Abstract
Background
Nutritional rickets is a disease which affects children, especially in low‐ and middle‐income countries. It causes problems such as skeletal deformities and impaired growth. The most common cause of nutritional rickets is vitamin D deficiency. Vitamin D administered with or without calcium is commonly regarded as the mainstay of treatment. In some sunny countries, however, where children are believed to have adequate vitamin D production from exposure to ultraviolet light, but who are deficient in calcium due to low dietary intake, calcium alone has also been used in the treatment of nutritional rickets. Therefore, it is important to compare the effects of vitamin D, calcium or a combination of vitamin D and calcium for the treatment of nutritional rickets in children living in different settings.
Objectives
To assess the effects of vitamin D, calcium or a combination of vitamin D and calcium for the treatment of nutritional rickets in children.
Search methods
We searched CENTRAL, MEDLINE, LILACS, WHO ICTRP Search Portal and ClinicalTrials.gov. The date of the last search of all databases was 25 July 2019. We applied no language restrictions.
Selection criteria
We included randomised controlled trials (RCT) involving children aged 0 to 18 years with nutritional rickets which compared treatment with vitamin D, calcium or a combination of vitamin D and calcium.
Data collection and analysis
Two review authors independently screened the title and abstracts of all studies, extracted data and assessed the risk of bias of included studies. We resolved any disagreements by consensus or recourse to a third review author. We conducted meta‐analyses for the outcomes reported by study authors. For dichotomous outcomes, we calculated the risk ratio (RR) and 95% confidence interval (CI) and, for continuous outcomes, we calculated mean differences (MD) with 95% CIs. We assessed the certainty of the evidence of the included studies using GRADE.
Main results
We identified 4562 studies; of these, we included four RCTs with 286 participants. The studies compared two or more of the following: vitamin D, calcium or vitamin D plus calcium. The number of participants randomised to receive vitamin D was 64, calcium was 102 and vitamin D plus calcium was 120. Two studies were conducted in India and two were conducted in Nigeria. None of the included studies had a low risk of bias in all domains. Three studies had a high risk of bias in at least one domain. The age of the participants ranged between six months and 14 years. The duration of follow‐up ranged between 12 weeks and 24 weeks.
Two studies compared vitamin D to calcium. There is low‐certainty evidence that, at 24 weeks' follow‐up, calcium alone improved the healing of rickets compared to vitamin D alone (RR 3.26, 95% CI 1.59 to 6.69; P = 0.001; 1 study, 71 participants). Comparing vitamin D to calcium showed no firm evidence of an advantage or disadvantage in reducing morbidity (fractures) (RR 0.27, 95% CI 0.03 to 2.32; P = 0.23; 1 study, 71 participants; very low‐certainty evidence). Adverse events were not reported.
Two studies compared vitamin D plus calcium to vitamin D at 12 or 24 weeks. Vitamin D plus calcium improved healing of rickets compared to vitamin D alone at 24 weeks' follow‐up (RR 3.06, 95% CI 1.49 to 6.29; P = 0.002; 1 study, 75 participants; low‐certainty evidence). There is no conclusive evidence in favour of either intervention for reducing morbidity (fractures) (RR 0.24, 95% CI 0.03 to 2.08; P = 0.20; 1 study, 71 participants; very low‐certainty evidence) or adverse events (RR 4.76, 95% CI 0.24 to 93.19; P = 0.30; 1 study, 39 participants; very low‐certainty evidence).
All four included studies compared vitamin D plus calcium to calcium at different follow‐up times. There is no conclusive evidence on whether vitamin D plus calcium in comparison to calcium alone improved healing of rickets at 24 weeks' follow‐up (RR 1.17, 95% CI 0.72 to 1.90; P = 0.53; 2 studies, 140 participants; very low‐certainty evidence). Evidence is also inconclusive for morbidity (fractures) (RR 0.89, 95% CI 0.06 to 13.76; P = 0.94; 1 study, 72 participants; very low‐certainty evidence) and adverse events (RR 4.29, 0.22 to 83.57; P = 0.34; 1 study, 37 participants; very low‐certainty evidence).
Most of the evidence in the review is low or very low certainty due to risk of bias, imprecision or both.
None of the included studies assessed all‐cause mortality, health‐related quality of life or socioeconomic effects. One study assessed growth pattern but this was not measured at the time‐point stipulated in the protocol of our review (one or more years after commencement of therapy).
Authors' conclusions
This review provides low‐certainty evidence that vitamin D plus calcium or calcium alone improve healing in children with nutritional rickets compared to vitamin D alone. We are unable to make conclusions on the effects of the interventions on adverse events or morbidity (fractures).
Plain language summary
Vitamin D, calcium or a combination of vitamin D and calcium for the treatment of nutritional rickets in children
Review question
To assess the effects of vitamin D, calcium or a combination of vitamin D and calcium for the treatment of nutritional rickets in children.
Background
Nutritional rickets is a disease of the bones that affects mostly children in low‐ and middle‐income countries. Children with nutritional rickets typically have deformed bones, may not grow well and experience other health problems. A lack of vitamin D is the most common cause of nutritional rickets. As such, nutritional rickets is usually treated by giving the child vitamin D with or without calcium. In some sunny countries, however, calcium alone has been used to treat nutritional rickets in children who are believed to have adequate vitamin D from their exposure to sunlight but who lack adequate calcium in their diet. This review was conducted to find out whether vitamin D, calcium or a combination of vitamin D and calcium is best for the treatment of nutritional rickets in children.
Study characteristics
We found four randomised controlled trials (clinical trials where people are randomly put into one of two or more treatment groups) that compared vitamin D, calcium or a combination of vitamin D and calcium for the treatment of nutritional rickets in children. The 286 children in the studies were aged six months to 14 years. Following treatment, the children were monitored for between 12 and 24 weeks.
This evidence is up to date as of 25 July 2019.
Key results
We found evidence that using calcium alone or vitamin D plus calcium to treat nutritional rickets may improve healing when compared to using vitamin D alone. We are uncertain about the effects on fractures of calcium alone compared to vitamin D alone. We are uncertain about the effects on fractures or other side effects of vitamin D plus calcium compared to vitamin D alone. We are uncertain about the effects of vitamin D plus calcium compared to calcium alone on healing of rickets, fractures and side effects.
None of the studies reported on growth pattern (differences in height, weight, height for age, weight for age), death from any cause, socioeconomic effects (cost of treatment, resources lost due to illness or due to absence of the caregiver from work, cost of visits to hospital or health facility) and health‐related quality of life.
Reliability of the evidence
The reliability of the evidence for all the outcomes in our review is low or very low. The reason for the uncertainty is mostly due to the low number of participants in the studies and the low number of studies included in the review. Imprecise results and the potential to arrive at wrong conclusions because of the way the trials were conducted in some of the studies also contributed to the level of uncertainty.
Summary of findings
Background
Description of the condition
Rickets is a disease of children caused by failure of the growing bone to calcify, leading to skeletal deformities, impaired growth, and other clinical features which vary depending on the age of the child and the stage of the disease. Most children are affected within the first 18 months of life (Pettifor 2004). Rickets is more prevalent among young children in low‐ and middle‐income countries and is a notable cause of deformities in children in Africa, the Indian subcontinent, Asia, the Middle East and parts of southern Europe (Prentice 2008). About 555,000 children in Bangladesh between the ages of one year and 15 years have deformities caused by rickets (UNICEF 2015). Rickets occurs in both dark‐ and light‐skinned children but dark‐skinned children are more commonly affected. Lerch 2007 distinguished three categories of children who are affected: infants with fair skin; infants with intermediate or dark skin living in their indigenous area and infants with intermediate or dark skin living in an area with lower ultraviolet B (UVB) irradiation than in their indigenous area.
Skeletal deformities associated with rickets are the result of delayed or failed mineralisation of the matrix at the growth plates in children before fusion of the epiphyses (Greenbaum 2011). Bowing of legs (genu varum), knock knees (genu valgum), and soft, asymmetrical, deformable skull (craniotabes) are the most common skeletal deformities associated with rickets. Other features include frontal bossing, rachitic rosary of the ribcage (swelling of the costochondral junction of the ribs), enlargement of the wrists and ankles. deformation of the limbs, hypocalcaemic convulsions, chest deformity (pigeon chest) and delayed motor movement (Agarwal 2009; Greenbaum 2011; Ozkan 2010; Weisberg 2004). Bone pain or tenderness in the arms, pelvis, legs or spine; delayed formation of teeth; loss of muscle strength (decreased muscle tone); impaired growth; increased bone fractures; skeletal deformities and abnormal spine curves (kyphosis or scoliosis) are also associated with rickets (Greenbaum 2011). Some skeletal deformities caused by rickets may require corrective surgery, positioning or bracing (Greenbaum 2011).
Vitamin D deficiency is the most common cause of rickets. There are two major types of rickets: calcipenic (hypocalcaemic) rickets and phosphopenic (hypophosphataemic) rickets. Calcipenic rickets is subdivided into nutritional rickets, vitamin D dependent rickets (type I or 1‐alpha‐hydroxylase deficiency; type II or hereditary resistance to vitamin D), and defects in vitamin D absorption or metabolism of calcium or vitamin D. Nutritional rickets may be caused by dietary deficiency of vitamin D, calcium or phosphorus; cases due to deficiency of calcium or phosphorus are less common (Pettifor 2012). Exclusively breastfed infants who do not receive vitamin D supplementation, dark‐skinned infants and infants born to mothers who were vitamin D deficient during pregnancy are most affected (Misra 2008; Pettifor 2004).
Rickets may be diagnosed clinically by physical examination and taking a medical history, and confirmed biochemically or radiographically (Nield 2006). Biochemical findings in rickets include normal or decreased blood levels of calcium; elevated blood levels of alkaline phosphatase (ALP) or parathyroid hormone; normal, decreased or increased blood levels of phosphate; and decreased blood levels of 25‐hydroxy vitamin D (25‐OHD) in vitamin D deficiency rickets (Pettifor 2005; Shaw 2004; Thacher 2003). Findings from radiographic investigations include cupping, flaring and fraying of the metaphysis, rachitic rosary, and angular deformities of the bones of the arms and legs (Greenbaum 2011; Hochberg 2003).
Although rickets was prevalent in Europe and the USA between the late nineteenth century and the early twentieth century, the discovery of the antirachitic properties of vitamin D and subsequent fortification of foods with vitamin D led to its eradication in the 1930s (Jessop 1950; Welch 2000). In more recent times, however, there has been a re‐emergence of rickets in these and other industrialised countries (Welch 2000; Wendling 2007). Callaghan 2006 reported an overall incidence of 7.5 cases per 100,000 per year in one survey in 2001 of children under five years of age in the UK (West Midlands). Most of the children were of black African or African‐Caribbean origin. The overall annual incidence rate in Canada in 2004 was 2.9 cases per 100,000.
Studies carried out to assess the prevalence of rickets in Asia and Africa show wide variation of prevalence rates from 42% in Ethiopian to 9% in Nigerian children aged six months to three years (Pfitzner 1988; Prentice 2008). The treatment of rickets depends on the type and cause, and usually includes supplementation with vitamin D, its metabolites, calcium or a combination. Goals of treatment are to relieve symptoms and correct the cause of the condition in order to prevent the disease from returning (Greenbaum 2011).
Description of the intervention
Rickets is treated by administration of vitamin D, calcium or both, or phosphorus depending on the underlying cause. With particular reference to nutritional rickets, vitamin D administered with or without calcium is commonly regarded as the mainstay of treatment (Greenbaum 2011; Reddy 2008). Vitamin D is administered orally or intramuscularly as ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). Various vitamin D preparations, dosages (high versus low), dosing schedules (single versus multiple) and administration routes (oral or intramuscular) are available. Where a child's compliance with a treatment regimen may be difficult, vitamin D may be given as a single administration of 100,000 IU to 600,000 IU over one to five days (high‐dose therapy or 'Stosstherapie', Balasubramanian 2013; Misra 2008; Pettifor 2014a). Adverse events such as hypercalcaemia and hypercalciuria have been reported with such therapy (Cesur 2003). Vitamin D may also be administered in smaller doses over several weeks. Exposure to ultraviolet (UV) radiation/sunlight and improved nutrition are also recommended. Rich dietary sources of vitamin D include liver, fish, milk, infant formula and other foods fortified with vitamin D such as margarine.
Calcium alone has also been used in the treatment of nutritional rickets especially in children who reside in sunny countries and who are believed to have adequate vitamin D production from exposure to UV light but are deficient in calcium due to low dietary intake. In supplements, the two main forms of calcium are calcium carbonate and calcium citrate. Calcium carbonate is inexpensive, convenient and readily available. It is highly dependent on stomach acid for absorption, and works more efficiently when taken with food. Calcium citrate is well absorbed and can be taken with or without food. Other forms of calcium in supplements or fortified foods are calcium phosphate, calcium lactate and calcium gluconate. These supplements contain varying amounts of elemental calcium. Calcium citrate contains 21% calcium, and calcium carbonate 40% calcium by weight. When these supplements are taken, the percentage of calcium absorbed by the body depends on the total amount of elemental calcium taken at one time. As the amount of elemental calcium taken increases, the percentage absorbed decreases. Absorption is highest with doses of elemental calcium 500 mg or less (Ross 2011).
Adverse effects of vitamin D or calcium
Adverse effects from vitamin D or calcium are uncommon if given in the correct dose. However, if doses of vitamin D or calcium are too high, hypercalcaemia (high levels of calcium in the blood), which can cause soft tissue calcification and kidney stones, and hypercalciuria (high levels of calcium in the urine) with varying degrees of renal insufficiency, can occur. High calcium intake can cause constipation, and may also interfere with the absorption of iron and zinc.
Vitamin D toxicity, also called hypervitaminosis D, is a rare but potentially serious condition that occurs when there are excessive amounts of vitamin D in the body due to very high doses of vitamin D supplements. Hypercalcaemia is responsible for most of the symptoms of vitamin D toxicity. Early symptoms of vitamin D toxicity include gastrointestinal disorders such as anorexia, diarrhoea, constipation, nausea and vomiting. Other symptoms include bone pain, drowsiness, irregular heartbeat, loss of appetite, muscle and joint pain, frequent urination, excessive thirst, weakness, nervousness, itching and kidney stones (Schwalfenberg 2007).
How the intervention might work
Vitamin D deficiency results in the failure of mineralisation of growing bones manifesting clinical and radiological features of rickets. Vitamin D replacement therapy in the presence of adequate dietary calcium results in the resolution of the features of rickets. Calcium deficiency (from inadequate dietary intake or malabsorption) increases the catabolism of vitamin D and ultimately results in vitamin D deficiency and rickets. In such settings where poor dietary calcium intake is the dominant cause of rickets, calcium replacement therapy (with or without vitamin D) will be needed to achieve resolution of the symptoms of rickets (Pettifor 2004).
Interventions for the treatment of nutritional rickets include supplementation of vitamin D, calcium supplementation or a combination of both. Educational interventions include nutritional counselling and advice on exposure to sunlight.
Why it is important to do this review
Rickets constitutes a significant public health problem, particularly in low‐ and middle‐income countries. In recent years there has been a re‐emergence of rickets in high‐income countries such as the UK and the USA where it was thought to have been eradicated (Allgrove 2004; Nield 2006; Pal 2001). Most occurrences of rickets are in children with dark skin or of non‐white origin such as African Americans and South East Asians.
Although vitamin D deficiency has been thought to be the predominant cause of nutritional rickets there is evidence that calcium deficiency is the major cause of rickets in Africa and some parts of Asia. It has been observed that where calcium deficiency is primarily responsible for the occurrence of rickets and where levels of 25‐OHD are normal, treatment with vitamin D alone may not result in resolving the disease (Thacher 1999). A combination of vitamin D and calcium or calcium alone has been recommended in these instances. There is evidence that low dietary calcium intakes play a significant role in the pathogenesis of rickets which has implications for the choice of appropriate treatment and preventive interventions (Pettifor 2014b).
A number of studies have been carried out to compare vitamin D and calcium in the treatment of nutritional rickets (both calcium deficiency and vitamin D deficiency rickets). Studies have also compared various regimens of vitamin D for the treatment of rickets. Thacher 2006 conducted a narrative review of non‐randomised studies that assessed the prevalence and causes of nutritional rickets. The objective and findings presented by Thacher 2006 did not include evaluation of the effectiveness of interventions for treating nutritional rickets. A search of major electronic health research databases found no systematic review or meta‐analysis of studies that assessed the effects of these interventions. Therefore, there is a need to carry out a systematic review to assess the effects of interventions for treating nutritional rickets.
Objectives
To assess the effects of vitamin D, calcium or a combination of vitamin D and calcium for the treatment of nutritional rickets in children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
Children up to 18 years of age with nutritional rickets.
Diagnostic criteria for (nutritional) rickets
Nutritional rickets refers to rickets confirmed by a combination of clinical, radiological and biochemical features, and shown to be due to any of the following aetiological categories (Pettifor 2004): vitamin D deficiency, calcium deficiency or a combination of vitamin D and calcium deficiency.
Clinical diagnosis is established by carrying out a complete physical and dental examination of the child and taking the medical, social and nutritional history to identify following features: enlargement of wrists, craniotabes (softening of the skull bones), rachitic rosary, bowing of legs, pigeon chest and frontal bossing. Other important clinical findings, apart from bone deformities, that will be used to establish clinical diagnosis, are hypocalcaemic convulsions, hypotonia (muscle weakness) and growth retardation. The diagnosis of rickets is supported by radiological and biochemical features characteristic of the disease.
Radiological investigations for diagnosis of rickets include radiographs of the wrists or knees. Key radiological findings are metaphyseal cupping or fraying and widening of epiphysis. Other radiological findings may include fractures, osteopenia or widened wrists and ankles.
Biochemical investigations include assessment of calcium, phosphorus and ALP blood levels. Rickets is characterised by low blood calcium levels (less than 8.5 mg/dL), low phosphorus levels (less than 4.5 mg/dL) and high ALP levels (greater than 461 U/L). There may be other abnormal biochemical findings such as elevated parathyroid hormone levels (greater than 55 pg/mL) and low vitamin D as evidenced by low 25‐OHD levels (less than 15 ng/dL).
Types of interventions
We investigated the following comparisons of intervention versus control/comparator.
Interventions and comparators
Calcium compared with vitamin D.
Vitamin D plus calcium compared with calcium or vitamin D.
Concomitant interventions had to be identical in both the intervention and comparator groups to establish fair comparisons. If a study included multiple groups, we included any group that met the inclusion criteria for this review. We investigated any type, dose and route of administration of vitamin D or calcium, as well as different forms of vitamin D or calcium. Furthermore, we distinguished between supplementation and fortification interventions.
Minimum duration of intervention
For calcium given alone or in combination with vitamin D, the minimum duration was at least eight weeks. This did not apply to studies that used one day high‐dose therapy.
Minimum duration of follow‐up
We included studies with a minimum duration of interventions of eight weeks.
We defined any follow‐up period going beyond the original time frame for the primary outcome measure as specified in the power calculation of the studies' protocols as an extended follow‐up period (also called open‐label extension study) (Buch 2011; Megan 2012).
Summary of specific exclusion criteria
Preterm children.
Children above 18 years of age.
Children with comorbidities such as HIV, sickle cell anaemia.
Quasi‐randomised trials and other non‐RCT study designs.
Types of outcome measures
We did not exclude studies because one or several of our primary or secondary outcome measures were not reported in the publication. When a study reported none of our primary or secondary outcomes, we did not include this study but planned to provide some basic information in an additional table.
We extracted the following outcomes, using the methods and time points specified below.
Primary outcomes
Healing of rickets.
Morbidity.
Adverse events.
Secondary outcomes
All‐cause mortality.
Health‐related quality of life.
Growth pattern.
Socioeconomic effects.
Method of outcome measurement
Healing of rickets: defined as resolution of clinical and radiological features of rickets.
Morbidity: defined as infections (such as acute lower respiratory tract infections, e.g. pneumonia), hypocalcaemic seizures, fractures.
Adverse events: such as hypercalcaemia, hypercalciuria, hypervitaminosis D.
All‐cause mortality: defined as death from any cause.
Health‐related quality of life: evaluated by a validated instrument such as Short Form 36 questionnaire (SF‐36).
Growth pattern: defined as differences in height, weight, height for age, weight for age and weight for height scores.
Socioeconomic effects: defined as cost of treatment, resources lost due to illness or due to absence of the caregiver from work, cost of visits to hospital or health facility.
Timing of outcome measurement
Healing of rickets and socioeconomic effects: measured at 12 or more weeks after commencement of therapy.
Morbidity: at any time from when the intervention was administered.
Adverse events: from commencement of the intervention to at least four weeks after stopping treatment.
All‐cause mortality: measured at any time during the study.
Health‐related quality of life: measured at any time during follow‐up.
Growth pattern: at one or more years after commencement of therapy.
Search methods for identification of studies
Electronic searches
We searched the following sources on 25 July 2019 from inception of each database to the specified date and placed no restrictions on the language of publication.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO), 25 July 2019.
MEDLINE Ovid (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE; from 1946 to 23 July 2019).
LILACS (Latin American and Caribbean Health Science Information database; from 1982 to "Last update: 15/07/2019").
ClinicalTrials.gov (www.clinicaltrials.gov), 25 July 2019.
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch/), 25 July 2019.
We did not include Embase in our search, as RCTs indexed in Embase are now prospectively added to CENTRAL via a highly sensitive screening process (Cochrane 2018).
We continuously applied a MEDLINE (via OvidSP) email alert service established by the Cochrane Metabolic and Endocrine Disorders (CMED) Group to identify newly published trials using the same search strategy as described for MEDLINE (Appendix 1).
Searching other resources
We tried to identify other potentially eligible studies or ancillary publications by searching the reference lists of included studies, systematic reviews, meta‐analyses and health technology assessment reports. In addition, we contacted authors of included studies to identify additional information on the retrieved studies and establish if further studies that we may have missed exist. We define grey literature as records detected in ClinicalTrials.gov or WHO ICTRP.
Data collection and analysis
Selection of studies
At least two review authors (MC, DG, JO) independently scanned the abstract, title or both, of every record we retrieved in the literature searches, to determine which studies we should assess further. We obtained the full text of all potentially relevant records. We resolved any disagreements through consensus or by recourse to a third review author (MM). If we could not resolve a disagreement, we categorised the study as a 'study awaiting classification' and contacted the study authors for clarification. We presented an adapted PRISMA flow diagram to shown the process of study selection (Liberati 2009).
We did not use abstracts or conference proceedings for data extraction because this information source does not fulfil CONSORT requirements which is "an evidence‐based, minimum set of recommendations for reporting randomised trials" (CONSORT; Scherer 2018).
Data extraction and management
For studies that fulfilled our inclusion criteria, two review authors (MC, JO) independently extracted key information on participants, interventions and comparators. We reported data on efficacy outcomes and adverse events using standardised data extraction sheets from the Cochrane Metabolic and Endocrine Disorders (CMED) Group. We resolved any disagreements by discussion, or if required, by consultation with a third review author (MM or DG) (for details, see Characteristics of included studies table; Table 4; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13; Appendix 14; Appendix 15; Appendix 16).
1. Overview of study populations.
| Study ID (design) | Intervention(s) and comparator(s) | Description of power and sample size calculation | Screened/eligible (n) | Randomised (n) | Analysed (n) | Finishing study (n) | Randomised finishing study (%) | Follow‐up |
| Thacher 2014 (parallel RCT) | I: calcium + vitamin D | "Based on the primary outcome measures of radiographic score and serum alkaline phosphatase values and SDs based on previous studies (1.6 for radiographic score and 150 U/L in alkaline phosphatase), 40 subjects in each treatment group would provide 80% power and 95% CI to detect a difference between groups of 1.0 in final radiographic score and 100 U/L in alkaline phosphatase." | 254 | 44 | 43 | 43 | 97.7 | 24 weeks |
| C: calcium + placebo | 28 | 25 | 25 | 89.3 | ||||
| total: | 72 | 68 | 68 | 94.4 | ||||
| Aggarwal 2013 (parallel RCT) | I1: calcium + vitamin D | — | 100 | 22 | 20 | 20 | 90.9 | 12 weeks |
| I2: calcium | 22 | 17 | 17 | 77.3 | ||||
| C: vitamin D | 23 | 19 | 19 | 82.6 | ||||
| total: | 67 | 56 | 56 | 83.6 | ||||
| Balasubramanian 2003 (parallel RCT) | I: calcium + vitamin D | — | — | 14 | 4 | 4 | 28.6 | 3 months |
| C: calcium | 10 | 4 | 4 | 40 | ||||
| total: | 24 | 8 | 8 | 33 | ||||
| Thacher 1999 (parallel RCT) | I1: calcium + vitamin D | — | 297 | 40 | 38 | 38 | 95 | 24 weeks |
| I2: calcium + placebo | 42 | 34 | 35 | 83.3 | ||||
| C: vitamin D + placebo | 41 | 37 | 37 | 90.2 | ||||
| total: | 123 | 109 | 110 | 89.4 | ||||
| Grand total | All interventions | 184 | 157 | |||||
| All comparators | 102 | 85 | ||||||
| All interventions and comparators | 286 | 242 | ||||||
— denotes not reported
C: comparator; CI: confidence interval; I: intervention; ITT: intention‐to‐treat; n: number of participants; RCT: randomised controlled trial; SD: standard deviation.
We planned to provided information, including the study identifier for potentially relevant ongoing studies in the Characteristics of ongoing studies table and in Appendix 7 entitled 'Matrix of study endpoint (publications and trial documents)'. We tried to find the protocol for each included study and reported primary, secondary and other outcomes from these protocols alongside the data from the study publications in Appendix 7.
We emailed all authors of included studies to enquire whether they would be willing to answer questions regarding their studies. We presented the results of this survey in Appendix 13. Thereafter, we sought relevant missing information on the study from the primary study author(s), if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximised the information yield by collating all available data and used the most complete dataset aggregated across all known publications. We listed duplicate publications, companion documents, multiple reports of a primary study and study documents of included studies (such as trial registry information) as secondary references under the study ID of the included study. Furthermore, we also listed duplicate publications, companion documents, multiple reports of a study and trial documents of excluded studies (such as trial registry information) as secondary references under the study ID of the excluded study.
Data from clinical trials registers
If data from included studies were available as study results in clinical trials registers, such as ClinicalTrials.gov or similar sources, we made full use of this information and extracted data. If there was also a full publication of the study, we collated and critically appraised all available data. If an included study was marked as a completed study in a clinical trial register, but no additional information was available, we added this study to the Characteristics of studies awaiting classification table.
Assessment of risk of bias in included studies
Two review authors (MC, DG) independently assessed the risk of bias of each included study. We resolved any disagreements by consensus or by consultation with a third review author (MM). In the case of disagreement, we consulted the rest of the review author team and made a judgement based on consensus. If adequate information was not available from study authors, study protocols or both, we contacted the study authors to request missing data on risk of bias items.
We used the Cochrane 'Risk of bias' assessment tool (Higgins 2019a), assigning assessments of low, high or unclear risk of bias (for details, see Appendix 2; Appendix 3). We evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions according to the criteria and associated categorisations contained therein (Higgins 2019a).
We presented a 'Risk of bias' graph and a 'Risk of bias' summary figure (Figure 1; Figure 2). We distinguished between self‐reported, investigator‐assessed and adjudicated outcome measures.
1.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials (blank cells indicate that the particular outcome was not measured in some trials).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial ((blank cells indicate that the particular outcome was not measured in some trials)
We considered the following self‐reported outcomes.
Morbidity.
Adverse events.
Health‐related quality of life.
Socioeconomic effects.
We considered the following outcomes to be investigator‐assessed.
Healing of rickets.
Morbidity.
Adverse events.
All‐cause mortality.
Growth pattern.
Socioeconomic effects.
Summary assessment of risk of bias
Risk of bias for a study across outcomes
Some risk of bias domains, such as selection bias (sequence generation and allocation sequence concealment), affect the risk of bias across all outcome measures in a study. In case of high risk of selection bias, all endpoints investigated in the associated study were marked as high risk. Otherwise, we did not perform a summary assessment of the risk of bias across all outcomes for a study.
Risk of bias for an outcome within a study and across domains
We assessed the risk of bias for an outcome measure by including all entries relevant to that outcome (i.e. both study level entries and outcome specific entries). We considered low risk of bias to denote a low risk of bias for all key domains, unclear risk to denote an unclear risk of bias for one or more key domains and high risk to denote a high risk of bias for one or more key domains.
Risk of bias for an outcome across studies and across domains
To facilitate our assessment of the certainty of evidence for key outcomes, we assessed risk of bias across studies and domains for the outcomes included in the 'Summary of finding' tables. We defined the evidence as being at low risk of bias when most information came from studies at low risk of bias, unclear risk of bias when most information came from studies at low or unclear risk of bias, and high risk of bias when a sufficient proportion of information came from studies at high risk of bias.
Measures of treatment effect
When at least two included studies are available for a comparison and a given outcome, we tried to express dichotomous data as a risk ratio (RR) or odds ratio (OR) with 95% confidence intervals (CIs). For continuous outcomes measured on the same scale (e.g. weight loss in kilograms), we estimated the intervention effect using the mean difference (MD) with 95% CIs. For continuous outcomes measuring the same underlying concept (e.g. health‐related quality of life) but using different measurement scales, we calculated the standardised mean difference (SMD). We expressed time‐to‐event data as hazard ratio (HR) with 95% CIs.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over studies, cluster‐randomised trials and multiple observations for the same outcome. If more than one comparison from the same study was eligible for inclusion in the same meta‐analysis, we either combined groups to create a single pair‐wise comparison or appropriately reduced the sample size so that the same participants did not contribute more than once (splitting the 'shared' group into two or more groups). While the latter approach offers some solution to adjusting the precision of the comparison, it does not account for correlation arising from the same set of participants being in multiple comparisons (Higgins 2019b).
We attempted to re‐analyse cluster‐RCTs that had not appropriately adjusted for potential clustering of participants within clusters in their analyses. The variance of the intervention effects was inflated by a design effect (DEFF). Calculation of a DEFF involves estimation of an intracluster correlation (ICC). We obtained estimates of ICCs through contact with authors, or imputed them using estimates from other included studies that reported ICCs, or using external estimates from empirical research (e.g. Bell 2013). We planned to examine the impact of clustering using sensitivity analyses.
Dealing with missing data
If possible, we obtained missing data from the authors of the included studies. We carefully evaluated important numerical data such as screened, randomly assigned participants as well as intention‐to‐treat, and as‐treated and per‐protocol populations. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals), and we critically appraised issues concerning missing data and imputation methods (e.g. last observation carried forward).
In studies where the standard deviation (SD) of the outcome was not available at follow‐up or could not be calculated, we standardised by the mean of the pooled baseline SD from those studies that reported this information.
Where included studies did not report means and SDs for outcomes and we not received the needed information from study authors, we imputed these values by estimating the mean and variance from the median, range, and the size of the sample (Hozo 2005).
We investigated the impact of imputation on meta‐analyses by performing sensitivity analyses, and we reported per outcome which studies were included with imputed SDs.
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we did not report study results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi² test with a significance level of α = 0.1 (Deeks 2019). In view of the low power of this test, we also considered the I² statistic – which quantifies inconsistency across studies – to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). When we identified heterogeneity, we attempted to determine the possible reasons for it by examining individual characteristics of the study and subgroups.
Assessment of reporting biases
If we included 10 or more studies that investigated a particular outcome, we used funnel plots to assess small study effects. Several explanations may account for funnel plot asymmetry, including true heterogeneity of effect with respect to study size, poor methodological design (and hence bias of small studies) and selective non‐reporting (Kirkham 2010). Therefore, we interpreted results carefully (Sterne 2011).
Data synthesis
We planned to undertake (or display) a meta‐analysis only if participants, interventions, comparisons and outcomes were sufficiently similar to ensure a result that was clinically meaningful. Unless good evidence shows homogeneous effects across studies, we primarily summarised data that were of low risk of bias using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration to the whole distribution of effects, ideally by presenting a prediction interval (Borenstein 2017a; Borenstein 2017b; Higgins 2009). A prediction interval needs at least three studies to be calculated and specifies a predicted range for the true treatment effect in an individual study (Riley 2011). For rare events (such as event rates below 1%), we used Peto's OR method, provided that there was no substantial imbalance between intervention and comparator group sizes and intervention effects are not exceptionally large. In addition, we also performed statistical analyses according to the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2019).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and we planned to carry out subgroup analyses for these, including investigation of interactions (Altman 2003).
Studies in low‐ and middle‐income countries compared to those in high‐income countries.
Dosing scheme.
Age.
Sex.
Type of supplementation/fortification.
Sensitivity analysis
We planned to perform sensitivity analyses to explore the influence of the following factors (when applicable) on effect sizes by restricting analysis to the following.
Published studies.
Taking into account risk of bias, as specified in the Assessment of risk of bias in included studies section.
Very long or large studies, to establish the extent to which they dominated the results.
We used the following filters, if applicable: diagnostic criteria, imputation used, language of publication (English versus other languages), source of funding (industry versus other) or country (depending on data).
We also tested the robustness of results by repeating the analyses using different measures of effect size (RR, OR, etc.) and different statistical models (fixed‐effect and random‐effects models).
Certainty of the evidence
We presented the overall certainty of evidence for each outcome specified below, according to the GRADE approach, which takes into account issues related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (such as directness of results). Two review authors (MC, DG) independently rated the certainty of evidence for each outcome. We resolved any differences in the assessment by discussion or consulting a third review author (MM).
We included an appendix entitled 'Checklist to aid consistency and reproducibility of GRADE assessments', to help with standardisation of the 'Summary of findings' tables (Meader 2014). Alternatively, we planned to use GRADEpro Guideline Development Tool (GDT) software and planned to present evidence profile tables as an appendix (GRADEpro GDT 2015). We presented results for the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we presented the results in a narrative format in the 'Summary of findings' table. We justified all decisions to downgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the Cochrane Review where necessary.
'Summary of findings' table
We presented a summary of the evidence in a 'Summary of findings' table. This provided key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies; the numbers of participants and studies addressing each important outcome; and a rating of overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019), along with Review Manager 5 software (Review Manager 2014). We presented in the 'Summary of findings' table the following interventions: calcium compared with vitamin D, vitamin D plus calcium compared with vitamin D and vitamin D plus calcium compared with calcium.
We reported the following outcomes, listed according to priority.
Healing of rickets.
Morbidity.
Adverse events.
All‐cause mortality.
Health‐related quality of life.
Growth pattern.
Socioeconomic effects.
Results
Description of studies
For a detailed description of studies, see Table 4; Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
Electronic search of databases and the continuous MEDLINE (via OvidSP) search yielded 4562 records. We did not identify any additional records through searching of non‐databases sources. After removal of duplicates, we obtained and screened 3952 unique records. Three review authors independently screened titles and abstracts of these records following which 3937 records were excluded. We assessed the full text of 15 studies for eligibility and four studies (five publications) met the inclusion criteria (see Figure 3).
3.

Trial flow diagram.
Included studies
Four studies met the inclusion criteria and were included in the review. A detailed description of the characteristics of included studies is presented elsewhere (see Characteristics of included studies table; Appendix 4; Appendix 5; Appendix 6). The following is a succinct overview.
Source of data
All data included in the review were obtained from reports published in medical journals except for some additional data which was obtained through correspondence with the study authors by email. Specific data obtained from study authors were:
baseline data on number of children randomised to study groups for Thacher 1999;
data on adverse events, serum alkaline phosphate values, serum 25‐OHD values and radiological scores at 12 and 24 weeks for Thacher 2014;
data on blinding of participants and personnel, study design and reasons for loss to follow‐up for Aggarwal 2013.
For details of correspondence with authors, see Appendix 13.
Comparisons
Two of the included studies had two groups which compared vitamin D plus calcium versus calcium alone or calcium with placebo (Balasubramanian 2003; Thacher 2014). The two other included studies had three groups which compared vitamin D plus calcium versus calcium (alone or with placebo) versus vitamin D (alone or with placebo) (Aggarwal 2013; Thacher 1999). Doses and route of administration of vitamin D varied between studies. Four studies/study arms administered calcium orally in the form of calcium lactate or calcium carbonate and the form was unclear in one study (Appendix 4).
Overview of study populations
Studies included 286 participants.
The number of participants randomised to intervention groups was 184 and comparator groups was 102.
The percentage of participants finishing the studies was 85.3% in the intervention groups and 83.3% in the comparator groups.
Individual sample size in the studies ranged from 24 to 123.
Study design
All included studies were parallel RCTs. Two studies had a two‐group parallel design (Balasubramanian 2003; Thacher 2014), and two studies had a three‐group parallel design (Aggarwal 2013; Thacher 1999).
Three studies had a superiority design (Balasubramanian 2003; Thacher 1999; Thacher 2014); Aggarwal 2013 had an equivalence design.
Two studies gave placebo in conjunction with an active treatment (Thacher 1999; Thacher 2014).
Two studies were performed at a single centre except for Balasubramanian 2003 that was performed at two centres and Thacher 1999 for which the number of centres was unclear.
One study was not blinded (Aggarwal 2013), and it was unclear whether participants and personnel were blinded in the remaining three studies.
Three studies blinded outcome assessors (Aggarwal 2013; Thacher 1999; Thacher 2014). It was unclear whether the assessors were blinded in the remaining study (Balasubramanian 2003).
Balasubramanian 2003 did not state the year in which the study was conducted. The remaining three studies were conducted between 1996 and 2009.
Mean duration of the intervention in the studies ranged from 12 weeks to 24 weeks while the duration of follow‐up also ranged from 12 weeks to 24 weeks.
There was no run‐in period for any of the included studies.
None of the studies was terminated before time due to benefit or harm.
Settings
Two of the studies were conducted in India (Aggarwal 2013; Balasubramanian 2003), and two in Nigeria (Thacher 1999; Thacher 2014). All were conducted in hospital facilities.
Participants
All participants were from low‐ to middle‐income countries.
Sixty‐eight per cent of participants were of African ethnicity (Thacher 1999; Thacher 2014), and 32% were Asian (Aggarwal 2013; Balasubramanian 2003).
Only one study reported the duration of rickets and this ranged from 0.5 months to 108 months (Thacher 2014).
One hundred and fifty‐five participants were girls and 131 were boys. Two studies recruited almost equal proportion of boys and girls (Aggarwal 2013; Balasubramanian 2003), while the other two studies recruited more girls (Thacher 1999; Thacher 2014).
The mean age of the participants ranged from six months to 14 years.
None of the studies reported comorbidities, cointerventions or comedications used by participants.
The major exclusion criteria in the included studies were a history of renal disease, liver diseases or tuberculosis; history of consuming calcium, vitamin D supplements or multivitamins in the preceding three months to six months; history of treatment with anticonvulsant or antiepileptic drugs and cases presenting with hypocalcaemic seizures.
Diagnosis
Diagnosis of rickets was confirmed biochemically or radiologically, or both. Aggarwal 2013 and Thacher 2014 diagnosed rickets radiologically (radiographs of the wrist and knee) using Thacher's 10‐point scale (Thacher 2000). Thacher's 10‐point scale is a scoring system to assess the radiographic changes in the wrists and knees of people with rickets, where 0 represents no rickets and 10 represents severe rickets. In Aggarwal 2013, a radiological score of greater than 1.5 indicated rickets, while Thacher 2014 diagnosed children with rickets as those with a radiographic score of at least 2.5 on a 10‐point scale. In addition, Aggarwal 2013 reported that based on currently accepted paediatric standards, children with serum 25‐OHD levels less than 20 ng/mL were considered to have vitamin D deficiency.
Balasubramanian 2003 confirmed the diagnosis of rickets if the children had characteristic radiological changes and elevated serum ALP level (SAP) (greater than 375 IU/L). However, the author did not describe how radiological changes were assessed. Thacher 1999 diagnosed rickets clinically and radiologically. Children with deformities characteristic of rickets (such as genu varum and genu valgum) had radiography of the wrists and knees and active rickets was diagnosed if the epiphyseal plate was wider than normal and there was concave cupping or fraying of the metaphyseal margins on the radiographs.
Interventions
None of the included studies reported administration of any treatment before the start of the study.
Two studies had three groups comparing vitamin D plus calcium versus calcium or vitamin D (Aggarwal 2013; Thacher 1999). The remaining two studies compared vitamin D plus calcium versus calcium (Balasubramanian 2003; Thacher 2014).
Three studies administered calcium orally, as calcium carbonate (Balasubramanian 2003; Thacher 1999; Thacher 2014), while one study did not specify the form of elemental calcium used (Aggarwal 2013). Two studies administered vitamin D orally (Balasubramanian 2003; Thacher 2014), and two administered vitamin D intramuscularly (Aggarwal 2013; Thacher 1999).
Total daily doses of calcium and vitamin D varied between studies (Appendix 4).
Two studies used a placebo (Thacher 1999; Thacher 2014). However, the placebo was not given alone but in conjunction with an active treatment (Appendix 4).
All studies used adequate interventions and comparators.
Outcomes
All four included studies explicitly stated a primary/secondary endpoint in their publications. Healing of rickets was the most commonly defined primary outcome (Appendix 7). All studies reported healing of rickets. Only one study reported on morbidity and growth pattern (Thacher 1999). None of the studies reported on all‐cause mortality, health‐related quality of life and socioeconomic effects. One study reported adverse events (Aggarwal 2013). Only two studies had trial registration documents (Aggarwal 2013; Thacher 2014). The endpoints reported in Aggarwal 2013 did not differ from the prespecified endpoints in the trial document. Some of the secondary outcomes specified by Thacher 2014 were not reported in the publication; however, these did not include any of the outcomes of interest. The number of outcomes of interest reported by the studies ranged between one and three. Definition of endpoint for healing of rickets based on radiological and biochemical criteria was provided by all the studies, although these varied. Adverse events, morbidity or growth pattern were not defined as study endpoints. Aggarwal 2013 reported specific adverse events and Thacher 1999 reported fractures (type of morbidity) at different time points in the study.
Excluded studies
We excluded nine studies after evaluation of the full publication. The main reasons for exclusion were that they were not RCTs (for further details, see Characteristics of excluded studies table).
Studies awaiting classification
One study is awaiting classification as we could not obtain a copy of the publication (El'chaninov 1969).
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
For details on the risk of bias of the included studies, see Characteristics of included studies table.
For an overview of review authors' judgements about each risk of bias item for individual studies and across all studies, see Figure 1 and Figure 2.
Allocation
One study was at low risk of selection bias (random sequence generation and allocation concealment) (Aggarwal 2013). In two studies, there was random sequence generation but it was unclear whether there was allocation concealment (Balasubramanian 2003; Thacher 2014). Although we judged allocation concealment to be adequate in Thacher 1999, the method of random sequence generation was unclear.
Blinding
None of the included studies stated explicitly that blinding of the participants and personnel was undertaken. However, we were able to confirm from the study authors that there was no blinding of participants or personnel in Aggarwal 2013. In three studies, there was insufficient information to determine whether participants and personnel were blinded (Balasubramanian 2003; Thacher 1999; Thacher 2014).
We judged blinding of participants and personnel to be adequate for three studies for healing of rickets (Aggarwal 2013; Thacher 1999; Thacher 2014). Only one study reported on adverse events (Aggarwal 2013). As neither the participants or personnel were blinded in this study, we judged blinding of participants and personnel for adverse events at high risk of performance bias. Similarly, only Thacher 1999 assessed and reported on morbidity (fractures). We judged the risk of performance bias to be low for this outcome. Two studies reported growth pattern (Thacher 1999; Thacher 2014). We considered the risk of performance bias for this outcome to be low.
One study stated explicitly that outcome assessors were blinded (Aggarwal 2013). Two studies blinded outcome assessors for some of the outcomes (Thacher 1999; Thacher 2014). Specifically, in Thacher 1999, study personnel who assessed healing of rickets were blinded. However, it was unclear whether personnel that assessed growth pattern and morbidity were blinded. Similarly in Thacher 2014, it was not clear whether personnel assessing growth pattern or healing of rickets were blinded. However, we judged detection bias for these studies to be low because the outcomes were objective and were unlikely to be affected by absence of blinding. One study did not provide enough information to enable us assess whether outcome assessors in the study were blinded (Balasubramanian 2003).
Overall, we judged detection bias for healing of rickets to be low. Three studies had low risk of detection bias (Aggarwal 2013; Thacher 1999; Thacher 2014), while we were unable to determine whether outcome assessors were blinded in one study (Balasubramanian 2003).
We judged the overall risk of detection bias to be low for adverse events, morbidity and growth pattern. Only one study reported on adverse events (Aggarwal 2013) and one on morbidity (Thacher 1999). Two studies reported on growth pattern (Thacher 1999; Thacher 2014).
Incomplete outcome data
Attrition rates varied widely, being 6% in Thacher 2014, 11% in Thacher 1999, 16% in Aggarwal 2013, and 67% in Balasubramanian 2003. All four included studies reported losses to follow‐up. One of the studies reported the reasons for loss to follow‐up as failure of participants to return (Thacher 2014). We were, however able to obtain reasons for loss to follow‐up for one additional study by contacting study authors (Aggarwal 2013). The reasons given by the study authors were the participants were not contactable, felt they were well or shifted treatment to another hospital.
We judged overall bias due to attrition to be unclear for most of the reported outcomes. However, Balasubramanian 2003 and Aggarwal 2013 had high loss to follow‐up and we judged this to have a possible impact on healing of rickets.
None of the studies used intention‐to‐treat analysis.
Selective reporting
Two studies had a published protocol (Aggarwal 2013; Thacher 2014). We judged the risk of reporting bias to be low for Aggarwal 2013 as all outcomes were reported as prespecified in the protocol. However, we considered the risk of reporting bias to be high for two studies (Balasubramanian 2003; Thacher 2014). Thacher 2014 stated the primary outcome 'combined attainment of a radiographic score of 1.5 or less and a SAP concentration of 350 U/L or less' in the publication but as 'XR [radiological] healing of rickets' in the protocol. Although there was no protocol for Balasubramanian 2003, the author did not report data on radiological measurements even though these were taken. We could not ascertain the risk of reporting bias for Thacher 1999. For full details of ORBIT (Outcome Reporting Bias In Trials), see Appendix 8.
Other potential sources of bias
We did not detect any other potential sources of bias for three studies (Aggarwal 2013; Thacher 1999; Thacher 2014). However, we judged the risk of bias for one study to be unclear because data for baseline characteristics of participants for the two groups were not separated and so it was not possible to assess if baseline imbalances existed across groups (Balasubramanian 2003).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Vitamin D or calcium for the treatment of nutritional rickets in children.
| Vitamin D or calcium for the treatment of nutritional rickets in children | ||||||
|
Patients: children with nutritional rickets Settings: outpatients Intervention: calcium Comparison: vitamin D | ||||||
| Outcomes | Vitamin D | Calcium | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Healing of rickets (number) Definition: normal alkaline phosphatase and bone radiograph Follow‐up: 24 weeks |
189 per 1000 | 617 per 1000 (301 to 1266) | RR 3.26 (1.59 to 6.69) | 71 (1) | ⊕⊕⊝⊝ Lowa | — |
|
Morbidity (number) Definition: fractures Follow‐up: 24 weeks |
108 per 1000 | 29 per 1000 (3 to 251) | RR 0.27 (0.03 to 2.32) | 71 (1) | ⊕⊝⊝⊝ Very lowb | — |
| Adverse events (number) | Not reported | — | ||||
| All‐cause mortality | Not reported | — | ||||
| Health‐related quality of life | Not reported | — | ||||
| Growth pattern | Not reported at time point stipulated in protocol (≥ 1 years after commencement of therapy) | — | ||||
| Socioeconomic effects | Not reported | — | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*Assumed risk was derived from the event rates in the comparator groups. aDowngraded two levels because of serious imprecision (small number of participants, one study only), see Appendix 14. bDowngraded three levels because of very serious imprecision (small number of participants, one study only, and CI consistent with benefit and harm), see Appendix 14.
Summary of findings 2. Vitamin D plus calcium versus vitamin D for the treatment of nutritional rickets in children.
| Vitamin D plus calcium versus vitamin D for the treatment of nutritional rickets in children | ||||||
|
Patients: children with nutritional rickets Settings: outpatients Intervention: vitamin D + calcium Comparison: vitamin D | ||||||
| Outcomes | Vitamin D | Vitamin D + calcium | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Healing of rickets (number) Definition: normal alkaline phosphatase and bone radiograph Follow‐up: 24 weeks |
189 per 1000 | 579 per 1000 (282 to 1190) | RR 3.06 (1.49 to 6.29) | 75 (1) | ⊕⊕⊝⊝ Lowa | — |
|
Morbidity (number) Definition: fractures Follow‐up: 24 weeks |
108 per 1000 | 26 per 1000 (3 to 225) | RR 0.24 (0.03 to 2.08) | 75 (1) | ⊕⊝⊝⊝ Very lowb | — |
|
Adverse events (number) Definition: asymptomatic hypercalcaemia and hypercalciuria Follow‐up: 12 weeks |
See comment | RR 4.76 (0.24 to 93.19) | 39 (1) | ⊕⊝⊝⊝ Very lowc | 2/20 children in the vitamin D + calcium group vs 0/19 children in the vitamin D alone group had an adverse event. | |
| All‐cause mortality | Not reported | — | ||||
| Health‐related quality of life | Not reported | — | ||||
| Growth pattern | Not reported at time point stipulated in protocol (≥ 1 years after commencement of therapy) | — | ||||
| Socioeconomic effects | Not reported | — | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*Assumed risk was derived from the event rates in the comparator groups. aDowngraded two levels because of serious imprecision (small number of participants, one study only), see Appendix 15. bDowngraded three levels because of very serious imprecision (small number of participants, one study only, and CI consistent with benefit and harm), see Appendix 15. cDowngraded three levels because of risk of bias (performance bias and attrition bias) and very serious imprecision (small number of participants, one study only, and CI consistent with benefit and harm), see Appendix 15.
Summary of findings 3. Vitamin D plus calcium versus calcium for the treatment of nutritional rickets in children.
| Vitamin D plus calcium versus calcium for the treatment of nutritional rickets in children | ||||||
|
Patients: children with nutritional rickets Settings: outpatients Intervention: vitamin D + calcium Comparison: calcium | ||||||
| Outcomes | Calcium | Vitamin D + calcium | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|
Healing of rickets (number) Definition: normal alkaline phosphatase and bone radiograph Follow‐up: 24 weeks |
542 per 1000 | 635 per 1000 (391 to 1031) | RR 1.17 (0.72 to 1.90) | 140 (2) | ⊕⊝⊝⊝ Very lowa | — |
|
Morbidity (number) Definition: fractures Follow‐up: 24 weeks |
See comment | RR 0.89 (0.06 to 13.76) | 72 (1) | ⊕⊝⊝⊝ Very lowb | 1/38 children had a fracture in the vitamin D + calcium group vs 1/34 children in the calcium alone group. | |
|
Adverse events (number) Asymptomatic hypercalcaemia and hypercalciuria Follow‐up: 12 weeks |
See comment | RR 4.29 (0.22 to 83.57) | 37 (1) | ⊕⊝⊝⊝ Very lowc | 2/20 children in the calcium + vitamin D group compared to 0/17 children in the calcium alone group had an adverse event. | |
| All‐cause mortality | Not reported | — | ||||
| Health‐related quality of life | Not reported | — | ||||
| Growth pattern | Not reported at time point stipulated in protocol (≥ 1 years after commencement of therapy) | — | ||||
| Socioeconomic effects | Not reported | — | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
*Assumed risk was derived from the event rates in the comparator groups. aDowngraded one level because of risk of bias (selective reporting) and by two levels because of serious imprecision (small number of studies and CI consistent with benefit and harm), see Appendix 16. bDowngraded three levels because of very serious imprecision (small number of participants, one study only, and CI consistent with benefit and harm), see Appendix 16. cDowngraded by three levels because of risk of bias (performance bias and potential reporting bias) and very serious imprecision (small number of participants, one study only, and CI consistent with benefit and harm), see Appendix 16.
Baseline characteristics
For details of baseline characteristics, see Appendix 5 and Appendix 6.
Vitamin D versus calcium
Two studies compared vitamin D to calcium (Aggarwal 2013; Thacher 1999). Thacher 1999 compared vitamin D plus placebo to calcium plus placebo while Aggarwal 2013 compared vitamin D alone to calcium alone.
Primary outcomes
Healing of rickets
Normal alkaline phosphatase and bone radiograph
Aggarwal 2013 reported healing of rickets based on normal ALP and bone radiograph at 12 weeks and Thacher 1999 reported normal ALP and bone radiograph at 24 weeks. At 12 weeks, 2/17 children were healed in the calcium group compared to 3/19 in the vitamin D group. There was no clear difference in the proportion of children healed of rickets between the two groups at 12 weeks (RR 0.75, 95% CI 0.14 to 3.94; P = 0.73; 1 study, 36 participants; very low‐certainty evidence; Analysis 1.1). At 24 weeks, the proportion of participants who were healed of rickets was higher in the calcium group compared to the vitamin D group (RR 3.26, 95% CI 1.59 to 6.69; P = 0.001; 1 study, 71 participants; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.
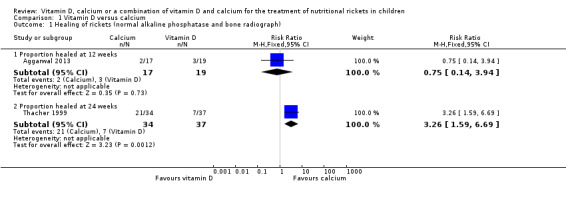
Comparison 1 Vitamin D versus calcium, Outcome 1 Healing of rickets (normal alkaline phosphatase and bone radiograph).
Serum alkaline phosphatase
Two studies assessed SAP (Aggarwal 2013; Thacher 1999). Both studies assessed SAP at 12 weeks but only one study assessed SAP at 24 weeks (Thacher 1999). At 12 weeks, there was no clear difference in the SAP of participants in the vitamin D and calcium groups (MD –37 U/L, 95% CI –129 to 56; P = 0.44; 2 studies, 107 participants; very low‐certainty evidence; Analysis 1.2), but at 24 weeks, the MD in SAP was –148 U/L in favour of calcium (95% CI –241 to –55; P = 0.002; 1 study, 71 participants; low‐certainty evidence).
1.2. Analysis.

Comparison 1 Vitamin D versus calcium, Outcome 2 Healing of rickets (biochemical parameters). serum alkaline phosphatase.
Serum 25‐hydroxy vitamin D
Two studies measured serum 25‐OHD at 12 weeks (Aggarwal 2013; Thacher 1999) and one study at 24 weeks (Thacher 1999). At 12 weeks, comparing vitamin D with calcium showed an MD in serum 25‐OHD of –8.5 ng/mL in favour of vitamin D (95% CI –13.9 to –3.0; P = 0.002; 2 studies, 107 participants; low‐certainty evidence; Analysis 1.3). At 24 weeks, vitamin D also improved serum 25‐OHD levels compared to calcium (MD –14.0 ng/mL, 95% CI –20.3 to –7.7; P < 0.001; 1 study, 71 participants; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1 Vitamin D versus calcium, Outcome 3 Healing of rickets (biochemical parameters): serum 25‐OHD.
Radiological score
At 12 and 24 weeks, there was no clear difference in the mean radiological score (Thacher's 10‐point scale) for participants in the vitamin D group when compared to the calcium group (at 12 weeks: MD 0.4, 95% CI –1.2 to 2.0; P = 0.60; 2 studies, 107 participants; very low‐certainty evidence; Analysis 1.4; at 24 weeks: MD –0.5, 95% CI –1.1 to 0.1; P = 0.10; 1 study, 71 participants; very low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1 Vitamin D versus calcium, Outcome 4 Healing of rickets (radiological).
Morbidity
Thacher 1999 reported on morbidity with regard to fractures. A comparison of vitamin D versus calcium showed no clear difference between the proportion of participants who had fractures in the two groups (RR 0.27, 95% CI 0.03 to 2.32; P = 0.23; 1 study, 71 participants, very low‐certainty evidence; Analysis 1.5). At 24 weeks, 1/34 children had a fracture in the calcium group compared to 4/37 children in the vitamin D group.
1.5. Analysis.

Comparison 1 Vitamin D versus calcium, Outcome 5 Morbidity (fractures).
Adverse events
Neither study reported adverse events.
Secondary outcomes
All‐cause mortality
Neither study reported all‐cause mortality.
Health‐related quality of life
Neither studies reported health‐related quality of life.
Growth pattern
Neither study reported growth pattern at the time point stipulated in our protocol (one or more years after commencement of therapy).
Socioeconomic effects
Neither study reported socioeconomic effects.
Vitamin D plus calcium versus vitamin D
Two studies compared vitamin D plus calcium versus vitamin D (Aggarwal 2013; Thacher 1999).
Primary outcomes
Healing of rickets
Normal alkaline phosphatase and bone radiograph
Two studies reported healing of rickets based on normal ALP and bone radiograph at 12 weeks (Aggarwal 2013), and at 24 weeks (Thacher 1999). At 12 weeks, there was a greater number of participants with healing of rickets in the vitamin D plus calcium group compared to vitamin D alone (RR 3.17, 95% CI 1.03 to 9.77; P = 0.05; 1 study, 39 participants; very low‐quality evidence; Analysis 2.1). At 24 weeks, there was also a greater number of participants with healing of rickets in the vitamin D plus calcium group compared to vitamin D alone (RR 3.06, 95% CI 1.49 to 6.29; P = 0.002; 1 study, 75 participants; low‐certainty evidence; Analysis 2.1). At 24 weeks, 22/38 children were healed in the vitamin D plus calcium group compared to 7/37 children in the vitamin D alone group.
2.1. Analysis.
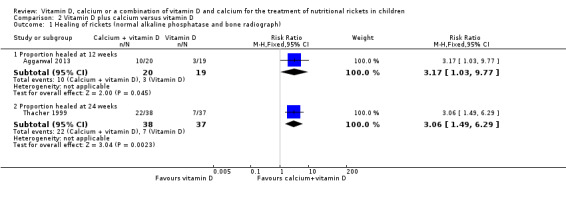
Comparison 2 Vitamin D plus calcium versus vitamin D, Outcome 1 Healing of rickets (normal alkaline phosphatase and bone radiograph).
Serum alkaline phosphatase
Aggarwal 2013 and Thacher 1999 reported SAP levels at 12 weeks, while only Thacher 1999 reported SAP at 24 weeks. At 12 weeks and 24 weeks, vitamin D plus calcium improved SAP compared with vitamin D (at 12 weeks: MD –157 U/L, 95% CI –245 to –68; P < 0.001; 2 studies, 114 participants; low‐certainty evidence; Analysis 2.2; at 24 weeks: MD –155 U/L, 95% CI –243 to –67; P < 0.001; 1 study, 75 participants; low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2 Vitamin D plus calcium versus vitamin D, Outcome 2 Healing of rickets (biochemical parameters): serum alkaline phosphatase.
Serum 25‐hydroxy vitamin D
A comparison of serum 25‐OHD levels for vitamin D plus calcium versus vitamin D at showed no clear difference between the two groups at 12 weeks or 24 weeks (at 12 weeks: MD 7.0 ng/mL, 95% CI –3.0 to 17.1; P = 0.17, 2 studies, 114 participants; very low‐certainty evidence; Analysis 2.3; at 24 weeks: MD 6.0 ng/mL, 95% CI –1.5 to 13.5; P = 0.12; 1 study, 75 participants; very low‐certainty evidence; Analysis 2.3).
2.3. Analysis.
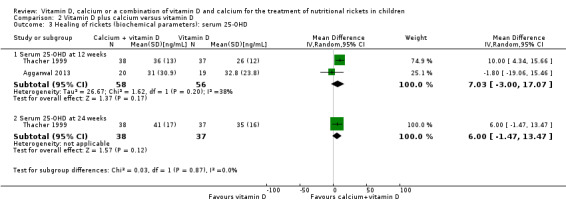
Comparison 2 Vitamin D plus calcium versus vitamin D, Outcome 3 Healing of rickets (biochemical parameters): serum 25‐OHD.
Radiological score
Aggarwal 2013 and Thacher 1999 reported radiological score (Thacher's 10‐point scale) at 12 weeks, but only Thacher 1999 reported the score at 24 weeks. At 12 and 24 weeks, the comparison favoured vitamin D plus calcium (at 12 weeks: MD –0.7, 95% CI –1.1 to –0.4; P < 0.001; 2 studies, 114 participants; low‐certainty evidence; Analysis 2.4; at 24 weeks: MD –1.0, 95% CI –1.6 to –0.4, P < 0.001; 1 study, 75 participants; low‐certainty evidence; Analysis 2.4).
2.4. Analysis.
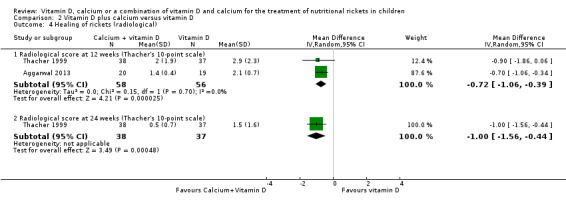
Comparison 2 Vitamin D plus calcium versus vitamin D, Outcome 4 Healing of rickets (radiological).
Morbidity
Only Thacher 1999 reported the number of participants with fractures. There was no clear difference between the proportion of participants with fractures in the vitamin D plus calcium group compared to the vitamin D group (RR 0.24, 95% CI 0.03 to 2.08; P = 0.20; 1 study, 75 participants; very low‐certainty evidence; Analysis 2.5). At 24 weeks, 1/38 children had a fracture in the vitamin D plus calcium group compared to 4/37 children in the vitamin D alone group.
2.5. Analysis.
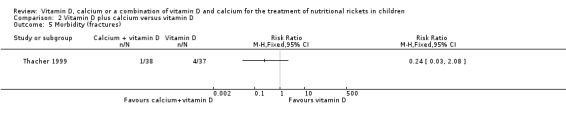
Comparison 2 Vitamin D plus calcium versus vitamin D, Outcome 5 Morbidity (fractures).
Adverse events
Aggarwal 2013 reported adverse events, namely asymptomatic hypercalcaemia and hypercalciuria. There was no clear difference in the number of adverse events in the two groups: 2/20 participants in the vitamin D plus calcium group compared to 0/19 participants in the vitamin D group had an adverse event (RR 4.76, 95% CI 0.24 to 93.19; P = 0.30; 1 study, 39 participants; very low‐certainty evidence; Analysis 2.6).
2.6. Analysis.

Comparison 2 Vitamin D plus calcium versus vitamin D, Outcome 6 Adverse events.
Secondary outcomes
All‐cause mortality
Neither study reported all‐cause mortality.
Health‐related quality of life
Neither study reported health‐related quality of life.
Growth pattern
Neither study reported growth pattern at the time point stipulated in our protocol (one or more years after commencement of therapy).
Socioeconomic effects
Neither study reported socioeconomic effects.
Vitamin D plus calcium versus calcium
Three studies compared vitamin D plus calcium versus calcium (Aggarwal 2013; Balasubramanian 2003; Thacher 2014).
Primary outcomes
Healing of rickets
Normal alkaline phosphatase and bone radiograph
Three studies measured the proportion of participants with normal ALP and bone radiograph at 12 weeks (Aggarwal 2013; Balasubramanian 2003; Thacher 2014). At 12 weeks, comparing the proportion of participants healed of rickets in the vitamin D plus calcium group with the calcium alone group showed a RR of 2.81 (95% CI 0.25 to 31.22; P = 0.40; 3 studies, 113 participants; very low‐quality evidence; Analysis 3.1). There was a high level of heterogeneity between the three studies. After elimination of Balasubramanian 2003, where all children in both the intervention and comparator group (4 in each group) experienced healing, the RR was 4.85 (95% CI 1.57 to 15.01) in favour of vitamin D plus calcium.
3.1. Analysis.

Comparison 3 Vitamin D plus calcium versus calcium, Outcome 1 Healing of rickets (normal alkaline phosphatase and bone radiograph).
Two studies measured the proportion of participants with normal ALP and bone radiograph at 24 weeks (Thacher 1999; Thacher 2014). At 24 weeks, the proportion of children healed of rickets did not differ between the vitamin D plus calcium group and the calcium group (RR 1.17, 95% CI 0.72 to 1.90; P = 0.53; 2 studies, 140 participants; very low‐certainty evidence, Analysis 3.1). At 24 weeks, 51/81 children were healed in the vitamin D plus calcium group compared to 32/59 children in the calcium alone group.
Serum alkaline phosphatase
Three studies assessed SAP levels at 12 weeks (Aggarwal 2013; Thacher 1999; Thacher 2014), and two studies at 24 weeks (Thacher 1999; Thacher 2014).
At 12 weeks, the participants who received vitamin D plus calcium had better SAP levels than those who received calcium alone (MD –110 U/L, 95% CI –183 to –36; P = 0.003; 3 studies, 177 participants; low‐certainty evidence; Analysis 3.2). At 24 weeks, however, there was no clear difference in SAP (MD –8 U/L, 95% CI –73 to 58; P = 0.82; 2 studies, 140 participants; very low‐certainty evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3 Vitamin D plus calcium versus calcium, Outcome 2 Healing of rickets (biochemical parameters): serum alkaline phosphatase.
Serum 25‐hydroxy vitamin D
Three studies measured serum 25‐OHD levels. Aggarwal 2013 measured serum 25‐OHD at 12 weeks, while Thacher 1999 and Thacher 2014 measured serum 25‐OHD levels at 12 weeks and 24 weeks.
At 12 weeks, there was no clear difference in serum 25‐OHD for children in the vitamin D plus calcium group compared to children in the calcium group (MD 10.4 ng/mL, 95% CI –0.8 to 21.7; P = 0.07; 3 studies, 177 participants; very low‐certainty evidence; Analysis 3.3). At 24 weeks, a comparison of serum 25‐OHD for vitamin D plus calcium versus calcium favoured calcium (MD 13.2 ng/mL, 95% CI 0.5 to 25.9; P = 0.04; 2 studies, 140 participants; low‐certainty evidence; Analysis 3.3).
3.3. Analysis.
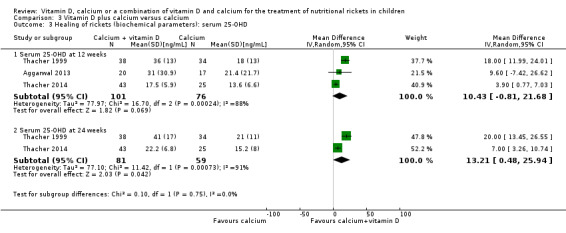
Comparison 3 Vitamin D plus calcium versus calcium, Outcome 3 Healing of rickets (biochemical parameters): serum 25‐OHD.
Radiological score
A comparison of vitamin D plus calcium with calcium alone at 12 and 24 weeks favoured vitamin D plus calcium (at 12 weeks: MD in radiological score (Thacher's 10‐point scale) –1.3, 95% CI –2.2 to –0.4; P = 0.004; 3 studies, 177 participants; low‐certainty evidence; Analysis 3.4; at 24 weeks: MD –0.6, 95% CI –0.9 to –0.2; P = 0.002; 2 studies, 140 participants; low‐certainty evidence; Analysis 3.4).
3.4. Analysis.

Comparison 3 Vitamin D plus calcium versus calcium, Outcome 4 Healing of rickets (radiological).
Morbidity
Only Thacher 1999 reported the number of participants with fractures. There was no clear difference in the number of fractures in the two groups (RR 0.89, 95% CI 0.06 to 13.76; P = 0.94; 1 study, 72 participants; very low‐quality evidence; Analysis 3.5). At 24 weeks, 1/38 children had a fracture in the vitamin D plus calcium group compared to 1/34 children in the calcium alone group.
3.5. Analysis.
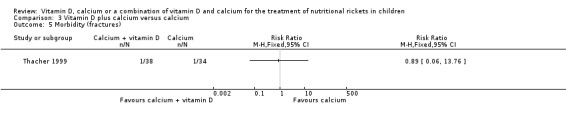
Comparison 3 Vitamin D plus calcium versus calcium, Outcome 5 Morbidity (fractures).
Adverse events
Only Aggarwal 2013 assessed adverse events. There was no clear difference in the risk of adverse events comparing vitamin D plus calcium to calcium alone (RR 4.29, 95% CI 0.22 to 83.57; P = 0.34; 1 study, 37 participants; very low‐quality evidence; Analysis 3.6). There was asymptomatic hypercalcaemia and hypercalciuria at 12 weeks in 2/20 children in the calcium plus vitamin D group compared to 0/17 children in the calcium alone group.
3.6. Analysis.
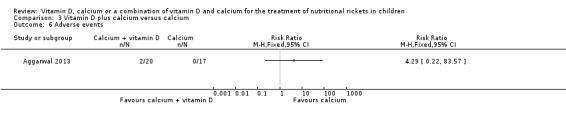
Comparison 3 Vitamin D plus calcium versus calcium, Outcome 6 Adverse events.
Secondary outcomes
All‐cause mortality
None of the studies reported all‐cause mortality.
Health‐related quality of life
None of the studies reported health‐related quality of life.
Growth pattern
None of the studies reported growth pattern at the time point stipulated in our protocol (one or more years after commencement of therapy).
Socioeconomic effects
None of the studies reported socioeconomic effects.
Subgroup analyses
We did not perform subgroups analyses because there were not enough studies to estimate effects in various subgroups and for some studies data were not presented in a manner that allowed for subgroup analyses.
Sensitivity analyses
We did not perform sensitivity analyses because of the low number of studies included in the review.
Assessment of reporting bias
We did not draw funnel plots due to limited number of studies.
Discussion
Summary of main results
This systematic review assessed the effects of vitamin D, calcium or a combination of vitamin D and calcium in children aged six months to 14 years with nutritional rickets. We found four studies with 286 randomised participants. The studies reported on healing of rickets, morbidity (fractures) and adverse events (non‐symptomatic hypercalcaemia and hypercalciuria). None of the studies reported all‐cause mortality or other patient‐important outcomes (health‐related quality of life and socioeconomic effects). One study reported on growth pattern (Thacher 1999), but this was not measured at time points specified in our protocol (one or more years after commencement of therapy) (Chibuzor 2017).
A summary of the main results for the outcomes reported by study authors is as follows.
In comparison to vitamin D, administering calcium to children with rickets may improve the healing of rickets (normal ALP and bone radiograph) (low‐certainty evidence).
We are uncertain whether calcium reduces the risk of fractures compared to vitamin D (very low‐certainty evidence).
Vitamin D plus calcium may lead to improvement in the healing of rickets compared to vitamin D (normal ALP and bone radiograph) (low‐certainty evidence).
We are uncertain whether vitamin D plus calcium reduces the risk of fractures compared to vitamin D (very low‐certainty evidence).
We are uncertain whether vitamin D plus calcium reduces the risk of adverse events compared to vitamin D (very low‐certainty evidence).
We are uncertain whether vitamin D plus calcium improves healing of rickets compared to calcium (very low‐certainty evidence).
We are uncertain whether vitamin D plus calcium reduces the risk of fractures compared to calcium (very low‐certainty evidence).
We are uncertain whether vitamin D plus calcium reduces the risk of adverse events compared with calcium (very low‐certainty evidence).
Overall completeness and applicability of evidence
We carried out an extensive search of biomedical databases to identify all relevant studies. We also searched the reference lists of all included studies but did not identify any additional studies from this source. The search identified four RCTs for inclusion in the review, but these studies had low number of participants. For additional information on baseline data, risk of bias domains, and outcomes, we contacted authors. Three study authors provided additional data. All the studies included participants of interest and compared vitamin D versus calcium, or vitamin D plus calcium versus calcium or vitamin D plus calcium versus calcium. The outcomes assessed by the studies were limited. Only healing of rickets, morbidity (fractures) and adverse events were assessed as indicated in our protocol by the study authors. Although growth pattern was measured and reported by one study (Thacher 1999), it was not measured at the time point stipulated in our protocol (one or more years after commencement of therapy).
All the studies were carried out in low‐ and middle‐income countries where rickets is mostly found; as such the findings of the review are applicable to people from regions most affected by rickets.
Quality of the evidence
The body of evidence provided by the four studies included in the review does not allow us to make a robust conclusion on whether vitamin D, calcium or a combination of vitamin D and calcium is most effective for the treatment of nutritional rickets in children aged 0 to 18 years. We rated the certainty of the evidence to be low to very low for all outcomes according to GRADE.
For vitamin D versus calcium, the certainty of the evidence was based on one RCT for two outcomes measured. We downgraded the certainty of the evidence for healing of rickets to low because of serious imprecision (small number of studies and sample size). We also downgraded the certainty of the evidence for morbidity to very low because of very serious imprecision (small number of studies, small sample size and CI consistent with benefit and harm).
For vitamin D plus calcium versus vitamin D, studies measured three outcomes: healing of rickets, morbidity and adverse events. The certainty of the evidence for healing of rickets and morbidity was based on one RCT (Thacher 1999), while the certainty of evidence for adverse events was based on one RCT (Aggarwal 2013). We considered the certainty of the evidence for healing of rickets to be low because of serious imprecision (small number of studies and sample size) and the certainty of the evidence for morbidity to be very low because of very serious imprecision (small number of studies, small sample size and CI consistent with benefit and harm). For adverse events, we rated the certainty of the evidence to be very low because of risk of bias (performance and attrition bias) and very serious imprecision (small number of studies, small sample size and CI consistent with benefit and harm).
The third comparison was vitamin D plus calcium versus calcium. Three studies provided evidence. Two studies provided evidence for healing of rickets (Thacher 1999; Thacher 2014), one study provided evidence for morbidity (Thacher 1999), and one study provided evidence for adverse events (Aggarwal 2013). We downgraded the certainty of the evidence for healing of rickets to very low because of risk of bias (selective reporting) and serious imprecision (small number of studies and CI consistent with benefit and harm). For morbidity, we considered the certainty of the evidence to be very low because of very serious imprecision (small number of studies, small sample size and CI consistent with benefit and harm). For adverse events, we downgraded the evidence to very low because of the risk of bias (performance bias and potential reporting bias) and very serious imprecision (small number of studies, small sample size and CI consistent with benefit and harm).
Potential biases in the review process
We made considerable effort to avoid bias in the review process. As such, we conducted a comprehensive search of relevant databases to identify studies for inclusion in the review and did not apply any language or date restrictions to the search. In addition, we adhered to the protocol (Chibuzor 2017): two review authors screened all abstracts identified by the search and assessed the risk of bias for the included studies. We rated the certainty of the evidence using GRADE methodology. There was some heterogeneity between the included studies but we could not explore the reasons for the heterogeneity using a funnel plot due to the small number of included studies.
Agreements and disagreements with other studies or reviews
The Global Consensus Recommendations on Prevention and Management of Nutritional Rickets recommends a combination of vitamin D and calcium for the treatment of nutritional rickets in children (Munns 2016). In agreement with this recommendation, our review found low‐certainty evidence that vitamin D plus calcium may lead to improvement in the healing of rickets compared to vitamin D alone. However, we are uncertain based on the findings of our review, whether vitamin D plus calcium improves healing or rickets compared to calcium alone. We are also uncertain whether vitamin D plus calcium reduces the risk of adverse events or morbidity when compared to vitamin D alone or calcium alone. We did not find any other published systematic review on vitamin D, calcium or a combination of vitamin D and calcium for treatment of nutritional rickets in children.
Authors' conclusions
Implications for practice.
Low‐certainty evidence showed that vitamin D plus calcium instead of vitamin D alone may lead to improvement in the healing of rickets. In comparison to vitamin D alone, administering calcium alone to children with rickets may improve the healing of rickets (low‐certainty evidence). We did not find sufficient evidence to support or discourage the current practice of vitamin D plus calcium for the treatment of nutritional rickets.
Implications for research.
We found only four studies with small sample sizes for inclusion in this review. The studies were conducted in lower‐ or middle‐income countries. Most of the studies did not measure any of the secondary outcomes including patient‐important outcomes. Although one study measured growth pattern, this outcome was measured at 24 weeks which was too early to determine the impact of the intervention on growth. The risk of bias in some of the included studies and imprecision affected our ability to make firm conclusions on the certainty of the evidence.
More large, well‐conducted randomised controlled trials conducted in lower‐, middle‐ and high‐income countries and which assess the interventions in the review are needed. Such studies should measure all‐cause mortality, growth pattern, health‐related quality of life and socioeconomic effects in addition to the primary outcomes to enable us make firm conclusions about the effects of the interventions for treatment of nutritional rickets in children. Studies should have a longer follow‐up period to enable the researchers to measure outcomes such as growth pattern at time points which can provide meaningful evidence for clinical decision making.
Notes
Portions of the background and methods sections, the appendices, additional tables and figures of this review are based on a standard template established by the Cochrane Metabolic and Endocrine Disorders Group.
Acknowledgements
The review authors thank the Cochrane Metabolic and Endocrine Disorders (CMED) Group's Information Specialist (Maria‐Inti Metzendorf) for the development of the search strategy. The review authors would like to thank the CMED Group for their support in the development of the review.
The review authors and the CMED editorial base are grateful to the peer reviewer Christian Lerch, Paediatrician, Garbsen, Germany, for his time and comments.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (Cochrane Register of Studies Online) |
|
Part 1: Condition 1. MESH DESCRIPTOR Rickets 2. rickets:TI,AB,KY 3. (rachitis or rachitides):TI,AB,KY 4. MESH DESCRIPTOR Vitamin D Deficiency 5. vitamin d defic*:TI,AB,KY 6. #1 OR #2 OR #3 OR #4 OR #5 Part 2: Intervention 7. MESH DESCRIPTOR Vitamin D EXPLODE ALL TREES 8. vitamin d:TI,AB,KY 9. vitamin d?:TI,AB,KY 10. cholecalciferol*:TI,AB,KY 11. calciol:TI,AB,KY 12. calcifediol:TI,AB,KY 13. hydroxycholecalciferol*:TI,AB,KY 14. dihydroxycholecalciferol*:TI,AB,KY 15. calciferol*:TI,AB,KY 16. hydroxyvitamin d:TI,AB,KY 17. hydroxyvitamin d?:TI,AB,KY 18. dihydrotachysterol*:TI,AB,KY 19. MESH DESCRIPTOR Calcium 20. MESH DESCRIPTOR Calcium, Dietary 21. MESH DESCRIPTOR Calcium Carbonate 22. MESH DESCRIPTOR Calcium Gluconate 23. calcium:TI,AB,KY 24. #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 Part 3: Population [adapted fromLeclercq 2013] 25. MESH DESCRIPTOR Adolescent 26. MESH DESCRIPTOR Child EXPLODE ALL TREES 27. MESH DESCRIPTOR Infant 28. MESH DESCRIPTOR Pediatrics 29. minors:TI,AB,KY 30. (boy or boys or boyhood):TI,AB,KY 31. girl*:TI,AB,KY 32. infant*:TI,AB,KY 33. (baby or babies):TI,AB,KY 34. toddler?:TI,AB,KY 35. (kid or kids):TI,AB,KY 36. (child or childs or children* or childhood* or childcare* or schoolchild*):TI,AB,KY 37. adolescen*:TI,AB,KY 38. juvenil*:TI,AB,KY 39. youth*:TI,AB,KY 40. (teen* or preteen*):TI,AB,KY 41. (underage* or under age*):TI,AB,KY 42. pubescen*:TI,AB,KY 43. p?ediatric*:TI,AB,KY 44. #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 Part 4: Condition + Intervention + Population 45. #6 AND #24 46. #45 AND #44 |
| MEDLINE (OvidSP) |
|
Part 1: Condition 1. Rickets/ 2. rickets.tw. 3. (rachitis or rachitides).tw. 4. Vitamin D Deficiency/ 5. vitamin d defic*.tw. 6. or/1‐5 Part 2: Intervention 7. exp Vitamin D/ 8. vitamin d.tw. 9. vitamin d?.tw. 10. cholecalciferol*.tw. 11. calciol.tw. 12. calcifediol.tw. 13. hydroxycholecalciferol*.tw. 14. dihydroxycholecalciferol*.tw. 15. calciferol*.tw. 16. hydroxyvitamin d.tw. 17. hydroxyvitamin d?.tw. 18. dihydrotachysterol*.tw. 19. Calcium/ 20. Calcium, Dietary/ 21. Calcium Carbonate/ 22. Calcium Gluconate/ 23. calcium.tw. 24. or/7‐23 Part 3: Population [adapted fromLeclercq 2013] 25. Adolescent/ 26. exp Child/ 27. Infant/ 28. Pediatrics/ 29. minors.tw. 30. (boy or boys or boyhood).tw. 31. girl*.tw. 32. infant*.tw. 33. (baby or babies).tw. 34. toddler?.tw. 35. (kid or kids).tw. 36. (child or childs or children* or childhood* or childcare* or schoolchild*).tw. 37. adolescen*.tw. 38. juvenil*.tw. 39. youth*.tw. 40. (teen* or preteen*).tw. 41. (underage* or under age*).tw. 42. pubescen*.tw. 43. p?ediatric*.tw. 44. or/25‐43 Part 4: Condition + Intervention + Population 45. 6 and 24 46. 45 and 44 Part 5: Cochrane Handbook 2008 RCT filter –sensitivity max. version] 47. randomized controlled trial.pt. 48. controlled clinical trial.pt. 49. randomi?ed.ab. 50. placebo.ab. 51. drug therapy.fs. 52. randomly.ab. 53. trial.ab. 54. groups.ab. 55. or/47‐54 56. exp animals/ not humans/ 57. 55 not 56 Part 6: [Wong 2006– systematic reviews filter – SensSpec version] 58. meta analysis.mp,pt. or review.pt. or search*.tw. Part 7: Part 4 + part 5 + part 6 59. 46 and 57 60. 46 and 58 61. 59 or 60 62. ..dedup 61 |
| LILACS (iAHx) |
| ((MH:"Rickets" OR rickets$ OR rachitis OR rachitides OR raquit$ OR MH:"Vitamin D Deficiency" OR (("vitamina d" OR "vitamin d" "vitamina d3" OR "vitamin d3") AND defic$)) AND (MH:"Vitamin D" OR "vitamin d" OR "vitamin d3" OR "vitamina d" OR "vitamina d3" OR cholecalciferol$ OR colecalciferol$OR calciol OR calcifediol OR hydroxycholecalciferol$ OR hidroxicolecalciferol$ OR dihydroxycholecalciferol$ OR dihidroxicolecalciferol$ OR calciferol$ OR "hydroxyvitamin d" OR "hidroxivitamina d" OR "hydroxyvitamin d3" OR "hidroxivitamina d3" OR dihydrotachysterol$ OR MH:"Calcium" OR MH:"Calcium, Dietary" OR MH:"Calcium Carbonate" OR MH:"Calcium Gluconate" OR calcium OR calcio) AND (MH:"Adolescent" OR MH:"Child" OR MH:"Pediatrics" OR MH:"Infant" OR minors OR infant$ OR boy OR boys OR girl$ OR kid OR kids OR child OR childs OR children$ OR childhood$ OR childcare$ OR schoolchild$ OR escolar$ OR adolescen$ OR preadolescen$ OR teen$ OR preteen$ OR baby OR babies OR bebe OR bebes OR toddler$ OR juvenil$ OR juventud$ OR youth$ OR teen$ OR preteen$ OR underage$ OR pubescen$ OR pubert$ OR OR paediatri$ OR pediatri$ OR joven$ OR jovem$ OR niños OR niñas OR crianca$ OR menin$ OR garot$ OR "menor de edad" OR "menores de edad" OR "menor de idade" OR "menores de idade")) + Controlled Clinical Trial |
| ClinicalTrials.gov (Advanced search) |
|
Conditions: rickets OR rachitis OR rachitides OR "vitamin d deficiency" OR "vitamin d deficient" Interventions: "vitamin d" OR calcium OR "vitamin d2" OR "vitamin d3" OR cholecalciferol OR calciol OR calcifediol OR hydroxycholecalciferol OR dihydroxycholecalciferol OR calciferol OR "hydroxyvitamin d" OR "hydroxyvitamin d2" OR "hydroxyvitamin d3" OR dihydrotachysterol Age Group: Child (birth‐17) |
| ICTRP Search Portal (Standard search) |
| rickets* OR rachiti* OR vitamin* AND deficien* AND adolescen* OR vitamin* AND deficien* AND child* OR vitamin* AND deficien* AND pediatr* OR vitamin* AND deficien* AND paediatr* OR vitamin* AND deficien* AND infant* OR vitamin* AND deficien* AND teen* OR vitamin* AND deficien* AND boy* OR vitamin* AND deficien* AND girl* OR vitamin* AND deficien* AND schoolchild* OR vitamin* AND deficien* AND bab* |
Appendix 2. 'Risk of bias' assessment
| 'Risk of bias' domains |
|
Random sequence generation (selection bias due to inadequate generation of a randomised sequence) For each included trial, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment (selection bias due to inadequate concealment of allocation prior to assignment) We described for each included trial the method used to conceal allocation to interventions prior to assignment and we assessed whether intervention allocation could have been foreseen in advance of or during recruitment or changed after assignment.
We also evaluated trial baseline data to incorporate assessment of baseline imbalance into the 'Risk of bias' judgement for selection bias (Corbett 2014). Chance imbalances may also affect judgements on the risk of attrition bias. In the case of unadjusted analyses, we distinguished between trials that we rated at low risk of bias on the basis of both randomisation methods and baseline similarity, and trials that we judged at low risk of bias on the basis of baseline similarity alone (Corbett 2014). We reclassified judgements of unclear, low or high risk of selection bias as specified in Appendix 3. Blinding of participants and study personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the trial) We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment) We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Incomplete outcome data (attrition bias due to quantity, nature or handling of incomplete outcome data) For each included trial or each outcome, or both, we described the completeness of data, including attrition and exclusions from the analyses. We stated whether the trial reported attrition and exclusions, and we reported the number of participants included in the analysis at each stage (compared with the number of randomised participants per intervention/comparator group). We also noted if the trial reported the reasons for attrition or exclusion, and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial groups).
Selective reporting (reporting bias due to selective outcome reporting) We assessed outcome reporting bias by integrating the results of Appendix 7 'Matrix of trial endpoints (publications and trial documents)' (Boutron 2014; Jones 2015; Mathieu 2009), with those of Appendix 8 'High risk of outcome reporting bias according to the Outcome Reporting Bias In Trials (ORBIT) classification' (Kirkham 2010). This analysis formed the basis for the judgement of selective reporting.
Other bias
|
Appendix 3. Selection bias decisions
| Selection bias decisions for trials that reported unadjusted analyses: comparison of results obtained using method details alone versus results obtained using method details and trial baseline informationa | |||
| Reported randomisation and allocation concealment methods | 'Risk of bias' judgement using methods reporting | Information gained from study characteristics data | 'Risk of bias' using baseline information and methods reporting |
| Unclear methods | Unclear risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited or no baseline details | Unclear risk | ||
| Would generate a truly random sample, with robust allocation concealment | Low risk | Baseline imbalances present for important prognostic variable(s) | Unclear riskb |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Low risk | ||
| No baseline details | Unclear risk | ||
| Sequence is not truly randomised or allocation concealment is inadequate | High risk | Baseline imbalances present for important prognostic variable(s) | High risk |
| Groups appear similar at baseline for all important prognostic variables | Low risk | ||
| Limited baseline details, showing balance in some important prognostic variablesc | Unclear risk | ||
| No baseline details | High risk | ||
| aTaken from Corbett 2014; judgements highlighted in bold indicate situations in which the addition of baseline assessments would change the judgement about risk of selection bias compared with using methods reporting alone. bImbalance was identified that appears likely to be due to chance. cDetails for the remaining important prognostic variables are not reported. | |||
Appendix 4. Description of interventions
| Study ID | Intervention(s) (route, frequency, total dose/day) | Comparator(s) (route, frequency, total dose/day) |
| Thacher 2014 | Oral calcium 3.5 g powdered limestone (containing approximately 938 mg of elemental calcium) twice daily + oral vitamin D2 as 50,000 IU (ergocalciferol) once every 4 weeks for 24 weeks. | Oral calcium, 3.5 g powdered limestone (containing approximately 938 mg of elemental calcium) twice daily + placebo, which was a single vitamin B complex tablet, once every 4 weeks for 24 weeks. |
| Aggarwal 2013 | I1: vitamin D 600,000 IU single intramuscular injection and elemental calcium 75 mg/kg in 3 divided doses per day for 12 weeks. | Vitamin D 600,000 IU single intramuscular injection. |
| I2: elemental calcium 75 mg/kg orally in 3 divided doses per day for 12 weeks. | ||
| Balasubramanian 2003 | Calcium carbonate 1 g as 3 divided doses per day + vitamin D either as 6000 IU oral daily for 3 months or 600,000 IU in a single oral dose. | Calcium carbonate 1 g orally as in 3 divided doses per day for 3 months. |
| Thacher 1999 | I1: vitamin D 600,000 U intramuscular injection at enrolment and after 12 weeks) and calcium carbonate 200 mg tablets – 2 tablets in morning and 3 in evening ≥ 30 minutes before eating (total dose, 1000 mg of elemental calcium daily). | Vitamin D 600,000 U intramuscular injection at enrolment and after 12 weeks and chewable placebo tablets (candy containing no calcium but similar in appearance to calcium tablets) 2 in the morning and 3 in the evening ≥ 30 minutes before eating. |
| I2: calcium carbonate 200 mg chewable tablets – 2 tablets in the morning and 3 in the evening ≥ 30 minutes before eating (total dose, 1000 mg of elemental calcium daily) and an injection of placebo (light mineral oil) at enrolment and after 12 weeks. | ||
| I: intervention. | ||
Appendix 5. Baseline characteristics (I)
| Study ID | Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) | Description of participants | Study period (year to year) | Country | Setting | Ethnic groups (%) | Duration of rickets |
| Thacher 2014 | I: oral calcium + oral vitamin D | 24 weeks (24 weeks) | Children with active rickets aged 15–144 months | 2004–2007 | Nigeria | Tertiary teaching hospital, outpatients | African | 0.5–108 months |
| C: oral calcium + placebo | ||||||||
| Aggarwal 2013 | I1: oral calcium + single dose intramuscular vitamin D | 12 weeks (12 weeks) | Children aged 6 months to 5 years with nutritional rickets | 2007–2009 | India | Tertiary teaching hospital, outpatients | Asian | — |
| I2: oral calcium alone | ||||||||
| C: single dose intramuscular vitamin D alone | ||||||||
| Balasubramanian 2003 | I: oral calcium + oral vitamin D | 3 months (3 months) | Children aged 11 months to 10 years with nutritional rickets | — | India | Hospital facility, outpatients | Asian | — |
| C: oral calcium alone | ||||||||
| Thacher 1999 | I1: oral calcium + intramuscular vitamin D | 24 weeks (24 weeks) | Children aged 1–14 years | 1996–1997 | Nigeria | Tertiary teaching hospital, outpatients | African | — |
| I2: oral calcium + placebo | ||||||||
| C: intramuscular vitamin D + placebo | ||||||||
| —: denotes not reported. C: comparator; I: intervention. | ||||||||
Appendix 6. Baseline characteristics (II)
| Study ID | Intervention(s) and comparator(s) | Sex (girls %) | Age (months, range) | Comedications/Cointerventions (% of participants) | Comorbidities (% of participants) |
| Thacher 2014 | I: calcium + vitamin D | 57 | 15–144 | — | — |
| C: calcium + placebo | 61 | 16–91 | |||
| Aggarwal 2013 | I1: calcium + vitamin D | 59 | 6–60 | — | — |
| I2: calcium alone | 41 | 6–60 | |||
| C: vitamin D | 43 | 6–58 | |||
| Balasubramanian 2003 | I: calcium + vitamin D | 54 | 11–120 | — | — |
| C: calcium | |||||
| Thacher 1999 | I1: calcium + vitamin D | Figure given for total group: 55 | Figure given for total group: 12–168 | — | — |
| I2: calcium + placebo | |||||
| C: vitamin D + placebo | |||||
| —: denotes not reported. C: comparator; I: intervention. | |||||
Appendix 7. Matrix of study endpoints (publications and trial documents)
| Study ID | Endpoints |
| Thacher 2014 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source:clinicaltrials.gov/ct2/show/NCT00949832 Primary outcome measure: XR healing of rickets (time frame: 6 months) Secondary outcome measures: alkaline phosphatase (time frame: 6 months), serum calcium (time frame: 6 months), 25‐hydroxy vitamin D (time frame: 6 months), 1,25‐dihydroxy vitamin D (time frame: 2 weeks), calcium absorption (time frame: 1 week) Other outcome measures: — Trial results available in trial register: no | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measure: combined attainment of a radiographic score of ≤ 1.5 and a serum alkaline phosphatase concentration of ≤ 350 U/L Secondary outcome measure: — Other outcome measures: 25‐hydroxy vitamin D, serum calcium | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measures: main outcome measure achievement of a 10‐point radiographic severity score ≤ 1.5 and serum alkaline phosphatase ≤ 350 U/L Secondary outcome measures: — Other outcome measure: 25‐hydroxy vitamin D | |
| Aggarwal 2013 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
|
Source: CTRI/2010/091/000448 (www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=1634&EncHid=&modid=&compid=%27,%271634det%27) Primary outcome measures: percentage of participants showing complete radiological and biochemical evidence of healing of rickets Secondary outcome measures: — Other outcome measures: — Trial results available in trial register: no | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measures: improvement in radiological score and biochemical parameters of healing of rickets at 12 weeks Secondary outcome measures: — Other outcome measure: adverse events | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measures: — Secondary outcome measures: — Other outcome measures: mean serum 25‐hydroxycholecalciferol D levels, radiological and biochemical evidence of healing rickets, combined endpoint of normal serum alkaline phosphatase and complete radiological healing at 12 weeks | |
| Balasubramanian 2003 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measures: — Secondary outcome measures: — Other outcome measure: healing of rickets | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measures: — Secondary outcome measures: — Other outcome measures: healing of rickets, 25 hydroxy vitamin D | |
| Thacher 1999 | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a,c |
| Source: NT | |
| Endpoints quoted in publication(s)b,c | |
|
Primary outcome measures: changes in serum calcium and alkaline phosphatase concentrations and radiographic scores Secondary outcome measures: — Other outcome measures: fractures, height, height‐for‐age, weight, serum albumin, serum phosphorus, serum 1,25‐dihydroxy vitamin D concentrations; % of children with the combined outcome of a serum alkaline phosphatase concentration ≤ 350 U/L and radiographic score ≤ 1.5 (indicating nearly complete resolution of the abnormalities) after 24 weeks of treatment) | |
| Endpoints quoted in abstract of publication(s)b,c | |
|
Primary outcome measures: — Secondary outcome measures: — Other outcome measures: serum calcium concentration, combined endpoint of a serum alkaline phosphatase concentration ≤ 350 U/L and radiographic evidence of nearly complete healing of rickets | |
| —: denotes not reported.
aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers).
bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary study).
cPrimary and secondary outcomes refer to verbatim specifications in publication/records. Unspecified outcome measures refer to all outcomes not described as primary or secondary outcome measures. EMA: European Medicines Agency; FDA: Food and Drug Administration (US); NT: no trial document available. | |
Appendix 8. High risk of outcome reporting bias according to ORBIT classification
| Study ID | Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d |
| Thacher 2014 | Adverse events | Yes | No | No | No |
| Growth pattern | Yes | No | No | No | |
| Aggarwal 2013 | ND | ||||
| Balasubramanian 2003 | ND | ||||
| Thacher 1999 | ND | ||||
|
aClear that outcome was measured and analysed; study report states that outcome was analysed but reports only that result was not significant (Classification 'A', table 2, Kirkham 2010).
bClear that outcome was measured and analysed; study report states that outcome was analysed but report no results (Classification 'D', table 2, Kirkham 2010).
cClear that outcome was measured but was not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results (Classification 'E', table 2, Kirkham 2010).
dUnclear whether outcome was measured; not mentioned, but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results (Classification 'G', table 2, Kirkham 2010). ND: none detected; ORBIT: Outcome Reporting Bias In Trials. | |||||
Appendix 9. Definition of endpoint measurementa
| Study ID | Healing of rickets | Morbidity | All‐cause mortality | Health‐related quality of life | Growth pattern | Socioeconomic effects | Severe/serious adverse events |
| Thacher 2014 | Achievement of a 10‐point radiographic severity score ≤ 1.5 and serum alkaline phosphatase ≤ 350 U/L (IO) | NR | NR | NR | NR | NR | NR |
| Aggarwal 2013 | Normal serum alkaline phosphatase and radiological evidence of complete healing Achievement of a 10‐point radiographic severity score ≤ 1.5 (IO) | NR | NR | NR | NR | NR | Asymptomactic hypercalcaemia and hypercalciuria (I/O) |
| Balasubramanian 2003 | ND | NR | NR | NR | NR | NR | NR |
| Thacher 1999 | Serum alkaline phosphatase concentration ≤ 350 U/L and a radiographic score ≤ 1.5 (IO) | Fractures (IO) | NR | NR | Height, weight and height for age (z score) (IO) | NR | NR |
|
aIn addition to definition of endpoint measurement, description who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self‐reported outcome measurement). ND: not defined; NR: not reported. | |||||||
Appendix 10. Adverse events (I)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (n) | Deaths (n) | Deaths (% of participants) | Participants with ≥ 1 adverse event (n) | Participants with ≥ 1 adverse event (%) | Participants with ≥ 1 severe/serious adverse event (n) | Participants with ≥ 1 severe/serious adverse event (%) |
| Thacher 2014 | I: calcium + vitamin D | 43 | — | — | — | — | — | — |
| C: calcium + placebo | 25 | — | — | — | — | — | — | |
| Aggarwal 2013 | I1: calcium + vitamin D | 20 | — | — | 2 | 9 | — | — |
| I2: calcium | 17 | — | — | 0 | 0 | — | — | |
| C: vitamin D | 19 | — | — | 0 | 0 | — | — | |
| Balasubramanian 2003 | I: calcium + vitamin D | — | — | — | — | — | — | — |
| C: calcium | — | — | — | — | — | — | — | |
| Thacher 1999 | I1: calcium + vitamin D | 38 | — | — | — | — | — | — |
| I2: calcium + placebo | 34 | — | — | — | — | — | — | |
| C: vitamin D + placebo | 37 | — | — | — | — | — | — | |
| —: denotes not reported. C: comparator; I: intervention; n: number of participants. | ||||||||
Appendix 11. Adverse events (II)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (n) | Participants discontinuing study due to an adverse event (n) | Participants discontinuing study due to an adverse event (%) | Participants with ≥ 1 hospitalisation (n) | Participants with ≥ 1 hospitalisation (%) | Participants with ≥ 1 outpatient treatment (n) | Participants with ≥ 1 outpatient treatment (%) |
| Thacher 2014 | I: calcium + vitamin D | 43 | — | — | — | — | — | — |
| C: calcium + placebo | 25 | — | — | — | — | — | — | |
| Aggarwal 2013 | I1: calcium + vitamin D | 20 | — | — | — | — | — | — |
| I2: calcium | 17 | — | — | — | — | — | — | |
| C: vitamin D | 19 | — | — | — | — | — | ||
| Balasubramanian 2003 | I: calcium + vitamin D | 4 | — | — | — | — | — | — |
| C: calcium | 4 | — | — | — | — | — | — | |
| Thacher 1999 | I1: calcium + vitamin D | 38 | — | — | — | — | — | — |
| I2: calcium + placebo | 34 | — | — | — | — | — | — | |
| C: vitamin D + placebo | 37 | — | — | — | — | — | ||
| —: denotes not reported. C: comparator; I: intervention; n: number of participants. | ||||||||
Appendix 12. Adverse events (III)
| Study ID | Intervention(s) and comparator(s) | Participants included in analysis (n) | Participants with a specific adverse event (description) | Participants with ≥ 1 specific adverse events (n) | Participants with ≥ 1 specific adverse event (%) |
| Thacher 2014 | I: calcium + vitamin D | 43 | — | — | — |
| C: calcium + placebo | 25 | — | — | — | |
| Aggarwal 2013 | I1: calcium + vitamin D | 20 | (1) Hypercalcaemia (2) Hypercalciuria |
(1) 1 (2) 1 |
(1) 0.05 (2) 0.05 |
| I2: calcium | 17 | 0 | 0 | 0 | |
| C: vitamin D | 19 | 0 | 0 | 0 | |
| Balasubramanian 2003 | I: calcium + vitamin D | 4 | — | — | — |
| C: calcium | 4 | — | — | — | |
| Thacher 1999 | I1: calcium + vitamin D | 38 | — | — | — |
| I2: calcium + placebo | 34 | — | — | — | |
| C: vitamin D + placebo | 37 | — | — | — | |
| —: denotes not reported. C: comparator; I: intervention; n: number of participants. |
|||||
Appendix 13. Survey of study investigators providing information on included trials
| Study ID | Date study author contacted | Date study author replied | Date study author was asked for additional information (short summary) | Date study author provided data (short summary) |
| Thacher 2014 | 19 September 2017 | 22 September 2017 | 19 September 2017 – information on adverse events requested 7 December 2017 – data on healing of rickets requested 8 February 2017 – blinding, allocation concealment and loss to follow‐up | 19 September 2017 8 December 2017 No answer |
| Aggarwal 2013 | 8 January 2018 | 11 January 2018 | 31 January 2018 – information on blinding, study design and loss to follow‐up requested | 9 February 2018 |
| Balasubramanian 2003 | 15 November 2017 | 15 November 2017 | 15 November 2017 – information on blinding, healing of rickets and demographic data requested | No answer |
| Thacher 1999 | 19 September 2017 | 22 September 2017 | 19 September 2017 – baseline data requested 25 September 2017 – baseline data requested 8 February 2018 – risk of bias, loss to follow‐up and demographic data requested | 22 September 2017 25 September 2018 No answer |
Appendix 14. Checklist to aid consistency and reproducibility of GRADE assessments (vitamin D versus calcium)
| (1) Healing of rickets (normal alkaline phosphatase and bone radiograph at 24 weeks) | (2) Morbidity (fractures at 24 weeks) | (3) Adverse events | (4) All‐cause mortality | (5) Growth pattern | (6) Health‐related quality of life | (7) Socioeconomic effects | ||
| Study limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | NR | NR | NR | NR | NR |
| Was allocation concealment used (i.e. no potential for selection bias)? | Yes | Yes | ||||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Unclear | Unclear | ||||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | ||||||
| Was an objective outcome used? | Yes | Yes | ||||||
| Were more than 80% of participants enrolled in studies included in the analysis (i.e. no potential reporting bias)?b | Yes | Yes | ||||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Unclear | Unclear | ||||||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | ||||||
| Did the studies end up as scheduled (i.e. not stopped early)? | Yes | Yes | ||||||
| Inconsistencyc | Point estimates did not vary widely? | NA | NA | |||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap ≥ 1 of the included studies point estimate; some: confidence intervals overlap but not all overlap ≥ 1 point estimate; no: ≥ 1 outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | NA | NA | ||||||
| Was the direction of effect consistent? | NA | NA | ||||||
| What was the magnitude of statistical heterogeneity (as measured by I² statistic) low (I² < 40%), moderate (I² 40–60%), high I² > 60%)? | NA | NA | ||||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | NA | NA | ||||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | |||||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | ||||||
| Was the included outcome not a surrogate outcome? | Yes | Yes | ||||||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | ||||||
| Were the conclusions based on direct comparisons? | Yes | Yes | ||||||
| Imprecisiond | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | Yes | No (↓) | |||||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100–300 participants, low: <100 participants)?e | Low (↓) | Low (↓) | ||||||
| What was the magnitude of the number of included studies (large: >10 studies, moderate: 5–10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | ||||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | Yes | ||||||
| Publication biase | Was a comprehensive search conducted? | Yes | Yes | |||||
| Was grey literature searched? | Yes | Yes | ||||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | ||||||
| There was no industry influence on studies included in the review? | Yes | Yes | ||||||
| There was no evidence of funnel plot asymmetry? | NA | NA | ||||||
| There was no discrepancy in findings between published and unpublished studies? | Unclear | Unclear | ||||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual studies.
bDepends on the context of the systematic review area.
cQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² statistic. dWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. eQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished studies. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); NA: not applicable; NR: not reported. | ||||||||
Appendix 15. Checklist to aid consistency and reproducibility of GRADE assessments (vitamin D plus calcium versus vitamin D)
| (1) Healing of rickets (normal alkaline phosphatase and bone radiograph at 24 weeks) | (2) Morbidity (fractures at 24 weeks) | (3) Adverse events | (4) All‐cause mortality | (5) Growth pattern | (6) Health‐related quality of life | (7) Socioeconomic effects | ||
| Study limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Yes | NR | NR | NR | NR |
| Was allocation concealment used (i.e. no potential for selection bias)? | Yes | Yes | Yes | |||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Unclear | Unclear | No (↓) | |||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | |||||
| Was an objective outcome used? | Yes | Yes | Yes | |||||
| Were more than 80% of participants enrolled in studies included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | No (↓) | |||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Unclear | Unclear | Yes | |||||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | |||||
| Did the studies end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | |||||
| Inconsistencyb | Point estimates did not vary widely? | NA | NA | NA | ||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap ≥ 1 of the included studies point estimate; some: confidence intervals overlap but not all overlap ≥ 1 point estimate; no: ≥ 1 outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | NA | NA | NA | |||||
| Was the direction of effect consistent? | NA | NA | NA | |||||
| What was the magnitude of statistical heterogeneity (as measured by I² statistic) low (I² < 40%), moderate (I² 40–60%), high I² > 60%)? | NA | NA | NA | |||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | NA | NA | NA | |||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | ||||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | |||||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | |||||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | |||||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | |||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | Yes | No (↓) | No (↓) | ||||
| What is the magnitude of the median sample size (high: > 300 participants, intermediate: 100–300 participants, low: < 100 participants)?e | Low (↓) | Low (↓) | Low (↓) | |||||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5–10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | Small (↓) | |||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | Yes | Yes | Yes | |||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | ||||
| Was grey literature searched? | Yes | Yes | Yes | |||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | |||||
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | |||||
| There was no evidence of funnel plot asymmetry? | NA | NA | NA | |||||
| There was no discrepancy in findings between published and unpublished studies? | Unclear | Unclear | Unclear | |||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual studies.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² statistic. cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished studies. eDepends on the context of the systematic review area. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); NA: not applicable; NR: not reported. | ||||||||
Appendix 16. Checklist to aid consistency and reproducibility of GRADE assessments (vitamin D plus calcium versus calcium)
| (1) Healing of rickets (normal alkaline phosphatase and bone radiograph at 24 weeks) | (2) Morbidity (fractures at 24 weeks) | (3) Adverse events | (4) All‐cause mortality | (5) Growth pattern | (6) Health‐related quality of life | (7) Socioeconomic effects | ||
| Study limitations (risk of bias)a | Was random sequence generation used (i.e. no potential for selection bias)? | Unclear | Unclear | Yes | NR | NR | NR | NR |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Yes | Yes | |||||
| Was there blinding of participants and personnel (i.e. no potential for performance bias) or outcome not likely to be influenced by lack of blinding? | Unclear | Yes | No (↓) | |||||
| Was there blinding of outcome assessment (i.e. no potential for detection bias) or was outcome measurement not likely to be influenced by lack of blinding? | Yes | Yes | Yes | |||||
| Was an objective outcome used? | Yes | Yes | Yes | |||||
| Were more than 80% of participants enrolled in studies included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | No (↓) | |||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | No (↓) | Unclear | Yes | |||||
| No other biases reported (i.e. no potential of other bias)? | Yes | Yes | Yes | |||||
| Did the studies end up as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | |||||
| Inconsistencyb | Point estimates did not vary widely? | Yes | NA | NA | ||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap ≥ 1 of the included studies point estimate; some: confidence intervals overlap but not all overlap ≥ 1 point estimate; no: ≥ 1 outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Some | NA | NA | |||||
| Was the direction of effect consistent? | Yes | NA | NA | |||||
| What was the magnitude of statistical heterogeneity (as measured by I² statistic) low (I² < 40%), moderate (I² 40–60%), high I² > 60%)? | Low | NA | NA | |||||
| Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | NA | NA | |||||
| Indirectness | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | ||||
| Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Highly applicable | |||||
| Was the included outcome not a surrogate outcome? | Yes | Yes | Yes | |||||
| Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | |||||
| Were the conclusions based on direct comparisons? | Yes | Yes | Yes | |||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | No (↓) | No (↓) | No (↓) | ||||
| What is the magnitude of the median sample size (high: > 300 participants, intermediate: 100–300 participants, low: < 100 participants)?e | Intermediate | Low (↓) | Low (↓) | |||||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5–10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | Small (↓) | |||||
| Was the outcome a common event (e.g. occurred more than 1/100)? | Yes | Yes | Yes | |||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | Yes | ||||
| Was grey literature searched? | Yes | Yes | Yes | |||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | |||||
| There was no industry influence on studies included in the review? | Yes | Yes | Yes | |||||
| There was no evidence of funnel plot asymmetry? | NA | NA | NA | |||||
| There was no discrepancy in findings between published and unpublished studies? | Unclear | Unclear | Unclear | |||||
|
aQuestions on risk of bias are answered in relation to the majority of the aggregated evidence in the meta‐analysis rather than to individual studies.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I² statistic. cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished studies. eDepends on the context of the systematic review area. (↓): key item for potential downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); NA: not applicable; NR: not reported. | ||||||||
Data and analyses
Comparison 1. Vitamin D versus calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Healing of rickets (normal alkaline phosphatase and bone radiograph) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Proportion healed at 12 weeks | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.14, 3.94] |
| 1.2 Proportion healed at 24 weeks | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [1.59, 6.69] |
| 2 Healing of rickets (biochemical parameters). serum alkaline phosphatase | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Serum alkaline phosphatase at 12 weeks | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐36.65 [‐129.30, 56.00] |
| 2.2 Serum alkaline phosphatase at 24 weeks | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐148.0 [‐241.47, ‐54.53] |
| 3 Healing of rickets (biochemical parameters): serum 25‐OHD | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Serum 25‐OHD at 12 weeks | 2 | 107 | Mean Difference (IV, Random, 95% CI) | ‐8.45 [‐13.89, ‐3.02] |
| 3.2 Serum 25‐OHD at 24 weeks | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐14.0 [‐20.34, ‐7.66] |
| 4 Healing of rickets (radiological) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Radiographic score at 12 weeks (Thacher's 10‐point scale) | 2 | 107 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐1.15, 1.99] |
| 4.2 Radiographic score at 24 weeks (Thacher's 10‐point scale) | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.10, 0.10] |
| 5 Morbidity (fractures) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Vitamin D plus calcium versus vitamin D.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Healing of rickets (normal alkaline phosphatase and bone radiograph) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Proportion healed at 12 weeks | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [1.03, 9.77] |
| 1.2 Proportion healed at 24 weeks | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [1.49, 6.29] |
| 2 Healing of rickets (biochemical parameters): serum alkaline phosphatase | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Serum alkaline phosphatase at 12 weeks | 2 | 114 | Mean Difference (IV, Random, 95% CI) | ‐156.52 [‐245.10, ‐67.93] |
| 2.2 Serum alkaline phosphatase at 24 weeks | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐155.0 [‐242.73, ‐67.27] |
| 3 Healing of rickets (biochemical parameters): serum 25‐OHD | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Serum 25‐OHD at 12 weeks | 2 | 114 | Mean Difference (IV, Random, 95% CI) | 7.03 [‐3.00, 17.07] |
| 3.2 Serum 25‐OHD at 24 weeks | 1 | 75 | Mean Difference (IV, Random, 95% CI) | 6.0 [‐1.47, 13.47] |
| 4 Healing of rickets (radiological) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Radiological score at 12 weeks (Thacher's 10‐point scale) | 2 | 114 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.06, ‐0.39] |
| 4.2 Radiological score at 24 weeks (Thacher's 10‐point scale) | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.56, ‐0.44] |
| 5 Morbidity (fractures) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Vitamin D plus calcium versus calcium.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Healing of rickets (normal alkaline phosphatase and bone radiograph) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Proportion healed at 12 weeks | 3 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.25, 31.22] |
| 1.2 Proportion healed at 24 weeks | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.72, 1.90] |
| 2 Healing of rickets (biochemical parameters): serum alkaline phosphatase | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Serum alkaline phosphatase at 12 weeks | 3 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐109.73 [‐183.14, ‐36.32] |
| 2.2 Serum alkaline phosphatase at 24 weeks | 2 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐7.75 [‐73.20, 57.70] |
| 3 Healing of rickets (biochemical parameters): serum 25‐OHD | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Serum 25‐OHD at 12 weeks | 3 | 177 | Mean Difference (IV, Random, 95% CI) | 10.43 [‐0.81, 21.68] |
| 3.2 Serum 25‐OHD at 24 weeks | 2 | 140 | Mean Difference (IV, Random, 95% CI) | 13.21 [0.48, 25.94] |
| 4 Healing of rickets (radiological) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Radiological score at 12 weeks (Thacher's 10‐point scale) | 3 | 177 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐2.18, ‐0.42] |
| 4.2 Radiological score at 24 weeks (Thacher's 10‐point scale) | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐0.91, ‐0.21] |
| 5 Morbidity (fractures) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aggarwal 2013.
| Methods | Study design: parallel randomised controlled trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: children aged 6 months to 5 years with clinical and radiological features of nutritional rickets Exclusion criteria: participants with features suggestive of non‐nutritional aetiology (renal or hepatic disease, malabsorption states, antiepileptic drug intake or any chronic illness), cases presenting with hypocalcaemic seizures or with history of consuming calcium or vitamin D supplements in the preceding 6 months Diagnostic criteria: radiographs of left wrist and knee obtained and evaluated by 2 separate observers using the method developed by Thacher 2000 on a 0‐ to 10‐point scale. A radiological score of > 1.5 indicated rickets. Setting: hospital Age group: children, adolescents Gender distribution: girls and boys Country where study was performed: India |
|
| Interventions |
Interventions: I1: vitamin D 600,000 IU single intramuscular injection and elemental calcium 75 mg/kg orally in 3 divided doses per day for 12 weeks I2: elemental calcium 75 mg/kg orally in 3 divided doses per day for 12 weeks Comparator: 600,000 IU vitamin D single intramuscular injection Duration of intervention: 12 weeks Duration of follow‐up: 12 weeks Number of study centres: 1 Run‐in period: none Extension period: none |
|
| Outcomes |
Outcomes reported
|
|
| Study details |
Study terminated early: no Trial identifier: CTRI/2010/091/000448 |
|
| Publication details |
Language of publication: English Funding: no funding support Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote: "We endeavoured to study the effect of supplementation with calcium, vitamin D or a combination of these two on healing of nutritional rickets in Indian children." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote (trials register CTRI/2010/091/000448): "Computer generated randomization." Quote: "the subjects were randomized using block randomization to one of the following three treatment arms …" (p129, 'randomization and treatment allocation', paragraph 1). |
| Allocation concealment (selection bias) | Low risk |
Quote: "allocation concealment was achieved using opaque sealed envelopes" (p129, 'randomization and treatment allocation', paragraph 1). Quote (trials register): "Sequentially numbered, sealed, opaque envelopes." |
| Blinding of participants and personnel (performance bias) – adverse events | High risk | Comment: participants and personnel were not blinded (information obtained from study author), outcome measure likely influenced by lack of blinding. |
| Blinding of participants and personnel (performance bias) – healing of rickets | Low risk | Comment: participants and personnel were not blinded (information obtained from study author), outcome measure unlikely influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) – adverse events | Low risk | Quote: "Both the radiologist and the biochemist were blinded to treatment protocol" (p129, 'randomization and treatment allocation', paragraph 1). |
| Blinding of outcome assessment (detection bias) – healing of rickets | Low risk | Quote: "Both the radiologist and the biochemist were blinded to treatment protocol" (p129, 'randomization and treatment allocation', paragraph 1). |
| Incomplete outcome data (attrition bias) – adverse events | High risk | Comment: attrition rates high and disparate attrition rates between interventions (calcium + vitamin D 9.1%, calcium 22.7%, vitamin D 17.4%) with possible impact on outcome measure. |
| Incomplete outcome data (attrition bias) – healing of rickets | High risk | Comment: attrition rates high and disparate attrition rates between interventions (calcium + vitamin D 9.1%, calcium 22.7%, vitamin D 17.4%) with possible impact on outcome measure. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes stated in protocol were assessed in the manner prespecified in the protocol. |
| Other bias | Low risk | Comment: we did not identify additional sources of bias. |
Balasubramanian 2003.
| Methods | Study design: parallel randomised controlled trial, randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: children and adolescents clinically suspected to have rickets or osteomalacia, attending the Endocrinology Department, Sanjay Gandhi Postgraduate Institute and the Pediatrics Department and Rehabilitation and Artificial Limb Center, King George's Medical College, all in Lucknow (latitude 26°N) in northern India. Rickets/osteomalacia confirmed if characteristic radiological changes were present and SAP > 375 IU/L in children, this being 3 times the upper limit of the normal for adults. Exclusion criteria: evidence of renal or liver disease, history of treatment with anticonvulsants, treatment with vitamin D or calcium or for tuberculosis in preceding 3 months Diagnostic criteria: rickets/osteomalacia confirmed if characteristic radiological changes were present and SAP > 375 IU/L in children, this being 3 times the upper limit of the normal for adults Setting: hospital Age group: children, adolescents Gender distribution: girls and boys Country where study was performed: India |
|
| Interventions |
Intervention: elemental calcium 1 g as calcium carbonate orally in 3 divided doses per day and vitamin D either as 6000 IU orally per day for 3 months or 600,000 IU vitamin D in a single oral dose Comparator: elemental calcium 1 g as calcium carbonate orally in 3 divided doses per day for 3 months Duration of intervention: 3 months Duration of follow‐up: 3 months Number of study centres: 2 Run‐in period: none Extension period: none |
|
| Outcomes |
Outcome reported
|
|
| Study details |
Study terminated early: no Trial ID: not stated |
|
| Publication details |
Language of publication: English Funding: not stated Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote: "We conducted this study to characterize calcium intake, sun exposure, and serum vitamin D levels in subjects with and without rickets, and to elucidate the etiology of nutritional rickets in young children and adolescents in our region." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "For group 1 cases were randomised by numbers generated by a random number table to receive one of two treatment protocols'' (p202, 'Treatment and follow‐up') Quote: "Similar randomization was planned initially for group 3 … randomization was abandoned and all were given vitamin D …" Comment: group 1 (childhood rickets) = preadolescent children; group 2 (childhood controls) = children presenting to the paediatrics outpatient department for acute illnesses, such as upper respiratory tract infection; group 3 (adolescent rickets) = adolescents (boys with testicular volume ≥ 4 mL and girls with Tanner breast stage ≥ 2 and aged ≤ 20 years); group 4 (adolescents controls) = adolescents drawn from children attending the endocrine outpatient clinic for exogenous obesity or euthyroid goitre. Comment: 2 comparison groups without rickets or osteomalacia were studied (group 2 vs group 4) Comment: group 1 received calcium (10 participants, group 1A) or calcium + vitamin D (14 participants, group 1B) |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) – healing of rickets | Unclear risk | Comment: not described |
| Blinding of outcome assessment (detection bias) – healing of rickets | Unclear risk | Comment: not described |
| Incomplete outcome data (attrition bias) – healing of rickets | High risk |
Quote: "Of the 24 children in group 1, 14 presented for follow‐up at 1 month and eight at 3 months of therapy." Comment: attrition rate was high, total attrition was about 33%. In addition, attrition rates varied between the groups (calcium + vitamin D 71.4%, calcium 60%). Reasons for loss of participants in each group were not given (p204, 'follow up'). |
| Selective reporting (reporting bias) | High risk | Comment: data for radiological measurements not reported. |
| Other bias | Unclear risk | Comment: unclear whether there were any other sources of bias. Data for baseline characteristics of participants in groups 1A and 1B are not separated so it was not possible to assess if baseline imbalances existed across groups. |
Thacher 1999.
| Methods | Study design: parallel randomised controlled trial, randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: children with deformities characteristic of rickets (genu varum, genu valgum and widened wrists) were recruited within and around Jos, Nigeria (population 360,100), through posters, radio announcements and word of mouth. Each child was examined, and a parent or guardian was interviewed. Children aged 1 to 14 years and who had clinical evidence of rickets underwent radiography of the wrists and knees. Rickets was considered active if the epiphyseal plate was wider than normal and there was concave cupping or fraying of the metaphyseal margins on the radiographs. Exclusion criteria: not stated Diagnostic criteria: children were eligible for enrolment if they had a radiographic score of ≥ 2.5 on a validated 10‐point scoring method that assessed the severity of rickets in the growth plates of the distal radius and ulna and around the knee. Setting: hospital Age group: children, adolescents Gender distribution: girls and boys Country where study was performed: Nigeria |
|
| Interventions |
Interventions: I1: vitamin D 600,000 U intramuscular injection at enrolment and after 12 weeks + calcium carbonate 200 mg tablets – 2 tablets in the morning and 3 in the evening ≥ 30 minutes before eating (total dose, 1000 mg of elemental calcium daily) I2: calcium carbonate 200 mg chewable tablets – 2 tablets in the morning and 3 in the evening at least 30 minutes before eating (total dose, 1000 mg of elemental calcium daily) and an injection of placebo (light mineral oil) at enrolment and after 12 weeks Comparator: vitamin D 600,000 U intramuscular injection at enrolment and after 12 weeks and chewable placebo tablets (candy containing no calcium but similar in appearance to calcium tablets) – 2 in the morning and 3 in the evening ≥ 30 minutes before eating Duration of intervention: 24 weeks Duration of follow‐up: 24 weeks Number of study centres: unclear Run‐in period: none Extension period: none |
|
| Outcomes |
Outcomes reported
|
|
| Study details |
Study terminated early: no Trial ID: not stated |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding (supported by a grant from the Thrasher Research Fund, Salt Lake City) Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote: "We report the results of a 24‐week controlled trial to test the hypothesis that calcium supplementation with or without vitamin D is superior to vitamin D alone for the treatment of rickets in Nigerian children." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Eligible children were randomly assigned in blocks of nine to receive vitamin D, calcium, or both" (p564, 'Study Protocol'). Comment: no details |
| Allocation concealment (selection bias) | Low risk | Quote: "Medication kits were serially numbered and contained the complete 24‐week treatment for each child. The randomization code was kept at the University of Utah and was not broken until all data had been collected. The medications were dispensed in sealed, opaque packets, and vitamin D or placebo was administered intramuscularly on two occasions by a nurse while the investigators were in a different room" (p564, 'study protocol'). |
| Blinding of participants and personnel (performance bias) – growth pattern | Low risk |
Quote: "The medications were dispensed in sealed, opaque packets, and vitamin D or placebo was administered intramuscularly on two occasions by a nurse while the investigators were in a different room". (P564, study protocol). Comment: outcome measure unlikely influenced by potential lack of blinding. |
| Blinding of participants and personnel (performance bias) – healing of rickets | Low risk |
Quote: "The medications were dispensed in sealed, opaque packets, and vitamin D or placebo was administered intramuscularly on two occasions by a nurse while the investigators were in a different room. Children assigned to the vitamin D group received an intramuscular injection of 600,000 U of vitamin D at enrollment and after 12 weeks. They were also provided chewable placebo tablets (candy containing no calcium but similar in appearance to calcium tablets) and instructed to take two in the morning and three in the evening at least 30 minutes before eating. Children assigned to the calcium group were supplied chewable 200‐mg tablets of calcium carbonate and were instructed to take two tablets in the morning and three in the evening at least 30 minutes before eating (total dose, 1000 mg of elemental calcium daily). They were given an injection of placebo (light mineral oil) at enrollment and after 12 weeks. The combination‐therapy group received both vitamin D and calcium in the doses given above." (p564, 'study protocol'). Comment: outcome measure unlikely influenced by potential lack of blinding. |
| Blinding of participants and personnel (performance bias) – morbidity | Low risk |
Quote: "The medications were dispensed in sealed, opaque packets, and vitamin D or placebo was administered intramuscularly on two occasions by a nurse while the investigators were in a different room. Children assigned to the vitamin D group received an intramuscular injection of 600,000 U of vitamin D at enrollment and after 12 weeks. They were also provided chewable placebo tablets (candy containing no calcium but similar in appearance to calcium tablets) and instructed to take two in the morning and three in the evening at least 30 minutes before eating. Children assigned to the calcium group were supplied chewable 200‐mg tablets of calcium carbonate and were instructed to take two tablets in the morning and three in the evening at least 30 minutes before eating (total dose, 1000 mg of elemental calcium daily). They were given an injection of placebo (light mineral oil) at enrollment and after 12 weeks. The combination‐therapy group received both vitamin D and calcium in the doses given above" (p564, 'study protocol'). Comment: outcome measure unlikely influenced by potential lack of blinding. |
| Blinding of outcome assessment (detection bias) – growth pattern | Low risk | Comment: unclear who assessed growth pattern (height and weight); however, this is an objective measurement and is unlikely to be influenced by absence of blinding. |
| Blinding of outcome assessment (detection bias) – healing of rickets | Low risk |
Quote: radiographs: "Each radiograph was independently scored by three physicians who were unaware of the child's treatment assignment, and the mean value of the three scores was used for the analysis" (p564, 'Data and Sample Collection'). Biochemical: "Biochemical testing was performed by Associated Regional University Pathologists (Salt Lake City)" (p564, 'Biochemical Measurements'). |
| Blinding of outcome assessment (detection bias) – morbidity | Low risk | Comment: unclear who assessed morbidity; however, in the case of this study, the outcome (fractures) was an objective measurement and unlikely to be influenced by absence of blinding. |
| Incomplete outcome data (attrition bias) – adverse events | Unclear risk | Comment: attrition rates differed between intervention groups (calcium + vitamin D 5%, calcium + placebo 16.7%, vitamin D + placebo 9.8%). Unclear whether the outcome measure was influenced by these attrition rates. |
| Incomplete outcome data (attrition bias) – growth pattern | Unclear risk | Comment: attrition rates differed between intervention groups (calcium + vitamin D 5%, calcium + placebo 16.7%, vitamin D + placebo 9.8%). Unclear whether the outcome measure was influenced by these attrition rates. |
| Incomplete outcome data (attrition bias) – healing of rickets | Unclear risk | Comment: attrition rates differed between intervention groups (calcium + vitamin D 5%, calcium + placebo 16.7%, vitamin D + placebo 9.8%). Unclear whether the outcome measure was influenced by these attrition rates. |
| Incomplete outcome data (attrition bias) – morbidity | Unclear risk | Comment: attrition rates differed between intervention groups. Unclear whether the outcome measure was influenced by these attrition rates. |
| Selective reporting (reporting bias) | Unclear risk | Comment: protocol for study not available. |
| Other bias | Low risk | Comment: no other sources of bias were identified. |
Thacher 2014.
| Methods | Study design: parallel randomised controlled trial, randomisation ratio 2:1 | |
| Participants |
Inclusion criteria: children with radiographic score ≥ 2.5 on a validated 10‐point scoring method that assessed the severity of rickets in the growth plates of the distal radius and ulna and around the knee Exclusion criteria: not specifically stated Diagnostic criteria: radiographic score ≥ 2.5 on a validated 10‐point scoring method that assessed the severity of rickets in the growth plates of the distal radius and ulna and around the knee Setting: hospital Age group: children, adolescents Gender distribution: girls and boys Country where study was performed: Nigeria |
|
| Interventions |
Intervention: oral calcium, 3.5 g powdered limestone (containing approximately 938 mg of elemental calcium) twice daily + vitamin D2 50,000 IU (ergocalciferol) orally once every 4 weeks for 24 weeks Comparator: oral calcium, 3.5 g powdered limestone (containing approximately 938 mg of elemental calcium) twice daily + placebo, which was a single vitamin B complex tablet, once every 4 weeks for 24 weeks Duration of intervention: 24 weeks Duration of follow‐up: 24 weeks Number of study centres: 1 Run‐in period: none Extension period: none |
|
| Outcomes |
Outcomes reported
|
|
| Study details |
Study terminated early: no Trial ID: NCT00949832 |
|
| Publication details |
Language of publication: English Funding: non‐commercial funding (this study was supported by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research.) Publication status: peer‐reviewed journal |
|
| Stated aim for study | Quote: "The objective of this randomised controlled trial was to compare the response of rickets to calcium treatment as limestone with and without vitamin D supplementation." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Enrolled children were randomised by coin toss (performed by (TDT)" (p808, 'Intervention'). |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment not described. |
| Blinding of participants and personnel (performance bias) – growth pattern | Low risk | Comment: unclear whether participants and personnel were blinded, outcome measure unlikely influenced by potential lack of blinding. |
| Blinding of participants and personnel (performance bias) – healing of rickets | Low risk | Comment: unclear whether participants and personnel were blinded, outcome measure unlikely influenced by potential lack of blinding. |
| Blinding of outcome assessment (detection bias) – growth pattern | Low risk | Comment: outcome measure unlikely influenced by potential lack of blinding. |
| Blinding of outcome assessment (detection bias) – healing of rickets | Low risk |
Quote: Radiographic assessment: "All radiographs were scored independently by 2 of the authors (TDT and PRF)" (p808, 'Statistical analysis'). Biochemical assessment: "Serum samples were stored at –20 °C until transported frozen to the Mayo Clinic for analysis" (p808, 'Intervention', 2nd paragraph). Comment: outcome measure unlikely influenced by potential lack of blinding. |
| Incomplete outcome data (attrition bias) – growth pattern | Unclear risk | Comment: proportion of participants lost to follow‐up was higher in the calcium group (calcium + vitamin D 2.3%, calcium + placebo 10.7%), unclear whether outcome was influenced by attrition rate. |
| Incomplete outcome data (attrition bias) – healing of rickets | Unclear risk | Comment: proportion of participants lost to follow‐up was higher in the calcium group (calcium + vitamin D 2.3%, calcium + placebo 10.7%), unclear whether outcome was influenced by attrition rate. |
| Selective reporting (reporting bias) | High risk |
Comment: primary outcome was stated as 'combined attainment of a radiographic score of ≤ 1.5 and a SAP concentration of ≤ 350 U/L in the publication' but as 'XR [radiological] healing of rickets' in ClinicalTrials.gov. Comment: adverse events and growth pattern were associated with high risk of outcome reporting bias in ORBIT (Outcome Reporting Bias In Trials), see Appendix 8. |
| Other bias | Low risk | Comment: no other bias detected. |
SAP: serum alkaline phosphatase. Note: where the judgement is 'Unclear' and the description is blank, the study did not report that particular outcome.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arya 2000 | Not a randomised trial but a commentary on 1 of the included studies. |
| Atabek 2013 | Open‐label non‐randomised prospective trial. |
| Barr 1969 | Not a randomised controlled trial. |
| Curtis 1983 | Not a randomised controlled trial. |
| Davis 1978 | Not a randomised controlled trial. |
| Kutluk 2002 | Quasi‐randomised controlled trial. |
| NCT01512537 | Intervention was ultraviolet radiation compared with calcium + vitamin D. |
| Rooze 2016 | Not a randomised controlled trial; participants not children with nutritional rickets. |
| Zauche 2017 | Case study. Not a randomised controlled trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
El'chaninov 1969.
| Methods | Unknown |
| Participants | Unknown |
| Interventions | Unknown |
| Outcomes | Unknown |
| Study details | Unknown |
| Publication details | Unknown |
| Stated aim of study | Unknown |
| Notes | We currently cannot obtain a copy of this publication; it is probably a conference paper ('The effect of videin D3 therapy of children with rickets'). |
Differences between protocol and review
We did not search Embase, as specified in the protocol (Chibuzor 2017), because this database is no longer available to us. Randomised controlled trials indexed in Embase are now prospectively added to CENTRAL via a highly sensitive screening process.
We did not perform subgroup or sensitivity analyses or draw funnel plots due to the limited number of studies.
Contributions of authors
All review authors contributed to, read and approved the final review draft.
Moriam T Chibuzor (MC): protocol draft, acquisition of study reports, study selection, data extraction, data analysis, data interpretation, review of drafts and review updates.
Diepiriye Graham‐Kalio (DG): protocol draft, acquisition of study reports, study selection, data extraction, data analysis, data interpretation and review updates.
Joy O Osaji (JO): protocol draft, acquisition of study reports, study selection, data extraction and review updates.
Martin M Meremikwu (MM): protocol draft, data analysis, data interpretation and review updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Effective Health Care Research Consortium, UK.
Grant: HRPC09 Evidence Building and Synthesis Research
Declarations of interest
MC: This review was partly funded by a grant from Department for International Development (DFID) through UKAid funding provided to the Effective Health Care Research Consortium at the Liverpool School of Tropical Medicine.
DG: none known.
JO: none known.
MM: none known.
New
References
References to studies included in this review
Aggarwal 2013 {published and unpublished data}
- Aggarwal V, Seth A, Marwaha RK, Sharma B, Sonkar P, Singh S, et al. Management of nutritional rickets in Indian children: a randomized controlled trial. Journal of Tropical Pediatrics 2013;59(2):127‐33. [DOI] [PubMed] [Google Scholar]
- CTRI/2010/091/000448. Role of calcium and vitamin D in nutritional rickets and its management. ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=1634&EncHid=&modid=&compid=%27,%271634det%27 (first received 1 October 2010).
- Marwaha RK, Aggarwal V, Seth A, Sonkar P, Aneja S, Sharma B, et al. Role of calcium and vitamin D in the etiology and management of nutritional rickets. Osteoporosis International 2011;22:S730. [Google Scholar]
Balasubramanian 2003 {published data only}
- Balasubramanian K, Rajeswari J, Govil YC, Agarwal AK, Kumar A, Bhatia V. Varying role of vitamin D deficiency in the etiology of rickets in young children vs. adolescents in northern India. Journal of Tropical Pediatrics 2003;49(4):201‐6. [DOI] [PubMed] [Google Scholar]
Thacher 1999 {published and unpublished data}
- Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Reading JC, et al. A comparison of calcium, vitamin D or both for nutritional rickets in Nigerian children. New England Journal of Medicine 1999;341(8):563‐8. [DOI] [PubMed] [Google Scholar]
Thacher 2014 {published and unpublished data}
- NCT00949832. Vitamin D and genetics in nutritional rickets. clinicaltrials.gov/ct2/show/NCT00949832 (first received 30 July 2009).
- Thacher TD, Fischer PR, Pettifor JM. Vitamin D treatment in calcium‐deficiency rickets: a randomised controlled trial. Archives of Disease in Childhood 2014;99:807‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Arya 2000 {published data only}
- Arya V. Calcium and the treatment of nutritional rickets. National Medical Journal of India 2000;13(1):23. [PubMed] [Google Scholar]
Atabek 2013 {published data only}
- Atabek ME. How should we manage vitamin D‐deficient adolescents?. Journal of Pediatric Endocrinology and Metabolism 2013;26(9‐10):1009‐10. [DOI] [PubMed] [Google Scholar]
Barr 1969 {published data only}
- Barr DG, Forfar JO. Oral calcium‐loading test in rickets and in neonatal tetany: effect of vitamin D. British Medical Journal 1969;3:150‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtis 1983 {published data only}
- Curtis JA, Kooh SW, Fraser DO, Greenberg M. Nutritional rickets in vegetarian children. Canadian Medical Association Journal 1983;128(2):150. [PMC free article] [PubMed] [Google Scholar]
Davis 1978 {published data only}
- Davies DP, Hughes CA, Moore JR. Rickets in preterm infants. Archives of Disease in Childhood 1978;53(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kutluk 2002 {published and unpublished data}
- Kutluk G, Çetinkaya F, Basak M. Comparisons of oral calcium, high dose vitamin D and a combination of these in the treatment of nutritional rickets in children. Journal of Tropical Pediatrics 2002;48(6):351‐3. [DOI] [PubMed] [Google Scholar]
NCT01512537 {published data only}
- NCT01512537. Alternative treatments of vitamin D deficiency. clinicaltrials.gov/ct2/show/NCT01512537 (first received 19 January 2012).
Rooze 2016 {published data only}
- Rooze S, Mathieu F, Claus W, Yangzom T, Yangzom D, Goyens P, et al. Effect of calcium and vitamin D on growth, rickets and Kashin–Beck disease in 0‐ to 5‐year‐old children in a rural area of central Tibet. Tropical Medicine & International Health 2016;21(6):768‐75. [DOI] [PubMed] [Google Scholar]
Zauche 2017 {published data only}
- Zauche LH. Rickets: not just a disease caused by vitamin D deficiency. Journal of Pediatric Health Care 2017;31(2):235‐40. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
El'chaninov 1969 {published data only}
- El'chaninov GM. The effect of videin D3 therapy of children with rickets. Voprosy Okhrany Materinstva i Detstva 1969;14(2):91. [PubMed] [Google Scholar]
Additional references
Agarwal 2009
- Agarwal A, Gulati D, Rath S, Walia M. Rickets: a cause of delayed walking in toddlers. Indian Journal of Pediatrics 2009;76(3):269‐72. [DOI] [PubMed] [Google Scholar]
Allgrove 2004
- Allgrove J. Is nutritional rickets returning?. Archives of Disease in Childhood 2004;89(1):699‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [PUBMED: 12543843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balasubramanian 2013
- Balasubramanian S, Dhanalakshmi K, Amperayani S. Vitamin D deficiency in childhood – a review of current guidelines on diagnosis and management. Indian Pediatrics 2013;50(7):669‐75. [DOI] [PubMed] [Google Scholar]
Bell 2013
- Bell ML, McKenzie JE. Designing psycho‐oncology randomised trials and cluster randomised trials: variance components and intra‐cluster correlation of commonly used psychosocial measures. Psycho‐oncology 2013;22(8):1738‐47. [DOI] [PubMed] [Google Scholar]
Borenstein 2017a
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta‐analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5‐18. [DOI] [PubMed] [Google Scholar]
Borenstein 2017b
- Borenstein M. Prediction intervals. www.meta‐analysis.com/prediction (accessed 3 July 2017).
Boutron 2014
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32(36):4120‐6. [DOI] [PubMed] [Google Scholar]
Buch 2011
- Buch MH, Aletaha D, Emery P, Smolen JS. Reporting of long‐term extension studies: lack of consistency calls for consensus. Annals of the Rheumatic Diseases 2011;70(6):886‐90. [DOI] [PubMed] [Google Scholar]
Callaghan 2006
- Callaghan AL, Moy RJ, Booth IW, Debelle G, Shaw NJ. Incidence of symptomatic vitamin D deficiency. Archives of Disease in Childhood 2006;91(7):606‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cesur 2003
- Cesur Y, Caksen H, Gündem A, Kirimi E, Odabaş D. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. Journal of Pediatric Endocrinology and Metabolism 2003;16(6):1105‐9. [DOI] [PubMed] [Google Scholar]
Cochrane 2018
- Cochrane. CENTRAL creation details. www.cochranelibrary.com/central/central‐creation (accessed 21 August 2018).
CONSORT
- The CONSORT statement. www.consort‐statement.org (accessed 19 May 2016).
Corbett 2014
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5(1):79‐85. [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta‐analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
GRADEpro GDT 2015 [Computer program]
- McMaster University, 2015 (developed by Evidence Prime, Inc.). GRADEpro GDT. Version accessed 28 February 2017. Hamilton (ON): McMaster University, 2015 (developed by Evidence Prime, Inc.), 2015.
Greenbaum 2011
- Greenbaum LA. Rickets and hypervitaminosis D. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF editor(s). Nelson Textbook of Pediatrics. 19th Edition. Philadelphia (PA): Elsevier Saunders, 2011. [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hochberg 2003
- Hochberg Z. Endocrine development. Vitamin D and Rickets. Vol. 6, Basel: Karger, 2003:220‐32. [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI: 10.1186/1471-2288-5-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jessop 1950
- Jessop WJ. Results of rickets surveys in Dublin. British Journal of Nutrition 1950;4(2‐3):289‐92. [DOI] [PubMed] [Google Scholar]
Jones 2015
- Jones CW, Keil LG, Holland WC, Caughey MC, Platts‐Mills TF. Comparison of registered and published outcomes in randomized controlled trials: a systematic review. BMC Medicine 2015;13:282. [DOI: 10.1186/s12916-015-0520-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Leclercq 2013
- Leclercq E, Leeflang MM, Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies in PubMed. Journal of Pediatrics 2013;162(3):629‐34. [PUBMED: 23084708] [DOI] [PubMed] [Google Scholar]
Lerch 2007
- Lerch C, Meissner T. Interventions for the prevention of nutritional rickets in term born children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006164.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathieu 2009
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302(9):977‐84. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI: 10.1186/2046-4053-3-82] [DOI] [PMC free article] [PubMed] [Google Scholar]
Megan 2012
- Megan B, Pickering RM, Weatherall M. Design, objectives, execution and reporting of published open‐label extension studies. Journal of Evaluation in Clinical Practice 2012;18(2):209‐15. [DOI] [PubMed] [Google Scholar]
Misra 2008
- Misra M, Pacaud D, Petryk A, Collett‐Solberg PF, Kappy M. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Paediatrics 2008;122(2):398‐417. [DOI] [PubMed] [Google Scholar]
Munns 2016
- Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. Hormone Research in Paediatrics 2016;85(2):83‐106. [DOI] [PubMed] [Google Scholar]
Nield 2006
- Nield LS, Mahajan P, Joshi A, Kamat D. Rickets: not a disease of the past. American Family Physician 2006;74(4):619‐26. [PubMed] [Google Scholar]
Ozkan 2010
- Ozkan B. Nutritional rickets. Journal of Clinical Research in Pediatric Endocrinology 2010;2(4):137‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pal 2001
- Pal BR, Shaw NJ. Rickets resurgence in the United Kingdom: improving antenatal management in Asians. Journal of Pediatrics 2001;139(2):337‐8. [DOI] [PubMed] [Google Scholar]
Pettifor 2004
- Pettifor JM. Nutritional rickets: deficiency of vitamin D, calcium, or both?. American Journal of Clinical Nutrition 2004;80(6 Suppl):1725S‐9S. [DOI] [PubMed] [Google Scholar]
Pettifor 2005
- Pettifor JM. Rickets and vitamin D deficiency in children and adolescents. Endocrinology and Metabolism Clinics of North America 2005;34(3):537‐53. [DOI] [PubMed] [Google Scholar]
Pettifor 2012
- Pettifor JM. Nutritional rickets. In: Glorieux FH, Pettifor JM, Juppner H editor(s). Pediatric Bone. 2nd Edition. London: Elsevier, 2012:625‐54. [Google Scholar]
Pettifor 2014a
- Pettifor JM. How should we manage vitamin D deficiency rickets?. Indian Pediatrics 2014;51(4):259‐60. [DOI] [PubMed] [Google Scholar]
Pettifor 2014b
- Pettifor JM. International perspectives of rickets. 2014 Meet‐The‐Professor: Endocrine Case Management. Washington (DC): Endocrine Society, 2014:45‐9. [Google Scholar]
Pfitzner 1988
- Pfitzner MA, Thacher TF, Pettifor JM, Zoakah AI, Lawson JO, Isichei CO. Absence of vitamin D deficiency in young Nigerian children. Journal of Pediatrics 1998;133(6):740‐4. [DOI] [PubMed] [Google Scholar]
Prentice 2008
- Prentice A. Vitamin D deficiency: a global perspective. Nutrition Reviews 2008;66(Suppl 2):S153‐64. [DOI] [PubMed] [Google Scholar]
Reddy 2008
- Reddy V, Lamb WH. Specific vitamin deficiencies. In: Stanfield P, Brueton M, Chan M, Parkin M, Waterson T editor(s). Diseases of Children in the Subtropics and Tropics: a Global Text. 4th Edition. Zurich (Switzerland): Jacobs Foundation, 2008:376‐8. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Ross 2011
- Ross AC, Taylor CL, Yaktine AL, Valle HB. Committee to review dietary reference intakes for vitamin D and calcium. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academy Press, 2011. [PubMed] [Google Scholar]
Scherer 2018
- Scherer RW, Meerpohl JJ, Pfeifer N, Schmucker C, Schwarzer G, Elm E. Full publication of results initially presented in abstracts. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.MR000005.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwalfenberg 2007
- Schwalfenberg G. Not enough vitamin D: health consequences for Canadians. Canadian Family Physician 2007;53(5):841‐54. [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the confidence in or quality of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Shaw 2004
- Shaw NJ, Kershaw M. Vitamin D deficiency in children. In: David TJ editor(s). Recent Advances in Paediatrics. London: Royal Society of Medicine Press, 2004:85‐100. [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Thacher 2000
- Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Manaster BJ, Reading JC. Radiographic scoring method for the assessment of the severity of nutritional rickets. Journal of Tropical Pediatrics 2000;46(3):132‐9. [DOI] [PubMed] [Google Scholar]
Thacher 2003
- Thacher TD. Calcium‐deficiency rickets. Endocrine Development 2003;6:105‐25. [DOI] [PubMed] [Google Scholar]
Thacher 2006
- Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Annals of Tropical Paediatrics 2006;26(1):1‐16. [DOI] [PubMed] [Google Scholar]
UNICEF 2015
- United Nations Children's Fund. Preventing bone disorders through better nutrition in Bangladesh. www.unicef.org/infobycountry/bangladesh_51457.html (accessed 30 March 2015).
Weisberg 2004
- Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. American Journal of Clinical Nutrition 2004;80(6 Suppl):1697S‐705. [DOI] [PubMed] [Google Scholar]
Welch 2000
- Welch TR, Bergstrom WH, Tsang RC. Vitamin D‐deficient rickets: the re‐emergence of a once‐conquered disease. Journal of Paediatrics 2000;137(2):143‐5. [DOI] [PubMed] [Google Scholar]
Wendling 2007
- Wendling P. Preventable nutritional rickets stages reemergence. Rheumatology News 2007;6(10):32. [Google Scholar]
Wong 2006
- Wong SS, Wilczynski NL, Haynes RB. Comparison of top‐performing search strategies for detecting clinically sound treatment studies and systematic reviews in MEDLINE and EMBASE. Journal of the Medical Library Association 2006;94(4):451‐5. [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Chibuzor 2017
- Chibuzor MT, Graham‐Kalio D, Meremikwu MM, Adukwu JO. Vitamin D, calcium or a combination of vitamin D and calcium for the treatment of nutritional rickets in children. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD012581] [DOI] [PMC free article] [PubMed] [Google Scholar]


