Abstract
Objective
To describe detection of severe acute respiratory syndrome (SARS)–coronavirus 2 (CoV-2) in seminal fluid of patients recovering from coronavirus disease 2019 (COVID-19) and to describe the expression profile of angiotensin-converting enzyme 2 (ACE2) and Transmembrane Serine Protease 2 (TMPRSS2) within the testicle.
Design
Observational, cross-sectional study.
Setting
Tertiary referral center.
Patient(s)
Thirty-four adult Chinese males diagnosed with COVID-19 through confirmatory quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) from pharyngeal swab samples.
Intervention(s)
None.
Main Outcome Measure(s)
Identification of SARS-CoV-2 on qRT-PCR of single ejaculated semen samples. Semen quality was not assessed. Expression patterns of ACE2 and TMPRSS2 in the human testis are explored through previously published single-cell transcriptome datasets.
Result(s)
Six patients (19%) demonstrated scrotal discomfort suggestive of viral orchitis around the time of COVID-19 confirmation. Severe acute respiratory syndrome–CoV-2 was not detected in semen after a median of 31 days (interquartile range, 29-36 days) from COVID-19 diagnosis. Single-cell transcriptome analysis demonstrates sparse expression of ACE2 and TMPRSS2, with almost no overlapping gene expression.
Conclusion(s)
Severe acute respiratory syndrome–CoV-2 was not detected in the semen of patients recovering from COVID-19 1 month after COVID-19 diagnosis. Angiotensin-converting enzyme 2–mediated viral entry of SARS–CoV-2 into target host cells is unlikely to occur within the human testicle based on ACE2 and TMPRSS2 expression. The long-term effects of SARS–CoV-2 on male reproductive function remain unknown.
Key Words: COVID-19, angiotensin-converting enzyme 2, coronavirus, semen, infertility, male
Abstract
No hay evidencia de síndrome respiratorio agudo severo - coronavirus 2 en semen de varones que se recuperan de la enfermedad del coronavirus 2019
Objetivo
Describir la detección del síndrome respiratorio agudo severo (SRAS) –coronavirus 2 (CoV-2) en el líquido seminal de pacientes en recuperación de la enfermedad por coronavirus 2019 (COVID-19) y describir el perfil de expresión de la enzima convertidora de angiotensina 2 (ACE2) y Serina Proteasa Transmembrana 2 (TMPRSS2) dentro del testículo.
Diseño experimental
Estudio observacional, transversal.
Lugar de realización
Centro de referencia terciario.
Pacientes
Treinta y cuatro varones chinos adultos diagnosticados con COVID-19 mediante la confirmación cuantitativa de la reacción en cadena de la polimerasa con transcriptasa reversa (qRT-PCR) de muestras de hisopado faríngeo.
Intervenciones
Ninguna.
Principal variable de medida
Identificación de SARS-CoV-2 a través de qRT-PCR de muestras de semen eyaculadas individuales. La calidad del semen no fue evaluada. Los patrones de expresión de ACE2 y TMPRSS2 en los testículos humanos se exploran a través de conjunto de datos de transcriptoma de célula única publicados previamente.
Resultados
Seis pacientes (19%) demostraron molestias escrotales relacionadas con la orquitis viral en el momento de la confirmación de COVID-19. El síndrome respiratorio agudo severo - CoV-2 no se detectó en semen después de una mediana de 31 días (rango intercuartil, 29-36 días) desde el diagnóstico de COVID-19. El análisis de transcriptoma de célula única demuestra una expresión escasa de ACE2 y TMPRSS2, casi sin expresión de genes superpuestos.
Conclusión
El síndrome respiratorio agudo severo-CoV-2 no se detectó en el semen de pacientes que se recuperaron de COVID-19 1 mes después del diagnóstico. La entrada viral mediada por la enzima convertidora de angiotensina 2 del SARS-CoV-2 en las células huésped diana es poco probable que ocurra dentro del testículo humano basado en la expresión de ACE2 y TMPRSS2. Los efectos a largo plazo del SARS-CoV-2 en la función reproductora masculina sigue siendo desconocida.
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/65727-30244
Severe acute respiratory syndrome coronavirus 2 (SARS–CoV-2), a novel coronavirus reported in late December 2019 in Wuhan, China, has spread world-wide with more than 1,000,000 coronavirus disease 2019 (COVID-19) cases reported (1, 2). New developments in molecular virology and immunobiology of SARS–CoV-2 improve our understanding about COVID-19 prevention, management, and possible long-term effects. Although viral transmission occurs predominantly through respiratory droplets, SARS–CoV-2 has been isolated in blood samples and feces from patients with COVID-19, raising questions about viral shedding in other bodily fluids, including semen, as well as alternative modes of transmission (3). Similar to SARS–CoV 2002, viral entry into target cells by SARS–CoV-2 likely is mediated by the interaction between the viral spike (S) protein and cellular angiotensin-converting enzyme 2 (ACE2) (4, 5). Angiotensin-converting enzyme 2 is expressed in multiple organ systems including type II alveolar cells of the lungs, intestine, heart, kidney, and the testis (4, 5). Transmembrane Serine Protease 2 (TMPRSS2) appears to prime the S protein to enhance ACE2-mediated viral entry (6). Interestingly, TMPRSS2 expression is identified in prostatic epithelial cells, with aberrant expression associated with tumorigenesis (7).
The male reproductive tract and the testicle may be involved after some systemic viral infections (e.g., mumps orchitis). Testicular immune privilege normally protects the immunogenic germ cells from the host response. However, certain viruses are able to cross the blood-testis barrier, enter cells of the male reproductive tract, and elicit an immune response within the testicle (8). Evidence regarding viral seeding and viral entry into cells of the male reproductive tract after SARS–CoV-2 infection is not well understood. Our objectives are to describe detection of SARS–CoV-2 in the semen of patients recovering from COVID-19 and to determine the expression profile of ACE2 and TMPRSS2 within the human testicle, thereby providing mechanistic insights into viral entry and the early impact on male reproductive function.
Methods
We identified adult Chinese male patients (range, 18–57 years) diagnosed with COVID-19 in Wuhan, China between January 26, 2020 and March 2, 2020. Local institutional review board approval was obtained prior to study initiation (Huazhong University of Science and Technology, Wuhan, China). A total of 34 adult male patients were recruited for this study after informed consent and were asked to provide one ejaculated semen sample. The duration of abstinence prior to obtaining the semen sample was not standardized.
Patients were diagnosed initially with COVID-19 based on clinical symptoms (fever, cough, pharyngodynia, and respiratory distress) confirmed with quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) of pharyngeal swab samples (Anda Gene Ltd,). Patients had one or more of these symptoms at the time of COVID-19 confirmation, but demonstrated generally milder symptoms. Severe acute respiratory syndrome–CoV-2 nucleic acid was extracted using the automatic nucleic acid extraction system (3DMed, Shanghai, China), per the manufacturer’s instructions. Two target genes, including open reading frame 1ab (ORF1ab) and nucleocapsid protein (N), were amplified and tested using real-time RT-PCR (Anda Gene Ltd). A cycle threshold value less than 37 cycles was defined as a positive test, and a value of 40 cycles or more was defined as a negative test, per recommendation of the Chinese National Institute for Viral Disease Control and Prevention. Samples with an equivocal cycle threshold were retested. Detection of SARS–CoV-2 in semen samples was performed using an identical protocol. Semen sample testing for SARS–CoV-2 was performed at the Huazhong University of Science and Technology. Given safety concerns regarding viral transmission from the semen specimens, comprehensive semen analyses were not performed on the samples.
To investigate the gene expression level of ACE2 and TMPRSS2 in different cells of the testes, we examined our prior published single-cell RNA-seq (scRNA-seq) dataset of human testicular cells at the University of Utah (9, 10). The scRNA-seq dataset was analyzed as described in prior work (9, 10). In brief, scRNA profiling experiments were performed by loading single testicular cells from the testes of three healthy, young adults to a 10X Genomics Chromium platform (10X Genomics, Pleasanton, CA) to generate libraries, which were sequenced using Illumina HiSeq 2500 (Illumina, San Diego, CA). The generated fastq files were then de-multiplexed and aligned using CellRanger (10X Genomics), which will generate the single-cell gene expression matrices. The gene expression matrices were then analyzed using dimension reduction algorithm with t-distributed stochastic neighbor embedding (t-SNE) and clustering approaches. Cells with similar transcriptomes were placed in proximity to each other with the same color. Known markers were used to help decode the major testicular cell type identities, as described in prior work (9, 10). Detailed downstream analysis was performed using customized R script. The expression levels of ACE2 and TMPRSS2 were examined and displayed by projecting them onto the t-SNE plot. In addition, co-localization of ACE2 and TMPRSS2 were reported, and their correlation was calculated.
Results
Table 1 presents characteristics of the 34 Chinese men recovering from COVID-19. Median age was 37 years (interquartile range [IQR], 31–49 years). Median body mass index (BMI) was 25.0 kg/m2 (IQR, 23.2–26.9), with 17 men (50%) classified as overweight (BMI >25 kg/m2). Three patients (9%) had a past medical history of hypertension. Baseline treatment of hypertension (for example, use of angiotensin converting enzyme [ACE] inhibitors or angiotensin receptor blockers) was unknown. Interestingly, six patients (19%) had scrotal discomfort around the time of COVID-19 confirmation suggestive of viral orchitis, although a comprehensive genitourinary examination was not performed on the entire cohort due to the pandemic. Generally, patients in the cohort demonstrated mild-moderate symptoms of COVID-19 at the time of disease confirmation. The median time from the collection of a semen sample from a confirmatory diagnosis of COVID-19 was 31 days (IQR, 29–36 days). Severe acute respiratory syndrome–CoV-2 was not detected in any ejaculated semen sample after a median of 31 days.
Table 1.
Individual characteristics for adult males with confirmed COVID-19 providing semen samples for SARS-CoV-2 testing.
| Patient | Age (y) | BMI (kg/m2) | Time between COVID-19 diagnosis and semen sample obtained (d) | SARS–CoV-2 semen result |
|---|---|---|---|---|
| 1 | 31 | 26 | 33 | Negative |
| 2 | 55 | 23.5 | 28 | Negative |
| 3 | 31 | 29.4 | 32 | Negative |
| 4 | 20 | 31.2 | 31 | Negative |
| 5 | 28 | 24.6 | 33 | Negative |
| 6 | 50 | 25.1 | 29 | Negative |
| 7 | 35 | 27 | 27 | Negative |
| 8 | 54 | 18.1 | 25 | Negative |
| 9 | 46 | 24.9 | 30 | Negative |
| 10 | 30 | 24.6 | 40 | Negative |
| 11 | 30 | 21.7 | 40 | Negative |
| 12 | 37 | 29.2 | 31 | Negative |
| 13 | 49 | 24.3 | 31 | Negative |
| 14 | 50 | 22.2 | 30 | Negative |
| 15 | 49 | 26.4 | 33 | Negative |
| 16 | 55 | 26.2 | 37 | Negative |
| 17 | 33 | 25.1 | 27 | Negative |
| 18 | 50 | 23.1 | 37 | Negative |
| 19 | 37 | 22 | 15 | Negative |
| 20 | 27 | 29.9 | 31 | Negative |
| 21 | 47 | 26.6 | 30 | Negative |
| 22 | 36 | 22.8 | 36 | Negative |
| 23 | 36 | 24.1 | 33 | Negative |
| 24 | 32 | 30.9 | 14 | Negative |
| 25 | 44 | 26.6 | 31 | Negative |
| 26 | 32 | 28.7 | 33 | Negative |
| 27 | 26 | 24.1 | 9 | Negative |
| 28 | 32 | 32 | 36 | Negative |
| 29 | 43 | 22.2 | 36 | Negative |
| 30 | 39 | 35.5 | 8 | Negative |
| 31 | 54 | 26 | 35 | Negative |
| 32 | 18 | 23 | 29 | Negative |
| 33 | 39 | 24.3 | 56 | Negative |
| 34 | 55 | 22.9 | 75 | Negative |
Note: BMI = body mass index; COVID-19 = coronavirus disease 2019; SARS–CoV-2 = severe acute respiratory syndrome coronavirus 2.
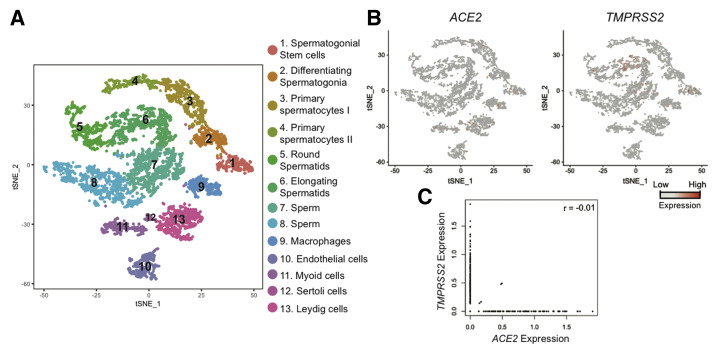
Figure 1 displays the expression of ACE2 and TMPRSS2 using published single-cell transcriptome profiles of human testicular cells. The dimension reduction approach (t-SNE) was used to present the single-cell transcriptome data from human testes (6,490 single cells), with each dot representing a single cell (Fig. 1A). Cells were colored based on cell identities (10). We examined the expression of ACE2 and TMPRSS2 by projecting their expression to the t-SNE plot, observing very low expression of both genes (Fig. 1B). Last, we examined co-expression of ACE2 and TMPRSS2 in every single testicular cell (Fig. 1C); only 4 of 6,490 cells displayed expression of both genes (Pearson correlation value = -0.01), suggesting limited overlap expression.
Figure 1.
Expression of ACE2 and TMPRSS2 in human testicular cells using single-cell RNA-seq dataset. (A) Dimension reduction (t-SNE) analysis of single-cell transcriptome data from human testes (n = 6,490) based on data from Guo et al. (10). Each dot represents a single cell and is colored according to its cluster identity, as indicated on the figure key. (B) Expression patterns of ACE2 and TMPRSS2 projected on the t-SNE plot. Red indicates high expression, and gray indicates low or no expression, as shown on the figure key. (C) Scatter plot to show the co-expression of ACE2 (x-axis) and TMPRSS2 (y-axis) in human testicular cells.
Discussion
In our study, we did not detect SARS–CoV-2 within the semen of adult Chinese males recovering from COVID-19. Nineteen percent of patients in our cohort had scrotal discomfort around the time of their COVID-19 confirmation, although the significance of this remains unclear. Additionally, in our scRNA-seq dataset of human testicular cells at the University of Utah, ACE2 and TMPRSS2 are expressed sparsely in the human testes, with almost no overlapping gene expression.
Testicular immune privilege protects the immunogenic germ cells from a host response and a systemic inflammatory response may alter this environment (8). Many viruses such as mumps, human immunodeficiency virus, human herpes virus, Ebola, and Zika can be detected in human semen after infection and can cause orchitis (11, 12). These viruses, through febrile illness, can have a negative impact on male reproductive function and spermatogenesis (11). Before our analysis, the impact of SARS–CoV-2 on male reproductive function was largely unknown. Previously, Xu et al. reported testicular pathology after autopsy from six males who died due to complications of SARS-CoV (13). The authors found widespread destruction of germ cells and sperm, in a background of complex inflammatory infiltrate. Although they were unable to isolate a genomic signature from SARS-CoV itself, they postulated that SARS-CoV caused orchitis and reproductive impairment. Angiotensin-converting enzyme 2, a likely receptor for viral entry by SARS–CoV-2, previously has been localized to human Leydig and Sertoli cells (14). Our single-cell transcriptome data suggest ACE2 RNA expression occurs at low levels. Therefore, ACE2-mediated viral entry of SARS–CoV-2 into target host cells is unlikely to occur within the human testicle. Investigation of alternative biologic mechanisms for alteration of the testicular microenvironment by SARS–CoV-2 is warranted.
There are certain limitations to this study. First, our results are impacted by the small sample size and selection bias because men with COVID-19 in this study are more likely to have demonstrated milder symptoms. Prior research has suggested that higher viral loads are associated with more severe disease symptoms, and it is plausible that viremia or a certain viral threshold is not achieved to cross the blood-testis barrier (15). Second, given safety concerns regarding viral transmission from the semen specimens, comprehensive semen analyses were not performed. We only were able to obtain a single semen sample for the purposes of this study, with only three patients providing a sample within 14 days of their COVID-19 diagnosis. This limits our ability to provide data on possible early viral shedding in the semen. Third, not all patients in our cohort had a comprehensive genitourinary examination, limiting interpretations regarding the incidence of scrotal findings consistent with orchitis during acute SARS–CoV-2 infection. Fourth, we were unable to assess hormone profiles in our cohort, including total testosterone, luteinizing hormone, and follicle-stimulating hormone, which could provide an assessment of testicular function. This preliminary data is unable to characterize the long-term impact of COVID-19 on fertility and testicular endocrine function. Finally, we only examined expression of ACE2 and TMPRSS2 at the RNA expression level. We understand that gene expression may not always reflect protein abundance, and cannot explicitly conclude that these proteins are absent. Further study of the distribution and roles of ACE2 and TMPRSS2 in regulating SARS–CoV-2 infection in the testes is indicated.
Conclusion
We did not detect SARS–CoV-2 within semen of adult Chinese males recovering from COVID-19, approximately 1 month after the initial COVID-19 confirmation. Unfortunately, we cannot definitively rule out the presence of SARS–CoV-2 in the seminal fluid during an acute infection with severe COVID-19 symptoms. Additionally, we did not find evidence of ACE2 and TMPRSS2 co-expression in high-fidelity RNA datasets from our prior published work at the University of Utah, indicating that SARS–CoV-2 likely would not be able to gain entry to testicular cells through an ACE2/TMPRSS2-mediated mechanism. Further research is needed to understand the long-term impact of SARS–CoV-2 on male reproductive function including fertility and testicular endocrine function.
Footnotes
F.P. has nothing to disclose. X.X. has nothing to disclose. J.G. has nothing to disclose. Y.S. has nothing to disclose. H.L. has nothing to disclose. D.P.P. has nothing to disclose. A.M.S. has nothing to disclose. J.P.A. has nothing to disclose. X.Z. has nothing to disclose. C.X. has nothing to disclose. P.S.L. has nothing to disclose. J.M.H. reports equity in startups NANONC, Andro360, and Inherent Bioscience, and grants from Endo Pharmaceuticals and Boston Scientific, outside of the submitted work. J.M.H. also has two patents pending for microfluidic sperm sorting.
F.P., X.X., J.G., Y.S., and H.L. should be considered similar in author order.
Supported by the National Natural Science Foundation of China of China (no. 81873854) of Feng Pan; National Natural Science Foundation of China (no. 81874091) to X.X.; and Covid-19 rapid response call of Huazhong University of Science and Technology (2020kfyXGYJ057) to H.L..
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.The Center for Systems Science and Engineering, Johns Hopkins. Coronavirus COVID-19 Global Cases. 2020. http://www.arcgis.com/apps/opsdashboard/index.html - /bda7594740fd40299423467b48e9ecf6 Available at:
- 3.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y.W., Lee M.S., Lucht A., Chou F.P., Huang W., Havighurst T.C., et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol. 2010;176:2986–2996. doi: 10.2353/ajpath.2010.090665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao S., Zhu W., Xue S., Han D. Testicular defense systems: immune privilege and innate immunity. Cell Mol Immunol. 2014;11:428–437. doi: 10.1038/cmi.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo J., Nie X., Giebler M., Mlcochova H., Wang Y., Grow E.J., et al. The dynamic transcriptional cell atlas of testis development during human puberty. Cell Stem Cell. 2020;26:262–276.e4. doi: 10.1016/j.stem.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J., Grow E.J., Mlcochova H., Maher G.J., Lindskog C., Nie X., et al. The adult human testis transcriptional cell atlas. Cell Res. 2018;28:1141–1157. doi: 10.1038/s41422-018-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W., Han R., Wu H., Han D. Viral threat to male fertility. Andrologia. 2018;50 doi: 10.1111/and.13140. [DOI] [PubMed] [Google Scholar]
- 12.Salam A.P., Horby P.W. The breadth of viruses in human semen. Emerging Infect Dis. 2017;23:1922–1924. doi: 10.3201/eid2311.171049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J., Qi L., Chi X., Yang J., Wei X., Gong E., et al. Orchitis: a complication of severe acute respiratory syndrome (SARS) Biol Reprod. 2006;74:410–416. doi: 10.1095/biolreprod.105.044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas G.C., O'Bryan M.K., Hedger M.P., Lee D.K., Yarski M.A., Smith A.I., et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M., et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/s1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]