Abstract
The outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has posed the world at a pandemic risk. Coronavirus-19 disease (COVID-19) is an infectious disease caused by SARS-CoV-2, which causes pneumonia, requires intensive care unit hospitalization in about 10% of cases and can lead to a fatal outcome. Several efforts are currently made to find a treatment for COVID-19 patients. So far, several anti-viral and immunosuppressive or immunomodulating drugs have demonstrated some efficacy on COVID-19 both in vitro and in animal models as well as in cases series. In COVID-19 patients a pro-inflammatory status with high levels of interleukin (IL)-1B, IL-1 receptor (R)A and tumor necrosis factor (TNF)-α has been demonstrated. Moreover, high levels of IL-6 and TNF-α have been observed in patients requiring intensive-care-unit hospitalization. This provided rationale for the use of anti-rheumatic drugs as potential treatments for this severe viral infection. Other agents, such as hydroxychloroquine and chloroquine might have a direct anti-viral effect. The anti-viral aspect of immunosuppressants towards a variety of viruses has been known since long time and it is herein discussed in the view of searching for a potential treatment for SARS-CoV-2 infection.
Keywords: SARS-CoV-2, Infection, COVID-19, Coronavirus, Autoimmunity, Rheumatic, Immunosuppressant. IL-1, IL-6, Biologics, DMARDs, tDMARDs
Highlights
-
•
COVID-19 is a potentially life-threatening condition characterized by interstitial pneumonia with acute respiratory distress and a cytokine storm in severe phases.
-
•
Some anti-rheumatic drugs including chloroquine, hydroxychloroquine and tocilizumab have proved, in preliminary studies, a beneficial effect.
-
•
Baricitinib may target the ACE2 receptor used by the virus to enter the cells and fedratinib may impair the Th17-mediated responses.
-
•
It is important to balance between the anti-viral effects and the immunosuppressive effects.
1. Introduction
The outbreak of the 2019-coronavirus (SARS-CoV-2) has posed the world at a pandemic risk [[1]]. Coronavirus-19 disease (COVID-19) is an infectious disease caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), an enveloped non-segmented positive-sense RNA β-coronavirus belonging to the same family as severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS) viruses, which causes pneumonia, requires intensive care unit hospitalization in about 10% of cases and can lead to a fatal outcome [[2], [3], [4], [5], [6]]. COVID-19 is characterized in severe cases by an acute respiratory distress syndrome (ARDS) and cytokine release syndrome and there is an immediate need for any agent before the development of a vaccine [2]. To date, there is no registered molecule for the treatment of COVID-19 patients. However, there are ongoing trials on the use of some anti-viral and immunosuppressive or immunomodulating drugs that have demonstrated efficacy on COVID-19 both in vitro and in animal models as well as in small cases series [7]. Certainly, previous experiences on viruses belonging to the same β-coronavirus family have formed the cornerstones of the current therapeutic strategy [8,9]. The emergency facing the scientific community in addressing the pandemic from COVID-19 provides the rationale for the use of drugs that have not yet been approved and with still preliminary scientific evidence. So far, therapeutic regimes include a combination of anti-viral drugs and supportive care.
Accumulating evidence suggests that SARS-CoV-2 infection is associated with a pro-inflammatory status characterized by high levels of different cytokines, including interleukin (IL)‐1β, IL‐1Rα, IL-2, IL‐10, fibroblast growth factor (FGF), granulocyte-macrophage colony stimulating factor (GM-CSF), granulocyte-colony stimulating factor (G-CSF), interferon-γ-inducible protein (IP10), monocyte chemoattractant protein (MCP1), macrophage inflammatory protein 1 alpha (MIP1A), platelet derived growth factor (PDGF), tumor necrosis factor (TNFα) and vascular endothelial growth factor (VEGF). Critically ill patients requiring admission to intense care unit (ICU) display markedly high concentration of IL-2, IL-10, G-CSF, IP10, MCP1, MIP1A, TNFα and IL-6. Interestingly, levels of IL-6 also correlated with increased mortality. Moreover, in severe COVID-19, a reduction of natural killer cells, CD4+ and CD8+ T lymphocytes and IFNγ expression in CD4+ cells, has been observed. Levels of IL-6, IL-10 and TNFα inversely correlates with lymphocyte count, suggesting that the cytokine release syndrome may hamper the adaptative immune response against SARS-CoV-2 infection [10,11]. Moreover, high levels of ferritin were demonstrated in patients requiring ICU hospitalization [2]. This provided rational for the use of several anti-rheumatic drugs as potential treatments for this severe viral infection, while other preliminary experiments suggested a direct anti-viral effect of some of them at least in vitro. For instance, chloroquine (CQ) and hydroxychloroquine (HCQ) are currently used to face COVID-19 [12]. Tocilizumab, an anti-IL-6 monoclonal antibody approved for the treatment of patients with rheumatoid arthritis (RA), has been used with encouraging results in particularly ill patients since a massive release of pro-inflammatory cytokines, especially IL-6, by may occurs in lung epithelium in severe cases [13]. Trials to test the efficacy of Tocilizumab on severe COVID-19 patients are ongoing [14,15] (Table 1 ).
Table 1.
Ongoing Clinical Trials on rheumatologic drugs in COVID-19 (last updated on the 1st of April 2020).
| Compound | Title | Study type [ID] | Status | Condition |
|---|---|---|---|---|
| Hydroxychloroquinea | Efficacy and Safety of Hydroxychloroquine for Treatment of Pneumonia Caused by 2019-nCoV (HC-nCoV) | Interventional RCT P/A [NCT04261517] |
Recruiting (phase 3) | COVID-19 pneumonia |
| Proflaxis for Healthcare Professionals Using Hydroxychloroquine Plus Vitamin Combining Vitamins A, C, D and Zinc During COVID-19 Pandemia | Observational case-control prospective [NCT04326725] | Recruiting (phase N/A) | Healthcare personnel exposed to COVID-19 | |
| Randomized Controlled Clinical Trials of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) | Interventional RCT P/A [NCT04307693] |
Recruiting (phase 2) | Mild COVID-19 | |
| Post-exposure Prophylaxis for SARS-Coronavirus-2: A Pragmatic Randomized Clinical Trial | Interventional RCT P/A [NCT04308668] |
Recruiting (phase 2, 3) | symptomatic COVID19 | |
| Norwegian Coronavirus Disease 2019 Study: An Open Labeled Randomized Controlled Pragmatic Trial to Evaluate the Antiviral Effect of Chloroquine in Adult Patients With SARS-CoV-2 Infection | Interventional RCT P/A [NCT04316377] |
Recruiting (phase 4) | established COVID-19 |
|
| Evaluation of the Safety and Clinical Efficacy of Hydroxychloroquine Associated With Azithromycin in Patients With Pneumonia Caused by Infection by the SARS-CoV2 Virus - Alliance COVID-19 Brasil II - Severely-ill Patients | Interventional RCT P/A [NCT04321278] |
Recruiting (phase 3) | COVID-19 pneumonia | |
| Multi-centre, Adaptive, Randomized Trial of the Safety and Efficacy of Treatments of COVID-19 in Hospitalized Adults | Interventional RCT P/A [NCT04315948] |
Recruiting (phase 3) | established COVID-19 | |
| Treatment of Non-severe Confirmed Cases of COVID-19 and Chemoprophylaxis of Their Contacts as Prevention Strategy: a Cluster Randomized Clinical Trial (PEP CoV-2 Study) | Interventional RCT P/A [NCT04304053] |
Recruiting (phase 3) | Non-severe COVID-19 and contacts | |
| Baricitinib | Baricitinib Combined With Antiviral Therapy in Symptomatic Patients Infected by COVID-19: an Open-label, Pilot Studyb | Interventional CT Non Randomized Crossover Assignment [NCT04320277] |
Recruiting (phase 3) | mild to moderate COVID-19 |
| Sarilumab | An Adaptive Phase 2/3, Randomized, Double-Blind, Placebo-Controlled Study Assessing Efficacy and Safety of Sarilumab for Hospitalized Patients With COVID-19 | Interventional RCT P/A[NCT04315298] |
Recruiting (phase 2, 3) | Severe/critical COVID-19 |
| Cohort Multiple Randomized Controlled Trials Open-label of Immune Modulatory Drugs and Other Treatments in COVID-19 Patients - Sarilumab Trial | Interventional RCT P/A [NCT04324073] |
Recruiting (phase 2, 3) | COVID-19 pneumonia | |
| Sarilumab/Tocilizumab | Effectiveness of Interleukin-6 Receptor Inhibitors in the Management of Patients With Severe SARS-CoV-2 Pneumonia: An Open-Label, Multicenter Sequential and Cluster Randomized Trial | Interventional RCT Sequential Assignment [ NCT04322773] |
Not yet recruiting (phase 2) | COVID-19 with respiratory failure |
| Tocilizumab | A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia | Interventional RCT P/A [ NCT04320615] |
Not yet recruiting (phase 3) | COVID-19 pneumonia |
| Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019-A Multicenter, Randomized and Controlled Clinical Trial Study | Interventional RCT P/A [NCT04310228] |
Recruiting (phase N/A) | COVID-19 pneumonia | |
| A Retrospective Study of Evaluating Safety and Efficacy of Tocilizumab Compared to Continuous Renal Replacement Therapy in Controlling CRS Triggered by COVID-19 | Observational Cohort Retrospective [NCT04306705] |
Recruiting (phase N/A) | Severe COVID-19 | |
| Multicenter Study on the Efficacy and Tolerability of Tocilizumab in the Treatment of Patients With COVID-19 Pneumonia | Interventional CT Single Group Assignment [NCT04317092] |
Recruiting (phase 2) | COVID-19 pneumonia | |
| Tocilizumab (RoActemra) as Early Treatment of Patients Affected by SARS-CoV2 Infection With Severe Multifocal Interstitial Pneumonia | Interventional CT Single Group Assignment [NCT04315480] |
Not yet recruiting (phase 3) | COVID-19 pneumonia | |
| Anakinra/Tocilizumab | A Prospective, Randomized, Factorial Design, Interventional Study to Compare the Safety and Efficacy of Combinations of Blockade of Interleukin-6 Pathway and Interleukin-1 Pathway | Interventional CT Factorial Assignment [NCT04330638] |
Not yet recruiting (phase 4) | COVID-19 pneumonia |
| Anakinra/Emapalumab | Efficacy and Safety of Emapalumab and Anakinra in Reducing Hyperinflammation and Respiratory Distress in Patients With COVID-19 Infection | Interventional RCT P/A [NCT04324021] |
Not yet recruiting (phase 2, 3) | COVID-19 with respiratory failure |
| Adalimumab | A randomized, open-label, controlled trial for the efficacy and safety of Adalimumab Injection in the treatment of patients with severe novel COVID-19 | Interventional RCT P/A [ChiCTR2000030089] |
Not yet recruiting (phase 4) | Severe/critical COVID-19 |
| IVIg | A Randomized, Open-label, Controlled, Single-centre Study to Evaluate the Efficacy of Intravenous Immunoglobulin Therapy in Patients With Severe 2019- nCoV Pneumonia | Interventional RCT P/A [NCT04261426] |
Recruiting (phase 2, 3) | COVID-19 pneumonia |
| Immunoglobulin From Cured 2019-nCoV Pneumonia Patients | An Exploratory Clinical Study on the Treatment of Acute Severe 2019-nCoV Pneumonia With Immunoglobulin From Cured 2019-nCoV Pneumonia Patients | Interventional CT Non Randomized P/A [NCT04264858] |
Phase N/A | COVID-19 pneumonia |
| IFN | Clinical study for combination of anti-viral drugs and type I interferon and inflammation inhibitor TFF2 in the treatment of novel coronavirus pneumonia | Interventional Sequential Assignment [ChiCTR2000030262] |
Phase N/A | COVID-19 pneumonia |
| GM-CSF | Sargramostim in Patients With Acute Hypoxic Respiratory Failure Due to COVID-19 (SARPAC) | Interventional RCT P/A [NCT04326920] |
Recruiting (phase 4) | COVID-19 with respiratory failure |
| Naproxene | Efficacy of Addition of Naproxen in the Treatment of Critically Ill Patients Hospitalized for COVID-19 Infection | Interventional RCT P/A [NCT04325633] |
Not yet recruiting (phase 3) | Severe/critical COVID-19 |
| Methylprednisolone/Dexamethasone | Efficacy and Safety of Corticosteroids in COVID-19: A Prospective Randomized Controlled Trails | Interventional CT Single Group Assignment [NCT04273321] |
Recruiting (phase N/A) | COVID-19 pneumonia |
| Glucocorticoid Therapy for Critically Ill Patients With Severe Acute Respiratory Infections Caused by Noval Coronovirus 2019-nCoV: a Prospective, Randomized Controlled Trial | Interventional RCT P/A [NCT04244591] |
Recruiting (phase 2, 3) | Severe/critical COVID-19 | |
| Phase 2, Randomized, Open-label Study to Compare Efficacy and Safety of Siltuximab vs. Corticosteroids in Hospitalized Patients With COVID19 | Interventional RCT P/A [NCT04329650] |
Not yet recruiting (phase 3) | COVID-19 pneumonia | |
| Prolonged Low Doses of Methylprednisolone for Patients With COVID-19 Severe Acute Respiratory Syndrome | Interventional CT Single Group Assignment [NCT04323592] |
Recruiting (phase 2, 3) | COVID-19 with respiratory failure | |
| An Open, Prospective/Retrospective, Randomized Controlled Cohort Study to Compare the Efficacy of Different Hormone Doses in the Treatment of 2019-nCoV Severe Pneumonia | Interventional RCT P/A [NCT04263402] |
Recruiting (phase 4) | Severe/critical COVID-19 | |
| Efficacy of Dexamethasone Treatment for Patients With ARDS Caused by COVID-19 | Interventional RCT P/A [NCT0432506] |
Not yet recruiting (phase 4) | COVID-19 with respiratory failure | |
| COVID-19-associated ARDS Treated With DEXamethasone: an Open-label, Randomized, Controlled Trial: CODEX (Alliance Covid-19 Brasil III) | Interventional RCT P/A [NCT04327401] |
Not yet recruiting (phase 3) | COVID-19 with respiratory failure |
ID: NCT, Clinicaltrial.gov identifier, ChiCTR, Chinese Clinical Trial Registry; P/A, parallel assignment; RCT, Randomized Clinical Trial; N/A, not applicable; IVIg, intravenous immunoglobulins; IFN, interferon.
Only trials in recruiting status were included about the use of Hydroxychloroquine.
Controls. All consecutive patients with mild to moderate COVID-19 infection, older than 18, admitted during the previous 2 weeks, who were treated with antiviral and/or hydroxychloroquine.
Nonetheless, in the last two decades, rheumatology has known an incredible revolution in terms of available treatment and outcome for patients with several molecules that can now be used in the armamentarium of the clinician [16]. These molecules are targeted towards specific mechanisms in immune pathogenesis of rheumatic diseases, either blocking/modulating inflammatory pathways and specific cell subpopulations, apoptosis, autophagy and other mechanisms that could hamper selective viral responses.
2. Glucocorticoids
Glucocorticoids (GCs) are among the most frequently used agent in rheumatologic clinical practice. The GCs have proved to be effective in the management of a broad spectrum of diseases such as RA, systemic lupus erythematosus (SLE), vasculitis both representing the main treatment or a bridging/supporting therapy to the immunosuppressant [[17], [18], [19], [20]].
Glucocorticoids are lipophilic molecules able to activate cytoplasm receptors and then, translocating into the cellular nucleus, to change the expression of several genes, including those responsible for inflammatory response. GCs promote anti-inflammatory proteins transcription such as IL-10 and, simultaneously, impair the expression of pro-inflammatory proteins such as IL-1, IL-2, IL-6, TNF- α and IFN- γ. GCs can also inhibit leukocytes migration to the inflammation site [[21], [22], [23], [24]].
Therefore, it is not surprising that GCS are burdened with numerous and well-known side effects, including the increased infection risk [[25], [26], [27], [28]]. For instance, in RA patients the use of 7.5 mg or more of daily prednisone can increase the risk of infection requiring hospitalization up to six times than in the control group [29,30]. On the other hand, an improved RA disease activity achievable with glucocorticoids is associated with lower serious infection rates [31]. Moreover, these drugs appear to be independent predictors of infection-related hospitalization [32]. Although most of the GC-related reported infections are bacterial, viral infections have been reported too, primarily represented by HSV and HBV reactivation [25,33]. The most debating question is the possible usage of GCs during COVID-19 pandemic and, in case, which, at which disease stage, and at which dosage/administration route. Hydrocortisone showed to improve respiratory and mortality outcome in bacterial severe community-acquired pneumonia [34] and inconstant efficacy in septic shock [35,36]. Budesinide might reduce post viral-inflammatory-cytokine production by human bronchial epithelial cells in vitro [37], while discordant results are reported in viral influenza pneumonia.
Based on the current evidences, as reported by the WHO regarding COVID-19, GCs should not be routinely given for treatment of viral pneumonia outside of clinical trials [38,39].
Various studies reported that GCs administration in patients with severe influenza pneumonia was associated with a higher rate of mortality [[40], [41], [42], [43], [44]]. A meta-analysis conducted with a total of 6548 patients with influenza pneumonia (H7N9 or H1N1), found the use of systemic GCs (methylprednisolone with different dose ranges, when reported) associated with higher mortality rate (risk ratio [RR] 1.75, 95% confidence interval [CI] 1.30–2.36, Z = 3.71, P = 0.0002), longer intensive care unit permanence and higher rate of secondary infection [[44], [45], [46]].
The use of systemic GCs, especially methylprednisolone, in MERS-CoV-infected patients, was found as one of the most significant factors that contributed to increased mortality, with an odd ratio of 3.85 [47]. Nonetheless, no information about dose and duration of the treatment were reported in this retrospective study. As reported in a recent Cochrane analysis, these data are predominantly based on observational studies and mostly of low quality [46,48]. In fact, Li et al. observed in a prospective trial, that the use of low to moderate dose of GCs on 2141 patient infected by H1N1 virus, 54.2% complicated by ARDS, significantly reduced both 30 and 60-day mortality (aHR 0.49 [95% CI 0.32–0.77] (aHR 0.51 [95% CI 0.33–0.78]) [49]. Analysing the outcome of MERS-CoV infected patients treated with GCs, Arabi et al. did not find a rise in death rates correlated to the use of the drug but found delayed lower respiratory tract clearance of MERS-CoV RNA [50]. Equally, the use of GCs seemed to protract SARS-CoV viral clearance [[51], [52], [53]].
Nonetheless, in reference to ARDS, at the moment there is very low evidence on the positive effect of the treatment in this critical condition [54]. There is not conclusive evidence that supports the use of GCs in the treatment of viral respiratory infection and their use remains controversial in COVID-19 [52]. An expert consensus form the Chinese Thoracic Society developed few basic principles for the use of GCs in COVID-19 patients [55,56]. There is evidence that in patients with confirmed ARDS, not infected with COVID-19, a benefit from dexamethasone is provided when used at a low dosage and for a limited period (10 days) in reducing the consequences of mortality [28] thus supporting the assistance of such therapy on elective indication.
As mentioned above, GCs inhibit immune reactions acting on migration and chemokines production. [[21], [22], [23], [24]]; thus, this might lead to prolonged viremia and can impair viral clearance. In addition, it is important not to forget the other corticosteroid-related adverse effects, especially on the cardio-vascular system, which can be another precipitating factor in these critically ill patients that are often already comorbid.
3. Nonsteroidal anti-inflammatory drugs
The nonsteroidal anti-inflammatory drugs (NSAIDs) are available over the counter for several indications and are prescribed widely for symptomatic relief for pain, fever, and inflammation.
The inhibitory effect of NSAIDs on prostaglandin synthases, also known as cyclo-oxygenases (COX) 1 and 2, reduces the production of prostaglandins (PGs). Many NSAIDs blocks both COXs, such as ibuprofen, while NSAIDs selective for COX-2, including celecoxib and diclofenac, selectively target COX2 thought to be of most relevance to prostanoid formation in inflammation. Acetaminophen belongs to NSAIDs but its effects on COX activity remain controversial being a weak, reversible, isoform-nonspecific COX inhibitor [57]. The COX-2 is an important mediator of inflammation in response to viral infection, and it contributes to viral replication, for CMV, HCV, and viruses belonging to the Flaviviridae family. Recently, it has been documented that compounds that significantly inhibit COX-2 enzymatic activities and prostaglandin E2 levels, associated with viral replication, showed therapeutic safety and efficacy in vitro and in vivo for Flaviviridae infection [58].
Evidence on the severe pneumonia related to the avian influenza A H7N9 virus documented that treatment with a combination of antiviral and COX2-selective NSAID was able to ameliorate pulmonary inflammation in mice [59]. Interestingly, the tumorigenic potential of COX-2 and PGE2 through eicosanoid receptors is described in several viral associated malignancies such as the Kaposi's sarcoma-associated herpes virus and support COX-2 as a therapeutic target with an antineoplastic purpose [60]. Several years ago, authors have shown that the spike protein of SARS-CoV activated the COX-2 expression via ERK/NF-kB and PI3K/JNK pathways [61]. These studies provided key findings on molecular mechanisms involved in the viral infection and the induction of inflammation by SARS-CoV. Few data on the inhibitory effects of Ibuprofen on ACE2 were reported in animal models [62].
Naproxen is an inhibitor of both COX-2 and of Influenza A virus nucleoprotein (NP) [63]. This drug may have the potential to present antiviral properties against SARS-CoV-2. A recent clinical trial showed that the combination of clarithromycin, naproxen and oseltamivir reduced mortality of patients hospitalized for H3N2 Influenza infection [64]. A phase 3 clinical trial is ongoing to explore the efficacy of addition of naproxen in the standard treatment of critical patients hospitalized for COVID-19 [65]. However, since the use of both NSAIDs and acetaminophen could be associated with a masking of the symptoms during COVID-19, and thus to a diagnostic delay and a prolonged illness, complications may be more common when NSAIDs are used—both respiratory and cardiovascular [66]. In patients with acute respiratory infection, NSAIDs have been widely used and evidence suggests that in these circumstances NSAIDs are associated with an increased risk of stroke and myocardial infarction [[67], [68], [69]]. Moreover, large randomized trials support that NSAIDs may cause more prolonged illness or complications when taken during respiratory tract infections [70,71]. Patients with COVID-19 have a higher risk of hospitalization, critical disease and mortality correlated with age and presence of comorbidities, particularly hypertension. Thus, besides further research evidence relating specifically to people with COVID-19 are awaited, regular symptomatic NSAIDs use should not be recommended as the first line option for managing COVID-19 [72,73].
4. Chloroquine and hydroxychloroquine
Chloroquine (CQ) and its derivative hydroxychloroquine (HCQ) are aminoquinolinic compounds currently registered for the treatment and prevention of malaria and for the treatment of a number of autoimmune diseases. CQ was developed in the 1930s and extensively used during World War II to prevent and treat malaria infection [74], but its use as an antimalarial is now very limited due to the widespread resistance of Plasmodium.
HCQ is currently part of first-line treatment for RA and SLE, due to its immunomodulatory effect. The mechanisms of action are still not fully elucidated, nonetheless some important aspects are well known. CQ is a weak base with a large distribution volume and accumulates at high concentrations in almost all body tissues, including the lungs [75]. After oral administration, it is efficiently absorbed and is present in the blood mainly as a protonated molecule. However, the circulating unprotonated portion can easily cross cell membranes and, due to its basic charge, tends to accumulate in acidic organelles such as lysosomes, Golgi vesicles and endosomes, where it binds to free protons, thus significantly increasing the pH. Thus, a large number of enzymes contained in these organelles, which optimally work in the acidic milieu, are inhibited [76,77]. This mechanism is presumably at the basis of the direct anti-parasitic, anti-viral, and immunomodulatory effects of the molecule. Although most of the evidence in this field regards CQ, there are some data showing similar effects by HCQ. Additionally, current data support that the mechanism of action of the two molecules is equivalent and HCQ appears to be significantly less toxic and has almost completely replaced the use of CQ in the treatment of rheumatic diseases [77,78].
Most of the data on the anti-viral activity of CQ derive from experiments performed on Human Immunodeficiency Virus (HIV), hepatitis A, and influenza viruses [76]. Some viruses, in fact, enter host cells via endocytosis and, following fusion of endosomes with lysosomes, cause the disruption of the host cells to release the nucleic acid and the enzymes required for replication. Similarly, some enveloped viruses require post-translational modifications of envelope glycoproteins by endosomal enzymes in order to complete the maturation process and shed from the host cell. Additionally, enzymatic activity is required in multiple intra-cytoplasmic signal cascade and post-translational modifications that lead to cytokine, such as TNFα and IL-6, and chemokine release [76].
After the outbreak of SARS in the early 2000s, studies have been performed to identify potential effective anti-viral treatments on SARS-CoV-2, including CQ. In fact, coronaviridae seem to require the above-mentioned steps to replicate. In vitro experiments showed that the CQ-treatment of infected Vero E6 cells – a cell lineage commonly used to assess viral replication in vitro –caused a significant inhibition of viral replication when the drug was added within the first 5 h post-infection, with more marked effects when administered before or within the first hour. Interestingly, the half maximal inhibitory concentration (IC50) doses range between 1 and 10 μM, that are easily and safely reachable in humans [79,80]. In addition to the mechanisms described above, CQ seems to inhibit the glycosylation process of angiotensin-converting enzyme (ACE)-2, which may have an effect on the affinity of SARS-CoV for its receptor, further contributing to the inhibition of virus cell entry [80]. Another possible effect through interfering with the bond between PICALM (phosphatidylinositol binding clathrin assembly protein) and clathrin impeding the endocytosis of the virus. Other in vitro data demonstrated that CQ, probably through its pH-dependent enzyme inhibitory effect, can significantly hamper phosphorylation of p38 mitogen-activated protein kinase (MAPK), thus, blocking replication of the human coronavirus HcoV-229E, similarly to a selective inhibitor of the same enzyme [81] (Fig. 1 ).
Fig. 1.
SARS-CoV-2 replicates in the cytoplasm of an infected host cell. SARS-CoV-2 binds to the host-cell with several receptors, among which is angiotensin converting enzyme (ACE)-2 and PICALM through interaction of the spike (S) glycoprotein. Virus entry into the host cell can occur through fusion with the surface of the host cell, with the subsequent release of the genomic RNA into the cytoplasm. Alternatively, SARS-CoV-2 can enter the host cell through the formation of endocytic vesicles, and genomic RNA is released into the cytoplasm following fusion with the vesicle membrane (not shown). Chloroquine and hydroxychloroquine can alter the glycosylation process of ACE-2, which may have an effect on the affinity of SARS-CoV-2. Moreover, chloroquine can interfere with the bond between PICALM and clathrin impeding the endocytosis of the virus. Finally, chloroquine may alter the pH of the lysosome blocking replication of the virus.
In February 2020, Wang et al. reported the first evidence of the in vitro efficacy of CQ on SARS-CoV-2 with an effective concentration (EC)90 value of 6.90 μM, which can be achievable with clinically used doses [82]. These data have been subsequently confirmed by other authors who compared the in vitro anti-viral activity of CQ and HCQ. HCQ was demonstrated to be more potent than CQ with lower EC50 values, which were even lower for longer incubation time. This effect may be due to the slow and progressive accumulation of HCQ at higher concentrations into the cells [83].
Nevertheless, in vitro data are obtained in ideal experimental conditions and do not necessarily imply effects in vivo. Although very few in vivo data are currently available on SARS-CoV or SARS-CoV-2, some studies have been performed on animal models of coronavirus-related diseases, reporting conflicting results. An interesting study tested the anti-viral activity of CQ against HcoV-OC43 infection in new-born mice [84]. Female pregnant C57BL/6 mothers were administered different solutions of CQ and five-day-old pups were infected with HcoV-OC43 after birth. The highest survival rate was depicted in the group of pups breastfed by CQ-treated mothers with survival rates declining in a dose-dependent manner. On the contrary, the mortality rate in the untreated cohort and the cohort of pups that received CQ transplacentally was 100% [84]. Moreover, other studies have demonstrated the immunomodulatory effect of CQ on TNFα, IL-1β and IL-6 in coronavirus-related feline infectious peritonitis [85], while others have failed to show any significant effect [86]. It is possible that these effects may contribute to the clinical efficacy in COVID-19, also given the rationale behind the use of IL-6 inhibitors.
Taking into account the potential beneficial effect of CQ and HCQ in vitro studies, clinical trials have been recently conducted to confirm these data in subjects affected by COVID-19 infection (Table 1). Preliminary results seem to confirm that CQ can improve COVID-19 associated pneumonia in terms of inhibition of pneumonia exacerbation, amelioration of lung imaging and shortening of disease course [87].
Based on this evidence, CQ and HCQ have been included in most treatment protocols in China and worldwide [88]. However, the risk of adverse events has been heavily underlined [89].
A recent systematic review included six articles and 23 ongoing clinical trials in China. CQ seems to be effective in limiting the replication of SARS-CoV-2 [90]. Gautret et al. reported twenty cases were treated for 6 days with HCQ at the dosage of 600 mg/day plus azithromycin that showed a significant reduction of the viral carriage compared to controls. The authors suggested that azithromycin added to HCQ was significantly more efficient for virus elimination [91].
In rheumatology practice, CQ and HCQ are among the most prescribed medications in patients with RA and connective tissue diseases and are very well tolerated. The potential adverse events, including retinopathy, cardiotoxicity and myelotoxicity, are rare and cumulative dose dependent. The risk of retinal toxicity in patients treated for 10 years with HCQ for SLE was shown to be around 7.5% and higher in patients treated for longer periods [92]. In the COVID-19 associated acute infection, CQ and HCQ are used for a very short time (5–20 days according to expert consensus) [88,93], with a probably negligible risk of adverse events. Nevertheless, acute adverse events, such as hypersensitivity and gastrointestinal intolerance, require attention, especially in critically ill patients that may develop similar clinical manifestations due to COVID-19. Additionally, CQ and HCQ can be safely used during pregnancy [94]. Nonetheless, the ideal dose regimen has not been established yet and most guidelines suggest the same protocols used in rheumatology, i.e. 500 mg BID of CQ or 200 mg BID of HCQ, including those from China and Italy [88,93]. Although clinical trials in vivo are needed to found the best dosing regimen, a physiologically-based pharmacokinetic model suggested a loading dose of HCQ 400 mg BID on the first day, followed by 200 mg BID in the next four days, which should allow to maintain an appropriate plasma concentration up to ten days [83].
To date, anti-malarials are one of the medications with the largest – albeit limited – scientific background warranting their use in COVID-19 treatment protocols. Soon, a significant amount of data should be available from clinical trials, in order to verify whether the theoretical strong effect of the drug has a real impact on survival and recovery of COVID-19 patients. Until then, due to the excellent safety profile and vast experience, their use remains a pillar of current treatment protocols.
5. Immunosuppressants
The other concern for the rheumatologist is that immunocompromised patients may be at higher risk of developing COVID-19, while turning the speech around immunosuppressant drugs may represent unpredictable weapons in this scenario. Indeed, Coronaviruses have not shown to cause a more severe disease in immunosuppressed patients. For this family of viruses, the host innate immune response appears the main driver of lung tissue damage during infection. A review of the mortality and morbidity reports published on SARS-CoV and MERS-CoV, no fatality was reported in patients undergoing transplantation, chemotherapy or other immunosuppressive treatments, at any age. Thus, in this second part of the review, we seek for any immunomodulatory agent that at any potential at least in in vitro studies of anti-viral activity, thus excluding others such as azathioprine [95].
6. Leflunomide
Leflunomide [N-(4-trifluoromethylphenyl)-methylisoxazol-4-carboxamide; HWA 486] (LEF) is a member of the malononitrile amide family of compounds and a marketed therapeutic agent for the treatment of RA, autoimmune disorders, and renal transplantation. Its active form, teriflunomide, approved for the treatment of multiple sclerosis [96], named also A77 1726 [N-(4-trifluoromethylphenyl)- 2-cyano-3-hydroxycrotoamide] exhibits two main mechanisms of action: inhibition of protein tyrosine kinase activity [97] and inhibition of T-cell proliferation. Indeed, it interferes with the de novo pathway of pyrimidine synthesis through inhibition of dihydroorotate dehydrogenase, a key enzyme in the biosynthesis of pyrimidine nucleotide triphosphates (pyNTP) [98]. However, additional mechanisms of activity may remain to be discovered yet.
Numerous studies have already demonstrated several anti-viral effects of LEF [99]. The most important is against cytomegalovirus (CMV) infection in solid organ transplant recipients. The American Society of Transplantation and the Transplantation Society International CMV Consensus Group reported LEF as a possible alternative or experimental therapy in case of extensive resistance to standard anti-viral treatment [100]. Even though randomized controlled trial are lacking, numerous case reports described the efficacy of LEF in treating drug-resistant CMV systemic and ocular infections, or in preventing the recurrence of viremia in transplant patients [101]. In one case report and in a retrospective study, LEF (100 mg for 5 days followed by a maintenance dose of 20 mg every 12 h) resulted to be effective in clearing low-dose viremia and long-term CMV suppression in lung transplantation. It must be noted that this small cohort was already taking other anti-viral agents [102]. Three case reports described that LEF could be able to induce a persistent clearance of CMV UL97 and UL54 (genes of resistance against classical anti-viral therapy) in serum and clinical resolution in transplant patients complicated by active CMV retinitis resistant to treatment with systemic and intra-vitreal foscarnet, ganciclovir and oral valganciclovir [103,104]. Other case reports in hemopoietic stem cell transplant recipients described the use of LEF, alone or in combination with other anti-viral drugs as a salvage therapy for recalcitrant CMV infection, with variable results [105]. In addition, LEF was reported in the treatment of acyclovir-resistant perianal Herpes simplex type 2 (HSV-2) infection in HIV patients [106].
The anti-viral mechanism against CMV is an “off target” mechanism and also applies against β-HSV-1. In fact, even restoring pyrimidine nucleotide triphosphates to normal levels, viral replication is persistently impaired. LEF reduces the in vitro spreading of CMV and HSV-1 infection by interfering with virion cytoplasmatic assembly in a dose-dependent manner [107,108]. This action appears independent of the levels of pyNTP and persists also in CMV strain D16 usually resistant to the action of ganciclovir, cidofovir and foscarnet [109]. In particular, the drug induces failure to acquire tegument and external membrane in the cytoplasm of infected cells. It has been proposed that LEF may interfere with tegument assembly by inhibiting protein phosphorylation of the phosphoproteins [107]. Thus, its anti-viral activity, specifically against CMV and HSV-1, occurs at a late stage of viral assembly, in contrast with traditional anti-CMV agents that inhibit viral DNA replication [110]. Viral protein synthesis does not seem to be affected, while two different studies reported discordant results on the viral DNA production Data from in vivo experiments document that LEF is able in reducing viral load in nude rats [107,108]. LEF can significantly reduce CMV-induced apoptosis of the infected cells, interfering with the spreading of the virus [111]. Bilger et al. showed that teriflunomide can have a role against Epstein-Barr virus (EBV) [112]. Drug concentrations lower than those normally used for treatment of RA can prevent EBV-transformed human B cell proliferation thanks to an “on target” mechanism on B cells and an “off target” action that alter EBV latency protein expression. In fact, an increased expression of EBV nuclear antigen-2 (EBNA2) and latent membrane protein-1 (LMP1), the latter able to inhibit B cell proliferation, were observed [112]. Furthermore, teriflunomide induces expression of p53 and cleavage level of PARP, promoting apoptosis of lymphocytes in vitro. No reduction of nuclear factor kappa-light-chain-enhancer of activated B cell (NF-kB) signaling by teriflunomide was reported in this setting. The drug can inhibit lytic EBV infection in vitro by preventing the initial steps of lytic viral reactivation by reducing immediate-early lytic protein and early lytic protein expression, in response to B-cell receptor, and by blocking lytic viral DNA replication [112].
Intriguingly, LEF has shown to influence HIV-1 replication in vitro. The drug downgrades viral proliferation inhibiting NF-kB activation. Nevertheless, LEF inhibits, through MEK, MAP kinase pathway and protein tyrosine kinase, thus resulting in the reduction of CD28 and ICOS activity; this leads to an increased viral load [113].
Reducing pyrimidine pool, teriflunomide can inhibit the replication of Junìn virus, the agent of Argentine hemorrhagic fever and an enveloped negative-sense ssRNA arenavirus, in a dose-dependent manner. The combination with ribavirin appeared to have a stronger anti-viral activity than monotherapy [114].
The same mechanism of action seems to explain the anti-viral activity of teriflunomide on the foot-and-mouth disease virus, an RNA virus member of the Picornaviridae family, usually affecting livestock breeding [115].
Martin et al. reported preliminary effect on several RNA viruses such us Newcastle disease virus, Rabies virus and Influenza A virus [116]. BK polyomavirus is a DNA virus member of polyomaviridae family and causative agent of infectious hemorrhagic cystitis in transplant recipients and of polyomavirus-associated nephropathy (PVAN), a potentially dreadful complication after kidney transplantation. For several years, LEF has been used as second-line therapy in refractory infection after reducing maintenance immunosuppression. Small case series and a prospective open-label trial analyzed the effect of LEF, used alone or in combination with other drugs (tacrolimus/everolimus, fluoroquinolones), in PVAN; they described a partial success in viremia clearance or better renal outcome [[117], [118], [119]]. Nonetheless, as reported by the American Society of Transplantation Infectious disease, true efficacy of these approaches has not been proved so far because of the lack of randomized controlled- trials. Additionally, a meta-analysis, albeit with various limitations, failed to document significant benefits of these adjunctive therapies [120,121].
No direct anti-viral effect on the Dengue virus was reported by LEF, despite the drug seem able to reduce dendritic cell migration and virus-induced cytokine releasing induced in vitro [122].
Enterovirus 71 (EV-A71) is a neurotropic virus that can cause severe complications such as encephalitis. As it happens in viral infections, such as Dengue fever and most recently COVID-19, a prominent role in the progression of tissue damage and of the severity of the disease appears to be secondary to the resulting cytokine release syndrome. Similarly, in EV-A71 infection, IL-6 seems to be closely correlated with the clinical severity of the disease [123,124]. Hung et al. observed that teriflunomide can significantly reduce EV-A71 yield in infected cells in vitro and is able to induce a 40–50% reduction of IL-6 levels, compared to controls [125].
Interestingly, LEF showed robust efficacy in vitro and in vivo even against respiratory syncytial virus (RSV), an RNA virus. Significant dose-dependent reduction of RSV induced syncytia formation in human Hep-2 has been reported, and when the drug was used on infected cotton rats (30 mg/kg/day), it reduced by > 3 log the pulmonary viral load compared to controls, similarly to what Chong et al. reported for CMV infected rats [126].
The anti-viral effect of high concentrations of the drug was comparable to that of ribavirin, though LEF showed greater anti-viral efficacy than ribavirin at lower concentration [127].
Dose-limiting side-effects of LEF therapy are liver damage, lung disease, immunosuppression, diarrhea, rash, and reversible alopecia [128]. In vivo, high dose of LEF appears to be a potent inhibitor of T cells but also a partial inhibitor of B cell responses and can totally suppress humoral immune response against various antigens [129]. The mechanisms by which LEF can hamper the immune response to viral infections in humans are not known. Although anti-viral effects of LEF against various agents gave been demonstrated in humans, very few data are available on the optimal dosage, treatment duration and target blood levels of teriflunomide for an effective anti-viral activity, and its usage in COVID-19 treatment are doubtful.
7. Mycophenolate mofetil
Mycophenolate mofetil (MMF) is an immunosuppressive agent commonly used for allograft rejections in different organs transplants and in the treatment of autoimmune diseases.
MMF and its active form mycophenolic acid (MA) inhibit inosine monophosphate dehydrogenase (IMPDH) and block the conversion of inosine monophosphate (IMP) to guanosine monophosphate (GMP), hampering lymphocyte proliferation [130]. This action is shared by ribavirin and it is almost the only anti-viral mechanism that has been demonstrated for MMF [131].
Different studies have shown anti-viral properties of MMF against various members of Orthomyxoviridae family, especially against influenza viruses. These are single stranded ribonucleic acid negative-sense (−)ssRNA viruses. MMF and MA have anti-viral activity against influenza viruses A (IAV); MA also against influenza viruses B (IBV). MA can prevent virus-induced cell death by IBV B/411 and by the pandemic IAV(H1N1)pdm09 virus H1/415. It interferes with the early stage of the viral replication, before the protein synthesis [132,133]. Other experiments demonstrated MA anti-viral activity against eight different clinical isolates of A(H1N1), A(H3N2), A(H7N9) and influenza B viruses (IC50 < 1 μM) [133].
Cho et al. demonstrated that MMF, blocking IMPDH, could completely inhibit A/Vietnam/1194/2004 (H5N1) virus mRNA replication and protein expression in Madin-Darby Canine Kidney (MDCK) cells and in infected mice. All infected lab rat treated survived, and the drug leaded to substantially reduced lung viral titers. Additionally, MMF treatment reduced the IL-1β, interferon (IFN)-β, IL-6, and IP-10 mRNA expression levels in MDCK infected cells [132].
Inconclusive results have been obtained against several other strains of influenza virus [134] and there are discordant evidences regarding the action of the drug against Zika virus (ZKV) [135,136]. ZKV is not the only Flavivirus against to MMF was tested. In one study the drug could significantly reduce cytopathic effect in West Nile-virus-infected Vero cells [137] and, in another one, MMF demonstrated also a significantly anti-viral activity against Japanese encephalitis virus in vitro and in vivo, protecting the infected mice with an IC50 of 3.1 μg/ml and a therapeutic index of 16 [138]. Other Flavivirus susceptible to MMF are dengue virus type 2, Yellow fever virus 17D, Modoc virus, and Montana myotis leukoencephalitis virus [[139], [140], [141]]. Some studies report anti-viral effect of the drug against other several different RNA viruses including Chikungunya [142,143], smallpox virus, foot-and-mouth disease virus (FMDV [144], peste des petits ruminants virus (PPRV) [145], Junin virus [146], norovirus [147], the arenavirus causative agent Lassa's hemorrhagic fever [148], the reovirus [149,150] and others [151,152].
The use of MA It has been also reported that the use of MA on rotavirus-infected-Caco2 cells, could induce a 99% inhibition of viral RNA production at the clinically relevant concentration (10 mg/ml), with a persisting effect in time [153].
The anti-viral effect against all these numerous viruses mentioned above appears to be due to the reduction of GMP cell pool, whereas and it can be reversible with the addiction of guanosine in a dose dependent manner [154,155]. Similarly, MMF inhibits viral RNA, mRNA protein synthesis, and it almost completely blocks virus entry and cytopathic effect of human parainfluenza virus type 2 (hPIV-2), but these restrictions are recovered by guanosine and S-(4-nitrobenzyl)-6- thioinosine administration [156]. The only evidence of the presence of another antiviral mechanism of MMF derives from a study on hepatitis C virus (HCV). It seems that the drug is able to inhibit in vitro and in vivo the virus replication by augmentation of IFN-stimulated gene expression [157], suppressing HuH-7 cell autophagy, decreasing autophagosome formation, and increasing p62 level expression in vitro [158]. MERS-CoV caused several fatal infections in the Middle East and traveler-associated cases in Europe and Africa with over 35% fatality since its emergence in 2012 [159]. A clinical retrospective analysis on 51 patients infected by MERS-CoV showed that treatment with MMF was one of the predictors of increased survival [160]. MA together with disulfiram and/or 6-thioguanine seemed able to synergistically inhibit MERS-CoV papain-like protease PLpr [161]. Chan et al. found that a concentration ≤0.063 μg/ml of MMF inhibited cytopathic effects, reduced the viral load and achieved a 100% plaque reduction in Vero cells infected. The combination of MA and IFN-1β showed synergistic activity [162]. Despite these evidences, the same author observed that MMF and MA were ineffective in coronaviridae-infected-animal models. In fact, in one study all MERS-CoV-infected marmosets treated with MMF developed severe and/or fatal disease with higher mean viral loads than the untreated animals, and higher mortality than the ones treated with lopinavir/ritonavir and IFN-1β [163]. Barnard et al. found the drug unable in preventing viral replication in the lungs of SARS-infected BALB/c mice, although it did not significantly increase it, as ribavirin did [164]; the same inefficacy against SARS-CoV replication has been previously reported [165].
Interestingly, MMF could act as a synergistic drug to potentiate the anti-viral effects of other drugs, through the depletion of the endogenous GTP pools [166]. MA increases the anti-HBV activity of penciclovir, lobucavir, 37-fluorodideoxyguanosine, diaminopurine dioxolane [167,168]. Similar results have been obtained with the combination of MMF with other classical anti-viral drugs, such as abacavir, didanosine and tenofovir, treating HSV-1, HSV-2, CMV and others [[169], [170], [171], [172], [173], [174], [175], [176]]. All these evidences could allow the clinician to use of MMF as a support-drug of other anti-viral drug, in specifically clinical contest, even thou the lack of in vivo clinical trial. This is a strong limitation, considering that frequently in vitro activity does not correspond to a satisfactory action in vivo. Nevertheless, MMF is a strong immunosuppressive agent, it reduces the immunity response to vaccination in transplant patients [177], and it is associated with increased risk of viral infections from human herpesvirus 6, herpes zoster (HZ) and CMV [178,179], although data remain inconclusive [180,181].
8. Methotrexate
Methotrexate (4-amino-10-methylfolic acid, MTX), an analogue and antagonist of folic acid, is the first-line disease-modifying anti-rheumatic drugs (DMARDs) in the treatment several rheumatic autoimmune diseases including RA, adult-onset Still's disease (AOSD), and Psoriatic Arthritis (PsA) [182]. Its effectiveness is related to its ability to inhibit key enzymes involved in the biosynthesis of purines and pyrimidines by interfering with dihydrofolate reductase (DHFR) pathway. MTX thus limits the viability of highly replicating cells. Moreover, MTX-induced reactive oxygen species generation modulates different cell functions, such as suppression of cytokine production and cell proliferation decreasing TNF-α, IL-1β, and adhesion molecules (E-selectin and VCAM-1) [183].
Interestingly, DHFR inhibitors have also been investigated to treat infectious diseases [184].
Current evidence supports MTX as a potential treatment of viruses mediated arthritis, specifically Parvovirus B19, HBV, HCV, and HIV [185]. Studies documented that pro-inflammatory mediators were markedly decreased after MTX treatment in virus-induced arthritis [186]. As a remark, recent studies suggest that hepatitis B reactivation or on accelerated liver disease are rather infrequent manifestations during long-term MTX treatment [187].
In vitro findings reported an anti-viral effect for MTX against ZKV through inhibition of DHFR pathway with mechanisms similar to other published results in related Flaviviruses [184].
It is notable that authors documented the efficacy of intravitreal MTX as treatment option for patients with necrotizing retinitis EBV-positive not responding to conventional anti-viral therapy [188]. MTX has a high risk of reactivation demonstrated for several viruses, including the abovementioned HBV and EBV, thus its usage as an anti-viral drug and as therapeutic weapon against COVID-19 may not be suitable.
9. Colchicine
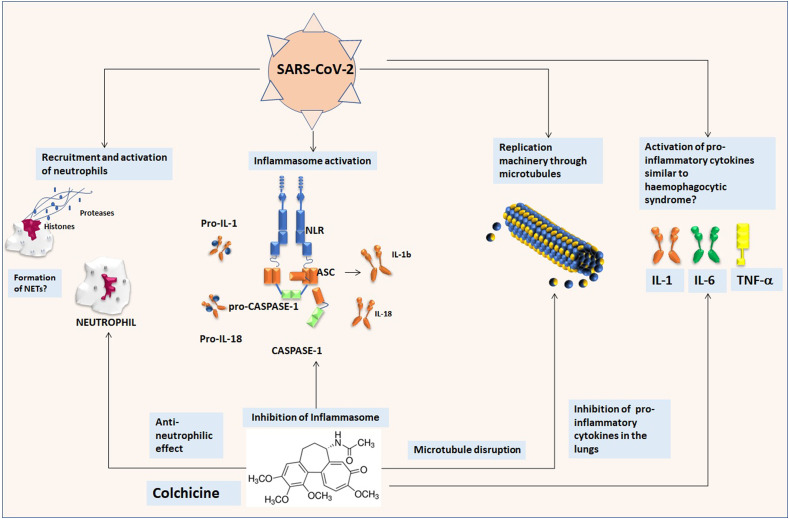
One of the oldest know drugs so far in the field of rheumatology is Colchicine. This is an inexpensive, orally administered, potent anti-inflammatory medication that was initially extracted from the autumn crocus and has been used for centuries [189]. Its mechanisms of actions are through the inhibition of tubulin polymerization and microtubule generation and, possibly, effects on cellular adhesion molecules, inflammatory chemokines, and the inflammasome [190,191]. Colchicine is currently indicated for the treatment of gout, familial Mediterranean fever, and pericarditis [[192], [193], [194]]. Indeed, this agent is the main treatment of several rheumatic conditions [195] in which the activation of the inflammasome and the release of pro-inflammatory cytokines are the key pathogenic events. It alters the organization of actin cytoskeleton by binding to tubulin monomers and inhibits polymer formation [196]. Colchicine drug has shown anti-inflammatory effects, more pronounced on the IL-1 and IL-6 axis. It is highly concentrated in neutrophils and macrophages prolonging its action [197]. Its ability to bind tubulin achieves multiple cellular actions, including inhibition of the assembly of the nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3) inflammasome [198]. In addition to its effect on neutrophils [199,200], colchicine impairs the release of IL-1β into neutrophil extracellular traps [201]. Many of the actions have been demonstrated in patients with coronary disease [202,203]. Colchicine blocks the NLRP3 inflammasome, a cytosolic complex responsible for the production of IL-1β and IL-18. In patients with acute coronary syndromes, in which IL-1β, IL-18, and downstream IL-6 that are key inflammatory cytokines, colchicine administration was able to significantly reduce transcoronary gradients of all 3 cytokines in by 40%–88% [204]. A similar reduction in IL-6 but also in IL-8 or TNF-α levels can be observed in mucocutaneous Behcet's disease patients [205]. Moreover, colchicine has anti-viral properties against Flaviviridae and against the recombinant demyelinating strain of mouse hepatitis virus RSA59 as demonstrated reducing virus replication since microtubules are crucial in cell entry and blocking neuronal transport [206,207]. It inhibits RSV replication reducing IL-6 and TNF-α levels [208]. Some studies have suggested that colchicine and colchicine derivatives may have some influence HIV viral load [209]. Certain subsets of the 2003-SARS coronavirus replication machinery have been shown to move in the cell in a manner that corresponds with microtubule-associated transport, inducing the formation of double-membrane vesicles in infected cells. SARS-CoV-2 is a similar virus to SARS-CoV and microtubule disruption may influence viral replication [210]. It was also documented that SARS-CoV and its accessory protein are potent activators of pro-IL-1β gene transcription and protein maturation, and thus are able to activate the NLRP3 inflammasome and that influenza virus M2 or encephalomyocarditis virus 2B proteins stimulate IL-1β secretion following activation of the NLRP3 inflammasome [211], thus making colchicine a promising treatment for these conditions.
Indeed, acute respiratory syndrome related to COVID-19-infection is associated with a wide release of pro-inflammatory cytokines, including interleukin IL-1 and IL-6 [212]. Specifically, the binding of COVID-19 to the toll like receptors leads to the lung inflammation, fever and fibrosis. Thus, it might be useful to obtain a suppression of pro-inflammatory IL-1 family members and IL-6 to reach a therapeutic effect in viral infections. Moreover, a dysregulated activation and inflammatory activity of myeloid cells is one the main pathogenic events characterizing the infection by coronaviruses [213,214]. Colchicine is a well- known inhibitor of the pro-inflammatory mechanisms induced by neutrophils thus it was proposed as an adjuvant treatment for RSV bronchiolitis [15]. Thus, colchicine has broad anti-inflammatory effects, anti-viral properties summarized in Fig. 2 , and it is not hampered by an immunosuppressant effect. It is cheap, which may be of great usefulness in the uneventful case of an outbreak in countries that may not sustain expensive biologic treatments. Colchicine has a narrow therapeutic index; gastrointestinal disturbance may be seen in up to 10% of patients possibly limiting its usage in COVID-19 due to the relatively frequent occurrence of diarrhea in these patients. Nonetheless, potential intravenous administration may reduce this side effect and increase its bioavailability. It could be suggested that colchicine may be trialed in an appropriate COVID-19 patient population to reduce both viral entry and inflammatory status. In this view, an open-label, phase 2 study enrolling patients with COVID-19 disease to evaluate the efficacy and safety of colchicine has been recently promoted by the Italian Society of Rheumatology (SIR), the Italian Society of Infectious and Tropical Diseases (SIMIT) and by the Italian Thoracic Society (AIPO) and has been approved by the Italian Drug Agency (AIFA).
Fig. 2.
Potential effects of colchicine in SARS-CoV-2 infection. a) SARS-CoV-2 provokes a dysregulated activation of the neutrophils that could contrasted by the anti-neutrophilic effect of colchicine. b) SARS-CoV-2 activates the inflammasome with release of pro-inflammatory cytokines including IL-1β, colchicine acts to inhibit the assembly of the nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3) inflammasome activation and lowers IL-1β levels and possible onset of a cytokine storm. c) SARS-CoV-2 may use microtubules for replication machinery for instance in the formation of double-membrane, colchicine is an inhibitor of tubulin polymerization and microtubule generation. d) During COVID-19-infection a wide release of pro-inflammatory cytokines, including IL-1, IL-6 and TNF, has been observed, a phenomena that can be antagonized by colchicine.
Encouraging results are provided by the case reported by Gandolfini et al. who treated a patient with signs of systemic inflammation (plasmatic IL-6: 108.2 pg/ml; normal range 0–10 pg/ml), who received colchicine (1 mg on day 8, and 0.5 mg/day thereafter) with benefit due to worsening pf respiratory functions [215]. So far, the patients is stable, reinforcing that this is a suitable option in patients with COVID-19 disease.
10. Anti-interleukin 1
As known, the innate immune response of the host against infections plays a crucial role in inducing pro-inflammatory host factors, leading to tissue damage. IL-1 is a pleiotropic cytokine with a key role in inflammation, immunity, and hemopoiesis. The major of IL-1 agonistic molecules are IL-1α and IL-1β, which bind to IL-1R type I (IL-1R1) inducing similar biologic functions. The precursor of IL-1α (ProIL-1α) is constitutively expressed in several tissue cells and its expression increases during stress or inflammation, whereas IL-1β is produced mainly by myeloid cells upon inflammatory stimulation and is active as a secreted molecule [216]. IL-1β is up-regulated during respiratory viral infections. Interestingly, community-acquired pneumonia (CAP) observed in children below 5 years of age in India has been related to a phenotype-genotype association with pro-inflammatory cytokines. Specifically, IL-1RA gene polymorphism and cytokine levels were linked with an adverse outcome in severe CAP [217].
Authors reported that IL-1β mediates virus-induced M2 muscarinic receptor dysfunction and airway hyper-reactivity during parainfluenza virus infection [218]. Data from the literature reported that IL-1β and IL-17A are key mediators of neutrophilic inflammation in influenza-induced exacerbations of chronic lung inflammation [219]. Using a mouse model of disease, authors demonstrated the crucial role of IL-1β in driving neutrophilic recruitment and inflammation during the whole phase of viral infection. Blocking of IL-17A or IL-1 resulted to be potential targets for therapeutic treatment of viral exacerbations of chronic lung inflammation [219]. Authors described that inflammasome complex/IL-1β are an essential signaling pathway, which is over activated and directly causes the severe liver disease during viral infection [220].
Anakinra (ANK), a human IL-1R antagonist, has been approved for the treatment of RA, AOSD, and other severe autoimmune and autoinflammatory disorders including tumor necrosis factor receptor-associated periodic syndrome (TRAPS) [221]. The objective of IL‐1Ra–based therapy is to occupy enough IL‐1 receptors with IL‐1Ra to block IL‐1 cell signaling [222]. Rilonacept and Canakinumab are other molecules currently used in clinical practice to treat autoinflammatory diseases. Rilonacept is a long-acting soluble decoy receptor that binds to both IL-1α and IL-1β and prevents their interaction with the IL-1 cell surface receptor [223]. It was approved in 2008 for the treatment of adults and children 12 years of age and older with cryopyrin-associated periodic syndromes (CAPS). Canakinumab is a fully human anti-IL-1β antibody that is used in the therapy of AOSD and other autoinflammatory conditions. Evidence documented that the control of the inflammation achieved with this drug, targeting the IL-1β innate immunity pathway, can reduce inflammatory tissue damage as it is demonstrated in cardiovascular disorders such as atherosclerosis and diabetes [224]. Currently, no evidence of anti-viral properties of these drugs exists but, as widely described, SARS-CoV is capable of inducing a storm of pro-inflammatory cytokines [225]. Several studies suggest that cells infected by SARS-CoV produce elevated levels of IL-1 β which strongly contribute to the immuno-mediated damage to the lungs and other organs, resulting in acute lung injury (ALI) and, subsequently, multi-organ dysfunction. Therefore, the application of IL-1 β antagonists may reduce the severity and mortality of SARS-CoV-2 infection [226].
11. Tumor necrosis factor α inhibitors
TNF-α is a vital cytokine mediating host immune response [227]. Inhibition of TNF leads to a significant decrease of the inflammatory response, and this approach is used in therapy of autoimmune conditions, most effectively in RA, juvenile idiopathic arthritis, PsA, Psoriasis (PsO), Spondyloarthritis (SpA) and inflammatory bowel disease [228].
Five TNF inhibitors (TNFi) have been developed and introduced in clinical medicine: a chimeric monoclonal antibody, infliximab, a soluble recombinant form of the TNF cellular receptor etanercept, two fully human monoclonal antibodies (adalimumab and golimumab) and a humanized Fab fragment of anti-TNF linked to polyethylene glycol, certolizumab pegol [229]. These TNFi show differences in molecular design, route of administration, and potency in clearing TNF-α [229].
TNF gene expression can be induced primarily in cells of the monocyte-macrophage lineage by a variety of inducers, including lipopolysaccharides (LPS) and viruses [230].
The role of specific TNF receptor (TNFR) family members in anti-viral immunity depends on the stage of the immune response being different in accordance with the virus type and its virulence [231]. Depending on conditions, TNFR induces cell survival/apoptosis, and programmed necrosis which may play a role in controlling viral infection [232]. The risks of bacterial and mycobacterial infection are increased in patients receiving biological DMARDs therapy, particularly TNFi [233]. These drugs are relevant immunosuppressants leading to reactivation of latent infections such as hepatitis B and tuberculosis [233]. For instance, hepatitis B and C assessment is required prior to start a TNFi and in patients with a positive HBsAg, infliximab has the most reported cases associated with HBV reactivation [234].
Receptors and ligands of the TNF family play key roles in controlling lymphocyte activation and survival during an immune response. TNF family ligands are being explored as adjuvants for viral vaccines, and agonistic antibodies to TNFR family members are being investigated for immunotherapy of chronic viral infection alone and in combination with checkpoint blockade.
Recent evidence demonstrated that TNFα has potent anti-rotavirus effects achieved by NFκB-regulated genes via the activation of classical NF-κB signaling and independent of type I IFN production. Moreover, the use of infliximab totally abrogates the anti-rotavirus effect of TNF-α [235]. TNFi have potent activity in several diseases marked by excessive production of pro-inflammatory cytokine. That effect may prove useful in viral infections highly characterized by immune activation and inflammation such as HIV-1 infections. HIV-1 infection results in the depletion of CD4+/CD8+T cells with abnormalities in the cytokine network in the infected individuals. HIV-1 uses the TNFα signaling for expanding its reservoir. Several HIV-1 proteins mimic and regulate the TNF-α signaling pathway. Thus, TNFi has been considered as an additional therapeutic option for HIV-1 infection [[236], [237], [238]]. Evidence shows that TNF-α is a key mediator of virus-induced M₂ muscarinic receptor dysfunction and airway hyper-responsiveness in influenza infected-animals [239]. Interestingly, in this model etanercept has been shown to be able in preventing virus-induced airway hyperresponsiveness and M₂ receptor dysfunction, without changing lung viral titres. Nonetheless, all these data do not support enough the use of these molecules as anti-viral drugs. The only potential beneficial mechanism in COVID-19 patients could be reduction of TNF levels that appears to be increased especially in the critical stages. Moreover, a possible role in COVID-19 is the interaction with the ACE2 receptor. Indeed, SARS-CoV-related severe respiratory distress sees associated with the ability of the spike protein of SARS-CoV to down-regulate ACE2 expression [240]. In addition, SARS-CoV modulates with its spike protein the TNF-α-converting enzyme (TACE)- activity by via the cytoplasmic domain of ACE2 leading to both the viral entry and the only potential beneficial mechanism in tissue damage through TNF-α production [241]. Since the downregulation of ACE2 by SARS-CoV spike protein was proposed as an etiological event in the severe lung injury, authors previously suggested that TACE antagonists can block SARS-CoV infection and attenuate the severe clinical outcome [242]. Other studies confirmed the role of TNFα in promoting pathogenesis in SARS-CoV infection through its receptors and suggested that inhibition of TNFα signaling may represent viable chance for effective therapies [243]. Accordingly, evidence reports markedly higher serum levels of TNF-α together with IL2R, IL-6, IL-10, in severe than in moderate cases of COVID-19 [244]. A phase 4 randomized, open-label, controlled trial for the efficacy and safety of Adalimumab especially in those patients with particularly elevated TNF levels in the critical stages with severe COVID-19 is ongoing in Shanghai with the main outcome of Time to Clinical Improvement [245].
12. Tocilizumab
IL-6 is a cytokine produced by innate immune cells, mostly monocytes and macrophages, and also by mesenchymal, endothelial cells and fibroblasts, after the recognition of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [246]. IL-6 stimulates acute-phase immune response and hematopoiesis and it is involved in the complex pathogenesis of autoimmune disease like RA, Giant Cell Arteritis (GCA), Systemic Juvenile Idiopathic Arthritis (SJIA), and of cytokine release syndrome (CRS). CRS is a possible consequence of the administration of chimeric antigen receptor engineered T cells (CAR-T) immunotherapy, recently approved for the treatment of B-cell leukemias and lymphomas [[246], [247], [248], [249]]. Tocilizumab, a humanized monoclonal antibody directed against IL-6 receptor, is capable to reduce and control the effects of the abnormal production of IL-6 and is approved by FDA and EMA for the treatment of RA and these diseases [250]. CRS related to CAR-T therapy can be caused directly by the infused CAR-T cells or through the activation of other immune cells and usually manifests with a clinical presentation that can range from a smolder condition to a multiple organ failure [251]. Since COVID-19 has some clinical similarities eventually progressing in the 15% of cases in ARDS, acute cardiac injury and acute kidney disease requiring ICU monitoring and a key role seems played by IL-6 that is markedly elevated along with TNF-α, IFN-γ, IL-2, IL-10, IL-8 and granulocyte macrophage–colony-stimulating factor (GM-CSF), the use of Tocilizumab (IV 8 mg/kg i.v.) was proposed [[252], [253], [254]].
Indeed, retrospective analysis of 452 patients admitted in the Tongji hospital for COVID-19 showed that inflammatory cytokine levels in patients with severe infection compared with non-severe, were significantly elevated, including IL-6, IL-2R, IL-8, IL-10, and TNF-α [214]. Analogous findings were reported by Lei et al. in a cohort of 29 Chinese patients: the levels of IL-2R and IL-6 in peripheral blood of patients with COVID-19 pneumonia were significantly increased and related to the severity of the disease [252].
Even though tocilizumab does not seem to have any direct anti-viral effect, the elevated serum levels of IL-6 in severe COVID and the evidence of a cytokine pattern similar to CRS led to the hypothesis of the use of this drug as treatment of severe respiratory infections whose manifestations could be correlated to the abnormal innate immune response stimulated by IL-6. A randomized controlled clinical trial is actually undergoing in China and another has been started in Italy, as abovementioned. Tocilizumab has already been used in the past weeks as an off-label drug in the absence of a specific therapy for the treatment of severe manifestations of COVID (ICU and not-ICU patients) in Italy during the epidemic outbreak that is leading to an emergency situation with more than 4000 victims [12,13]. Notably, another anti-IL6 drug is currently available for the treatment of RA, Sarilumab, a human monoclonal antibody against the IL-6 receptor. Tocilizumab is available both IV as well as subcutaneously, while Sarilumab only subcutaneously, possibly limiting its usage due to the slower effect in an acute scenario as that of ARDS or pre-ARDS from SARS-CoV-2 infection.
13. IVIg
Intravenous (IV) immunoglobulins (Ig), therapeutic preparations of pooled normal serum derived poly-specific IgG are widely used for the treatment of several immune-mediated conditions and rheumatologic diseases [255]. The immunomodulating properties of IVIg seem to be related to a complex and not yet understood direct action both on innate immune components (complement, monocytes, macrophages, natural killer, cells) and on adaptive immune cells such as CD4 T-cells and B-cells [255].
Far before the use of IVIg as immunomodulating therapy, serum Ig were identified as one of the cardinal constituents of the adaptive immune system against microorganisms (especially viruses, bacteria). The bond of Ig to viral superficial antigen is capable to prevent virus cell penetration, induce phagocytosis and clearance of viral structure by macrophages and neutrophils and finally to activate NK cells to eliminate infected cells. Enriched Ig preparation extracted from human placenta and then from human serum can be considered the first anti-viral drug and the first example of the use of immunotherapy against an infection [256].
Intramuscular Ig were first used to prevent HAV and measles in the ‘40s later in the ‘80s of intravenous preparation with reduced anaphylaxis risk, specific Ig preparations developed from donors with high titers of desired antibodies (the so called hyperimmune preparations) were used to prevent and treat a variety of viral infections (HAV, rabies, CMV, varicella zoster virus (VZV), Measles etc.) [257].
As derivatives from blood of healthy donors, IVIg contains a panel of polyclonal Ig directed against a wide variety of common microorganisms. In an interesting work Krause et al. evaluated the presence of specific antimicrobial activity in 5 commercial kit of IVIg showing that all of these preparations have significant activities against HSV, CMV, VZV, EBV, measles, mumps, rubella, parvovirus B19 [258]. For these reasons, IVIg are employed as a substitute treatment for patients with hypogammaglobulinemia (primary or secondary deficiency] and childhood acquired immunodeficiency syndrome (AIDS) to prevent common opportunistic viral and bacterial infection [259,260]. Many examples in literature were reported about the use of IVIg as prevention or treatment for opportunistic infections in immunocompromised patient secondary to immunosuppressive therapy, AIDS and bone marrow transplantation (BMT).
CMV is a common herpes virus that infects around 90% of the general population. In transplant patients, IVIg administered with acyclovir and ganciclovir seem to be capable of preventing CMV related complications [261]. A retrospective study by Ljungman et al. showed no additional benefit of IVIg in combination to the standard anti-viral therapy compared to anti-viral therapy alone, in the treatment of CMV gastrointestinal disease in BMT [262]. Some case series showed that IVIg could be a possible treatment strategy for active CMV infections in immunocompromised patients [263].
Common respiratory viruses like human metapneumovirus (hMPV), adenovirus (ADV) and RSV emerged as important etiological players in serious respiratory infections in immunocompromised patients following BMT and renal transplantation. IVIg demonstrated direct anti-viral activity in vitro against hMPV and RSV [264]. Many case reports and case series highlighted the efficacy of a regimen which included ribavirin or cidofovir and IVIg with complete resolution of symptoms in pneumonia caused by the abovementioned viruses [[265], [266], [267], [268]].
IVIg were also used as prophylaxis for VZV infection in newborns exposed to the virus after birth [269] and were effective for the treatment of a disseminated VZV infection [270].
IVIg seem able to reduce the recurrence of genital manifestations of HSV 2 [271] and were shown to reduce the burden of BK polyomavirus in a proportion of transplant patients [272,273].
West Nile (WN) fever is caused by a flavivirus and provokes encephalitis and meningitis. In a mouse model, Commercial IVIg from Israel, in which the disease is endemic, showed efficacy to prevent the development of such disease if administrated during the viremic phase (i.e., administered 1 day before and after the inoculation of the virus). The efficacy was directly proportional to the dose administered. Interestingly, IVIg derived from USA donors, in which the disease is not endemic, showed no protective effect [274].
On the other way, Ig specific serum antibodies could be also associated with an increased risk of infection by promoting viral entry in susceptible cells through a mechanism of antibody-dependent enhancement (ADE), firstly described for Flaviviridae, HIV and Ebola viruses, which involves specific cellular receptors like Fc receptor (FcR) or complement receptor type 3 (CR3) [275,276]. This process has been recently proposed by Fu et al. as a possible mechanism of lung damage in SARS-CoV pneumonia [277]. In particular, the viral surface antigen S-host antibodies (anti-Spike IgG) complex promotes the FcR-mediated internalization of the virus in macrophages (ADE) and activates intracellular signaling of FcR. This interaction results in the up-regulation and release of the pro-inflammatory cytokines responsible for severe lung disease. Moreover, it was observed that the appearance of anti-viral IgG coincided in 80% of patients with the onset of acute respiratory disease [278]. The same study identified IVIg as an agent able to saturate FcR and possibly prevent the lung damage in SARS-CoV infection [279]. Despite a systematic review of SARS treatment showed inconclusive results in a retrospective analysis of IVIg treated patients, a possible therapeutic role of IVIg in COVID-19 could be hypothesized [280].
14. Janus kinases inhibitors
Janus kinases (JAKs) are intracellular signaling components that function downstream of many cytokines [281]. Four members are included in the JAK family: JAK1, JAK2, JAK3, and non-receptor tyrosine-protein kinase (TYK2). All JAKs play a key role in immune response, and JAK1/2 are also involved in hematopoiesis [282]. JAK-1 and JAK-2 (JAK 1/2) kinase are found in the cell cytoplasm and are associated with key cytokine receptors and activating kinases. Upon activation, JAK 1/2 induce downstream activation of signal transducer and activator of transcription (STAT) protein, with transduction of the signal and the final transcription of several genes involved in immune cells proliferation, differentiation, activation/inhibition, and survival/apoptosis [283].
In the immune response to pathogens including viruses, the signaling occurs via the JAK-STAT pathway, including type I (regulated by JAK1-TYK2 complexes) and type II IFNs (mediated via JAK1-JAK2 complexes) [284]. The IFNs are a primary defense against pathogens because of their strong anti-viral activities. As known, IFN-α is an important therapeutic option for the successful treatment of HBV and HCV and have effects against hepatitis E infection and for chronic delta hepatitis [285].
JAK1 is required for both type I (α and β) and type II (γ) IFN signaling pathways, while JAK3 is crucial for lymphocyte development and function [281]. Viral infections induce the expression of pro-inflammatory cytokines such as IL-1, IL-6, TNF, IL-8, as well as chemokines [282]. Generally, IFN-α/β would be considerably upregulated by viral infections: studies found that distinct anti-viral activities correlate with virus-specific expression levels of Interferon-stimulated genes (ISGs) subsets. For instance, some ISGs have strong anti-viral effects while others promote viral replication in vitro [283]. Macrophages produce significant levels of IFN-α/β, IL-1β, TNF-α, chemokines, and IL-18 [284]. Dendritic cells (DCs), especially plasmacytoid DCs, respond to virus infections dose dependently by inducing high expression of IFN-α/β or on the contrary by a rapid pDC apoptosis. Moreover, IFN-α downregulates IFN-γ production by NK and CD8+ T cells through IL-10 production, leading to a rapid induction of an adaptive immune response [285].
JAK inhibitors (JAKi) are small molecules targeting the intracellular cytokine signaling [286]. There are a number of JAKi currently both used and under investigation for use in several autoimmune systemic diseases such as RA, PsO, PsA, some of which are also approved or under investigation in other indications [287]. Tofacitinib has specificity for JAK3 and JAK1 over JAK2 and baricitinib mainly inhibits JAK1 and JAK2 [288,289] while upadacitinib that will be soon marketed demonstrated a 74- and 58-fold greater selectivity for JAK1 over JAK2 and JAK3, respectively [290].
JAKi have anti-viral potential since may lower the pro-inflammatory response mediated by viruses. For instance, baricitinib induces a dose dependent inhibition of IL-6 [291]. Intriguingly, baricitinib inhibits the AP2-associated protein kinase 1 (AAK1) that is a regulator of endocytosis that the SARS-CoV-2 uses to infect lung cells through binding with ACE2. This may possibly interrupt the passage of the virus into cells and also the intracellular assembly of virus particles. Baricitinib, at therapeutic dosing (either as 2 mg or 4 mg once daily) is sufficient to inhibit AAK1, thus it has been suggested to be trialed in an appropriated COVID population [6,292]. On the other hand, therapies targeting JAK complexes may interfere with normal anti-viral response including inhibition of IFN-γ activity and may potentially increase the risk of infection and/or reactivation of several viral infectious diseases [[293], [294], [295]], including a dose dependent risk for VZV observed for tofacitinib and baricitinib [296]. Thus, their usage should be carefully evaluated. Moreover, selective JAKi, such as fedratinib, a JAK2 targeted JAKi able to inhibit Th17-mediated response implicated in the disease cytokine release syndrome, may be more beneficial than others [297].
15. Granulocyte-monocyte colony stimulating factor
Granulocyte-monocyte colony stimulating factor (GM-CSF) is a fundamental cytokine and growth factor involved in the proliferation and maturation of myeloid precursors, such as granulocytes, monocytes and macrophages that express its receptor. In fact, it is widely used to reverse neutropenia induced by cytotoxic agents. However, there is extensive evidence that GM-CSF is able to prime macrophages to transcribe and secrete cytokines, such as IL-6 and TNF-α, responsible for common adverse events such as fever and myalgia [298].
Following the outbreak of COVID-19, Huang et al. demonstrated that patients display markedly increased plasma levels of GM-CSF compared to healthy population, suggesting GM-CSF may play a role in the context of CRS [2]. Additionally, pathologic studies demonstrated a significant macrophage infiltration in lungs of patients who died with SARS-CoV infection, which shares significant similarities with COVID-19 [299].
Recently, a more detailed analysis has been performed on T cells from peripheral blood of COVID-19 patients. Very interestingly, high levels of CD4+ cells expressing both GM-CSF and IL-6 were found in COVID-19 patients, along with GM–CSF–secreting CD14+CD16+, compared to healthy controls. Even more intriguingly, Th1 cells expressing both GM-CSF and IFN-γ were detected exclusively in ICU patients, thus suggesting a potential contribution of GM-CSF to CRS [300]. These data are further supported by the evidence that human pulmonary microvascular endothelial cells (HPMEC) are able to secrete GM-CSF following an inflammatory stimulus such as LPS, suggesting a potential important role in the pathogenesis of ARDS, in which increased levels of GM-CSF are present in BAL fluid [301]. Additionally, animal models have demonstrated that GM-CSF neutralization is effective in reducing the severity of neuroinflammation induced by CAR-T therapy, with no negative effect on its efficacy [302].
Multiple GM-CSF and GM-CSF receptor inhibitors (namilumab, mavrilimumab, otilimab) are currently under investigation for the treatment of RA and preliminary data seem promising [303,304]. The blockade of this pathway may represent an additional interesting experimental approach to treat CRS in COVID-19 patients.
16. Concluding remarks
The fight against SARS-CoV-2 is one of the greatest challenges not only for medical science but that the entire human community had to face in the last century. The search and discovery of any weapon that could possibly ameliorate this disease is eagerly awaited and a crack in the door has been opened by already existing drugs from the rheumatologist armamentarium. Table 2 summarizes the currently used agents with an anti-viral effect and their supposed mechanism of action. Moreover, we aimed at giving new ideas towards a rheumatologic approach at the treatment of the SARS-CoV-2 infection. Notwithstanding that this fight will be won only together with the all the professional figures collaborating every day in treating COVID-19 patients [[305], [306], [307]].
Table 2.
Ongoing Clinical Trials on rheumatologic drugs in COVID-19 (last updated on the April 1, 2020).
| Compound | Approved indications for clinical practice in Rheumatology | Potential target viruses | Anti-viral mechanism of action |
|---|---|---|---|
| Glucocorticoids | Several including rheumatoid arthritis, systemic lupus erythematosus, vasculitis | H1N1 | Controversy. Low dose for short time (10 days) may improve acute respiratory distress syndrome. |
| Nonsteroidal anti-inflammatory drugs | Several | CMV, HCV, Flaviviridae, H7N9, H3N2, SARS-CoV | COX-2 inhibition impairs viral replication Spike protein of SARS-CoV inhibited by COX-2 via ERK/NF-kB and PI3K/JNK pathways Ibuprofen effect ACE2 receptor |
|
Chloroquine Hydroxychloroquine |
Rheumatoid arthritis Juvenile idiopathic arthritis Discoid lupus erythematosus Systemic lupus erythematosus |
HIV, HAV, Influenza, HcoV-OC43, FIPV, HcoV-229E, SARS-CoV, SARS-CoV-2 | Increases pH of host cell organelles, impairing enzymatic activity for virus antigen-binding, replication and pro-inflammatory cytokine production and secretion |
| Leflunomide (and teriflunomide) | Rheumatoid arthritis Psoriatic arthritis |
CMV, HSV-1, HSV-2, EBV (teriflunomide), HIV-1, Argentinian mammarenavirus, FMDV, EBOV, Influenza A, Polyomavirus, NDV, EV-A71, RSV | Tyrosine kinase inhibition Inhibition of pyrimidine de novo synthesis through the blockade of dihydroorotate dehydrogenase Possible reduction of IL-6 in vitro |
| Mycophenolate Mofetil | Severe Autoimmune disease (Systemic lupus erythematosus) | Influenza, ZKV, West Nile-virus, Chikungunya, smallpox virus, FMDV, PPRV, Junin virus, norovirus, Lassa's hemorrhagic fever, reovirus, rotavirus, HCV, MERS-CoV | Inhibition IMPDH and conversion of inosine monophosphate to guanosine monophosphate hampering lymphocyte proliferation Increased IFN-stimulated gene expression Synergizes the anti-viral effects penciclovir, lobucavir, 37-fluorodideoxyguanosine, diaminopurine dioxolane of through the depletion of the endogenous GTP pools |
| Methotrexate | ZKV | Inhibition of dihydrofolate reductase | |
| Colchicine | Gout treatment and prevention Acute and recurrent pericarditis Familial Mediterranean fever |
Flaviviridae, RSA59, M − CoV, RSV | Alters cytoskeleton organization via microtubules assembly inhibition Inhibition of the inflammasome Reduction of IL-1 and IL-6 Anti-neutrophilic action |
|
Anakinra Canakinumab Rilonacept |
Rheumatoid arthritis Cryopirin Associated Periodic Syndromes Systemic Juvenile idiopathic arthritis Adult onset Still's disease |
No evidence of direct anti-viral activity Indirect evidence on reduction of inflammatory response in infections by arthritogenic alphavirus and parainfluenza |
IL-1 receptor inhibitor (Anakinra), IL-1β inhibitor (Canakinumab), IL-1α/IL-1β inhibitor (Rilonacept), inhibition of the inflammasome |
| TNFα-inhibitors | Rheumatoid arthritis Juvenile idiopathic arthritis Non-radiographic axial spondyloarthritis Ankylosing spondylitis Psoriasis Psoriatic arthritis |
Rotavirus and indirect evidence on reduction of inflammatory response in infections by influenza viruses | Reduction of inflammatory response |
| Tocilizumab | Rheumatoid arthritis Juvenile idiopathic arthritis |
No evidence of direct anti-viral activity. Indirect evidence on reduction of inflammatory response in COVID-19 |
IL-6 inhibition, inhibition of the cytokine storm? |
| IVIg | Primary immunodeficiency syndromes with impaired antibody production Hypogammaglobulinemia Primary immune thrombocytopenia (ITP) Kawasaki disease |
HAV, HSV, CMV, VZV, EBV, measles, mumps, rubella, parvovirus B19, CMV, hMPV, ADV, RSV, HSV 2, Flaviviridae, HIV, Ebola, SARS-CoV | Direct action both on innate immune components (complement, monocytes, macrophages, natural killer, cells), and on adaptive immune cells (CD4 T-cells and B-cells) Saturate FcR |
| JAK inhibitors | Rheumatoid arthritis Psoriatic arthritis Ulcerative colitis |
No evidence of direct anti-viral activity SARS-CoV-2? Potential reduction of inflammatory response in ALI |
Inhibition of JAK/STAT pathway, reduction of pro-inflammatory cytokines Potential inhibition of AP2-associated protein kinase 1 (AAK1) by baricitinib that SARS-CoV-2 uses to infect lung cells through binding with ACE2 |
| GM-CSFi | Rheumatoid arthritis | Potential reduction of inflammatory response in ALI | GM-CSF neutralization effective in reducing the severity of inflammation |
List of abbreviations: HIV: Human Immunodeficiency Virus, HAV: Hepatitis A Virus, HcoV-OC43: Human coronavirus-OC43, FIPV: Feline infectious peritonitis virus, HcoV-229E: Human coronavirus, SARS-CoV: Severe acute respiratory syndrome-coronavirus, SARS-CoV-2: Severe acute respiratory syndrome-coronavirus-2, CMV: cytomegalovirus, HSV-1: Herpes Simplex Virus-1, HSV-2: Herpes Simplex Virus-2, EBV: Epstein-Barr Virus: FMDV: Foot-and-mouth disease virus, EBOV: Zaire Ebolavirus, NDV: Newcastle disease virus, EV-A71: Non-Polio Enterovirus, RSV: Respiratory Syncytial Virus, ZKV: Zika virus, RSA59: Neurotropic demyelinating strain of MHV, PPRV: peste des petits ruminants virus, HCV: Hepatitis C Virus, MERS-CoV: Middle East respiratory syndrome coronavirus, human metapneumovirus: hMPV, VZV: Varicella Zoster Virus, ADV: adenovirus: ADV; IMPDH: inosine monophosphate dehydrogenase, IFN: interferon, GTP: guanosine triphosphate, ACE2: Angiotensin-converting enzyme 2, interleukin: IL, FcR: Fc receptor, Acute lung injury: ALI
References
- 1.World Health Organization Novel coronavirus [cited 2020 Jan 16] https://www.who.int/westernpacific/emergencies/novel-coronavirus Available from:
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020 doi: 10.1016/S0140-6736(20)30627-9. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., Liao J., Yang H., Hou W., Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID-19: combining anti-viral and anti-inflammatory treatments. Lancet Infect. Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCloskey B., Heymann D.L. SARS to novel coronavirus: old lessons and new lessons. Epidemiol. Infect. 2020;22:e22. doi: 10.1017/S0950268820000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilder-Smith A., Chiew C.J., Lee V.J. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30129-8. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen F., Ho Y.C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020 doi: 10.1172/JCI137647. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro anti-viral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiaoling X., Mingfeng H., Tiantian L. ChinaXiv; 2003. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TOCIVID-19 https://www.aifa.gov.it/sperimentazioni-cliniche-covid-19
- 16.Sarzi-Puttini P., Ceribelli A., Marotto D., Batticciotto A., Atzeni F. Systemic rheumatic diseases: from biological agents to small molecules. Autoimmun. Rev. 2019;18:583–592. doi: 10.1016/j.autrev.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Smolen J.S., Landewé R.B.M., Bijlsma J.W.J., Burmester G.R., Dougados M., Kerschbaumer A. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2019-216655. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Hellmich B., Agueda A., Monti S., Buttgereit F., De Boysson H., Brouwer E. Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2018;79(2020):19–30. doi: 10.1136/annrheumdis-2019-215672. [DOI] [PubMed] [Google Scholar]
- 19.Fanouriakis A., Kostopoulou M., Alunno A., Aringer M., Bajema I., Boletis J.N. Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019;78:736–745. doi: 10.1136/annrheumdis-2019-215089. 2019. [DOI] [PubMed] [Google Scholar]
- 20.Ehlers L., Askling J., Bijlsma H.W.J., Cid M.C., Cutolo M., Dasgupta B. EULAR recommendations for a core data set to support observational research and clinical care in giant cell arteritis. Ann. Rheum. Dis. 2018;78:1160–1166. doi: 10.1136/annrheumdis-2018-214755. 2019. [DOI] [PubMed] [Google Scholar]
- 21.Strehl C., Ehlers L., Gaber T., Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front. Immunol. 2019;10:1744. doi: 10.3389/fimmu.2019.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belvisi M.G., Wicks S.L., Battram C.H., Bottoms S.E.W., Redford J.E., Woodman P. Therapeutic benefit of a dissociated glucocorticoid and the relevance of in vitro separation of transrepression from transactivation activity. J. Immunol. 2001;166:1975–1982. doi: 10.4049/jimmunol.166.3.1975. [DOI] [PubMed] [Google Scholar]
- 23.Schäcke H., Schottelius A., Döcke W.D., Strehlke P., Jaroch S., Schmees N. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc. Natl. Acad. Sci. U.S.A. 2004;101:227–232. doi: 10.1073/pnas.0300372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tullar C., Issa F. Immune regulation by glucocorticoids can be linked to cell type-dependent transcriptional responses. Transplantation. 2019;103:651. doi: 10.1084/jem.20180595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widdifield J., Bernatsky S., Paterson J.M., Gunraj N., Thorne J.C., Pope J. Serious infections in a population-based cohort of 86,039 seniors with rheumatoid arthritis. Arthritis Care Res. 2013;65:353–361. doi: 10.1002/acr.21812. [DOI] [PubMed] [Google Scholar]
- 26.Doran M.F., Crowson C.S., Pond G.R., O'Fallon W.M., Gabriel S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–2293. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 27.Michaud K., Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2007;21:885–906. doi: 10.1016/j.berh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Dixon W.G., Suissa S., Hudson M. The association between systemic glucocorticoid therapy and the risk of infection in patients with rheumatoid arthritis: systematic review and meta-analyses. Arthritis Res. Ther. 2011;13:R139. doi: 10.1186/ar3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Au K., Reed G., Curtis J.R., Kremer J.M., Greenberg J.D., Strand V. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2011;70:785–791. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 30.Listing J., Gerhold K., Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology. 2013;52:53–61. doi: 10.1093/rheumatology/kes305. [DOI] [PubMed] [Google Scholar]
- 31.Accortt N.A., Lesperance T., Liu M., Rebello S., Trivedi M., Li Y. Impact of sustained remission on the risk of serious infection in patients with rheumatoid arthritis. Arthritis Care Res. 2018;70:679–684. doi: 10.1002/acr.23426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin J., Lunt M., Bunn D., Symmons D., Silman A. Risk and predictors of infection leading to hospitalisation in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann. Rheum. Dis. 2007;66:308–312. doi: 10.1136/ard.2006.057265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smalls D.J., Kiger R.E., Norris L.A.B., Bennett C.L., Love B.L. Hepatitis B virus reactivation: risk factors and current management strategies. Pharmacotherapy. 2019;39:1190–1203. doi: 10.1002/phar.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Confalonieri M., Urbino R., Potena A., Piattella M., Parigi P., Puccio G. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am. J. Respir. Crit. Care Med. 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 35.Keh D., Boehnke T., Weber-Cartens S., Schulz C., Ahlers O., Bercker S. Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am. J. Respir. Crit. Care Med. 2003;167:512–520. doi: 10.1164/rccm.200205-446OC. [DOI] [PubMed] [Google Scholar]
- 36.Lamontagne F., Rochwerg B., Lytvyn L., Guyatt G.H., Møller M.H., Annane D. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ. 2018;362:1–15. doi: 10.1136/bmj.k3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Homma T., Fukuda Y., Uchida Y., Uno T., Jinno M., Kishino Y. Inhibition of virus-induced cytokine production from airway epithelial cells by the late addition of budesonide. Medicina (Kaunas) 2020;56 doi: 10.3390/medicina56030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Who W. 2020. WHO-2019-nCoV-clinical-2020.4-eng; pp. 1–21. [Google Scholar]
- 39.Clinical W.H.O., Who W. 2020. WHO-nCoV-Clinical-2020.3-eng; pp. 1–10. [Google Scholar]
- 40.Moreno G., Rodríguez A., Reyes L.F., Gomez J., Sole-Violan J., Díaz E. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med. 2018;44:1470–1482. doi: 10.1007/s00134-018-5332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarda C., Palma P., Rello J. Severe influenza: overview in critically ill patients. Curr. Opin. Crit. Care. 2019;25:449–457. doi: 10.1097/MCC.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 42.Hui D.S.C., Ng S.S.S. Recommended hospital preparations for future cases and outbreaks of novel influenza viruses. Expet Rev. Respir. Med. 2020;14:41–50. doi: 10.1080/17476348.2020.1683448. [DOI] [PubMed] [Google Scholar]
- 43.Delaney J.W., Pinto R., Long J., Lamontagne F., Adhikari N.K., Kumar A. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit. Care. 2016;20:1–11. doi: 10.1186/s13054-016-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao B., Gao H., Zhou B., Deng X., Hu C., Deng C. Adjuvant corticosteroid treatment in adults with influenza a (H7N9) viral pneumonia. Crit. Care Med. 2016;44:e318–328. doi: 10.1097/CCM.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 45.Ni Y.N., Chen G., Sun J., Liang B.M., Liang Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit. Care. 2019;23:1–9. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lansbury L.E., Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Shen Lim W. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta-analysis. Crit. Care Med. 2020;48:e98–106. doi: 10.1097/CCM.0000000000004093. [DOI] [PubMed] [Google Scholar]
- 47.Alfaraj S.H., Al-Tawfiq J.A., Assiri A.Y., Alzahrani N.A., Alanazi A.A., Memish Z.A. Clinical predictors of mortality of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: a cohort study. Trav. Med. Infect. Dis. 2019;29:48–50. doi: 10.1016/j.tmaid.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chow E.J., Doyle J.D., Uyeki T.M. Influenza virus-related critical illness: prevention, diagnosis, treatment. Crit. Care. 2019;23:1–11. doi: 10.1186/s13054-019-2491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H., Yang S.G., Gu L., Zhang Y., Yan X.X., Liang Z.A. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other. Resp. Viruses. 2017;11:345–354. doi: 10.1111/irv.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 51.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:1525–1531. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis S.R., Pritchard M.W., Thomas C.M., Smith A.F. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst. Rev. 2019;23:7. doi: 10.1002/14651858.CD004477.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao J.P., Hu Y., Du R.H., Chen Z., Jin Y., Zhou M., Zhang J., Qu J., Cao B. Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia (in Chinese) Zhonghua Jiehe He Huxi Zazhi. 2020;43:E007. doi: 10.3760/cma.j.issn.1001-0939.2020.0007. [DOI] [PubMed] [Google Scholar]
- 57.Catella-Lawson F., Reilly M.P., Kapoor S.C., Cucchiara A.J., DeMarco S., Tournier B., Vyas S.N., FitzGerald G.A. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 58.Chuang F.K., Huang S.M., Liao C.L., Lee A.R., Lien S.P., Chiu Y.L., Chang T.H., Tsai P.L., Lin R.J., Shih C.C., Tsai Y.J., Lin G.J., Yen L.C. Anti-inflammatory compound shows therapeutic safety and efficacy against flavivirus infection. Antimicrob. Agents Chemother. 2019;64 doi: 10.1128/AAC.00941-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li C., Li C., Zhang A.J., To K.K., Lee A.C., Zhu H., Wu H.W., Chan J.F., Chen H., Hung I.F., Li L., Yuen K.Y. Avian influenza A H7N9 virus induces severe pneumonia in mice without prior adaptation and responds to a combination of zanamivir and COX-2 inhibitor. PloS One. 2014;9 doi: 10.1371/journal.pone.0107966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paul A.G., Chandran B., Sharma-Walia N. Cyclooxygenase-2-prostaglandin E2-eicosanoid receptor inflammatory axis: a key player in Kaposi's sarcoma-associated herpes virus associated malignancies. Transl. Res. 2013;162:77–92. doi: 10.1016/j.trsl.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu M., Yang Y., Gu C., Yue Y., Wu K.K., Wu J., Zhu Y. Spike protein of SARS-CoV stimulates cyclooxygenase-2 expression via both calcium-dependent and calcium-independent protein kinase C pathways. Faseb. J. 2007;21:1586–1596. doi: 10.1096/fj.06-6589com. [DOI] [PubMed] [Google Scholar]
- 62.Qiao W., Wang C., Chen B., Zhang F., Liu Y., Lu Q. Ibuprofen attenuates cardiac fibrosis in Streptozotocin-induced diabetic rats. Cardiology. 2015;131:97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- 63.Dilly S., Fotso Fotso A., Lejal N., Zedda G., Chebbo M., Rahman F., Companys S., Bertrand H.C., Vidic J., Noiray M., Alessi M.C., Tarus B., Quideau S., Riteau B., Slama-Schwok A. From naproxen repurposing to naproxen analogues and their antiviral activity against influenza A virus. J. Med. Chem. 2018;61:7202–7217. doi: 10.1021/acs.jmedchem.8b00557. [DOI] [PubMed] [Google Scholar]
- 64.Mazzetti A., Neary J.D. Outcome switching in triple therapy in influenza A(H3N2) infection: an open-label randomized, controlled, phase IIb/III trial. Chest. 2018;154:998–999. doi: 10.1016/j.chest.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 65.ClinicalTrialsgov Identifier: NCT04325633.
- 66.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 67.Moore N. Coronary risks associated with diclofenac and other NSAIDs: an update. Drug Saf. 2020;43:301–318. doi: 10.1007/s40264-019-00900-8. [DOI] [PubMed] [Google Scholar]
- 68.Wen Y.C., Hsiao F.Y., Lin Z.F., Fang C.C., Shen L.J. Risk of stroke associated with use of nonsteroidal anti-inflammatory drugs during acute respiratory infection episode. Pharmacoepidemiol. Drug Saf. 2018;27:645–651. doi: 10.1002/pds.4428. [DOI] [PubMed] [Google Scholar]
- 69.Wen Y.C., Hsiao F.Y., Chan K.A., Lin Z.F., Shen L.J., Fang C.C. Acute respiratory infection and use of nonsteroidal anti-inflammatory drugs on risk of acute myocardial infarction: a nationwide case-crossover study. J. Infect. Dis. 2017;215:503–509. doi: 10.1093/infdis/jiw603. [DOI] [PubMed] [Google Scholar]
- 70.Little P., Moore M., Kelly J. PIPS Investigators. Ibuprofen, paracetamol, and steam for patients with respiratory tract infections in primary care: pragmatic randomised factorial trial. BMJ. 2013;347 doi: 10.1136/bmj.f6041. 347 f6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Little P., Stuart B., Andreou P. Primary care randomised controlled trial of a tailored interactive website for the self-management of respiratory infections (internet doctor) BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;368:m1185. doi: 10.1136/bmj.m1185. [DOI] [PubMed] [Google Scholar]
- 73.Day M. Covid-19: European drugs agency to review safety of ibuprofen. BMJ. 2020;368:m1168. doi: 10.1136/bmj.m1168. [DOI] [PubMed] [Google Scholar]
- 74.Shukla A.M., Wagle Shukla A. Expanding horizons for clinical applications of chloroquine, hydroxychloroquine, and related structural analogues. Drugs Context. 2019;8 doi: 10.7573/dic.2019-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adelusi S.A., Salako L.A. Kinetics of the distribution and elimination of chloroquine in the rat. Gen. Pharmacol. 1982;13:433–437. doi: 10.1016/0306-3623(82)90110-0. [DOI] [PubMed] [Google Scholar]
- 76.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Bari M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017;5 doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vincent M.J. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kono M. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Anti-viral Res. 2008;77:150–152. doi: 10.1016/j.antiviral.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sarma P, Kaur H, Kumar H. Virological and clinical cure in Covid-19 patients treated with hydroxychloroquine: A systematic review and meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keyaerts E. Anti-viral activity of chloroquine against human coronavirus OC43 infection in newborn mice. Antimicrob. Agents Chemother. 2009;53:3416–3421. doi: 10.1128/AAC.01509-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takano T., Katoh Y., Doki T., Hohdatsu T. Effect of chloroquine on feline infectious peritonitis virus infection in vitro and in vivo. Anti-viral Res. 2013;99:100–107. doi: 10.1016/j.antiviral.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barnard D.L. Evaluation of immunomodulators, interferons and known in vitro SARS-CoV inhibitors for inhibition of SARS-coV replication in BALB/c mice. Antivir. Chem. Chemother. 2006;17:275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- 87.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 88.J Multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia. [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia] Tuberc. Respir. Dis. 2020;43:185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. Zhonghua Jie He He Hu Xi Za Zhi Zhonghua Jiehe He Huxi Zazhi Chin. [DOI] [PubMed] [Google Scholar]
- 89.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Anti-viral Res. 2020;177:104762. doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gautret P., Lagier J.C., Parola P., Hoang V.T., Medded L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D., Sr. Hydroxychloroquine and Azithromycin as a treatment of COVID-19: preliminary results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Melles R.B., Marmor M.F. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453–1460. doi: 10.1001/jamaophthalmol.2014.3459. [DOI] [PubMed] [Google Scholar]
- 93.SIMIT Società Italiana di Malattie Infettive e Tropicali SEZIONE REGIONE LOMBARDIA. Vademecum per la cura delle persone con malattia da COVID-19 - versione 2.0 2020.
- 94.Skorpen C.G. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann. Rheum. Dis. 2016;75:795–810. doi: 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]
- 95.D’Antiga A. Coronaviruses and Immunosuppressed Patients. The Facts during the Third Epidemic. Liver Transplant. 2020 doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 96.He D., Zhang C., Zhao X., Zhang Y., Dai Q., Li Y., Chu L. Teriflunomide for multiple sclerosis Summary of findings for the main comparison. Cochrane Database Syst. Rev. 2016;3:CD009882. doi: 10.1002/14651858.CD009882.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu X., Williams J., Bremer E., Finnegan A., Chong A. Inhibition of protein tyrosine phosphorylation in T cells by a novel immunosuppressive agent, leflunomide. J. Biol. Chem. 1995;270:12398–12403. doi: 10.1074/jbc.270.21.12398. [DOI] [PubMed] [Google Scholar]
- 98.Williamson R., Yea C., Robson P., Curnock A., Gadher S., Hambleton A., Woodward K., Bruneau J., Hambleton P., Moss D., Thomson T., Spinella-Jaegle S., Morand P., Courtin O., Saute's C., Westwood R., Hercend T., Kuo E., Ruuth E. Dihydroorotate dehydrogenase is a high affinity binding protein for A77 1726 and mediator of a range of biological effects of the immunomodulatory compound. J. Biol. Chem. 1995;270:22467–22472. doi: 10.1074/jbc.270.38.22467. [DOI] [PubMed] [Google Scholar]
- 99.Munier-Lehmann H., Vidalain P.O., Tangy F., Janin Y.L. On dihydroorotate dehydrogenases and their inhibitors and uses. J. Med. Chem. 2013;56:3148–3167. doi: 10.1021/jm301848w. [DOI] [PubMed] [Google Scholar]
- 100.Kotton C., Kumar D., Caliendo A., Åsberg A., Chou S., Snydman D., Allen U., Humar A. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]
- 101.Eid A., Arthurs S., Deziel P., Wilhelm M., Razonable R. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clin. Transplant. 2008;22:162–170. doi: 10.1111/j.1399-0012.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 102.Silva J., Pérez-González V., Lopez-Medrano F., Alonso-Moralejo R., Fernández-Ruiz M., San-Juan R., Brañas P., Folguera M., Aguado J., De Pablo-Gafas A. Experience with leflunomide as treatment and as secondary prophylaxis for cytomegalovirus infection in lung transplant recipients: a case series and review of the literature. Clin. Transplant. 2018;32:1–7. doi: 10.1111/ctr.13176. [DOI] [PubMed] [Google Scholar]
- 103.Rifkin L., Minkus C., Pursell K., Jumroendararasame C., Goldstein D. Utility of leflunomide in the treatment of drug resistant cytomegalovirus retinitis. Ocul. Immunol. Inflamm. 2017;25:93–96. doi: 10.3109/09273948.2015.1071406. [DOI] [PubMed] [Google Scholar]
- 104.Dunn J., Weinberg A., Chan L., Mandava N., Levi M., Olson J. Long-term suppression of multidrug-resistant cytomegalovirus retinitis with systemically administered leflunomide. JAMA Ophthalmology. 2013;131:958–960. doi: 10.1001/jamaophthalmol.2013.1589. [DOI] [PubMed] [Google Scholar]
- 105.El Chaer F., Mori N., Shah D., Oliver N., Wang E., Jan A., Doan V., Tverdek F., Tayar J., Ariza-Heredia E., Chemaly R. Adjuvant and salvage therapy with leflunomide for recalcitrant cytomegalovirus infections in hematopoietic cell transplantation recipients: a case series. Antivir. Res. 2016;135:91–96. doi: 10.1016/j.antiviral.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 106.Henao-Martínez A., Weinberg A., Waldman W., Levi M. Successful treatment of acyclovir-resistant herpes simplex virus type 2 proctitis with leflunomide in an HIV-infected man. J. Clin. Virol. 2012;54:276–278. doi: 10.1016/j.jcv.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 107.Waldman W., Knight D., Blinder L., Shen J., Lurain N., Miller D., Sedmak D., Williams J., Chong A. Inhibition of cytomegalovirus in vitro and in vivo by the experimental immunosuppressive agent leflunomide. Intervirology. 1999;42:412–418. doi: 10.1159/000053979. [DOI] [PubMed] [Google Scholar]
- 108.Knight D., Hejmanowski A., Dierksheide J., Williams J., Chong A., Waldman W. Inhibition of herpes simplex virus type 1 by the experimental immunosuppressive agent leflunomide. Transplant. 2001;71:170–174. doi: 10.1097/00007890-200101150-00031. [DOI] [PubMed] [Google Scholar]
- 109.Tatarowicz W., Lurain N., Thompson K. A ganciclovir-resistant clinical isolate of human cytomegalovirus exhibiting cross-resistance to other DNA polymerase inhibitors. J. Infect. Dis. 1992;166:904–907. doi: 10.1093/infdis/166.4.904. [DOI] [PubMed] [Google Scholar]
- 110.Chacko B., John G. Leflunomide for cytomegalovirus: bench to bedside. Transpl. Infect. Dis. 2012;14:111–120. doi: 10.1111/j.1399-3062.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 111.Qi R., Hua-Song Z., Xiao-Feng Z. Leflunomide inhibits the apoptosis of human embryonic lung fibroblasts infected by human cytomegalovirus. Eur. J. Med. Res. 2013;18:3. doi: 10.1186/2047-783X-18-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bilger A., Plowshay J., Ma S., Nawandar D., Barlow E., Romero-Masters J., Bristol J., Li Z., Tsai M., Delecluse H., Kenney S. Leflunomide/teriflunomide inhibit Epstein-barr virus (EBV)-induced lymphoproliferative disease and lytic viral replication. Oncotarget. 2017;8:44266–44280. doi: 10.18632/oncotarget.17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou X., Kubo M., Nishitsuji H., Kurihara K., Ikeda T., Ohashi T., Azuma M., Masuda T., Kannagi M. Inducible-costimulator-mediated suppression of human immunodeficiency virus type 1 replication in CD4+ T lymphocytes. Virology. 2004;325:252–263. doi: 10.1016/j.virol.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 114.Sepúlveda C., García C., Damonte E. Antiviral activity of A771726, the active metabolite of leflunomide, against Junín virus. J. Med. Virol. 2018;90:819–827. doi: 10.1002/jmv.25024. [DOI] [PubMed] [Google Scholar]
- 115.Mei-jiao G., Shi-fang L., Yan-yan C., Jun-jun S., Yue-feng S., Ting-ting R., Yong-Guang Z., Hui-Yun C. Antiviral effects of selected IMPDH and DHODH inhibitors against foot and mouth disease virus. Biomed. Pharmacother. 2019;118:1–7. doi: 10.1016/j.biopha.2019.109305. [DOI] [PubMed] [Google Scholar]
- 116.Martin S., Chiramel A., Schmidt M., Chen Y., Whitt N., Watt A., Dunham M.E., Shifflett K., Traeger S., Leske A., Buehler E., Martellaro C., Brandt J., Wendt L., Groseth A., Feldmann H., Hoenen T. A genome-wide siRNA screen identifies a druggable host pathway essential for the Ebola virus life cycle. Genome Med. 2018;10:1–14. doi: 10.1186/s13073-018-0570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cuellar-Rodriguez J., Stephany B., Poggio E., Mossad S., Goldfarb D., Lard M., Askar M., Farica R., Srinivas T., Braun W., Shoskes D., Flecher S., Schmitt S., Shrestha R., Avery R. Contrasting patterns of viral load response in transplant recipients with BK polyomavirus DNAemia on leflunomide therapy. Clin. Transplant. 2013;27:230–237. doi: 10.1111/ctr.12110. [DOI] [PubMed] [Google Scholar]
- 118.Wu M., Chen Y., Hung L., Lee C., Chen H., Hsu Y., Wu M. Successful treatment for BK virus nephropathy by leflunomide in a kidney transplant patient: a case report. Transplant. Proc. 2019;51:1472–1474. doi: 10.1016/j.transproceed.2019.01.146. [DOI] [PubMed] [Google Scholar]
- 119.Faguer S., Hirsch H., Kamar N., Guilbeau-Frugier C., Ribes D., Guitard J., Esposito L., Cointault O., Modesto A., Lavit M., Mengelle C., Rostaing L. Leflunomide treatment for polyomavirus BK-associated nephropathy after kidney transplantation. Transpl. Int. 2007;20:962–969. doi: 10.1111/j.1432-2277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- 120.Hirsch H., Randhawa P. BK polyomavirus in solid organ transplantation—guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019;33:1–19. doi: 10.1111/ctr.13528. [DOI] [PubMed] [Google Scholar]
- 121.Johnston O., Jaswal D., Gill J., Doucette S., Fergusson A., Knoll G. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplant. 2010;89:1057–1070. doi: 10.1097/TP.0b013e3181d0e15e. [DOI] [PubMed] [Google Scholar]
- 122.Wu W., Ho L., Chen P., Tsai Y., Hsu S., Chang D., Lai J. Immunosuppressive effects and mechanisms of leflunomide in dengue virus infection of human dendritic cells. J. Clin. Immunol. 2011;31:1065–1078. doi: 10.1007/s10875-011-9578-7. [DOI] [PubMed] [Google Scholar]
- 123.Khong W., Foo D., Trasti S., Tan E., Alonso S. Sustained high levels of interleukin-6 contribute to the pathogenesis of Enterovirus 71 in a neonate mouse model. J. Virol. 2011;85:3067–3076. doi: 10.1128/JVI.01779-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin T., Hsia S., Huang Y., Wu C., Chang L. Proinflammatory cytokine reactions in Enterovirus 71 infections of the central nervous system. Clin. Infect. Dis. 2003;36:269–274. doi: 10.1086/345905. [DOI] [PubMed] [Google Scholar]
- 125.Hung H., Shih S., Chang T., Fang M., Hsu J. The combination effects of LiCL and the active leflunomide metabolite, A771726, on viral-induced interleukin 6 production and EV-A71 replication. PloS One. 2014;9:e111331. doi: 10.1371/journal.pone.0111331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chong A., Zeng H., Knight D., Shen J., Meister G., Williams J., Waldman W. Concurrent antiviral and immunosuppressive activities of leflunomide in vivo. Am. J. Transplant. 2006;6:69–75. doi: 10.1111/j.1600-6143.2005.01152.x. [DOI] [PubMed] [Google Scholar]
- 127.Dunn M., Knight D., Waldman W. Inhibition of respiratory syncytial virus in vitro and in vivo by the immunosuppressive agent leflunomide. Antivir. Ther. 2011;16:309–317. doi: 10.3851/IMP1763. [DOI] [PubMed] [Google Scholar]
- 128.Sanders S., Harisdangkul V. Leflunomide for the treatment of rheumatoid arthritis and autoimmunity. Am. J. Med. Sci. 2002;323:190–193. doi: 10.1097/00000441-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 129.Pinschewer D., Ochsenbein A., Fehr T., Zinkernagel R. Leflunomide-mediated suppression of antiviral antibody and T cell responses: differential restoration by uridine. Transplant. 2001;72:712–719. doi: 10.1097/00007890-200108270-00026. [DOI] [PubMed] [Google Scholar]
- 130.Dasgupta A. Therapeutic drug monitoring of mycophenolic acid. Adv. Clin. Chem. 2016;76:165–184. doi: 10.1016/bs.acc.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 131.Leyssen P., Balzarini J., De Clercq E., Neyts J. The predominant mechanism by which ribavirin exerts its antiviral activity in vitro against Flaviviruses and paramyxoviruses is mediated by inhibition of IMP dehydrogenase. J. Virol. 2005;79:1943–1947. doi: 10.1128/JVI.79.3.1943-1947.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cho J., Yi H., Jang E., Lee M., Lee J., Kang C., Lee C., Kim K. Mycophenolic mofetil, an alternative antiviral and immunomodulator for the highly pathogenic avian influenza H5N1 virus infection. Biochem. Biophys. Res. Commun. 2017;494:298–304. doi: 10.1016/j.bbrc.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 133.To K., Mok K., Chan A., Cheung N., Wang P., Lui Y., Chan J., Chen H., Chan K., Kao R., Yuen K. Mycophenolic acid, an immunomodulator, has potent and broad-spectrum in vitro antiviral activity against pandemic, seasonal and avian influenza viruses affecting humans. J. Gen. Virol. 2016;97:1807–1817. doi: 10.1099/jgv.0.000512. [DOI] [PubMed] [Google Scholar]
- 134.Park J., Ávila-Pérez G., Nogales A., Blanco-Lobo P., de la Torre J.C., Martínez-Sobrido L. Identification and characterization of novel compounds with broad spectrum antiviral activity against influenza A and B viruses. J. Virol. 2020;94 doi: 10.1128/JVI.02149-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Adcock R., Chu Y., Golden J., Chung D. Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antivir. Res. 2017;138:47–56. doi: 10.1016/j.antiviral.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 136.Goebel S., Snyder B., Sellati T., Saeed M., Ptak R., Murray M., Bostwickb R., Raynerc J., Koidea F., Kalkeri R. A sensitive virus yield assay for evaluation of Antivirals against Zika Virus. J. Virol. Met. 2016;238:13–20. doi: 10.1016/j.jviromet.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 137.Morrey J., Smee D., Sidwell R., Tseng C. Identification of active antiviral compounds against a New York isolate of West Nile virus. Antivir. Res. 2002;55:107–116. doi: 10.1016/s0166-3542(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 138.Sebastian L., Madhusudana S., Ravi V., Desai A. Mycophenolic acid inhibits replication of Japanese encephalitis virus. Chemotherapy. 2011;57:56–61. doi: 10.1159/000321483. [DOI] [PubMed] [Google Scholar]
- 139.Takhampunya R., Ubol S., Houng H., Cameron C., Padmanabhan R. Inhibition of dengue virus replication by mycophenolic acid and ribavirin. J. Gen. Virol. 2006;87:1947–1952. doi: 10.1099/vir.0.81655-0. [DOI] [PubMed] [Google Scholar]
- 140.Leyssen P., Van Lommel A., Drosten C., Schmitz H., De Clercq E., Neyts J. A novel model for the study of the therapy of flavivirus infections using the Modoc virus. Virology. 2001;279:27–37. doi: 10.1006/viro.2000.0723. [DOI] [PubMed] [Google Scholar]
- 141.Charlier N., Leyssen P., Paeshuyse J., Drosten C., Schmitz H., Van Lommel A., De Clercq E., Neyts J. Infection of SCID mice with Montana Myotis leukoencephalitis virus as a model for flavivirus encephalitis. J. Gen. Virol. 2002;83:1887–1896. doi: 10.1099/0022-1317-83-8-1887. [DOI] [PubMed] [Google Scholar]
- 142.Khan M., Dhanwani R., Patro I.K., Rao P.V.L., Parida M. Cellular IMPDH enzyme activity is a potential target for the inhibition of Chikungunya virus replication and virus induced apoptosis in cultured mammalian cells. Antivir. Res. 2011;89:1–8. doi: 10.1016/j.antiviral.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 143.Smee D., Hurst B., Evans W., Clyde N., Wright S., Jung K., Peterson C., Day C. Viruses that exhibit different cytopathogenic properties. J. Virol. Met. 2018;246:51–57. doi: 10.1016/j.jviromet.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mei-jiao G., Shi-fang L., Yan-yan C., Jun-jun S., Yue-feng S., Ting-ting R., Guang Y., Zhang Yun H. Chang. Antiviral effects of selected IMPDH and DHODH inhibitors against foot and mouth disease virus. Biomed. Pharmacother. 2019;118:1–7. doi: 10.1016/j.biopha.2019.109305. [DOI] [PubMed] [Google Scholar]
- 145.Chang Q., Guo F., Li X., Zhou J., Cai X., Pan Q., Ma X. The IMPDH inhibitors, ribavirin and mycophenolic acid, inhibit peste des petits ruminants virus infection. Vet. Res. Commun. 2018;42:309–313. doi: 10.1007/s11259-018-9733-1. [DOI] [PubMed] [Google Scholar]
- 146.Sepúlveda C., García C., Fascio M., D'Accorso N., Docampo Palacios M., Pellón R., Damonte E. Inhibition of Junin virus RNA synthesis by an antiviral acridone derivative. Antivir. Res. 2012;93:16–22. doi: 10.1016/j.antiviral.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 147.Dang W., Yin Y., Wang Y., Wang W., Su J., Sprengers D., Van der Laan L., Felczak K., Pankiewicz K., Chang K., Koopmans M., Metselaar H., Peppelenbosch M., Pan Q. Inhibition of calcineurin or IMP dehydrogenase exerts moderate to potent antiviral activity against norovirus replication. Antimicrob. Agents Chemother. 2017;61:1–17. doi: 10.1128/AAC.01095-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ölschläger S., Neyts J., Günther S. Depletion of GTP pool is not the predominant mechanism by which ribavirin exerts its antiviral effect on Lassa virus. Antivir. Res. 2011;91:89–93. doi: 10.1016/j.antiviral.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 149.Robertson C., Hermann L., Coombs K. Mycophenolic acid inhibits avian reovirus replication. Antivir. Res. 2004;64:55–61. doi: 10.1016/j.antiviral.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 150.Hermann L., Coombs K. Mycophenolic acid inhibits replication of Type 2 Winnipeg, a cerebrospinal fluid-derived reovirus isolate. Can. J. Infect Dis. 2004;15:261–265. doi: 10.1155/2004/387272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Livonesi M., Moro De Sousa R., Moraes Figueiredo L. In vitro study of antiviral activity of mycophenolic acid on Brazilian orthobunyaviruses. Intervirology. 2007;50:204–208. doi: 10.1159/000099219. [DOI] [PubMed] [Google Scholar]
- 152.Marroquí L., Estepa A., Perez L. Inhibitory effect of mycophenolic acid on the replication of infectious pancreatic necrosis virus and viral hemorrhagic septicemia virus. Antivir. Res. 2008;80:332–338. doi: 10.1016/j.antiviral.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 153.Yin Y., Wang Y., Dang W., Xu L., Su J., Zhou X., Wang W., Felczak K., Van der Laan L., Pankiewicz K., Van der Eijk A., Bijvelds M., Sprengers D., De Jonge H., Koopmans M., Metselaar H., Peppelenbosch M., Pan Q. Mycophenolic acid potently inhibits rotavirus infection with a high barrier to resistance development. Antivir. Res. 2016;133:41–49. doi: 10.1016/j.antiviral.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 154.Diamond M., Zachariah M., Harris E. Mycophenolic acid inhibits dengue virus infection by preventing replication of viral RNA. Virology. 2002;304:211–221. doi: 10.1006/viro.2002.1685. [DOI] [PubMed] [Google Scholar]
- 155.Takhampunya R., Ubol S., Houng H., Cameron C., Padmanabhan R. Inhibition of dengue virus replication by mycophenolic acid and ribavirin. J. Gen. Virol. 2006;87:1947–1952. doi: 10.1099/vir.0.81655-0. [DOI] [PubMed] [Google Scholar]
- 156.Uematsu J., Sakai-Sugino K., Kihira-Nakanishi S., Yamamoto H., Hirai K., Kawano M., Nishio M., Tsurudome M., O'Brien M., Komada H. Inhibitions of human parainfluenza virus type 2 replication by ribavirin and mycophenolate mofetil are restored by guanosine and S-(4-nitrobenzyl)-6-thioinosine. Drug Discov. Theroy. 2019;13:314–321. doi: 10.5582/ddt.2019.01084. [DOI] [PubMed] [Google Scholar]
- 157.Pan Q., De Ruiter P., Metselaar H., Kwekkeboom J., De Jonge J., Tilanus H., Janssen H., Van der Laan L. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C Virus infection in vitro and in vivo. Hepatology. 2012;55:1673–1683. doi: 10.1002/hep.25562. [DOI] [PubMed] [Google Scholar]
- 158.Fang S., Su J., Liang B., Li X., Li Y., Jiang J., Huang J., Zhou B., Ning C., Li J., Ho W., L Y, Chen H., Liang H., Ye L. Suppression of autophagy by mycophenolic acid contributes to inhibition of HCV replication in human hepatoma cells. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep44039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chan J., Lau S., To K., Cheng V., Woo P., Yue K. Middle East Respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Al Ghamdi M., Alghamdi K., Ghandoora Y., Alzahrani A., Salah F., Alsulami A., Bawayan M., Vaidya D., Perl T., Sood G. Treatment outcomes for patients with middle eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the kingdom of Saudi Arabia. BMC Infect. Dis. 2016;16:1–7. doi: 10.1186/s12879-016-1492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lin M., Moses D., Hsieh C., Cheng S., Chen Y., Sun C., Chen Y., Sun C., Chou C. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir. Res. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chan J., Chan K., Kao R., To K., Zheng B., Li C., Li P., Dai J., Mok F., Chen H., Hayden F., Yuen K. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J. Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chan J., Yao Y., Yeung M., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P., Cai J.P., Chu H., Zhou J., Chen H., Qin C., Yuen K. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Barnard D., Day C., Bailey K., Heiner M., Montgomery R., Lauridsen L., Winslowa S., Hoopesb J., Li J., Lee J., Carson D., Cottam H., Sidwell R. Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antivir. Res. 2006;71:53–63. doi: 10.1016/j.antiviral.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neyts J., Andrei G., De Clercq E. The novel immunosuppressive agent mycophenolate mofetil markedly potentiates the antiherpesvirus activities of acyclovir, ganciclovir, and penciclovir in vitro and in vivo. Antimicrob. Agents Chemother. 1998;42:216–222. doi: 10.1128/aac.42.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ying C., Colonno R., De Clercq E., Neyts J. Ribavirin and mycophenolic acid markedly potentiate the anti-hepatitis B virus activity of entecavir. Antivir. Res. 2007;73:192–196. doi: 10.1016/j.antiviral.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 168.Ying C., De Clercq E., Neyts J. Ribavirin and mycophenolic acid potentiate the activity of guanine- and diaminopurine-based nucleoside analogues against hepatitis B virus. Antivir. Res. 2000;48:117–124. doi: 10.1016/s0166-3542(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 169.Planterose D. Antiviral and cytotoxic effects of mycophenolic acid. J. Gen. Virol. 1969;4:629–630. doi: 10.1099/0022-1317-4-4-629. [DOI] [PubMed] [Google Scholar]
- 170.Reis A., Reinhard T., Voiculescu A., Mayer K., Sundmacher R. Hochaktive antivirale und immunsuppressive kombinationstherapie mit acyclovir und mycophenolatmofetil nach keratoplastik bei herpetischer grunderkrankung. Klin Monbl Augenheilkd. 2001;218:183–186. doi: 10.1055/s-2001-13078. [DOI] [PubMed] [Google Scholar]
- 171.Margolis D., Heredia A., Gaywee J., Oldach D., Drusano G., Redfield R. Abacavir and mycophenolic acid, an inhibitor of inosine monophosphate dehydrigenase, have profound and synergistic anti-HIV acrivity. J. Acquir. Immune Defic. Syndr. 1999;21:362–370. [PubMed] [Google Scholar]
- 172.Hossain M., Coull J., Drusano G., Margolis D. Dose proportional inhibition of HIV-1 replication by mycophenolic acid and synergistic inhibition in combination with abacavir, didanosine, and tenofovir. Antivir. Res. 2002;55:41–52. doi: 10.1016/s0166-3542(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 173.Margolis D., Knew S., Coull J., Ylisastigui L., Turner D., Wise H., Hossain M., Lanier E., Shaw L., Back D. The addition of mycophenolate mofetil to antiretroviral therapy including abacavir is associated with depletion of intracellular deoxyhanosine triphosphate and a decrease in plasma HIV-1 RNA. J. Acquir. Immune Defic. Syndr. 2002;31:45–49. doi: 10.1097/00126334-200209010-00006. [DOI] [PubMed] [Google Scholar]
- 174.Borroto-Esoda K., Myrick F., Feng J., Jeffrey J., Furman P. In vitro combination of amdoxovir and the inosine monophosphate dehydrogenase inhibitors mycophenolic acid and ribavirin demonstrates potent activity against wild-type and drug-resistant variants of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2004;48:4387–4394. doi: 10.1128/AAC.48.11.4387-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sankatsing S., Jurriaans S., Van Swieten P., Van Leth F., Cornelissen M., Miedema F., Lange J., Schuitemaker H., Prins J. Highly active antiretroviral therapy with or without mycophenolate mofetil in treatment-naive HIV-1 patients. AIDS. 2004;18:1925–1931. doi: 10.1097/00002030-200409240-00008. [DOI] [PubMed] [Google Scholar]
- 176.Sebastian L., Desai A., Yogeeswari P., Sriram D., Madhusudana S.N., Ravi V. Combination of N-methylisatin-β-thiosemicarbazone derivative (SCH16) with ribavirin and mycophenolic acid potentiates the antiviral activity of SCH16 against Japanese encephalitis virus in vitro. Lett. Appl. Microbiol. 2012;55:234–239. doi: 10.1111/j.1472-765X.2012.03282.x. [DOI] [PubMed] [Google Scholar]
- 177.Scharpé J., Evenepoel P., Maes B., Bammens B., Claes K., Osterhaus A., Vanrenterghem Y., Peetermans E. Influenza vaccination is efficacious and safe in renal transplant recipients. Am. J. Transplant. 2008;8(2):332–337. doi: 10.1111/j.1600-6143.2007.02066.x. [DOI] [PubMed] [Google Scholar]
- 178.Inui Y., Yakushijin K., Okamura A., Tanaka Y., Shinzato I., Nomura T., Ichikawa H., Mizutami Y., Kitao A., Kurata K., Kakiuchi S., Miyata Y., Sanada Y., Kitagawa K., Uryu K., Kawamoto S., Yamamoto K., Matsuoka H., Murayama T., Ito M., Minami H. Human herpesvirus 6 encephalitis in patients administered mycophenolate mofetil as prophylaxis for graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2019;21:1–8. doi: 10.1111/tid.13024. [DOI] [PubMed] [Google Scholar]
- 179.Kim W., Kim S., Oh J., Jeong Y., Rhu J., Kim K., Lee J., Choi G., Kim J., Joh J. Incidence and risk factors for herpes zoster after adult liver transplantation. Ann. Surg. Treat. Res. 2019;96:95–99. doi: 10.4174/astr.2019.96.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Monlezun D., John M., Dortonne I., Gaines I., Carsky K., Chernobylsky D., Ferrin B., Paramesh A., Zhang R., McDermott C., Parker G., Darden M., Buell J. Postrenal transplant infection: what is the effect of specific immunosuppressant agents? Surgery. 2018;164:895–899. doi: 10.1016/j.surg.2018.05.056. [DOI] [PubMed] [Google Scholar]
- 181.Holmes M., Caplin B., Atkinson C., Smith C., Harber M., Sweny P., Haque T. Prospective monitoring of epstein-barr virus DNA in adult renal transplant recipients during the early posttransplant period: role of mycophenolate mofetil. Transplantation. 2009;87:852–856. doi: 10.1097/TP.0b013e318199f983. [DOI] [PubMed] [Google Scholar]
- 182.Brown P.M., Pratt A.G., Isaacs J.D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 2016;12:731–742. doi: 10.1038/nrrheum.2016.175. [DOI] [PubMed] [Google Scholar]
- 183.Bedoui Y., Guillot X., Sélambarom J., Guiraud P., Giry C., Jaffar-Bandjee M.C., Ralandison S., Gasque P. Methotrexate an old drug with new tricks. Int. J. Mol. Sci. 2019;20(20) doi: 10.3390/ijms20205023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Beck S., Zhu Z., Oliveira M.F., Smith D.M., Rich J.N., Bernatchez J.A., Siqueira-Neto J.L. Mechanism of action of methotrexate against Zika virus. Viruses. 2019;11:4. doi: 10.3390/v11040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Marks M., Marks J.L. Viral arthritis. Clin. Med. 2016;16:129–134. doi: 10.7861/clinmedicine.16-2-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Amaral J.K., Sutaria R., Schoen R.T. Treatment of chronic Chikungunya arthritis with methotrexate: a systematic review. Arthritis Care Res. 2018;70:1501–1508. doi: 10.1002/acr.23519. [DOI] [PubMed] [Google Scholar]
- 187.Fukuda W., Hanyu T., Katayama M., Mizuki S., Okada A., Miyata M., Handa Y., Hayashi M., Koyama Y., Arii K., Kitaori T., Hagiyama H., Urushidani Y., Yamasaki T., Ikeno Y., Suzuki T., Omoto A., Sugitani T., Morita S., Inokuma S. Incidence of hepatitis B virus reactivation in patients with resolved infection on immunosuppressive therapy for rheumatic disease: a multicentre, prospective, observational study in Japan. Ann. Rheum. Dis. 2017;76:1051–1056. doi: 10.1136/annrheumdis-2016-209973. [DOI] [PubMed] [Google Scholar]
- 188.Mashima A., Usui Y., Umazume K., Muramatsu D., Goto H. Successful treatment of necrotizing retinitis with Epstein-Barr virus-positive ocular fluid by intravitreal methotrexate injection. Ocul. Immunol. Inflamm. 2019;3:1–4. doi: 10.1080/09273948.2019.1609047. [DOI] [PubMed] [Google Scholar]
- 189.Slobodnick A., Shah B., Krasnokutsky S., Pillinger M.H. Update on colchicine. Rheumatology. 2017;57(2018):i4–i11. doi: 10.1093/rheumatology/kex453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tardif J.C., Kouz S., Waters D.D., Bertrand O.F., Diaz R., Maggioni A.P., Pinto F.J., Ibrahim R., Gamra H., Kiwan G.S., Berry C., López-Sendón J., Ostadal P., Koenig W., Angoulvant D., Grégoire J.C., Lavoie M.A., Dubé M.P., Rhainds D., Provencher M., Blondeau L., Orfanos A., L'Allier P.L., Guertin M.C., Roubille F. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 191.Perico N., Ostermann D., Bontempeill M. Colchicine interferes with L-selectin and leukocyte function-associated antigen- 1 expression on human T lymphocytes and inhibits T cell activation. J. Am. Soc. Nephrol. 1996;7:594–601. doi: 10.1681/ASN.V74594. [DOI] [PubMed] [Google Scholar]
- 192.Pope R.M., Tschopp J. The role of interleukin- 1 and the inflammasome in gout: implications for therapy. Arthritis Rheum. 2007;56:3183–3188. doi: 10.1002/art.22938. [DOI] [PubMed] [Google Scholar]
- 193.Imazio M., Bobbio M., Cecchi E. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation. 2005;112:2012–2016. doi: 10.1161/CIRCULATIONAHA.105.542738. [DOI] [PubMed] [Google Scholar]
- 194.Cerquaglia C., Diaco M., Nucera G., La Regina M., Montalto M., Manna R. Pharmacological and clinical basis of treatment of Familial Mediterranean Fever (FMF) with colchicine or analogues: an update. Curr. Drug Targets - Inflamm. Allergy. 2005;4:117–124. doi: 10.2174/1568010053622984. [DOI] [PubMed] [Google Scholar]
- 195.Cacoub P.P. Colchicine for treatment of acute or recurrent pericarditis. Lancet. 2014;383:2193–2194. doi: 10.1016/S0140-6736(14)60113-6. [DOI] [PubMed] [Google Scholar]
- 196.Ravelli R.B., Gigant B., Curmi P.A. Insight into tubulin regulation from a complex with colchicine and a stathminlike domain. Nature. 2004;428:198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 197.Fordham J.N., Kirwan J., Cason J., Currey H.L. Prolonged reduction in polymorphonucler adhesion following oral colchicine. Ann. Rheum. Dis. 1981;40:605–608. doi: 10.1136/ard.40.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Misawa T., Takahama M., Kozaki T. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14:454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 199.Tong D.C., Wilson A.M., Layland J. 102 Heart. 2016. pp. 995–1002. (Colchicine in Cardiovascular Disease: an Ancient Drug with Modern Tricks). [DOI] [PubMed] [Google Scholar]
- 200.Papageorgiou N., Briasoulis A., Lazaros G. Colchicine for prevention and treatment of cardiac diseases: a meta-analysis. Cardiovasc. Theroy. 2017;35:10–18. doi: 10.1111/1755-5922.12226. [DOI] [PubMed] [Google Scholar]
- 201.Apostolidou E., Skendros P., Kambas K. Neutrophil extracellular traps regulate IL-1(-mediated inflammation in familial Mediterranean fever. Ann. Rheum. Dis. 2016;75:269–277. doi: 10.1136/annrheumdis-2014-205958. [DOI] [PubMed] [Google Scholar]
- 202.Martínez G.J., Robertson S., Barraclough J. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Robertson S., Martinez G.J., Payet C.A. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin. Sci. (Lond.) 2016;130:1237–1246. doi: 10.1042/CS20160090. [DOI] [PubMed] [Google Scholar]
- 204.Nidorf S.M., Verma S. Is there a role for colchicine in acute coronary syndromes? J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sun A., Wang Y.P., Chia J.S., Liu B.Y., Chiang C.P. Treatment with levamisole and colchicine can result in a significant reduction of IL-6, IL-8 or TNF-alpha level in patients with mucocutaneous type of Behcet's disease. J. Oral Pathol. Med. 2009;38:401–405. doi: 10.1111/j.1600-0714.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 206.Richter M., Boldescu V., Graf D., Streicher F., Dimoglo A., Bartenschlager R., Klein C.D. Synthesis, biological evaluation, and molecular docking of combretastatin and colchicine derivatives and their hCE1-activated prodrugs as anti-viral agents. ChemMedChem. 2019;14:469–483. doi: 10.1002/cmdc.201800641. [DOI] [PubMed] [Google Scholar]
- 207.Biswas K., Das Sarma J. Effect of microtubule disruption on neuronal spread and replication of demyelinating and nondemyelinating strains of mouse hepatitis virus in vitro. J. Virol. 2014;88:3043–3079. doi: 10.1128/JVI.02545-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lu N., Yang Y., Liu H., Ding X., Ou Y., Xia J., Du Y. Inhibition of respiratory syncytial virus replication and suppression of RSV-induced airway inflammation in neonatal rats by colchicine. Biotechnology. 2019;9:392. doi: 10.1007/s13205-019-1917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Worachartcheewan A., Songtawee N., Siriwong S., Prachayasittikul S., Nantasenamat C., Prachayasittikul V. Rational design of colchicine derivatives as anti-HIV agents via QSAR and molecular docking. Med. Chem. 2019;15:328–340. doi: 10.2174/1573406414666180924163756. [DOI] [PubMed] [Google Scholar]
- 210.Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio. 2013;4 doi: 10.1128/mBio.00524-13. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by COVID-19: anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 213.Channappanavar R., Perlman S. Evaluation of activation and inflammatory activity of myeloid cells during pathogenic human coronavirus infection. Methods Mol. Biol. 2020;2099:195–204. doi: 10.1007/978-1-0716-0211-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gandolfini I., Delsante M., Fiaccadori E., Zaza G., Manenti L., Degli Antoni A., Peruzzi L., Riella L.V., Cravedi P., Maggiore U. COVID-19 in kidney transplant recipients. Am. J. Transplant. 2020 doi: 10.1111/ajt.15891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Apte R.N., Voronov E. Immunotherapeutic approaches of IL-1 neutralization in the tumor microenvironment. J. Leukoc. Biol. 2017;102:293–306. doi: 10.1189/jlb.3MR1216-523R. [DOI] [PubMed] [Google Scholar]
- 217.Awasthi S., Yadav K.K., Pandey M., Mahdi A.A., Awasthi N. Pediatr. Pulmonol. 2018;53:1276–1283. doi: 10.1002/ppul.24090. [DOI] [PubMed] [Google Scholar]
- 218.Sichelstiel A., Yadava K., Trompette A., Salami O., Iwakura Y., Nicod L.P., Marsland B.J. Targeting IL-1β and IL-17A driven inflammation during influenza-induced exacerbations of chronic lung inflammation. PloS One. 2014;9 doi: 10.1371/journal.pone.0098440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wolf S., Taylor A., Zaid A., Freitas J., Herrero L.J., Rao S., Suhrbier A., Forwood M.R., Bucala R., Mahalingam S. Inhibition of interleukin-1β signaling by anakinra demonstrates a critical role of bone loss in experimental arthritogenic alphavirus infections. Arthritis Rheum. 2019;71:1185–1190. doi: 10.1002/art.40856. [DOI] [PubMed] [Google Scholar]
- 220.Guo S., Yang C., Diao B., Huang X., Jin M., Chen L., Yan W., Ning Q., Zheng L., Wu Y., Chen Y. The NLRP3 inflammasome and IL-1β accelerate immunologically mediated pathology in experimental viral fulminant hepatitis. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.De Benedetti F., Gattorno M., Anton J., Ben-Chetrit E., Frenkel J., Hoffman H.M., Koné-Paut I. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N. Engl. J. Med. 2018;378:1908–1919. doi: 10.1056/NEJMoa1706314. [DOI] [PubMed] [Google Scholar]
- 222.Arend W.P., Malyak M., Guthridge C.J., Gabay C. Interleukin‐1 receptor antagonist: role in biology. Annu. Rev. Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 223.Dubois E.A., Rissmann R., Cohen A.F. Rilonacept and canakinumab. Br. J. Clin. Pharmacol. 2011;71:639–641. doi: 10.1111/j.1365-2125.2011.03958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ridker P.M., MacFadyen J.G., Thuren T., Everett B.M., Libby P., Glynn R.J., CANTOS Trial Group. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 225.He L., Ding Y., Zhang Q., Che X., He Y., Shen H., Wang H., Li Z., Zhao L., Geng J., Deng Y., Yang L., Li J., Cai J., Qiu L., Wen K., Xu X., Jiang S. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Davignon J.L., Rauwel B., Degboé Y., Constantin A., Boyer J.F., Kruglov A., Cantagrel A. Modulation of T-cell responses by anti-tumor necrosis factor treatments in rheumatoid arthritis: a review. Arthritis Res. Ther. 2018;20:229. doi: 10.1186/s13075-018-1725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li P., Zheng Y., Chen X. Drugs for autoimmune inflammatory diseases: from small molecule compounds to anti-TNF biologics. Front. Pharmacol. 2017;8:460. doi: 10.3389/fphar.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rutherford A.I., Patarata E., Subesinghe S., Hyrich K.L., Galloway J.B. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology. 2018;57:997–1001. doi: 10.1093/rheumatology/key023. [DOI] [PubMed] [Google Scholar]
- 229.Willeaume V., Kruys V., Mijatovic T., Huez G. Tumor necrosis factor-alpha production induced by viruses and by lipopolysaccharides in macrophages: similarities and differences. J. Inflamm. 1995-1996;46(1):1–12. [PubMed] [Google Scholar]
- 230.Cui J., Chen Y., Wang H.Y., Wang R.F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccines Immunother. 2014;10:3270–3285. doi: 10.4161/21645515.2014.979640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wortzman M.E., Clouthier D.L., McPherson A.J., Lin G.H., Watts T.H. The contextual role of TNFR family members in CD8(+) T-cell control of viral infections. Immunol. Rev. 2013;255:125–148. doi: 10.1111/imr.12086. [DOI] [PubMed] [Google Scholar]
- 232.Huang J., Yu S., Ji C., Li J. Structural basis of cell apoptosis and necrosis in TNFR signaling. Apoptosis. 2015;20(2):210–215. doi: 10.1007/s10495-014-1061-5. [DOI] [PubMed] [Google Scholar]
- 233.Chiu Y.M., Chen D.Y. Infection risk in patients undergoing treatment for inflammatory arthritis: non-biologics versus biologics. Expet Rev. Clin. Immunol. 2020;16:207–228. doi: 10.1080/1744666X.2019.1705785. [DOI] [PubMed] [Google Scholar]
- 234.Lee Y.H., Bae S.C., Song G.G. Hepatitis B virus reactivation in HBsAg-positive patients with rheumatic diseases undergoing anti-tumor necrosis factor therapy or DMARDs. Int. J. Rheum. Dis. 2013;16:527–531. doi: 10.1111/1756-185X.12154. [DOI] [PubMed] [Google Scholar]
- 235.Hakim M.S., Ding S., Chen S., Yin Y., Su J., van der Woude C.J., Fuhler G.M., Peppelenbosch M.P., Pan Q., Wang W. TNF-α exerts potent anti-rotavirus effects via the activation of classical NF-κB pathway. Virus Res. 2018;253:28–37. doi: 10.1016/j.virusres.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 236.Kumar A., Coquard L., Herbein G. Targeting TNF-alpha in HIV-1 infection. Curr. Drug Targets. 2016;17:15–22. doi: 10.2174/1573399811666150615145824. [DOI] [PubMed] [Google Scholar]
- 237.Kumar A., Abbas W., Herbein G. The use of TNF inhibitors can have significant impact on HIV disease progression. TNF and TNF receptor superfamily members in HIV infection: new cellular targets for therapy? Mediat. Inflamm. 2013:484378. doi: 10.1155/2013/484378. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Filippi J., Roger P.M., Schneider S.M., Durant J., Breittmayer J.P., Benzaken S., Bernard A., Dellamonica P., Hébuterne X. Groupe d'Etude Niçois Polyvalent en Infectiologie (GENPI). Infliximab and human immunodeficiency virus infection: viral load reduction and CD4+ T-cell loss related to apoptosis. Arch. Intern. Med. 2006;166:1783–1784. doi: 10.1001/archinte.166.16.1783. [DOI] [PubMed] [Google Scholar]
- 239.Nie Z., Scott G.D., Weis P.D., Itakura A., Fryer A.D., Jacoby D.B. Role of TNF-α in virus-induced airway hyperresponsiveness and neuronal M₂ muscarinic receptor dysfunction. Br. J. Pharmacol. 2011;164:444–452. doi: 10.1111/j.1476-5381.2011.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005 Jul 7;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Black R.A., Rauch C.T., Kozlosky C.J., Peschon J.J., Slack J.L., Wolfson M.F., Castner B.J., Stocking K.L., Reddy P., Srinivasan S., Nelson N., Boiani N., Schooley K.A., Gerhart M., Davis R., Fitzner J.N., Johnson R.S., Paxton R.J., March C.J., Cerretti D.P. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 242.Haga S., Nagata N., Okamura T., Yamamoto N., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antivir. Res. 2010;85:551–555. doi: 10.1016/j.antiviral.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.McDermott J.E., Mitchell H.D., Gralinski L.E., Eisfeld A.J., Josset L., Bankhead A., 3rd, Neumann G., Tilton S.C., Schäfer A., Li C., Fan S., McWeeney S., Baric R.S., Katze M.G., Waters K.M. The effect of inhibition of PP1 and TNFα signaling on pathogenesis of SARS coronavirus. BMC Syst. Biol. 2016;23:93. doi: 10.1186/s12918-016-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. pii; 2020 Mar 27. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019; p. 137244. J. Clin. Invest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chinese Clinical Trial Registry, Registration number: ChiCTR2000030089.
- 246.Tanaka T., Kishimoto T. The biology and medical implications of interleukin-6. Canc. Immunol. Res. 2014;2:288–294. doi: 10.1158/2326-6066.CIR-14-0022. [DOI] [PubMed] [Google Scholar]
- 247.Xu X.J., Tang Y.M. Cytokine release syndrome in cancer immunotherapy with chimeric antigen receptor engineered T cells. Canc. Lett. 2014;343:172–178. doi: 10.1016/j.canlet.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 248.Brudno J.N., Kochenderfer J.N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee D.W., Gardner R., Porter D.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kotch C., Barret D., Theachey D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expet Rev. Clin. Immunol. 2019;15:813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Le R.Q., Li L., Yuan W. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncol. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China medical treatment expert group for covid-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chen L., Liu H.G., Liu W. Zhonghua Jie He He Hu Xi Za Zhi; 2020. Analysis of Clinical Features of 29 Patients with 2019 Novel Coronavirus Pneumonia. (Articolo in cinese) [DOI] [PubMed] [Google Scholar]
- 254.Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: what we know. Int. J. Infect. Dis. 2020 Mar 11 doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Galeotti C., Kaveri S.V., Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int. Immunol. 2017;29:491–498. doi: 10.1093/intimm/dxx039. [DOI] [PubMed] [Google Scholar]
- 256.DesJardin J.A., Snydman D.R. Antiviral immunotherapy: a review of current status. BioDrugs Clin. Immunother. Biopharm. Gene Theroy. 1998;9:487–507. doi: 10.2165/00063030-199809060-00006. [DOI] [PubMed] [Google Scholar]
- 257.Krause I., Wu R., Sherer Y., Patanik M., Peter J.B., Shoenfeld Y. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations--a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfus. Med. Oxf. Engl. 2002;12:133–139. doi: 10.1046/j.1365-3148.2002.00360.x. [DOI] [PubMed] [Google Scholar]
- 258.Buckley R., Schiff R. The use of intravenous immune globulin in immunodiciencies disease. N. Engl. J. Med. 1991;110:7. doi: 10.1056/NEJM199107113250207. [DOI] [PubMed] [Google Scholar]
- 259.Deener A., Mehra A., Bernstein L., Shliozberg J., Rubinstein A. Intravenous gammaglobulin treatment in HIV-1 infection. Immunol. Allergy Clin. 2008;28:851–859. doi: 10.1016/j.iac.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 260.Dickinson B.I., Gora-Harper M.L., McCraney S.A., Gosland M. Studies evaluating high-dose acyclovir, intravenous immune globulin, and cytomegalovirus hyperimmunoglobulin for prophylaxis against cytomegalovirus in kidney transplant recipients. Ann. Pharmacother. 1996;30:1452–1464. doi: 10.1177/106002809603001215. [DOI] [PubMed] [Google Scholar]
- 261.Ljungman P., Cordonnier C., Einsele H., Bender-Götze C., Bosi A., Dekker A. Use of intravenous immune globulin in addition to antiviral therapy in the treatment of CMV gastrointestinal disease in allogeneic bone marrow transplant patients: a report from the European Group for Blood and Marrow Transplantation (EBMT). Infectious Diseases Working Party of the EBMT. Bone Marrow Transplant. 1998;21:473–476. doi: 10.1038/sj.bmt.1701113. [DOI] [PubMed] [Google Scholar]
- 262.Sarmiento E., Lanio N., Gallego A., Rodriguez-Molina J., Navarro J., Fernandez-Yañez J. Immune monitoring of anti cytomegalovirus antibodies and risk of cytomegalovirus disease in heart transplantation. Int. Immunopharm. 2009;9:649–652. doi: 10.1016/j.intimp.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 263.Wyde P.R., Chetty S.N., Jewell A.M., Boivin G., Piedra P.A. Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antivir. Res. 2003;60:51–59. doi: 10.1016/s0166-3542(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 264.Bonney D., Razali H., Turner A., Will A. Successful treatment of human metapneumovirus pneumonia using combination therapy with intravenous ribavirin and immune globulin. Br. J. Haematol. 2009;145:667–669. doi: 10.1111/j.1365-2141.2009.07654.x. [DOI] [PubMed] [Google Scholar]
- 265.Shachor-Meyouhas Y., Ben-Barak A., Kassis I. Treatment with oral ribavirin and IVIG of severe human metapneumovirus pneumonia (HMPV) in immune compromised child. Pediatr. Blood Canc. 2011;57:350–351. doi: 10.1002/pbc.23019. [DOI] [PubMed] [Google Scholar]
- 266.Park U.J., Hyun S.K., Kim H.T., Cho W.H., Han S.Y. Successful treatment of disseminated adenovirus infection with ribavirin and intravenous immunoglobulin in an adult renal transplant recipient: a case report. Transplant. Proc. 2015;47:791–793. doi: 10.1016/j.transproceed.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 267.Saquib R., Melton L.B., Chandrakantan A., Rice K.M., Spak C.W., Saad R.D. Disseminated adenovirus infection in renal transplant recipients: the role of cidofovir and intravenous immunoglobulin. Transpl. Infect. Dis. 2010;12:77–83. doi: 10.1111/j.1399-3062.2009.00452.x. [DOI] [PubMed] [Google Scholar]
- 268.Ghosh S., Champlin R.E., Ueno N.T., Anderlini P., Rolston K., Raad I. Respiratory syncytial virus infections in autologous blood and marrow transplant recipients with breast cancer: combination therapy with aerosolized ribavirin and parenteral immunoglobulins. Bone Marrow Transplant. 2001;28:271–275. doi: 10.1038/sj.bmt.1703131. [DOI] [PubMed] [Google Scholar]
- 269.Huang Y.C., Lin T.Y., Lin Y.J., Lien R.I., Chou Y.H. Prophylaxis of intravenous immunoglobulin and acyclovir in perinatal varicella. Eur. J. Pediatr. 2001;160:91–94. doi: 10.1007/s004310000640. [DOI] [PubMed] [Google Scholar]
- 270.Lu Y.-C., Fan H.-C., Wang C.-C., Cheng S.-.N. Concomitant use of acyclovir and intravenous immunoglobulin rescues an immunocompromised child with disseminated varicella caused multiple organ failure. J. Pediatr. Hematol. Oncol. 2011;33:e350–351. doi: 10.1097/MPH.0b013e3181ec0efb. [DOI] [PubMed] [Google Scholar]
- 271.Masci S., De Simone C., Famularo G., Gravante M., Ciancarelli M., Andreassi M. Intravenous immunoglobulins suppress the recurrences of genital herpes simplex virus: a clinical and immunological study. Immunopharmacol. Immunotoxicol. 1995;17:33–47. doi: 10.3109/08923979509052718. [DOI] [PubMed] [Google Scholar]
- 272.Randhawa P., Pastrana D.V., Zeng G., Huang Y., Shapiro R., Sood P. Commercially available immunoglobulins contain virus neutralizing antibodies against all major genotypes of polyomavirus BK. Am. J. Transplant. 2015;15:1014–1020. doi: 10.1111/ajt.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sener A., House A.A., Jevnikar A.M., Boudville N., McAlister V.C., Muirhead N. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81:117–120. doi: 10.1097/01.tp.0000181096.14257.c2. [DOI] [PubMed] [Google Scholar]
- 274.Ben-Nathan D., Lustig S., Tam G., Robinzon S., Segal S., Rager-Zisman B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J. Infect. Dis. 2003;188:5–12. doi: 10.1086/376870. [DOI] [PubMed] [Google Scholar]
- 275.Takada A., Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev. Med. Virol. 2003;13:387–398. doi: 10.1002/rmv.405. [DOI] [PubMed] [Google Scholar]
- 276.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol. Sin. 2020 doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Peiris J.S.M., Chu C.M., Cheng V.C.C., Chan K.S., Hung I.F.N., Poon L.L.M. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet Londn. Engl. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jaume M., Yip M.S., Cheung C.Y., Leung H.L., Li P.H., Kien F. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J. Virol. 2011;85:10582–10597. doi: 10.1128/JVI.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wang S.-.F., Tseng S.-.P., Yen C.-.H., Yang J.-.Y., Tsao C.-.H., Shen C.-.W. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 2014;451:208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Stockman L.J., Bellamy R., Garner P.S.A.R.S. Systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ghoreschi K., Laurence A., O'Shea J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yamaoka K., Saharinen P., Pesu M., Holt V.E., 3rd, Silvennoinen O., O'Shea J.J. The janus kinases (jaks) Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhang S., Carriere J., Lin X., Xie N., Feng P. Interplay between cellular metabolism and cytokine responses during viral infection. Viruses. 2018;10:10. doi: 10.3390/v10100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Schoggins J.W., Rice C.M. Interferon-stimulated genes and their anti-viral effector functions. Curr. Opin. Virol. 2011;1(6):519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Igaz P., Tóth S., Falus A. Biological and clinical significance of the JAK-STAT pathway; lessons from knockout mice. Inflamm. Res. 2001;50:435–441. doi: 10.1007/PL00000267. [DOI] [PubMed] [Google Scholar]
- 286.Jamilloux Y., El Jammal T., Vuitton L., Gerfaud-Valentin M., Kerever S., Sève P. JAK inhibitors for the treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2019;18:102390. doi: 10.1016/j.autrev. [DOI] [PubMed] [Google Scholar]
- 287.Fragoulis G.E., McInnes I.B., Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology. 2019;58:i43–i54. doi: 10.1093/rheumatology/key276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Dhillon S. Drugs. Tofacitinib: Rev. Rheumatoid Arthr. 2017;77:1987–2001. doi: 10.1007/s40265-017-0835-9. [DOI] [PubMed] [Google Scholar]
- 289.Al-Salama Z.T., Baricitinib Scott L.J. A review in rheumatoid arthritis. Drugs. 2018;78:761–772. doi: 10.1007/s40265-018-0908-4. [DOI] [PubMed] [Google Scholar]
- 290.Genovese M.C., Fleischmann R., Combe B., Hall S., Rubbert-Roth A., Zhang Y., Zhou Y., Mohamed M.F., Meerwein S., Pangan A.L. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391:2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 291.Kuchipudi S.V. The complex role of STAT3 in viral infections. J. Immunol. Res. 2015;2015:272359. doi: 10.1155/2015/272359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bechman K., Subesinghe S., Norton S., Atzeni F., Galli M., Cope A.P., Winthrop K.L., Galloway J.B. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology. 2019;58:1755–1766. doi: 10.1093/rheumatology/kez087. [DOI] [PubMed] [Google Scholar]
- 294.Chiu Y.M., Chen D.Y. Infection risk in patients undergoing treatment for inflammatory arthritis: non-biologics versus biologics. Expet Rev. Clin. Immunol. 2020;16:207–228. doi: 10.1080/1744666X.2019.1705785. [DOI] [PubMed] [Google Scholar]
- 295.Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology. 2019;58:i34–i42. doi: 10.1093/rheumatology/key287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Colombel J.F. Herpes zoster in patients receiving JAK inhibitors for ulcerative colitis: mechanism, epidemiology, management, and prevention. Inflamm. Bowel Dis. 2018;24:2173–2182. doi: 10.1093/ibd/izy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bergamini A., Bolacchi F., Bongiovanni B., Cepparulo M., Ventura L., Capozzi M., Sarrecchia C., Rocchi G. Granulocyte-macrophage colony-stimulating factor regulates cytokine production in cultured macrophages through CD14-dependent and -independent mechanisms. Immunology. 2000;101:254–261. doi: 10.1046/j.1365-2567.2000.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., Yan K.W., Chan K.H., Tsang N.C., Guan Y., Yuen K.Y., Peiris J.S. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa041. Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Burg J., Krump-Konvalinkova V., Bittinger F., Kirkpatrick C.J. GM-CSF expression by human lung microvascular endothelial cells: in vitro and in vivo findings. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L460–L467. doi: 10.1152/ajplung.00249.2001. [DOI] [PubMed] [Google Scholar]
- 302.Sterner R.M., Sakemura R., Cox M.J., Yang N., Khadka R.H., Forsman C.L., Hansen M.J., Jin F., Ayasoufi K., Hefazi M., Schick K.J., Walters D.K., Ahmed O., Chappell D., Sahmoud T., Durrant C., Nevala W.K., Patnaik M.M., Pease L.R., Hedin K.E., Kay N.E., Johnson A.J., Kenderian S.S. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709. doi: 10.1182/blood-2018-10-881722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Kerschbaumer A., Sepriano A., Smolen J.S., van der Heijde D., Dougados M., van Vollenhoven R., McInnes I.B., Bijlsma J.W.J., Burmester G.R., de Wit M., Falzon L., Landewé R. Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2019-216656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Crotti C., Agape E., Becciolini A., Biggioggero M., Favalli E.G. Targeting granulocyte-monocyte colony-stimulating factor signaling in rheumatoid arthritis: future prospects. Drugs. 2019;79:1741–1755. doi: 10.1007/s40265-019-01192-z. [DOI] [PubMed] [Google Scholar]
- 305.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., H L H Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ferro F., Elefante E., Baldini C., Bartoloni E. Covid-19: the new challenge for rheumatologists. Clin. Exp. Rheumatol. 2020;38:175–180. Puxeddu I, talarico R, mosca M, bombardieri S. [PubMed] [Google Scholar]
- 307.Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G., Antinori S., Galli M. Covid-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38:337–3422020. Mar 22. [PubMed] [Google Scholar]