mulla M. Response to emergence of Middle East respiratory syndrome coronavirus, Abu Dhabi, United Arab Emirates, 2013–2014. Emerg Infect Dis. 2016;22:1162–1168. doi: 10.3201/eid2207.160040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alfaraj SH, Al-Tawfiq JA, Altuwaijri TA. Middle East respiratory syndrome coronavirus transmission among health care workers: implication for infection control. Am J Infect Control. 2017 doi: 10.1016/j.ajic.2017.08.010. published online Sept 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SW, Park JW, Jung HD. Risk factors for transmission of Middle East respiratory syndrome coronavirus infection during the 2015 outbreak in South Korea. Clin Infect Dis. 2017;64:551–557. doi: 10.1093/cid/ciw768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bin SY, Heo JY, Song MS. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin Infect Dis. 2016;62:755–760. doi: 10.1093/cid/civ1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang CK, Song KH, Choe PG. Clinical and epidemiologic characteristics of spreaders of Middle East respiratory syndrome coronavirus during the 2015 outbreak in Korea. J Korean Med Sci. 2017;32:744–749. doi: 10.3346/jkms.2017.32.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SH, Chang SY, Sung M. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hunter JC, Nguyen D, Aden B. Transmission of Middle East respiratory syndrome coronavirus infections in healthcare settings, Abu Dhabi. Emerg Infect Dis. 2016;22:647–656. doi: 10.3201/eid2204.151615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho SY, Kang J-M, Ha YE. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016;388:994–1001. doi: 10.1016/S0140-6736(16)30623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.WHO WHO MERS-CoV global summary and assessment of risk. 21 July 2017. http://www.who.int/emergencies/mers-cov/risk-assessment-july-2017.pdf?ua=1 (accessed Nov 5, 2017).
- 59.Alraddadi BM, Al-Salmi HS, Jacobs-Slifka K. Risk factors for Middle East respiratory syndrome coronavirus infection among healthcare personnel. Emerg Infect Dis. 2016;22:1915–1920. doi: 10.3201/eid2211.160920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Majumder MS, Brownstein JS, Finkelstein SN, Larson RC, Bourouiba L. Nosocomial amplification of MERS-coronavirus in South Korea, 2015. Trans R Soc Trop Med Hyg. 2017;111:261–269. doi: 10.1093/trstmh/trx046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi S, Jung E, Choi BY, Hur YJ, Ki M. High reproduction number of Middle East respiratory syndrome coronavirus in nosocomial outbreaks: mathematical modelling in Saudi Arabia and South Korea. J Hosp Infect. 2017 doi: 10.1016/j.jhin.2017.09.017. published online Sept 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song JY, Cheong HJ, Choi MJ. Viral shedding and environmental cleaning in Middle East respiratory syndrome coronavirus infection. Infect Chemother. 2015;47:252–255. doi: 10.3947/ic.2015.47.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corman VM, Albarrak AM, Omrani AS. Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2016;62:477–483. doi: 10.1093/cid/civ951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park GE, Ko JH, Peck KR. Control of an outbreak of Middle East respiratory syndrome in a tertiary hospital in Korea. Ann Intern Med. 2016;165:87–93. doi: 10.7326/M15-2495. [DOI] [PubMed] [Google Scholar]
- 65.Moon SY, Son JS. Infectivity of an asymptomatic patient with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis. 2017;64:1457–1458. doi: 10.1093/cid/cix170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.WHO . World Health Organization; Geneva: 2015. Infection prevention and control during health care for probable or confirmed cases of Middle East respiratory syndrome coronavirus (MERS-CoV) infection Interim guidance.http://www.who.int/csr/disease/coronavirus_infections/ipc-mers-cov/en/ (accessed Jan 17, 2018). [Google Scholar]
- 67.WHO . World Health Organization; Geneva: 2009. WHO guidelines on natural ventilation for infection control in health-care settings.http://whqlibdoc.who.int/publications/2009/9789241547857_eng.pdf (accessed Nov 11, 2017). [PubMed] [Google Scholar]
- 68.WHO Management of asymptomatic persons who are RTPCR positive for Middle East respiratory syndrome coronavirus (MERS-CoV) http://apps.who.int/iris/bitstream/10665/180973/1/WHO_MERS_IPC_15.2_eng.pdf?ua=1&ua=1 Interim Guidance. Updated Jan 3, 2018. (accessed Jan 16, 2018).
- 69.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) http://www.who.int/mediacentre/factsheets/mers-cov/en/ Fact sheet. Updated May 2017. (accessed Jan 16, 2018).
- 70.Chu DK, Chan SM, Perera RA. MERS-CoV in Arabian camels in Africa and Central Asia. Virus Evol. 2017;3(suppl 1) doi: 10.1093/ve/vew036.045. vew036.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miguel E, Perera RA, Baubekova Absence of Middle East respiratory syndrome coronavirus in camelids, Kazakhstan, 2015. Emerg Infect Dis. 2016;22:555–557. doi: 10.3201/eid2203.151284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Tawfiq JA, Zumla A, Memish ZA. Coronaviruses: severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus in travelers. Curr Opin Infect Dis. 2014;27:411–417. doi: 10.1097/QCO.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 74.Hui DS, Perlman S, Zumla A. Spread of MERS to South Korea and China. Lancet Respir Med. 2015;3:509–510. doi: 10.1016/S2213-2600(15)00238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Memish ZA, Zumla A, Alhakeem RF. Hajj: infectious disease surveillance and control. Lancet. 2014;383:2073–2082. doi: 10.1016/S0140-6736(14)60381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zumla A, Rustomjee R, Ntoumi F. Middle East respiratory syndrome—need for increased vigilance and watchful surveillance for MERS-CoV in sub-Saharan Africa. Int J Infect Dis. 2015;37:77–79. doi: 10.1016/j.ijid.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zumla A, Hui DS. Infection control and MERS-CoV in health-care workers. Lancet. 2014;383:1869–1871. doi: 10.1016/S0140-6736(14)60852-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hui DS, Zumla A. Advancing priority research on the Middle East respiratory syndrome coronavirus. J Infect Dis. 2014;209:173–176. doi: 10.1093/infdis/jit591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.