Abstract
Viruses can infect all cell-based organisms, from bacteria to humans, animals, and plants. They are responsible for numerous cases of hospitalization, many deaths, and widespread crop destruction, all of which result in an enormous medical, economical, and biological burden. Each of the currently used decontamination methods has important drawbacks. Cold plasma (CP) has entered this field as a novel, efficient, and clean solution for virus inactivation. We present recent developments in this promising field of CP-mediated virus inactivation, and describe the applications and mechanisms of the inactivation. This is particularly relevant because viral pandemics, such as COVID-19, highlight the need for alternative virus inactivation methods to replace, complement, or upgrade existing procedures.
Keywords: virus, virus inactivation, cold plasma, reactive oxygen species, reactive nitrogen species
Highlights
Pathogenic viruses are becoming an increasing burden for health, agriculture, and the global economy. Classic disinfection methods have several drawbacks, and innovative solutions for virus inactivation are urgently needed.
CP can be used as an environmentally friendly tool for virus inactivation. It can inactivate different human, animal, and plant viruses in various matrices.
When using CP for virus inactivation it is important to set the correct parameters and to choose treatment durations that allow particles to interact with the contaminated material.
Reactive oxygen and/or nitrogen species have been shown to be responsible for virus inactivation through effects on capsid proteins and/or nucleic acids. The development of more accurate methods will provide information on which plasma particles are crucial in each experiment, and how exactly they affect viruses.
When Viruses Meet Plasma
Viruses are the most abundant and diverse microbes on our planet. They have inhabited the Earth for billions of years [1], have adapted to various environments, and are now found across all ecosystems. Viruses have contributed to the evolution of life on Earth, and can be beneficial for preserving ecosystems and important natural Earth cycles such as the carbon cycle in the sea [2]. On the other hand, pathogenic viruses cause tens to hundreds of millions of plant, animal, and human infections annually, which result in high crop losses and numerous deaths (Box 1 ). Therefore, inactivating harmful viruses is crucial for better quality of life.
Box 1. Viruses and Methods for Their Disinfection.
Viruses are microscopic agents that can infect all forms of cellular life. Their classification as living organisms has historically been a question of philosophical debate, but they are unquestionably one of the most powerful engines of evolution on the planet [51]. Most viruses are not harmful, and some are even beneficial for their hosts [52]. In recent years viruses have been increasingly used to promote human wellbeing. For example, lentiviruses [53] and adeno-associated viruses [54] are being genetically engineered to formulate state-of-the-art gene therapies. Nevertheless, viruses have a bad reputation as causative agents of various human, animal, and plant diseases. This is no surprise because they have been the main players in numerous epidemics and pandemics throughout history (https://www.who.int/emergencies/diseases/managing-epidemics/en/). Several viral agents have contributed to the well-deserved ‘biohazard’ fame of viruses, including influenza, Ebola, HIV ,and coronavirus SARS-CoV-2. Despite not being such ‘viral celebrities’, waterborne viruses pose increasingly serious health and economic burdens in the present era that is threatened by climate change and the scarcity of potable water.
Different physical and chemical treatments have traditionally been applied for inactivation of viruses. Chlorine, alcohols, acids, alkalis, and bleach are examples of chemical disinfectants, whereas UV irradiation, filtration, pressure, and temperature are physical treatments [55]. The method of choice depends on the matrix to be disinfected and on the virus targeted for inactivation. Waterborne viruses, including enteric viruses [56] and plant tobamoviruses [57], are among the most stable of all viruses. To inactivate such stable viruses in a delicate matrix, the disinfection method needs to be strong enough to inactivate the virus but at the same time it needs to be nontoxic to maintain the quality and properties of the water. It is now known that chlorination, a traditionally used method for water disinfection, does not efficiently inactivate some viruses, and in the long term can pose a risk to human health through release of toxic byproducts [58]. In recent years novel waterborne virus inactivation technologies have been developed, such as membrane filtration, reverse osmosis, UV and ozone treatments, and hydrodynamic cavitation, each of which has their own pros and cons. The frequent disadvantages of these technologies are cost inefficiency, scalability problems, and unsustainable power usage. Laboratory-scale studies suggest that CP has potential to overcome these problems, but confirmation will require studies focused on pilot or industrial scale deployment of plasma-based disinfection devices.
Alt-text: Box 1
Viruses can be transmitted directly from one infected individual to another or indirectly via contaminated intermediates such as surfaces, objects, air, food, and water. Transmission via contaminated surfaces and aerosols has shown to be of great importance in the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. Water is also an increasingly important transmission route for pathogenic viruses. This has arisen from global climate change and the continued increase in water demand, combined with inefficient virus removal by traditional water treatments and reuse of wastewater for irrigation purposes [4,5]. Pathogenic waterborne viruses are important contributors to one of the most important global risks we are facing today, the scarcity of potable water [6]. Various virus inactivation (see Glossary) methods are used to prevent viral spread in different matrices, but unfortunately the ideal method has yet to be discovered (Box 1). Thus, there is an urgent need for an environmentally friendly treatment that produces neither waste nor toxic byproducts, does not use toxic chemicals, is easy and safe to work with, and is also efficient in terms of virus inactivation. The emergence of CP treatments for virus inactivation aims to provide a solution to all of these features.
Plasma is the fourth state of matter. It is a partially or fully ionized gas where the atoms and/or molecules are stripped of their outer-shell electrons (Box 2 ) [7]. Among its complex constituents, the emission of UV radiation and reactive oxygen and/or nitrogen species (RONS) have the most important antimicrobial properties [8]. UV can damage nucleic acids [9], whereas RONS can oxidize nucleic acids, proteins, and lipids, with different affinities that depend on the species [10]. These inherent properties of plasma, and more specifically of CP, have motivated extensive studies on the use of CP for inactivation of various pathogenic microorganisms. The main target has been bacteria, with investigations across different fields such as food production [11], medicine, and dentistry [12]. These have even extended to oncotherapy applications, where cancer cells are targeted instead of pathogenic microorganisms [13].
Box 2. Let’s Talk about Plasma.
Plasma is the most abundant state of matter and comprises 99% of the visible universe. The sun and other stars, nebulae, solar winds, lightning, and aurora borealis are all in the plasma state. Plasma TVs as well as neon and fluorescent lights are the best-known man-made uses of plasma. Generally, plasma contains free electrons, atoms, and molecules in neutral, ionized, and/or excited states (including ROS/RNS). Plasma of many gases represents an extensive source of UV and vacuum UV radiation [59]. The possibility to use a particular or a combination of constituents makes plasma a unique material-treatment technique.
Plasma can be roughly divided into thermal or equilibrium plasma, where all particles have approximately the same temperature (average kinetic energy of random motion), and nonthermal, nonequilibrium, or CP, where light electrons have much higher temperatures compared with heavy atoms and molecules, which often remain close to room temperature. In other words, CP is at the point of application at room temperature, and is therefore suitable for treating diverse biological material including solids, liquids, and aerosols. CP can be further classified into low pressure and atmospheric pressure. The latter is limited to the volume where there are large electric fields, whereas low-pressure plasma spreads in a large volume [60]. CP is usually sustained with an electrical discharge. The gas temperature is usually almost unaffected, but the chemical reactivity is vast compared with the source gas because of the presence of reactive species. In most cases of virus inactivation, atmospheric pressure plasma has been used because of practical considerations ([61] for more information on various plasma sources used in microbial decontamination).
Plasmas are used in various industries, mainly for tailoring the surfaces of solids (e.g., oxidation, cleaning, nanostructuring, binding different atom/molecule groups), but also for the destruction of microorganisms such as viruses. Plasma can also be used for treatment of liquids; however, inactivation of viruses in liquid media is more challenging than for surfaces because plasma cannot be sustained in liquids, and is only present in gaseous bubbles inside the liquid or above the liquid surface. Depending on the place of their generation, RONS interact with either the bubble surface or the surface of the liquid, where many dissolve. They can then diffuse within the liquid, and may eventually interact with the virus. Furthermore, UV radiation penetrates liquids with a penetration depth that depends greatly on the wavelength, and the concentration, and type of impurities [62]. There are various techniques for measuring both long- and short-lived RONS in liquids [63], but these are not used frequently by researchers working on the destruction of viruses. Many authors state the discharge parameters (voltage, current, power) rather than the plasma parameters (concentration of reactive species) which are necessary to compare various plasma sources. The plasma–virus scientific niche is therefore in its infancy at present.
Alt-text: Box 2
Plasma-mediated virus inactivation is a relatively young field of research (reviewed in [14,15]) which started only about 20 years ago [16]. This is despite the decades-old knowledge that ozone, that is usually synthesized from O2 subjected to plasma conditions, can inactivate viruses [17]. However, over the past few years the number of publications in the CP–virus field has doubled, and research has expanded from only defining the virucidal properties of plasma to describing its modes of inactivation.
This review offers a comprehensive overview of the latest progress and achievements in the CP–virus field. We also describe and discuss the modes of CP-mediated virus inactivation and the reactive species that are responsible.
CP Inactivation of Viruses
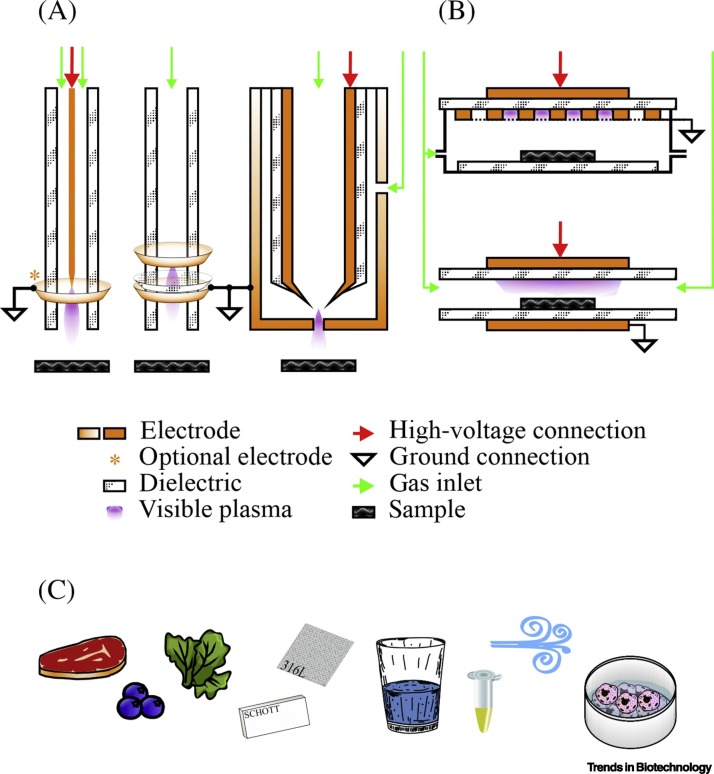
Almost every study on CP inactivation of viruses is unique because they either use a specific plasma source [e.g., dielectric barrier discharge (DBD), plasma (micro)jet s] (Figure 1) with different characteristics (e.g., power, gas, treatment time) or they deal with the treatment of different liquid volumes (from microliters to several milliliters), matrices (e.g., water, other solutions, surfaces, cells), and viruses (surrogates of human viruses, human, animal, and plant viruses). Such wide diversity makes it difficult to directly compare these studies and to define the mechanistic conclusions or any universal inactivation parameters. To simplify these complexities, we consider here the individual types of viruses that have been subjected to CP treatments. A complete list of the treatments published to date is given in Table S1, Table S2 in the supplemental information online.
Figure 1.
Examples of Commonly Used Plasma Sources for Virus Inactivation in Different Matrices.
These include different types of (micro)jets (A) and dielectric barrier discharges (B). (C) Various matrices inoculated with viruses and treated using cold plasma: (left to right) meat, blueberries, lettuce, glass, stainless steel, water, buffer or other liquid medium, air, cells.
Human Viruses
Enteric Viruses
CP treatments have been often focused on enteric viruses such as norovirus, adenovirus, and hepatitis A virus. These are the leading causes of acute gastroenteritis, the second most common infectious disease worldwide, which is responsible for high levels of hospitalization and mortality [18]. Working with human viruses can pose serious health hazards, and such studies require specialized laboratories and equipment. Moreover, infectivity assessments of important enteric viruses, such as norovirus, have been limited owing to a lack of cultivation methods [19]. For these reasons, these viruses are often replaced by surrogate viruses.
Animal viruses such as feline calicivirus (FCV), murine norovirus (MNV), and Tulane virus (TV) have been used as surrogates for norovirus owing to their similar sizes, morphologies, and genetic material. Furthermore, these surrogate viruses are easy to culture/reproduce, and are safe to work with [19]. Opinions are divided over which of these surrogate viruses best resembles the stability of norovirus, and the final choice strongly depends on the inactivation method used and the environmental properties [13., 14., 15.]. In addition to animal viruses, bacteriophages (viruses that infect bacteria) can be used as surrogates for enteric viruses and other human pathogens (Box 3 ).
Box 3. Bacteriophages as Surrogates, and an Alternative CP Treatment.
Bacteriophages are the first choice in many studies to establish proof of concept for virus inactivation methods because of their many advantages. They are relatively inexpensive to culture/produce, easy and safe to work with, they can be produced in large quantities, and plaque-based infectivity assays are time-efficient [64]. However, care must be taken when interpreting the results because they do not always correlate with the response of the actual virus to the inactivation method.
The first study that triggered the expansion of the plasma–virus field was conducted on bacteriophages [16]. In recent years, bacteriophages have been used to test the use of CP for air purification [41,49] and to study CP effects on waterborne viral pathogens [46,49].
Bacteriophages have been successfully inactivated in water, where almost complete inactivation of MS2 was obtained after 3 minutes using a plasma microjet [49]. Waterborne MS2, T4, and ⌽174 were treated directly with surface DBD or indirectly with CP-activated water [46]. All three bacteriophages were successfully inactivated with both treatments, but shorter treatment times were needed for inactivation of ⌽174 and MS2 than for T4 (Table S1). In general, CP-activated liquids are gaining increasing attention [65] because they can be produced in more controlled ways than can direct CP treatments. Such a strategy is likely to be a better choice when working with irregular and sensitive samples because CP-activated liquids can be applied evenly and can reduce potentially unwanted mechanical changes in a treated material [66].
Bacteriophages have been successfully inactivated in water, where almost complete inactivation of MS2 was obtained after 3 minutes using a plasma microjet [49]. Waterborne MS2, T4, and ⌽174 were treated directly with surface DBD or indirectly with CP-activated water [46]. All three bacteriophages were successfully inactivated with both treatments, but shorter treatment times were needed for inactivation of ⌽174 and MS2 than for T4 (Table S1). In general, CP-activated liquids are gaining increasing attention [65] because they can be produced in more controlled ways than can direct CP treatments. Such a strategy is likely to be a better choice when working with irregular and sensitive samples because CP-activated liquids can be applied evenly and can reduce potentially unwanted mechanical changes in a treated material [66].
Airborne human viral pathogens pose a serious threat to human health. In two studies, aerosolized MS2 bacteriophages were successfully inactivated by CP after only 0.12 s [49] or 0.25 s [41] of contact time of the aerosol with the plasma. Although these are very promising results, one of the biggest concerns when using plasma for air purification is the production of ozone because it can be hazardous at high concentrations. Future applications of plasma should consider this, and thus aim to lower ozone concentrations below the recommended limit [67].
Another plasma-based alternative to protect against aerosolized pathogenic viruses would be a protective mask equipped with a miniature plasma source. Such a mask would have the potential to stop the spread of various viruses (e.g., SARS-CoV-2) that are transmitted by droplets because droplets are ideal for dissolution of radicals owing to their large surface-to-volume ratio. However, the problem again arises because radicals such as ozone and nitric oxides would be inhaled, and a mask would need to include a radical catalyzer or, even better, a two-membrane design in which the first plasma membrane inactivates the virus, and the second membrane serves as a catalyzer to remove toxic species created by the plasma. We believe that such an innovative mask configuration could be highly beneficial in future outbreaks.
Alt-text: Box 3
Enteric viruses and their surrogates have been successfully treated in aqueous solutions [20., 21., 22.] and other liquid media [23], and also on the surfaces of food [24., 25., 26.], stainless steel [25,27], and glass [28,29]. Three studies reported on the comparative use of both surrogates and enteric viruses [23,25,27]. It was shown that the inactivation of a chosen surrogate virus is more efficient than that of the target enteric virus [23,27], suggesting that the effects of CP on such surrogates might not always mirror the effects on their enteric virus counterparts, and should thus be interpreted with caution.
Norovirus is one of the most, if not the most, problematic enteric virus. Its inactivation in comparison with FCV as its surrogate has been investigated using CP for diluted stool samples on a stainless steel surface and lettuce leaves. Because no infectivity assays are available to date for norovirus, inactivation was determined using ethidium monoazide-coupled reverse-transcription quantitative real-time PCR (EMA-RT-qPCR) [25]. A decrease of ~2.6 log units of gene copies was observed after 5 minutes of treatment with DBD on both surfaces. The inactivation of the surrogate FCV measured by EMA-RT-qPCR in the same medium on the stainless steel surface was similar to that of norovirus, although the cell culture-based infectivity assays showed complete FCV inactivation after 3 min (also confirmed in [20]). The underestimation of FCV inactivation based on EMA-RT-qPCR in comparison with the infectivity assay might also indicate underestimation of norovirus inactivation. Because FCV and norovirus were similarly affected by the CP treatment, FCV can be considered as an appropriate surrogate. However, it remains to be determined if this would also apply when using different treatment conditions.
FCV was also inactivated by a DBD plasma torch on a glass surface [28], indicating that both this device, and the previously mentioned DBD, have good potential to inactivate enteric viruses on various surfaces. On the other hand, inactivation of adenovirus on a glass surface with a pulsed high-voltage source that sustains plasma at 0.5 bar was not as successful and would thus not be as suitable as DBD for this purpose [29].
One of the most successful events of inactivation in liquid medium, including work on bacteriophages (Box 3), was achieved by a 15 s treatment of FCV using a plasma jet [21,22]. This extremely short treatment time indicates that plasma jets could be an important tool for enteric virus inactivation in liquids; however, based on its present configuration, it would be limited to the treatment of smaller objects contaminated with potentially infected droplets.
Different CP sources have also been applied to the surfaces of various foods, such as blueberries [24], lettuce [25,26], and meat [30], and viruses were successfully inactivated without altering the appearance of the treated food. It was also shown that DBD could be used to treat packaged food [26]; however, inactivation was not as good as for nonpackaged food, and this process would therefore require further improvement before implementation. Application of CP in food industry for decontamination has multiple advantages over the most widely used thermal processing of food because it can sustain the freshness and quality of food with minimal impact on the environment because of shorter treatment times and energy requirements [11]. One must be careful when using CP for treating sensitive material such as food because, although CP is generally at room temperature at the point of application, the temperatures can rise in some cases because of the specific CP generation conditions. To prevent thermal damage during treatment of sensitive materials, the CP discharge needs to be placed far enough from the treated material [24] and/or have additional cooling provided. Another option is to use indirect treatments with plasma-activated liquids.
Respiratory Viruses
Treatment of the respiratory viruses influenza A and B ([14] for review) and respiratory syncytial virus (RSV) [31] have only been performed with the already mentioned pulsed high-voltage CP source. RSV is the most frequent causative agent of lower respiratory tract infections in infants, and is one of the most important viruses in pediatric medicine, particularly because it spreads easily through contact with contaminated surfaces [32]. Even though CP treatment completely inactivated RSV on a glass surface after 5 min [31], a simpler and more portable plasma configuration would be needed for efficient decontamination of hospital surfaces, and the previously described method would be practical only for decontamination of tools and smaller objects. Some respiratory viruses can also remain stable as aerosols for longer periods of time (e.g., SARS-CoV-2) and, to stop their spread, it is crucial to treat the air and not only surfaces (see the section on Animal Viruses and Box 3).
Sexually Transmitted Viruses
HIV is one of the most important sexually transmitted pathogens, and one of the greatest challenges to public health in general (https://www.who.int/news-room/facts-in-pictures/detail/hiv-aids). Three shots for a total of 45 s with a plasma jet were applied to macrophages before infection with HIV [33]. Upon infection, this treatment reduced viral reverse transcriptase activity by over two-thirds, and impaired the other steps required for successful virus infection, without any cytotoxic effects on the macrophages. By contrast, another study reported increasing cytotoxicity of the treated cells with decreasing virus concentration [34].
Despite these promising results, there are some limitations to deploying such a strategy in real-life scenarios, including the extraction of macrophages from affected individuals to treat them by CP, and their delivery back into the body. Such issues will need to be solved before CP can be considered as an alternative HIV treatment option in the future.
Animal Viruses
Three important animal pathogens have been treated with CP: avian influenza virus (AIV), Newcastle disease virus (NDV), and porcine reproductive and respiratory syndrome virus (PRRSv). All three viruses pose a significant threat to global food security and economic stability. Some strains of AIV can cause up to 100% mortality in chickens (https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm), and some strains of NDV can cause up to 100% mortality in different avian species [35]. Prevention of their spread by vaccination is essential. Vaccines against both viruses would benefit from improvements that would allow them to be more cost-effective, provide higher immune protection, and decrease the risk of disease development by ensuring complete virus inactivation without affecting the antigens responsible for inducing the immune response [36,37]. For this purpose, CP was used as a possible inactivation step in vaccine preparation [35]. Complete inactivation was achieved after a 2-min treatment with a plasma jet. This was shown to be ideal for vaccine preparation because longer treatment times can alter the antigen determinants responsible for immunogenicity. Both vaccines have been used to successfully induce the production of specific antibodies, and the NDV vaccine induced even higher antibody titers than the traditionally inactivated vaccines. Additional prevention methods to stop the spread of these viruses include decontamination of surfaces and tools that are in contact with potentially infected poultry by using CP-activated solutions. It has been shown that, at specific ratios, CP-activated distilled water, 0.9% NaCl, and 0.3% H2O2 completely inactivated viruses, and the chicken embryos attained 100% survival [38].
PRRSv is economically one of the most important pathogens in the pork industry, and can be transmitted as aerosols and remain infective after traveling long distances, making it a potential threat even to distant barns [39,40]. Most commonly used methods for air treatment in general rely either on physically limiting virus transmission (e.g., the use of various filters) or on lowering virus infectivity (e.g., UV irradiation). CP could potentially achieve both goals by stopping viral spread and abolishing virus infectivity, by charge-driven filtration and RONS, respectively [39., 40., 41.]. Aerosolized PRRSv has been treated in two studies by different DBDs [39,40]. Promising results with complete virus inactivation (~3.5 log reduction) were achieved with one DBD system [39], whereas the other system was only partly successful [40], and authors have suggested potential improvements that would increase inactivation efficiency. Based on these and the results on bacteriophages (Box 3), we can conclude that CP has great potential to be used for direct air disinfection, which could also be utilized in the fight against COVID-19-like outbreaks. Nevertheless, issues such as high ozone production (Box 3) will need to be addressed and solved before such treatment becomes a part of regular practice.
Plant Viruses
Plant viruses were the first viruses to be discovered [42]. Although most virus-to-plant transmission occurs via insects [42], the increasing reuse of untreated wastewater and the use of closed irrigation systems as an answer to water scarcity are promoting viral spread. Plant viruses can result in tremendous economic losses, estimated at ~30 billion US dollars annually [43]. Despite this, there are only two reports of their deactivation by CP treatments. Inactivation of the most important potato viral pathogen, potato virus Y (PVY), in water samples was achieved using plasma jet [7]. This water-transmissible virus [44] was successfully inactivated in polluted and clean water with treatments of only 5-min and 1-min, respectively. Other economically relevant plant viruses that are highly stable, resistant to classic inactivation methods, and water-transmissible are the members of the genus Tobamovirus, such as tobacco mosaic virus (TMV). Despite the inherent stability of TMV, a 10 min treatment by DBD was shown to be sufficient to inactivate it [45].
Because enormous quantities of water are being used for irrigation (up to 70% of global water usage), closed irrigation systems or reuse of wastewater are increasingly utilized, enabling the spread of plant pathogens and high crop losses. Based on results that CP can achieve efficient inactivation of important resilient plant viral pathogens, we believe that the use of plasma as a decontamination tool in agriculture has high potential and deserves additional attention, especially in the upcoming global warming scenario.
Proposed Mechanisms of Inactivation
Understanding of the underlying mechanisms of virus inactivation by CP will be crucial for fine-tuning CP treatments before their deployment in industrial, medical, and agricultural environments, and to more easily predict all possible outcomes including the formation of undesired byproducts that do not contribute to inactivation.
Reactive Species Responsible for Inactivation
The main consensus emerging from diverse studies to date is that the formation of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) is the main feature of CP that contributes to virus inactivation, whereas UV irradiation and temperature changes are only minor contributors or have no effect. Different methods have been used to measure and identify the RONS (Table 1 ), but this is a challenging task because of their short lifespan.
Table 1.
Mechanisms of Virus Inactivation by Plasmaa
| Virus | ROS/RNS involved in inactivationb | Mode of virus inactivation |
Methods for identification of virus inactivation |
Methods used for CP characterizationc | Refs | ||
|---|---|---|---|---|---|---|---|
| Protein degradation | DNA/RNA degradation | Protein degradation | DNA/RNA degradation | ||||
| Bacteriophages | |||||||
| λ | NA | Yes | Yes | SDS-PAGE alone or in combination with in vitro packaging | Agarose gel electrophoresis alone or in combination with in vitro packaging | Optical emission spectroscopy | [50] |
| MS2d | ↑O | Yes | Yes | SDS-PAGE | RT-PCR, agarose gel electrophoresis | Optical emission spectroscopy | [49] |
| MS2 | O3e | NA | No | Not measured | RT-qPCR | Ozone sensor | [41] |
| T4 | 1O2f | Yes | Yes | SDS-PAGE | Agarose gel electrophoresis | H2O2/peroxidase assay kit, nitrite/nitrate colorimetric assay kit, electron spin resonance | [46] |
| Animal surrogates of enteric viruses | |||||||
| FCVg | 1O2 or ONOOH (in acidic conditions)f, O3h, H2O2, NO2−e. | Yes | NA | SDS-PAGE, LC-MS/MS | Not measured | Colorimetric assay with titanium sulfate, Griess assay, LC/MS equipped with an electrospray ionization ion source, fluorescence probe, spectrophotometry | [22] |
| FCV | 1O2 and ONOO− or ONOOH (acidic conditions)f | NA | Yes | Not measured | RT-qPCR | Optical emission spectroscopy, UV test strips, Griess assay, H2O2 test strips | [28] |
| FCV | 1O2f, O3h | Yes | Yes | EMA-RT-qPCR, EMA-RT-PCR, SDS-PAGE | RT-PCR, RT-qPCR, sequencing | Indirect measurements with LC-MS/MS | [21] |
| FCV | NOx, O3f | NA | NA | Not measured | Not measured | UV light meter, UV absorption spectroscopy, Griess assay | [20] |
| Human viruses | |||||||
| Adenovirus | H2O2e | No | Yes | Immunochromatography and Western blotting | PCR, qPCR | H2O2, nitrite, and nitrate test strips | [29] |
| Adenovirus | O3e | NA | NA | Not measured | Not measured | Optical spectrometer, UV power meter, photometric ozone analyzer | [47] |
| Influenza A and B virusesi | H2O2f | Yes | Yes | Hemagglutination assays, ELISA, Western blotting | RT-qPCR | Chemical indicator strips, multichannel spectrophotometer, gas detector | [48] |
| RSV | H2O2f | No | Yes | Immunochromatography kits | RT-PCR, RT-qPCR | Active O2 test strips | [31] |
| HIV | ↑O2+, O, NO, N2 (second positive), N2+ | NA | Yes | Not measured | qPCR | Optical emission spectroscopy | [33] |
| Animal viruses | |||||||
| NDV | ↑ Oxidation/reduction potential, H2O2, OH•, NO• | Yes | Yes | Bradford protein assay kits | Agilent 2100 bioanalyzer | Oxidation/reduction potential probe, H2O2 assay kit, electrical conductivity meter, electron spin resonance | [38] |
| NDV, AIV | ↑ Oxidation/ reduction potential, O, NO, OH | NA | NA | Not measured | Not measured | Oxidation/reduction potential probe, optical emission spectroscopy | [35] |
| Plant viruses | |||||||
| TMV | ↑ H2O2, NO3−, HNO2, N2O2, NO2− | No | Yes | Western blotting | RT-PCR | Optical absorption spectroscopy, chemical probe | [45] |
| PVY | H2O2e, ↑OH, O | NA | Yes | Not measured | RT-PCR | Optical emission spectroscopy, H2O2 test strips | [7] |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; EMA, ethidium monoazide; FT-IR, Fourier-transform infrared; LC-MS, liquid chromatography-mass spectrometry; MS/MS, tandem mass spectrometry; NA, not applicable.
↑, The increase of RNS/ROS was measured but their importance for inactivation was not determined.
Measurements of pH and temperature are excluded, as are scavenger experiments and other methods used for indirect identification of RONS.
Methods to determine modes of virus inactivation were applied only for treated solutions.
Plays a role in inactivation, but its importance was not defined.
Major role in the inactivation.
Methods to determine modes of virus inactivation were applied only for plasma ignited in 99% Ar and 1% O2.
Important but does not have a main role in inactivation.
The only group that reported degradation of viral envelope using FT-IR spectrophotometry. ELISA was performed only for influenza B; western blotting, RT-qPCR, hemagglutination, and FT-IR only for Influenza A.
Singlet O2 (1O2) was shown to be the most important ROS for inactivation of FCV [21,22,28] and bacteriophage T4 [46]. 1O2 causes oxidative modification of histidine residues and a shift in molecular mass of methionine residues [21]. It also reacts with cysteine, tyrosine, and tryptophan, and oxidizes guanine [46]. Ozone (O3) has been reported as the main [20] or additional inactivation factor [21,22] in FCV treatment, and it was proposed to also have roles in the inactivation of the bacteriophage MS2 [41] and adenovirus [47]. Hydrogen peroxide (H2O2) has been suggested to be crucial for inactivation of RSV [31] and influenza A virus [48], but to have a secondary role in the inactivation of FCV [22], PVY [7], and adenovirus [29]. RNS have been proposed as the principal inactivation factors only in studies with FCV, where the main RNS species were ONOOH (in an acidic environment) [22,28], ONOO− [28], and NOx [20]. Other groups have measured increases in different RONS during CP treatments [7,33,35,38,45,49] (Table 1), but these studies did not expand their research to determine the precise involvement of each of the RONS in virus inactivation.
In summary, RONS are the main contributors to CP-mediated virus inactivation; however, the particular reactive species that are responsible vary and are highly dependent on the experimental conditions, such as the gas used for the CP generation, the matrix, the virus treated, and the method used for RONS determination. Increased availability and development of more accurate methods for measurement of RONS and UV intensity will enable determination of the exact CP properties that are crucial for virus inactivation. In addition to determining the CP properties that contribute most to virus inactivation, for a better mechanistic understanding of the inactivation process it is also important to explore which virus component is disrupted.
Modes of Virus Inactivation
The viral capsid, or envelope, is the first contact point with the host and, for efficient recognition of a virus by the cell receptors, it is important that their outer structure is more or less intact. Once inside the target cell, the viral genome takes over the process of replication. Therefore, these are the most important components for evaluating virus inactivation (Table 1).
Capsid protein damage and nucleic acid degradation were reported for bacteriophages T4 [46], MS2 [49], and λ [50], as well as for NDV [38] and FCV [21]. In the case of the enveloped virus influenza A, in addition to capsid and nucleic acid damage, changes in lipid components from the envelope have been reported [48]. Only in the case of bacteriophage λ [50] and FCV [21] has it been shown that the main mode of inactivation is degradation of the capsid proteins, which preceded the degradation of nucleic acids. In other studies it was not possible to determine which degradation path contributed more to the decay in viral infectivity. The aforementioned detailed study of FCV [21] identified primary targets of CP oxidation, which included specific amino acids in different regions of the capsid protein, and specific functional peptide residues in the capsid protein region that were responsible for virus attachment and entry into the host cell. CP treatments resulted in nucleic acid degradation for FCV [28] and PVY [7] (although protein degradation was not measured), as well as for adenovirus [29], RSV [31], and TMV [45], where nucleic acid degradation was indicated as the only mode of inactivation (the viral proteins or their subunits remained intact).
It is evident that the high oxidative power of CP derivatives can disrupt virus integrity at both the structural and genomic levels by affecting both proteins and nucleic acids. Minor disruption or conformational changes of the capsid proteins (or the lipid envelope when present) caused by RONS can result in loss of viral infectivity owing to disruption of the virus binding to receptors on the host cell surface. In cases where genomic nucleic acids are damaged, viruses will no longer be infective because intact genetic material is necessary for virus genome translation and replication. Even in cases where the damage was shown to be inflicted only to nucleic acids, it is likely that RONS also damaged or disrupted the outer protein layer to some extent because otherwise it would not be possible for the RONS to penetrate the virus and reach the genetic material.
One challenge in the study of the modes of virus inactivation is the selection of the appropriate method. Methods used for determination of protein degradation are either not as sensitive as the molecular methods used to determine nucleic acid degradation, or they target only specific protein subunits, and hence can sometimes overlook other changes to proteins. Future studies using combinations of the state-of-the-art methods to assess both types of damage will help with more accurate interpretation of how the damage occurs. These include cryo-electron microscopy, mass spectrometry, and long-read sequencing, as well as methods based on nucleic acid amplification such as quantitative PCR and digital PCR.
Concluding Remarks
Diverse CP sources can completely inactivate or significantly reduce the infectivity of numerous human, animal, and plant pathogenic viruses on or in various matrices (Figure 2, Key Figure). However, as indicated from various studies (Table S1), virus inactivation is highly dependent on the treatment properties, and the optimal parameters need to be chosen on a case-by-case basis.
Figure 2.
Key Figure. Inactivation of Viruses Using Cold Plasma (CP).
(A) Morphologically different viruses treated with CP. (B) Close-up of CP properties responsible for virus inactivation. The most essential moieties in virus inactivation are reactive oxygen and/or nitrogen species (RONS), although UV radiation and charged particles (e.g., ions, electrons) can also play a role. Molecules in the ground state are neutral and do not have any effects on virus inactivation. CP can target both viral proteins and nucleic acids (or even the virus envelope, when present). (C) After CP treatment, the virus particles and nucleic acids are partly or completely degraded to noninfective particles that cannot cause harm to their hosts.
Based on the recent developments in the CP–virus field described here, we anticipate that CP will be one of the most effective and environmentally friendly tools for inactivating different viruses in the near future. Ultimately, its use should lead to reduced human, animal, and plant infections, as well as lower economic and biological burdens. We believe that one of the fields of virus inactivation where plasma can represent a more significant breakthrough is water decontamination. CP could inactivate problematic enteric viruses and resilient plant viruses for either human consumption and/or for agricultural purposes. In any case, it will first be necessary to evaluate the potential adverse genotoxic and cytotoxic effects of plasma-activated water on humans and plants. In addition, a field of CP application that may gain relevance as a response to viral outbreaks (e.g., SARS-CoV-2), would in our opinion be CP-mediated air purification and incorporation of CP into protective masks and respirators (Box 3), which could help to palliate the sanitary burden caused by any future outbreaks. There is also potential in decontaminating small-surface objects such as tools and food. Although initial results are promising, the use of CP in medicinal treatments or vaccine preparation will require significant research before implementation.
Despite the high efficiency of virus inactivation, the exact modes of action and the plasma functionality in scaled-up systems remain largely unexplored (see Outstanding Questions), and further research needs to be focused in this direction. Insufficient knowledge of plasma/virus interactions present the biggest obstacle to expansion of this field. To understand these interactions, it is important to know the flux of reactive species (RONS or radiation) on the surface of the virus, the probability that a particular type of reactive species inactivates the virus, and synergetic effects between different reactive species for viral deactivation. None of these parameters are currently understood completely. Another issue to be dealt with is how to scale up CP reactors without altering the composition and amount of reactive species achieved at small scale. This could be overcome by a scale-out approach, where several small-scale reactors could be used in parallel, thus increasing the amount of treated material but maintaining the desired plasma composition. Such an approach would also abolish the need for specialized equipment for characterizing plasma chemistry in scaled-up systems because they would be the same as those already characterized at laboratory scale.
In view of environmental protection, novel environmentally friendly decontamination methods are needed. We suggest that CP should replace current chemical decontamination practices because it does not produce excessive waste and can efficiently inactivate viruses in or on different media and surfaces. CP usage will likely spread in different directions to help in coping with upcoming global challenges such as the scarcity of clean water and the detrimental effects of future viral epidemics or pandemics such as COVID-19. CP in combination with other existing technologies could help to improve virus inactivation through synergistic effects, thus providing an ultimate decontamination tool.
Outstanding Questions.
What CP source conditions will enable optimal efficiency of targeted virus inactivation in terms of the required treatment times and energy consumption?
Which types of RONS are the most relevant for inactivation of a given virus in a given matrix, how can we optimize production of such relevant RONS, and which methods should be used for their accurate determination?
Does UV radiation have a synergistic effect with RONS in virus inactivation?
What are the main viral components that are affected by different CP-mediated virus inactivation strategies, and which viral characterization methods should be used in each experiment to get a precise answer? Should a standardized protocol be developed for this purpose?
What is the scale-up potential of CP treatments?
Could CP cause cytotoxic or genotoxic damage when used for virus inactivation in specific matrices that will come in contact with human, plant, and animal tissues?
Would the combination of CP with already established methods, such as chlorine treatment, or new environmentally friendly methods such as cavitation, have a synergistic effect on virus inactivation? Would such synergy contribute to shorter treatment times, lower energy consumption, and decreased environmental burden?
Alt-text: Outstanding Questions
Acknowledgments
This work was financially supported by the Slovenian Research Agency [research core funding P4–0407, project L4-9325, and program for young researchers in the accordance with agreement on (co) financing research activity in 2019 1000-18-0105], the Ministry of Agriculture, Forestry, and Food, and JP Centralna Čistilna Naprava Domžale-Kamnik d.o.o. wastewater treatment plant. We thank Assoc. Prof. Dr Jana Žel for her valuable input to the manuscript.
Glossary
- Acute gastroenteritis
inflammation of the gastrointestinal tract that is mainly caused by viruses, especially rotaviruses and noroviruses. The most common symptoms are vomiting, diarrhea, and abdominal pain.
- Dielectric barrier discharge (DBD)
plasma is created when the processing gas is guided between an insulator with electrodes on opposite sides.
- Enteric viruses
a diverse group of human viruses that are most commonly transmitted via the fecal/oral route (including contaminated food and water), including norovirus, rotavirus, hepatitis A, sapovirus, astrovirus, and adenovirus. They infect the gastrointestinal tract, where they replicate and are then excreted in high concentrations. They can cause illness at low doses, and they can survive in the environment for long periods of time because they are resistant to physiological changes such as pH and temperature.
- Ethidium monoazide-coupled reverse-transcription qPCR (EMA-RT-qPCR)
this method combines a nucleic acid-intercalating dye that polymerizes nucleic acid upon exposure to light (ethidium monoazide), which blocks the targeted part of the genome from PCR amplification. As a result, the EMA-RT-qPCR method only detects infectious viruses with an intact capsid after treatment. This has been proposed to be used instead of infectivity assays.
- Plasma (micro)jet
plasma is created by blowing a gas next to or through an electrode.
- Reactive oxygen and nitrogen species (RONS)
reactive oxygen species (ROS; e.g., O3, O, O2*, H2O2, OH•, 1O2) are partially reduced or excited forms of oxygen, and reactive nitrogen species (RNS; e.g., N, N2*, NO, NO2, NO2−, NO3−, ONOO−, ONOOH) are the most common nitrogen- and nitric oxide-derived compounds. RONS have crucial and versatile roles in maintaining the normal function of different cells in most organisms.
- Virus inactivation
decreased host infection by a virus. The most reliable method to determine inactivation efficiency is an infectivity assay in which appropriate hosts (e.g., bacterial or eukaryotic cells, plants, chicken embryos) are inoculated with a virus. Inoculation is followed by the observation/measurement of different factors such as plaque formation, cytopathic effects, and symptoms in plants, embryo survival, or the integrity of viral nucleic acids.
Footnotes
Supplemental information associated with this article can be found online at https://doi.org/10.1016/j.tibtech.2020.04.003.
Supplemental Information
Experimental properties of cold plasma-virus treatments.
Properties of cold plasma sources used for virus inactivation.
References
- 1.Nasir A., Caetano-Anollés G. A phylogenomic data-driven exploration of viral origins and evolution. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilhelm S.W., Suttle C.A. Viruses and nutrient cycles in the sea aquatic food webs. Bioscience. 1999;49:781–788. [Google Scholar]
- 3.van Doremalen N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrestha S. Virological quality of irrigation water sources and pepper mild mottle virus and tobacco mosaic virus as index of pathogenic virus contamination level. Food Environ. Virol. 2018;10:107–120. doi: 10.1007/s12560-017-9324-2. [DOI] [PubMed] [Google Scholar]
- 5.Mehle N., Ravnikar M. Plant viruses in aqueous environment – survival, water mediated transmission and detection. Water Res. 2012;46:4902–4917. doi: 10.1016/j.watres.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Franco E.G. 15th edn. World Economic Forum; 2020. The Global Risks Report 2020. [Google Scholar]
- 7.Filipić A. Cold atmospheric plasma as a novel method for inactivation of potato virus Y in water samples. Food Environ. Virol. 2019;11:220–228. doi: 10.1007/s12560-019-09388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo J. Bactericidal effect of various non-thermal plasma agents and the influence of experimental conditions in microbial inactivation: a review. Food Control. 2015;50:482–490. [Google Scholar]
- 9.US Environmental Protection Agency Office of Water . US EPA; 2006. Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule. [Google Scholar]
- 10.Mittler R. ROS are good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Bourke P. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol. 2018;36:615–626. doi: 10.1016/j.tibtech.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Sakudo A. Disinfection and sterilization using plasma technology: fundamentals and future perspectives for biological applications. Int. J. Mol. Sci. 2019;20:5216. doi: 10.3390/ijms20205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai X. The emerging role of gas plasma in oncotherapy. Trends Biotechnol. 2018;36:1183–1198. doi: 10.1016/j.tibtech.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Puligundla P., Mok C. Non-thermal plasmas (NTPs) for inactivation of viruses in abiotic environment. Res. J. Biotechnol. 2016;11:91–96. [Google Scholar]
- 15.Weiss M. Virucide properties of cold atmospheric plasma for future clinical applications. J. Med. Virol. 2017;89:952–959. doi: 10.1002/jmv.24701. [DOI] [PubMed] [Google Scholar]
- 16.Kelly-Wintenberg K. Use of a one atmosphere uniform glow discharge plasma to kill a broad spectrum of microorganisms. J. Vac. Sci. Technol. A. 1999;17:1539–1544. [Google Scholar]
- 17.Burleson G.R. Inactivation of viruses and bacteria by ozone, with and without sonication. Appl. Microbiol. 1975;29:340–344. doi: 10.1128/am.29.3.340-344.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMinn B.R. Bacteriophages as indicators of faecal pollution and enteric virus removal. Lett. Appl. Microbiol. 2017;65:11–26. doi: 10.1111/lam.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cromeans T. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl. Environ. Microbiol. 2014;80:5743–5751. doi: 10.1128/AEM.01532-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nayak G. Reactive species responsible for the inactivation of feline calicivirus by a two-dimensional array of integrated coaxial microhollow dielectric barrier discharges in air. Plasma Process. Polym. 2018;15:1–12. [Google Scholar]
- 21.Aboubakr H.A. Cold argon-oxygen plasma species oxidize and disintegrate capsid protein of feline calicivirus. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aboubakr H.A. Inactivation of virus in solution by cold atmospheric pressure plasma: identification of chemical inactivation pathways. J. Phys. D. Appl. Phys. 2016;49:1–17. [Google Scholar]
- 23.Takamatsu T. Microbial inactivation in the liquid phase induced by multigas plasma jet. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacombe A. Nonthermal inactivation of norovirus surrogates on blueberries using atmospheric cold plasma. Food Microbiol. 2017;63:1–5. doi: 10.1016/j.fm.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 25.Aboubakr H.A. In situ inactivation of human norovirus GII.4 by cold plasma: ethidium monoazide (EMA)-coupled RT-qPCR underestimates virus reduction and fecal material suppresses inactivation. Food Microbiol. 2020;85 doi: 10.1016/j.fm.2019.103307. [DOI] [PubMed] [Google Scholar]
- 26.Min S.C. Dielectric barrier discharge atmospheric cold plasma inhibits Escherichia coli O157:H7, Salmonella, Listeria monocytogenes, and Tulane virus in Romaine lettuce. Int. J. Food Microbiol. 2016;237:114–120. doi: 10.1016/j.ijfoodmicro.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Park S.Y., Do Ha S. Assessment of cold oxygen plasma technology for the inactivation of major foodborne viruses on stainless steel. J. Food Eng. 2018;223:42–45. [Google Scholar]
- 28.Yamashiro R. Key role of singlet oxygen and peroxynitrite in viral RNA damage during virucidal effect of plasma torch on feline calicivirus. Sci. Rep. 2018;8:17947. doi: 10.1038/s41598-018-36779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakudo A. Nitrogen gas plasma generated by a static induction thyristor as a pulsed power supply inactivates adenovirus. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae S.C. Inactivation of murine norovirus-1 and hepatitis A virus on fresh meats by atmospheric pressure plasma jets. Food Res. Int. 2015;76:342–347. doi: 10.1016/j.foodres.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 31.Sakudo A. Crucial roles of reactive chemical species in modification of respiratory syncytial virus by nitrogen gas plasma. Mater. Sci. Eng. C. 2017;74:131–136. doi: 10.1016/j.msec.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Toivonen L. Respiratory syncytial virus infections in children 0–24 months of age in the community. J. Infect. 2019;80:69–75. doi: 10.1016/j.jinf.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Volotskova O. Cold atmospheric plasma inhibits HIV-1 replication in macrophages by targeting both the virus and the cells. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amiran M.R. In vitro assessment of antiviral activity of cold atmospheric pressure plasma jet against the human immunodeficiency virus (HIV) J. Med. Microbiol. Infec. Dis. 2016;4:62–67. [Google Scholar]
- 35.Wang G. Non-thermal plasma for inactivated-vaccine preparation. Vaccine. 2016;34:1126–1132. doi: 10.1016/j.vaccine.2015.10.099. [DOI] [PubMed] [Google Scholar]
- 36.Dimitrov K.M. Newcastle disease vaccines - a solved problem or a continuous challenge? Vet. Microbiol. 2017;206:126–136. doi: 10.1016/j.vetmic.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang H. Efficacy and synergy of live-attenuated and inactivated influenza vaccines in young chickens. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su X. Inactivation efficacy of nonthermal plasma-activated solutions against Newcastle disease virus. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.02836-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nayak G. Rapid inactivation of airborne porcine reproductive and respiratory syndrome virus using an atmospheric pressure air plasma. Plasma Process. Polym. 2020 doi: 10.1002/ppap.201900269. Published online February 24, 2020. [DOI] [Google Scholar]
- 40.Xia T. Inactivation of airborne porcine reproductive and respiratory syndrome virus (PRRSv) by a packed bed dielectric barrier discharge non-thermal plasma. J. Hazard. Mater. 2020;393:122266. doi: 10.1016/j.jhazmat.2020.122266. [DOI] [PubMed] [Google Scholar]
- 41.Xia T. Inactivation of airborne viruses using a packed bed non-thermal plasma reactor. J. Phys. D. Appl. Phys. 2019;52:255201. doi: 10.1088/1361-6463/ab1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefeuvre P. Evolution and ecology of plant viruses. Nat. Rev. Microbiol. 2019;17:632–644. doi: 10.1038/s41579-019-0232-3. [DOI] [PubMed] [Google Scholar]
- 43.Nicaise V. Crop immunity against viruses: outcomes and future challenges. Front. Plant Sci. 2014;5:660. doi: 10.3389/fpls.2014.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehle N. Survival and transmission of potato virus Y, pepino mosaic virus, and potato spindle tuber viroid in water. Appl. Environ. Microbiol. 2014;80:1455–1462. doi: 10.1128/AEM.03349-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanbal S.E. Atmospheric pressure plasma irradiation can disrupt tobacco mosaic virus particles and RNAs to inactivate their infectivity. Arch. Virol. 2018;163:2835–2840. doi: 10.1007/s00705-018-3909-4. [DOI] [PubMed] [Google Scholar]
- 46.Guo L. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Appl. Environ. Microbiol. 2018;84:1–10. doi: 10.1128/AEM.00726-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimmermann J.L. Effects of cold atmospheric plasmas on adenoviruses in solution. J. Phys. D. Appl. Phys. 2011;44:505201. [Google Scholar]
- 48.Sakudo A. N2 gas plasma inactivates influenza virus mediated by oxidative stress. Front. Biosci. 2014;6:69–79. doi: 10.2741/e692. [DOI] [PubMed] [Google Scholar]
- 49.Wu Y. MS2 virus inactivation by atmospheric-pressure cold plasma using different gas carriers and power levels. Appl. Environ. Microbiol. 2015;81:996–1002. doi: 10.1128/AEM.03322-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yasuda H. Biological evaluation of DNA damage in bacteriophages inactivated by atmospheric pressure cold plasma. Plasma Process. Polym. 2010;7:301–308. [Google Scholar]
- 51.Koonin E.V., Starokadomskyy P. Are viruses alive? The replicator paradigm sheds decisive light on an old but misguided question. Stud. Hist. Phil. Biol. Biomed. Sci. 2016;59:125–134. doi: 10.1016/j.shpsc.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roossinck M.J., Baz E.R. Symbiosis: viruses as intimate partners. Annu. Rev. Virol. 2017;4:123–139. doi: 10.1146/annurev-virology-110615-042323. [DOI] [PubMed] [Google Scholar]
- 53.Milone M.C., O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang D. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehle N. Water-mediated transmission of plant, animal, and human viruses. In: Malmstrom C.M., editor. Advances in Virus Research. Academic Press; 2018. pp. 85–128. [DOI] [PubMed] [Google Scholar]
- 56.Staggemeier R. Animal and human enteric viruses in water and sediment samples from dairy farms. Agric. Water Manag. 2015;152:135–141. [Google Scholar]
- 57.Zhang T. RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol. 2006;4:0108–0118. doi: 10.1371/journal.pbio.0040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyon B.A. Integrated chemical and toxicological investigation of UV-chlorine/ chloramine drinking water treatment. Environ. Sci. Technol. 2014;48:6743–6753. doi: 10.1021/es501412n. [DOI] [PubMed] [Google Scholar]
- 59.Machala Z., Pavlovich M.J. A new phase in applied biology. Trends Biotechnol. 2018;36:577–578. doi: 10.1016/j.tibtech.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Mozetič M. Introduction to plasma and plasma diagnostics. In: Thomas S., editor. Non-Thermal Plasma Technology for Polymeric Materials: Applications In Composites, Nanostructured Materials and Biomedical Fields. Elsevier; 2019. pp. 23–65. [Google Scholar]
- 61.Ehlbeck J. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D. Appl. Phys. 2011;44 [Google Scholar]
- 62.Bruggeman P.J. Plasma-liquid interactions: a review and roadmap. Plasma Sources Sci. Technol. 2016;25 [Google Scholar]
- 63.Labay C. Production of reactive species in alginate hydrogels for cold atmospheric plasma-based therapies. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-52673-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMinn B.R. Bacteriophages as indicators of faecal pollution and enteric virus removal. Lett. Appl. Microbiol. 2017;65:11–26. doi: 10.1111/lam.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Machala Z. Chemical and antibacterial effects of plasma activated water: correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D. Appl. Phys. 2019;52 [Google Scholar]
- 66.Alekseev O. Nonthermal dielectric barrier discharge (DBD) plasma suppresses herpes simplex virus type 1 (HSV-1) replication in corneal epithelium. Transl. Vis. Sci. Technol. 2014;3:2. doi: 10.1167/tvst.3.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jakober C., Phillips T. Air Resources Board, California Environmental Protection Agency; 2008. Evaluation of Ozone Emissions from Portable Indoor Air Cleaners: Electrostatic Precipitators and Ionizers. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental properties of cold plasma-virus treatments.
Properties of cold plasma sources used for virus inactivation.