Summary
Together with influenza, the non-influenza RNA respiratory viruses (NIRVs), which include respiratory syncytial virus, parainfluenza viruses, coronavirus, rhinovirus, and human metapneumovirus, represent a considerable global health burden, as recognised by WHO's Battle against Respiratory Viruses initiative. By contrast with influenza viruses, little is known about the contemporaneous global diversity of these viruses, and the relevance of such for development of pharmaceutical interventions. Although far less advanced than for influenza, antiviral drugs and vaccines are in different stages of development for several of these viruses, but no interventions have been licensed. This scarcity of global genetic data represents a substantial knowledge gap and impediment to the eventual licensing of new antiviral drugs and vaccines for NIRVs. Enhanced genetic surveillance will assist and boost research and development into new antiviral drugs and vaccines for these viruses. Additionally, understanding the global diversity of respiratory viruses is also part of emerging disease preparedness, because non-human coronaviruses and paramyxoviruses have been listed as priority concerns in a recent WHO research and development blueprint initiative for emerging infectious diseases. In this Personal View, we explain further the rationale for expanding the genetic database of NIRVs and emphasise the need for greater investment in this area of research.
Introduction
Lower respiratory tract infections are the fourth most common cause of death, globally, after ischaemic heart disease, chronic obstructive pulmonary disease, and stroke, all of which are non-infectious chronic conditions.1 In patients with these non-infective diseases, respiratory infections often trigger life-threatening exacerbations. Therefore, respiratory infections are the leading cause of death due to infection, worldwide.
The rationale for investment in the global surveillance of influenza viruses is driven by the need to ensure the efficacy of seasonal influenza vaccines, and to monitor circulating strains for pandemic potential or resistance against antiviral drugs. However, the importance and feasibility of surveillance is less well established for non-influenza respiratory viruses (NIRVs), including respiratory syncytial virus, parainfluenza virus, human metapneumovirus, rhinovirus, and coronavirus, despite scientific consensus that the burden of disease attributable to these infections is considerable.
NIRVs
Similar to influenza, NIRVs are also RNA viruses that have a relatively high mutation rate because proofreading—an inherent process in the replication of DNA genomes—is absent. In combination, these viruses are responsible for greater annual morbidity and mortality than influenza viruses, across all age groups.2, 3, 4, 5 Higher annual morbidity and mortality is particularly evident when the full range of mild to severe respiratory illnesses is taken into account, from common colds (mostly due to rhinovirus and coronavirus infections) that affect people of all ages, to more severe respiratory illness that require hospital admission.6
WHO's Battle against Respiratory Viruses initiative7 has highlighted the need for enhanced clinical and epidemiological surveillance for respiratory viruses, with a focus on the development of a vaccine for respiratory syncytial virus.8 This initiative acknowledges the considerable health burden of respiratory syncytial virus,3, 9, 10, 11, 12 which is now one of the highest priorities for intervention of all NIRVs. The initiative recommends improving the diagnosis of severe acute respiratory illness using deep sequencing (to detect all viral populations present in the sample), which is explicitly listed as a research priority. We would like to emphasise and expand upon this point.
Burden of diagnostic testing
A key reason for the scarcity of global data about the burden of NIRVs is the small number of cost-effective, sensitive assays that can be used routinely in everyday diagnostic settings. Many health-care facilities will have some capacity to test for influenza virus and respiratory syncytial virus (particularly where paediatric services are provided), whereas the other common respiratory viruses might not be routinely screened for. Transmission of respiratory viruses has caused outbreaks among patients in intensive care units and oncology wards,13, 14 and timely testing might decrease the unnecessary use of antimicrobials and importantly, restrict transmission by effective isolation of patients with infections.15, 16, 17
In some regions (eg, Canada, Mongolia, Hong Kong, Rwanda, Côte D'Ivoire, Madagascar, and South Africa) specialised centres can offer multiplex PCR-based testing, but the cost of the diagnostic testing compared with the small effect it is perceived to have on clinical decision making precludes widespread use. Nevertheless, these multiplex molecular assays and the high-throughput sequencing protocols that are now being piloted enable a wider spectrum of respiratory viruses to be assessed from a single sample.18, 19, 20 These advances have offered an unprecedented opportunity to focus on the burden of these NIRVs.
Disease surveillance and rationale
How could surveillance be implemented for these other NIRVs? An important starting point is whom and how to sample. Groups at higher risk of disease associated with seasonal influenza virus and NIRV infections are similar and include children,21, 22, 23, 24, 25 elderly individuals,26, 27, 28, 29, 30 immunocompromised individuals,31, 32, 33 and individuals with chronic comorbidities (eg, asthma, chronic obstructive pulmonary disease, cardiac and renal failure, or diabetes). Both influenza and NIRV infections can result in hospital admission due to exacerbation of these conditions.4, 34, 35, 36, 37, 38, 39 In these at-risk populations, co-infections with more than one respiratory virus are not uncommon, although the clinical significance of multiple co-infections is still unclear.40, 41, 42, 43, 44, 45
Many studies46, 47, 48, 49, 50, 51, 52 have shown that in young children, NIRVs, especially respiratory syncytial virus, are the predominant viral cause of respiratory morbidity and mortality, with accumulating evidence to suggest that infection in early childhood with NIRVs, such as respiratory syncytial virus and rhinovirus, in predisposed children, might result in the development of increased airways sensitivity and asthma later in life. This observation provides an example of the effect of both the acute and more chronic respiratory virus-associated health-care burden. Taken together, we conclude that enhanced surveillance for these other NIRVs is warranted.
One key factor that questions the usefulness of such surveillance is that no agreed interventions are available. Nevertheless, current research and development interest into NIRV therapeutics will benefit from the availability of more large-scale, full-genome NIRV sequences. Although a small survey of the clinical trial websites for the USA and European Union shows a substantially greater research and development investment into influenza viruses than NIRVs, a few clinical trials targeting NIRVs are in progress (table ).
Table.
Ongoing clinical trials associated with vaccine and antiviral drug development for the different respiratory viruses
| Influenza | Respiratory syncytial virus | Human parainfluenze virus | Human metapneumovirus | Coronavirus | Rhinovirus | |
|---|---|---|---|---|---|---|
| USA | 1561 vaccine trials; 190 antiviral drug trials | 49 vaccine trials; 33 antiviral drug trials | 13 vaccine trials; 0 antiviral drug trials | 3 vaccine trials; 0 antiviral drug trials | 4 vaccine trials; 4 antiviral drug trials | 12 vaccine trials; 3 antiviral drug trials |
| European Union | 357 vaccine trials; 11 antiviral drug trials | 4 vaccine trials; 13 antiviral drug trials | 1 vaccine trial; 0 antiviral drug trials | 0 vaccine trials; 0 antiviral drug trials | 0 vaccine trials; 0 antiviral drug trials | 1 vaccine trial; 0 antiviral drug trials |
Genetic diversity of respiratory viruses
Mutational pressure
With the progression of antiviral drugs and vaccines, we believe that understanding the global genomic diversity of these viruses is increasingly important. Not all antiviral targets are subject to the same mutational pressure, with some being highly conserved, and some highly variable, such as the fusion protein of respiratory syncytial virus and attachment proteins of human metapneumovirus, respectively.53, 54
Antiviral drug and vaccine development
Several candidate respiratory syncytial virus and parainfluenza virus vaccines are in various stages of clinical evaluation,55, 56, 57, 58, 59 and assessing the effect of vaccine-induced immune responses in the context of continued viral evolution and the subsequent potential for vaccine escape will be an essential consideration when determining the annual vaccine composition.
For antiviral drug development, since modern rational drug design allows for the development of new antivirals that target specific viral proteins, assessing the mutation rates and genetic diversity of these therapeutic targets is crucial to ensure the long-lasting effectiveness of these drugs. Thus, this baseline data will provide an estimate of the naturally occurring mutation rate for the individual genes in each of these viruses, which might then affect the choice of target for the development of any future specific antiviral drugs or vaccines.
Studies60, 61, 62, 63 have shown that with influenza treatment, patients who are immunocompromised on long-term antiviral treatment (or prophylaxis) are more susceptible to the rapid evolution and development of drug resistance, either in total or in subpopulations of viruses within the host. Therefore, any antiviral agent developed for the NIRVs might have similar characteristics unless it can be specifically designed to act on a more stable viral protein. Drug resistance has already been reported for palivizumab, a humanised monoclonal antibody directed against the respiratory syncytial virus fusion protein. Despite relatively rare usage of the antibody and the highly conserved nature of its viral protein target, resistance has been reported due to variations in the fusion protein binding site.64, 65, 66, 67, 68
One study69 investigated the human monoclonal antibody MPE8, which cross-neutralises respiratory syncytial virus and human metapneumovirus by binding to two highly conserved anti-parallel β-strands on the pre-fusion viral fusion protein. The investigators found naturally occurring antibodies with this same target specificity in some patient serum samples, and therefore proposed this pre-fusion viral fusion protein as a potential vaccine candidate.69 A wider, global genetic survey of this target in respiratory syncytial virus would help to confirm its suitability for development into a global respiratory syncytial virus vaccine.
For rhinoviruses, the expansion of the small amount of complete viral genome data available—only around 200 full genomes are available compared with over 5000 for influenza A H1N1 pdm09 and over 6000 for influenza A H3N2—is essential to understand further the natural genetic diversity of these viruses and underlying evolutionary forces (ie, viral gene mutation and recombination driven by selection pressure), as a foundation for designing new antiviral drugs and vaccines.70, 71, 72
Research regarding coronavirus infections has been dominated by studies on severe acute respiratory syndrome-associated coronaviruses and Middle East respiratory syndrome-associated coronaviruses. However, the development of an antiviral drug that is effective against the coronavirus family as a whole will also be effective against the milder, but much more prevalent common cold viruses (eg, coronavirus OC43, 229E, NL63, and HKU1).73 A study74 using a mouse hepatitis coronavirus found a relatively small repertoire of resistance mutations to an experimental compound that inhibits the action of a coronavirus fusion protein. This fusion protein, heptad repeat 2, is required to enter the host cell and its inhibition will block infection. The investigators found that after multiple passages in vitro, most mutations that conferred resistance were located within a small 19 aminoacid region of the related heptad repeat 1 region in the mouse hepatitis coronavirus spike protein.74
Should the development of such a heptad repeat 2 fusion inhibitor progress further, global surveillance will be essential to establish whether any of these heptad repeat 1 mutations that confer resistance exist naturally, because this would indicate an increased potential for the emergence of resistant mutants.
These examples further highlight the need for baseline genetic information about NIRVs, and ongoing monitoring for the emergence of drug resistance, once such antiviral drugs become available.
Genetic surveillance
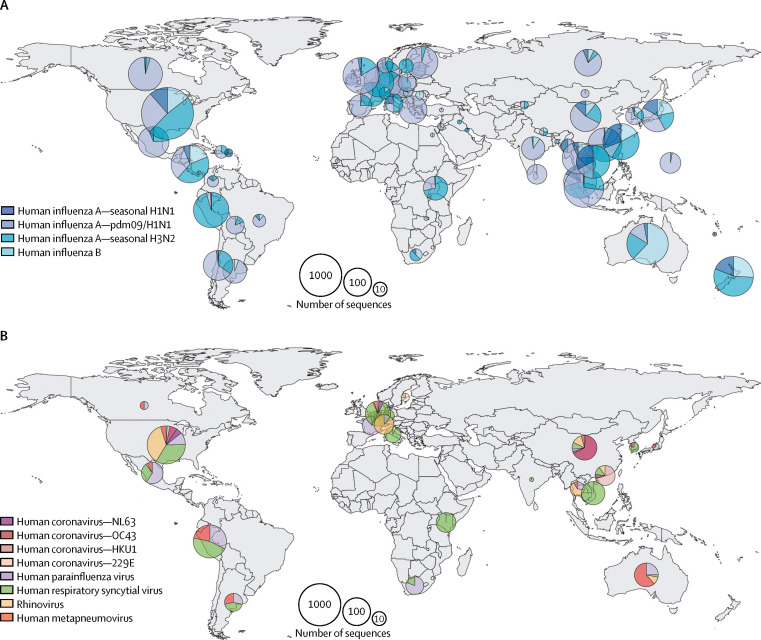
From a drug design viewpoint, the selection of an initial antiviral target from a well conserved genomic region that mutates only very slowly is imperative. This careful selection can only be achieved with a large-scale characterisation of the natural genetic diversity of these NIRVs. Such an enhanced genetic surveillance approach to NIRVs, together with comprehensive conventional epidemiological data, will naturally fuel the search for new drugs and vaccines to combat these viruses. However, when reviewing the currently available genomic information in GenBank, the total number of whole genome influenza sequences available is approximately ten times higher than that of the combined total for NIRVs (figure ). We believe that this represents a large data gap that needs to be filled with some urgency.
Figure.
Geographical sources of genome sequence data for different respiratory RNA viruses
Only field strains with complete genome sequences (>80% of full-length) and geographical information in GenBank were included. Counts of human influenza A and B viruses (A). Counts of four human coronavirus species (NL63, OC43, HKU1, and 229E), human parainfluenza virus, human respiratory syncytial virus, rhinovirus, and human metapneumovirus (B). Radius of pie chart is equal to log2 of the total number of sequences from the country or region.
Additionally, genetic surveillance has many other important public health implications, such as the identification of novel respiratory viruses that can clinically mimic other more common NIRVs. With the emergence of several novel respiratory viruses able to cause multiple outbreaks with varying degrees of potential for person-to-person transmission (eg, severe acute respiratory syndrome coronavirus, influenza A H1N1 pdm09, avian influenza A H5N1 and A H7N9, and Middle East respiratory syndrome coronavirus), the surveillance of both mild versus severe and community versus acute respiratory infections requiring hospital admission is becoming more crucial for the detection of such novel viruses.
Implementation and funding of large-scale sequencing
Genetic sequence diversity
The rapid development of quicker and cheaper high-throughput sequencing platforms now readily allows the profiling of pathogen genetic sequence diversity directly from clinical samples. This technique could be applied to characterise the global population of NIRVs, but will require substantial funding support and collaboration between clinical, public health, and research experts. In principle, two options are available, but they are not necessarily mutually exclusive. A preliminary large-scale whole-genome sequencing project funded by either government or private research institutions to investigate feasibility and utility would be the most likely initial source of funding. As the clinical and public health benefits of this approach become well established, governmental funding is likely to follow, perhaps with support from commercial companies, such as those developing antiviral drugs or vaccines against NIRVs.
An example of this funding strategy is the Pandemic Influenza Preparedness Framework,75 which includes an annual contribution from vaccine and diagnostic pharmaceutical companies towards this partnership with WHO. The most direct benefit and savings to the health-care system from a successful vaccine will be fewer admissions to hospital and visits to general practitioners, because fewer people will become infected and develop disease from these NIRVs. Failing this reduction in patient number, effective antivirals will reduce morbidity and mortality for people who require hospital admission because of NIRV infections, or might prevent the need for admission if such drugs are available and prescribed in a timely manner by their general practioner, subsequently reducing absences from school or work. These benefits will strengthen the case for the cost-effectiveness of this investment and support the argument for its longer-term maintenance and sustainability.
In view of an average reagent cost per sample of £200 (roughly US$246) for high-throughput sequencing, with an average of 24 samples per year (ie, two samples per month) from ten global sampling sites, for each of these five groups of NIRVs (respiratory syncytial virus, parainfluenza virus, human metapneumovirus, coronavirus, rhinovirus excluding individual species and subtypes), and excluding additional staff, overheads, and dry-ice sample shipments to the nearest laboratory with high-throughput sequencing capability, over a 5-year surveillance period, the cost of this venture would be in the region of £1·2 million (roughly $1·48 million), but would generate 6000 complete genomes of NIRVs, which would expand the current sequence datasets by three times. With the inclusion of individual viral subtypes and species (eg, parainfluenza virus type 1–4 and coronavirus OC43, 229E, NL63, and HKU1), the cost becomes £2·64 million (roughly $3·25 million). Increasing the number of global sampling sites from ten to 20 would double the cost to £5·28 million (roughly $6·49 million).
At sites where samples are already collected for routine influenza surveillance, the residual volume from these samples could be used relatively easily for the surveillance of NIRVs, where the presence of these viruses has been detected through routine diagnostic testing, as for influenza. Further savings can be achieved by batching these samples for periodic, large-scale, sequencing runs to ensure that all lanes are filled to capacity.
The greater number of viruses and the more sites sampled will enhance the resolution and our understanding of viral diversity. If this surveillance can be maintained (as with influenza) on an annual, global basis, this could capture the most important viral variation that has the potential to affect the clinical effectiveness of any antiviral drug or vaccine developed for these viruses. An analysis starting with a greater number of sites might lead to a subsequent reduction in the number of sites and samples if similar patterns of genetic diversity are seen within samples obtained from neighbouring regions or populations. A minimum number of samples could then be obtained from fewer key sentinel sites (that might not necessarily all match the same sites used for influenza surveillance), which would make for a much more affordable and efficient annual surveillance programme, similar to the present situation for global influenza surveillance.
The actionable outcomes of such a project would include, but are not limited to: a greater understanding of the pattern and spectrum of genetic diversity for each of these viruses in different populations in different parts of the world, which will also identify conserved genomic regions that could then be targets for the development of antiviral drugs and vaccines with long-lasting effectiveness; the identification of viral strains with specific mutations that might link to possible increases in clinical virulence, leading to the development of routine diagnostic assays to detect such strains in patients, allowing clinicians and public health teams to prepare for any potential system effects (eg, increased emergency department visits, and poorer clinical outcomes); and the identification of any unusual patterns of increased mutation rates for any of these viruses in specific populations or regions (ie, mutant hotspot regions for any of these viruses) that might warrant particular attention and perhaps even customised antiviral and vaccine development (eg, combination therapy or multi-epitope vaccine targets) to control the emergence of mutant viruses that could then spread worldwide.
Conclusion
Thus, considering a catch-all approach to the surveillance of NIRVs might be more cost-effective than the single pathogen path, particularly for cases in which residual samples from existing influenza surveillance testing can be used. NIRVs affect all age groups, either directly or indirectly, via the exacerbation of pre-existing comorbidities. The development of specific antiviral drugs and vaccines for these viruses will have a substantial beneficial effect on the health and wellbeing of children and elderly individuals. For this reason, we should strive together, as an increasingly interconnected global community, to make this happen.
For more on clinical trials in the European Union see https://www.clinicaltrialsregister.eu/ctr-search/search
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on June 21, 2017
Contributors
JWT, MPK, and WIL developed the concept and drafted the text. MPK, TTL, and HZ conceptualised and produced the figure and table. SJD and TFH critically reviewed and edit the manuscript. J-MH reviewed the first draft of the manuscript and coined the acronym INSPIRE for our colloaborative network. All authors critically reviewed the final manuscript.
Declaration of interests
We declare no competing interests.
Contributor Information
Julian W Tang, Email: jwtang49@hotmail.com.
INSPIRE investigators:
Ashta Mary Abraham, Amal Baraket, Seweryn Bialasiewicz, Miguela A Caniza, Paul KS Chan, Cheryl Cohen, André Corriveau, Benjamin J Cowling, Steven J Drews, Marcela Echavarria, Ron Fouchier, Pieter LA Fraaij, Todd F Hachette, Jean-Michel Heraud, Hamid Jalal, Lance Jennings, Alice Kabanda, Herve A Kadjo, Mohammed Rafiq Khanani, Evelyn SC Koay, Marion P Koopmans, Mel Krajden, Tommy T Lam, Hong Kai Lee, W. Ian Lipkin, Julius Lutwama, David Marchant, Hidekazu Nishimura, Pagbajabyn Nymadawa, Benjamin A Pinsky, Sanjiv Rughooputh, Joseph Rukelibuga, Taslimarif Saiyed, Anita Shet, Theo Sloots, JJ Muyembe Tamfum, Julian W Tang, Stefano Tempia, Sarah Tozer, Florette Treurnicht, Matti Waris, Aripuana Watanabe, and Emile Okitolonda Wemakoy
Supplementary Material
References
- 1.WHO. The top 10 causes of death, http://www.who.int/mediacentre/factsheets/fs310/en/, (accessed May 6, 2016).
- 2.Matthew J, Pinto Pereira LM, Pappas TE. Distribution and seasonality of rhinovirus and other respiratory viruses in a cross-section of asthmatic children in Trinidad, West Indies. Ital J Pediatr. 2009;35:16. doi: 10.1186/1824-7288-35-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair H, Nokes DJ, Gessner BD. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545-55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcone DN, Durand LO, Azziz-Baumgartner E. Incidence of viral respiratory infections in a prospective cohort of outpatient and hospitalized children aged ≤5 years and its associated cost in Buenos Aires, Argentina. BMC Infect Dis. 2015;15:447. doi: 10.1186/s12879-015-1213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei L, Chan KH, Ip DK. Burden, seasonal pattern and symptomatology of acute respiratory illnesses with different viral aetiologies in children presenting at outpatient clinics in Hong Kong. Clin Microbiol Infect. 2015;21:861-66. doi: 10.1016/j.cmi.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg SB. Rhinovirus and coronavirus infections. Semin Respir Crit Care Med. 2007;28:182-92. doi: 10.1055/s-2007-976490. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Research needs for the Battle against Respiratory Viruses (BRaVe) 2013. http://www.who.int/influenza/patient_care/clinical/BRaVe_Research_Agenda_2013.pdf (accessed March 26, 2016). [Google Scholar]
- 8.Modjarrad K, Giersing B, Kaslow DC, Smith PG, Moorthy VS. WHO consultation on respiratory syncytial virus vaccine development report from a World Health Organization meeting held on 23–24 March 2015. Vaccine. 2016;34:190-97. doi: 10.1016/j.vaccine.2015.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair H, Verma VR, Theodoratou E. An evaluation of the emerging interventions against respiratory syncytial virus (RSV)-associated acute lower respiratory infections in children. BMC Public Health. 2011;11(suppl 3):S30. doi: 10.1186/1471-2458-11-S3-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang JW, Loh TP. Correlations between climate factors and incidence—a contributor to RSV seasonality. Rev Med Virol. 2014;24:15-34. doi: 10.1002/rmv.1771. [DOI] [PubMed] [Google Scholar]
- 11.Mazur NI, Martinón-Torres F, Baraldi E. Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med. 2015;3:888–900. doi: 10.1016/S2213-2600(15)00255-6. [DOI] [PubMed] [Google Scholar]
- 12.Campbell H, Bont L, Nair H. Respiratory syncytial virus (RSV) disease—new data needed to guide future policy. J Glob Health. 2015;5:020101. doi: 10.7189/jogh.05.020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoellein A, Hecker J, Hoffmann D. Serious outbreak of human metapneumovirus in patients with hematologic malignancies. Leuk Lymphoma. 2016;57:623-27. doi: 10.3109/10428194.2015.1067699. [DOI] [PubMed] [Google Scholar]
- 14.Midgley CM, Watson JT, Nix WA. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir Med. 2015;3:879-87. doi: 10.1016/S2213-2600(15)00335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen MY, Lin YE, Su IJ. Using an integrated infection control strategy during outbreak control to minimize nosocomial infection of severe acute respiratory syndrome among healthcare workers. J Hosp Infect. 2006;62:195-99. doi: 10.1016/j.jhin.2005.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldmann DA. Transmission of viral respiratory infections in the home. Pediatr Infect Dis J. 2000;19(suppl 10):S97–S102. doi: 10.1097/00006454-200010001-00002. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Zhao B, Yang X, Li Y. Role of two-way airflow owing to temperature difference in severe acute respiratory syndrome transmission: revisiting the largest nosocomial severe acute respiratory syndrome outbreak in Hong Kong. J R Soc Interface. 2011;8:699–710. doi: 10.1098/rsif.2010.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HK, Oh SH, Yun KA, Sung H, Kim MN. Comparison of Anyplex II RV16 with the xTAG respiratory viral panel and Seeplex RV15 for detection of respiratory viruses. J Clin Microbiol. 2013;51:1137-41. doi: 10.1128/JCM.02958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prachayangprecha S, Schapendonk CM, Koopmans MP. Exploring the potential of next-generation sequencing in detection of respiratory viruses. J Clin Microbiol. 2014;52:3722–3730. doi: 10.1128/JCM.01641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somerville LK, Ratnamohan VM, Dwyer DE, Kok J. Molecular diagnosis of respiratory viruses. Pathology. 2015;47:243–249. doi: 10.1097/PAT.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams JV, Edwards KM, Weinberg GA. Population-based incidence of human metapneumovirus infection among hospitalized children. J Infect Dis. 2010;201:1890-98. doi: 10.1086/652782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristoffersen AW, Nordbø SA, Rognlien AG, Christensen A, Døllner H. Coronavirus causes lower respiratory tract infections less frequently than RSV in hospitalized Norwegian children. Pediatr Infect Dis J. 2011;30:279-83. doi: 10.1097/INF.0b013e3181fcb159. [DOI] [PubMed] [Google Scholar]
- 23.Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets. 2012;12:92-97. doi: 10.2174/187152612800100099. [DOI] [PubMed] [Google Scholar]
- 24.Tempia S, Walaza S, Viboud C. Mortality associated with seasonal and pandemic influenza and respiratory syncytial virus among children <5 years of age in a high HIV prevalence setting—South Africa, 1998–2009. Clin Infect Dis. 2014;58:1241–1249. doi: 10.1093/cid/ciu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz J, Morales-Romero J, Pérez-Gil G. Viral coinfection in acute respiratory infection in Mexican children treated by the emergency service: a cross-sectional study. Ital J Pediatr. 2015;41:33. doi: 10.1186/s13052-015-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mlinaric-Galinovic G, Falsey AR, Walsh EE. Respiratory syncytial virus infection in the elderly. Eur J Clin Microbiol Infect Dis. 1996;15:777-81. doi: 10.1007/BF01701518. [DOI] [PubMed] [Google Scholar]
- 27.Falsey AR, McCann RM, Hall WJ. The “common cold” in frail older persons: impact of rhinovirus and coronavirus in a senior daycare center. J Am Geriatr Soc. 1997;45:706-11. doi: 10.1111/j.1532-5415.1997.tb01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection-associated hospitalizations among older adults. J Infect Dis. 2002;185:1338-41. doi: 10.1086/339881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179:25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 30.Gorse GJ, O'Connor TZ, Hall SL, Vitale JN, Nichol KL. Human coronavirus and acute respiratory illness in older adults with chronic obstructive pulmonary disease. J Infect Dis. 2009;199:847-57. doi: 10.1086/597122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wendt CH, Hertz MI. Respiratory syncytial virus and parainfluenza virus infections in the immunocompromised host. Semin Respir Infect. 1995;10:224-31. [PubMed] [Google Scholar]
- 32.Liu V, Dhillon GS, Weill D. A multi-drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart-lung transplant recipients. Transpl Infect Dis. 2010;12:38-44. doi: 10.1111/j.1399-3062.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- 33.Godet C, Le Goff J, Beby-Defaux A. Human metapneumovirus pneumonia in patients with hematological malignancies. J Clin Virol. 2014;61:593-96. doi: 10.1016/j.jcv.2014.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MS, Walker RE, Mendelman PM. Medical burden of respiratory syncytial virus and parainfluenza virus type 3 infection among US children. Implications for design of vaccine trials. Hum Vaccin. 2005;1:6–11. doi: 10.4161/hv.1.1.1424. [DOI] [PubMed] [Google Scholar]
- 35.Falsey AR. Human metapneumovirus infection in adults. Pediatr Infect Dis J. 2008;27(suppl 10):S80–S83. doi: 10.1097/INF.0b013e3181684dac. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez JA. RSV infection in the adult population. Manag Care. 2008;17(suppl 12):13–15. [PubMed] [Google Scholar]
- 37.Villaran MV, Garcí J, Gomez J. Human parainfluenza virus in patients with influenza-like illness from Central and South America during 2006–2010. Influenza Other Respir Viruses. 2014;8:217-27. doi: 10.1111/irv.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Ji W, Chen Z, Yan YD, Shao X, Xu J. Comparison of severe pneumonia caused by human metapneumovirus and respiratory syncytial virus in hospitalized children. Indian J Pathol Microbiol. 2014;57:413-17. doi: 10.4103/0377-4929.138735. [DOI] [PubMed] [Google Scholar]
- 39.Widmer K, Griffin MR, Zhu Y, Williams JV, Talbot HK. Respiratory syncytial virus- and human metapneumovirus-associated emergency department and hospital burden in adults. Influenza Other Respir Viruses. 2014;8:347–352. doi: 10.1111/irv.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefanska I, Romanowska M, Donevski S, Gawryluk D, Brydak LB. Co-infections with influenza and other respiratory viruses. Adv Exp Med Biol. 2013;756:291-301. doi: 10.1007/978-94-007-4549-0_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kouni S, Karakitsos P, Chranioti A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin Microbiol Infect. 2013;19:772–777. doi: 10.1111/1469-0691.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goka E, Vallely P, Mutton K. Influenza A viruses dual and multiple infections with other respiratory viruses and risk of hospitalisation and mortality. Influenza Other Respir Viruses. 2013;7:1079-87. doi: 10.1111/irv.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asner SA, Science ME, Tran D. Clinical disease severity of respiratory viral co-infection versus single viral infection: a systematic review and meta-analysis. PLoS One. 2014;9:e99392. doi: 10.1371/journal.pone.0099392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goka EA, Vallely PJ, Mutton KJ. Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol Infect. 2015;143:37–47. doi: 10.1017/S0950268814000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asner SA, Rose W, Petrich A. Is virus coinfection a predictor of severity in children with viral respiratory infections? Clin Microbiol Infect. 2015;21:264.e1–264.e6. doi: 10.1016/j.cmi.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rakes GP, Arruda E, Ingram JM. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 47.Korppi M, Kotaniemi-Syrjänen A, Waris M, Vainionpää R, Reijonen TM. Rhinovirus-associated wheezing in infancy: comparison with respiratory syncytial virus bronchiolitis. Pediatr Infect Dis J. 2004;23:995-99. doi: 10.1097/01.inf.0000143642.72480.53. [DOI] [PubMed] [Google Scholar]
- 48.Jartti T, Lehtinen P, Vanto T. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25:482-88. doi: 10.1097/01.inf.0000215226.69696.0c. [DOI] [PubMed] [Google Scholar]
- 49.Emuzyte R, Firantiene R, Petraityte R, Sasnauskas K. Human rhinoviruses, allergy, and asthma: a clinical approach. Medicina. 2009;45:839-47. [PubMed] [Google Scholar]
- 50.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? J Allergy Clin Immunol. 2010;125:1202–1205. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Jartti T, Korppi M. Rhinovirus-induced bronchiolitis and asthma development. Pediatr Allergy Immunol. 2011;22:350-55. doi: 10.1111/j.1399-3038.2011.01170.x. [DOI] [PubMed] [Google Scholar]
- 52.Ruotsalainen M, Hyvärinen MK, Piippo-Savolainen E, Korppi M. Adolescent asthma after rhinovirus and respiratory syncytial virus bronchiolitis. Pediatr Pulmonol. 2013;48:633-39. doi: 10.1002/ppul.22692. [DOI] [PubMed] [Google Scholar]
- 53.Agrawal AS, Roy T, Ghosh S, Chawla-Sarkar M. Genetic variability of attachment (G) and fusion (F) protein genes of human metapneumovirus strains circulating during 2006–2009 in Kolkata, eastern India. Virol J. 2011;8:67. doi: 10.1186/1743-422X-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papenburg J, Carbonneau J, Isabel S. Genetic diversity and molecular evolution of the major human metapneumovirus surface glycoproteins over a decade. J Clin Virol. 2013;58:541–547. doi: 10.1016/j.jcv.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein DI, Malkin E, Abughali N. Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr Infect Dis J. 2012;31:109-14. doi: 10.1097/INF.0b013e31823386f1. [DOI] [PubMed] [Google Scholar]
- 56.Englund JA, Karron RA, Cunningham CK. Safety and infectivity of two doses of live-attenuated recombinant cold-passaged human parainfluenza type 3 virus vaccine rHPIV3cp45 in HPIV3-seronegative young children. Vaccine. 2013;31:5706-12. doi: 10.1016/j.vaccine.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nelson CL, Tang RS, Stillman EA. Genetic stability of RSV-F expression and the restricted growth phenotype of a live attenuated PIV3 vectored RSV vaccine candidate (MEDI-534) following restrictive growth in human lung cells. Vaccine. 2013;31:3756-62. doi: 10.1016/j.vaccine.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 58.Li C, Zhou X, Zhong Y. A recombinant G protein plus cyclosporine A-based respiratory syncytial virus vaccine elicits humoral and regulatory T cell responses against infection without vaccine-enhanced disease. J Immunol. 2016;196:1721-31. doi: 10.4049/jimmunol.1502103. [DOI] [PubMed] [Google Scholar]
- 59.Neuzil KM. Progress toward a respiratory syncytial virus vaccine. Clin Vaccine Immunol. 2016;23:186-88. doi: 10.1128/CVI.00037-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campanini G, Piralla A, Rovida F. First case in Italy of acquired resistance to oseltamivir in an immunocompromised patient with influenza A/H1N1v infection. J Clin Virol. 2010;48:220-22. doi: 10.1016/j.jcv.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 61.Ujike M, Ejima M, Anraku A. Monitoring and characterization of oseltamivir-resistant pandemic (H1N1) 2009 virus, Japan, 2009–2010. Emerg Infect Dis. 2011;17:470-79. doi: 10.3201/eid1703.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suhaila M, Tang JW, Lee HK. Mixtures of oseltamivir-sensitive and -resistant pandemic influenza A/H1N1/2009 viruses in immunocompromised hospitalized children. Pediatr Infect Dis J. 2011;30:625-27. doi: 10.1097/INF.0b013e31820929ab. [DOI] [PubMed] [Google Scholar]
- 63.Hurt AC, Chotpitayasunondh T, Cox NJ. WHO consultation on pandemic influenza A (H1N1) 2009 virus resistance to antivirals. Antiviral resistance during the 2009 influenza A H1N1 pandemic: public health, laboratory, and clinical perspectives. Lancet Infect Dis. 2012;12:240–248. doi: 10.1016/S1473-3099(11)70318-8. [DOI] [PubMed] [Google Scholar]
- 64.Adams O, Bonzel L, Kovacevic A, Mayatepek E, Hoehn T, Vogel M. Palivizumab-resistant human respiratory syncytial virus infection in infancy. Clin Infect Dis. 2010;51:185-88. doi: 10.1086/653534. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Q, McAuliffe JM, Patel NK. Analysis of respiratory syncytial virus preclinical and clinical variants resistant to neutralization by monoclonal antibodies palivizumab and/or motavizumab. J Infect Dis. 2011;203:674-82. doi: 10.1093/infdis/jiq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia Q, Zhou L, Peng C. Detection of respiratory syncytial virus fusion protein variants between 2009 and 2012 in China. Arch Virol. 2014;159:1089–1098. doi: 10.1007/s00705-013-1870-9. [DOI] [PubMed] [Google Scholar]
- 67.Bates JT, Keefer CJ, Slaughter JC, Kulp DW, Schief WR, Crowe JE., Jr. Escape from neutralization by the respiratory syncytial virus-specific neutralizing monoclonal antibody palivizumab is driven by changes in on-rate of binding to the fusion protein. Virology. 2014;454–55:139–144. doi: 10.1016/j.virol.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oliveira DB, Iwane MK, Prill MM. Molecular characterization of respiratory syncytial viruses infecting children reported to have received palivizumab immunoprophylaxis. J Clin Virol. 2015;65:26–31. doi: 10.1016/j.jcv.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corti D, Bianchi S, Vanzetta F. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501:439-43. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- 70.Kistler AL, Webster DR, Rouskin S. Genome-wide diversity and selective pressure in the human rhinovirus. Virol J. 2007;4:40. doi: 10.1186/1743-422X-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis-Rogers N, Bendall ML, Crandall KA. Phylogenetic relationships and molecular adaptation dynamics of human rhinoviruses. Mol Biol Evol. 2009;26:969-81. doi: 10.1093/molbev/msp009. [DOI] [PubMed] [Google Scholar]
- 72.Waman VP, Kolekar PS, Kale MM, Kulkarni-Kale U. Population structure and evolution of rhinoviruses. PLoS One. 2014;9:e88981. doi: 10.1371/journal.pone.0088981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zumla A, Chan JF, Azhar EI, Hui DS, Yuen KY. Coronaviruses-drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327-47. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bosch BJ, Rossen JW, Bartelink W. Coronavirus escape from heptad repeat 2 (HR2)-derived peptide entry inhibition as a result of mutations in the HR1 domain of the spike fusion protein. J Virol. 2008;82:2580-85. doi: 10.1128/JVI.02287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.WHO. Pandemic influenza preparedness framework for the sharing of influenza viruses and access to vaccines and other benefits. 2011. http://www.who.int/influenza/resources/pip_framework/en/ (accessed March 31, 2017). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.