Summary
Background
A range of public health measures have been implemented to suppress local transmission of coronavirus disease 2019 (COVID-19) in Hong Kong. We examined the effect of these interventions and behavioural changes of the public on the incidence of COVID-19, as well as on influenza virus infections, which might share some aspects of transmission dynamics with COVID-19.
Methods
We analysed data on laboratory-confirmed COVID-19 cases, influenza surveillance data in outpatients of all ages, and influenza hospitalisations in children. We estimated the daily effective reproduction number (Rt) for COVID-19 and influenza A H1N1 to estimate changes in transmissibility over time. Attitudes towards COVID-19 and changes in population behaviours were reviewed through three telephone surveys done on Jan 20–23, Feb 11–14, and March 10–13, 2020.
Findings
COVID-19 transmissibility measured by Rt has remained at approximately 1 for 8 weeks in Hong Kong. Influenza transmission declined substantially after the implementation of social distancing measures and changes in population behaviours in late January, with a 44% (95% CI 34–53%) reduction in transmissibility in the community, from an estimated Rt of 1·28 (95% CI 1·26–1·30) before the start of the school closures to 0·72 (0·70–0·74) during the closure weeks. Similarly, a 33% (24–43%) reduction in transmissibility was seen based on paediatric hospitalisation rates, from an Rt of 1·10 (1·06–1·12) before the start of the school closures to 0·73 (0·68–0·77) after school closures. Among respondents to the surveys, 74·5%, 97·5%, and 98·8% reported wearing masks when going out, and 61·3%, 90·2%, and 85·1% reported avoiding crowded places in surveys 1 (n=1008), 2 (n=1000), and 3 (n=1005), respectively.
Interpretation
Our study shows that non-pharmaceutical interventions (including border restrictions, quarantine and isolation, distancing, and changes in population behaviour) were associated with reduced transmission of COVID-19 in Hong Kong, and are also likely to have substantially reduced influenza transmission in early February, 2020.
Funding
Health and Medical Research Fund, Hong Kong.
Introduction
The first wave of coronavirus disease 2019 (COVID-19) in China, outside of Hubei province, was addressed with the implementation of aggressive public health measures.1 These measures relied heavily on massive mobility restrictions, universal fever screening in all settings, and neighbourhood-based, household-focused social distancing that was enforced by large teams of community workers, as well as pervasive deployment of artificial intelligence-based social media applications and the use of big data.2 Whether some or all of these measures would be acceptable and feasible in settings outside of mainland China has been questioned.3
Hong Kong is a Special Administrative Region of China that operates with a large degree of autonomy. It is located outside the mainland on the southern coast of China, neighbouring Guangdong province—which has recorded the largest number of confirmed cases of COVID-19 (1490 cases as of March 31, 2020) outside of Hubei. Having been one of the most heavily affected epicentres during the severe acute respiratory syndrome (SARS) epidemic in 2003, the community in Hong Kong has been prepared to respond to emerging infectious diseases. A range of public health measures have been implemented to delay and reduce local transmission of COVID-19, and there have been major changes in population behaviour.
The initial containment or current suppression measures used to control COVID-19 in Hong Kong include intense surveillance for infections, not only in incoming travellers but also in the local community, with around 400 outpatients and 600 inpatients tested each day in early March, 2020. Once individuals are identified to be positive for COVID-19, they are isolated in hospital until they recover and cease virus shedding. Their close contacts are traced (from 2 days before illness onset) and quarantined in special facilities, including holiday camps and newly constructed housing estates. Because not every infected person will be identified, containment measures only work if social distancing measures or behavioural changes also reduce so-called silent transmission in the community as a whole.
Research in context.
Evidence before this study
Coronavirus disease 2019 (COVID-19) was first identified in late December 2019, in a cluster of cases of atypical pneumonia in Wuhan. Infections increased through January until the implementation of a lockdown of Wuhan and other affected cities. Since January, 2020, COVID-19 cases have been reported outside China in increasing numbers, with many countries not taking strong control measures, such as lockdowns, until relatively larger numbers of cases had been reported. In Hong Kong, Singapore, and Taiwan public health measures to prevent community epidemics were quickly implemented and were able to avoid the need for complete lockdowns. We searched PubMed on March 31, 2020, for studies reporting the impact of alternative public health measures against COVID-19 using keywords including “COVID-19”, “2019-nCoV”, “novel coronavirus-infected pneumonia”, “SARS-CoV-2”, “lockdown”, “social distancing”, “isolation”, “contact tracing”, and “quarantine”. We scanned 227 published studies and found six that estimated the impact of public health measures in Wuhan or elsewhere in mainland China, one study that described the early application of testing and contact tracing in Singapore, and one study reporting the impact of quarantine on transmission on the Diamond Princess cruise ship.
Added value of this study
We estimated the effective reproduction number of COVID-19 in Hong Kong as a measure of transmissibility over time and found that it has remained at approximately 1 for the past 8 weeks. We described the public health measures that have been introduced to contain COVID-19 transmission and the behavioural changes reported by the population, and found that distancing measures and changes in behaviour were associated with rapid declines in influenza activity. The speed of decline in influenza activity in 2020 was quicker than in previous years in which school closures were implemented but there were no other social distancing measures or voluntary changes in behaviour.
Implications of all the available evidence
The experience in Hong Kong indicates that COVID-19 transmission can be contained with a combination of testing and isolating cases, plus tracing and quarantining their close contacts, along with some degree of social distancing to reduce community transmission from unidentified cases.
Hong Kong offers an opportunity to study the impact of public health interventions and population behavioural changes that could be rolled out in resource-sufficient settings in other countries. We aimed to quantify the effect of containment measures on COVID-19. In addition, to identify whether social distancing and behavioural changes have been associated with reducing silent transmission of COVID-19, we analysed data on influenza activity as a proxy for potential changes in transmission of infection in line with the interventions implemented, assuming a similar mode and efficiency of spread of influenza and COVID-19. The specific objective of this study was to quantify population behavioural changes in Hong Kong during the COVID-19 outbreak, and to describe the likely impact of the behavioural changes and public health measures on COVID-19 transmission and influenza transmission in the community.
Methods
Data collection
Data on laboratory-confirmed COVID-19 cases were obtained from the Hong Kong Centre for Health Protection, which provides daily updates with individual case data on a dedicated webpage.
We obtained sentinel surveillance data on influenza-like illnesses in a network of around 60 general outpatient clinics from the Centre for Health Protection. These include weekly reports on the proportion of outpatient consultations that were in patients with influenza-like illness, defined as fever plus cough or sore throat. We obtained laboratory surveillance data from the Public Health Laboratory Services on influenza testing results on specimens from public hospitals and sentinel surveillance sites, including the weekly number of specimens tested and the number testing positive for influenza by type and subtype. Data on the current population of Hong Kong by age and sex were obtained from the Census and Statistics Department of the Hong Kong Government. We obtained the daily hospitalisation rates for influenza-positive cases among children in Hong Kong using the daily hospital admissions for influenza to the paediatric departments of two large hospitals in Hong Kong and the relevant catchment populations.4
We did three cross-sectional telephone surveys among the general adult population in Hong Kong, on Jan 20–23, Feb 11–14, and March 10–13, 2020. The methods and survey instruments used were similar to those used for surveys during the SARS epidemic in 2003,5, 6 the influenza A H1N1 pandemic in 2009,7 and the influenza A H7N9 outbreak in China in 2013.8 Participants were recruited using random-digit dialling of both landline and mobile telephone numbers. Telephone numbers were randomly generated by a computer system. Calls were made during both working and non-working hours by trained interviewers to avoid over-representation of non-working groups. Respondents were required to be at least 18 years old and able to speak Cantonese Chinese or English. New respondents were recruited for each survey round. Within each household, an eligible household member with the nearest birthday was invited to participate in the survey, which was not necessarily the person that initially answered the telephone. Survey items included measures of risk perception, attitudes towards COVID-19, and behaviours taken against contracting COVID-19, including hygiene, face masks, and reduction of social contact. In the second and third surveys, respondents who were parents of school-age children were asked to answer additional questions about social contact patterns of their children because schools were closed at the time of the interviews. Ethical approval for this study was obtained from the Institutional Review Board of the University of Hong Kong. All participants gave verbal informed consent.
Statistical analysis
Means and proportions of survey responses were directly weighted by sex and age to the general population. Categorical variables with ordinal Likert-type response scales, including risk perception and attitudes towards COVID-19, were first dichotomised as either above or below a threshold. Responses to perceived susceptibility were dichotomised as 1 (likely, very likely, or certain) versus 0 (never, very unlikely, unlikely, or even chance); responses to perceived severity were dichotomised as 1 (serious or very serious) versus 0 (very mild, mild, or moderate); responses about worry were dichotomised as 1 (moderately worried or very worried) versus 0 (not at all worried or slightly worried); responses about attitudes towards COVID-19 were dichotomised as 1 (agree or strongly agree) versus 0 (strongly disagree, disagree, or neutral).
We estimated changes in COVID-19 transmissibility over time via the effective reproduction number (R t), which represents the mean number of secondary infections that result from a primary case of infection at time t. Values of R t exceeding 1 indicate that the epidemic will tend to grow, whereas values below 1 indicate that the epidemic will tend to decline. We estimated the time-varying reproduction numbers from serial intervals and incidence of COVID-19 cases over time,9, 10 assuming a serial interval of 7·5 days.11 We extended the approach used by Thompson and colleagues10 to allow for undetected cases due to censoring and imperfect detection of local cases. We assumed that 99% of imported cases and 80% of local cases would be detected. We developed a data-augmented Markov chain Monte Carlo algorithm to jointly estimate the time-varying reproduction number, the delay distribution from onset to reporting which can be used to inform censoring and undetected cases. Time-varying estimates of reproduction numbers were made with a 7-day sliding window.
To measure changes in influenza transmissibility over time, we first calculated the influenza proxy12, 13 for influenza A H1N1 during the 2019–20 winter by multiplying the weekly influenza-like illness consultation rates by the weekly proportions of specimens positive for influenza A H1N1, which was the predominant strain. This influenza proxy is a better correlate of the incidence of influenza virus infections in the community than either influenza-like illness rates or laboratory detection rates alone.13 We interpolated daily influenza proxy values from the weekly influenza proxy values with use of flexible cubic splines.14
Using the daily influenza proxy, we estimated daily transmissibility via the daily effective reproduction number, R t. We used a simple branching process model for epidemic spread to estimate the time-varying intensity of transmission.15 We assumed the serial interval distribution for influenza followed a gamma distribution with a mean of 2·85 days and SD of 0·93 days.16 We repeated these analyses for the daily influenza A H1N1 hospitalisation rates among children in two large local hospitals (Queen Mary Hospital and Princess Margaret Hospital). We evaluated the changes in transmissibility by comparing the R t values during the 2 weeks before and after the start of the school closures (including the Chinese New Year holidays) for the 2019–20 winter influenza season. The 95% CIs for the change in R t were calculated using Fieller's theorem.17 We compared the reductions in 2019–20 with reductions in previous years when the Chinese New Year holidays occurred during influenza epidemics. All analyses were done with R version 3.6.2.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
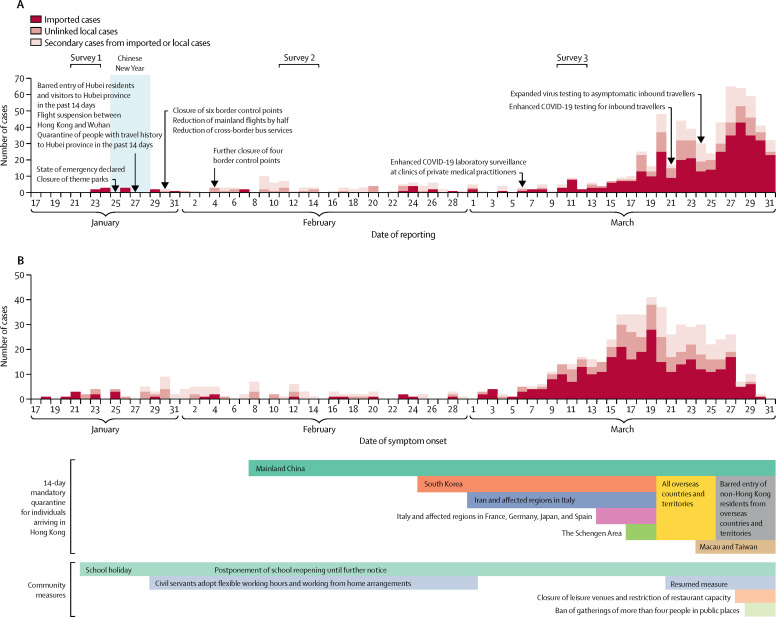
As of March 31, 2020, Hong Kong had confirmed 715 cases of SARS coronavirus 2 (SARS-CoV-2) infection, including 386 individuals that were presumed to have acquired infection outside of Hong Kong (imported cases), 142 cases that could not be linked to any other case (unlinked local cases), and 187 cases that were linked to the other known cases (secondary cases; appendix p 1). Among these 715 cases of SARS-CoV-2 infection, 94 were asymptomatic infections and 621 were symptomatic infections. Figure 1 shows the timeline of interventions that were implemented by the government in Hong Kong, including travel restrictions and bans, flexible working arrangements, and school closures from kindergartens up to tertiary and post-tertiary institutions, including tutorial centres. Some religious organisations cancelled services from Feb 13 onwards, and many conferences and other local mass gatherings have been cancelled. Quarantine orders have been issued to the close contacts of individuals with confirmed infection, as well as travellers arriving from affected countries (figure 1).
Figure 1.
COVID-19 cases in Hong Kong by date of reporting (A) and date of symptom onset (B)
The Chinese New Year, a major winter festival in Hong Kong, was on Jan 25, and there were public holidays on Jan 25–28. Most schools started holidays on Jan 22 and were scheduled to resume on Feb 3. The Hong Kong Government has deferred class resumption several times and closures are now until further notice without an expected resumption date. 94 asymptomatic cases are not shown in panel B. All dates are in 2020. COVID-19=coronavirus disease 2019.
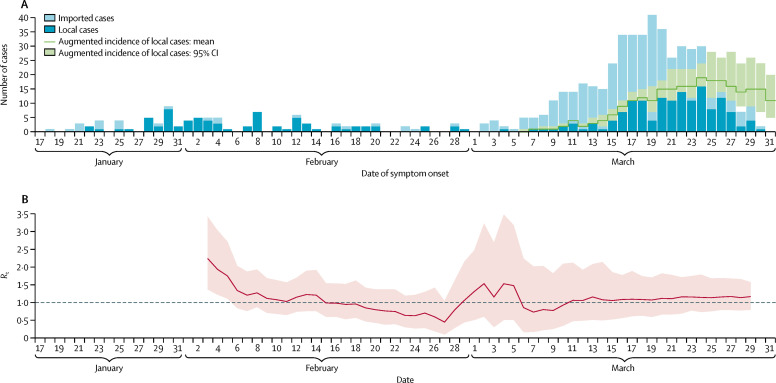
Although unlinked COVID-19 cases have been detected in increasing numbers since early March, transmissibility (R t) remains around the critical threshold of 1 (figure 2 ). Increases in local cases could be attributed to the transmission of infections into the community, resulting from an increasing number of imported infections since early March. With a local effective reproduction number of 1, a gradual increase in the incidence of local infections would be expected.
Figure 2.
Incidence and transmissibility of COVID-19 in Hong Kong
(A) Incidence of local COVID-19 cases in Hong Kong (dark blue bars) and cases infected overseas but detected locally (light blue bars). Augmented incidence includes estimated additional cases that have occurred but have not yet been identified due to reporting delays. (B) Estimates of the daily Rt of COVID-19 over time. The pink shaded area indicates 95% CIs. The dashed line indicates the critical threshold of Rt=1. All dates are in 2020. COVID-19=coronavirus disease 2019. Rt=effective reproduction number.
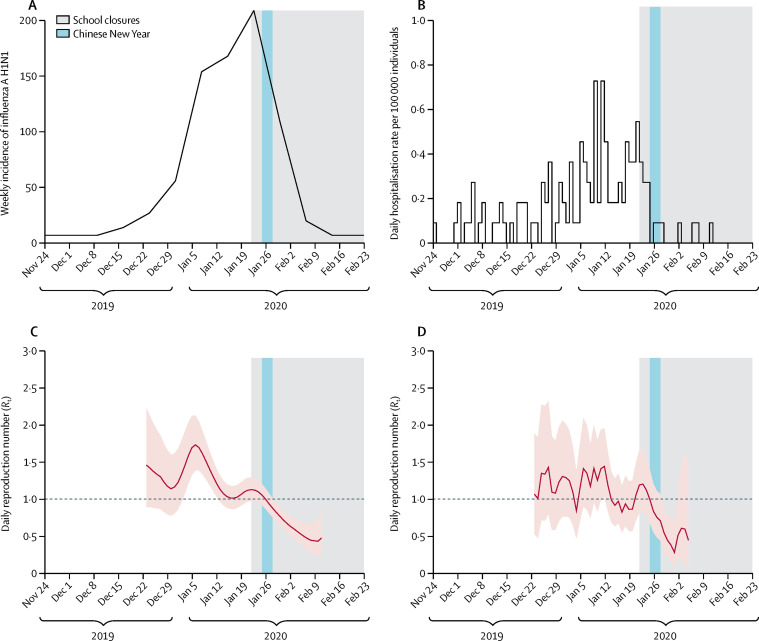
Data on influenza activity based on the community influenza proxy were consistent with the rate of hospitalisation of children with influenza A H1N1 in Hong Kong (figure 3A, B ). Influenza incidence peaked in the third week of January, with influenza A H1N1 predominating, and declined to low levels by the second week of February. The R t for influenza A H1N1 gradually declined from the second week of January but was greater than 1 before the start of the school closures and Chinese New Year. The R t then declined to less than 1 shortly after the school closures and continued to decrease until early February (figure 3C, D). The estimated R t was 1·28 (95% CI 1·26–1·30) during the 2-week period before the start of the school closures and 0·72 (0·70–0·74) during the first 2 weeks of school closures, corresponding to a 44% (34–53%) reduction in transmissibility (figure 3C). Similarly, the R t calculated from hospitalisation data was 1·10 (1·06–1·12) before the start of the school closures and reduced to 0·73 (0·68–0·77) after school closures, corresponding to a 33% (24–43%) reduction in transmissibility (figure 3D).
Figure 3.
Incidence, hospitalisation rate, and Rt of influenza A (H1N1) in 2019–20
(A) Weekly incidence, calculated as the weekly consultation rate multiplied by the proportion of laboratory specimens testing positive for influenza A (H1N1). (B) Daily hospitalisation rate with influenza A (H1N1) in children in two large hospitals in Hong Kong. (C) Estimated Rt in Hong Kong based on the influenza proxy data, with 95% CIs indicated by the pink shaded region. (D) Estimated Rt in Hong Kong based on the hospitalisation data, with 95% CIs indicated by the pink shaded region. We stopped estimating Rt when the local epidemic ended, indicated by a reduction in influenza proxy to very low levels and no further influenza hospitalisations in children. Dashed lines indicate the critical threshold of Rt=1. Shaded bars show the dates of Chinese New Year (light blue) and school closures (grey). Rt=effective reproduction number.
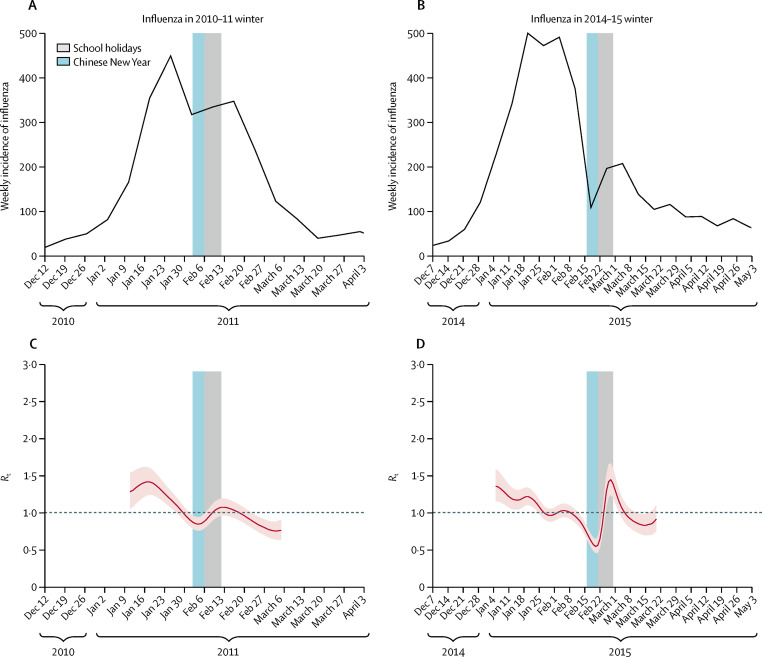
In comparison, during the 2010–11 winter, we estimated the reduction in transmissibility of influenza associated with the Chinese New Year holidays to be 15% (95% CI 11–19%), declining from an R t of 1·10 (1·08–1·13) in the 11-day period before the start of the school holidays to 0·95 (0·92–0·96) during the 11-day school holiday (figure 4 ). In the 2014–15 winter, the reduction in transmissibility associated with the Chinese New Year holidays was 14% (7–22%), declining from an R t of 0·94 (0·93–0·96) in the 11-day period before the start of the school holidays to 0·81 (0·75–0·86) during the 11-day school holiday.
Figure 4.
Incidence and Rt of influenza in 2010–11 and 2014–15
Weekly incidence of influenza (all type and subtypes) was calculated as the weekly consultation rate multiplied by the proportion of laboratory specimens testing positive for influenza in the winter influenza season of 2010–11 (A) and 2014–15 (B). Rt in Hong Kong was estimated based on the influenza proxy data, with 95% CIs indicated by the pink shaded region, for the winter influenza season of 2010–11 (C) and 2014–15 (D). Dashed lines indicate the critical threshold of Rt=1. Shaded bars show the dates of Chinese New Year (light blue) and school holidays (grey). Rt=effective reproduction number.
We interviewed 1008 participants on Jan 20–23 (22% response rate), 1000 participants on Feb 11–14 (23% response rate), and 1005 participants on March 10–13 (response rate 15%; appendix p 3). Respondents included a broad cross-section of the adult population of Hong Kong (appendix p 2). Respondents perceived that they had similar susceptibility to COVID-19 as they did to seasonal influenza, but that COVID-19 was a much more serious infection, and around half of the respondents reported worrying about being infected with COVID-19 compared with around a third of respondents for seasonal influenza (table ). In survey 2, 76·4% of respondents agreed with the statement that complete border closure would be effective in preventing the spread of COVID-19 to Hong Kong, 84·1% were worried about the availability of medical supplies, including face masks, but only 27·7% were worried about the availability of living supplies, including food and household goods, in Hong Kong (table).
Table.
Public attitudes, risk perceptions, and behavioural responses towards COVID-19 and seasonal influenza in three telephone surveys in Hong Kong
| Survey 1, Jan 20–23 (n=1008) | Survey 2, Feb 11–14 (n=1000) | Survey 3, March 10–13 (n=1005) | |
|---|---|---|---|
| Risk perception of COVID-19 | |||
| Perceived susceptibility to COVID-19* | 186 (18·9%; 16·0–21·9) | 185 (17·4%; 14·8–20·1) | 140 (15·2%; 12·6–17·8) |
| Perceived severity of COVID-19† | 916 (89·6%; 85·8–93·3) | 902 (90·5%; 86·4–94·7) | 829 (82·0%; 78·6–85·4) |
| Worried about being infected with COVID-19‡ | 551 (52·5%; 48·7–56·3) | 558 (53·9%; 49·9–57·9) | 471 (46·5%; 42·9–50·0) |
| Risk perception of seasonal influenza | |||
| Perceived susceptibility to seasonal influenza* | 260 (25·1%; 22·0–28·3) | 231 (22·5%; 19·4–25·6) | NA |
| Perceived severity of seasonal influenza† | 406 (42·3%; 38·4–46·3) | 311 (32·7%; 28·9–36·6) | NA |
| Worried about being infected with seasonal influenza‡ | 370 (36·5%; 32·9–40·0) | 283 (30·3%; 26·6–33·9) | NA |
| Attitudes towards COVID-19§ | |||
| I'm confident that I can take measures to protect myself against COVID-19 | 518 (50·5%; 46·6–54·4) | 594 (59·2%; 54·9–63·5) | 679 (68·0%; 64·3–71·7) |
| I believe that the Hong Kong Government can take effective measures to control the spread of COVID-19 in Hong Kong | 338 (33·5%; 29·9–37·1) | 271 (31·8%; 27·8–35·8) | 336 (35·8%; 32·2–39·3) |
| I believe that the Central Chinese Government can take effective measures to control COVID-19 | 308 (31·7%; 28·1–35·3) | 352 (39·0%; 34·8–43·2) | NA |
| I believe that complete border closure is an effective measure to prevent COVID-19 spreading from mainland China to Hong Kong | NA | 784 (76·4%; 72·3–80·5) | NA |
| Complete border closure will seriously affect the life of citizens | NA | 298 (33·2%; 29·2–37·1) | NA |
| I worry about medical supplies, such as face masks, in Hong Kong | NA | 860 (84·1%; 80·0–88·2) | NA |
| I worry about the living supplies in Hong Kong due to border closure | NA | 244 (27·7%; 24·0–31·4) | NA |
| Preventive measures taken against COVID-19¶ | |||
| Avoided going to crowded places | 627 (61·3%; 57·2–65·4) | 920 (90·2%; 86·2–94·2) | 860 (85·1%; 81·7–88·4) |
| Avoided visiting mainland China | 800 (78·1%; 73·9–82·2) | NA | NA |
| Avoided contact with people with respiratory symptoms | 687 (66·8%; 62·7–70·9) | 834 (80·0%; 76·0–84·0) | 806 (78·7%; 75·3–82·1) |
| Used face masks | 778 (74·5%; 70·4–78·6) | 976 (97·5%; 93·5–100·0) | 992 (98·8%; 96·0–100·0) |
| Washed hands more often (including using hand sanitiser) | 726 (71·1%; 67·0–75·2) | 938 (92·5%; 88·6–96·5) | 941 (93·0%; 90·0–96·0) |
| Avoided touching public objects or used protective measures when touching public objects (eg, use tissue) | 387 (36·4%; 32·3–40·5) | 767 (73·8%; 69·8–77·9) | 746 (73·1%; 69·6–76·7) |
| House disinfection | NA | 897 (89·3%; 85·2–93·4) | 899 (89·6%; 86·4–92·8) |
| Used serving utensils when eating | NA | 686 (66·0%; 61·9–70·1) | 692 (67·7%; 64·1–71·3) |
| Stayed at home as much as possible | NA | 894 (88·0%; 83·9–92·1) | 868 (83·8%; 80·5–87·1) |
| Avoided going to health-care facilities | NA | 832 (81·0%; 77·0–85·1) | 759 (74·7%; 71·1–78·3) |
Data are n (%; 95% CI). Proportions were weighted by age and sex to the adult population in Hong Kong. All dates are in 2020. NA=not applicable (question was not asked in the survey). COVID-19=coronavirus disease 2019.
Numbers and proportions represent respondents that answered likely, very likely, or certain, rather than never, very unlikely, unlikely, or even chance.
Numbers and proportions represent respondents that answered serious or very serious, rather than very mild, mild, or moderate.
Numbers and proportions represent respondents that answered moderately worried or very worried, rather than not at all worried or slightly worried.
Numbers and proportions represent respondents that answered agree or strongly agree to these statements, rather than strongly disagree, disagree, or neutral.
Numbers and proportions represent respondents who had taken the measure in the previous 7 days to prevent contracting COVID-19.
We identified considerable increases in the use of preventive measures in response to the threat of COVID-19. In recent years, face masks have mainly been used by individuals in the general community who are ill and by those who feel particularly susceptible to infection and want to protect themselves. We found that 74·5%, 97·5%, and 98·8% of respondents wore masks when going out; 61·3%, 90·2%, and 85·1% avoided going to crowded places; and 71·1%, 92·5%, and 93·0% reported washing or sanitising their hands more often, in surveys 1, 2, and 3, respectively (table). In surveys 2 and 3, 88·0% and 83·8% reported staying at home as much as possible.
In surveys 2 and 3, we asked the subset of respondents who were parents of school-age children about their support for school closures and the activities of their children during the school closures. Among respondents who were parents, 249 (95·4%) of 261 and 192 (93·7%) of 205 agreed or strongly agreed that school closure was needed as a control measure for COVID-19 in Hong Kong, and 209 (80·1%) of 261 and 141 (68·8%) of 205 responded that their children had no contact with people other than their household members on the preceding day.
Discussion
Our findings suggest that the package of public health interventions (including border entry restrictions, quarantine and isolation of cases and contacts, and population behaviour changes, such as social distancing and personal protective measures) that Hong Kong has implemented since late January, 2020, is associated with reduced spread of COVID-19. In the 10 weeks (corresponding to about ten generation times) since the first known individual with COVID-19 in Hong Kong began to show symptoms, there has been little sustained, local transmission of the disease. Our findings strongly suggest that social distancing and population behavioural changes—that have a social and economic impact that is less disruptive than total lockdown—can meaningfully control COVID-19.
The increasing number of imported infections in March poses a challenge to suppression efforts. This increase has occurred at the same time as relaxation of some voluntary avoidance behaviours in the general community. Without a strengthening of social distancing measures, local infections are likely to continue to occur, given that the effective reproduction number is approximately 1 or slightly higher than 1. Travel measures and testing, tracing, and treating efforts are particularly important in maintaining suppression, although these measures will be increasingly difficult to implement as case numbers increase.
In addition to the identification of cases with isolation, contact tracing, and quarantine, social distancing has also likely played an important role in suppressing transmission. We found that control measures and changes in population behaviour coincided with a substantial reduction in influenza transmission in early February, 2020. This observation suggests that the same measures would also have affected COVID-19 transmission in the community, because there will be some similarities—as well as some differences—in the modes of transmission of influenza and COVID-19. The potentially higher basic reproduction number for COVID-19 indicates that it might be more difficult to control than influenza.11 Because a variety of measures were used simultaneously, we were not able to disentangle the specific effects of each one, although this may become possible in the future if some measures are strengthened or relaxed locally, or with use of cross-national or subnational comparisons of the differential application of these measures.
The estimated 44% reduction in influenza transmission in the general community in February, 2020, was much greater than the estimated 10–15% reduction in transmission associated with school closures alone during the 2009 pandemic,18 and the 16% reduction in transmission of influenza B associated with school closures during the 2017–18 winter in Hong Kong.19 We therefore estimate that the other social distancing measures and avoidance behaviours have had a substantial effect on influenza transmission in addition to the effect of school closures. However, if the basic reproduction number of COVID-19 in Hong Kong exceeds 2, (it was 2·2 in Wuhan),11 we would need more than a 44% reduction in COVID-19 transmission to completely avert a local epidemic. A reduction of this magnitude could, however, substantially flatten the peak of and area under the epidemic curve, thus reducing the risk of exceeding the capacity of the health-care system, and potentially saving many lives, especially among older adults.
The postponement of class resumption in local schools in Hong Kong after the Chinese New Year holiday is technically a class dismissal or suspension rather than a school closure, because most teachers are still required to go to school premises to plan e-learning activities and set homework. Full school closures have been implemented locally in previous years, including during the SARS epidemic in 2003,6 during the influenza pandemic in 2009,18 and to control seasonal influenza epidemics in 2008 and 2018.19, 20 Although school closures can have considerable effects on influenza transmission, their role in reducing COVID-19 transmission would depend on the susceptibility of children to infection and their infectiousness if infected. Both of these factors are major unanswered questions at present.21, 22 Despite this acknowledged uncertainty, our surveys revealed considerable local support for school closures.
Individual behaviours in the Hong Kong population have changed in response to the threat of COVID-19. People have been choosing to stay at home, and in our most recent survey, 85% of respondents reported avoiding crowded places and 99% reported wearing face masks when leaving home. Using similar surveys, face mask use during the SARS outbreak in 2003 was 79%,5 and it reached a maximum of 10% during the influenza A (H1N1) pandemic in 2009.7 These changes in behaviour indicate the level of concern among the population about this particular infection, and the extent of voluntary social distancing in addition to the distancing created by school closures. However, we did identify evidence of reductions in voluntary social distancing behaviours in our third survey in March, perhaps indicating some fatigue with these measures.
Our study has some limitations. First, we could not identify which measure was potentially the most effective and whether border restrictions, quarantine and isolation, social distancing, or behavioural changes are most important in suppressing COVID-19 transmission. It is likely that each plays a part. Unlinked cases have been identified in the community and will continue to be identified, indicating that not every chain of transmission has been identified by contact tracing from known cases. Although we have noted major effects of control measures and behavioural changes on influenza transmission, the effects could be of a different magnitude for COVID-19 because of differences in transmission dynamics. Second, our surveys of population behaviours could have been affected by response bias, because we relied on self-reported data. They also could have been affected by selection bias away from working adults, although this should have been reduced by conducting surveys in non-working as well as working hours—we were unable to assess potential selection bias. Without a baseline survey before Jan 23, 2020, we could not compare changes in behaviours, although the results of similar surveys from previous epidemics can be used for comparison.6, 7, 8, 11 Finally, although we identified reductions in the incidence of influenza virus infections in outpatients and paediatric inpatients, it is possible that these time series were affected by reduced health-care-seeking behaviours and limited health-care access that probably resulted from private clinic closure, which occurred around the period of the Chinese New Year holiday.
In conclusion, our study suggests that measures taken to control the spread of COVID-19 have been effective and have also had a substantial impact on influenza transmission in Hong Kong. Although the transmission dynamics and modes of transmission of COVID-19 have not been precisely elucidated, they are likely to share at least some characteristics with influenza virus transmission, because both viruses are directly transmissible respiratory pathogens with similar viral shedding dynamics.23 The measures implemented in Hong Kong are less drastic than those used to contain transmission in mainland China, and are probably more feasible in many other locations worldwide. If these measures and population responses can be sustained, avoiding fatigue among the general population, they could meaningfully mitigate the impact of a local epidemic of COVID-19.
Data sharing
Data on the incidence of coronavirus disease 2019 and influenza activity are freely available from the Centre for Health Protection website (https://www.chp.gov.hk/en/index.html). Survey data are available from the corresponding author on request.
Acknowledgments
Acknowledgments
This project was supported by a commissioned grant from the Health and Medical Research Fund, Food and Health Bureau, Government of the Hong Kong Special Administrative Region.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
BJC and GML designed the study. STA, TWYN, MWF, QL, MYWK, SLL, and SSC collected the data. STA, TWYN, TKT, JCML, and MWF analysed the data. BJC, STA, TWYN, PW, and GML interpreted the data. BJC wrote the first draft. All authors contributed to the final draft.
Declaration of interests
BJC reports honoraria from Sanofi Pasteur and Roche. All other authors declare no competing interests.
Supplementary Material
References
- 1.Leung K, Wu JT, Liu D, Leung GM. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures and second-wave scenario planning: a modelling impact assessment. Lancet. 2020 doi: 10.1016/S0140-6736(20)30746-7. published online April 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong R, Mosur P. To tame coronavirus, Mao-style social control blankets China. Feb 20, 2020. https://www.nytimes.com/2020/02/15/business/china-coronavirus-lockdown.html
- 3.Kavanagh MM. Authoritarianism, outbreaks, and information politics. Lancet Public Health. 2020;5:135–136. doi: 10.1016/S2468-2667(20)30030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu SS, Kwan MY, Feng S. Early season estimate of influenza vaccination effectiveness against influenza hospitalisation in children, Hong Kong, winter influenza season 2018/19. Euro Surveill. 2019;24 doi: 10.2807/1560-7917.ES.2019.24.5.1900056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung GM, Quah S, Ho LM. A tale of two cities: community psychobehavioral surveillance and related impact on outbreak control in Hong Kong and Singapore during the severe acute respiratory syndrome epidemic. Infect Control Hosp Epidemiol. 2004;25:1033–1041. doi: 10.1086/502340. [DOI] [PubMed] [Google Scholar]
- 6.Leung GM, Ho LM, Chan SK. Longitudinal assessment of community psychobehavioral responses during and after the 2003 outbreak of severe acute respiratory syndrome in Hong Kong. Clin Infect Dis. 2005;40:1713–1720. doi: 10.1086/429923. [DOI] [PubMed] [Google Scholar]
- 7.Cowling BJ, Ng DM, Ip DK. Community psychological and behavioral responses through the first wave of the 2009 influenza A(H1N1) pandemic in Hong Kong. J Infect Dis. 2010;202:867–876. doi: 10.1086/655811. [DOI] [PubMed] [Google Scholar]
- 8.Wu P, Fang VJ, Liao Q. Responses to threat of influenza A(H7N9) and support for live poultry markets, Hong Kong, 2013. Emerg Infect Dis. 2014;20:882–886. doi: 10.3201/eid2005.131859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cori A. EpiEstim: estimate time varying reproduction numbers from epidemic curves. 2019. https://CRAN.R-project.org/package=EpiEstim
- 10.Thompson RN, Stockwin JE, van Gaalen RD. Improved inference of time-varying reproduction numbers during infectious disease outbreaks. Epidemics. 2019;29 doi: 10.1016/j.epidem.2019.100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein E, Viboud C, Charu V, Lipsitch M. Improving the estimation of influenza-related mortality over a seasonal baseline. Epidemiology. 2012;23:829–838. doi: 10.1097/EDE.0b013e31826c2dda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong JY, Wu P, Nishiura H. Infection fatality risk of the pandemic A(H1N1)2009 virus in Hong Kong. Am J Epidemiol. 2013;177:834–840. doi: 10.1093/aje/kws314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali ST, Wu P, Cauchemez S. Ambient ozone and influenza transmissibility in Hong Kong. Eur Respir J. 2018;51 doi: 10.1183/13993003.00369-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cori A, Ferguson NM, Fraser C, Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178:1505–1512. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallinga J, Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. Proc Biol Sci. 2007;274:599–604. doi: 10.1098/rspb.2006.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fieller EC. Some problems in interval estimation. J R Stat Soc B. 1954;16:175–185. [Google Scholar]
- 18.Wu JT, Leung K, Perera RA. Inferring influenza infection attack rate from seroprevalence data. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali ST, Cowling BJ, Lau EHY, Fang VJ, Leung GM. Mitigation of influenza B epidemic with school closures, Hong Kong, 2018. Emerg Infect Dis. 2018;24:2071–2073. doi: 10.3201/eid2411.180612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowling BJ, Ho LM, Leung GM. Effectiveness of control measures during the SARS epidemic in Beijing: a comparison of the Rt curve and the epidemic curve. Epidemiol Infect. 2008;136:562–566. doi: 10.1017/S0950268807008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowling BJ, Leung GM. Epidemiological research priorities for public health control of the ongoing global novel coronavirus (2019-nCoV) outbreak. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.6.2000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of COVID-19—studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 23.Zou L, Ruan F, Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data on the incidence of coronavirus disease 2019 and influenza activity are freely available from the Centre for Health Protection website (https://www.chp.gov.hk/en/index.html). Survey data are available from the corresponding author on request.