Abstract
Background
Transmission of infection through international travel is a growing health issue, and the frequency of imported infection is increasing in China. We aimed to quantify the total number of infections imported into mainland China by arriving travellers.
Methods
We actively surveyed arriving travellers at all 272 international entry–exit ports in mainland China. Suspected cases were detected through fever screening, medical inspection, self-declaration, and reporting by on-board staff. Participants completed a standardised questionnaire with questions about demographics, their travel itinerary (including detailed information about all countries or regions visited), and clinical manifestations. Nasopharyngeal swabs, sputum samples, faecal samples, vomitus, blood, and serum were collected as appropriate for diagnoses. Diagnosis was made by specific laboratory tests according to the national technical guidelines. Infections were classified as respiratory, gastrointestinal, vector-borne, blood-transmitted and sex-transmitted, or mucocutaneous. We divided arriving travellers into two groups: travellers coming from countries other than China, and travellers coming from Hong Kong, Macau, and Taiwan. We integrated surveillance data for 2014–16, calculated incidences of travel-related infections, and compared the frequency of infections among subgroups.
Findings
Between Jan 1, 2014, and Dec 31, 2016, 22 797 cases were identified among 805 993 392 arriving travellers—an overall incidence of 28·3 per million. 45 pathogens were detected in participants: 18 respiratory (19 662 cases), ten gastrointestinal (189 cases), seven vector-borne (831 cases), seven blood-transmitted and sex-transmitted (1531 cases), and three mucocutaneous (584 cases). Both the overall number and incidence of infection were more than five times higher in 2016 than in 2014. Case numbers and incidences also varied substantially by province, autonomous region, and municipality. Overall, 17 643 (77%) infections were detected by fever screening, but 753 (49%) blood-transmitted and sex-transmitted infections were identified through medical inspection. 14 305 (73%) cases of respiratory infection and 96 (51%) of gastrointestinal infections were in tourists. Tuberculosis, hepatitis A virus, vector-borne, and blood-transmitted and sex-transmitted infections were common among Chinese labourers who worked abroad. Dengue and malaria were most commonly diagnosed in travellers arriving from Africa. 12 126 (93%) of the 12 985 cases arriving from Hong Kong, Macau, or Taiwan were respiratory infections. Hand, foot, and mouth disease accounted for 2·90% of infections in travellers from Hong Kong, Macau, or Taiwan and 0·31% of infections in international travellers.
Interpretation
This report is the first to characterise the profile of travel-related infections among arriving travellers in mainland China. Our findings should increase public awareness of the potential risk of imported infections, and help health-care providers to make evidence-based health recommendations to travellers.
Funding
The Natural Science Foundation of China.
Introduction
The number of international travellers has more than doubled worldwide during the past two decades, from 527 million in 1995 to 1·186 billion in 2015.1 This rapid increase in cross-border travelling has become the main driver of the global spread of infections, as exemplified by international transmission of severe acute respiratory syndrome, dengue, 2009 influenza A (H1N1), and Zika virus.2, 3, 4, 5 Timely identification of infections among arriving travellers can help to alert the medical and public health communities of outbreak threats before they affect the general population of that country. Surveillance of travel-related infections is important for global public health as international travel continues to increase worldwide.6, 7
China has become the world's fourth most popular destination in terms of arrivals, with 57 million tourists in 2015,1 and has inevitably been affected by travel-related infections. Malaria and dengue have been introduced by arriving travellers from countries where those diseases are endemic.8, 9, 10 An outbreak of Chikungunya virus caused by infected travellers has been recorded in mainland China,11 and Zika virus, yellow fever, and Rift Valley fever have all been imported within the past 8 years.12, 13, 14, 15
Research in context.
Evidence before this study
We searched PubMed and ISI Web of Science with the terms “travel” and “infection” or “infectious disease”, and “global spread” and “infection” or “infectious disease” for work published in any language between Jan 1, 2000, and Oct 31, 2017. We noted that previous studies mainly focused on surveillance of post-travel illness, which was usually based on clinician-based surveillance systems—eg, GeoSentinel, which tracks infectious diseases and other adverse health outcomes related to travel. Several identified studies were about the epidemiological features of imported malaria or about febrile illnesses or HIV infection at one port of China. We did not find any studies of active surveillance for various infections among arriving travellers at all entry–exit ports throughout mainland China.
Added value of this study
To the best of our knowledge, our report is the first to characterise the spectrum of travel-related infections, and reveals variety in frequency of each infection by traveller type, exposure country or region, and arrival provinces of mainland China in 2014–16, representing the pattern of travel-associated infections during travel.
Implications of all the available evidence
Our findings imply that health-care providers should make evidence-based health recommendations to travellers before travel, and do destination-specific medical assessments of arriving travellers when they are ill, based on our estimates of the incidence of infections. Active surveillance at entry ports can identify imported cases with emerging or re-emerging infections to prevent or at least postpone local transmission. In addition to entry surveillance upon arrival, follow-up surveillance (especially contact tracing of highly communicable infections) is needed to better understand the whole profile of travel-related infections. Overall, our findings should help to increase public health awareness about the potential risk of imported infections to mainland China.
In response to the increasing risk of imported infections, the General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China (AQSIQ) obliged all air and surface ports to do active surveillance of infections among arriving travellers. Systematic surveillance began in 2014 to reduce risk of autochthonous transmission and help to inform evidence-based advice for travellers. We integrated the data from all entry–exit ports in mainland China to characterise travel-related infections, define the demographic features of imported cases, identify risk groups and exposure countries or regions, and assess the effectiveness of surveillance for further improvement.
Methods
Study design
We did an active surveillance study of all travel-related infections from Jan 1, 2014, to Dec 31, 2016, at all 272 international entry–exit ports to mainland China, comprising 69 airports, 128 water (sea and river) ports, and 75 land (highway and railway) entry–exit stations (appendix p 2). We also gathered data for the total number of people arriving in each of the 22 provinces, five autonomous regions, and four municipalities of mainland China. All patient data were anonymised. Because this study constituted public health surveillance rather than research in human beings, ethical approval from institutional review boards was not required. Participants or their guardians provided written informed consent for collection of biological samples (appendix p 4). People who were suspected of having an infection but who did not consent to collection of samples were made known to local centres for disease control and prevention for potential further follow-up.
Data collection and diagnosis
Active surveillance was done among all arriving travellers at a quarantine station before they passed through customs at each entry–exit port. Suspected cases were detected through four approaches: fever screening, medical inspection, self-declaration, and reporting by on-board staff (appendix p 3). All people with suspected infections were quarantined according to WHO's International Health Regulations and the Rules for the Implementation of Frontier Health and Quarantine Law of the People's Republic of China. Participants completed a standardised questionnaire with questions about demographics, their travel itinerary (including detailed information about all countries or regions visited), and clinical manifestations.
Infections were diagnosed by laboratory testing at the international travel health-care centre of each provincial entry–exit Inspection or the Quarantine Bureau, per the surveillance technical scheme developed by AQSIQ.16 Quarantine officers at entry–exit ports suggested which tests should be done on the basis of clinical manifestations in each suspected patient. They collected nasopharyngeal swabs, sputum samples, faecal samples, vomitus, blood, or serum samples as appropriate for diagnoses. According to core capacity requirements for surveillance and response in WHO's International Health Regulations and the Frontier Health and Quarantine Law of China, AQSIQ listed 28 required and 40 recommended infectious diseases for causative testing.17, 18 Diagnosed infections were classified as respiratory, gastrointestinal, vector-borne, blood-transmitted and sex-transmitted, or mucocutaneous according to standard clinical practice in China (appendix pp 5–6). Patients with infections were informed of their diagnosis, and recommended for treatment. We also informed local centres for disease control and prevention of each case, and close contacts were informed for prevention and quarantine, if necessary, according to WHO's International Health Regulations and the Frontier Health and Quarantine Law of China.
We excluded people without specific diagnoses, people with ambiguous itineraries, and those whose final diagnosis had been identified before travelling. We used EPIDATA 3.0 to establish a structured database. Each case was geo-referenced to a world map with ArcGIS (ESRI, Redlands, CA, USA) according to the exposure location where patients might have been infected. The exposure location was defined as the country or region that the participant travelled from. For travellers who visited several destinations, exposure location was established according to their itinerary on the basis of incubation period or known patterns of endemicity. Arriving travellers were classified into two groups: travellers coming from countries other than China, and travellers coming from Hong Kong, Macau, and Taiwan. The latter group included citizens of mainland China who visited Hong Kong, Macau, or Taiwan and returned to China, and those who resided in Hong Kong, Macau, or Taiwan and arrived on mainland China.
Statistical analysis
Descriptive statistics were calculated for all variables. Continuous variables were summarised as median and range. We estimated the annual incidence of each infection at national and provincial levels. Proportions were calculated according to various categories. We constructed graphs to show distribution patterns of proportion among different subgroups, and created thematic maps according to entry province and exposure countries.
Role of the funding source
The study funder had no role in study design; data collection, analysis, or interpretation; or writing of the report. W-CC and L-QF had full access to all data in the study, and had final responsibility for the decision to submit for publication.
Results
Of 805 993 392 arriving travellers at the 272 entry–exit ports of mainland China, 24 819 had suspected infections and underwent diagnosis. A specific diagnosis could not be made in 1949 people, and in a further 79 people, the diagnosed infection had been identified before travel. Thus, 22 797 cases were included in our study, corresponding to an overall incidence of 28·3 per million for 2014–16. Most cases had clinical manifestations of disease upon arrival (appendix pp 9–10). The median age was 30 years (IQR 10–45; range 1–94), and 15 541 (68%) were male (table ). 16 779 (73·6%) cases were citizens of mainland China, 2035 (9%) were citizens of Hong Kong, Macau, or Taiwan, and 3983 (17%) were citizens of other countries (table). The most frequent reason for travel was tourism (15 346 [67%]; table). Land entry–exit ports were the most common site of infection diagnosis, accounting for 10 218 (45%) cases (table). A roughly four-times increase in both the number and incidence of infections was noted between 2014 and 2015 (table). Between 2014 and 2016, an almost six-times increase in the number of infections was noted, whereas a five-times increase in incidence of infections was recorded (table).
Table.
Characteristics of travel-related infections in mainland China, 2014–16
| Overall (n=22 797) | Respiratory (n=19 662) | Gastrointestinal (n=189) | Vector-borne (n=831) | Blood-transmitted and sex-transmitted (n=1531) | Mucocutaneous (n=584) | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 15 541 (68%) | 13 128 (67%) | 116 (61%) | 660 (79%) | 1198 (78%) | 439 (75%) |
| Female | 7256 (32%) | 6534 (33%) | 73 (39%) | 171 (21%) | 333 (22%) | 145 (25%) |
| Age, years | ||||||
| Median (IQR) | 30 (10–45) | 30 (9–45) | 33 (24–46) | 34 (25–44) | 39 (31–48) | 5 (4–26) |
| ≤9 | 5397 (24%) | 4948 (25%) | 20 (11%) | 25 (3%) | 2 (<1%) | 402 (69%) |
| 10–19 | 1501 (7%) | 1392 (7%) | 8 (4%) | 68 (8%) | 30 (2%) | 3 (1%) |
| 20–39 | 8104 (36%) | 6759 (34%) | 87 (46%) | 436 (52%) | 730 (48%) | 92 (16%) |
| 40–59 | 5862 (26%) | 4850 (25%) | 65 (34%) | 281 (34%) | 599 (39%) | 67 (11%) |
| ≥60 | 1767 (8%) | 1627 (8%) | 9 (5%) | 14 (2%) | 97 (6%) | 20 (3%) |
| Missing | 166 (1%) | 86 (<1%) | 0 (0%) | 7 (1%) | 73 (5%) | 0 (0%) |
| Citizenship | ||||||
| Mainland China | 16 779 (74%) | 15 039 (76%) | 122 (65%) | 528 (64%) | 628 (41%) | 462 (79%) |
| Other countries | 3983 (17%) | 2714 (14%) | 60 (32%) | 301 (36%) | 838 (55%) | 70 (12%) |
| Hong Kong, Macau, or Taiwan regions | 2035 (9%) | 1909 (10%) | 7 (4%) | 2 (<1%) | 65 (4%) | 52 (9%) |
| Case-finding approach | ||||||
| Fever screening | 17 643 (77%) | 16 237 (83%) | 76 (40%) | 553 (67%) | 397 (26%) | 380 (65%) |
| Medical inspection | 3967 (17%) | 2862 (15%) | 34 (18%) | 155 (19%) | 753 (49%) | 163 (28%) |
| Self-declaration | 690 (3%) | 248 (1%) | 46 (24%) | 89 (11%) | 293 (19%) | 14 (2%) |
| Reported by on-board staff | 497 (2%) | 315 (2%) | 33 (17%) | 34 (4%) | 88 (6%) | 27 (5%) |
| Reason for travel | ||||||
| Tourism | 15 346 (67%) | 14 305 (73%) | 96 (51%) | 228 (27%) | 279 (18%) | 438 (75%) |
| Labour | 3214 (14%) | 1974 (10%) | 56 (30%) | 440 (53%) | 735 (48%) | 9 (2%) |
| Sailor | 2346 (10%) | 1793 (9%) | 8 (4%) | 24 (3%) | 412 (27%) | 109 (19%) |
| Business | 1144 (5%) | 971 (5%) | 20 (11%) | 70 (8%) | 74 (5%) | 9 (2%) |
| Visiting friends or relatives | 428 (2%) | 348 (2%) | 4 (2%) | 53 (6%) | 7 (<1%) | 16 (3%) |
| Student | 319 (1%) | 271 (1%) | 5 (3%) | 16 (2%) | 24 (2%) | 3 (1%) |
| Entry–exit port | ||||||
| Air | 6606 (29%) | 5680 (29%) | 91 (48%) | 535 (64%) | 265 (17%) | 35 (6%) |
| Water (sea and river) | 5973 (26%) | 5254 (27%) | 19 (10%) | 56 (7%) | 471 (31%) | 173 (30%) |
| Land (highway and railway) | 10 218 (45%) | 8728 (44%) | 79 (42%) | 240 (29%) | 795 (52%) | 376 (64%) |
| Number of infections (incidence*) | ||||||
| 2014 | 2068 (8·2) | 1706 (6·7) | 22 (0·1) | 139 (0·5) | 163 (0·6) | 38 (0·2) |
| 2015 | 8977 (33·1) | 7771 (28·6) | 52 (0·2) | 417 (1·5) | 478 (1·8) | 259 (1·0) |
| 2016 | 11 752 (41·7) | 10 185 (36·2) | 115 (0·4) | 275 (1·0) | 890 (3·2) | 287 (1·0) |
Data are n (%), unless otherwise specified.
Incidence is the number of infections per million arriving travellers per year.
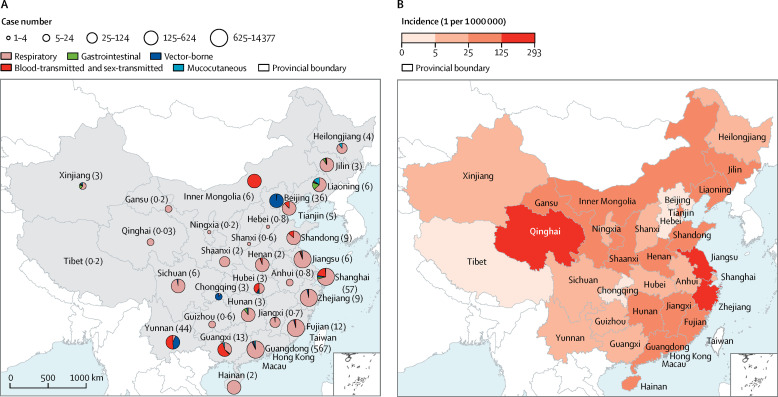
The number of cases imported varied greatly by province and region. Most infected people presented in coastal provinces of eastern and southeastern China, including Shanghai municipality, Shandong, Jiangsu, Zhejiang, Fujian, and Guangdong provinces. Large numbers of cases also presented in Yunnan in southern China, which has a long inland border with other countries (figure 1A ). Incidence was highest in Qinghai, Jiangsu, and Zhejiang provinces (figure 1B). The five types of infections were diagnosed in substantially different proportions in different provinces. Nearly all people diagnosed in the Inner Mongolia autonomous region and slightly more than half those diagnosed in Yunnan province and Guangxi autonomous region had blood-transmitted and sex-transmitted infections (figure 1A). 142 (98%) of the 145 cases imported to Beijing were vector-borne infections.
Figure 1.
Case number and type (A), and incidence (B), of travel-related infections by province in mainland China, 2014–16
In (A), the total number of travellers (in millions) who arrived in each province, autonomous region, and municipality is shown in parentheses.
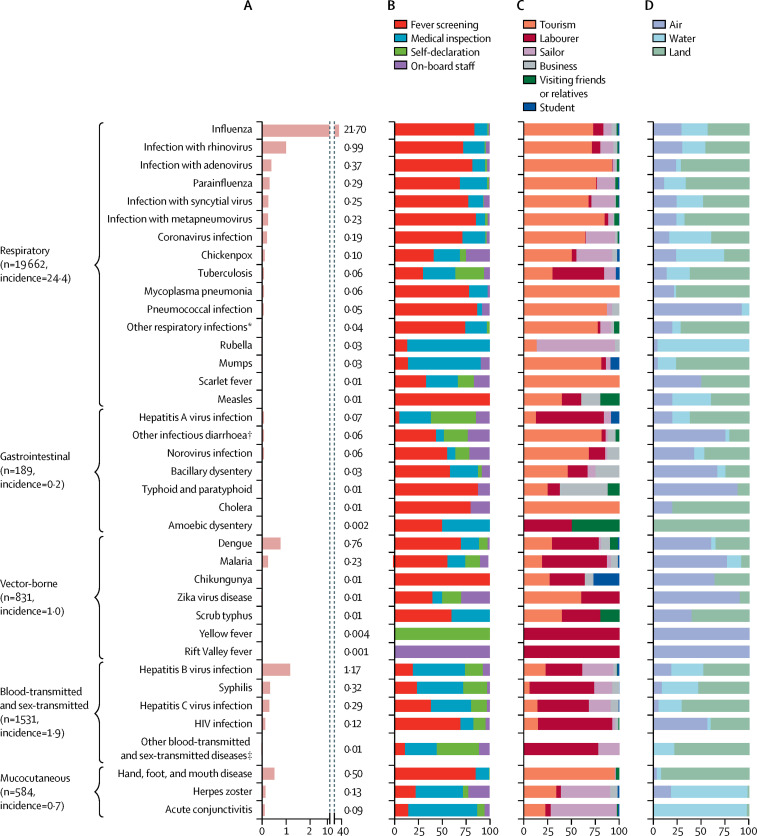
45 types of pathogens were detected in participants: 18 respiratory pathogens (19 662 cases), ten gastrointestinal pathogens (189 cases), seven vector-borne pathogens (831 cases), seven blood-transmitted and sex-transmitted pathogens (1531 cases), and three mucocutaneous pathogens (584 cases; figure 2A ). The most frequent respiratory infection was influenza virus, followed by rhinovirus (figure 2A). Dengue was the most common vector-borne disease, hepatitis B virus infection was the most common blood-transmitted and sex-transmitted infection, and hand, foot, and mouth disease was the most common mucocutaneous infection (figure 2A).
Figure 2.
Incidence of (A), and case-finding approach (B), reason for travel (C), and type of entry–exit port (D) for, travel-related infections in travellers arriving to mainland China, by infection type
Incidence is per 1 million travellers. *Including Chlamydia pneumoniae, Legionella pneumophila, and Bocavirus. †Caused by rotavirus, Escherichia coli O157:H7, Vibrio parahemolyticus, or Staphylococcus aureus. ‡Including gonorrhoea, papillomavirus infection, and genital chlamydial infection.
16 237 (83%) respiratory infections, 76 (40%) gastrointestinal infections, 553 (67%) vector-borne infections, and 346 (85%) of the 406 cases of hand, foot, and mouth disease were detected by fever screening (figure 2B). Rubella, mumps, hepatitis B virus infection, herpes zoster virus infection, and acute conjunctivitis were mainly detected by medical inspection (figure 2B). Quite high proportions of tuberculosis and hepatitis A cases, and all cases of yellow fever, were self-declared (figure 2B), whereas about quarter of chickenpox and norovirus infections (and the only case of Rift Valley fever) were reported by on-board staff (figure 2B).
14 305 (73%) respiratory cases, 96 (51%) gastrointestinal infections, and 386 (95%) of the 406 cases of hand, foot, and mouth disease were detected among tourists (figure 2C). Most cases of tuberculosis, hepatitis A and C virus infections, malaria, syphilis, HIV, all three cases of yellow fever, and the only case of Rift Valley fever were in labourers who worked abroad and returned to mainland China (figure 2C). Most cases of herpes zoster virus infection, acute conjunctivitis, and rubella occurred among sailors (figure 2C). Cases of pneumococcal infection; acute diarrhoea including bacillary dysentery, typhoid, and paratyphoid; and vector-borne infections were detected mainly at airports, whereas most cases of chickenpox, rubella, herpes zoster virus infection, and acute conjunctivitis were identified at water ports (figure 2D).
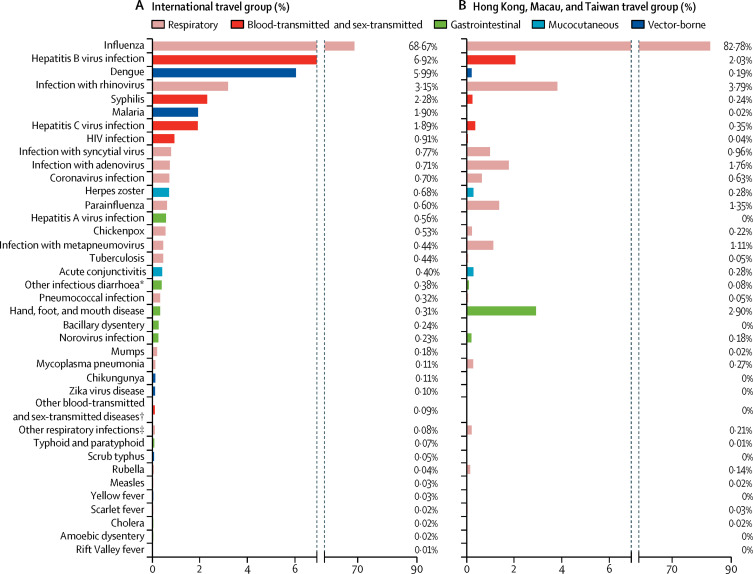
9812 (43%) of participants arrived from a different country (140 countries represented), whereas 12 985 (57%) came from Hong Kong, Macau, or Taiwan. The types and distribution of infection differed substantially between these two groups (figure 3 ). 12 126 (93%) of the 12 985 cases arriving from Hong Kong, Macau, or Taiwan were respiratory infections. Influenza was the most common infection in both groups, accounting for 6738 (69%) cases in the international group and 10 749 (83%) cases in the Hong Kong, Macau, and Taiwan group (figure 3). However, hepatitis B and C virus infections, syphilis, HIV, malaria, and dengue were substantially more common in the international group, whereas respiratory infections with rhinovirus, parainfluenza virus and metapneumovirus, and hand, foot, and mouth disease were more common in travellers from Hong Kong, Macau, and Taiwan (figure 3).
Figure 3.
Proportions of each infection among international travellers (A), and travellers arriving from Hong Kong, Macau, or Taiwan (B)
*Caused by rotavirus, Escherichia coli O157:H7, Vibrio parahemolyticus, or Staphylococcus aureus. †Including gonorrhoea, papillomavirus infection, and genital chlamydial infection. ‡Including Chlamydia pneumoniae, Legionella pneumophila, and Bocavirus.
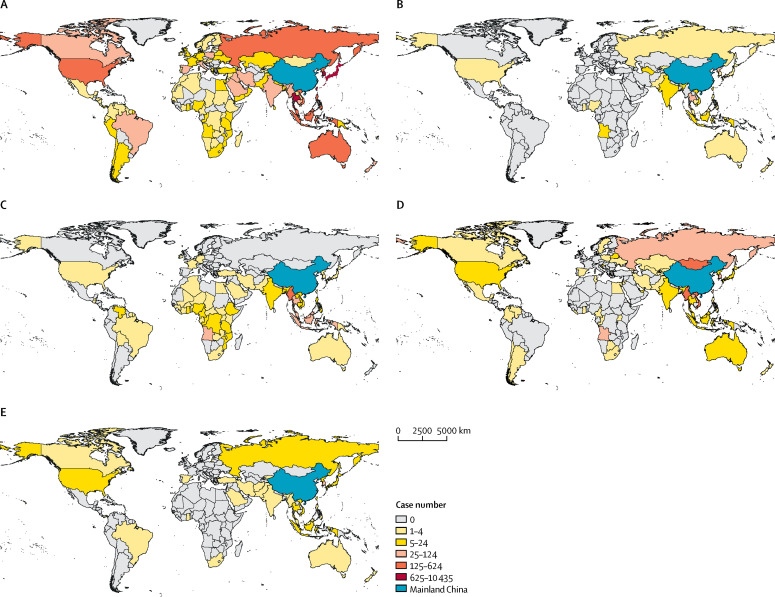
In the international travel group, infection was acquired in the Western Pacific region by 5153 (53%) people, in the South-East Asia region by 2666 (27%), in the European region by 838 (9%), in the American region by 432 (4%), in the African region by 448 (5%), and in the Eastern Mediterranean region by 275 (3%). Influenza accounted for more than two-thirds of cases among travellers who acquired infections in the Western Pacific, South-East Asia, European, American, and Eastern Mediterranean regions (appendix p 7). People with gastrointestinal infections mainly travelled from countries in the South-East Asia and Western Pacific regions (figure 4B ). Vector-borne diseases, including dengue and malaria, were pre-dominantly diagnosed among travellers from countries in western and central Africa and the South-East Asia region (figure 4C). Cases with mucocutaneous infections mostly come from countries in the Western Pacific region and eastern Europe (figure 4).
Figure 4.
Geographical distribution of respiratory (A), gastrointestinal (B), vector-borne (C), blood-transmitted and sex-transmitted (D), and mucocutaneous (E) infections
We noted seasonal patterns of travel-related infections, which differed between the international travellers (infection peak in November and December) and those who travelled from Hong Kong, Macau, or Taiwan (respiratory infections peak in February and June, mucocutaneous infection peak in May and July; appendix p 8).
Discussion
To our knowledge, ours is the first multicentre study of travel-related infections among travellers arriving in mainland China. We characterised the range of travel-related infections, and showed how they varied by traveller type, exposure country or region, and province of arrival to mainland China. Our findings, which are based on surveillance data for 45 infections among 22 797 travellers who arrived at the 272 entry–exit ports to the mainland, are helpful for increasing public health awareness about the potential risk of imported infections.
Most previous studies about this topic in mainland China explored the epidemiological features of imported malaria, or febrile illnesses or HIV infection at a single entry–exit port.8, 9, 19, 20, 21, 22 By comparison with GeoSentinel, a clinician-based global surveillance system that tracks infectious diseases and other adverse health outcomes related to travel,23 we actively surveyed infections among travellers upon arrival in mainland China. This method enabled us not only to profile imported infections, identify risk groups, and formulate optimum advice for travellers (as done previously on the basis of data from GeoSentinel or its linked European surveillance network, EuroTravNet),24, 25, 26 but also to estimate the incidence of each infection by using the total number of arrivals as denominator. Additionally, active surveillance of arriving travellers enables timely identification of imported infections, allowing for alerting of public health authorities of threats before autochthonous transmission.
Both case numbers and incidence of infection have increased over time among travellers arriving in mainland China (table). This increase probably reflects the worldwide epidemic trend of infectious diseases.27 Improvements in diagnostic procedures and rapid tests might have also contributed to the increase. Case numbers and incidence varied greatly between the 31 provinces, autonomous regions, and municipalities (figure 1), which implies that each province should optimise a unique strategy for surveillance of, and response to, specific infections.
The diagnostics used in our study were based on the surveillance technical scheme developed by AQSIQ,16 whereby diagnosis was based on recommended specific laboratory tests. Accordingly, we classified diagnosis according to the main transmission route of each infection: respiratory, gastrointestinal, vector-borne, blood-transmitted and sex-transmitted, and mucocutaneous. Over four-fifths of respiratory infections and about two-thirds of vector-borne and mucocutaneous infections were detected by fever screening (table), suggesting that fever screening is essential to identification of febrile patients. Our findings also suggest that medical inspection should be sustained to identify people with mucocutaneous infections other than hand, foot, and mouth disease or blood-transmitted and sex-transmitted infections, which are often associated with obvious clinical manifestations such as rashes, vomiting, and jaundice (appendix pp 9–10). Additionally, on-board staff should be encouraged to report patients they suspect of being infected for timely identification.
Chinese labourers (ie, Chinese people who work overseas, mostly in the manufacturing, construction, forestry, fishing, transportation, and catering industries, although some are highly skilled workers) abroad seemed prone to tuberculosis, hepatitis A virus infection, vector-borne infections, and blood-transmitted and sex-transmitted infections (figure 2C). Pre-travel advice to labourers should focus on such public health threats. Additionally, we suggest that on-site health education and primary health care should be provided to Chinese labourers in the countries where they work. Rubella, acute conjunctivitis, and herpes zoster virus infections were frequently detected in sailors (figure 2C), suggesting that close contact and working in humid environments are risk factors for transmission of these infections.
Although the infection profiles of international travellers and travellers from Hong Kong, Macau, and Taiwan differed substantially, influenza was the most common infection in both groups (figure 3). Our findings show the epidemic pattern of influenza in different global regions,28 and imply that vaccination should be considered if travelling to a region where influenza transmission is ongoing.29 People with blood-transmitted and sex-transmitted diseases were mostly male labourers and sailors (table). Although the reasons for high risk in these populations were unclear, our study shows the sociodemographic characteristics of people with these diseases arriving in mainland China, which were significantly associated with travel-associated sexually transmitted infections in data gathered by GeoSentinel travel medicine clinics worldwide.30 Further research is required to investigate effective intervention measures.
Dengue and malaria were frequent among international travellers arriving from Africa (appendix p 7). Imported dengue has caused autochthonic transmission in new areas of China, Europe, and the USA.31, 32, 33, 34 In our study, we also noted the arrival of patients with Zika virus, yellow fever virus, and Rift Valley fever to mainland China since February, 2016.12, 13, 14, 15 Governments and the public health community should be aware of these new threats to prevent possible local epidemics.
In travellers from Hong Kong, Macau, and Taiwan, the incidence of hand, foot, and mouth disease was high (figure 3). We could not establish where these travellers were infected, because they usually travel frequently to and from mainland China. Detailed data about itineraries should be collected to help to clarify the infection exposure site. The warm and humid climate in Hong Kong, Macau, Taiwan, and southern China could favour transmission of hand, foot, and mouth disease. Fortunately, a vaccine against the EV71, the most prevalent virus strain, is commercially available and has a good protective effect.35
We mapped travel-related infections globally to show differences in terms of exposure country and region (figure 4). Health-care professionals should be aware of the specific risks to travellers, and should provide targeted pre-travel consultations. Accordingly, country-specific or region-specific vaccination and prophylaxis are strongly recommended to reduce the burden of travel-related infections.
Our study had several limitations. Because our findings were based on surveillance of travellers when entering mainland China, most cases had clinical manifestations of disease upon arrival (appendix pp 9–10). Patients who were infected but did not develop symptoms until after arrival were not included in our study. Thus, the frequency of travel-related infections are underestimated. By contrast, for infections such as tuberculosis, hepatitis B and C virus infections, HIV, and syphilis, which have long latent or incubation periods, the location of acquisition is difficult to establish. For example, Chinese citizens who travelled overseas and were diagnosed upon arrival back to mainland China could have acquired the infection before travelling. Thus, the frequency of such infection in arriving travellers could have been overestimated. Although we estimated the overall incidence of each infection, would could not calculate the numerical risk for travellers coming from a particular country or region because of the lack of denominator data. Furthermore, we could not compare the demographic characteristics of the 22 797 people with an infection to those without an infection, because demographic data were not collected for all arriving travellers.
In conclusion, our findings suggest that health-care providers should provide evidence-based health recommendations to travellers before travel and should do destination-specific medical assessments of arriving travellers who are ill. Active surveillance at entry–exit ports can help with timely identification of people with emerging or re-emerging infections to prevent or at least postpone local transmission.36, 37 In addition to entry surveillance, follow-up surveillance—especially contact tracing of highly communicable infections—will be necessary to understand the entire profile of travel-related infections.
Acknowledgments
Acknowledgments
We thank the medical inspection officers, on-board staff, and laboratory-test personnel who contributed to the detection, epidemiological investigation, and diagnosis of all suspected infections. This work was supported by the Natural Science Foundation of China (81621005), the Basic Work on Special Program for Science & Technology Research (2013FY114600), the Special Program for Prevention and Control of Infectious Diseases in China (2017ZX10303401), and the National Key Research and Development Program (2016YFC1201300).
Contributors
L-QF, YS, Y-JT, and W-CC conceived and designed the study. L-QF, G-PZ, L-JL, Z-JJ, Z-WF, J-XW, YJ, M-JM, JT, YZ, PY, KL, YS, Y-JT, and W-CC did the main data collection and analysis. All authors contributed to the writing, review, and revision of the Article.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.World Tourism Organization UNWTO tourism highlights. https://www.e-unwto.org/doi/pdf/10.18111/9789284418145 2016 edition.
- 2.Olsen SJ, Chang HL, Cheung TY. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 3.Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- 4.Khan K, Arino J, Hu W. Spread of a novel influenza A (H1N1) virus via global airline transportation. N Engl J Med. 2009;361:212–214. doi: 10.1056/NEJMc0904559. [DOI] [PubMed] [Google Scholar]
- 5.Bogoch II, Brady OJ, Kraemer MUG. Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia-Pacific region: a modelling study. Lancet Infect Dis. 2016;16:1237–1245. doi: 10.1016/S1473-3099(16)30270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thwaites GE, Day NP. Approach to fever in the returning traveler. N Engl J Med. 2017;376:548–560. doi: 10.1056/NEJMra1508435. [DOI] [PubMed] [Google Scholar]
- 7.Harvey K, Esposito DH, Han P. Surveillance for travel-related disease–GeoSentinel Surveillance System, United States, 1997–2011. MMWR Surveill Summ. 2013;62:1–23. [PubMed] [Google Scholar]
- 8.Li Z, Zhang Q, Zheng C. Epidemiologic features of overseas imported malaria in the People's Republic of China. Malar J. 2016;15:141. doi: 10.1186/s12936-016-1188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai S, Wardrop NA, Huang Z. Plasmodium falciparum malaria importation from Africa to China and its mortality: an analysis of driving factors. Sci Rep. 2016;6:39 524. doi: 10.1038/srep39524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai S, Huang Z, Zhou H. The changing epidemiology of dengue in China, 1990–2014: a descriptive analysis of 25 years of nationwide surveillance data. BMC Med. 2015;13:100. doi: 10.1186/s12916-015-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D, Wu J, Zhang Q. Chikungunya outbreak in Guangdong Province, China, 2010. Emerg Infect Dis. 2012;18:493–495. doi: 10.3201/eid1803.110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng YQ, Zhao H, Li XF. Isolation, identification and genomic characterization of the Asian lineage Zika virus imported to China. Sci China Life Sci. 2016;59:428–430. doi: 10.1007/s11427-016-5043-4. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Xiong Y, Wu W. Zika virus in a traveler returning to China from Caracas, Venezuela, February 2016. Emerg Infect Dis. 2016;22:1133–1136. doi: 10.3201/eid2206.160273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Zhou P, Fu X. Yellow fever virus: increasing imported cases in China. J Infect. 2016;73:377–380. doi: 10.1016/j.jinf.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Sun F-J, Tong Y-G, Zhang S-Q, Cao W-C. Rift Valley fever virus imported into China from Angola. Lancet Infect Dis. 2016;16:1226. doi: 10.1016/S1473-3099(16)30401-7. [DOI] [PubMed] [Google Scholar]
- 16.General Administration of Quality Supervision. Inspection and Quarantine of the People's Republic of China Basic technical scheme for the active surveillance and medical response for infectious diseases at entry-exit ports. May 20, 2008. http://www.aqsiq.gov.cn/xxgk_13386/zvfg/gfxwj/wsjy/201502/P020150204561220143984.pdf (in Mandarin).
- 17.WHO International health regulations. 2005. http://www.who.int/ihr/publications/9789241580496/en/ 3rd edn.
- 18.The State Council of the People's Republic of China Rules for the Implementation of Frontier Health and Quarantine Law of the People's Republic of China. 2016. http://www.gov.cn/gongbao/content/2016/content_5139369.htm (in Mandarin).
- 19.Zhou S, Li Z, Cotter C. Trends of imported malaria in China 2010–2014: analysis of surveillance data. Malar J. 2016;15:39. doi: 10.1186/s12936-016-1093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Lai S, Zheng C. The epidemiology of Plasmodium vivax and Plasmodium falciparum malaria in China, 2004–2012: from intensified control to elimination. Malar J. 2014;13:419. doi: 10.1186/1475-2875-13-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi L, Fu S, Wang L. Surveillance of mosquito-borne infectious diseases in febrile travelers entering China via Shenzhen ports, China, 2013. Travel Med Infect Dis. 2016;14:123–130. doi: 10.1016/j.tmaid.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Liang Y, Feng Y. HIV-1 prevalence and subtype/recombinant distribution among travelers entering China from Vietnam at the HeKou port in the Yunnan province, China, between 2003 and 2012. J Med Virol. 2015;87:1500–1509. doi: 10.1002/jmv.24202. [DOI] [PubMed] [Google Scholar]
- 23.Torresi J, Leder K. Defining infections in international travellers through the GeoSentinel surveillance network. Nat Rev Microbiol. 2009;7:895–901. doi: 10.1038/nrmicro2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman DO, Weld LH, Kozarsky PE. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–130. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 25.Leder K, Torresi J, Libman MD. GeoSentinel surveillance of illness in returned travelers, 2007–2011. Ann Intern Med. 2013;158:456–468. doi: 10.7326/0003-4819-158-6-201303190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlagenhauf P, Weld L, Goorhuis A. Travel-associated infection presenting in Europe (2008–12): an analysis of EuroTravNet longitudinal, surveillance data, and evaluation of the effect of the pre-travel consultation. Lancet Infect Dis. 2015;15:55–64. doi: 10.1016/S1473-3099(14)71000-X. [DOI] [PubMed] [Google Scholar]
- 27.WHO Emergencies: disease outbreaks. http://www.who.int/emergencies/diseases/en/
- 28.Bedford T, Riley S, Barr IG. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature. 2015;523:217–220. doi: 10.1038/nature14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boggild AK, Castelli F, Gautret P. Latitudinal patterns of travel among returned travelers with influenza: results from the GeoSentinel Surveillance Network, 1997–2007. J Travel Med. 2012;19:4–8. doi: 10.1111/j.1708-8305.2011.00579.x. [DOI] [PubMed] [Google Scholar]
- 30.Matteelli A, Schlagenhauf P, Carvalho ACC. Travel-associated sexually transmitted infections: an observational cross-sectional study of the GeoSentinel surveillance database. Lancet Infect Dis. 2013;13:205–213. doi: 10.1016/S1473-3099(12)70291-8. [DOI] [PubMed] [Google Scholar]
- 31.Cheng Q, Jing Q, Spear RC, Marshall JM, Yang Z, Gong P. Climate and the timing of imported cases as determinants of the dengue outbreak in Guangzhou, 2014: evidence from a mathematical model. PLoS Negl Trop Dis. 2016;10:e0004417. doi: 10.1371/journal.pntd.0004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Centers for Disease Control and Prevention Locally acquired dengue—Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:577–581. [PubMed] [Google Scholar]
- 33.Gjenero-Margan I, Aleraj B, Krajcar D. Autochthonous dengue fever in Croatia, August–September 2010. Euro Surveill. 2011;16:19 805. [PubMed] [Google Scholar]
- 34.La Ruche G, Souares Y, Armengaud A. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15:19 676. [PubMed] [Google Scholar]
- 35.Zhu F, Xu W, Xia J. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370:818–828. doi: 10.1056/NEJMoa1304923. [DOI] [PubMed] [Google Scholar]
- 36.Cowling BJ, Lau LL, Wu P. Entry screening to delay local transmission of 2009 pandemic influenza A (H1N1) BMC Infect Dis. 2010;10:82. doi: 10.1186/1471-2334-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Read JM, Diggle PJ, Chirombo J, Solomon T, Baylis M. Effectiveness of screening for Ebola at airports. Lancet. 2015;385:23–24. doi: 10.1016/S0140-6736(14)61894-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.