Summary
We present a case of subacute sclerosing panencephalitis that developed in a previously healthy 29-year-old pregnant woman who had returned from a trip to rural India shortly before the onset of symptoms. She was admitted to hospital at 27 weeks' gestation with a history of cognitive decline and difficulty completing simple tasks. She had no clinical signs of infection. The working diagnosis was autoimmune encephalitis, although extensive investigations did not lead to a final classifying diagnosis. The patient became comatose and developed hypertension, and an emergency caesarean section was done at 31 weeks to deliver the child, who seemed healthy. The patient died about 6 weeks after the onset of symptoms. The patient was found to have had subacute sclerosing panencephalitis at autopsy. In this Grand Round, we review the clinical features and treatment of subacute sclerosing panencephalitis, and the epidemiological and public health aspects of the case.
Introduction
Subacute sclerosing panencephalitis is the most devastating consequence of an often remote measles infection. Subacute sclerosing panencephalitis occurs almost exclusively in children and usually years after the primary measles infection. The long latency of its development, its aspecific presentation, and the fact that laboratory and imaging findings can be deceptively normal all make for a difficult diagnosis, and the disease is often misdiagnosed or diagnosed late, only after a long list of differential diagnoses has been excluded. Once diagnosed, subacute sclerosing panencephalitis is not easily treated, but progresses relentlessly until death. While subacute sclerosing panencephalitis cannot be cured, it can be prevented through measles vaccination, and it has become a very rare disease, most likely through the worldwide use of effective vaccines. Here, we report the case of an adult patient who was admitted to our hospital with a rapidly progressive encephalopathy and behavioural changes.
Case presentation
The patient was born at a military hospital in rural southern India in 1985 and migrated to Canada in 2009. Her past medical history was unremarkable, and she received routine childhood vaccinations, including measles vaccination at age 1 year. As an adult she had mild environmental asthma and suffered miscarriages at 18 gestational weeks in 2012 and at 12 gestational weeks in 2013. Her prenatal serology was typical: positive measles IgG (30·5 IU/mL); positive rubella IgG (87·2 IU/mL); positive varicella IgG; negative hepatitis B surface antigen; negative syphilis antibody screen; cervical swabs negative for Chlamydia trachomatis, Neisseria gonorrhoeae, bacterial vaginosis, and trichomonas; and negative HIV serology.
She became pregnant for the third time and at 21 weeks' gestation she and her husband travelled to the state of Tamil Nadu in India, where they stayed in a small village and visited the port city of Tuticorin. During her stay she was exposed to chickens and wild pigeons on her parents' property and had several mosquito bites. She drank only bottled water and avoided raw fruits and vegetables as much as possible. She did not take malaria prophylaxis. Her 4 week stay was noteworthy only for a mild upper respiratory illness with coryza, cough, rhinorrhoea, and malaise for several days, which had resolved by the time she returned to Canada.
7 days after her return to Canada she developed mild confusion and intermittently unbalanced gait. Over the next few days she developed difficulty with common tasks such as starting and driving her car. 5 days after the onset of her initial symptoms, she developed bizarre behaviour, disorientation, disturbed sleep, and incomprehensible speech. Her husband brought her to the hospital for further evaluation, where she was seen 7 days after symptom onset.
In the emergency room, she was found to be afebrile and haemodynamically stable with no abnormalities on routine blood tests, including a normal complete blood count and differential. The neurology service was consulted. In the neurological exam, the patient was unable to recall and name routine objects and had difficulty following commands. The motor exam showed normal strength with mildly increased tone, which was more noticeable in the right compared with the left extremities, and brisk deep tendon reflexes throughout. In the cerebellar exam, the patient had difficulties with rapid alternating movements in both arms and a wide-based gait with a tendency to veer to the left. The rest of the neurological and physical examination was normal, which included ultrasound and biophysical profile assessment of her pregnancy. She was admitted to the neurology service for further investigation.
Results from an extensive diagnostic investigation during her hospital stay showed few abnormalities. Cerebrospinal fluid was tested on three occasions and showed no abnormalities in cell counts, protein content, and glucose content (table 1 ). The cerebrospinal fluid was negative for oligoclonal bands. Several infectious and autoimmune causes were investigated in cerebrospinal fluid and found to be negative: viral nucleic acid testing; herpes simplex virus 1 and 2; varicella zoster virus; enterovirus; parvovirus; fungal culture; Crypotoccus neoformans antigen testing; Gram stain; acid fast bacilli staining; bacterial and mycobacterial culture; antibodies against N-methyl D-aspartate receptor (NMDA) NR1 subunit, leucine-rich, glioma inactivated-1 receptor, contactin-associated protein-like 2 autoantigen, aquaporin-4, and the gangliosides GM1, GM2, GM3, GD1a, GD1b, GQ1b, and GT1b. Blood and urine cultures were negative for bacterial, viral, or fungal pathogens. In view of her travel history, malaria thick and thin smears were done and were negative. Serological analyses involved tests for Eastern equine encephalitis virus (negative), Powassan virus (titre 1/20), chikungunya virus (IgM negative), Jamestown Canyon virus (IgM negative), snowshoe hare virus (IgM negative), dengue virus (IgM negative and IgG positive suggestive of remote infection), Japanese encephalitis virus (titre 1/80), Mycoplasm pneumoniae (IgM negative), rubella virus (IgM negative and IgG positive, 5·6 IU/mL), West Nile virus (IgM negative and IgG positive with high avidity suggestive of remote infection), brucella (negative), Leptospira species (negative), and Orientia tsutsugamushi (negative). Nucleic acid testing for enterovirus, parechovirus, and West Nile virus viraemia was negative. Nucleic acid testing of the bronchial alveolar lavage specimens was negative for influenza A and B RNA, respiratory syncytial virus RNA, parainfluenza virus RNA, human metapneumovirus RNA, enterovirus RNA, rhinovirus RNA, human coronavirus (229E, NL63, OC42, HKU1) DNA, and adenovirus DNA. Legionella antigen testing on urine was negative. PCR for rabies in a saliva sample was negative.
Table 1.
Cerebrospinal fluid analyses
|
Day of stay in hospital |
|||
|---|---|---|---|
| 1 | 10 | 27 | |
| Glucose (2·2–3·9 mmol/L)* | 3·2 | 4·5 | 4·0 |
| Protein (0·15–0·45 g/L) | 0·26 | 0·22 | 0·23 |
| Total IgG (0·000–0·058 g/L) | Not tested | 0·118 | Not tested |
| Red blood cells (0·0–5·0×106 cells per L) | 0·6 | 36·1 | 2·8 |
| White blood cells (0·0–5·0×106 cells per L) | 0·0 | 3·3 | 2·2 |
| Xanthochromia | Negative | Negative | Negative |
Cerebrospinal fluid measurements on day 1, 10, and 27 of stay in hospital (normal range).
Blood glucose (2·5–11·0 mmol/L) day 1 of hospital stay 5·0, on day 10 of hospital stay 6·2, and on day 27 of hospital stay 7·5.
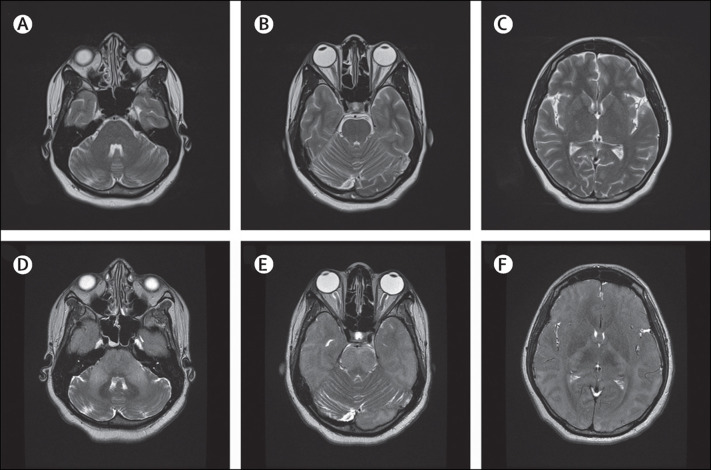
MRI of the brain showed no abnormalities in the first weeks after symptom onset (figure 1 ), and results from electroencephalogram (EEG) registrations showed no epileptiform changes.
Figure 1.
T2 weighted MRI scans from the time of admission and shortly before death
Normal findings early in the course of the disease at time of admission (A–C). Brain oedema and diffuse hyperintensity in the cerebral cortex, particularly in the occipital lobe, and throughout the brainstem shortly before death (D–F).
The patient was treated empirically for viral encephalitis and bacterial meningitis until the appropriate test and cultures were reported negative. A working diagnosis of autoimmune encephalitis was then made and she was treated with high dose intravenous methylprednisolone. 3 days after admission to hospital, she became somnolent and developed bilateral upgoing plantar reflex responses. The patient became hypoxic shortly afterwards because of an aspiration pneumonia; she was intubated and admitted to the intensive care unit, and given broad spectrum antibiotics for possible infectious causes. She became comatose despite treatment with intravenous immunoglobulins, plasmapheresis, rituximab, and antiseizure medications. At 31 weeks gestation, she developed hypertension and pre-eclampsia was suspected. Because of this complication, the child was delivered by emergency caesarean section. She was delivered of a healthy boy, and the hypertension resolved soon afterwards. She then developed acute tubular necrosis, which was confirmed with a renal biopsy, and was started on haemodialysis. She remained comatose and repeat cranial MRI showed diffuse hyperintensity in the brainstem and cerebral cortex, brain oedema, and brain herniation (figure 1). She died soon afterwards, 6 weeks after symptom onset.
Findings at autopsy
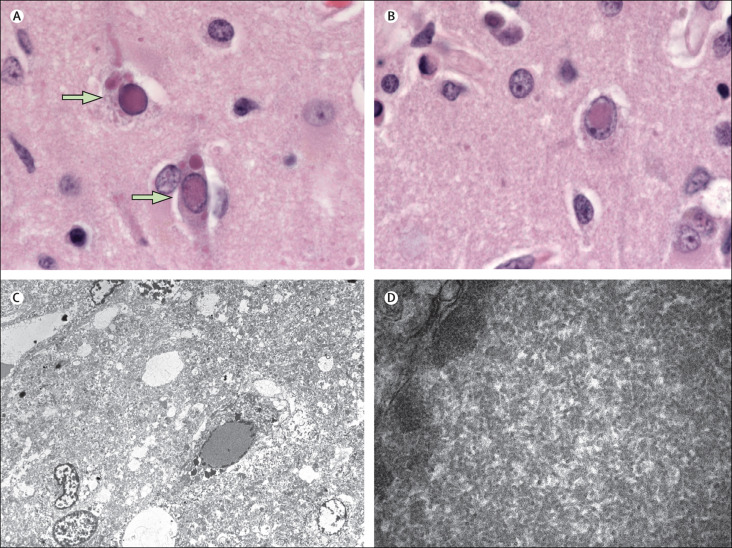
Gross examination of the brain showed generalised oedema and central herniation with associated temporo-occipital acute cerebral infarction. Diffuse encephalitis with associated astrocytic gliosis and neuropil loss was present in multiple areas of the cortex and thalamus. The brain had numerous eosinophilic viral inclusions, which were both nuclear and cytoplasmic. Transmission electron microscopy images showed that the intranuclear inclusion bodies comprised viral nucleocapsid filaments typical of paramyxoviruses (figure 2 ). Results of RT-PCR testing showed strongly positive results for measles virus. The virus strain was classified as a genotype D6 by genotyping done at the National Microbiology Laboratory in Winnipeg, MB, Canada. Results from sequencing of the virus at the Alberta Provincial Laboratory in Calgary, AB, Canada, showed that there were several mutations in the wild-type virus consistent with previously described mutations in cases of subacute sclerosing panencephalitis.1 The autopsy revealed a left breast fibroadenoma and uterine changes secondary to the patient's pregnancy and caesarean section. The kidneys were autolytic; no viral inclusions were identified. No noteworthy pathological changes were identified in other organ systems.
Figure 2.
Microscopy findings
Haematoxylin and eosin stained sections (A, B) showing ovoid-to-elongated eosinophilic nuclear and cytoplasmic inclusions, some of which showed a characteristic Cowdry type A morphology (arrows; magnification × 1000). Transmission electron microscopy image (C) of the specimen showing intranuclear inclusions (right) comprised of nucleocapsid filaments (magnification × 700). High magnification (× 25 000) transmission electron microscopy image (D) of the nucleus showing nucleocapsid filaments.
Discussion
The differential diagnosis of a subacute encephalopathy in pregnancy is broad. A detailed clinical discussion was published in 2005.2 Major causes include infections, autoimmune disease, and neoplasm. Infectious causes include viral encephalitides caused by varicella zoster virus, herpes simplex virus, enteroviruses, and arbovirus infections (such as West Nile virus, Japanese encephalitis virus, tick-borne encephalitis), and rabies. Progressive multifocal leukoencephalopathy is caused by the papova virus known as the John Cunningham virus, which typically affects patients with HIV and other patients who are immunocompromised. Infective endocarditis can have cerebral involvement through cerebral embolism. Autoimmune disorders such as acute disseminated encephalomyelitis, neurosarcoidosis, cerebral lupus, Behçet's syndrome, paraneoplastic limbic encephalitis, and primary cerebral angiitis can present as a progressive encephalopathy. Neoplastic disorders such as leptomeningeal metastasis of solid tumours, CNS lymphoma, and rarely intravascular lymphoma (angioendotheliomatosis) could lead to similar presentations and sequelae.
Measles-related neurological syndromes can be divided into four different diagnoses: primary measles encephalitis, acute post-measles encephalitis, measles inclusion body encephalitis, and subacute sclerosing panencephalitis.3 Primary measles encephalitis takes place during primary infection with onset typically during the rash phase of illness. Primary measles encephalitis affects one to three in 1000 patients with measles. The underlying pathophysiology seems to be a primary viral infection of CNS, because the cerebrospinal fluid usually has high titres of measles antibodies.4 Vaccination and primary encephalitis are not clearly related; investigators of a case series from 1963 to 1971 reported only 45 cases of primary encephalitis within 6–15 days of vaccination out of 50·9 million doses.5 Furthermore, findings from a prospective 14 year follow-up study from 1982 to 1996 showed that four of 1·8 million individuals had neurological complications attributed to the measles vaccine. Two cases of measles encephalitis after vaccination were reported in patients who were immunocompromised and two other infants younger than 1 year developed encephalitis 9–13 days after vaccination that could not be attributed to any specific cause.6
Acute post-measles encephalitis is the most common CNS sign of measles virus infection and takes place immediately after resolution of the primary infection. This disease is an autoimmune-mediated inflammatory disorder and occurs in one individual per 1000 cases of measles7 and in one to three per 10 million live measles vaccinations.8 The mortality associated with acute post-measles encephalitis is up to 25% in adults and 5% in children and the first-line treatment is high dose steroids with subsequent intravenous immunoglobulin.
Measles inclusion body encephalitis is associated with immunodeficiency (such as in patients with HIV infection) and can occur within 1 year of primary measles infection or vaccination. Measles inclusion body encephalitis has a mortality rate of 75% and survivors are often left with neurological deficits.3, 9 Disease pathogenesis is not completely understood, but is thought to involve infection of cerebral endothelial cells, and subsequent slow spread of the infection to CNS cells such as glial cells and neurons. One case of measles inclusion body encephalitis caused by a vaccine strain was reported in a child who was subsequently diagnosed with immunodeficiency.10 Viral mutations typically found in subacute sclerosing panencephalitis are also found in the measles inclusion body encephalitis.11
Subacute sclerosing panencephalitis is the most severe consequence of measles infection and happens after a longer latency period. In subacute sclerosing panencephalitis, mutated and defective measles virus proliferate within CNS cells.12 The favoured view is that the virus mutates after invading the CNS, where it is incapable of generating infectious virions. Viral genome replication and translation of viral proteins takes place and presumably spreads to other neurons by axonal transport.13 Symptoms typically occur 6–8 years after primary infection.14
Because subacute sclerosing panencephalitis develops some years after natural measles infection, and the typical symptoms of cognitive decline and behavioural changes develop slowly, the disease is difficult to diagnose. The symptoms typically progress to dementia, general convulsions, coma, and death over a period of 1–3 years.15, 16 Many cases of the disorder probably remain undiagnosed because of the difficulty of diagnosis and the variable practices in brain biopsies and autopsies.
Most patients with subacute sclerosing panencephalitis had their primary measles infection when younger than 2 years;2 those who are infected with measles virus when younger than 1 year are 16 times more likely to develop subacute sclerosing panencephalitis.2 The disease most commonly affects children, although it can occur in adults. In adults, the mean age at presentation is 25 years (with the oldest patient aged 35 years) and ocular symptoms are more common than in children.2 Clinical presentation of the disease is quite variable, but is usually characterised by progressive dementia, abnormal movements, and myoclonic jerks. An attempt to classify subacute sclerosing panencephalitis into four clinical stages has been made (appendix).17
Impaired immune responses seem to predispose individuals to the development of subacute sclerosing panencephalitis. Immaturity of the immune system in the first 2 years of life is thought to prevent a successful cell mediated immune response that would eradicate the virus, which allows the mutated virus to enter a persistent dormant state in CNS cells.18 The viral isolates associated with subacute sclerosing panencephalitis were investigated in a series of 11 cases.19 Genotypes D7 and D1 were the most common isolates, which were present in four of 11 cases each. One of the 11 reported cases (diagnosed in Glasgow, UK in 1999) was caused by the genotype D6 isolate, the same genotype found in the patient described in this Grand Round. Genotype D6 is known to be responsible for outbreaks of disease in many European countries from 1996–2002.20 A review12 in 2002 assessed subacute sclerosing panencephalitis cases in the USA. Among the PCR-proven cases, genotype D3 viral subtype was the most prevalent, followed by several cases of genotype E. Genotype D6 had not been reported in Canada since 1997,21 and has not been seen anywhere in the world since 2007,22 which supports our contention that our patient was infected decades ago.
Review of case reports in families suggests a genetic susceptibility of subacute sclerosing panencephalitis, because the disease has been reported in twins,23 siblings,23, 24 and members of the same extended family.25 Several studies have reviewed the genetics of patients with subacute sclerosing panencephalitis and of the viral strains implicated. However, such research is difficult in view of the rarity of the disease, and its results should be regarded as hypothesis-generating rather than confirmatory of genetic susceptibility. The programmed cell death-1 (PD-1) gene is thought to contribute to genetic susceptibility to subacute sclerosing panencephalitis. A statistically significant difference in PD-1 gene polymorphisms has been found in patients with subacute sclerosing panencephalitis and healthy controls in Turkey.26 PD-1 is a co-inhibitory molecule and a member of the CD28 family that acts as a negative immunomodulator in the suppression of T lymphocytes.27 Results from a study28 from Japan suggest that mutations in toll-like receptor 3 (TLR3) are positively associated with the disease. TLR3 recognises the dsRNA of measles virus and triggers the immunological cascade that results in the production of type 1 interferon, which is important for viral clearance.28 Mutations in TLR3 are proposed to decrease the recognition of measles virus and the production of interferon. Mutations in the sodium channel α-1 subunit gene, which are implicated in epilepsy, are thought to increase cerebral neuron vulnerability to measles infection.29
Measles virus is a member of the Morbillivirus genus, family Paramyxoviridae. The genome consists of a single strand non-segmented RNA of negative polarity and is about 16 kb long.13, 14 Measles viral strains that are associated with subacute sclerosing panencephalitis have defective envelope associated proteins; specifically haemagglutinin, fusion, and matrix protein.30 Proteins encoded by the matrix gene are essential for viral replication; therefore, crucial mutations impair the ability of the virus to produce viral progeny outside of the infected cell.31, 32 The D6 isolate, the cause of subacute sclerosing panencephalitis in the patient described in this Grand Round, had mutations characteristic of the disease strains for these essential viral proteins.19, 33
The risk of subacute sclerosing panencephalitis is estimated to be 0·2–1·0 per 100 000 measles cases worldwide with a higher risk in males (male-to-female ratio 2–4:1).34 The disease is more common in rural areas and in poor communities. Authors of an epidemiological study34 of subacute sclerosing panencephalitis done in Germany reported a risk of developing the disease after measles infection before 5 years of age of between one in 1700 and one in 3300. Most case reports of subacute sclerosing panencephalitis are described in non-vaccinated patients; however, similar to the patient described in this paper, some reports describe cases of the disease despite adequate vaccination.35, 36 A 7-year-old patient had rapid onset of subacute sclerosing panencephalitis with a documented vaccination history of measles, mumps, and rubella (MMR) vaccination at 15 months. The patient was presumed to have been exposed to measles before his vaccination, which might have been the case in our patient.35 Alternatively vaccine failure has been reported in a small number of patients, who were typically immunised before 12 months of age and did not seroconvert.37
Subacute sclerosing panencephalitis during pregnancy is very rare. Our review of the scientific literature yielded 21 prior cases (table 2 ). The most recent case report46 describes two 20-year-old patients with similar clinical signs and biochemical and radiological investigations to the patient described here. The diagnosis of presumed subacute sclerosing panencephalitis was made on the basis of clinical judgment of the care providers and the patients were treated; one patient was given interferon alfa-2b for 5 weeks and the other was given intrathecal interferon alfa-2 and inosinepranobex for 8 weeks; no clear benefits were seen before both patients succumbed to illness. Most other reported cases were not diagnosed until after death; most patients were treated for a presumed infectious and paraneoplastic encephalopathy. Only two case reports of subacute sclerosing panencephalitis in pregnancy presented with typical symptoms: a 30-year-old woman at 30 weeks' gestation (pregnant three times, with one livebirth) and a 27-year-old woman at 24 weeks' gestation (pregnant once, with one livebirth).2, 41 Both patients presented with visual symptoms and subsequently developed confusion, hemianopia, and focal seizures. Cerebrospinal fluid test results showed expected pleocytosis and oligoclonal bands. Findings from EEGs in both cases showed non-specific slowing and no radiological abnormalities in MRI. Despite the typical presentation, both cases presented diagnostic challenges and were treated empirically for infectious and inflammatory causes. At autopsy, both patients tested positive for the measles genotype D1 isolate.
Table 2.
Age and outcomes of patients with subacute sclerosing panencephalitis cases during pregnancy
| Time of delivery (gestational weeks) | Patient presentation | Treatment | Patient outcome | Child outcome | |
|---|---|---|---|---|---|
| 14 years16 | 33 | Progressive confusion and visual loss | Interferon alfa (6 weeks) | Subacute sclerosing panencephalitis stage 4 | Healthy at 5 months |
| 15 years 38 | Not reported | Confusion | None | Subacute sclerosing panencephalitis stage 4 | Healthy at 3 years |
| 15 years 39 | 30 | Confusion | Not reported | Alive; subacute sclerosing panencephalitis stage not reported | Healthy infant |
| 16 years 40 | 30 | Progressive confusion and visual loss | None | Death | Death 4 days after birth |
| 18 years 41 | Last trimester | Confusion | None | Death | Healthy |
| 18 years 42 | Last trimester | Confusion | None | Death | Intrauterine death |
| 19 years 43 | 36 | Progressive confusion and visual loss | None | Death | Intrauterine death |
| 19 years 39 | 30 | Confusion | Not reported | Death | Healthy |
| 19 years 43 | 38 | Progressive confusion and visual loss | None | Death | Healthy at 5 years |
| 20 years 44 | 26 | Progressive confusion and visual loss | None | Death | Intrauterine death |
| 20 years 43 | Last trimester | Progressive confusion and visual loss | None | Death | Healthy |
| 20 years 45 | 35 | Confusion | Prednisone | Stabilised subacute sclerosing panencephalitis stage 4 | Healthy at 15 months |
| 20 years 46 | 40 | Confusion and myoclonus | Interferon alfa-2b (5 weeks) | Death | Not reported |
| 20 years 47 | 28 | Progressive confusion and visual loss | Interferon alfa (6 weeks) | Stabilised subacute sclerosing panencephalitis stage 4 | Healthy at 7 months |
| 21 years 48 | 34 | Confusion | None | Subacute sclerosing panencephalitis stage 4 | Death within 24 h |
| 21 years 49 | 31 | Myoclonus | Isoprinosine | Death | Death at 3 years |
| 24 years 42 | Second month | Confusion | None | Death | Intrauterine death |
| 27 years 41 | 33 | Progressive confusion and visual loss | None | Stabilised subacute sclerosing panencephalitis stage 4 | Healthy |
| 29 years (patient described in this Grand Round) | 31 | Confusion | Plasmapheresis; intravenous immunoglobulin; rituximab | Death | Healthy at 1 year |
| 34 years 50 | 40 | Progressive confusion and visual loss | Interferon alfa-2b (6 weeks) | Stabilised subacute sclerosing panencephalitis stage 3 | Healthy |
| Age not specified “young”2 | 33 | Confusion | Methylprednisone; dexamethasone | Death | Healthy |
| Age not specified “young”51 | Third trimester | Not reported | Not reported | Not reported | Not reported |
The youngest reported patient with subacute sclerosing panencephalitis in pregnancy was a 14-year-old girl who had a history of 1 year onset of progressive unilateral visual impairment. At 31 weeks' gestation, she had rapid progressive cognitive decline in 1 week. Test results showed periodic spikes and slow wave complexes on EEG and a cortical abnormality in brain MRI.16 Patients who presented in their late 20s and 30s had a rapid neurological decline with mean survival of 1–2 months.16
One report41 describes an 18-year-old woman who developed post-partum fulminant subacute sclerosing panencephalitis. A few days after delivery, she developed rapidly progressing confusion and akinetic mutism followed by hyperpyrexia, tachycardia, hypertension and tachycardia, and eventual death. However, she might have shown early symptoms during the second and third trimesters of her pregnancy.
Pregnancy is known to alter the immune response of women, which can potentially affect susceptibility to some infectious diseases. Although many studies suggest systemic suppression of immunity takes place, which is mainly related to progesterone, this has not been proven. An immunomodulatory effect probably takes place, which results in a different response to microorganisms and inflammatory cascade, and is probably associated with the onset of subacute sclerosing panencephalitis during pregnancy.52
Most children born to mothers with subacute sclerosing panencephalitis have been free of the disease and medical complications. One report17 described a newborn baby who had measles during the perinatal period and developed subacute sclerosing panencephalitis during the first year of life. A case report47 from Germany describes the outcome of 13 infants of maternal subacute sclerosing panencephalitis cases. Intrauterine death occurred in four cases, two infants died in the first days of life and remaining children were reported as healthy. Importantly, this study had no long-term follow-up and it is therefore unknown if later complications occurred. The child born to the patient in this Grand Round is healthy at age 1 year, and continues to be followed up by a paediatrician.
Misdiagnosis of subacute sclerosing panencephalitis as infection or autoimmune encephalitis, as was the case here, is common, especially in adult patients. Authors of a review53 from the National Institute of Mental Health and Neurosciences in India show that an accurate initial diagnosis of subacute sclerosing panencephalitis was only made in 21% of cases. Attempts to formulate diagnostic criteria for the disease have been made; however, the gold standard for diagnosis is a positive brain biopsy suggestive of panencephalitis. Measles antibody detection using indirect immunofluorescent assay has been successful in establishing cerebrospinal fluid to serum ratios.37, 54 Cerebrospinal fluid analysis for both anti-measles virus IgG titres and intrathecal synthesis of virus-specific IgG index is diagnostic for subacute sclerosing panencephalitis.37, 54 Intrathecal synthesised IgG is typically markedly elevated with oligoclonal bands; however, IgG might not be solely specific for measles virus but to other viruses at lower titres. Detection of anti-measles IgM in cerebrospinal fluid has been suggested to lead to an extended course of subacute sclerosing panencephalitis. Measles RNA is rarely identified, but it is diagnostic for subacute sclerosing panencephalitis.37, 54 Investigators of one study55 found that patients with the disease had cerebrospinal fluid-to-serum ratios between 1:80 and 1:5. This represents the antibody index, which calculates the cerebrospinal fluid-to-serum antibody ratio that could differentiate between blood-derived and brain-derived pathogenesis. Normalisation of cerebrospinal fluid-to-serum ratios that account for albumin and total IgG has been introduced to improve the specificity and sensitivity of diagnosis.56, 57 Cerebrospinal fluid was not tested for measles-specific antibody in the patient described in this paper, but the measles IgG titre in serum was relatively high in the absence of a reported recent infection or vaccination; in hindsight this result might have been a clue to the diagnosis.
Diagnostic criteria proposed by Dyken in 198558 suggest that three of the five criteria are needed for a diagnosis of probable subacute sclerosing panencephalitis. Diagnostic criteria described in 201059 define major and minor criteria needed for diagnosis (panel ).58 The sensitivity and specificity of these criteria have not been assessed in either the general population or pregnant women, because the disease is very rare. Results from EEG analysis of patients with subacute sclerosing panencephalitis in India showed 98% of patients had delta burst patterns that were most commonly seen in the second stage of the disease.60 Periodic slowing seemed to be a common EEG finding in earlier stages of disease. Changes in MRI imaging are non-specific and, as was the case in the patient here, only present when the disease is advanced.2 Diffusion weighted MRI has been assessed as an aid to diagnosis of subacute sclerosing panencephalitis. The apparent diffusion coefficient values from six different areas of the brain were significantly higher in the patients with subacute sclerosing panencephalitis and enabled differentiation of disease stages.61
Panel. Diagnostic criteria for subacute sclerosing panencephalitis.
-
•
Mental status: progressive, subacute mental deterioration
-
•
EEG: periodic, sterotyped high voltage discharges
-
•
Cerebrospinal fluid: immunoglobulin or oligoclonal pattern
-
•
Serum: raised titres of measles antibody (≥1/256), cerebrospinal fluid (≥1/4), or both
-
•
Brain biopsy: suggestive of panencephalitis
Proposed subacute sclerosing panencephalitis diagnostic criteria, 2010 59
Major
-
•
Cerebrospinal fluid: elevated measles antibody titres
-
•
Typical history: acute progressive, subacute progressive, chronic progressive, chronic relapsing-remitting
-
•
Atypical history: seizures, prolonged stage 1, unusual age (infant or adult)
Minor
-
•
Typical EEG: periodic sterotyped high voltage discharges
-
•
Brain biopsy: cortical and subcortical perivascular infiltration of inflammatory cells
-
•
Specials: molecular diagnostic testing for mutated viral genome
EEG=electroencephalogram.
Treatment efficacy in subacute sclerosing panencephalitis cannot easily be established in clinical trials because the disease is rare. Although there is no known cure for the disease, several therapies, which might delay progression of disease or provide symptomatic relief, have been tested in non-randomised settings. In the Infectious Disease Society of America (IDSA) guidelines, intrathecal ribavirin is classed as level C-III level of evidence with no known efficacy for other treatment regimens investigated, such as interferon alfa, intravenous immunoglobulin, and isoprinosine.62, 63 The use of isoprinosine, interventricular interferon alfa, and ribavirin with 18F-fluorodeoxyglucose PET have been investigated in various case reports. Cortical metabolism was preserved and neurological prognosis was good at 3 years after diagnosis.64 One report65 noted an improvement of myoclonus with carbamazepine use, although this treatment had no effect on mortality.
In the past 10 years, fewer cases of subacute sclerosing panencephalitis have been reported, which corresponds to a decreased incidence of primary measles infection. Epidemiological data have shown that successful vaccination programmes have directly and indirectly protected the population against the disease through eradication and prevention of measles transmission.14, 22, 34, 66
Subacute sclerosing panencephalitis is related to wild-type measles infection, usually before age 2 years. Since the introduction of the measles vaccine, the disease has become exceedingly rare in North America. In Alberta, Canada, subacute sclerosing panencephalitis became reportable in 1983 and six cases were reported between 1983 and 1994. A monovalent live measles vaccination programme was introduced in Alberta in July, 1970, and the universal infant MMR programme was introduced in 1982.67 The MMR second dose campaign for children aged 4–6 years was introduced in 1996 and a catch-up programme (age 14–15 years) was introduced between 1997 and 1998.68 In India, an immunisation programme was introduced in 1978 as a WHO Expanded Programme of Immunization initiative.69 This programme gained momentum in 1985, and was expanded in a phased manner to become a universal immunisation programme covering all districts by 1990. The programme consisted of seven vaccines for preventable diseases, including measles. Two doses of measles vaccine were given to children at age 9–12 months and a second dose at 15–18 months.69 A dose of MMR at 5 years of age can be recommended depending on the district. Measles deaths in India decreased from 106 000 in 2005 to 65 000 in 2010 after a measles catch-up campaign that provided vaccination for 135 million children.70 In Japan, the addition of a mass immunisation campaign in 1982 resulted in a gradual reduction in subacute sclerosing panencephalitis cases from 24 cases reported in 1981 to 15 in 1985.66 Similar trends have been reported in Israel.14 These findings emphasise the importance of herd immunity in the maintenance of a population free of measles, because infants are susceptible to measles infection until the MMR vaccination can be given at 12 months old.
Conclusion
The outcome of our patient draws attention to the most serious complication of measles infection. Diagnosis of subacute sclerosing panencephalitis is difficult because of the broad differential and rarity of the disease. Subacute sclerosing panencephalitis should be considered in the diagnosis of progressive encephalitis, especially in view of increased migration and global travel. Although no treatment exists for the disease, early diagnosis and therapeutic intervention with off-label treatment candidates could help in the development of a management strategy.
Perhaps the most important lesson learned from our patient is the importance of the prevention of subacute sclerosing panencephalitis through immunisation and prevention of measles virus infection. Mass immunisation campaigns in many countries have resulted in the rapid reduction of primary measles infection and subsequent reduction of cases of subacute sclerosing panencephalitis. In the USA, the incidence of the disease has dropped from 0·61 to 0·01 per million people per year.14 Areas with successful vaccination programmes have had a steady decline in subacute sclerosing panencephalitis. Primary prevention of measles infection through the creation, improvement, and continuation of vaccination programmes remains our best chance to prevent this devastating disease.
Search strategy and selection criteria
Citations were identified through PubMed searches with no publication date restrictions using the search terms (including variations), “subacute sclerosing panencephalitis (SSPE)”, “SSPE AND pregnancy”, “SSPE AND adult”, combined with study filters for case reports, case series, cohort studies, review articles, and original research. Additional articles were identified from the reference lists of identified papers. Only papers published in English were reviewed. The final reference list was generated on the basis of originality, quality, and relevance.
Acknowledgments
Acknowledgments
We thank the patient's family for providing background information and allowing us to publish this Grand Round.
Contributors
MHC, BM, and MWK all participated in the care of the patient and wrote the first draft of the manuscript. JTJ did the brain autopsy, identified the virus, submitted tissue for PCR analysis, and helped prepare the pathology text, pathology figures, and the pathology figure legends. AN helped to prepare the pathology text, pathology figures, and the pathology figure legends. KC, KF, JM, KP, RT, and SW took part in the investigations for the patient. All authors contributed to the writing and revision of the manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Cattaneo R, Schmid A, Rebmann G. Accumulated measles virus mutations in a case of subacute sclerosing panencephalitis: interrupted matrix protein reading frame and transcription alteration. Virology. 1986;154:97–107. doi: 10.1016/0042-6822(86)90433-2. [DOI] [PubMed] [Google Scholar]
- 2.Joseph F, Dawson K, Betmouni S, Moss T, Smith P. The Bath Advanced Neurology Course 2003: progressive neurological decline in pregnancy. Pract Neurol. 2005;5:168–175. [Google Scholar]
- 3.Fisher DL, Defres S, Solomon T. Measles-induced encephalitis. QJM. 2015;108:177–182. doi: 10.1093/qjmed/hcu113. [DOI] [PubMed] [Google Scholar]
- 4.Patterson CE, Daley JK, Echols LA, Lane TE, Rall GF. Measles virus infection induces chemokine synthesis by neurons. J Immunol. 2003;171:3102–3109. doi: 10.4049/jimmunol.171.6.3102. [DOI] [PubMed] [Google Scholar]
- 5.Landrigan PJ, Witte JJ. Neurologic disorders following live measles-virus vaccination. JAMA. 1973;223:1459–1462. [PubMed] [Google Scholar]
- 6.Patja A, Davidkin I, Kurki T, Kallio M, Valle M, Petola H. Serious adverse events after measles-mumps-rubella vaccination during a 14-year prospective follow-up. Pediatr Infect Dis J. 2000;19:1127–1134. doi: 10.1097/00006454-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan R, Bonthius DJ. Measles virus and associated central nervous system sequelae. Semin Pediatr Neurol. 2012;19:107–114. doi: 10.1016/j.spen.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Bennetto L, Scolding N. Inflammatory/post-infectious encephalomyelitis. J Neurol Neurosurg Psychiatry. 2004;75(suppl 1):i22–i28. doi: 10.1136/jnnp.2003.034256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mustafa MM, Weitman SD, Winick NJ, Bellini WJ, Timmons CF, Siegel JD. Subacute measles encephalitis in the young immunocompromised host: report of two cases diagnosed by polymerase chain reaction and treated with ribavirin and review of the literature. Clin Infect Dis. 1993;16:654–660. doi: 10.1093/clind/16.5.654. [DOI] [PubMed] [Google Scholar]
- 10.Bitnun A, Shannon P, Durward A. Measles inclusion-body encephalitis caused by the vaccine strain of measles virus. Clin Infect Dis. 1999;29:855–861. doi: 10.1086/520449. [DOI] [PubMed] [Google Scholar]
- 11.Rima BK, Duprex WP. Molecular mechanisms of measles virus persistence. Virus Res. 2005;111:132–147. doi: 10.1016/j.virusres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Bellini WJ, Rota JS, Lowe LE. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J Infect Dis. 2005;192:1686–1693. doi: 10.1086/497169. [DOI] [PubMed] [Google Scholar]
- 13.Griffin DE. Measles virus. In: Knipe D, Howley P, editors. Fields virology. Wolters Kluwer; Philadelphia, PA: 2013. pp. 1042–1069. [Google Scholar]
- 14.Gadoth N. Subacute sclerosing panencephalitis (SSPE) the story of a vanishing disease. Brain Dev. 2012;34:705–711. doi: 10.1016/j.braindev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Campbell H, Andrews N, Brown KE, Miller E. Review of the effect of measles vaccination on the epidemiology of SSPE. Int J Epidemiol. 2007;36:1334–1348. doi: 10.1093/ije/dym207. [DOI] [PubMed] [Google Scholar]
- 16.Jayawant S, Feyi-Waboso A, Wallace S. Retinitis and dementia in a pregnant girl: an unusual case. Eur J Paediatr Neurol. 2000;4:177–179. doi: 10.1053/ejpn.2000.0296. [DOI] [PubMed] [Google Scholar]
- 17.Garg RK. Subacute sclerosing panencephalitis. J Neurol. 2008;255:1861–1871. doi: 10.1007/s00415-008-0032-6. [DOI] [PubMed] [Google Scholar]
- 18.Yentür SP, Gürses C, Demirbilek V. Alterations in cell-mediated immune response in subacute sclerosing panencephalitis. J Neuroimmunol. 2005;170:179–185. doi: 10.1016/j.jneuroim.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Jin L, Beard S, Hunjan R, Brown DW, Miller E. Characterization of measles virus strains causing SSPE: a study of 11 cases. J Neurovirol. 2002;8:335–344. doi: 10.1080/13550280290100752. [DOI] [PubMed] [Google Scholar]
- 20.Rota PA, Liffick SL, Rota JS. Molecular epidemiology of measles viruses in the United States, 1997–2001. Emerg Infect Dis. 2002;8:902–908. doi: 10.3201/eid0809.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tipples G, Gray A, Garbutt M, Rota P, for the Canadian Measles Surveillance Program Genotyping of measles virus in Canada: 1979–2002. J Infect Dis. 2004;189(suppl 1):171–176. doi: 10.1086/377716. [DOI] [PubMed] [Google Scholar]
- 22.Rota PA, Brown K, Mankertz A. Global distribution of measles genotypes and measles molecular epidemiology. J Infect Dis. 2011;204(suppl 1):S514–S523. doi: 10.1093/infdis/jir118. [DOI] [PubMed] [Google Scholar]
- 23.Tuxhorn IE. Familial subacute sclerosing panencephalitis in two siblings. Pediatr Neurol. 2004;31:291–294. doi: 10.1016/j.pediatrneurol.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Vieker S, Schmitt JJ, Behrens C, Weissbrich B, Hartmann H. Subacute sclerosing panencephalitis in two brothers. Neuropediatrics. 2003;34:326–329. doi: 10.1055/s-2003-44672. [DOI] [PubMed] [Google Scholar]
- 25.Sharma V, Gupta VB, Eisenhut M. Familial subacute sclerosing panencephalitis associated with short latency. Pediatr Neurol. 2008;38:215–217. doi: 10.1016/j.pediatrneurol.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Piskin IE, Calık M, Abuhandan M, Kolsal E, Celik SK, Iscan A. PD-1 gene polymorphism in children with subacute sclerosing panencephalitis. Neuropediatrics. 2013;44:187–190. doi: 10.1055/s-0033-1338134. [DOI] [PubMed] [Google Scholar]
- 27.Ishizaki Y, Yukaya N, Kusuhara K. PD1 as a common candidate susceptibility gene of subacute sclerosing panencephalitis. Hum Genet. 2010;127:411–419. doi: 10.1007/s00439-009-0781-z. [DOI] [PubMed] [Google Scholar]
- 28.Ishizaki Y, Takemoto M, Kira R. Association of toll-like receptor 3 gene polymorphism with subacute sclerosing panencephalitis. J Neurovirol. 2008;14:486–491. doi: 10.1080/13550280802298120. [DOI] [PubMed] [Google Scholar]
- 29.Garg RK. Are SCN1A gene mutations responsible for genetic susceptibility to subacute sclerosing panencephalitis? Med Hypotheses. 2012;78:247–249. doi: 10.1016/j.mehy.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 30.Forcić D, Baricević M, Zgorelec R. Detection and characterization of measles virus strains in cases of subacute sclerosing panencephalitis in Croatia. Virus Res. 2004;99:51–56. doi: 10.1016/j.virusres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Jiang DP, Ide YH, Nagano-Fujii M, Shoji I, Hotta H. Single-point mutations of the M protein of a measles virus variant obtained from a patient with subacute sclerosing panencephalitis critically affect solubility and subcellular localization of the M protein and cell-free virus production. Microbes Infect. 2009;11:467–475. doi: 10.1016/j.micinf.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Moulin E, Beal V, Jeantet D, Horvat B, Wild TF, Waku-Kouomou D. Molecular characterization of measles virus strains causing subacute sclerosing panencephalitis in France in 1977 and 2007. J Med Virol. 2011;83:1614–1623. doi: 10.1002/jmv.22152. [DOI] [PubMed] [Google Scholar]
- 33.Santak M, Baricević M, Mazuran R, Forcić D. Intra- and intergenotype characterization of D6 measles virus genotype. Infect Genet Evol. 2007;7:645–650. doi: 10.1016/j.meegid.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Schönberger K, Ludwig MS, Wildner M, Weissbrich B. Epidemiology of subacute sclerosing panencephalitis (SSPE) in Germany from 2003 to 2009: a risk estimation. PLoS One. 2013;8:e68909. doi: 10.1371/journal.pone.0068909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik B, Sharma FJ, Bhardwaj AK, Sharma A. Sub acute sclerosing pan encephalitis despite adequate vaccination. Australas Med J. 2012;5:359–361. doi: 10.4066/AMJ.2012.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Har-Even R, Aichenbaum S, Rabey JM, Livne A, Bistritzer T. Measles-vaccinated Israeli boy with subacute sclerosing panencephalitis. Pediatr Neurol. 2011;44:467–470. doi: 10.1016/j.pediatrneurol.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Anlar B, Yalaz K. Measles virus infection and subacute sclerosing panecephalitis. In: Jackson AC, editor. viral infections of the human nervous system, Birkhauser advances in infectious diseases. Springer; Basel: 2013. pp. 3–22. [Google Scholar]
- 38.Gaines KJ, Jabbour JT, Whitaker JN, Sever J. Subacute sclerosing panencephalitis during pregnancy. A surviving normal infant. Arch Neurol. 1979;36:314–316. doi: 10.1001/archneur.1979.00500410092016. [DOI] [PubMed] [Google Scholar]
- 39.Miller C, Andrews N, Rush M, Munro H, Jin L, Miller E. The epidemiology of subacute sclerosing panencephalitis in England and Wales 1990–2002. Arch Dis Child. 2004;89:1145–1148. doi: 10.1136/adc.2003.038489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mańko-Juraszek R, Kasperek S. Subacute sclerosing panencephalitis pregnancy. Neurol Neurochir Pol. 1992;26:711–715. (in Polish). [PubMed] [Google Scholar]
- 41.Wirguin I, Steiner I, Kidron D. Fulminant subacute sclerosing panencephalitis in association with pregnancy. Arch Neurol. 1988;45:1324–1325. doi: 10.1001/archneur.1988.00520360042009. [DOI] [PubMed] [Google Scholar]
- 42.Tan E, Namer IJ, Ciger A, Zileli T, Kucukali T. The prognosis of subacute sclerosing panencephalitis in adults. Report of 8 cases and review of the literature. Clin Neurol Neurosurg. 1991;93:205–209. doi: 10.1016/s0303-8467(05)80004-6. [DOI] [PubMed] [Google Scholar]
- 43.Sobczyk W, Kulczycki J, Tarnowska-Dziduszko E, Stroińska-Kuś B, Nowacki P. Subacute sclerosing panencephalitis (SSPE) in women during pregnancy. Neurol Neurochir Pol. 1992;26:291–296. (in Polish). [PubMed] [Google Scholar]
- 44.Cole AJ, Henson JW, Roehrl MH, Frosch MP. Case records of the Massachusetts General Hospital. Case 24–2007. A 20-year-old pregnant woman with altered mental status. N Engl J Med. 2007;357:589–600. doi: 10.1056/NEJMcpc079018. [DOI] [PubMed] [Google Scholar]
- 45.Glasner H, Kirsch W. Pathogenetic and therapeutic aspects of subacute sclerosing encephalitis. Arch Psychiatr Nervenkr. 1975;221:29–38. (in German). [PubMed] [Google Scholar]
- 46.Rana M, Rana VM, Singh A, Singhal S. Subacute sclerosing panencephalitis: a rare neurological disorder in pregnancy. J Evol Med Dent Sci. 2015;4:4033–4036. [Google Scholar]
- 47.Thiel A, Nau R, Fischer F. Healthy infant delivered by a mother with subacute sclerosing panencephalitis during pregnancy. Neurology. 1996;47:1604. doi: 10.1212/wnl.47.6.1604. [DOI] [PubMed] [Google Scholar]
- 48.Nelson RF, Dennery JM, Montpetit V, Furesz J. S. S. P. E. and pregnancy. Lancet. 1972;1:1289. doi: 10.1016/s0140-6736(72)91013-6. [DOI] [PubMed] [Google Scholar]
- 49.Nagar VS, Sawarkar R, Dhekne D, Hedau M, Kadu R. Subacute sclerosing panencephelitis in pregnancy. J Assoc Physicians India. 2015;63:76. [PubMed] [Google Scholar]
- 50.Gascon GG, Frosch MP. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 15–1998. A 34-year-old woman with confusion and visual loss during pregnancy. N Engl J Med. 1998;338:1448–1456. doi: 10.1056/NEJM199805143382008. [DOI] [PubMed] [Google Scholar]
- 51.Roman G, Navarro L, Toro G, Vergara I. Subacute sclerosing panencephalitis in South America. Lancet. 1976;2:1352–1353. doi: 10.1016/s0140-6736(76)91995-4. [DOI] [PubMed] [Google Scholar]
- 52.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javali M, Menon R, Chakor R. Adult onset subacute sclerosing panencephalitis—lessons learnt from an atypical presentation. J Neurosci Rural Pract. 2014;5:310–313. doi: 10.4103/0976-3147.133637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anlar B. Subacute sclerosing panencephalitis and chronic viral encephalitis. In: Dulac O, Lassonde M, Sarnat HB, editors. Handbook of clinical neurology, volume 112. Pediatric neurology part II. Elsevier; Amsterdam: 2013. pp. 1183–1189. [DOI] [PubMed] [Google Scholar]
- 55.Manayani DJ, Abraham M, Gnanamuthu C, Solomon T, Alexander M, Sridharan G. SSPE—the continuing challenge: a study based on serological evidence from a teritary care centre in India. Indian J Med Microbiol. 2002;20:16–18. [PubMed] [Google Scholar]
- 56.Reiber H, Lange P. Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem. 1991;37:1153–1160. [PubMed] [Google Scholar]
- 57.Jacobi C, Lange P, Reiber H. Quantitation of intrathecal antibodies in cerebrospinal fluid of subacute sclerosing panencephalitis, herpes simplex encephalitis and multiple sclerosis: discrimination between microorganism-driven and polyspecific immune response. J Neuroimmunol. 2007;187:139–146. doi: 10.1016/j.jneuroim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Dyken PR. Subacute sclerosing panencephalitis. Current status. Neurol Clin. 1985;3:179–196. [PubMed] [Google Scholar]
- 59.Gutierrez J, Issacson RS, Koppel BS. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol. 2010;52:901–907. doi: 10.1111/j.1469-8749.2010.03717.x. [DOI] [PubMed] [Google Scholar]
- 60.Praveen-kumar S, Sinha S, Taly AB. Electroencephalographic and imaging profile in a subacute sclerosing panencephalitis (SSPE) cohort: a correlative study. Clin Neurophysiol. 2007;118:1947–1954. doi: 10.1016/j.clinph.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 61.Abuhandan M, Cece H, Calik M, Karakas E, Dogan F, Karakas O. An evaluation of subacute sclerosing panencephalitis patients with diffusion-weighted magnetic resonance imaging. Clin Neuroradiol. 2013;23:25–30. doi: 10.1007/s00062-012-0163-0. [DOI] [PubMed] [Google Scholar]
- 62.Tunkel AR, Glaser CA, Bloch KC. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327. doi: 10.1086/589747. [DOI] [PubMed] [Google Scholar]
- 63.Garg RK. Subacute sclerosing panencephalitis. Postgrad Med J. 2002;78:63–70. doi: 10.1136/pmj.78.916.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohya T, Yamashita Y, Shibuya I. A serial 18FDG-PET study of a patient with SSPE who had good prognosis by combination therapy with interferon alpha and ribavirin. Eur J Paediatr Neurol. 2014;18:536–539. doi: 10.1016/j.ejpn.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Ravikumar S, Crawford JR. Role of carbamazepine in the symptomatic treatment of subacute sclerosing panencephalitis: a case report and review of the literature. Case Rep Neurol Med. 2013;2013:327647. doi: 10.1155/2013/327647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okuno Y, Nakao T, Ishida N. Incidence of subacute sclerosing panencephalitis following measles and measles vaccination in Japan. Int J Epidemiol. 1989;18:684–689. doi: 10.1093/ije/18.3.684. [DOI] [PubMed] [Google Scholar]
- 67.Alberta Health and Wellness. Government of Alberta Public health notifiable disease management guidelines. Subacute sclerosing panencephalitis. August, 2011. http://www.health.alberta.ca/documents/Guidelines-Subacute-Sclerosing-Panencephalitis-SSPE-2011.pdf (accessed Jan 1, 2016).
- 68.Alberta Health and Wellness. Government of Alberta Alberta immunization policy appendix 2. 2007–2014. http://www.health.alberta.ca/documents/AIP-Appendices.pdf (accessed Jan 1, 2016).
- 69.Government of India. Ministry for Health and Family Welfare Universal Immunization Program. http://www.mohfw.nic.in/WriteReadData/l892s/Immunization_UIP.pdf (accessed Jan 1, 2016).
- 70.Gupta A. India's Universal immunization programme. GAVI Alliance Board Meeting 2012. http://www.gavi.org/About/Governance/GAVI-Board/Minutes/2012/12-june/Presentations/16—Country-presentation—India/ (accessed Jan 1, 2016).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.