Summary
Despite extensive global efforts in the fight against killer infectious diseases, they still cause one in four deaths worldwide and are important causes of long-term functional disability arising from tissue damage. The continuing epidemics of tuberculosis, HIV, malaria, and influenza, and the emergence of novel zoonotic pathogens represent major clinical management challenges worldwide. Newer approaches to improving treatment outcomes are needed to reduce the high morbidity and mortality caused by infectious diseases. Recent insights into pathogen–host interactions, pathogenesis, inflammatory pathways, and the host's innate and acquired immune responses are leading to identification and development of a wide range of host-directed therapies with different mechanisms of action. Host-directed therapeutic strategies are now becoming viable adjuncts to standard antimicrobial treatment. Host-directed therapies include commonly used drugs for non-communicable diseases with good safety profiles, immunomodulatory agents, biologics (eg monoclonal antibodies), nutritional products, and cellular therapy using the patient's own immune or bone marrow mesenchymal stromal cells. We discuss clinically relevant examples of progress in identifying host-directed therapies as adjunct treatment options for bacterial, viral, and parasitic infectious diseases.
Introduction
Infectious diseases are leading causes of morbidity and mortality worldwide.1 In high-income countries, mortality from respiratory tract infections remains high despite access to quality health services and availability of antibiotic therapy.1 The intermittent emergence of new zoonotic pathogens and the increasing incidence of treatment-resistant infections draws attention to the limits of the current antimicrobial treatment portfolio and the urgent need for alternative management strategies.
In evolutionary terms, host–pathogen interactions are dependent on the microbe surviving without causing harm to the host. The host's innate and adaptive immune surveillance mechanisms govern whether the infection will be contained or progress to clinical disease with either recovery or death. Several host factors affect antimicrobial treatment outcome and are responsible for progression of disease after infection, poor treatment response, tissue damage, long-term functional disability, and increased mortality. These factors include immune dysregulation from any cause (aberrant or excess host inflammatory response to infection, stress, immunosuppressive drugs, poor living conditions, socioeconomic factors, micronutrient deficiencies, HIV, malnutrition, and alcohol or substance misuse) and comorbidity with non-communicable diseases such as diabetes, cancer, smoking, and chronic obstructive pulmonary disease.1
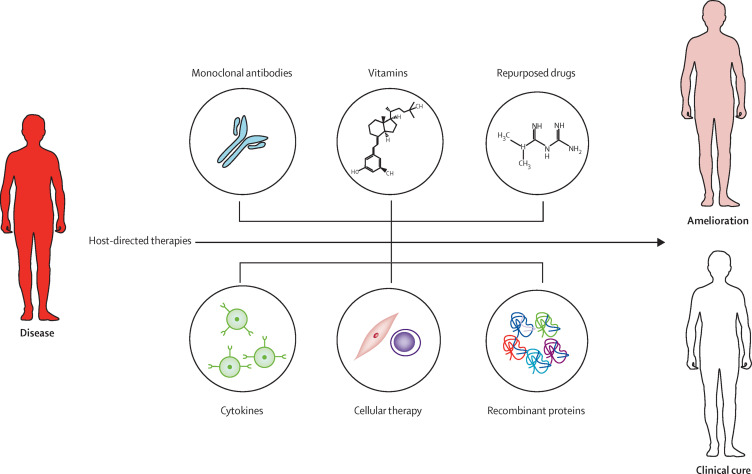
During the past 4 years, a renaissance of scientific research strategies targeting host factors, rather than pathogen components directly, has opened up novel treatment approaches termed host-directed therapies. A host-directed therapy is any product that can augment host defence mechanisms or modulate excessive inflammation, or both, leading to improved clinical treatment outcomes as shown by reduced morbidity, mortality, and end-organ damage, and long-term functional recovery. A range of host-directed therapies have been identified with different mechanisms of action (figure 1 ), and they are now regarded as viable adjuncts to standard antimicrobial treatment. Host-directed therapies can improve host cellular responses to pathogens, target disease-causing virulence factors (figure 2 ), and activate innate and adaptive immune responses and immunological memory (figure 3 ).2 Examples of host-directed therapies include commonly used and affordable drugs for non-communicable diseases with good safety profiles, immunomodulatory agents, biologics, nutritional products, and cellular therapy using the patient's own immune or mesenchymal stromals cells (table 1 ). See appendix for discussion of potential host factors for targets of host-directed therapy against infectious disease.
Figure 1.
The main types of host-directed therapies
Host-directed therapies focus on ameliorating the severity of disease and improving treatment outcomes. Host-directed therapies constitute a range of therapeutic agents such as repurposed drugs, small molecules, synthetic nucleic acids, biologics (such as monoclonal antibodies), cytokines, cellular therapy, recombinant proteins, and micronutrients.
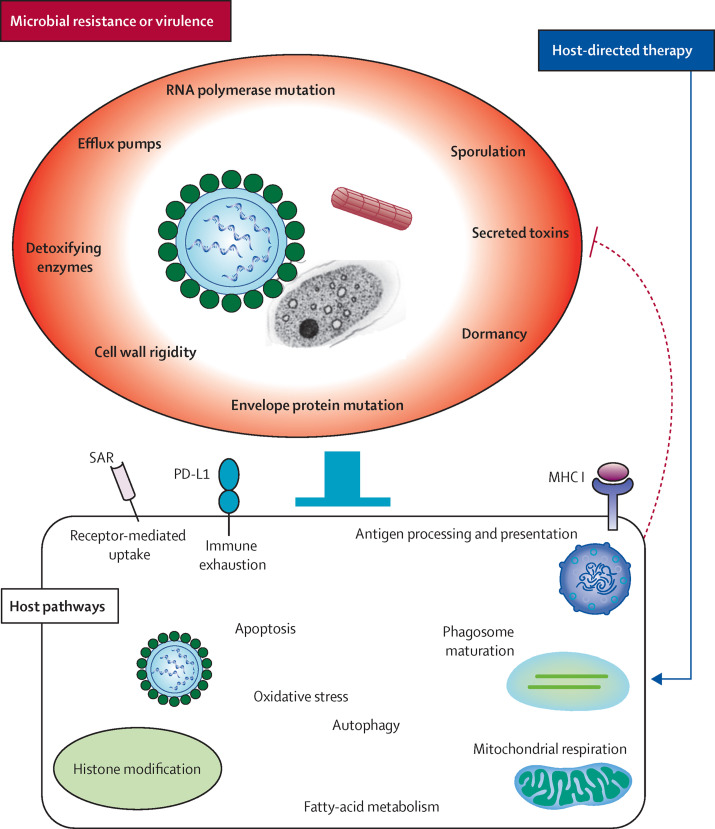
Figure 2.
Host-directed therapies as a means to counteract antimicrobial resistance
Pathogens develop resistance to antimicrobial therapy via various factors, including modification of cell-surface proteins and intracellular enzymes (bacteria and parasites), modification of envelope proteins (viruses), secretion of toxins (bacteria and parasites), sporulation and dormancy (bacteria, viruses, and fungi), activation of efflux pumps (bacteria, fungi, and parasites), and decreased permeability of cell wall (bacteria and fungi). These virulence factors impede cellular functions (solid blockade), which are required to successfully eradicate the pathogen. Host-directed therapies can counter these mechanisms by targeting impaired intracellular processes in affected host cells (blue arrow), by mechanisms such as activation of autophagy and apoptosis, induction of oxidative and nitrosative stress, and increased antigen processing and presentation, which in turn trigger necessary adaptive immune responses. Novel host-directed therapeutic strategies target host surface receptors, such as programmed death-ligand 1 (PD-L1; involved in immune exhaustion) and sialic acid-containing receptor (SAR; enhances entry of pathogens into host cells). Histone modification is done by targeting genes involved in pathogen replication and induction of apoptosis, autophagy, and antigen processing and presentation. Fatty-acid metabolism might have a role in maintenance of memory CD8 cytotoxic T-lymphocyte pools in the host. Responses induced by host-directed therapies might counteract microbial virulence factors (dotted blockade), in addition to neutralising tissue damage.
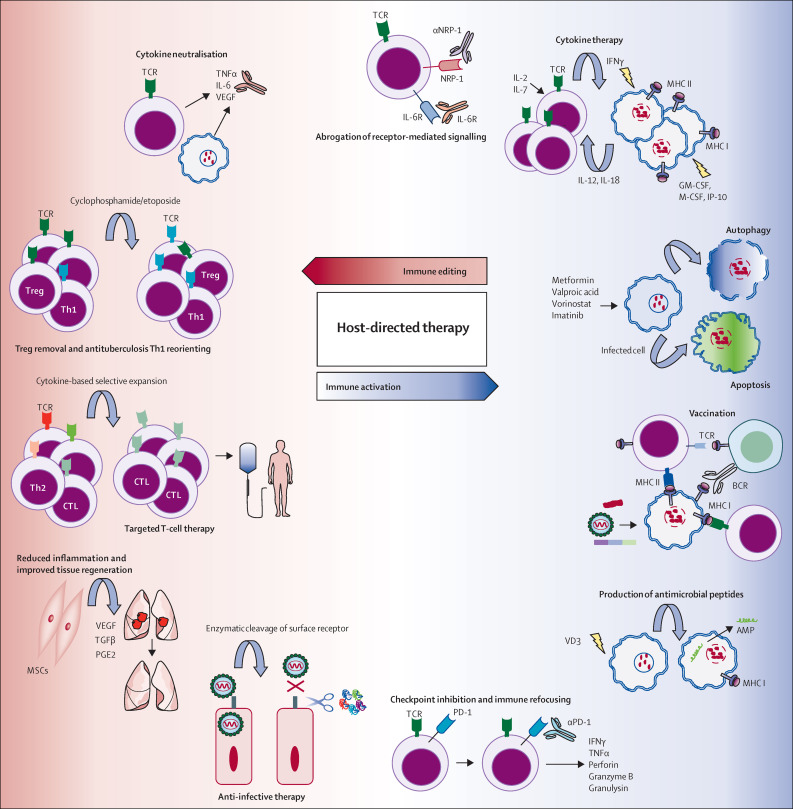
Figure 3.
Possible biological pathways and mechanisms for host-directed interventions against infectious diseases
Pharmacological activation of autophagy or apoptosis, or both, drives improved intracellular killing of pathogens and enhanced antigen presentation. Activation and recruitment of antigen-presenting cells (ie, dendritic cells and macrophages) via therapy with the pro-inflammatory cytokines interferon γ (IFNγ), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IFNγ-induced protein (IP-10), among others, could amplify the antimicrobial immune response. Several anticancer drugs (ie, cisplatin, gemcitabine, and paclitaxel) can potentiate antigen-specific CD8 cytotoxic T-lymphocyte (CTL) responses in patients by inducing production of interleukin (IL) 12, tumour necrosis factor γ (TNFγ), and IL 6. Immune checkpoint inhibition by blocking the programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway activates antigen-specific T cells. In-vitro selection and expansion of pathogen-specific autologous T-cell subsets (antigen-specific CD4 T cells and CD8 CTLs) can allow for reinfusion into the patient after confirmation of activity. Blockade of cell surface-bound signalling molecules, such as the receptors for IL 6 and neuropilin 1 (NRP-1), may potentiate specific T-cell responses. Removal of excess inflammatory cytokines by use of monoclonal antibodies, or depletion of regulatory T cells (Treg) with cytotoxic agents (eg, cyclophosphamide and etoposide) dampens destructive inflammation in the target organs, and might re-orientate Mycobacterium tuberculosis-targeted immune responses (T-helper-1 [Th1] and CD8 CTLs). Histone deacetylase inhibitors, valproic acid, and vorinostat, might reprogramme non-productive Th2 cells to antigen-specific Th1 cells. Infusion of autologous mesenchymal stromal cells (MSCs) could neutralise the local cytokine milieu, promote tissue repair, and orchestrate antigen-specific T-cell responses, in multidrug-resistant tuberculosis. Host-cell surface receptors used by pathogens for entry could be targeted by host-directed therapies. AMP=antimicrobial peptide. BCR=B-cell receptor. M-CSF=macrophage colony-stimulating factor. PGE2=prostaglandin E2. TCR=T-cell receptor. TGF=transforming growth factor. TGFβ=TGF β. VD3=vitamin D3. VEGF=vascular endothelial growth factor.
Table 1.
Developmental pipeline of host-directed therapies for infectious diseases
| Examples of host-directed therapy | Mechanism of action | Developmental stage | ||
|---|---|---|---|---|
| Bacterial infections | ||||
| Mycobacterium tuberculosis | ||||
| Repurposed drug* | Imatinib, verapamil, metformin, ibuprofen | Modulation of inflammation and activation of intracellular antimicrobial defences | Preclinical/clinical (early phase) | |
| Cytokine therapy* | Interleukin 2, GM-CSF, interferon γ, interleukin 12 (early stage) | Induction of pro-inflammatory cell signalling | Clinical (late phase) | |
| Monoclonal antibody* | Anti-TNFα, anti-interleukin 6, anti-VEGF | Reduction of tissue-destructive inflammation by cytokine neutralisation | Preclinical/clinical (early phase) | |
| Monoclonal antibody* | Anti-PD-1, anti-LAG3, anti-CTLA-4 | Activation and mobilisation of antigen-specific T cells by immune checkpoint inhibition | Preclinical | |
| Vitamin* | Vitamin D3 | Activation and augmentation of intracellular antimicrobial defences (via interferon γ and interleukin-15 signalling) | Clinical (late phase) | |
| Cellular therapy* | Autologous mesenchymal stromal cells, T cells | Neutralisation of tissue-destructive inflammation, enhancement of organ repair, and potentiation of antigen-specific immune responses | Clinical (early phase) | |
| Streptococcus pneumoniae | ||||
| Repurposed drug3 | Prednisone | Reduction of tissue-destructive inflammation by activating the glucocorticoid pathway | Clinical (late phase; also in current practice) | |
| Repurposed drug4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 | Ibuprofen, statins, indometacin, aspirin | Reduction of tissue-destructive inflammation by inhibiting prostaglandin release via cyclooxygenase inhibition, regulation of MHC molecules | Clinical (late phase) | |
| Repurposed drug20 | Glibenclamide | An oral hypoglycaemic agent that modulates voltage-gated calcium channels, leading to immunomodulatory effects | Clinical (early phase) | |
| Antibiotic4, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 | Azithromycin, erythromycin | Reduces local tissue inflammation through anti-inflammatory activities | Clinical (current practice) | |
| Helicobacter pylori | ||||
| Monoclonal antibody33 | Anti-interleukin 1β, anti-TNFα (late stage) | Reduction of tissue-destructive inflammation by cytokine neutralisation | Preclinical | |
| Vitamin34 | Vitamin D3 | Activation and augmentation of intracellular antimicrobial defences (via interferon γ and interleukin-15 signalling) | Preclinical | |
| Bordetella pertussis | ||||
| Repurposed drug35 | Fingolimod | Activates the sphingosine-1-phosphate pathway to improve antigen-specific lymphocyte responses, as well as reduced hyper-inflammation | Preclinical | |
| Monoclonal antibody36, 37, 38 | Antipertussis toxin antibodies | Reduces toxin load via infusion of intravenous immunoglobulins | Clinical (in current practice) | |
| Neisseria gonorrhoeae | ||||
| Repurposed drug39, 40 | Sulforaphane | Increased histone acetylation to enhance gene transcription | Preclinical | |
| Recombinant protein39, 40 | Secretory leucocyte protease inhibitor, β-defensin 2 | Host-derived antimicrobial peptides with bactericidal effects | Preclinical | |
| Viral infections | ||||
| HIV | ||||
| Repurposed drug41, 42, 43 | Valproic acid, vorinostat | Reactivation of latent HIV infection and making new viral progeny susceptible to ART and immune attack by enhancing gene transcription | Clinical (early phase) | |
| Monoclonal antibody44, 45, 46 | Anti-PD-1 | Activation and mobilisation of antigen-specific T cells via immune checkpoint blockade | Preclinical | |
| Cellular therapy47 | MSCs | Reduction of destructive inflammation and enhancement of tissue regeneration and organ repair | Not yet tested in HIV infection | |
| Epstein-Barr virus | ||||
| Cellular therapy48, 49, 50, 51 | CD19 CAR (for Epstein-Barr virus [EBV] B-cell lymphoma), in-vitro-expanded EBV-specific CD8 CTLs | Depletion of viral reservoirs to deter progression to lymphoma | Clinical (mid phase) | |
| Cytomegalovirus | ||||
| Monoclonal antibody52 | Viral envelope protein-targeted IgG | Neutralises virus and reduces viral load | In clinical use | |
| Cellular therapy49, 50, 51, 53, 54 | In-vitro-expanded cytomegalovirus-specific CD8 CTLs | Depletion of viral reservoirs to avoid fulminant viraemia in immunocompromised individuals | In clinical use | |
| Adenovirus | ||||
| Cellular therapy49, 50, 51, 55 | In-vitro-expanded adenovirus-specific CD8 CTLs | Depletion of viral reservoirs to avoid fulminant viraemia in immunocompromised individuals | In clinical use | |
| Hepatitis C virus | ||||
| Repurposed drug56, 57, 58, 59 | Miravirsen (SPC3649) | Antisense RNA targeting miR-122 for modulation of fatty acid metabolism to reduce viral burden in host cells | Clinical (early phase) | |
| Monoclonal antibody60 | Anti-PD-1 | Activation and mobilisation of antigen-specific T cells via immune checkpoint blockade | Clinical (early phase) | |
| Cytokine therapy61 | Pegylated interferon α and β | Potentiation of pro-inflammatory antiviral immune response | In clinical use | |
| Influenza viruses | ||||
| Repurposed drug62, 63, 64, 65 | Metformin | Induction of autophagy and improved antigen processing and presentation; improves maintenance of memory CD8 CTLs | Preclinical | |
| Repurposed drug62, 63, 64, 65 | Glitazones (PPAR-γ), fibrates (PPAR-α) | Pleiotropic effects, including blockade of angiogenesis and pro-inflammatory signalling | Preclinical | |
| Repurposed drug62, 63, 64, 65 | Sartans | Angiotensin-II-receptor blocker that reduce inflammation and allow tissue remodelling | Clinical (mid-late phase) | |
| Repurposed drug62, 63, 64, 65 | Atorvastatin | Angiotensin-converting enzyme blocker that reduces pro-inflammatory signalling and improves tissue repair | Clinical (mid-late phase) | |
| Recombinant protein66, 67 | Sialidase fusion peptide DAS181 | Reduces infectivity of influenza viruses by cleaving surface receptors on host epithelia | Clinical (early phase) | |
| Ebola virus | ||||
| Repurposed drug68, 69 | Irbesartan | Angiotensin-II-receptor blockers that reduce inflammation and allow tissue remodelling | Clinical (early phase) | |
| Repurposed drug68, 69 | Atorvastatin | Angiotensin-converting enzyme blocker that reduces pro-inflammatory signalling and improves tissue repair | Clinical (early phase) | |
| Cytokine therapy70 | Pegylated interferon α and β | Potentiation of pro-inflammatory antiviral immune response | Clinical (early phase) | |
| Monoclonal antibody71 | ZMAb | Antibody neutralises virus and reduces viral load | Preclinical | |
| Recombinant protein72 | rNAPc2 | Blocks blood coagulation to reduce vasculopathy; also reduces release of pro-inflammatory cytokines | Preclinical | |
| Dengue virus | ||||
| Repurposed drug73 | Lovastatin | Angiotensin-converting enzyme inhibitor that reduces pro-inflammatory signalling and improves tissue repair | Preclinical | |
| Repurposed drug73, 74 | Dasatinib | Tyrosine kinase inhibitor that inhibits viral replication via blockade of host proto-oncogene kinase (Fyn) | Preclinical | |
| Repurposed drug73, 74 | Ciclosporin | Cyclophilin inhibitor that reduces viral replication via blockade of host cyclophilin A | Preclinical | |
| Cytokine therapy75 | Pegylated interferon α and β, interferon γ | Potentiation of pro-inflammatory antiviral immune response | Not yet tested in dengue fever | |
| Recombinant protein72 | rNAPc2 | Blocks blood coagulation to reduce vasculopathy; also reduces release of pro-inflammatory cytokines | Preclinical | |
| Middle East respiratory syndrome coronavirus (MERS-CoV) | ||||
| Repurposed drug76, 77 | Sitagliptin, vildagliptin | Incretin-based inhibitors or blockers of host DPP-4 surface receptors that inhibit virus entry into host cells | Preclinical | |
| Monoclonal antibody76, 78 | Anti-interleukin 17A, anti-interleukin 23 | Cytokine neutralisation of tissue-destructive inflammation | Not yet tested in MERS-CoV disease | |
| Parasitic diseases | ||||
| Malaria | ||||
| Repurposed drug79 | Desferrioxamine | Ferrochelatase inhibitor reduces Plasmodium sp replication in erythrocytes | Preclinical | |
| Recombinant protein80, 81 | IDR-1018 | Innate defence regulator peptide enables balanced cytokine release, which allows for pathogen killing without excessive inflammation | Preclinical | |
| Leishmaniasis | ||||
| Repurposed drug82 | Imiquimod, resiquimod | TLR agonist that induces B-cell activation and pro-inflammatory cytokine signalling | In clinical use | |
| Cytokine therapy83 | Interferon γ, interleukin 2, interleukin 12 (early stage) | Induction of pro-inflammatory immune responses and intracellular antimicrobial activity | Not yet tested in leishmaniasis | |
| Monoclonal antibody84 | Anti-TNFα (late stage) | Cytokine neutralisation reduces tissue-destructive inflammation | Not yet tested in leishmaniasis | |
| African trypanosomiasis | ||||
| Cytokine therapy85, 86 | Interferon γ, interleukin 2, TNFα (early stage) | Induction of pro-inflammatory immune responses and intracellular antimicrobial activity | Preclinical | |
| Monoclonal antibody85, 86 | Anti-TNFα (late stage) | Cytokine neutralisation to reduce tissue-destructive inflammation | Not yet tested in trypanosomiasis | |
| Recombinant protein or cytokine therapy85, 86 | Interferon-γ-induced apolipoprotein 1 | Cytokine-induced protein that can directly engage and kill trypanosomes | Preclinical | |
| Cellular therapy85, 86 | MSCs (late stage) | Neutralisation of tissue-destructive inflammation and enhancement of organ repair | Not yet tested in trypanosomiasis | |
| Schistosomiasis | ||||
| Cytokine therapy87 | Interleukin 2, interferon γ (early stage) | Induction of pro-inflammatory immune responses and intracellular antimicrobial activity | Not yet tested in schistosomiasis | |
| Recombinant protein88 | Peroxiredoxin (as adjuvant to vaccine candidate) | Regulation of hydrogen peroxide concentrations in the host; induction of antigen-specific B-cell responses | Preclinical | |
| Cellular therapy51 | In-vitro-expanded schistosoma-specific CD8 CTLs | Potentiation of parasite-specific cellular immune responses to deter progression to clinical disease | Not yet tested in schistosomiasis | |
GM-CSF=granulocyte-macrophage colony-stimulating factor. TNFα=tumour necrosis factor α. VEGF=vascular endothelial growth factor. LAG3=lymphocyte-activation gene 3. CTLA-4=cytotoxic-T-lymphocyte-associated antigen 4. MSCs=mesenchymal stromal cells. ART=antiretroviral therapy. PD-1=programmed cell death 1. CAR=chimeric antigen receptor. CTLs=cytotoxic T lymphocytes. miR-122=microRNA 122. PPAR=peroxisome proliferator-activated receptor. rNAPc2=recombinant nematode anticoagulant protein c2. DPP-4=dipeptidyl peptidase 4. TLR=Toll-like receptor.
See table 2 for more details.
Key messages.
-
•
Despite the availability of antimicrobial drugs, infectious diseases are leading causes of morbidity and mortality worldwide.
-
•
The widespread emergence of antimicrobial resistance calls for novel interventions in addition to new antimicrobial development.
-
•
A range of host factors are responsible for development of disease, poor treatment response, and increased mortality. These include immune dysregulation from any cause and comorbidity with non-communicable diseases such as diabetes, cancer, smoking, and chronic obstructive pulmonary disease.
-
•
During the past 4 years, a renaissance of scientific research strategies targeting host factors—rather than pathogen components directly—is leading to development of a wide range of host-directed therapies that target and modify biological pathways to achieve a positive clinical treatment outcome.
-
•
Host-directed therapies can augment host cellular responses to pathogens, target disease-causing virulence factors, activate innate and adaptive protective immune responses, or modulate excessive inflammation, leading to reduced morbidity, mortality, and end-organ damage.
-
•
Host-directed therapies include commonly used, safe, and cheap drugs for non-communicable diseases; biologics; nutritional products; and cellular therapy, using the patient's own immune or mesenchymal stromal cells.
-
•
The broad spectrum efficacy of host-directed therapies could also be useful for treatment of infectious diseases with epidemic potential, which are associated with high mortality.
-
•
Host-directed therapies have the additional unique benefit of preventing or reducing the development of antibiotic resistance.
Studies of host-directed therapies also enable new insights into underlying mechanisms of pathogenesis, inflammatory pathways, and the host's innate and acquired immune responses. In this Review we discuss clinically relevant examples of progress in identification of candidate host-directed therapies as adjunct treatment options for bacterial, viral, and parasitic infectious diseases.
Bacterial infections
Tuberculosis is the most common cause of death from an infectious disease worldwide.89 Since the declaration of tuberculosis as a global emergency in 1993 by WHO, there has been a major focus on development of new drugs that target Mycobacterium tuberculosis, the causative pathogen. For decades, the notion that M tuberculosis is the sole cause of the continuing worldwide tuberculosis pandemic has led to a focus on treatment of patients with tuberculosis with WHO-recommended combination antituberculosis antibiotic therapy. This focus still thrives, although there are about 2 billion people in the world with latent tuberculosis infection who do not develop active disease.89 Furthermore, the substantial decline in tuberculosis in Europe and North America in the first half of the 19th century occurred well before the antibiotic era. In 2014, an estimated 1·5 million people died of tuberculosis and there were an estimated 450 000 cases of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis,89 suggesting that host factors have an important role in achieving anti-M tuberculosis immune responses. M tuberculosis is largely intracellular in nature, and intact T-cell responses (T-helper-1 [Th1] CD4 cytotoxic lymphocytes, CD8 cytotoxic lymphocytes, and natural killer T cells) and interferon-γ production are needed to restrict M tuberculosis growth.90 Pulmonary tissue pathology, substantial tissue destruction, and subdued protective anti-M tuberculosis immune responses are noted in patients with tuberculosis who are predominantly affected by tumour necrosis factor (TNF)-γ-mediated inflammation.91
Improving treatment for both drug-sensitive and MDR tuberculosis is a high priority. Few new anti-M tuberculosis drugs are in clinical assessment and some have substantial safety concerns. Furthermore, resistance is likely to develop against new tuberculosis drugs. The greatest clinical needs for tuberculosis treatment are interventions that could reduce the lengthy duration of tuberculosis therapy (currently 6 months in patients with drug-sensitive tuberculosis and 18–24 months in patients with MDR or XDR tuberculosis), thus improving patient compliance and reducing long-term toxicity; invigorate immune responses to eradicate or contain M tuberculosis; dampen excessive inflammation and repair tissue damage to prevent long-term pulmonary damage and functional disability; and reduce the high mortality from MDR and XDR tuberculosis.
There is an expanding portfolio of host-directed therapies for use as adjunct treatments to tuberculosis therapy for improving treatment outcomes, shortening the duration of therapy, and reducing lung pathology and long-term functional disability for drug-sensitive and drug-resistant tuberculosis (table 2 ).91, 133 Other host-directed therapies may decrease local inflammatory tissue pathology, including that caused by tuberculosis-associated immune reconstitution inflammatory syndrome. Examples of host-directed therapies currently being developed are cellular therapy using the patient's own bone marrow-derived mesenchymal stromal cells;133 repurposing commonly used drugs for diabetes, epilepsy, peptic ulcers, hypercholesterolaemia, asthma, cancer, and arthritis; micronutrients and other immune-modulators; antimicrobial peptide inducers and checkpoint inhibitors; specific immune-based therapies; and therapeutic vaccines. Multinational consortia have been established to take these therapies forward in controlled clinical trials.
Table 2.
Developmental pipeline of host-directed therapies for adjunct treatment of drug-sensitive and drug-resistant tuberculosis, by host pathway
| Class or type | Mechanism of action | Host effect | Developmental stage for tuberculosis | |
|---|---|---|---|---|
| Mitochondrial respiration and fatty acid oxidation | ||||
| Metformin92, 93, 94 | Biguanide | Interrupts the mitochondrial respiratory chain and induces ROS production; increases mitochondrial biogenesis and respiration | Enhanced killing of intracellular Mycobacterium tuberculosis via ROS production; improved control of bacterial burden and reduced lung pathology in mice; enhanced T-cell responses; might improve maintenance of memory CD8 T cells via increased FAO; promotes generation of CD8 T-cell memory against tumour engraftment in experimental TRAF6-deficient mice by restoring FAO, possibly via AMPK activation; increases mitochondrial biogenesis and hence respiration in rabbit renal proximal tubular cells | Preclinical |
| Niraparib95 | PARP inhibitor | Inhibition of PARP-1 and PARP-2 activity, and impairs repair of DNA single strand breaks | Restores mitochondrial respiratory function in human myotubes, also by improved FAO; might promote maintenance of antituberculosis memory CD8 T cells | Preclinical |
| Interleukin 1596, 97 | Cytokine | Involved in maintenance and possibly proliferation of CD8 T cells | Increases mitochondrial mass and FAO in memory CD8 T cells to prolong survival in experimental mice | Preclinical |
| Arachidonic acid metabolism | ||||
| Aspirin98 | NSAID | Increased lipoxin A4 production to reduce TNFα levels and achieve eicosanoid balance during chronic inflammation | Dampening of TNFα-induced hyperinflammation to aid tissue repair and control burden of M tuberculosis | Preclinical |
| Zileuton99 | Leukotriene synthesis inhibitor | Blocks leukotriene production by disrupting lipooxygenase activity; promotes prostaglandin production via cyclooxygenase activation | Increases PGE2 levels and augments interleukin-1β-mediated immune control of tuberculosis in mice; promotes reduced lung M tuberculosis burden and pathology | Preclinical |
| Ibuprofen100, 101 | NSAID | Blocks production of prostaglandins possibly by inhibiting cyclooxygenase activity | Reduces lung pathology and mycobacterial burden in a highly susceptible mouse model of tuberculosis | Clinical (early phase) |
| Corticosteroid metabolism | ||||
| Prednisone102 | Glucocorticoid receptor antagonist | Forms a complex with glucocorticoid receptor and triggers transcription of several important host genes (ie, iNOS, cyclooxygenase-2, collagenase) | Use in patients with community-acquired pneumonia showed improved survival; results in patients with tuberculosis require further validation | Clinical (mid-late phase) |
| Histone acetylation | ||||
| Valproic acid and vorinostat41, 103 | Histone deacetylase inhibitor | Acetylation of lysine residues on histones to promote DNA unwinding and gene transcription | Valproic acid and vorinostat can activate latent HIV reservoirs and increase ART efficacy as well as CD8 T-cell activity; both drugs can improve efficacy of isoniazid and rifampicin against intracellular M tuberculosis | Preclinical |
| Phenylbutyrate104, 105 | Histone deacetylase inhibitor | Acetylation of lysine residues on histones to promote DNA unwinding and gene transcription | Augments vitamin D3 activity, cathelicidin production, and MAPK signalling to kill intracellular M tuberculosis | Clinical (early phase) |
| Host cell cytotoxicity | ||||
| Cyclophosphamide106, 107 | Alkylating agent | CYP450 metabolism of cyclophosphamide produces chemical species that can alkylate DNA guanine to reduce cell proliferation. Cells highly expressing ALDH are resistant to cyclophosphamide | Abrogation of regulatory T-cell responses, and potentiation of RCC vaccine candidate efficacy in clinical trials, with induction of CD8 T-cell responses; might increase efficacy of the BCG vaccine | Not yet tested in tuberculosis |
| Etoposide108, 109 | Topoisomerase inhibitor | Blockade of DNA topoisomerase II to prevent re-ligation of nascent DNA strands | Depletion of pathogenic inflammatory T cells in influenza-induced HLH | Preclinical |
| Modulation of ion efflux channels | ||||
| Verapamil110 | Calcium-channel blocker | Modulation of voltage-gated calcium-channel activity for maintenance of cellular ionic homeostasis | Improves efficacy of conventional and novel antituberculosis drugs in M tuberculosis-infected mice | Preclinical |
| Carbamazepine111 | Sodium-channel blocker | Anticonvulsant; acts via voltage-gated sodium-channel downmodulation and activation of GABA receptors for reduced sensitivity to neuropathic pain. Activates AMPK to induce autophagy | Shown to induce inositol depletion-dependent autophagic killing of intracellular M tuberculosis in macrophages; augments reduced lung pathology and improved immune responses in the mouse model of tuberculosis | Preclinical |
| Statins112, 113 | Inhibitors of 3-hydroxy-3-methylglutaryl coenzyme reductase | Block biosynthesis of endogenous cholesterol | Simvastatin can reduce M tuberculosis CFUs (human macrophages and mice) | Preclinical |
| Inhibition of tyrosine kinases | ||||
| Imatinib mesylate114 | Inhibitor of BCR-ABL tyrosine kinase | Induces apoptotic death of cancerous B cells, and cells expressing related kinases | Reduces CFU load and pathology in lungs of M tuberculosis-infected mice; induces myelopoiesis | Preclinical (about to enter early phase clinical trials) |
| Innate immune defences | ||||
| Vitamin D3115, 116 | Vitamin | Induces cathelicidin production, improves antigen processing and presentation, augments response to interferon-γ signalling | Kills intracellular M tuberculosis and improves T-cell responses | Clinical (late phase) |
| Immune activation | ||||
| GM-CSF, interleukin 2, and interferon γ117 | Cytokine | Contribute to proliferation and activation of macrophages, dendritic cells, monocytes, T cells | Variable results but with a generally positive outcome following treatment, coupled with reduction in sputum AFB | Clinical (mid-late phase) |
| Immune checkpoint inhibition | ||||
| Ipilimumab (anti-CTLA-4)118, 119 | Monoclonal antibody | Blockade of CTLA-4 to undo T-cell exhaustion; restores interleukin-2 secretion and signalling | CTLA-4 inhibition in melanoma increases CD8 T-cell activity and tumour regression; might improve CD8 T-cell activity against M tuberculosis-infected cells | Preclinical |
| Nivolumab or pembrolizumab (anti-PD-1)120, 121, 122 | Monoclonal antibody | Blockade of PD-1 to restore lymphocyte functionality. Also, PD-L1 blockade on the surface of APCs contributes to T-cell activation | PD-1 blockade potentiates in-vitro killing of M tuberculosis-infected macrophages by CD4 T cells in an interferon-γ-dependent manner and prevents apoptosis of T cells; downregulation of PD-1 on CD4 T cells is commensurate with antituberculosis treatment | Preclinical |
| Anti-Tim3123, 124 | Monoclonal antibody | Modulation of Tim3–Gal9 interaction to induce targeted T-cell responses | M tuberculosis-infected human CD14 monocytes shown to have reduced Tim3 expression with extent of tuberculosis disease in patients; Tim3–Gal9 interaction induces interleukin-1β-driven immune control of M tuberculosis infection in vitro | Preclinical |
| Anti-LAG3125, 126 | Monoclonal antibody | Blockade of LAG3 to abrogate regulatory T-cell interaction with activated effector CD4 and CD8 T cells | Blockade of LAG3 can potentiate targeted CD8 CTL responses in patients with solid tumours. In tuberculosis, low LAG3 expression may be reflective of successful containment of tuberculosis infection | Preclinical |
| Cytokine neutralisation | ||||
| Adalimumab (anti-TNFα)127 | Monoclonal antibody | Removal of excess TNFα from tissue and circulation | Successfully used salvage therapy in a patient with severe pulmonary tuberculosis | Clinical (compassionate use) |
| Siltuximab (anti-interleukin 6)128, 129 | Monoclonal antibody | Removal of excess interleukin 6 from tissue and circulation | Effective against arthritis and Castleman's disease; used prospectively in patients with HIV/tuberculosis co-infection may reduce mortality from tuberculosis-associated IRIS | Preclinical |
| Angiogenesis inhibition | ||||
| Bevacizumab (anti-VEGF)130, 131 | Monoclonal antibody | Blockade of VEGF-induced neovascularisation in tissue | Disrupts neovascularisation within lung granulomas in a rabbit model of tuberculosis; improves small-molecule penetration into granulomas and increases air supply, might therefore improve antituberculosis drug efficacy | Preclinical |
| Reduction of inflammation and improved tissue regeneration | ||||
| BM-MSCs132 | Cell-based therapy | BM-MSCs can reduce destructive inflammation, regenerate tissue, and restore positive modulation of immune responses, secretion of soluble factors, and activation of regulatory T cells | Autologous MSC reinfusion in a phase 1 trial in Belarus of patients with multidrug-resistant tuberculosis was safe and reconstituted anti-M tuberculosis T cell responses; a phase 1 study is underway in Durban, South Africa | Clinical (early phase) |
ROS=reactive oxygen species. FAO=fatty acid oxidation. TRAF6=tumour necrosis factor receptor-associated factor 6. AMPK=5' adenosine monophosphate-activated protein kinase. PARP=poly (ADP-ribose) polymerase. NSAID=non-steroidal anti-inflammatory drug. TNFα=tumour necrosis factor α. PGE2=prostaglandin E2. iNOS=inducible nitric oxide synthase. ART=antiretroviral therapy. MAPK=mitogen-activated protein kinase. CYP450=cytochrome P450. ALDH=aldehyde dehydrogenase. RCC=renal cell carcinoma. HLH=haemophagocytic lymphohistiocytosis. GABA=γ-aminobutyric acid. CFUs=colony forming units. GM-CSF=granulocyte-macrophage colony-stimulating factor. AFB=acid-fast bacilli. CTLA-4=cytotoxic-T-lymphocyte-associated antigen 4. PD-1=programmed cell death 1. PD-L1=programmed death-ligand 1. APCs=antigen-presenting cells. Tim3=T-cell immunoglobulin and mucin-domain containing-3. Gal9=galectin 9. LAG3=lymphocyte-activation gene 3. IRIS=immune reconstitution inflammatory syndrome. VEGF=vascular endothelial growth factor. BM-MSCs=bone marrow-derived mesenchymal stromal cells. BCR-ABL=breakpoint cluster-Abelson tyrosine kinase.
Streptococcus pneumoniae is a Gram-positive bacterium that remains a major cause of childhood and adult morbidity and mortality worldwide,134 despite the availability of effective antibiotic therapy. It is largely associated with community-acquired pneumonia, and often causes invasive pneumococcal disease, affecting any organ in the body.135 Both cell-mediated and humoral immune responses operate in preventing disease in human beings.136 The pathogenesis of pneumonia is associated with overt inflammatory responses that eventually cause lung damage and death.137 Thus, several adjunctive host-directed interventions are being investigated.
The use of corticosteroids in pneumonia remains controversial, and data to support the use of corticosteroids in cases of community-acquired pneumonia are limited. In a prospective randomised clinical study of 785 patients with community-acquired pneumonia, prednisolone led to overall improved survival after treatment, concomitant with slightly shorter hospital stay and reduced need for mechanical ventilators, compared with placebo.3 Corticosteroids can also prevent hearing loss and other neurological sequelae in bacterial meningitis.138
Although the clinical use of macrolide antibiotics specifically targets the causative bacteria, macrolides might have an additional host-directed effect in treating community-acquired pneumonia. Preclinical assessment of azithromycin in mice after secondary S pneumoniae infection following primary exposure to influenza A virus potentiated anti-inflammatory effects marked by reduction in neutrophil influx, and promoted dampening of immunopathological outcome in the lungs.21 Retrospective and prospective studies22, 23, 24, 25, 26, 27 have shown that macrolide-containing antibiotic regimens decrease mortality in patients with community-acquired pneumonia, although other studies28, 29, 30 have shown no significant benefit from these regimens. Addition of a macrolide to a fluoroquinolone seems to provide some improvement in survival,31 suggesting a host-directed effect. Analyses in patients with community-acquired pneumonia with bacteraemia of all causes32 or community-acquired pneumonia with severe sepsis24 showed that benefit specific to macrolides was not only restricted to pneumococcal bacteraemia but was also shown for Gram-negative bacterial infections.24, 32 Findings suggest that macrolides provide benefit mainly to patients with more severe illness.4, 22, 24, 26, 27
The use of non-steroidal anti-inflammatory drugs (NSAIDs) for the treatment of pneumonia in people has yielded conflicting results.5, 6 A study investigating the effects of ibuprofen in patients with sepsis (50% had pneumonia) showed some improvement in gas exchange,7 albeit without any effect on mortality.139
Statins (3-hydroxyl-3-methyl-glutaryl-CoA reductase inhibitors) might have a role as adjunct treatment of community-acquired pneumonia via their pleiotropic anti-inflammatory, anti-oxidative, and immunomodulatory effects; however, their effects need to be defined in randomised controlled trials. Observational studies of patients on statins before development of pneumonia or other infection were less likely to develop sepsis, die from sepsis, or have complications leading to intensive care unit admission.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 No study has examined the addition of statins as an adjunctive therapy once pneumonia has developed.
Oral hypoglycaemic agents, such as glitazones, may have anti-inflammatory effects similar to corticosteroids in patients with community-acquired pneumonia, and glyburide has been associated with significantly lower mortality in patients with severe melioidosis than in patients without diabetes or patients taking other diabetes agents.140 These findings warrant clinical investigation to establish whether oral hypoglycaemic agents are potential host-directed therapies for severe community-acquired pneumonia.
Helicobacter pylori has emerged as a major human pathogen because of its role in gastric cancer (classified as a type 1 carcinogen)141 and gastric ulcers. More than 50% of the world's population is infected with the pathogen, which has co-evolved with human beings for almost 60 000 years.142 Raised concentrations of interleukin 1β and TNFγ in the gut of individuals with H pylori infection have been postulated as risk factors for inflammatory tissue transformation and damage, and carcinogenesis.33 Efforts to develop host-directed therapies against H pylori infection have focused on neutralisation of these pro-inflammatory cytokines with monoclonal antibodies (eg, anti-interleukin 1β, gevokizumab, anti-TNFγ, and adalimumab) during the course of antibiotic treatment to clear the infection.33 Vitamin D3 also has potential for use as a host-directed therapy for H pylori infection. In a preclinical study34 with a gastric epithelial cell line (GES-1) infected with H pylori, vitamin D3 supplement augmented killing of intracellular bacteria via induction of cathelicidin and β-defensin 4.
Bordetella pertussis remains an important cause of morbidity and mortality. Antibiotics do not substantially affect the course of whooping cough disease unless treatment is started early after symptom onset. Many patients develop long-term pulmonary damage. Immunotherapy with antipertussis toxin antibodies might confer protection against more severe forms of whooping cough. Halperin and colleagues36 investigated the use of multiple doses of intravenous antipertussis immunoglobulin in 25 infants with pertussis infection and noted an increase in serum antipertussis antibody titres, a decline in lymphocytosis, and a reduction in paroxysmal coughing compared with baseline; however, findings from more recent studies37 of antipertussis immunoglobulin were not promising. Manipulation of the sphingosine-1-phosphate signalling pathway, involved in several immunological processes including lymphocyte trafficking, might have therapeutic benefits in reversing the pathological outcome in pertussis disease (appendix).35
The number of infections with antibiotic-resistant Neisseria gonorrhoeae is increasing worldwide. The histone deacetylase inhibitor sulforaphane, which induces expression of antimicrobial peptides (eg, secretory leucocyte protease inhibitor and β-defensin 2), has been shown to augment the activity of antibiotics against multidrug-resistant N gonorrhoeae, thus showing potential as a host-directed therapy.39, 40 Supernatants from human endocervical carcinoma cells pre-treated with sulforaphane potentiated better bacterial killing in combination with sublethal doses of ciprofloxacin and cefixime40 compared with antibiotics alone.39 Furthermore, sulforaphane treatment in N gonorrhoeae-infected female mice resulted in better control of bacterial load and reduced inflam—mation.39 Treatment of cervical cells with sulforaphane in combination with antibiotic therapy might reduce the amount of antibiotic needed to eradicate N gonorrhoeae.40 See appendix for discussion of host-directed therapies for bacterial sepsis.
Viral infections
HIV targets and infects human CCR5-positive T cells143 and causes AIDS, impeding CD4 T-cell-mediated responses to a wide range of microbes.144 In 2014, co-infection with HIV accounted for 12% of the 1·5 million deaths from tuberculosis worldwide.89 Although HIV-reactive CD8 cytotoxic T lymphocytes and antibodies are present in individuals with HIV infection, the protective role of CD8 T cells and humoral immune responses are rather limited when CD4 T-cell numbers are low.144 Moreover, expression of programmed cell death protein 1 (PD-1) by circulating HIV-specific CD8 cytotoxic T lymphocytes isolated from patients with AIDS compromises their responsiveness to antigenic stimuli because of cellular exhaustion.44 Antiretroviral therapy (ART) promotes immune reconstitution (increase in CD4 T-cell numbers) in individuals undergoing treatment, in addition to reducing viral load145 and restoring a diverse T-cell receptor repertoire. This immune reconstitution, however, does not purge latent viral reservoirs in the host, nor sustain HIV-specific CD8 cytotoxic T-lymphocyte repertoires.145 Immune reconstitution inflammatory syndrome is an important clinical manifestation in patients with HIV–tuberculosis co-infection early after initiation of ART.146 Overt Th1-mediated immune responses result in pro-inflammatory cytokine storms (of which interleukin 6 is an important component147) and hyperactivation of immune cells, mediating extensive tissue damage. At present, repurposing of histone deacetylase inhibitors—eg, vorinostat, panobinostat, and valproic acid—has shown promise as host-directed therapy for improved clinical management of HIV/AIDS. These clinically approved drugs are able to reactivate latent virus reservoirs in the host and expose new virus progeny to ART as well as immune attack.148 The encouraging results of early-phase clinical trials of histone deacetylase inhibitor treatment of latent HIV infection could revolutionise ART.148 Since PD-1 expression on HIV-specific CD8 cytotoxic T lymphocytes is a barrier to effective antiviral immune responses in patients with AIDS,44 timely blockade of the PD-1/programmed death-ligand 1 (PD-L1) pathway could be a viable option to pursue.45 In-vitro blockade of PD-L1 has already been shown to improve anti-HIV CD8 cytotoxic T-lymphocyte responses, marked by increased proliferation and production of cytokines and cytotoxic molecules.149 Faster recovery of immune competence in lymphopenic hosts has been seen in HIV-positive patients after treatment with recombinant interleukin 7.150
Epstein-Barr virus (EBV) is a human herpesvirus, which is ubiquitous and remains largely latent in nature; at least 95% of the world's population is infected with the virus. EBV has tropism for B cells. It causes a wide range of clinical syndromes, from self-limited infectious mononucleosis to lymphoproliferative syndromes and B-cell lymphomas. Chimeric antigen receptors designed against the B-cell surface antigen CD19 are currently approved for eliminating latent viral reservoirs in the patient to decrease chances of developing B-cell lymphomas.48 This approach is also feasible for patients with EBV-associated B-cell lymphoma. Alternatively, transfer of EBV-specific CD8 cytotoxic T lymphocytes initially isolated from patients, and cultured and expanded ex vivo, has been clinically tested with much success.49, 50 Not only CD8, but also CD4 T-cell responses have been shown to mediate control of EBV-infected cells. Interleukin 21, produced by CD4 T cells, has been shown to be involved in the EBV nuclear antigen 2 (EBNA-2)-independent expression of latent membrane protein 1 (LMP-1) in EBV-carrying type 2 cells.151
Cytomegalovirus is another ubiquitous human herpesvirus that infects the lungs, eyes, CNS, and gastrointestinal tract, but can cause serious disease in adults and children. In particular, patients who have recently undergone haemopoietic stem-cell transplantation (HSCT) are at increased risk of developing clinical cytomegalovirus disease53 associated with the cytomegalovirus status of the donor and the recipient of of HSCT. Immune control is largely attributed to antigen-specific CD8 cytotoxic T lymphocytes, although cell activation is noticeably subdued after HSCT as a result of immunosuppressive therapy.53 For host-directed therapies, transfer of autologous or allogeneic antigen-specific CD8 cytotoxic T lymphocytes has been investigated, mainly in HSCT settings.49, 50 The timing of this strategy is crucial to avoid cytomegalovirus-associated immune reconstitution inflammatory syndrome.
Hepatitis C virus (HCV) causes at least 3% of liver diseases worldwide, and is the leading cause of liver cirrhosis and hepatocellular carcinoma.152 Acute and chronic stages of HCV infection of the liver promote T-cell exhaustion, which, as in HIV infection, is characterised by PD-1 expression on virus-specific CD4 and CD8 cytotoxic T lymphocytes.153 Another immune checkpoint, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), is also expressed on exhausted T cells and reduces the immune reactivity of anti-HCV effector T cells to virus-infected tissue in patients.154 The current first-line treatment for HCV infection is a combination regimen of direct-acting antiviral drugs, which mandatorily includes the nucleotide analogue sofosbuvir.155 Despite their high efficacy, the high financial cost of direct-acting antiviral drugs is a barrier to provision of HCV treatment.
The anti-PD-1 monoclonal antibody nivolumab has been shown to induce productive clinical responses in patients with HCV infection, marked by pronounced viral load reduction.60 No adverse side-effects were noted, although some important observations were reported. In one patient receiving nivolumab only, a transient, grade 4 rise in alanine transaminase concentration was concomitant with maximum HCV viral load reduction (4·55 log IU/mL) 22 days after treatment. Another patient, who also had diabetes mellitus and was receiving metformin treatment, had an increase in blood glucose concentrations, requiring insulin therapy. Anti-PD-1 therapy has been used for treatment of melanoma and other solid cancers,156 including HCV-induced hepatocellular carcinoma in combination with anti-CTLA-4 (NCT01658878). HCV-infected hepatocytes secrete newly formed viruses bound to apolipoprotein B (apoB),157 thus making increased apoB expression a risk factor for infection and progression to active hepatitis, and a potential target for host-directed therapy. MicroRNA-122 (miR-122) is highly expressed in the liver and has an important role in fatty-acid metabolism. Notably, increased levels of circulating miR-122 in serum samples from patients with HCV infection (genotypes 1 and 3) qualifies it as a disease biomarker and target for host-directed therapy.56 A phase 2 clinical trial of miravirsen (SPC3649; Santaris Pharma, Hørsholm Municipality, Denmark), an antisense oligonucleotide targeting miR-122, in patients with chronic HCV infection, has recently been completed (NCT01200420), and previous preclinical assessment showed promising antiviral effects.57
Influenza virus has caused several pandemics with millions of deaths worldwide. Many patients with influenza succumb to extensive lung pathology or secondary bacterial pneumonia, and resistance to current neuraminidase inhibitors presents a major hurdle to management of future pandemics. Immunosuppressive treatment with etoposide to dampen the cytokine storm-induced lung pathology was clinically beneficial in patients with severe influenza infection.108, 109 DAS181 (fludase; Ansun Biopharma, San Diego, CA, USA) is a sialidase fusion peptide that cleaves off the sialic acid residues on host epithelial cell-surface receptors. Following approval by the US Food and Drug Administration (FDA), DAS181 has been used in two HSCT recipients with parainfluenza infection; the first patient died whereas the second recovered.66 Clinical benefit and reduction of viral load has been reported from a phase 2 clinical trial of DAS181 in patients with pneumonia caused by either influenza B or the 2009 H1N1 pandemic strain.67 Several immunomodulatory drugs,62, 63 statins,62 angiotensin-II-receptor blockers, angiotensin-converting enzyme inhibitors, peroxisome proliferator-activated receptor γ (PPARγ) and PPARγ agonists (glitazones and fibrates, respectively), and adenosine monophosphate-activated kinase agonists (eg, metformin) have been suggested to modify the host response to severe influenza to improve survival,62 since these interventions have been shown to reduce mortality in mice infected with influenza virus. In an observational study of more than 3000 patients admitted to hospital with laboratory-confirmed influenza, statins reduced the number of deaths in hospital and within 30 days of discharge by 41%.64 An observational study showed that inpatient treatment with angiotensin-II-receptor blockers, angiotensin-converting enzyme inhibitors, and statins reduced 30-day pneumonia mortality by 53%, 42%, and 32%, respectively.158
In the 2014–15 epidemic of Ebola virus disease, this disease caused more than 28 639 cases and 11 316 deaths in three west African countries.159 Clinical trials of experimental antiviral agents, antibody preparations, and vaccines were completed. The clinical success of treating patients with Ebola virus infection with convalescent plasma from individuals who survived the 1976 and 1995 disease outbreaks in the Democratic Republic of the Congo prompted use of this strategy in the 2014 Ebola outbreak in west Africa.160 Although this intervention provided some survival benefit, acute kidney or lung injury were reported; however, these adverse effects could not be directly attributed to convalescent plasma transfusion.160
New treatment strategies targeting host factors in Ebola virus disease are in development. Recombinant nematode anticoagulant protein c2 (rNAPc2), an anticoagulant with FDA approval for treatment of thrombosis, has shown promising preclinical data in Ebola virus-infected monkeys,72 although no clinical trials are currently listed. Endothelial dysfunction causes the fluid and electrolyte imbalances seen in patients with Ebola virus infection; in-vitro studies have shown that statins and angiotensin-II-receptor blockers preserve or restore endothelial barrier integrity.62, 68 These drugs could be considered for treating the host response in these patients. In Sierra Leone, about 100 patients with Ebola virus infection were treated with this combination, and reports suggest substantial extension of survival.69
Dengue virus belongs to the genus of flaviviruses, which also includes yellow fever virus, West Nile virus, tick-borne encephalitis virus, and Zika virus; all are arthropod-transmitted infections.161 Four different serotypes of dengue virus exist, and infection with one serotype does not protect against the other. Dengue virus infection can lead to establishment of severe haemorrhagic disease, and in some cases leads to shock syndrome, which is fatal.162 As a host-directed therapy, lovastatin, a known modulator of cholesterol metabolism, was shown to inhibit replication of dengue virus in A549 human epithelial cells.73 Other repurposed drugs have also been clinically tested: ivermectin, dasatinib, and ciclosporin.73 Additionally, use of type 1 interferon (γ or β) and interferon γ has shown promising results in animal models (non-human primates).75 rNAPc2 could also be useful in managing vasculopathy during dengue haemorrhagic fever or shock syndrome, but this approach requires clinical investigation.
Middle East respiratory syndrome coronavirus (MERS-CoV) was first isolated in June, 2012, in Jeddah, Saudi Arabia, from a patient who died of severe respiratory infection and multiorgan failure.78 MERS-CoV is associated with high mortality in patents with co-morbidities and there are no effective anti-MERS-CoV antiviral agents or therapeutics. The lung pathology seen in patients with MERS probably represents the end result of a fine balance of host immune and MERS-CoV interactions. In-vitro laboratory investigation identified the membrane-bound form of dipeptidyl peptidase 4 to be the cardinal host-cell receptor for virus entry.76 Pneumonia is a common feature in patients with MERS and the high mortality caused by MERS-CoV is attributable to acute lung injury or development of acute respiratory distress syndrome (ARDS). ARDS is associated with leaky alveolar–capillary interfaces with pulmonary oedema, hypoxia, polymorphonuclear leucocytes or lymphocytic cellular infiltrates, and an aberrant immune response, with upregulation of pro-inflammatory cytokines, including interferon γ, which results in further tissue damage and deterioration of lung function. Analysis of serum and bronchoalveolar lavage fluid samples from patients who died from MERS-CoV infection showed non-productive inflammatory immune responses and induction of interleukin 6 and interleukin 17A.76 Patients with acute lung injury or ARDS died from the disease. Blockade of the pro-inflammatory cytokines interleukin 17A and interleukin 6 during severe disease might be useful as adjunct therapy and needs to be assessed in clinical trials. Additionally, reinfusion of bone marrow mesenchymal stem cells might also help ameliorate lung pathology in critically ill patients.78 Potential host-directed therapies to improve treatment outcomes of MERS are shown in table 1.
Parasitic diseases
Plasmodium falciparum malaria kills up to 1 million people worldwide every year. Individuals with P falciparum infection often develop severe clinical symptoms such as brain damage and multiple organ failure. Up to 25% of cases of severe clinical malaria are fatal even with access to the best health care, partly because the parasite triggers inflammation that damages vital organs. Case fatality rates for severe malaria remain high even in the best clinical settings because antimalarial drugs act against the parasite without alleviating life-threatening inflammation.163 Drug resistance now threatens efficacy of artemisinin-based therapies.164
Ferrochelatase, an enzyme important for haem biosynthesis in human erythrocytes, has been reported to be instrumental in parasite survival. Human erythrocytes deficient for ferrochelatase (from patients with erythropoietic protoporphyria) are more resistant to P falciparum growth, and pharmacological inhibition of host ferrochelatase in vitro abrogated parasite replication in healthy human erythrocytes.79 Desferrioxamine is a potent inhibitor of ferrochetalase,165 and could be considered for repurposing in human malaria.
Excess TNFγ production is involved in the pathogenesis of severe malaria.166 In a clinical study that included 20 Gambian children in malaria-associated coma, treatment with an anti-TNFγ antibody reduced parasite load in a dose-dependent manner and had noteworthy antipyrogenic effects.167 The use of anti-TNFγ drugs (eg, adalimumab, etanercept) in severe malaria need to be further investigated in clincial trials.
A small synthetic peptide known as innate defence regulator (IDR)-1018 seems to have broad therapeutic potential, including in-vivo activity in murine models by enhancement of wound healing and protection against Staphylococcus aureus, multidrug-resistant M tuberculosis, herpes simplex virus, and inflammatory disorders, including cerebral malaria.80 Recent studies of the Plasmodium berghei ANKA model of experimental cerebral malaria showed that IDR peptides prevented CNS inflammation and protected mice from experimental cerebral malaria, improving survival.81 IDR peptides enhance the beneficial aspects of the initial immune response, while dampening harmful tissue damage by downregulating the secretion of pro-inflammatory cytokines including TNFγ and interleukin 1β. Co-administration of IDR-1018 with standard first-line antimalarial drugs (pyrimethamine and chloroquine) increased survival in infected mice. Thus, IDR peptides could serve as adjunctive host-directed therapy for severe disease in combination with antimalarial treatment.80, 81
Leishmania spp cause a range of clinical disease including cutaneous, mucocutaneous, and visceral involvement.168 Like most intracellular pathogens, Leishmania spp parasites are difficult to kill because their localisation protects against immune responses and chemotherapy. Drug treatments have limited efficacy, have to be used for lengthy periods of time, and the systemic side-effects sometimes outweigh any clinical benefits. Thus, successful treatment of diseases caused by intracellular pathogens might need combination therapies and effective delivery systems. Imiquimod and resiquimod are currently used for treatment of leishmaniasis; both trigger Toll-like receptor (TLR)-7-mediated innate immune responses, inducing production of interleukin 6, type 1 interferons, and TNFγ,82 and thus act in a host-directed manner. Overproduction of TNFγ in Leishmania braziliensis infection contributes to mucosal tissue damage, consequently leading to development of mucocutaneous leishmaniasis.84 In this case, a combination of antileishmanial drugs and anti-TNFγ (adalimumab) during active L braziliensis infection might yield a clinical response. In preclinical studies, delivery of nanocapsulated doxorubicin (in a formulation that included chondroitin sulfate) to hamsters increased killing of leishmanial promastigotes via augmentation of Th1-mediated immune responses via induction of interferon γ, TNFγ, and interleukin 2 release in addition to direct antiparasitic activity.83 Sequential chemoimmunotherapy, with a single low dose of liposomal amphotericin B and a novel T-cell-epitope-enriched DNA vaccine candidate (LEISHDNAVAX; Mologen AG, Germany) was tested as host-directed therapy. The vaccine candidate boosted the efficacy of a single suboptimal dose of liposomal amphotericin B in C57BL/6 mice.169 Polyhexanide is a cationic polymer, which is able to directly kill Leishmania major and to enhance host-directed killing by improving the delivery of immunomodulatory nucleic acids. Polyhexanide spontaneously binds CpG ODN (short synthetic oligodeoxynucleotides comprising cytosine triphosphate [C] and guanine triphosphate [G] residues in sequential order), forming stable nanopolyplexes that enhanced uptake of CpG ODN, potentiated antimicrobial killing, and reduced host-cell toxicity of polyhexanide.170 These findings warrant further investigation.
Trypanosoma spp cause human trypanosomiasis, two important vector-borne diseases: human African trypanosomiasis (also known as sleeping sickness; caused by Trypanosoma brucei gambiense and T brucei rhodesiense) and Chagas disease (caused by Trypanosoma cruzi).171 Severe pathology in patients with human African trypanosomiasis can lead to fatal meningoencephalopathy and in many cases coma.172 In Chagas disease, patients are prone to infectious myocarditis or meningoencephalitis, or both, which are often life-threatening, and progressive damage of the autonomic nervous system occurs with organ enlargement and failure.171
In mouse models of T brucei infections, equilibrium between early onset of Th1 responses (interferon γ, TNFγ) and late Th2 responses (interleukin 4, interleukin 10) can control parasitaemia and associated pathology.85 Additionally, interferon-γ-driven nitric oxide, MHC-I antigen processing and presentation, and CD8 cytotoxic T-lymphocyte activation have a role in eliminating parasite reservoirs in macrophages.85 In human beings, cytokine analysis of cerebrospinal fluid specimens showed that patients with late-stage human African trypanosomiasis have raised levels of pro-inflammatory cytokines, including TNFγ, interleukin 6, interleukin 8, monocyte chemotactic protein 1 (MCP-1; also known as CCL2), and macrophage inflammatory protein (MIP)-1γ, among others.85 This destructive inflammation might be amenable to cellular therapy in late-stage human African trypanosomiasis, whereas immunostimulatory treatment with vitamin D3, interferon γ, and interleukin 2 could be useful at early stages. Interferon-γ-induced apolipoprotein 1 is a known host factor with antitrypanosomal activity. Thus, activating the immune system at an early stage with interferon γ could help control burden of parasitaemia via different effector mechanisms. Since the immune response profile in Chagas disease is similar to that in human African trypanosomiasis,171 host-directed therapies relevant to human African trypanosomiasis might also benefit patients with T cruzi infection, in addition to antiparasitic therapy.
Schistosomiasis affects more than 250 million people in 78 countries,173 and is caused by the trematode parasites of the genus Schistosoma. Major clinical manifestations arise from pathology due to granulomatous reaction around the ova in all major organs of the body, especially the urinary and gastrointestinal tracts.173 The antischistosomal immune response milieu mainly consists of Th1 cytokines (interferon γ, TNFγ, interleukin 12p40) and interleukin 17. Interleukin 6 and interleukin 1β also seem to have an important role early after infection with Schistosoma spp cercariae.87 In interleukin-10-deficient mice repeatedly infected with Schistosoma mansoni, CD4 T-cell activity was more pronounced, targeted, and efficient, upon exposure to schistosoma antigen preparation.174 This finding, if extrapolated to human beings, would suggest that multiple exposures to S mansoni might reduce T-cell responsiveness in an interleukin-10-dependent manner and hence, weaken productive cellular immune responses. Use of interleukin 2 and interferon γ might help to recover T-cell responses in patients in endemic countries. Notably, the use of Th2 cytokines such as interleukin 25 and interleukin 33 in a mixture with schistosomal glyceraldehyde-3-phosphate dehydrogenase and peroxiredoxin in a post-exposure vaccination attempt resulted in immense reduction of migrating cercariae in S mansoni-infected mice.88
Conclusions
Host-directed therapies targeting host immune and inflammatory pathways to enhance immune responses and alleviate immunopathology could benefit treatment outcomes in a range of bacterial, viral, and parasitic diseases. The variability in the potential of adjunct host-directed therapies to deliver clinically meaningful benefit for each pathogen demands definition. This definition will in part depend on how effective the standard antimicrobial therapy is, and whether tissue damage or other events represent therapeutic targets that otherwise are not addressed by conventional treatment. The focus on host-directed therapeutic strategies across various infectious diseases will require more investment for multidisciplinary research collaborations between academic and industrial partners to develop and take forward the assessment of host-directed therapies.
Search strategy and selection criteria
We searched PubMed, the Cochrane Library, Embase, and Google Scholar for articles published in English between Nov 15, 2000, and Jan 15, 2016, with the terms “infectious diseases”, “parasitic diseases” and combined with the terms “host”, “host-directed therapy”, “host-directed treatment”, “adjunct therapy”, “adjunct treatment”, “immunotherapy”, “cellular therapy”, “repurposed drugs”, “therapeutic advances”, “treatment”, “treatment regimens”, “trials”, “clinical trials”, and “animal models”. We also found publications from searches of websites of manufacturers of anti-infective drugs and immune-based therapies. We also reviewed studies (published between Nov 15, 2000, and Jan 15, 2016) cited by articles identified by this search strategy and selected those we identified as relevant. Selected review articles are cited to provide readers with more details and references than this Review can accommodate.
Acknowledgments
Acknowledgments
AZ receives support from the European Union FW7 RiD-RTI Project, European Developing Countries Clinical Trials Partnership (TB-NEAT), and the National Institute for Health Research Biomedical Research Centre at University College of London Hospital, London, UK. CV receives support from ISCIII-Subdirección General de Evaluación and Fondo-EU de Desarrollo Regional (FEDER) contract CP13/00174. MH is supported by BmBF grants, EDCTP–Pan African Consortium for the Evaluation of Anti-tuberculosis Antibiotics (PanACEA) 2007 32011 013. MM is supported by the EDCTP, Vetenskapsradet, Vinnova, and the Heart and Lung Foundation, Sweden.
Contributors
AZ, MM, and MR developed the first drafts of the manuscript, and the draft of the revisions. All authors contributed to the writing of subsequent and final drafts.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol. 2012;10:243–254. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- 3.Blum CA, Nigro N, Briel M. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385:1511–1518. doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez A, Mendia A, Sirvent JM, the CAPUCI Study Group Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35:1493–1498. doi: 10.1097/01.CCM.0000266755.75844.05. [DOI] [PubMed] [Google Scholar]
- 5.Hanly PJ, Roberts D, Dobson K, Light RB. Effect of indomethacin on arterial oxygenation in critically ill patients with severe bacterial pneumonia. Lancet. 1987;1:351–354. doi: 10.1016/s0140-6736(87)91727-2. [DOI] [PubMed] [Google Scholar]
- 6.Ferrer M, Torres A, Baer R, Hernández C, Roca J, Rodriguez-Roisin R. Effect of acetylsalicylic acid on pulmonary gas exchange in patients with severe pneumonia: a pilot study. Chest. 1997;111:1094–1100. doi: 10.1378/chest.111.4.1094. [DOI] [PubMed] [Google Scholar]
- 7.Bernard GR, Wheeler AP, Russell JA, the Ibuprofen in Sepsis Study Group The effects of ibuprofen on the physiology and survival of patients with sepsis. N Engl J Med. 1997;336:912–918. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- 8.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortensen EM, Pugh MJ, Copeland LA. Impact of statins and angiotensin-converting enzyme inhibitors on mortality of subjects hospitalised with pneumonia. Eur Respir J. 2008;31:611–617. doi: 10.1183/09031936.00162006. [DOI] [PubMed] [Google Scholar]
- 10.Schlienger RG, Fedson DS, Jick SS, Jick H, Meier CR. Statins and the risk of pneumonia: a population-based, nested case-control study. Pharmacotherapy. 2007;27:325–332. doi: 10.1592/phco.27.3.325. [DOI] [PubMed] [Google Scholar]
- 11.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The effect of prior statin use on 30-day mortality for patients hospitalized with community-acquired pneumonia. Respir Res. 2005;6:82. doi: 10.1186/1465-9921-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 2007;131:1006–1012. doi: 10.1378/chest.06-1997. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers JD, Singanayagam A, Murray MP, Hill AT. Prior statin use is associated with improved outcomes in community-acquired pneumonia. Am J Med. 2008;121:1002. doi: 10.1016/j.amjmed.2008.06.030. 07.e1. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sørensen HT. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29900 patients. Arch Intern Med. 2008;168:2081–2087. doi: 10.1001/archinte.168.19.2081. [DOI] [PubMed] [Google Scholar]
- 15.Liappis AP, Kan VL, Rochester CG, Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–1357. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen RW, Hundborg HH, Johnsen SP. Statin use and mortality within 180 days after bacteremia: a population-based cohort study. Crit Care Med. 2006;34:1080–1086. doi: 10.1097/01.CCM.0000207345.92928.E4. [DOI] [PubMed] [Google Scholar]
- 17.Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2009;18:269–275. doi: 10.1002/pds.1715. [DOI] [PubMed] [Google Scholar]
- 18.Douglas I, Evans S, Smeeth L. Effect of statin treatment on short term mortality after pneumonia episode: cohort study. BMJ. 2011;342:d1642. doi: 10.1136/bmj.d1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yende S, Milbrandt EB, Kellum JA. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med. 2011;39:1871–1878. doi: 10.1097/CCM.0b013e31821b8290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh GC, Weehuizen TA, Breitbach K. Glyburide reduces bacterial dissemination in a mouse model of melioidosis. PLoS Negl Trop Dis. 2013;7:e2500. doi: 10.1371/journal.pntd.0002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlström A, Heston SM, Boyd KL, Tuomanen EI, McCullers JA. Toll-like receptor 2 mediates fatal immunopathology in mice during treatment of secondary pneumococcal pneumonia following influenza. J Infect Dis. 2011;204:1358–1366. doi: 10.1093/infdis/jir522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001;161:1837–1842. doi: 10.1001/archinte.161.15.1837. [DOI] [PubMed] [Google Scholar]
- 23.Weiss K, Low DE, Cortes L. Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults. Can Respir J. 2004;11:589–593. doi: 10.1155/2004/461392. [DOI] [PubMed] [Google Scholar]
- 24.Restrepo MI, Mortensen EM, Waterer GW, Wunderink RG, Coalson JJ, Anzueto A. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Respir J. 2009;33:153–159. doi: 10.1183/09031936.00054108. [DOI] [PubMed] [Google Scholar]
- 25.Mufson MA, Stanek RJ. Bacteremic pneumococcal pneumonia in one American City: a 20-year longitudinal study, 1978–1997. Am J Med. 1999;107:34S–43S. doi: 10.1016/s0002-9343(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 26.Martinez JA, Horcajada JP, Almela M. Addition of a macrolide to a β-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2003;36:389–395. doi: 10.1086/367541. [DOI] [PubMed] [Google Scholar]
- 27.Baddour LM, Yu VL, Klugman KP, the International Pneumococcal Study Group Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am J Respir Crit Care Med. 2004;170:440–444. doi: 10.1164/rccm.200311-1578OC. [DOI] [PubMed] [Google Scholar]
- 28.Aspa J, Rajas O, Rodriguez de Castro F, the Pneumococcal Pneumonia in Spain Study Group Impact of initial antibiotic choice on mortality from pneumococcal pneumonia. Eur Respir J. 2006;27:1010–1019. doi: 10.1183/09031936.06.00126004. [DOI] [PubMed] [Google Scholar]
- 29.Harbarth S, Garbino J, Pugin J, Romand JA, Pittet D. Lack of effect of combination antibiotic therapy on mortality in patients with pneumococcal sepsis. Eur J Clin Microbiol Infect Dis. 2005;24:688–690. doi: 10.1007/s10096-005-0018-6. [DOI] [PubMed] [Google Scholar]
- 30.Dwyer R, Ortqvist A, Aufwerber E. Addition of a macrolide to an β-lactam in bacteremic pneumococcal pneumonia. Eur J Clin Microbiol Infect Dis. 2006;25:518–521. doi: 10.1007/s10096-006-0183-2. [DOI] [PubMed] [Google Scholar]
- 31.Brown RB, Iannini P, Gross P, Kunkel M. Impact of initial antibiotic choice on clinical outcomes in community-acquired pneumonia: analysis of a hospital claims-made database. Chest. 2003;123:1503–1511. doi: 10.1378/chest.123.5.1503. [DOI] [PubMed] [Google Scholar]
- 32.Metersky ML, Ma A, Houck PM, Bratzler DW. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest. 2007;131:466–473. doi: 10.1378/chest.06-1426. [DOI] [PubMed] [Google Scholar]
- 33.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo L, Chen W, Zhu H. Helicobacter pylori induces increased expression of the vitamin D receptor in immune responses. Helicobacter. 2014;19:37–47. doi: 10.1111/hel.12102. [DOI] [PubMed] [Google Scholar]
- 35.Skerry C, Scanlon K, Rosen H, Carbonetti NH. Sphingosine-1-phosphate receptor agonism reduces Bordetella pertussis-mediated-lung pathology. J Infect Dis. 2015;211:1883–1886. doi: 10.1093/infdis/jiu823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halperin SA, Vaudry W, Boucher FD, Mackintosh K, Waggener TB, Smith B, the Pediatric Investigators Collaborative Network on Infections in Canada Is pertussis immune globulin efficacious for the treatment of hospitalized infants with pertussis? No answer yet. Pediatr Infect Dis J. 2007;26:79–81. doi: 10.1097/01.inf.0000247103.01075.cc. [DOI] [PubMed] [Google Scholar]
- 37.Scanlon KM, Skerry C, Carbonetti NH. Novel therapies for the treatment of pertussis disease. Pathog Dis. 2015;73:ftv074. doi: 10.1093/femspd/ftv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruss JB, Siber GR. Protective effects of pertussis immunoglobulin (P-IGIV) in the aerosol challenge model. Clin Diagn Lab Immunol. 1999;6:464–470. doi: 10.1128/cdli.6.4.464-470.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yedery R, Jerse A. Augmentation of cationic antimicrobial peptide production with histone deacetylase inhibitors as a novel epigenetic therapy for bacterial infections. Antibiotics. 2015;4:44–61. doi: 10.3390/antibiotics4010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leduc I, Jerse A. Host-directed therapeutics as adjunctive therapy for antibiotic-resistant Neisseria gonorrhoeae. Sex Transm Infect. 2015;91(suppl 2) asbtr 38. [Google Scholar]
- 41.Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, Margolis DM. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen TA, Tolstrup M, Moller HJ. Activation of latent human immunodeficiency virus by the histone deacetylase inhibitor panobinostat: a pilot study to assess effects on the central nervous system. Open Forum Infect Dis. 2015;2:ofv037. doi: 10.1093/ofid/ofv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matalon S, Rasmussen TA, Dinarello CA. Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir. Mol Med. 2011;17:466–472. doi: 10.2119/molmed.2011.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day CL, Kaufmann DE, Kiepiela P. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 45.Velu V, Shetty RD, Larsson M, Shankar EM. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology. 2015;12:14. doi: 10.1186/s12977-015-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrovas C, Casazza JP, Brenchley JM. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Fu J, Xu X. Safety and immunological responses to human mesenchymal stem cell therapy in difficult-to-treat HIV-1-infected patients. AIDS. 2013;27:1283–1293. doi: 10.1097/QAD.0b013e32835fab77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cruz CR, Micklethwaite KP, Savoldo B. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerdemann U, Katari UL, Papadopoulou A. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21:2113–2121. doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uhlin M, Gertow J, Uzunel M. Rapid salvage treatment with virus-specific T cells for therapy-resistant disease. Clin Infect Dis. 2012;55:1064–1073. doi: 10.1093/cid/cis625. [DOI] [PubMed] [Google Scholar]
- 51.Parida SK, Poiret T, Zhenjiang L. T-cell therapy: options for infectious diseases. Clin Infect Dis. 2015;61(suppl 3):S217–S224. doi: 10.1093/cid/civ615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander BT, Hladnik LM, Augustin KM. Use of cytomegalovirus intravenous immune globulin for the adjunctive treatment of cytomegalovirus in hematopoietic stem cell transplant recipients. Pharmacotherapy. 2010;30:554–561. doi: 10.1592/phco.30.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ariza-Heredia EJ, Nesher L, Chemaly RF. Cytomegalovirus diseases after hematopoietic stem cell transplantation: a mini-review. Cancer Lett. 2014;342:1–8. doi: 10.1016/j.canlet.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Brestrich G, Zwinger S, Roemhild A. Generation of HCMV-specific T-cell lines from seropositive solid-organ-transplant recipients for adoptive T-cell therapy. J Immunother. 2009;32:932–940. doi: 10.1097/CJI.0b013e3181b88fda. [DOI] [PubMed] [Google Scholar]
- 55.Ljungman P. Treatment of adenovirus infections in the immunocompromised host. Eur J Clin Microbiol Infect Dis. 2004;23:583–588. doi: 10.1007/s10096-004-1165-x. [DOI] [PubMed] [Google Scholar]
- 56.Oliveira KG, Malta FM, Nastri AC. Increased hepatic expression of miRNA-122 in patients infected with HCV genotype 3. Med Microbiol Immunol. 2015 doi: 10.1007/S00430-015-0431-0. published online Aug 14. [DOI] [PubMed] [Google Scholar]
- 57.Ottosen S, Parsley TB, Yang L. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob Agents Chemother. 2015;59:599–608. doi: 10.1128/AAC.04220-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hovingh K, Besseling J, Kastelein J. Efficacy and safety of mipomersen sodium (Kynamro) Expert Opin Drug Saf. 2013;12:569–579. doi: 10.1517/14740338.2013.793670. [DOI] [PubMed] [Google Scholar]
- 59.Reiner Ž. Management of patients with familial hypercholesterolaemia. Nat Rev Cardiol. 2015;12:565–575. doi: 10.1038/nrcardio.2015.92. [DOI] [PubMed] [Google Scholar]
- 60.Gardiner D, Lalezari J, Lawitz E. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foster GR. Pegylated interferons for the treatment of chronic hepatitis C: pharmacological and clinical differences between peginterferon-alpha-2a and peginterferon-alpha-2b. Drugs. 2010;70:147–165. doi: 10.2165/11531990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 62.Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417–435. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Fedson DS. Confronting the next influenza pandemic with anti-inflammatory and immunomodulatory agents: why they are needed and how they might work. Influenza Other Respi Viruses. 2009;3:129–142. doi: 10.1111/j.1750-2659.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vandermeer ML, Thomas AR, Kamimoto L. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205:13–19. doi: 10.1093/infdis/jir695. [DOI] [PubMed] [Google Scholar]
- 65.Fedson DS. How will physicians respond to the next influenza pandemic? Clin Infect Dis. 2014;58:233–237. doi: 10.1093/cid/cit695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chalkias S, Mackenzie MR, Gay C. DAS181 treatment of hematopoietic stem cell transplant patients with parainfluenza virus lung disease requiring mechanical ventilation. Transpl Infect Dis. 2014;16:141–144. doi: 10.1111/tid.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moss RB, Hansen C, Sanders RL, Hawley S, Li T, Steigbigel RT. A phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. J Infect Dis. 2012;206:1844–1851. doi: 10.1093/infdis/jis622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fedson DS, Jacobson JR, Rordam OM, Opal SM. Treating the host response to Ebola virus disease with generic statins and angiotensin receptor blockers. MBio. 2015;6:e00716. doi: 10.1128/mBio.00716-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fedson DS, Rordam OM. Treating Ebola patients: a ‘bottom up’ approach using generic statins and angiotensin receptor blockers. Int J Infect Dis. 2015;36:80–84. doi: 10.1016/j.ijid.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 70.WHO Categorization and prioritization of drugs for consideration for testing or use in patients infected with Ebola. Feb 18, 2015. http://www.who.int/medicines/ebola-treatment/2015-0218_tables_of_ebola_drugs_updated.pdf (accessed Feb 10, 2016).
- 71.Qiu X, Audet J, Wong G. Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb. Sci Rep. 2013;3:3365. doi: 10.1038/srep03365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geisbert TW, Hensley LE, Jahrling PB. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362:1953–1958. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 73.Rothwell C, Lebreton A, Young Ng C. Cholesterol biosynthesis modulation regulates dengue viral replication. Virology. 2009;389:8–19. doi: 10.1016/j.virol.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 74.de Wispelaere M, LaCroix AJ, Yang PL. The small molecules AZD0530 and dasatinib inhibit dengue virus RNA replication via Fyn kinase. J Virol. 2013;87:7367–7381. doi: 10.1128/JVI.00632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krishnan MN, Garcia-Blanco MA. Targeting host factors to treat West Nile and dengue viral infections. Viruses. 2014;6:683–708. doi: 10.3390/v6020683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nauck MA, Vilsbøll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies: viewpoints on the way to consensus. Diabetes Care. 2009;32(suppl 2):S223–S231. doi: 10.2337/dc09-S315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses—drug discovery and therapeutic options. Nat Rev Drug Discov. 2016 doi: 10.1038/nrd.2015.37. published online Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith CM, Jerkovic A, Puy H. Red cells from ferrochelatase-deficient erythropoietic protoporphyria patients are resistant to growth of malarial parasites. Blood. 2015;125:534–541. doi: 10.1182/blood-2014-04-567149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mansour SC, de la Fuente-Nunez C, Hancock RE. Peptide IDR-1018: modulating the immune system and targeting bacterial biofilms to treat antibiotic-resistant bacterial infections. J Pept Sci. 2015;21:323–329. doi: 10.1002/psc.2708. [DOI] [PubMed] [Google Scholar]
- 81.Achtman AH, Pilat S, Law CW. Effective adjunctive therapy by an innate defense regulatory peptide in a preclinical model of severe malaria. Sci Transl Med. 2012;4:135ra64. doi: 10.1126/scitranslmed.3003515. [DOI] [PubMed] [Google Scholar]
- 82.Collier MA, Gallovic MD, Peine KJ. Delivery of host cell-directed therapeutics for intracellular pathogen clearance. Expert Rev Anti Infect Ther. 2013;11:1225–1235. doi: 10.1586/14787210.2013.845524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaurasia M, Pawar VK, Jaiswal AK, Dube A, Paliwal SK, Chourasia MK. Chondroitin nanocapsules enhanced doxorubicin induced apoptosis against leishmaniasis via Th1 immune response. Int J Biol Macromol. 2015;79:27–36. doi: 10.1016/j.ijbiomac.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 84.Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2010;8:419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 85.Bucheton B, MacLeod A, Jamonneau V. Human host determinants influencing the outcome of Trypanosoma brucei gambiense infections. Parasite Immunol. 2011;33:438–447. doi: 10.1111/j.1365-3024.2011.01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 87.El Ridi RA, Tallima HA. Novel therapeutic and prevention approaches for schistosomiasis: review. J Adv Res. 2013;4:467–478. doi: 10.1016/j.jare.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El Ridi R, Tallima H. Vaccine-induced protection against murine schistosomiasis mansoni with larval excretory-secretory antigens and papain or type-2 cytokines. J Parasitol. 2013;99:194–202. doi: 10.1645/GE-3186.1. [DOI] [PubMed] [Google Scholar]
- 89.WHO . Global tuberculosis report 2015. World Health Organization; Geneva: 2015. http://www.who.int/tb/publications/global_report/en/ (accessed Feb 10, 2016). [Google Scholar]
- 90.O'Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. The immune response in tuberculosis. Annu Rev Immunol. 2013;31:475–527. doi: 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 91.Zumla A, Rao M, Parida SK. Inflammation and tuberculosis: host-directed therapies. J Intern Med. 2015;277:373–387. doi: 10.1111/joim.12256. [DOI] [PubMed] [Google Scholar]
- 92.Singhal A, Jie L, Kumar P. Metformin as adjunct antituberculosis therapy. Sci Transl Med. 2014;6:263ra159. doi: 10.1126/scitranslmed.3009885. [DOI] [PubMed] [Google Scholar]
- 93.Pearce EL, Walsh MC, Cejas PJ. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beeson CC, Beeson GC, Schnellmann RG. A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem. 2010;404:75–81. doi: 10.1016/j.ab.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pirinen E, Cantó C, Jo YS. Pharmacological Inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van der Windt GJ, Everts B, Chang CH. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Montoya D, Inkeles MS, Liu PT. IL-32 is a molecular marker of a host defense network in human tuberculosis. Sci Transl Med. 2014;6:250ra114. doi: 10.1126/scitranslmed.3009546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tobin DM, Roca FJ, Ray JP, Ko DC, Ramakrishnan L. An enzyme that inactivates the inflammatory mediator leukotriene b4 restricts mycobacterial infection. PLoS One. 2013;8:e67828. doi: 10.1371/journal.pone.0067828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mayer-Barber KD, Andrade BB, Oland SD. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vilaplana C, Marzo E, Tapia G, Diaz J, Garcia V, Cardona PJ. Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J Infect Dis. 2013;208:199–202. doi: 10.1093/infdis/jit152. [DOI] [PubMed] [Google Scholar]
- 101.Ivanyi J, Zumla A. Nonsteroidal antiinflammatory drugs for adjunctive tuberculosis treatment. J Infect Dis. 2013;208:185–188. doi: 10.1093/infdis/jit153. [DOI] [PubMed] [Google Scholar]
- 102.Critchley JA, Young F, Orton L, Garner P. Corticosteroids for prevention of mortality in people with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:223–237. doi: 10.1016/S1473-3099(12)70321-3. [DOI] [PubMed] [Google Scholar]
- 103.Shan L, Deng K, Shroff NS. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coussens AK, Wilkinson RJ, Martineau AR. Phenylbutyrate is bacteriostatic against Mycobacterium tuberculosis and regulates the macrophage response to infection, synergistically with 25-hydroxy-vitamin D3. PLoS Pathog. 2015;11:e1005007. doi: 10.1371/journal.ppat.1005007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kulkarni NN, Yi Z, Huehnken C, Agerberth B, Gudmundsson GH. Phenylbutyrate induces cathelicidin expression via the vitamin D receptor: linkage to inflammatory and growth factor cytokines pathways. Mol Immunol. 2015;63:530–539. doi: 10.1016/j.molimm.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 106.Hall AG, Tilby MJ. Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev. 1992;6:163–173. doi: 10.1016/0268-960x(92)90028-o. [DOI] [PubMed] [Google Scholar]
- 107.Walter S, Weinschenk T, Stenzl A. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 108.Henter JI, Chow CB, Leung CW, Lau YL. Cytotoxic therapy for severe avian influenza A (H5N1) infection. Lancet. 2006;367:870–873. doi: 10.1016/S0140-6736(06)68232-9. [DOI] [PubMed] [Google Scholar]
- 109.Henter JI, Palmkvist-Kaijser K, Holzgraefe B, Bryceson YT, Palmér K. Cytotoxic therapy for severe swine flu A/H1N1. Lancet. 2010;376:2116. doi: 10.1016/S0140-6736(10)61345-1. [DOI] [PubMed] [Google Scholar]
- 110.Gupta S, Tyagi S, Bishai WR. Verapamil increases the bactericidal activity of bedaquiline against Mycobacterium tuberculosis in a mouse model. Antimicrob Agents Chemother. 2015;59:673–676. doi: 10.1128/AAC.04019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schiebler M, Brown K, Hegyi K. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol Med. 2015;7:127–139. doi: 10.15252/emmm.201404137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parihar SP, Guler R, Khutlang R. Statin therapy reduces the Mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis. 2014;209:754–763. doi: 10.1093/infdis/jit550. [DOI] [PubMed] [Google Scholar]
- 113.Catron DM, Lange Y, Borensztajn J, Sylvester MD, Jones BD, Haldar K. Salmonella enterica serovar Typhimurium requires nonsterol precursors of the cholesterol biosynthetic pathway for intracellular proliferation. Infect Immun. 2004;72:1036–1042. doi: 10.1128/IAI.72.2.1036-1042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Napier RJ, Norris BA, Swimm A. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog. 2015;11:e1004770. doi: 10.1371/journal.ppat.1004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Daley P, Jagannathan V, John KR. Adjunctive vitamin D for treatment of active tuberculosis in India: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15:528–534. doi: 10.1016/S1473-3099(15)70053-8. [DOI] [PubMed] [Google Scholar]
- 116.Rahman S, Rehn A, Rahman J, Andersson J, Svensson M, Brighenti S. Pulmonary tuberculosis patients with a vitamin D deficiency demonstrate low local expression of the antimicrobial peptide LL-37 but enhanced FoxP3+ regulatory T cells and IgG-secreting cells. Clin Immunol. 2015;156:85–97. doi: 10.1016/j.clim.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 117.Kaufmann SH, Lange C, Rao M. Progress in tuberculosis vaccine development and host-directed therapies—a state of the art review. Lancet Respir Med. 2014;2:301–320. doi: 10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- 118.Leung J, Suh WK. The CD28-B7 family in anti-tumor immunity: emerging concepts in cancer immunotherapy. Immune Netw. 2014;14:265–276. doi: 10.4110/in.2014.14.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh A, Mohan A, Dey AB, Mitra DK. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon γ-producing T cells from apoptosis in patients with pulmonary tuberculosis. J Infect Dis. 2013;208:603–615. doi: 10.1093/infdis/jit206. [DOI] [PubMed] [Google Scholar]
- 121.Jurado JO, Alvarez IB, Pasquinelli V. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol. 2008;181:116–125. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 122.Hassan SS, Akram M, King EC, Dockrell HM, Cliff JM. PD-1, PD-L1 and PD-L2 gene expression on T-cells and natural killer cells declines in conjunction with a reduction in PD-1 protein during the intensive phase of tuberculosis treatment. PLoS One. 2015;10:e0137646. doi: 10.1371/journal.pone.0137646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ngiow SF, Teng MW, Smyth MJ. Prospects for TIM3-targeted antitumor immunotherapy. Cancer Res. 2011;71:6567–6571. doi: 10.1158/0008-5472.CAN-11-1487. [DOI] [PubMed] [Google Scholar]
- 124.Sada-Ovalle I, Chávez-Galán L, Torre-Bouscoulet L. The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. J Immunol. 2012;189:5896–5902. doi: 10.4049/jimmunol.1200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Phillips BL, Mehra S, Ahsan MH, Selman M, Khader SA, Kaushal D. LAG3 expression in active Mycobacterium tuberculosis infections. Am J Pathol. 2015;185:820–833. doi: 10.1016/j.ajpath.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3—potential mechanisms of action. Nat Rev Immunol. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 127.Wallis RS, van Vuuren C, Potgieter S. Adalimumab treatment of life-threatening tuberculosis. Clin Infect Dis. 2009;48:1429–1432. doi: 10.1086/598504. [DOI] [PubMed] [Google Scholar]
- 128.Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21:1248–1257. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- 129.Ravimohan S, Tamuhla N, Steenhoff AP. Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect Dis. 2015;15:429–438. doi: 10.1016/S1473-3099(15)70008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Datta M, Via LE, Kamoun WS. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci USA. 2015;112:1827–1832. doi: 10.1073/pnas.1424563112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oehlers SH, Cronan MR, Scott NR. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2015;517:612–615. doi: 10.1038/nature13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Skrahin A, Ahmed RK, Ferrara G. Autologous mesenchymal stromal cell infusion as adjunct treatment in patients with multidrug and extensively drug-resistant tuberculosis: an open-label phase 1 safety trial. Lancet Respir Med. 2014;2:108–122. doi: 10.1016/S2213-2600(13)70234-0. [DOI] [PubMed] [Google Scholar]
- 133.Zumla A, Maeurer M, Chakaya J, the Host-Directed Therapies Network Towards host-directed therapies for tuberculosis. Nat Rev Drug Discov. 2015;14:511–512. doi: 10.1038/nrd4696. [DOI] [PubMed] [Google Scholar]
- 134.O'Brien KL, Wolfson LJ, Watt JP, the Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 135.Hortal M, Camou T, Palacio R, Dibarboure H, Garcia A. Ten-year review of invasive pneumococcal diseases in children and adults from Uruguay: clinical spectrum, serotypes, and antimicrobial resistance. Int J Infect Dis. 2000;4:91–95. doi: 10.1016/s1201-9712(00)90100-0. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L, Li Z, Wan Z, Kilby A, Kilby JM, Jiang W. Humoral immune responses to Streptococcus pneumoniae in the setting of HIV-1 infection. Vaccine. 2015;33:4430–4436. doi: 10.1016/j.vaccine.2015.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morton B, Pennington SH, Gordon SB. Immunomodulatory adjuvant therapy in severe community-acquired pneumonia. Expert Rev Respir Med. 2014;8:587–596. doi: 10.1586/17476348.2014.927736. [DOI] [PubMed] [Google Scholar]
- 138.Brouwer MC, McIntyre P, Prasad K, van de Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2013;6 doi: 10.1002/14651858.CD004405.pub4. CD004405. [DOI] [PubMed] [Google Scholar]
- 139.Voiriot G, Dury S, Parrot A, Mayaud C, Fartoukh M. Nonsteroidal antiinflammatory drugs may affect the presentation and course of community-acquired pneumonia. Chest. 2011;139:387–394. doi: 10.1378/chest.09-3102. [DOI] [PubMed] [Google Scholar]
- 140.Koh GC, Maude RR, Schreiber MF. Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis. 2011;52:717–725. doi: 10.1093/cid/ciq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 142.Linz B, Balloux F, Moodley Y. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bruner KM, Hosmane NN, Siliciano RF. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol. 2015;23:192–203. doi: 10.1016/j.tim.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kaufmann SH, McMichael AJ. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat Med. 2005;11(suppl):S33–S44. doi: 10.1038/nm1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Imami N, Gotch F. Prospects for immune reconstitution in HIV-1 infection. Clin Exp Immunol. 2002;127:402–411. doi: 10.1046/j.1365-2249.2002.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lai RP, Meintjes G, Wilkinson RJ. HIV-1 tuberculosis-associated immune reconstitution inflammatory syndrome. Semin Immunopathol. 2015 doi: 10.1007/S00281-015-0532-2. published online Sept 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goovaerts O, Jennes W, Massinga-Loembé M, the TB-IRIS Study Group LPS-binding protein and IL-6 mark paradoxical tuberculosis immune reconstitution inflammatory syndrome in HIV patients. PLoS One. 2013;8:e81856. doi: 10.1371/journal.pone.0081856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rasmussen TA, Tolstrup M, Søgaard OS. Reversal of latency as part of a cure for HIV-1. Trends Microbiol. 2016;24:90–97. doi: 10.1016/j.tim.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 149.Trautmann L, Janbazian L, Chomont N. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 150.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Konforte D, Simard N, Paige CJ. Interleukin-21 regulates expression of key Epstein-Barr virus oncoproteins, EBNA2 and LMP1, in infected human B cells. Virology. 2008;374:100–113. doi: 10.1016/j.virol.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 152.Ghasemi F, Rostami S, Meshkat Z. Progress in the development of vaccines for hepatitis C virus infection. World J Gastroenterol. 2015;21:11984–12002. doi: 10.3748/wjg.v21.i42.11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154–3160. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakamoto N, Cho H, Shaked A. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog. 2009;5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Panel AIHG, the AASLD/IDSA HCV Guidance Panel Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 156.Swaika A, Hammond WA, Joseph RW. Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy. Mol Immunol. 2015;67(2 pt A):4–17. doi: 10.1016/j.molimm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 157.Nahmias Y, Goldwasser J, Casali M. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. 2008;47:1437–1445. doi: 10.1002/hep.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mortensen EM, Nakashima B, Cornell J. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis. 2012;55:1466–1473. doi: 10.1093/cid/cis733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.WHO Ebola situation report. Aug 12, 2015. http://apps.who.int/ebola/current-situation/ebola-situation-report-17-february-2016 (accessed Feb 26, 2016).
- 160.Winkler AM, Koepsell SA. The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease. Curr Opin Hematol. 2015;22:521–526. doi: 10.1097/MOH.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 161.Blitvich BJ, Firth AE. Insect-specific flaviviruses: a systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses. 2015;7:1927–1959. doi: 10.3390/v7041927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carabali M, Hernandez LM, Arauz MJ, Villar LA, Ridde V. Why are people with dengue dying? A scoping review of determinants for dengue mortality. BMC Infect Dis. 2015;15:301. doi: 10.1186/s12879-015-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.WHO World malaria report 2014. World Health Organization; Geneva: 2014. http://www.who.int/malaria/publications/world_malaria_report_2014/en/ (accessed Feb 10, 2016). [Google Scholar]
- 164.Cui L, Mharakurwa S, Ndiaye D, Rathod PK, Rosenthal PJ. Antimalarial drug resistance: literature review and activities and findings of the ICEMR Network. Am J Trop Med Hyg. 2015;93(suppl):57–68. doi: 10.4269/ajtmh.15-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jacobs A. Iron chelation therapy for iron loaded patients. Br J Haematol. 1979;43:1–5. doi: 10.1111/j.1365-2141.1979.tb03713.x. [DOI] [PubMed] [Google Scholar]
- 166.Kinra P, Dutta V. Serum TNF alpha levels: a prognostic marker for assessment of severity of malaria. Trop Biomed. 2013;30:645–653. [PubMed] [Google Scholar]
- 167.Kwiatkowski D, Molyneux ME, Stephens S. Anti-TNF therapy inhibits fever in cerebral malaria. Q J Med. 1993;86:91–98. [PubMed] [Google Scholar]
- 168.Gurung P, Kanneganti TD. Innate immunity against Leishmania infections. Cell Microbiol. 2015;17:1286–1294. doi: 10.1111/cmi.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Seifert K, Juhls C, Salguero FJ, Croft SL. Sequential chemoimmunotherapy of experimental visceral leishmaniasis using a single low dose of liposomal amphotericin B and a novel DNA vaccine candidate. Antimicrob Agents Chemother. 2015;59:5819–5823. doi: 10.1128/AAC.00273-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Firdessa R, Good L, Amstalden MC. Pathogen- and host-directed antileishmanial effects mediated by polyhexanide (PHMB) PLoS Negl Trop Dis. 2015;9:e0004041. doi: 10.1371/journal.pntd.0004041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bern C. Chagas' disease. N Engl J Med. 2015;373:456–466. doi: 10.1056/NEJMra1410150. [DOI] [PubMed] [Google Scholar]
- 172.Franco JR, Simarro PP, Diarra A, Jannin JG. Epidemiology of human African trypanosomiasis. Clin Epidemiol. 2014;6:257–275. doi: 10.2147/CLEP.S39728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weerakoon KG, Gobert GN, Cai P, McManus DP. Advances in the diagnosis of human schistosomiasis. Clin Microbiol Rev. 2015;28:939–967. doi: 10.1128/CMR.00137-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Prendergast CT, Sanin DE, Cook PC, Mountford AP. CD4+ T cell hyporesponsiveness after repeated exposure to Schistosoma mansoni larvae is dependent upon interleukin-10. Infect Immun. 2015;83:1418–1430. doi: 10.1128/IAI.02831-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.