Summary
Background Human thymic stromal lymphopoietin (TSLP) is expressed in the human asthmatic lung and activates dendritic cells (DCs) to strongly induce proallergic T‐helper type 2 (Th2) cell responses, suggesting that TSLP plays a critical role in the pathophysiology of human asthma. Th2 cells are predominantly involved in mild asthma, whereas a mixture of Th1 and Th2 cells with neutrophilic inflammation, probably induced by Th17, affects more severe asthmatic disease. Exacerbation of asthmatic inflammation is often triggered by airway‐targeting RNA viral infection; virus‐derived double‐stranded RNA, Toll‐like receptor (TLR)3 ligand, activates bronchial epithelial cells to produce pro‐inflammatory mediators, including TSLP.
Objective Because TSLPR‐expressing DCs express TLR3, we examined how the relationship between TSLP and TLR3 ligand stimulation influences DC activation.
Methods CD11c+DCs purified from adult peripheral blood were cultured in TLR ligands containing media with or without TSLP and then co‐cultured with allogeneic naïve CD4+T cells.
Results CD11c+ DCs responded to a combination of TSLP and TLR3 ligand, poly(I : C), to up‐regulate expression of the functional TSLP receptor and TLR3. Although TSLP alone did not induce IL‐23 production by DCs, poly(I : C) alone primed DCs for the production of IL‐23, and a combination of TSLP and poly(I : C) primed DCs for further production of IL‐23. The addition of poly(I : C) did not inhibit TSLP‐activated DCs to prime naïve CD4+ T cells to differentiate into inflammatory Th2 cells. Furthermore, DCs activated by a combination of TSLP and poly(I : C) primed more naïve CD4+ T cells to differentiate into Th17‐cytokine–producing cells with a central memory T cell phenotype compared with DCs activated by poly(I : C) alone.
Conclusions These results suggest that through DC activation, human TSLP and TLR3 ligands promote differentiation of Th17 cells with the central memory T cell phenotype under Th2‐polarizing conditions.
Keywords: dendritic cells, double‐stranded RNA, IL‐23, T cell differentiation, Th17 cells, Th2 cells, Toll‐like receptor 3 ligand, TSLP
Introduction
Human thymic stromal lymphopoietin (TSLP) activates CD11c+ dendritic cells (DCs) to give rise to proallergic T cell responses [1, 2]. TSLP activates DCs to strongly up‐regulate the expression of costimulatory molecules and to uniquely produce T helper (Th)2 cell‐attracting chemokines, thymus and activation‐regulated chemokine (TARC), and macrophage‐derived chemokine (MDC) – but not the pro‐inflammatory cytokines IL‐1β, IL‐6, IL‐12, or TNF‐α [3]. CD4+ T cells are primed by TSLP‐activated DCs to differentiate into inflammatory Th2 cells that produce the proallergic cytokines IL‐4, IL‐5, IL‐13, and TNF‐α, while down‐regulating IL‐10 and IFN‐γ [3, 4, 5]. In addition, naïve CD8+ T cells are primed by TSLP‐activated DCs to produce IL‐5 and IL‐13, but exhibit poor cytolytic activity [6]. Moreover, human TSLP is produced by bronchial epithelial cells, bronchial smooth muscle cells, lung fibroblasts, and mast cells [1, 2, 3]. TSLP expression is increased in asthmatic airways and correlates with the expression of Th2‐attracting chemokines and disease severity [7, 8]. These data suggest that TSLP is involved in the pathogenesis of human asthma [9, 10, 11, 12].
TSLP plays a critical role in asthmatic inflammatory responses not only in humans but also in mice and monkeys. TSLP concentrations are increased in mice with antigen‐mediated airway inflammatory disease, and lung TSLP concentrations correlate with the severity of eosinophilia [13]. Furthermore, TSLPR‐deficient mice develop severely attenuated antigen‐mediated airway inflammatory disease [13, 14]. In the rhesus monkey model of dust‐mite‐induced allergic asthma, TSLP is expressed on airway respiratory cells and smooth muscle cells in sensitized monkeys [15]. Inhibition of OX40L, which is a critical downstream mediator in TSLP‐mediated immune responses [4, 5], inhibits Th2 inflammation in the monkey model of asthma [15]. These data suggest that TSLP plays a critical role in the development of asthma.
Th17 cells–characterized by the production of IL‐17/IL‐17A, IL‐17F, IL‐6, TNF‐α, and IL‐22–mediate protection against extracellular microbes and link to the development of autoimmune diseases in vivo [16, 17]. TGF‐β, IL‐1β, IL‐6, IL‐22, and IL‐23 play critical roles in IL‐17 production in human T cells [18, 19, 20, 21], and the IL‐23–IL‐17 axis is involved in human psoriasis and inflammatory bowel diseases, suggesting an important role for Th17 cytokines in chronic inflammatory diseases [17, 18, 22]. Although asthma is characterized by chronic dysregulated Th2 inflammation in the airways, recent human studies have shown that IL‐17 is also expressed in the airways of patients with asthma, and IL‐17 expression is increased in patients with moderate‐to‐severe asthma, compared with patients with mild asthma and with normal controls [23, 24, 25]. Thus, Th2 cells are predominantly involved in mild asthma, whereas a mixture of Th2 and Th1 cells with neutrophilic inflammation probably induced by Th17 cells affects more severe asthmatic disease [26]. Still, it has remained unclear how Th17 lineage commitment is mediated in Th2‐predominant inflammation, such as human asthma.
Exacerbation of asthmatic inflammation is often triggered by infection of airway‐targeting viruses such as the rhinovirus, coronavirus, influenza virus, respiratory syncytial virus, and adenovirus [27, 28, 29]. Bronchial epithelial cells express several Toll‐like receptors (TLRs); virus‐derived double‐stranded RNA activates bronchial epithelial cells through TLR3 to produce pro‐inflammatory mediators, including TSLP [30, 31, 32].
Because TSLPR‐expressing DCs express TLR3 [3, 33, 34], in this study we examined how the relationship between TSLP and TLR3 ligand stimulation influences DC activation. We show that a combination of TSLP and TLR3 ligands activated human CD11c+ DCs to produce IL‐23. In addition, the addition of poly(I : C) did not inhibit TSLP‐activated DCs to prime naïve CD4+ T cells to differentiate into inflammatory Th2 cells. Furthermore, DCs activated by a combination of TSLP and poly(I : C) primed more naïve CD4+ T cells to differentiate into Th17‐cytokine–producing cells with a central memory T cell phenotype compared with DCs activated by poly(I : C) alone. These results suggest that through DC activation, human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory T cell phenotype under Th2‐polarizing conditions.
Materials and methods
DC purification and culture
This study was approved by the Institutional Review Board for Human Research at the Graduate School of Medicine, Kyoto University. Peripheral blood mononuclear cells (PBMCs) were obtained from the adult buffy coat of healthy donors (kindly provided by Kyoto Red Cross Blood Center, Kyoto, Japan). CD11c+ DCs were isolated from PBMCs as described previously [34, 35]. CD11c+lineage− cells were isolated by a FACS Aria™ (BD Biosciences, San Jose, CA, USA) to reach >99% purity. CD11c+ DCs were cultured immediately after being sorted in RPMI 1640 (Gibco BRL, Grand Island, NY, USA), supplemented with 5% human AB serum (Sigma, St Louis, MO, USA), penicillin G, streptomycin, 10 mm HEPES, and 1 mm sodium pyruvate (Gibco BRL; referred to as complete medium). Cells were seeded at a density of 1 × 106 cells/mL in round‐bottomed 96‐well plates in the presence of 25 μg/mL of poly(I : C) (InvivoGen, San Diego, CA, USA), 1 μg/mL of lipopolysaccharide (LPS) (Sigma) or 5 μg/mL of Escherichia coli RNA/LyoVec (InvivoGen), with or without 15 ng/mL of TSLP (R&D Systems, Minneapolis, MN, USA). After 24 h of culture, viable DCs were counted by trypan blue exclusion of dead cells.
Real‐time quantitative reverse transcription polymerase chain reaction (RT‐PCR)
Total RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, CA, USA) and treated with DNase I (Qiagen) according to the manufacturer's instructions. Reverse transcription was performed with SuperScript™ II (Invitrogen, Carlsbad, CA, USA). Real‐time quantitative reactions were performed with an ABI Prism 7300 detection system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's instructions. Values are expressed as arbitrary units relative to glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) × 105. The following primers were used: GAPDH: 5′‐CCACATCGCTCAGACACCAT‐3′ and 5′‐GGCAACAATATCCACTTTACCAGAGT‐3′; TSLPR: 5′‐AGAGCAGCCAGACGACATT‐3′ and 5′‐CAGCGACTTGCGGTGAAAAC‐3′; IL‐7Rα: 5′‐GGATGAAAACAAATGGACGCATG‐3′ and 5′‐GCTGCCGGTTGGAGCTTTCT‐3′; TLR3: 5′‐TGACTGAACTCCATCTCATGTCC‐3′ and 5′‐CCATTATGAGACAGATCTAATGTG‐3′; TLR4: 5′‐CTGCGTGAGACCAGAAAGC‐3′ and 5′‐TTCAGCTCCATGCATTGATAA‐3′; TLR8: 5′‐TCTGCATGAGGTTGTCGATGA‐3′ and 5′‐TGCGCTACCACCTTGAAGAGA‐3′; T‐bet: 5′‐AACACAGGAGCGCACTGGAT‐3′ and 5′‐TGCTCTCCTGGCTGCAGAC‐3′; GATA‐3: 5′‐ACCGGCTTCGGATGCAA‐3′ and 5′‐TGCTCTCCTGGCTGCAGAC‐3′; RORc: 5′‐TTTTCCGAGGATGAGATTGC‐3′ and 5′‐CTTTCCACATGCTGGCTACA‐3′; CCR4: 5′‐CAGAAAAGCAAGAAGCTGCTTCTG‐3′ and 5′‐CGAGGGTGGTATCTGCTATATC‐3′; and CCR6: 5′‐GGCTGCAAATTTGGGTAAAA‐3′ and 5′‐CACAGGAGAAGCCTGAGGAC‐3′.
DC cytokine production
After a 24‐h culture of DCs at a concentration of 1 × 106 cell/mL, DC culture supernatants were collected and analysed with protein ELISA kits for TARC, MDC, IL‐12p70 (from R&D Systems), and for IL‐1β, IL‐6, IL‐23p19/p40, TNF‐α, and TGF‐β1 (all from eBioscience, San Diego, CA, USA). Diluted supernatants (1 : 10, 1 : 10, 1 : 1, 1 : 1, 1 : 5, 1 : 1, 1 : 1, and 1 : 1) were used for ELISA for TARC, MDC, IL‐12p70, IL‐1β, IL‐6, IL‐23p19/p40, TNF‐α, and TGF‐β1, respectively.
Analysis of cell surface markers of DCs
To determine the cell surface markers characteristic of activated DCs, DCs were incubated with various stimuli for 24 h. DCs were subsequently stained with fluorescein isothiocyanate (FITC)‐conjugated anti‐CD80 (eBioscience). Finally, they were analysed with a FACS Calibur™ (BD Biosciences).
DC T cell co‐culture
After 24 h of culture, CD11c+ DCs were collected and washed three times to remove any cytokines. Viable DCs were counted by trypan blue exclusion of dead cells. CD4+CD45RA+ naïve T cells were isolated from PBMC using a FACS Aria™ to reach >99% purity, as described previously [3, 35]. The remaining DCs were co‐cultured with 2.5 × 104 freshly purified allogeneic naïve CD4+ T cells in round‐bottomed 96‐well culture plates in complete medium. Cells were cultured in triplicate at a DC :T cell ratio of 1 : 5. After 7 days of culture, viable cells were counted by trypan blue exclusion of dead cells.
T cell cytokine production
After 7 days of co‐culture, DC‐primed CD4+ T cells were restimulated for 24 h with plate‐bound 10 μg/mL anti‐CD3 (UCHT1, BD Biosciences) and soluble 1 μg/mL anti‐CD28 (CD28.2, BD Biosciences) at a concentration of 1 × 106 cell/mL. Cytokine production was assessed in the culture supernatant by protein ELISA for IL‐10, IL‐13, IL‐17, IFN‐γ and TNF‐α (all from eBioscience), and IL‐22 (from R&D Systems). Diluted supernatants (1 : 20, 1 : 2, 1 : 1, 1 : 100, 1 : 20, and 1 : 5) were used for ELISA for IL‐10, IL‐13, IL‐17, IFN‐γ TNF‐α, and IL‐22, respectively. For intracellular cytokine production, T cells were collected on day 7 of the co‐culture, washed twice, and restimulated with 50 ng/mL PMA (Sigma) +2 μg/mL ionomycin (Sigma) in flat‐bottomed 96‐ or 48‐well plates at a concentration of 1 × 106 cell/mL. After 3.5 h, brefeldin A (Sigma) was added at 10 μg/mL. After 2.5 h, cells were collected and stained for cell‐surface molecules. Cells were fixed and permeabilized using a Fix & Perm Cell Permeabilization Kit (Caltag Laboratories, An Der Grub, Austria), and stained with phycoerythrin‐conjugated mAbs to IL‐13, TNF‐α IL‐17, and FITC‐conjugated anti IFN‐γ (all from eBioscience). Stained cells were analysed on a FACS Calibur.
Analysis of cell‐surface markers of T cells
To determine the cell‐surface markers characteristic of CD4+ T cells, after 7 days of co‐culture, DC‐primed CD4+ T cells were stained with FITC‐conjugated anti‐CD45RA, anti‐CD45RO, anti‐CD62L (all from eBioscience), or purified mouse anti‐CCR7 (BD Bioscience) and FITC‐conjugated anti‐mouse IgM (AbD Serotec, Kidlington, UK), followed by allophycocyanin‐conjugated anti‐CD4 (eBioscience). In secondary culture, T cells were cultured for 3 days with plate‐bound 10 μg/mL anti‐CD3 mAb and soluble 1 μg/mL anti‐CD28 mAb with 2 ng/mL IL‐2 (R&D Systems). Finally, they were analysed with a FACS Calibur.
CD4+ T cell purification
CD4+CD45RA+ naïve T cells and CD4+CD45RA− memory T cells (purity, >99%) were isolated by cell sorting as described above [35]. Purified T cells were cultured at 2.5 × 106 cells/mL in complete medium. In primary culture, T cells were cultured with plate‐bound 10 μg/mL anti‐CD3 mAb and soluble 1 μg/mL anti‐CD28 mAb in the presence of 10 ng/mL of IL‐6 and IL‐23, and 10 μg/mL of anti‐IL‐4 and anti‐IFN‐γ neutralizing Abs (all from R&D Systems) with or without 15 ng/mL of TSLP. After 3 days of culture, activated T cells were expanded for 4 days with 10 μg/mL plate‐bound anti‐CD3, soluble 1 μg/mL anti‐CD28 mAbs, and 2 ng/mL of IL‐2. Culture supernatants were measured by protein ELISA for IL‐17 and IL‐22. Diluted supernatants (1 : 10, 1 : 3) were used for ELISA for IL‐17 and IL‐22, respectively.
Results
TSLP and TLR3 ligands synergistically enhance the expression of their own receptors
Human CD11c+ immature DCs respond to the stimulation of TLR ligands (TLRLs) and TSLP [3, 33, 34]. First, to examine the effects of TLRLs and TSLP on the expression levels of TSLPR, IL‐7Rα chain, and TLRs in activated DCs, we isolated CD11c+ immature DCs from blood buffy coats and cultured them for 24 h in TLRLs containing media with or without TSLP. Using real‐time quantitative RT‐PCR, we found that human CD11c+ DCs cultured with medium alone expressed both TSLPR and IL‐7Rα chain genes and that their expressions were enhanced by stimulation with TSLP and TLR3, 4, and 7/8 ligand agonist, poly(I : C), LPS, and LyoVec, respectively (Fig. 1a). TSLP did not enhance LPS‐ or LyoVec‐induced expression of TSLPR or IL‐7Rα chain genes. However, TSLP significantly up‐regulated poly(I : C)‐induced expression of both TSLPR and IL‐7Rα chain genes (Fig. 1a). Moreover, although TSLP alone did not affect TLR3 gene expression, it significantly enhanced poly(I : C)‐induced TLR3 gene expression in DCs (Fig. 1b). In contrast, TSLP did not enhance gene expression of TLR4 and TLR8 induced by LPS or LyoVec. These data indicate that TSLP had a synergistic enhancing action only with the TLR3 ligand on expression of their own receptor genes.
Figure 1.
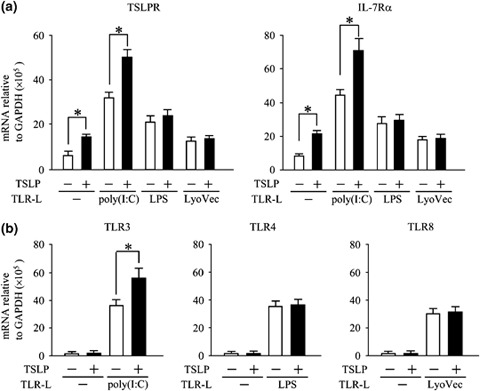
TSLP and TLR3 ligands synergistically enhanced expression of their own receptors. Blood CD11c+ DCs were activated by TLR‐ligands, poly(I : C), LPS, or LyoVec with (closed bar) or without TSLP (open bar) for 24 h. DCs were used for real‐time quantitative RT‐PCR analyses for TSLPR and IL‐7Rα chain mRNA expressions (a) and for TLR3, TLR4, and TLR8 mRNA expressions (b). Closed bar and horizontal error bars indicate the mean and SD of three independent experiments, respectively. Student's t‐test for unpaired data was used to compare the values between two groups. * P<0.05. TSLP, thymic stromal lymphopoietin; TLR, Toll‐like receptor; DCs, dendritic cells; LPS, lipopolysaccharide.
TSLP enhances TLR3 ligand‐induced production of IL‐23 by DCs
Because cytokine and chemokine production by activated DCs plays a critical role in the differentiation of primed T cells, we next assessed cytokine and chemokine production in activated DCs in the culture supernatant by protein ELISA. After 24 h of culture, TSLP‐primed DCs produced high levels of TARC and MDC, while TSLP did not stimulate DC production of pro‐inflammatory cytokines IL‐1β, IL‐6, IL‐12, IL‐23, or TNF‐α, demonstrating one of the unique properties of TSLP in DC activation (Fig. 2a, left panels) [3, 34]. In contrast, poly(I : C) alone and LPS alone primed DCs to produce pro‐inflammatory cytokines such as IL‐1β, IL‐6, IL‐12, IL‐23, and TNF‐α (Fig. 2a, middle panels). TSLP did not affect poly(I : C)‐ or LPS‐induced production of IL‐1β, IL‐6, IL‐12, or TGF‐β1, whereas TSLP marginally enhanced poly(I : C)‐ and LPS‐induced increase in TNF‐α production (Fig. 2a, middle panels and 2c). However, TSLP significantly up‐regulated poly(I : C)‐induced increase of IL‐23 production (Fig. 2a, middle panels and 2b), which can induce CD4+ T cell differentiation into Th17 cells in humans [18, 19, 20]. Although TLRLs alone did not produce large amounts of TARC or MDC in DCs, TSLP strongly induced production of the Th2‐cell attracting chemokines, TARC and MDC, regardless of the presence of the TLRLs (Fig 2a, lower panels). Taken together, these data suggest that TSLP may maintain its unique ability in DC activation regardless of the presence of TLRLs.
Figure 2.
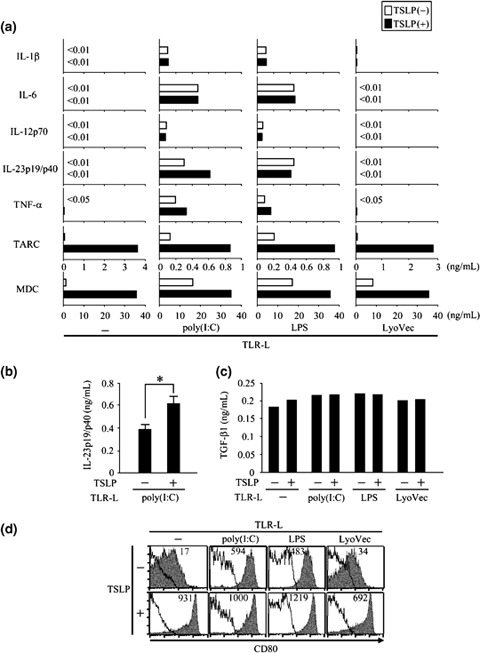
TSLP conserved its unique abilities in DC activation in the presence of TRLs and enhanced TLR3 ligand induced IL‐23 production. Blood CD11c+ DCs were activated by TLR‐ligands, poly(I : C), LPS, or LyoVec, with or without TSLP for 24 h. (a), IL‐1β, IL‐6, IL‐12p70, IL‐23p19/p40, TNF‐α TARC, and MDC were measured in the culture supernatant by protein ELISA. (b), IL‐23p19/p40 production measured by protein ELISA. Closed bar and horizontal error bars indicate the mean and SD of five independent experiments, respectively. Student's t‐test for unpaired data was used to compare the values between two groups. * P<0.05. (c), TGF‐β1 concentration measured by protein ELISA. (d), Cell‐surface marker phenotypes of DCs were determined by flow cytometry. Filled histograms represent staining of CD80; open histograms represent the isotype control. Numbers above histograms indicate the mean fluorescence intensity for CD80. Data represent one of five independent experiments. TSLP, thymic stromal lymphopoietin; TLR, Toll‐like receptor; DCs, dendritic cells; MDC, macrophage‐derived chemokine.
TSLP conserves its unique ability in DC activation in the presence of TLRLs
One of the characteristics of TSLP in DC activation is its capability to induce strong cell‐surface expression of costimulatory molecules, such as CD80 and CD86, in DCs [3, 34, 35]. To examine whether TSLP retains this ability in the presence of TLRLs, we analysed the surface expression of CD80 on DCs by flow cytometry. Poly(I : C), LPS, LyoVec, and TSLP all stimulated surface CD80 expression in DCs (Fig. 2d). Notably, TSLP enhanced surface CD80 expression in the presence of TLRLs to an extent similar to that of TSLP alone. Another trait of TSLP stimulation in DCs is enhancement of DC survival [3, 35]. After 24 h of culture, TSLP augmented the number of viable DCs induced by the TLRLs (data not shown). Taken together, these data suggest that TSLP maintains its unique abilities in DC activation regardless of the presence of TLRLs.
TSLP+poly(I : C)‐activated DCs [TSLP+poly(I : C)‐DCs] induce strong CD4+ T cell expansion
Both virus‐derived TLRL stimulation and epithelial‐cell–derived TSLP appear to be involved in exacerbating asthma [7, 8, 27, 28, 29, 30, 31, 32]. In addition, we demonstrated in this study that TSLP+poly(I : C) stimulation resulted in unique activation of DCs (Fig. 2). Subsequently, we examined how CD4+ T cells expand and differentiate by DCs activated with TSLP+poly(I : C). CD11c+ DCs purified from adult peripheral blood were cultured in poly(I : C)‐containing media with or without TSLP and then co‐cultured with allogeneic naïve CD4+ T cells at a 1 : 5 ratio of DCs: T cells. After 7 days of co‐culture, CD4+ T cells co‐cultured with TSLP+poly(I : C)‐DCs expanded significantly more than those co‐cultured with DCs activated with poly(I : C) alone, suggesting that TSLP+poly(I : C)‐DCs have a stronger effect on CD4+ T cell expansion than do poly(I : C)‐DCs (Fig. 3a).
Figure 3.
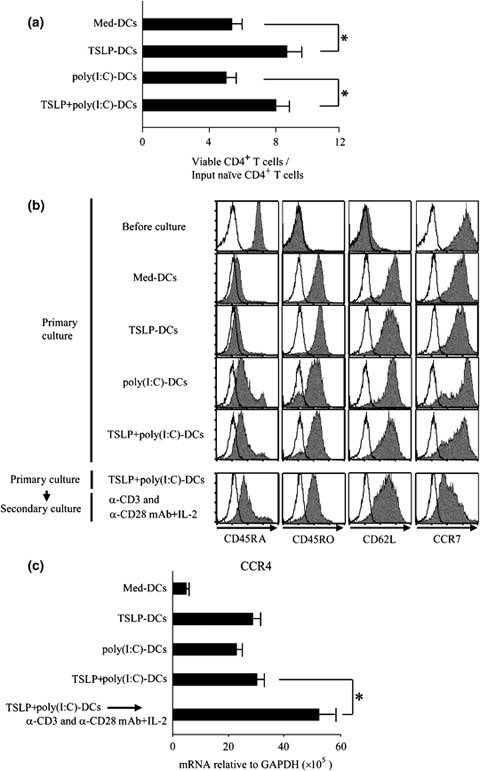
TSLP+poly(I : C)‐DCs efficiently induced naïve CD4+ T‐cell expansion and converted naïve to central memory T cells. CD11c+ DCs cultured medium alone or activated with TSLP, poly(I : C), or the combination of TSLP and poly(I : C) were used to prime naïve CD4+ T cells for 7 days. For secondary culture, T cells primed by TSLP+poly(I : C)‐DCs were subsequently stimulated for 3 days with anti‐CD3, anti‐CD28 mAbs, and IL‐2. (a), Fold expansion of CD4+ T cells cultured with indicated DCs compared with the initial T cell number. Viable T cells were counted by trypan blue exclusion of dead cells, and cell‐surface marker phenotypes were determined by flow cytometry. Closed bar and vertical error bars indicate the mean and SD of five independent experiments, respectively. Student's t‐test for unpaired data was used to compare the values between two groups. * P<0.05. (b), Cell‐surface marker phenotypes of CD4+ T cells before and after culture with indicated DCs. Cell‐surface marker phenotypes were determined by flow cytometry. Filled histograms represent staining of T cells with indicated markers; open histograms represent isotype controls. Data represent one of five experiments. (c), CCR4 expression of various CD4+ T cells. Expression level of CCR4 mRNA was measured using real‐time quantitative RT‐PCR. Closed bar and horizontal error bars indicate the mean and SD of three independent experiments, respectively. Student's t‐test for unpaired data was used to compare the values between two groups. * P<0.05. TSLP, thymic stromal lymphopoietin; TLR, Toll‐like receptor; DCs, dendritic cells.
T cells primed with TSLP+poly(I:C)‐DCs show the central memory T cell phenotype
To determine the cell‐surface markers characteristic of CD4+ T cells primed by TSLP+poly(I : C)‐DCs, we used flow cytometry to analyse naïve CD4+ T cells before and after culture for 7 days with activated DCs. After isolation, naïve CD4+ T cells decreased their CD62L expression, possibly due to cell isolation processes (Fig. 3b) [35]. Priming with TSLP+poly(I : C)‐DCs down‐regulated CD45RA and up‐regulated CD45RO in CD4+ T cells, acquiring a CD45RA−CD45RO+CD62L+CCR7+ central memory T cell phenotype, a phenotype similar to TSLP‐DC‐induced memory Th2 cells [5]. Restimulation with anti‐CD3, anti‐CD28 mAbs, and IL‐2 for 3 days converted these T cells to a CD45RA−CD45RO+CD62LlowCCR7low effector memory T cell phenotype. In addition, these effector memory T cells showed enhanced expression of CCR4, which is a receptor for the Th2 cell‐attracting chemokines–TARC and MDC (Fig. 3c). These data suggest that T cells expanded with TSLP+poly(I : C)‐DCs can rapidly acquire effector functions.
TSLP+poly(I : C)‐DCs programme naïve CD4+ T cells more to differentiate into Th17 cells, and do not alter development of the inflammatory Th2 cells
Next, we examined the cytokine‐producing capacity of CD4+ T cells primed by DCs. CD4+ T cells were primed for 7 days by DCs activated with TSLP, poly(I : C), or a combination of both stimuli. After washing the cells, half of the expanded CD4+ T cells were collected to measure the expression levels of mRNA encoding lineage‐specific transcription factors such as GATA‐3, RORc, and T‐bet, as well as Th‐17 cell‐expressing CCR6 [17, 36]. The remaining CD4+ T cells were restimulated for a further 24 h with anti‐CD3 and anti‐CD28 mAbs, and T cell‐derived cytokines were measured in the culture supernatant using protein ELISA.
CD4+ T cells primed with TSLP‐DCs expressed a high level of GATA‐3 mRNA but not RORc, CCR6, or T‐bet (Fig. 4a–c) and produced only the inflammatory Th2 cytokines IL‐13 and TNF‐α (Fig. 4d–f). In contrast, poly(I : C)‐DCs enhanced expression of all the lineage‐specific transcription factors and production of various cytokines, including IL‐17, IL‐22, IFN‐γ, and IL‐10 from CD4+ T cells. In comparison with priming of poly(I : C)‐DCs, TSLP+poly(I : C)‐DCs primed CD4+ T cells to express a decreased level of T‐bet (Fig. 4c) and increased levels of GATA‐3, RORc, and CCR6 mRNA (Fig. 4a and b). In addition, CD4+ T cells primed with TSLP+poly(I : C)‐DCs enhanced the production of IL‐17 and IL‐22, while they reduced the production of IFN‐γ and IL‐10 compared with CD4+ T cells primed with poly(I : C)‐DCs (Fig. 4e and f). Interestingly, when compared with TSLP‐DCs, TSLP+poly(I : C)‐DCs failed to induce further production of IL‐13 and TNF‐α from primed CD4+ T cells (Fig. 4d). These data suggest that TSLP+poly(I : C)‐DCs prime naïve CD4+T cells more to differentiate into Th17 cells compared with poly(I : C)‐DCs, and do not alter development of the inflammatory Th2 cells compared with TSLP‐DCs.
Figure 4.
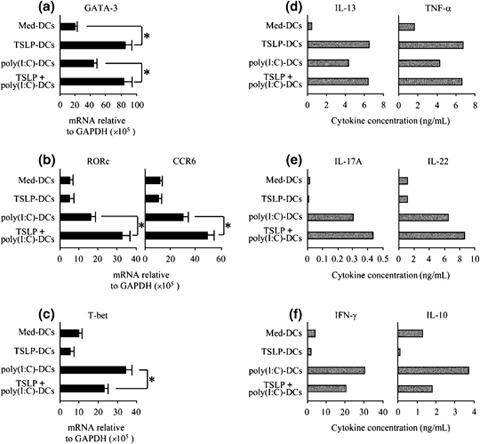
TSLP+poly(I : C)‐activated DCs primed naïve CD4+ T cells to produce IL‐17 and IL‐22, but failed to induce further development of the inflammatory Th2 cells. Naïve CD4+ T cells were primed for 7 days by DCs activated with TSLP, poly(I : C), or the combination of both stimuli. (a–c), Expression levels of mRNA encoding GATA‐3 (a), RORc and CCR6 (b), and T‐bet (c). Expression levels were measured using real‐time quantitative RT‐PCR. Closed bar and vertical error bars indicate the mean and SD of five independent experiments, respectively. Student's t‐test for unpaired data was used to compare the values between two groups. * P<0.05. (d–f), Cytokine production of primed T cells. After 7 days of co‐culture, T cells were restimulated for 24 h with anti‐CD3 and anti‐CD28 mAbs. IL‐13 and TNF‐α (d), IL‐17A, and IL‐22 (e), and IFN‐γ and IL‐10 (c), were measured in the culture supernatant using protein ELISA. Data represent one of five independent experiments. TSLP, thymic stromal lymphopoietin; DCs, dendritic cells.
TSLP+poly(I : C)‐DCs reduce IL‐17 and IFN‐γ double‐producing T cells and increase IL‐17 single‐producing T cells
We further evaluated cytokine production capacity using intracellular cytokine staining of primed T cells restimulated with PMA plus ionomycin. Intracellular cytokine staining of T cells demonstrated that the percentage of TNF‐α‐producing cells primed with TSLP+poly(I : C)‐DCs was similar to those with poly(I : C)‐DCs and TSLP‐DCs (Fig. 5a and b, left panels). In contrast, the percentage of IL‐17‐producing cells primed with TSLP+poly(I : C)‐DCs was greater than those with poly(I : C)‐DCs and TSLP‐DCs (Fig. 5a and b, middle panels). Importantly, the percentage of IL‐13‐producing cells primed with TSLP+poly(I : C)‐DCs was greater than that primed with poly(I : C)‐DCs, but similar to that with TSLP‐DCs (Fig. 5a and b, right panels).
Figure 5.
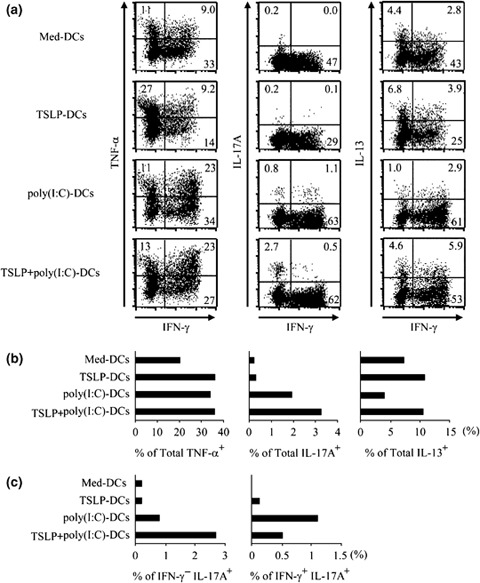
TSLP+poly(I : C)‐DCs reduced IL‐17 and IFN‐γ double‐producing T cells and increased IL‐17 single‐producing T cells. CD11c+ DCs cultured with TSLP, poly(I : C), a combination of both stimuli, or medium alone were used to prime naïve CD4+ T cells. After 7 days of co‐culture, T cells were restimulated for 6 h with PMA plus ionomycin, and production of indicated T cell‐derived cytokines was determined by intracellular cytokine staining. (a), Dot blots profiles of indicated cytokines. Numbers indicate percent of cells in each quadrant. Data represent one of three independent experiments. (b), Percentage of TNF‐α– IL‐17–, IL‐13 – producing cells. Data represent numbers in (a). (c), Percentage of IL‐17 single‐producing cells (IFN‐γ−IL‐17+), and IFN‐γ and IL‐17 double‐producing cells (IFN‐γ+IL‐17+). Data represents numbers in (a). TSLP, thymic stromal lymphopoietin; DCs, dendritic cells.
In addition, priming with TSLP+poly(I : C)‐DCs increased IL‐17 single‐producing T cells more prominently than priming with poly(I : C)‐DCs (Fig. 5c, left panels). In contrast, IL‐17 and IFN‐γ double‐producing (Th17/Th1) cells decreased by priming with TSLP+poly(I : C)‐DC as compared with those primed by poly(I : C)‐DCs (Fig. 5c, right panels). These findings suggest that TSLP+poly(I : C)‐DCs may induce conversion of Th17/Th1 cells to Th17 cells.
TSLP can directly prime human memory CD4+ T cells to enhance the production of Th17 cytokines
Although freshly isolated peripheral blood human CD4+ T cells do not show any response to TSLP, we recently found that TCR stimulation allows a potent response of CD4+ T cells to TSLP and that TSLP can directly enhance T cell expansion [37, 38]. To examine the possibility that the direct action of TSLP on CD4+ T cells is involved in differentiation of Th17 cells, purified naïve CD4+CD45RA+ T cells and memory CD4+CD45RA− T cells were activated with Th17‐cell differentiation media, anti‐CD3, and anti‐CD28 with neutralizing Abs for IL‐4, and IFN‐γ with or without IL‐6 and IL‐23, in the presence or absence of TSLP. After 3 days of culture, cells were further expanded for 4 days with IL‐2, and the production of IL‐17 and IL‐22 was measured in the culture supernatant using protein ELISA. TSLP had no effect on IL‐17 or IL‐22 production of naïve CD4+CD45RA+ T cells cultured even in Th17‐cell differentiation media (Fig. 6). However, TSLP significantly enhanced IL‐17 and IL‐22 production of memory CD4+CD45RA− T cells in Th17‐cell differentiation media. These data suggest that in addition to indirectly priming naïve CD4+ T cells to differentiate into Th17 cells with a memory phenotype, TSLP may also directly act on these memory T cells to accelerate further expansion and production of IL‐17.
Figure 6.
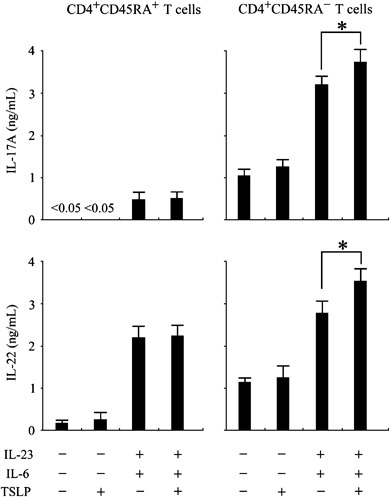
TSLP directly acted on human memory CD4+ T cells to enhance production of Th17 cytokines. Purified CD4+CD45RA+ naïve T cells and CD4+CD45RA− memory T cells were cultured with anti‐CD3, anti‐CD28, anti‐IL‐4, and anti‐IFN‐γ Abs in the presence or absence of IL‐6 and IL‐23 with or without TSLP. After 3 days of culture, cells were further cultured for 4 days with anti‐CD3, anti‐CD28, and IL‐2. Production of IL‐17 and IL‐22 was measured in the culture supernatant using protein ELISA. Closed bar and horizontal error bars indicate the mean and SD of three independent experiments, respectively. Student's t‐test for unpaired data was used to compare the values between two groups. * P<0.05. TSLP, thymic stromal lymphopoietin.
Discussion
In the present study, we demonstrated for the first time that TSLP and TLR3 ligands activated human blood CD11+ DCs to produce more IL‐23 and more programmed naïve CD4+ T cells to differentiate into Th17 cells compared with the TLR3 ligand alone, and a combination of TSLP and TLR3 ligands did not alter development of the inflammatory Th2 cells compared with TSLP alone. These results suggest that through DC activation, human TSLP and TLR3 ligands promote Th17‐cell differentiation under Th2‐polarizing conditions. If Th2 cells predominate in driving inflammation in mild asthma, there is evidence that a more severe asthmatic phenotype is regulated through a mixture of Th1 and Th2 cells with neutrophilic inflammation probably induced by Th17 cells in the lungs [26]. TSLP‐mediated Th17‐cell commitment under Th2‐polarizing conditions may be involved in the development of an inflammatory mixture of Th2 and Th17 cells, a unique profile that resembles severe asthma.
In humans, CD4+ memory T cells have been defined as CD45RO+CD62L+CCR7+ central memory cells and CD45RO+CD62L−CCR7− effector memory cells by their distinct surface phenotype, homing capacity, and effector functions [39, 40]. These T cell subsets can be further divided into subsets that are prone to produce Th1 or Th2 cytokines according to their differential expression of chemokine and tissue‐homing receptors [40, 41, 42], linking memory T cell subsets with a polarized Th1 or Th2 function. TSLP‐DCs play important roles not only in Th2 priming but also in the maintenance and further polarization of Th2 central memory cells in allergic diseases [5]. In this study, we show that DCs activated by a combination of TSLP and poly(I : C) primed naïve CD4+ T cells to produce IL‐17 and IL‐22 and priming with TSLP+poly(I : C)‐DCs down‐regulated CD45RA and up‐regulated CD45RO in CD4+ T cells, acquiring a CD45RA−CD45RO+CD62L+CCR7+ central memory T cell phenotype.Th17 cells are linked to the development of autoimmune diseases in vivo [16, 17] and the IL‐23–IL‐17 axis is involved in human psoriasis and inflammatory bowel diseases, suggesting an important role for Th17 cytokines in chronic inflammatory diseases [17, 18, 22]. Therefore, IL‐23–mediated generation of Th17 central memory cells by TSLP+poly(I : C)‐DCs may be involved in maintenance of chronic inflammation of allergic diseases.
In addition, restimulation with anti‐CD3, anti‐CD28 mAbs, and IL‐2 rapidly converts TSLP+poly(I : C)‐DCs–induced central memory T cells into effector memory T cells highly expressing CCR4 (Fig. 3), suggesting that T cells expanded with TSLP+poly(I : C)‐DCs can rapidly acquire effector functions. Thus, through DC activation, TSLP and TLR3 ligands may facilitate the link between differentiation and maturation of naïve, memory, and effector T cells in chronic inflammation of allergic diseases.
Previous studies have reported that TLR3 ligand, virus‐derived double‐stranded RNA activates bronchial epithelial cells to produce pro‐inflammatory mediators, including TSLP [30, 31, 32]. In this study, we showed that TSLP and TLR3 ligand stimulation induced strong and unique activation of DCs. In addition, exacerbation of asthmatic inflammation is often triggered by infection involving airway‐targeting RNA viruses [27, 28, 29], and TSLP expression correlates with disease severity [7, 8]. Therefore, the TLR3 ligand may play dual roles in exacerbation of asthmatic inflammation through induction of TSLP by epithelial cells and activation of DCs in the presence of TSLP.
In human blood, the other unique DC subsets are immature plasmacytoid DCs. Bratke et al. [43] demonstrated that not only human myeloid CD11c+ DCs but also plasmacytoid DCs infiltrate bronchoalveolar lavage fluid in the lung during segmental allergen challenge using allergens such as birch, rye, and Dermatophagoides pteronyssinus. We examined whether the TSLP and/or the TLR3 ligand can activate immature plasmacytoid DCs. However, stimulation of the TSLP and/or the TLR3 ligand did not directly induce CD80 up‐regulation and IFN‐α secretion in immature plasmacytoid DCs (data not shown). In mice, plasmacytoid DCs can differentiate into myeloid DCs after viral infection and poly(I : C) injection to mice through type‐I IFN production [44]. Therefore, in humans, TLR3 ligands may play an additional role in exacerbation of asthmatic inflammation indirectly through conversion of plasmacytoid DCs to TSLPR‐expressing CD11c+ DCs.
IL‐23 consists of a unique p19 subunit coupled to the p40 subunit of IL‐12. However, enhanced DC cytokine production by TSLP and poly(I : C) stimulation were demonstrated only in IL‐23 and not in IL‐12 (Fig. 2a). Using real‐time quantitative RT‐PCR, we found that priming of DCs with TSLP and poly(I : C) enhanced gene expression of IL‐23p19 but not that of IL‐12p40 and IL‐12p35, another subunit of bioactive IL‐12 (data not shown). These data suggest that increased expression of IL‐23p19 but not IL‐12p35 and IL‐12p40 may enhance the production of IL‐23 but not of IL‐12 in DCs primed with TSLP and poly(I : C).
In conclusion, we demonstrated that TSLP and TLR3 ligands activate human blood CD11+ DCs to produce more IL‐23 and program naïve CD4+ T cells more to differentiate into Th17 cells compared with the TLR3 ligand alone, and a combination of TSLP and TLR3 ligands does not alter development of the inflammatory Th2 cells compared with TSLP alone. These results suggest that through DC activation, human TSLP and TLR3 ligands promote Th17‐cell differentiation under Th2‐polarizing conditions, implying that in allergic diseases, virus‐derived double‐stranded RNA and TSLP may play a role in Th17 lineage commitment, even under Th2‐predominant polarizing conditions.
Acknowledgements
We thank Dr Dovie Wylie and Ms Yoshimi Yamakawa for assistance with preparation of the manuscript. This work was supported by Grants‐in‐aid for Scientific Research, 17659212, 18012029, 18015028, 18209027, 18590679, and 20390207 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, a Grant‐in‐Aid for Research on Measures for Intractable Diseases, and Research on Advanced Medical Technology from the Ministry of Health, Labor, and Welfare, Japan, and a Grant‐in‐Aid by the Uehara Memorial Foundation, Takeda Science Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Novartis Foundation for the Promotion of Science, Astellas Foundation for Research on Metabolic Disorders, Yakult Bioscience Research Foundation, and the Naito Foundation. None of the authors have competing interests.
References
- 1. Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol 2006; 7:709–14. [DOI] [PubMed] [Google Scholar]
- 2. Liu YJ, Soumelis V, Watanabe N et al TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol 2007; 25:193–219. [DOI] [PubMed] [Google Scholar]
- 3. Soumelis V, Reche PA, Kanzler H et al Human epithelial cells trigger dendritic cell‐mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3:673–80. [DOI] [PubMed] [Google Scholar]
- 4. Ito T, Wang YH, Duramad O et al TSLP‐activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 2005; 202:1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang YH, Ito T, Wang YH et al Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin‐activated dendritic cells. Immunity 2006; 24:827–38. [DOI] [PubMed] [Google Scholar]
- 6. Gilliet M, Soumelis V, Watanabe N et al Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med 2003; 197:1059–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ying S, O'Connor B, Ratoff J et al Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2‐attracting chemokines and disease severity. J Immunol 2005; 174:8183–90. [DOI] [PubMed] [Google Scholar]
- 8. Préfontaine D, Hamid Q. Airway epithelial cells in asthma. J Allergy Clin Immunol 2007; 120:1475–8. [DOI] [PubMed] [Google Scholar]
- 9. Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell‐mediated allergic inflammation. J Allergy Clin Immunol 2007; 120:238–44. [DOI] [PubMed] [Google Scholar]
- 10. Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 2008; 8:183–92. [DOI] [PubMed] [Google Scholar]
- 11. Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol 2008; 8:193–204. [DOI] [PubMed] [Google Scholar]
- 12. Holgate ST. Pathogenesis of asthma. Clin Exp Allergy 2008; 38:872–97. [DOI] [PubMed] [Google Scholar]
- 13. Zhou B, Comeau MR, De Smedt T et al Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 2005; 6:1047–53. [DOI] [PubMed] [Google Scholar]
- 14. Al‐Shami A, Spolski R, Kelly J et al A role for TSLP in the development of inflammation in an asthma model. J Exp Med 2005; 202:829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seshasayee D, Lee WP, Zhou M et al In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest 2007; 117:3868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bettelli E, Oukka M, Kuchroo VK. TH‐17 cells in the circle of immunity and autoimmunity. Nat Immunol 2007; 8:345–50. [DOI] [PubMed] [Google Scholar]
- 17. Annunziato F, Cosmi L, Santarlasci V et al Phenotypic and functional features of human Th17 cells. J Exp Med 2007; 20:1849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson NJ, Boniface K, Chan JR et al Development, cytokine profile and function of human interleukin 17–producing helper T cells. Nat Immunol 2007; 8:950–7. [DOI] [PubMed] [Google Scholar]
- 19. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)‐17 cells requires transforming growth factor‐β and induction of the nuclear receptor RORγt. Nat Immunol 2008; 9:641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Volpe E, Servant N, Zollinger R et al A critical function for transforming growth factor‐β, interleukin 23 and proinflammatory cytokines in driving and modulating human TH‐17 responses. Nat Immunol 2008; 9:650–7. [DOI] [PubMed] [Google Scholar]
- 21. Yang L, Anderson DE, Baecher‐Allan C et al IL‐21 and TGF‐β are required for differentiation of human T(H)17 cells. Nature 2008; 454:350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laurence A, O'Shea JJ. TH‐17 differentiation: of mice and men. Nat Immunol 2007; 8:903–5. [DOI] [PubMed] [Google Scholar]
- 23. Molet S, Hamid Q, Davoine F et al IL‐17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 2001; 108:430–8. [DOI] [PubMed] [Google Scholar]
- 24. Chakir J, Shannon J, Molet S et al Airway remodeling‐associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 2003; 111:1293–8. [DOI] [PubMed] [Google Scholar]
- 25. Bullens DM, Truyen E, Coteur L et al IL‐17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 2006; 7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shannon J, Ernst P, Yamauchi Y et al Differences in airway cytokine profile in severe asthma compared to moderate asthma. Chest 2008; 133:420–6. [DOI] [PubMed] [Google Scholar]
- 27. Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993; 307:982–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schaller M, Hogaboam CM, Lukacs N et al Respiratory viral infections drive chemokine expression and exacerbate the asthmatic response. J Allergy Clin Immunol 2000; 118:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Falsey AR, Hennessey PA, Formica MA et al Respiratory syncytial virus infection in elderly and high‐risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 30. Greene CM, McElvaney NG. Toll‐like receptor expression and function in airway epithelial cells. Arch Immunol Ther Exp 2005; 53:418–27. [PubMed] [Google Scholar]
- 31. Sha Q, Truong‐Tran AQ, Plitt JR et al Activation of airway epithelial cells by toll‐like receptor agonists. Am J Respir Cell Mol Biol 2004; 31:358–64. [DOI] [PubMed] [Google Scholar]
- 32. Kato A, Favoreto S Jr, Avila PC et al TLR3‐ and Th2 cytokine‐dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 2007; 179:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kadowaki N, Ho S, Antonenko S et al Subsets of human dendritic cell precursors express different Toll‐like receptors and respond to different microbial antigens. J Exp Med 2001; 194:863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watanabe N, Wang YH, Lee HK et al Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 2005; 436:1181–5. [DOI] [PubMed] [Google Scholar]
- 35. Watanabe N, Hanabuchi S, Soumelis V et al Human thymic stromal lymphopoietin promotes dendritic cellmediated CD4+ T cell homeostatic expansion. Nat Immunol 2004; 5:426–34. [DOI] [PubMed] [Google Scholar]
- 36. Ho IC, Glimcher LH. Transcription: tantalizing times for T cells. Cell 2002; 109:S109–20. [DOI] [PubMed] [Google Scholar]
- 37. Rochman I, Watanabe N, Arima K et al Direct action of thymic stromal lymphopoietin on activated human CD4+T cells. J Immunol 2007; 178:6720–4. [DOI] [PubMed] [Google Scholar]
- 38. Akamatsu T, Watanabe N, Kido M et al Human TSLP directly enhances expansion of CD8+T cells. Clin Exp Immunol 2008; 154:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sallusto F, Lenig D, Förster R et al Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708–12. [DOI] [PubMed] [Google Scholar]
- 40. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745–63. [DOI] [PubMed] [Google Scholar]
- 41. Campbell JJ, Murphy KE, Kunkel EJ et al CCR7 expression and memory T cell diversity in humans. J Immunol 2001; 166:877–84. [DOI] [PubMed] [Google Scholar]
- 42. Rivino L, Messi M, Jarrossay D et al Chemokine receptor expression identifies pre‐T helper (Th)1, pre‐Th2, and nonpolarized cells among human CD4+ central memory T cells. J Exp Med 2004; 200:725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bratke K, Lommatzsch M, Julius P et al Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax 2007; 62:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zuniga EI, McGavern DB, Pruneda‐Paz JL et al Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol 2004; 5:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


